2. Study Area
Maiduguri is the capital and the largest city of Borno State in north-eastern Nigeria. The city sits along the seasonal Ngadda River, which disappears into the Firki swamps in the areas around Lake Chad. Maiduguri was founded in 1907 as a military outpost by the British. It has since grown rapidly, with a population exceeding a million by 2007. Maiduguri is the capital of Borno State, Nigeria. It is located at 11.85° latitude N|E and 13.16° longitude N|E and it is situated at an elevation of 325 meters above sea level. Maiduguri has a population of 1,112,449, making it the biggest city in Borno. It operates on the WAT time zone. [
10]. The climate of the study area is harsh, and that made it suitable for the study.
In 2025, the temperatures range from 32 °C (89 °F) in August to a scorching 42 °C (107 °F) in April [
11]. Notably, the duration of the hot season in Maiduguri is 2.4 months, from March to May, with an average daily high temperature of above
38.9 °C [
12]
. The hottest month of the year is usually May, with an average high temperature of 39.4 oC and a low of 26 oC [
13].
2.1. The Sources of bOPV Used in the Study
In Nigeria, there are three different levels of health care vaccination centres (federal, state, and local government). The Epidemiological Unit (EPU) of the Borno State Ministry of Health represents the State’s vaccination center, serving as the central storage facility in the State. Fifty vials of bOPV, representing two batches by the same manufacturer, were randomly selected from the EPU, Borno State Ministry of Health. At the EPU, vials of bOPV are usually stored at -15-25 °C. The center is empowered with standby generators and Solar refrigerators and freezers in addition to the National electricity supply. The following particulars were documented during the collection of the vaccine samples: date of manufacture and expiry, date of arrival at the storage facility, batch number, company