Submitted:
16 October 2025
Posted:
16 October 2025
You are already at the latest version
Abstract
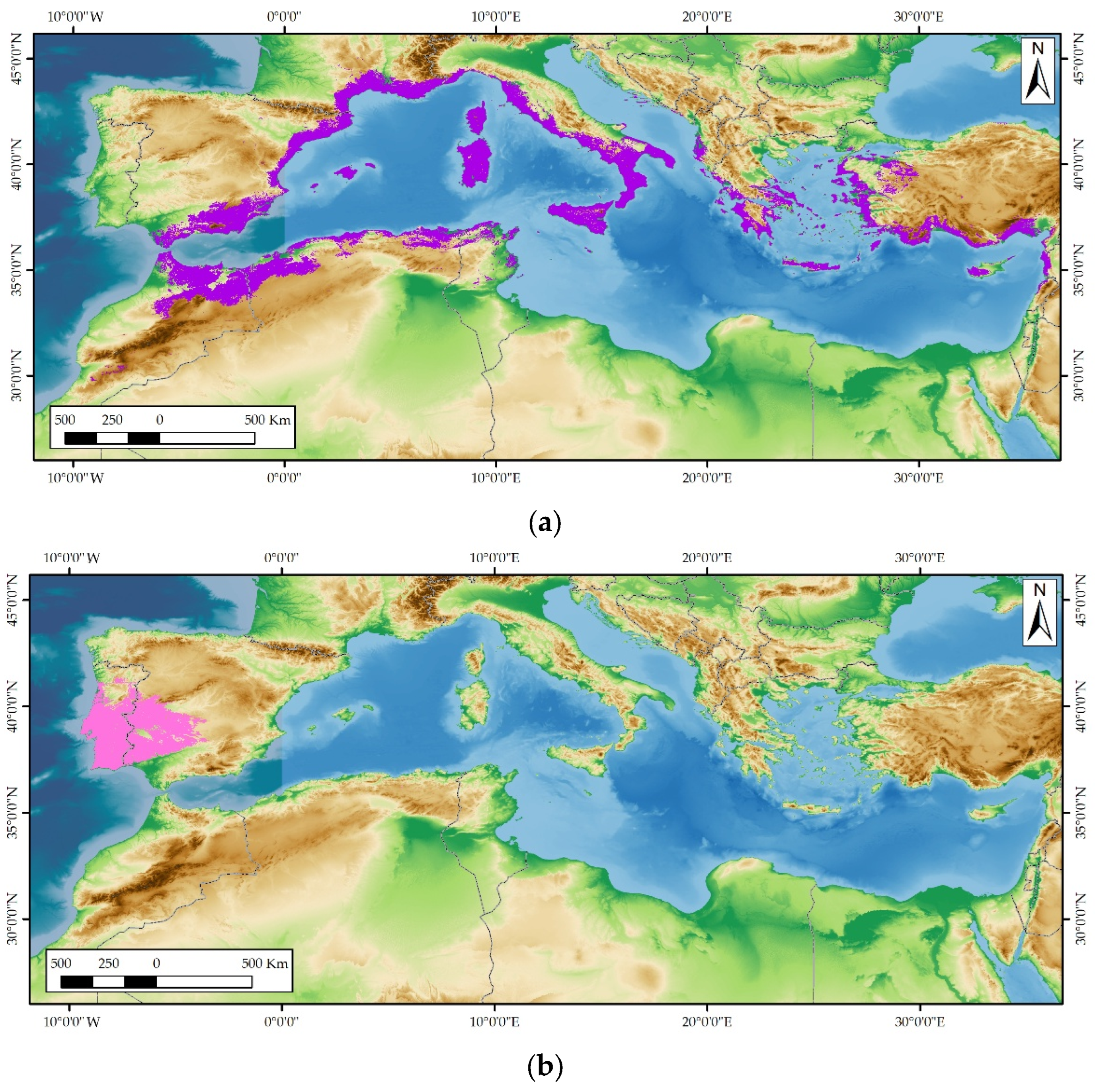
Lavandula stoechas subsp. luisieri (Rozeira) Rozeira is an aromatic shrub endemic to the south-west of the Iberian Peninsula. It is distinguished by being the only species of lavender that contains necrodol derivatives in its essential oil. This study aims to evaluate the diversity of the chemical composition of L. stoechas subsp. luisieri essential oil and how it differs from essential oils of other lavender species with which it shares its habitat and with which it can hybridize. The variability in the chemical composition of L. stoechas subsp. luisieri essential oil has been evaluated across 66 populations distributed among 14 areas in southwestern Iberian Peninsula. The main compounds present in the essential oil of Lavandula stoechas subsp. luisieri are trans-α-necrodyl acetate (20.68% ± 4.17%), 1,8-cineole (7.79% ± 7.14%) and trans-α-necrodol (8.66% ± 2.18%). Other compounds may occasionally be present in percentages greater than 6.00%, such as α-cadinol, linalool, lavandulyl acetate, fenchone and camphor. Two chemotypes have been identified in the essential oil of L. stoechas subsp. luisieri: 1) trans-α-necrodyl acetate – 1,8-cineole, and 2) trans-α-necrodyl acetate, with little or no presence of 1,8-cineole. Furthermore, the absence or very low percentage of camphor (0.16 – 7.61%) and fenchone (0.00 – 7.39%) has been confirmed as a unique characteristic of this essential oil.
Keywords:
1. Introduction
2. Results
2.1. Essential Oil Yield
2.2. Chemical Composition
2.3. Cluster Analysis
3. Discussion
4. Materials and Methods
4.1. Collection of Plant Materials
4.2. Essential Oil Extraction
4.3. Chemical Characterization of Essential Oils
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Upson, T.M.; Andrews, S. The Genus Lavandula. A Botanical Magazine Monograph. Royal Botanic Gardens, Kew, 2004, 442 pp.
- Govaerts, R.; Paton, A.; Harvey, Y.; Navarro, T.; Del Rosario, M. World Checklist of Lamiaceae; The Royal Botanic Gardens, Kew: Richmond, UK, 2020; Available online: http://wcsp.science.kew.org/ (accessed on 20 July 2025).
