Introduction
The incidence of neurodegenerative diseases is steadily increasing worldwide. According to the WHO, the number of patients with dementia may exceed 130 million by 2050 [
1]. Dementia can be caused by various diseases or injuries leading to brain damage, mostly Alzheimer disease (AD) or stroke [
2,
3]. Not only age and the development of AD contribute to the progression of cognitive impairments and dementia, but also other diseases, such as arterial hypertension, atherosclerosis, and diabetes.
Despite significant advances in understanding the pathogenesis of these diseases, their early diagnosis remains a challenge, as there are no clear diagnostic criteria, and the clinical diagnosis is based on a combination of clinical, psychometric, tomographic, and other data and may be subjective [
4]. Traditional diagnostic methods, such as magnetic resonance imaging (MRI) and cerebrospinal fluid analysis, are quite accurate but have significant disadvantages—first of all the invasiveness and high costs of examination. These factors limit their appropriateness for widespread screening. We have previously investigated and described the problem of diagnosing cognitive impairments in AD [
5,
7].
There still remains the problem of finding and developing non-invasive and accessible methods for the early diagnosis of cognitive impairments in dementia caused by neurovascular pathologies, which is the second most common form of dementia after AD [
6]. Unlike neurodegenerative dementias, vascular cognitive disorders are potentially preventable as they are associated with manageable and most common risk factors of cardiovascular diseases [
8].
Peripheral tissues, particularly the buccal epithelium, are of special interest due to their accessibility and ability to express key molecules involved in the pathogenesis of neurodegenerative diseases and other brain disorders. Many signaling molecules, which are involved in the cascade regulation of neuroimmune and endocrine cell interactions in the central nervous system, also play a key role in the evolvement of neurodegeneration mechanisms. As shown earlier, changes in the levels of β-amyloid, tau protein, NF-kB, and other markers are observed in the buccal epithelium, which correlate with pathological processes in the central nervous system [
9].
The purpose of this study was to develop a diagnostically viable panel of signaling molecules (biomarkers), which are expressed in the buccal epithelium, for the screening of cognitive impairments in dementia caused by neurovascular disorders.
Materials and Methods
The expression of signaling molecules (molecular markers) involved in the neurodegeneration process in buccal epithelial cells was studied in patients with dementia caused by neurovascular disorders, and in age-matched volunteers without such pathologies. The study included only patients with vascular dementia diagnosed multidisciplinary in accordance with current European ((EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia) and national guidelines [
10]. In examining the patients, we also relied on the criteria of the VICCCS (Vascular Impairment of Cognition Classification Consensus Study) [
11], which provide for a comprehensive assessment of clinical syndromes, neuroimaging signs of cerebrovascular diseases and their causal link with cognitive decline.
The study participants were selected in accordance with the expert assessment of medical records (analysis of diagnoses, anamnestic data, results of biochemical, functional, imaging examinations, psychometric tests), their geriatric status based on comprehensive geriatric assessment, and, where necessary, additional follow-up examination and testing based on experts’ recommendations and using rigorous diagnostic algorithms.
The following inclusion criteria were defined for the study: age over 50 years; the presence of clinical manifestations of objective cognitive disorders of vascular origin. The exclusion criteria for the main and control groups were the following: oncological or hematological diseases with no remission, conditions requiring emergency care; refusal of study participants or their legal representatives to participate in the study. While selecting control group participants, a psychometric examination was also carried out, since they could have preclinical cognitive disorders in the absence of complaints and clinically significant manifestations, which prevented them from being included in the control group.
Following the preliminary assessments, 146 study participants were selected, of whom 57 (39%) were male and 89 (61%) were female. The average age of the patients with vascular dementia was 79.7±9.8 years, and that of the volunteers was 61.7±7.6 years. The subjects were divided into 2 groups:
- 1)
patients with clinically diagnosed vascular dementia (100 individuals, of whom 26% were male and 74% were female);
- 2)
volunteers without clinical manifestations of neuropsychiatric disorders (46 individuals, of whom 32.6% were male and 67.4% were female).
We used the dynamic cohort method which provides for the formation and reformation of groups and subgroups in the study based on the variation of cognitive dysfunctions and the presence of comorbidity, which allows for carrying out correlation analysis of participants with various mixed cognitive disorders.
The potentially polyfactorial nature of dementia and the presence of its mixed forms in one participant did not allow the formation of stable groups for the study, but required an approach for dynamic formation of groups and subgroups among the participants.
An immunocytochemical method was used to investigate the expression of signaling molecules (molecular markers) involved in the neurodegeneration process in buccal epithelial cells in patients with dementia caused by neurovascular pathology and in age-matched volunteers without such pathology.
