1. Introduction
Affibody molecules, engineered derivatives of the Z-domain from
Staphylococcus aureus protein A, offer promising alternatives to conventional antibodies. Their small size (~6.5 kDa), high stability, and engineerable binding interfaces provide key advantages, including efficient tissue penetration, thermostability, and tunable affinity/specificity for diverse targets such as proteins, peptides, and small molecules.[
1,
2] These properties position Affibodies as valuable tools in targeted drug delivery, molecular imaging, diagnostics, biosensor development, and proteomics research.
Despite these strengths, monomeric Affibody constructs have inherent limitations, including rapid renal clearance and short
in vivo half-life.[
2,
3] To address these, researchers have shifted toward multivalent supramolecular architectures. Constructed via strategic non-covalent interactions, these architectures mitigate monomeric shortcomings while enabling new functions, such as enhanced target avidity, improved pharmacokinetics, controlled therapeutic release, and amplified diagnostic signals.[
4,
5,
6] Moreover, they facilitate integration of multiple functionalities, supporting advanced therapeutic delivery systems and biomaterials.
This review focuses on recent advances in Affibody-based supramolecular architectures, with emphasis on their applications in diagnostics, targeted therapeutics, and biomaterial development.
2. Brief History and Development of Affibodies
Affibody technology originated in the early 1990s at the Royal Institute of Technology (KTH) in Stockholm, Sweden, to address limitations of conventional antibodies.[
7] While antibodies provide high specificity, their large size, poor tissue penetration, and complex production hinder many applications. The key innovation involved engineering the Z-domain of Staphylococcal Protein A into small proteins that retain high specificity and affinity while offering superior properties.[
1,
2,
3,
7,
8]
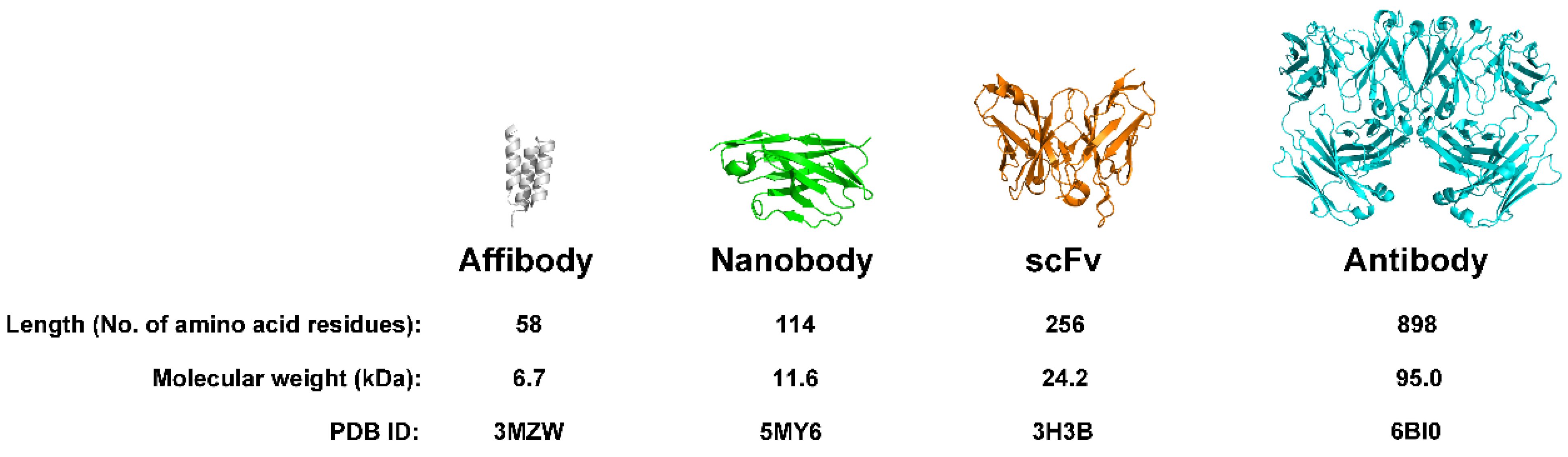
At approximately 6.5 kDa, Affibodies are roughly one-twenty-fifth the size of conventional antibodies (
Figure 1), conferring advantages including improved tissue penetration and simplified production through recombinant DNA technology.[
7] The pivotal development involved modifying the Z-domain to introduce variability at specific binding regions, enabling creation of diverse Affibody libraries capable of recognizing various target proteins with high specificity.[
1,
2,
3,
7,
8]
The evolution of Affibody technology accelerated through advances in protein engineering and molecular biology. Key developments included implementation of phage display and yeast surface display techniques, enabling generation of diverse Affibody libraries with picomolar binding affinities for critical targets including human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR) which are important markers in cancer diagnostics and therapeutics.[
7,
8,
9,
10,
11,
12,
13,
14]
Affibodies rapidly gained traction across multiple scientific and clinical domains. In targeted therapeutics, they facilitate delivery of cytotoxic agents directly to cancer cells expressing specific biomarkers, minimizing off-target effects while enhancing treatment efficacy.[
8,
9,
10,
15,
16,
17] In molecular imaging, Affibodies labelled with radionuclides have proven valuable for positron emission tomography (PET) and single-photon emission computed tomography (SPECT), enabling precise visualization of disease-associated targets
in vivo.[
17,
18,
19,
20,
21] Additionally, these molecules contribute significantly to biosensor development, where their specificity and stability enhance detection sensitivity and reliability.[
8,
9,
10,
17]
The continuous innovation in protein engineering, molecular biology, and biotechnological applications has driven substantial development of Affibody technology since its inception. The unique properties of Affibodies (i.e. small size, high stability, and exceptional binding specificity) combined with scalable production methods and versatile application potential, have established them as promising platforms for diagnostic and therapeutic applications. Ongoing research continues expanding the capabilities and applications of these molecules, underscoring their importance in molecular medicine and biotechnology.