- Pohrib, E.-L.; Nistor, E. Spikes of Azure Bloom: Lavander – History… and Stories. Sci. Papers Ser. A. Agron. 2012, 55, 396–405. [Google Scholar]
- Farsam, H.; Attari, S.A.; Khalaj, A.; Kamalinejad, M.; Shahrokh, R.; Ahmadian-Attari, M.M. The Story of Stoechas: from Antiquity to the Present Day. Res. Hist. Med. 2016, 5, 69–86. [Google Scholar]
- Civilyte, A.; Karanikola, Kramer, A. From antiquity to modern hygiene: the archaeological and medicinal legacy of lavender as a promising antimicrobial agent. GMS Hyg. Infect. Control 2025, 20, Doc21. [Google Scholar] [CrossRef]
- El-Sabier, G.; Oluwafemi, J.; Wasef, L.; Shaheen, H.M. , Akomolafe, A.P.; Ayandeyi, T.K., Al-kuraishy, H.M.; Al-Garbeeb, A.; Alexiou, A. A review of the bioactive components and pharmacological properties of Lavandula species. N-S Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Kadam, A.J.; Balkete, R.R.; Chauhan, A.G.; Jadhav, A.A.; Kamble, A.A. A Review on Lavander. Int. J. Curr. Sci., 2023, 13, 45–58. [Google Scholar]
- Giray, F.H. An Analysis of World Lavender Oil Markets and Lessons for Turkey. J. Essent. Oil Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Crișan, I.; Ona, A.; Vârban, D.; Muntean, L.; Vârban, R.; Stoie, A.; Mihăiescu, T.; Morea, A. Current Trends for Lavender (Lavandula angustifolia Mill.) Crops and Products with Emphasis on Essential Oil Quality. Plants 2023, 12, 357. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules 2023, 28, 2943. [Google Scholar] [CrossRef] [PubMed]
- Cáceres-Cevallos, G.J.; Quílez, M.; Ortiz de Elguea-Culebras, G.; Melero-Bravo, E.; Sánchez-Vioque, R.; Jordán, M.J. Agronomic Evaluation and Chemical Characterization of Lavandula latifolia Medik. under the Semiarid Conditions of the Spanish Southeast. Plants 2023, 12, 1986. [Google Scholar] [CrossRef]
- Habán, M.; Korczyk-Szabó, J.; Čerteková, S.; Ražná, K. Lavandula Species, Their Bioactive Phytochemicals, and Their Biosynthetic Regulation. Int. J. Mol. Sci. 2023, 24, 8831. [Google Scholar] [CrossRef] [PubMed]
- Ez zoubi, Y.; Bousta, D.; Farah, A. A Phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.D.; Paradowska, K. Phytochemical Profiling, Antioxidant and Anti-Inflammatory Activity of Plants Belonging to the Lavandula Genus. Molecules 2023, 28, 256. [Google Scholar] [CrossRef]
- Morales, R. Lavandula L. In: Flora iberica. Plantas vasculares de la península ibérica e islas Baleares. Vol. XII (Verbenaceae-Labiatae-Callitrichaceae), Castroviejo S. (Coord.), CSIC Real Jardín Botánico. Madrid, Spain, 2010, pp. 484–496.
- Vázquez-Pardo, F.M.; Márquez-García, F.; García-Alonso, D.; Nogales-Gómez, L. Aproximación al conocimiento del género Lavandula L. sección stoechas Ging., (Lamiaceae) en el SW de la península ibérica. Fol. Bot. Extrem. 2022, 16, 55–94. [Google Scholar]
- Kokkalou, E. The Constituents of the Essential Oil from Lavandula stoechas Growing Wild in Greece. Planta Med. 1988, 54, 58–59. [Google Scholar] [CrossRef] [PubMed]
- Skoula, M.; Abidi, C.; Kokkalou, E. Essential oil variation of Lavandula stoechas L. ssp. stoechas growing wild in Crete (Greece). Biochem. Syst. Ecol. 1996, 24, 255–260. [Google Scholar] [CrossRef]
- Ristorcelli, D.; Tomi, F.; Casanova. J. 13C-NMR as a tool for identification and enantiomeric differentiation of major terpenes exemplified by the essential oil of Lavandula stoechas L. ssp. Stoechas. Flavour Fragr. J. 1998, 13, 154–158. [Google Scholar] [CrossRef]