Buccal epithelial swab samples were collected non-invasively using sterile cytobrushes. For immunocytochemical analysis (ICC test), we used monoclonal antibodies to the following markers: β-amyloid, NF-κB, tau protein, S100 protein, claudin, synuclein, RAGE, and PTEN-induced kinase 1 (PINK1) — all Abcam.
To visualize the ICC test, a reagent kit based on the NovoLink polymer and peroxidase, RE7150-K (NovoCastra) was used. Images of buccal epithelium preparations with a magnification of 400x were obtained using computer analysis systems for microscopic images, including the Olympus BX46 microscope, VideoZavrStandartVZ-18C23-B digital camera, PC (AMD Ryzen 3 3200G), and VideoZavrCatalog software.
The results of the ICC test were assessed with light microscopy, and a comparative quantitative assessment of the expression of signaling molecules was performed using morphometry and computer analysis of microscopic images. Morphometry allows for quantitative measurements of the expression of signaling molecules (biomarkers) and thereby provides an objective assessment of the synthesis of specific molecules in the cell samples under study. The number of immunopositive cells and the total number of cells were estimated manually. The so-called “cell specific weight” (the proportion of the number of immunopositive cells to the number of all cells in the field of view expressed as a percentage) was determined with a total number of cells in preparation > 100. The ImageJ application allows to calculate the area occupied by immunopositive cells and enables us to calculate the area of protein expression and find correlations of marker expression.
The Shapiro-Wilk test was used in statistical data processing to verify the normality of the distribution. The results were described using parametric and nonparametric methods. If the data matched the normal distribution (only for age in the comparison groups), the typical value was presented as the mean and standard deviation (M±SD), and the groups were compared by using Student’s t-test. If the data did not match the normal distribution (all extensive indicators characterizing the proportion of cells with marker expression), the typical value was presented as a median, the deviation was characterized by an interquartile range of Ме(Q1–Q3), and the 95% confidence interval was calculated by using the Wilson method. The overall confidence interval was calculated using the Wilson formula.
The groups were compared by using the Mann-Whitney U-test. In a paired comparison, the null hypothesis was rejected at a significance level less than 0.05.
Results
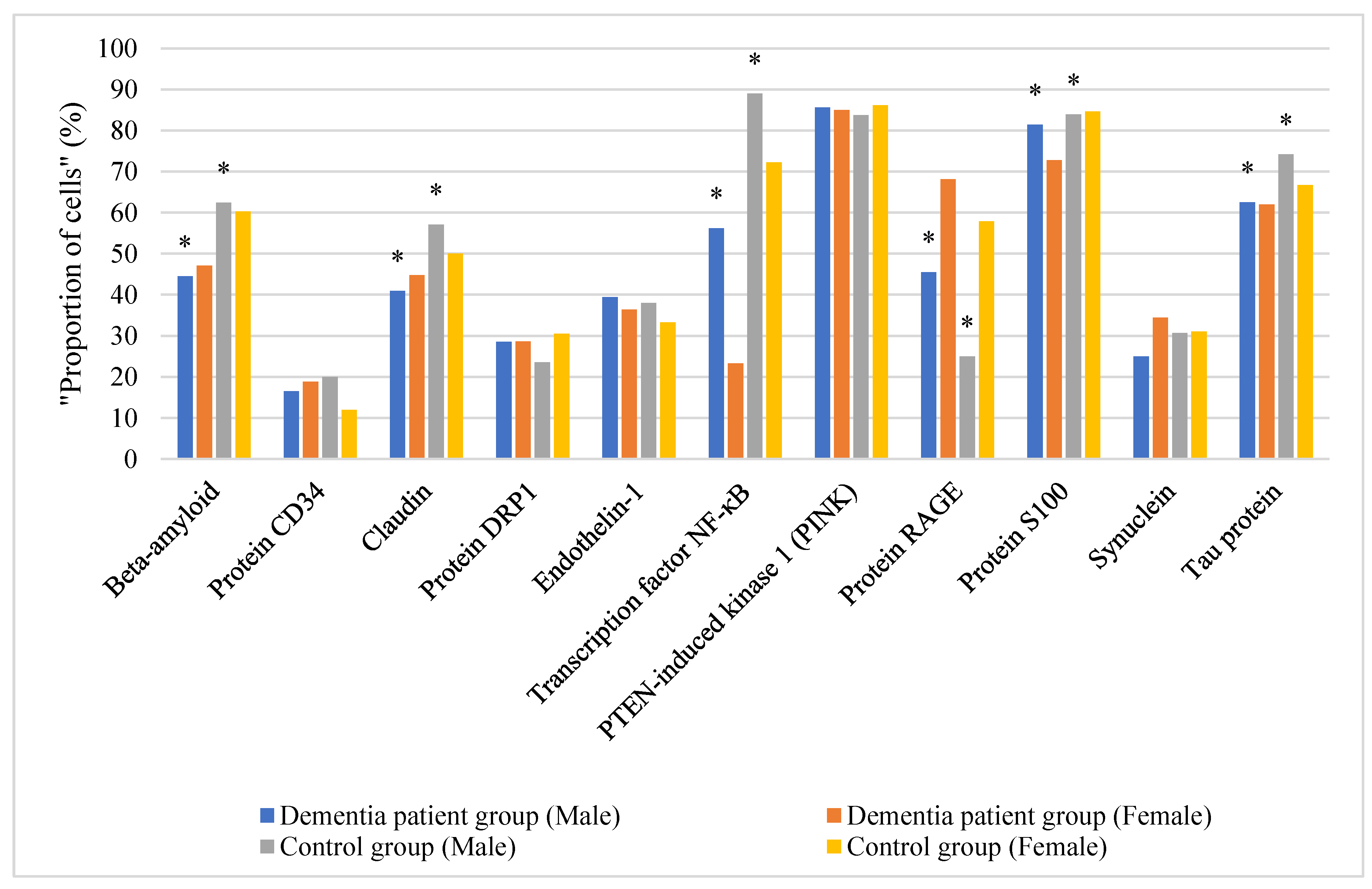
Analysis of the expression of key signaling molecules in the buccal epithelium revealed significant differences between the groups of patients with vascular dementia and the control group (