3. Molecular Structure and Scaffold Description
Affibody molecules represent a distinctive class of engineered proteins derived from the Z-domain of Staphylococcal Protein A.[
7] They are characterized by their remarkably compact structure, comprising 58 amino acids with a molecular mass of approximately 6.5 kDa.[
7]
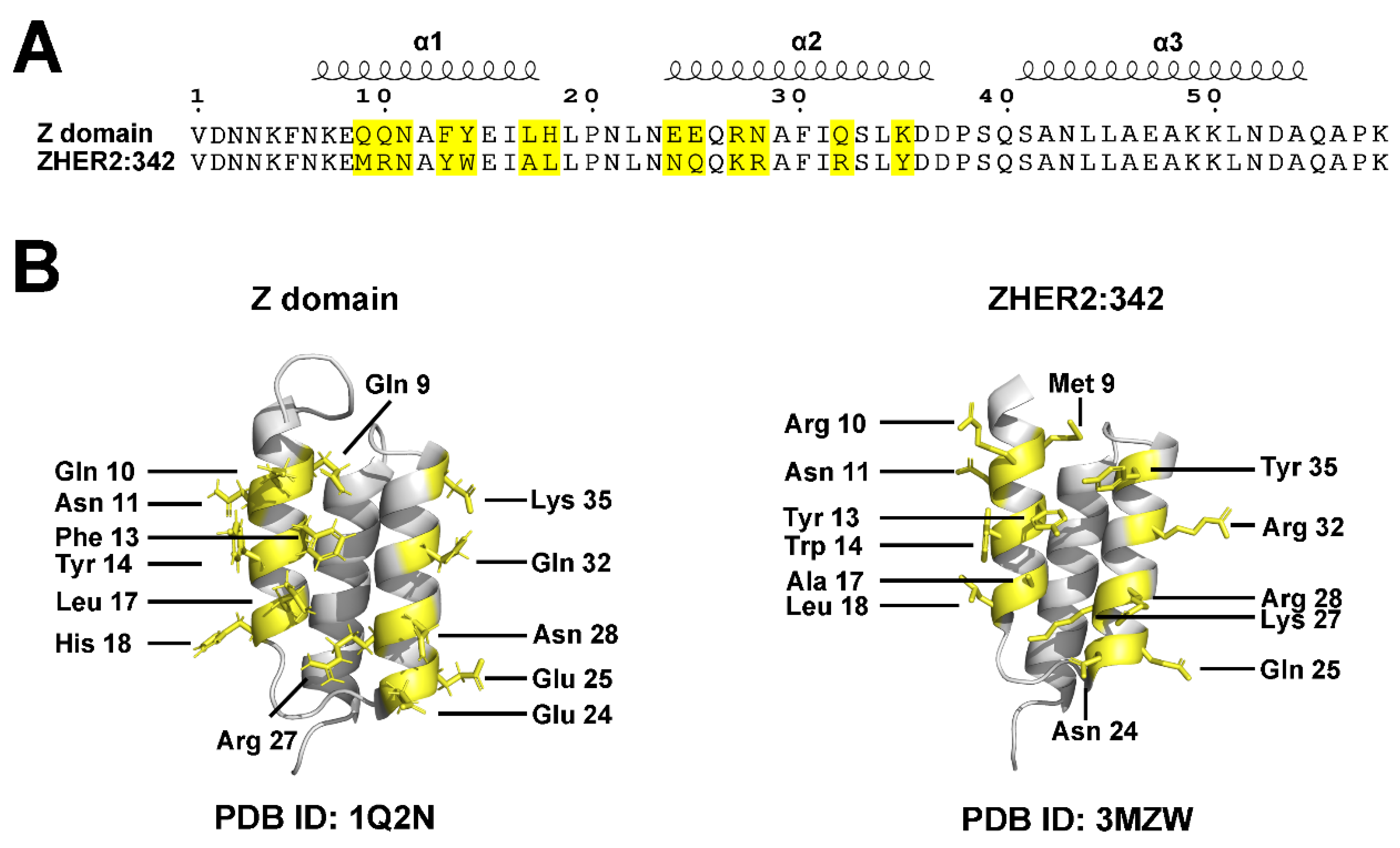
The molecular architecture features a characteristic three-helix bundle configuration (α1, α2, and α3) stabilized by a central hydrophobic core of strategically positioned nonpolar amino acids (
Figure 2).[
22,
23,
24] Notably, this stable structure does not require disulfide bonds, distinguishing Affibodies from many other protein scaffolds and contributing to their robust stability under reducing conditions.
A defining structural feature of Affibody molecules is their adaptable terminal regions. Both N- and C-termini serve as versatile attachment points for various functional groups, enabling precise molecular engineering while preserving the essential three-helix bundle conformation.[
13,
24,
25,
26] This structural characteristic renders Affibodies highly adaptable for diverse applications requiring site-specific modifications.
3.1. Binding Properties and Affinity
Affibody molecules achieve high specificity and affinity through engineered surface binding interfaces. Their binding properties primarily derive from modifications to 13 specific amino acid residues located on the first two helices (α1 and α2) of the three-helix bundle (
Figure 2 and
Figure 3).[
22,
23,
24] These residues constitute the primary binding interface and serve as key interaction points with target proteins. Through targeted mutagenesis of these 13 positions, either through random mutations or rational design, researchers generate diverse Affibody libraries exhibiting wide-ranging binding specificities and affinities.[
1,
2]
,[
8,
11]
-[
14,
22]
-[
24,
27] This flexibility enables Affibodies to recognize and bind a broad spectrum of targets, including receptors, enzymes, and clinically relevant proteins, with remarkable precision.[
2,
3]
,[
8,
17]
,[
28,
29] Recent studies have demonstrated that next-generation Affibody libraries with optimized randomization strategies can achieve binding affinities in the low picomolar range, rivalling those of monoclonal antibodies while maintaining the advantageous properties of small protein scaffolds.[
29]
3.2. Stability
Affibody molecules exhibit exceptional structural resilience and stability, maintained even when extensive modifications are introduced at the thirteen binding residues. This robustness stems from the inherent stability of the Z-domain-derived scaffold, featuring a well-conserved folding pattern and hydrophobic core composed of nonpolar amino acid residues.[
22,
23,
24,
27] This architecture creates a stable framework that accommodates significant sequence variability without compromising the molecule's overall conformation. Consequently, Affibody molecules retain their functional and structural integrity despite extensive engineering, ensuring reliable performance across diverse applications.[
24,
27]
Affibodies demonstrate remarkable stability under challenging environmental conditions, including broad pH tolerance (pH 2-11), resistance to reducing conditions (due to the absence of disulfide bonds), compatibility with organic solvents including DMSO and ethanol, high thermal stability (up to 90 °C), and resistance to proteolytic degradation.[
2,
29] These properties make them suitable for a wide range of therapeutic and diagnostic applications requiring stability under physiological and non-physiological conditions.
3.3. Half-life
The small size of Affibody molecules promotes rapid renal clearance, resulting in short circulatory half-life.[
1,
2,
3,
17] While this characteristic proves advantageous for diagnostic applications requiring swift elimination from non-target tissues, it presents limitations for therapeutic applications where extended circulation time is desirable.[
1,
2,
3,
17] To address this constraint, researchers have developed modification strategies, including polyethylene glycol (PEG) conjugation and incorporation of albumin-binding domains, to extend the
in vivo half-life of Affibodies while preserving their binding functionality.[
3,
30,
31] Recent work by Zhang et al. (2024) has demonstrated that ABD-fusion leads to significantly higher tumor uptake compared to other half-life extension strategies like PASylation and XTENylation, while maintaining favourable biodistribution profiles for therapeutic applications.[
31] This represents an important advancement in addressing one of the key limitations of Affibody molecules for therapeutic use.
4. Basic Affibody Monomeric Constructs
Basic Affibody monomeric constructs fall into two primary categories: monopartite and multipartite designs. Monopartite Affibody constructs consist solely of the engineered Affibody domain, emphasizing simplicity in production and characterization. These designs find application in receptor inhibition, biosensor development, and neutralizing inhibition strategies.[
1,
2,
3,
8,
17,
29]
Multipartite Affibody designs expand upon the basic structure by incorporating a linker, either functional or non-functional, and at least one additional functional unit. These units may include peptides, proteins, organic molecules, or detection probes. This modular architecture enables diverse applications, including bispecific targeting, drug conjugation with optimized release kinetics, enzyme attachment for localized therapeutic activity, and imaging applications using chelators such as DOTA for PET/SPECT imaging.[
1,
2,
3,
8,
17,
20,
21,
29] Additionally, these constructs can be integrated into advanced protein degradation systems, including Proteolysis Targeting Chimeras (PROTAC).[
32,
33]
Functionalization of Affibodies can be achieved through either chemical conjugation, where moieties are attached via chemical linkers, or genetic incorporation, where functional groups are introduced during protein synthesis.[
1,
2,
3] Each approach offers distinct advantages depending on the specific application requirements.
