Submitted:
27 October 2025
Posted:
28 October 2025
You are already at the latest version
Abstract
Keywords:
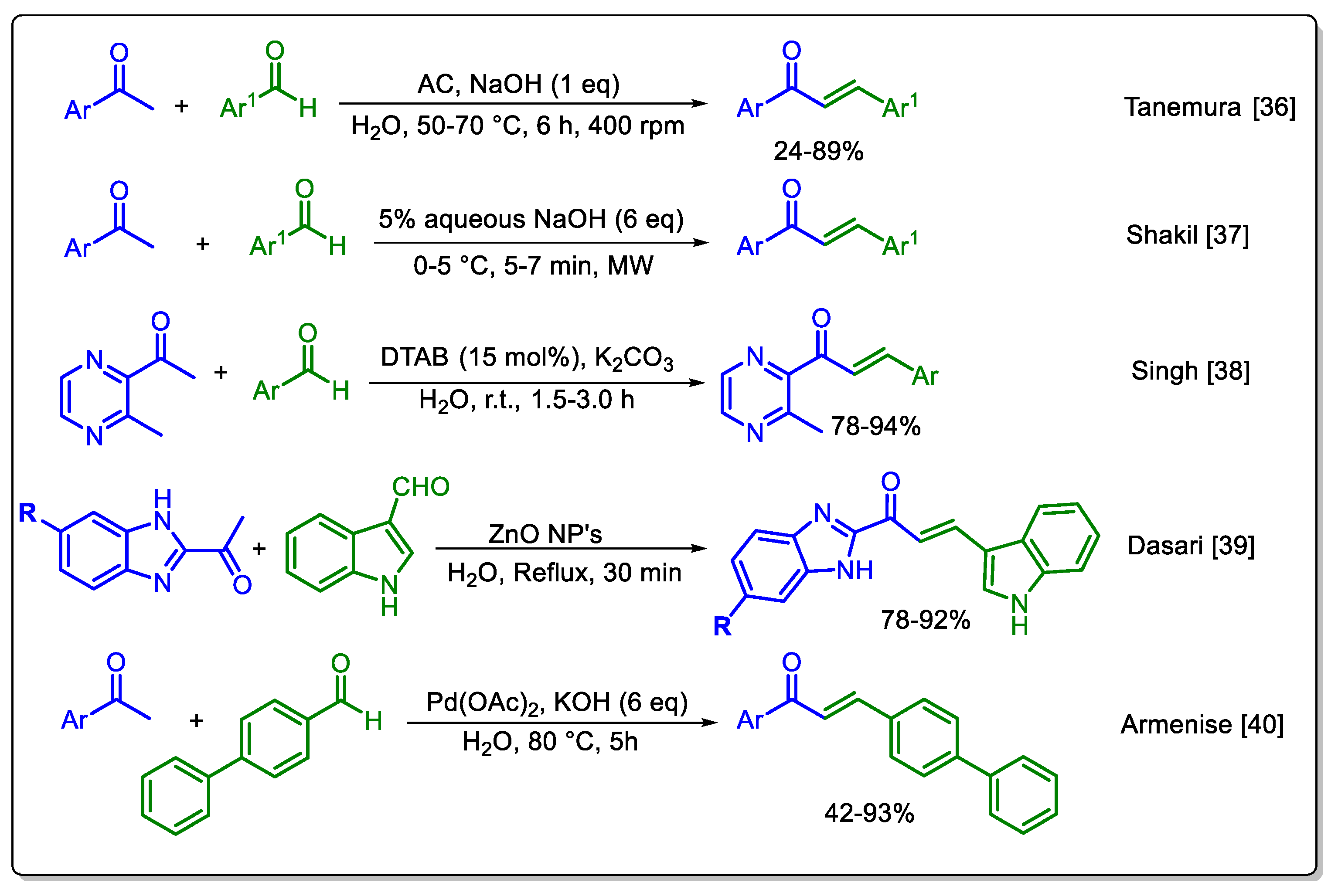
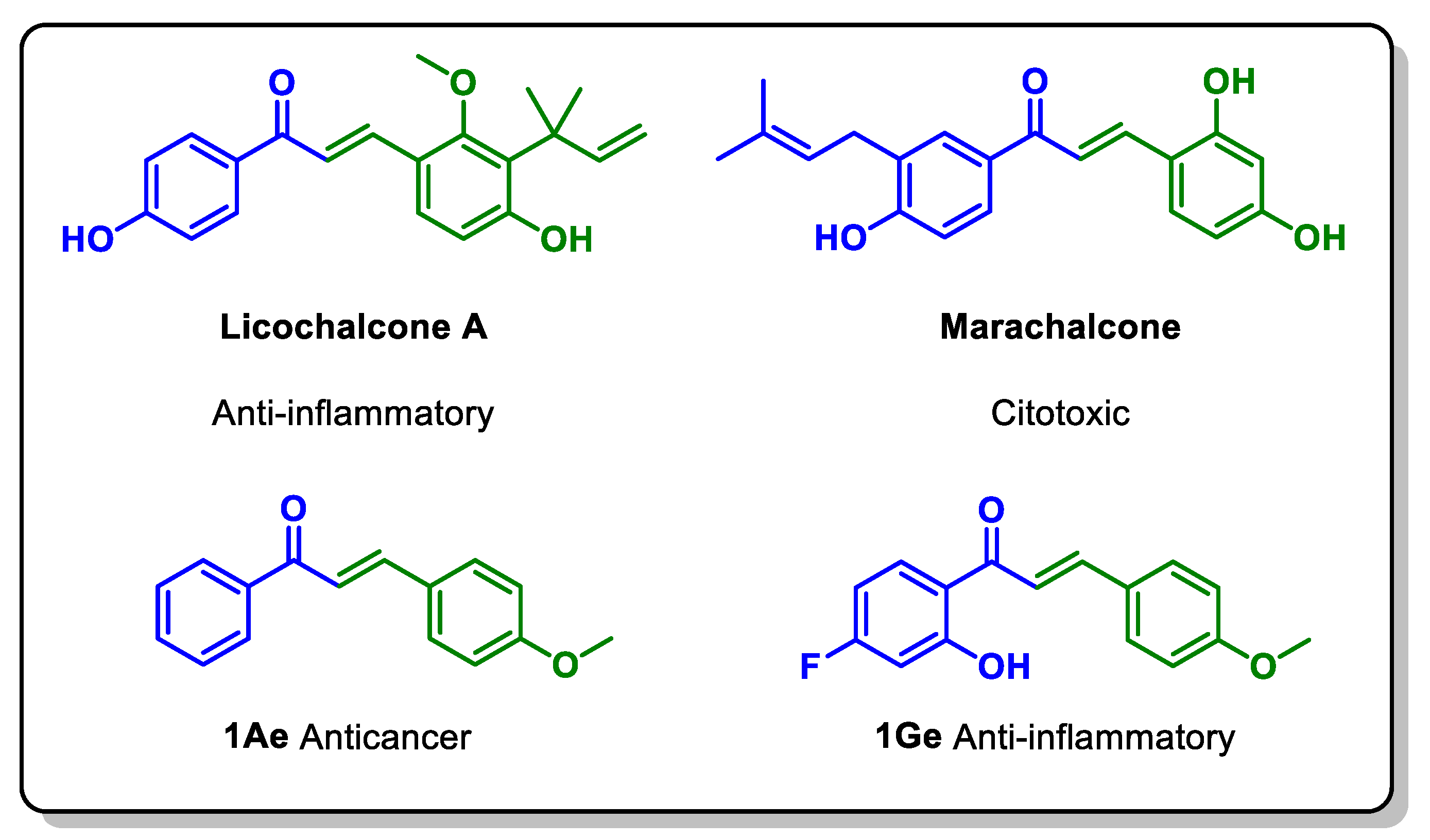
1. Introduction
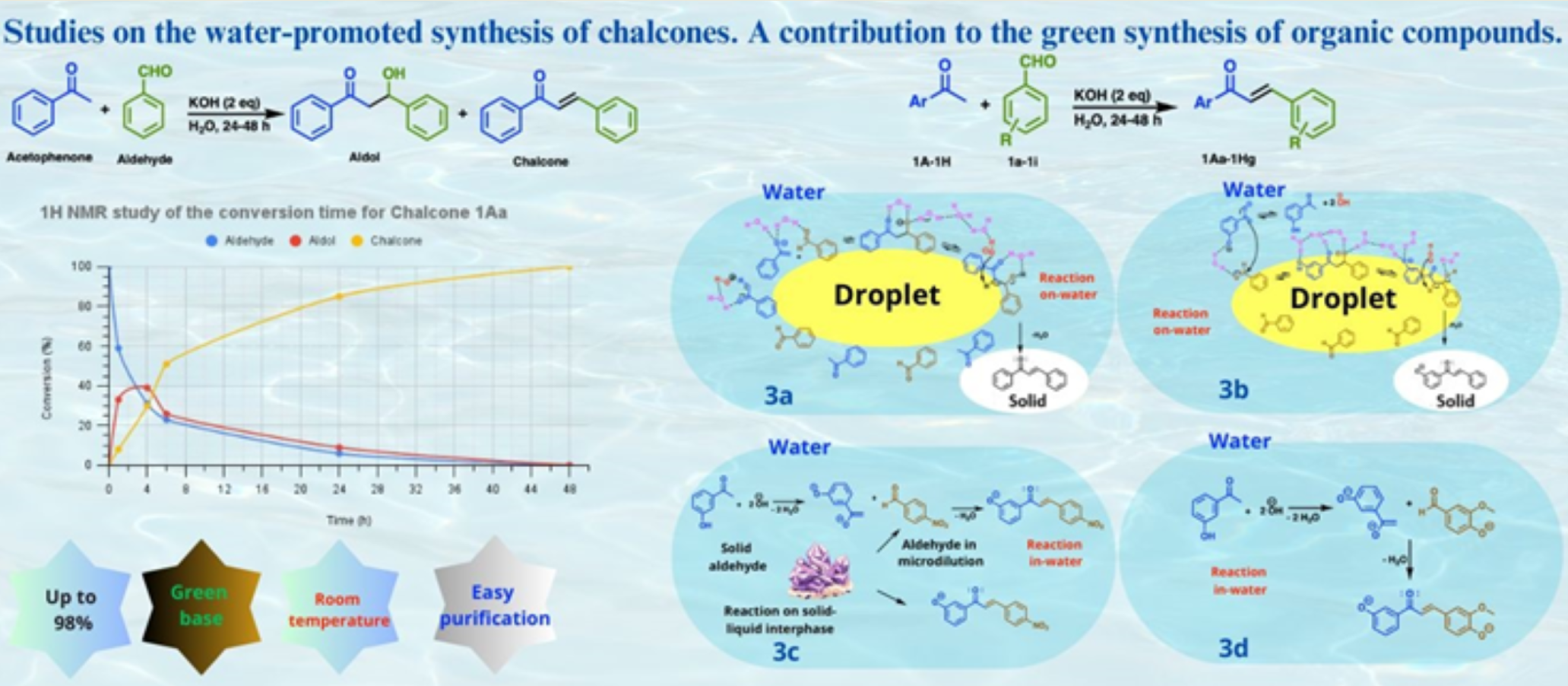
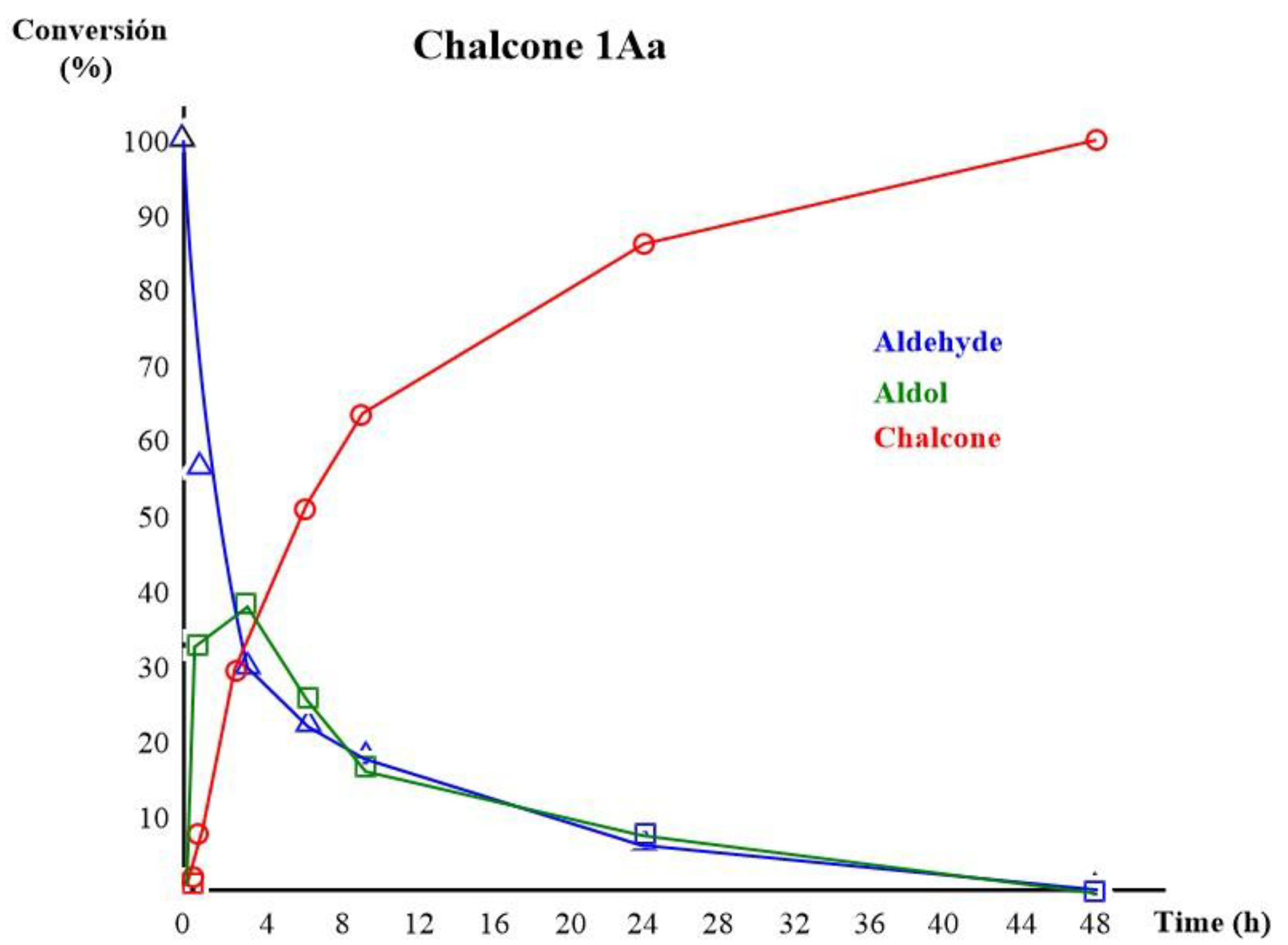
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Miranda, C. L.; Maier, C. S.; Stevens, J. F. Flavonoids. In eLS; John Wiley & Sons, Ltd, 2012. [CrossRef]
- Rosa, G. P.; Seca, A. M. L.; Barreto, M. D. C.; Silva, A. M. S.; Pinto, D. C. G. A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Ninomiya, M.; Koketsu, M. Minor Flavonoids (Chalcones, Flavanones, Dihydrochalcones, and Aurones). In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K. G., Mérillon, J.-M., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013. [Google Scholar] [CrossRef]
- Elkanzi, N. A. A.; Hrichi, H.; Alolayan, R. A.; Derafa, W.; Zahou, F. M.; Bakr, R. B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, H.; Santos, C.; Cavaleiro, J.; Silva, A. Chalcones as Versatile Synthons for the Synthesis of 5- and 6-Membered Nitrogen Heterocycles. Curr. Org. Chem. 2014, 18, 2750–2775. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A. O.; Caruntu, C.; Leyva-Gómez, G.; Dey, A.; Martorell, M.; Calina, D.; López, V.; Les, F. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Mezgebe, K.; Melaku, Y.; Mulugeta, E. Synthesis and Pharmacological Activities of Chalcone and Its Derivatives Bearing N -Heterocyclic Scaffolds: A Review. ACS Omega 2023, 8, 19194–19211. [Google Scholar] [CrossRef]
- Aoki, N.; Muko, M.; Ohta, E.; Ohta, S. C-Geranylated Chalcones from the Stems of Angelica Keiskei with Superoxide-Scavenging Activity. J. Nat. Prod. 2008, 71, 1308–1310. [Google Scholar] [CrossRef] [PubMed]
- Nasir Abbas Bukhari, S.; Jasamai, M.; Jantan, I.; Ahmad, W. Review of Methods and Various Catalysts Used for Chalcone Synthesis. Mini-Rev. Org. Chem. 2013, 10, 73–83. [Google Scholar] [CrossRef]