manufacturing the vaccine, and whether the vaccine was thermo-stabilized with 1M magnesium chloride. Before subjecting the vaccines to different temperatures, each vaccine vial was physically examined using the following criteria: appearance, consistency, colour, transparency, and any visible particles. All the vials of vaccines collected from EPU were transported in a cold box packed with ice packs to the WHO National Polio Laboratory, University of Maiduguri Teaching Hospital, Maiduguri, Borno State, Nigeria, where the test was performed.
2.2. Exposure at Refrigerator Temperatures (2-8 °C)
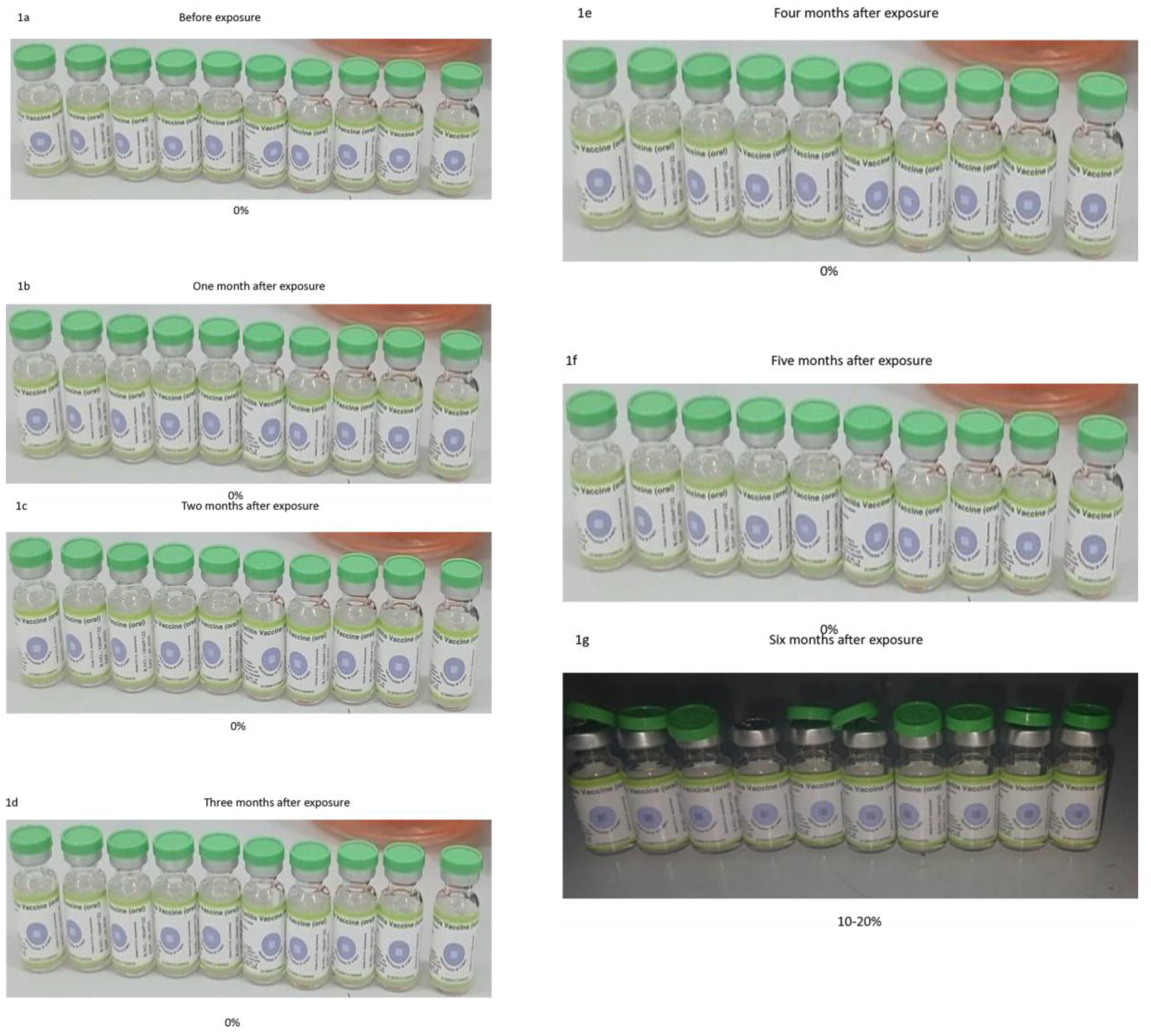
Ten vials of bOPV (type 1 and type 3) were stored in the refrigerator for six months. Before exposure, the vaccines were titrated to obtain the initial titers using the standardized protocol described below. The temperature of the fridge was recorded daily, and average weekly readings were obtained (Appendix 1). A specific quantity of the vaccine in each vial was aspirated using a disposable insulin syringe for monthly titration while the vaccines remained stored in the refrigerator (Appendix 2). The colour of the VVM on the vial of each vaccine was monitored and documented with pictures taken every month. Monthly titration and the picture of the VVM were performed on the same day. The exercise was terminated when the colour of the VVM reached stage 4 or 100% degradation.
2.3. Exposure at Room Temperatures
Ten vials of bOPV (type 1 and type 3) were placed on a Styrofoam and kept on a shelf in a room (Appendix 2). The daily temperatures of the room were used to calculate the average weekly values (Appendix 1). A specific quantity of the vaccine in each vial was aspirated using a disposable insulin syringe for weekly titration while the vaccine remained in the room. The colour of the VVM on the vial of each vaccine was monitored and documented with pictures taken every week. The exercise was terminated when the colour of the VVM reached stage 4 or 100% degradation.
