1. Introduction
Oral lichen planus (OLP) is a chronic, non-infectious inflammatory condition affecting the oral mucosa, characterized by erythematous lesions, papules, white plaques, and ulcerations [
1,
2]. Although not a malignant condition, OLP can cause symptoms such as burning, pain, and discomfort during food and beverage intake, significantly compromising patients’ quality of life [
3].
The etiology of OLP remains unknown, but it is believed to be an immune-mediated condition triggered by genetic factors, stress, viral infections, and hypersensitivity reactions [
4]. The pathogenesis involves an alteration in immunoregulation, with the activation of cytotoxic T lymphocytes that induce apoptosis of basal keratinocytes [
5]. Currently, there is no curative therapy for OLP, and treatment mainly aims to alleviate symptoms and prevent complications. Standard therapeutic options include topical application of high-potency corticosteroids, immunosuppressants, and laser therapy [
6]. However, these treatments may be associated with side effects and recurrences after discontinuation. Zinc-L-carnosine is a chelated compound consisting of L-carnosine and zinc, which has demonstrated anti-inflammatory properties and a potential role in promoting mucousal healing [
7,
8]. Previous studies have suggested a potential role for zinc in the pathogenesis and treatment of OLP, showing a correlation between serum zinc levels and disease severity [
9,
10].
This randomized, double-blind clinical study aims to evaluate the efficacy of Hepilor, a zinc-L-carnosine-based mouthwash, in reducing pain and improving oral mucosal health in patients with OLP.
2. Materials and Methods
Study Design and Participants
The study involved 19 patients with a clinical and histological diagnosis of symptomatic OLP, recruited from the Department of Oral Pathology and Oral Medicine and Diagnostic Odontostomatology at the UOC Clinical Dentistry, University of Padova. Inclusion criteria included symptomatic patients with OLP, while exclusion criteria excluded asymptomatic patients, patients receiving corticosteroids, antifungals, or laser photobiomodulation within the last 3 months, and patients with clinical signs of oral candidiasis. A randomized, double-blind, crossover clinical trial was conducted. Patients were randomly assigned to two groups that sequentially received either Hepilor mouthwash or placebo mothwash for four weeks, separated by a four-week washout period.
