Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
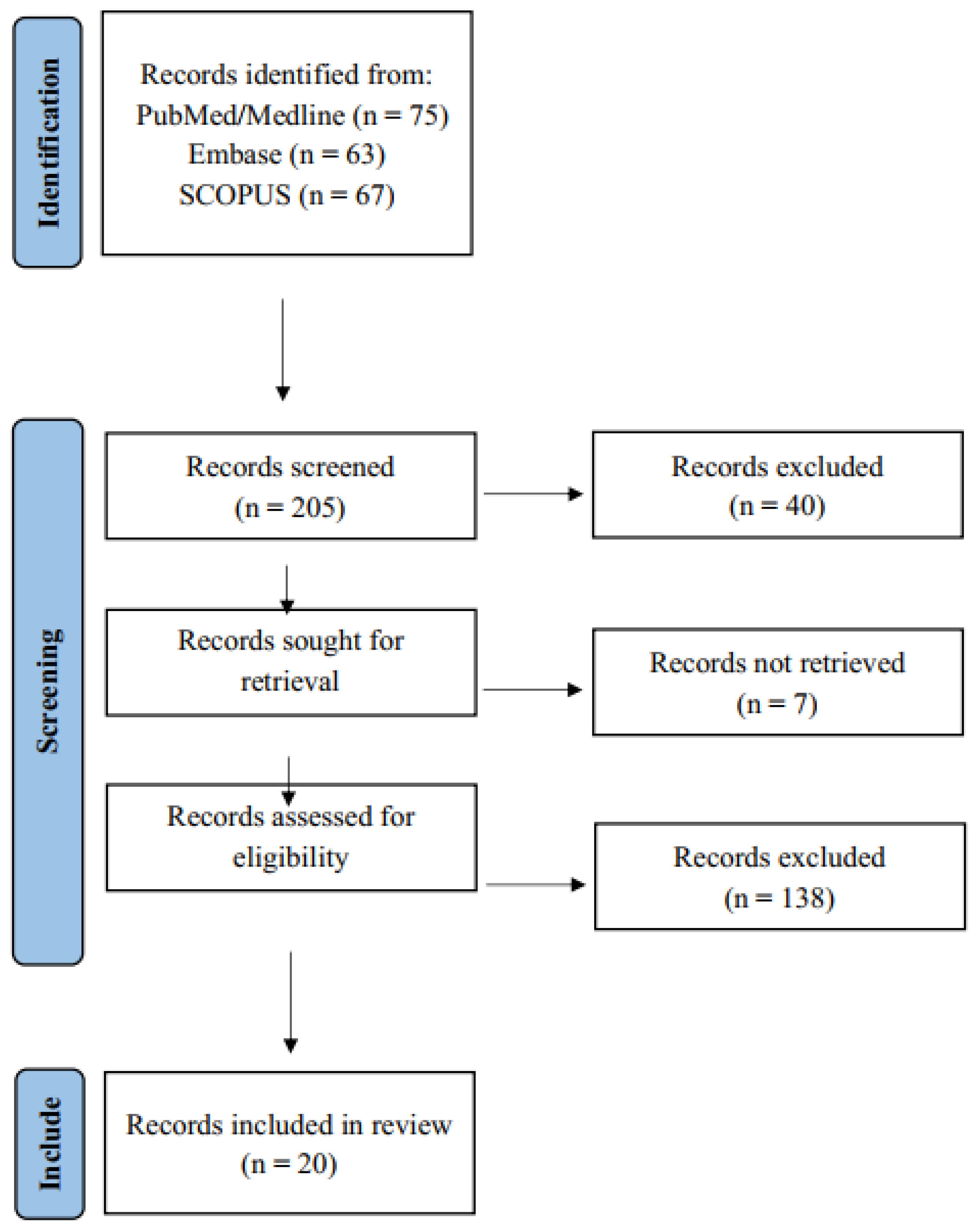
2.1. Search Strategy
2.2. Article Selection
2.1.1. Inclusion Criteria
- Topical Relevance: Articles were included based on a review of their title and abstract, provided their primary focus was CVR in pregnant women, ensuring their relevance to the objective of this review.
- Study Design: Priority was given to original research studies (e.g., cohort studies), as well as systematic and narrative reviews that addressed the topic from clinical, epidemiological, or preventive medicine perspectives.
- Target Population: Studies focusing exclusively on pregnant women were included. Studies involving mixed populations (e.g., men or non-pregnant women) were included only if results for pregnant women were reported separately.
- Language: Only articles published in English or Spanish were selected to ensure complete comprehension and rigorous analysis.
- Publication Date: Articles published between 2019 and 2024 were included to ensure the current relevance of the studies.
2.1.2. Exclusion Criteria
- Lack of Thematic Relevance: Articles that, although mentioning CVR and pregnancy, focused on unrelated topics such as oncology, autoimmune diseases, or other conditions outside of pregnancy were excluded.
- Limited Population Representativeness: Studies with small sample sizes (under 60 participants) or those focused on particular geographic regions or ethnic groups were excluded if their results were not generalizable to the broader context of CVR in pregnant women.
- Low Methodological Quality or Inadequate Design: Editorials, letters to the editor, conference abstracts, and case reports were excluded, as were articles lacking structural integrity or presenting incomplete data.
- After applying these criteria, the final selection included n = 20 articles for inclusion in this review.
- General Considerations of CVR during Pregnancy. This category encompasses publications that address foundational concepts related to CVR during gestation, including epidemiological data and the conceptual underpinnings of CVR in pregnant populations.
