Submitted:
25 October 2025
Posted:
27 October 2025
You are already at the latest version
Abstract
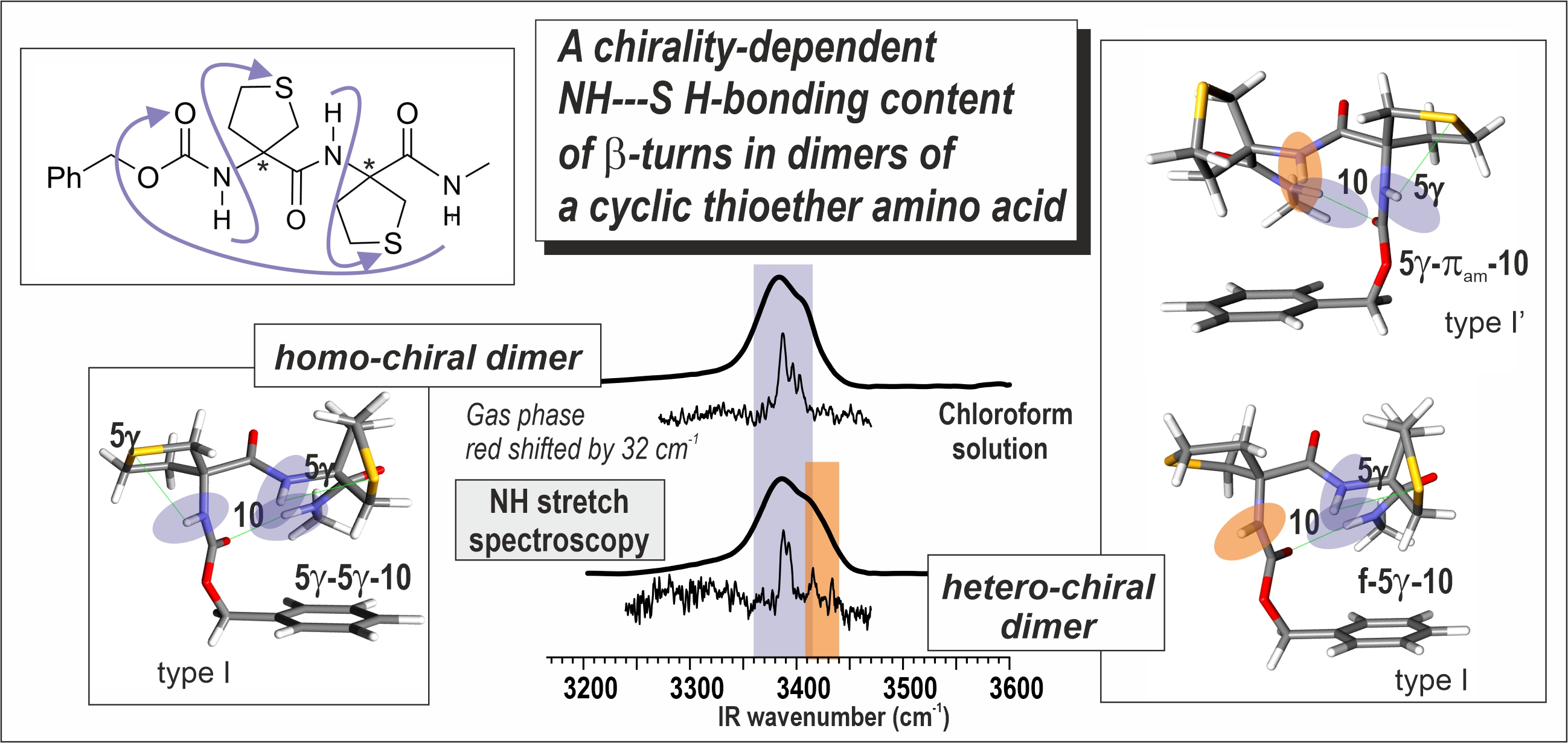
Keywords:
1. Introduction
2. Results
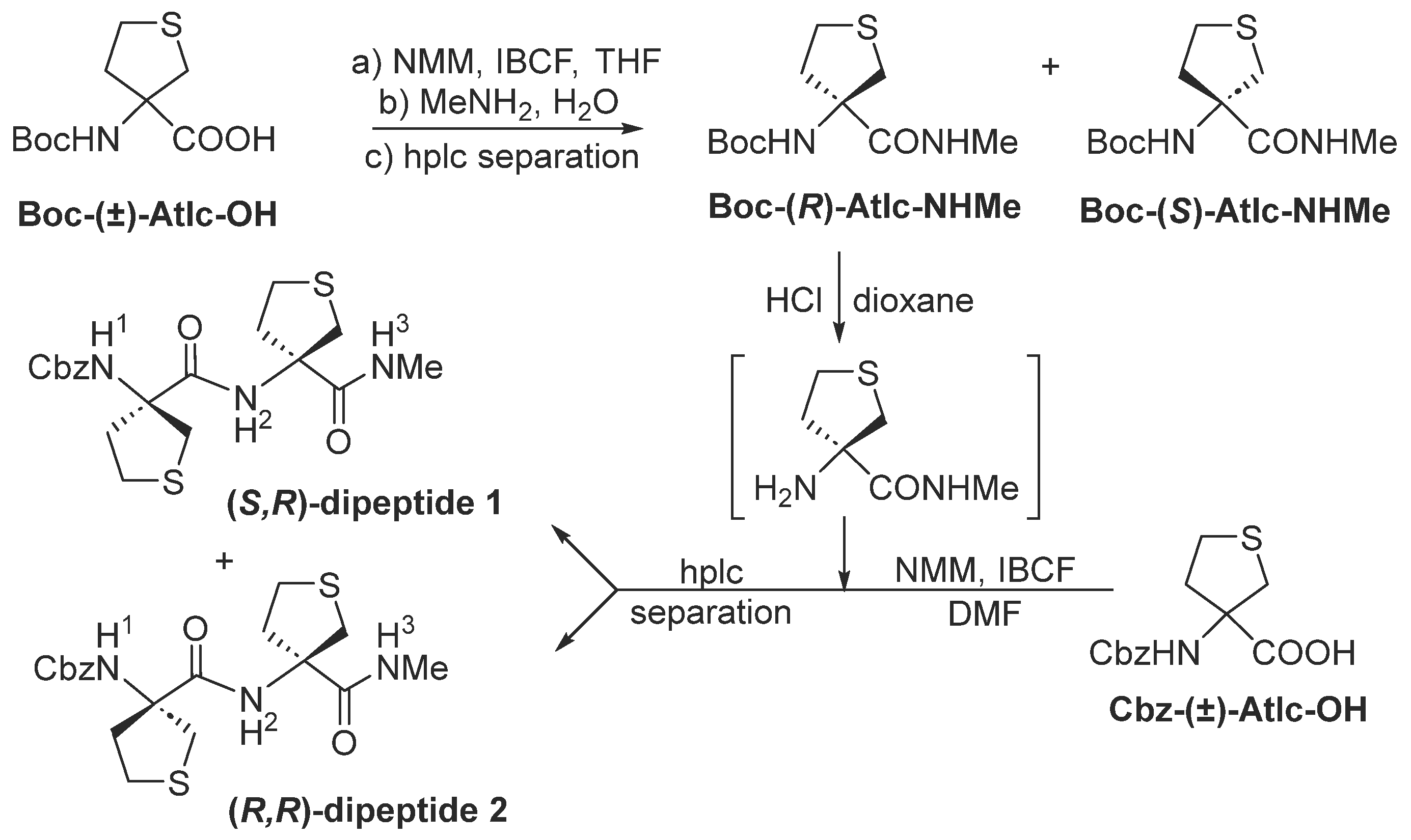
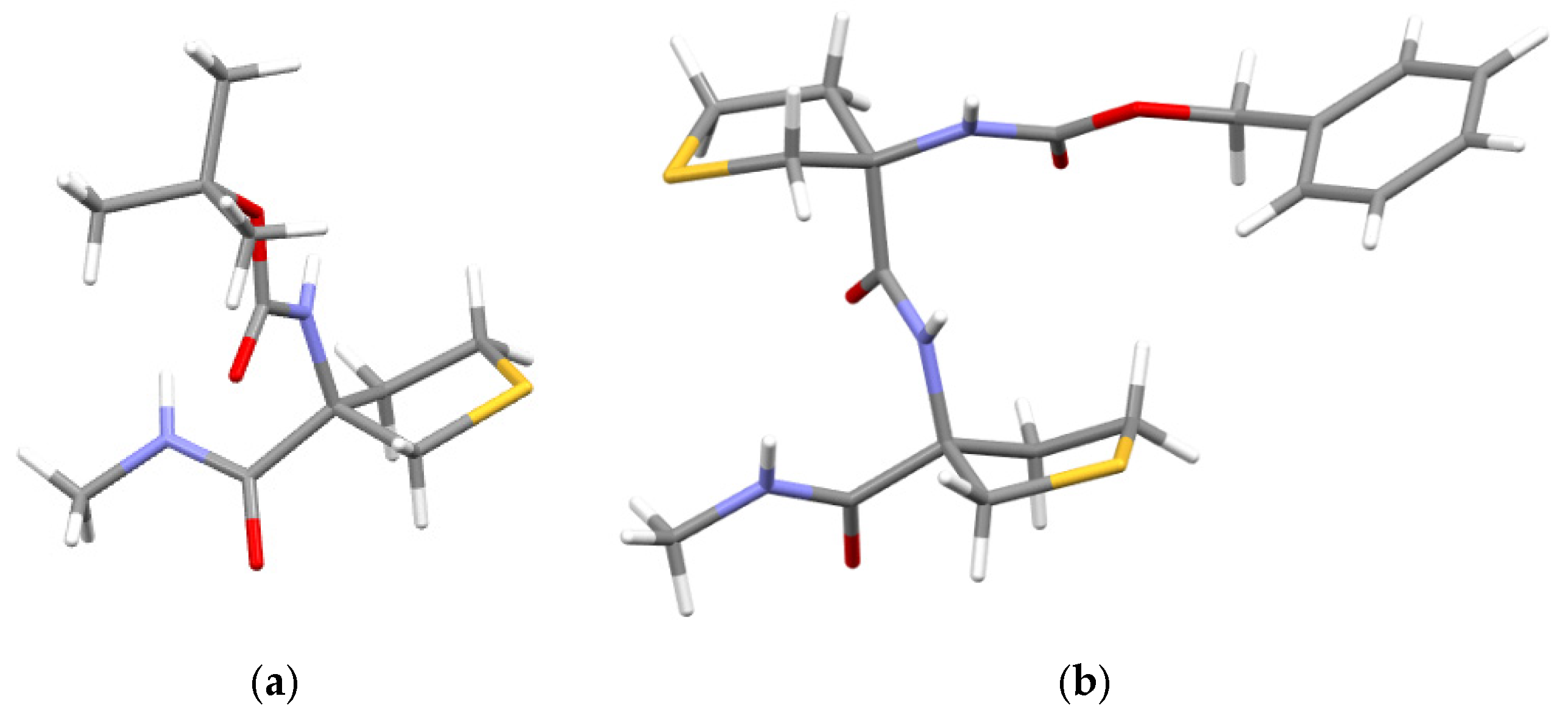
