1. Introduction
Olfactory and taste disorders (OTDs) are associated with a variety of conditions, not only those directly affecting the nasal or oral cavity mucosa (e.g., rhinosinusitis, chronic sinusitis) but also others such as neurodegenerative disorders, diabetes, thyroid diseases, vitamin deficiencies, medications, surgical interventions of the head and neck, and prior head trauma [
1,
2]. The prevalence of olfactory disorders in the general population is estimated as 12–25%, increasing with age, with approximately 40% of people over 65 years of age experiencing them. Functional anosmia, where a person’s olfactory function is lowered, affects about 5% of the general population [
1,
2]. The prevalence of taste disorders is 17–19% and also increases with age [
3,
4]. The presence of an OTD can affect quality of life, cause eating and anxiety disorders and depressive states [
1,
2,
5].
In clinical practice, the acuity of the sense of smell is investigated subjectively using a variety of olfactory tests, both screening and diagnostic. Most tests involve the orthonasal olfactory sensation associated with air flowing through the anterior nostrils into the olfactory epithelium. A few tests use the retronasal olfactory sensation caused by flow of air past the soft palate into the nasopharynx, as in swallowing or nasal exhalation. This latter mode is involved when detecting the aroma of food while eating [
1,
2,
6].
The aim of this study was to develop an application (or app) that allows a patient to self-administer an olfactory and taste test at home. We validated its performance by comparing the results with an existing validated screening test.
2. Materials and Methods
This paper presents the results of a prospective study performed by two consortium collaborators. The study protocol, consent forms, and patient brochure were approved by the Bioethics Committee of the Institute of Physiology and Pathology of Hearing (number BC.IFPS 17/2021) and complied with the World Medical Association’s Declaration of Helsinki. Participation in the study was voluntary and free of charge.
Measures
Smell Test (ST)
The study used an olfactory test based on the smell test (ST) used in the Sensory Testing Capsule (STC) [
7], which can be used for olfactory screening. It consists of 6 odour-coated strips encapsulated in microcapsules carrying the scents of cinnamon, banana, smoke, leather, chocolate, and petrol. The strips come in four combinations having different order of the scents. The strips are clipped together in a fan and packaged in an envelope with a QR code, which allows a computer system to recognise the combination and assess the test result. A result is considered a pass if the patient can identify 5 or 6 odours correctly.
Sniffin Sticks Test (SST)
The SST is a common test for assessing the sense of smell. It was originally developed in 1997, and a Polish version was published in 2014 [
8,
9]. The test uses sticks soaked in different fragrances. During the test, the sticks are brought close to the patient’s nostrils for about 3 seconds. An extended version of the test consists of three parts: a threshold test, a discrimination test, and an identification test, but in our study we only used the identification test (iSST). The iSST consists of 16 sticks with different odors. The patient’s task is to indicate the correct scent from among four suggested answers. A maximum of 16 points can be obtained in the test. In our study, a score of 12 or more was taken as the norm.
Taste Test (TT)
This study used a taste test based on the TT used in the STC [
7], which can be used for taste screening. It consists of four paper strips soaked in sweet (40% glucose), salty (25% sodium chloride), bitter (0.6% quinine), and sour (30% citric acid) solutions, as well as one control strip with no taste. The control strip allows the taste and texture of the paper to be familiarised before the actual test begins. The other strips are placed by the patient in the middle of the tongue and removed after about 30 seconds. The patient’s task is to identify the taste. A correct result is recorded if the patient recognises all samples correctly. All strips are packaged in a cardboard insert, which is placed in an envelope carrying a QR code. The QR code allows the test version number to be identified (4 variants are available) and the test result to be automatically read by a computer.
Taste Strips (TS)
TS is a test, validated in 2009, to evaluate the sense of taste based on psychophysical methods [
10]. The test kit consists of a set of jars containing strips saturated with substances of four basic tastes (sweet, salty, bitter, sour) in various concentrations. To perform the test, the tester places the strip in the middle of the patient’s tongue and asks them to identify the taste. The manufacturer of the test also offers a version for screening purposes, consisting of strips carrying just the highest concentration of the taste substances, and we used this version in our study. The result was considered correct if the patient correctly identifies all four tastes.
