Submitted:
26 September 2025
Posted:
29 September 2025
Read the latest preprint version here
Abstract
Keywords:
1. Introduction
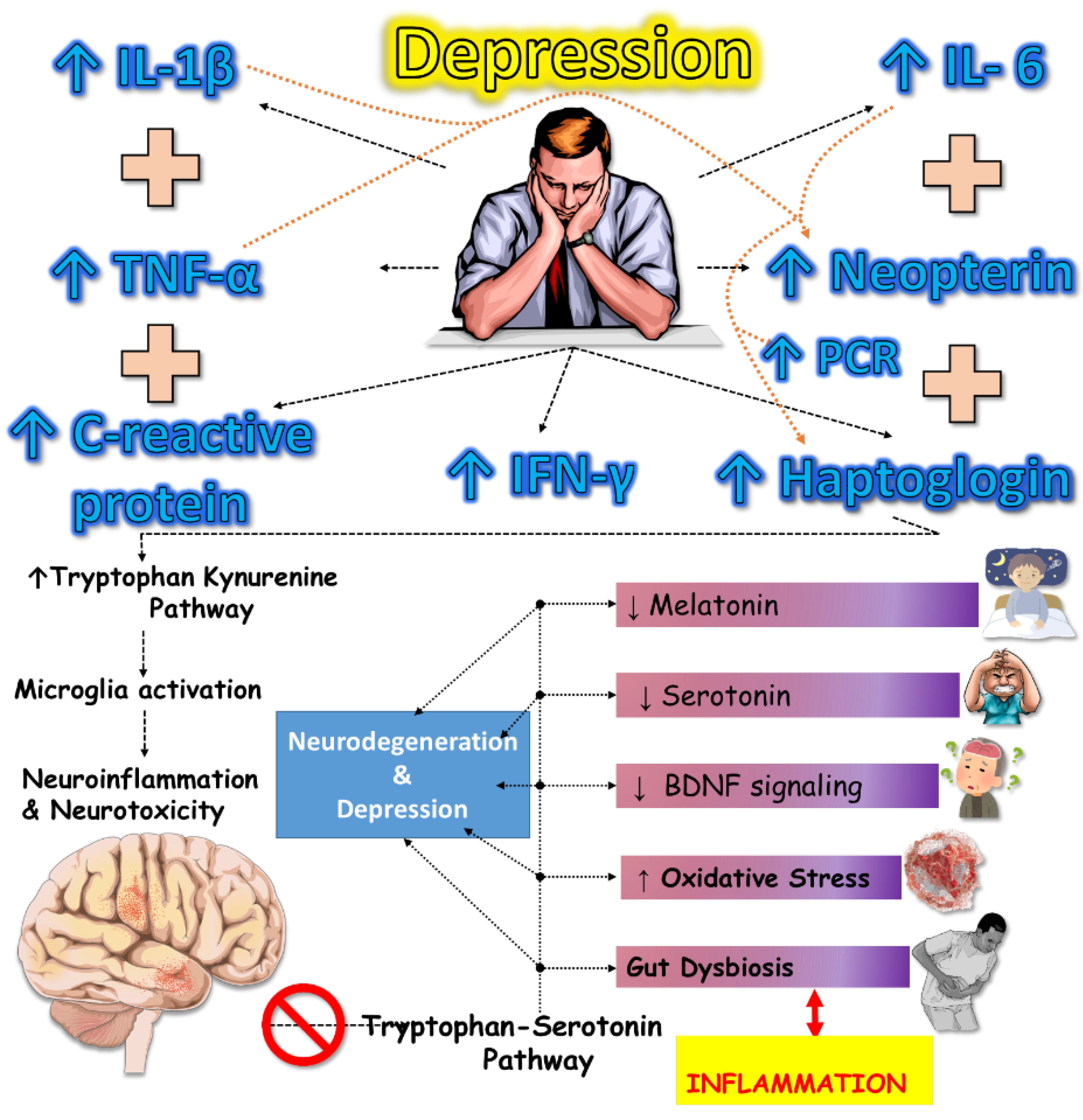
2. Neurobiology of Depression: Beyond Serotonin (5-HT)
2.1. Microglia Activation and Neuroinflammation
2.2. Neurogenesis Impairment and Hippocampal Atrophy in Depressive Pathology
2.3. The Link between Inflammation, Triptophan (Trp) metabolism, and Depressive Symptoms
3. Tryptophan (Trp) Metabolism: Central Pathways and Peripheral Influences
3.1. Serotonin (5HT) and Kynurenine (KYN) Metabolic Pathways: Fundamental Metabolic Routes
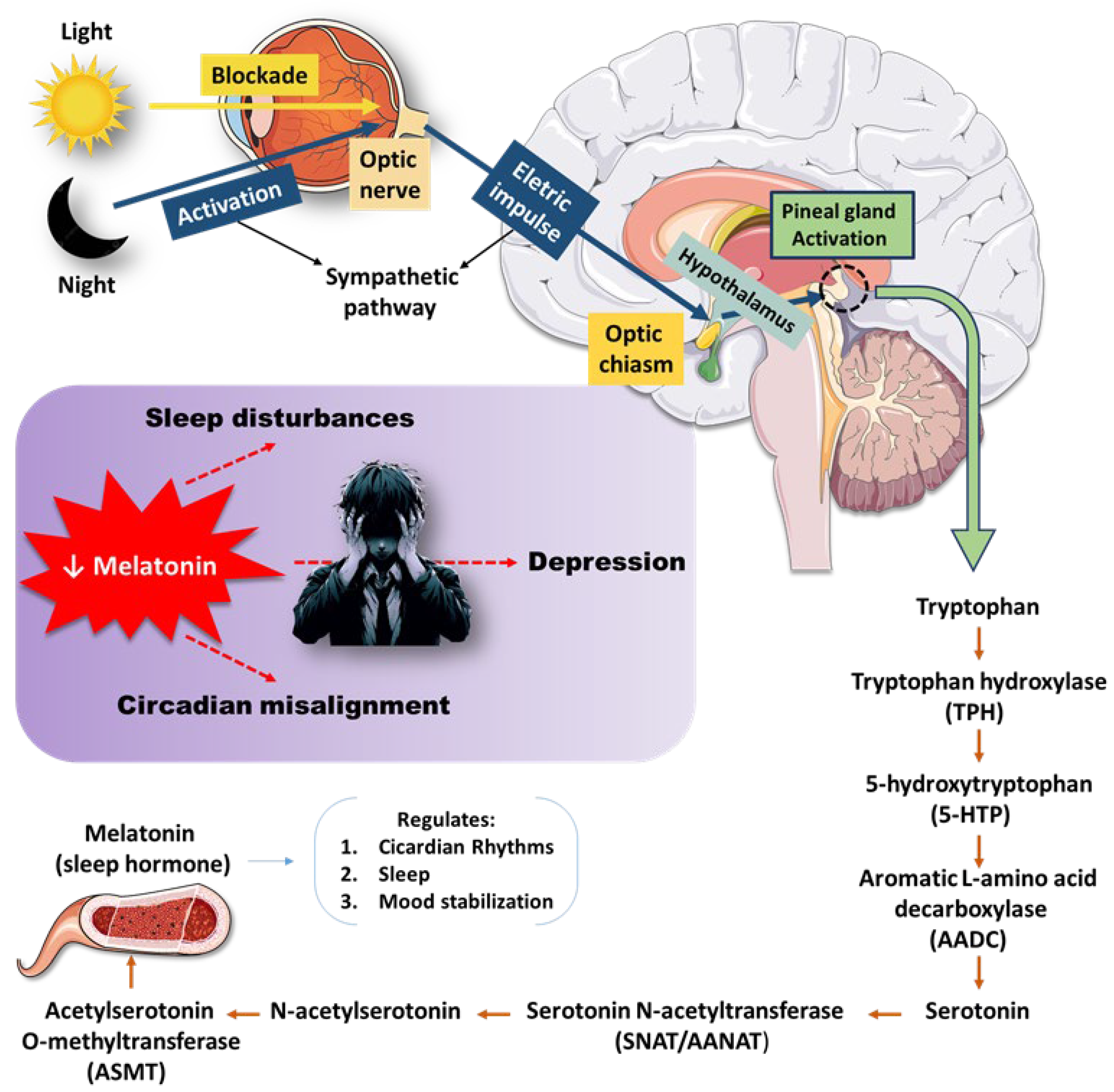
3.2. Oxidative Stress and Inflammation: Drivers of Kynurenine (KYN) Pathway Activation
3.3. Gut-Brain Axis and Intestinal Microbiota: Peripheral Modulators of Tryptophan (Trp) Metabolism
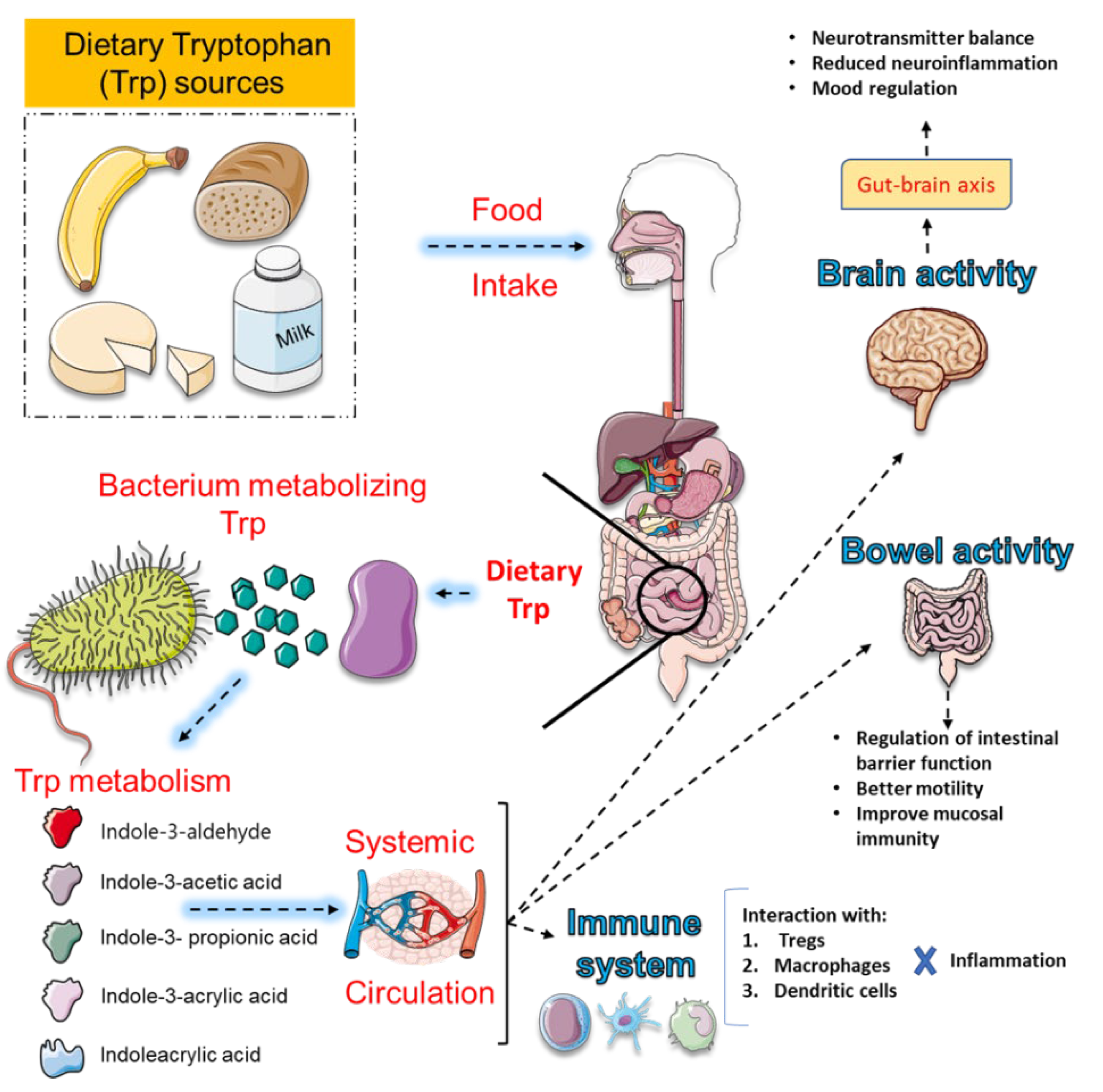
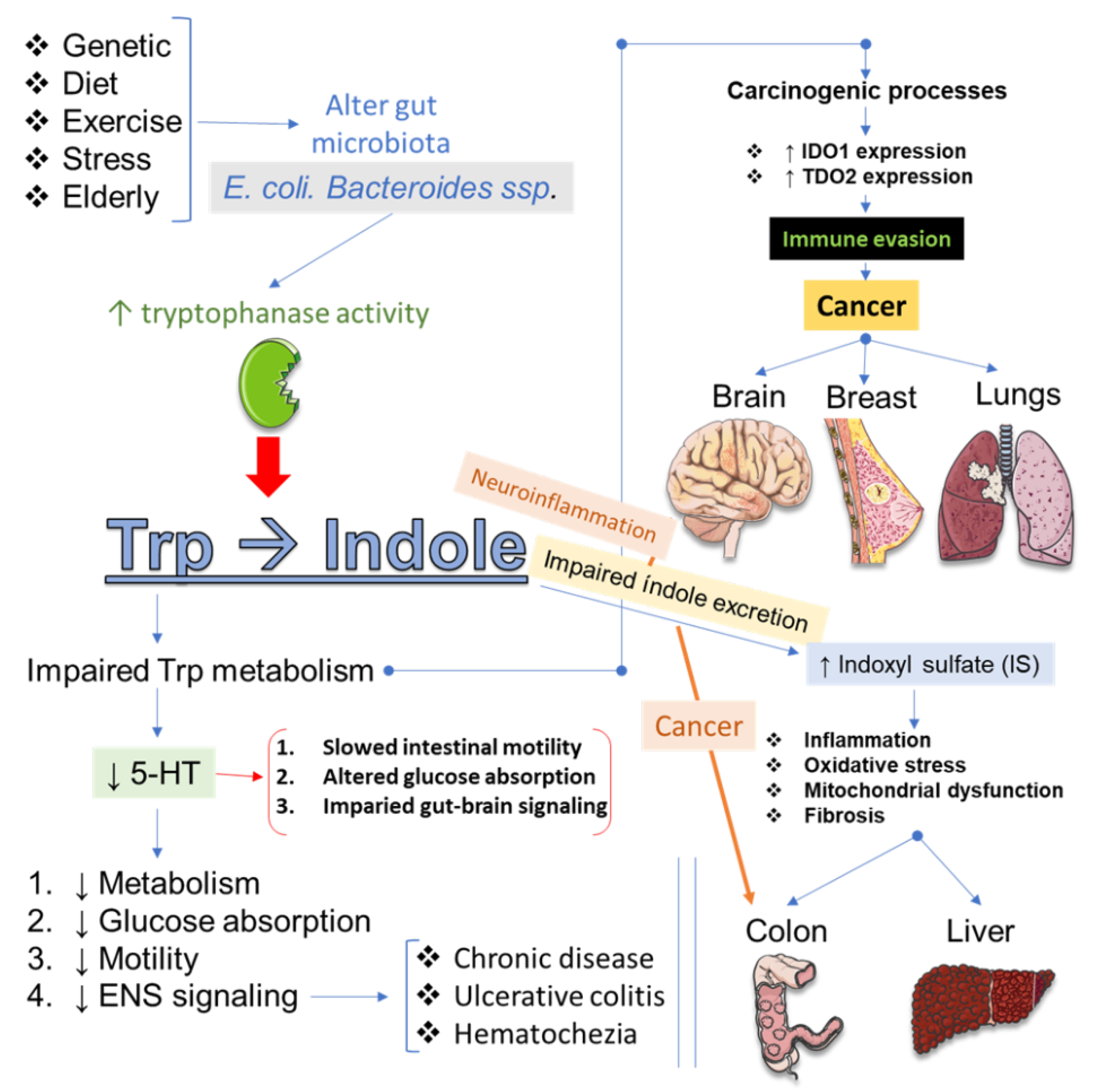
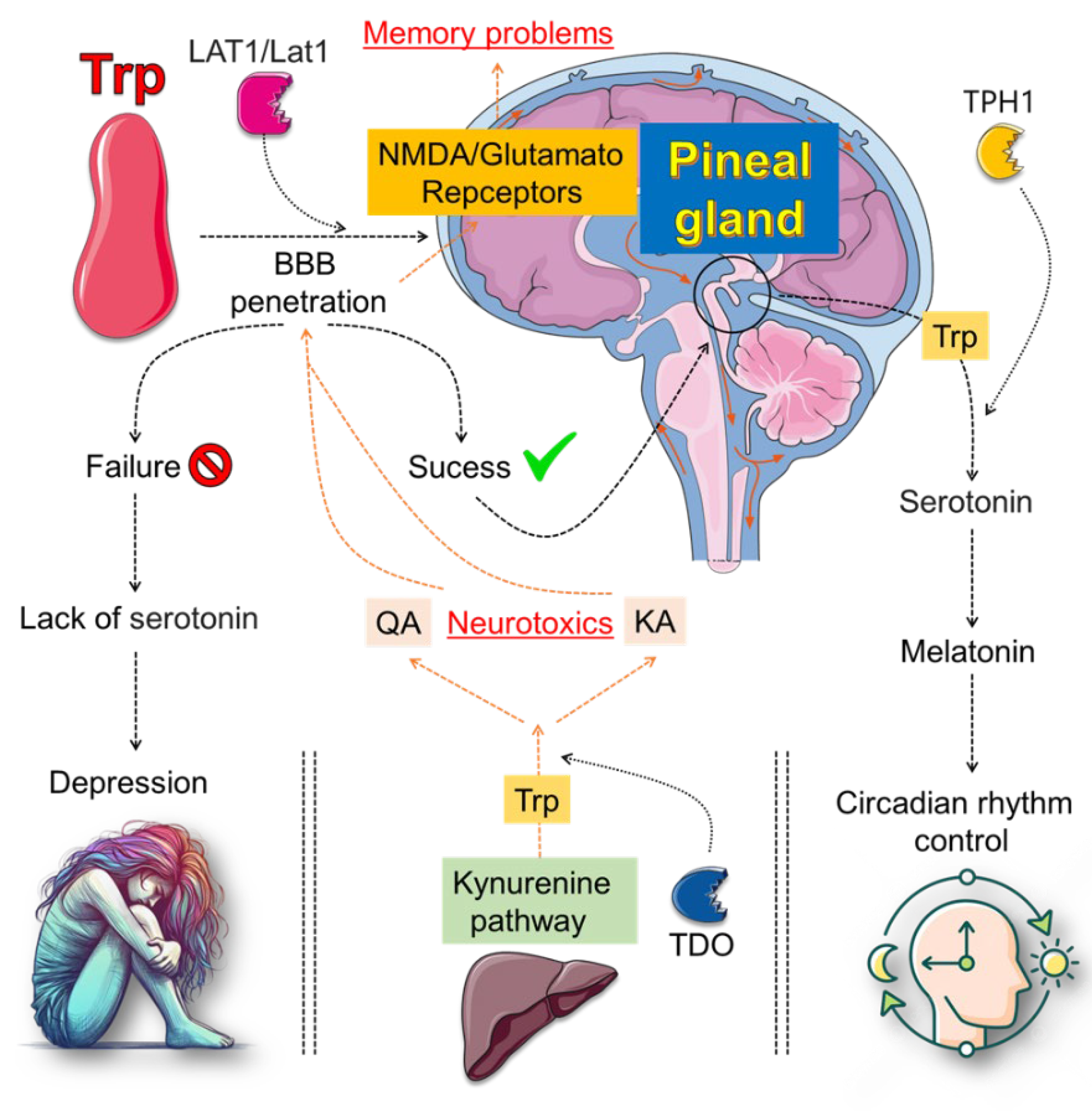
4. Plant-Derived Compounds as Modulators of Tryptophan (Trp) Metabolism
4.1. Clinical Evidence for Plant-Derived Modulators of Tryptophan (Trp) Metabolism
| Recovered / Remitted Depression | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 23 recovered depressed vs. 20 healthy controls (NL) | α-lactalbumin vs. casein | ↑ TRP/LNAA, improved memory; no mood effect | Enhanced cognition, no mood change | [227] |
| RCT, DB, 29 stress-vulnerable vs. 29 stress-invulnerable adults (NL) | α-lactalbumin vs. casein | Slight mood improvement (POMS) in stress vulnerable | Small antidepressant-like effect under stress | [228] |
| RCT, DB, 43 MDD in remission (UK) | [229]. TRP+ | No mood change overall; ↑ thalamic/caudate activation in MDD | ATD altered neural responses to distractors | [230] |
| Clinically Depressed Patients | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 30 MDD (MY) | Talbinah (TRP-rich barley) vs. control | ↓ depression & anxiety (DASS, POMS) | Multidomain improvement | [231] |
| RCT, DB, 70 MDD (IN) | 5-HTP vs. fluoxetine (8 wks) | Both ↓ HDRS (by wk 2) | Comparable efficacy | [232] |
| RCT, DB, 25 Parkinson’s disease (IT) | 5-HTP (50 mg) vs. placebo (12 wks) | ↓ depression (BDI-II, HDRS) ; no effect on apathy | 5-HTP improved depressive symptoms but not apathy in PD | [233] |
| High-Risk / Vulnerable Groups | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 29 high vs. low stress vulnerability (NL) | α-lactalbumin (TRP-rich) vs. casein | TRP improved mood (POMS), ↓ cortisol in all; mood benefit only in vulnerable | Enhanced stress resilience in high-vulnerability group | [228] |
| RCT, DB, 38 high vs. low cognitive reactivity (NL) | TRP-rich hydrolysate vs. casein | TRP improved positive mood, ↓ cortisol after stress | Both groups benefited physiologically, mood benefit more pronounced in vulnerable | [234] |
| RCT, DB, 25 high-risk (family history) vs. low-risk (US) | ATD vs. control | High-risk: ↑ sad mood, slower RT to sad words after ATD | ATD amplified vulnerability markers; no effect in low-risk | [235] |
| Bulimia | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 64 26 ♂ bulimia ± 13 ♂ bulimia on SSRIs vs. healthy controls (CA) | ATD vs. control (balanced amino acids) | ATD ↓ mood in all; ↑ urge to binge eat in bulimia + SSRIs | Serotonin depletion linked to bulimic relapse, especially with SSRI use | [236] |
| Healthy Volunteers | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 38 healthy adults (UK) | TRP (1g, 3x/day, 14d) vs. placebo | TRP increased recognition of happy faces, reduced negative bias (females) | Positive emotional bias, effect stronger in women | [237] |
| RCT, DB, 20 healthy adults (CA/NL) | TRP (3g/day, 3wks) vs. placebo (crossover) | ↑ plasma/urine kynurenine & indoles; no mood/GI changes | TRP activated immunomodulatory metabolic pathways | [238] |
| RCT, DB, 47 healthy adults (Netherlands) | TRP (2.8g/day, 6d) vs. placebo | No main effect on social fairness, but ↑ rejection of unfair offers | Prolonged TRP may affect social decision-making | [239] |
| RCT, DB, 18 healthy adults (NL) | TRP-rich hydrolyzed protein vs. ALAC, placebo, pure TRP, synthetic peptide | HPROT and pure TRP improved mood; HPROT fastest ↑ in plasma TRP/LNAA | Hydrolyzed TRP protein most effective for mood and serotonin | [240] |
| Elderly | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, SB, 35 elderly (ES) | TRP-enriched cereals vs. control | ↑ sleep quality, ↓ anxiety & depression | Improved sleep efficiency, reduced fragmentation | [241] |
| RCT, DB, 25 elderly with mild cognitive impairment (IT) | DHA-phospholipids + melatonin + TRP vs. placebo, 12 weeks | ↑ cognitive function, ↑ nutrition, ↑ olfactory sensitivity | MMSE improved, positive trend for verbal fluency | [242] |
| RCT, DB, 14 elderly (NL) | TRP, nicotinic acid, nicotinamide vs. control, 32 days | No effect on mitochondrial or muscle function | ↑ NAD+ metabolism, no functional benefit | [243] |
| RCT, SB, 40 elderly with depression/sleep disorders (PL) | TRP-rich diet (25 mg/kg/d) vs. usual diet, 12 weeks | ↓ depression & insomnia scores, improved TRP metabolism | ISI and HAM-D scores halved post-intervention | [244] |
| Fibromyalgia | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| RCT, DB, 22 ♂ fibromyalgia (ES) | Mediterranean diet enriched with high-dose TRP (60 mg) and Mg (60 mg) vs. standard Mediterranean diet | ↓ anxiety, ↓ mood disturbance, ↓ eating disorder symptoms, ↓ body image dissatisfaction; no improvement in sleep quality | Significant improvements in psychological variables; intervention was safe | [207,220] |
| Large-Scale Observational | ||||
| Study Design & Population | Intervention / Control | Main Outcomes | Key Notes | Ref. |
| Clinical trial, 29,687 adults (US) (NHANES 2001–2012) | Mean TRP intake: 826 mg/d (observational, no intervention) | Higher TRP intake associated with ↓ depression severity (PHQ-9), ↑ sleep duration | No adverse effects on liver, kidney, or metabolism; intake well above requirement | [229,245] |
4.2. Specific Tryptophan (Trp)-Rich Phytocompounds and Associated Clinical Outcomes
4.3. Concise Summaries: Key Outcomes from Clinical Trials
5. Gaps and Controversies in Current Research
5.1. Identified Gaps in Current Research
5.2. Controversies Regarding Clinical Efficacy and Bioavailability
6. Clinical Translation: From Bench to Bedside
6.1. Practical Considerations for Clinical Application
6.2. Personalized Medicine Approaches
7. Future Perspectives and Research Directions
7.1. Advancing Clinical Evidence through Robust Study Designs
7.2. Combined Pharmacological and Dietary Intervention Strategies
7.3. Leveraging Biotechnological Innovations for Personalized Depression Management
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HK | 3-hydroxykynurenine |
| 5-HT | serotonin |
| 5-HTP | 5-hydroxytryptophan |
| AhR | aryl hydrocarbon receptor |
| ASMT | acetylserotonin O-methyltransferase |
| ATD | acute tryptophan depletion |
| BDNF | brain-derived neurotrophic factor |
| DOAJ | directory of open access journals |
| HPA | hypothalamic–pituitary–adrenal |
| IAld | indole-3-aldehyde |
| IAA | indole-3-acetic acid |
| IPA | indole-3-propionic acid |
| IDO | indoleamine 2,3-dioxygenase |
| IFN-γ | interferon-gamma |
| IL | interleukin |
| IS | indoxyl sulfate |
| KYN | kynurenine |
| KYNA | kynurenic acid |
| LAT1 | large neutral amino acid transporter 1 |
| LD | linear dichroism |
| LNAA | large neutral amino acid |
| MDD | major depressive disorder |
| QA | quinolinic acid |
| RCT | randomized controlled trial |
| ROS | reactive oxygen species |
| SCN | suprachiasmatic nucleus |
| SNAT | serotonin N-acetyltransferase |
| SSRI | selective serotonin reuptake inhibitor |
| TDO | tryptophan 2,3-dioxygenase |
| TLA | three letter acronym |
| TNF-α | tumor necrosis factor-alpha |
| Trp | tryptophan |
| TPH | tryptophan hydroxylase |
| TPH1 | tryptophan hydroxylase 1 |
| TPH2 | tryptophan hydroxylase 2 |
References
- Filatova, E.V.; Shadrina, M.I.; Slominsky, P.A. Major Depression: One Brain, One Disease, One Set of Intertwined Processes. Cells 2021, 10. [Google Scholar] [CrossRef]
- Fries, G.R.; Saldana, V.A.; Finnstein, J.; Rein, T. Molecular pathways of major depressive disorder converge on the synapse. Mol. Psychiatry 2023, 28, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Proudman, D.; Greenberg, P.; Nellesen, D. The Growing Burden of Major Depressive Disorders (MDD): Implications for Researchers and Policy Makers. Pharmacoeconomics 2021, 39, 619–625. [Google Scholar] [CrossRef]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Simes, M.; Berman, R.; Koenigsberg, S.H.; Kessler, R.C. The Economic Burden of Adults with Major Depressive Disorder in the United States (2010 and 2018). Pharmacoeconomics 2021, 39, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ruan, M.; Chen, J.; Fang, Y. Major Depressive Disorder: Advances in Neuroscience Research and Translational Applications. Neurosci. Bull. 2021, 37, 863–880. [Google Scholar] [CrossRef]
- Pu, J.; Liu, Y.; Zhang, H.; Tian, L.; Gui, S.; Yu, Y.; Chen, X.; Chen, Y.; Yang, L.; Ran, Y.; et al. An integrated meta-analysis of peripheral blood metabolites and biological functions in major depressive disorder. Mol. Psychiatry 2021, 26, 4265–4276. [Google Scholar] [CrossRef]
- Kouba, B.R.; de Araujo Borba, L.; Borges de Souza, P.; Gil-Mohapel, J.; Rodrigues, A.L.S. Role of Inflammatory Mechanisms in Major Depressive Disorder: From Etiology to Potential Pharmacological Targets. Cells 2024, 13. [Google Scholar] [CrossRef]
- Athira, K.V.; Bandopadhyay, S.; Samudrala, P.K.; Naidu, V.G.M.; Lahkar, M.; Chakravarty, S. An Overview of the Heterogeneity of Major Depressive Disorder: Current Knowledge and Future Prospective. Curr. Neuropharmacol. 2020, 18, 168–187. [Google Scholar] [CrossRef]
- Ilavská, L.; Morvová, M., Jr.; Paduchová, Z.; Muchová, J.; Garaiova, I.; Ďuračková, Z.; Šikurová, L.; Trebatická, J. The kynurenine and serotonin pathway, neopterin and biopterin in depressed children and adolescents: an impact of omega-3 fatty acids, and association with markers related to depressive disorder. A randomized, blinded, prospective study. Front. Psychiatry 2024, 15, 1347178. [Google Scholar] [CrossRef]
- Correia, A.S.; Vale, N. Tryptophan Metabolism in Depression: A Narrative Review with a Focus on Serotonin and Kynurenine Pathways. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Fang, X.; Wu, Y.; Dai, Y.; Xiao, H.; Li, S.; Chen, X.; Yuan, M.; Guo, Y.; Ma, L.; Lin, D.; et al. In Situ Recovery of Serotonin Synthesis by a Tryptophan Hydroxylase-Like Nanozyme for the Treatment of Depression. J. Am. Chem. Soc. 2025, 147, 9111–9121. [Google Scholar] [CrossRef]
- Wang, D.; Wu, J.; Zhu, P.; Xie, H.; Lu, L.; Bai, W.; Pan, W.; Shi, R.; Ye, J.; Xia, B.; et al. Tryptophan-rich diet ameliorates chronic unpredictable mild stress induced depression- and anxiety-like behavior in mice: The potential involvement of gut-brain axis. Food Res. Int. 2022, 157, 111289. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhao, M.; Chen, X.; Zhang, R.; Le, A.; Hong, M.; Zhang, Y.; Jia, L.; Zang, W.; Jiang, C.; et al. Tryptophan Metabolism in Central Nervous System Diseases: Pathophysiology and Potential Therapeutic Strategies. Aging Dis. 2023, 14, 858–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.W.; Gao, C.S.; Zhang, H.; Yang, J.; Wang, Y.P.; Pan, L.B.; Yu, H.; He, C.Y.; Luo, H.B.; Zhao, Z.X.; et al. Morinda officinalis oligosaccharides increase serotonin in the brain and ameliorate depression via promoting 5-hydroxytryptophan production in the gut microbiota. Acta Pharm. Sin. B 2022, 12, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo Godoy, A.C.; Frota, F.F.; Araújo, L.P.; Valenti, V.E.; Pereira, E.; Detregiachi, C.R.P.; Galhardi, C.M.; Caracio, F.C.; Haber, R.S.A.; Fornari Laurindo, L.; et al. Neuroinflammation and Natural Antidepressants: Balancing Fire with Flora. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Cowen, P.J. SSRIs in the Treatment of Depression: A Pharmacological CUL-DE-SAC? Curr. Top. Behav. Neurosci. 2024, 66, 1–19. [Google Scholar] [CrossRef]
- Gabriel, F.C.; de Melo, D.O.; Fráguas, R.; Leite-Santos, N.C.; Mantovani da Silva, R.A.; Ribeiro, E. Pharmacological treatment of depression: A systematic review comparing clinical practice guideline recommendations. PLoS One 2020, 15, e0231700. [Google Scholar] [CrossRef]
- Stachowicz, K.; Sowa-Kućma, M. The treatment of depression - searching for new ideas. Front. Pharmacol. 2022, 13, 988648. [Google Scholar] [CrossRef]
- Dudek, D.; Chrobak, A.A.; Krupa, A.J.; Gorostowicz, A.; Gerlich, A.; Juryk, A.; Siwek, M. TED-trazodone effectiveness in depression: a naturalistic study of the effeciveness of trazodone in extended release formulation compared to SSRIs in patients with a major depressive disorder. Front. Pharmacol. 2023, 14, 1296639. [Google Scholar] [CrossRef]
