1. Introduction
The Black Vulture (Coragyps atratus) and Turkey Vulture (Cathartes aura) play a critical ecological role as obligate scavengers [
1]. These birds consume carcasses. In doing so, they perform a vital ecological function by removing decomposing organic matter. This scavenging behavior helps prevent the accumulation of pathogens in the environment. As a result, they reduce disease transmission risks for both wildlife and humans [
2,
3]. Although these species often share the same habitats, their foraging strategies differ significantly. Anatomical and behavioral studies suggest that Turkey Vultures rely primarily on a well-developed sense of smell to locate carrion [
4,
5]. In contrast, Black Vultures depend more on sharp vision and social cues, often following other Black Vultures or Turkey Vultures to a carcass [
6,
7]. However, Turkey Vultures also possess acute vision comparable to that of Black Vultures [
8], and Black Vultures can detect carrion by smell at distances up to 50 meters [
9].
Building on their distinct sensory strategies, it is paradoxical that the same behavioral and physiological traits that help vultures thrive also contribute to conflict in human-modified environments. Black and Turkey Vultures are among the few vulture species whose populations are currently increasing worldwide [
2]. With minimal predation pressure, vultures exhibit limited anti-predator behaviors, typically restricted to death-feigning (thanatosis), in which the bird becomes motionless to mimic death, and chemically deterrent regurgitation, releasing foul-smelling gastric contents to repel threats [
10,
11,
12,
13]. These traits reflect their ecological niche and have likely contributed to their survival and increasing population numbers in human-modified environments. As their numbers grow, their large body size, flocking behavior, and soaring flight patterns, often concentrated near landfills, roadways, and open fields, heighten the risk of aircraft collisions [
14,
15,
16]. Such events often result in serious injuries, underscoring the need for both veterinary intervention and preventative research. One promising approach involves studying their visual systems through electroretinography (ERG), which provides insight into sensory processing [
17]. Because ERG requires prolonged immobilization, developing a safe and reliable anesthetic protocol is essential for advancing both clinical care and research.
The avian brain has followed a distinct evolutionary trajectory, diverging from the mammalian lineage for over 300 million years. Despite this divergence, birds retain a complex neural architecture capable of supporting consciousness, the principal target of general anesthetic drugs [
19,
20]. Consciousness, in the context of anesthesia, refers to the brain’s capacity to integrate and respond to sensory input [
19,
20]. This includes proprioception, muscle tone, and protective reflexes against noxious stimuli, all of which reflect coordinated activity across multiple neurochemical systems [
19,
20]. These functions are maintained by foundational neurotransmitter pathways, GABAergic (gamma-aminobutyric acid), adrenergic, opioidergic, and glutamatergic systems, that are conserved across vertebrate taxa [
19,
20,
21,
22,
23,
24,
25]. Anesthetic agents act on these systems to disrupt consciousness and produce the desired clinical endpoints: unconsciousness, muscle relaxation, antinociception, and amnesia [
19,
20]. Because each anesthetic class targets a specific neurotransmitter pathway, a multimodal approach, combining agents from different pharmacologic classes, can create synergistic effects that more reliably achieve these endpoints.
Multimodal anesthesia thus offers a strategic advantage: it allows for lower doses of individual drugs, reducing side effects while enhancing physiological stability [
20].
In contrast, mono-modal techniques, often relying on high concentrations of inhalants, can lead to pronounced cardiorespiratory depression, including bradycardia, hypotension, and respiratory compromise [
18]. Attempts to mitigate these effects by lowering inhalant doses may induce significant oscillations in anesthetic depth, resulting in the so-called “motor-boating” effect, characterized by fluctuating cardiovascular depression and unstable anesthesia planes [
18]. This instability demands constant vigilance, monitoring, and rapid intervention.