- Bouzouita, N.; Kachouri, F.; Hamdi, M.; Chaabouni, M.M.; Ben Aissa, R.; Zgoulli, S. Volatile Constituents and Antimicrobial Activity of Lavandula stoechas L. Oil from Tunisia. J. Essent. Oil Res. 2005, 17, 584–586. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Dob, T.; Dahmane, D.; Agli, M.; Chelghoum, C. Essential Oil Composition of Lavandula stoechas from Algeria. Pharm. Biol. 2006, 44, 60–64. [Google Scholar] [CrossRef]
- Giray, E.S.; Kırıcı, S.; Kaya, D.A.; Türk, M.; Sönmez, Ö.; İnan, M. Comparing the effect of sub-critical water extraction with conventional extraction methods on the chemical composition of Lavandula stoechas. Talanta 2008, 74, 930–935. [Google Scholar] [CrossRef]
- Kirmizibekmez, H.; Demirci, B.; Yeşilada, E.; Başer, K.H.; Demirci, F. Chemical composition and antimicrobial activity of the essential oils of Lavandula stoechas L. ssp. stoechas growing wild in Turkey. Nat. Prod. Commun. 2009, 4, 1001–1006. [Google Scholar] [CrossRef]
- Tzakou, O.; Bazos, I.; Yannitsaros, A. Essential Oil Composition and Enantiomeric Distribution of Fenchone and Camphor of Lavandula cariensis and L. stoechas subsp. stoechas grown in Greece. Nat. Prod. Commun. 2009, 4, 1103–1106. [Google Scholar] [CrossRef]
- Benabdelkader,T. ; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential Oils from Wild Populations of Algerian Lavandula stoechas L.: Composition, Chemical Variability, and in vitro Biological Properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef]
- Zohra, M.; Fawzia, A. Pouvoir Antifongique Et Antioxydant De L’huile Essentielle De Lavandula Stoechas L. Rev. Nat. Technol. 2012, 6, 34–39. [Google Scholar]
- Sebai, H.; Selmi, S.; Rtibi, K.; Souli, A.; Gharbi, N.; Sakly, M. Lavender (Lavandula stoechas L.) essential oils attenuate hyperglycemia and protect against oxidative stress in alloxan-induced diabetic rats. Lipids Health Dis. 2013, 12, 189. [Google Scholar] [CrossRef] [PubMed]
- La Bella, S.; Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.N.; Potorti, A.G.; Fede, M.R.; Virga, G.; Leone, R.; D'Anna, E.; Licata, M. Composition and Variability of the Essential Oil of the Flowers of Lavandula stoechas from Various Geographical Sources. Nat. Prod. Commun. 2015, 10, 2001–2004. [Google Scholar] [CrossRef]
- Tuttolomondo, T.; Dugo, G.; Ruberto, G.; Leto, C.; Napoli, E.N.; Potorti, A.G.; Fede, M.R.; Virga, G.; Leone, R.; D'Anna, E.; et al. Agronomical evaluation of Sicilian biotypes of Lavandula stoechas L. spp. stoechas and analysis of the essential oils. J. Essent. Oil Res. 2015, 27, 115–124. [Google Scholar] [CrossRef]
- Hassiotis, C.N.; Orfanoudakis, M. The impact of Lavandula stoechas L. degradation on arbuscular mycorrhizal fungi, in a Mediterranean ecosystem. Appl. Soil Ecol. 2018, 126, 182–188. [Google Scholar] [CrossRef]
- Barhouchi, B.; Aouadi, S.; Abdi, A. Comparative Chemical Profile of Lavandula stoechas L. Essential Oils Isolated from Flowers and Leaves Native to Algeria. Phytotherapie 2019, 17, 240–248. [Google Scholar] [CrossRef]
- Chaouchi, O.; Farida, F. Antimicrobial and Antioxidant Activities of Algerian Lavandula stoechas Essential Oil. Eurasia Proc. Sci. Technol. Eng. Math. 2024, 29, 16–21. [Google Scholar] [CrossRef]
- Syaj, H.; Aboalhaija, N.; Afifi, F.; Abu-Dahab, R.; Abusulieh, S.; Amro, R. Phytochemistry and Antiproliferative Potential of a Naturalized Plant in Jordan: Lavandula stoechas. Chem. Biodivers. 2025, 22, e202500181. [Google Scholar] [CrossRef]