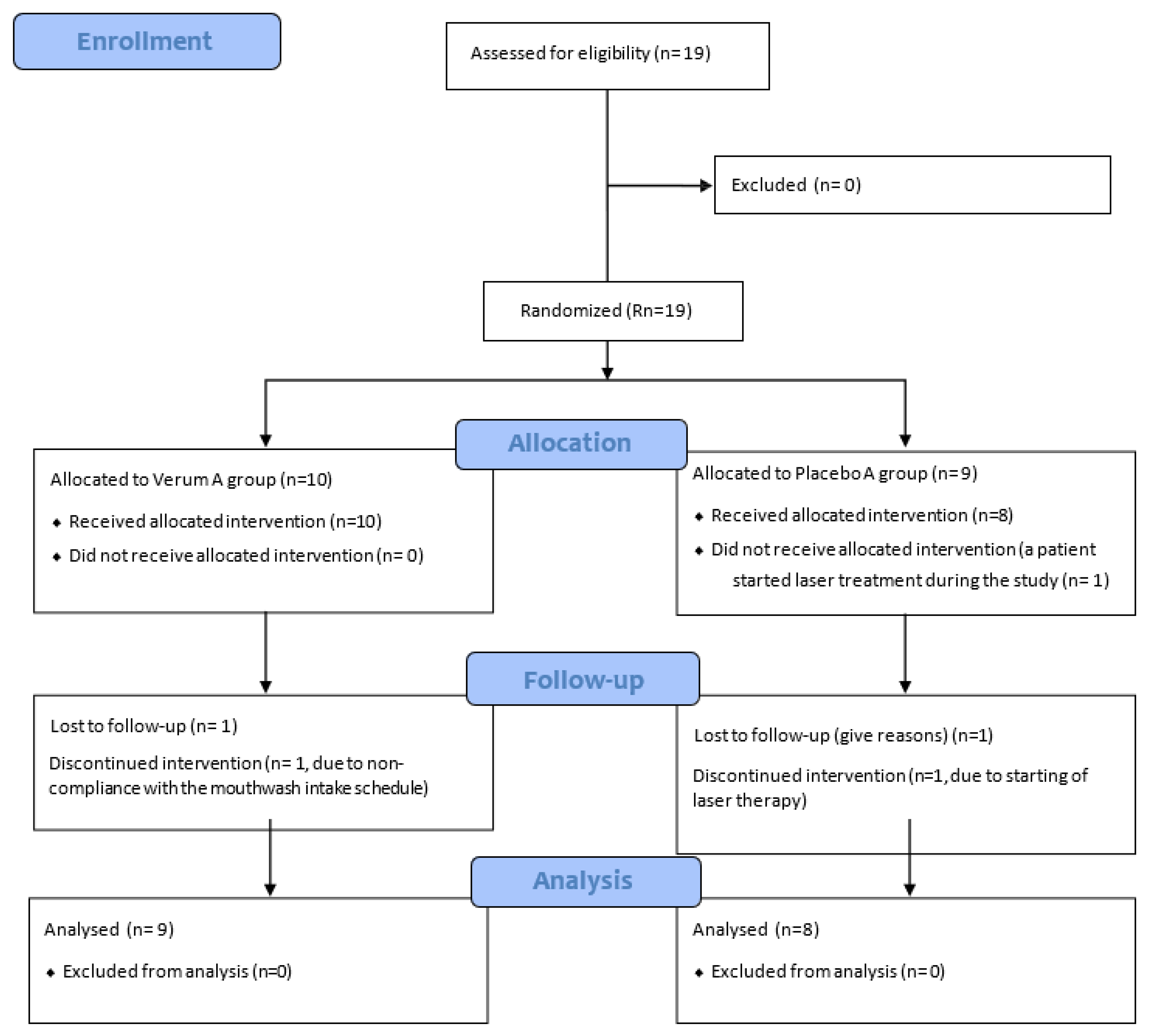
Flow diagram of the RCT study, following the CONSORT 2010 guidelines, is presented below in
Figure 1 [
11].
Intervention
The Hepilor medical device mouthwash contains Zinc-L-carnosine (39.53 mg per dose), sodium alginate, magnesium hydroxide, glycerin, sucralose, sweet orange essential oil, flavoring agents, methyl-p-hydroxybenzoate, and purified water. The placebo had same composition but lacked zinc-L-carnosine. Patients were instructed to rinse their mouths with 10 mL of mouthwash three times a day after meals and after oral hygiene, holding it in their mouths for at least two minutes before spitting it out.
Outcome Measures
Patients’ discomfort was assessed using the Visual Analogue Scale (VAS) for pain and the Oral Lichen Planus Symptom Severity Measure (LPOSSM) questionnaire at the beginning and end of each treatment period. The VAS consists of a 10-cm straight line, where 0 represents "no pain" and 10 represents "worst possible pain." Patients indicated their level of pain by marking a point on the line.
The LPOSSM questionnaire evaluates the severity of OLP symptoms during the performance of seven daily activities (tooth brushing, eating, drinking, smiling, breathing through the mouth, speaking, and touching the mucosa). Each activity is rated on a scale from 0 (no discomfort) to 4 (preventing the activity), with a total score ranging from 0 to 28 [
12].
Statistical Analysis
Data were analyzed using the paired Student’s t-test to assess the significance of the difference between VAS and LPOSSM scores before and after treatment in both study phases. A one-way analysis of variance (ANOVA) was also conducted to investigate the effect of time and treatment on VAS and LPOSSM scores.
3. Results
The study results were collected in both Phase 1 and Phase 2 and summarized in
Table 1 and
Table 2.
