Submitted:
28 September 2025
Posted:
29 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Overview of Fishery Anesthetics
3. Pharmacological Actions and Safety Assessment
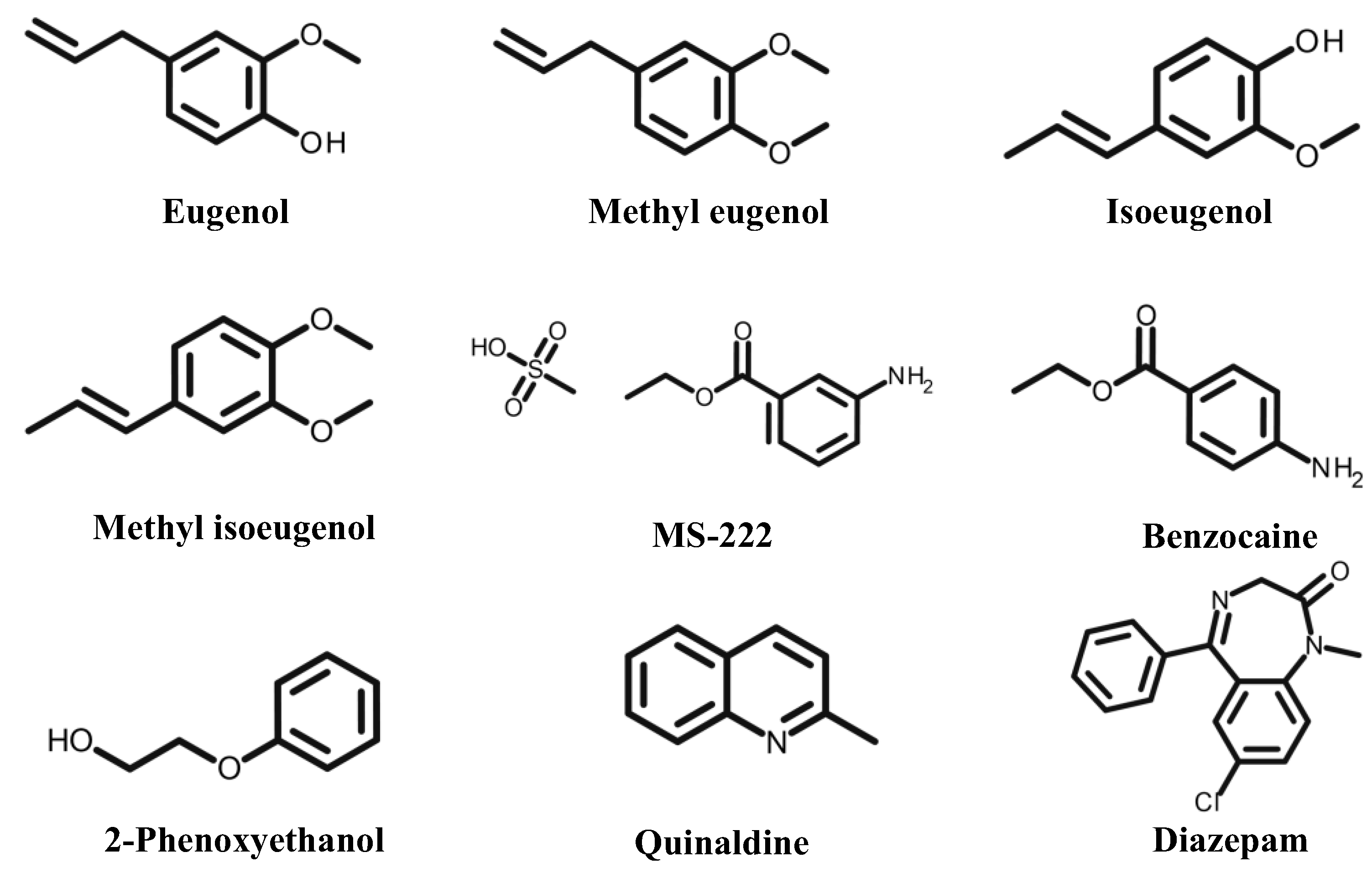
3.1. Eugenol
3.2. MS-222
3.3. Benzocaine
3.5. Diazepam
3.6. Quinaldine
4. Analytical Methods for Residue Detection
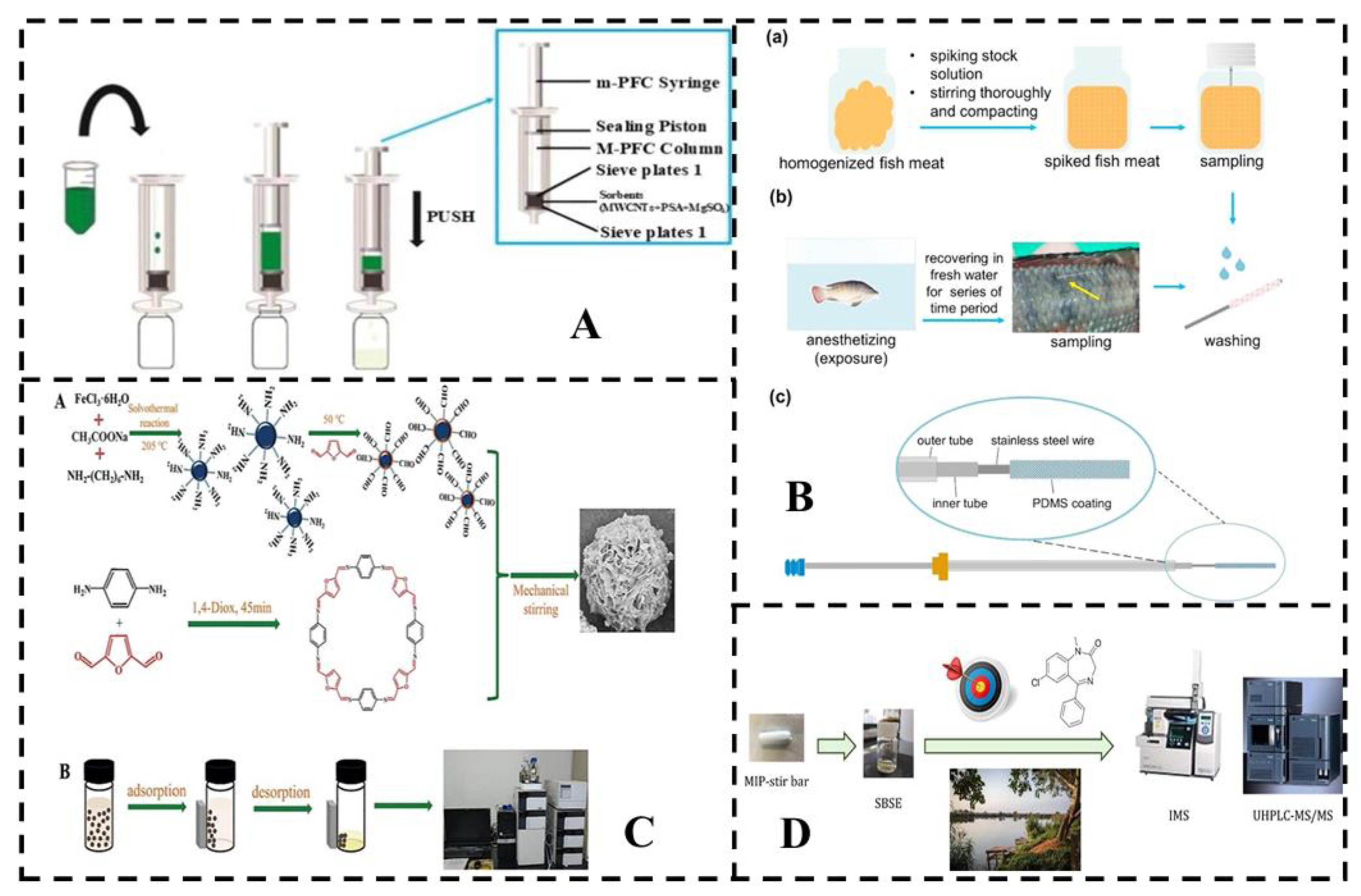
4.1. Instrument detection
5.1.1. GAS chromatography (GC, GC-MS)
4.1.2. Liquid Chromatograph (LC or HPLC)
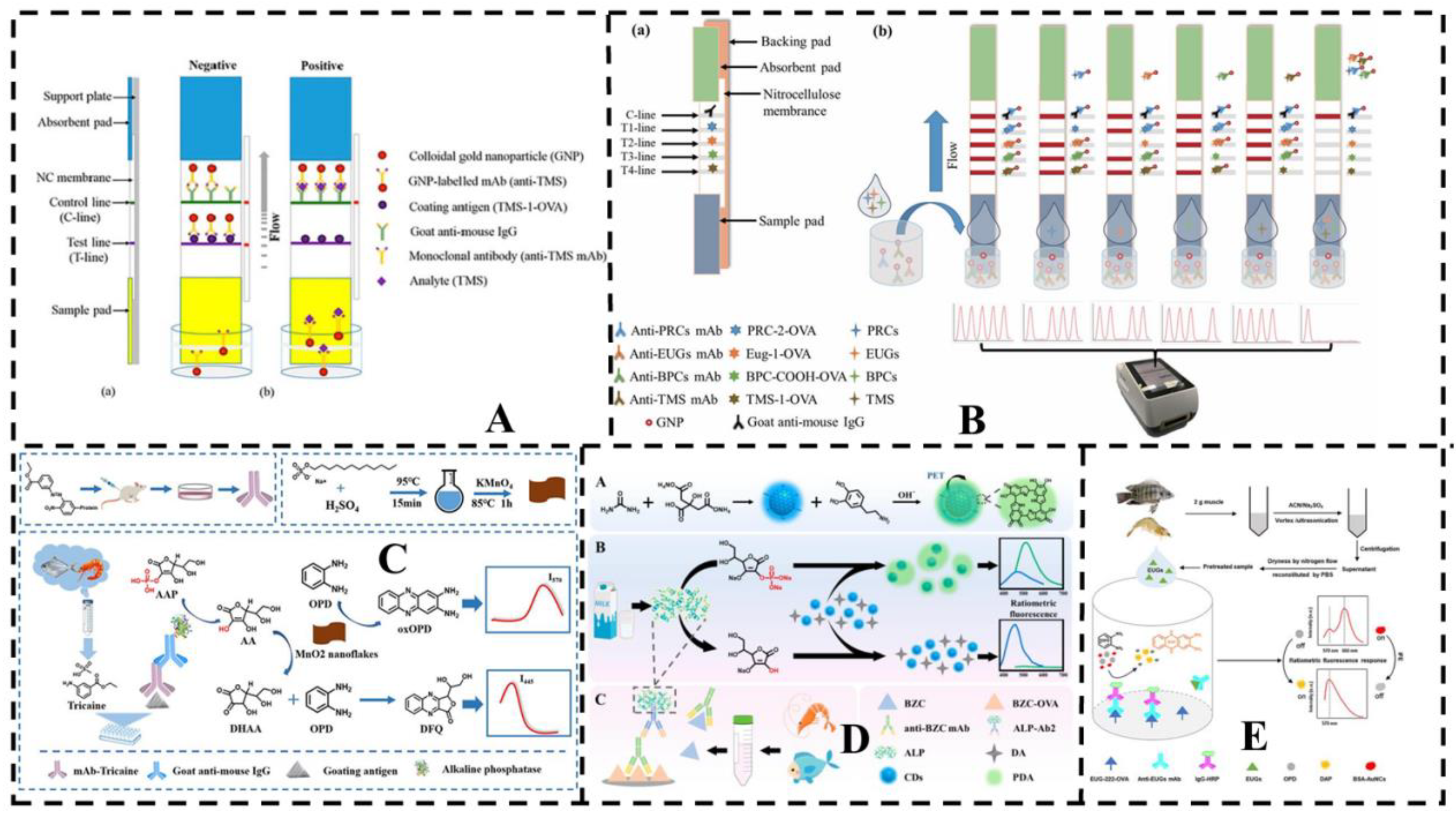
4.2. Rapid detection