Table 1).
Distribution of the proportion of cells with the expression of signaling molecules in the buccal epithelium by gender depending on the comparison group is shown in
Figure 1.
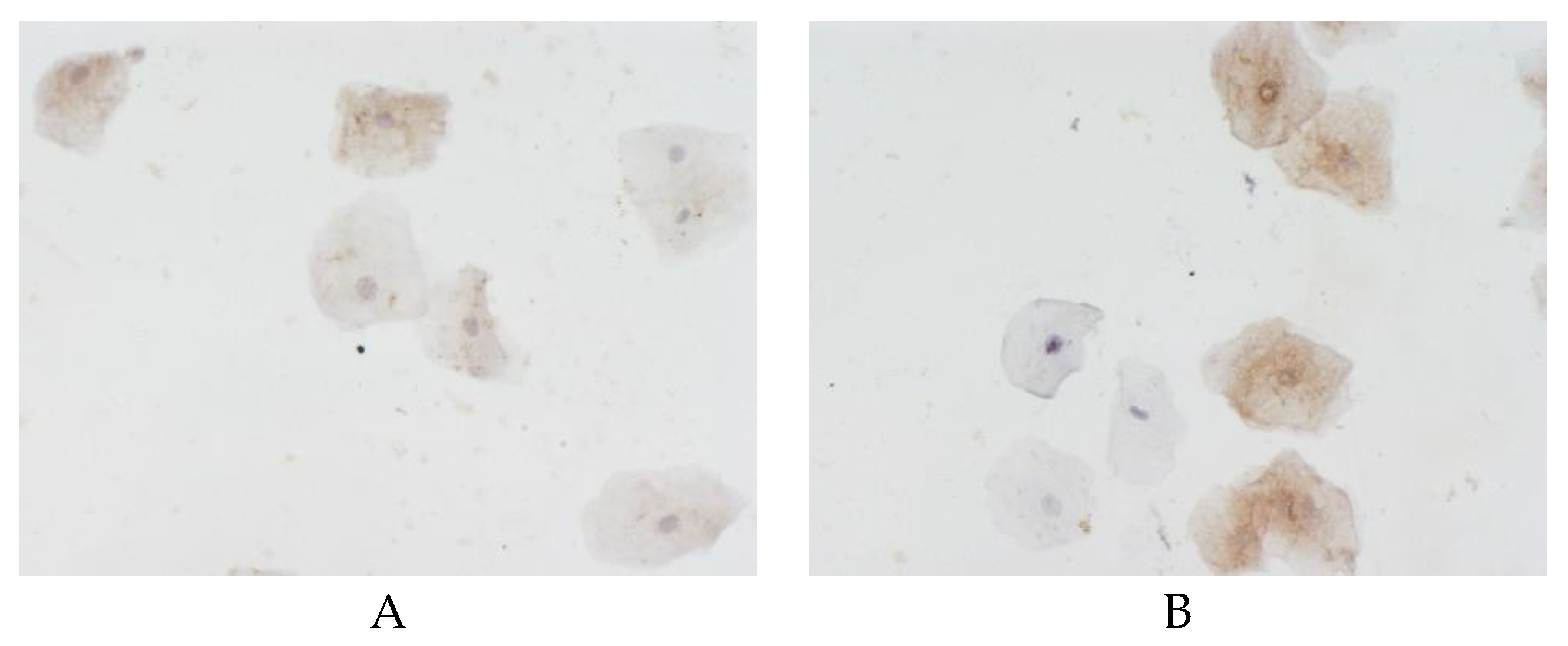
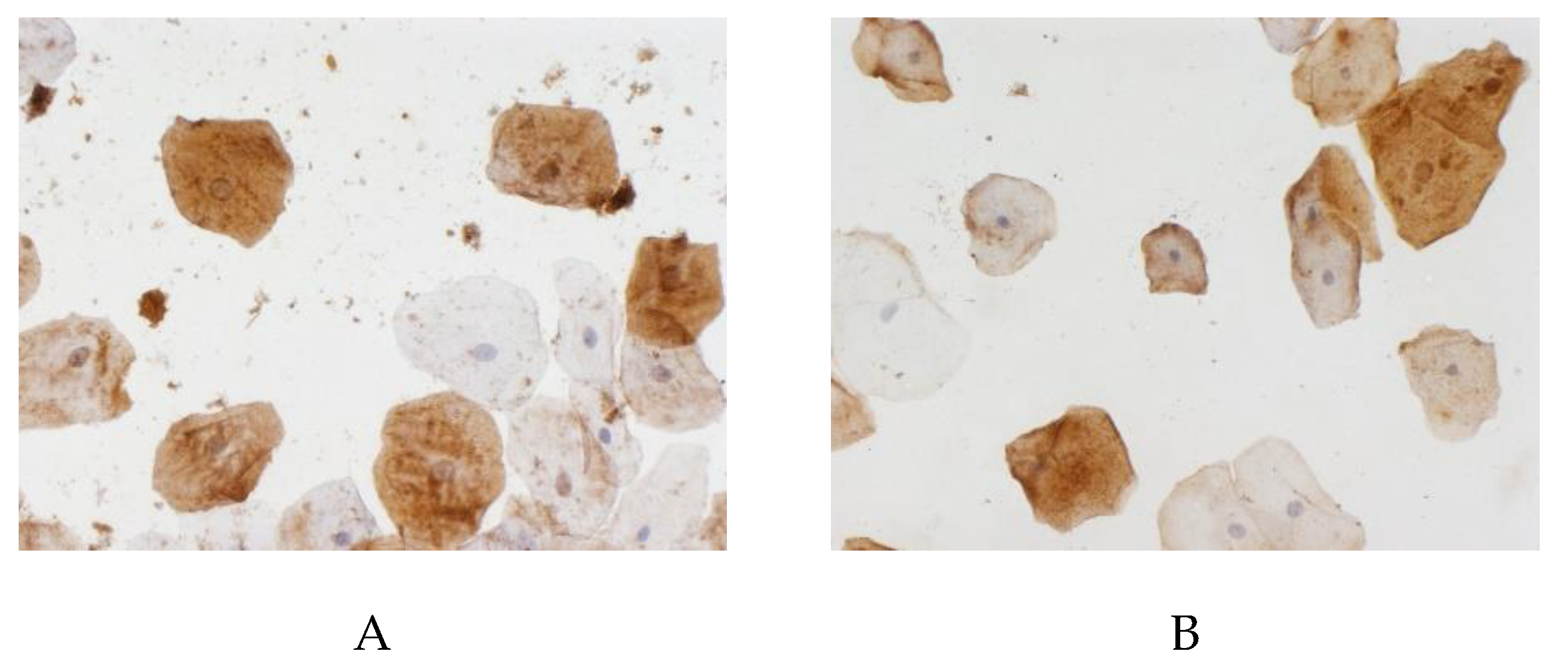
The proportion of cells with beta-amyloid expression in the buccal epithelium was highest (60.7% (50%–67.9%)) in the control group, as compared to 45.9% (31.3%–60%) in the vascular dementia group (p < 0.05). The area of beta-amyloid expression in the buccal epithelium in the patients with vascular dementia was statistically significantly lower than in the controls (
Figure 2). As follows from the data presented in
Table 1, in all the comparison groups, the differences in beta-amyloid expression in the buccal epithelium between males and females were within the margin of error.
The proportion of cells with CD34 protein expression in the buccal epithelium was higher (18.8% (9.8%–23.1%)) in the vascular dementia group than among the controls (15.9% (10.3%–28.8%)). However, no statistically significant differences were found in the proportion of cells with CD34 expression in the buccal epithelium between the comparison groups (p>0.05). Gender differences in CD34 protein expression in the buccal epithelium between males and females were statistically insignificant.