- Pregnancy Complications and CVR. This group includes studies examining pregnancy-specific complications, such as preeclampsia and gestational diabetes mellitus, and their association with both immediate and long-term cardiovascular outcomes.
- Genetics and Molecular Biology of CVR Factors in Pregnancy. This thematic area encompasses investigations into the genetic, molecular, and pathophysiological mechanisms underlying CVR during pregnancy, providing a biomedical perspective to the broader clinical understanding.
- Clinical Management of CVR during Pregnancy. This section comprises literature addressing strategies for preventing, diagnosing, and managing CVR in pregnant individuals, with an emphasis on evidence-based guidelines and implications for obstetric care.
2.4. Construction of Summary Tables: Original Research Articles
- Author and Year of Publication: For citation and identification purposes.
- Study Design: Type of research conducted (e.g., cohort, cross-sectional, case-control).
- Inclusion and Exclusion Criteria: The Criteria used to define the study population are relevant for assessing external validity.
- Gestational Period Analyzed: Specific trimester(s) or pregnancy stage(s) addressed.
- Study Objective and Primary Variables: The main research aim and the variables employed to evaluate it.
- Principal Findings: Primary results, emphasizing outcomes related to CVR.
- Limitations: The authors reported methodological limitations or identified them during critical appraisal, which aids in assessing study quality and internal validity.
2.5. Construction of Summary Tables: Review Articles
- Author and Year of Publication, Study Design, Gestational Period, Primary Objective, Key Findings, and Limitations: As in the original studies, these fields ensure consistent reporting and comparability.
- Main Focus: Summary of the central topic or hypothesis addressed in the review.
- Number of Included Studies and Time Frame Covered: Total articles reviewed and the temporal scope of the literature search.
- Quality of Evidence: An assessment of the methodological rigor of included studies and the review process, based on criteria such as the Newcastle-Ottawa Scale or other validated quality appraisal tools.
- Secondary Findings: Additional observations or findings beyond the primary objective of the review.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFE | Amniotic fluid embolism |
| APO | Adverse pregnancy outcome |
| BMI | Body mass index |
| BP | Blood pressure |
| CAD | Coronary artery disease |
| CCLM | Clinical Chemistry and Laboratory Medicine |
| CHD | Congenital heart disease |
| CS | Cardiogenic shock |
| CVD | Cardiovascular disease |
| CVR | Cardiovascular risk |
| CVRF | Cardiovascular risk factor |
| CVS | Cardiovascular system |
| ECG | Electrocardiogram |
| ECMO | Extracorporeal membrane oxygenation |
| GDM | Gestational diabetes mellitus |
| HDP | Hypertensive disorders of pregnancy |
| HR | Hazard ratio |
| HTN | Hypertension |
| IABP | Intra-aortic balloon pump |
| ICD | International Classification of Diseases |
| ICU | Intensive care unit |
| IUGR | Intrauterine growth restriction |
| LDL | Low-density lipoprotein |
| LMWH | Low molecular weight heparin |
| LVM | Left ventricular mass |
| MACE | Major adverse cardiovascular events |
| MI | Myocardial infarction |
| NSTEMI | Non–ST-segment elevation myocardial infarction |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| OSF | Open Science Framework |
| PAAD | Pregnancy-associated arterial dissection |
| PE | Preeclampsia |
| PPCM | Peripartum cardiomyopathy |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| qPCR | Quantitative polymerase chain reaction |
| RR | Relative risk |
| SCAI | Society for Cardiovascular Angiography and Interventions |
| SCAD | Spontaneous coronary artery dissection |
| SBP | Systolic blood pressure |
| SMM | Severe maternal morbidity |
| STEMI | ST-segment elevation myocardial infarction |
| ST2/IL-33 | Suppression of tumorigenicity 2 / Interleukin-33 receptor |
| T2DM | Type 2 diabetes mellitus |
References
- Múnera-Echeverri, A.G. Enfermedad Cardíaca y Embarazo. Revista Colombiana de Cardiología 2018, 25, 49–58. [CrossRef]
- Hall, M.E.; George, E.M.; Granger, J.P. El Corazón Durante El Embarazo. Rev Esp Cardiol 2011, 64, 1045–1050. [CrossRef]
- Roos-Hesselink, J.W.; Stein, J.I. Embarazo y Enfermedad Cardiaca. Rev Esp Cardiol 2017, 70, 78–80. [CrossRef]
- Hogan, M.C.; Foreman, K.J.; Naghavi, M.; Ahn, S.Y.; Wang, M.; Makela, S.M.; Lopez, A.D.; Lozano, R.; Murray, C.J. Maternal Mortality for 181 Countries, 1980–2008: A Systematic Analysis of Progress towards Millennium Development Goal 5. The Lancet 2010, 375, 1609–1623. [CrossRef]
- Instituto Nacional de Estadística Estadística de Defunciones Según La Causa de Muerte. Año 2024. Datos Provisionales Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736176780&menu=ultiDatos&idp=1254735573175 (accessed on 29 July 2025).
