Submitted:
01 October 2025
Posted:
02 October 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Surface Characterization (Materials)
- -
- -
- Stainless-steel consumables table (AISI 304, mirror/matte finish) — Reflectivity: polished stainless steel reflects a portion of incident UVC, increasing irradiance in some directions but also producing specular reflections and potential shadow cancellation; smooth surfaces minimize micro-sheltering [14,15,16].
- -
2.2.1. Quantitative Surface Property Assessment
- -
- Surface Roughness (Ra): The arithmetic average of the absolute values of the profile height deviations from the mean line (Ra) was measured in triplicate on each material using a calibrated profilometer (Mitutoyo Surftest SJ-210). Measurements were taken across the grain on the wood, along the lay on the metal, and across a flat area on the dental unit polymer to quantify the micro-topography that can create “micro-shadowing” effects.
- -
- Contact Angle: The static contact angle was measured in triplicate for each surface using the sessile drop method with a goniometer (KRÜSS DSA100) and 5 μL of deionized water. This measurement indicates the surface’s hydrophobicity, which influences how contaminants and water-based aerosols adhere, potentially affecting the UVC dose received by microorganisms.
- -
- Spectral Reflectance (254 nm): The percentage of 254 nm UVC light reflected by each surface was measured using a calibrated spectroradiometer with an integrating sphere accessory. This helps determine how material reflectivity (e.g., for the stainless steel) might enhance the germicidal dose via scattered radiation.
2.3. UVC Device and Dosimetry
- -
- 5 min (300 s): 90 × 300 / 1000 = 27 mJ/cm²
- -
- 10 min (600 s): 90 × 600 / 1000 = 54 mJ/cm²
- -
- Instrument: Solar Light PMA2100 Radiometer (Serial Number: 2024-A-7215)
- -
- Spectral range: 200–280 nm
- -
- Measurement resolution: ±1 µW/cm²
- -
- Calibration: Traceable to the National Institute of Standards and Technology (NIST); most recent calibration performed on 25 March 2025.
- -
- Lamp type: Dual low-pressure mercury lamps (2 × 40 W)
- -
- Emission peak: 253.7 nm
- -
- Lamp height above samples: 50 cm
- -
- Irradiance at 50 cm: 90 µW/cm² (mean of three measurements)
- -
- Operating hours at time of test: ~250 hours
