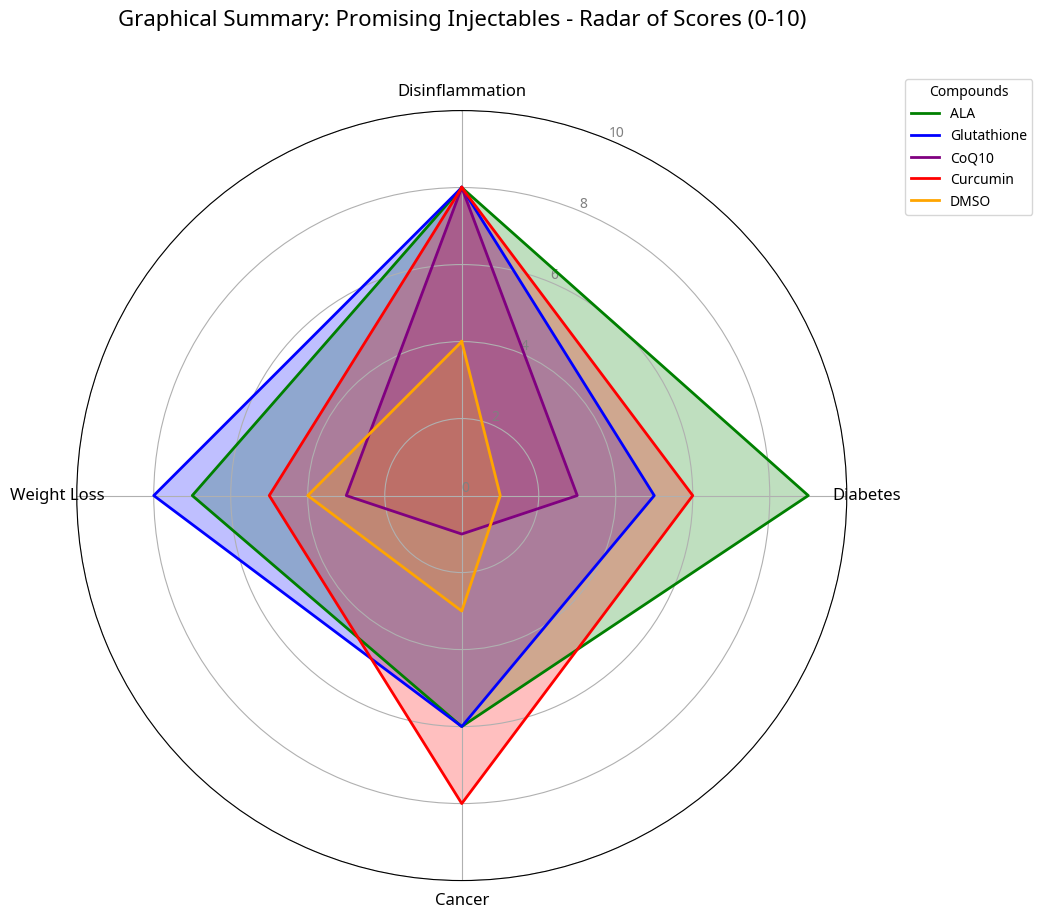
Introduction
Chronic low-grade inflammation constitutes a common
mechanistic link between cancer, obesity, and type 2 diabetes (T2D). Central
inflammatory pathways such as NF-κB and JNK orchestrate the production of
pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) contributing to insulin
resistance, tumor progression, and adipose tissue dysfunction [1–4]. MicroRNAs, especially miR-146a and miR-155, regulate
these processes post-transcriptionally by fine-tuning signaling components such
as IRAK1 and TRAF6 [5–8]. Dysregulation of these miRNAs correlates with exacerbated inflammatory
states in these diseases [9–11]. Injectable therapies delivering DMSO, CoQ10, ALA, curcumin, and
miR-146a mimics optimize delivery, overcoming limitations of oral
administration, enhancing therapeutic effects [12–16]. Phytotherapeutics rich in these compounds modulate miRNA expression and
provide additional anti-inflammatory benefits [17–20]. This article reviews molecular and clinical data supporting these
integrated injectable therapies.