- Badreddine, B.S.; Olfa, E.; Samir, D.; Hnia, C.; Lahbib, B.J.M. Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae). Asian Pac. J. Trop. Med. 2015, 8, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Asghari, J.; Sadani, S.; Ghaemi, E.; Tehrani, M.M. Investigation of Composition and Antimicrobial Properties of Lavandula stoechas Essential Oil Using Disk Diffusion and Broth Microdilution. mljgoums 2016, 10, 53–58. [Google Scholar] [CrossRef]
- Sadani, S.; Shakeri, A. Antimicrobial activity of the essential oils of Lavandula stoechas flowers extracted by microwave. J. Med. Plants Stud. 2016, 4, 224–228. [Google Scholar]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, antibacterial and antioxidant activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Karan, T. Metabolic profile and biological activities of Lavandula stoechas L. Cell. Mol. Biol. 2018, 64, 1–7. [Google Scholar] [CrossRef]
- Insawang, S.; Pripdeevech, P.; Tanapichatsakul, C.; Khruengsai, S.; Monggoot, S.; Nakham, T.; Artrod, A.; D’Souza, P.E.; Panuwet, P. Essential Oil Compositions and Antibacterial and Antioxidant Activities of Five Lavandula stoechas Cultivars Grown in Thailand. Chem. Biodivers. 2019, 16, e1900371. [Google Scholar] [CrossRef]
- Küçük, S.; Altıntaş, A.; Demirci, B.; Koca, F.; Can Başer, K.H. Morphological, Anatomical and Phytochemical Characterizations of Lavandula stoechas L. subsp. stoechas Growing in Turkey. Nat. Volatiles & Essent. Oils 2019, 6, 9–19. [Google Scholar]
- Chograni, H.; Raihi, L.; Messaoud, C. Variability of qualitative and quantitative secondary metabolites traits among wild genetic resources of Lavandula stoechas L. Biochem. Syst. Ecol. 2021, 98, 104327. [Google Scholar] [CrossRef]
- Ghalbane, I.; Alahyane, H.; Aboussaid, H.; Chouikh, N.-e.; Costa, J.; Romane, A.; El Messoussi, S. Chemical Composition and Insecticidal Properties of Moroccan Lavandula dentata and Lavandula stoechas Essential Oils Against Mediterranean Fruit Fly, Ceratitis capitata. Neotrop. Entomol. 2022, 51, 628–636. [Google Scholar] [CrossRef]
- Abdel-Baki, A.-A.S.; Aboelhadid, S.M.; Al-Quraishy, S.; Hassan, A.O.; Daferera, D.; Sokmen, A.; Kamel, A.A. Cytotoxic, Scolicidal, and Insecticidal Activities of Lavandula stoechas Essential Oil. Separations 2023, 10, 100. [Google Scholar] [CrossRef]
- Benali, T.; Lemhadri, A.; Harboul, K.; Chtibi, H.; Khabbach, A.; Jadouali, S.M.; Quesada-Romero, L.; Louahlia, S.; Hammani, K.; Ghaleb, A.; et al. Chemical Profiling and Biological Properties of Essential Oils of Lavandula stoechas L. Collected from Three Moroccan Sites: In Vitro and In Silico Investigations. Plants 2023, 12, 1413. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Sudha, T.; Darwish, N.H.E.; Chader, H.; Belkadi, A.; Rajabi, M.; Houche, A.; Benkebailli, F.; Oudjida, F.; Mousa, S.A. A New Eucalyptol-Rich Lavender (Lavandula stoechas L.) Essential Oil: Emerging Potential for Therapy against Inflammation and Cancer. Molecules 2020, 25, 3671. [Google Scholar] [CrossRef]
- Özcan, M.M.; Starovic, M.; Aleksic, G.; Figueredo, G.; Al Juhaimi, F.; Chalchat, J.-C. Chemical Composition and Antifungal Activity of Lavender (Lavandula stoechas) Oil. Nat. Prod. Commun. 2018, 13, 895–898. [Google Scholar] [CrossRef]
