Submitted:
25 October 2025
Posted:
28 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
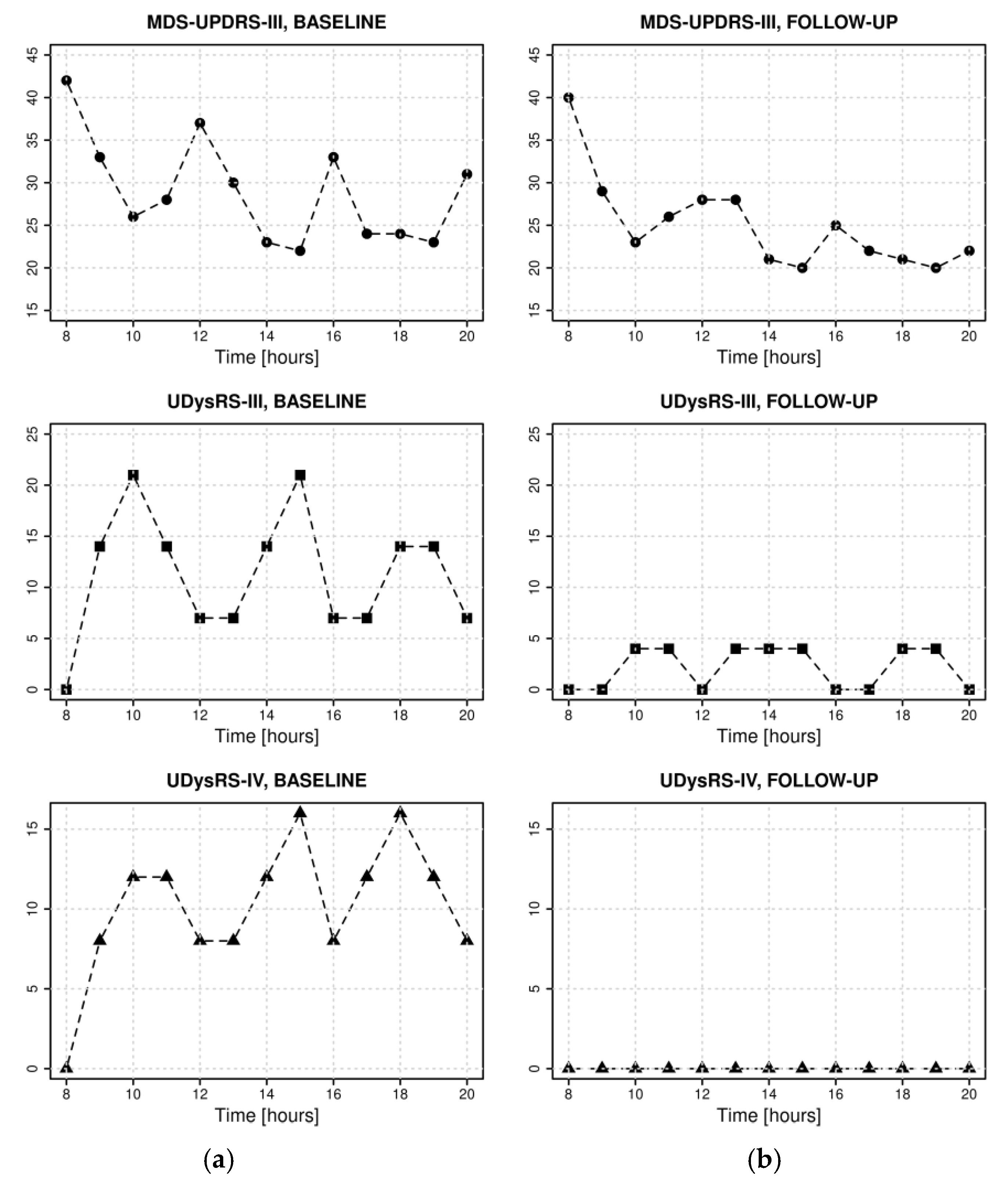
2. Case Report
3. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borgohain R, Szasz J, Stanzione P, et al. Randomized trial of safinamide add-on levodopa in Parkinson’s disease with motor fluctuations. Mov Disord. 2014;29:229-237. [CrossRef]
- Schapira AH. Safinamide in the treatment of Parkinson’s disease. Expert Opin Pharmacother. 2010;11:2261-2268. [CrossRef]
- Cattaneo C, La Ferla R, Bonizzoni E, Sardina M. Long-term effects of safinamide on dyskinesia in mid- to late-stage Parkinson’s disease: a post-hoc analysis. J Parkinsons Dis. 2015;5:475-481. [CrossRef]
- Bette S, Mollenhauer B, Trenkwalder C. Safinamide in the management of patients with Parkinson’s disease. Ther Adv Neurol Disord. 2018;11:1756286418789431. [CrossRef]
- Antonini A, Martinez-Martin P, Chaudhuri RK, et al. Wearing-off scales in Parkinson’s disease: critique and recommendations. Mov Disord. 2011;26(12):2169-2175. [CrossRef]
- Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord. 2015;30:1591-1601. [CrossRef]
- Abbruzzese G, Antonini A, Barone P, et al. Linguistic, psychometric validation and diagnostic ability assessment of an Italian version of a 19-item wearing-off questionnaire for wearing-off detection in Parkinson’s disease. Neurol Sci. 2012;33:1319-1327. [CrossRef]
- Christopher G, Glenn T, Theeuwes A, et al. Temporal stability of the Unified Dyskinesia Rating Scale. Mov Disord. 2011;26:—. [CrossRef]
- Schrag A, Quinn N. Dyskinesias and motor fluctuations in Parkinson's disease: a community-based study. Brain. 2000;123(Pt 11):2297-2305. [CrossRef]