- Fox, R.; Kitt, J.; Leeson, P.; Aye, C.Y.L.; Lewandowski, A.J. Preeclampsia: Risk Factors, Diagnosis, Management, and the Cardiovascular Impact on the Offspring. Journal of Clinical Medicine 2019, Vol. 8, Page 1625 2019, 8, 1625. [CrossRef]
- Herández-Ruíz, S.; Solano-Ceh, A.; Villarreal-Ríos, E.; Pérez, M.O.C.; Galicia-Rodríguez, L.; Elizarrarás-Rivas, J.; Jiménez-Reyes, O.H. Prevalence of Gestational Diabetes and Gestational Hypertension in Pregnant Women with Pregestational Obesity. Ginecol Obstet Mex 2023, 91, 85–91. [CrossRef]
- Ives, C.W.; Sinkey, R.; Rajapreyar, I.; Tita, A.T.N.; Oparil, S. Preeclampsia—Pathophysiology and Clinical Presentations: JACC State-of-the-Art Review. J Am Coll Cardiol 2020, 76, 1690–1702. [CrossRef]
- Rana, S.; Lemoine, E.; Granger, J.; Karumanchi, S.A. Preeclampsia: Pathophysiology, Challenges, and Perspectives. Circ Res 2019, 124, 1094–1112. [CrossRef]
- Lende, M.; Rijhsinghani, A. Gestational Diabetes: Overview with Emphasis on Medical Management. International Journal of Environmental Research and Public Health 2020, Vol. 17, Page 9573 2020, 17, 9573. [CrossRef]
- Farahi, N.; Oluyadi, F.; Dotson, A.B. Hypertensive Disorders of Pregnancy. Am Fam Physician 2024, 109, 251–260.
- Moutquin, J.M.; Rainville, C.; Giroux, L.; Raynauld, P.; Amyot, G.; Bilodeau, R.; Pelland, N. A Prospective Study of Blood Pressure in Pregnancy: Prediction of Preeclampsia. Am J Obstet Gynecol 1985, 151, 191–196. [CrossRef]
- Sammour, M.B.; El-Kabarity, H.; Fawzy, M.M.; Schindler, A.E. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia. WHO Recommendations for Prevention and Treatment of Pre-Eclampsia and Eclampsia 2011, 97, 439–440.
- Metoki, H.; Iwama, N.; Hamada, H.; Satoh, M.; Murakami, T.; Ishikuro, M.; Obara, T. Hypertensive Disorders of Pregnancy: Definition, Management, and out-of-Office Blood Pressure Measurement. Hypertension Research 2022 45:8 2022, 45, 1298–1309. [CrossRef]
- Maul, H.; Longo, M.; Saade, G.R.; Garfield, R.E. Nitric Oxide and Its Role During Pregnancy: From Ovulation to Delivery. Curr Pharm Des 2005, 9, 359–380. [CrossRef]
- Sladek, S.M.; Magness, R.R.; Conrad, K.P. Nitric Oxide and Pregnancy. Am J Physiol Regul Integr Comp Physiol 1997, 272, 441–463. [CrossRef]
- Catalano, P.M.; Shankar, K. Obesity and Pregnancy: Mechanisms of Short Term and Long Term Adverse Consequences for Mother and Child. BMJ 2017, 356, 1. [CrossRef]
- Giouleka, S.; Tsakiridis, I.; Koutsouki, G.; Kostakis, N.; Mamopoulos, A.; Kalogiannidis, I.; Athanasiadis, A.; Dagklis, T. Obesity in Pregnancy: A Comprehensive Review of Influential Guidelines. Obstet Gynecol Surv 2023, 78, 50–68. [CrossRef]
- Riley, L.; Wertz, M.; McDowell, I. Obesity in Pregnancy: Risks and Management. Am Fam Physician 2018, 97, 559–561.
- Lin, J.; Gu, W.; Huang, H. Effects of Paternal Obesity on Fetal Development and Pregnancy Complications: A Prospective Clinical Cohort Study. Front Endocrinol (Lausanne) 2022, 13, 826665. [CrossRef]
- Alfadhli, E.M. Gestational Diabetes Mellitus. Saudi Med J 2015, 36, 399–406. [CrossRef]
- Zhu, Y.; Zhang, C. Prevalence of Gestational Diabetes and Risk of Progression to Type 2 Diabetes: A Global Perspective. Curr Diab Rep 2016, 16, 1–11. [CrossRef]
- International Diabetes Federation Diabetes Atlas; 2025.
- Zhang, C.; Ning, Y. Effect of Dietary and Lifestyle Factors on the Risk of Gestational Diabetes: Review of Epidemiologic Evidence. Am J Clin Nutr 2011, 94, S1975–S1979. [CrossRef]
- Regitz-Zagrosek, V.; Roos-Hesselink, J.W.; Bauersachs, J.; Blomström-Lundqvist, C.; Cífková, R.; De Bonis, M.; Iung, B.; Johnson, M.R.; Kintscher, U.; Kranke, P.; et al. 2018 ESC Guidelines for the Management of Cardiovascular Diseases during Pregnancy: The Task Force for the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 2018, 39, 3165–3241. [CrossRef]
- Kepley, J.M.; Bates, K.; Mohiuddin, S.S. Physiology, Maternal Changes. StatPearls 2023.
