1. Introduction
Type-1 diabetes mellitus (T1D) and autoimmune thyroid disease (ATD) frequently coexist as polyglandular syndrome type 3 variant (APS3v), sharing a pathogenic basis in autoreactive T-cell–mediated destruction of endocrine cells [
1,
2]. The worldwide incidence of T1D, especially in children < 5 years, is rising and is often accompanied by ATD, underscoring the need for therapies that preserve residual β-cells and thyrocytes [
3]. The substitutive administration of the deficient hormones, i.e. insulin and levo-thyroxine (L-T4), is the standard treatment that, however, does not halt the autoimmune process and does not rescue the residual hormone producing cells [
4]. Identification of innovative therapeutic interventions, especially aimed to preserve the residual cells, is of crucial importance in the expectation of quality of life in pediatric patients [
5].
Recently, the potential pathophysiological role played in several autoimmune conditions, including T1D and APS3v by the
PTPN22) C1858T mutation [
6] has been demonstrated.
PTPN22 encodes the protein tyrosine-protein phosphatase non-receptor type 22 (also named lymphoid phosphatase, Lyp), which is a negative regulator of T cell antigen receptor (TCR) signaling, acting in concert with C-Src kinase. In Lyp, the C1858T variant produces the replacement of arginine 620 with a tryptophan residue (R620W), which leads to a gain of function with paradoxical reduced T cell activation [
7].
The variant LypR620W has effect on both innate and adaptive immune responses. In the light of this, may be a valid drug target for the treatment of T1D and APS3v patients. We therefore implemented a novel immunotherapy based on the use of lipoplexes delivering siRNA that cause variant allele selective inhibition instead of complete gene knockdown [
8,
9,
10,
11,
12]. We already demonstrated efficacy and tolerability of lipoplexes in peripheral blood mononuclear cells (PBMC) of T1D patients harbouring the variant
PTPN22. Furthermore, we developed strategies of functionalization of lipoplexes to improve selective delivery to specific immunocytes in particular, the possibility to expose Fab of monoclonal antibodies [
12] to achieve specific silencing in B lymphocytes and cytotoxic T cells. An alternative strategy of functionalization to target several immunocytes in the peripheral blood was exploited using high affinity Siglec 10 sialoside mimetic (SAM, PEG-lipid-F9) [
12,
13,
14]. The Siglec family of sialic-acid binding proteins is primarily expressed on cells of the immune system, which mediate innate and adaptive responses [