A review of the veterinary literature reveals a notable lack of information on effective anesthetic protocols for Black Vultures, Turkey Vultures, and related species [
18]. Given their unique physiology and the evolutionary conservation of key neurotransmitter systems, it is essential to evaluate anesthetic strategies that engage these pathways in a balanced, multimodal fashion. Doing so will not only improve clinical outcomes but also support ethical research practices by ensuring predictable and safe anesthetic responses in New World vultures.
The primary objective of this study was to evaluate the efficacy and safety of a specific multimodal anesthetic protocol in adult Black Vultures (Coragyps atratus) and Turkey Vultures (Cathartes aura), and to characterize its associated cardiorespiratory effects. The protocol involved injectable premedication with dexmedetomidine, midazolam, butorphanol, and ketamine (DMBK), followed by induction and maintenance using isoflurane.
To support this objective, the study pursued several focused aims centered on protocol assessment and species-specific characterization. These included developing a quantifiable scoring system to objectively evaluate sedation quality and recovery, monitoring key cardiorespiratory parameters including heart rate (HR), respiratory rate (RR), blood pressure (BP), and oxygen saturation (SpO2) throughout the anesthetic period, determining the isoflurane concentration required to maintain a stable plane of immobilization, and comparing physiological responses and anesthetic requirements between Black and Turkey Vultures to identify clinically relevant, species-specific considerations.
3. Results
During initial pre-sedation handling, individual vultures exhibited a range of alert behaviors, spanning from overt defensiveness to active aggression. Common responses included biting at the handler’s leather gloves, vigorous wing flapping, forceful leg kicking, pronounced postural tension, and low-pitched cooing vocalizations. These behaviors reflected heightened arousal and stress induced by physical restraint, indicating a strong aversive response to handling.
Five of the fifteen vultures (33%) displayed liquid regurgitation and/or defecation responses. These behaviors ceased upon the onset of sedation, frequently occurring within three minutes following IM injection. All vultures became profoundly sedated at the end of the 15 minutes, with a uniformly smooth transition and final sedation scores of 4 (Good) or 5 (Excellent) based on a standardized scoring system. This level of sedation allowed for calm and cooperative handling during transport to the procedure room. No discernible differences were noted between Black Vultures and Turkey Vultures in their awake behavior, response to sedation, or baseline HR.
Although sedation facilitated easy beak manipulation and a clear view of the glottis, initial intubation attempts were unsuccessful in all vultures due to a strong swallowing reflex triggered by the endotracheal tube. To proceed, anesthesia was induced via face mask with isoflurane, resulting in a rapid onset (1–2 minutes), successful intubation, and a smooth transition to maintenance anesthesia.
The mean body weights for Black and Turkey Vultures were similar, and no statistically significant differences were found between the species for induction score, endotracheal tube size, total anesthesia time, ERG recording time, or recovery score (
Table 1). The median Et-Iso required for maintenance during the ERG procedures was 1.4% across the study.
Awake baseline HR and RR were measured in eleven Black Vultures and four Turkey Vultures, with no statistically significant differences observed between species (
Table 1). Following DMBK administration, HR decreased significantly from baseline in both Black Vultures (F33,261 = 16.94, p < 0.001) and Turkey Vultures (F35,76 = 5.27, p < 0.001). This significant reduction was evident immediately at induction and persisted throughout the entire monitoring period (
Table 2).
After induction, all vultures were transitioned to pressure-controlled mechanical ventilation (10 breaths/min; 6 cmH2O PIP). This intervention also caused a statistically significant reduction in RR from baseline in both species, stabilizing at an estimated marginal mean of 7–13 breaths per minute in Black Vultures (F33,262 = 88.7, p < 0.001) and 8–12.5 breaths per minute in Turkey Vultures (F35,75 = 14.9, p < 0.001). No interspecies differences in RR were noted during ventilation, as both groups were maintained on identical settings, though this comparison was not statistically tested.