4.2.1. Immunoassays
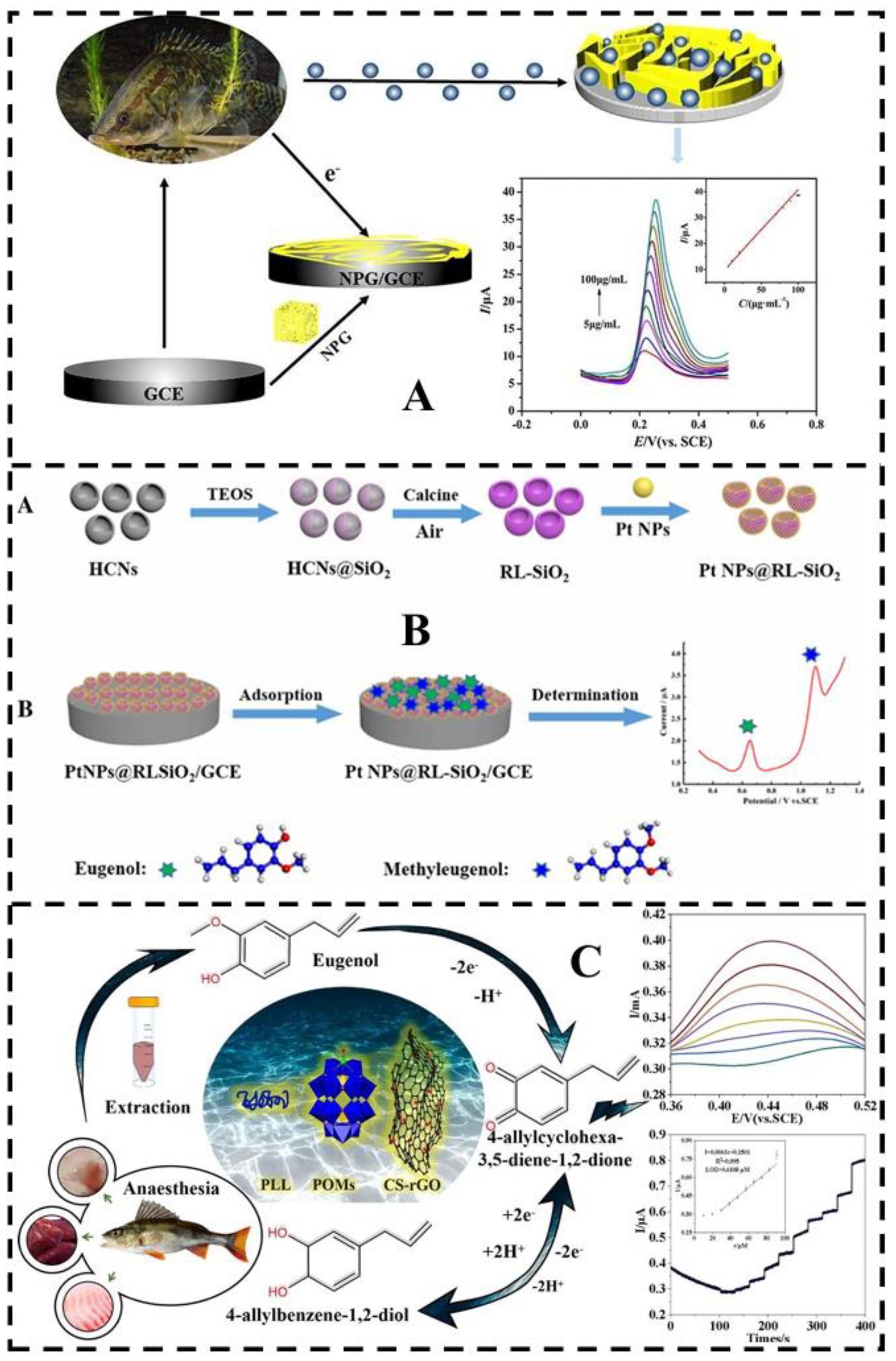
4.2.2. Electrochemical Sensor
5. Conclusion
- Bao-Zhu Jia: Investigation, Methodology, Writing – original draft. Xue-Ying Rui: Data curation, Investigation, Methodology, Editing the draft. Yu Wang: Writing – review & editing, Resources. Xi Zeng: Writing – review & editing. Shu-Jing Sheng: Supervision, Writing – review & editing. Bi-Jian Zeng: Conceptualization, Funding acquisition. Zhen-Lin Xu: Resources, Funding acquisition. Lin Luo: Investigation, Resources, Funding acquisition.
