1. Introduction
Moyamoya disease is a chronic cerebrovascular disorder characterized by progressive stenosis or occlusion of the terminal portion of the internal carotid artery (ICA) and the proximal portions of the anterior and middle cerebral arteries (MCA), accompanied by an abnormal vascular network known as "Moyamoya-like collateral vessels." When similar vascular findings are observed in patients with known underlying conditions, the diagnosis is termed “Moyamoya syndrome” [
1]. Previously, “head trauma” had been listed as a possible underlying cause of Moyamoya syndrome; however, the revised Japanese diagnostic criteria for Moyamoya disease [
2] published in 2021 omitted this association, and a causal relationship is currently considered unlikely. Notably, case reports describing Moyamoya syndrome secondary to head trauma are extremely rare [
3,
4]. Since the 2021 removal, accumulating well-documented post-traumatic cases may provide important insights into the possible underrecognized subtypes of Moyamoya syndrome. A scientific statement in 2023 by the American Heart Association noted that head trauma, excluded by the Research Committee on Moyamoya Disease guidelines, is still debated as a potentially associated condition in Moyamoya syndrome [
1]. Therefore, there may be no international consensus regarding this exclusion.
Here, we report a rare case of a middle-aged man who developed chronic subdural hematoma (CSDH) following head trauma, underwent burr-hole drainage, and subsequently developed progressive stenosis of the ipsilateral ICA distal segment to the MCA, potentially due to unilateral moyamoya syndrome. The use of Basi-Parallel Anatomical Scanning (BPAS) [
5] enabled the visualization of fine cerebral vessels in distinguishing structural abnormalities or arterial wall changes in the anterior circulation from simple stenosis. Given the rarity of moyamoya syndrome observed through long-term post-traumatic follow-up and the detailed imaging follow-up, we believe that this case has significant clinical and academic value.
2. Case Description
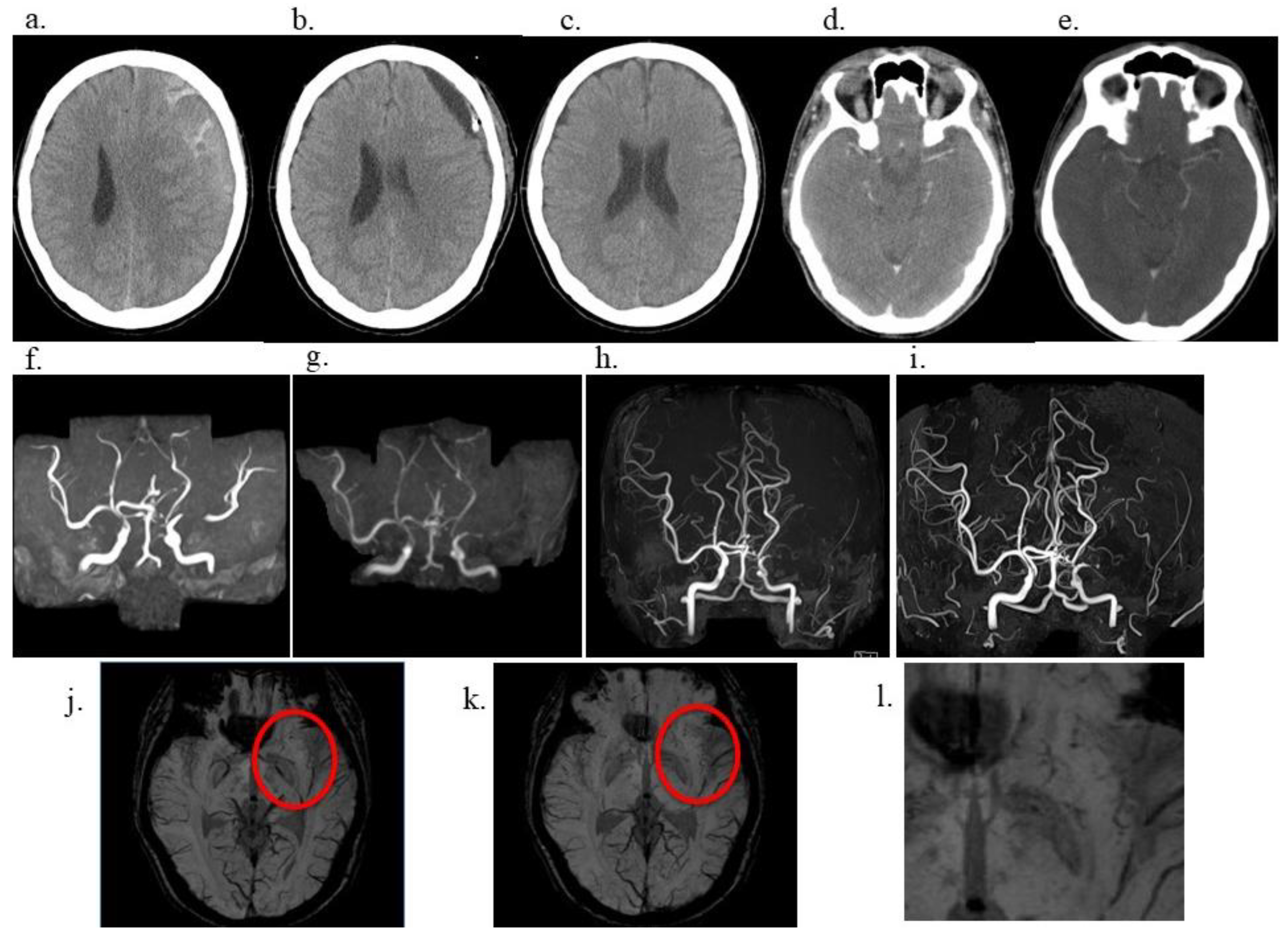
A 40-year-old male government employee sustained a head trauma while playing soccer. Approximately 2 months later, he experienced a gradually worsening headache and presented to our hospital. Head computed tomography (CT) revealed left-sided CSDH (