- Donaire-Arias, A.; Poulsen, M. L.; Ramón-Costa, J.; Montagut, A. M.; Estrada-Tejedor, R.; Borrell, J. I. An Improved High-Yield and Substituent-Independent Protocol for an Old Structure. Molecules 2023, 28, 7576. [Google Scholar] [CrossRef]
- Radwan, M. A. A.; Alshubramy, M. A.; Abdel-Motaal, M.; Hemdan, B. A.; El-Kady, D. S. Synthesis, Molecular Docking and Antimicrobial Activity of New Fused Pyrimidine and Pyridine Derivatives. Bioorganic Chem. 2020, 96, 103516. [Google Scholar] [CrossRef]
- Torres-Sauret, Q.; Vilchis-Reyes, M. A.; Martínez, R.; Romero-Ceronio, N.; Alarcon-Matus, E.; Hernández-Abreu, O.; Vázquez Cancino, R.; Alvarado Sánchez. , C. Crossing Borders: On-Water Synthesis of Flavanones. ChemistrySelect 2022, 7, e202202567. [Google Scholar] [CrossRef]
- El-Hashash, M. A. E-A.; Gohma, S. M.; El-Arab, E. Z. Utility of Pyrazolylchalcone Synthon to Synthesize Azolopyrimidines under Grindstone Technology. Chem Pharm Bull (Tokyo). 2017, 65, 90–96. [Google Scholar] [CrossRef]
- Gomha, S. M.; Abdallah, M. A.; Abbas, I. M.; Kazem, M. S. H. Synthesis, Cytotoxicity Evaluation, Molecular Docking and Utility of Novel Chalcones as Precursors for Heterocycles Incorporating Pyrazole Moiety. Med. Chem. Shariqah United Arab Emir. 2018, 14, 344–355. [Google Scholar] [CrossRef]
- Janković, T.; Turković, N.; Kotur-Stevuljević, J.; Vujić, Z.; Ivković, B. Differences in Antioxidant Potential of Chalcones in Human Serum: In Vitro Study. Chem. Biol. Interact. 2020, 324, 109084. [Google Scholar] [CrossRef]
- Bale, A. T.; Salar, U.; Khan, K. M.; Chigurupati, S.; Fasina, T.; Ali, F.; Ali, M.; Nanda, S. S.; Taha, M.; Perveen, S. Chalcones and Bis-Chalcones Analogs as DPPH and ABTS Radical Scavengers. Lett. Drug Des. Discov. 2021, 18, 249–257. [Google Scholar] [CrossRef]
- Mahapatra, D. K.; Bharti, S. K.; Asati, V. Chalcone Scaffolds as Anti-Infective Agents: Structural and Molecular Target Perspectives. Eur. J. Med. Chem. 2015, 101, 496–524. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Yoshimura, M.; Yamaguchi, F.; Kouchi, T.; Tsuji, R.; Saito, M.; Obata, A.; Kikuchi, M. Anti-Allergic Activity of Naringenin Chalcone from a Tomato Skin Extract. Biosci. Biotechnol. Biochem. 2004, 68, 1706–1711. [Google Scholar] [CrossRef] [PubMed]