Declaration of Competing Interest:
Data availability:
Acknowledgments
References
- Zapata-Guerra, N.A.; Rueda-Gomez, D.S.; Lozano-Villegas, K.J.; Herrera-Sanchez, M.P.; Uribe-Garcia, H.F.; Rondon-Barragan, I.S. Menthol as anaesthetic for red-bellied pacu (Piaractus brachypomus) and its effect onHIF1aandGlucoRgene expression. Aquaculture Research 2020, 51, 4421-4429. [CrossRef]
- Park, I.-S. The Anesthetic Effects of Clove Oil and MS-222 on Far Eastern Catfish, Silurus asotus. Development & reproduction 2019, 23, 183-191. [CrossRef]
- Suski, C.D. Development of Carbon Dioxide Barriers to Deter Invasive Fishes: Insights and Lessons Learned from Bigheaded Carp. Fishes 2020, 5. [CrossRef]
- Atsumi, T.; Fujisawa, S.; Tonosaki, K. A comparative study of the antioxidant/prooxidant activities of eugenol and isoeugenol with various concentrations and oxidation conditions. Toxicology in Vitro 2005, 19, 1025-1033. [CrossRef]
- Gressler, L.T.; Riffel, A.P.K.; Parodi, T.V.; Saccol, E.M.H.; Koakoski, G.; da Costa, S.T.; Pavanato, M.A.; Heinzmann, B.M.; Caron, B.; Schmidt, D.; et al. Silver catfish Rhamdia quelen immersion anaesthesia with essential oil of Aloysia triphylla (L'Herit) Britton or tricaine methanesulfonate: effect on stress response and antioxidant status. Aquaculture Research 2014, 45, 1061-1072. [CrossRef]
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Reviews in Fisheries Science & Aquaculture 2018, 26, 417-442. [CrossRef]
- da Paz, C.A.; da Costa, B.M.P.A.; Hamoy, M.K.O.; dos Santos, M.F.; da Rocha, L.L.; da Silva Deiga, Y.; de Sousa Barbosa, A.; do Amaral, A.L.G.; Câmara, T.M.; Barbosa, G.B.; et al. Establishing a safe anesthesia concentration window for Nile tilapia (Oreochromis niloticus) (Linnaeus 1758) by monitoring cardiac activity in eugenol immersion baths. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2024, 278, 109839. [CrossRef]
- Rucinque, D.S.; Ferreira, P.F.; Leme, P.R.P.; Lapa-Guimarães, J.; Viegas, E.M.M. Ocimum americanum and Lippia alba essential oils as anaesthetics for Nile tilapia: Induction, recovery of apparent unconsciousness and sensory analysis of fillets. Aquaculture 2021, 531, 735902. [CrossRef]
- Gladden, J.N.; Brainard, B.M.; Shelton, J.L.; Camus, A.C.; Divers, S.J. Evaluation of isoeugenol for anesthesia in koi carp (Cyprinus carpio). American Journal of Veterinary Research 2010, 71, 859-866. [CrossRef]
- Speare, R.; Speare, B.; Muller, R.; Bishop, P. ANESTHESIA OF TADPOLES OF THE SOUTHERN BROWN TREE FROG (LITORIA EWINGII) WITH ISOEUGENOL (AQUI-S). Journal of Zoo and Wildlife Medicine 2014, 45, 492-496. [CrossRef]
- Pattanasiri, T.; Taparhudee, W.; Suppakul, P. Acute toxicity and anaesthetic effect of clove oil and eugenol on Siamese fighting fish, Betta splendens. Aquaculture International 2017, 25, 163-175. [CrossRef]
- Hobani, Y.H.; Mohan, S.; Shaheen, E.; Abdelhaleem, A.; Faruque Ahmad, M.; Bhatia, S.; Abou-Elhamd, A.S. Gastroprotective effect of low dose Eugenol in experimental rats against ethanol induced toxicity: Involvement of antiinflammatory and antioxidant mechanism. Journal of Ethnopharmacology 2022, 289, 115055. [CrossRef]
- Tago, A.; Yokoyama, S.; Ishikawa, M.; Koshio, S. Pharmacokinetics of Eugenol in Japanese Flounder, Paralichthys olivaceus. Journal of the World Aquaculture Society 2018, 49, 780-787. [CrossRef]
- Kiessling, A.; Johansson, D.; Zahl, I.H.; Samuelsen, O.B. Pharmacokinetics, plasma cortisol and effectiveness of benzocaine, MS-222 and isoeugenol measured in individual dorsal aorta-cannulated Atlantic salmon (Salmo salar) following bath administration. Aquaculture 2009, 286, 301-308. [CrossRef]
- Tang, Y.; Zhang, H.; Yang, G.; Fang, C.; Kong, C.; Tian, L.; Huang, X. Pharmacokinetics studies of eugenol in Pacific white shrimp (Litopenaeus vannamei) after immersion bath. Bmc Veterinary Research 2022, 18. [CrossRef]
- Ke, C.; Liu, Q.; Huang, K.; Mo, M.; Chen, H.; Cheng, B. Effect of Water Temperature on the Depletion of Eugenol in Sea Bass under the Simulated Settings in Handling and Transport. Applied Sciences 2021, 11. [CrossRef]
- Priya, P.S.; Guru, A.; Meenatchi, R.; Haridevamuthu, B.; Velayutham, M.; Seenivasan, B.; Pachaiappan, R.; Rajagopal, R.; Kuppusamy, P.; Juliet, A.; et al. Syringol, a wildfire residual methoxyphenol causes cytotoxicity and teratogenicity in zebrafish model. Science of The Total Environment 2023, 864, 160968. [CrossRef]
- Tao, Y.; Du, C.; Duan, B.; Wang, W.; Guo, H.; Feng, J.; Xu, H.; Li, Y. Eugenol exposure inhibits embryonic development and swim bladder formation in zebrafish. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2023, 268, 109602. [CrossRef]
- Hume, W.R. Basic Biological Sciences Effect of Eugenol on Respiration and Division in Human Pulp, Mouse Fibroblasts, and Liver Cells in vitro. Journal of Dental Research 1984, 63, 1262-1265. [CrossRef]
- Jeng, J.H.; Hahn, L.J.; Lu, E.; Wang, Y.; Kuo, M.Y.P. Eugenol Triggers Different Pathobiological Effects on Human Oral Mucosal Fibroblasts 1. Journal of Dental Research 1994, 73, 1050-1055. [CrossRef]
- Thompson, D.C.; Constantin-Teodosiu, D.; Moldéus, P. Metabolism and cytotoxicity of eugenol in isolated rat hepatocytes. Chemico-Biological Interactions 1991, 77, 137-147. [CrossRef]
- Escobar-Garcia, M.; Rodriguez-Contreras, K.; Ruiz-Rodriguez, S.; Pierdant-Perez, M.; Cerda-Cristerna, B.; Pozos-Guillen, A. Eugenol Toxicity in Human Dental Pulp Fibroblasts of Primary Teeth. Journal of Clinical Pediatric Dentistry 2016, 40, 312-318.