2.4. Exposure at Incubator Temperature (36 ± 1 °C)
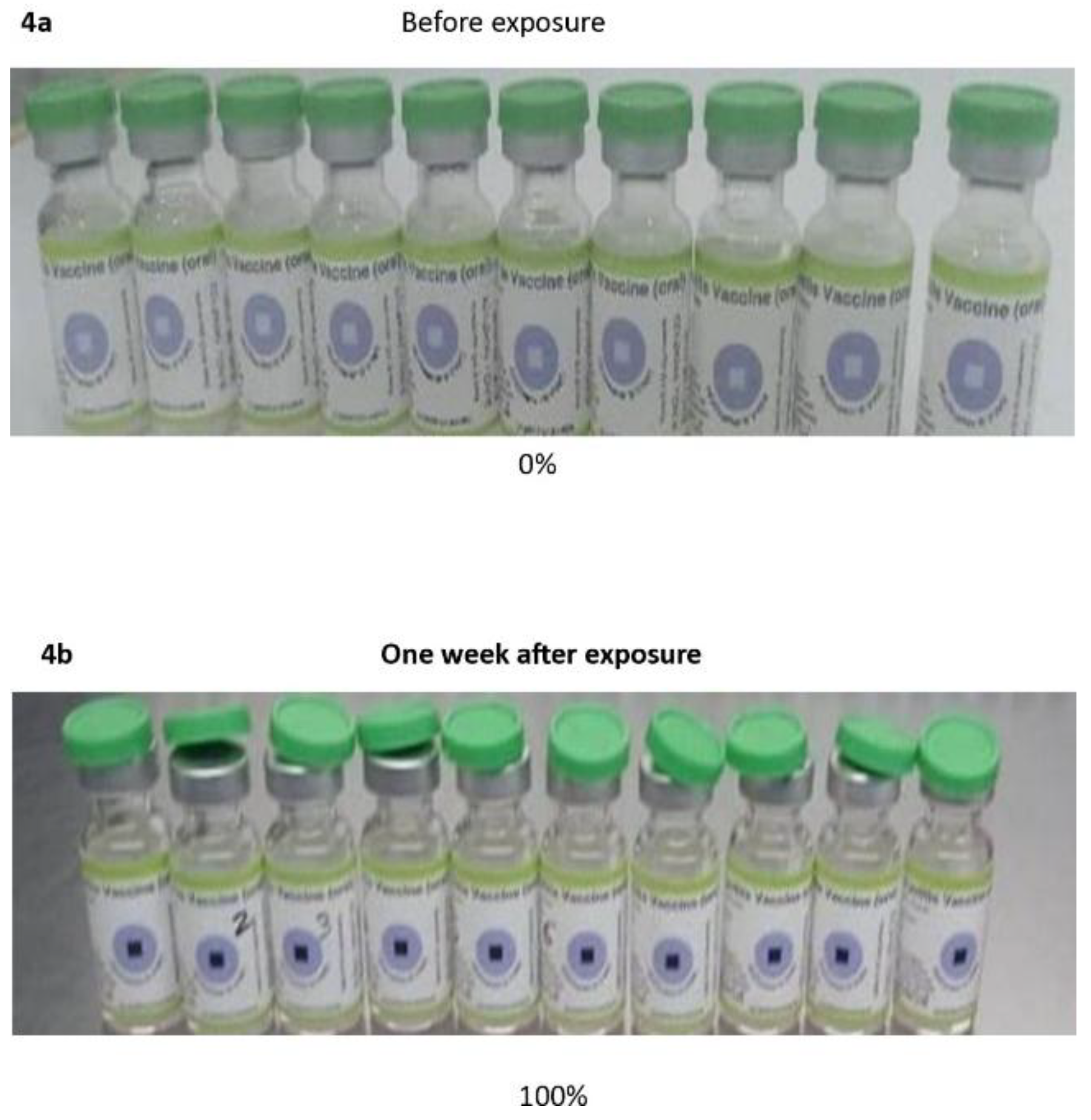
Twenty vials of bOPV (type 1 and type 3) were placed in the incubator. A trough filled with water was placed at the bottom of the incubator to provide humidity (Appendix 2). The daily temperatures of the incubator were used to calculate the average weekly values (Appendix 1). A specific quantity of the vaccine in each vial was aspirated using a disposable insulin syringe for weekly titration while the vaccine remained in the incubator. The colour of the VVM on the vial of each vaccine was monitored and documented with pictures taken every week. The exercise was terminated when the colour of the VVM reached stage 4 (Figure 4).
2.5. Exposure to the Atmospheric Temperatures
Exposure of bOPV (type 1 and type 3) was achieved by placing the vaccine vials on a small Styrofoam and keeping them inside a locally perforated carton. The carton was placed on a stool in an open space, and the top of the carton was covered with another Styrofoam (Appendix 2). Such packaging prevented the vaccine from being blown away by strong winds, or soaked by rain, or contaminated by droppings, and helped to reduce the direct effect of the sun on the vaccine. A digital thermometer was placed inside the perforated carton, and daily atmospheric temperatures were recorded. The readings were taken twice a day- morning (6.30 to 7 am) and afternoon (1 pm to 2 pm), and average weekly temperatures were calculated (Appendix 1). A specific quantity of the vaccine was aspirated using a disposable insulin syringe for weekly titration while the vaccine remained in its storage conditions. The colour of the VVM on the vial of each vaccine was monitored and documented with pictures taken every week. The exercise was terminated when the colour of the VVM reached stage 4 or 100% degradation.