Figure 1a), prompting admission. The patient had a medical history of right inguinal hernia repair, right-hand fracture, and papillary thyroid carcinoma at the ages of 10, 16, and 37 years, respectively, for which he underwent surgical resection only, with no radiation therapy, resulting in cure. Importantly, the patient had no prior history of radiotherapy, which is a known risk factor for moyamoya-like vasculopathy. Similarly, he had no history of hyperthyroidism. At 40 years of age, he was diagnosed with mild hypertension and treated with amlodipine monotherapy. Moreover, he had a smoking history of 10 cigarettes per day from the age of 17 to 38 years and occasionally consumed approximately one bottle of beer. There was no family history of Moyamoya disease or other cerebrovascular disorders. The patient underwent left burr hole irrigation and drainage on admission. His headache resolved promptly, and his CSDH was sufficiently drained (
Figure 1b). He was discharged home 1 week later with a modified Rankin Scale (mRS) score of 0, and no CSDH was observed on postoperative month 1 (
Figure 1c). After surgery for CSDH, the patient was relocated to work and continued to follow up at another hospital. A review of contrast-enhanced CT performed at the age of 37 years for the evaluation of a thyroid nodule 3 years before the head trauma demonstrated a symmetrical depiction of the MCA, with no evidence of vascular abnormalities (
Figure 1d); additionally, imaging performed at 41 years of age (1 year after the CSDH) exhibited no evidence of MCA stenosis (
Figure 1e). However, magnetic resonance angiography (MRA) revealed mild stenosis of the left MCA at the age of 43 years (3 years since CSDH) (
Figure 1f), which gradually progressed over time. The stenosis had become severe at the age of 46 (6 years from CSDH) (
Figure 1g). Moreover, at the age of 47 (7 years from CSDH) (
Figure 1h, i), the left MCA was no longer visible on MRA, a finding that persisted at the age of 50 (10 years from CSDH). Susceptibility-weighted imaging (SWI) demonstrated multiple fine, punctate, and linear low-signal structures in the left basal ganglia (
Figure 1j, k, l), consistent with Moyamoya-like collateral vessels characteristic of moyamoya-type vasculopathy.
