Submitted:
23 October 2025
Posted:
27 October 2025
You are already at the latest version
Abstract
Keywords:
Introduction
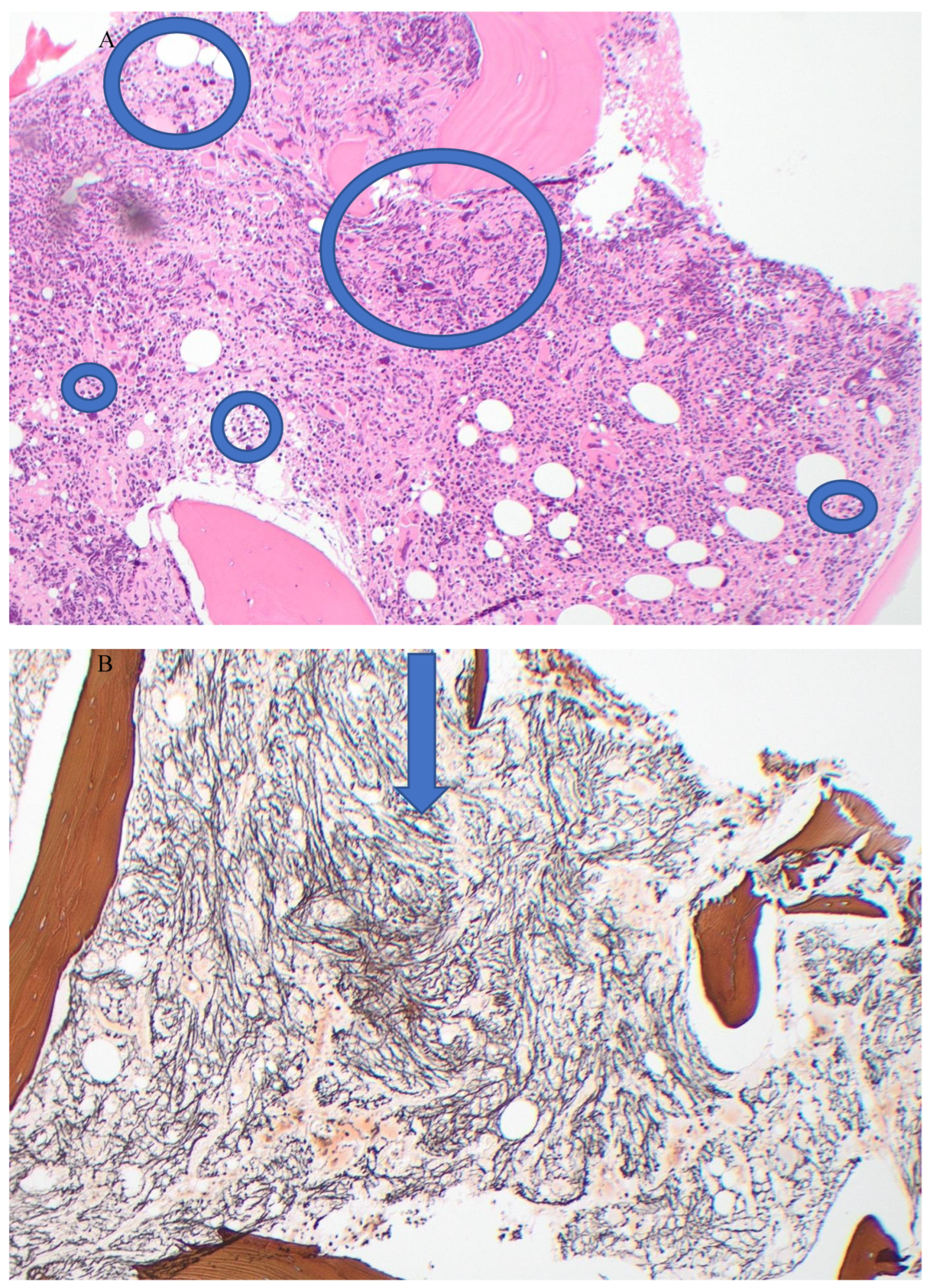
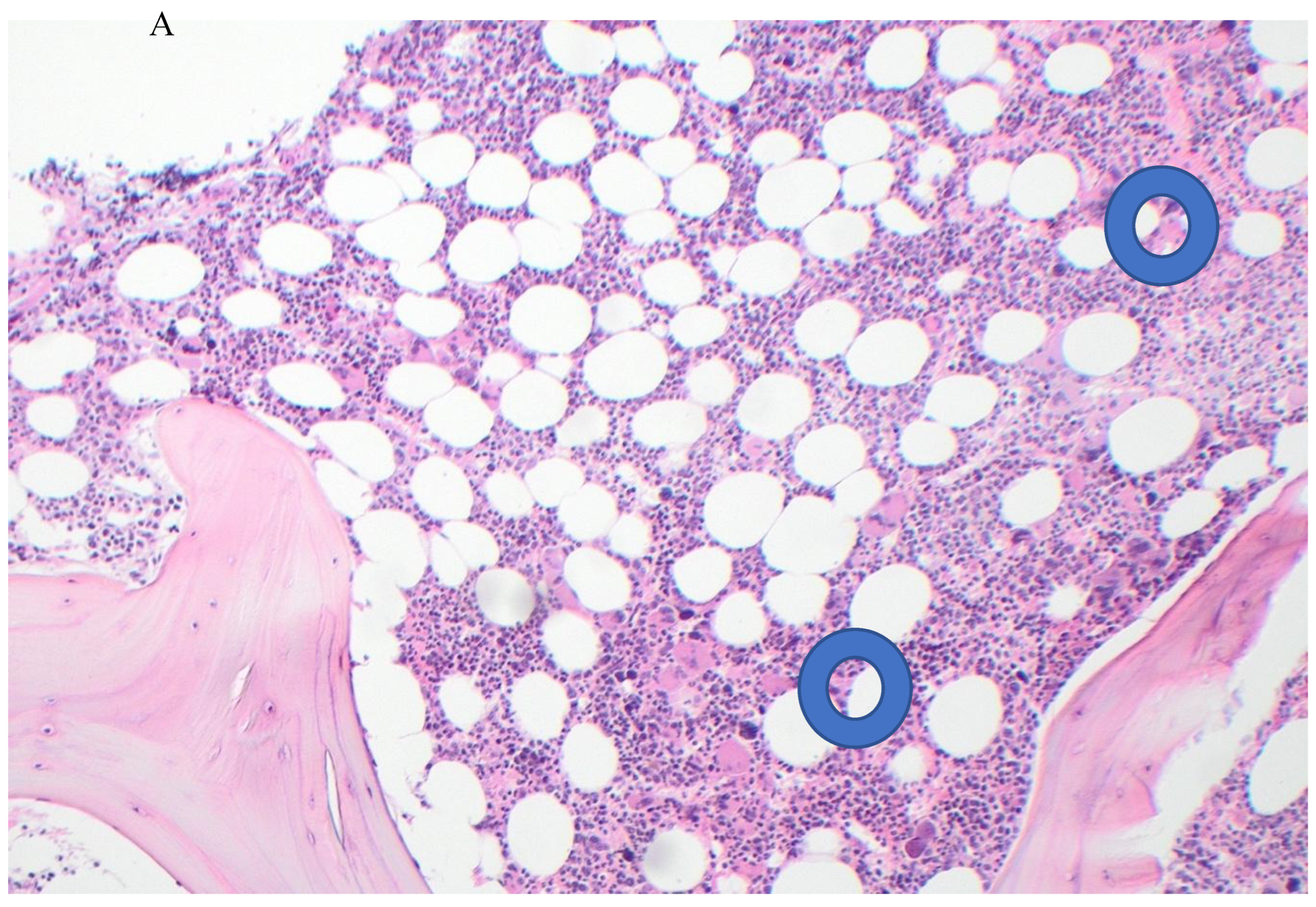
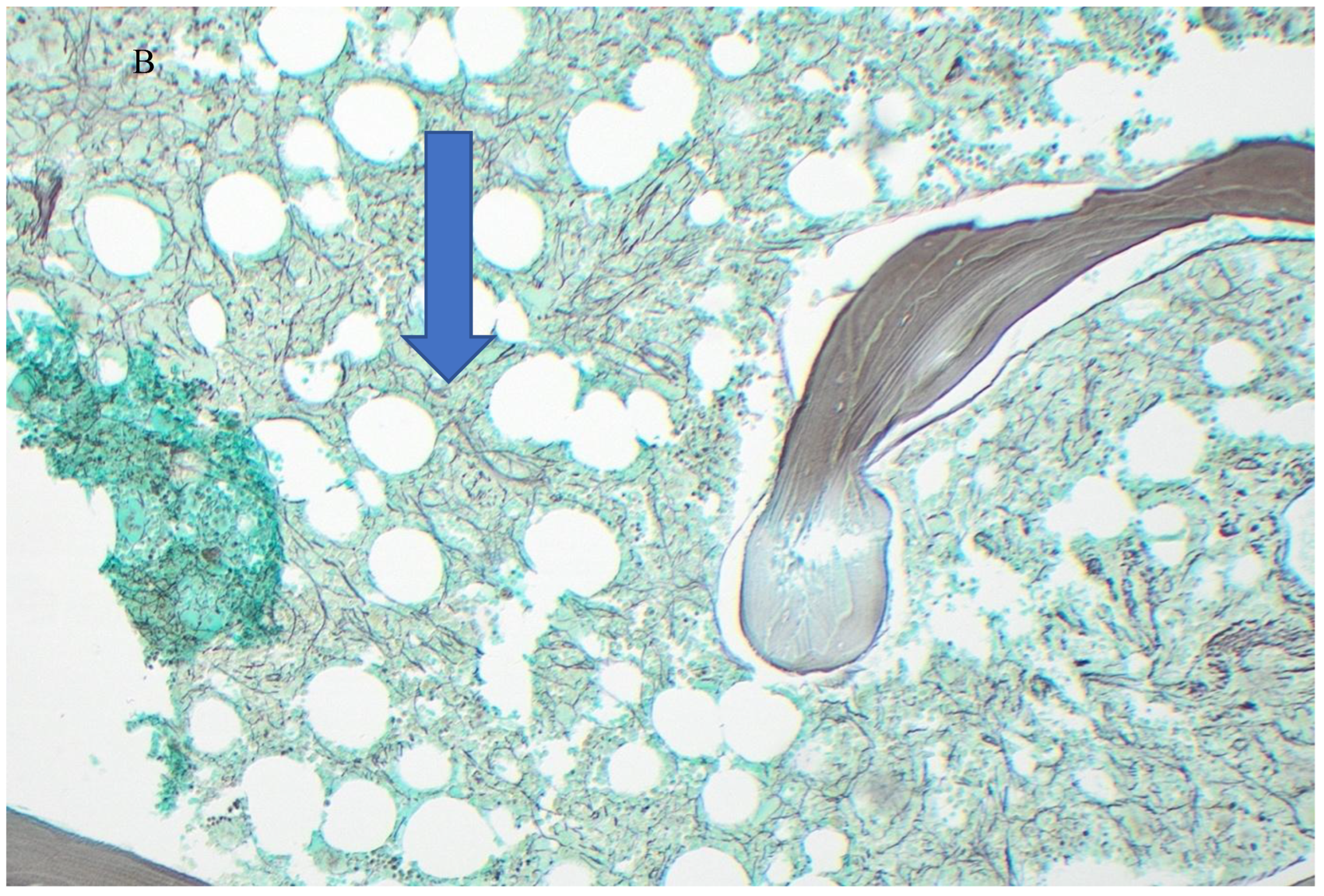
Case Report
Discussion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Thiele, J.; Kvasnicka, H.M.; Orazi, A.; et al. The International Consensus Classification of Myeloid Neoplasms and Acute Leukemia: Myeloproliferative Neoplasms. Am J Hematol. 2023, 98, 166–179. [Google Scholar] [CrossRef]
- Tefferi, A. Myeloproliferative Neoplasms: A Decade of Discoveries and Treatment Advances. Am J Hematol. 2016, 92, 50–58. [Google Scholar] [CrossRef]
- Tefferi, A.; Wassie, E.A.; Lasho, T.L.; et al. Calreticulin Mutations and Long-term Survival in Essential Thrombocythemia. Leukemia, 2014. [CrossRef]
- Barbui, T.; Thiele JPassamonti, F.; et al. Survival and Disease Progression in Essential Thrombocythemia Are Significantly Influenced by Accurate Morphologic Diagnosis: An International Study. J Clin Oncol. 2011, 29, 3179–84. [Google Scholar] [CrossRef]
- Al Assaf, C.; Van Olbergh, F.; Billiet, J.; et al. Analysis of Phenotype and Outcome in Essential Thrombocythemia with CALR or JAK2 Mutations. Haematologica 2015, 100, 893–897. [Google Scholar] [CrossRef]
- Erdos, K.; Lee, N.; Lebbe, A.; et al. Low Thrombosis Risk with CALR Mutation Confer Higher Risk of ET Progression. Blood 2023, 142, 1819. [Google Scholar] [CrossRef]
- Santaliestra, M.; Garrote, M.; Noya, M.S.; et al. Prognostic Value of Response to IPSET Stratification in Essential Thrombocythemia. Leukemia 2024, 38, 2636–2643. [Google Scholar] [CrossRef] [PubMed]