Study Plan
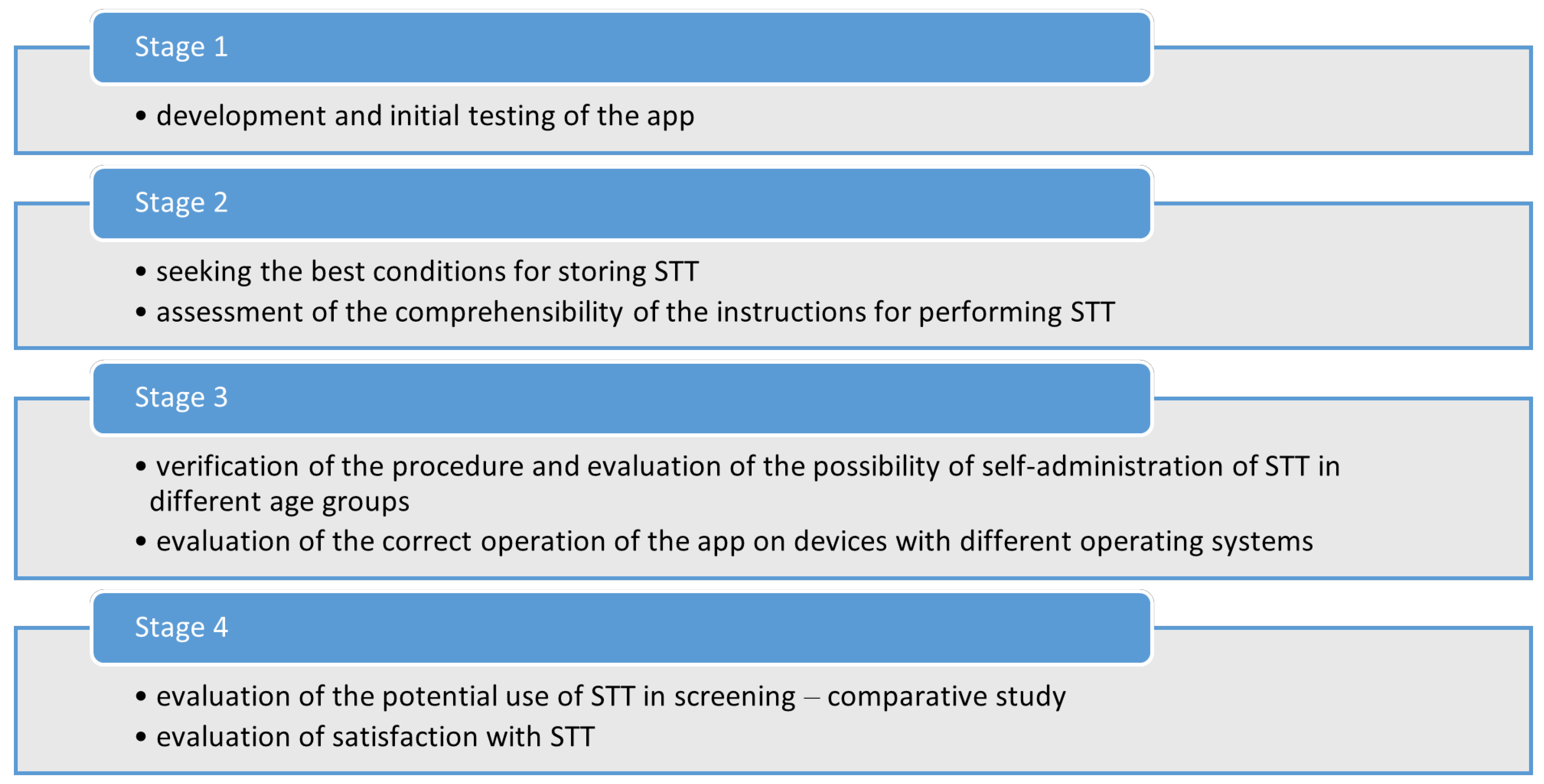
The development of a system to allow the patient to self-test their sense of smell and taste at home involved 4 stages.