- Iwamura, C.; Shinoda, K.; Yoshimura, M.; Watanabe, Y.; Obata, A.; Nakayama, T. Naringenin Chalcone Suppresses Allergic Asthma by Inhibiting the Type-2 Function of CD4 T Cells. Allergol. Int. 2010, 59, 67–73. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone Derivatives: Role in Anticancer Therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef]
- Syam, S.; Abdelwahab, S. I.; Al-Mamary, M. A.; Mohan, S. Synthesis of Chalcones with Anticancer Activities. Molecules 2012, 17, 6179–6195. [Google Scholar] [CrossRef] [PubMed]
- Okolo, E. N.; Ugwu, D. I.; Ezema, B. E.; Ndefo, J. C.; Eze, F. U.; Ezema, C. G.; Ezugwu, J. A.; Ujam, O. T. New Chalcone Derivatives as Potential Antimicrobial and Antioxidant Agent. Sci. Rep. 2021, 11, 21781. [Google Scholar] [CrossRef]
- Henry, E. J.; Bird, S. J.; Gowland, P.; Collins, M.; Cassella, J. P. Ferrocenyl Chalcone Derivatives as Possible Antimicrobial Agents. J. Antibiot. (Tokyo) 2020, 73, 299–308. [Google Scholar] [CrossRef]
- M. Gomha, S.; M. Riyadh, S.; M. Abdalla, M. Solvent-Drop Grinding Method: Efficient Synthesis, DPPH Radical Scavenging and Anti-Diabetic Activities of Chalcones, Bis-Chalcones, Azolines, and Bis-Azolines. Curr. Org. Synth. 2015, 12, 220–228. [Google Scholar] [CrossRef]
- Zhuang, C.; Zhang, W.; Sheng, C.; Zhang, W.; Xing, C.; Miao, Z. Chalcone: A Privileged Structure in Medicinal Chemistry. Chem. Rev. 2017, 117, 7762–7810. [Google Scholar] [CrossRef]
- Farooq, S.; Ngaini, Z. Recent Synthetic Methodologies for Chalcone Synthesis (2013-2018). Curr. Organocatalysis 2019, 6, 184–192. [Google Scholar] [CrossRef]
- Rammohan, A.; Reddy, J. S.; Sravya, G.; Rao, C. N.; Zyryanov, G. V. Chalcone Synthesis, Properties and Medicinal Applications: A Review. Environ. Chem. Lett. 2020, 18, 433–458. [Google Scholar] [CrossRef]