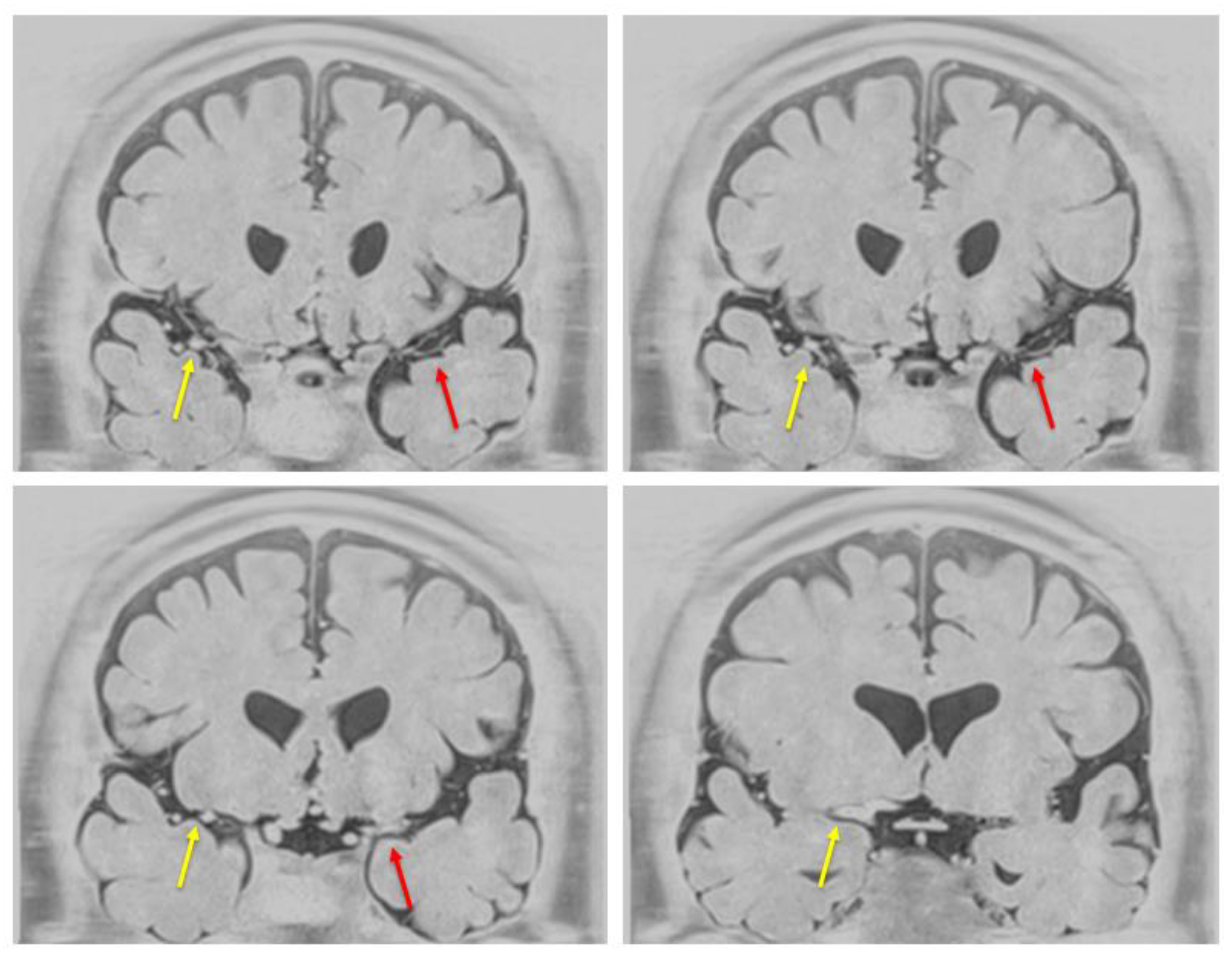
At the age of 53 years (13 years from CSDH), the patient remained neurologically stable, with an mRS score of 0 and no history of transient ischemic attacks or overt ischemic stroke. Resting single-photon emission computed tomography demonstrated a mild reduction (<5%) in cerebral blood flow on the affected side. Additionally, BPAS was performed to assess the arterial wall structure of the anterior circulation (
Figure 2). Notably, the left MCA appeared remarkably thinner than the contralateral side, indicating a significant reduction in its outer diameter. These structural changes were not apparent on conventional time-of-flight (TOF)-MRA, underscoring the added value of BPAS for detecting subtle arterial wall abnormalities that may be difficult to distinguish from simple luminal stenosis on TOF-MRA alone.