- Barosi, G.; Mesa, R.A.; Thiele, J.; et al. Proposed Criteria for the Diagnosis of Post-Polycythemia Vera and Post-essential Thrombocythemia Myelofibrosis: A consensus Statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia 2008, 22, 437–8. [Google Scholar] [CrossRef] [PubMed]
- Passamonti, F.; Mora, B. Myelofibrosis. Blood 2023, 141, 1954–70. [Google Scholar] [CrossRef] [PubMed]
- Puglianini, O.C.; Peker, D.; Zhang, L.; et al. Essential Thrombocythemia and Post-essential Thrombocythemia Myelofibrosis: Updates on Diagnosis, Clinical Aspects and Management. Laboratory Medicine 2023, 54, 13–22. [Google Scholar] [CrossRef]
- Thiele, J.; Krosnicka, H.M.; Facchetti, F.; et al. European Consensus on Grading of Bone Marrow Fibrosis and Assessment of Cellularity. Haematologica 2005, 90, 1128–1132. [Google Scholar]
- Manoharan, A.; Gemmel, R.; Cavanaugh, L.; et al. Thrombosis in Myeloproliferative Neoplasms: A Single Center Experience of Using Whole Blood Platelet Aggregation Studies for Risk Assessment and Thromboprophylaxis. Clin Appl Thr/Hemo. 2022, 28, 1076029622111. [Google Scholar] [CrossRef]
- Schag, C.C.; Heinrich, R.L.; Gang, P.A. Kornofsky Performance Status Revisited: Reliability, Validity and Guidelines. J Clin Oncol 1984, 2, 187–193. [Google Scholar] [CrossRef]
- Mesa, R.A.; Miller, C.B.; Thyne, M.; et al. Differences in Treatment Goal and Perception of Symptoms Burden between Patients with Myeloproliferative Neoplasms (MPNs) and Hematologist/Oncologists in the United States. Findings from the MPN Landmark Survey. Cancer 2017, 123, 449–58. [Google Scholar]