- Stantliff, T. M.; Hill, A.; Kuo, M. E.; Neal, H. E.; Harrod, T. C.; Goens, K.; Mashuta, M.; Christianson, A. M.; Krzysiak, A. J. Flexibility in the Bridge of Chalcone Derivatives Is Important for the Inhibition of Cellular Growth. Bioorg. Med. Chem. Lett. 2023, 95, 129467. [Google Scholar] [CrossRef] [PubMed]
- Iftikhar, S.; Khan, S.; Bilal, A.; Manzoor, S.; Abdullah, M.; Emwas, A.-H.; Sioud, S.; Gao, X.; Chotana, G. A.; Faisal, A.; Saleem, R. S. Z. Synthesis and Evaluation of Modified Chalcone Based P53 Stabilizing Agents. Bioorg. Med. Chem. Lett. 2017, 27, 4101–4106. [Google Scholar] [CrossRef]
- Neha, K.; Wakode, S. Contemporary Advances of Cyclic Molecules Proposed for Inflammation. Eur. J. Med. Chem. 2021, 221, 113493. [Google Scholar] [CrossRef]
- Eucerin: Nuestras investigaciones| Base de Datos de Ingredientes. https://www.eucerin.es/nuestras-investigaciones/base-de-datos-de-ingredientes/licochalcone-a (accessed 2024-08-20).
- Mei-Ing Chung; Mei-Hsun Lai; Ming-Hong Yen; Ru-Rong Wu; Chun-Nan Lin. Phenolics from Hypericum Geminiflorum. Phytochemistry 1997, 44, 943–947. [Google Scholar] [CrossRef]
- Marotta, L.; Rossi, S.; Ibba, R.; Brogi, S.; Calderone, V.; Butini, S.; Campiani, G.; Gemma, S. The Green Chemistry of Chalcones: Valuable Sources of Privileged Core Structures for Drug Discovery. Front. Chem. 2022, 10. [Google Scholar] [CrossRef]
- Kitanosono, T.; Kobayashi, S. Reactions in Water Involving the “On-Water” Mechanism. Chem. – Eur. J. 2020, 26, 9408–9429. [Google Scholar] [CrossRef]
- Shaik, A. B.; Bhandare, R. R.; Nissankararao, S.; Edis, Z.; Tangirala, N. R.; Shahanaaz, S.; Rahman, M. M. Design, Facile Synthesis and Characterization of Dichloro Substituted Chalcones and Dihydropyrazole Derivatives for Their Antifungal, Antitubercular and Antiproliferative Activities. Molecules 2020, 25, 3188. [Google Scholar] [CrossRef] [PubMed]
- Tanemura, K.; Rohand, T. Activated Charcoal-Mediated Synthesis of Chalcones Catalyzed by NaOH in Water. Tetrahedron Lett. 2021, 71, 152918. [Google Scholar] [CrossRef]