14] with function in the discrimination of self and non-self. Thus, these endocytic receptors became attractive immunotherapeutic targets [
14]. We proved internalization and low toxicity of Lipo-siRNAR620W-PEGF9 (LiposiRNA-PEGF9) in PBMC and their efficacy in halting variant mRNA expression, as well as their functional efficacy by rescuing IL-2 secretion in PBMC of C1858T
PTPN22 T1D patients.
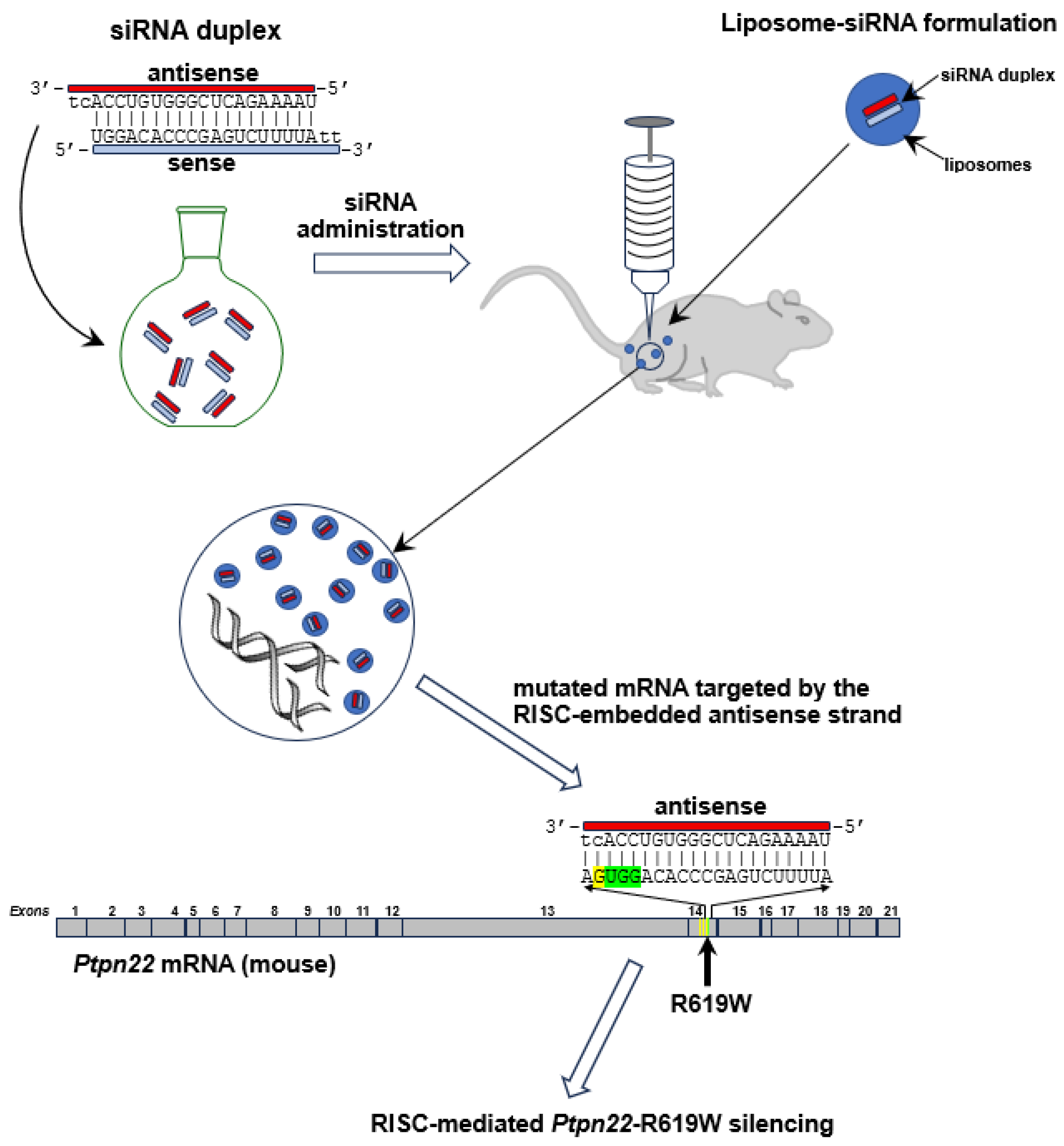
In view of the prospective use of lipoplexes as immunotherapy of T1D and APS3v, a fundamental step forward is to conduct preclinical studies in animal models. To this extend, according to international regulations, biodistribution of lipoplexes and their functional derivatives has to be assessed by injection of fluorescently labelled compounds in murine strains such as C57BL/6 mice or BALB/c, commonly used for this kind of evaluation [
15]. Furthermore, safety and efficacy in delaying or halting the development of disease have to be ascertained in an animal model of disease. As regard the NOD/ShiLtJ mouse strain (non-obese diabetic mouse), being polygenic for the development of autoimmune type 1 diabetes, is genetically predisposed to the development of the disease and most closely mimics the human model of type 1 diabetes [
16,
17,
18]. The NOD/ShiLtJ mouse thus appears to be the ideal model for preclinical studies to further investigate the influence of the R620W gene variant that causes the human disease, and to evaluate the efficacy of the novel pharmacological treatment. In particular, phenotyping of the NOD model confirmed a 90% incidence of diabetic females by 30 weeks of age. The ideal NOD model carrying the R619W-
Ptpn22 variant, equivalent to the human R620W, was generated using CRISPR-Cas9 technology [
19]. This transgenic animal exhibits higher levels of anti-insulin antibodies, an earlier onset of the disease, and a higher prevalence in females.
In unravelling the feasibility of undertaking the proposed line of experimentation to ascertain efficacy and safety of lipoplexes in the NOD transgenic mouse, we preliminarily aimed to effectively demonstrate the possibility of inhibiting the murine variant in vitro by using lipoplexes carrying respective siRNA duplexes in the R619W transfected L929 murine fibroblast cell line.
2. Results
2.1. Transfection of the R619W-Ptpn22 Variant in the L929 Mouse Fibroblast Cell Line
The mouse L929 cell line (ATCC® CCL-1™, American Type Culture Collection, Manassas, VA, USA) was transfected with the PF62-pLentiPtpn22-R619W plasmid (Aurogene S.r.l., Rome, Italy).
The quantitative Real-Time PCR (qRT-PCR) results of transfection with the PF62-pLenti
Ptpn22-R619W plasmid
versus empty vector (EV) are shown in the table below (
Table 1).