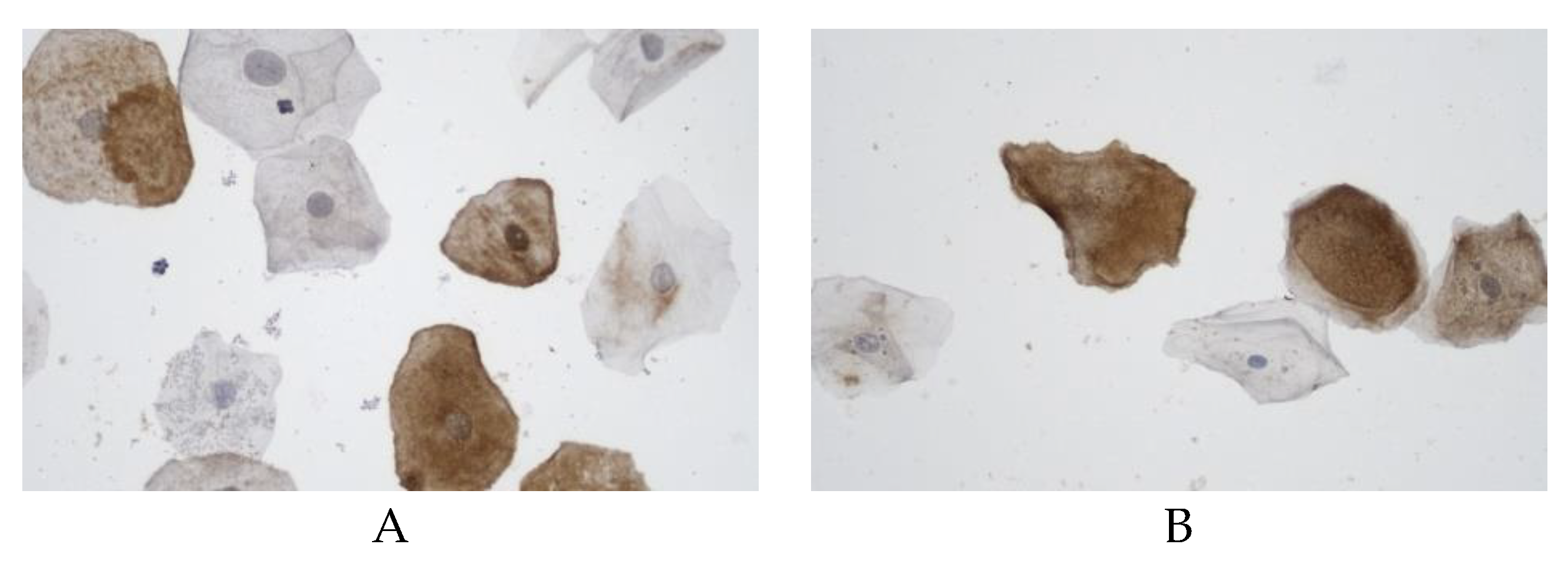
The proportion of cells with claudin expression in the buccal epithelium was statistically significantly higher (52.8% (46.9–57.1%)) among the controls than in the vascular dementia group (44.2% (40–46.8%)) (
Figure 3). The differences in claudin expression in the buccal epithelium in males and females were statistically insignificant in all the comparison groups.
The proportion of cells with DRP1 protein expression in the buccal epithelium was higher (29.4% (18.2–46.2%)) among the controls than in the vascular dementia group (28.6% (17.9–50%)). There were no statistically significant differences in the relative DRP1 expression in the buccal epithelium between the comparison groups (p>0.05). The differences in DRP1 protein expression in the buccal epithelium in males and females were statistically insignificant.
The proportion of cells with endothelin expression in the buccal epithelium was slightly higher (37.5% (14.3–57.7%)) in the vascular dementia group than among the controls (35.4 % (8.8%–66.7%)). No statistically significant differences were found (p>0.05).