- Shakil, N. A.; Singh, M. K.; Kumar, J.; Sathiyendiran, M.; Kumar, G.; Singh, M. K.; Pandey, R. P.; Pandey, A.; Parmar, V. S. Microwave Synthesis and Antifungal Evaluations of Some Chalcones and Their Derived Diaryl-Cyclohexenones. J. Environ. Sci. Health Part B 2010, 45, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kitawat, B. S.; Singh, M.; Kale, R. K. Robust Cationic Quaternary Ammonium Surfactant-Catalyzed Condensation Reaction for ( E )-3-Aryl-1-(3-Alkyl-2-Pyrazinyl)-2-Propenone Synthesis in Water at Room Temperature. ACS Sustain. Chem. Eng. 2013, 1, 1040–1044. [Google Scholar] [CrossRef]
- Dasari, G. K.; Sunkara, S.; Gadupudi, P. C. R. Green and Ecofriendly Synthesis of Indole-condensed Benzimidazole Chalcones in Water and Their Antimicrobial Evaluations. J. Heterocycl. Chem. 2020, 57, 1201–1210. [Google Scholar] [CrossRef]
- Armenise, N.; Malferrari, D.; Ricciardulli, S.; Galletti, P.; Tagliavini, E. Multicomponent Cascade Synthesis of Biaryl-Based Chalcones in Pure Water and in an Aqueous Micellar Environment. Eur. J. Org. Chem. 2016, 2016, 3177–3185. [Google Scholar] [CrossRef]
- Shen, Y. R.; Ostroverkhov, V. Sum-Frequency Vibrational Spectroscopy on Water Interfaces: Polar Orientation of Water Molecules at Interfaces. Chem. Rev. 2006, 106, 1140–1154. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Yu, J.; Kincaid, J. R. A.; Walde, P.; Gallou, F.; Lipshutz, B. H. Water as the Reaction Medium in Organic Chemistry: From Our Worst Enemy to Our Best Friend. Chem. Sci. 2021, 12, 4237–4266. [Google Scholar] [CrossRef]
- Antonio-Arias, J. E.; Díaz-Oliva, V. del C.; Romero-Ceronio, N.; Gómez-Rivera, A.; Aguilar-Mariscal, H.; Fuente, L. F. R. la; Lobato-García, C. E. Monomodal vs Multimodal Microwave Irradiation Applied in the Synthesis of Fluorochalcones. American Journal of Organic Chemistry 2018, 8, 8–12. [Google Scholar]