Stage 1
In the first stage, a literature review of the available olfactory and taste tests on the market was carried out. We selected those tests that were available in Polish and would allow self-testing at home, without the need for a researcher or travel to a medical facility [
11]. Work then began on developing an app by which the patient could create an individual account, order and perform the ST and/or TT, view the results, and have access to them later. Care was also taken to ensure that registration and data collection complied with current data protection legislation (RODO). To verify the correct functioning of the app, 20 volunteers, who had no olfactory or taste disorders, were asked to perform one ST and one TT. These two tests, when performed together, are hereafter referred to as STT.
Stage 2
In the second stage, the best storage conditions for STT were investigated. The STT samples was divided into three groups, with each group stored under different conditions: an unventilated room at a temperature of about 28°C, an air-conditioned room with a temperature of 20°C, and a refrigerator with a temperature of about 4°C. After 8 months, a group of 7 healthy people performed an STT from each group and assessed the degree to which the odours and tastes had been retained based on questions about the detectability of the tastes/odours, their intensity, and their identifiability.
A questionnaire was then developed to assess how well the instructions for performing the STT were understood. The questionnaire was given to 30 randomly selected patients of the Institute of Hearing Physiology and Pathology aged 18–65 years who were asked to read the multimedia instructions, register the app, and order the STT.
Stage 3
Stage 3 tested how well patients of different ages and levels of electronic literacy could self-administer the STT. Sixty subjects in each of the following age groups were asked to participate: children up to 7 years of age, school children, adolescents, adults up to 50 years of age, adults 50–60 years old, adults 60–70 years old, and adults over 70 (420 subjects in all). The subjects were asked to complete the SST in the presence of a researcher who observed the subject’s behaviour. The researchers assessed whether the instructions and messages in the app were understandable to the user, and whether any of the steps had caused problems. The tests were performed on different devices and different operating systems (MS Windows, Mac OS, Android). The correctness of the QR codes printed on the STT envelopes was also verified.