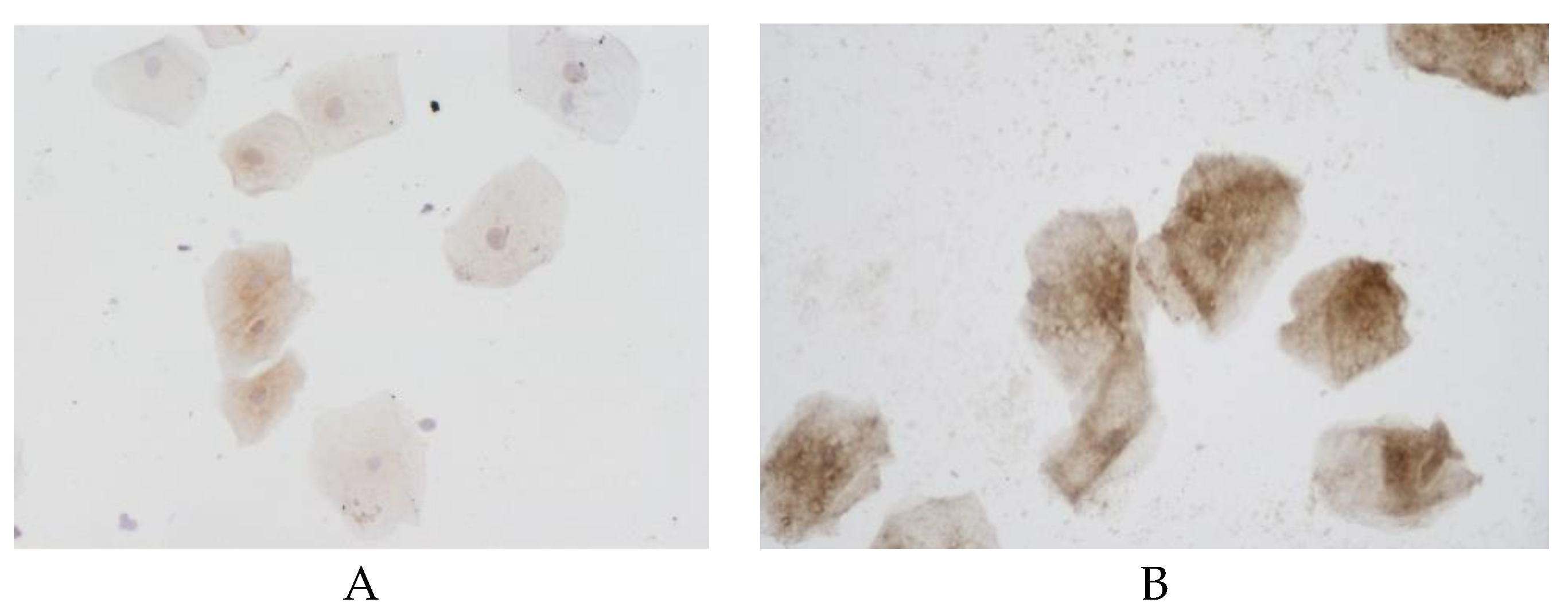
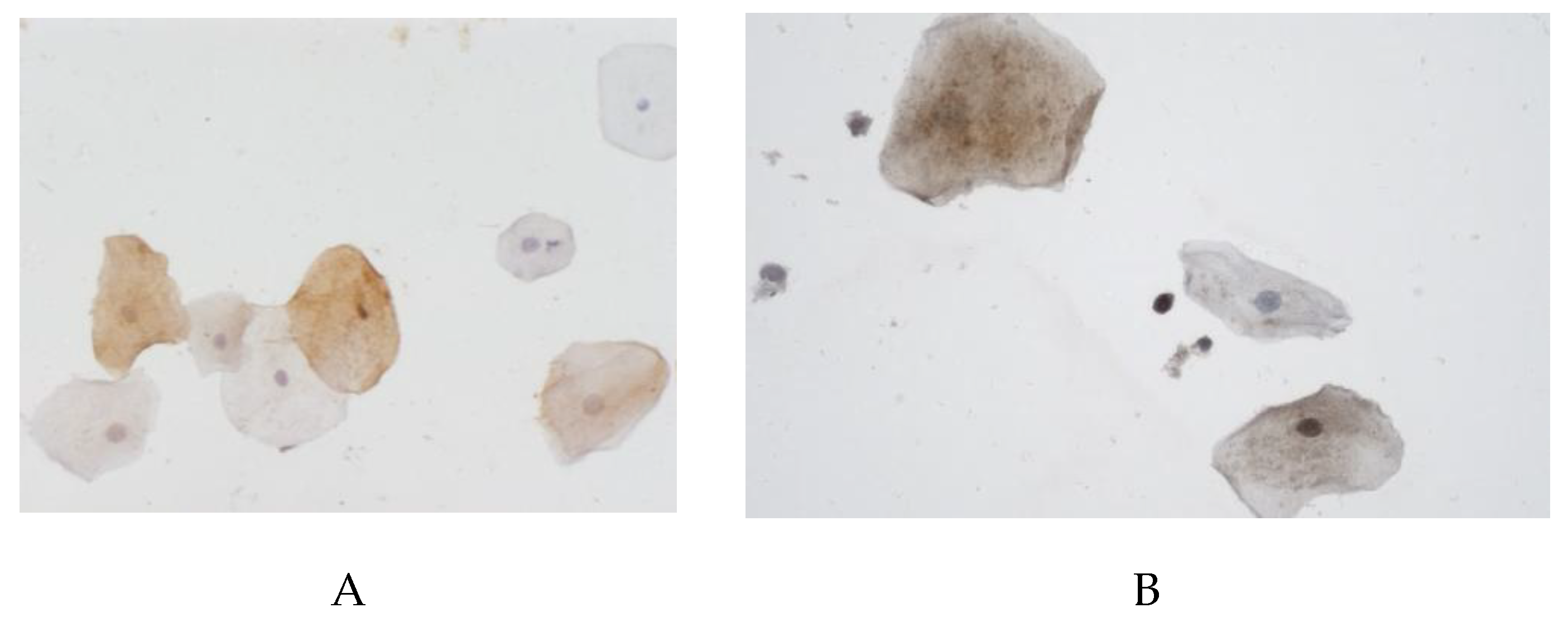
The proportion of cells with NF-kB expression in the buccal epithelium was statistically significantly lower in the group with vascular dementia (54.3% (21.4-57.1%)) as compared to the controls (79.2% (61.5–81.3%)) (
Figure 4).