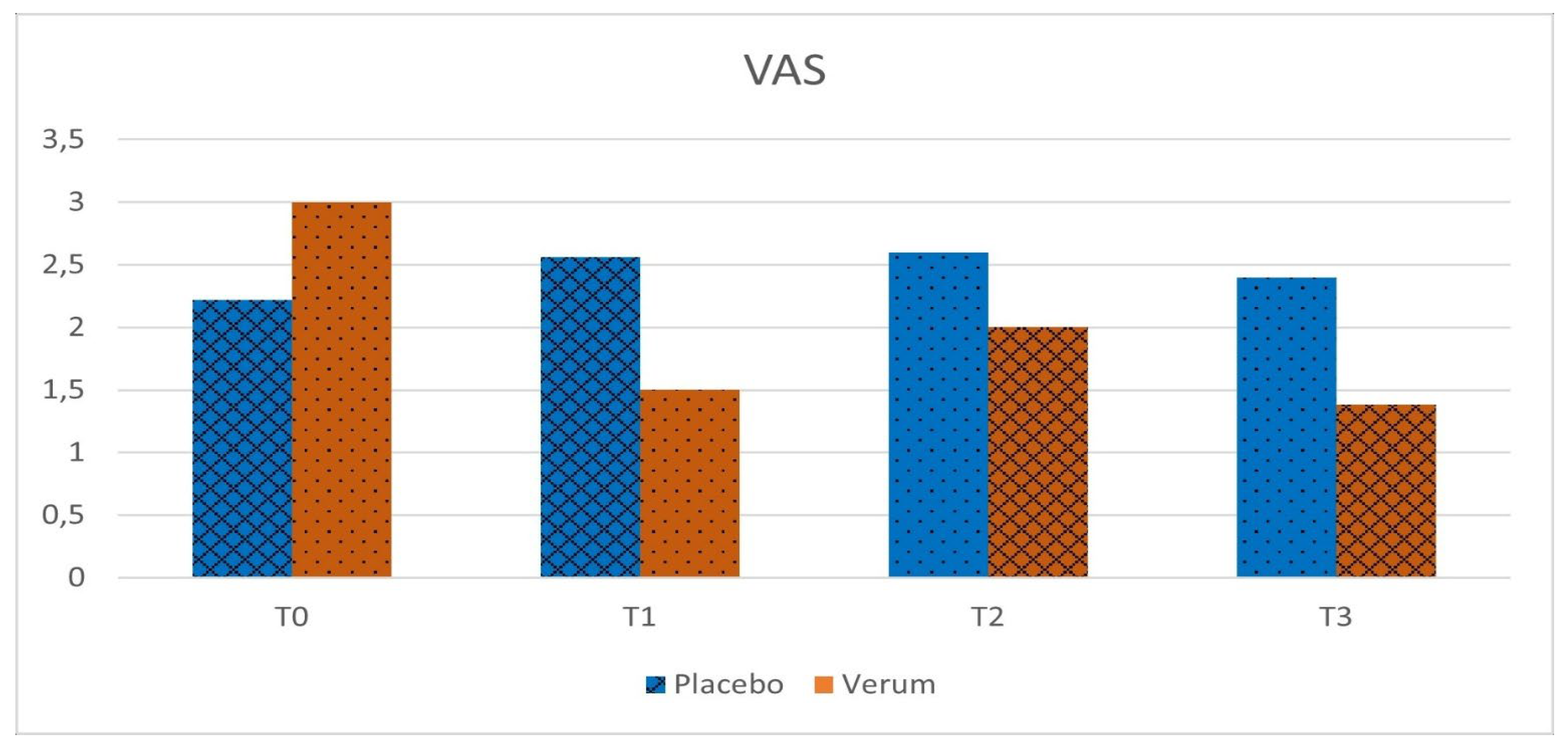
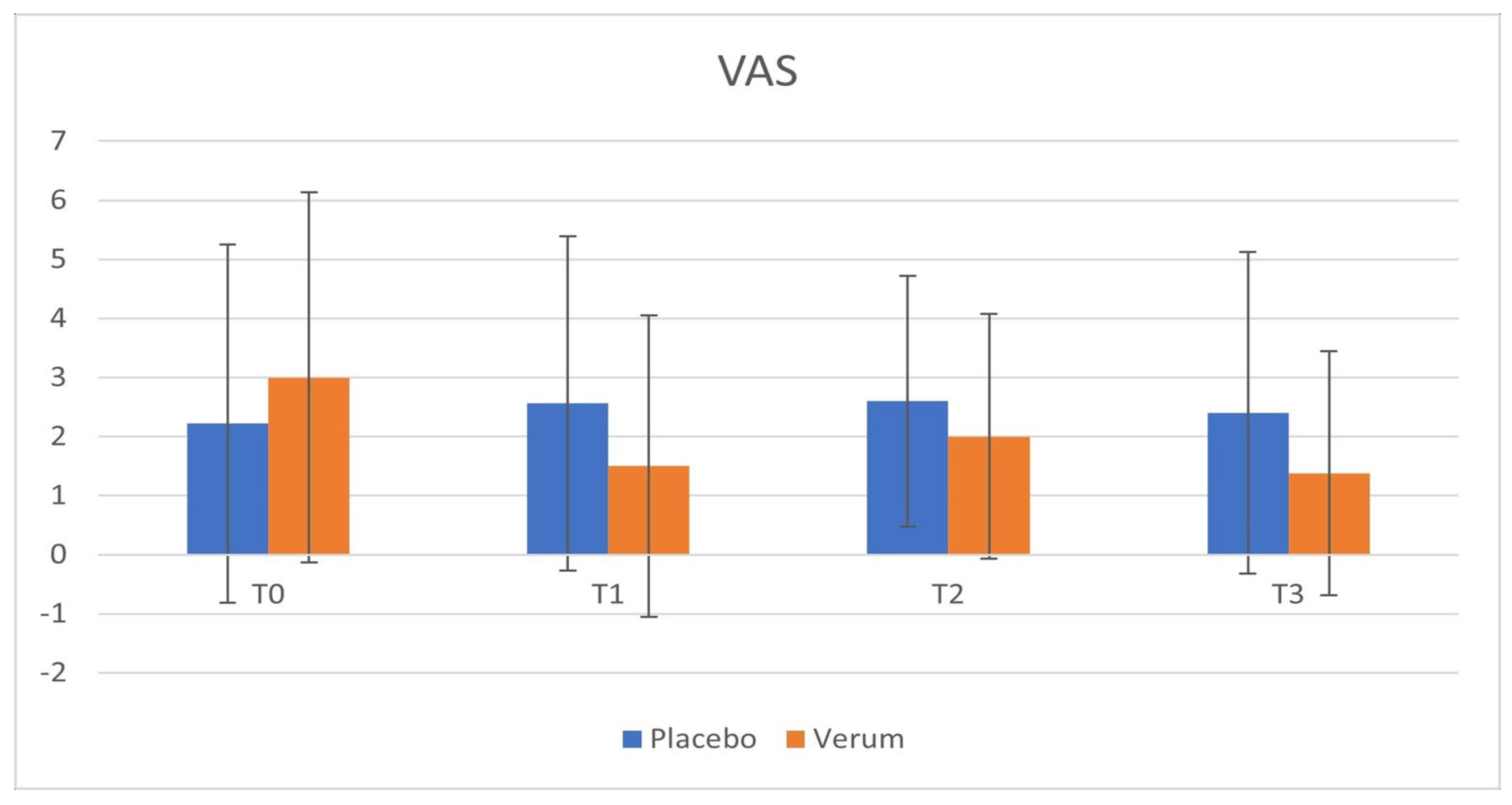
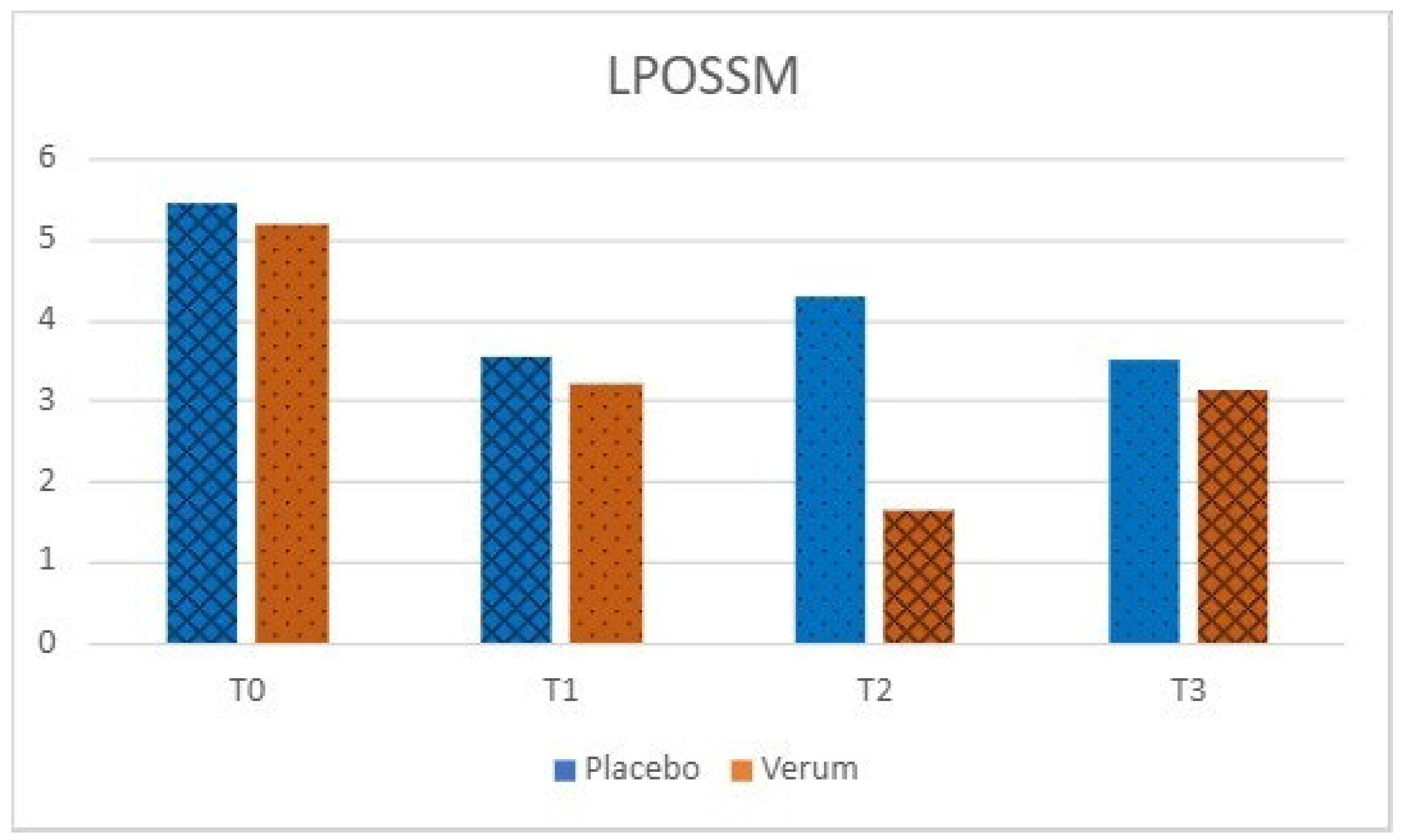
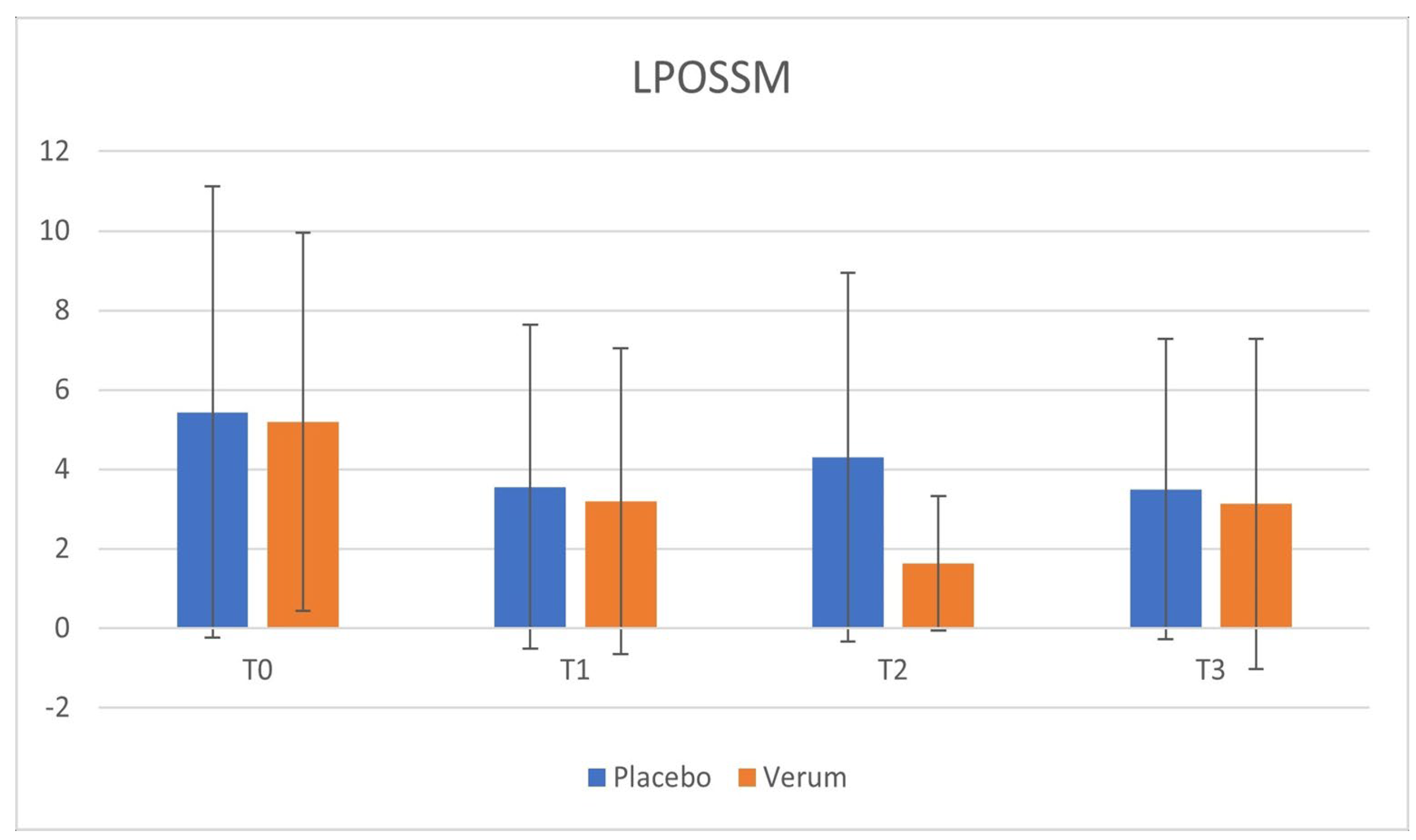
Phase 1: in the placebo group, no statistically significant differences were observed in VAS (p = 0.563) and LPOSSM (p = 0.051) scores before and after treatment. In the Hepilor-treated group, VAS scores decreased from 3.00 ± 3.13 to 1.50 ± 2.55, although not statistically significant (p = 0.067). Similarly, LPOSSM scores decreased from 5.20 ± 4.76 to 3.20 ± 3.85, but did not reach statistical significance (p = 0.082).
Phase 2: neither the placebo nor the Hepilor group showed statistically significant differences in VAS (placebo: p = 0.758; Hepilor: p = 0.217) and LPOSSM (placebo: p = 0.223; Hepilor: p = 0.328) scores before and after treatment.