The patient expressed surprise at the progressive nature of the vascular findings but was relieved to have remained neurologically intact and able to maintain his occupational and daily activities without restriction. Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
3. Discussion
Moyamoya syndrome is diagnosed in individuals who exhibit Moyamoya-like cerebrovascular changes in association with an identifiable underlying condition [
2,
6]. The present case is extremely rare because it involved unilateral progressive stenosis of the MCA and the development of abnormal vascular networks observed long-term following head trauma and surgical intervention for CSDH. Notably, BPAS was crucial in detecting outer diameter narrowing of the anterior circulation vessels, particularly the left MCA, which were not clearly visualized on conventional TOF-MRA, highlighting the complementary value of anterior circulation BPAS in evaluating subtle arterial wall changes and structural abnormalities in Moyamoya-like conditions.
3.1. Previous Context Reported and Uniqueness of This Case
Previous reports have described only two similar conditions, including a pediatric case reported by Fernandez-Alvarez et al. in 1979, in which Moyamoya disease developed 3 years after head trauma [
3], and another case reported by Zaletel et al. in 2011, in which Moyamoya changes were observed 24 years after traumatic injury [
4]. Nonetheless, in both cases, the extended interval between the initial trauma and vascular pathology onset limits the ability to draw a strong causal inference. Conversely, our case demonstrated progressive vascular changes over a relatively short period following ipsilateral burr hole drainage for CSDH. The vascular lesion extended from the distal ICA to the MCA, and Moyamoya-like collateral vessels were identified on SWI. Although digital subtraction angiography was not performed, Moyamoya-like collateral vessels were observed on SWI, which have been reported to reflect moyamoya-like vascular networks in previous studies [
7]. Additionally, anterior circulation BPAS demonstrated a remarkably thinner left MCA compared with the contralateral side, indicating a significant reduction in its outer diameter. Therefore, post-traumatic inflammation or alterations in cerebral hemodynamics may contribute to pathological vascular remodeling.
3.2. Potential Etiologies and Risk Factors
Several pathophysiological mechanisms may be considered. Cigarette smoking has been reported as a potential contributor to intracranial large artery stenosis, particularly involving the ICA. Nevertheless, the development of moyamoya-like collaterals and progressive M1 stenosis limited to the ipsilateral side following trauma suggests that additional factors beyond smoking may have contributed to the pathophysiology of this case.
One possibility may be the endothelial injury hypothesis, in which trauma-induced damage to the vascular endothelium triggers chronic inflammation and progressive stenosis. The hemodynamic redistribution hypothesis may indicate that trauma-induced changes in cerebral perfusion promote vascular remodeling and collateral formation. The third possibility may be the chronic inflammation hypothesis, whereby a sustained inflammatory environment resulting from CSDH stimulates cytokine-mediated vascular changes. Although traumatic intracranial artery dissection can cause focal stenosis or occlusion, it typically presents with abrupt vascular narrowing, pseudoaneurysm formation, or intimal flap, most commonly affecting the proximal M1 segment. Conversely, this case demonstrated gradual, long-segment progressive stenosis extending from the distal ICA to the MCA over a decade, without imaging findings suggestive of dissection such as vessel wall irregularity, aneurysmal dilatation, or mural hematoma. Furthermore, BPAS imaging demonstrated that the reduction involved both the luminal narrowing and an actual decrease in the outer diameter of the vessels. These findings collectively may support the diagnosis of moyamoya-like vasculopathy rather than post-traumatic arterial dissection.