- Masmoudi, N.; Chtourou, M. Green and Ultrasound-Assisted Synthesis of 1,3-Diaryl-2-Propenones Catalyzed by Amberlyte IRA-410 and Amberlyte IRA-400 Basic Resins. Lett. Org. Chem. 2023, 20, 362–369. [Google Scholar] [CrossRef]
- Tang, L.; Gao, Y.; Chen, J.; Yang, L.; Xiao, B.; Shen, G.; Ouyang, Y.; Han, W. Ligand-Free Palladium-Catalyzed Substoichiometric Base Mediated Carbonylation of Aryl Iodides with Alkenylboronic Acids under Ambient Conditions. Synlett 2023, 34, 1280–1284. [Google Scholar] [CrossRef]
- Karaman, İ.; Gezegen, H.; Gürdere, M. B.; Dingil, A.; Ceylan, M. Screening of Biological Activities of a Series of Chalcone Derivatives against Human Pathogenic Microorganisms. Chem. Biodivers. 2010, 7, 400–408. [Google Scholar] [CrossRef]
- Zhao, P.-L.; Liu, C.-L.; Huang, W.; Wang, Y.-Z.; Yang, G.-F. Synthesis and Fungicidal Evaluation of Novel Chalcone-Based Strobilurin Analogues. J. Agric. Food Chem. 2007, 55, 5697–5700. [Google Scholar] [CrossRef]
- Chintakrindi, A. S.; Gohil, D. J.; Kothari, S. T.; Chowdhary, A. S.; Kanyalkar, M. A. Design, Synthesis and Evaluation of Chalcones as H1N1 Neuraminidase Inhibitors. Med. Chem. Res. 2018, 27, 1013–1025. [Google Scholar] [CrossRef]
- Jacob, K. C.; Jadhav, G. V.; Vakharia, M. N. Synthesis of 2-Hydroxy-4-Methyl-5-Chlorochalcones and Their Derivatives. Pesticides 1972, 6, 94–96. [Google Scholar]
- Qiu, X. Y.; Li, S. Z.; Shi, A. R.; Yue, Q. L. Synthesis, Characterized and Biological Activities of Chalcone Derivatives. Adv. Mater. Res. 2012, 535–537, 2540–2543. [Google Scholar] [CrossRef]
- Balaji, S.; Manikandan, V.; Senbagam, R.; Vijayakumar, R.; Rajarajan, M.; Vanangamudi, G.; Thirunarayanan, G. Synthesis, Evaluation of Antimicrobial Activities of some (e)-1-(5-chloro- 2-hydroxyphenyl)-3-phenylprop-2-en-1-one compounds. Med. Sci. 2016, 5. [Google Scholar]
- Ammaji, S.; Masthanamma, S.; Bhandare, R. R.; Annadurai, S.; Shaik, A. B. Antitubercular and Antioxidant Activities of Hydroxy and Chloro Substituted Chalcone Analogues: Synthesis, Biological and Computational Studies. Arab. J. Chem. 2022, 15. [Google Scholar] [CrossRef]