The advantages of monomeric Affibody constructs include their small size facilitating rapid tissue penetration, high affinity and specificity for targeted binding, straightforward production methods, and exceptional stability.[
1,
2,
3,
17,
29] However, a significant limitation remains their short serum half-life due to rapid renal clearance, particularly problematic for therapeutic applications requiring prolonged circulation.[
1,
2,
3] Strategies such as PEGylation, PASylation, XTENylation, and incorporation of albumin-binding domains are employed to extend their
in vivo half-life while maintaining functional activity.[
3,
30,
31]
5. Multivalent Assembly of Affibody Molecules
Despite their small size and high binding affinity, Affibody molecules face a critical
in vivo limitation: short circulatory half-life due to rapid renal clearance. Supramolecular assembly strategies address this by enhancing pharmacokinetic profiles and enabling versatile, multivalent systems for therapeutic and diagnostic applications.[
4,
5,
6,
34,
35,
36,
37]
Supramolecular chemistry leverages non-covalent interactions, including hydrogen bonding, π–π stacking, and van der Waals forces, to construct highly organized, functional biomolecular architectures.[
38] Researchers harness these to integrate Affibody molecules, combining their target-binding strengths with the adaptability, stability, and multivalency of supramolecular frameworks.[
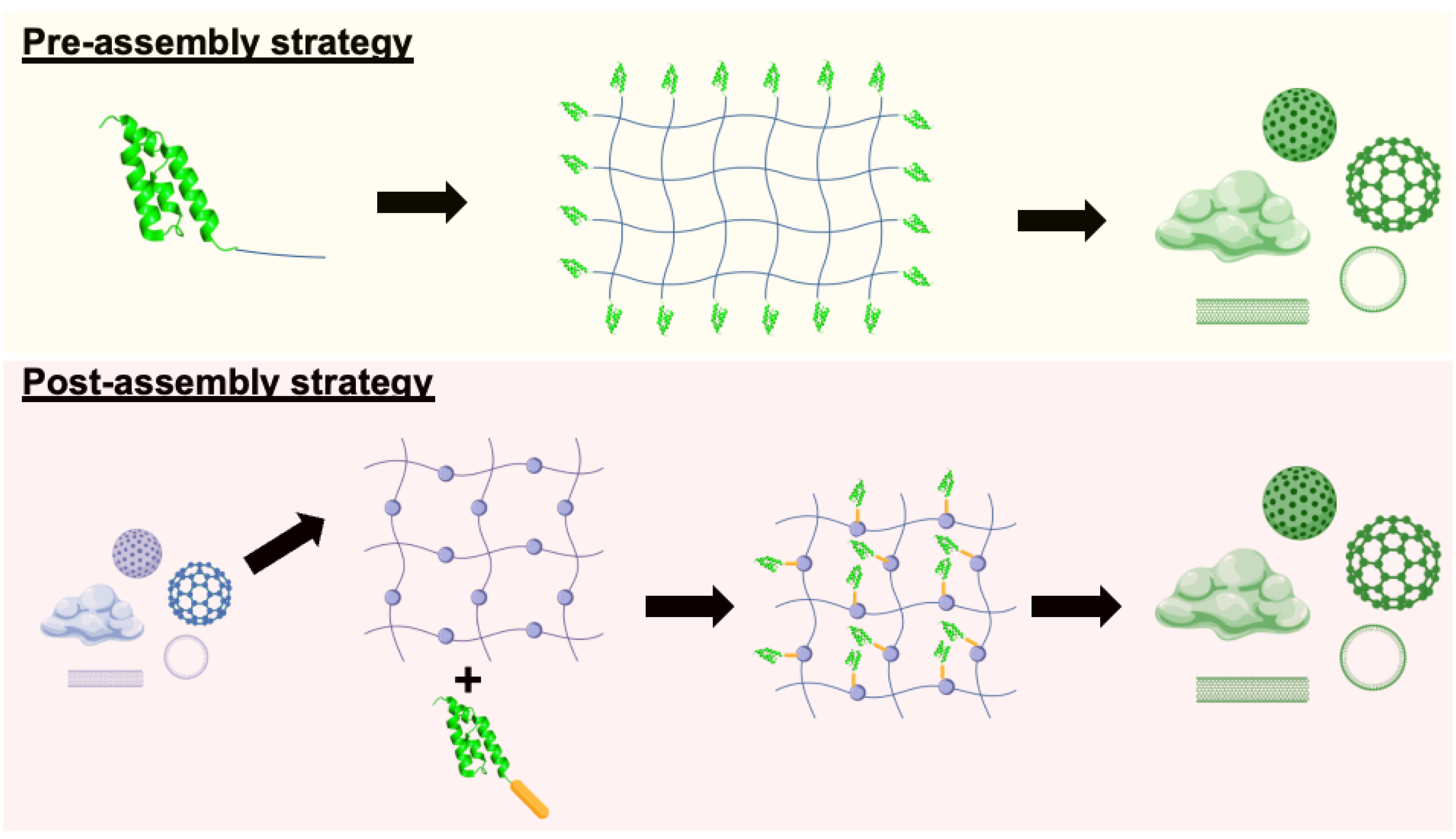
34] To the best of our knowledge, pre-assembly and post-assembly strategies are the two primary approaches that guide Affibody integration into supramolecular scaffolds (
Figure 4).
5.1. Pre-assembly Incorporation Strategies
In pre-assembly strategies, Affibody molecules are modified through genetic fusion or post-expression chemical and biological methods to incorporate self-assembling units (e.g., peptides, polymers, or DNA scaffolds) before scaffold formation. The key advantage lies in enabling precise, site-specific integration through the introduction of reactive groups, such as azides, alkynes, or thiol, without interfering with the function of these self-assembling units. For instance, site-directed mutagenesis can introduce cysteine residues for thiol-maleimide reactions, while genetic code expansion enables the incorporation of non-natural amino acids for bioorthogonal click chemistry. High-affinity non-covalent partners, like biotin-streptavidin, serve as effective alternatives to these covalent methods. As the modified Affibodies self-organize into micelles, vesicles, fibers, or hydrogels, they achieve optimized positioning for target binding.
This method provides exceptional spatial precision, enabling researchers to adjust Affibody orientation and distribution. Binding domains thus remain accessible and functional, which is essential for targeted therapeutics and high-resolution imaging.[
39,
40,
41,
42] Moreover, uniform modifications ensure consistent functionality across batches, improving experimental reproducibility. Pre-assembly also supports multivalent configurations, where multiple Affibody units synergize to boost target recognition via avidity effects.[
3,
5]
However, this approach requires rigorous chemistry to preserve Affibody folding and binding functionality. Scalability challenges may arise when complex purification steps prove difficult to replicate at industrial scale. Additionally, Affibodies must be protected from destabilizing assembly conditions (such as heat, pH shifts, or organic solvents) that could impair molecular recognition.
5.2. Post-assembly Incorporation Strategies
Post-assembly strategies involve attaching Affibodies to preformed supramolecular scaffolds, offering enhanced design flexibility. The process begins with synthesizing and characterizing nanostructures (such as liposomes, polymeric micelles, protein cages, or inorganic nanoparticles) focusing on key parameters like size distribution, surface charge, and stability.[
43]
Affibodies are attached to these preformed scaffolds using covalent bonds or usually high-affinity non-covalent interactions functionally equivalent to covalent bonds, enabling sequential functionalization. Covalent attachment methods, including thiol–maleimide chemistry, click reactions, EDC/NHS coupling, SpyTag/SpyCatcher systems, and HaloTag technology, provide robust long-term stability through irreversible linkages. Alternatively, non-covalent approaches such as biotin-streptavidin pairs, electrostatic forces, hydrophobic interactions, or molecular recognition motifs can also be used to functionalized these preformed scaffold.