The patient had a history of thyroid papillary carcinoma. Elevated thyroid autoantibodies and thyroid function are independently associated with Moyamoya disease [
8]. Recent studies have suggested that RNF213, a known susceptibility gene for Moyamoya disease in East Asian populations, may also have immunological functions, including antimicrobial defense, via the ubiquitination of bacterial lipopolysaccharide [
1]. These findings suggest a potential link between genetic predisposition and the immune-related pathophysiology of Moyamoya syndrome. Furthermore, autoimmune thyroid diseases, particularly the presence of thyroid autoantibodies such as anti-thyroid peroxidase and anti-thyroglobulin, have been reported to be significantly associated with moyamoya-type vasculopathy [
9,
10]. Although our patient had no clinical hyperthyroidism, the history of thyroid carcinoma warrants consideration of potential immune involvement. Despite no clinical evidence of hyperthyroidism, the possibility remains that autoimmune thyroid factors or subclinical thyroid dysfunction may have contributed to the disease pathogenesis. Evaluation of thyroid autoantibodies and thyroid function may help clarify this association.
3.3. Utility of BPAS in Detecting Moyamoya-like Vascular Changes
Here, conventional TOF-MRA was limited in visualizing the fine vascular structures within the anterior circulation. Nonetheless, anterior circulation BPAS [
5] provided a clear delineation of the outer contours of the left MCA, revealing a notable reduction in outer diameter and confirming the presence of subtle yet significant vascular narrowing. BPAS imaging may be particularly advantageous for detecting changes in the vessel wall and identifying hypoplastic or structurally compromised arteries that may be difficult to evaluate with conventional luminal imaging techniques, such as TOF-MRA. In this case, BPAS allowed for the detection of fine collateral vessels and better characterization of the affected segment, potentially indicating a Moyamoya-like vasculopathy diagnosis rather than simple atherosclerotic stenosis. The ability of BPAS to complement MRA by highlighting extracranial vessel morphology may offer important diagnostic value, especially in atypical cases such as unilateral Moyamoya syndrome or secondary vascular changes following trauma or surgery. Therefore, this imaging approach may aid in early detection, differentiation, and longitudinal monitoring of Moyamoya-related cerebrovascular changes.
3.4. Limitations and Hypothesis
Genetic testing for RNF213 variants [
11], which are strongly associated with moyamoya disease in East Asian populations, may provide insights into any underlying genetic predisposition. Although genetic testing for RNF213 variants was not performed in this case due to facility limitations, such an analysis could provide valuable insights into genetic susceptibility. Here, no family history or bilateral vascular changes were observed. Although rare, such cases may serve as a basis for reevaluating the current exclusion of head trauma from the classification of moyamoya syndrome in future guidelines. Further accumulation of similar cases is essential to better understand the pathophysiological mechanisms and clinical significance of trauma-associated moyamoya-like vasculopathy.
This case report was prepared according to the CAse REport (CARE) guidelines to ensure transparency and completeness of clinical reporting.
4. Conclusions
This case highlights a rare clinical course of progressive unilateral Moyamoya-like vascular changes following head trauma and burr-hole drainage for CSDH. The temporal sequence and radiological findings warrant further attention. Notably, the use of anterior circulation BPAS may facilitate the detection of structural arterial changes not apparent on conventional MRA, indicating its potential utility in evaluating such vascular abnormalities.