A detailed table for RR overtime, analogous to
Table 2 for HR, is not presented. As all vultures were transitioned to pressure-controlled mechanical ventilation, the RR was externally controlled and remained stable throughout the anesthetic period. This is consistent with the estimated marginal means (7–13 breaths/min for Black Vultures; 8–12.5 breaths/min for Turkey Vultures), which were, as noted above, significantly lower than awake baseline values.
The statistical results from the general linear mixed models are presented in
Table 3, with the corresponding model-predicted values (Estimated Marginal Means) detailed in
Table 4.
Analysis showed that oxygen saturation (SpO
2,
Supplementary Figure S2-e) remained stable and consistent over time and between species, with values consistently maintained near 100%. Other variables demonstrated significant changes over time. End-tidal carbon dioxide, for instance, showed no species-specific difference but increased steadily in both groups throughout the procedure (
Supplementary Figure S2-f). Similarly, body temperature exhibited a significant decline over time in both species. While a visual trend suggested that Turkey Vulture temperatures dropped more rapidly (
Supplementary Figure S2-d), this interaction was not statistically significant.
Most notably, a highly significant Time × Species interaction was observed for all three blood pressure parameters: systolic, diastolic, and mean. This interaction indicates that cardiovascular trends diverged over time; pressures in Turkey Vultures tended to decrease, whereas pressures in Black Vultures tended to increase (
Supplementary Figure S2-a–c). Statistical significance for these effects is summarized in
Table 3.
The analysis of blood pressure also revealed more complex, species-specific responses. No significant differences were observed between groups for systolic or mean arterial pressure. However, Turkey Vultures maintained a significantly higher overall diastolic blood pressure throughout the procedures, as reflected in the mean values (
Table 4).
4. Discussion
This study found that DMBK premedication, followed by isoflurane maintenance, provides an effective anesthetic regimen for both Black and Turkey Vultures, characterized by stable cardiorespiratory parameters and smooth recoveries.
The 12–16-hour fasting period used in this study appeared appropriate, as no regurgitation occurred during premedication, anesthesia, or recovery. Regurgitant observed during initial awake handling consisted primarily of liquid with minimal solid content, suggesting effective gastric emptying. Captive vultures in this study consumed 6 to 8 oz (approximately 170–225 grams) of meat daily, provided
ad libitum. Vultures of both species are capable of fasting for several days without deleterious effect [
25,
26]. Although gastric emptying times are not well documented in either species, their unique physiology, including a large crop and gorge-famine feeding behavior [
3], may predispose them to perioperative regurgitation via drug-induced emesis or anesthetic-related esophageal relaxation. In this context, appropriate fasting and maintenance of anesthetic depth are critical to minimizing aspiration risk, especially given that endotracheal tube cuffs were intentionally left deflated.
Vultures are also known to regurgitate crop contents as a potent “fight or flight” mechanism [
12,
13,
27]. In this study, 33% of the combined cohort exhibited such behavior during initial physical restraint. However, no regurgitation occurred after IM injection, indicating that the sedative and anxiolytic effects of the drug combination were sufficient to suppress this stress-induced response. The absence of regurgitation during the anesthetic period further supports the safety and efficacy of the protocol in managing both physiological and behavioral risks during immobilization.
A key finding was that the vultures in this study required notably lower drug dosages than those typically reported for other raptors. For instance, while published doses for dexmedetomidine in raptors range from 25–75 µg/kg [
28], the vultures in this study were effectively sedated with only 5 µg/kg. Similarly, recommended ketamine doses often range from 10–20 mg/kg [
18], whereas this study used 5 mg/kg. Midazolam is frequently used at 0.25–0.5 mg/kg (18), but a dose of 0.2 mg/kg was sufficient here. Finally, butorphanol is commonly cited at 1–6 mg/kg for analgesia in raptors [
29], a range substantially higher than the 0.2 mg/kg used in this protocol.