A major strength of post-assembly is its modularity, allowing sequential addition of distinct Affibodies to create multifunctional systems for dual-targeting or combined imaging and therapy. This adaptability supports customization for specific research or clinical needs while preserving the scaffold's structural integrity and functional properties throughout functionalization.[
44,
45] For instance, functionalizing existing nanoparticle systems such as gold nanoparticles and quantum dots can enhance imaging signal intensity or improve drug delivery precision.[
42,
46,
47,
48,
49] Similarly, attaching Affibodies to protein cages, including virus-like particles, can enhance pharmacokinetic control and therapeutic efficacy.[
17,
50,
51]
Despite this versatility, post-assembly offers less precise control over Affibody spatial distribution and orientation compared to pre-assembly, which may lead to heterogeneous binding. Achieving uniform functionalization across scaffolds can be challenging, underscoring the need for optimized conjugation protocols and rigorous characterization to ensure reliable performance.
6. Recent progress in Affibody-based supramolecular architectures
6.1. Examples on Pre-assembly Incorporation in Affibody-Based Supramolecular Architectures
Pre-assembly incorporation involves integrating Affibody molecules into supramolecular structures during the initial formation process, allowing for precise control over architecture, stoichiometry, and functionality. This strategy contrasts with post-assembly methods by embedding the targeting moiety within the scaffold, often enhancing stability, multivalency, and pharmacokinetic properties. Below, we highlight recent examples that demonstrate the versatility of pre-assembly in therapeutic and diagnostic applications.
One exemplary approach is to integrate Affibodies into virus-like particles (VLPs) for targeted delivery, leveraging genetic fusion for seamless incorporation during assembly. In a study by Nishimura et al. (2013), HER2-specific Affibodies were genetically fused to hepatitis B core (HBc) proteins. During recombinant expression in
E. coli, these fusion proteins self-assembled into hollow HBc particles. This pre-assembly conferred high specificity for HER2-overexpressing breast cancer cells (e.g., SK-BR-3), with enhanced cellular uptake.[
52]
Building on well-known amphiphile self-assembly mechanisms, Affibody-cytotoxin conjugates have been pre-incorporated into nanoagents for targeted cancer therapy, capitalizing on the conjugates' inherent ability to form nanostructures. For instance, Yang et al. (2025) described EGFR-targeted Affibodies conjugated to monomethyl auristatin E (MMAE) through thiol-maleimide chemistry. Due to the amphiphilic nature, the Affibody-MMAE conjugates self-assembled into nanoagents. The resulting nanoagents exhibited rapid internalization, potent cytotoxicity in EGFR-positive cancer cells, as well as strong tumour targeting and antitumor effects
in vivo. This pre-assembly method improved tumour penetration and reduced systemic toxicity compared to free conjugates, highlighting its promise for precise cancer targeted therapy.[
53] Similarly, Xia et al. (2024) conjugated HER2-targeted Affibodies to epothilone B via thiol-maleimide chemistry. The amphiphilic Affibody-drug conjugates self-assembled into core-shell nanoagents for enhanced release and efficacy in HER2-positive breast and ovarian tumors.[
54] Recent advances have also leveraged self-assembling Affibody-PROTAC conjugates for targeted delivery. For PROTAC-based systems, Gao et al. (2024) and Li et al. (2024) described amphiphilic conjugates where EGFR- or HER2-targeted Affibodies (ZEGFR:1907 or ZHER2:342) were linked to PROTACs (MS28 or MZ1) via bioresponsive linkers. These amphiphilic Affibody-PROTAC conjugates self-assembled into nanoagents (e.g., ADCN or APCN) in aqueous solutions. This enhanced tumour accumulation, controlled release, and degradation of targets like cyclin D1 or BRD4 in EGFR- or HER2-positive models, achieving high biosafety and antitumor efficacy.[
32,
33]
Apart from amphiphile self-assembly systems, thermoresponsive self-assembly systems have also been designed to integrate Affibody molecules into supramolecular structures. For example, Li et al. (2024) fused HER2-targeted Affibodies (ZHER2:342) to elastin-like polypeptides (ELP) and conjugated them to MMAE, forming thermoresponsive nanomicelles during self-assembly above the transition temperature. This pre-assembly improved HER2-mediated endocytosis and tumour penetration in ovarian cancer xenografts.[
55]
Peptide-driven self-assembly offers another avenue to generate Affibody supramolecular architectures for diagnostic applications. In Liu et al. (2022), Affibodies targeting alpha-fetoprotein (AFP), a biomarker for liver cancer, were pre-assembled with β-sheet-forming peptides to create bioactive nanofibril aggregates. The Affibodies were genetically fused to self-assembling peptides prior to aggregation, resulting in structures with enhanced stability and improved immunoassay signal amplification activity. This system achieved limits of detection as low as 9.7 ng mL⁻¹ AFP, far surpassing traditional ELISA methods, and underscores the role of Affibody-based supramolecular architectures in biosensing platforms.[
56]
DNA-based nanostructures further expand pre-assembly possibilities for synergistic therapies using Affibody supramolecular architectures. Zhang et al. (2022) reported HER2-targeted Affibodies modified onto G-quadruplex DNA micellar prodrugs, which self-assembled into G-quadruplex DNA micelles loaded with polymeric 5-fluorodeoxyuridine (5-FdU) and curcumin during micelle formation. The pre-incorporated Affibodies facilitated receptor-mediated endocytosis in HER2-positive gastric cancer cells, enabling dual-drug release that synergistically inhibited tumour growth
in vivo.[
57] Similarly, Zhang et al. (2020) utilized Affibody-conjugated RALA amphipathic peptides to form nanoparticles encapsulating oligomeric 5-FdU. Pre-assembly ensured uniform distribution of the targeting ligands, leading to superior efficacy in HER2-overexpressing gastric cancer models.[
58] These innovative applications demonstrate the broad utility of Affibody-based supramolecular architectures in enhancing drug delivery systems and enabling controlled release of multiple therapeutic agents.
Finally, hydrogel-based systems represent a culminative evolution of these pre-assembly strategies, incorporating Affibodies into biocompatible matrices for long-term therapeutic delivery in regenerative contexts. Teal et al. (2022) incorporated Affibody-modified methylcellulose into biopolymer-based hydrogels to achieve sustained and independent release of therapeutic proteins over seven days. This methodology demonstrated significant applications in tissue regeneration and retinal degenerative disease treatment, offering versatile strategies for simultaneous delivery of multiple therapeutics.[
59]
6.2. Examples on Post-assembly Incorporation in Affibody-Based Supramolecular Architectures
Post-assembly incorporation refers to the attachment of Affibody molecules to pre-formed supramolecular structures, such as nanoparticles, beads, or bubbles, after their initial synthesis or assembly. This approach allows for modular functionalization, enabling precise targeting while preserving the core properties of the scaffold, including stability, imaging capabilities, and biocompatibility. In contrast to pre-assembly methods, post-assembly strategies often simplify production and facilitate the addition of multiple ligands for multimodal applications. Below, we highlight recent examples that showcase the versatility of post-assembly in diagnostic, imaging, and therapeutic contexts.