- -
- All experiments were performed under controlled ambient conditions(22.5 ∘°C, 55% RH).
2.4. Sampling Protocol
2.5. Culture and Identification
2.6. Data Handling and Statistics
2.7. Limitations (Stated Within Methods)
3. Results
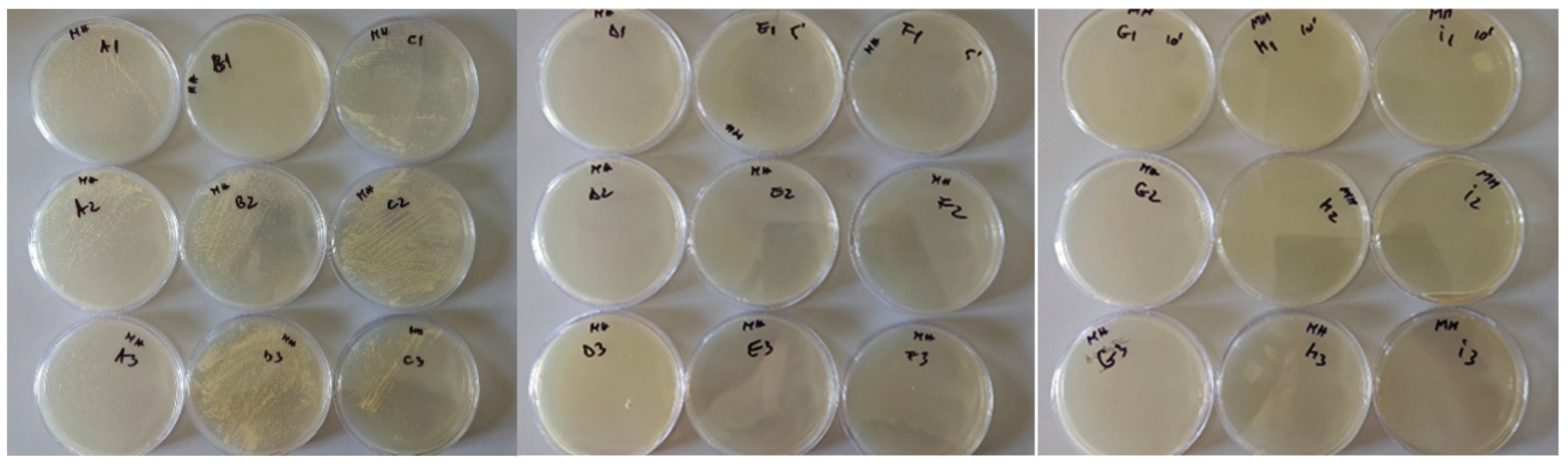
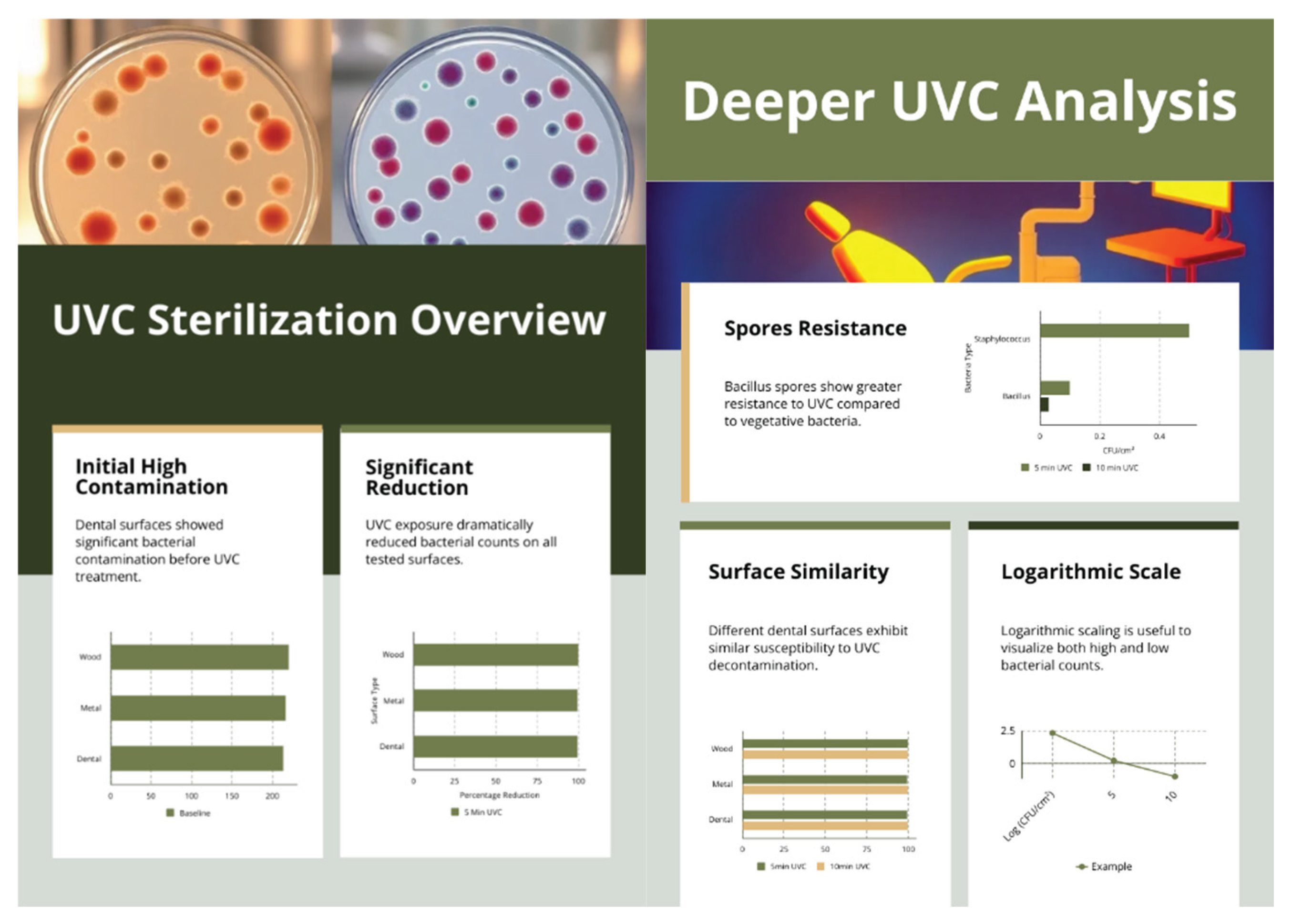
3.1. Baseline Contamination
3.2. Reduction After UVC Exposure
- -
- Limit of detection (LOD) = 0.4 CFU/cm² (one colony on a plate → CFU/cm² = 10/25 = 0.4).
- -
- Example calculation (Wood, 10 min with no colonies): log₁₀(220.0 / 0.4) = 2.74 → reported as ≥2.74.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Szymańska, J.; Sitkowska, J.; Dutkiewicz, J. Microbial Contamination of Dental Unit Waterlines. Ann. Agric. Environ. Med. 2008, 15, 173–179. [Google Scholar]
- Pardo, A.; Fiorini, V.; Zangani, A.; Faccioni, P.; Signoriello, A.; Albanese, M.; Lombardo, G. Topical Agents in Biofilm Disaggregation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2024, 13, 2179. [Google Scholar] [CrossRef]
- Marsh, P.D.; Moter, A.; Devine, D.A. Dental Plaque Biofilms: Communities, Conflict and Control. Periodontol. 2000 2011, 55, 16–35. [Google Scholar] [CrossRef]
- Al-Hammadi, S.; Al-Shehari, W.A.; Edrees, W.H.; et al. Prevalence of Methicillin-Resistant Staphylococcus aureus (MRSA) among Patients with Oral Infections in Sana’a City, Yemen. BMC Oral Health 2025, 25, 1047. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Disinfection, Sterilization, and Antisepsis: Principles, Practices, Current Issues, New Research, and New Technologies. Am. J. Infect. Control 2019, 47S, A1–A2. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, W. Ultraviolet Germicidal Irradiation Handbook; Springer, 2009. [Google Scholar]
- Buonanno, M.; Welch, D.; Shuryak, I.; Brenner, D.J. Far-UVC Light (222 nm) Efficiently and Safely Inactivates Airborne Human Coronaviruses. Sci. Rep. 2020, 10, 10285. [Google Scholar] [CrossRef]
- Kebbi, Y.; Muhammad, A.I.; Sant’Ana, A.S.; do Prado-Silva, L.; Liu, D.; Ding, T. Recent Advances on the Application of UV-LED Technology for Microbial Inactivation: Progress and Mechanism. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3501–3527. [Google Scholar] [CrossRef]
