1. Introduction
Colorectal cancer remains a leading cause of global morbidity and mortality, with surgical resection as the cornerstone of curative treatment (1). Treatment of colorectal cancer has evolved exponentially over time. In the UK, Colorectal Cancer is the fourth most common cancer. Depending on the stage and course of the disease, multiple treatment options exist. However, surgical resection remains the cornerstone of curative treatment. The advent of minimally invasive techniques—laparoscopic and robotic—has revolutionised colorectal surgery, offering reduced trauma, faster recovery, superior oncological clearance, and improved cosmesis. The importance of minimally invasive surgery is paramount, not only for patient outcomes, but for surgeons as well.
The emergence of robotics came with the promise of enhancing operative times as well as recovery, reduce the post-operative complications of patients as well as aid speed, surgical technique and avoid fatigue for the surgeon (2). The other technique of minimally invasive surgery is laparoscopic surgery. Laparoscopic surgery is a more familiar method of surgery for surgeons due to its earlier conception. Laparoscopic surgery emerged as the minimally invasive option when open surgery was the sole, standard procedure. Both techniques have been analysed closely in this study to ascertain the benefit, if any, of robotic surgery over its laparoscopic counterpart.
Surgical skill and human factors are without a doubt the most variable factor in this equation. A high-performing surgeon can yield the same results from different types of surgery (3).
Standardisation of surgical techniques is critical to mitigate variability and optimise outcomes (4). While prior studies have compared robotic and laparoscopic methods, heterogeneity in technique and surgeon variability has hindered the ability to determine if there is a direct link between surgical and approach and outcome when comparing the minimally invasive methods. Having a standardised approach not only allows for increased proficiency during planning of surgery but also reduces the learning curve and enable quicker development of surgical skills for trainees. It is crucial to ensure standardisation when new techniques of surgery, especially as it will inform the training pathway of future surgeons (4).
Therefore, we designed a single-surgeon study to compare outcomes using a standardised technique. Our aim was to standardise framework to minimise confounders inherent in multi-surgeon analyses, such as variation in technique, preference, and approach. This design facilitates a direct comparison of robotic versus laparoscopic colorectal resection outcomes (2). The extended five-year data collection window (2019–2024) further ensures temporal consistency in surgical methods, strengthening the validity of the findings.
2. Methods
2.1. Study Design and Population
We performed a retrospective analysis of 250 consecutive patients undergoing colorectal resection (2019–2024) by a single high-volume surgeon in the department of colorectal surgery in our institute. Procedures included hemicolectomies, anterior resections, and abdominoperineal resections. Standardisation involves the creation of Conses driven protocols for various surgical procedures, a stepwise approach during the procedure quality assessment of specimens, reduction of operative variance and improvement of training programs (5). In keeping with this, stepwise surgical techniques were used for each procedure. Published videos demonstrating both the laparoscopic and robotic techniques used are available for further details (6–9).
2.2. Data Collection
Demographic information was obtained from patient notes. Operative metrics such as conversion and intraoperative complications were found in the operative note. Operative time was recorded either on the anaesthetic chart for laparoscopic surgery or by the console for robotic surgery. For robotic cases, the console time does not include time setting up and docking the robot, or time for anastomosis completion and skin closure, therefore overall time is likely underestimated.
Postoperative complications were identified in the patient notes and discharge summaries. Complications were graded by the modified Clavien-Dindo score (10). Pathology data such as tumour staging and resection margins was obtained from the histology reports.
2.3. Statistical Analysis
Analyses were performed in R (v4.3.0). Continuous variables were compared using the t-test for normally distributed data, otherwise by the Mann-Whitney U test. Categorical
Variables were compared using chi-square or Fisher’s exact tests, the latter used when any of the expected values were less than five. Correlation analyses were performed using Pearson’s correlation coefficient. Graphs were produced by ggplot2 version 3.4.4. A p-value of less than 0.05 was considered statistically significant.
3. Results
3.1. Patient demographics were comparable between groups
A total of 250 patients were included in the study with similar baseline characteristics (
Table 1), with the exception that a higher proportion of patients undergoing laparoscopic surgery had been prescribed neoadjuvant therapy (0.49 vs. 0.3,
p = 0.006). Overall, the distribution of ASA grades differed between the two groups (
Supplementary Table 1)
, with the robotic group having a slightly higher proportion of ASA grade 1 patients (0.12 vs. 0.02,
p = 0.05). The distribution of resection types was similar between the two groups (
Supplementary Figure 1), with most operations being right hemicolectomies or anterior resections (high or low).