2.2. siRNAs Are Delivered in R619W-L929 Cell Line by a Commercial Transfection System and Efficiently Block the Variant Ptpn22 Expression
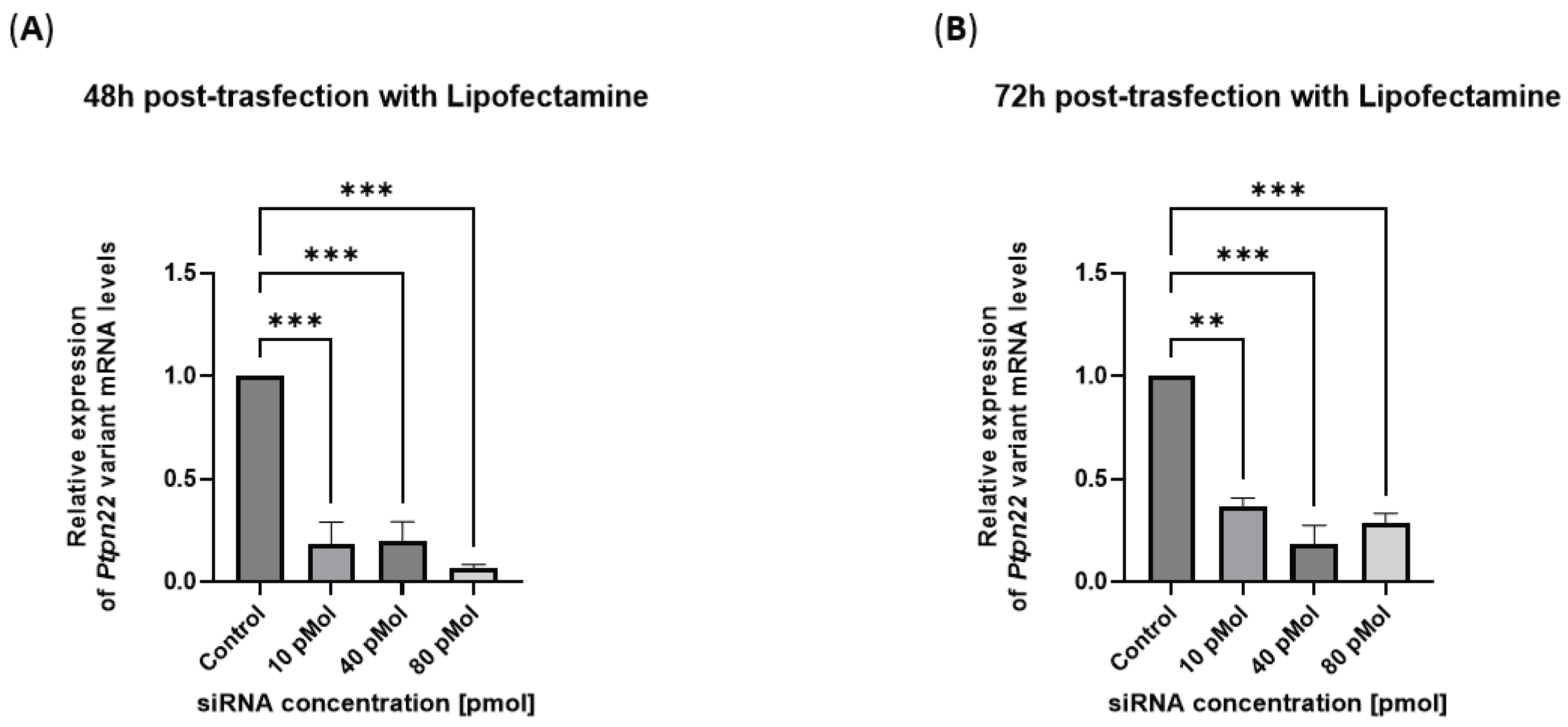
In the experiment performed using the transfection kit to vehicle siRNA sense/antisense (s/a) duplex with Lipofectamine (Lipofectamine™ RNAiMAX, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), having higher affinity for the target mRNA sequence, after 48 hours (h) of transfection we observed a maximal inhibition of R619W mRNA in the range between 10 and 80 pmols of siRNA in respect to control untreated cells. After 72 hours from the beginning of the transfection, a higher inhibition persisted at 40 pmol concentration of siRNA duplex (
Figure 1 A and B).
These results indicate the specificity of the siRNA molecule chosen for the subsequent experiments of lipoplexes variant inhibition reported below (vide infra).