- Houdkova, M.; Rondevaldova, J.; Doskocil, I.; Kokoska, L. Evaluation of antibacterial potential and toxicity of plant volatile compounds using new broth microdilution volatilization method and modified MTT assay. Fitoterapia 2017, 118, 56-62. [CrossRef]
- Kasugai, S.; Hasegawa, N.; Ogura, H. Application of the MTT Colorimetric Assay to Measure Cytotoxic Effects of Phenolic Compounds on Established Rat Dental Pulp Cells. Journal of Dental Research 1991, 70, 127-130. [CrossRef]
- Chang, Y.-C.; Tai, K.-W.; Huang, F.-M.; Huang, M.-F. Cytotoxic and Nongenotoxic Effects of Phenolic Compounds in Human Pulp Cell Cultures. Journal of Endodontics 2000, 26, 440-443. [CrossRef]
- Kozam, G.; Mantell, G.M. The Effect of Eugenol on Oral Mucous Membranes. Journal of Dental Research 1978, 57, 954-957. [CrossRef]
- Ke, C.; Liu, Q.; Li, L.; Chen, J.; Wang, X.; Huang, K. Simultaneous determination of eugenol, isoeugenol and methyleugenol in fish fillet using gas chromatography coupled to tandem mass spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences 2016, 1031, 189-194. [CrossRef]
- Li, J.; Zhang, J.; Liu, Y. Optimization of solid-phase-extraction cleanup and validation of quantitative determination of eugenol in fish samples by gas chromatography-tandem mass spectrometry. Analytical and Bioanalytical Chemistry 2015, 407, 6563-6568. [CrossRef]
- Zhao, D.-H.; Ke, C.-L.; Liu, Q.; Wang, X.-F.; Wang, Q.; Li, L.-D. Elimination kinetics of eugenol in grass carp in a simulated transportation setting. Bmc Veterinary Research 2017, 13. [CrossRef]
- Mohammadi Nejad, S.; Ozgunes, H.; Basaran, N. Pharmacological and Toxicological Properties of Eugenol. Turkish journal of pharmaceutical sciences 2017, 14, 201-206. [CrossRef]
- Hu, Q.; Zhou, M.F.; Wei, S.Y. Progress on the Antimicrobial Activity Research of Clove Oil and Eugenol in the Food Antisepsis Field. Journal of Food Science 2018, 83, 1476-1483. [CrossRef]
- Carter, K.M.; Woodley, C.M.; Brown, R.S. A review of tricaine methanesulfonate for anesthesia of fish. Reviews in Fish Biology and Fisheries 2011, 21, 51-59. [CrossRef]
- Matthews, M.; Varga, Z.M. Anesthesia and Euthanasia in Zebrafish. ILAR Journal 2012, 53, 192-204. [CrossRef]
- Matsche, M.A. Evaluation of tricaine methanesulfonate (MS-222) as a surgical anesthetic for Atlantic Sturgeon Acipenser oxyrinchus oxyrinchus. Journal of Applied Ichthyology 2011, 27, 600-610. [CrossRef]
- Fraser, T.W.K.; Mayer, I.; Skjaeraasen, J.E.; Hansen, T.; Fjelldal, P.G. The effect of triploidy on the efficacy and physiological response to anesthesia with MS 222 and isoeugenol in Atlantic salmon post-smolts. Aquaculture International 2014, 22, 1347-1359. [CrossRef]
- Park, G.D.; Mitchel, J.T. Working with the US Food and Drug Administration to obtain approval of products under the Animal Rule. In Countermeasures against Chemical Threats, Laskin, J.D., Ed.; Annals of the New York Academy of Sciences; 2016; Volume 1374, pp. 10-16.
- Kolanczyk, R.C.; Fitzsimmons, P.N.; McKim, J.M.; Erickson, R.J.; Schmieder, P.K. Effects of anesthesia (tricaine methanesulfonate, MS222) on liver biotransformation in rainbow trout (Oncorhynchus mykiss). Aquatic Toxicology 2003, 64, 177-184. [CrossRef]
- Popovic, N.T.; Strunjak-Perovic, I.; Coz-Rakovac, R.; Barisic, J.; Jadan, M.; Berakovic, A.P.; Klobucar, R.S. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. Journal of Applied Ichthyology 2012, 28, 553-564. [CrossRef]
- Zou, N.; Chen, R.; Qin, Y.; Song, S.; Tang, X.; Pan, C. Comparison of pulse glow discharge-ion mobility spectrometry and liquid chromatography with tandem mass spectrometry based on multiplug filtration cleanup for the analysis of tricaine mesylate residues in fish and water. Journal of Separation Science 2016, 39, 3638-3646. [CrossRef]
- Bernstein, P.S.; Digre, K.B.; Creel, D.J. Retinal toxicity associated with occupational exposure to the fish anesthetic MS-222. American journal of ophthalmology 1997, 124, 843-844. [CrossRef]
- Rairat, T.; Chi, Y.; Hsieh, C.Y.; Liu, Y.K.; Chuchird, N.; Chou, C.C. Determination of Optimal Doses and Minimum Effective Concentrations of Tricaine Methanesulfonate, 2-Phenoxyethanol and Eugenol for Laboratory Managements in Nile Tilapia (Oreochromis niloticus). Animals 2021, 11. [CrossRef]
- Ferreira, A.L.; de Souza e Silva, W.; Neves, L.d.C.; Ferreira, N.S.; Takata, R.; Luz, R.K. Benzocaine and menthol as anesthetics for the African cichlid Aulonocara nyassae. Aquaculture International 2020, 28, 1837-1846. [CrossRef]
- Gomes, L.C.; Chippari-Gomes, A.R.; Lopes, N.P.; Roubach, R.; Araujo-Lima, C.A.R.M. Efficacy of benzocaine as an anesthetic in juvenile tambaqui Colossoma macropomum. Journal of the World Aquaculture Society 2001, 32, 426-431. [CrossRef]
- Stehly, G.R.; Meinertz, J.R.; Gingerich, W.H. Effects of temperature on the elimination of benzocaine and acetylated benzocaine residues from the edible fillet of rainbow trout (Oncorhynchus mykiss). Food additives and contaminants 2000, 17, 387-392. [CrossRef]
- Meinertz, J.R.; Gingerich, W.H.; Allen, J.L. Metabolism and elimination of benzocaine by rainbow trout, Oncorhynchus mykiss. Xenobiotica; the fate of foreign compounds in biological systems 1991, 21, 525-533. [CrossRef]
- Jiwa, N.; Ibe, U.; Beri, R. Benzocaine Spray-Induced Methemoglobinemia. American Journal of Respiratory and Critical Care Medicine 2018, 197.