3.2. Analysis of intraoperative outcomes shows that conversion to open surgery was less likely with robotic surgery
No robotic cases required conversion to open surgery (
Table 2), a significant difference even compared to the low frequency of conversion of laparoscopic cases. The operative time was not significantly different between modalities. However, limited data was available in the laparoscopic group due to unavailability of the anaesthetic records, on account of the age of the cases.
We had hypothesised at the beginning of the study that operative time for the robotic cases would show a downward trend over time, due to the increased experience of the surgeon and the wider surgical team. However, this was not the case and operative time tended to either not change or even to increase (
Supplementary Figure 2).
3.3. Generic postoperative outcomes were not different between modalities
We analysed a range of postoperative outcomes including the length of hospital stay, the requirement for reoperation, mortality statistics, and the proportion experiencing a postoperative complication. None of these domains showed a significant difference between laparoscopic and robotic surgery (
Table 3).
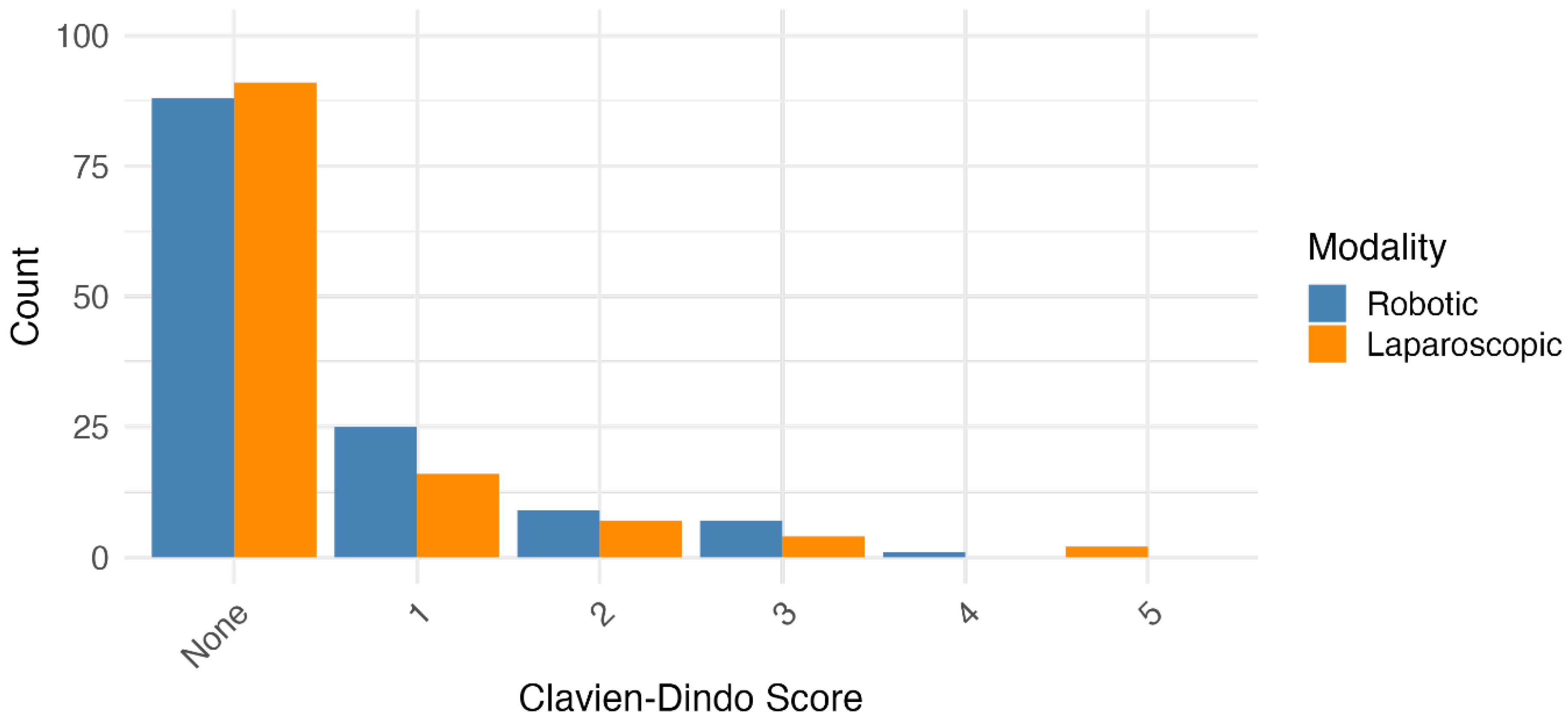
3.4. Severity and nature of postoperative complications were similar between surgical modalities
Data were collected on postoperative complications and broadly categorised (
Supplementary Table 2). There was no overall difference in the types of complications experienced by patients having laparoscopic vs. robotic surgery. Secondly, the severity of complications was graded using the Clavien-Dindo score. A comparison of the distribution of severities found no significant difference between the two groups (
Figure 1 and
Supplementary Table 3).