Author Contributions
Conceptualization, S.W. and Y.S.; methodology, S.W. and Y.S..; software, S.W.; validation, Y.S.; resources, S.W.; data curation, S.W.; writing—original draft preparation, S.W.; writing—review and editing, all authors.; supervision, E.I.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from a subject involved in the study, and written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| BPAS |
Basiparallel Anatomic Scanning magnetic resonance imaging |
| CARE |
CAse REport |
| CSDH |
Chronic Subdural Hematoma |
| CT |
Computed Tomography |
| ICA |
Internal Carotid Artery |
| MCA |
Middle Cerebral Artery |
| MRA |
Magnetic Resonance Angiography |
| TOF |
Time-of-Flight |
| mRS |
modified Rankin Scale |
| SWI |
Susceptibility-Weighted Imaging |
References
- Gonzalez, N.R.; Amin-Hanjani, S.; Bang, O.Y.; Coffey, C.; Du, R.; Fierstra, J.; Fraser, J.F.; Kuroda, S.; Tietjen, G.E.; Yaghi, S.; et al. Adult Moyamoya disease and syndrome: current perspectives and future directions: A scientific statement from the American Heart Association/American Stroke Association. Stroke 2023, 54, e465–e479. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, S.; Fujimura, M.; Takahashi, J.; Kataoka, H.; Ogasawara, K.; Iwama, T.; Tominaga, T.; Miyamoto, S.; Research Committee on Moyamoya Disease (Spontaneous Occlusion of Circle of Willis) of the Ministry of Health, Labor, and Welfare, Japan. Diagnostic criteria for Moyamoya disease—2021 revised version. Neurol Med Chir (Tokyo) 2022, 62, 307–312. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Alvarez, E.; Pineda, M.; Royo, C.; Manzanares, R. ‘Moya-moya’ disease caused by cranial trauma. Brain Dev 1979, 1, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Zaletel, M.; Surlan-Popović, K.; Pretnar-Oblak, J.; Zvan, B. Moyamoya syndrome with arteriovenous dural fistula after head trauma. Acta Clin Croat 2011, 50, 115–120. [Google Scholar] [PubMed]
- Nagahata, M.; Abe, Y.; Ono, S.; Hosoya, T.; Uno, S. Surface appearance of the vertebrobasilar artery revealed on basiparallel anatomic scanning (BPAS)-MR imaging: its role for brain MR examination. AJNR Am J Neuroradiol 2005, 26, 2508–2513. [Google Scholar] [PubMed]
- Rifino, N.; Hervé, D.; Acerbi, F.; Kuroda, S.; Lanzino, G.; Vajkoczy, P.; Bersano, A. Diagnosis and management of adult moyamoya angiopathy: an overview of guideline recommendations and identification of future research directions. Int J Stroke 2025, 20, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Horie, N.; Morikawa, M.; Nozaki, A.; Hayashi, K.; Suyama, K.; Nagata, I. ‘Brush Sign’ on susceptibility-weighted MR imaging indicates the severity of Moyamoya disease. AJNR Am J Neuroradiol 2011, 32, 1697–1702. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Wu, B.; Ma, Z.; Zhang, S.; Liu, M. Association of Moyamoya disease with thyroid autoantibodies and thyroid function: a case-control study and meta-analysis. Eur J Neurol 2014, 21, 996–1001. [Google Scholar] [CrossRef]
- Otten, E.G.; Werner, E.; Crespillo-Casado, A.; Boyle, K.B.; Dharamdasani, V.; Pathe, C.; Santhanam, B.; Randow, F. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature 2021, 594, 111–116. [Google Scholar] [CrossRef]
- Kim, S.J.; Heo, K.G.; Shin, H.Y.; Bang, O.Y.; Kim, G.M.; Chung, C.S.; Kim, K.H.; Jeon, P.; Kim, J.S.; Hong, S.C.; et al. Association of thyroid autoantibodies with moyamoya-type cerebrovascular disease: a prospective study. Stroke 2010, 41, 173–176. [Google Scholar] [CrossRef]
- Liu, W.; Morito, D.; Takashima, S.; Mineharu, Y.; Kobayashi, H.; Hitomi, T.; Hashikata, H.; Matsuura, N.; Yamazaki, S.; Toyoda, A.; et al. Identification of RNF213 as a susceptibility gene for Moyamoya disease and its possible role in vascular development. PLOS One 2011, 6, e22542. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).