2.1. Synthesis
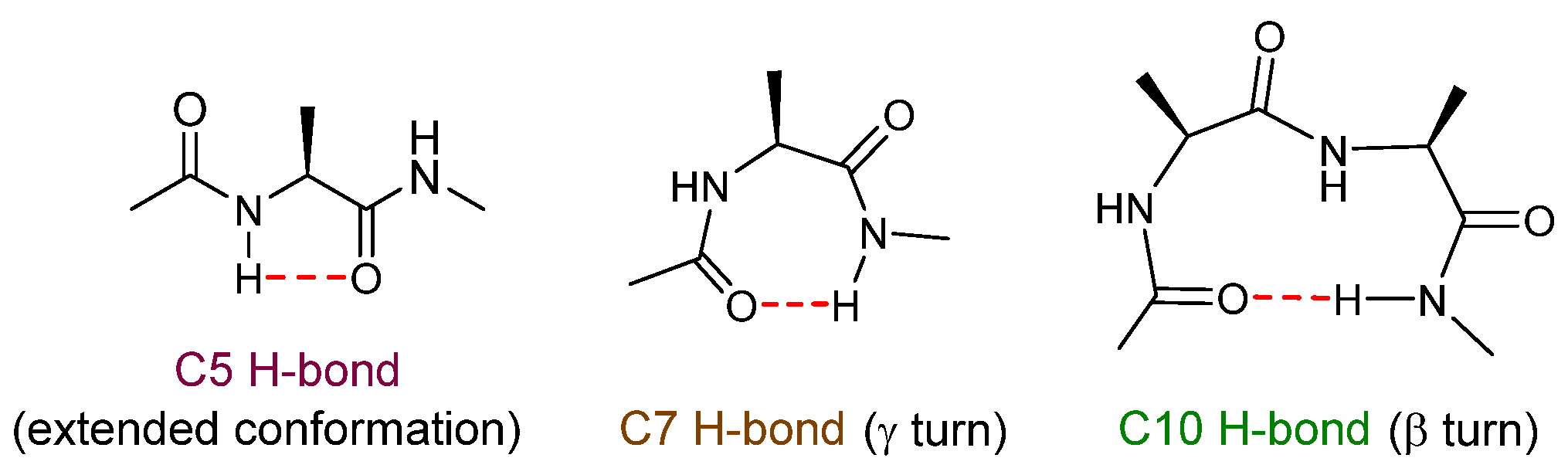
2.2. Gas Phase Conformational Analysis
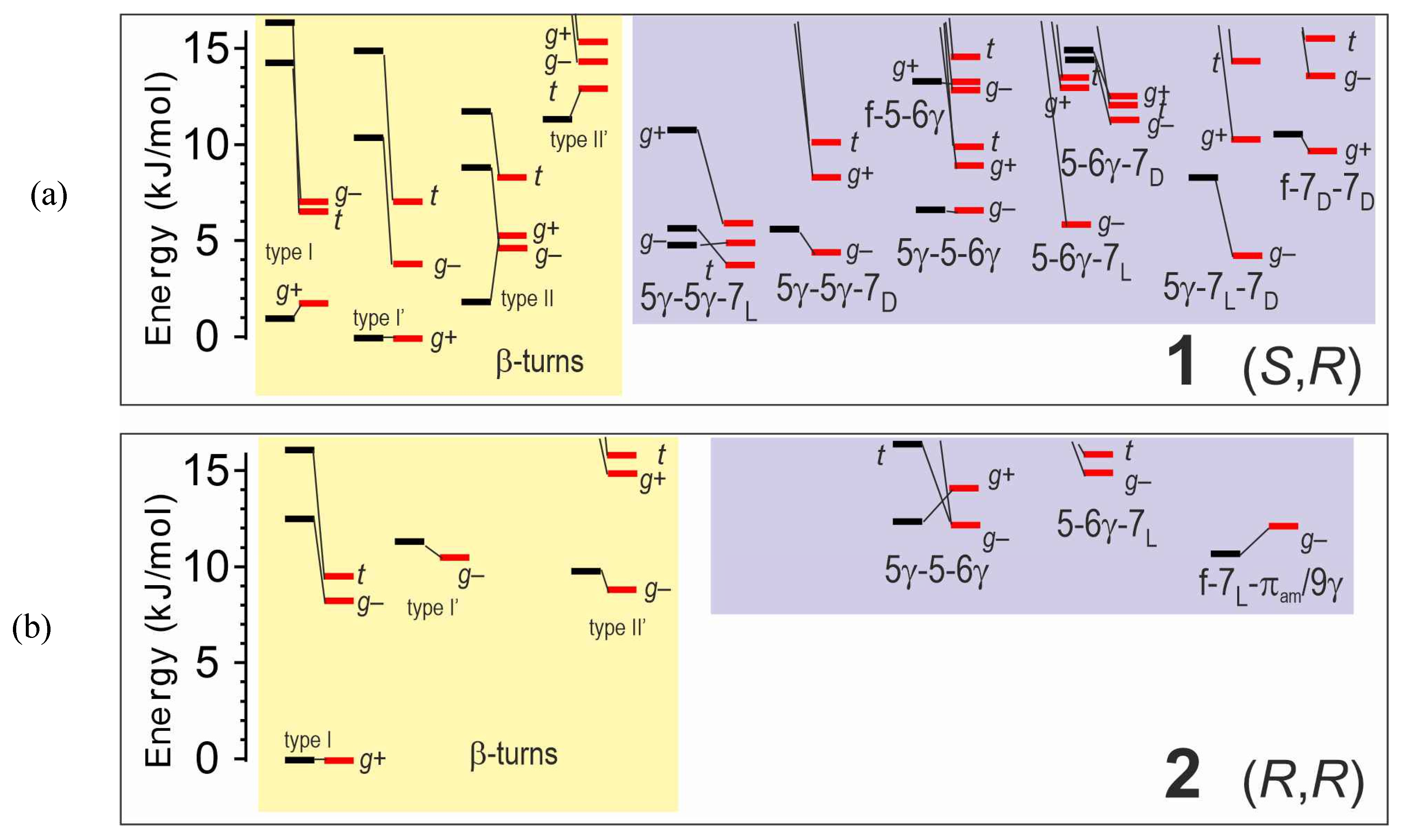
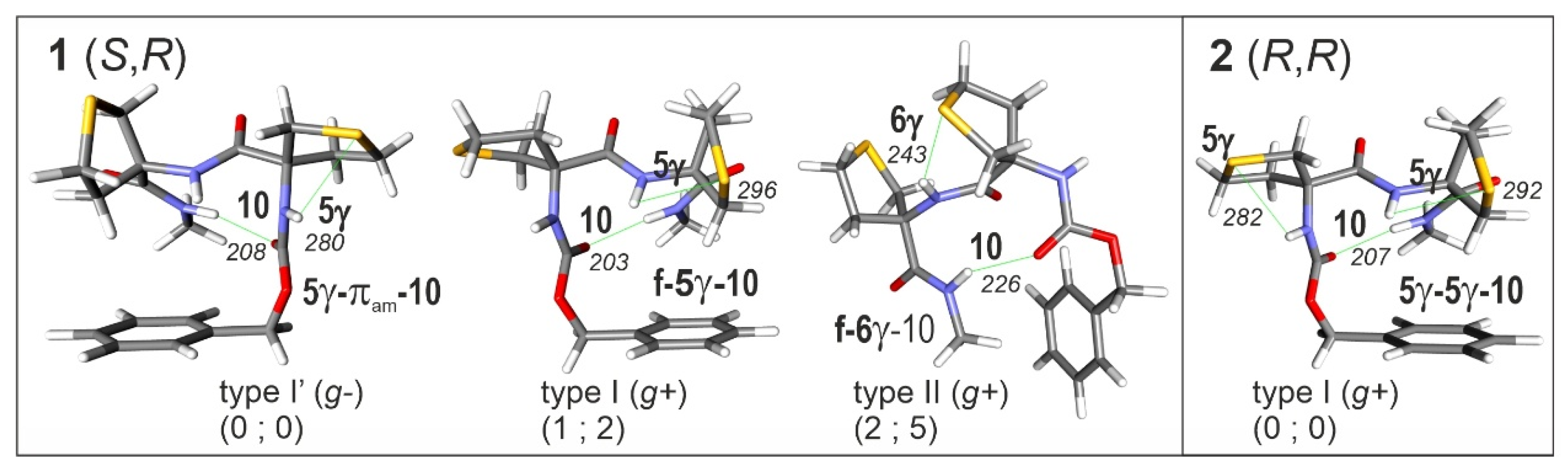
2.1.1. Theoretical Landscapes and Structures
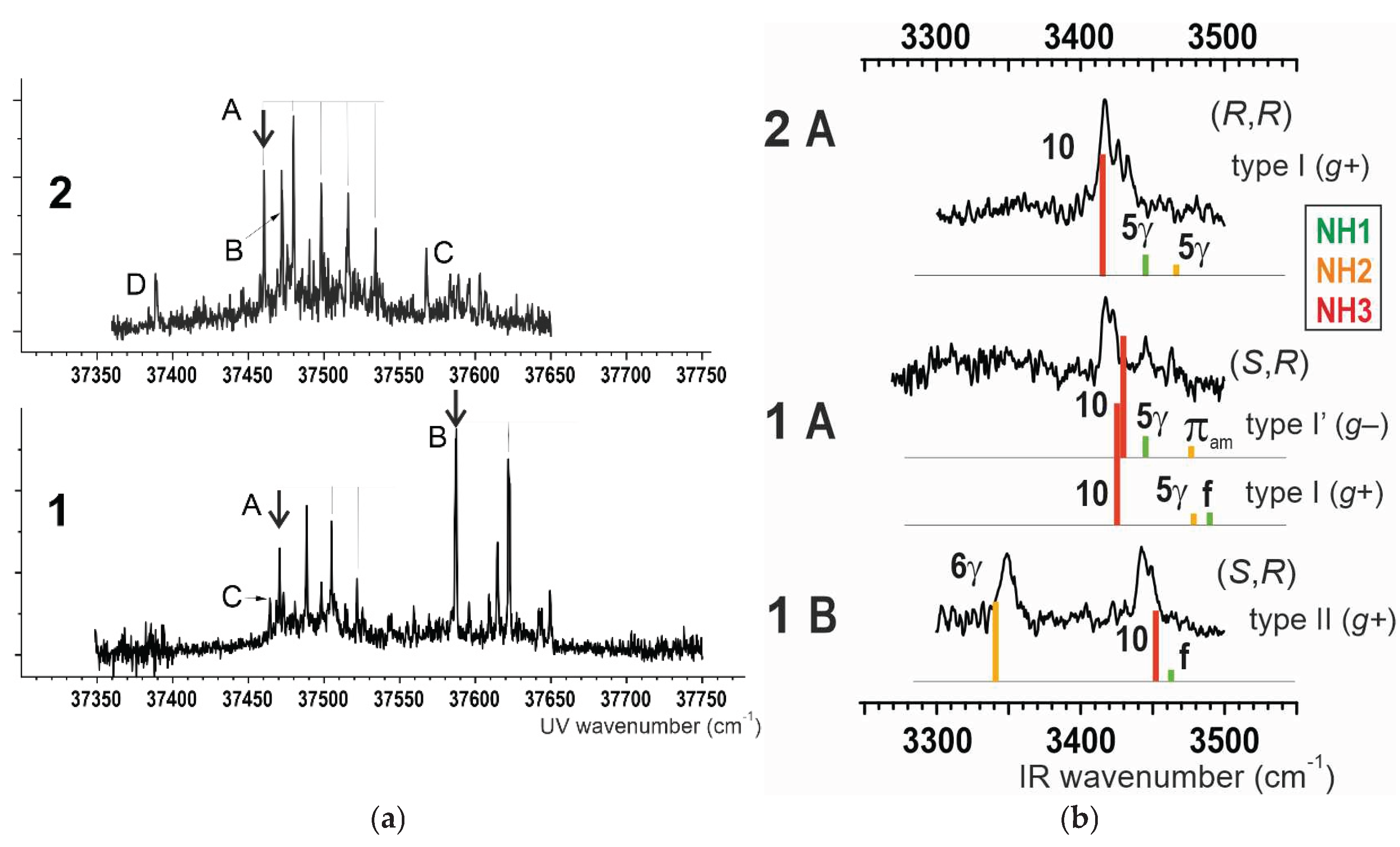