2.3. Efficient Silencing of Variant Ptpn22 in R619W-L929 Cells by siRNA Delivery Using Lipoplexes
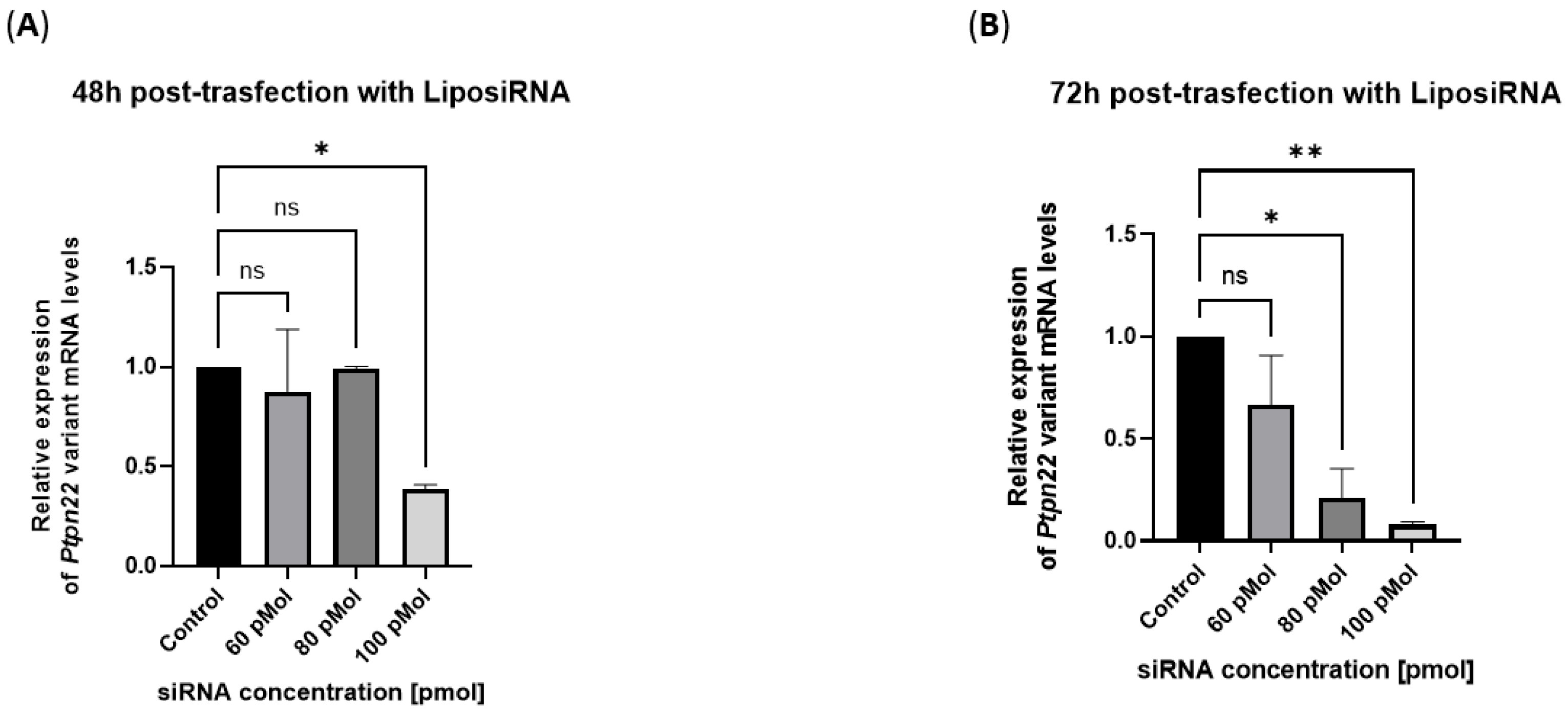
Lipoplexes efficiently block the variant Ptpn22 expression in R619W transfected L929 cells.
Within the range of siRNA doses between 60 and 100 pmols, the highest inhibition of variant mRNA was obtained with 100 pmol of siRNA after 48 hours of transfection (
Figure 2A). The efficiency increased after 72 hours from the beginning of transfection and was shown even at the lower doses of 60 and 80 pmols of siRNA (
Figure 2B).
3. Discussion
According to regulatory authorities in Europe (EMA, European Medicinal Agency) and US (FDA, Food and Drug Administration), interpretation of results from animal studies is necessary to support future clinical application of novel therapeutic modalities. Indeed, preclinical safety and efficacy assessment of new medicinal products ensures that benefits will outweigh risks associated with their use [
15].