- Barbui, T.; Vannucchi, A.M.; Buxhofer-Ausch, V.; et al. Practice -relevant revision of IPSET=thrombosis Based on 1019 Patients with WHO-Defined Essential Thrombocythemia. Blood Cancer J. 2015, 5, e369. [Google Scholar] [CrossRef] [PubMed]
- Haider, M.; Gangat, N.; Lasho, T.; et al. Validation of the Revised International Prognostic Score of Thrombosis for Essential Thrombocythemia (IPSET-thrombosis) in 585 Mayo Clinic Patients. Am J Hematol. 2016, 91, 390–4. [Google Scholar] [CrossRef] [PubMed]
- Barbui, T.; Vannucchi, A.; Finazzi, G.; et al. A Reappraisal of the Benefit-Risk Profile of Hydroxyurea in Polycythemia Vera: A Propensity-matched Study. Am J Hematol. 2017, 92, 1131–1136. [Google Scholar] [CrossRef]
- Tefferi, A.; Vannucchi, A.M.; Barbui, T. Essential Thrombocythemia: 2024 Update on Diagnosis, Risk Stratification and Management. Am J Hematol. 2024, 99, 697–718. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Trillos, A.; Gaya, A.; Maffioli, M.; et al. Efficacy and Tolerability of Hydroxyurea in the Treatment of Hyperproliferative Manifestations of Myelofibrosis: Results in 40 Patients. Ann Hematol. 2010, 89, 1233–1237. [Google Scholar] [CrossRef]
- Buyukasik, Y.; Ali, R.; Turgut, M.; et al. Patterns of Hydroxyurea Prescription and Use in Routine Clinical Management of Polycythemia Vera: A Multicentre Chart Review Study. Turkish J Hematol. 2020, 37, 177. [Google Scholar] [CrossRef]
- Grunwald, M.; Kutes, D.J.; Altomare, I.; et al. Treatment Patterns and Blood Counts in Patients with Polycythemia Vera Treated with Hydroxyurea in the United States: An Analysis from the REVEAL Study. Clin Lymphoma Myeloma and Leuk. 2020, 20, 219–225. [Google Scholar] [CrossRef]