Despite these low doses, the protocol provided safe and effective sedation. This suggests the synergistic activity of this multimodal combination reduced the required dose of each agent, that these vulture species are inherently more sensitive to these drugs than other raptors, or a combination of both factors.
In this study, intravenous (IV) catheter placement and fluid administration were left out following risk-benefit analysis tailored to the species, procedure, and patient condition. All vultures were classified as ASA I (healthy) and confirmed to be well-hydrated prior to the 12–16-hour fasting period. Given that ERG is a non-invasive procedure with no blood loss and minimal fluid loss, the potential risks of catheterization, including hematoma formation, vessel thrombosis, and infection, were deemed to outweigh the limited benefits of prophylactic fluid therapy. The procedure duration was approximately two hours, and all patients remained clinically stable throughout. Cardiovascular parameters, particularly BP, were consistently within normal limits during anesthesia, indicating no evidence of hypotension or compromise. The absence of adverse effects or hemodynamic instability suggests that omitting IV fluids and catheterization did not result in any clinically significant or catastrophic outcome and supports that this is an alternative approach for non-invasive procedures in healthy avian patients.
A critical component of this protocol was the management of the avian respiratory system, which differs markedly from that of mammals [
18]. Unlike the elastic lungs of mammals, vultures possess rigid pulmonary structures ventilated by a series of air sacs [
18,
30,
31]. Under anesthesia, spontaneous breathing in birds often leads to hypoventilation and variable elevations in arterial carbon dioxide, which can disrupt retinal perfusion and neuronal activity [
32], compromising the precision required for ERG recordings. To eliminate this variability and maintain consistent physiological conditions across individuals, controlled mechanical ventilation was implemented. Pressure-controlled ventilation was specifically selected over volume-controlled modes due to its compatibility with the non-compliant nature of avian lungs, minimizing the risk of barotrauma [
30,
31]. In this study, a low PIP of approximately 6-8 cmH
2O provided stable and effective ventilation throughout the procedure. Anesthetic depth was carefully tapered to suppress spontaneous respiratory efforts (“bucking”) during controlled ventilation, abolish nystagmus, and limit the corneal reflex, thereby ensuring stable respiratory support and complete immobilization for high-fidelity ERG acquisition.
Following airway establishment, selecting an appropriately sized endotracheal tube is a critical step in avian anesthesia. Unlike mammals, birds such as vultures possess complete, non-expandable tracheal rings, rendering them highly susceptible to pressure-related tracheal injury (18, 30, 31). Over-inflation of a standard cuffed endotracheal tube can result in mucosal necrosis, tracheal rupture, or stricture. To mitigate this risk, non-cuffed tubes are frequently recommended for avian species (18). However, they often fail to achieve a complete seal, leading to anesthetic gas leakage and complicating the delivery of controlled ventilator pressures.
Before selecting a cuffed tube, we evaluated the use of a Cole tube, which features a tapered distal end designed to seal at the glottis [
18]. This approach proved unsuitable for vultures requiring mechanical ventilation, as substantial leakage occurred immediately upon applying positive pressure. We therefore adopted a third strategy by placing a cuffed endotracheal tube with the cuff left deflated [
18]. This method provided a secure airway with minimal leakage, likely due to the deflated cuff acting as a passive baffle that disrupted direct airflow escape through the glottis. Crucially, it also eliminated the risk of cuff-related tracheal injury, an important safety consideration during prolonged procedures.