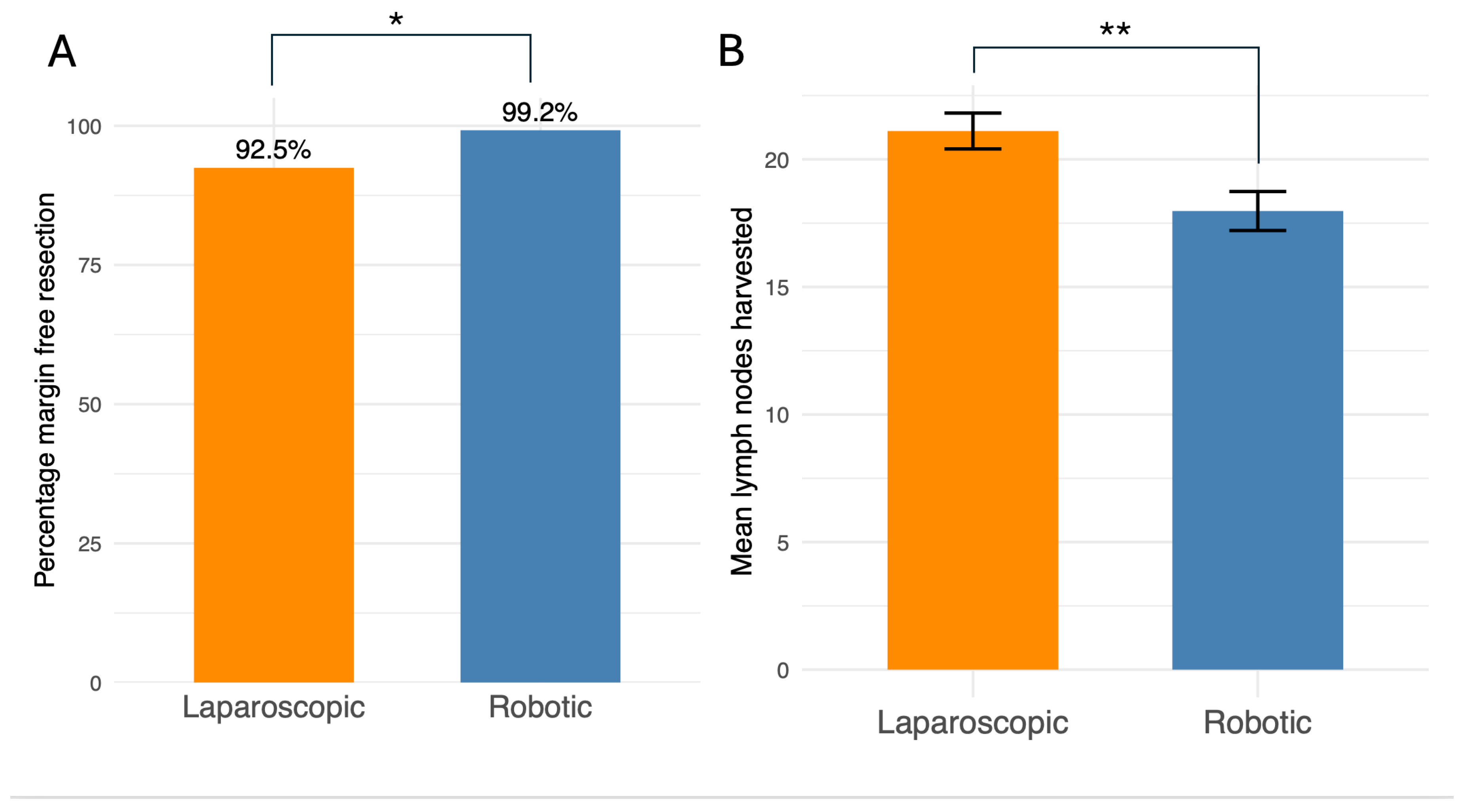
4. Robotic surgery was more likely to achieve complete resection of tumours
Most operations in this study were performed for the treatment of colorectal cancer. A key outcome of this surgery is complete histological resection of the tumour denoted as “R0” on the pathology report. In our dataset, robotic surgery was more likely to result in tumour-free margins than laparoscopic surgery (99.2 % vs. 92.5 %,
p = 0.013) (
Figure 2A). The staging of tumours as defined by the pathology reports did not differ significantly between groups (
Supplementary Table 4). The number of lymph nodes harvested is also important and a minimum of 12 nodes is generally recommended (11). Both groups achieved this threshold but on average the laparoscopic technique harvested more lymph nodes (21.1 vs. 18.0 nodes,
p = 0.003) (
Figure 2B).