The proportion of cells with PINK expression in the buccal epithelium was 86.1% (81.2%–90.7%)) in the control group and 85.6% (76.6–91.3%) in the vascular dementia group (p>0.05). The differences in PINK expression in the buccal epithelium in males and females were statistically insignificant in all the comparison groups.
The proportion of cells with RAGE expression in the buccal epithelium was 64.2% (21.8–77.1%) in the vascular dementia group, and 41.4 % (10–70.8 %) among the controls (p>0.05). The differences in RAGE expression in the buccal epithelium in males and females were statistically insignificant in all the comparison groups.
The proportion of cells with S100 protein expression in the buccal epithelium was high (84.2% (76.2–88%)) among the controls, while in the vascular dementia group it was 72.8% (67.9–92.3%) (
Figure 5). The differences were statistically significant (p>0.05). The differences in S100 protein expression in the buccal epithelium in males and females were statistically insignificant in all the comparison groups.
The proportion of cells with endothelin expression in the buccal epithelium was slightly higher (32.1 % (10.3–50 %)) in the vascular dementia group, and among the controls it was 31% (17.2–50%). No statistically significant differences were found between the groups (p>0.05), as in the comparison groups between males and females.
The proportion of cells with tau protein expression in the buccal epithelium in the group of patients with vascular dementia was statistically significantly lower (62.5% (45.5–71.4%)) than in the control group (69.6% (53.1–85.7%)) (
Figure 6).
Analyzing the results of tau protein verification, it should be noted that we previously verified the expression of phosphorylated (pathological) tau protein and other signaling molecules (biomarkers) in lymphocytes and buccal epithelium in patients with clinically diagnosed Alzheimer’s disease (AD) [
5].
Given these findings, in this study, it was decided to use antibodies to non-phosphorylated (normal) tau protein and to check the possibility of its expression as a negative control in buccal epithelial samples from volunteers as compared to a group of patients with vascular dementia.
Verification of non-phosphorylated tau protein in buccal epithelial samples and the presence of statistically significant differences in patients in the compared groups, confirms our previously described findings and allows to recommend the inclusion of phosphorylated tau protein in the diagnostic panel of molecular markers for neurodegenerative diseases.
The most pronounced and statistically significant changes in expression were observed for the following markers:
Beta-amyloid showed a statistically significant decrease in expression in patients with vascular dementia (both male and female) as compared to healthy controls (male: 44.5% and 62.4%, respectively, p<0.05; female: 47.1% and 60.3%, respectively, p<0.05).
The transcription factor NF-kB demonstrated gender differences in the vascular dementia group: its levels were higher in men (56.2%) than in women (23.3%), both figures being significantly lower than the control values (p<0.05).
Claudin was decreased in patients with vascular dementia (40.9% in male and 44.8% in female) as compared to the control group (57.1% and 50%, respectively, p<0.05).
The S100 protein showed the following dynamics: while maintaining high values in patients with vascular dementia (81.4% in male and 72.8% in female), these figures were statistically significantly lower than those of the controls (p<0.05).
Tau protein was expressed at lower levels in patients with vascular dementia (62.5% in male and 62% in female) as compared to the control group (74.2% and 66.7%, respectively, p<0.05).
It should be noted that no statistically significant differences in the expression of some markers (DRP1 protein, endothelin-1, PINK, RAGE, and synuclein) were found between the group of patients with vascular dementia and the control group, which points to their lower diagnostic value for screening of this pathology.