- Srifuengfung, M.; Pennington, B.R.T.; Lenze, E.J. Optimizing treatment for older adults with depression. Ther. Adv. Psychopharmacol. 2023, 13, 20451253231212327. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, D.; Wang, J.; Zhou, D.; Liu, A.; Sun, Y.; Yuan, Y.; Li, J.; Guo, W. The pharmacological mechanism of chaihu-jia-longgu-muli-tang for treating depression: integrated meta-analysis and network pharmacology analysis. Front. Pharmacol. 2023, 14, 1257617. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Zhang, H.; Wu, Y.; Yu, C. The efficacy and safety of St. John’s wort extract in depression therapy compared to SSRIs in adults: A meta-analysis of randomized clinical trials. Adv. Clin. Exp. Med. 2023, 32, 151–161. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, X.; Liang, Y.; Zhang, R.; Liu, X.; Lu, L.; Huang, Y. Electroacupuncture as a rapid-onset and safer complementary therapy for depression: A systematic review and meta-analysis. Front. Psychiatry 2022, 13, 1012606. [Google Scholar] [CrossRef]
- Shafiee, A.; Jafarabady, K.; Seighali, N.; Mohammadi, I.; Rajai Firouz Abadi, S.; Abhari, F.S.; Bakhtiyari, M. Effect of Saffron Versus Selective Serotonin Reuptake Inhibitors (SSRIs) in Treatment of Depression and Anxiety: A Meta-analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, e751–e761. [Google Scholar] [CrossRef]
- Zhichao, H.; Ching, L.W.; Huijuan, L.; Liang, Y.; Zhiyu, W.; Weiyang, H.; Zhaoxiang, B.; Linda, Z.L.D. A network meta-analysis on the effectiveness and safety of acupuncture in treating patients with major depressive disorder. Sci. Rep. 2021, 11, 10384. [Google Scholar] [CrossRef]
- Nitzan, K.; David, D.; Franko, M.; Toledano, R.; Fidelman, S.; Tenenbaum, Y.S.; Blonder, M.; Armoza-Eilat, S.; Shamir, A.; Rehavi, M.; et al. Anxiolytic and antidepressants’ effect of Crataegus pinnatifida (Shan Zha): biochemical mechanisms. Transl. Psychiatry 2022, 12, 208. [Google Scholar] [CrossRef]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines (Basel) 2020, 8. [Google Scholar] [CrossRef]
- Vrânceanu, M.; Galimberti, D.; Banc, R.; Dragoş, O.; Cozma-Petruţ, A.; Hegheş, S.C.; Voştinaru, O.; Cuciureanu, M.; Stroia, C.M.; Miere, D.; et al. The Anticancer Potential of Plant-Derived Nutraceuticals via the Modulation of Gene Expression. Plants (Basel) 2022, 11. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Wang, H.; Song, Y.X.; Lan, W.Y.; Li, J.; Wang, F. Natural saponins and macrophage polarization: Mechanistic insights and therapeutic perspectives in disease management. Front. Pharmacol. 2025, 16, 1584035. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Song, Y.; Ai, Z.; Liu, Y.; Li, H.; Xu, W.; Chen, L.; Zhu, G.; Yang, M.; Su, D. Pulsatilla chinensis saponins ameliorated murine depression by inhibiting intestinal inflammation mediated IDO1 overexpression and rebalancing tryptophan metabolism. Phytomedicine 2023, 116, 154852. [Google Scholar] [CrossRef]
- Liaqat, H.; Parveen, A.; Kim, S.Y. Neuroprotective Natural Products’ Regulatory Effects on Depression via Gut-Brain Axis Targeting Tryptophan. Nutrients 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, S.; Azhar, A.; Tikmani, P.; Rafique, H.; Khan, A.; Mesiya, H.; Saeed, H. A randomized clinical trial to test efficacy of chamomile and saffron for neuroprotective and anti-inflammatory responses in depressive patients. Heliyon 2022, 8, e10774. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.H.; Li, C.X.; Zhang, R.B.; Shen, Y.; Xu, X.J.; Yu, Q.M. A review of the pharmacological action and mechanism of natural plant polysaccharides in depression. Front. Pharmacol. 2024, 15, 1348019. [Google Scholar] [CrossRef] [PubMed]
- Tartt, A.N.; Mariani, M.B.; Hen, R.; Mann, J.J.; Boldrini, M. Dysregulation of adult hippocampal neuroplasticity in major depression: pathogenesis and therapeutic implications. Mol. Psychiatry 2022, 27, 2689–2699. [Google Scholar] [CrossRef]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21. [Google Scholar] [CrossRef]
- Sun, X.; Zu, Y.; Li, X.; Zhao, S.; Sun, X.; Li, L.; Zhang, X.; Wang, W.; Liang, Y.; Wang, W.; et al. Corticosterone-induced Hippocampal 5-HT Responses were Muted in Depressive-like State. ACS Chem. Neurosci. 2021, 12, 845–856. [Google Scholar] [CrossRef]
- Lee, M.T.; Peng, W.H.; Kan, H.W.; Wu, C.C.; Wang, D.W.; Ho, Y.C. Neurobiology of Depression: Chronic Stress Alters the Glutamatergic System in the Brain-Focusing on AMPA Receptor. Biomedicines 2022, 10. [Google Scholar] [CrossRef]
- He, J.G.; Zhou, H.Y.; Wang, F.; Chen, J.G. Dysfunction of Glutamatergic Synaptic Transmission in Depression: Focus on AMPA Receptor Trafficking. Biol. Psychiatry Glob. Open Sci. 2023, 3, 187–196. [Google Scholar] [CrossRef]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-Life Stress Induces Depression-Like Behavior and Synaptic-Plasticity Changes in a Maternal Separation Rat Model: Gender Difference and Metabolomics Study. Front. Pharmacol. 2020, 11, 102. [Google Scholar] [CrossRef]
- Yan, Y.; Xu, X.; Chen, R.; Wu, S.; Yang, Z.; Wang, H.; Zhang, T. Down-regulation of MST1 in hippocampus protects against stress-induced depression-like behaviours and synaptic plasticity impairments. Brain Behav. Immun. 2021, 94, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, L.R.; Phillips, A.G. Neuroplasticity as a convergent mechanism of ketamine and classical psychedelics. Trends Pharmacol. Sci. 2021, 42, 929–942. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.A.; Gould, T.D. Targeting metaplasticity mechanisms to promote sustained antidepressant actions. Mol. Psychiatry 2024, 29, 1114–1127. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S. Dualistic Dynamics in Neuropsychiatry: From Monoaminergic Modulators to Multiscale Biomarker Maps. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Li, B.; Yang, W.; Ge, T.; Wang, Y.; Cui, R. Stress induced microglial activation contributes to depression. Pharmacol. Res. 2022, 179, 106145. [Google Scholar] [CrossRef]
- Woodburn, S.C.; Bollinger, J.L.; Wohleb, E.S. The semantics of microglia activation: neuroinflammation, homeostasis, and stress. J. Neuroinflammation 2021, 18, 258. [Google Scholar] [CrossRef]
- Wang, H.; He, Y.; Sun, Z.; Ren, S.; Liu, M.; Wang, G.; Yang, J. Microglia in depression: an overview of microglia in the pathogenesis and treatment of depression. J. Neuroinflammation 2022, 19, 132. [Google Scholar] [CrossRef]
- Kokkosis, A.G.; Madeira, M.M.; Hage, Z.; Valais, K.; Koliatsis, D.; Resutov, E.; Tsirka, S.E. Chronic psychosocial stress triggers microglial-/macrophage-induced inflammatory responses leading to neuronal dysfunction and depressive-related behavior. Glia 2024, 72, 111–132. [Google Scholar] [CrossRef]
- Sugama, S.; Kakinuma, Y. Stress and brain immunity: Microglial homeostasis through hypothalamus-pituitary-adrenal gland axis and sympathetic nervous system. Brain Behav. Immun. Health 2020, 7, 100111. [Google Scholar] [CrossRef]
- Picard, K.; Bisht, K.; Poggini, S.; Garofalo, S.; Golia, M.T.; Basilico, B.; Abdallah, F.; Ciano Albanese, N.; Amrein, I.; Vernoux, N.; et al. Microglial-glucocorticoid receptor depletion alters the response of hippocampal microglia and neurons in a chronic unpredictable mild stress paradigm in female mice. Brain Behav. Immun. 2021, 97, 423–439. [Google Scholar] [CrossRef] [PubMed]
- Asveda, T.; Talwar, P.; Ravanan, P. Exploring microglia and their phenomenal concatenation of stress responses in neurodegenerative disorders. Life Sci. 2023, 328, 121920. [Google Scholar] [CrossRef] [PubMed]
- Biswas, K. Microglia mediated neuroinflammation in neurodegenerative diseases: A review on the cell signaling pathways involved in microglial activation. J. Neuroimmunol. 2023, 383, 578180. [Google Scholar] [CrossRef]
- Bolton, J.L.; Short, A.K.; Othy, S.; Kooiker, C.L.; Shao, M.; Gunn, B.G.; Beck, J.; Bai, X.; Law, S.M.; Savage, J.C.; et al. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Rep. 2022, 38, 110600. [Google Scholar] [CrossRef] [PubMed]
- Reemst, K.; Kracht, L.; Kotah, J.M.; Rahimian, R.; van Irsen, A.A.; Congrains Sotomayor, G.; Verboon, L.N.; Brouwer, N.; Simard, S.; Turecki, G. Early-life stress lastingly impacts microglial transcriptome and function under basal and immune-challenged conditions. Translational psychiatry 2022, 12, 507. [Google Scholar] [CrossRef]
- Smail, M.A.; Lenz, K.M. Developmental functions of microglia: Impact of psychosocial and physiological early life stress. Neuropharmacology 2024, 258, 110084. [Google Scholar] [CrossRef]
- Shen, S.Y.; Liang, L.F.; Shi, T.L.; Shen, Z.Q.; Yin, S.Y.; Zhang, J.R.; Li, W.; Mi, W.L.; Wang, Y.Q.; Zhang, Y.Q.; et al. Microglia-Derived Interleukin-6 Triggers Astrocyte Apoptosis in the Hippocampus and Mediates Depression-Like Behavior. Adv Sci (Weinh) 2025, 12, e2412556. [Google Scholar] [CrossRef]
- Klawonn, A.M.; Fritz, M.; Castany, S.; Pignatelli, M.; Canal, C.; Similä, F.; Tejeda, H.A.; Levinsson, J.; Jaarola, M.; Jakobsson, J.; et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity 2021, 54, 225–234.e226. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wu, H.R.; Zhang, S.S.; Xiao, H.L.; Yu, J.; Ma, Y.Y.; Zhang, Y.D.; Liu, Q. Catalpol ameliorates depressive-like behaviors in CUMS mice via oxidative stress-mediated NLRP3 inflammasome and neuroinflammation. Transl. Psychiatry 2021, 11, 353. [Google Scholar] [CrossRef]
- Xia, C.Y.; Guo, Y.X.; Lian, W.W.; Yan, Y.; Ma, B.Z.; Cheng, Y.C.; Xu, J.K.; He, J.; Zhang, W.K. The NLRP3 inflammasome in depression: Potential mechanisms and therapies. Pharmacol. Res. 2023, 187, 106625. [Google Scholar] [CrossRef]
- Wang, L.; Hauenstein, A.V. The NLRP3 inflammasome: Mechanism of action, role in disease and therapies. Mol. Aspects Med. 2020, 76, 100889. [Google Scholar] [CrossRef]
- Cattaneo, A.; Ferrari, C.; Turner, L.; Mariani, N.; Enache, D.; Hastings, C.; Kose, M.; Lombardo, G.; McLaughlin, A.P.; Nettis, M.A.; et al. Whole-blood expression of inflammasome- and glucocorticoid-related mRNAs correctly separates treatment-resistant depressed patients from drug-free and responsive patients in the BIODEP study. Transl. Psychiatry 2020, 10, 232. [Google Scholar] [CrossRef]
- Xu, Y.; Liang, J.; Sun, Y.; Zhang, Y.; Shan, F.; Ge, J.; Xia, Q. Serum cytokines-based biomarkers in the diagnosis and monitoring of therapeutic response in patients with major depressive disorder. Int. Immunopharmacol. 2023, 118, 110108. [Google Scholar] [CrossRef] [PubMed]
- Bodnár, K.; Hermán, L.; Zsigmond, R.; Réthelyi, J. Investigation of cytokine imbalance in schizophrenia, assessment of the possible role of serum cytokine levels in predicting treatment response, prognosis and psychotic relapses. European Psychiatry 2024, 67, S685–S685. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, Y.; Zhou, R.; Li, Y.; Gao, Y.; Tu, D.; Wilson, B.; Song, S.; Feng, J.; Hong, J.S.; et al. A novel role of NLRP3-generated IL-1β in the acute-chronic transition of peripheral lipopolysaccharide-elicited neuroinflammation: implications for sepsis-associated neurodegeneration. J. Neuroinflammation 2020, 17, 64. [Google Scholar] [CrossRef] [PubMed]
- Moraes, C.A.; Hottz, E.D.; Dos Santos Ornellas, D.; Adesse, D.; de Azevedo, C.T.; d’Avila, J.C.; Zaverucha-do-Valle, C.; Maron-Gutierrez, T.; Barbosa, H.S.; Bozza, P.T.; et al. Microglial NLRP3 Inflammasome Induces Excitatory Synaptic Loss Through IL-1β-Enriched Microvesicle Release: Implications for Sepsis-Associated Encephalopathy. Mol. Neurobiol. 2023, 60, 481–494. [Google Scholar] [CrossRef]
- Xu, W.; Huang, Y.; Zhou, R. NLRP3 inflammasome in neuroinflammation and central nervous system diseases. Cell Mol. Immunol. 2025, 22, 341–355. [Google Scholar] [CrossRef]
- Lu, S.; Wu, C.; Jia, L.; Fang, Z.; Lu, J.; Mou, T.; Hu, S.; He, H.; Huang, M.; Xu, Y. Increased plasma levels of IL-6 are associated with striatal structural atrophy in major depressive disorder patients with anhedonia. Front. Psychiatry 2022, 13, 1016735. [Google Scholar] [CrossRef]
- Sanders, A.F.P.; Tirado, B.; Seider, N.A.; Triplett, R.L.; Lean, R.E.; Neil, J.J.; Miller, J.P.; Tillman, R.; Smyser, T.A.; Barch, D.M.; et al. Prenatal exposure to maternal disadvantage-related inflammatory biomarkers: associations with neonatal white matter microstructure. Transl. Psychiatry 2024, 14, 72. [Google Scholar] [CrossRef]
- Lim, J.; Sohn, H.; Kwon, M.S.; Kim, B. White Matter Alterations Associated with Pro-inflammatory Cytokines in Patients with Major Depressive Disorder. Clin. Psychopharmacol. Neurosci. 2021, 19, 449–458. [Google Scholar] [CrossRef]
- Yin, R.; Zhang, K.; Li, Y.; Tang, Z.; Zheng, R.; Ma, Y.; Chen, Z.; Lei, N.; Xiong, L.; Guo, P.; et al. Lipopolysaccharide-induced depression-like model in mice: meta-analysis and systematic evaluation. Front. Immunol. 2023, 14, 1181973. [Google Scholar] [CrossRef]