Nanoparticle-based systems exemplify post-assembly's utility in multimodal imaging, where Affibodies are conjugated to pre-formed cores to boost targeting specificity and signal amplification. For instance, Jokerst et al. (2011) functionalized gold–silica nanoparticles (SERS NPs) with EGFR-specific Affibodies after silica shell formation, linking them via a short PEG-maleimide cross-linker to thiol groups on the surface. This enabled Raman molecular imaging of EGFR-positive A431 tumours, achieving a signal nearly 35-fold higher in positive tumours compared to EGFR-negative ones.[
46] Similarly, Yang et al. (2013) modified gold-iron oxide heteronanostructures with EGFR-specific Affibodies post-assembly, incorporating radiolabels for PET, optical, and magnetic resonance imaging (MRI) of EGFR-expressing tumours, resulting in high tumour uptake and multimodal contrast via maleimide chemistry.[
42] Gao et al. (2012) also explored Affibody-conjugated quantum dots and iron oxide nanoparticles for imaging HER2-expressing cells and tumours. Incorporating anti-HER2 Affibodies onto these supramolecular architectures using maleimide chemistry resulted in high specificity and affinity for HER2-positive cancer cells, making them effective for both fluorescence and MRI-based diagnostic applications. This dual-modality approach enhances tumour detection precision, providing comprehensive diagnostic capabilities.[
34] These imaging-focused examples illustrate how post-assembly leverages plasmonic or magnetic properties for sensitive detection, paving the way for sensitive cancer diagnosis.
Building on these imaging foundations, nanoparticle systems have evolved to incorporate post-assembly Affibodies for targeted therapy, combining diagnostic capabilities with potent antitumor effects. Extending to photothermal applications, Shipunova et al. (2022) conjugated HER2-specific Affibodies (ZHER2:342) to pre-synthesized silver nanoparticles via a PEG linker, enabling targeted photothermal therapy (PTT) in HER2-overexpressing tumours. The post-assembled Ag-PEG-HER2 NPs demonstrated efficient ROS generation and heating under light irradiation, leading to complete tumour regression in xenograft models.[
60] Complementing this, Shipunova et al. (2021) decorated pre-formed PLGA nanoparticles with anti-HER2 Affibodies post-assembly for targeted delivery of photosensitizers, inducing photoactivated cell death upon light irradiation in HER2-positive breast cancer cells with high specificity and minimal off-target effects.[
61]
Bubble-based architectures offer innovative post-assembly strategies for ultrasound imaging and combined photodynamic treatments. Yang et al. (2015) conjugated biotinylated anti-HER2 Affibodies to pre-formed phospholipid nanobubbles via streptavidin-biotin bridging, producing targeted ultrasound contrast agents for HER2-overexpressing breast tumours, with peak intensities of 104.5 dB and high tumour selectivity. This innovation improved molecular ultrasound imaging specificity and offers potential for real-time monitoring of therapeutic responses using ultrasound imaging.[
62] Similarly, enhancing this targeted nanobubble concept with combined photodynamic therapy (PDT), Cai et al. (2023) conjugated HER2-specific Affibodies to pre-formed nanobubbles loaded with IR783 and HPPH through a biotin-streptavidin system to form nanobubble-IR783-HPPH-Affibody (NIHA) complexes, enabling laser-activated PDT for HER2-positive breast cancer, with significant increases in ROS production and tumour cell death
in vitro and
in vivo.[
63] The combination of PDT with targeted delivery significantly reduced tumour viability, highlighting the potential of Affibody-functionalized nanoparticles in non-invasive cancer treatment.
Protein nanocage-based systems further advance post-assembly by integrating Affibodies into porous protein-based scaffolds for enhanced delivery and synergistic effects. In a study by Kim et al. (2021), Gd(III)-DOTA protein cage nanoparticles were post-functionalized with multiple HER2 or EGFR-specific Affibodies using a SpyTag/SpyCatcher system, enabling target-switchable T1 contrast in high-field MRI with selective binding to HER2 or EGFR-overexpressing cells for highly specific and sensitive cancer diagnosis.[
64] Similarly, Eom et al. (2024) engineered porous SpyCatcher-mi3 protein cage nanoparticles as modular delivery platforms, post-displaying Affibodies for ligand-targeted cargo release in therapeutic applications, enabling precise targeting and delivery of chemotherapeutic agents to EGFR-overexpressing cancer cells.[
65] Extending to protein nanocage nanoparticle systems for intracellular drug delivery, Jun et al. (2022) displayed both TRAIL and EGFR-specific Affibodies on pre-formed lumazine synthase protein cage nanoparticles post-assembly, synergistically inducing apoptosis and suppressing tumour growth in EGFR-overexpressing models through combined receptor targeting and death ligand activation.[
50]
Apart from protein nanocage systems, metal-organic framework (MOF) systems are another promising nanoparticle-based vehicle for drug delivery. Oh et al. (2023) coated pre-formed Zr-based MOF nanoparticles (PCN-224) with GST-fused HER2- or EGFR-specific Affibodies to form a protein-MOF hybrid system, creating a protective protein corona shield that minimized serum protein adsorption and enabled targeted delivery of camptothecin for synergistic PDT and chemotherapy in breast cancer models.[
66]
DNA-based nanostructures further expand post-assembly possibilities for targeted drug delivery using Affibody-incorporated supramolecular architectures. Yu et al. (2023) developed CytoDirect, a disulfide-modified DNA origami nanodevice functionalized with HER2 Affibodies for efficient cytosolic delivery of therapeutic payloads. By integrating Affibodies post-assembly through an oligonucleotide handle, CytoDirect achieved high specificity and enhanced cellular uptake in HER2-positive cancer cells, facilitating precise delivery of therapeutic oligonucleotides and doxorubicin.[
67]
Affibody-incorporated supramolecular architectures also contribute to the area of controlled drug release. Dorogin et al. (2023) investigated controlled delivery of bone morphogenetic protein-2 (BMP-2) for bone regeneration by conjugating BMP-2-specific Affibodies to polyethylene glycol-maleimide hydrogels. This system enabled precise modulation of BMP-2 release kinetics, enhancing osteogenic stimulation while minimizing adverse effects and offering promising strategies for improving bone healing outcomes.[
68] In addition to covalent linkages, Liang et al. (2018) developed a novel therapeutic approach targeting HER2-positive tumours by co-assembling drug-peptide amphiphiles with anti-HER2 Affibodies to form a supramolecular nanofiber hydrogel embedded with Affibodies. The resulting nanofibers demonstrated significant suppression of HER2⁺ tumour growth
in vivo, showing improved accumulation and retention in tumours.[
36]
7. Conclusion and Future Outlook
Affibody-based supramolecular architectures represent a pivotal advancement in protein engineering, transforming monomeric constructs into versatile multivalent systems with profound biomedical potential. By addressing inherent limitations like rapid renal clearance, these assemblies expand applications in therapeutics, diagnostics, biosensing, and biomaterials. Pre- and post-assembly strategies provide complementary tools for integration: pre-assembly excels in delivering precise spatial control, predictable multivalency, and uniform functionality, making it ideal for ordered arrays in complex scaffolds such as peptide assemblies or nucleic acid nanostructures. In contrast, post-assembly offers superior flexibility for sequential functionalization and scaffold preservation, enhancing existing platforms like nanoparticles and protein cages, though it may trade off some stability and precision.