Time Effect: the analysis of the time effect on VAS and LPOSSM scores did not show statistically significant differences between treatment groups during the study. All results are reported in
Table 3.
A marked heterogeneity of the data was observed, as evidenced by the high standard deviation values. Data are shown in
Figure 2,
Figure 3,
Figure 4 and
Figure 5.
4. Discussion
This randomized, double-blind clinical study evaluated the efficacy of Hepilor, a zinc-L-carnosine-based mouthwash, in reducing pain associated with OLP. Although the results did not reach statistical significance, a trend toward a reduction in VAS and LPOSSM scores was observed in the Hepilor-treated group during Phase 1 of the study.
The crossover design adopted in this study allowed for a direct comparison of the effect of each treatment within the same individual, increasing statistical power and reducing the number of participants needed. Furthermore, the double-blind design helped minimize biases related to the expectations of participants and researchers [
13].
However, the study had some limitations. First, the relatively small sample size may have influenced the ability to detect statistically significant differences. Additionally, the heterogeneity of the data, as evidenced by the high standard deviations, suggests the presence of underlying complex underlying factors influencing the variables under examination.
Another limitation is the lack of measurement of serum zinc levels in patients. Since zinc plays a role in immunomodulation, it is possible that patients with an initial zinc deficiency may benefit more from treatment with zinc-L-carnosine, as it could contribute to restoring an optimal inflammatory response [
14].
Furthermore, the study focused solely on the evaluation of symptoms, while other studies have analyzed the size of oral lesions. Assessing the extent of lesions could provide a more objective evaluation of the treatment’s efficacy in promoting the healing of damaged mucous membranes [
15].
Another potential limitation concerns the standardization of subjective evaluation methods. Although the VAS and LPOSSM questionnaires are validated tools, providing more detailed guidance to participants could ensure greater consistency in responses.
Finally, the statistical approach adopted may not have been adequate to account for the heterogeneity of the data. The use of non-parametric tests or more sophisticated statistical models could yield more robust results. Despite these limitations, the study showed a trend toward symptom reduction in patients treated with zinc-L-carnosine during Phase 1, suggesting a potential beneficial effect of this compound in the treatment of OLP.