- Dofe, V. S.; Sarkate, A. P.; Kathwate, S. H.; Gill, C. H. Synthesis, Antimicrobial Activity and Anti-Biofilm Activity of Novel Tetrazole Derivatives. Heterocycl. Commun. 2017, 23, 325–330. [Google Scholar] [CrossRef]
- Dofe, V. S.; Sarkate, A. P.; Lokwani, D. K.; Shinde, D. B.; Kathwate, S. H.; Gill, C. H. Novel O-Alkylated Chromones as Antimicrobial Agents: Ultrasound Mediated Synthesis, Molecular Docking and ADME Prediction. J. Heterocycl. Chem. 2017, 54, 2678–2685. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Vijeender, K.; Reddy, K. V. New Synthesis of Flavanones Catalyzed by L-Proline. Tetrahedron Lett. 2005, 46, 6991–6993. [Google Scholar] [CrossRef]
- Devia, A. C.; Ferretti, F. H.; Ponce, C. A.; Tomás, F. Conformational Equilibrium and Intramolecular Hydrogen Bond of 4′X and 4X Substituted 2′(OH)Chalcones. J. Mol. Struct. THEOCHEM 1999, 493, 187–197. [Google Scholar] [CrossRef]
- Muller, B. M.; Mai, J.; Yocum, R. A.; Adler, M. J. Impact of Mono- and Disubstitution on the Colorimetric Dynamic Covalent Switching Chalcone/Flavanone Scaffold. Org. Biomol. Chem. 2014, 12, 5108–5114. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A. R.; Marques, A. P.; Rauter, A. P. An Easy Approach to Dihydrochalcones via Chalcone in Situ Hydrogenation. Pure Appl. Chem. 2016, 88, 349–361. [Google Scholar] [CrossRef]
- De, R.; Savarimuthu, A.; Ballav, T.; Singh, P.; Nanda, J.; Hasija, A.; Chopra, D.; Bera, M. K. DBU-Catalyzed Rearrangement of Secondary Propargylic Alcohols: An Efficient and Cost-Effective Route to Chalcone Derivatives. Synlett 2020, 31, 1587–1592. [Google Scholar] [CrossRef]
- Ciupa, A.; Mahon, M. F.; Bank, P. A. D.; Caggiano, L. Simple Pyrazoline and Pyrazole “Turn on” Fluorescent Sensors Selective for Cd2+ and Zn2+ in MeCN. Org. Biomol. Chem. 2012, 10, 8753–8757. [Google Scholar] [CrossRef]
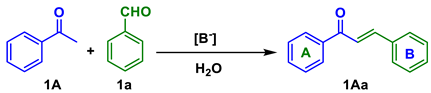 | ||||
| Entry | Base | Base (eq) | Time (h) | Yield (%) |
| 1 | KOH | 1 | 4 | ND |
| 2 1 | KOH | 1 | 4 | 88 |