- Hunter, S.; Robson, S.C. Adaptation of the Maternal Heart in Pregnancy. Heart 1992, 68, 540–543. [CrossRef]
- Poppas, A.; Shroff, S.G.; Korcarz, C.E.; Hibbard, J.U.; Berger, D.S.; Lindheimer, M.D.; Lang, R.M. Serial Assessment of the Cardiovascular System in Normal Pregnancy: Role of Arterial Compliance and Pulsatile Arterial Load. Circulation 1997, 95, 2407–2415. [CrossRef]
- Harville, E.W.; Crook, C.E.; Bazzano, L.A.; Woo, J.G.; Burns, T.L.; Raitakari, O.; Urbina, E.M.; Venn, A.; Jacobs, D.R.; Steinberger, J.; et al. Cardiovascular Risk Factors before and during Pregnancy: Does Pregnancy Unmask or Initiate Risk? Journal of Obstetrics and Gynaecology Research 2021, 47, 3849–3856. [CrossRef]
- Beyer, S.E.; Dicks, A.B.; Shainker, S.A.; Feinberg, L.; Schermerhorn, M.L.; Secemsky, E.A.; Carroll, B.J. Pregnancy-Associated Arterial Dissections: A Nationwide Cohort Study. Eur Heart J 2020, 41, 4234–4242. [CrossRef]
- Essa, A.; Kovell, L.C.; Wilkie, G.L. Mode of Delivery and Perinatal Outcomes by Modified World Health Organization Classification of Maternal Cardiovascular Risk in Pregnancy. Am J Obstet Gynecol MFM 2023, 5, 101034. [CrossRef]
- Ferreira, A.F.; Azevedo, M.J.; Morais, J.; Trindade, F.; Saraiva, F.; Diaz, S.O.; Alves, I.N.; Fragão-Marques, M.; Sousa, C.; Machado, A.P.; et al. Cardiovascular Risk Factors during Pregnancy Impact the Postpartum Cardiac and Vascular Reverse Remodeling. Am J Physiol Heart Circ Physiol 2023, 325, H774–H789. [CrossRef]
- Li, W.; Ruan, W.; Lu, Z.; Wang, D. Parity and Risk of Maternal Cardiovascular Disease: A Dose–Response Meta-Analysis of Cohort Studies. Eur J Prev Cardiol 2019, 26, 592–602. [CrossRef]
- Honigberg, M.C.; Zekavat, S.M.; Aragam, K.; Klarin, D.; Bhatt, D.L.; Scott, N.S.; Peloso, G.M.; Natarajan, P. Long-Term Cardiovascular Risk in Women With Hypertension During Pregnancy. J Am Coll Cardiol 2019, 74, 2743–2754. [CrossRef]
- Brown, H.L.; Smith, G.N. Pregnancy Complications, Cardiovascular Risk Factors, and Future Heart Disease. Obstet Gynecol Clin North Am 2020, 47, 487–495. [CrossRef]
- Graves, M.; Howse, K.; Pudwell, J.; Smith, G.N. Pregnancy-Related Cardiovascular Risk Indicators: Primary Care Approach to Postpartum Management and Prevention of Future Disease. Canadian Family Physician 2019, 65, 883.
- Staff, A.C.; Costa, M.L.; Dechend, R.; Jacobsen, D.P.; Sugulle, M. Hypertensive Disorders of Pregnancy and Long-Term Maternal Cardiovascular Risk: Bridging Epidemiological Knowledge into Personalized Postpartum Care and Follow-Up. Pregnancy Hypertens 2024, 36, 101127. [CrossRef]
- Greer, O.Y.O.; Anandanadesan, R.; Shah, N.M.; Price, S.; Johnson, M.R. Cardiogenic Shock in Pregnancy. BJOG 2024, 131, 127–139. [CrossRef]
- Quesada, O.; Scantlebury, D.C.; Briller, J.E.; Michos, E.D.; Aggarwal, N.R. Markers of Cardiovascular Risk Associated with Pregnancy. Curr Cardiol Rep 2023, 25, 77–87. [CrossRef]
- Fürniss, H.E.; Stiller, B. Arrhythmic Risk during Pregnancy in Patients with Congenital Heart Disease. Herzschrittmachertherapie und Elektrophysiologie 2021, 32, 174–179. [CrossRef]
- Gédéon, T.; Akl, E.; D’Souza, R.; Altit, G.; Rowe, H.; Flannery, A.; Siriki, P.; Bhatia, K.; Thorne, S.; Malhamé, I. Acute Myocardial Infarction in Pregnancy. Curr Probl Cardiol 2022, 47, 101327. [CrossRef]
- Abu-Awwad, S.A.; Craina, M.; Gluhovschi, A.; Ciordas, P.D.; Marian, C.; Boscu, L.; Bernad, E.; Iurciuc, M.; Abu-Awwad, A.; Iurciuc, S.; et al. Linking Pregnancy and Long-Term Health: The Impact of Cardiovascular Risk on Telomere Shortening in Pregnant Women. Medicina 2023, Vol. 59, Page 1012 2023, 59, 1012. [CrossRef]
- Tschiderer, L.; van der Schouw, Y.T.; Burgess, S.; Bloemenkamp, K.W.M.; Seekircher, L.; Willeit, P.; Onland-Moret, C.; Peters, S.A.E. Hypertensive Disorders of Pregnancy and Cardiovascular Disease Risk: A Mendelian Randomisation Study. Heart 2024, 110, 710–717. [CrossRef]
- Aldo, C.; Martina, Z.; Alberto, A.; Mario, P. Cardiovascular Risk Evaluation in Pregnancy: Focus on Cardiac Specific Biomarkers. Clin Chem Lab Med 2024, 62, 581–592. [CrossRef]
- Marschner, S.; Mukherjee, S.; Watts, M.; Min, H.; Beale, A.L.; O’brien, J.; Juneja, A.; Tremmel, J.A.; Zaman, S. Prevention of Cardiovascular Disease in Women With Pregnancy-Related Risk Factors: A Prospective Women’s Heart Clinic Study. J Am Heart Assoc 2023, 12, 30015. [CrossRef]
- Man, A.W.C.; Zhou, Y.; Xia, N.; Li, H. Dietary Supplements and Vascular Function in Hypertensive Disorders of Pregnancy. Pflügers Archiv - European Journal of Physiology 2023 475:7 2023, 475, 889–905. [CrossRef]
- Lumsden, R.H.; Pagidipati, N. Management of Cardiovascular Risk Factors during Pregnancy. Heart 2022, 108, 1438–1444. [CrossRef]
- Shah, A.; Patel, J.; Isath, A.; Virk, H.U.H.; Jneid, H.; Zelop, C.M.; Mehta-Lee, S.; Economy, K.E.; Gulati, M.; Krittanawong, C. Cardiovascular Complications in Pregnancy. Curr Treat Options Cardiovasc Med 2023, 25, 391–414. [CrossRef]
| Reference Number and Author | Methodology | Main Objective and Variables | Primary Outcome | Secondary Outcomes | Limitations |
| 29. Harville et al. (2021) | Prospective observational cohort study Data collection likely occurred several years before 2021; cohorts began in 2009. Sample: 296 women from the International Childhood Cardiovascular Cohort Consortium. Mean age: 27 years. Inclusion: Women with at least one pre-pregnancy and one pregnancy visit with available data. Exclusion: Missing pre-pregnancy data or incomplete pregnancy information. Pregnancy period: Focus on first and second trimesters. |