- Khair-ul-Bariyah, S.; Arshad, M.; Ali, M.; Din, M.I.; Sharif, A.; Ahmed, E. Benzocaine: Review on a Drug with Unfold Potential. Mini-Reviews in Medicinal Chemistry 2020, 20, 3-11. [CrossRef]
- Brock, W.J.; Bell, T.A. The In Vitro and In Vivo Genotoxicity of Benzocaine: A Brief Communication. International Journal of Toxicology 2012, 31, 222-227. [CrossRef]
- Zealand, F.S.A.N. MAXIMUM RESIDUE LIMITS–BENZOCAINE (LOCAL ANAESTHETIC) Available online: https://www.foodstandards.gov.au/sites/default/files/food-standards-code/applications/Documents/A538_Benzocaine_FAR.pdf (accessed on.
- Svacina, P.; Priborsky, J.; Blecha, M.; Policar, T.; Velisek, J. Haematological and biochemical response of burbot (Lota lota L.) exposed to four different anaesthetics. Czech Journal of Animal Science 2016, 61, 414-420. [CrossRef]
- Imamura-Kojima, H.; Takashima, F.; Yoshida, T. Absorption, distribution and excretion of 2-phenoxyetanol in rainbow trout. Nippon Suisan Gakkaishi 1987, 53, 1339-1342. [CrossRef]
- Burka, J.F.; Hammell, K.L.; Horsberg, T.E.; Johnson, G.R.; Rainnie, D.J.; Speare, D.J. Drugs in salmonid aquaculture – A review. Journal of Veterinary Pharmacology and Therapeutics 1997, 20, 333-349. [CrossRef]
- Öğretmen, F.; Gökçek, C. Comparative Efficacy of Three Anesthetic Agents on Juvenile African Catfish, Clarias gariepinus (Burchell, 1822). Turkish Journal of Fisheries and Aquatic Sciences 2013, 13, 51-56. [CrossRef]
- Coyle, S.D.; Durborow, R.M.; Tidwell, J.H. Anesthetics in aquaculture; Southern Regional Aquaculture Center Texas: 2004; Volume 3900.
- Priborsky, J.; Velisek, J. A Review of Three Commonly Used Fish Anesthetics. Reviews in Fisheries Science & Aquaculture 2018, 26.
- Zahl, I.H.; Samuelsen, O.; Kiessling, A. Anaesthesia of farmed fish: implications for welfare. Fish Physiology and Biochemistry 2012, 38, 201-218. [CrossRef]
- Uçar, A.; Atamanalp, M. The Effects of Natural (Clove Oil) and Synthetical (2-phenoxyethanol) Anesthesia Substances on Hematology Parameters of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta fario). Journal of Animal and Veterinary Advances - J ANIM VET ADV 2010, 9, 1925-1933. [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; et al. RIFM fragrance ingredient safety assessment, 2-phenoxyethanol, CAS Registry Number 122-99-6. Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association 2019, 130 Suppl 1, 110629. [CrossRef]
- Hwang, J.-h.; Jeong, H.; Jung, Y.-o.; Nam, K.T.; Lim, K.-M. Skin irritation and inhalation toxicity of biocides evaluated with reconstructed human epidermis and airway models. Food and Chemical Toxicology 2021, 150, 112064. [CrossRef]
- Troutman, J.A.; Rick, D.L.; Stuard, S.B.; Fisher, J.; Bartels, M.J. Development of a physiologically-based pharmacokinetic model of 2-phenoxyethanol and its metabolite phenoxyacetic acid in rats and humans to address toxicokinetic uncertainty in risk assessment. Regulatory Toxicology and Pharmacology 2015, 73, 530-543. [CrossRef]
- Velíšek, J.; Svobodova, Z. Anaesthesia of common carp (Cyprinus carpio L.) with 2-phenoxyethanol: acute toxicity and effects on biochemical blood profile. Acta Veterinaria Brno 2004, 73, 247-252. [CrossRef]
- Crestani, F.; Löw, K.; Keist, R.; Mandelli, M.-J.; Möhler, H.; Rudolph, U. Molecular Targets for the Myorelaxant Action of Diazepam. Molecular Pharmacology 2001, 59, 442. [CrossRef]
- Zhang, X.C.Y.L.H.Y.R.H.X.Z.X. Risk analysis and source investigation of diazepam residue in cultured freshwater fish in a region of East China. Quality and Safety of Agro-Products 2024, 52-59.
- Mandelli, M.; Tognoni, G.; Garattini, S. Clinical Pharmacokinetics of Diazepam. Clinical Pharmacokinetics 1978, 3, 72-91. [CrossRef]
- Seddon, T.; Michelle, I.; Chenery, R.J. Comparative drug metabolism of diazepam in hepatocytes isolated from man, rat, monkey and dog. Biochemical Pharmacology 1989, 38, 1657-1665. [CrossRef]
- Affairs, H.P.D.o.A.a.R. The notice on the results of the second supervision and sampling inspection of key agricultural product quality and safety. Available online: https://agri.hunan.gov.cn/agri/ztzl/c102414/c102416/202408/t20240806_33425197.html (accessed on.
- Li, J.; Zhang, J.; Liu, H.; Wu, L. A comparative study of primary secondary amino (PSA) and multi-walled carbon nanotubes (MWCNTs) as QuEChERS absorbents for the rapid determination of diazepam and its major metabolites in fish samples by high-performance liquid chromatography–electrospray ionisation–tandem mass spectrometry. Journal of the Science of Food and Agriculture 2016, 96, 555-560. [CrossRef]
- Kamble, A.; Kennady, C.J.; Badiye, A.; Kapoor, N. Detection of diazepam in spiked drink using thin-layer chromatography. JPC – Journal of Planar Chromatography – Modern TLC 2022, 35, 543-546. [CrossRef]
- Carmona Araújo, A.; Casal, R.J.; Goulão, J.; Martins, A.P. Misuse of psychoactive medicines and its consequences in the European Union – a scoping review. Journal of Substance Use 2024, 29, 629-640. [CrossRef]
- Muench, B. Quinaldine, a new anesthetic for fish. The Progressive Fish-Culturist 1958, 20, 42-44.
- Hasan, M.; Bart, A.N. Improved survival of rohu, Labeo rohita (Hamilton-Buchanan) and silver carp, Hypophthalmichthys molitrix (Valenciennes) fingerlings using low-dose quinaldine and benzocaine during transport. Aquaculture Research 2007, 38, 50-58. [CrossRef]
- Bircan-Yildirim, Y.; Genc, E.; Turan, F.; Cek, S.; Yanar, M. The anaesthetic effects of quinaldine sulphate, muscle relaxant diazepam and their combination on convict cichlid, Cichlasoma nigrofasciatum (Günther, 1867) juveniles. 2010.