Nevertheless, the main challenge in developing proof of concept for advancing to a clinical trial is, in most circumstances, the lack of appropriate animal models with similar functional characteristics to the intended human disorder.
A current line of investigation in our laboratory is to exploit the feasibility of the novel immunotherapy for T1D and APS3v based on the use of lipoplexes targeting the C1858T
PTPN22 variant [
9,
10,
11,
12]. In the light of the arguments raised (
vide supra), preclinical assessment is requested at this stage of the investigation to ascertain the safety and efficacy of lipoplexes in the ideal NOD mice model made transgenic for the variant R619W-
Ptpn22 through CRISPR-Cas9 technology [
19]. To this aim, it was necessary to have in preliminary settings confirmation of the possibility to block the variant R619W mRNA of
Ptpn22 in the transgenic L929 fibroblast cell line with lipoplexes carrying the appropriate siRNA duplex. The murine line was transfected with the PF62-pLenti
Ptpn22-R619W plasmid vector, commercially available. Lipoplexes carrying siRNA duplexes against the variant efficiently reduced the R619W variant
Ptpn22 expression as revealed in qRT-PCR, although with less efficacy than with the standard Lipofectamine system used as control. Indeed, lipoplexes produced a significant effect of inhibition of variant mRNA expression at higher doses (80-100 pmols of siRNA concentration) and with a longer time interval for internalization than with the Lipofectamine control of transfection.
These results encourage to proceed to assessment through animal experimentation in vivo of lipoplexes, whose results will possibly allow future advancement to Phase I/II clinical trials in adults of T1D immunotherapy that are necessary before a pediatric investigation plan can be implemented.
4. Materials and Methods
4.1. siRNA Design
Authentic siRNA sequences were designed (Design ID: ABHSP52) to specifically target the C1858T Ptpn22 murine gene variant (Life Technologies, Thermo Fisher, Milan).
4.2. Liposome/Lipoplexes Preparation
Phospholipids [1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), N-[1-(2,3-Dioleoyloxy) propyl]-N, N, N-trimethylammonium chloride (DOTAP), 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [maleimide (polyethylene glycol)-2000] (ammonium salt) (DSPE-PEG2000-Maleimide)] were purchased from Avanti Polar Lipids (Alabaster, AL). Liposomal solutions were prepared in 100 mM phosphate buffer (PBS, Euroclone, Milan, Italy) at pH 7.4. DOTAP/DOPE/DSPEPEG2000-Maleimeide (47.5/47.5/5 molar ratio) liposomes were prepared by the lipid film method as previously described [
11]. Briefly, phospholipids, previously dissolved in chloroform, were mixed and the organic solvent removed under a stream of nitrogen gas to obtain a homogeneous film. Then, the film was hydrated with 1.0 mL of 100 mM PBS (pH = 7.4) in order to obtain a concentration of 800 μM. The suspension was sonicated for 30 minutes (min) and successively extruded 10 times at room temperature (RT), using a thermo barrel extruder system (Northern Lipids Inc, Vancouver, BC, Canada) under nitrogen through a polycarbonate membrane (Nucleopore Track Membrane 25 mm, Whatman, Brentford, UK) having 0.1 μm pore size. 500 μL of a siRNA solution (5.2 μM in water) was added to an equivalent volume of liposomes and the resulting mixture was stirred at RT for 3 h.
Lipoplexes were prepared by incubating liposomal solution with siRNA at RT for 3 h. In this preparation, equal volumes of liposomes and siRNA solutions were used. The siRNA concentration in the starting solution was 2.6 μM.
Physico-chemical characterization of lipoplexes was conducted according to previously established protocols [
12](
Figure 3).
4.3. Generation of R619W Transfected L929 Murine Fibroblast Cell Line
The L929 mouse line was transfected with the R619W mouse variant using PF62-pLentiPtpn22-R619W plasmid available from Aurogene S.r.l. (Rome).
4.4. L929 Cell Culture
R619W-Ptpn22 transfected L929 cells (ATCC® CCL-1™, American Type Culture Collection, Manassas, VA, USA) were cultured at 2x105 cells/mL density in T75 flasks (Falcon Labware Becton Dickinson (BD) Biosciences, Oxnard, CA). Cells were maintained in complete Dulbecco’s Modified Eagle Medium high glucose medium (DMEM HG, Euroclone, Milan, Italy) supplemented with 10% FBS (Hyclone, South Logan, UT), L-glutamine (2 mM, Euroclone, Milan, Italy) and 1% penicillin/streptomycin (pen/strep) (Euroclone), incubated at 37˚C in a humidified atmosphere containing 5% CO2 and sub-cultured twice per week at the same seeding density.