To determine whether pregnancy reveals latent cardiovascular damage or independently initiates it. Variables: BMI, SBP, DBP, LDL/HDL cholesterol, triglycerides, glucose, insulin (pre-pregnancy vs. during pregnancy). |
Most risk parameters showed correlation (r = 0.29–0.54) between pre-pregnancy and pregnancy measurements, suggesting cardiovascular risk during pregnancy reflects pre-pregnancy levels. | Glucose and insulin showed lower correlations. Stronger correlations were observed in the first and second trimesters compared to the third trimester. | Relatively small sample size; variable time between pre-pregnancy and pregnancy measures; lack of data on health status during pregnancy. |
| 30. Beyer et al. (2020) | Observational cohort study (2010–2015). Sample: 18,151,897 pregnant/postpartum women in the U.S.; 993 diagnosed with pregnancy-associated arterial dissection (PAAD). Data from U.S. National Readmission Database. Inclusion: Women ≥12 years hospitalized during pregnancy, delivery, or within 42 days postpartum. Exclusion: Records missing discharge date or from out-of-state hospitals. Pregnancy period: Focus on postpartum. |
To assess risk factors, timing, anatomical distribution, and outcomes of PAAD. Primary variable: Incidence of PAAD. Secondary: Age, chronic hypertension, tobacco use, GDM, hormone therapy. |
Incidence: 5.5 per 100,000. Most common: coronary dissections. 61.5% occurred within 30 days postpartum. Higher risk among women with PAAD: age 32.8 vs. 28; chronic HTN 19% vs. 3.1%; tobacco 13.5% vs. 7.8%, etc. |
Incidence increased from 4.46 (2010) to 6.2 (2015). In-hospital mortality: 3.7% with dissections (p<0.001). Highest mortality in aortic dissection (8.6%), followed by coronary (4.2%). |
Based on ICD-9 coded administrative data, potentially misclassifying cases. Limited to early postpartum (42 days), lacking long-term follow-up. |
| 31. Essa et al. (2023) | Retrospective cohort study (Jan 2017 – May 2022). Sample: 108 pregnant women with known cardiovascular disease (CVD); low risk (n=41), moderate/high risk (n=67). Mean age: 32.1 years. Inclusion: Pregnant women >18 years with CVD and at least one prenatal/postpartum echocardiogram. Exclusion: Incomplete data or echocardiograms outside the perinatal period. Pregnancy period: First trimester to 12 weeks postpartum. |
To assess delivery mode and maternal CVD risk. Primary variable: Delivery mode. Secondary: Adverse perinatal outcomes. |
No significant difference in delivery mode by CVD risk group. Among well-compensated high-risk women, delivery mode did not affect severe maternal morbidity (SMM). |
Higher incidence of SMM, ICU admissions, and earlier gestational age at delivery in moderate/high-risk group. BMI was significantly higher pre-pregnancy in the high-risk group. |
Small sample size; single academic center; limits generalizability. Possible underpowering to detect differences. |
| 32. Ferreira et al. (2023) | Prospective cohort study (Feb 2019 – Jul 2022). Sample: 130 pregnant women (54 with CV risk factors: HTN, GDM, obesity; 76 healthy). Inclusion: Pregnant women >18 years with/without CV risk factors. Exclusion: Multiple gestations, severe chronic diseases, or pregnancy losses. Pregnancy period: First to third trimester, plus follow-ups at 1, 6, and 12 months postpartum. |
To assess CV remodeling and its postpartum reversal in women with CV risk factors, and identify predictive biomarkers. Main variables: LV mass (LVM), diastolic function (E/e’), arterial stiffness (pulse wave velocity), and biomarkers: CRP, ST2/IL-33 receptor, lysyl oxidase. |
LVM and ventricular filling pressures increased during pregnancy and normalized postpartum. HTN and obesity predicted less postpartum LVM reversal; obesity associated with concentric remodeling. |
GDM linked to long-term arterial stiffness. Elevated ST2/IL-33 correlated with better recovery. CRP in third trimester linked to poor LVM reversal. Lysyl oxidase levels rose in third trimester and 6-month follow-up. |
Small sample size; many participants enrolled in third trimester. COVID-19 pandemic led to follow-up losses. |
| Ref. No. & Author | Design | Main Focus | Number of Studies & Coverage Period | Evidence Quality | Pregnancy Period Considered | Main Objective | Key Findings | Secondary Findings | Limitations |
| 33. Li et al. (2019) | Meta-analysis of cohort studies | Quantitative assessment of the association between parity and cardiovascular risk | 10 cohort studies; total of 3,089,929 participants and 150,512 cardiovascular disease (CVD) cases. Included studies published from 1978 to June 1, 2018 | Newcastle–Ottawa Scale scores ranged from 7 to 9, indicating high-quality evidence | Not focused on a specific gestational period; parity is considered as a cumulative reproductive history | To determine the relationship between parity and long-term cardiovascular risk, including dose-response analysis | Parity was associated with a 14% increased risk of CVD compared to nulliparous women (RR: 1.14). Each additional birth was associated with a 4% increase in CVD risk. A J-shaped relationship was observed between number of births and CVD risk. | Similar associations were found for increased risk of ischemic heart disease and stroke. Subgroup analyses revealed variability in risk depending on geographic location and CVD subtype. | Significant heterogeneity in the included studies regarding parity comparisons and dose-response analysis. Not all studies adjusted for potential confounders such as lifestyle, ethnicity, or age at first birth. |
| Reference No. & Author | Methodology | Objective & Key Variables | Primary Outcome | Secondary Outcomes | Limitations |