Stage 4
The fourth stage assessed the feasibility of using STT for olfactory and taste screening. The aim of the work was to gather information on the level of difficulty of performing the STT on a larger group of subjects and to compare results performed using the app with commercially available smell and taste tests (SST and TS). A total of 1100 people were recruited to take part in the study. In addition, 100 randomly selected patients were asked to complete a questionnaire about their satisfaction with the STT.
Figure 1.
Workflow for creating the app.
Figure 1.
Workflow for creating the app.
3. Results
Stage 1
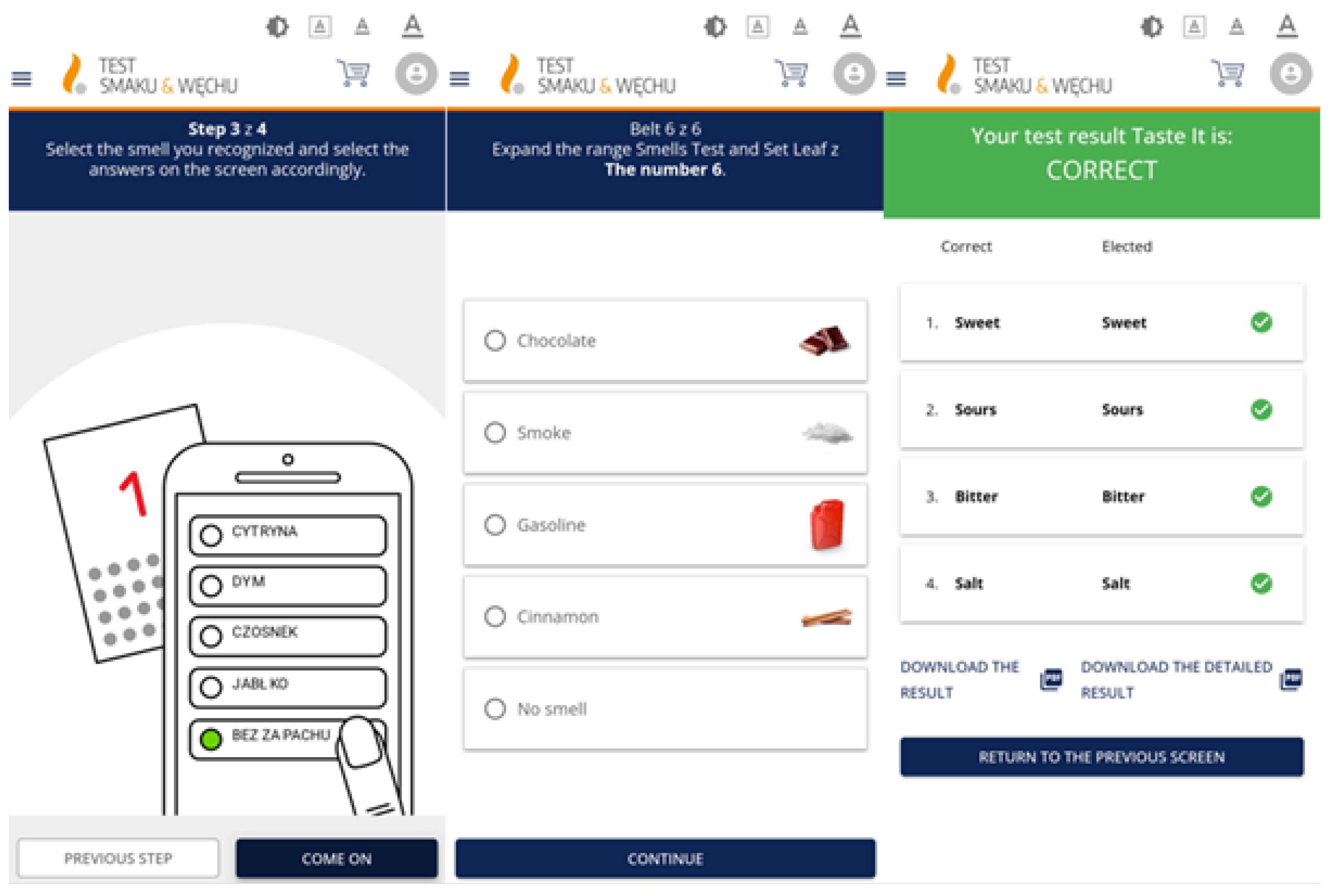
After reviewing the available literature, the feasibility of using different olfactory and taste tests in the app was evaluated, taking into account the time taken to perform a test, how well it performed, and whether it was feasible to mail the test to the patient’s home. A suitable test needed to be done quickly and able to be performed remotely in the subject’s home without having to travel to a medical facility. Development of the app involved steps of registration, ordering, performing the STT, and read-out of results. Simple instructions for performing the STT were created using graphics to illustrate the steps involved (see
Figure 2). The answer form, which appeared on the screen, contained the names of the different smells or tastes, together with icons indicating the correct answer. The result of each test included the names of the selected odour and taste. The results can be saved in pdf format.
Figure 2 shows some screenshots.
Initial tests were aimed at assessing the overall performance of the app: its registration, selection of type of test, and whether all steps could be performed satisfactorily. We found that 95% of the olfactory tests and 90% of the taste tests were performed correctly (
Table 1).
Stage 2
The results of the STT carried out by the group of testers is summarised in
Table 2, from which we conclude that suitable conditions for storing STT test kits are an air-conditioned room at a temperature of around 20°C. Samples stored under these conditions retain their odours and tastes.
On the basis of the group survey, the comprehensibility of the app’s instructions for performing the STT were good, with the instructions clear and easy to use for 29 of 30 people (97%). One person reported difficulties in creating an account due to the complex password required.