- Lasselin, J.; Schedlowski, M.; Karshikoff, B.; Engler, H.; Lekander, M.; Konsman, J.P. Comparison of bacterial lipopolysaccharide-induced sickness behavior in rodents and humans: Relevance for symptoms of anxiety and depression. Neurosci. Biobehav. Rev. 2020, 115, 15–24. [Google Scholar] [CrossRef]
- Su, J.; Pan, Y.W.; Wang, S.Q.; Li, X.Z.; Huang, F.; Ma, S.P. Saikosaponin-d attenuated lipopolysaccharide-induced depressive-like behaviors via inhibiting microglia activation and neuroinflammation. Int. Immunopharmacol. 2020, 80, 106181. [Google Scholar] [CrossRef] [PubMed]
- Tomaz, V.S.; Chaves Filho, A.J.M.; Cordeiro, R.C.; Jucá, P.M.; Soares, M.V.R.; Barroso, P.N.; Cristino, L.M.F.; Jiang, W.; Teixeira, A.L.; de Lucena, D.F.; et al. Antidepressants of different classes cause distinct behavioral and brain pro- and anti-inflammatory changes in mice submitted to an inflammatory model of depression. J. Affect. Disord. 2020, 268, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.S.; Zou, J.J.; Meng, L.; Chen, H.M.; Hong, Z.Q.; Liu, X.F.; Farooq, U.; Chen, M.X.; Lin, Z.R.; Zhou, W.; et al. Ultrasound Stimulation of Prefrontal Cortex Improves Lipopolysaccharide-Induced Depressive-Like Behaviors in Mice. Front. Psychiatry 2022, 13, 864481. [Google Scholar] [CrossRef]
- Kappelmann, N.; Lewis, G.; Dantzer, R.; Jones, P.B.; Khandaker, G.M. Antidepressant activity of anti-cytokine treatment: a systematic review and meta-analysis of clinical trials of chronic inflammatory conditions. Mol. Psychiatry 2018, 23, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, X.L.; Shi, H.; Meng, L.Q.; Quan, H.F.; Yan, L.; Yang, H.F.; Peng, X.D. Betaine Inhibits NLRP3 Inflammasome Hyperactivation and Regulates Microglial M1/M2 Phenotypic Differentiation, Thereby Attenuating Lipopolysaccharide-Induced Depression-Like Behavior. J. Immunol. Res. 2022, 2022, 9313436. [Google Scholar] [CrossRef]
- Planchez, B.; Lagunas, N.; Le Guisquet, A.M.; Legrand, M.; Surget, A.; Hen, R.; Belzung, C. Increasing Adult Hippocampal Neurogenesis Promotes Resilience in a Mouse Model of Depression. Cells 2021, 10. [Google Scholar] [CrossRef]
- Jones, K.L.; Zhou, M.; Jhaveri, D.J. Dissecting the role of adult hippocampal neurogenesis towards resilience versus susceptibility to stress-related mood disorders. NPJ Sci. Learn. 2022, 7, 16. [Google Scholar] [CrossRef]
- Fang, S.; Wu, Z.; Guo, Y.; Zhu, W.; Wan, C.; Yuan, N.; Chen, J.; Hao, W.; Mo, X.; Guo, X.; et al. Roles of microglia in adult hippocampal neurogenesis in depression and their therapeutics. Front. Immunol. 2023, 14, 1193053. [Google Scholar] [CrossRef]
- Tang, C.; Wang, Q.; Shen, J.; Wang, C.; Ding, H.; Wen, S.; Yang, F.; Jiao, R.; Wu, X.; Li, J.; et al. Neuron stem cell NLRP6 sustains hippocampal neurogenesis to resist stress-induced depression. Acta Pharm. Sin. B 2023, 13, 2017–2038. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Deng, Q.; Zhu, Q.; Hu, Z.L.; Long, L.H.; Wu, P.F.; He, J.G.; Chen, H.S.; Yue, Z.; Lu, J.H.; et al. Cell type-specific NRBF2 orchestrates autophagic flux and adult hippocampal neurogenesis in chronic stress-induced depression. Cell Discov. 2023, 9, 90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, F.; Zhai, M.; He, M.; Hu, Y.; Feng, L.; Li, Y.; Yang, J.; Wu, C. Hyperactive neuronal autophagy depletes BDNF and impairs adult hippocampal neurogenesis in a corticosterone-induced mouse model of depression. Theranostics 2023, 13, 1059. [Google Scholar] [CrossRef] [PubMed]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav. Immun. 2021, 91, 24–47. [Google Scholar] [CrossRef]
- Parul; Mishra, A. ; Singh, S.; Singh, S.; Tiwari, V.; Chaturvedi, S.; Wahajuddin, M.; Palit, G.; Shukla, S. Chronic unpredictable stress negatively regulates hippocampal neurogenesis and promote anxious depression-like behavior via upregulating apoptosis and inflammatory signals in adult rats. Brain Res. Bull. 2021, 172, 164–179. [Google Scholar] [CrossRef]
- Zhang, J.; Rong, P.; Zhang, L.; He, H.; Zhou, T.; Fan, Y.; Mo, L.; Zhao, Q.; Han, Y.; Li, S.; et al. IL4-driven microglia modulate stress resilience through BDNF-dependent neurogenesis. Sci. Adv. 2021, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, M.; Sun, N.; Wang, H.; Fan, H. Melatonin attenuates chronic stress-induced hippocampal inflammatory response and apoptosis by inhibiting ADAM17/TNF-α axis. Food Chem. Toxicol. 2022, 169, 113441. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Wang, P.; Fan, C.; Zhang, P.; Shen, J.; Yu, S.Y. Ginsenoside-Rg1 Rescues Stress-Induced Depression-Like Behaviors via Suppression of Oxidative Stress and Neural Inflammation in Rats. Oxid. Med. Cell Longev. 2020, 2020, 2325391. [Google Scholar] [CrossRef]
- Li, J.; Gao, W.; Zhao, Z.; Li, Y.; Yang, L.; Wei, W.; Ren, F.; Li, Y.; Yu, Y.; Duan, W.; et al. Ginsenoside Rg1 Reduced Microglial Activation and Mitochondrial Dysfunction to Alleviate Depression-Like Behaviour Via the GAS5/EZH2/SOCS3/NRF2 Axis. Mol. Neurobiol. 2022, 59, 2855–2873. [Google Scholar] [CrossRef]
- Amanollahi, M.; Jameie, M.; Heidari, A.; Rezaei, N. The Dialogue Between Neuroinflammation and Adult Neurogenesis: Mechanisms Involved and Alterations in Neurological Diseases. Mol. Neurobiol. 2023, 60, 923–959. [Google Scholar] [CrossRef]
- Zhang, J.; He, H.; Qiao, Y.; Zhou, T.; He, H.; Yi, S.; Zhang, L.; Mo, L.; Li, Y.; Jiang, W.; et al. Priming of microglia with IFN-γ impairs adult hippocampal neurogenesis and leads to depression-like behaviors and cognitive defects. Glia 2020, 68, 2674–2692. [Google Scholar] [CrossRef]
- Zhao, R.; Tian, X.; Xu, H.; Wang, Y.; Lin, J.; Wang, B. Aerobic Exercise Restores Hippocampal Neurogenesis and Cognitive Function by Decreasing Microglia Inflammasome Formation Through Irisin/NLRP3 Pathway. Aging Cell 2025, 24, e70061. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Wang, L.; Li, Y.; Lan, T.; Wang, C.; Chen, X.; Chen, S.; Yu, S. Ginsenoside-Rg1 synergized with voluntary running exercise protects against glial activation and dysregulation of neuronal plasticity in depression. Food Funct. 2023, 14, 7222–7239. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.P.; Tanaka, M.; Lamas, C.B.; Quesada, K.; Detregiachi, C.R.P.; Araújo, A.C.; Guiguer, E.L.; Catharin, V.; de Castro, M.V.M.; Junior, E.B.; et al. Vascular Impairment, Muscle Atrophy, and Cognitive Decline: Critical Age-Related Conditions. Biomedicines 2024, 12. [Google Scholar] [CrossRef] [PubMed]
- Liloia, D.; Zamfira, D.A.; Tanaka, M.; Manuello, J.; Crocetta, A.; Keller, R.; Cozzolino, M.; Duca, S.; Cauda, F.; Costa, T. Disentangling the role of gray matter volume and concentration in autism spectrum disorder: A meta-analytic investigation of 25 years of voxel-based morphometry research. Neurosci. Biobehav. Rev. 2024, 164, 105791. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.; Di Benedetto, S. Neuroimmune crosstalk in chronic neuroinflammation: microglial interactions and immune modulation. Front. Cell Neurosci. 2025, 19, 1575022. [Google Scholar] [CrossRef]
- Navabi, S.P.; Badreh, F.; Khombi Shooshtari, M.; Hajipour, S.; Moradi Vastegani, S.; Khoshnam, S.E. Microglia-induced neuroinflammation in hippocampal neurogenesis following traumatic brain injury. Heliyon 2024, 10, e35869. [Google Scholar] [CrossRef]
- Kwon, H.S.; Koh, S.H. Neuroinflammation in neurodegenerative disorders: the roles of microglia and astrocytes. Transl. Neurodegener. 2020, 9, 42. [Google Scholar] [CrossRef]
- Sanchez-Molina, P.; Almolda, B.; Giménez-Llort, L.; González, B.; Castellano, B. Chronic IL-10 overproduction disrupts microglia-neuron dialogue similar to aging, resulting in impaired hippocampal neurogenesis and spatial memory. Brain Behav. Immun. 2022, 101, 231–245. [Google Scholar] [CrossRef]
- Izzy, S.; Yahya, T.; Albastaki, O.; Abou-El-Hassan, H.; Aronchik, M.; Cao, T.; De Oliveira, M.G.; Lu, K.J.; Moreira, T.G.; da Silva, P.; et al. Nasal anti-CD3 monoclonal antibody ameliorates traumatic brain injury, enhances microglial phagocytosis and reduces neuroinflammation via IL-10-dependent T(reg)-microglia crosstalk. Nat. Neurosci. 2025, 28, 499–516. [Google Scholar] [CrossRef]
- Witcher, K.G.; Bray, C.E.; Chunchai, T.; Zhao, F.; O’Neil, S.M.; Gordillo, A.J.; Campbell, W.A.; McKim, D.B.; Liu, X.; Dziabis, J.E.; et al. Traumatic Brain Injury Causes Chronic Cortical Inflammation and Neuronal Dysfunction Mediated by Microglia. J. Neurosci. 2021, 41, 1597–1616. [Google Scholar] [CrossRef]
- Lawrence, J.M.; Schardien, K.; Wigdahl, B.; Nonnemacher, M.R. Roles of neuropathology-associated reactive astrocytes: a systematic review. Acta Neuropathol. Commun. 2023, 11, 42. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, X.; Gu, Y. Evaluation of serum brain-derived neurotrophic factor, IL-6, IL-10, and TNF-a cognitive function, and sleep quality in elderly patients with major depressive disorder and somatic symptoms. J. Med. Biochem. 2025, 44, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Skelly, D.T.; Griffin, É.W.; Murray, C.L.; Harney, S.; O’Boyle, C.; Hennessy, E.; Dansereau, M.A.; Nazmi, A.; Tortorelli, L.; Rawlins, J.N.; et al. Acute transient cognitive dysfunction and acute brain injury induced by systemic inflammation occur by dissociable IL-1-dependent mechanisms. Mol. Psychiatry 2019, 24, 1533–1548. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Guo, A.; Guan, K.; Chen, C.; Xu, S.; Tang, Y.; Li, X.; Huang, Z. Lactobacillus rhamnosus GG attenuates depression-like behaviour and cognitive deficits in chronic ethanol exposure mice by down-regulating systemic inflammatory factors. Addict. Biol. 2024, 29, e13445. [Google Scholar] [CrossRef]
- Zhang, J.C.; Yao, W.; Hashimoto, K. Brain-derived Neurotrophic Factor (BDNF)-TrkB Signaling in Inflammation-related Depression and Potential Therapeutic Targets. Curr. Neuropharmacol. 2016, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Rossetti, A.C.; Racagni, G.; Gass, P.; Riva, M.A.; Molteni, R. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Front. Cell Neurosci. 2014, 8, 430. [Google Scholar] [CrossRef]
- Carniel, B.P.; da Rocha, N.S. Brain-derived neurotrophic factor (BDNF) and inflammatory markers: Perspectives for the management of depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 108, 110151. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, J.; Xiao, C.; Su, D.; Li, L.; Yang, C.; Zhao, Z.; Jiang, W.; You, Z.; Zhou, T. Akebia saponin D protects hippocampal neurogenesis from microglia-mediated inflammation and ameliorates depressive-like behaviors and cognitive impairment in mice through the PI3K-Akt pathway. Front. Pharmacol. 2022, 13, 927419. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, W.; Yun, Y.; Ma, T.; An, H.; Fan, N.; Wang, J.; Wang, Z.; Yang, F. Multiomics analysis reveals aberrant tryptophan-kynurenine metabolism and immunity linked gut microbiota with cognitive impairment in major depressive disorder. J. Affect. Disord. 2025, 373, 273–283. [Google Scholar] [CrossRef]
- de Lima, E.P.; Laurindo, L.F.; Catharin, V.C.S.; Direito, R.; Tanaka, M.; Jasmin Santos German, I.; Lamas, C.B.; Guiguer, E.L.; Araújo, A.C.; Fiorini, A.M.R.; et al. Polyphenols, Alkaloids, and Terpenoids Against Neurodegeneration: Evaluating the Neuroprotective Effects of Phytocompounds Through a Comprehensive Review of the Current Evidence. Metabolites 2025, 15. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Laurindo, L.F.; de Oliveira Zanuso, B.; da Silva, R.M.S.; Gallerani Caglioni, L.; Nunes Junqueira de Moraes, V.B.F.; Fornari Laurindo, L.; Dogani Rodrigues, V.; da Silva Camarinha Oliveira, J.; Beluce, M.E.; et al. AdipoRon’s Impact on Alzheimer’s Disease-A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2025, 26. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res. Rev. 2022, 75, 101573. [Google Scholar] [CrossRef]
- Tanaka, M.; Tóth, F.; Polyák, H.; Szabó, Á.; Mándi, Y.; Vécsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Bano, S.; Badawy, A.A. Inflammation and serotonin deficiency in major depressive disorder: molecular docking of antidepressant and anti-inflammatory drugs to tryptophan and indoleamine 2,3-dioxygenases. Biosci. Rep. 2022, 42. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Paramanik, V. Role of Probiotics in Depression: Connecting Dots of Gut-Brain-Axis Through Hypothalamic-Pituitary Adrenal Axis and Tryptophan/Kynurenic Pathway involving Indoleamine-2,3-dioxygenase. Mol. Neurobiol. 2025, 62, 7230–7241. [Google Scholar] [CrossRef]
- Sublette, M.E.; Postolache, T.T. Neuroinflammation and depression: the role of indoleamine 2,3-dioxygenase (IDO) as a molecular pathway. Psychosom. Med. 2012, 74, 668–672. [Google Scholar] [CrossRef]
- Tanaka, M.; Battaglia, S. From Biomarkers to Behavior: Mapping the Neuroimmune Web of Pain, Mood, and Memory. 2025, 13, 2226.