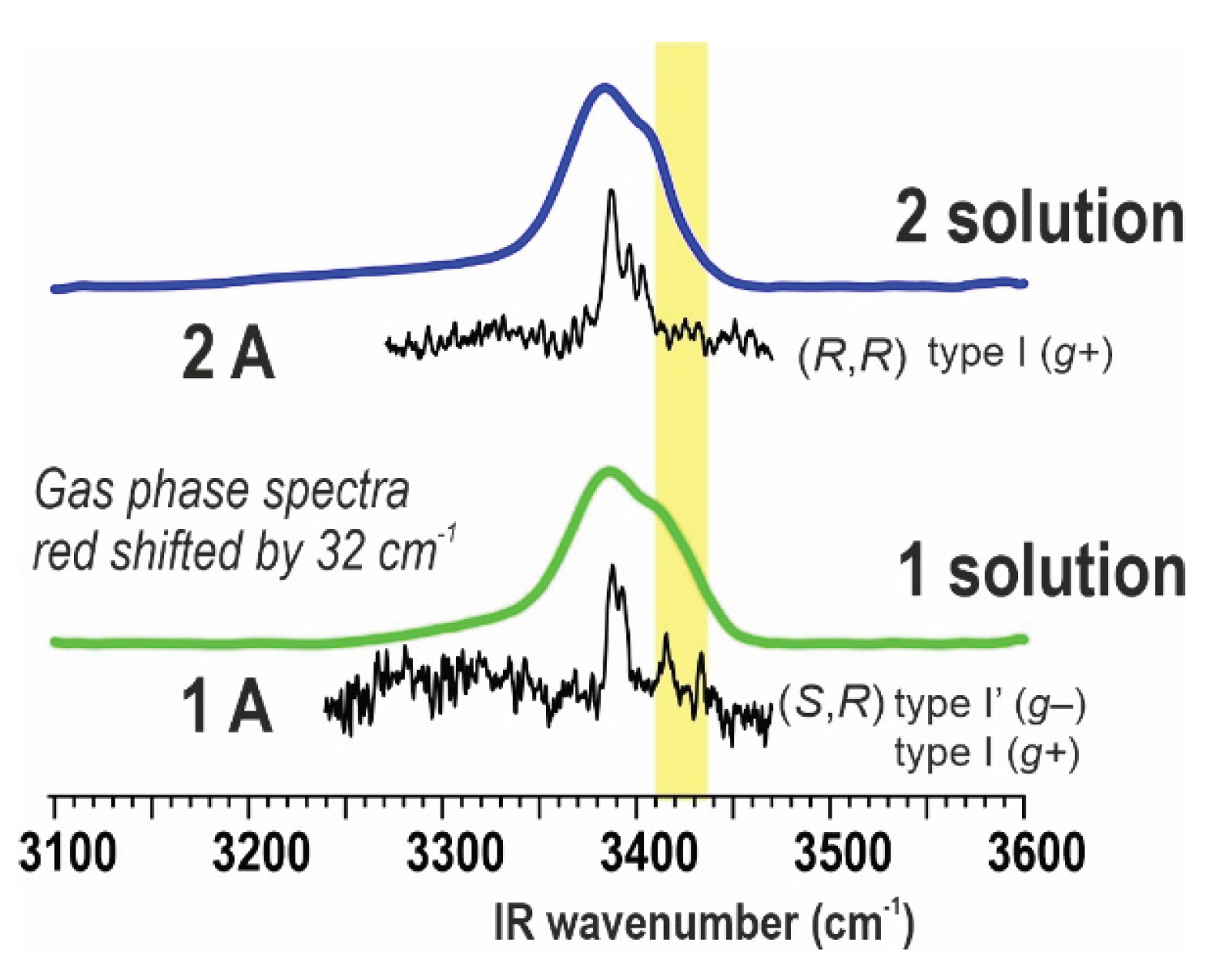
2.1.2. Gas Phase Laser Spectroscopy
2.3. Solution Phase Conformational Analysis
2.3.1. Theoretical Landscapes
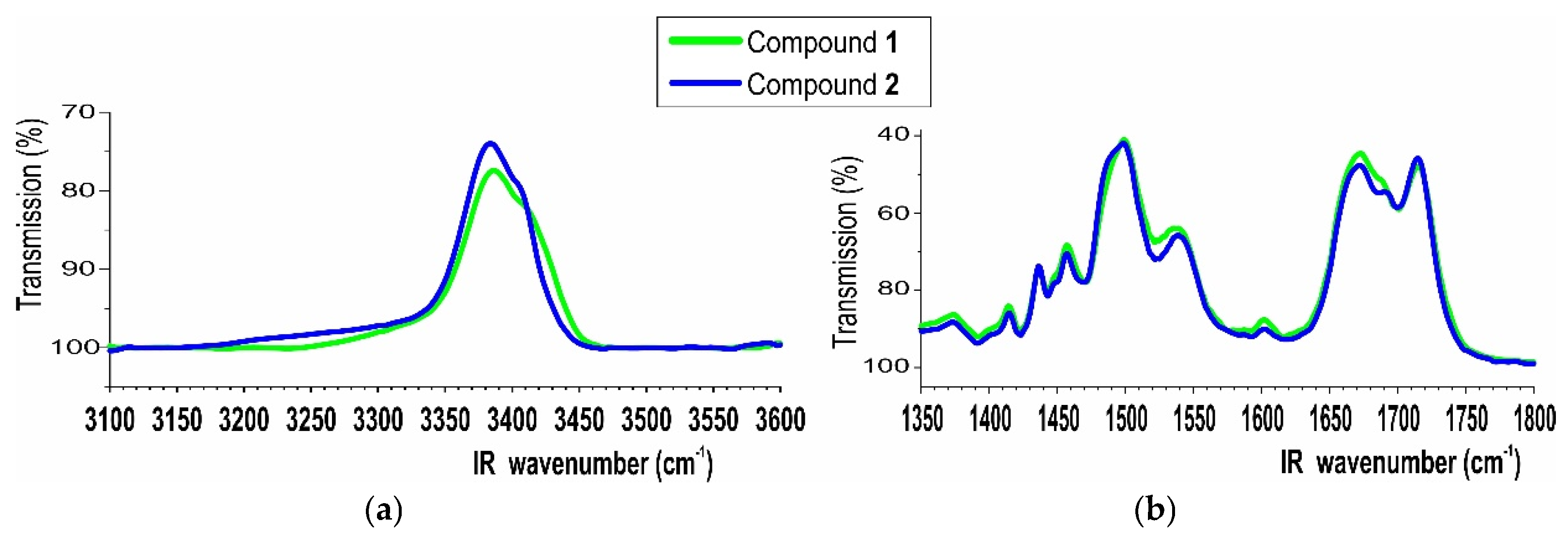
2.3.2. IR Spectroscopy
2.3.3. NMR Spectroscopy
2.4. Concluding Remarks
3. Materials and Methods
3.1. Synthesis and Structure Characterization
3.1.1. General Information
3.1.2. Preparation of Boc–(R)-Atlc–NHMe and Boc–(S)-Atlc–NHMe
3.1.3. Preparation of (S,R)-Dipeptide 1 and (R,R)-Dipeptide 2
3.1.4. X-Ray Diffraction Studies
3.2. Theoretical Chemistry
3.3. Gas Phase Experimental
3.4. Solution Phase Spectroscopic Studies
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karshikoff, A., Non-covalent Interactions in Proteins. Imperial College Press: London, UK, 2006.