Stage 3
STT were conducted on 420 people, of whom 404 (96%) performed the test correctly. In terms of age groups, the procedure was performed correctly by at least 90% of the volunteers in each group. Older people, i.e., over 70 years of age, performed STT slightly slower, reflecting less experience with electronic devices. A bug with some operating systems was identified in which the graphical interface did not display as intended; appropriate fixes were made.
Stage 4
Some 1,100 complete test results were obtained (SST, TS, ST, and TT), based on which the potential of ST and TT performed with the Application for use in clinical practice as screening tests could be assessed.
Table 3 indicates that satisfactory parameters were obtained.
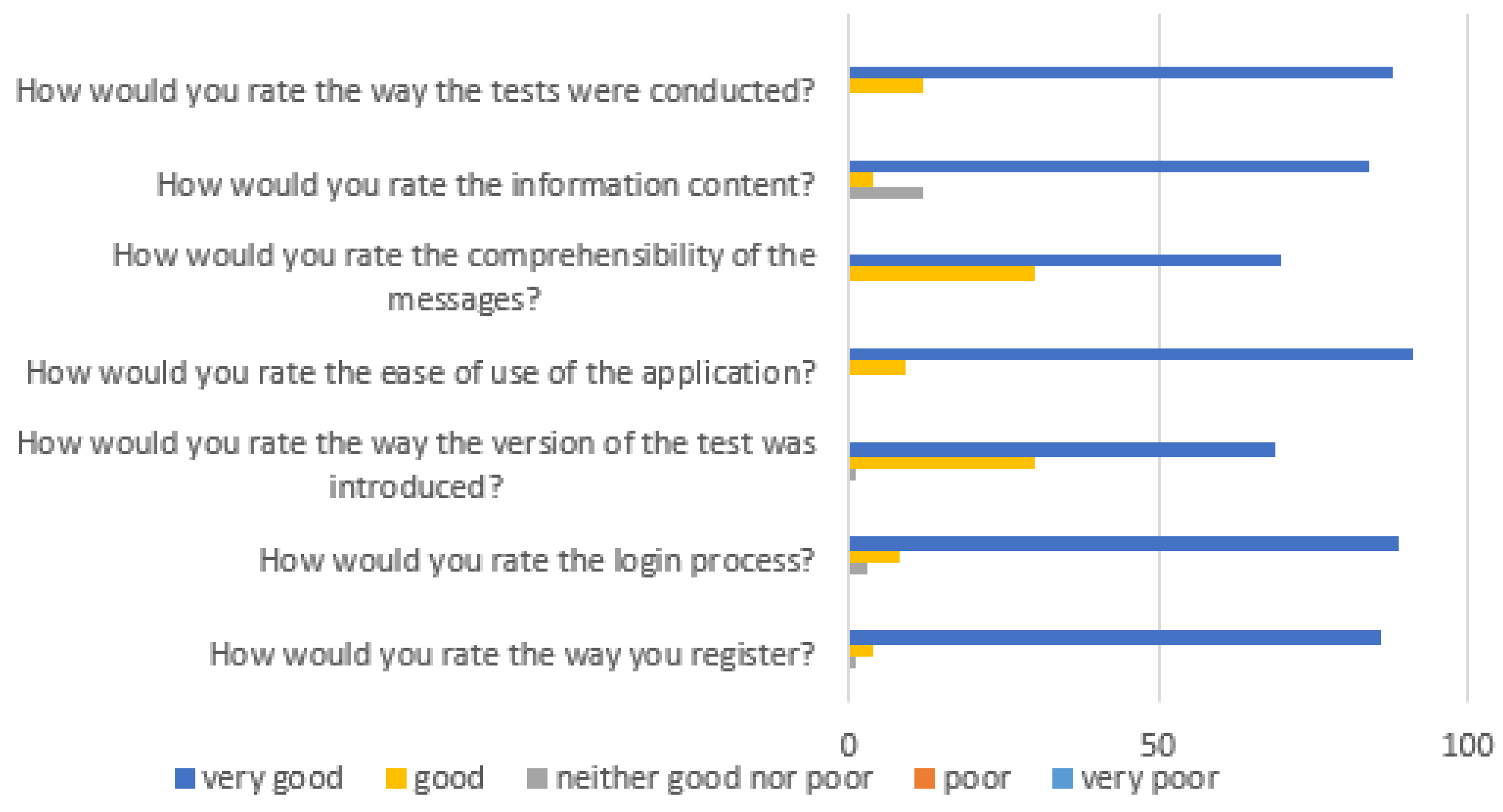
Based on the responses to the satisfaction survey, we conclude that the app was easy to use for most respondents, the messages in it were understandable, and the information displayed was of interest. The results of the survey are shown in
Figure 3.