- Stone, T.W.; Williams, R.O. Tryptophan metabolism as a ‘reflex’ feature of neuroimmune communication: Sensor and effector functions for the indoleamine-2, 3-dioxygenase kynurenine pathway. J. Neurochem. 2024, 168, 3333–3357. [Google Scholar] [CrossRef]
- Juhász, L.; Spisák, K.; Szolnoki, B.Z.; Nászai, A.; Szabó, Á.; Rutai, A.; Tallósy, S.P.; Szabó, A.; Toldi, J.; Tanaka, M.; et al. The Power Struggle: Kynurenine Pathway Enzyme Knockouts and Brain Mitochondrial Respiration. J. Neurochem. 2025, 169, e70075. [Google Scholar] [CrossRef]
- Szabó, Á.; Galla, Z.; Spekker, E.; Szűcs, M.; Martos, D.; Takeda, K.; Ozaki, K.; Inoue, H.; Yamamoto, S.; Toldi, J.; et al. Oxidative and Excitatory Neurotoxic Stresses in CRISPR/Cas9-Induced Kynurenine Aminotransferase Knockout Mice: A Novel Model for Despair-Based Depression and Post-Traumatic Stress Disorder. Front Biosci (Landmark Ed) 2025, 30, 25706. [Google Scholar] [CrossRef]
- Fiore, A.; Murray, P.J. Tryptophan and indole metabolism in immune regulation. Curr. Opin. Immunol. 2021, 70, 7–14. [Google Scholar] [CrossRef]
- Alfaro-Rodríguez, A.; Reyes-Long, S.; Roldan-Valadez, E.; González-Torres, M.; Bonilla-Jaime, H.; Bandala, C.; Avila-Luna, A.; Bueno-Nava, A.; Cabrera-Ruiz, E.; Sanchez-Aparicio, P.; et al. Association of the Serotonin and Kynurenine Pathways as Possible Therapeutic Targets to Modulate Pain in Patients with Fibromyalgia. Pharmaceuticals (Basel) 2024, 17. [Google Scholar] [CrossRef] [PubMed]
- Hunt, C.; Macedo, E.C.T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms - A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Almulla, A.F.; Supasitthumrong, T.; Tunvirachaisakul, C.; Algon, A.A.A.; Al-Hakeim, H.K.; Maes, M. The tryptophan catabolite or kynurenine pathway in COVID-19 and critical COVID-19: a systematic review and meta-analysis. BMC Infect. Dis. 2022, 22, 615. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Peng, Y.; Shen, Y.; Zhang, Y.; Liu, L.; Yang, X. Dietary polyphenols: regulate the advanced glycation end products-RAGE axis and the microbiota-gut-brain axis to prevent neurodegenerative diseases. Crit. Rev. Food Sci. Nutr. 2023, 63, 9816–9842. [Google Scholar] [CrossRef]
- Ghimire, S.; Cady, N.M.; Lehman, P.; Peterson, S.R.; Shahi, S.K.; Rashid, F.; Giri, S.; Mangalam, A.K. Dietary Isoflavones Alter Gut Microbiota and Lipopolysaccharide Biosynthesis to Reduce Inflammation. Gut Microbes 2022, 14, 2127446. [Google Scholar] [CrossRef]
- Winiarska-Mieczan, A.; Kwiecień, M.; Jachimowicz-Rogowska, K.; Donaldson, J.; Tomaszewska, E.; Baranowska-Wójcik, E. Anti-Inflammatory, Antioxidant, and Neuroprotective Effects of Polyphenols-Polyphenols as an Element of Diet Therapy in Depressive Disorders. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Ma, Y.; Li, X.; Zhang, J.; Zhao, L. Supplementation with soy isoflavones alleviates depression-like behaviour via reshaping the gut microbiota structure. Food Funct. 2021, 12, 4995–5006. [Google Scholar] [CrossRef]
- Chatterjee, A.; Kumar, S.; Roy Sarkar, S.; Halder, R.; Kumari, R.; Banerjee, S.; Sarkar, B. Dietary polyphenols represent a phytotherapeutic alternative for gut dysbiosis associated neurodegeneration: A systematic review. J. Nutr. Biochem. 2024, 129, 109622. [Google Scholar] [CrossRef]
- Tayab, M.A.; Islam, M.N.; Chowdhury, K.A.A.; Tasnim, F.M. Targeting neuroinflammation by polyphenols: A promising therapeutic approach against inflammation-associated depression. Biomed. Pharmacother. 2022, 147, 112668. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Fakhri, S.; Khan, H. Targeting apoptosis and autophagy following spinal cord injury: Therapeutic approaches to polyphenols and candidate phytochemicals. Pharmacol. Res. 2020, 160, 105069. [Google Scholar] [CrossRef]
- Grifka-Walk, H.M.; Jenkins, B.R.; Kominsky, D.J. Amino Acid Trp: The Far Out Impacts of Host and Commensal Tryptophan Metabolism. Front. Immunol. 2021, 12, 653208. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhou, M.; Wang, J.; Yao, J.; Yu, J.; Liu, W.; Wu, L.; Wang, J.; Gao, R. Involvement of the microbiota-gut-brain axis in chronic restraint stress: disturbances of the kynurenine metabolic pathway in both the gut and brain. Gut Microbes 2021, 13, 1–16. [Google Scholar] [CrossRef]
- Tutakhail, A.; Boulet, L.; Khabil, S.; Nazari, Q.A.; Hamid, H.; Coudoré, F. Neuropathology of kynurenine pathway of tryptophan metabolism. Current pharmacology reports 2020, 6, 8–23. [Google Scholar] [CrossRef]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front Endocrinol (Lausanne) 2019, 10, 158. [Google Scholar] [CrossRef]
- Fujigaki, H.; Yamamoto, Y.; Saito, K. L-Tryptophan-kynurenine pathway enzymes are therapeutic target for neuropsychiatric diseases: Focus on cell type differences. Neuropharmacology 2017, 112, 264–274. [Google Scholar] [CrossRef]
- Messaoud, A.; Mensi, R.; Douki, W.; Neffati, F.; Najjar, M.F.; Gobbi, G.; Valtorta, F.; Gaha, L.; Comai, S. Reduced peripheral availability of tryptophan and increased activation of the kynurenine pathway and cortisol correlate with major depression and suicide. World J. Biol. Psychiatry 2019, 20, 703–711. [Google Scholar] [CrossRef]
- Chivite, M.; Leal, E.; Míguez, J.M.; Cerdá-Reverter, J.M. Distribution of two isoforms of tryptophan hydroxylase in the brain of rainbow trout (Oncorhynchus mykiss). An in situ hybridization study. Brain Struct. Funct. 2021, 226, 2265–2278. [Google Scholar] [CrossRef]
- Maffei, M.E. 5-Hydroxytryptophan (5-HTP): Natural Occurrence, Analysis, Biosynthesis, Biotechnology, Physiology and Toxicology. Int. J. Mol. Sci. 2020, 22. [Google Scholar] [CrossRef]
- Xue, C.; Li, G.; Zheng, Q.; Gu, X.; Shi, Q.; Su, Y.; Chu, Q.; Yuan, X.; Bao, Z.; Lu, J.; et al. Tryptophan metabolism in health and disease. Cell Metab. 2023, 35, 1304–1326. [Google Scholar] [CrossRef]
- Zakrocka, I.; Urbańska, E.M.; Załuska, W.; Kronbichler, A. Kynurenine Pathway after Kidney Transplantation: Friend or Foe? International Journal of Molecular Sciences 2024, 25, 9940. [Google Scholar] [CrossRef] [PubMed]
- Perez--Castro, L.; Garcia, R.; Venkateswaran, N.; Barnes, S.; Conacci--Sorrell, M. Tryptophan and its metabolites in normal physiology and cancer etiology. The FEBS journal 2023, 290, 7–27. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Fathi, M.; Vakili, K.; Yaghoobpoor, S.; Tavasol, A.; Jazi, K.; Mohamadkhani, A.; Klegeris, A.; McElhinney, A.; Mafi, Z.; Hajiesmaeili, M.; et al. Dynamic changes in kynurenine pathway metabolites in multiple sclerosis: A systematic review. Front. Immunol. 2022, 13, 1013784. [Google Scholar] [CrossRef]
- Ou, W.; Chen, Y.; Ju, Y.; Ma, M.; Qin, Y.; Bi, Y.; Liao, M.; Liu, B.; Liu, J.; Zhang, Y.; et al. The kynurenine pathway in major depressive disorder under different disease states: A systematic review and meta-analysis. J. Affect. Disord. 2023, 339, 624–632. [Google Scholar] [CrossRef]
- Kozieł, K.; Urbanska, E.M. Kynurenine Pathway in Diabetes Mellitus-Novel Pharmacological Target? Cells 2023, 12. [Google Scholar] [CrossRef]
- Tanaka, M.; Szabó, Á.; Vécsei, L. Redefining Roles: A Paradigm Shift in Tryptophan-Kynurenine Metabolism for Innovative Clinical Applications. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Skorobogatov, K.; De Picker, L.; Verkerk, R.; Coppens, V.; Leboyer, M.; Müller, N.; Morrens, M. Brain Versus Blood: A Systematic Review on the Concordance Between Peripheral and Central Kynurenine Pathway Measures in Psychiatric Disorders. Front. Immunol. 2021, 12, 716980. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Schwarcz, R.; Erhardt, S. Neuroactive Kynurenines as Pharmacological Targets: New Experimental Tools and Exciting Therapeutic Opportunities. Pharmacol. Rev. 2024, 76, 978–1008. [Google Scholar] [CrossRef]
- Zhang, S.; Sakuma, M.; Deora, G.S.; Levy, C.W.; Klausing, A.; Breda, C.; Read, K.D.; Edlin, C.D.; Ross, B.P.; Wright Muelas, M.; et al. A brain-permeable inhibitor of the neurodegenerative disease target kynurenine 3-monooxygenase prevents accumulation of neurotoxic metabolites. Commun. Biol. 2019, 2, 271. [Google Scholar] [CrossRef]
- Jones, S.P.; Franco, N.F.; Varney, B.; Sundaram, G.; Brown, D.A.; de Bie, J.; Lim, C.K.; Guillemin, G.J.; Brew, B.J. Expression of the Kynurenine Pathway in Human Peripheral Blood Mononuclear Cells: Implications for Inflammatory and Neurodegenerative Disease. PLoS One 2015, 10, e0131389. [Google Scholar] [CrossRef]
- Sathyasaikumar, K.V.; Notarangelo, F.M.; Kelly, D.L.; Rowland, L.M.; Hare, S.M.; Chen, S.; Mo, C.; Buchanan, R.W.; Schwarcz, R. Tryptophan Challenge in Healthy Controls and People with Schizophrenia: Acute Effects on Plasma Levels of Kynurenine, Kynurenic Acid and 5-Hydroxyindoleacetic Acid. Pharmaceuticals (Basel) 2022, 15. [Google Scholar] [CrossRef]
- Hestad, K.; Alexander, J.; Rootwelt, H.; Aaseth, J.O. The Role of Tryptophan Dysmetabolism and Quinolinic Acid in Depressive and Neurodegenerative Diseases. Biomolecules 2022, 12. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Tuka, B.; Tanaka, M.; Vécsei, L.; Telegdy, G. Memory Enhancement with Kynurenic Acid and Its Mechanisms in Neurotransmission. Biomedicines 2022, 10. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Szatmári, I.; Vécsei, L. Quinoline Quest: Kynurenic Acid Strategies for Next-Generation Therapeutics via Rational Drug Design. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef] [PubMed]
- Mor, A.; Tankiewicz-Kwedlo, A.; Krupa, A.; Pawlak, D. Role of Kynurenine Pathway in Oxidative Stress during Neurodegenerative Disorders. Cells 2021, 10. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. From Microbial Switches to Metabolic Sensors: Rewiring the Gut-Brain Kynurenine Circuit. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. Revolutionizing our understanding of Parkinson’s disease: Dr. Heinz Reichmann’s pioneering research and future research direction. J Neural Transm (Vienna) 2024, 131, 1367–1387. [Google Scholar] [CrossRef]
- Cheong, J.E.; Sun, L. Targeting the IDO1/TDO2-KYN-AhR Pathway for Cancer Immunotherapy - Challenges and Opportunities. Trends Pharmacol. Sci. 2018, 39, 307–325. [Google Scholar] [CrossRef]
- Ye, Z.; Yue, L.; Shi, J.; Shao, M.; Wu, T. Role of IDO and TDO in Cancers and Related Diseases and the Therapeutic Implications. J. Cancer 2019, 10, 2771–2782. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Zhang, C.; Zhang, J.; Su, W.; Wang, G.; Wang, Z. The Kynurenine Pathway and Indole Pathway in Tryptophan Metabolism Influence Tumor Progression. Cancer Med. 2025, 14, e70703. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Williams, R.O. Interactions of IDO and the Kynurenine Pathway with Cell Transduction Systems and Metabolism at the Inflammation-Cancer Interface. Cancers (Basel) 2023, 15. [Google Scholar] [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Shayan, M.; Niazi Shahraki, F.; Hosseini, Y.; Momtaz, S.; Abdolghaffari, A.H. IDO/Kynurenine; novel insight for treatment of inflammatory diseases. Cytokine 2023, 166, 156206. [Google Scholar] [CrossRef] [PubMed]
- Labadie, B.W.; Bao, R.; Luke, J.J. Reimagining IDO Pathway Inhibition in Cancer Immunotherapy via Downstream Focus on the Tryptophan-Kynurenine-Aryl Hydrocarbon Axis. Clin. Cancer Res. 2019, 25, 1462–1471. [Google Scholar] [CrossRef]
- Campesato, L.F.; Budhu, S.; Tchaicha, J.; Weng, C.H.; Gigoux, M.; Cohen, I.J.; Redmond, D.; Mangarin, L.; Pourpe, S.; Liu, C.; et al. Blockade of the AHR restricts a Treg-macrophage suppressive axis induced by L-Kynurenine. Nat. Commun. 2020, 11, 4011. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Kato, S.; Nesline, M.K.; Conroy, J.M.; DePietro, P.; Pabla, S.; Kurzrock, R. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat. Rev. 2022, 110, 102461. [Google Scholar] [CrossRef]
- Bertollo, A.G.; Mingoti, M.E.D.; Ignácio, Z.M. Neurobiological mechanisms in the kynurenine pathway and major depressive disorder. Rev. Neurosci. 2025, 36, 169–187. [Google Scholar] [CrossRef]
- Gong, X.; Chang, R.; Zou, J.; Tan, S.; Huang, Z. The role and mechanism of tryptophan - kynurenine metabolic pathway in depression. Rev. Neurosci. 2023, 34, 313–324. [Google Scholar] [CrossRef]
- Anderson, E.W.; Fishbein, J.; Hong, J.; Roeser, J.; Furie, R.A.; Aranow, C.; Volpe, B.T.; Diamond, B.; Mackay, M. Quinolinic acid, a kynurenine/tryptophan pathway metabolite, associates with impaired cognitive test performance in systemic lupus erythematosus. Lupus Sci. Med. 2021, 8. [Google Scholar] [CrossRef]
- Wang, L.; Feng, Z.; Zheng, T.; Dai, G.; Wang, M.; Zhou, L.; Zheng, Y.; Chen, G. Associations between the kynurenine pathway and the brain in patients with major depressive disorder-A systematic review of neuroimaging studies. Prog. Neuropsychopharmacol. Biol. Psychiatry 2023, 121, 110675. [Google Scholar] [CrossRef]
- Ogyu, K.; Kubo, K.; Noda, Y.; Iwata, Y.; Tsugawa, S.; Omura, Y.; Wada, M.; Tarumi, R.; Plitman, E.; Moriguchi, S.; et al. Kynurenine pathway in depression: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 90, 16–25. [Google Scholar] [CrossRef]
- Marx, W.; McGuinness, A.J.; Rocks, T.; Ruusunen, A.; Cleminson, J.; Walker, A.J.; Gomes-da-Costa, S.; Lane, M.; Sanches, M.; Diaz, A.P.; et al. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a meta-analysis of 101 studies. Mol. Psychiatry 2021, 26, 4158–4178. [Google Scholar] [CrossRef] [PubMed]
- Joisten, N.; Ruas, J.L.; Braidy, N.; Guillemin, G.J.; Zimmer, P. The kynurenine pathway in chronic diseases: a compensatory mechanism or a driving force? Trends Mol. Med. 2021, 27, 946–954. [Google Scholar] [CrossRef] [PubMed]
- Martos, D.; Lőrinczi, B.; Szatmári, I.; Vécsei, L.; Tanaka, M. Decoupling Behavioral Domains via Kynurenic Acid Analog Optimization: Implications for Schizophrenia and Parkinson’s Disease Therapeutics. Cells 2025, 14. [Google Scholar] [CrossRef]
- Inam, M.E.; Fernandes, B.S.; Salagre, E.; Grande, I.; Vieta, E.; Quevedo, J.; Zhao, Z. The kynurenine pathway in major depressive disorder, bipolar disorder, and schizophrenia: a systematic review and meta-analysis of cerebrospinal fluid studies. Braz. J. Psychiatry 2023, 45, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Liloia, D. Navigating Neurodegeneration: Integrating Biomarkers, Neuroinflammation, and Imaging in Parkinson’s, Alzheimer’s, and Motor Neuron Disorders. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Więdłocha, M.; Marcinowicz, P.; Janoska-Jaździk, M.; Szulc, A. Gut microbiota, kynurenine pathway and mental disorders - Review. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 106, 110145. [Google Scholar] [CrossRef]
- Chen, C.Y.; Wang, Y.F.; Lei, L.; Zhang, Y. Impacts of microbiota and its metabolites through gut-brain axis on pathophysiology of major depressive disorder. Life Sci. 2024, 351, 122815. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef]