Thus, this study has identified and validated a panel of key molecular biomarkers that are pathogenetically associated with the onset of vascular dementia. Our findings demonstrate that the buccal epithelium is a promising material for the non-invasive diagnosis of cognitive impairments.
A comprehensive assessment of β-amyloid, NF-kB, claudin, S100 protein, and tau protein expression in the buccal epithelium may become an effective tool for identifying cognitive impairments in dementia due to neurovascular pathologies. The revealed gender-specific changes in NF-kB expression are particularly significant and require additional investigation in further studies.
Conclusion
The conducted study enabled us to verify the expression of key neurotropic signaling molecules and their direct pathogenetic link with the development of dementia of various origins in peripheral tissue (buccal epithelial cells).
Due to its accessibility and informativeness, the buccal epithelium is a promising material for the non-invasive diagnosis of cognitive impairments. The obtained data provides evidence that a comprehensive assessment of the expression of such signaling molecules as β-amyloid, transcription factor NF-kB, claudin, S100 and tau protein in the buccal epithelium can be an effective non-invasive method for identifying cognitive impairments, and these molecules should be recommended for use as an informative diagnostic panel of molecular biomarkers.
References
- Abdul Y, Karakaya E, Chandran R, Jamil S, Ergul A. Endothelin A receptors contribute to senescence of brain microvascular endothelial cells. Can J Physiol Pharmacol. 2022;100(12):1087-1096. [CrossRef]
- Kurmyshev M.V., Ivko O.M., Ponomarev A.S., Gavrilova A.A., Fesenko E.V., Prashchayeu K.I., Litvinov M.S. Clinical determinants for the targeted effects of neurocognitive rehabilitation in elderly and senile patients with mild cognitive impairment. Advancement of gerontology. 2024. Vol. 37, № 6. P. 731–736. [CrossRef]
- Baecker J, Wartchow K, Sehm T, Ghoochani A, Buchfelder M, Kleindienst A. Treatment with the Neurotrophic Protein S100B Increases Synaptogenesis after Traumatic Brain Injury. J Neurotrauma. 2020;37(8):1097-1107. [CrossRef]
- Langeh U, Singh S. Targeting S100B Protein as a Surrogate Biomarker and its Role in Various Neurological Disorders. Curr. Neuropharmacol. 2021;19(2):265-277. [CrossRef]
- Kvetnoy I.M., Hernandez-Yago J., Kvetnaia T.V., Khavinson V. Kh., Malinin V.V., Yarilin A.A. Tau-protein expression in human blood lymphocytes: promising marker and suitable sample for life-time diagnosis of Alzheimer’s disease. Neuroendocrinol. Lett., 2000, V.21, р. 313-318.
- Barton M, Yanagisawa M. Endothelin: 30 Years from Discovery to Therapy. Hypertension. 2019;74(6):1232-1265. [CrossRef]
- Kvetnoy I., Kheifets O., Safaniev L., Kheifets V., Mironova E., Prashchayeu K. Alzheimer’s Desease: Non-Invasive Method and An Immunocytochemical Panel of Molecular Makers for Screening and Life-Time Diagnosis. Am. J. of Biomedical Sciences & Research. 2025; 28(2):251-256.
- Markus HS, Hunt D, Palmer K, Enzinger C, Schmidt H, Schmidt R. Markers of endothelial and hemostatic activation and progression of cerebral white matter hyperintensities: longitudinal results of the Austrian Stroke Prevention Study. Stroke. 2005;36(7):1410–1414.
- Yuan K, Park BM, Choi YT, Kim JH, Cho KW, Kim SH. Effects of endothelin family on ANP secretion. Peptides. 2016;82:12-19. [CrossRef]
- Sorbi S, Hort J, Erkinjuntti T, Fladby T, Gainotti G, Gurvit H, Nacmias B, Pasquier F, Popescu BO, Rektorova I, Religa D, Rusina R, Rossor M, Schmidt R, Stefanova E, Warren JD, Scheltens P; EFNS Scientist Panel on Dementia and Cognitive Neurology. EFNS-ENS Guidelines on the diagnosis and management of disorders associated with dementia. Eur J Neurol. 2012 Sep;19(9):1159-79. [CrossRef] [PubMed]
- Skrobot OA, O’Brien J, Black S, Chen C, DeCarli C, Erkinjuntti T, Ford GA, Kalaria RN, Pantoni L, Pasquier F, Roman GC, Wallin A, Sachdev P, Skoog I; VICCCS group; Ben-Shlomo Y, Passmore AP, Love S, Kehoe PG. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimer’s Dement. 2017 Jun;13(6):624-633. Epub 2016 Dec 10. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).