2.6. Monitoring of the VVM
The pictures of all the vials of both bOPV (type 1 and type 3) with VVM affixed to the vaccine bottles were taken before exposure to different temperatures. The colour of the VVM of each vaccine stored at different temperatures was observed with pictures taken and recorded, along with temperature readings on a weekly/monthly basis, until it reached stage 4. Notably, VVM registers the cumulative heat exposure over time. During the exposure, the combined effects of time and temperature caused the inner square of the VVM to darken gradually and irreversibly. Overall, the vaccine is considered safe or potent when the center square is lighter than the surrounding circle. However, as soon as the square becomes the same color as the background or is darker, it implies that, the heat may have damaged the vaccine, and the vial should be discarded. Thus, the VVM was classified as: 0%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 100% based on the colour changes as the exposure progressed. In parallel with each potency test (virus titer), the VVM at different temperatures was evaluated and classified before and after exposure using the above VVM grading.
2.7. Validation of the Protocol Using the Reference Strains
The protocol described in the Polio Laboratory Manual, 4th Edition [
14], for vaccine testing and cell sensitivity, was adopted for this study. Notably, this protocol is standard for determining the potency of OPV, both for total virus content (type 1 + type 3) and individual serotypes separately [
14,
15,
16]. The reference Sabin 1 (01/528) and Sabin 3 (01/532), kindly supplied by the National Institute of Biological Standards (NIBSC), UK, were used to validate the protocol. The production of the local virus stock (LVS) for each Poliovirus serotype from the reference strains, identification of the virus isolates (LVS), and the determination of the titer of the LVS in parallel with the reference strains were performed as described in Polio Laboratory Manual [
14]
2.8. Validity Conditions
The average titers of the reference strains were within ±0.5 log of the expected titers specified by the WHO. The expected average titre of the LVS fell within the upper and lower range of the reference strains.
2.9. Titration of the bOPV Using the Validated Protocol
Before commencing vaccine testing, the adopted protocol was validated by obtaining a valid titer for a previously stored LVS (-80 °C). Each vial of the vaccine was appropriately labeled (10 vials were labeled 1-10), and a picture of the VVM was taken. A single-use insulin syringe was used to aspirate 1ml of each vaccine into appropriately labeled cryovials (labelled 1-10 to correspond to the labeled vaccine vials). Maintenance medium (MM) was dispensed into corresponding dilution tubes labeled 1-10 as follows: 1800 μL of MM was added to tube 1and 900ul into tubes 2-8. 200ul of the vaccine from cryovial 1 was added to tube one. After vortexing, 100ul was transferred from tube one into tube 2, and serial dilution was carried out till tube 10; thereafter, 100ul was discarded. The same process of serial dilution was repeated for vaccines 2 to 10. Dilutions 10
-1 and 10
-2 (tubes 1 and 2) of each vaccine were discarded. Using a single-channel pipette, 100 μL of the dilution (10
-3) from tube 3 of vaccine 1 (after vortexing), was dispensed into the first 5 wells of the appropriately labeled microtitre plate. The procedure was repeated for tubes 4 to 10 (10
-4 to 10
-10). 100 μL of L20B cell line (1.2X10
5) was added to all the wells of the microtiter plates. This procedure was repeated for all the vaccines. Each microtiter plate had the control wells, which contained only the medium and the cells. The microtitre plate was sealed, lightly rocked to mix the content, incubated at 36
oC and examined daily for 7 days. The plates were read microscopically using an inverted microscope to observe the cytopathic effect (CPE), which indicates the infected cells rounding up, shrinking, and marking nuclear pyknosis, becoming refractile, degenerating, and falling off the surface[
17]. The titration of the vaccine under different temperatures was terminated immediately the VVM degradation was at 100% (Stage 4). The vaccine titre per 0.1ml was calculated using Kaerber’s formula [
18].
Log CCID50 = L −d(S−0.5)
where L is the log of the lowest dilution used in the test, d is the difference between logarithmic dilution steps (1 for bOPV and nOPV2), and S is the sum of the proportion of positive tests (i.e., cultures showing CPE). The viral titer (CCID50®) is the smallest amount of virus capable of causing cytopathic effects in 50% of infected cells.
2.10. Criteria Considered During the Validation of the Protocol and Testing of the Vaccine
(a)The lowest dilution used (10-3) should have 100% CPE wells (the five wells used should have the CPE). (b) As the virus titer declines, at least three wells of this dilution must show 100% CPE (c)The highest dilutions (10-8, 10-9, 10-10,) depending on the titer of virus used) should not have any CPE (d) One or more positive (with CPE) wells in the highest dilutions (10-8 or 10-9 or 10-10,) were considered invalid (e). The cell control wells (without any virus) must not have any CPE.