- Nerandzic, M.M.; Thota, P.; Sankar, C.T.; et al. Evaluation of a Pulsed Xenon Ultraviolet Disinfection System for Reduction of Healthcare-Associated Pathogens in Hospital Rooms. Infect. Control Hosp. Epidemiol. 2015, 36, 192–197. [Google Scholar] [CrossRef]
- Di Martino, G.; Pasqua, S.; Douradinha, B.; Monaco, F.; Di Bartolo, C.; Conaldi, P.G.; D’Apolito, D. Efficacy of Three Commercial Disinfectants in Reducing Microbial Surfaces’ Contaminations of Pharmaceuticals Hospital Facilities. Int. J. Environ. Res. Public Health 2021, 18, 779. [Google Scholar] [CrossRef]
- Duanis-Assaf, D.; Kenan, E.; Sionov, R.; Steinberg, D.; Shemesh, M. Proteolytic Activity of Bacillus subtilis upon κ-Casein Undermines Its “Caries-Safe” Effect. Microorganisms 2020, 8, 221. [Google Scholar] [CrossRef] [PubMed]
- Sliney, D.H.; Stuck, B.E. A Need to Revise Human Exposure Limits for Ultraviolet UV-C Radiation. Photochem. Photobiol. 2021, 97, 485–492. [Google Scholar] [CrossRef]
- Reed, N.G. The History of Ultraviolet Germicidal Irradiation for Air Disinfection. Public Health Rep. 2010, 125, 15–27. [Google Scholar] [CrossRef]
- Kujundzic, E.; Matalkah, F.; Howard, C.J.; Hernandez, M.; Miller, S.L. UV Air Cleaners and Upper-Room Air Ultraviolet Germicidal Irradiation for Controlling Airborne Bacteria and Fungal Spores. J. Occup. Environ. Hyg. 2006, 3, 536–546. [Google Scholar] [CrossRef]
- Kowalski, W.; Bahnfleth, W.; Hernandez, M. A Genomic Model for Predicting the Ultraviolet Susceptibility of Viruses and Bacteria. IUVA News 2006, 8, 33–38. [Google Scholar]
- Albertini, R.; Colucci, M.E.; Coluccia, A.; et al. An Overview on the Use of Ultraviolet Radiation to Disinfect Air and Surfaces. Acta Biomed. 2023, 94, e2023165. [Google Scholar] [CrossRef]
- Jeon, M.-J.; Choi, Y.-S.; Seo, D.-G. Bactericidal Effects of Ultraviolet-C Light-Emitting Diode Prototype Device Through Thin Optical Fiber. Appl. Sci. 2025, 15, 4504. [Google Scholar] [CrossRef]
- Kvam, E.; Benner, K. Mechanistic Insights into UV-A Mediated Bacterial Disinfection via Endogenous Photosensitizers. J. Photochem. Photobiol. B 2020, 209, 111899. [Google Scholar] [CrossRef] [PubMed]
- Imada, K.; Tanaka, S.; Ibaraki, Y.; Yoshimura, K.; Ito, S. Antifungal Effect of 405-nm Light on Botrytis cinerea. Lett. Appl. Microbiol. 2014, 59, 670–676. [Google Scholar] [CrossRef]