Together, these approaches harness the robust binding specificity, stability, and adaptability of Affibodies to create multifunctional platforms that outperform traditional methods. They enable precise targeting, extended pharmacokinetics, minimized off-target effects, and avidity-enhanced binding, driving innovations in cancer therapy, molecular imaging, and drug delivery. For instance, multivalent presentations facilitate efficient cellular uptake and therapeutic efficacy, while modular designs support hybrid systems for theranostics.
As the field progresses, ongoing refinements in conjugation chemistry, scaffold engineering, and protein design coupled with computational modelling, advanced fabrication, and deeper biological insights will yield increasingly tailored architectures. Researchers are exploring hybrid covalent-non-covalent methods to balance stability and reversibility, alongside strategies for controlling assembly homogeneity, reducing immunogenicity, and scaling production. These developments position Affibody-based systems at the vanguard of personalized medicine and molecular diagnostics, promising adaptable solutions for diverse clinical challenges and accelerating transformative translations from bench to bedside.
Acknowledgments
This research was supported in part by Xi’an Jiaotong-Liverpool University Research Development Fund (RDF-23-01-007 and RDF-23-01-008) and the 2025 GenScript Life Science Research Grant. S.L and A.K would also like to acknowledge the support of Jiangsu Province Higher Education Key Laboratory of Cell Therapy Nanoformulation, Suzhou Municipal Key Lab for Metabolic Syndrome Drug Research and the Suzhou Municipal Key Lab of Biomedical Sciences and Translational Immunology.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Frejd, F.Y.; Kim, K.-T. Affibody molecules as engineered protein drugs. Exp. Mol. Med. 2017, 49, e306–e306. [CrossRef]
- Löfblom, J.; Feldwisch, J.; Tolmachev, V.; Carlsson, J.; Ståhl, S.; Frejd, F.Y. Affibody molecules: Engineered proteins for therapeutic, diagnostic and biotechnological applications. FEBS Lett. 2010, 584, 2670–2680. [CrossRef]
- Ståhl, S.; Gräslund, T.; Eriksson Karlström, A.; Frejd, F.Y.; Nygren, P.-Å.; Löfblom, J. Affibody Molecules in Biotechnological and Medical Applications. Trends Biotechnol. 2017, 35, 691–712. [CrossRef]
- Ashford, M.B.; England, R.M.; Akhtar, N. Highway to Success—Developing Advanced Polymer Therapeutics. Adv. Ther. 2021, 4, 2000285. [CrossRef]
- Cole, J.T.; Holland, N.B. Multifunctional nanoparticles for use in theranostic applications. Drug Deliv. Transl. Res. 2015, 5, 295–309. [CrossRef]
- Fang, C.; Zhang, M. Nanoparticle-based theragnostics: Integrating diagnostic and therapeutic potentials in nanomedicine. J. Control. Release 2010, 146, 2–5. [CrossRef]
- Nord, K.; Gunneriusson, E.; Ringdahl, J.; Ståhl, S.; Uhlén, M.; Nygren, P.Å. Binding proteins selected from combinatorial libraries of an α-helical bacterial receptor domain. Nat. Biotechnol. 1997, 15, 772–777. [CrossRef]
- Feldwisch, J.; Tolmachev, V. Engineering of affibody molecules for therapy and diagnostics. Therapeutic Proteins: Methods and Protocols 2012:103-26.
- Orlova, A.; Magnusson, M.; Eriksson, T.L.J.; Nilsson, M.; Larsson, B.; Höidén-Guthenberg, I.; Widström, C.; Carlsson, J.; Tolmachev, V.; Ståhl, S.; et al. Tumor Imaging Using a Picomolar Affinity HER2 Binding Affibody Molecule. Cancer Res. 2006, 66, 4339–4348. [CrossRef]
- Sörensen, J.; Sandberg, D.; Sandström, M.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Åström, G.; Lubberink, M.; Garske-Román, U.; Carlsson, J.; et al. First-in-Human Molecular Imaging of HER2 Expression in Breast Cancer Metastases Using the 111In-ABY-025 Affibody Molecule. J. Nucl. Med. 2014, 55, 730–735. [CrossRef]
- Friedman, M.; Nordberg, E.; Höidén-Guthenberg, I.; Brismar, H.; Adams, G.; Nilsson, F.; Carlsson, J.; Ståhl, S. Phage display selection of Affibody molecules with specific binding to the extracellular domain of the epidermal growth factor receptor. Protein Eng. Des. Sel. 2007, 20, 189–199. [CrossRef]
- Gast, V.; Sandegren, A.; Dunås, F.; Ekblad, S.; Güler, R.; Thorén, S.; Mohedano, M.T.; Molin, M.; Engqvist, M.K.M.; Siewers, V. Engineering Saccharomyces cerevisiae for the production and secretion of Affibody molecules. Microb. Cell Factories 2022, 21, 1–15. [CrossRef]
- Ståhl, S.; Lindberg, H.; Hjelm, L.C.; Löfblom, J.; Leitao, C.D. Engineering of Affibody Molecules. Cold Spring Harb. Protoc. 2023, 2024. [CrossRef]
- Grimm, S.; Yu, F.; Nygren, P. Ribosome Display Selection of a Murine IgG1 Fab Binding Affibody Molecule Allowing Species Selective Recovery Of Monoclonal Antibodies. Mol. Biotechnol. 2011, 48, 263–276. [CrossRef]
- Ekerljung, L.; Steffen, A.-C.; Carlsson, J.; Lennartsson, J. Effects of HER2-Binding Affibody Molecules on Intracellular Signaling Pathways. Tumor Biol. 2006, 27, 201–210. [CrossRef]
- Altai, M.; Liu, H.; Orlova, A.; Tolmachev, V.; Gräslund, T. Influence of molecular design on biodistribution and targeting properties of an Affibody-fused HER2-recognising anticancer toxin. Int. J. Oncol. 2016, 49, 1185–1194. [CrossRef]
- Orlova, A.; Feldwisch, J.; Abrahmsén, L.; Tolmachev, V. Update: Affibody Molecules for Molecular Imaging and Therapy for Cancer. Cancer Biotherapy Radiopharm. 2007, 22, 573–584. [CrossRef]
- Sörensen, J.; Velikyan, I.; Sandberg, D.; Wennborg, A.; Feldwisch, J.; Tolmachev, V.; Orlova, A.; Sandström, M.; Lubberink, M.; Olofsson, H.; et al. Measuring HER2-Receptor Expression In Metastatic Breast Cancer Using [68Ga]ABY-025 Affibody PET/CT. Theranostics 2016, 6, 262–271. [CrossRef]
- Eissler, N.; Altena, R.; Alhuseinalkhudhur, A.; Bragina, O.; Feldwisch, J.; Wuerth, G.; Loftenius, A.; Brun, N.; Axelsson, R.; Tolmachev, V.; et al. Affibody PET Imaging of HER2-Expressing Cancers as a Key to Guide HER2-Targeted Therapy. Biomedicines 2024, 12, 1088. [CrossRef]
- Tolmachev, V.; Orlova, A. Affibody Molecules as Targeting Vectors for PET Imaging. Cancers 2020, 12, 651. [CrossRef]
- Wållberg, H.; Orlova, A.; Altai, M.; Hosseinimehr, S.J.; Widström, C.; Malmberg, J.; Ståhl, S.; Tolmachev, V. Molecular Design and Optimization of 99mTc-Labeled Recombinant Affibody Molecules Improves Their Biodistribution and Imaging Properties. J. Nucl. Med. 2011, 52, 461–469. [CrossRef]
- Högbom, M.; Eklund, M.; Nygren, P.; Nordlund, P. Structural basis for recognition by an in vitro evolved affibody. Proc. Natl. Acad. Sci. 2003, 100, 3191–3196. [CrossRef]
- Lendel C, Dogan J, Härd T. Structural basis for molecular recognition in an affibody: affibody complex. Journal of molecular biology 2006;359:1293-304.