| 34. Honigberg et al. (2019) | Prospective observational cohort study based on the UK Biobank cohort. Data collected between 2006 and 2010; study published in December 2019. Average follow-up period of 7 years post-data collection. | Population: 220,024 women aged 40–69 years with ≥1 live birth; 2,808 (1.3%) had a history of hypertensive disorders of pregnancy (HDP). Inclusion Criteria: Women with and without HDP. Exclusion Criteria: Women with congenital heart disease. Pregnancy Period: Postpartum; focused on histories of gestational hypertension, preeclampsia, eclampsia, and HELLP syndrome. |
To determine the relationship between a history of HDP and the development of cardiovascular disease (CVD), compared to women without HDP. Primary Variable: Incidence of CVD, including coronary artery disease (CAD), heart failure, aortic stenosis, and mitral regurgitation. HR: 1.3 (95% CI: 1.04–1.60; p = 0.02). |
HDP associated with increased risk of: - CAD (HR: 1.8) - Heart failure (HR: 1.7) - Aortic stenosis (HR: 2.9) - Mitral regurgitation (HR: 5.0) No significant associations with atrial fibrillation, peripheral artery disease, or venous thromboembolism. |
- Self-reported history of HDP may introduce recall bias. - Selection bias due to the UK Biobank cohort. - Lack of data to stratify different types of HDP. |
| Author (Year) | Design | Main Focus | No. of Articles / Period Covered | Quality of Evidence | Pregnancy Period | Main Objective | Key Findings | Secondary Findings | Limitations |
| 35. Brown & Smith (2020) | Narrative review | Association between pregnancy complications and future CV risk | 51 articles (2000–2019) | Epidemiological studies, meta-analyses, large cohorts | Entire pregnancy | Identify pregnancy complications increasing CV risk; emphasize postpartum care | History of preeclampsia, GDM, and preterm/low birth weight linked to higher future CV morbidity and mortality | Breastfeeding linked to long-term CV protection; CV risk monitoring should begin postpartum | Non-systematic review; dependent on selected studies’ quality and heterogeneity |
| 36. Graves et al. (2019) | Narrative clinical review | Pregnancy complications as early CV risk indicators | 54 articles (2001–2018) | Clinical guidelines, systematic reviews, cohort studies | Pregnancy and early postpartum | Identify CV risk after HDP and suggest strategies in primary care | HDP increases HTN risk x4 and CVD x2; GDM increases T2DM risk x7 | Follow-up at 6 and 12 months postpartum including BP, lipids, lifestyle counseling; awareness posters proposed | No formal quantitative analysis or meta-analysis; generalizability limited |
| 37. Staff et al. (2024) | Narrative review | HDP and maternal CV risk | 88 articles (1976–2024) | Varied; includes population-based and systematic reviews | Entire pregnancy and immediate postpartum | Assess HDP and future CV risk; propose follow-up strategies | Women with HDP have 2–4x higher long-term CVD risk | Increased risk of T2DM, kidney disease, and offspring CV risk; use of angiogenic biomarkers and lifestyle strategies recommended | Article selection method not detailed; studies focus on high-income countries |
| 38. Greer et al. (2024) | Narrative review | Cardiogenic shock in pregnancy | 80 articles (2005–2023) | Observational records, national registries, prior reviews | Pregnancy and delivery | Guide identification and management of cardiogenic shock | PPCM, AFE, SCAD as major causes; associated with maternal and neonatal morbidity and preterm birth | SCAI classification adapted for pregnancy; multidisciplinary care essential (e.g., ECMO, IABP) | Lacks systematic review protocol; evidence mostly observational |
| 39. Quesada et al. (2023) | Narrative review | Adverse pregnancy outcomes (APOs) and CV risk | 101 articles (2005–2022) | Includes robust meta-analysis (13+ million women), plus small, lower-quality studies | Pregnancy through puerperium | Identify APOs as CV risk factors; recommend prevention strategies | Preeclampsia: +63% lifetime CVD risk; GDM: x2 CVD risk; preterm birth: x1.79 CV mortality | APOs double risk of stroke, HF, and CV mortality; breastfeeding reduces HTN, T2DM, and future CVD | Unclear definition of APO; study heterogeneity; mechanisms insufficiently detailed |
| 40. Fürniss & Stiller (2021) | Narrative review | Arrhythmia risk in congenital heart disease (CHD) during pregnancy | 34 references (1995–2019) | Multicenter registries, observational studies | Entire pregnancy and up to 6 months postpartum | Identify arrhythmia risk and guide management in CHD | Arrhythmias in 7–9% of CHD pregnancies; >30% in complex CHD; mostly supraventricular | Preconception CV evaluation and Holter monitoring advised; restrict antiarrhythmic drugs in first trimester | Focus on moderate/severe CHD; lack of data on mild CHD; limited evidence on pharmacologic management |
| 41. Gédéon et al. (2022) | Narrative review | Myocardial infarction (MI) in pregnancy | 166 articles (2005–2022) | Observational studies, meta-analyses, registries | Third trimester and immediate postpartum | Update on MI in pregnancy, including risk, diagnosis, and man agement | Pregnancy increases MI risk 3–5x vs. nonpregnant peers; SCAD most common cause; cardiac troponins effective | Vaginal delivery preferred unless instability; conservative management in SCAD; standard STEMI/NSTEMI treatment applied | MI is rare; most studies small and heterogeneous; RCT data lacking |
| Reference and Author | Methodology | Objectives and Main Variables | Primary Outcome | Secondary Outcomes | Limitations |