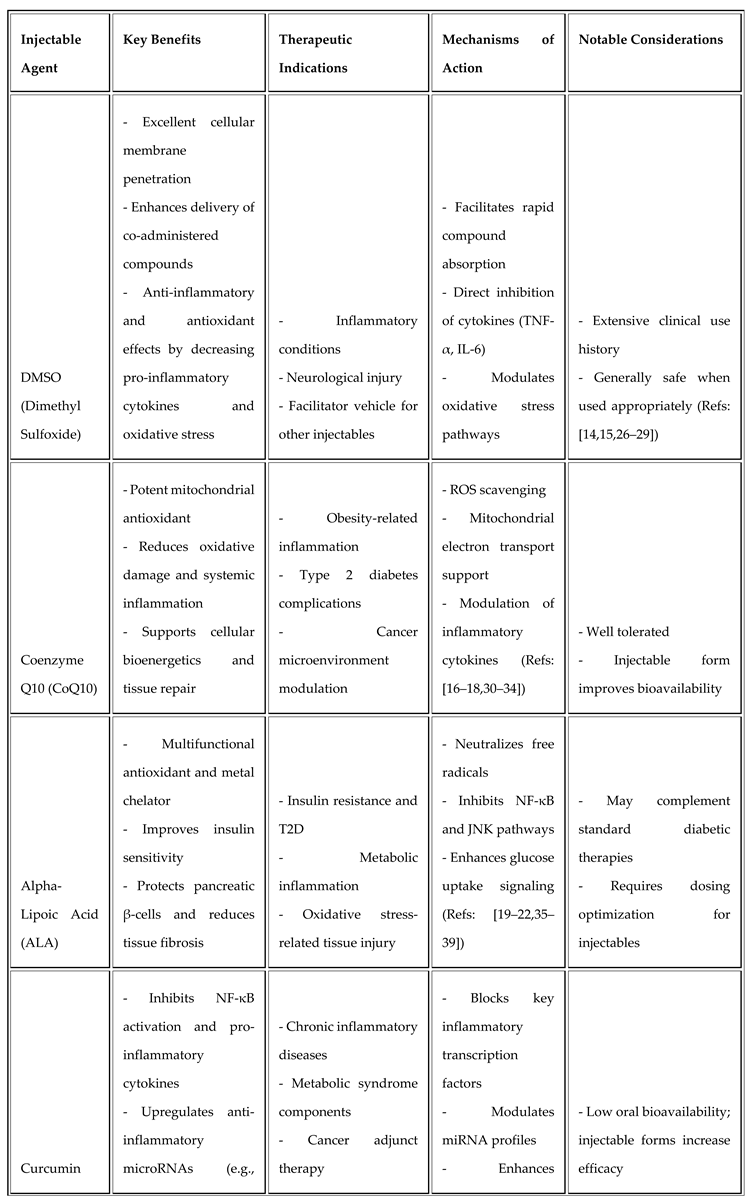
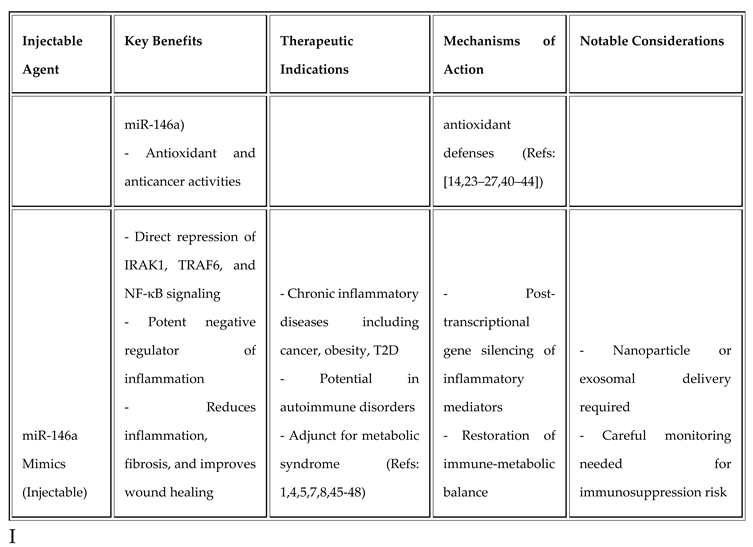
Injectable Therapeutics and Their Rationale
Injectable delivery bypasses gastrointestinal degradation and first-pass metabolism, ensuring higher systemic bioavailability and faster pharmacodynamic effects [
12,21]. It allows precise dosing and co-administration of multiple agents, crucial for multimodal therapy [22]. Nanocarriers and exosomal formulations facilitate the delivery of miRNA mimics like miR-146a, protecting them from degradation and enhancing cellular uptake [
23,24,25].
Specific Injectable Agents
Dimethyl Sulfoxide (DMSO)
DMSO is a membrane-penetrating solvent enhancing absorption of co-administered compounds and exhibits independent anti-inflammatory, antioxidant effects by reducing pro-inflammatory cytokines and oxidative stress [26,27,28]. Clinical experience supports its safety in inflammation and neurological conditions [29].
- DOI: 10.1007/s11010-011-0727-3
- PMID: 21674015
- PMC: PMC3106211
Coenzyme Q10 (CoQ10)
CoQ10 is a mitochondrial electron transporter with potent antioxidant activity, decreasing reactive oxygen species related to inflammation and preserving mitochondrial function [30,31,32,33]. Injectable CoQ10 ameliorates metabolic inflammation in obesity and diabetes models [34].