- Calon F, Rajput AH, Hornykiewicz O, Bédard PJ, Di Paolo T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol Dis. 2003;14:404-416. [CrossRef]
- Luginger E, Wenning GK, Bösch S, Poewe W. Beneficial effects of amantadine on L-dopa-induced dyskinesias in Parkinson’s disease. Mov Disord. 2000;15:873-878. [CrossRef]
- Grégoire L, Jourdain VA, Townsend M, Roach A, Di Paolo T. Safinamide reduces dyskinesias and prolongs L-DOPA antiparkinsonian effect in parkinsonian monkeys. Parkinsonism Relat Disord. 2013;19:508-514. [CrossRef]
- Guerra A, Asci F, Zampogna A, D’Onofrio V, Suppa A, Fabbrini G, Berardelli A. Long-term changes in short-interval intracortical facilitation modulate motor cortex plasticity and L-dopa-induced dyskinesia in Parkinson’s disease. Brain Stimul. 2022;15:99-108. [CrossRef]
- Kwon DK, Kim A, et al. Levodopa-Induced Dyskinesia in Parkinson’s Disease: mechanisms and management. Cells. 2022;11(23):3736. [CrossRef]
- Yang K, et al. Circuit mechanisms of L-DOPA-induced dyskinesia. Front Neurosci. 2021;15:614412. [CrossRef]
- Bhidayasiri R, et al. Sustained response in early responders to safinamide (post-hoc SETTLE). Front Neurol. 2023;14:1147008. [CrossRef]
- Nishikawa N, et al. Safinamide as adjunctive therapy to levodopa in older patients: motor, non-motor and quality-of-life outcomes. J Neurol Sci. 2024;451:123726. [CrossRef]
| Experience symptoms | Symptoms that improve after the next dose |
|||||
| WOQ-19 | Total score | Non-motor symptoms |
Motor symptoms | Total score | Non-motor symptoms |
Motor symptoms |
| Baseline | 11 | 6 | 5 | 8 | 5 | 3 |
| Follow-up | 6 | 3 | 3 | 3 | 2 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