- Wahlberg, E.; Lendel, C.; Helgstrand, M.; Allard, P.; Dincbas-Renqvist, V.; Hedqvist, A.; Berglund, H.; Nygren, P.; Härd, T. An affibody in complex with a target protein: Structure and coupled folding. Proc. Natl. Acad. Sci. 2003, 100, 3185–3190. [CrossRef]
- Lindgren J, Wahlström A, Danielsson J, Markova N, Ekblad C, Gräslund A, et al. N-terminal engineering of amyloid-β-binding Affibody molecules yields improved chemical synthesis and higher binding affinity. Protein Science 2010;19:2319-29.
- Engfeldt, T.; Renberg, B.; Brumer, H.; Nygren, P.Å.; Karlström, A.E. Chemical Synthesis of Triple-Labelled Three-Helix Bundle Binding Proteins for Specific Fluorescent Detection of Unlabelled Protein. ChemBioChem 2005, 6, 1043–1050. [CrossRef]
- Feldwisch, J.; Tolmachev, V.; Lendel, C.; Herne, N.; Sjöberg, A.; Larsson, B.; Rosik, D.; Lindqvist, E.; Fant, G.; Höidén-Guthenberg, I.; et al. Design of an Optimized Scaffold for Affibody Molecules. J. Mol. Biol. 2010, 398, 232–247. [CrossRef]
- Grönwall, C.; Ståhl, S. Engineered affinity proteins—Generation and applications. J. Biotechnol. 2009, 140, 254–269. [CrossRef]
- Zhang, L.; Zhang, H. Recent advances of affibody molecules in biomedical applications. Bioorganic Med. Chem. 2024, 113, 117923. [CrossRef]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; Ekblad, C.; Frejd, F.Y.; Tolmachev, V. Site-Specific Radiometal Labeling and Improved Biodistribution Using ABY-027, A Novel HER2-Targeting Affibody Molecule–Albumin-Binding Domain Fusion Protein. J. Nucl. Med. 2013, 54, 961–968. [CrossRef]
- Zhang, J.; Bodenko, V.; Larkina, M.; Bezverkhniaia, E.; Xu, T.; Liao, Y.; Abouzayed, A.; Plotnikov, E.; Tretyakova, M.; Yuldasheva, F.; et al. Half-life extension via ABD-fusion leads to higher tumor uptake of an affibody-drug conjugate compared to PAS- and XTENylation.. J. Control. Release 2024, 370, 468–478. [CrossRef]
- Gao, W.; Xia, X.; Yang, X.; Li, Q.; Xia, X.; Huang, W.; Yan, D. Amphiphilic Affibody-PROTAC conjugate Self-Assembled nanoagents for targeted cancer therapy. Chem. Eng. J. 2024, 495. [CrossRef]
- Li, Q.; Yang, X.; Zhao, M.; Xia, X.; Gao, W.; Huang, W.; Xia, X.; Yan, D. A self-assembled affibody-PROTAC conjugate nanomedicine for targeted cancer therapy. Nano Res. 2024, 17, 9954–9964. [CrossRef]
- Gao, J.; Chen, K.; Miao, Z.; Ren, G.; Chen, X.; Gambhir, S.S.; Cheng, Z. Affibody-based nanoprobes for HER2-expressing cell and tumor imaging. Biomaterials 2011, 32, 2141–2148. [CrossRef]
- Schardt, J.S.; Oubaid, J.M.; Williams, S.C.; Howard, J.L.; Aloimonos, C.M.; Bookstaver, M.L.; Lamichhane, T.N.; Sokic, S.; Liyasova, M.S.; O’nEill, M.; et al. Engineered Multivalency Enhances Affibody-Based HER3 Inhibition and Downregulation in Cancer Cells. Mol. Pharm. 2017, 14, 1047–1056. [CrossRef]
- Liang, C.; Zhang, L.; Zhao, W.; Xu, L.; Chen, Y.; Long, J.; Wang, F.; Wang, L.; Yang, Z. Supramolecular Nanofibers of Drug-Peptide Amphiphile and Affibody Suppress HER2+ Tumor Growth. Adv. Heal. Mater. 2018, 7, e1800899. [CrossRef]
- Radford, D.C.; Yang, J.; Doan, M.C.; Li, L.; Dixon, A.S.; Owen, S.C.; Kopeček, J. Multivalent HER2-binding polymer conjugates facilitate rapid endocytosis and enhance intracellular drug delivery. J. Control. Release 2020, 319, 285–299. [CrossRef]
- Talreja, S.; Tiwari, S. Supramolecular Chemistry: Unveiling the Fascinating World of Non-Covalent Interactions and Complex Assemblies. J. Pharm. Pharmacol. Res. 2023, 07, 133–139. [CrossRef]
- Xia, X.; Yang, X.; Huang, W.; Xia, X.; Yan, D. Self-Assembled Nanomicelles of Affibody-Drug Conjugate with Excellent Therapeutic Property to Cure Ovary and Breast Cancers. Nano-Micro Lett. 2021, 14, 1–16. [CrossRef]
- Fu, R.; Carroll, L.; Yahioglu, G.; Aboagye, E.O.; Miller, P.W. Antibody Fragment and Affibody ImmunoPET Imaging Agents: Radiolabelling Strategies and Applications. ChemMedChem 2018, 13, 2466–2478. [CrossRef]
- Persson, J.; Puuvuori, E.; Zhang, B.; Velikyan, I.; Åberg, O.; Müller, M.; Nygren, P.; Ståhl, S.; Korsgren, O.; Eriksson, O.; et al. Discovery, optimization and biodistribution of an Affibody molecule for imaging of CD69. Sci. Rep. 2021, 11, 1–11. [CrossRef]
- Yang, M.; Cheng, K.; Qi, S.; Liu, H.; Jiang, Y.; Jiang, H.; Li, J.; Chen, K.; Zhang, H.; Cheng, Z. Affibody modified and radiolabeled gold–Iron oxide hetero-nanostructures for tumor PET, optical and MR imaging. Biomaterials 2013, 34, 2796–2806. [CrossRef]
- Justino CI, Duarte AC, Rocha-Santos TA. Analytical applications of affibodies. TrAC Trends in Analytical Chemistry 2015;65:73-82.
- Abd Ellah NH, Abouelmagd SA. Surface functionalization of polymeric nanoparticles for tumor drug delivery: approaches and challenges. Expert opinion on drug delivery 2017;14:201-14.