- Ohara, T.E.; Hsiao, E.Y. Microbiota-neuroepithelial signalling across the gut-brain axis. Nat. Rev. Microbiol. 2025, 23, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Bonfili, L.; Wei, T.; Eleuteri, A.M. Understanding the Gut-Brain Axis and Its Therapeutic Implications for Neurodegenerative Disorders. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Ullah, H.; Arbab, S.; Tian, Y.; Liu, C.Q.; Chen, Y.; Qijie, L.; Khan, M.I.U.; Hassan, I.U.; Li, K. The gut microbiota-brain axis in neurological disorder. Front. Neurosci. 2023, 17, 1225875. [Google Scholar] [CrossRef]
- Ma, Q.; Xing, C.; Long, W.; Wang, H.Y.; Liu, Q.; Wang, R.F. Impact of microbiota on central nervous system and neurological diseases: the gut-brain axis. J. Neuroinflammation 2019, 16, 53. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef]
- Góralczyk-Bińkowska, A.; Szmajda-Krygier, D.; Kozłowska, E. The Microbiota-Gut-Brain Axis in Psychiatric Disorders. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Han, Y.; Wang, B.; Gao, H.; He, C.; Hua, R.; Liang, C.; Zhang, S.; Wang, Y.; Xin, S.; Xu, J. Vagus Nerve and Underlying Impact on the Gut Microbiota-Brain Axis in Behavior and Neurodegenerative Diseases. J. Inflamm. Res. 2022, 15, 6213–6230. [Google Scholar] [CrossRef]
- Naufel, M.F.; Truzzi, G.M.; Ferreira, C.M.; Coelho, F.M.S. The brain-gut-microbiota axis in the treatment of neurologic and psychiatric disorders. Arq. Neuropsiquiatr. 2023, 81, 670–684. [Google Scholar] [CrossRef]
- Loh, J.S.; Mak, W.Q.; Tan, L.K.S.; Ng, C.X.; Chan, H.H.; Yeow, S.H.; Foo, J.B.; Ong, Y.S.; How, C.W.; Khaw, K.Y. Microbiota-gut-brain axis and its therapeutic applications in neurodegenerative diseases. Signal Transduct. Target. Ther. 2024, 9, 37. [Google Scholar] [CrossRef]
- Rutsch, A.; Kantsjö, J.B.; Ronchi, F. The Gut-Brain Axis: How Microbiota and Host Inflammasome Influence Brain Physiology and Pathology. Front. Immunol. 2020, 11, 604179. [Google Scholar] [CrossRef]
- Long-Smith, C.; O’Riordan, K.J.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2020, 60, 477–502. [Google Scholar] [CrossRef]
- Lee, S.H.; Han, C.; Shin, C. IUPHAR review: Microbiota-gut-brain axis and its role in neuropsychiatric disorders. Pharmacol. Res. 2025, 216, 107749. [Google Scholar] [CrossRef]
- Zhou, M.; Fan, Y.; Xu, L.; Yu, Z.; Wang, S.; Xu, H.; Zhang, J.; Zhang, L.; Liu, W.; Wu, L.; et al. Microbiome and tryptophan metabolomics analysis in adolescent depression: roles of the gut microbiota in the regulation of tryptophan-derived neurotransmitters and behaviors in human and mice. Microbiome 2023, 11, 145. [Google Scholar] [CrossRef]
- Zhao, H.; Qiu, X.; Wang, S.; Wang, Y.; Xie, L.; Xia, X.; Li, W. Multiple pathways through which the gut microbiota regulates neuronal mitochondria constitute another possible direction for depression. Front. Microbiol. 2025, 16, 1578155. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Chen, X.; Zhang, Y.; Zhang, H.; Xie, P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine 2023, 90, 104527. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; He, T.; Johnston, L.J.; Ma, X. Host-microbiome interactions: the aryl hydrocarbon receptor as a critical node in tryptophan metabolites to brain signaling. Gut Microbes 2020, 11, 1203–1219. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A. Activation of aryl hydrocarbon receptor (AhR) in Alzheimer’s disease: role of tryptophan metabolites generated by gut host-microbiota. J Mol Med (Berl) 2023, 101, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Jiang, Y.; Xu, K.; Cui, M.; Ye, W.; Zhao, G.; Jin, L.; Chen, X. The progress of gut microbiome research related to brain disorders. J. Neuroinflammation 2020, 17, 25. [Google Scholar] [CrossRef]
- You, M.; Chen, N.; Yang, Y.; Cheng, L.; He, H.; Cai, Y.; Liu, Y.; Liu, H.; Hong, G. The gut microbiota-brain axis in neurological disorders. MedComm (2020) 2024, 5, e656. [Google Scholar] [CrossRef]
- Zatorska, O. Impact of gut microbiota on the central nervous system relevance in neurodegenerative and psychiatric diseases. Current Problems of Psychiatry 2024, 25, 239–247. [Google Scholar] [CrossRef]
- Varesi, A.; Campagnoli, L.I.M.; Chirumbolo, S.; Candiano, B.; Carrara, A.; Ricevuti, G.; Esposito, C.; Pascale, A. The brain-gut-microbiota interplay in depression: A key to design innovative therapeutic approaches. Pharmacol. Res. 2023, 192, 106799. [Google Scholar] [CrossRef] [PubMed]
- Palepu, M.S.K.; Dandekar, M.P. Remodeling of microbiota gut-brain axis using psychobiotics in depression. Eur. J. Pharmacol. 2022, 931, 175171. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Kanoujia, J.; Mohana Lakshmi, S.; Patil, C.R.; Gupta, G.; Chellappan, D.K.; Dua, K. Role of Brain-Gut-Microbiota Axis in Depression: Emerging Therapeutic Avenues. CNS Neurol. Disord. Drug Targets 2023, 22, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Generoso, J.S.; Giridharan, V.V.; Lee, J.; Macedo, D.; Barichello, T. The role of the microbiota-gut-brain axis in neuropsychiatric disorders. Braz. J. Psychiatry 2021, 43, 293–305. [Google Scholar] [CrossRef]
- Zhang, Q.; Bi, Y.; Zhang, B.; Jiang, Q.; Mou, C.K.; Lei, L.; Deng, Y.; Li, Y.; Yu, J.; Liu, W.; et al. Current landscape of fecal microbiota transplantation in treating depression. Front. Immunol. 2024, 15, 1416961. [Google Scholar] [CrossRef]
- Qiao, Y.; Yu, J.; Zhang, Z.; Hou, Q.; Guo, Z.; Wang, Y. Regulatory effects of Lactobacillus zhachilii HBUAS52074(T) on depression-like behavior induced by chronic social defeat stress in mice: modulation of the gut microbiota. Food Funct. 2025, 16, 691–706. [Google Scholar] [CrossRef]
- Li, W.; Xu, M.; Liu, Y.; Zhang, S.; Wang, J.; Zhang, Z.; Xiao, G.; Wang, R.; Zhang, J.; Xue, H. Lactiplantibacillus plantarum GOLDGUT-HNU082 Alleviates CUMS-Induced Depressive-like Behaviors in Mice by Modulating the Gut Microbiota and Neurotransmitter Levels. Foods 2025, 14. [Google Scholar] [CrossRef]
- Xiong, R.G.; Li, J.; Cheng, J.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Gan, R.Y.; Li, H.B. The Role of Gut Microbiota in Anxiety, Depression, and Other Mental Disorders as Well as the Protective Effects of Dietary Components. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Sanada, K.; Nakajima, S.; Kurokawa, S.; Barceló-Soler, A.; Ikuse, D.; Hirata, A.; Yoshizawa, A.; Tomizawa, Y.; Salas-Valero, M.; Noda, Y.; et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 2020, 266, 1–13. [Google Scholar] [CrossRef]
- Ng, Q.X.; Lim, Y.L.; Yaow, C.Y.L.; Ng, W.K.; Thumboo, J.; Liew, T.M. Effect of Probiotic Supplementation on Gut Microbiota in Patients with Major Depressive Disorders: A Systematic Review. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Hofmeister, M.; Clement, F.; Patten, S.; Li, J.; Dowsett, L.E.; Farkas, B.; Mastikhina, L.; Egunsola, O.; Diaz, R.; Cooke, N.C.A.; et al. The effect of interventions targeting gut microbiota on depressive symptoms: a systematic review and meta-analysis. CMAJ Open 2021, 9, E1195–e1204. [Google Scholar] [CrossRef] [PubMed]
- Esmeeta, A.; Adhikary, S.; Dharshnaa, V.; Swarnamughi, P.; Ummul Maqsummiya, Z.; Banerjee, A.; Pathak, S.; Duttaroy, A.K. Plant-derived bioactive compounds in colon cancer treatment: An updated review. Biomed. Pharmacother. 2022, 153, 113384. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Wu, Y.; Maruyama, W.; Shamoto-Nagai, M. Phytochemicals Modulate Biosynthesis and Function of Serotonin, Dopamine, and Norepinephrine for Treatment of Monoamine Neurotransmission-Related Psychiatric Diseases. Int. J. Mol. Sci. 2025, 26. [Google Scholar] [CrossRef]
- Rahman, M.A.; Hannan, M.A.; Dash, R.; Rahman, M.H.; Islam, R.; Uddin, M.J.; Sohag, A.A.M.; Rahman, M.H.; Rhim, H. Phytochemicals as a Complement to Cancer Chemotherapy: Pharmacological Modulation of the Autophagy-Apoptosis Pathway. Front. Pharmacol. 2021, 12, 639628. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Paterson, J.; Bucknall, M.; Arcot, J. Interactions between phytochemicals from fruits and vegetables: Effects on bioactivities and bioavailability. Crit. Rev. Food Sci. Nutr. 2018, 58, 1310–1329. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Chen, L.; Gao, Z.; Lin, M.; Wang, Y.; Xiao, Z.; Chen, Y.; Huang, X. Phytomedicine Fructus Aurantii-derived two absorbed compounds unlock antidepressant and prokinetic multi-functions via modulating 5-HT(3)/GHSR. J. Ethnopharmacol. 2024, 323, 117703. [Google Scholar] [CrossRef]
- Cheng, S.; Zhu, Z.; Li, H.; Wang, W.; Jiang, Z.; Pan, F.; Liu, D.; Ho, R.C.M.; Ho, C.S.H. Rifaximin ameliorates depression-like behaviour in chronic unpredictable mild stress rats by regulating intestinal microbiota and hippocampal tryptophan metabolism. J. Affect. Disord. 2023, 329, 30–41. [Google Scholar] [CrossRef]
- Martínez-Rodríguez, A.; Rubio-Arias, J.; Ramos-Campo, D.J.; Reche-García, C.; Leyva-Vela, B.; Nadal-Nicolás, Y. Psychological and Sleep Effects of Tryptophan and Magnesium-Enriched Mediterranean Diet in Women with Fibromyalgia. Int. J. Environ. Res. Public. Health 2020, 17. [Google Scholar] [CrossRef]
- Patel, A.; Cheung, J. The effect of mediterranean diet and chrononutrition on sleep quality: a scoping review. Nutr. J. 2025, 24, 31. [Google Scholar] [CrossRef]
- Scoditti, E.; Tumolo, M.R.; Garbarino, S. Mediterranean Diet on Sleep: A Health Alliance. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Godos, J.; Ferri, R.; Lanza, G.; Caraci, F.; Vistorte, A.O.R.; Yelamos Torres, V.; Grosso, G.; Castellano, S. Mediterranean Diet and Sleep Features: A Systematic Review of Current Evidence. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Gantenbein, K.V.; Kanaka-Gantenbein, C. Mediterranean Diet as an Antioxidant: The Impact on Metabolic Health and Overall Wellbeing. Nutrients 2021, 13. [Google Scholar] [CrossRef] [PubMed]
- Godos, J.; Ferri, R.; Caraci, F.; Cosentino, F.I.I.; Castellano, S.; Galvano, F.; Grosso, G. Adherence to the Mediterranean Diet is Associated with Better Sleep Quality in Italian Adults. Nutrients 2019, 11. [Google Scholar] [CrossRef] [PubMed]
- Casas, I.; Nakaki, A.; Pascal, R.; Castro-Barquero, S.; Youssef, L.; Genero, M.; Benitez, L.; Larroya, M.; Boutet, M.L.; Casu, G.; et al. Effects of a Mediterranean Diet Intervention on Maternal Stress, Well-Being, and Sleep Quality throughout Gestation-The IMPACT-BCN Trial. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Booij, L.; Merens, W.; Markus, C.R.; Van der Does, A.J. Diet rich in alpha-lactalbumin improves memory in unmedicated recovered depressed patients and matched controls. J. Psychopharmacol. 2006, 20, 526–535. [Google Scholar] [CrossRef]
- Markus, C.R.; Olivier, B.; Panhuysen, G.E.; Van Der Gugten, J.; Alles, M.S.; Tuiten, A.; Westenberg, H.G.; Fekkes, D.; Koppeschaar, H.F.; de Haan, E.E. The bovine protein alpha-lactalbumin increases the plasma ratio of tryptophan to the other large neutral amino acids, and in vulnerable subjects raises brain serotonin activity, reduces cortisol concentration, and improves mood under stress. Am. J. Clin. Nutr. 2000, 71, 1536–1544. [Google Scholar] [CrossRef]
- Procópio Pinheiro, R.; Gaubeur, M.A.; Itezerote, A.M.; Saleh, S.O.; Hojaij, F.; Andrade, M.; Jacomo, A.L.; Akamatsu, F.E. Anatomical Study of the Innervation of the Masseter Muscle and Its Correlation with Myofascial Trigger Points. J. Pain. Res. 2020, 13, 3217–3226. [Google Scholar] [CrossRef]
- Porter, R.J.; Phipps, A.J.; Gallagher, P.; Scott, A.; Stevenson, P.S.; O’Brien, J.T. Effects of acute tryptophan depletion on mood and cognitive functioning in older recovered depressed subjects. The American journal of geriatric psychiatry 2005, 13, 607–615. [Google Scholar] [CrossRef]
- Badrasawi, M.M.; Shahar, S.; Abd Manaf, Z.; Haron, H. Effect of Talbinah food consumption on depressive symptoms among elderly individuals in long term care facilities, randomized clinical trial. Clin. Interv. Aging 2013, 8, 279–285. [Google Scholar] [CrossRef]
- Jangid, P.; Malik, P.; Singh, P.; Sharma, M.; Gulia, A.K. Comparative study of efficacy of l-5-hydroxytryptophan and fluoxetine in patients presenting with first depressive episode. Asian J. Psychiatr. 2013, 6, 29–34. [Google Scholar] [CrossRef]
- Meloni, M.; Puligheddu, M.; Carta, M.; Cannas, A.; Figorilli, M.; Defazio, G. Efficacy and safety of 5-hydroxytryptophan on depression and apathy in Parkinson’s disease: a preliminary finding. Eur. J. Neurol. 2020, 27, 779–786. [Google Scholar] [CrossRef]
- Firk, C.; Markus, C.R. Mood and cortisol responses following tryptophan-rich hydrolyzed protein and acute stress in healthy subjects with high and low cognitive reactivity to depression. Clin. Nutr. 2009, 28, 266–271. [Google Scholar] [CrossRef]
- Benkelfat, C.; Ellenbogen, M.A.; Dean, P.; Palmour, R.M.; Young, S.N. Mood-lowering effect of tryptophan depletion. Enhanced susceptibility in young men at genetic risk for major affective disorders. Arch. Gen. Psychiatry 1994, 51, 687–697. [Google Scholar] [CrossRef]
- Bruce, K.R.; Steiger, H.; Young, S.N.; Kin, N.M.; Israël, M.; Lévesque, M. Impact of acute tryptophan depletion on mood and eating-related urges in bulimic and nonbulimic women. J. Psychiatry Neurosci. 2009, 34, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Longhitano, C.; Ayres, R.E.; Cowen, P.J.; Harmer, C.J. Tryptophan supplementation induces a positive bias in the processing of emotional material in healthy female volunteers. Psychopharmacology (Berl) 2006, 187, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Rueda, G.H.; Causada-Calo, N.; Borojevic, R.; Nardelli, A.; Pinto-Sanchez, M.I.; Constante, M.; Libertucci, J.; Mohan, V.; Langella, P.; Loonen, L.M.P.; et al. Oral tryptophan activates duodenal aryl hydrocarbon receptor in healthy subjects: a crossover randomized controlled trial. Am. J. Physiol. Gastrointest. Liver Physiol. 2024, 326, G687–g696. [Google Scholar] [CrossRef] [PubMed]
- Cerit, H.; Schuur, R.J.; de Bruijn, E.R.; Van der Does, W. Tryptophan supplementation and the response to unfairness in healthy volunteers. Front. Psychol. 2015, 6, 1012. [Google Scholar] [CrossRef]
- Markus, C.R.; Firk, C.; Gerhardt, C.; Kloek, J.; Smolders, G.F. Effect of different tryptophan sources on amino acids availability to the brain and mood in healthy volunteers. Psychopharmacology (Berl) 2008, 201, 107–114. [Google Scholar] [CrossRef]
- Bravo, R.; Matito, S.; Cubero, J.; Paredes, S.D.; Franco, L.; Rivero, M.; Rodríguez, A.B.; Barriga, C. Tryptophan-enriched cereal intake improves nocturnal sleep, melatonin, serotonin, and total antioxidant capacity levels and mood in elderly humans. Age (Dordr) 2013, 35, 1277–1285. [Google Scholar] [CrossRef]
- Rondanelli, M.; Opizzi, A.; Faliva, M.; Mozzoni, M.; Antoniello, N.; Cazzola, R.; Savarè, R.; Cerutti, R.; Grossi, E.; Cestaro, B. Effects of a diet integration with an oily emulsion of DHA-phospholipids containing melatonin and tryptophan in elderly patients suffering from mild cognitive impairment. Nutr. Neurosci. 2012, 15, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Connell, N.J.; Grevendonk, L.; Fealy, C.E.; Moonen-Kornips, E.; Bruls, Y.M.H.; Schrauwen-Hinderling, V.B.; de Vogel, J.; Hageman, R.; Geurts, J.; Zapata-Perez, R.; et al. NAD+-Precursor Supplementation With L-Tryptophan, Nicotinic Acid, and Nicotinamide Does Not Affect Mitochondrial Function or Skeletal Muscle Function in Physically Compromised Older Adults. J. Nutr. 2021, 151, 2917–2931. [Google Scholar] [CrossRef]