- Miethling, R.; Hecht, V.; Deckwer, W.D. Microbial degradation of quinoline: Kinetic studies with Comamonas acidovorans DSM 6426. Biotechnology and bioengineering 1993, 42, 589-595. [CrossRef]
- Kildea, M.A.; Allan, G.L.; Kearney, R.E. Accumulation and clearance of the anaesthetics clove oil and AQUI-S™ from the edible tissue of silver perch (Bidyanus bidyanus). Aquaculture 2004, 232, 265-277. [CrossRef]
- Ye, L. Development and validation of a LC-MS/MS method for the determination of isoeugenol in finfish. Food Chemistry 2017, 228, 70-76. [CrossRef]
- WU, S.; OUYANG, L.; MENG, P.; HE, M.; LIN, Q.; CHEN, Y.; LIU, W.; SU, X.; DAI, M. Determination of 18 caine anesthetics in animal meat using solid phase extraction combined with ultra-performance liquid chromatography-tandem mass spectrometry. Chinese Journal of Chromatography 2023, 41, 434-442. [CrossRef]
- Abreu, D.C.P.; Botrel, B.M.C.; Bazana, M.J.F.; e Rosa, P.V.; Sales, P.F.; Marques, M.d.S.; Saczk, A.A. Development and comparative analysis of single-drop and solid-phase microextraction techniques in the residual determination of 2-phenoxyethanol in fish. Food Chemistry 2019, 270, 487-493. [CrossRef]
- Wang, M.; Qiao, Y.; Luo, Z.; Guo, E.; Ma, W.; Wang, K.; Guo, A.; Lian, K. Development of a QuEChERS combined with LC-MS/MS method for determining 24 sedatives and anesthetics in animal-derived foods. Journal of Food Composition and Analysis 2024, 127, 106000. [CrossRef]
- Sun, H.; Lai, J.-P.; Chen, F.; Zhu, D.-R. Molecularly imprinted microspheres synthesized by a simple, fast, and universal suspension polymerization for selective extraction of the topical anesthetic benzocaine in human serum and fish tissues. Analytical and Bioanalytical Chemistry 2015, 407, 1745-1752. [CrossRef]
- Li, J.C.; Liu, H.; Wang, C.Y.; Wu, L.D.; Liu, D. Determination of eugenol in fish and shrimp muscle tissue by stable isotope dilution assay and solid-phase extraction coupled gas chromatography-triple quadrupole mass spectrometry. Analytical and Bioanalytical Chemistry 2016, 408, 6537-6544. [CrossRef]
- Liang, X.; Feng, T.T.; Wu, J.H.; Du, M.; Qin, L.; Wang, Z.Y.; Xu, X.B. Vortex-Assisted Liquid-Liquid Micro-extraction Followed by Head Space Solid Phase Micro-extraction for the Determination of Eugenol in Fish Using GC-MS. Food Analytical Methods 2018, 11, 790-796. [CrossRef]
- Huang, Y.X.; Li, Q.; Zhang, Y.L.; Meng, Z.J.; Yuan, X.X.; Fan, S.F.; Zhang, Y. Determination of Six Eugenol Residues in Aquatic Products by Gas Chromatography-Orbitrap Mass Spectrometry. Journal of Food Quality 2021, 2021. [CrossRef]
- Zhao, D.-H.; Wang, Q.; Wang, X.-F.; Li, Z.-G.; Li, Y.-X.; Huang, K.; Li, L.-D. Determination of MS-222 in Water Samples by Solid-phase Extraction Coupled with Liquid Chromatography/Tandem Mass Spectrometry. Journal of Chromatographic Science 2017, 55, 813-817. [CrossRef]
- Huang, S.Y.; Xu, J.Q.; Wu, J.Y.; Hong, H.J.; Chen, G.S.; Jiang, R.F.; Zhu, F.; Liu, Y.; Ouyang, G.F. Rapid detection of five anesthetics in tilapias by in vivo solid phase microextraction coupling with gas chromatography-mass spectrometry. Talanta 2017, 168, 263-268. [CrossRef]
- Cheng, X.H.; Hochlowski, J. Current application of mass spectrometry to combinatorial chemistry. Analytical Chemistry 2002, 74, 2679-2690. [CrossRef]
- Scherpenisse, P.; Bergwerff, A.A. Determination of residues of tricaine in fish using liquid chromatography tandem mass spectrometry. Analytica Chimica Acta 2007, 586, 407-410. [CrossRef]
- Xie, C.N.; Li, Q.; Han, G.; Liu, H.; Yang, J.; Li, J.C. Stable isotope dilution assay for the accurate determination of tricaine in fish samples by HPLC-MS-MS. Biomedical Chromatography 2019, 33. [CrossRef]
- Xia, G.; Ruan, G.; Huang, Y.; Hu, H.; Yu, S.; Lai, B.; Li, Z.; Zhang, Y.; Tang, N. Highly efficient enrichment of eugenol anesthetics in aquatic products using magnetic nanospheres decorated covalent organic framework microflowers. Microchemical Journal 2023, 195, 109362. [CrossRef]
- Sorribes-Soriano, A.; Albert Esteve-Turrillas, F.; Armenta, S.; Manuel Herrero-Martínez, J. Molecularly imprinted polymer –stir bar sorptive extraction of diazepam from natural water. Microchemical Journal 2023, 186, 108354. [CrossRef]
- Liang, H.-W.; Jia, B.-Z.; Zhang, W.-F.; Wang, X.-X.; Zhou, K.; Lei, H.-T.; Xu, Z.-L.; Luo, L. Ratiometric Fluorescence Immunoassay Based on MnO2 Nanoflakes for Sensitive and Accurate Detection of Tricaine. Journal of Agricultural and Food Chemistry 2023, 71, 7575-7583. [CrossRef]
- Luo, L.; Luo, S.-Z.; Jia, B.-Z.; Zhang, W.-F.; Wang, H.; Wei, X.-Q.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L.; Yang, J.-Y. A high-resolution colorimetric immunoassay for tyramine detection based on enzyme-enabled growth of gold nanostar coupled with smartphone readout. Food Chemistry 2022, 396, 133729. [CrossRef]
- Lin, L.; Wu, X.; Cui, G.; Song, S.; Kuang, H.; Xu, C. Colloidal Gold Immunochromatographic Strip Assay for the Detection of Azaperone in Pork and Pork Liver. Acs Omega 2020, 5, 1346-1351. [CrossRef]
- Li, C.; Zhang, Y.; Eremin, S.A.; Yakup, O.; Yao, G.; Zhang, X. Detection of kanamycin and gentamicin residues in animal-derived food using IgY antibody based ic-ELISA and FPIA. Food Chemistry 2017, 227, 48-54. [CrossRef]
- Can-Herrera, L.A.; Oliva, A.I.; Dzul-Cervantes, M.A.A.; Pacheco-Salazar, O.F.; Cervantes-Uc, J.M. Morphological and Mechanical Properties of Electrospun Polycaprolactone Scaffolds: Effect of Applied Voltage. Polymers 2021, 13. [CrossRef]
- Shen, X.; Wu, X.; Liu, L.; Kuang, H. Development of a colloidal gold immunoassay for the detection of four eugenol compounds in water. Food and Agricultural Immunology 2019, 30, 1318-1331. [CrossRef]