- Jeffrey, G. A.; Sänger, W., Hydrogen Bonding in Biological Structures. Springer-Verlag: Berlin, Germany, 1991.
- Perrin, C. L.; Nielson, J. B., ‘’Strong’’ hydrogen bonds in chemistry and biology. Annu. Rev. Phys. Chem. 1997, 48, 511-544. [CrossRef]
- Baker, E. N.; Hubbard, R. E., Hydrogen-bonding in globular-proteins. Progr. Biophys. Mol. Biol. 1984, 44, 97-179. [CrossRef]
- Toniolo, C., Intramolecularly Hydrogen-Bonded Peptide Conformations. CRC Crit.Rev.Biochem. 1980, 9, 1-44. [CrossRef]
- Rose, G. D.; Gierasch, L. M.; Smith, J. A., Turns in Peptides and Proteins. Adv. Protein Chem. 1985, 37, 1-109. [CrossRef]
- Peggion, C.; Moretto, A.; Formaggio, F.; Crisma, M.; Toniolo, C., Multiple, Consecutive, Fully-Extended 2.0(5)-Helix Peptide Conformation. Biopolymers 2013, 100, 621-636. [CrossRef]
- Crisma, M.; Formaggio, F.; Alemán, C.; Torras, J.; Ramakrishnan, C.; Kalmankar, N.; Balaram, P.; Toniolo, C., The fully-extended conformation in peptides and proteins. Pep. Sci. 2018, 110, e23100. [CrossRef]
- Milner-White, E. J., Situations of gamma-turns in proteins - their relation to alpha-helices, beta-sheets and ligand-binding sites. J. Mol. Biol. 1990, 216, 385-397. [CrossRef]
- Crisma, M.; De Zotti, M.; Moretto, A.; Peggion, C.; Drouillat, B.; Wright, K.; Couty, F.; Toniolo, C.; Formaggio, F., Single and multiple peptide γ-turns: literature survey and recent progress. New J. Chem. 2015, 39, 3208-3216. [CrossRef]
- Smith, J. A.; Pease, L. G., Reverse Turns in Peptides and Proteins. CRC Crit.Rev.Biochem. 1980, 8, 315-399. [CrossRef]
- Wilmot, C. M.; Thornton, J. M., Analysis and prediction of the different types of beta-turn in proteins. J. Mol. Biol. 1988, 203, 221-232. [CrossRef]
- de Brevern, A. G., Extension of the Classical Classification of Beta-Turns. Sci. Rep. 2016, 6, 33191. [CrossRef]
- Bordo, D.; Argos, P., The role of side-chain hydrogen-bonds in the formation and stabilization of secondary structure in soluble-proteins. J. Mol. Biol. 1994, 243, 504-519. [CrossRef]
- Eswar, N.; Ramakrishnan, C., Deterministic Features of Side-Chain Main-Chain Hydrogen Bonds in Globular Protein Structures. Protein Eng. 2000, 13, 227-238. [CrossRef]
- Vijayakumar, M.; Qian, H.; Zhou, H. X., Hydrogen bonds between short polar side chains and peptide backbone: Prevalence in proteins and effects on helix-forming propensities. Proteins 1999, 34, 497-507. [CrossRef]
- Wan, W. Y.; Milner-White, E. J., A Natural Grouping of Motifs with an Aspartate or Asparagine Residue Forming Two Hydrogen Bonds to Residues Ahead in Sequence: Their Occurrence at Alpha-Helical N Termini and in Other Situations. J. Mol. Biol. 1999, 286, 1633-1649. [CrossRef]
- D’Mello, V. C.; Goldsztejn, G.; Mundlapati, V. R.; Brenner, V.; Gloaguen, E.; Charnay-Pouget, F.; Aitken, D. J.; Mons, M., Characterization of Asx Turn Types and Their Connate Relationship with β-Turns. Chem. Eur. J. 2022, 28. [CrossRef]
- Zhou, P.; Tian, F. F.; Lv, F. L.; Shang, Z. C., Geometric characteristics of hydrogen bonds involving sulfur atoms in proteins. Proteins 2009, 76, 151-163. [CrossRef]
- Avignon, M.; Huong, P. V.; Lascombe, J.; Marraud, M.; Néel, J., Infrared spectroscopy of some model peptide conformations. Biopolymers 1969, 8, 69-89. [CrossRef]
- Cung, M. T.; Marraud, M.; Néel, J. Etude expérimentale de la conformation de molécules dipeptidiques - Comparaison avec les résultats théoriques. Ann. Chimie 1972, 7, 183–209. [Google Scholar]
- Ribeiro, A. A.; Goodman, M.; Naider, F. Preferred conformations of protected homodi-to homoheptamethionine peptides - H1-NMR study in deuterochloroform medium. Int. J. Pept. Protein Res. 1979, 14, 414–436. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, M.; Darin, S.; Bonora, G. M.; Toniolo, C. Linear oligopeptides .29. Infrared conformational-analysis of homo-oligopeptides in solid-state and in solution. Macromol. Chem. Phys. 1976, 177, 1477–1492. [Google Scholar] [CrossRef]