- Chojnacki, C.; Gąsiorowska, A.; Popławski, T.; Konrad, P.; Chojnacki, M.; Fila, M.; Blasiak, J. Beneficial Effect of Increased Tryptophan Intake on Its Metabolism and Mental State of the Elderly. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Agarwal, S.; Fulgoni, V.L. , 3rd. Tryptophan Intake in the US Adult Population Is Not Related to Liver or Kidney Function but Is Associated with Depression and Sleep Outcomes. J. Nutr. 2016, 146, 2609s–2615s. [Google Scholar] [CrossRef] [PubMed]
- Nematolahi, P.; Mehrabani, M.; Karami-Mohajeri, S.; Dabaghzadeh, F. Effects of Rosmarinus officinalis L. on memory performance, anxiety, depression, and sleep quality in university students: A randomized clinical trial. Complement. Ther. Clin. Pract. 2018, 30, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh Rahbardar, M.; Hosseinzadeh, H. Therapeutic effects of rosemary (Rosmarinus officinalis L.) and its active constituents on nervous system disorders. Iran. J. Basic. Med. Sci. 2020, 23, 1100–1112. [Google Scholar] [CrossRef]
- Li, T.; Wang, W.; Guo, Q.; Li, J.; Tang, T.; Wang, Y.; Liu, D.; Yang, K.; Li, J.; Deng, K.; et al. Rosemary (Rosmarinus officinalis L.) hydrosol based on serotonergic synapse for insomnia. J. Ethnopharmacol. 2024, 318, 116984. [Google Scholar] [CrossRef]
- Kenda, M.; Kočevar Glavač, N.; Nagy, M.; Sollner Dolenc, M. Medicinal Plants Used for Anxiety, Depression, or Stress Treatment: An Update. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Can, S.; Yildirim Usta, Y.; Yildiz, S.; Tayfun, K. The effect of lavender and rosemary aromatherapy application on cognitive functions, anxiety, and sleep quality in the elderly with diabetes. Explore (NY) 2024, 20, 103033. [Google Scholar] [CrossRef]
- Ghasemi, A.; Sharafabadi, F.M.; Marani, E. The Effect of Inhalation Aromatherapy with Essential Oils of Various Plants on Anxiety and Sleep Quality in Diabetic Patients: A Review Study. Iranian journal of diabetes and obesity 2025. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Quintero-Rincón, P.; Olivero-Verbel, J. Aromatherapy and Essential Oils: Holistic Strategies in Complementary and Alternative Medicine for Integral Wellbeing. Plants (Basel) 2025, 14. [Google Scholar] [CrossRef]
- Mathews, I.M.; Eastwood, J.; Lamport, D.J.; Cozannet, R.L.; Fanca-Berthon, P.; Williams, C.M. Clinical Efficacy and Tolerability of Lemon Balm (Melissa officinalis L.) in Psychological Well-Being: A Review. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Wei, Z.; Jiang, N.; Chen, Y.; Wang, Y.; Li, S.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. Soy isoflavones protects against cognitive deficits induced by chronic sleep deprivation via alleviating oxidative stress and suppressing neuroinflammation. Phytother. Res. 2022, 36, 2072–2080. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.R.; Manhães-de-Castro, R.; de Santana, B.; Olegário da Silva, L.; Toscano, A.E.; Guzmán-Quevedo, O.; Galindo, L.C.M. Effects of flavonols on emotional behavior and compounds of the serotonergic system: A preclinical systematic review. Eur. J. Pharmacol. 2022, 916, 174697. [Google Scholar] [CrossRef] [PubMed]
- Hamsalakshmi; Alex, A. M.; Arehally Marappa, M.; Joghee, S.; Chidambaram, S.B. Therapeutic benefits of flavonoids against neuroinflammation: a systematic review. Inflammopharmacology 2022, 30, 111–136. [Google Scholar] [CrossRef]
- Scuto, M.; Majzúnová, M.; Torcitto, G.; Antonuzzo, S.; Rampulla, F.; Di Fatta, E.; Trovato Salinaro, A. Functional Food Nutrients, Redox Resilience Signaling and Neurosteroids for Brain Health. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Li, F.; An, L. Berberine alleviates postoperative cognitive dysfunction by suppressing neuroinflammation in aged mice. Int. Immunopharmacol. 2016, 38, 426–433. [Google Scholar] [CrossRef]
- Premkumar, L.S. Transient receptor potential channels as targets for phytochemicals. ACS Chem. Neurosci. 2014, 5, 1117–1130. [Google Scholar] [CrossRef]
- Pham, D.C.; Shibu, M.A.; Mahalakshmi, B.; Velmurugan, B.K. Effects of phytochemicals on cellular signaling: reviewing their recent usage approaches. Crit. Rev. Food Sci. Nutr. 2020, 60, 3522–3546. [Google Scholar] [CrossRef]
- Rao, P.P. Phytochemicals in Obesity Management: Mechanisms and Clinical Perspectives. Curr. Nutr. Rep. 2025, 14, 17. [Google Scholar] [CrossRef]
- Sarris, J.; Ravindran, A.; Yatham, L.N.; Marx, W.; Rucklidge, J.J.; McIntyre, R.S.; Akhondzadeh, S.; Benedetti, F.; Caneo, C.; Cramer, H.; et al. Clinician guidelines for the treatment of psychiatric disorders with nutraceuticals and phytoceuticals: The World Federation of Societies of Biological Psychiatry (WFSBP) and Canadian Network for Mood and Anxiety Treatments (CANMAT) Taskforce. World J. Biol. Psychiatry 2022, 23, 424–455. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.Z.; Ma, Q.Y.; Tao, G.; Huang, J.Q.; Chen, J.X. Oral coniferyl ferulate attenuated depression symptoms in mice via reshaping gut microbiota and microbial metabolism. Food Funct. 2021, 12, 12550–12564. [Google Scholar] [CrossRef]
- Mannino, G.; Serio, G.; Gaglio, R.; Maffei, M.E.; Settanni, L.; Di Stefano, V.; Gentile, C. Biological Activity and Metabolomics of Griffonia simplicifolia Seeds Extracted with Different Methodologies. Antioxidants (Basel) 2023, 12. [Google Scholar] [CrossRef]
- Ribas, R.; Oliveira, D.; Silva, P. The different roles of Griffonia simplicifolia in the treatment of depression: a narrative review. Int. J. Complement. Alt. Med. 2021, 14, 167–172. [Google Scholar]
- Moharir, Y. 5-HTP for Improved Sleep, Mood and Weight Loss.
- Fellows, L.E.; Bell, E.A. 5-Hydroxy-L-tryptophan, 5-hydroxytryptamine and L-tryptophan-5-hydroxylase in Griffonia simplicifolia. Phytochemistry 1970, 9, 2389–2396. [Google Scholar] [CrossRef]
- Vigliante, I.; Mannino, G.; Maffei, M.E. Chemical Characterization and DNA Fingerprinting of Griffonia simplicifolia Baill. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Jędrejko, K.; Sułkowska-Ziaja, K.; Ziaja, M.; Kała, K.; Muszyńska, B. Edible Mushrooms as a Potential Component of Dietary Interventions for Major Depressive Disorder. Foods 2022, 11. [Google Scholar] [CrossRef]
- Lu, C.; Wei, Z.; Wang, Y.; Li, S.; Tong, L.; Liu, X.; Fan, B.; Wang, F. Soy isoflavones alleviate lipopolysaccharide-induced depressive-like behavior by suppressing neuroinflammation, mediating tryptophan metabolism and promoting synaptic plasticity. Food Funct. 2022, 13, 9513–9522. [Google Scholar] [CrossRef]
- Lu, C.; Gao, R.; Zhang, Y.; Jiang, N.; Chen, Y.; Sun, J.; Wang, Q.; Fan, B.; Liu, X.; Wang, F. S-equol, a metabolite of dietary soy isoflavones, alleviates lipopolysaccharide-induced depressive-like behavior in mice by inhibiting neuroinflammation and enhancing synaptic plasticity. Food Funct. 2021, 12, 5770–5778. [Google Scholar] [CrossRef] [PubMed]
- McLaren, S.; Seidler, K.; Neil, J. Investigating the Role of 17β-Estradiol on the Serotonergic System, Targeting Soy Isoflavones as a Strategy to Reduce Menopausal Depression: A Mechanistic Review. J. Am. Nutr. Assoc. 2024, 43, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Sekikawa, A.; Wharton, W.; Butts, B.; Veliky, C.V.; Garfein, J.; Li, J.; Goon, S.; Fort, A.; Li, M.; Hughes, T.M. Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Birru, R.; Snitz, B.E.; Ihara, M.; Lopresti, B.J.; Aizenstein, H.J.; Lopez, O.L.; Mathis, C.; Miyamoto, Y.; Kuller, L.H. P3--615: EFFECTS OF SOY ISOFLAVONES ON COGNITIVE FUNCTION: A SYSTEMATIC REVIEW AND META--ANALYSIS OF RANDOMIZED CONTROLLED TRIALS. Alzheimer’s & Dementia 2018, 14, P1365–P1366. [Google Scholar]
- Huang, M.; He, Y.; Tian, L.; Yu, L.; Cheng, Q.; Li, Z.; Gao, L.; Gao, S.; Yu, C. Gut microbiota-SCFAs-brain axis associated with the antidepressant activity of berberine in CUMS rats. J. Affect. Disord. 2023, 325, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yu, X.; Wang, Z.; Kong, L.; Jiang, Z.; Shang, R.; Zhong, X.; Lv, S.; Zhang, G.; Gao, H.; et al. Microbiota-gut-brain axis: Natural antidepressants molecular mechanism. Phytomedicine 2024, 134, 156012. [Google Scholar] [CrossRef]
- Xiao, W.; Su, J.; Gao, X.; Yang, H.; Weng, R.; Ni, W.; Gu, Y. The microbiota-gut-brain axis participates in chronic cerebral hypoperfusion by disrupting the metabolism of short-chain fatty acids. Microbiome 2022, 10, 62. [Google Scholar] [CrossRef]
- Qian, X.H.; Xie, R.Y.; Liu, X.L.; Chen, S.D.; Tang, H.D. Mechanisms of Short-Chain Fatty Acids Derived from Gut Microbiota in Alzheimer’s Disease. Aging Dis. 2022, 13, 1252–1266. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, H.; Ju, Y.; Liu, J.; Wang, M.; Liu, B.; Zhang, Y. Gut microbiota-derived short-chain fatty acids and depression: deep insight into biological mechanisms and potential applications. Gen. Psychiatr. 2024, 37, e101374. [Google Scholar] [CrossRef]
- Zhao, Z.X.; Fu, J.; Ma, S.R.; Peng, R.; Yu, J.B.; Cong, L.; Pan, L.B.; Zhang, Z.G.; Tian, H.; Che, C.T.; et al. Gut-brain axis metabolic pathway regulates antidepressant efficacy of albiflorin. Theranostics 2018, 8, 5945–5959. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Qin, Z.; Shi, D.D.; Li, W.; Cheng, D.; Zhang, Y.D.; Zhang, S.; Tsoi, B.; Zhao, J.; Wang, Z.; Zhang, Z.J. Berberine ameliorates depression-like behaviors in mice via inhibiting NLRP3 inflammasome-mediated neuroinflammation and preventing neuroplasticity disruption. J. Neuroinflammation 2023, 20, 54. [Google Scholar] [CrossRef]
- Tang, C.F.; Wang, C.Y.; Wang, J.H.; Wang, Q.N.; Li, S.J.; Wang, H.O.; Zhou, F.; Li, J.M. Short-Chain Fatty Acids Ameliorate Depressive-like Behaviors of High Fructose-Fed Mice by Rescuing Hippocampal Neurogenesis Decline and Blood-Brain Barrier Damage. Nutrients 2022, 14. [Google Scholar] [CrossRef]
- Dudek, K.A.; Dion-Albert, L.; Lebel, M.; LeClair, K.; Labrecque, S.; Tuck, E.; Ferrer Perez, C.; Golden, S.A.; Tamminga, C.; Turecki, G.; et al. Molecular adaptations of the blood-brain barrier promote stress resilience vs. depression. Proc. Natl. Acad. Sci. U S A 2020, 117, 3326–3336. [Google Scholar] [CrossRef] [PubMed]
- Knox, E.G.; Aburto, M.R.; Clarke, G.; Cryan, J.F.; O’Driscoll, C.M. The blood-brain barrier in aging and neurodegeneration. Mol. Psychiatry 2022, 27, 2659–2673. [Google Scholar] [CrossRef] [PubMed]
- Grabska-Kobyłecka, I.; Szpakowski, P.; Król, A.; Książek-Winiarek, D.; Kobyłecki, A.; Głąbiński, A.; Nowak, D. Polyphenols and Their Impact on the Prevention of Neurodegenerative Diseases and Development. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, B.N.; Parylak, S.L.; Gage, F.H. Mechanisms of dietary flavonoid action in neuronal function and neuroinflammation. Mol. Aspects Med. 2018, 61, 50–62. [Google Scholar] [CrossRef]
- Calderaro, A.; Patanè, G.T.; Tellone, E.; Barreca, D.; Ficarra, S.; Misiti, F.; Laganà, G. The Neuroprotective Potentiality of Flavonoids on Alzheimer’s Disease. Int. J. Mol. Sci. 2022, 23. [Google Scholar] [CrossRef]
- Pontifex, M.G.; Malik, M.; Connell, E.; Müller, M.; Vauzour, D. Citrus Polyphenols in Brain Health and Disease: Current Perspectives. Front. Neurosci. 2021, 15, 640648. [Google Scholar] [CrossRef]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; López-Valdés, H.E. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef]
- Moosavi, F.; Hosseini, R.; Saso, L.; Firuzi, O. Modulation of neurotrophic signaling pathways by polyphenols. Drug Des. Devel Ther. 2016, 10, 23–42. [Google Scholar] [CrossRef]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24. [Google Scholar] [CrossRef]
- Carey, C.C.; Lucey, A.; Doyle, L. Flavonoid Containing Polyphenol Consumption and Recovery from Exercise-Induced Muscle Damage: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 1293–1316. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Du, P.; Xie, Q.; Wang, N.; Li, H.; Smith, E.E.; Li, C.; Liu, F.; Huo, G.; Li, B. Protective effects of tryptophan-catabolizing Lactobacillus plantarum KLDS 1.0386 against dextran sodium sulfate-induced colitis in mice. Food Funct. 2020, 11, 10736–10747. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Analysis, Nutrition, and Health Benefits of Tryptophan. Int. J. Tryptophan Res. 2018, 11, 1178646918802282. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; Elsaraf, M.; Mindt, M.; Wendisch, V.F. l-Serine Biosensor-Controlled Fermentative Production of l-Tryptophan Derivatives by Corynebacterium glutamicum. Biology (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Guo, Y.W.; Liu, X.J.; Yuan, J.; Li, H.J.; Mahmud, T.; Hong, M.J.; Yu, J.C.; Lan, W.J. l-Tryptophan Induces a Marine-Derived Fusarium sp. to Produce Indole Alkaloids with Activity against the Zika Virus. J. Nat. Prod. 2020, 83, 3372–3380. [Google Scholar] [CrossRef]
- Henarejos-Escudero, P.; Méndez-García, F.F.; Hernández-García, S.; Martínez-Rodríguez, P.; Gandía-Herrero, F. Design, Synthesis and Gene Modulation Insights into Pigments Derived from Tryptophan-Betaxanthin, Which Act against Tumor Development in Caenorhabditis elegans. Int. J. Mol. Sci. 2023, 25. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Chen, Q.; Zhou, N.; Wang, X.; Zhang, A.; Chen, K.; Ouyang, P. Construction of cell factory capable of efficiently converting L-tryptophan into 5-hydroxytryptamine. Microb. Cell Fact. 2022, 21, 47. [Google Scholar] [CrossRef]
- Shaw, K.; Turner, J.; Del Mar, C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst. Rev. 2002, Cd003198. [Google Scholar] [CrossRef]
- Herrington, R.N.; Bruce, A.; Johnstone, E.C. Comparative trial of L-tryptophan and E.C.T. in severe depressive illness. Lancet 1974, 2, 731–734. [Google Scholar] [CrossRef]
- Kikuchi, A.M.; Tanabe, A.; Iwahori, Y. A systematic review of the effect of L-tryptophan supplementation on mood and emotional functioning. J. Diet. Suppl. 2021, 18, 316–333. [Google Scholar] [CrossRef]
- Tian, P.; Chen, Y.; Zhu, H.; Wang, L.; Qian, X.; Zou, R.; Zhao, J.; Zhang, H.; Qian, L.; Wang, Q.; et al. Bifidobacterium breve CCFM1025 attenuates major depression disorder via regulating gut microbiome and tryptophan metabolism: A randomized clinical trial. Brain Behav. Immun. 2022, 100, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Su, T.; Wu, P.; Dai, Y.; Chen, Y.; Wang, Q.; Cao, C.; Chen, F.; Wang, Q.; Wang, S. Identification of paeoniflorin from Paeonia lactiflora pall. As an inhibitor of tryptophan 2,3-dioxygenase and assessment of its pharmacological effects on depressive mice. J. Ethnopharmacol. 2023, 317, 116714. [Google Scholar] [CrossRef] [PubMed]