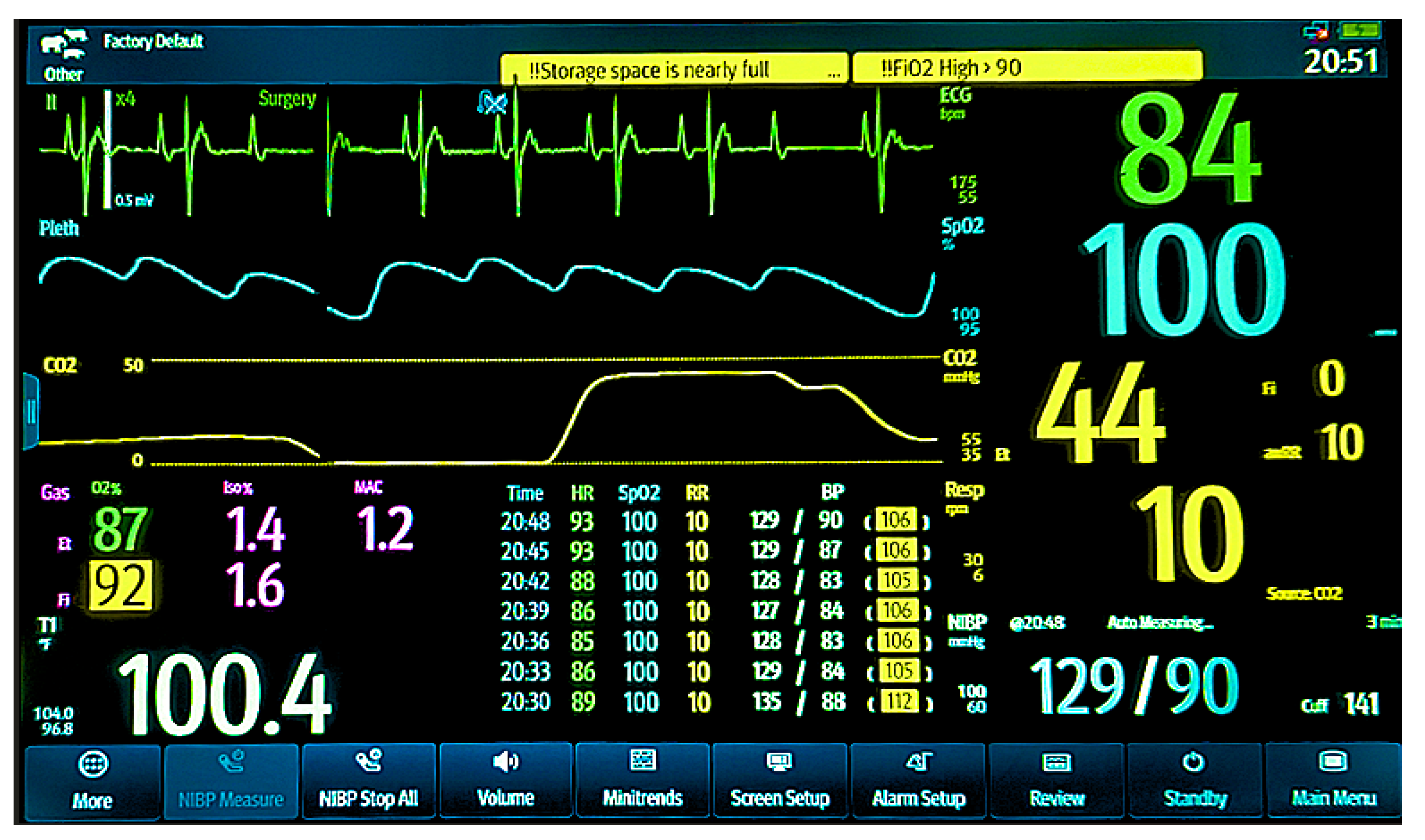
One significant challenge in avian anesthesia involves adapting standard veterinary monitoring equipment, which was originally designed for mammals, to suit the distinct anatomical features of birds. In this study, we introduced several important modifications to establish a consistent monitoring protocol for vultures. Among these adjustments, cardiac rhythm monitoring produced a stable and interpretable ECG waveform using a Lead II configuration. This arrangement involved placing nontraumatic alligator clips on the wings to serve as the right and left arm leads, and on the left toe to represent the left leg lead. The resulting signal allowed for clear rhythm analysis with minimal interference, confirming its reliability for use in large raptors. The clinical value of this approach was demonstrated when it enabled immediate detection and continuous tracking of a second-degree atrioventricular (AV) block in one Black Vulture (
Figure 1). This arrhythmia was identified as a Type I (Wenckebach) block, characterized by a progressive lengthening of the P-R interval until a QRS complex is dropped. This setup allowed us to monitor the arrhythmia’s stability throughout the procedure without requiring intervention.