- Néel, J. Experimental study of the influence of specific intramolecular interactions on the conformation of model molecules. (Peptides and oligopeptides). Pure Appl. Chem. 1972, 31, 201–225. [Google Scholar] [CrossRef] [PubMed]
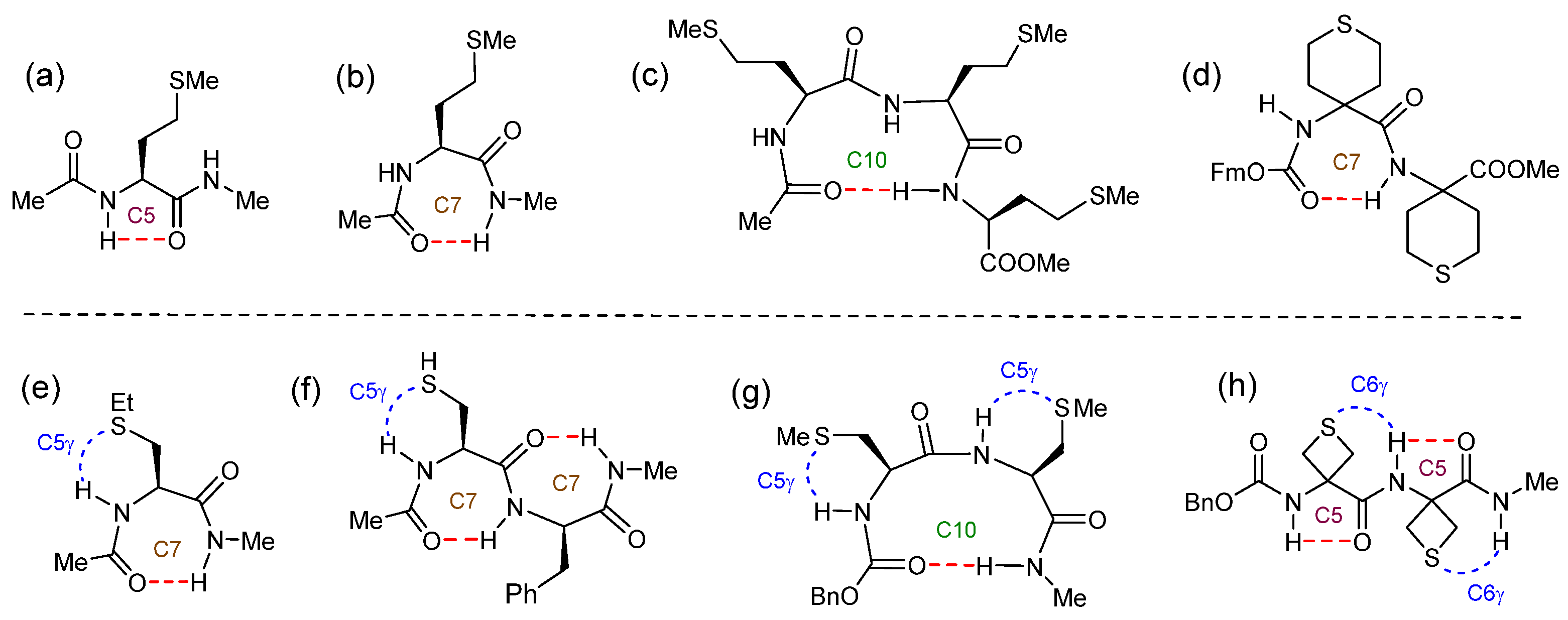
- Alauddin, M.; Biswal, H. S.; Gloaguen, E.; Mons, M., Intra-residue interactions in proteins: interplay between serine or cysteine side chains and backbone conformations, revealed by laser spectroscopy of isolated model peptides. Phys. Chem. Chem. Phys. 2015, 17, 2169-2178. [CrossRef]
- Biswal, H. S.; Gloaguen, E.; Loquais, Y.; Tardivel, B.; Mons, M., Strength of NH...S Hydrogen Bonds in Methionine Residues Revealed by Gas-Phase IR/UV Spectroscopy. J. Phys. Chem. Lett. 2012, 3, 755-759. [CrossRef]
- Mundlapati, V. R.; Imani, Z.; Goldsztejn, G.; Gloaguen, E.; Brenner, V.; Le Barbu-Debus, K.; Zehnacker-Rentien, A.; Baltaze, J. P.; Robin, S.; Mons, M.; Aitken, D. J., A theoretical and experimental case study of the hydrogen bonding predilection of S-methylcysteine. Amino Acids 2021, 53, 621-633. [CrossRef]
- De Zotti, M.; Clayden, J., Extended Diethylglycine Homopeptides Formed by Desulfurization of Their Tetrahydrothiopyran Analogues. Org. Lett. 2019, 21, 2209-2212. [CrossRef]
- Paradisi, M. P.; Torrini, I.; Zecchini, G. P.; Lucente, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G. Gamma-turn conformation induced by alpha,alpha-disubstituted amino-acids with a cyclic 6-membered side-chain. Tetrahedron-Asymmetry 1995, 51, 2379–2386. [Google Scholar] [CrossRef]
- Torrini, I.; Zecchini, G. P.; Paradisi, M. P.; Lucente, G.; Mastropietro, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G.; Traniello, S.; Spisani, S., Modified chemotactic peptides: Synthesis, conformation, and activity of HCO-Thp-Ac(6)c-Phe-OMe. Biopolymers 1996, 39, 327-337. [CrossRef]
- Torrini, I.; Zecchini, G. P.; Paradisi, M. P.; Lucente, G.; Gavuzzo, E.; Mazza, F.; Pochetti, G.; Traniello, S.; Spisani, S.; Cerichelli, G., Modified chemotactic peptides - synthesis, conformation, and biological-activity of For-Thp-Leu-delta(Z)Phe-OMe Biopolymers 1994, 34, 1291-1302. [CrossRef]
- Mundlapati, V. R.; Imani, Z.; D’mello, V. C.; Brenner, V.; Gloaguen, E.; Baltaze, J.-P.; Robin, S.; Mons, M.; Aitken, D. J., N-H···X interactions stabilize intra-residue C5 hydrogen bonded conformations in heterocyclic α-amino acid derivatives. Chem. Sci. 2021, 12, 14826-14832. [CrossRef]
- Imani, Z.; Mundlapati, V. R.; Goldsztejn, G.; Brenner, V.; Gloaguen, E.; Guillot, R.; Baltaze, J. P.; Le Barbu-Debus, K.; Robin, S.; Zehnacker, A.; Mons, M.; Aitken, D. J., Conformation Control Through Concurrent N-H···S and N-H···C Hydrogen Bonding and Hyperconjugation Effects. Chem. Sci. 2020, 11, 9191-9197. [CrossRef]
- Liu, D. Y.; Bardaud, J. X.; Imani, Z.; Robin, S.; Gloaguen, E.; Brenner, V.; Aitken, D. J.; Mons, M., Length-Dependent Transition from Extended to Folded Shapes in Short Oligomers of an Azetidine-Based α-Amino Acid: The Critical Role of NH···N H-Bonds. Molecules 2023, 28. [CrossRef]
- Imani, Z.; Mundlapati, V. R.; Brenner, V.; Gloaguen, E.; Le Barbu-Debus, K.; Zehnacker-Rentien, A.; Robin, S.; Aitken, D. J.; Mons, M., Non-covalent interactions reveal the protein chain δ conformation in a flexible single-residue model. Chem. Commun. 2023, 59, 1161-1164. [CrossRef]
- Schäfer, G.; Bode, J. W., Synthesis of Sterically Hindered N-Acylated Amino Acids from N-Carboxyanhydrides. Org. Lett. 2014, 16, 1526-1529. [CrossRef]