- Kałużna-Czaplińska, J.; Gątarek, P.; Chirumbolo, S.; Chartrand, M.S.; Bjørklund, G. How important is tryptophan in human health? Crit. Rev. Food Sci. Nutr. 2019, 59, 72–88. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, C.N.; Loh, W.W.; Toh, D.W.K.; Lee, D.P.S.; Kim, J.E. Association Between Dietary Protein Intake and Sleep Quality in Middle-Aged and Older Adults in Singapore. Front. Nutr. 2022, 9, 832341. [Google Scholar] [CrossRef]
- Sutanto, C.N.; Loh, W.W.; Kim, J.E. The impact of tryptophan supplementation on sleep quality: a systematic review, meta-analysis, and meta-regression. Nutr. Rev. 2022, 80, 306–316. [Google Scholar] [CrossRef]
- Mohajeri, M.H.; Wittwer, J.; Vargas, K.; Hogan, E.; Holmes, A.; Rogers, P.J.; Goralczyk, R.; Gibson, E.L. Chronic treatment with a tryptophan-rich protein hydrolysate improves emotional processing, mental energy levels and reaction time in middle-aged women. Br. J. Nutr. 2015, 113, 350–365. [Google Scholar] [CrossRef]
- Chen, M.; Liu, Y.; Xiong, S.; Wu, M.; Li, B.; Ruan, Z.; Hu, X. Dietary l-tryptophan alleviated LPS-induced intestinal barrier injury by regulating tight junctions in a Caco-2 cell monolayer model. Food Funct. 2019, 10, 2390–2398. [Google Scholar] [CrossRef]
- Scott, S.A.; Fu, J.; Chang, P.V. Microbial tryptophan metabolites regulate gut barrier function via the aryl hydrocarbon receptor. Proc. Natl. Acad. Sci. U S A 2020, 117, 19376–19387. [Google Scholar] [CrossRef]
- Liu, W.; Mi, S.; Ruan, Z.; Li, J.; Shu, X.; Yao, K.; Jiang, M.; Deng, Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J. Food Sci. 2017, 82, 562–567. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Dai, Z.; Kou, J.; Sun, K.; Chen, J.; Yang, Y.; Wu, G.; Wu, Z. Dietary l-Tryptophan Supplementation Enhances the Intestinal Mucosal Barrier Function in Weaned Piglets: Implication of Tryptophan-Metabolizing Microbiota. Int. J. Mol. Sci. 2018, 20. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S. Tryptophan Dietary Impacts Gut Barrier and Metabolic Diseases. Front. Immunol. 2019, 10, 2113. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol. 2018, 8, 13. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Yang, H.; Wang, R.; Wang, X.; Zhang, X. Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer-Depression Comorbidity Management. Nutrients 2025, 17. [Google Scholar] [CrossRef]
- Trzeciak, P.; Herbet, M. Role of the Intestinal Microbiome, Intestinal Barrier and Psychobiotics in Depression. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Nunes, Y.C.; Mendes, N.M.; Pereira de Lima, E.; Chehadi, A.C.; Lamas, C.B.; Haber, J.F.S.; Dos Santos Bueno, M.; Araújo, A.C.; Catharin, V.C.S.; Detregiachi, C.R.P.; et al. Curcumin: A Golden Approach to Healthy Aging: A Systematic Review of the Evidence. Nutrients 2024, 16. [Google Scholar] [CrossRef]
- Nabavi, S.M.; Daglia, M.; Braidy, N.; Nabavi, S.F. Natural products, micronutrients, and nutraceuticals for the treatment of depression: A short review. Nutr. Neurosci. 2017, 20, 180–194. [Google Scholar] [CrossRef]
- Fernstrom, J.D. Effects and side effects associated with the non-nutritional use of tryptophan by humans. J. Nutr. 2012, 142, 2236s–2244s. [Google Scholar] [CrossRef]
- Hiratsuka, C.; Fukuwatari, T.; Sano, M.; Saito, K.; Sasaki, S.; Shibata, K. Supplementing healthy women with up to 5.0 g/d of L-tryptophan has no adverse effects. J. Nutr. 2013, 143, 859–866. [Google Scholar] [CrossRef]
- Zhu, X.; Tao, Q.; Sun-Waterhouse, D.; Li, W.; Liu, S.; Cui, C. γ-[Glu]n-Trp ameliorates anxiety/depression-like behaviors and its anti-inflammatory effect in an animal model of anxiety/depression. Food Funct. 2019, 10, 5544–5554. [Google Scholar] [CrossRef]
- Medsger, T.A., Jr. Tryptophan-induced eosinophilia-myalgia syndrome. N. Engl. J. Med. 1990, 322, 926–928. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.M.; Park, J.E.; Heo, I.K.; Shin, Y.U.; Kim, Y.H.; Son, W.C. Safety concerns regarding impurities in L-Tryptophan associated with eosinophilia myalgia syndrome. Food Chem. Toxicol. 2023, 179, 113946. [Google Scholar] [CrossRef]
- Mayeno, A.N.; Gleich, G.J. Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale. Trends Biotechnol. 1994, 12, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.H., Jr.; Caudill, S.P.; Philen, R.M.; Bailey, S.L.; Flanders, W.D.; Driskell, W.J.; Kamb, M.L.; Needham, L.L.; Sampson, E.J. Contaminants in L-tryptophan associated with eosinophilia myalgia syndrome. Arch. Environ. Contam. Toxicol. 1993, 25, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Eidson, M.; Philen, R.M.; Sewell, C.M.; Voorhees, R.; Kilbourne, E.M. L-tryptophan and eosinophilia-myalgia syndrome in New Mexico. Lancet 1990, 335, 645–648. [Google Scholar] [CrossRef]
- Hertzman, P.A.; Blevins, W.L.; Mayer, J.; Greenfield, B.; Ting, M.; Gleich, G.J. Association of the eosinophilia-myalgia syndrome with the ingestion of tryptophan. N. Engl. J. Med. 1990, 322, 869–873. [Google Scholar] [CrossRef]
- Das, Y.T.; Bagchi, M.; Bagchi, D.; Preuss, H.G. Safety of 5-hydroxy-L-tryptophan. Toxicol. Lett. 2004, 150, 111–122. [Google Scholar] [CrossRef]
- Martin, R.W.; Duffy, J.; Engel, A.G.; Lie, J.T.; Bowles, C.A.; Moyer, T.P.; Gleich, G.J. The clinical spectrum of the eosinophilia-myalgia syndrome associated with L-tryptophan ingestion. Clinical features in 20 patients and aspects of pathophysiology. Ann. Intern. Med. 1990, 113, 124–134. [Google Scholar] [CrossRef]
- Verity, M.A.; Bulpitt, K.J.; Paulus, H.E. Neuromuscular manifestations of L-tryptophan-associated eosinophilia-myalgia syndrome: a histomorphologic analysis of 14 patients. Hum. Pathol. 1991, 22, 3–11. [Google Scholar] [CrossRef]
- Munir, S.; Shahid, A.; Aslam, B.; Ashfaq, U.A.; Akash, M.S.H.; Ali, M.A.; Almatroudi, A.; Allemailem, K.S.; Rajoka, M.S.R.; Khurshid, M. The Therapeutic Prospects of Naturally Occurring and Synthetic Indole Alkaloids for Depression and Anxiety Disorders. Evid. Based Complement. Alternat Med. 2020, 2020, 8836983. [Google Scholar] [CrossRef]
- Kanova, M.; Kohout, P. Serotonin-Its Synthesis and Roles in the Healthy and the Critically Ill. Int. J. Mol. Sci. 2021, 22. [Google Scholar] [CrossRef]
- Guzel, T.; Mirowska-Guzel, D. The Role of Serotonin Neurotransmission in Gastrointestinal Tract and Pharmacotherapy. Molecules 2022, 27. [Google Scholar] [CrossRef]
- Mokhtari, T. Targeting autophagy and neuroinflammation pathways with plant-derived natural compounds as potential antidepressant agents. Phytother. Res. 2022, 36, 3470–3489. [Google Scholar] [CrossRef] [PubMed]
- Jamu, I.M.; Okamoto, H. Recent advances in understanding adverse effects associated with drugs targeting the serotonin receptor, 5-HT GPCR. Front. Glob. Womens Health 2022, 3, 1012463. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.L. Tryptophan supplementation and serotonin function: genetic variations in behavioural effects. Proc. Nutr. Soc. 2018, 77, 174–188. [Google Scholar] [CrossRef] [PubMed]
- Averina, O.V.; Poluektova, E.U.; Zorkina, Y.A.; Kovtun, A.S.; Danilenko, V.N. Human Gut Microbiota for Diagnosis and Treatment of Depression. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Lin, P.; Li, D.; Shi, Y.; Li, Q.; Guo, X.; Dong, K.; Chen, Q.; Lou, X.; Li, Z.; Li, P.; et al. Dysbiosis of the Gut Microbiota and Kynurenine (Kyn) Pathway Activity as Potential Biomarkers in Patients with Major Depressive Disorder. Nutrients 2023, 15. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, H.; Yuan, Y.; Guan, X.; Xie, Q.; Dong, Z. Baseline gut microbiota profiles affect treatment response in patients with depression. Front. Microbiol. 2024, 15, 1429116. [Google Scholar] [CrossRef]
- Liu, L.; Wang, H.; Zhang, H.; Chen, X.; Zhang, Y.; Wu, J.; Zhao, L.; Wang, D.; Pu, J.; Ji, P.; et al. Toward a Deeper Understanding of Gut Microbiome in Depression: The Promise of Clinical Applicability. Adv Sci (Weinh) 2022, 9, e2203707. [Google Scholar] [CrossRef]
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51. [Google Scholar] [CrossRef]
- Wang, M.; Song, Z.; Lai, S.; Tang, F.; Dou, L.; Yang, F. Depression-associated gut microbes, metabolites and clinical trials. Front. Microbiol. 2024, 15, 1292004. [Google Scholar] [CrossRef]
- Clerici, L.; Bottari, D.; Bottari, B. Gut Microbiome, Diet and Depression: Literature Review of Microbiological, Nutritional and Neuroscientific Aspects. Curr. Nutr. Rep. 2025, 14, 30. [Google Scholar] [CrossRef]
- Jiang, Y.; Qu, Y.; Shi, L.; Ou, M.; Du, Z.; Zhou, Z.; Zhou, H.; Zhu, H. The role of gut microbiota and metabolomic pathways in modulating the efficacy of SSRIs for major depressive disorder. Transl. Psychiatry 2024, 14, 493. [Google Scholar] [CrossRef]
- Pawlowski, T.; Malyszczak, K.; Pawlak, D.; Inglot, M.; Zalewska, M.; Grzywacz, A.; Radkowski, M.; Laskus, T.; Janocha-Litwin, J.; Frydecka, D. HTR1A, TPH2, and 5-HTTLPR Polymorphisms and Their Impact on the Severity of Depressive Symptoms and on the Concentration of Tryptophan Catabolites during Hepatitis C Treatment with Pegylated Interferon-α2a and Oral Ribavirin (PEG-IFN-α2a/RBV). Cells 2023, 12. [Google Scholar] [CrossRef]
- Duan, K.M.; Wang, S.Y.; Yin, J.Y.; Li, X.; Ma, J.H.; Huang, Z.D.; Zhou, Y.Y.; Yu, H.Y.; Yang, M.; Zhou, H.H.; et al. The IDO genetic polymorphisms and postpartum depressive symptoms: an association study in Chinese parturients who underwent cesarean section. Arch. Womens Ment. Health 2019, 22, 339–348. [Google Scholar] [CrossRef]
- Fasching, P.A.; Faschingbauer, F.; Goecke, T.W.; Engel, A.; Häberle, L.; Seifert, A.; Voigt, F.; Amann, M.; Rebhan, D.; Burger, P.; et al. Genetic variants in the tryptophan hydroxylase 2 gene (TPH2) and depression during and after pregnancy. J. Psychiatr. Res. 2012, 46, 1109–1117. [Google Scholar] [CrossRef]
- MUSHTAQ, R. TRYPTOPHAN HYDROXYLASE GENE POLYMORPHISM AND 5HT TRANSPORTER GENE PROMOTER REGION POLYMORPHISM IN ANXIETY AND DEPRESSIVE DISORDER-COMPREHENSIVE LITERATURE REVIEW. INTERNATIONAL JOURNAL OF RESEARCH IN MEDICAL SCIENCES 2024, 12, 3957–3961. [Google Scholar] [CrossRef]
- Ottenhof, K.W.; Sild, M.; Lévesque, M.L.; Ruhé, H.G.; Booij, L. TPH2 polymorphisms across the spectrum of psychiatric morbidity: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2018, 92, 29–42. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Battaglia, S.; Giménez-Llort, L.; Chen, C.; Hepsomali, P.; Avenanti, A.; Vécsei, L. Innovation at the Intersection: Emerging Translational Research in Neurology and Psychiatry. Cells 2024, 13. [Google Scholar] [CrossRef] [PubMed]
- Platten, M.; Nollen, E.A.A.; Röhrig, U.F.; Fallarino, F.; Opitz, C.A. Tryptophan metabolism as a common therapeutic target in cancer, neurodegeneration and beyond. Nat. Rev. Drug Discov. 2019, 18, 379–401. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, D.; Yu, J.; Song, X.J.; Li, X.; Cui, Y.L. Tryptophan Metabolism Disorder-Triggered Diseases, Mechanisms, and Therapeutic Strategies: A Scientometric Review. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.J.; Adnan, M.; Saxena, J.; Alam, M.J.; Abdelgadir, A.; Badraoui, R.; Singh, R. Therapeutic Potential of Plant- and Marine-Derived Bioactive Compounds in Prostate Cancer: Mechanistic Insights and Translational Applications. Pharmaceuticals (Basel) 2025, 18. [Google Scholar] [CrossRef] [PubMed]
- Modoux, M.; Rolhion, N.; Mani, S.; Sokol, H. Tryptophan Metabolism as a Pharmacological Target. Trends Pharmacol. Sci. 2021, 42, 60–73. [Google Scholar] [CrossRef]
- Sadee, W.; Wang, D.; Hartmann, K.; Toland, A.E. Pharmacogenomics: Driving Personalized Medicine. Pharmacol. Rev. 2023, 75, 789–814. [Google Scholar] [CrossRef]
- Heinken, A.; Basile, A.; Hertel, J.; Thinnes, C.; Thiele, I. Genome-Scale Metabolic Modeling of the Human Microbiome in the Era of Personalized Medicine. Annu. Rev. Microbiol. 2021, 75, 199–222. [Google Scholar] [CrossRef]
- Nowicka, A.; Tomczak, H.; Szałek, E.; Karbownik, A.; Gil, L. Microbial Crosstalk with Therapy: Pharmacomicrobiomics in AML-One Step Closer to Personalized Medicine. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Li, D.; Yu, S.; Long, Y.; Shi, A.; Deng, J.; Ma, Y.; Wen, J.; Li, X.; Liu, S.; Zhang, Y.; et al. Tryptophan metabolism: Mechanism-oriented therapy for neurological and psychiatric disorders. Front. Immunol. 2022, 13, 985378. [Google Scholar] [CrossRef]
- Ge, P.Y.; Qu, S.Y.; Ni, S.J.; Yao, Z.Y.; Qi, Y.Y.; Zhao, X.; Guo, R.; Yang, N.Y.; Zhang, Q.C.; Zhu, H.X. Berberine ameliorates depression-like behavior in CUMS mice by activating TPH1 and inhibiting IDO1-associated with tryptophan metabolism. Phytother. Res. 2023, 37, 342–357. [Google Scholar] [CrossRef]
- Hu, M.; Shi, X.; Song, P.X. Collaborative inference for treatment effect with distributed data-sharing management in multicenter studies. Stat. Med. 2024, 43, 2263–2279. [Google Scholar] [CrossRef]
- Adhikari, B.M.; Jahanshad, N.; Shukla, D.; Turner, J.; Grotegerd, D.; Dannlowski, U.; Kugel, H.; Engelen, J.; Dietsche, B.; Krug, A.; et al. A resting state fMRI analysis pipeline for pooling inference across diverse cohorts: an ENIGMA rs-fMRI protocol. Brain Imaging Behav. 2019, 13, 1453–1467. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Chen, X.; Xavier Castellanos, F.; Thompson, P.M.; Zuo, X.N.; Zang, Y.F.; Yan, C.G. The power of many brains: Catalyzing neuropsychiatric discovery through open neuroimaging data and large-scale collaboration. Sci Bull (Beijing) 2024, 69, 1536–1555. [Google Scholar] [CrossRef] [PubMed]
- Llovera, G.; Langhauser, F.; Isla Cainzos, S.; Hoppen, M.; Abberger, H.; Mohamud Yusuf, A.; Mencl, S.; Heindl, S.; Ricci, A.; Haupeltshofer, S.; et al. Stroke of Consistency: Streamlining Multicenter Protocols for Enhanced Reproducibility of Infarct Volumes in Preclinical Stroke Research. Stroke 2024, 55, 2522–2527. [Google Scholar] [CrossRef] [PubMed]
- Sheller, M.J.; Edwards, B.; Reina, G.A.; Martin, J.; Pati, S.; Kotrotsou, A.; Milchenko, M.; Xu, W.; Marcus, D.; Colen, R.R.; et al. Federated learning in medicine: facilitating multi-institutional collaborations without sharing patient data. Sci. Rep. 2020, 10, 12598. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, N.; Zhang, L.; Cheng, C.W.; Liu, Z.; Liang, F.; Xiong, W.; Deng, J.; Shi, D.; Cui, W.; et al. Reporting assessment of multicenter clinical trial protocols: A cross-sectional study. J. Evid. Based Med. 2023, 16, 16–18. [Google Scholar] [CrossRef]
- Chen, N.; Lee, J.J. Bayesian cluster hierarchical model for subgroup borrowing in the design and analysis of basket trials with binary endpoints. Stat. Methods Med. Res. 2020, 29, 2717–2732. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, R.; Chen, X.; Yan, F. Adaptive Bayesian information borrowing methods for finding and optimizing subgroup-specific doses. Clin. Trials 2024, 21, 308–321. [Google Scholar] [CrossRef]
- Wang, G.; Levis, A.; Steingrimsson, J.; Dahabreh, I. Efficient estimation of subgroup treatment effects using multi-source data. arXiv preprint arXiv:2402.02684, arXiv:2402.02684 2024.