4.5. Mutant Ptpn22 Gene Silencing in Transfected L929 Fibroblast Line (R619W-L929)
4.5.1. siRNA Transfection with Commercial Transfection System
Selected siRNA sense/antisense (s/a) duplexes (R619W-Forward: 5’-TCCCCTCCGAATAGTGCTGA-3’ - R619W-Reverse: 5’-CATTCAGGGAGTGGCGG-3’) with R619W mRNA target affinity, were first validated by testing their ability of silencing L929 cells by using a commercial transfection system (Lipofectamine™ RNAiMAX, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) and observing the resulting decrease of mRNA levels on qRT-PCR.
Briefly on day 1, L929 cells were harvested from T75 culture flasks using trypsin/EDTA solution (Euroclone), washed in PBS (Euroclone) and seeded at 1.5 x105 per well in 12-well plates (Falcon, Corning Incorporated, Corning NY) in 1 mL of DMEM HG (Euroclone) supplemented only with 1% pen/strep and L-glutamine (2 mM) and incubated for 24 hours at 37˚C in a humidified atmosphere containing 5% CO2.
On day 2, the culture medium was removed, cells washed with PBS (Euroclone), then treated with different concentrations of R619W-Ptpn22 siRNA duplex (10, 40, 60, 80 pmols of siRNA) in transfection medium (Gibco, Thermo Fisher Scientific) and incubated overnight (O/N) at 37˚C in humidified atmosphere containing 5% CO2. For control of siRNA internalization efficiency cells were incubated with the fluorescent (Alexa Fluor 555-conjugated) siRNA provided by the manufacturer.
On day 3, the O/N incubation was stopped by adding complete DMEM HG medium (Euroclone) supplemented with 20% FBS and 2% pen/strep. The control group of cells treated with the fluorescent siRNA was harvested and analyzed by flow-cytometry on FACS Canto II instrument (Becton and Dickinson, Sunnyvale, CA) and PC FACS Diva software (BD Biosciences, San Jose, CA).
On day 4, after 48 hours, and day 5, after 72 hours from the beginning of each transfection with different concentrations of R619W-Ptpn22 siRNA duplex, the experimental sets of cells were harvested, washed with PBS and centrifuged at 500 x g for 5 minutes. Each experimental condition included at least two independent determinations. Cells were pelleted and lysed in RLT buffer (Qiagen, Hilden, Germania) for subsequent RNA extraction.
4.5.2. Custom Liposome Transfection Protocol for R619W-L929 Cells
On day 1, L929 cells were harvested from T75 culture flasks using trypsin/EDTA solution (Euroclone) and cultured at 0.7x105 cells per well in 24-well plates (Falcon) in 500 μL of DMEM HG (Euroclone) supplemented with 10% FBS, 1% pen/strep and L-glutamine (2 mM) for 24 hours at 37˚C in a humidified atmosphere containing 5% CO2.
On day 2, cells were washed in PBS (Euroclone) and cultured with the same FBS and pen/strep free medium in a final volume of 500 μL, this last one containing different amount (60, 80, 100 pmols) of siRNA duplex against the Ptpn22 gene variant (vide supra) complexed with liposomes (lipoplexes). Cells were incubated O/N with the complexes at 37˚C in humidified atmosphere containing 5% CO2. Appropriate controls were set up by growing cells with complete DMEM HG (Euroclone) medium alone. Each experimental condition included at least three independent determinations (triplicates). At the end of the incubation period, cells were harvested from cell culture plates, washed in PBS by centrifugation at 500 x g for 5 minutes and cultured in complete DMEM HG (Euroclone) at the final volume of 1 mL.
On day 4, after 48 hours, and on day 5, after 72 hours from the beginning of each transfection, cells were washed in PBS by centrifugation at 500 x g for 5 minutes and then lysed in RLT buffer (Qiagen) for subsequent RNA extraction.
4.5.3. qRT-PCR
Relative gene expression was analyzed using quantitative Real-Time PCR (qRT-PCR).
Cells were lysed in RLT-Buffer at 48- and 72-hours post-treatment. After RNA extraction (RNeasy Plus Mini Kit, Qiagen), quantification was performed following the manufacturer’s instructions by spectrophotometry (Nanodrop), followed by reverse transcription (SuperScript™ IV First-Strand Synthesis System) and qRT-PCR amplification (PowerUp™ SYBR™ Green Master Mix) using the following primers to amplify the murine R619W-Ptpn22 variant:
Murine GAPDH was used as the internal housekeeping control. The following primers were used:
Relative expression levels were calculated using the 2–ΔΔCt method.