| 42. Abu-Awwad et al. (2023) | Prospective observational study conducted between January 1, 2020, and December 31, 2022, published in 2023. Sample: 68 pregnant women aged 18–40 years, divided into: Group 1: 38 women without cardiovascular or vascular disease. Group 2: 30 women with cardiovascular risk or established disease. Inclusion: Pregnant women in 2nd or 3rd trimester with ≥1 cardiovascular risk factor, undergoing elective cesarean delivery. Exclusion: History of substance abuse, psychiatric disorders, thromboembolic or infectious diseases, participation in other studies within the past 3 months, or use of telomere-shortening drugs. Gestational period: 2nd and 3rd trimesters. |
To evaluate whether telomere length is reduced in pregnant women with cardiovascular risk factors (CVRFs) compared to those without. Main variable: Telomere length measured via quantitative polymerase chain reaction (qPCR). |
Women with CVRFs exhibited significantly shorter telomere lengths (mean: 0.3537 megabases) compared to those without CVRFs (mean: 0.5728 megabases). Statistically significant difference (p = 0.0458). |
In the CVRF group, 32% of fetuses presented with intrauterine growth restriction (IUGR). Telomere length may serve as a biomarker of cardiovascular risk in pregnant women. Shorter telomeres are associated with increased morbidity and mortality, including various cancers, cognitive decline, depression, and infections. |
Small sample size and potential selection bias due to recruitment from a single hospital. Other factors influencing telomere length, such as diet, lifestyle, and stress, were not controlled. |
| 43. Tschiderer et al. (2024) | Prospective Mendelian randomization study based on cohort data. Utilized UK Biobank data recruited from 2006 to 2010; analysis conducted in 2023. Sample: 221,155 pregnant women, 41,506 nulliparous women, and 223,025 men as negative controls. Inclusion: Women with available genetic data and pregnancy history. Exclusion: Incomplete data or no pregnancy history. Focus on long-term post-pregnancy cardiovascular outcomes. |
To analyze the association between genetic predisposition to hypertensive pregnancy disorders (HPDs) and cardiovascular risk (CVR). Main variables: Myocardial infarction (MI), stroke, systolic/diastolic blood pressure (SBP/DBP), and age at hypertension diagnosis. |
Genome-wide association study identified links between genetic predisposition to preeclampsia/eclampsia and a 20% increased CVD risk. Gestational hypertension associated with a 24% increased CVD risk. Genetic predisposition also linked to increased BP and earlier hypertension diagnosis. |
Comparison with nulliparous women and men suggests that risk is not solely attributable to pregnancy, indicating other underlying biological mechanisms. Elevated cholesterol, triglycerides, and metabolic markers are also associated with genetic predisposition to HPDs. |
Potential genetic bias due to predominantly European ancestry in the UK Biobank sample. Lack of control over other genetic or environmental confounders. |
| Reference and Author | Design | Main Focus | Number of Articles and Time Frame | Quality of Evidence | Gestational Period | Main Objective | Key Findings | Secondary Outcomes | Limitations |
| 44. Aldo et al. (2024) | Narrative review published in Clinical Chemistry and Laboratory Medicine (CCLM) | Analysis of the role of specific cardiac biomarkers such as natriuretic peptides and troponins in cardiovascular risk evolution during both normal and complicated pregnancies. | 102 articles spanning from 1981 to 2023 | Findings are based on observational studies, previous reviews, and small-scale clinical trials. The article highlights methodological heterogeneity, lack of standardization, and small sample sizes, which limit the generalizability of the results. | All pregnancy stages: first, second, third trimesters, as well as peri- and postpartum. | To determine whether the measurement of specific cardiac biomarkers can improve cardiovascular risk assessment in pregnant women, aiming to identify early cardiovascular complications. | NT-proBNP and BNP: levels increase during the first trimester, decrease in the second and third trimesters, and rise again during delivery. Values >200 ng/L of NT-proBNP are associated with heart failure and hypertensive disorders in women with pre-existing heart conditions. High-sensitivity cardiac troponins remain unchanged in uncomplicated pregnancies but increase in conditions like hypertensive disorders and other cardiovascular complications. Troponins are proposed as risk assessment tools, though further validation is needed. |
Measurement of natriuretic peptides and cardiac troponins, when combined with other tests such as ECG, may assist in cardiovascular risk stratification in pregnancy, though with limited utility. Routine screening for these biomarkers is not recommended in early pregnancy for women with hypertension or other cardiovascular diseases. |
Heterogeneity in biomarker measurement methods and studied populations. Limited number of studies evaluating the combined use of biomarkers in pregnant women. Lack of consensus on pregnancy-specific reference values for cardiac biomarkers. Need for further research to substantiate the evidence linking biomarkers to cardiovascular risk in pregnancy, as current evidence is weak. |
| Reference and Author | Methodology | Objectives and Main Variables | Primary Outcome | Secondary Outcomes | Limitations |