- Khavarpour, M.; Vahdat, S.M.; Kazemi, S.; Moghadamnia, A.A.; Hasanzadeh, O.; Salimi, Z.; Rahmanpour, N. Chemical Composition, Antibacterial and Analgesic Activity of Lavandula stoechas Flowers from North of Iran. Int. J. Eng. 2019, 32, 1065–1073. [Google Scholar] [CrossRef]
- Gören, A.; Topçu, G.; Bilsel, G.; Bilsel, M.; Aydoğmusç, Z.; Pezzuto, J. The Chemical Constituents and Biological Activity of Essential Oil of Lavandula stoechas ssp. stoechas. Z. Naturforsch. C. 2002, 57, 797–800. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- García-Vallejo, M.I. Aceites esenciales de las lavandulas ibéricas. Ensayo de la quimiotaxonomia. PhD Thesis, Department of Plant Biology, University Complutense de Madrid, Madrid, 1992. [Google Scholar]
- García-Vallejo, M.I.; García-Vallejo, M.C.; Sanz, J.; Bernabe, M.; Velasco-Negueruela, A. Necrodane (1,2,2,3,4-pentamethylcyclopentane) derivatives in Lavandula luisieri, new compounds to the plant kingdom. Phytochemistry 1994, 36, 43–45. [Google Scholar] [CrossRef]
- Lavoine-Hanneguelle, S.; Casabianca, H. New Compounds from the Essential Oil and Absolute of Lavandula luisieri L. J. Essent. Oil Res. 2004, 16, 445–448. [Google Scholar] [CrossRef]
- Pombal, S.; Rodrigues, C.F.; Araújo, J.P.; Rocha, P.M.; Rodilla, J.M.; Diez, D.; Granja, Á.P.; Gomes, A.C.; Silva, L.A. Antibacterial and antioxidant activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and its relation with their chemical composition. SpringerPlus 2016, 5, 1711. [Google Scholar] [CrossRef] [PubMed]
- Julio, L.F.; Díaz, C.E.; Aissani, N.; Valcarcel, F.; Burillo, J.; Olmeda, S.; González-Coloma, A. Ixodicidal compounds from pre-domesticated Lavandula luisieri. Ind. Crops Prod. 2017, 110, 83–87. [Google Scholar] [CrossRef]
- Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules 2023, 28. [Google Scholar] [CrossRef]
- González-Coloma, A.; Delgado, F.; Rodilla, J.M.; Silva, L.; Sanz, J.; Burillo, J. Chemical and biological profiles of Lavandula luisieri essential oils from western Iberia Peninsula populations. Biochem. Syst. Ecol. 2011, 39, 1–8. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Pintado, C.S. Essential Oils of Lavandula stoechas subsp. luisieri as Antifungal Agent against Fungi from Strawberry Tree Fruit. J. Pharm. Pharmacol. 2021, 9, 98–106. [Google Scholar] [CrossRef]
- Domingues, J.; Goulão, M.; Delgado, F.; Gonçalves, J.C.; Gonçalves, J.; Pintado, C.S. Essential Oils of Two Portuguese Endemic Species of Lavandula as a Source of Antifungal and Antibacterial Agents. Processes 2023, 11, 1165. [Google Scholar] [CrossRef]
- Giménez-Rota, C.; Lorán, S.; Mainar, A.M.; Hernáiz, M.J.; Rota, C. Supercritical Carbon Dioxide Antisolvent Fractionation for the Sustainable Concentration of Lavandula luisieri (Rozeira) Riv.-Mart Antimicrobial and Antioxidant Compounds and Comparison with Its Conventional Extracts. Plants 2019, 8, 455. [Google Scholar] [CrossRef]
- García-Custodio, M.d.C.; Márquez-García, F.; García-Alonso, D.; Brieva-Trejo, C.D.; Vázquez Pardo, F.M. Antioxidant and Antifungal Effects of Six Plant Essential Oils Against Penicillium digitatum and Penicillium italicum. Microorganisms 2025, 13, 2042. [Google Scholar] [CrossRef]
- Matos, F.; Miguel, M.G.; Duarte, J.; Venâncio, F.; Moiteiro, C.; Correia, A.I.D.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antioxidant Capacity of the Essential Oils from Lavandula luisieri, L. stoechas subsp. lusitanica, L. stoechas subsp. lusitanica x L. luisieri and L. viridis Grown in Algarve (Portugal). J. Essent. Oil Res. 2009, 21, 327–336. [Google Scholar] [CrossRef]