- Ahmed, S.; Ning, J.N.; Peng, D.P.; Chen, T.; Ahmad, I.; Ali, A.; Lei, Z.X.; Shabbir, M.A.; Cheng, G.Y.; Yuan, Z.H. Current advances in immunoassays for the detection of antibiotics residues: a review. Food and Agricultural Immunology 2020, 31, 268-290. [CrossRef]
- Maragos, C. Fluorescence Polarization Immunoassay of Mycotoxins: A Review. Toxins 2009, 1, 196-207. [CrossRef]
- Rivas, L.; de la Escosura-Muñiz, A.; Serrano, L.; Altet, L.; Francino, O.; Sánchez, A.; Merkoçi, A. Triple lines gold nanoparticle-based lateral flow assay for enhanced and simultaneous detection of Leishmania DNA and endogenous control. Nano Research 2015, 8, 3704-3714. [CrossRef]
- Guo, L.; Wu, X.; Liu, L.; Kuang, H.; Xu, C. Gold Nanoparticle-Based Paper Sensor for Simultaneous Detection of 11 Benzimidazoles by One Monoclonal Antibody. Small 2018, 14, 1701782. [CrossRef]
- Lei, X.; Xu, X.; Liu, L.; Kuang, H.; Xu, L.; Hao, C. Immunochromatographic test strip for the rapid detection of tricaine in fish samples. Food and Agricultural Immunology 2020, 31, 687-699. [CrossRef]
- Lei, X.; Xu, X.; Wang, L.; Zhou, W.; Liu, L.; Xu, L.; Kuang, H.; Xu, C. A quadruplex immunochromatographic assay for the ultrasensitive detection of 11 anesthetics. Nano Research 2023, 16, 11269-11277. [CrossRef]
- Luo, L.; Lei, H.-T.; Yang, J.-Y.; Liu, G.-L.; Sun, Y.-M.; Bai, W.-D.; Wang, H.; Shen, Y.-D.; Chen, S.; Xu, Z.-L. Development of an indirect ELISA for the determination of ethyl carbamate in Chinese rice wine. Analytica Chimica Acta 2017, 950, 162-169. [CrossRef]
- Luo, L.; Jia, B.-Z.; Wei, X.-Q.; Xiao, Z.-L.; Wang, H.; Sun, Y.-M.; Shen, Y.-D.; Lei, H.-T.; Xu, Z.-L. Development of an inner filter effect-based fluorescence immunoassay for the detection of acrylamide using 9-xanthydrol derivatization. Sensors and Actuators B: Chemical 2021, 332, 129561. [CrossRef]
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sensors and Actuators B: Chemical 2020, 302, 127176. [CrossRef]
- Huang, X.; Song, J.; Yung, B.C.; Huang, X.; Xiong, Y.; Chen, X. Ratiometric optical nanoprobes enable accurate molecular detection and imaging. Chemical Society Reviews 2018, 47, 2873-2920. [CrossRef]
- Lin, S.-Q.; Jia, B.-Z.; Luo, W.; Wang, H.; Lei, H.-T.; Zhang, W.-F.; Xu, Z.-L.; Luo, L. Controllable formation of polydopamine on carbon dots for ultrasensitive detection of alkaline phosphatase and ratiometric fluorescence immunoassay of benzocaine. Food Chemistry 2023, 426, 136582. [CrossRef]
- Luo, L.; He, Z.-X.; Jia, B.-Z.; Kang, R.-Y.; Zhang, W.-F.; Huang, R.-M.; Xu, Z.-L. Gold nanocluster-based ratiometric fluorescence immunoassay for broad-spectrum screening of five eugenols. Analytica Chimica Acta 2024, 1310, 342723. [CrossRef]
- Wang, J.; Xu, X.; Li, Z.; Li, M.; Qiu, B. Sensitive electrochemical detection of benzocaine based on hollow carbon nanobowl modified electrode. Journal of Electroanalytical Chemistry 2024, 952, 117893. [CrossRef]
- Mahdi, M.A.; Yousefi, S.R.; Jasim, L.S.; Salavati-Niasari, M. Green synthesis of DyBa2Fe3O7.988/DyFeO3 nanocomposites using almond extract with dual eco-friendly applications: Photocatalytic and antibacterial activities. International Journal of Hydrogen Energy 2022, 47, 14319-14330. [CrossRef]
- Felix de Lima, R.M.; de Oliveira Silva, M.D.; Felix, F.S.; Angnes, L.; Pio dos Santos, W.T.; Saczk, A.A. Determination of Benzocaine and Tricaine in Fish Fillets Using BIA with Amperometric Detection. Electroanalysis 2018, 30, 283-287. [CrossRef]
- Cai, S.; Chen, X.; Liu, J.; Wang, L.; Liu, G.; Gu, Y. Highly efficient detection of Tricaine methanesulfonate based on the nanoporous gold electrochemical sensor. Materials Letters 2021, 301. [CrossRef]
- Shi, Z.; Xia, L.; Li, G.; Hu, Y. Platinum nanoparticles-embedded raspberry-liked SiO2 for the simultaneous electrochemical determination of eugenol and methyleugenol. Microchimica Acta 2021, 188, 241. [CrossRef]
- Chen, X.; Wei, J.; Li, J.; Jiao, T.; Wang, L.; Chen, Q. Rapid detection of eugenol in perch utilizing electrochemical method by transition metal substituted polyoxometalates. Food Chemistry 2023, 426, 136584. [CrossRef]
- Pysarevska, S.; Dubenska, L.; Plotycya, S.; Švorc, Ľ. A state-of-the-art approach for facile and reliable determination of benzocaine in pharmaceuticals and biological samples based on the use of miniaturized boron-doped diamond electrochemical sensor. Sensors and Actuators B: Chemical 2018, 270, 9-17. [CrossRef]
- Wang, Z.; Yu, L.; Huang, Y.; Chen, Y.; Cao, H. Highly sensitive determination of anesthetic drug benzocaine based on hydroxypropyl-β-cyclodextrin–carbon black nanohybrids. Analytical Methods 2022, 14, 900-906. [CrossRef]
- Ghosh, D. Food safety regulations in Australia and New Zealand Food Standards. Journal of the Science of Food and Agriculture 2014, 94, 1970-1973. [CrossRef]
- Kaartinen, L. Scientific Advisory Group on Antimicrobials (SAGAM) of the Committee for Medicinal Products for Veterinary Use - Mandate and work plan. International Journal of Medical Microbiology 2006, 296, 9-10. [CrossRef]
- Raymond, C.A.; Davies, N.W.; Larkman, T. GC-MS method validation and levels of methyl eugenol in a diverse range of tea tree (Melaleuca alternifolia) oils. Analytical and Bioanalytical Chemistry 2017, 409, 1779-1787. [CrossRef]
- Wagner, E.; Arndt, R.; Hilton, B. Physiological stress responses, egg survival and sperm motility for rainbow trout broodstock anesthetized with clove oil, tricaine methanesulfonate or carbon dioxide. Aquaculture 2002, 211, 353-366. [CrossRef]