- Mikelonis, A.M.; Abdel-Hady, A.; Aslett, D.; Ratliff, K.; Touati, A.; Archer, J.; Serre, S.; Mickelsen, L.; Taft, S.; Calfee, M.W. Comparison of Surface Sampling Methods for an Extended Duration Outdoor Biological Contamination Study. Environ. Monit. Assess. 2020, 192, 455. [Google Scholar] [CrossRef]
- Setlow, B.; Korza, G.; Blatt, K.M.; Fey, J.P.; Setlow, P. Mechanism of Bacillus subtilis Spore Inactivation by and Resistance to Supercritical CO₂ plus Peracetic Acid. J. Appl. Microbiol. 2016, 120, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Hessling, M.; Haag, R.; Sieber, N.; Vatter, P. The Impact of Far-UVC Radiation (200–230 nm) on Pathogens, Cells, Skin, and Eyes—A Collection and Analysis of a Hundred Years of Data. GMS Hyg. Infect. Control 2021, 16, Doc07. [Google Scholar] [CrossRef]
- Blau, K.; Gallert, C. Efficacy of UV-C 254 nm Light and a Sporicidal Surface Disinfectant in Inactivating Spores from Clostridioides difficile Ribotypes In Vitro. Pathogens 2024, 13, 965, Öberg, R.; Sil, T.B.; Johansson, A.C.; Malyshev, D.; Landström, L.; Johansson, S.; Andersson, M.; Andersson, P.O. UV-Induced Spectral and Morphological Changes in Bacterial Spores for Inactivation Assessment. J. Phys. Chem. B 2024, 128, 1638–1646. https://doi.org/10.1021/acs.jpcb.3c07062. [Google Scholar] [CrossRef] [PubMed]
- Hashemzadeh, F.; Geppert, A.; Jackson, L.J.; Harrison, J.J.; Achari, G. Hydrogen Peroxide Augments the Disinfection Efficacy of 280 nm Ultraviolet LEDs against Antibiotic-Resistant Uropathogenic Otherwise Tolerant to Germicidal Irradiation. ACS Omega 2025, 10, 29558–29568. [Google Scholar] [CrossRef]
- Duque-Sarango, P.; Delgado-Armijos, N.; Romero-Martínez, L.; Cruz, D.; Pinos-Vélez, V. Advancing Waterborne Fungal Spore Control: UV-LED Disinfection Efficiency and Post-Treatment Reactivation Analysis. Water 2025, 17, 922. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Liu, J.; Wang, Q. Effectiveness of ultraviolet-C disinfection systems for reduction of multi-drug resistant organism infections in healthcare settings: A systematic review and meta-analysis. Epidemiol Infect. 2023, 151, e149. [Google Scholar] [CrossRef]