- Pereira, F.; Baptista, R.; Ladeiras, D.; Madureira, A.M.; Teixeira, G.; Rosado, C.; Fernandes, A.S.; Ascensão, L.; Oliveira-Silva, C.; Pinto-Reis, C.; et al. Production and characterization of nanoparticles containing methanol extracts of Portuguese Lavenders. Measurement 2015, 74, 170–177. [Google Scholar] [CrossRef]
- Giménez-Rota, C.; Langa, E.; Urieta, J.S.; Hernáiz, M.J. , Mainar, A.M. Supercritical antisolvent fractionation of antioxidant compounds from Lavandula luisieri (Rozeira) Riv.-Mart. J. Supercrit. Fluids 2020, 161, 104821. [Google Scholar] [CrossRef]
- Neves, A.; Rosa, S.; Gonçalves, J.; Rufino, A.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Screening of Five Essential Oils for Identification of Potential Inhibitors of IL-1-induced Nf-κB Activation and NO Production in Human Chondrocytes: Characterization of the Inhibitory Activity of α-Pinene. Planta Med. 2010, 76, 303–308. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ferreira, I.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Differential effects of the essential oils of Lavandula luisieri and Eryngium duriaei subsp. juresianum in cell models of two chronic inflammatory diseases. Pharm. Biol. 2015, 53, 1220–1230. [Google Scholar] [CrossRef]
- Zuzarte, M.; Soussa, C.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. The Anti-Inflammatory Response of Lavandula luisieri and Lavandula pedunculata Essential Oils. Plants 2022, 11, 370. [Google Scholar] [CrossRef]
- Videira, R.; Castanheira, P.; Grãos, M.; Sagueiro, L.; Faro, C.; Cavaleiro, C. A necrodane monoterpenoid from Lavandula luisieri essential oil as a cell-permeable inhibitor of BACE-1, the β-secretase in Alzheimer's disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Costa, S.; Cavadas, C.; Cavaleiro, C.; Salgueiro, L.; do Céu, M. In vitro susceptibility of Trypanosoma brucei brucei to selected essential oils and their major components. Exp. Parasitol. 2018, 190, 34–40. [Google Scholar] [CrossRef]
- Bailén, M.; Illescas, C.; Quijada, M.; Martínez-Díaz, R.A.; Ochoa, E.; Gómez-Muñoz, M.T.; Navarro-Rocha, J.; González-Coloma, A. Anti-Trypanosomatidae Activity of Essential Oils and Their Main Components from Selected Medicinal Plants. Molecules 2023, 28, 1467. [Google Scholar] [CrossRef]
- Machado, M.; Martins, N.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Lavandula Luisieri and Lavandula Viridis Essential Oils as Upcoming Anti-Protozoal Agents: A Key Focus on Leishmaniasis. Appl. Sci. 2019, 9, 3056. [Google Scholar] [CrossRef]
- González-Coloma, A.; Martín-Benito, D.; Mohamed, N.; García-Vallejo, M.C.; Soria, A.C. Antifeedant effects and chemical composition of essential oils from different populations of Lavandula luisieri L. Biochem. Syst. Ecol. 2006, 34, 609–616. [Google Scholar] [CrossRef]
- Julio, L.F.; Martín, L.; Muñoz, R.; Mainar, A.M.; Urieta, J.S.; Sanz, J.; Burillo, J.; González-Coloma, A. Comparative chemistry and insect antifeedant effects of conventional (Clevenger and Soxhlet) and supercritical extracts (CO2) of two Lavandula luisieri populations. Ind. Crops Prod. 2014, 58, 25–30. [Google Scholar] [CrossRef]