3. Interpretation of the Results
The manufacturer’s specified titer prescribed on the vials of the vaccines tested were: bOPV (type 1) ≥10
6CCID
50/0.1ml; bOPV (type 3) =≥10
5.8CCID
50/0.1ml
. The weekly titer of the vaccines obtained after exposure to specific temperatures was considered within a range of +/- 0.5 units (upper and lower) of the manufacturers’ specified minimum titers (WHO-recommended minimum titer). A vial of bOPV vaccine was considered potent if the viral titer was 10
5.8 CCID
50/.1 mL +/- 0.5 (5.3-6.3) [
19]. This range (vaccine titer +/- 0.5) was prescribed for cell sensitivity testing in the WHO Laboratory Manual [
14]. However, the titers of the vaccine were interpreted in conjunction with the color changes on the VVM. A vial of bOPV vaccine was considered not potent if the viral titer was < 10
5.3 CCID
50/.1 mL [
19].
3.1. Statistical Analysis Data
The data analysis methods used in this study included both descriptive and inferential statistical techniques. Descriptive statistics, such as mean, standard deviation, and range of titers, were employed to summarize the potency data at each time point.
To assess changes in potency over time, repeated measures analysis of variance (ANOVA) was conducted, allowing for the examination of linear declines in potency within each storage condition. A one-way ANOVA was used to compare mean potency reductions across different storage conditions, followed by post-hoc Tukey’s Honest Significant Difference (HSD) test to identify which conditions caused the most significant potency loss.
Correlation and regression analyses were performed to explore the relationship between storage temperature and potency loss. Pearson’s correlation coefficient quantified the strength of this relationship, while simple linear regression estimated the effect of temperature on potency reduction.
Logistic regression was used to estimate the odds of potency preservation under each storage condition, with odds ratios calculated for each condition relative to refrigeration. A Chi-Square test of independence was conducted to examine the association between storage condition and potency preservation.
Survival analysis assessed the time-to-potency loss for vaccines stored under different conditions, with median and mean times to potency loss calculated. The log-rank test was used to compare survival rates across storage conditions.
Lastly, linear trend analysis was performed to quantify the rate of potency decline over time for each storage condition, with slope coefficients representing the rate of potency reduction per time point. Statistical analysis was carried out using R software, version 4.4.1.
3.2. Ethical Approval
This study was conducted as part of the evaluation of EPU with respect to poliomyelitis vaccination activities in Borno State and was carried out through a partnership between the Emergency Operation Center for the National Primary Health Care Development Agency (EOC-NPHCDA), Borno State. The study has no ethical associated issues.
5. Discussion
The potency of a bOPV is determined by the amount of live, attenuated poliovirus it contains, and this is measured in infectious units (CCID
50). Precisely, higher viral titers within the vaccine indicate a higher dose of the active virus, which corresponds to increased potency and a greater ability to induce an immune response [
20,
21,
22]. Notably, the total poliovirus content does not provide an estimate of potency for the two serotypes present in bOPV. Therefore, if potency declines, it is not possible to state which of the two serotypes is out of specification [
17].
The 50 vials of bOPV tested before exposure to different temperatures were potent, with titers above the manufacturer’s recommended minimum titers, in correlation with previous reports in Iran [
24] and Nigeria (15)(Muhammed et al., 2010). The Sustained potency of vaccines tested before exposure to different temperatures in this study confirms the ideal storage conditions of bOPV in the Borno State Epidemiological Unit.
Notably, previous studies used different standardized values to estimate the potency of the bOPV. For instance, in 2025, an adequate bOPV vaccine titer was ≥10
5.8 CCID50/0.1 mL [
24,
25]. However, in 2014, it was the calculated titer with an allowance of +0.5 log units [
26]. The value adopted in this study was 10
5.8 CCID50/0.1 mL ± 0.5, in agreement with Eswaran et al. [
19]. With the latter, the upper and lower limits (10
5.3 to 10
6.3) were determined, and only the vaccine sample titers that fell within these limits were considered potent. The vaccine titers were assessed along with the color changes on the VVM, which was rated from 0% (Stage 1) to 100% (Stage 4) according to the degree of degradation.