| 3 | Na2CO3 | 2 | 7 | 0 |
| 4 | K2CO3 | 2 | 7 | 0 |
| 5 | KOH | 2 | 2 | 88 |
| 6 | KOH | 3 | 2 | 88 |
| 7 | NaOH | 2 | 2 | 88 |
| 8 | KOH/piperidine | 2/0.1 M | 2 | 85 |
| 9 2 | KOH | 2 | 1 | - |
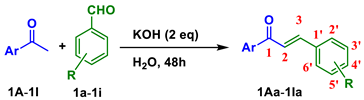 | |||
| Entry | Ar | R |
Chalcone Yield (%) |
| 1 | 1A Ph | 1a H | 1Aa (88) |
| 2 | 1b 3ʹ-F | 1Ab (81) | |
| 3 | 1c 4ʹ-F | 1Ac (88) | |
| 4 | 1d 4ʹ-NO2 | 1Ad (82) | |
| 5 | 1e 4ʹ-OMe | 1Ae (78) | |
| 6 |
1B 4-Cl-Ph |
1a H | 1Ba (86) |
| 7 | 1b 3ʹ-F | 1Bb (81) | |
| 8 | 1c 4ʹ-F | 1Bc (80) | |
| 9 | 1d 4ʹ-NO2 | 1Bd (66) | |
| 10 | 1e 4ʹ-OMe | 1Be (79) | |
| 11 |
1C 3-OH-Ph |
1a H | 1Ca (76) |
| 12 | 1b 3ʹ-F | 1Cb (78) | |
| 13 | 1c 4ʹ-F | 1Cc (83) | |
| 14 | 1d 4ʹ-NO2 | 1Cd (77) | |
| 15 | 1e 4ʹ-OMe | 1Ce (80) | |
| 16 | 1f 3ʹ-OMe,4ʹ-OH | 1Cf (68) | |
| 17 |
1D 4-NO2-Ph |
1a H | 1Da (93) |
| 18 | 1c 4ʹ-F | 1Dc (98) | |
| 19 | 1d 4ʹ-NO2 | 1Dd (40) | |
| 20 | 1e 4ʹ-OMe | 1De (78) | |
| 21 |
1E 2-OH-5-Cl-Ph |
1c 4ʹ-F | 1Ec (96) |
| 22 | 1g 2ʹ-OMe | 1Eg (83) | |
| 23 | 1h 3ʹ-OMe | 1Eh (67) | |
| 24 |
1F 2-OH-4-Me-5-Cl-Ph |
1b 3ʹ-F | 1Fb (83) |
| 25 | 1c 4ʹ-F | 1Fc (94) | |
| 26 | 1e 4ʹ-OMe | 1Fe (96) | |
| 27 | 1g 2ʹ-OMe | 1Fg (75) | |
| 28 | 1i 2ʹ-F | 1Fi (56) | |
| 29 |
1G 2-OH-4-F-Ph |
1a H | 1Ga (65) |
| 30 | 1b 3ʹ-F | 1Gb (72) | |
| 31 | 1e 4ʹ-OMe | 1Ge (72) | |
| 32 | 1g 2ʹ-OMe | 1Gg (86) | |
| 33 | 1h 3ʹ-OMe | 1Gh (85) | |
| 34 | 1i 2ʹ-F | 1Gi (75) | |
| 35 | 1H 2-OH-5-F-Ph | 1c 4ʹ-F | 1Hc (95) |
| 36 | 1e 4ʹ-OMe | 1He (85) | |
| 37 | 1I Furane | 1a H | 1Ia (81) |
| 38 | 1J Pyridine | 1a H | 1Ja (85) |
| 39 1 | 1A | 1a H | 1Aa (87) |
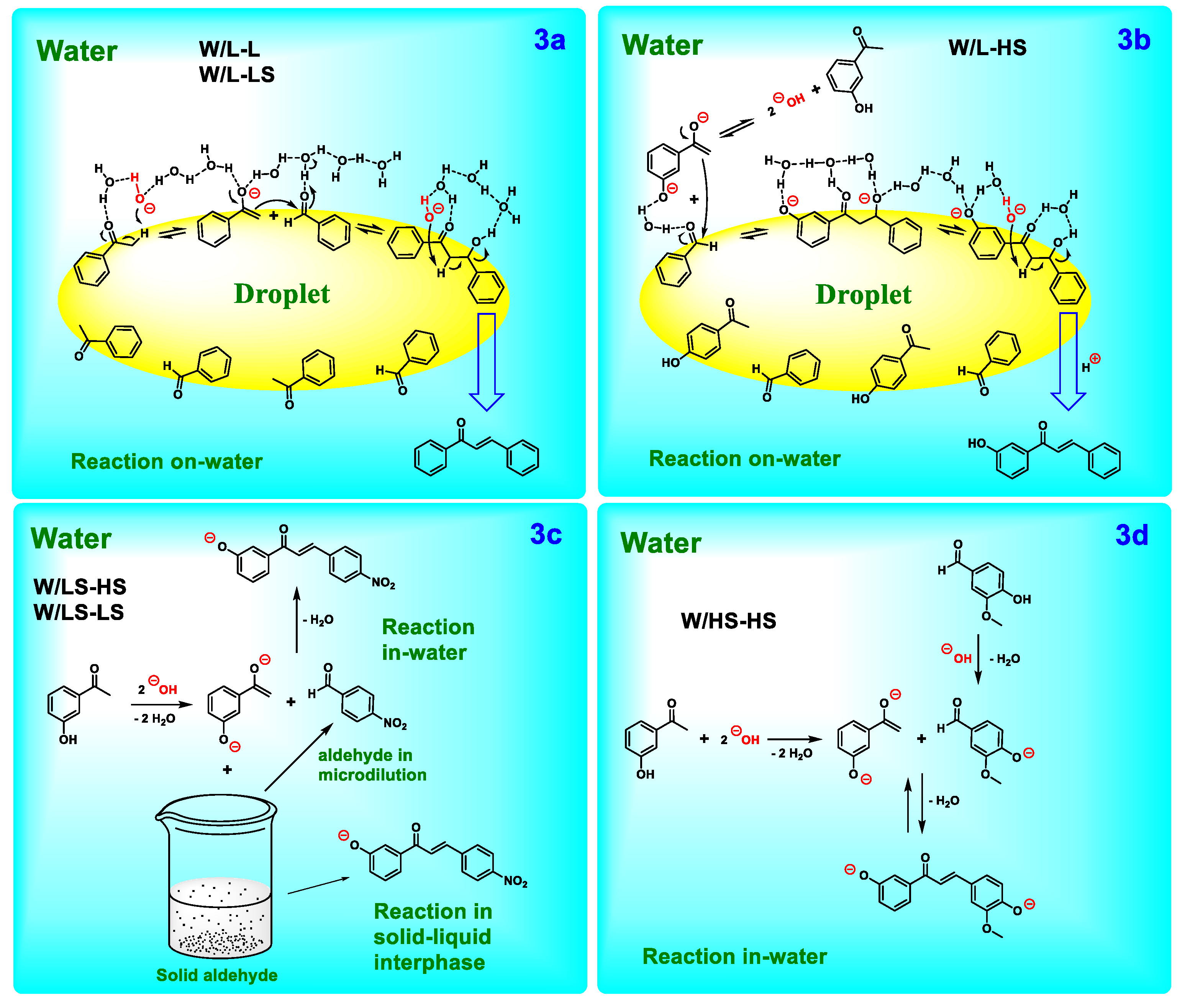
| Ketone | Aldehyde | System | ||
| Liquid (L) |
1A,1B,1G,1I | L | 1a-1c, 1e, 1g,1h | W/L-L |
| LS | 1d | W/L-LS | ||
| Lipophilic Solid (LS) |
1D |
L |
1a-1c, 1e, 1g,1h | W/L-LS |
| LS | 1d | W/LS-LS | ||
| Hydrophilic Solid (HS) |
1C, 1E-1H |
L |
1a-1c, 1e, 1g,1h | W/L-HS |
| LS | 1d | W/HS-LS | ||
| HS | 1f | W/HS-HS | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).