4. Discussion
Telemedicine makes it possible to perform various tests without the patient having to travel to a medical facility. So far, a screening tool for the sense of smell that can be performed independently at home by the patient without the need for contact with medical staff has not been described. In this study, we chose previously used ST and TT as tests that could be used in conjunction with the newly developed mobile application.
Traditionally, ST and TT require travel to a medical centre where the sense of smell and taste can be tested, either alone or with the help of staff. In many case, the need for assistance may arise due to medical conditions (chronic conditions or acute infections requiring isolation) or socioeconomic conditions (distance from home, travel time and cost). Building STT into an app allows olfactory and/or taste tests to be performed in the patient’s home. For children or people with physical or intellectual disabilities, the test could be performed with the help of a carer.
During the development process, particular attention was given to the clarity of the instructions, ease of use, and reliability of the application. The conducted usability studies demonstrated that the application is intuitive and user-friendly. Minor difficulties were observed mainly among elderly participants, who generally have less experience with modern technologies; however, they constituted a small percentage of users. Additionally, the optimal storage conditions for the smell and taste test materials (approximately 20°C) were determined and included in the information leaflet accompanying each test set.
The effectiveness of the application in detecting olfactory and gustatory disorders was also evaluated by comparing the results of the ST and TT with reference tests SST and TS. Analysis of over 1,100 complete test results showed a high level of agreement between the app-based screening tests and the standard diagnostic procedures. The obtained diagnostic parameters (TPR, TNR, PPV, NPV) confirmed that both ST and TT exhibit high sensitivity and specificity, indicating their usefulness in clinical practice. These findings demonstrate that mobile technology can provide a reliable alternative to conventional screening methods for assessing the sense of smell and taste.
Moreover, using the application and comparing results over time, it is possible to monitor the sense of smell and taste over the course of nasal and sinus disease, upper respiratory tract infection, and neurodegenerative conditions. The app could also be useful in the assessment of smell and taste by speech therapists before implementing multisensory therapy and used in occupational medicine to monitor the state of the sense of smell and taste in people who are occupationally exposed to toxic substances.
5. Conclusions
The app could also be useful in the assessment of smell and taste by speech therapists before implementing multisensory therapy and used in occupational medicine to monitor the state of the sense of smell and taste in people who are occupationally exposed to toxic substances.
Author Contributions
Conceptualization, M.B, P.H.S. ;methodology, M.B, P.H.S. software, M.B validation, M.B, P.H.S..; formal analysis, M.B, P.H.S., I.T.H..; investigation, M.B, P.H.S., I.T.H., M.T.; resources, P.H.S.; data curation, M.B.; writing—original draft preparation, M.B, P.H.S., I.T.H., M.T.; writing—review and editing, M.B, P.H.S., I.T.H.., M.T.; project administration, P.H.S.; . All authors have read and agreed to the published version of the manuscript
Funding
This research was funded by the project “System for detecting taste and smell disorders based on a self-administered test performed at the patient’s home” (No. POIR.04.01.04-00.0139/19-00), implemented under the Intelligent Development Operational Program 2014–2020, co-financed by the European Regional Development Fund, Priority Axis IV – Increasing the scientific and research potential, Measure 4.1 – Scientific research and development work, under a funding agreement concluded between the National Centre for Research and Development (NCBR) and the Institute of Physiology and Pathology of Hearing together with GNP Magnusson Aparatura Medyczna Sp. z o.o. (GNP Magnusson Medical Equipment Ltd.).