5. Discussion
Our single-surgeon study analysis shows that both minimally invasive techniques provide similar postoperative outcomes in the management of colorectal malignancies with robotic surgery having more favourable intraoperative outcomes than laparoscopic. Our findings show that when using a standardised technique and limiting confounding factors by focusing on the results of a single surgeon, outcomes are comparable between both surgical modalities.
Operation times and conversion rates to open surgery are key intra-operative outcome markers, as conversion is linked to longer hospital stays, post-operative ileus, increased morbidity, and higher overall costs (12,13). The laparoscopic group had a conversion rate of 5.8% and the robotic group had a significantly lower conversion rate of 0%. Additionally, our R0 rates were 92.5% and 99.2% for laparoscopic and robotic surgery respectively.
The literature reported a 17.9 +/- 10% conversion rate from laparoscopic to open surgery during elective colorectal resection (15), whereas our laparoscopic group's rate is notably lower. Other studies also report lower complication and conversion rates with robotic surgery (16,17). This could be due to increased precision, articulating instruments and 3D clearer field of vision due to stereoscopic optics of robotic surgeries, which proves particularly useful in complex surgeries that would otherwise lead to open surgery.
Although our patient selection was comparable in age, gender and BMI; the data suggests that the laparoscopic group had more advanced disease than the robotic group, which is a confounding factor that may have led to our increased conversion rate.
Whilst our intraoperative outcomes were favourable towards the robotic surgery, operation times and post-operative outcomes such as complications, length of stay and mortality showed no difference between both modalities.
There was no difference in operative time between the two modalities, although it is possible that robotic surgery takes longer when considering the set up and closure time not included in this study. An important caveat was that operative time data was limited in the laparoscopic group. Furthermore, there was no evidence that robotic surgery becomes faster with the surgeon’s experience, at least in the first few years of operating. It remains possible that with a longer period of training or developments in the equipment itself that operative time could improve. Although right hemicolectomies appeared to get slower over time, this is likely to be due to a transition from extracorporeal to intracorporeal anastomoses throughout the study.
The current research is mixed in its findings regarding operative times for robotic procedures. Whilst, Yang et al., 2021 found no significant difference in times and thus replicated our findings, other studies found that there were faster times in the laparoscopic group compared to the robotic in their meta-analyses. This could be associated with the time required for setting up and docking time as well as the learning curve of starting robotic surgery (16–18).
A comprehensive assessment of perioperative outcomes was undertaken, evaluating 30-day mortality, postoperative complications, length of hospital stay, and 30-day re-operation rates. No significant differences were observed between the robotic and laparoscopic cohorts, suggesting comparable short-term surgical effectiveness and safety profiles for both techniques (19). Although the study was underpowered to establish statistical significance, the absence of mortality in the robotic cohort is notable.
While the overall severity of complications, as classified by the Clavien-Dindo system, was similar between groups, it is noteworthy that only the laparoscopic cohort experienced complications of grade III or higher. This discrepancy is likely attributable to baseline differences in patient population characteristics, wherein a greater prevalence of advanced disease within the laparoscopic group may have increased susceptibility to major morbidities.