- Cook, D.C.; Olsen, M.; Tronstad, O.; et al. Ultraviolet-C-based sanitization is a cost-effective option for hospitals to manage health care-associated infection risks from high touch mobile phones. Front Health Serv. 2025, 4, 1448913. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, T.; Gomes Filho, N.; Padrão, J.; Zille, A. A Comprehensive Analysis of the UVC LEDs’ Applications and Decontamination Capability. Materials 2022, 15, 2854. [Google Scholar] [CrossRef]
- Welch, D.; Aquino de Muro, M.; Buonanno, M.; Brenner, D.J. Wavelength-Dependent DNA Photodamage in a 3-D Human Skin Model over the Far-UVC and Germicidal UVC Wavelength Ranges from 215 to 255 nm. Photochem. Photobiol. 2022, 98, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Abushahba, F.; Algahawi, A.; Areid, N.; Vallittu, P.K.; Närhi, T. Efficacy of biofilm decontamination methods of dental implant surfaces: A systematic review of in vitro studies. Eur J Oral Sci. 2025, 133, e70005. [Google Scholar] [CrossRef]
- Seneviratne, C.J.; Khan, S.A.; Zachar, J.; Yang, Z.; Kiran, R.; Walsh, L.J. Efficacy of Ultrasonic Cleaning Products with Various Disinfection Chemistries on Dental Instruments Contaminated with Bioburden. Int. Dent. J. 2025, 75, 1632–1639. [Google Scholar] [CrossRef]
- Szabó, S.; Feier, B.; Capatina, D.; Tertis, M.; Cristea, C.; Popa, A. An Overview of Healthcare Associated Infections and Their Detection Methods Caused by Pathogen Bacteria in Romania and Europe. J. Clin. Med. 2022, 11, 3204. [Google Scholar] [CrossRef]
- Velazquez-Meza, M.E.; Galarde-López, M.; Cornejo-Juárez, P.; Carrillo-Quiroz, B.A.; Velázquez-Acosta, C.; Bobadilla-del-Valle, M.; Ponce-de-León, A.; Alpuche-Aranda, C.M. Multidrug-Resistant Staphylococcus sp. and Enterococcus sp. in Municipal and Hospital Wastewater: A Longitudinal Study. Microorganisms 2024, 12, 645. [Google Scholar] [CrossRef]
- Razavifar, M.; Abdi, A.; Nikooee, E.; et al. Quantifying the Impact of Surface Roughness on Contact Angle Dynamics under Varying Conditions. Sci. Rep. 2025, 15, 16611. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, R.A.; Navne, J.; Adelmark, M.V.; Shkondin, E.; Crovetto, A.; Hansen, O.; Bachmann, J.; Taboryski, R. Understanding the Light Induced Hydrophilicity of Metal-Oxide Thin Films. Nat. Commun. 2024, 15, 124. [Google Scholar] [CrossRef] [PubMed]