| 45. Marschner et al. (2023) | Prospective cohort study conducted between May 2021 and October 2022. Sample: 156 women aged 30 to 55 years (mean age: 41 years) with a history of pregnancy complications. Inclusion: Women diagnosed with hypertensive disorders of pregnancy (HDP), gestational diabetes mellitus (GDM), or intrauterine growth restriction (IUGR) between January 2013 and December 2020. Exclusion: Women with known cardiovascular disease, unstable medical conditions, cognitive impairment, recent breastfeeding, or planning pregnancy during the study. Postpartum follow-up of women with prior pregnancy complications. |
To determine whether a postpartum follow-up and management program improves the cardiovascular profile of women with a history of pregnancy-related cardiovascular complications. Main variables: Blood pressure (BP) and total cholesterol/HDL-cholesterol ratio. |
After 6 months, 80.5% of women enrolled in the cardiovascular risk management program achieved BP targets (<140/90 mmHg or <130/80 mmHg for those with type 2 diabetes), compared to 69.2% of women who did not follow the program. LDL and total cholesterol levels were reduced, alongside improvements in BMI and waist circumference. Findings support the utility of women-focused cardiovascular health programs for effective risk factor control and CVD prevention. |
Participants in the follow-up program also showed improved lifestyle habits, including increased consumption of fish and olive oil, and reduced intake of fast food. | The study was non-randomized, which may introduce bias and limits causal inference. Potential selection bias due to high educational levels among participants. The intervention was conducted by the Women’s Heart Clinic in Australia, so results may be specific to that regional healthcare setting. |
| Reference No. and Author | Design | Main Focus | No. of Articles and Time Frame | Evidence Quality | Gestational Period | Main Objective | Key Findings | Secondary Findings | Limitations |
| 46. Man et al. (2023) | Narrative review published in Pflügers Archiv - European Journal of Physiology. | Effect of dietary supplements on vascular function in pregnant women and hypertensive disorders of pregnancy (HDP). | Over 250 articles from 1980 to 2023. | Includes meta-analyses providing robust evidence for calcium and omega-3 supplementation effects, supported by clinical trials and observational studies. Animal models included but with limited clinical applicability. | Entire pregnancy, with emphasis on early to mid-gestation and early postpartum. | To investigate how dietary supplements improve vascular function and cardiovascular risk in pregnant women, focusing on oxidative stress, endothelial dysfunction, and angiogenic factors. | L-arginine and L-citrulline enhance NO availability, lowering BP and improving vascular function. Omega-3 reduces inflammation and oxidative stress, improving placental angiogenesis. Calcium supplementation reduces preeclampsia incidence by 32%. Resveratrol shows promise but needs further validation. Some supplements exhibit epigenetic effects. | Preeclampsia is linked to imbalanced angiogenic factors and oxidative stress, leading to vascular/placental dysfunction. Supplements like vitamins D, C, and E reduce inflammation, though results are inconsistent. Animal studies support L-citrulline to prevent hypertension and placental dysfunction. | High heterogeneity among studies; causality of nutritional deficiencies unclear; limited human data; key findings from animal studies. |
| 47. Lumsden (2022) | Narrative review. | Overview of main CV risk factors during pregnancy and strategies for management. | 94 references from 1982 to 2021. | Many recommendations based on international guidelines, offering strong evidence. Some are from retrospective studies with weaker support. | Entire pregnancy, from preconception to immediate postpartum, focusing on second and third trimesters. | Provide guidance for managing CV risk factors in pregnant women through early identification and trimester-specific interventions. | Hypertension: differentiated management with safe medications (methyldopa, labetalol). Diabetes/obesity: strict glycemic control and weight/nutrition management. Dyslipidemia: lifestyle changes; statins limited. Thromboembolism: prophylaxis in at-risk women. | CV risk management prevents severe conditions (eclampsia, preterm birth) and improves long-term CV health. | Lack of data for subpopulations (e.g., racial differences); no clear selection/review methodology for included studies. |
| 48. Shah et al. (2023) | Narrative review published in Current Cardiology Reports. | Approach to common CV complications during pregnancy and their management. | 133 articles from 2000 to 2023. | Strong evidence for hypertension and preeclampsia based on AHA guidelines. Limited evidence for hyperlipidemia and MI management. | All pregnancy stages, including preconception and postpartum. | Provide comprehensive guidance for diagnosis, treatment, and prevention of pregnancy-related CV complications. | Chronic HTN: increases risk for preeclampsia and preterm birth. Managed with BP control and low-dose aspirin. HDP: requires intensive monitoring and antihypertensive therapy. Severe cases need magnesium sulfate. Hyperlipidemia: monitor lipids preconceptionally. MI: rare but serious; similar initial treatment as in non-pregnant women. PE: managed with LMWH. | Multidisciplinary cardio-obstetric teams recommended. Lifestyle counseling reduces maternal morbidity. | Limited studies on some topics; need for more clinical trials; some data derived from guidelines, others from observational studies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