- Rafson, J.P.; Turnipseed, S.B.; Casey, C.; De Bono, A.; Madson, M.R. Analysis and Stability Study of Isoeugenol in Aquaculture Products by Headspace Solid-Phase Microextraction Coupled to Gas Chromatography–Mass Spectrometry. Journal of Agricultural and Food Chemistry 2024, 72, 14411-14418. [CrossRef]
- Haji Abdolrasouli, M.; Roostaie, A.; Abedi, H.; Mohammadiazar, S. Determination of Lorazepam and Diazepam Using Modified Nanocrystalline Cellulose for Ultrasonic-Assisted Dispersive Solid Phase Microextraction (UA-DSPME) and Gas Chromatography-Mass Spectrometry (GC-MS). Analytical Letters 2024, 57, 2085-2102. [CrossRef]
- Klimánková, E.; Riddellová, K.; Hajšlová, J.; Poustka, J.; Kolářová, J.; Kocourek, V. Development of an SPME–GC–MS/MS procedure for the monitoring of 2-phenoxyethanol in anaesthetised fish. Talanta 2008, 75, 1082-1088. [CrossRef]
- Liu, Y.; Ai, X.; Li, L.; Li, J.; Yang, H. A fast and accurate isotope dilution GC-IT-MS/MS method for determination of eugenol in different tissues of fish: Application to a depletion study in mandarin fish. Biomedical Chromatography 2018, 32, e4163. [CrossRef]
- Li, J.; Liu, H.; Yu, M.; Wu, L.; Wang, Q.; Lv, H.; Ma, B.; Song, Y. Rapid determination of tricaine mesylate residues in fish samples using modified QuEChERS and high-performance liquid chromatography-tandem mass spectrometry. Anal. Methods 2014, 6, 9124-9128. [CrossRef]
- Xie, C.; Li, Q.; Han, G.; Liu, H.; Yang, J.; Li, J. Stable isotope dilution assay for the accurate determination of tricaine in fish samples by HPLC–MS–MS. Biomedical Chromatography 2019, 33, 4512. [CrossRef]
- Shen, K.; Zou, X.; Wang, J. Simultaneous determination of the four key fluoroquinolones and two antipsychotics in fish and shrimp by LC-MS/MS. Food Additives & Contaminants: Part A 2022, 39, 678-686. [CrossRef]
- Meinertz, J.R.; Hess, K.R. Evaluation of analytical techniques to determine AQUI-S (R) 20E (eugenol) concentrations in water. Aquaculture 2014, 418, 62-66. [CrossRef]
- Mu, S.; Wang, C.; Liu, H.; Han, G.; Wu, L.; Li, J. Development and evaluation of a novelty single-step cleanup followed by HPLC-QTRAP-MS/MS for rapid analysis of tricaine, tetracaine, and bupivacaine in fish samples. Biomedical Chromatography 2021, 35, 5176. [CrossRef]
- Huang, Q.; Zhou, H.; Wu, X.; Song, C.; Zheng, J.; Lei, M.; Mu, P.; Wu, P. Simultaneous determination of the residues of anesthetics and sedatives in fish using LC-QLIT-MS/MS combined with DSPE. Food Chemistry 2023, 403, 134407. [CrossRef]
- Abad, M.O.K.; Masrournia, M.; Javid, A. Synthesis of novel MOF-on-MOF composite as a magnetic sorbent to dispersive micro solid phase extraction of benzodiazepine drugs prior to determination with HPLC-UV. Microchemical Journal 2024, 197, 109797. [CrossRef]
| Anesthetic | MRL | Withdrawal period | Reference | |
| Eugenol | New Zealand | 100ng mL-1 | — | [123] |
| Japan | 50ng mL-1 | 7 d | [28] | |
| Isoeugenol | European Conformity (CE) |
6mg kg-1 | — | [124] |
| AQUI-S | Australia and Chile | — | 0d | [125] |
| MS-222 | FDA | 1µg mL-1 | 21 d | [126] |
| Canada | — | 7 d | [39] | |
| Methods | Anesthetic | Sample | Pretreatment | Linearity | Repeatability / Reproducibility* | Detection limits | Reference |
| GC-MS | eugenol | fish back, fish belly, and fish tail | VALLME/HS-SPME | 15.0-750.0 μg kg-1 | RSD<20%, n=3 | 0.5 μg kg-1 | [81] |
| eugenol | carp muscle tissues | SPE | 5.0-500.0 μg L-1 | RSD<12%, n=6 | 2.5 μg kg-1 | [28] | |
| Isoeugenol | shrimp, tilapia, and salmon | Headspace solid-phase microextraction | 0-160 ng g-1 | RSD 5-13%, n=9 | below 15ng g-1 | [120] | |
| GC-MS/MS | diazepam | water samples | dispersive solid-phase microextraction | 10-1000 ng mL-1 | RSD=6%, n=5 | 3 ng mL-1 | [121] |
| eugenol, isoeugenol‚ and methyleugenol | groupers | SPE | 5–500μg L-1 | RSD 2.18%-15.5%, n=4 | eugenol 0.4μg kg-1, isoeugenol 1.2μg kg-1 ‚ and methyleugenol 0.2μg kg-1 | [27] | |
| 2-Phenoxyethanol | rainbow trout | SPME | 0.1-250 mg kg-1 | RSD 3%-11%, n=5 | 0.03mg kg-1 | [122] | |
| GC-IT-MS/MS | eugenol | mandarin | QuEChERS | 5-1000μg L-1 | RSD 1.82%-9.74%, n=6 | 5.0 μg /kg | [123] |
| Orbitrap GC-MS | eugenols | prawns | m-PFC column | 0.001–0.1 μg mL-1 | RSD 1.2%–7.5%, n=6 | 2-10 μg kg-1 | [82] |
| HPLC-MS/MS | MS-222 | carp and eel | QuEChERS | 2–1000 μg L-1 | RSD<6%, n=3 | 2.5μg kg-1 | [124] |
| / | SPE | 0.05-10μg L-1 | RSD<9.36%, n=5 | 0.01μg/ L | [83] | ||
| finfish | extracted with acetone using a tissue homogenizer, followed by derivatization with dansyl chloride | 2.5-40.0 ng g-1 | RSD 2.6%–8.0%, n=6 | 0.2-1μg kg-1 | [75] | ||
| marine fish and freshwater fish | isotope dilution assay | 2.0-200.0 μg L-1 | inter- and intra-assay relative standard deviations (RSD values) were 0.39-3.01 and 0.85-2.77% | 1μg kg-1 | [125] | ||
| LC-MS/MS | diazepam | fish and shrimp tissue | C18 cartridge solid-phase extraction | 0.05-5ng mL-1 | RSD<4.9%, n=3 | 0.01μg kg-1 | [126] |
| LC | AQUI-S® 20E (eugenol) | standard water containing fish feed | SPE | 5-500mg L-1 | RSD<0.7%, n=3 | 0.0011 mg L-1 | [127] |
| HPLC-QTRAP-MS/MS | tricaine, tetracaine, and bupivacaine | fish samples | QuEChERS | 1.0–50.0 μg L-1 | RSD<15%, n=3 | 2.0 μg kg-1 | [128] |
| PGD-IMS)/LC-MS/MS | MS-222 | fish-raising water samples | m-PFC | 0.005-0.2 mg L-1 | PGD-IMS RSD 6.9%–10.3%, n=5 LC-MS/MS RSD 1.3%–3.4%, n=5 |
6μg kg-1/0.6μg kg-1 | [39] |
| LC-QLIT-MS/MS | eugenol | fish samples | dispersive solid-phase extraction (DSPE) | 1–100 μg kg-1 | RSD 1.9%–8.9%, n=6 | 0.03-0.4 μg kg-1 | [129] |
| HPLC-UV | Diazepam | water samples | Dispersive micro solid phase extraction | 0.3-450 ng mL-1 | RSD 3.42-3.75%, n=3 | 0.09ng mL-1 | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