Nonetheless, other observational studies and meta-analyses have compared robotic and laparoscopic surgery approaches in terms of complication rates and have found that both approaches have comparable post operative complication rates. Gahunia et al. (2025) reported superiority of robotic surgery to the laparoscopic approach in terms of conversion rates especially in obese individuals and male patients with low rectal tumours while reporting comparable outcomes in complication rates and blood loss (20). Furthermore, Asklid et al. (2022) also reported comparable post operative complication rates including Cavien- Dindo grades 1-5 for robotic versus laparoscopic approaches, 35.9% compared to 31.2% respectively (21). Robotic surgery is still in the early phases of recognition and acceptability, but it would be prudent to say that it is equally safe when compared to the laparoscopic approach.
Regarding oncological efficacy, the laparoscopic group was associated with a higher lymph node yield. Despite this, no concomitant difference in pathological staging was identified, a finding consistent with the results reported by Yang et al. (2021) (22). This contrasts with the systematic analysis by Waters et al. (2020), which concluded a higher lymph node harvest was achievable with robotic assistance, highlighting an ongoing discourse within the literature (19).
Within our study, the similar findings between the postoperative outcomes and operative times of both modalities supports our claim that standardisation is the key driver in ensuring consistent patient outcomes as opposed to differing surgical modalities. The work of a single surgeon remained unaffected using different modalities despite the technical advantages offered using robotic procedures even after developing proficiency over the years using the technique.
Standardisation in surgery and how it ultimately affects outcomes has been a topic of much debate. It allows for a more consistent high-quality care and ensures that a reduction in the complication rate could be achieved (5). Robotic surgery is no different to these integral components of standardisation. Along with standardisation of surgical technique, a structured training program is imperative in achieving optimum results. Coleman et al. (2011) demonstrated that through their National Training Program for Laparoscopic Colorectal Surgery (LapCo) that a standardised training program achieves optimum results without compromising patient safety (23). Similarly, Koc et al. (2024) demonstrated that using a structured and standardised pathway for robotic colorectal surgery, they were able to achieve favourable short term oncological and clinical outcomes (4). Furthermore, a structured training program also elucidates the significant reduction in the time to overcome learning process without doing any patient harm as reported by Panteleimonitis et al. (2021), where different surgeons were able to achieve competency and overcome the learning process in a short matter of time while achieving good oncological and safe clinical outcomes (24).
A primary limitation of this study is its retrospective design. The collation of data was contingent upon the availability and completeness of historical patient records. Notably, critical variables such as operative times were frequently absent, potentially due to omissions in initial data entry. A prospective study design would mitigate these issues of missing data and facilitate the collection of more granular perioperative metrics, such as estimated blood loss, time to first flatus, and time to first oral intake, which are established indicators of bowel recovery (22).
Baseline differences in the populations of both cohort groups that suggest the slightly worse postoperative outcomes such as the 30-day mortality rate and higher operative times were due to confounding factors rather than the implication that robotic surgery is safer than laparoscopic. The robotic group contained a greater proportion of healthier patients with 13% of patients having an ASA Grade 1 compared to just 0.02% in the laparoscopic group. They also had fewer patients that required neoadjuvant therapy (28% patients compared to 43% in the laparoscopic group) suggesting that their malignancies were less advanced.
Although distinct patterns of complications were observed between the operative cohorts, the present study was not powered to determine a statistically significant association between complication type and surgical approach. Future research with larger sample sizes is warranted to elucidate these trends.
As a cohort of patients treated by a single surgeon within a single institute, this study provides significant strength by controlling for technical variability and standardising the surgical approach, though this may limit the generalisability of the findings. Nevertheless, this highly standardised methodology enables a valuable comparison of intraoperative and postoperative outcomes. It underscores the critical principle that robotic and laparoscopic platforms are tools to facilitate minimally invasive surgery, and that the surgeon's standardised technique is paramount for achieving consistent results (4,24,25). This work thus offers a foundational understanding to guide future clinical management and inform the priorities of larger, multi-centre research (26).