4.6. Statistical Analysis
Statistical analyses were conducted using GraphPad Prism 10.0 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA), followed by Šidák’s post hoc test for multiple comparisons, was employed to determine significant differences among groups.
5. Conclusions
Allele-specific inhibition via siRNA-containing lipoplexes represents a promising strategy for selectively silencing the PTPN22 genetic mutation C1858T in immunocytes implicated in the pathogenesis of APS3v/T1D.
In vitro results shown in this manuscript confirm the possibility of targeting the pathogenic (R619W-Ptpn22) allele in the CRISPR-modified NOD/ShiLtJ mice, which harbors the variant, paving the way for preclinical translation. These findings provide a solid basis for future biodistribution, pharmacokinetic, and efficacy studies as requested by regulatory authorities in Europe before undertaking clinical investigations.
6. Patents
Italian Patent 102018000005182 issued on 26.05.2020; PCT/IT2019/050095 filed on 8.05.2019 extended in Europe, USA and Hong Kong, issued in USA July 2025. Inventor: Alessandra Fierabracci MD PhD.
Author Contributions
Conceptualization, A.F.; methodology, AF, I.M., A.A. and C.D.; software, I.M. and E.B.; validation, A.A. and A.F.; formal analysis, I.M., E.B. and A.F.; investigation, I.M., A.A., L.F., C.D. and A.F.; resources, A.A.; data curation, I.M, L.F. and A.A.; writing—original draft preparation, I.M., A.A., and A.F.; writing—review and editing, I.M., C.D. and A.F.; visualization, E.B.; supervision, A.F.; project administration, A.F.; funding acquisition, A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health with funding of Ricerca Finalizzata Grant RF-2019-12369889 to Alessandra Fierabracci and current research founds.
Institutional Review Board Statement
not applicable
Informed Consent Statement
not applicable
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Acknowledgments
We acknowledge Anna Lo Russo and Alessia Palma for technical assistance in cell culture and molecular biology experiments. Carlo Diaferia acknowledge the grant CN00000041 “National Center for Gene Therapy and Drugs based on RNA Technology” (concession number 1035 of 17 June 2022-PNRR MUR - M4C2 - Investment 1.4 Call "National Centers”, financed by EU- NextGenerationEU), project code MUR:CN00000041–CUP UNINA: E63C22000940007.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Ptpn22 |
Protein tyrosine phosphatase N22 |
| Lyp |
Lymphoid phosphatase |
| NOD |
Non-Obese Diabetic mice |
| siRNA |
Small interfering RNA |
| T1D |
Type 1 Diabetes |
| ATD |
Autoimmune Thyroid Disease |
| Ct |
Cycle Threshold |
| APS3v |
Autoimmune Polyglandular Syndrome type 3 variant |
| L-T4 |
Levotiroxine |
| Arg |
Arginine |
| Trp |
Tryptophan |
| SAM |
Siglec 10 sialoside mimetic |
| CRISPR- Cas9 |
Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) associated protein 9 (Cas9) |
| RISC |
RNA-Induced Silencing Complex |
| s/a |
sense/antisense |
| EMA |
European Medicinal Agency |
| FDA |
Food and Drug Administration |
| DOPE |
Phospholipids [1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOTAP |
N-[1-(2,3-Dioleoyloxy) propyl]-N, N, N-trimethylammonium chloride |
| DSPE-PEG2000-Maleimide |
1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N- [maleimide (polyethylene glycol)-2000] (ammonium salt) |
| Min. |
Minutes |
| ATCC |
American Type Culture Collection |
| DMEM HG |
Dulbecco’s Modified Eagle Medium High Glucose |
References
- Redondo, M.J.; Morgan, N.G. Heterogeneity and endotypes in type 1 diabetes mellitus. Nat Rev Endocrinol 2023, 9, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Dittmar, M.; Kahaly, G.J. Genetics of the autoimmune polyglandular syndrome type 3 variant. Thyroid 2010, 20, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. ; Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol 2020, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Rapini, N.; Schiaffini, R.; Fierabracci, A. Immunotherapeutic Strategies for the Prevention and Treatment of Distinct Stages of Type 1 Diabetes: An Overview. Int J Mol Sci 2020, 21, 2103. [Google Scholar] [CrossRef] [PubMed]