- Ferrer-Marin, F.; Hernandez-Boluda, J.C.; Alvarez-Larran, A. Essential Thrombocythemia: A Contemporary Approach with New Drugs on the Horizon. Br J Haematol. 2024, 204, 1605–1616. [Google Scholar] [CrossRef] [PubMed]
- Kuykendall, A.T. Treatment of Hydroxyurea-Resistant/Intolerant Polycythemia Vera: A Discussion of Best Practices. Ann Hematol. 2023, 102, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Nejadnik, B.; Mascarenhas, J.; Rappaport, K.M.; et al. Treatment of Essential Thrombocythemia Patients Intolerant/Resistant to Hydroxyurea. J Clin Oncol. 2017, 35, e. 18565. [Google Scholar] [CrossRef]
- Manoharan, A.; Enggist, S.I. Hydroxyurea: An Old Drug in Need of New Clinical Trials in Myeloproliferative Neoplasms? Int Internal Med J. 2024, 2, 1–6. [Google Scholar]
- Tracewell, W.G.; Trump, D.L.; Vaughan, W.P.; et al. Population Pharmacokinetics of Hydroxyurea in Cancer Patients. Cancer Chemotherapy and Pharmacology 1995, 35, 417–422. [Google Scholar] [CrossRef]
- Gwilt, P.R.; Tracewell, W.G. Pharmacokinetics and Pharmacodynamics of Hydroxyurea. Clin Pharmacokinetics 1998, 34, 347–358. [Google Scholar] [CrossRef]
- Passamonti, F.; Giorgino, T.; Mora, B.; et al. A Clinical-Molecular Prognostic Model to Predict Survival in Patients with Post Polycythemia Vera and Post Essential Thrombocythemia Myelofibrosis. Leukemia 2017, 31, 2726–31. [Google Scholar] [CrossRef]