Patients benefit from either approach and the decision of whether to use laparoscopic or robotic techniques cannot be easily approached from the perspective of clinical outcomes. To assess which patients benefit more from either technique, other factors such as patient or surgeon preferences and cost-benefit analyses rather than clinical outcomes alone should be used. A 2023 metanalysis found a 17% increase in median hospitalisation cost for the robotic group (27). Amidst the growing concerns regarding cost-effectiveness in the NHS, such research would be very useful in assessing whether centres that do not have robotic machines yet, would benefit from them.
6. Conclusions
Robotic colorectal resection demonstrates trends toward improved intraoperative and pathological outcomes compared to laparoscopy, though perioperative morbidity remains comparable. Standardisation mitigates technical variability, demonstrating that for skilled surgeons, the choice of platform may be influenced more by economic factors and preference than by major differences in clinical efficacy.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Funding
This study was supported by Northampton General Hospital.
Conflicts of Interest
The authors declare no conflicts of interest.
Ethics
No identifiable patient information was used in this study
References
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021 May;71(3):209–49. [CrossRef]
- Wang W, Liu J, Wang J, Li L, Kong D, Wang J. Comparative study of robotic-assisted vs. laparoscopic surgery for colorectal cancer: a single-center experience. Front Oncol. 2025 Jan 7;14. [CrossRef]
- Butnari V, Sultana M, Mansuri A, Rao C, Kaul S, Boulton R, et al. Comparison of early surgical outcomes of robotic and laparoscopic colorectal cancer resection reported by a busy district general hospital in England. Sci Rep. 2024 Apr 22;14(1):9227. [CrossRef]
- Koc MA, Thomas MS, Mavrantonis S, Panteleimonitis S, Harper M, Sanjay C, et al. Structured training pathway for robotic colorectal surgery: Short-term outcomes from five UK centres. Colorectal Dis. 2024 Nov;26(11):1965–70. [CrossRef]
- Eto K, Urashima M, Kosuge M, Ohkuma M, Noaki R, Neki K, et al. Standardization of surgical procedures to reduce risk of anastomotic leakage, reoperation, and surgical site infection in colorectal cancer surgery: a retrospective cohort study of 1189 patients. Int J Colorectal Dis. 2018 Jun;33(6):755–62. [CrossRef]
- Walmsley J, Max S, Hughes G, Ahmed J. https://youtu.be/06q5JPCOjYo?feature=shared. 2021 [cited 2025 Sep 23]. A Stepwise Approach to Laparoscopic Right Hemi-Colectomy with D2 Excision. Available online: https://youtu.be/06q5JPCOjYo?feature=shared.
- Walmsley J, Marcu V, Riaz S, Ahmed J. https://youtu.be/spJKtqKjYb0?feature=shared. 2020 [cited 2025 Sep 23]. A Stepwise Approach to LAR. An Illustrated Video for Training Colorectal Surgeons. Available online: https://youtu.be/spJKtqKjYb0?feature=shared.
- Rehman A, Rehman M, Malik K, Ahmed J. https://youtu.be/1n3hJ5XPJv8?feature=shared. 2024 [cited 2025 Sep 23]. Robotic Right Hemicolectomy: CME Approach and D3 Lymphadenectomy. Available online: https://youtu.be/1n3hJ5XPJv8?feature=shared.
- Khan F, Rehman A, Ur-Rehman M, Ahmed J. https://youtu.be/uFL4aIKy_yE?feature=shared. 2025 [cited 2025 Sep 23]. Robotic Low Anterior Resection. Available online: https://youtu.be/uFL4aIKy_yE?feature=shared.
- Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications. Ann Surg. 2004 Aug;240(2):205–13. [CrossRef]
- Liu Q, Huang M, Yang J, Jiang M, Zhao Z, Zhao H, et al. Association between the number of retrieved lymph nodes and demographic/tumour-related characteristics in colorectal cancer: a systematic review and meta-analysis. BMJ Open. 2023 Dec 22;13(12):e072244. [CrossRef]
- Allaix ME, Furnée EJB, Mistrangelo M, Arezzo A, Morino M. Conversion of laparoscopic colorectal resection for cancer: What is the impact on short-term outcomes and survival? World J Gastroenterol. 2016 Oct 7;22(37):8304–13. [CrossRef]
- Cleary RK, Mullard AJ, Ferraro J, Regenbogen SE. The cost of conversion in robotic and laparoscopic colorectal surgery. Surg Endosc. 2018 Mar;32(3):1515–24. [CrossRef]
- Giglio MC, Celentano V, Tarquini R, Luglio G, De Palma GD, Bucci L. Conversion during laparoscopic colorectal resections: a complication or a drawback? A systematic review and meta-analysis of short-term outcomes. Int J Colorectal Dis. 2015 Nov;30(11):1445–55. [CrossRef]
- Clancy C, O’Leary DP, Burke JP, Redmond HP, Coffey JC, Kerin MJ, et al. A meta-analysis to determine the oncological implications of conversion in laparoscopic colorectal cancer surgery. Colorectal Dis. 2015 Jun;17(6):482–90. [CrossRef]
- Wang X, Cao G, Mao W, Lao W, He C. Robot-assisted versus laparoscopic surgery for rectal cancer: A systematic review and meta-analysis. J Cancer Res Ther. 2020;16(5):979. [CrossRef]
- Flynn J, Larach JT, Kong JCH, Rahme J, Waters PS, Warrier SK, et al. Operative and oncological outcomes after robotic rectal resection compared with laparoscopy: a systematic review and meta-analysis. ANZ J Surg. 2023 Mar 10;93(3):510–21. [CrossRef]
- Cuk P, Kjær MD, Mogensen CB, Nielsen MF, Pedersen AK, Ellebæk MB. Short-term outcomes in robot-assisted compared to laparoscopic colon cancer resections: a systematic review and meta-analysis. Surg Endosc. 2022 Jan 1;36(1):32–46. [CrossRef]
- Waters PS, Cheung FP, Peacock O, Heriot AG, Warrier SK, O’Riordain DS, et al. Successful patient-oriented surgical outcomes in robotic vs. laparoscopic right hemicolectomy for cancer – a systematic review. Colorectal Disease. 2020 May 4;22(5):488–99. [CrossRef]
- Gahunia S, Wyatt J, Powell SG, Mahdi S, Ahmed S, Altaf K. Robotic-assisted versus laparoscopic surgery for colorectal cancer in high-risk patients: a systematic review and meta-analysis. Tech Coloproctol. 2025 Apr 8;29(1):98. [CrossRef]
- Asklid D, Ljungqvist O, Xu Y, Gustafsson UO. Short-term outcome in robotic vs. laparoscopic and open rectal tumor surgery within an ERAS protocol: a retrospective cohort study from the Swedish ERAS database. Surg Endosc. 2022 Mar;36(3):2006–17. [CrossRef]
- Hu DP, Zhu XL, Wang H, Liu WH, Lv YC, Shi XL, et al. Robotic-assisted versus conventional laparoscopic surgery for colorectal cancer: Short-term outcomes at a single center. Indian J Cancer. 2021;58(2):225–31. [CrossRef]
- Coleman MG, Hanna GB, Kennedy R, National Training Programme Lapco. The National Training Programme for Laparoscopic Colorectal Surgery in England: a new training paradigm. Colorectal Dis. 2011 Jun;13(6):614–6. [CrossRef]
- Panteleimonitis S, Miskovic D, Bissett-Amess R, Figueiredo N, Turina M, Spinoglio G, et al. Short-term clinical outcomes of a European training programme for robotic colorectal surgery. Surg Endosc. 2021 Dec;35(12):6796–806. [CrossRef]
- Panteleimonitis S, Popeskou S, Aradaib M, Harper M, Ahmed J, Ahmad M, et al. Implementation of robotic rectal surgery training programme: importance of standardisation and structured training. Langenbecks Arch Surg. 2018 Sep 20;403(6):749–60. [CrossRef]
- Negrut RL, Cote A, Caus VA, Maghiar AM. Systematic Review and Meta-Analysis of Laparoscopic versus Robotic-Assisted Surgery for Colon Cancer: Efficacy, Safety, and Outcomes-A Focus on Studies from 2020-2024. Cancers (Basel). 2024 Apr 18;16(8). [CrossRef]
- Park JS, Lee SM, Choi GS, Park SY, Kim HJ, Song SH, et al. Comparison of Laparoscopic Versus Robot-Assisted Surgery for Rectal Cancers. Ann Surg. 2023 Jul;278(1):31–8. [CrossRef]
- Dindo D, Demartines N, Clavien PA. Classification of Surgical Complications. Ann Surg. 2004 Aug;240(2):205–13. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).