Building on the adaptations developed for cardiac monitoring, one of the most significant challenges in avian anesthesia involves the limitations of pulse oximetry. Although the toe web proved to be a viable site for placing the oxygen saturation probe, standard pulse oximeters are calibrated for mammalian hemoglobin rather than avian. This discrepancy can lead to misleading readings, often underestimating oxygenation at higher saturation levels and overestimating it at lower levels [
18,
33]. These inaccuracies arise from physiological differences, as avian hemoglobin typically exhibits a higher affinity for oxygen compared to its mammalian counterpart. As a result, algorithms designed for human patients may misinterpret light absorption data, potentially concealing true hypoxemia behind a seemingly acceptable oxygen saturation value above 92 percent.
A similar issue occurs with arterial blood gas analysis. While partial pressure of oxygen (PaO
2) can be measured directly, the calculated oxygen saturation remains unreliable due to the use of human-based algorithms in standard analyzers. Given these limitations and the impracticality of frequent arterial sampling, we chose to monitor oxygen saturation trends rather than rely on absolute values. Focusing on directional changes, particularly stable or rising patterns, proved to be a more practical and informative approach, even when the numerical values were physiologically skewed. This trend-based monitoring is clearly illustrated in the historical record shown in
Figure 1.
To further support this assessment, we continuously monitored both FiO2 and EtO2. Observing consistently high values, typically above 85 percent, alongside a stable SpO2 trend provided strong indirect evidence that the vulture’s lungs were receiving oxygen-enriched gas, as shown in
Figure 1. This combined approach, which interpreted oxygen saturation trends in the context of delivered oxygen concentrations, offered a practical and more reliable method for evaluating oxygenation status in anesthetized vultures.
Obtaining consistent non-invasive blood pressure (NIBP) readings in avian species is notoriously challenging [
35]. However, we leveraged the large size of vultures to overcome this issue by placing a cuff, with a width approximately 40% of the limb’s circumference [
36], on the leg shaft near the tibiotarsus joint. This approach yielded consistent SBP, DBP, and MBP measurements, enabling us to effectively monitor cardiovascular trends (
Figure 1). These findings confirm that this specific technique and location are critical for assessing cardiovascular stability in vultures under anesthesia.
In addition to anatomical considerations, our protocol was tailored to address the distinctive physiological demands of avian patients. One key component was the use of side-stream capnography configured to a low-flow neonate setting (50 ml/min). This adjustment was essential, as standard aspiration rates designed for larger domestic animals can easily exceed a vulture’s tidal volume, resulting in sample dilution and inaccurate measurements of EtCO
2 and Et-Iso. This issue is well-documented in avian anesthesia literature, where high sampling rates have been shown to underestimate EtCO
2 due to anatomical dead space and low respiratory volumes [
36,
37,
38]. Despite these limitations, EtCO
2 remains a valuable tool for monitoring ventilation trends and detecting hyperventilation or hypoventilation [
36,
37,
38]. Additionally, placing the vultures on pressure-controlled ventilation likely helped compensate for any mismatch between delivered and measured ventilation.