- Julio, L.F.; Barrero, A.F.; Herrador del Pino, M.M.; Arteaga, J.F.; Burillo, J.; Andres, M.F.; Díaz, C.E.; González-Coloma, A. Phytotoxic and Nematicidal Components of Lavandula luisieri. J. Nat. Prod. 2016, 79, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Baldovini, N.; Lavoine-Hanneguelle, S.; Ferrando, G.; Dusart, G.; Lizzani-Cuvelier, L. Necrodane Monoterpenoids from Lavandula luisieri. Phytochemistry 2005, 66, 1651–1655. [Google Scholar] [CrossRef] [PubMed]
- Perrier, X.; Jacquemoud-Collet, J.P. Dissimilarity Analysis and Representation for Windows; CIRAD-BIOS UMR AGAP: Montpellier, France, 2014. [Google Scholar]
- Perrier, X.; Flori, A.; Bonnot, F. Data analysis methods. In Genetic diversity of cultivated tropical plants; Hamon, P., Seguin, M., Perrier, X., Glaszmann, J.C., Eds.; CIRAD-Science Publishers, Plymouth, UK, 2003; pp. 43-76. [CrossRef]

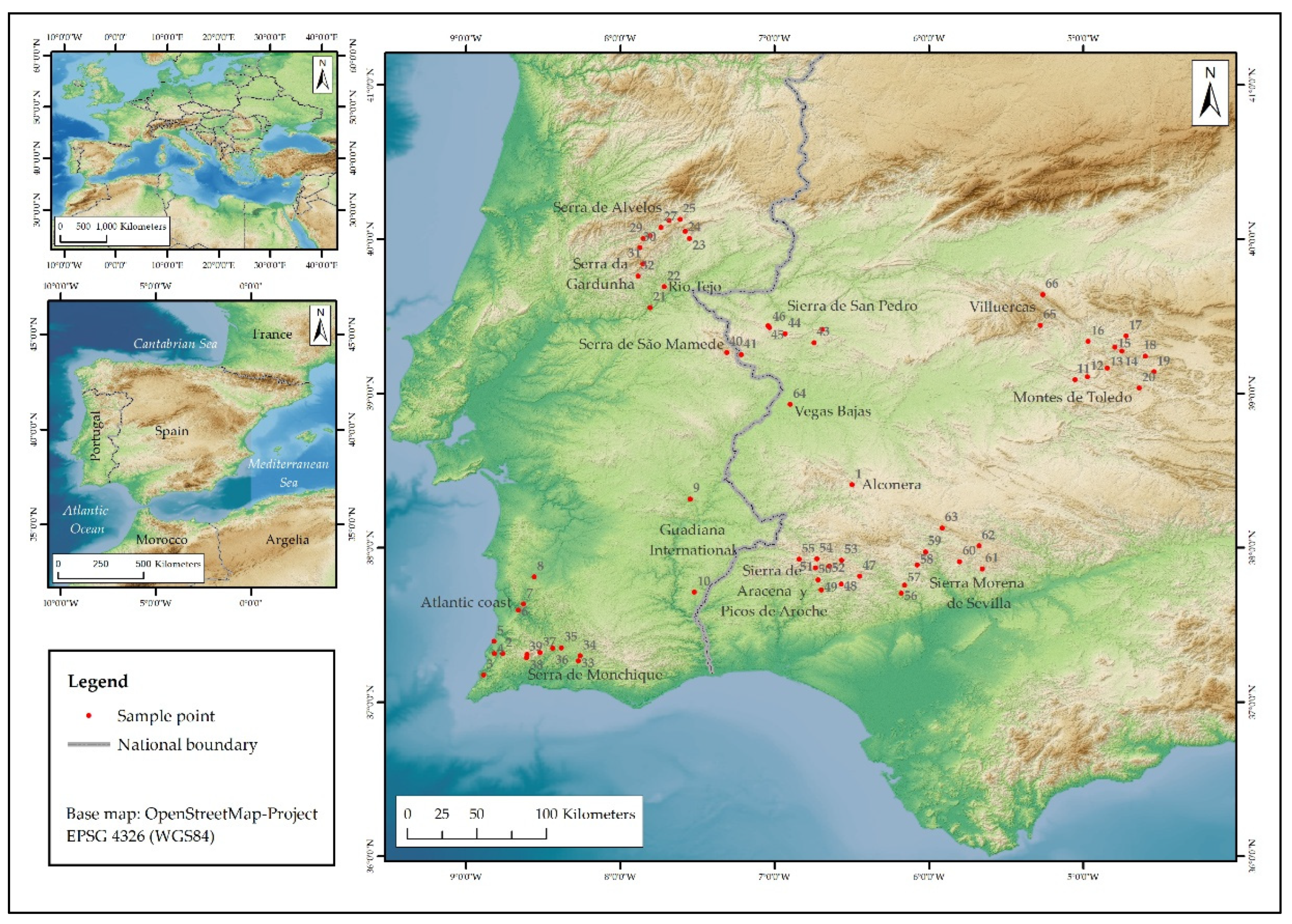
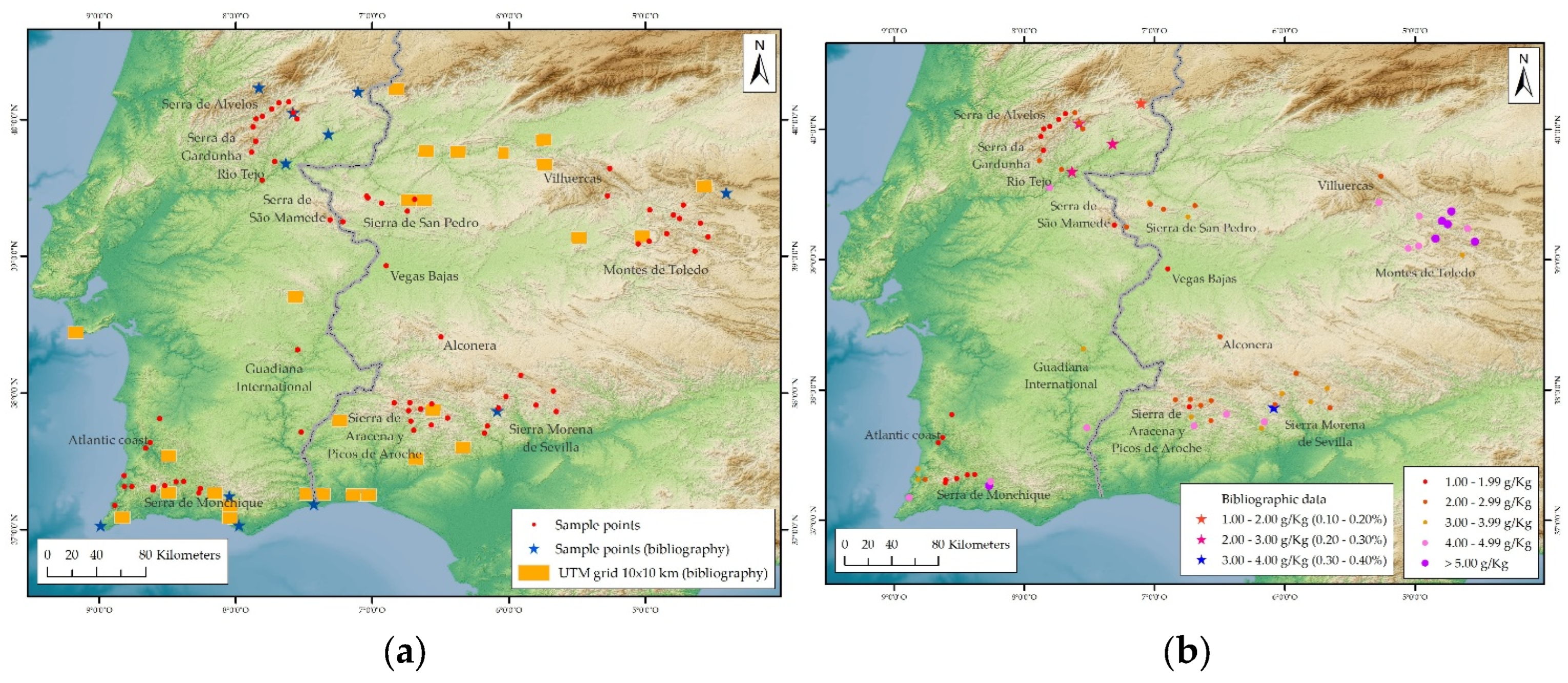
| Zone | Type | Country | N | IDs | Elev. |
| Alconera | MR | ES | 1 | 1 | 608 |
| Atlantic coast | CZ | PT | 7 | 2-8 | 36-112 |
| Guadiana International | AP | PT | 2 | 9-10 | 192-194 |
| Montes de Toledo | MR | ES | 10 | 11-20 | 515-824 |
| Rio Tejo | AP | PT | 2 | 21-22 | 204-373 |
| Serra da Gardunha | MR | PT | 5 | 23-27 | 425-617 |
| Serra de Alvelos | MR | PT | 5 | 28-32 | 311-829 |
| Serra de Monchique | MR | PT | 7 | 33-39 | 104-764 |
| Serra de São Mamede | MR | PT-ES | 2 | 40-41 | 362-526 |
| Sierra de San Pedro | MR | ES | 5 | 42-46 | 362-500 |
| Sierra de Aracena y Picos de Aroche | MR | ES | 9 | 47-55 | 260-709 |
| Sierra Morena de Sevilla | MR | ES | 8 | 56-63 | 296-709 |
| Vegas Bajas | AP | ES | 1 | 64 | 179 |
| Villuercas | MR | ES | 2 | 65-66 | 593-912 |
| Zone | N | Yield (g/kg) | ||
| µ ± σ | min. | max. | ||
| Alconera | 1 | 2.65 | ||
| Atlantic coast | 7 | 2.67 ± 1.24 | 1.42 | 4.43 |
| Guadiana International | 2 | 4.25 ± 1.07 | 3.46 | 4.97 |
| Montes de Toledo | 10 | 5.20 ± 1.29 | 3.01 | 8.17 |
| Rio Tejo | 2 | 3.31 ± 1.40 | 2.34 | 4.30 |
| Serra da Gardunha | 5 | 2.23 ± 0.60 | 1.42 | 2.95 |
| Serra de Alvelos | 5 | 1.46 ± 0.45 | 1.08 | 2.05 |
| Serra de Monchique | 7 | 2.62 ± 1.79 | 1.34 | 5.94 |
| Serra de São Mamede | 2 | 2.30 ± 0.47 | 1.97 | 2.64 |
| Sierra de San Pedro | 5 | 2.78 ± 0.46 | 2.20 | 3.27 |
| Sierra de Aracena y Picos de Aroche | 9 | 2.87 ± 0.87 | 1.79 | 4.25 |
| Sierra Morena de Sevilla | 8 | 3.11 ± 0.66 | 2.10 | 4.14 |
| Vegas Bajas | 1 | 1.75 | 1.75 | |
| Villuercas | 2 | 3.42 ± 0.82 | 2.80 | 4.03 |
| 66 | 3.08 ± 1.42 | 1.077 | 8.17 | |
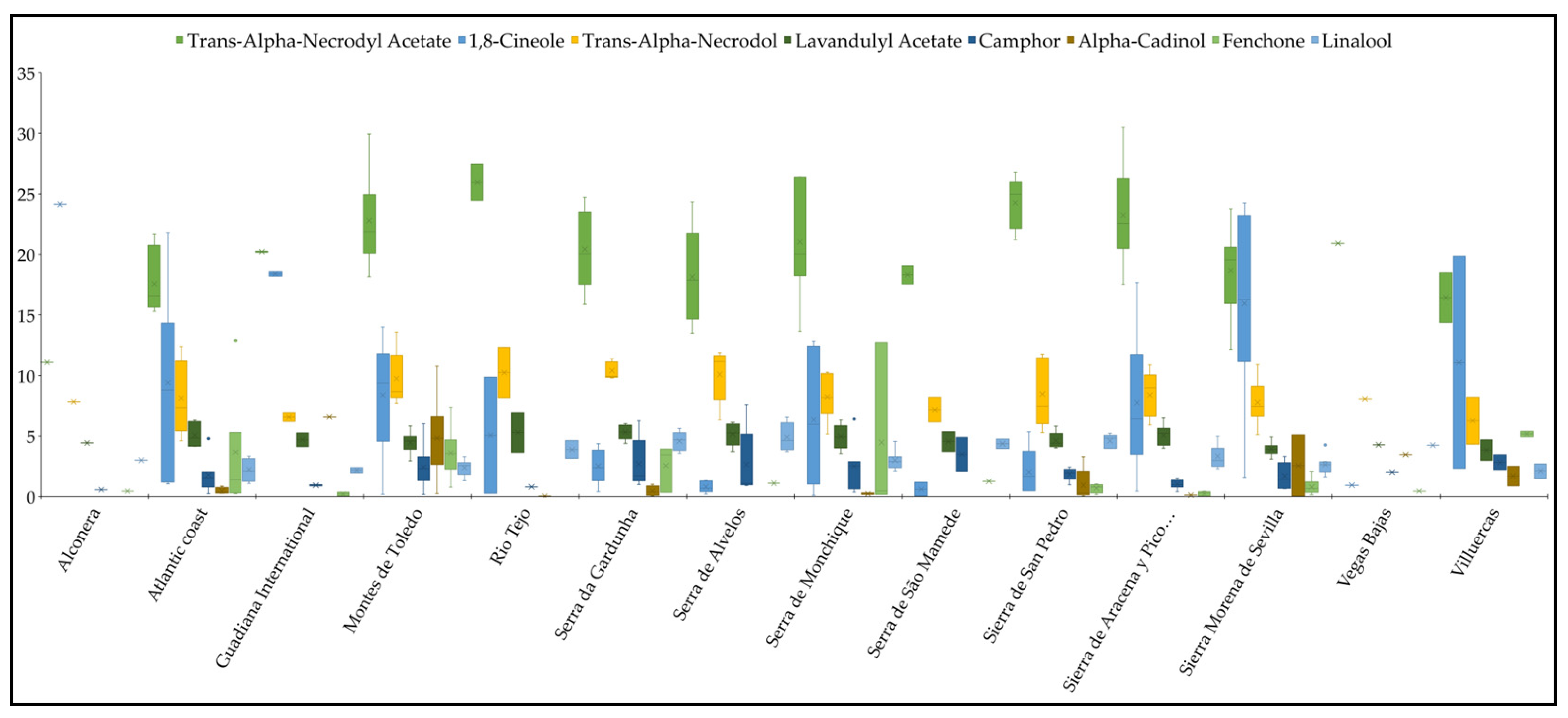
| Components | µ ± σ | min. | max. | N | N1 | N2 | N3 | N4 | N5 |
| trans-α-Necrodyl Acetate | 20.68 ± 4.17 | 11.11 | 30.50 | 66 | 60 | 6 | 0 | 0 | 0 |
| 1,8-Cineole | 7.79 ± 7.14 | 0.04 | 24.24 | 45 | 6 | 20 | 7 | 12 | 21 |
| trans-α-Necrodol | 8.66 ± 2.18 | 4.32 | 13.57 | 66 | 0 | 37 | 22 | 7 | 0 |
| Lavandulyl Acetate | 4.74 ± 0.91 | 2.95 | 6.96 | 66 | 0 | 1 | 22 | 43 | 0 |
| Camphor | 2.01 ± 1.55 | 0.16 | 7.61 | 30 | 0 | 1 | 3 | 26 | 36 |
| α-Cadinol | 2.24 ± 2.76 | 0.02 | 10.78 | 13 | 0 | 1 | 3 | 9 | 21 |
| Fenchone | 2.23 ± 2.91 | 0.01 | 12.92 | 17 | 0 | 0 | 6 | 11 | 31 |
| Linalool | 3.25 ± 1.19 | 1.09 | 6.58 | 62 | 0 | 0 | 3 | 59 | 3 |
| 5-Methylene-2,3,4,4-tetrame-2-Cyclopentenone | 2.73 ± 0.70 | 1.26 | 4.13 | 58 | 0 | 0 | 0 | 58 | 8 |
| Cymene Isomer | 2.01 ± 0.79 | 0.09 | 3.29 | 50 | 0 | 0 | 0 | 50 | 15 |
| Viridiflorol | 1.96 ± 0.81 | 0.01 | 3.43 | 36 | 0 | 0 | 0 | 36 | 28 |