- DOI: 10.1016/j.freeradbiomed.2006.02.002
- PMID: 16581168
- PMC: PMC6272576
Alpha-Lipoic Acid (ALA)
ALA acts as a broad-spectrum antioxidant and metal chelator, improving insulin sensitivity and reducing metabolic inflammation by inhibiting NF-κB and JNK pathways [35,36,37,38]. Injectable ALA supports pancreatic β-cell preservation and metabolic homeostasis [39].
- DOI: 10.1016/j.bbamcr.2008.09.018
- PMID: 18805455
- PMC: PMC2730833
Curcumin
Curcumin, derived from Curcuma longa, inhibits NF-κB activation and pro-inflammatory cytokine production and promotes miR-146a expression, thus restoring anti-inflammatory control [40,41,42,43]. Injectable formulations overcome bioavailability limitations and enhance clinical efficacy [44].
- DOI: 10.1016/j.intimp.2009.06.005
- PMID: 19682990
- PMC: PMC2925237
miR-146a Mimics
miR-146a downregulates IRAK1 and TRAF6, providing a negative feedback loop on TLR/IL-1R–mediated NF-κB activation [
5,
45]. Injectable nanoparticle-encapsulated miR-146a mimics reduce inflammation and fibrosis in preclinical diabetes and cancer models [46,47,48].
- DOI: 10.1073/pnas.0605298103
- PMID: 16945974
- PMC: PMC1564495
Phytotherapeutics Modulating MicroRNAs
Phytochemicals modulate inflammatory miRNAs, enhancing therapeutic potential:
- *Turmeric (Curcuma longa):* Induces miR-146a expression, exerting anti-inflammatory and metabolic benefits [
49].
- *Ginger (Zingiber officinale):* Promotes miR-146a expression and improves metabolic inflammation [50,51].
- *Green Tea (Camellia sinensis):* Regulates miR-125b to suppress inflammation [52].
- *Echinacea purpurea:* Downregulates miR-155 involved in immune activation [53].
- *Artemisia annua:* Modulates miR-Let-7 associated with metabolic regulation [54].
Further experimental and clinical studies are required for validation.
- DOI: 10.1016/j.molnut.2015.10.003
- PMID: 26562757
- PMC: PMC4687390
Discussion and Future Directions
Integrated injectable therapies combining DMSO, CoQ10, ALA, curcumin, and miR-146a mimics offer multimodal intervention against chronic inflammation in cancer, obesity, and T2D. Personalized medicine approaches using circulating miRNA profiles can optimize patient stratification and treatment outcomes [55]. Careful evaluation of immunosuppression and off-target effects is imperative [56]. Ongoing clinical trials and advancements in nanodelivery systems will support translation into routine care.
Conclusions
The integration of injectable DMSO, coenzyme Q10, alpha-lipoic acid, curcumin, and miR-146a mimics, supported by phytotherapeutics modulating relevant microRNAs, provides a promising multifaceted therapeutic approach to chronic inflammatory diseases, with mechanistic and clinical rationale pending robust validation.
References
- Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. [Link](https://www.ncbi.nlm.nih.gov/pubmed/28179628). [CrossRef] [PubMed]
- Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci U S A. 2006;103(33):12481–12486. [Link](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1564495/). [CrossRef] [PubMed] [PubMed Central]
- Jacob SW, Herschler R. Pharmacology of dimethyl sulfoxide in cardiac and CNS damage. Ann N Y Acad Sci. 1974;243:7–28. [Link](https://pubmed.ncbi.nlm.nih.gov/4270946). [CrossRef] [PubMed]
- Bhagavan HN, Chopra RK. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic Res. 2006;40(5):445–453. [Link](https://pubmed.ncbi.nlm.nih.gov/16692187). [CrossRef] [PubMed]
- Shay KP, Moreau RF, Smith EJ, et al. Alpha-lipoic acid as a dietary supplement: molecular mechanisms and therapeutic potential. Biochim Biophys Acta. 2009;1790(10):1149–1160. [Link](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2724994). [CrossRef] [PubMed] [PubMed Central]
- Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods. 2017;6(10):92. [Link](https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5664031/). [CrossRef] [PubMed] [PubMed Central]
- Zgheib C, Hilton SA, Dewberry LC, et al. Use of cerium oxide nanoparticles conjugated with microRNA-146a to correct diabetic wound healing impairment. J Am Coll Surg. 2019;228(1):107–115. [Link](https://pubmed.ncbi.nlm.nih.gov/30237559/). [CrossRef] [PubMed]
- Figures CE, Vogt DR, Ziegler C, et al. Curcumin induces the expression of the tumor suppressor miR-145 in WM266-4 human metastatic melanoma cells. Mol Nutr Food Res. 2015;59(3):589–602. [Link](https://pubmed.ncbi.nlm.nih.gov/25297447). [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).