Across all storage environments, there was a statistically significant reduction in mean titers from the initial to the final measurements over time, with p-values well below 0.001. This reduction indicated highly robust evidence of potency loss regardless of the storage condition. The refrigerator-maintained potency the longest, with a median time to potency loss of 6 months and a mean of 6.3 months, indicating high survival rates initially but dropping to 40% by month 3. It also showed the smallest mean reduction (–1.85) with a large effect size (Cohen’s d = 1.62). Although some fluctuation was observed (final titer 4.6 ± 0.9, minimum 2.7), the preservation of bOPV potency through refrigeration was consistent with WHO guidelines [
28]. Our results compared favorably with a similar study in Iran, where both the vaccine titer and VVM met the minimum requirements after storage at +2-8 °C for 60 days [
23]. Notably, not all the vials of the vaccines stored in this condition retain the same potency after six months of storage, especially when the temperature was beyond 8 °C for longer duration. The refrigerator temperatures > 8 °C observed in this study were consistent with previous studies in different parts of the world [
1,
28]. The major reason for this observation was attributed to frequent opening of the refrigerator, especially if it is designated exclusively for vaccine storage. The log-rank test confirms that a 2 °C to 10 °C temperature change could affect the time to vaccine potency loss. In this study, the F-statistic of 12.45 (df = 5, 45, p < 0.001) demonstrated a significant linear decline of vaccine potency over six months. To quantify the rate of potency, decline per time point, the refrigerator demonstrated a steady monthly decrease with a slope of –0.25 log units per month.
Vaccines stored at room temperature (27–29 °C, four weeks) had a moderate loss in potency (–2.2), with final titers similar to the incubator group but achieved after four weeks. This indicates that while room temperature is less damaging than 35–36 °C, potency still declined steadily, highlighting the risks of prolonged storage outside cold chains. In Nigeria, the storage of bOPV in primary/community health centers in the rural districts or remote areas during campaigns may be a challenge. This is because these environments may lack an electrical power supply or an alternative source of energy for the maintenance of the cold chain, as previously reported in India [
29,
30]. These environments may experience power failures for hours, days, or weeks, and sustaining the recommended storage conditions of the bOPV in such conditions could be very challenging. The findings of this study have demonstrated that bOPV potency remains stable at 27-29 °C for three weeks, in contrast to a report that bOPV was only stable at 22-25 °C for less than 10 days [
23]. Majorly, the discrepancy in both studies could be attributed to the value used in defining a potent vaccine. In the previous study, ≥10
6 CCID50/0.1m [
23] was the minimal protective titer, while this study used 10
5.8 CCID50/0.1m +/- 0.5. Incidentally, the defined vaccine titer and the degradation of the VVM did not align in the previous study because at 21 days, the titer of the vaccine was reported as 10
5.67 CCID50/0.1 mL while the VVM rate was 30%. In this study, the average titer of 10
5.46 CCID50/0.1mL was obtained after storage at 27-29 °C for 21 days, and the VVM was rated 40%. Overall, moderate reductions (2.2) with overlapping confidence intervals and a slightly slower weekly decline (–0.19 log units) over four weeks (p< 0.001) were observed at room temperature.
The vaccine samples (Batch 1 and Batch 2), placed in the incubator (35–36 °C) experienced markedly rapid reductions over just three weeks. Batch 1, which started at 6.5, dropped to a final mean of 4.2, while Batch 2, with a higher starting titer (7.4 ± 0.2) showed the steepest decline (–3.2), ending also at 4.2 ± 0.4 after three weeks of storage. Although the starting potency varies across batches, sustained high-temperature exposure could accelerate degradation significantly and erode stability faster than other conditions. Overall, both batches of bOPV were stable under these storage conditions for one week, in contrast to a report of only five days [
23]. The discrepancy between the studies could be attributed to the value considered as the defined vaccine titer [
23]. If our minimum protective titer was used in the previous study, the vaccines would have been reported stable at these temperatures for a week. Additionally, after one week of storage in the incubator, the degradation of the VVM was rated 30% in the previous study, indicating that the vaccine was stable and could be used. Although 8 (40%) of 20 vials retained the minimum protective titers that aligned with the VVM rating, it is advisable to restrict the use of the vaccine in this storage condition to 7 days.