Institutional Review Board Statement
The study protocol, consent forms, and patient brochure were approved by the Bioethics Committee of the Institute of Physiology and Pathology of Hearing (number BC.IFPS 17/2021, dated 23 September 2021) and complied with the World Medical Association’s Declaration of Helsinki.
Informed Consent Statement
All patients signed an informed consent form.
Data Availability Statement
Data are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no personal conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OTDs |
Olfactory and taste disorders |
| STC |
Sensory Testing Capsule |
| ST |
Smell Test |
| STT |
Sniffin Sticks Test |
| TT |
Taste Test |
| TS |
Taste Strip |
| STT |
Smell Taste Test |
References
- Hummel, T.; Whitcroft, K.L.; Andrews, P.; Altundag, A.; Cinghi, C.; Costanzo, R.M.; Damm, M.; Frasnelli, J.; Gudziol, H.; Gupta, N.; et al. Position Paper on Olfactory Dysfunction. Rhin 2017, 54, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; T. Liu, D.; A. Müller, C.; A. Stuck, B.; Welge-Lüssen, A.; Hähner, A. Olfactory Dysfunction: Etiology, Diagnosis, and Treatment. Dtsch Arztebl Int 2023, 120, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.; Hoffman, H.J.; Bainbridge, K.E.; Huedo-Medina, T.B.; Duffy, V.B. Prevalence and Risk Factors of Self-Reported Smell and Taste Alterations: Results from the 2011–2012 US National Health and Nutrition Examination Survey (NHANES). Chem Senses 2016, 41, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Zong, G.; Doty, R.L.; Sun, Q. Prevalence and Risk Factors of Taste and Smell Impairment in a Nationwide Representative Sample of the US Population: A Cross-Sectional Study. BMJ Open 2016, 6, e013246. [Google Scholar] [CrossRef] [PubMed]
- Bigman, G. Age-Related Smell and Taste Impairments and Vitamin D Associations in the U. S. Adults National Health and Nutrition Examination Survey. Nutrients 2020, 12, 984. [Google Scholar] [CrossRef]
- Heilmann, S.; Strehle, G.; Rosenheim, K.; Damm, M.; Hummel, T. Clinical Assessment of Retronasal Olfactory Function. Arch Otolaryngol Head Neck Surg 2002, 128, 414–418. [Google Scholar] [CrossRef] [PubMed]
- Skarzynski, H.; Krupa, A.; Kutyba, J.; Czajka, N.; Skarzynski, P.H. The Sensory Examination Capsule: Simultaneous Testing of Multiple Sensory Organs. J Hear Sci 2022, 11, 11–16. [Google Scholar] [CrossRef]
- Sorokowska, A.; Hummel, T. Polska wersja testu Sniffin’ Sticks – adaptacja i normalizacja. Otolaryngologia Polska 2014, 68, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Hummel, T.; Sekinger, B.; Wolf, S.R.; Pauli, E.; Kobal, G. “Sniffin” Sticks’: Olfactory Performance Assessed by the Combined Testing of Odor Identification, Odor Discrimination and Olfactory Threshold. Chem senses 1997, 22, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Landis, B.N.; Welge-Luessen, A.; Brämerson, A.; Bende, M.; Mueller, C.A.; Nordin, S.; Hummel, T. “Taste Strips” – A Rapid, Lateralized, Gustatory Bedside Identification Test Based on Impregnated Filter Papers. J Neurol 2009, 256, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Buksińska, M.; Tomaszewska-Hert, I.; Skarżyński, P.H. Subiektywne metody badania węchu – przegląd wybranych narzędzi diagnostycznych. Now Audiofonol 2024, 13, 7–19. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).