- Griffin, D.T.; Gourlay, T.; Maclean, M. The Antibacterial Efficacy of Far-UVC Light: A Combined-Method Study Exploring the Effects of Experimental and Bacterial Variables on Dose–Response. Pathogens 2024, 13, 698. [Google Scholar] [CrossRef] [PubMed]
- Duering, H.; Westerhoff, T.; Kipp, F.; Stein, C. Short-Wave Ultraviolet-Light-Based Disinfection of Surface Environment Using Light-Emitting Diodes: A New Approach to Prevent Health-Care-Associated Infections. Microorganisms 2023, 11, 386. [Google Scholar] [CrossRef]

| Surface | Porosity /Roughness | Typical UVC Reflectance (254 nm) | Likely effect on decontamination | References |
| Varnished oak (wood) | Moderate porosity; grain-aligned micro-crevices even after varnish | 5–12% (varies with coating thickness and pigment) | Absorption and “micro-shadowing” may protect cells lodged in pores or along fibres | [11,12,13] |
| Stainless steel (AISI 304) | Ra ≈ 0.05–0.2 µm (polished) | 25–30% | High reflectivity can enhance fluence on adjacent points; minimal micro-sheltering if free of debris | [14,15,16] |
| Dental-unit polymer/composite | Grooves and seams; matte areas | < 8% (most plastics); varies by pigment | Absorption and irregular topology reduce direct fluence; seams may shield bacteria | [17,18,19] |
| Surface Material | Surface Roughness (Ra) | Static Contact Angle | Spectral Reflectance (254 nm) |
| Wood (Varnished Oak) | 1.5±0.2 μm | 68.4∘±2.5∘ | 7.2%±0.4% |
| Stainless Steel (AISI 304) | 0.08±0.01 μm | 83.6∘±1.8∘ | 28.5%±1.2% |
| Dental Unit Polymer | 0.9±0.1 μm | 76.2∘±3.1∘ | 5.1%±0.3% |
| Surface | Condition | Mean CFU/cm² ± SD | 95% CI | p vs baseline |
| Wood | Baseline | 220.0 ± 10.0 | 209.6–230.4 | — |
| 5 min UVC | 0.7 ± 0.6 | 0.1–1.3 | < 0.001 | |
| 10 min UVC | 0.0 ± 0.0 | 0.0–0.0 | < 0.001 | |
| Metal | Baseline | 216.7 ± 7.6 | 208.8–224.5 | — |
| 5 min UVC | 1.0 ± 1.0 | 0.0–2.1 | < 0.001 | |
| 10 min UVC | 0.0 ± 0.0 | 0.0–0.0 | < 0.001 | |
| Dental | Baseline | 213.3 ± 7.6 | 205.5–221.2 | — |
| 5 min UVC | 1.0 ± 1.0 | 0.0–2.1 | < 0.001 | |
| 10 min UVC | 0.3 ± 0.6 | 0.0–0.9 | < 0.001 |
| Baseline mean (CFU/cm²) | 5 min mean (CFU/cm²) | 5 min log₁₀ reduction | 10 min mean (CFU/cm²) | 10 min log₁₀ reduction | |
| Wood | 220.0 | 0.7 | 2.50 | 0.0 (<LOD) | ≥2.74 |
| Metal | 216.7 | 1.0 | 2.34 | 0.0 (<LOD) | ≥2.73 |
| Dental | 213.3 | 1.0 | 2.33 | 0.3 | 2.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