Finally, Atmospheric conditions, characterized by fluctuating temperatures (22–44 °C), produced a rapid and substantial potency reduction (–2.22) within just one week. The associated effect size (d = 5.81) was comparable to incubator exposure, demonstrating that fluctuating heat stress destabilizes vaccine potency almost immediately. The wide fluctuations in daily temperature likely exacerbated instability, causing vaccines to lose viability almost immediately after exposure. Our observation corroborated a previous study [
31] which reported the potency stability of mOPV1 after exposure to the atmospheric temperature of 47.1 °C for 96.9 hours. Although we did not test the potency of vaccine samples stored at this temperature on an daily basis, the VVM degradation after 72 hours (three days) was still within the acceptable stage. Nevertheless, the ten vials of vaccines stored at this temperature changed to 100% degradation from day four of storage. Thus, the potency of bOPV can be retained for a few hours of exposure at ambient temperatures of 44
oC
This study has established the fact that storage condition and duration have a decisive effect on vaccine potency. Therefore, potency decline was not random but strongly associated with the type of storage condition and time of exposure. Room temperature storage, while inferior to refrigeration, showed greater stability than both atmospheric and incubator storage, making it a comparatively better alternative when refrigeration is not feasible. The analysis confirms a clear hierarchy of stability, with refrigeration offering the strongest protection against potency loss, room temperature providing moderate stability, atmospheric and incubator Batch 1 performing poorly, and incubator Batch 2 being the most detrimental.
The correlation and regression analysis showed a strong positive relationship between storage temperature and vaccine potency reduction. Pearson’s correlation coefficient (r = 0.78, 95% CI [0.62, 0.89], p < 0.001) indicated that higher temperatures were strongly associated with greater reductions in potency. The simple linear regression model further quantified this relationship with the slope coefficient (β = 0.25, 95% CI [0.18, 0.32], p < 0.001), suggesting that for every 1 °C increase in storage temperature, the vaccine loses approximately 0.25 log units of potency. According to this model, 61% of the variability in potency reduction (R² = 0.61), demonstrating that temperature is a major determinant of vaccine stability. The intercept is not statistically significant (β = –0.45, p = 0.24), indicating that predicted potency reduction at zero temperature is not meaningful in this context.
Furthermore, the logistic regression analysis revealed that, the odds of potency being maintained at the refrigerator temperature are high (OR = 7.46, 95% CI: 1.75–31.9, p = 0.007). All other storage conditions in this study significantly reduced the odds of potency preservation. Incubator Batch 1 decreased the odds by 84% (OR = 0.16, 95% CI: 0.05–0.52, p = 0.004), while incubator Batch 2 had the greatest negative effect, reducing the odds by 94% (OR = 0.06, 95% CI: 0.02–0.23, p < 0.001). Room temperature storage also significantly decreased the odds by 86% (OR = 0.14, 95% CI: 0.04–0.48, p = 0.001), and atmospheric conditions reduced the odds by 88% (OR = 0.12, 95% CI: 0.03–0.46, p = 0.002). These findings confirm that refrigeration provides the highest probability of maintaining vaccine potency for a longer period, while all other tested conditions substantially compromised potency stability. The limitations of the study lie in our inability to provide an hourly titre of the vaccine after exposure. The storage conditions used may not reflect the full range of temperature fluctuations experienced during real-life storage (e.g, room temperatures are subjective). Also, the VVM color change, which measures the cumulative heat exposure, may not correlate with the actual viral titer, allowing subtle losses of vaccine titer before it attains 100% degradation. Environmental factors such as humidity and light exposure were not strictly controlled, and these may have affected vaccine potency at the storage conditions studied. Possible inaccuracies in temperature monitoring devices or titration methods could introduce minor errors in the vaccine potency.
Abbreviations
OPV: Oral Polio vaccine, bOPV: bivalent Oral Polio vaccine (serotype 1 and 3), tOPV: trivalent oral Polio vaccine ( serotype 1,2,3), VVM: vaccine vial monitor, VPD: vaccine preventable diseases, WHO: World Health Organization, GPEI: Global Polio Eradication Initiative, EPU: Epidemiological Unit, cVDPV: circulating vaccine-derived poliovirus, AFP: Acute flaccid paralysis, MMC: Maiduguri Metropolitan Council, LGA: Local government area, OBR: outbreak response, NIBSC: National Institute of Biological Standards, UK: United Kingdom, LVS: Local virus stock, GM: Growth medium, MEM: Minimum essential medium, L20B: a mouse cell line genetically engineered to express the human poliovirus receptor CD155m, CC: Cell culture control, CCID50: Cell culture infectious dose 50, PV: Poliovirus, CPE: Cytopathic effect, IOR: Interquantile rangers, OR: odd ratio.