5. Conclusions
In conclusion, this randomized, double-blind clinical study did not demonstrate statistically significant efficacy of the zinc-L-carnosine-based Hepilor mouthwash in reducing pain associated with OLP. However, a trend toward a reduction in VAS and LPOSSM scores was observed in the Hepilor-treated group during Phase 1 of the study.
Further studies with larger sample sizes, standardized evaluation methods, and more robust statistical approaches could establish a stronger relationship between the use of zinc-L-carnosine and its efficacy in treating OLP. Additionally, it would be beneficial to investigate serum zinc levels in patients and evaluate the extent of oral lesions to obtain a more comprehensive assessment of the treatment’s efficacy.
Author Contributions
Conceptualization, A.B. and C.M.; methodology, A.B.; software, M.T.; validation, C.B., C.M. and I.D.; formal analysis, C.B.; investigation, A.B..; resources, A.B.; data curation, C.M.; writing—original draft preparation, A.B.; writing—review and editing, I.D.; visualization, M.T..; supervision, C.B.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Padova (Ethics Committee for Clinical Trials of the Province of Padua) (CESC code 5611/AO/22, URC code AOP2799 and date of approval 19/01/2023).”
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OLP |
Oral Lichen Planus |
| LPOSSM |
Oral Lichen Planus Symptom Severity Measure |
| VAS |
Visual Analogue Scale |
References
- Mutafchieva, M.Z.; Draganova-Filipova, M.N.; Zagorchev, P.I.; Tomov, G.T. Oral Lichen Planus - Known and Unknown: A Review. Folia Med. 2018, 60, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Alrashdan, M.S.; Cirillo, N.; McCullough, M. Oral lichen planus: A literature review and update. Arch Dermatol Res. 2016, 308, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus - a review. J Oral Pathol Med. 2010, 39, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Andabak-Rogulj, A.; Vindiš, E.; Aleksijević, L.H.; Škrinjar, I.; Juras, D.V.; Aščić, A.; et al. Different Treatment Modalities of Oral Lichen Planus—A Narrative Review. Dent J. 2023, 11. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Goss, E.; Carrozzo, M.; Castellano, S.; Conrotto, D.; Broccoletti, R.; et al. Systemic and topical corticosteroid treatment of oral lichen planus: A comparative study with long-term follow-up. J Oral Pathol Med. 2003, 32, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Sh, N.; Sh, G.; Poorfar, K.H. Evaluation of the Serum Zinc Level in Erosive and Non-Erosive Oral Lichen Planus. J Dent Shiraz Univ Med Sci. 2014, 15. [Google Scholar]
- Bahramian, A.; Rahbaran, M.; Bahramian, M.; Bohlouli, S.; Katebi, K. Effect of zinc supplementation as an adjuvant to corticosteroid treatment in patients with oral lichen planus: A systematic review. J Adv Periodontol Implant Dent. 2023, 15, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Hewlings, S.; Kalman, D. A review of zinc-L-carnosine and its positive effects on oral mucositis, taste disorders, and gastrointestinal disorders. Nutrients. 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Skovlund, E.; Breivik, H. Analysis of pain-intensity measurements. Scand J Pain. 2016, 13, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.B.; Brennan, M.T.; Ni Riordain, R.; Madsen, L.S. Novel Oral Lichen Planus Symptom Severity Measure for assessing patients’ daily symptom experience. Oral Dis. 2019, 25, 1564–1572. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. Trials 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Gholizadeh, N.; Sheykhbahaei, N. Micronutrients Profile in Oral Lichen Planus: A Review Literature. Biol Trace Elem Res. 2021, 199, 912–924. [Google Scholar] [CrossRef]
- Bacci, C.; Vanzo, V.; Frigo, A.C.; Stellini, E.; Sbricoli, L.; Valente, M. Topical tocopherol for treatment of reticular oral lichen planus: A randomized, double-blind, crossover study. Oral Dis. 2017, 23, 62–68. [Google Scholar] [CrossRef]
- Lin, P.H.; Sermersheim, M.; Li, H.; Lee, P.H.U.; Steinberg, S.M.; Ma, J. Zinc in wound healing modulation. Nutrients. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Nukaly, H.Y.; Halawani, I.R.; Alghamdi, S.M.S.; Alruwaili, A.G.; Binhezaim, A.; Algahamdi, R.A.A.; et al. Oral Lichen Planus: A Narrative Review Navigating Etiologies, Clinical Manifestations, Diagnostics, and Therapeutic Approaches. J Clin Med. 2024, 13. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).