Continuous monitoring of EtCO
2 and Et-Iso trends provided consistent, real-time feedback on ventilatory status and anesthetic depth. This approach aligned with observed anesthetic requirements during ERG procedures, where the median Et-Iso concentration needed to maintain a stable plane of anesthesia was 1.4%. Although no published minimum anesthetic concentration (MAC) exists for Black or Turkey Vultures, the reported MAC for the Cinereous Vulture (Aegypius monachus) is 1.06% [
39]. Our findings suggest that the actual MAC for the species studied may be higher than both the published value and the median concentration used. This is likely due to two factors: premedication with a multimodal DMBK combination, which has anesthetic-sparing effects, and the non-noxious nature of ERG procedures, which require immobilization rather than deep analgesia. Accordingly, the Et-Iso concentration used here would be expected to fall below the true MAC, which is defined by response to a noxious stimulus.
A significant reduction in HR from the awake baseline was observed in both species following DMBK premedication and isoflurane anesthesia. This bradycardia is a well-documented and anticipated effect of dexmedetomidine, a potent alpha-2 adrenergic agonist [
18,
31,
32]. Its centrally mediated sympatholytic action, combined with a peripherally mediated vasoconstriction that triggers a baroreceptor reflex, routinely slows HR. In this combination, the potent bradycardic effects of dexmedetomidine clearly superseded the known tachycardic properties of ketamine. Critically, this bradycardia was not associated with hypotension or other adverse events, suggesting it represented a stable, compensatory response rather than a sign of cardiovascular decompensation.
A related and notable finding was the maintenance of MBP at levels that would be considered hypertensive in commonly anesthetized domestic species, such as dogs and cats [
18,
31,
32]. This hypertension is the direct consequence of the profound peripheral vasoconstriction induced by dexmedetomidine, an effect likely compounded by the sympathomimetic properties of ketamine. While an initial hypertensive phase is common in mammals, the
sustained nature of this elevation, even during maintenance with isoflurane, may represent a significant species-specific difference in cardiovascular response. A statistically significant difference in DBP was observed between species (p=0.033), with Turkey Vultures maintaining a higher mean DBP. This finding, however, should be interpreted with extreme caution due to the very small sample size for the Turkey Vulture group (n=4), which makes the result highly susceptible to individual variation. Biologically, this difference could also suggest a genuine, species-specific variation in cardiovascular response to the anesthetic protocol, such as a more pronounced vasoconstrictive response to dexmedetomidine or a differing sensitivity to isoflurane-induced vasodilation. Clinically, the ≈13 mmHg mean difference may be of limited significance, as the DBP was markedly high in both Black Vultures (126.18 mmHg) and Turkey Vultures (139.16 mmHg).
Taken together, the cardiovascular profile observed in these vultures, characterized by significant bradycardia and concurrent hypertension, differs markedly from the hypotensive trends often managed in small animal anesthesia. The fact that all vultures remained physiologically stable without intervention suggests that these values may be well-tolerated or even represent a normal anesthetic plane for these avian species. This highlights the unreliability of extrapolating from mammalian anesthetic norms and underscores the necessity of establishing species-specific baseline data for wild, non-traditional patients.
It is important to acknowledge several limitations of this study. The primary limitation is the small sample size, particularly for Turkey Vultures, which restricts the statistical power of interspecies comparisons and may not capture the full range of individual physiological responses. Furthermore, while the monitoring techniques were effective for clinical trend analysis, their absolute accuracy has known constraints. Specifically, the NIBP values were not validated against the gold standard of direct arterial pressure, and the pulse oximetry readings are subject to the inherent inaccuracies of devices calibrated for human hemoglobin. Additionally, the absence of IV catheterization, while justified by the stable cardiovascular performance of the vultures, meant that immediate IV access was unavailable in the unlikely event of an adverse anesthetic reaction. Respiratory function was also controlled throughout the procedures to maintain a consistent and appropriate range of arterial partial pressure of CO2 (PaCO2), which was necessary for reliable ERG recordings. As a result, any anesthetic protocol–induced respiratory depression could not be properly evaluated under these conditions. Finally, the scope of the study was focused, as the anesthetic protocol was evaluated only during a non-painful procedure in healthy adult vultures. Consequently, its efficacy for more invasive surgeries, compromised patients, or other raptor species remains to be determined.