| Unknown Sesquiterpenol | 2.03 ± 0.86 | 0.14 | 3.71 | 30 | 0 | 0 | 0 | 30 | 24 |
| cis-α-Necrodol | 1.73 ± 0.27 | 1.10 | 2.29 | 29 | 0 | 0 | 0 | 29 | 37 |
| α-Pinene | 1.73 ± 0.98 | 0.41 | 5.36 | 20 | 0 | 0 | 0 | 20 | 46 |
| Arbozol | 1.69 ± 0.38 | 0.84 | 2.57 | 20 | 0 | 0 | 0 | 20 | 46 |
| Unknown Sesquiterpenol | 1.52 ± 0.70 | 0.09 | 2.89 | 16 | 0 | 0 | 0 | 16 | 46 |
| Unknown Mw=153 | 1.56 ± 0.40 | 0.57 | 2.31 | 13 | 0 | 0 | 0 | 13 | 43 |
| Unknown Ester | 1.60 ± 0.22 | 1.11 | 2.10 | 9 | 0 | 0 | 0 | 9 | 47 |
| Eudesma-3,7(11)-diene | 1.07 ± 0.59 | 0.12 | 3.24 | 6 | 0 | 0 | 0 | 6 | 60 |
| Unknown Mw=154 | 1.80 ± 0.26 | 1.24 | 2.08 | 6 | 0 | 0 | 0 | 6 | 4 |
| cis-α-Necrodyl Acetate | 1.38 ± 0.28 | 0.82 | 1.92 | 5 | 0 | 0 | 0 | 5 | 61 |
| Unknown Sesquiterpenol | 1.38 ± 0.90 | 0.66 | 3.60 | 3 | 0 | 0 | 0 | 3 | 7 |
| Copaborneol | 1.01 ± 0.37 | 0.04 | 1.78 | 1 | 0 | 0 | 0 | 1 | 59 |
| Unknown Sesquiterpenol | 0.80 ± 0.42 | 0.05 | 2.09 | 1 | 0 | 0 | 0 | 1 | 53 |
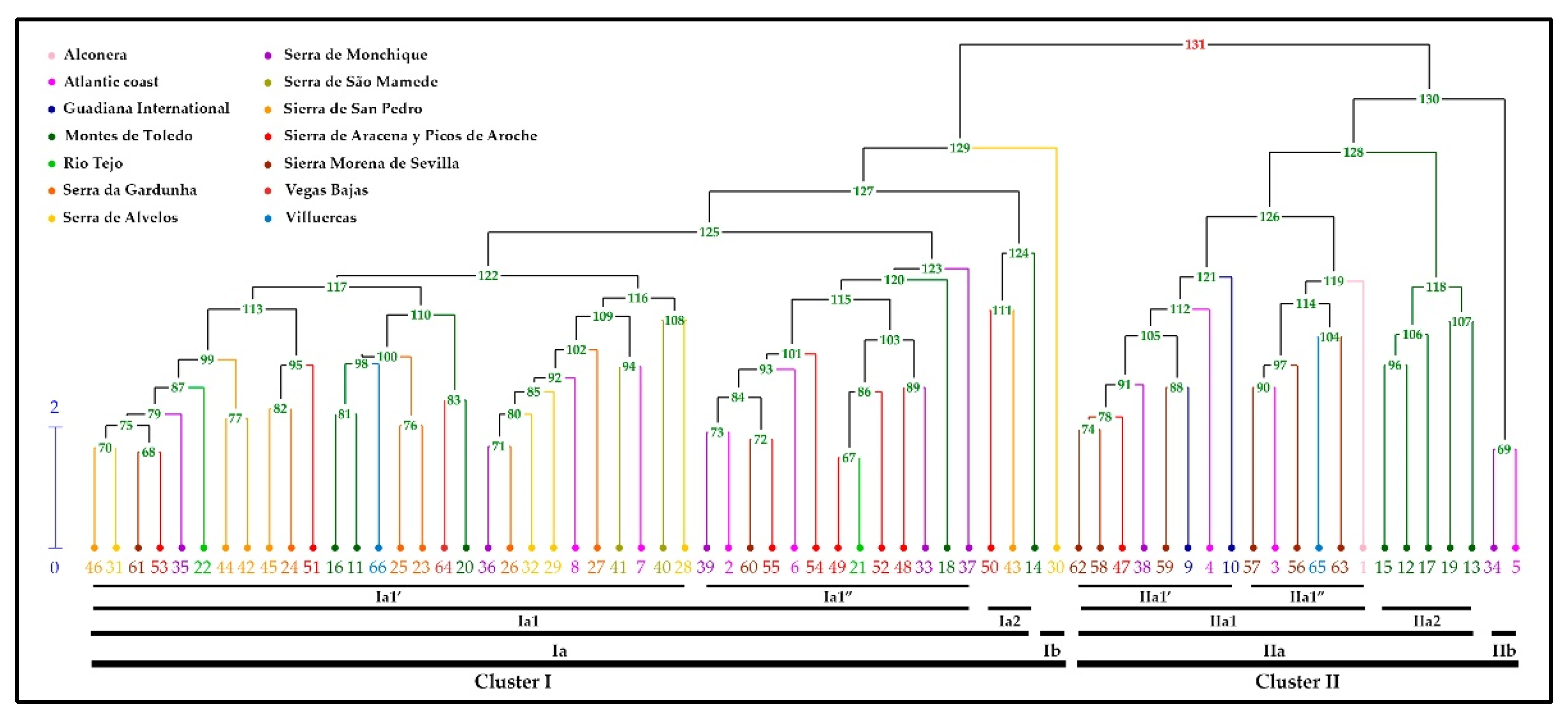
| Cluster I | Cluster II | ||||||||||||||
| Components | Ia1’ | Ia1” | Ia2 | Ib | IIa1’ | IIa1” | IIa2 | IIb | |||||||
| min. | max. | min. | max. | min. | max. | min. | max. | min. | max. | min. | max. | min. | max. | ||
| α-Pinene | 0.41 | 3.62 | 0.74 | 2.12 | 1.81 | 2.40 | 4.77 | 0.93 | 1.96 | 1.01 | 3.23 | 1.92 | 5.36 | 1.45 | 1.50 |
| Cymene Isomer | 0.09 | 3.29 | 0.17 | 3.16 | 0.26 | 1.37 | 1.91 | 1.85 | 2.76 | 1.80 | 2.36 | a | 2.31 | 2.04 | 2.06 |
| 1,8-Cineole | 0.04 | 5.63 | 5.38 | 12.84 | 1.35 | 7.56 | 1.33 | 12.42 | 18.60 | 19.84 | 24.24 | 10.50 | 14.01 | 12.86 | 13.06 |
| Fenchone | a | 7.39 | a | 1.40 | 0.39 | 2.75 | a | a | 5.31 | 0.34 | 5.41 | 2.32 | 4.88 | 12.76 | 12.92 |
| Camphor | 0.38 | 6.27 | 0.64 | 6.42 | 0.16 | 1.90 | 7.61 | 0.23 | 2.90 | 0.58 | 3.45 | 1.36 | 6.00 | 1.56 | 2.72 |
| Linalool | 1.52 | 6.58 | 1.89 | 4.99 | 1.72 | 5.25 | 4.62 | 1.26 | 2.91 | 1.09 | 3.01 | 1.31 | 2.87 | 2.10 | 2.10 |
| trans-α-Necrodyl Acetate | 15.30 | 26.42 | 17.54 | 28.15 | 26.83 | 30.50 | 13.48 | 16.59 | 20.46 | 11.11 | 19.48 | 18.17 | 26.04 | 13.64 | 15.68 |
| Lavandulyl Acetate | 3.72 | 6.96 | 3.65 | 6.35 | 3.92 | 6.53 | 3.73 | 3.75 | 5.28 | 3.00 | 4.43 | 2.95 | 5.83 | 3.55 | 4.15 |
| cis-α-Necrodyl Acetate | 0.91 | 1.80 | 1.09 | 1.81 | 1.21 | 1.92 | 0.84 | 0.95 | 1.67 | 0.84 | 1.38 | 1.10 | 1.72 | 0.82 | 1.16 |
| Arbozol | 0.84 | 2.57 | 1.25 | 2.55 | 1.33 | 1.67 | 1.39 | 2.24 | 1.11 | 1.70 | 1.16 | 1.96 | 1.57 | 1.58 | |
| 5-Methylene-2,3,4,4-tetrame-2-Cyclopentenone | 1.57 | 4.00 | 1.59 | 3.51 | 1.26 | 2.56 | 3.04 | 1.89 | 4.13 | 1.83 | 3.15 | 1.33 | 1.77 | 2.23 | 2.23 |
| trans-α-Necrodol | 4.59 | 12.38 | 5.92 | 13.57 | 6.71 | 10.48 | 6.36 | 6.22 | 10.93 | 4.32 | 7.84 | 7.70 | 8.85 | 5.16 | 5.43 |
| cis-α-Necrodol | 1.58 | 2.29 | 1.34 | 2.22 | 1.32 | 1.88 | 1.10 | 1.36 | 1.96 | 1.27 | 1.69 | 1.27 | 1.55 | 1.46 | 1.46 |
| Eudesma-3,7(11)-diene | 0.20 | 2.29 | 0.12 | 2.69 | 0.14 | 0.99 | 3.24 | 0.53 | 1.39 | 0.40 | 1.31 | 0.40 | 2.01 | 1.42 | 1.76 |
| Viridiflorol | 0.44 | 3.43 | a | 3.09 | 0.14 | 2.50 | 2.96 | 0.56 | 2.73 | 1.12 | 2.07 | a | 0.92 | 1.44 | 1.45 |
| Copaborneol | a | 1.78 | a | 1.43 | a | 0.82 | 1.48 | 0.19 | 1.43 | 0.54 | 0.95 | 0.04 | 0.45 | 0.71 | 0.72 |
| α-Cadinol | a | 4.85 | a | 0.25 | a | 6.09 | a | a | 6.60 | a | 5.10 | 2.99 | 10.78 | 0.31 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