- Coulter, A. W.; Lombardini, J. B.; Sufrin, J. R.; Talalay, P. Structural and conformational analogs of L-methionine as inhibitors of enzymatic-synthesis of S-adenosyl-L-methionine .3. Carbocyclic and heterocyclic amino-acids. Mol. Pharmacol. 1974, 10, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, M.; Ishimaru, T., Synthesis and configuration of 3-aminotetrahydrothiophene-3-carboxylic acids. Bull. Chem. Soc. Jpn. 1973, 46, 2515-2519. [CrossRef]
- Morimoto, Y.; Achiwa, K. Enzymes and catalysts .2. Pig-liver esterase-catalyzed asymmetric-synthesis of (-)-cucurbitine and (+)-cucurbitine and its (-)-thio analog. Chem. Pharm. Bull. 1987, 35, 3845–3849. [Google Scholar] [CrossRef] [PubMed]
- Lavrador, K.; Guillerm, D.; Guillerm, G., A new series of cyclic amino acids as inhibitors of S-adenosyl L-methionine synthetase. Bioorg. Med. Chem. Lett. 1998, 8, 1629-1634. [CrossRef]
- Oba, M.; Shimabukuro, A.; Ono, M.; Doi, M.; Tanaka, M., Synthesis of both enantiomers of cyclic methionine analogue: (R)- and (S)-3-aminotetrahydrothiophene-3-carboxylic acids. Tetrahedron-Asymmetry 2013, 24, 464-467. [CrossRef]
- Eichkorn, K.; Weigend, F.; Treutler, O.; Ahlrichs, R., Auxiliary basis sets for main row atoms and transition metals and their use to approximate Coulomb potentials. Theor. Chem. Acc. 1997, 97, 119-124. [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H., A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [CrossRef]
- Rappoport, D.; Furche, F., Property-optimized Gaussian basis sets for molecular response calculations. J. Chem. Phys. 2010, 133, 134105. [CrossRef]
- Gloaguen, E.; Mons, M.; Schwing, K.; Gerhards, M., Neutral Peptides in the Gas Phase: Conformation and Aggregation Issues. Chem. Rev. 2020, 120, 12490-12562. [CrossRef]
- Zwier, T. S., Laser probes of conformational isomerization in flexible molecules and complexes. J. Phys. Chem. A 2006, 110, 4133-4150. [CrossRef]
- Rijs, A. M.; Oomens, J., IR Spectroscopic Techniques to Study Isolated Biomolecules. In Gas-Phase IR Spectroscopy and Structure of Biological Molecules, Rijs, A. M.; Oomens, J., Eds. 2015; Vol. 364, pp 1-42.
- Sheldrick, G. M. SHELXS-97, Program for Crystal Structure Solution, University of Göttingen: Göttingen, Germany, 1997.
- Sheldrick, G. M., A short history of SHELX. Acta Crystallogr. A 2008, 64, 112-122. [CrossRef]
- Farrugia, L. J. WinGX suite for smallmolecule single-crystal crystallography. J. Appl. Cryst. 1999, 32, 837. [Google Scholar] [CrossRef]
- Parsons, S.; Flack, H. D.; Wagner, T., Use of intensity quotients and differences in absolute structure refinement. Acta Crystallogr. B 2013, 69, 249-259. [CrossRef]
- Macromodel Schrödinger, LLC,: New York, NY, Schrödinger Release 2019-3.
- Turbomole V7.2, 2017, a development of University of Karlsruhe and Forschungszentrum Karlsruhe GmbH, 1989-2007, Turbomole GmbH, since 2007; available from http://www.turbomole.com.
- Klamt, A.; Schuurmann, G., COSMO - A New Approach To Dielectric Screening In Solvents With Explicit Expressions For The Screening Energy And Its Gradient. J. Chem. Soc. Perkin Trans. 2 1993, 799-805. [CrossRef]
- Gloaguen, E.; Loquais, Y.; Thomas, J. A.; Pratt, D. W.; Mons, M., Spontaneous Formation of Hydrophobic Domains in Isolated Peptides. J. Phys. Chem. B 2013, 117, 4945-4955. [CrossRef]
| Amino acid | Monomer (gas phase) |
Monomer (solution) |
Dimer (gas phase) |
Dimer (solution) |
| Attc | 5-6γ [33] | 5-6γ [33] | Extended 5-6γ/5-6γ [33] | Semi-extended/extended forms + f-π-10 [34] |
| Cys(Me) | 5-6γ [27] | 5γ-πam [27] | (R,R) 5γ-5γ-10 (I) (+ 5γ-5γ-7 as minor) [27] |
(R,R) 5γ-5γ-10 (I) (+ minor with a free NH) [27] |
| Atlc | 5-6γ [35] | 5γ-πam / f-πam [35] | (R,R) 5γ-5γ-10 (I) [this work] (S,R) 5γ-π am-10 (I’), f-5γ-10 (I) and f-6γ-10 (II) [this work] |
(R,R) 5γ-5γ-10 (I) [this work] (S,R) 5γ-π am-10 (I’) and f-5γ-10 (I) [this work] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