- Woittiez, N.J.C.; Roep, B.O. Impact of disease heterogeneity on treatment efficacy of immunotherapy in Type 1 diabetes: different shades of gray. Immunotherapy 2015, 7, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Gianchecchi, E.; Palombi, M.; Fierabracci, A. The putative role of the C1858T polymorphism of protein tyrosine phosphatase PTPN22 gene in autoimmunity. Autoimmun Rev 2013, 12, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Vang, T.; Congia, M.; Macis, M.D.; Musumeci, L.; Orrú, V.; Zavattari, P.; Nika, K.; Tautz, L.; Taskén, K.; Cucca, F.; et al. Autoimmune-associated lymphoid tyrosine phosphatase is a gain-of-function variant. Nat Genet 2005, 37, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Bottini, N.; Musumeci, L.; Alonso, A.; Rahmouni, S.; Nika, K.; Rostamkhani, M.; MacMurray, J.; Meloni, G.F.; Lucarelli, P.; Pellacchia, M.; et al. A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 2004, 36, 337–338. [Google Scholar] [CrossRef] [PubMed]
- Perri, V.; Pellegrino, M.; Ceccacci, F.; Scipioni, A.; Petrini, S.; Gianchecchi, E.; Lo Russo, A.; De Santis, S.; Mancini, G.; Fierabracci, A. Use of short interfering RNA delivered by cationic liposomes to enable efficient down-regulation of PTPN22 gene in human T lymphocytes. PLoS One 2017, 12, e0175784. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, M.; Ceccacci, F.; Petrini, S.; Scipioni, A.; De Santis, S.; Cappa, M.; Mancini, G.; Fierabracci, A. Exploiting novel tailored immunotherapies of type 1 diabetes: Short interfering RNA delivered by cationic liposomes enables efficient down-regulation of variant PTPN22 gene in T lymphocytes. Nanomedicine 2019, 18, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Belcastro, E.; Accardo, A.; Sandomenico, A.; Pagliarosi, O.; Rosa, E.; Petrini, S.; Conti, L.A.; Giorda, E.; Corsetti, T.; et al. Preparation and In Vitro Evaluation of RITUXfab-Decorated Lipoplexes to Improve Delivery of siRNA Targeting C1858T PTPN22 Variant in B Lymphocytes. I J Mol Sci 2021, 23, 408. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Belcastro, E.; Ceccacci, F.; Petrini, S.; Conti, L.A.; Pagliarosi, O.; Giorda, E.; Sennato, S.; Schiaffini, R.; Wang, P.; et al. Improvement of Lipoplexes With a Sialic Acid Mimetic to Target the C1858T PTPN22 Variant for Immunotherapy in Endocrine Autoimmunity. Front Immunol 2022, 13, 838331. [Google Scholar] [CrossRef] [PubMed]
- Büll, C.; Heise, T.; Adema, G.J.; Boltje, T.J. Sialic Acid Mimetics to Target the Sialic Acid-Siglec Axis. Trends Biochem Sci 2016, 41, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Rillahan, C.D.; Schwartz, E.; McBride, R.; Fokin, V.V.; Paulson, J.C. Click and pick: Identification of sialoside analogues for siglec-based cell targeting. Angew Chem Int Ed Engl 2012, 51, 11014–11018. [Google Scholar] [CrossRef] [PubMed]
- Cavagnaro, J.; Silva Lima, B. Regulatory acceptance of animal models of disease to support clinical trials of medicines and advanced therapy medicinal products. Eur J Pharmacol 2015, 759, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Bernard, N.F.; Ertug, F.; Margolese, H. High incidence of thyroiditis and anti-thyroid autoantibodies in NOD mice. Diabetes 1992, 41, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Beales, P. E.; Castri, F.; Valiant, A.; Rosignoli, G.; Buckley, L.; Pozzilli, P. Adrenalitis in the non-obese diabetic mouse. Autoimmunity 2002, 35, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Thayer, T.C.; Wen, L.; Wong, F.S. Mouse Models of Autoimmune Diabetes: The Nonobese Diabetic (NOD) Mouse. Methods Mol Biol 2020, 2128, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Pelletier, S.; Gingras, S.; Rigaud, S.; Maine, C.J.; Marquardt, K.; Dai, Y.D. , Sauer, K.; Rodriguez, A.R.; Martin, G. et al. CRISPR-Cas9-Mediated Modification of the NOD Mouse Genome With Ptpn22R619W Mutation Increases Autoimmune Diabetes. Diabetes 2016, 65, 2134–2138. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).