- Hernandez-Boluda, J.-C.C.; Pereira, A.; Correa, J.-G.G.; et al. Performance of the Myelofibrosis Secondary to PV and ET-Prognostic Model (MYSEC-PM) in A Series of 262 Patients from the Spanish Registry of Myelofibrosis. Leukemia 2018, 32, 553–5. [Google Scholar] [CrossRef]
- Martino, M.; Pitea, M.; Sgarlata, A.; et al. Treatment Strategies Used in Treating Myelofibrosis: State of the Art. Hematol Rep. 2024, 16, 698–713. [Google Scholar] [CrossRef] [PubMed]
- Thaw, K.; Harrison, C.N.; Srikandarajah, P. JAK Inhibitors for Myelofibrosis: Strengths and Limitations. Curr Hematol Malig Rep. 2024, 19, 264–275. [Google Scholar] [CrossRef]
- Jacobson, R.J.; Salo, A.; Fialkow, P.J. Agnogenic Myeloid Metaplasia: A Clonal Proliferation of Hemopoietic Stem Cells with Secondary Myelofibrosis. Blood 1978, 51, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Zahr, A.A.; Salama, M.E.; Correau, N.; et al. Bone Marrow Fibrosis in Myelofibrosis: Pathogenesis, Prognosis and Targeted Strategies. Haematologica 2016, 101, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Manoharan, A.; Pitney, W.R. Chemotherapy Resolves Symptoms and Reverses Marrow Fibrosis in Myelofibrosis. Scand J Haematol. 1984, 33, 453–459. [Google Scholar] [CrossRef]
- Editorial. Reversible Myelofibrosis?. Lancet, 1985; 325: 497-498.
- Manoharan, A.; Chen, C.F.; Wilson, L.S.; et al. Ultrasonic Characterization of Splenic Tissue in Myelofibrosis: Further Evidence for Reversal of Fibrosis with Chemotherapy. Eur J Haematol. 1988, 40, 149–154. [Google Scholar] [CrossRef]
- Lofvenberg, E.; Wahlin, A.; Roos, G.; et al. Reversal of Myelofibrosis by Hydroxyurea. Eur J Haematol. 1990, 44, 33–38. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).