- Distaffen, H.E.; Jones, C.W.; Abraham, B.L.; Nilsson, B.L. Multivalent display of chemical signals on self-assembled peptide scaffolds. Pept. Sci. 2021, 113, e24224. [CrossRef]
- Jokerst, J.V.; Miao, Z.; Zavaleta, C.; Cheng, Z.; Gambhir, S.S. Affibody-Functionalized Gold–Silica Nanoparticles for Raman Molecular Imaging of the Epidermal Growth Factor Receptor. Small 2011, 7, 625–633. [CrossRef]
- Bang, J.; Park, H.; Choi, W.I.; Sung, D.; Lee, J.H.; Lee, K.Y.; Kim, S. Sensitive detection of dengue virus NS1 by highly stable affibody-functionalized gold nanoparticles. New J. Chem. 2018, 42, 12607–12614. [CrossRef]
- Zhang, Y.; Zhao, N.; Qin, Y.; Wu, F.; Xu, Z.; Lan, T.; Cheng, Z.; Zhao, P.; Liu, H. Affibody-functionalized Ag2S quantum dots for photoacoustic imaging of epidermal growth factor receptor overexpressed tumors. Nanoscale 2018, 10, 16581–16590. [CrossRef]
- Al-Ani, A.W.; Zamberlan, F.; Ferreira, L.; Bradshaw, T.D.; Thomas, N.R.; Turyanska, L. Near-infrared PbS quantum dots functionalized with affibodies and ZnPP for targeted imaging and therapeutic applications. Nano Express 2021, 2, 040005. [CrossRef]
- Jun, H.; Jang, E.; Kim, H.; Yeo, M.; Park, S.G.; Lee, J.; Shin, K.J.; Chae, Y.C.; Kang, S.; Kim, E. TRAIL & EGFR affibody dual-display on a protein nanoparticle synergistically suppresses tumor growth. J. Control. Release 2022, 349, 367–378. [CrossRef]
- Kim, K.R.; Lee, A.S.; Kim, S.M.; Heo, H.R.; Kim, C.S. Virus-like nanoparticles as a theranostic platform for cancer. Front. Bioeng. Biotechnol. 2023, 10, 1106767. [CrossRef]
- Nishimura, Y.; Mimura, W.; Suffian, I.F.M.; Amino, T.; Ishii, J.; Ogino, C.; Kondo, A. Granting specificity for breast cancer cells using a hepatitis B core particle with a HER2-targeted affibody molecule. J. Biochem. 2012, 153, 251–256. [CrossRef]
- Yang, X.; Xia, X.; Li, Q.; Zhao, M.; Gao, W.; Xia, X.-X.; Huang, W.; Yan, D. An Affibody-Cytotoxin Conjugate-Based Nanoagent for EGFR Targeting and Precise Therapy of Epidermal Squamous Cell Carcinoma. ACS Appl. Nano Mater. 2025. [CrossRef]
- Xia, X.; Yang, X.; Gao, W.; Huang, W.; Xia, X.; Yan, D. A novel HER2 targeting nanoagent self-assembled from affibody-epothilone B conjugate for cancer therapy. J. Nanobiotechnology 2024, 22, 1–12. [CrossRef]
- Li, Q.; Yang, X.; Xia, X.; Xia, X.-X.; Yan, D. Affibody-Functionalized Elastin-like Peptide–Drug Conjugate Nanomicelle for Targeted Ovarian Cancer Therapy. Biomacromolecules 2024, 25, 6474–6484. [CrossRef]
- Liu, J.; Jiang, Y.; Chen, X.; Chen, L.; Zhang, X.; Cui, D.; Li, Y.; Liu, Z.; Zhao, Q.; Diao, A. Development of active affibody aggregates induced by a self-assembling peptide for high sensitive detection of alpha-fetoprotein. Chem. Eng. J. 2022, 436. [CrossRef]
- Zhang, C.; Fu, S.; Zhang, F.; Han, M.; Wang, X.; Du, J.; Zhang, H.; Li, W. Affibody Modified G-quadruplex DNA Micelles Incorporating Polymeric 5-Fluorodeoxyuridine for Targeted Delivery of Curcumin to Enhance Synergetic Therapy of HER2 Positive Gastric Cancer. Nanomaterials 2022, 12, 696. [CrossRef]
- Zhang, F.; Yin, J.; Zhang, C.; Han, M.; Wang, X.; Fu, S.; Du, J.; Zhang, H.; Li, W. Affibody-Conjugated RALA Polymers Delivering Oligomeric 5-Fluorodeoxyuridine for Targeted Therapy of HER2 Overexpressing Gastric Cancer. Macromol. Biosci. 2020, 20, e2000083. [CrossRef]
- Teal, C.J.; Hettiaratchi, M.H.; Ho, M.T.; Ortin-Martinez, A.; Ganesh, A.N.; Pickering, A.J.; Golinski, A.W.; Hackel, B.J.; Wallace, V.A.; Shoichet, M.S. Directed Evolution Enables Simultaneous Controlled Release of Multiple Therapeutic Proteins from Biopolymer-Based Hydrogels. Adv. Mater. 2022, 34, e2202612. [CrossRef]
- Shipunova, V.O.; Belova, M.M.; Kotelnikova, P.A.; Shilova, O.N.; Mirkasymov, A.B.; Danilova, N.V.; Komedchikova, E.N.; Popovtzer, R.; Deyev, S.M.; Nikitin, M.P. Photothermal Therapy with HER2-Targeted Silver Nanoparticles Leading to Cancer Remission. Pharmaceutics 2022, 14, 1013. [CrossRef]
- Shipunova VO, Sogomonyan AS, Zelepukin IV, Nikitin MP, Deyev SM. PLGA Nanoparticles decorated with anti-HER2 affibody for targeted delivery and photoinduced cell death. Molecules 2021;26:3955.
- Yang, H.; Cai, W.; Xu, L.; Lv, X.; Qiao, Y.; Li, P.; Wu, H.; Yang, Y.; Zhang, L.; Duan, Y. Nanobubble–Affibody: Novel ultrasound contrast agents for targeted molecular ultrasound imaging of tumor. Biomaterials 2015, 37, 279–288. [CrossRef]
- Cai, W.; Lv, W.; Meng, L.; Duan, Y.; Zhang, L. The Combined Effect of Nanobubble-IR783-HPPH-Affibody Complex and Laser on HER2-Positive Breast Cancer. Int. J. Nanomed. 2023, ume 18, 339–351. [CrossRef]
- Kim, H.; Jin, S.; Choi, H.; Kang, M.; Park, S.G.; Jun, H.; Cho, H.; Kang, S. Target-switchable Gd(III)-DOTA/protein cage nanoparticle conjugates with multiple targeting affibody molecules as target selective T1 contrast agents for high-field MRI. J. Control. Release 2021, 335, 269–280. [CrossRef]
- Eom, S.; Jun, H.; Kim, E.; Min, D.; Kim, H.; Kang, S. Developing Porous Protein Cage Nanoparticles as Cargo-Loadable and Ligand-Displayable Modular Delivery Nanoplatforms. ACS Appl. Mater. Interfaces 2024, 16, 58464–58476. [CrossRef]
- Oh JY, Choi E, Jana B, Go EM, Jin E, Jin S, et al. Protein-precoated surface of metal-organic framework nanoparticles for targeted delivery. Small 2023;19:2300218.
- Yu, L.; Xu, Y.; Amin, A.; Jiang, S.; Sample, M.; Prasad, A.; Stephanopoulos, N.; Šulc, P.; Yan, H. CytoDirect: A Nucleic Acid Nanodevice for Specific and Efficient Delivery of Functional Payloads to the Cytoplasm. J. Am. Chem. Soc. 2023, 145, 27336–27347. [CrossRef]
- Dorogin, J.; Hochstatter, H.B.; Shepherd, S.O.; Svendsen, J.E.; Benz, M.A.; Powers, A.C.; Fear, K.M.; Townsend, J.M.; Prell, J.S.; Hosseinzadeh, P.; et al. Moderate-Affinity Affibodies Modulate the Delivery and Bioactivity of Bone Morphogenetic Protein-2. Adv. Heal. Mater. 2023, 12, e2300793. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).