- Tanaka, M. Parkinson’s Disease: Bridging Gaps, Building Biomarkers, and Reimagining Clinical Translation. Cells 2025, 14. [Google Scholar] [CrossRef]
- Tanaka, M. From Serendipity to Precision: Integrating AI, Multi-Omics, and Human-Specific Models for Personalized Neuropsychiatric Care. Biomedicines 2025, 13. [Google Scholar] [CrossRef]
- Tanaka, M. Beyond the boundaries: Transitioning from categorical to dimensional paradigms in mental health diagnostics. Adv. Clin. Exp. Med. 2024, 33, 1295–1301. [Google Scholar] [CrossRef]
- Pagotto, G.L.O.; Santos, L.; Osman, N.; Lamas, C.B.; Laurindo, L.F.; Pomini, K.T.; Guissoni, L.M.; Lima, E.P.; Goulart, R.A.; Catharin, V.; et al. Ginkgo biloba: A Leaf of Hope in the Fight against Alzheimer’s Dementia: Clinical Trial Systematic Review. Antioxidants (Basel) 2024, 13. [Google Scholar] [CrossRef]
- Tanaka, M.; Vécsei, L. A Decade of Dedication: Pioneering Perspectives on Neurological Diseases and Mental Illnesses. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Chahla, J.; Cinque, M.E.; Piuzzi, N.S.; Mannava, S.; Geeslin, A.G.; Murray, I.R.; Dornan, G.J.; Muschler, G.F.; LaPrade, R.F. A Call for Standardization in Platelet-Rich Plasma Preparation Protocols and Composition Reporting: A Systematic Review of the Clinical Orthopaedic Literature. J. Bone Joint Surg. Am. 2017, 99, 1769–1779. [Google Scholar] [CrossRef]
- Nueraihemaiti, N.; Dilimulati, D.; Baishan, A.; Hailati, S.; Maihemuti, N.; Aikebaier, A.; Paerhati, Y.; Zhou, W. Advances in Plant-Derived Extracellular Vesicle Extraction Methods and Pharmacological Effects. Biology (Basel) 2025, 14. [Google Scholar] [CrossRef] [PubMed]
- Lezoul, N.E.H.; Belkadi, M.; Habibi, F.; Guillén, F. Extraction Processes with Several Solvents on Total Bioactive Compounds in Different Organs of Three Medicinal Plants. Molecules 2020, 25. [Google Scholar] [CrossRef] [PubMed]
- Mew, E.J.; Monsour, A.; Saeed, L.; Santos, L.; Patel, S.; Courtney, D.B.; Watson, P.N.; Szatmari, P.; Offringa, M.; Monga, S.; et al. Systematic scoping review identifies heterogeneity in outcomes measured in adolescent depression clinical trials. J. Clin. Epidemiol. 2020, 126, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Centanni, M.; Friberg, L.E. Model-Based Biomarker Selection for Dose Individualization of Tyrosine-Kinase Inhibitors. Front. Pharmacol. 2020, 11, 316. [Google Scholar] [CrossRef]
- Yap, C.; Solovyeva, O.; de Bono, J.; Rekowski, J.; Patel, D.; Jaki, T.; Mander, A.; Evans, T.R.J.; Peck, R.; Hayward, K.S.; et al. Enhancing reporting quality and impact of early phase dose-finding clinical trials: CONSORT Dose-finding Extension (CONSORT-DEFINE) guidance. Bmj 2023, 383, e076387. [Google Scholar] [CrossRef]
- Schwarz, A.J. The Use, Standardization, and Interpretation of Brain Imaging Data in Clinical Trials of Neurodegenerative Disorders. Neurotherapeutics 2021, 18, 686–708. [Google Scholar] [CrossRef]
- Carini, C.; Seyhan, A.A. Tribulations and future opportunities for artificial intelligence in precision medicine. J. Transl. Med. 2024, 22, 411. [Google Scholar] [CrossRef]
- Chen, X.; He, R.; Chen, X.; Jiang, L.; Wang, F. Optimizing dose-schedule regimens with bayesian adaptive designs: opportunities and challenges. Front. Pharmacol. 2023, 14, 1261312. [Google Scholar] [CrossRef]
- Cottrell, T.R.; Lotze, M.T.; Ali, A.; Bifulco, C.B.; Capitini, C.M.; Chow, L.Q.M.; Cillo, A.R.; Collyar, D.; Cope, L.; Deutsch, J.S.; et al. Society for Immunotherapy of Cancer (SITC) consensus statement on essential biomarkers for immunotherapy clinical protocols. J. Immunother. Cancer 2025, 13. [Google Scholar] [CrossRef]
- Ciobanu-Caraus, O.; Aicher, A.; Kernbach, J.M.; Regli, L.; Serra, C.; Staartjes, V.E. A critical moment in machine learning in medicine: on reproducible and interpretable learning. Acta Neurochir (Wien) 2024, 166, 14. [Google Scholar] [CrossRef]
- Kim, J.W.; Choi, E.C.; Lee, K.J. Standardizing the approach to clinical-based human microbiome research: from clinical information collection to microbiome profiling and human resource utilization. Osong Public. Health Res. Perspect. 2025, 16, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, S.; Mahmoud, N.N.; Voke, E.; Landry, M.P.; Mahmoudi, M. Importance of Standardizing Analytical Characterization Methodology for Improved Reliability of the Nanomedicine Literature. Nanomicro Lett. 2022, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Araujo, M.; Voelkl, B.; Laloux, C.; Novak, J.; Koopmans, B.; Waldron, A.M.; Seiffert, I.; Stirling, H.; Aulehner, K.; Janhunen, S.K.; et al. Systematic assessment of the replicability and generalizability of preclinical findings: Impact of protocol harmonization across laboratory sites. PLoS Biol. 2022, 20, e3001886. [Google Scholar] [CrossRef] [PubMed]
- Lukić, I.; Ivković, S.; Mitić, M.; Adžić, M. Tryptophan metabolites in depression: Modulation by gut microbiota. Front. Behav. Neurosci. 2022, 16, 987697. [Google Scholar] [CrossRef]
- Hoepner, C.T.; McIntyre, R.S.; Papakostas, G.I. Impact of Supplementation and Nutritional Interventions on Pathogenic Processes of Mood Disorders: A Review of the Evidence. Nutrients 2021, 13. [Google Scholar] [CrossRef]
- Thomson, J.; Rankin, H.; Ashcroft, G.W.; Yates, C.M.; McQueen, J.K.; Cummings, S.W. The treatment of depression in general practice: a comparison of L-tryptophan, amitriptyline, and a combination of L-tryptophan and amitriptyline with placebo. Psychol. Med. 1982, 12, 741–751. [Google Scholar] [CrossRef]
- Kazemi, A.; Noorbala, A.A.; Azam, K.; Eskandari, M.H.; Djafarian, K. Effect of probiotic and prebiotic vs placebo on psychological outcomes in patients with major depressive disorder: A randomized clinical trial. Clin. Nutr. 2019, 38, 522–528. [Google Scholar] [CrossRef]
- Jia, L.; Xiao, L.; Fu, Y.; Shao, Z.; Jing, Z.; Yuan, J.; Xie, Y.; Guo, J.; Wang, Y.; Geng, W. Neuroprotective effects of probiotics on anxiety- and depression-like disorders in stressed mice by modulating tryptophan metabolism and the gut microbiota. Food Funct. 2024, 15, 2895–2905. [Google Scholar] [CrossRef]
- Donoso, F.; Cryan, J.F.; Olavarría-Ramírez, L.; Nolan, Y.M.; Clarke, G. Inflammation, Lifestyle Factors, and the Microbiome-Gut-Brain Axis: Relevance to Depression and Antidepressant Action. Clin. Pharmacol. Ther. 2023, 113, 246–259. [Google Scholar] [CrossRef]
- Nikdasti, A.; Khodadadi, E.S.; Ferdosi, F.; Dadgostar, E.; Yahyazadeh, S.; Heidari, P.; Ehtiati, S.; Vakili, O.; Khatami, S.H. Nutritional Strategies in Major Depression Disorder: From Ketogenic Diet to Modulation of the Microbiota-Gut-Brain Axis. Mol. Neurobiol. 2025, 62, 2973–2994. [Google Scholar] [CrossRef] [PubMed]
- Merino Del Portillo, M.; Clemente-Suárez, V.J.; Ruisoto, P.; Jimenez, M.; Ramos-Campo, D.J.; Beltran-Velasco, A.I.; Martínez-Guardado, I.; Rubio-Zarapuz, A.; Navarro-Jiménez, E.; Tornero-Aguilera, J.F. Nutritional Modulation of the Gut-Brain Axis: A Comprehensive Review of Dietary Interventions in Depression and Anxiety Management. Metabolites 2024, 14. [Google Scholar] [CrossRef] [PubMed]
- Parletta, N.; Zarnowiecki, D.; Cho, J.; Wilson, A.; Bogomolova, S.; Villani, A.; Itsiopoulos, C.; Niyonsenga, T.; Blunden, S.; Meyer, B.; et al. A Mediterranean-style dietary intervention supplemented with fish oil improves diet quality and mental health in people with depression: A randomized controlled trial (HELFIMED). Nutr. Neurosci. 2019, 22, 474–487. [Google Scholar] [CrossRef] [PubMed]
- Opie, R.S.; O’Neil, A.; Jacka, F.N.; Pizzinga, J.; Itsiopoulos, C. A modified Mediterranean dietary intervention for adults with major depression: Dietary protocol and feasibility data from the SMILES trial. Nutr. Neurosci. 2018, 21, 487–501. [Google Scholar] [CrossRef]
- Firth, J.; Marx, W.; Dash, S.; Carney, R.; Teasdale, S.B.; Solmi, M.; Stubbs, B.; Schuch, F.B.; Carvalho, A.F.; Jacka, F.; et al. The Effects of Dietary Improvement on Symptoms of Depression and Anxiety: A Meta-Analysis of Randomized Controlled Trials. Psychosom. Med. 2019, 81, 265–280. [Google Scholar] [CrossRef]
- Xu, Y.; Zeng, L.; Zou, K.; Shan, S.; Wang, X.; Xiong, J.; Zhao, L.; Zhang, L.; Cheng, G. Role of dietary factors in the prevention and treatment for depression: an umbrella review of meta-analyses of prospective studies. Transl. Psychiatry 2021, 11, 478. [Google Scholar] [CrossRef]
- Schefft, C.; Kilarski, L.L.; Bschor, T.; Köhler, S. Efficacy of adding nutritional supplements in unipolar depression: A systematic review and meta-analysis. Eur. Neuropsychopharmacol. 2017, 27, 1090–1109. [Google Scholar] [CrossRef]
- Thurfah, J.N.; Christine; Bagaskhara, P. P.; Alfian, S.D.; Puspitasari, I.M. Dietary Supplementations and Depression. J. Multidiscip. Healthc. 2022, 15, 1121–1141. [Google Scholar] [CrossRef]
- Sarris, J.; Murphy, J.; Mischoulon, D.; Papakostas, G.I.; Fava, M.; Berk, M.; Ng, C.H. Adjunctive Nutraceuticals for Depression: A Systematic Review and Meta-Analyses. Am. J. Psychiatry 2016, 173, 575–587. [Google Scholar] [CrossRef]
- Mötteli, S.; Vetter, S.; Colla, M.; Hotzy, F. Are probiotics effective in reducing the metabolic side effects of psychiatric medication? A scoping review of evidence from clinical studies. Transl. Psychiatry 2024, 14, 26. [Google Scholar] [CrossRef] [PubMed]
- Jacka, F.N.; O’Neil, A.; Opie, R.; Itsiopoulos, C.; Cotton, S.; Mohebbi, M.; Castle, D.; Dash, S.; Mihalopoulos, C.; Chatterton, M.L.; et al. A randomised controlled trial of dietary improvement for adults with major depression (the ‘SMILES’ trial). BMC Med. 2017, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Swainson, J.; Reeson, M.; Malik, U.; Stefanuk, I.; Cummins, M.; Sivapalan, S. Diet and depression: A systematic review of whole dietary interventions as treatment in patients with depression. J. Affect. Disord. 2023, 327, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, T.; Bondza, E.; Lethin, C. Evidence of the Importance of Dietary Habits Regarding Depressive Symptoms and Depression. Int. J. Environ. Res. Public. Health 2020, 17. [Google Scholar] [CrossRef]
- Marx, W.; Lane, M.; Hockey, M.; Aslam, H.; Berk, M.; Walder, K.; Borsini, A.; Firth, J.; Pariante, C.M.; Berding, K.; et al. Diet and depression: exploring the biological mechanisms of action. Mol. Psychiatry 2021, 26, 134–150. [Google Scholar] [CrossRef]
- Marx, W.; Moseley, G.; Berk, M.; Jacka, F. Nutritional psychiatry: the present state of the evidence. Proc. Nutr. Soc. 2017, 76, 427–436. [Google Scholar] [CrossRef]
- Levitan, R.D.; Shen, J.H.; Jindal, R.; Driver, H.S.; Kennedy, S.H.; Shapiro, C.M. Preliminary randomized double-blind placebo-controlled trial of tryptophan combined with fluoxetine to treat major depressive disorder: antidepressant and hypnotic effects. J. Psychiatry Neurosci. 2000, 25, 337–346. [Google Scholar]
- Frost, R.; Zamri, A.; Mathew, S.; Salame, A.; Bhanu, C.; Bhamra, S.K.; Bazo-Alvarez, J.C.; Heinrich, M.; Walters, K. Understanding the research landscape of over-the-counter herbal products, dietary supplements, and medications evaluated for depressive symptoms in adults: a scoping review. Front. Pharmacol. 2025, 16, 1609605. [Google Scholar] [CrossRef]
- Simon, M.S.; Weidinger, E.; Burger, B.; Kisla, Y.; Niedeggen, J.; Thaler, P.; Zaudig, M.; Voderholzer, U.; Schwarz, M.; Müller, N. Tryptophan metabolites predict response after cognitive behavioral therapy for depression: A single-arm trial. Journal of Affective Disorders Reports 2023, 11, 100464. [Google Scholar] [CrossRef]
- Kuwano, N.; Kato, T.A.; Setoyama, D.; Sato-Kasai, M.; Shimokawa, N.; Hayakawa, K.; Ohgidani, M.; Sagata, N.; Kubo, H.; Kishimoto, J.; et al. Tryptophan-kynurenine and lipid related metabolites as blood biomarkers for first-episode drug-naïve patients with major depressive disorder: An exploratory pilot case-control study. J. Affect. Disord. 2018, 231, 74–82. [Google Scholar] [CrossRef]
- Sha, Q.; Madaj, Z.; Keaton, S.; Escobar Galvis, M.L.; Smart, L.; Krzyzanowski, S.; Fazleabas, A.T.; Leach, R.; Postolache, T.T.; Achtyes, E.D.; et al. Cytokines and tryptophan metabolites can predict depressive symptoms in pregnancy. Transl. Psychiatry 2022, 12, 35. [Google Scholar] [CrossRef]
- von Glischinski, M.; von Brachel, R.; Thiele, C.; Hirschfeld, G. Not sad enough for a depression trial? A systematic review of depression measures and cut points in clinical trial registrations. J. Affect. Disord. 2021, 292, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Dawson, G.R.; Post, A.; Smart, T.S.; Browning, M.; Harmer, C.J. Accuracy in recognising happy facial expressions is associated with antidepressant response to a NOP receptor antagonist but not placebo treatment. J. Psychopharmacol. 2021, 35, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Hershenberg, R.; McDonald, W.M.; Crowell, A.; Riva-Posse, P.; Craighead, W.E.; Mayberg, H.S.; Dunlop, B.W. Concordance between clinician-rated and patient reported outcome measures of depressive symptoms in treatment resistant depression. J. Affect. Disord. 2020, 266, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Bain, E.E.; Shafner, L.; Walling, D.P.; Othman, A.A.; Chuang-Stein, C.; Hinkle, J.; Hanina, A. Use of a Novel Artificial Intelligence Platform on Mobile Devices to Assess Dosing Compliance in a Phase 2 Clinical Trial in Subjects With Schizophrenia. JMIR Mhealth Uhealth 2017, 5, e18. [Google Scholar] [CrossRef]
- Steinkamp, J.M.; Goldblatt, N.; Borodovsky, J.T.; LaVertu, A.; Kronish, I.M.; Marsch, L.A.; Schuman-Olivier, Z. Technological Interventions for Medication Adherence in Adult Mental Health and Substance Use Disorders: A Systematic Review. JMIR Ment. Health 2019, 6, e12493. [Google Scholar] [CrossRef]
- Straczkiewicz, M.; Wisniewski, H.; Carlson, K.W.; Heidary, Z.; Knights, J.; Keshavan, M.; Onnela, J.P.; Torous, J. Combining digital pill and smartphone data to quantify medication adherence in an observational psychiatric pilot study. Psychiatry Res. 2022, 315, 114707. [Google Scholar] [CrossRef]
- Fava, M.; Pani, L.; De Martin, S.; Cutler, A.J.; Gorodetzky, C.W.; Vocci, F.J.; Sapienza, F.L.; Kosten, T.R.; Kröger, C.; Champasa, P.; et al. Long-Term Safety and Efficacy of Esmethadone in Patients With Major Depressive Disorder: Findings From a 12-Month Open-Label Study. J. Clin. Psychiatry 2025, 86. [Google Scholar] [CrossRef]
- Rutherford, B.R.; Sneed, J.R.; Roose, S.P. Does study design influence outcome?. The effects of placebo control and treatment duration in antidepressant trials. Psychother. Psychosom. 2009, 78, 172–181. [Google Scholar] [CrossRef]
- Henssler, J.; Kurschus, M.; Franklin, J.; Bschor, T.; Baethge, C. Long-Term Acute-Phase Treatment With Antidepressants, 8 Weeks and Beyond: A Systematic Review and Meta-Analysis of Randomized, Placebo-Controlled Trials. J. Clin. Psychiatry 2018, 79. [Google Scholar] [CrossRef]
- Owoyemi, E.P.; Agwaza, K.P.; Doris, O.K.; Egwuatu, D.C.; Ogunye, R.O.; Anene, P.C.; Azubuike, E.O.; Ojobor, J.F.C.; Odanibeh, D.; Asenuga, O.O. Nutritional Genomics: The Use of Personalized Diets for Managing Depression. Journal of Complementary and Alternative Medical Research 2024, 25, 23–37. [Google Scholar] [CrossRef]
- Singar, S.; Nagpal, R.; Arjmandi, B.H.; Akhavan, N.S. Personalized Nutrition: Tailoring Dietary Recommendations through Genetic Insights. Nutrients 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Julian, C.; Shen, N.; Molusky, M.; Hu, L.; Gopu, V.; Gorakshakar, A.; Patridge, E.; Antoine, G.; Connell, J.; Keiser, H. The effectiveness of precision supplements on depression symptoms in a US population. medRxiv, 2004. [Google Scholar]
- Zelada, M.I.; Garrido, V.; Liberona, A.; Jones, N.; Zúñiga, K.; Silva, H.; Nieto, R.R. Brain-Derived Neurotrophic Factor (BDNF) as a Predictor of Treatment Response in Major Depressive Disorder (MDD): A Systematic Review. Int. J. Mol. Sci. 2023, 24. [Google Scholar] [CrossRef] [PubMed]
- Suktas, A.; Ekalaksananan, T.; Aromseree, S.; Bumrungthai, S.; Songserm, N.; Pientong, C. Genetic polymorphism involved in major depressive disorder: a systemic review and meta-analysis. BMC Psychiatry 2024, 24, 716. [Google Scholar] [CrossRef] [PubMed]
- Ugartemendia, L.; Bravo, R.; Reuter, M.; Castaño, M.Y.; Plieger, T.; Zamoscik, V.; Kirsch, P.; Rodríguez, A.B. SLC6A4 polymorphisms modulate the efficacy of a tryptophan-enriched diet on age-related depression and social cognition. Clin. Nutr. 2021, 40, 1487–1494. [Google Scholar] [CrossRef]
- van Dalfsen, J.H.; Markus, C.R. The serotonin transporter gene-linked polymorphic region (5-HTTLPR) and the sleep-promoting effects of tryptophan: A randomized placebo-controlled crossover study. J. Psychopharmacol. 2019, 33, 948–954. [Google Scholar] [CrossRef]
- Nedic Erjavec, G.; Sagud, M.; Nikolac Perkovic, M.; Svob Strac, D.; Konjevod, M.; Tudor, L.; Uzun, S.; Pivac, N. Depression: Biological markers and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 105, 110139. [Google Scholar] [CrossRef]
- Milic, J.; Jovic, S.; Sapic, R. Advancing Depression Management Through Biomarker Discovery with a Focus on Genetic and Epigenetic Aspects: A Comprehensive Study on Neurobiological, Neuroendocrine, Metabolic, and Inflammatory Pathways. Genes (Basel) 2025, 16. [Google Scholar] [CrossRef]
- Beer, C.; Rae, F.; Semmler, A.; Voisey, J. Biomarkers in the Diagnosis and Prediction of Medication Response in Depression and the Role of Nutraceuticals. Int. J. Mol. Sci. 2024, 25. [Google Scholar] [CrossRef]
- Magzal, F.; Turroni, S.; Fabbrini, M.; Barone, M.; Vitman Schorr, A.; Ofran, A.; Tamir, S. A personalized diet intervention improves depression symptoms and changes microbiota and metabolite profiles among community-dwelling older adults. Front. Nutr. 2023, 10, 1234549. [Google Scholar] [CrossRef]
- Chen, L.; Liu, B.; Ren, L.; Du, H.; Fei, C.; Qian, C.; Li, B.; Zhang, R.; Liu, H.; Li, Z.; et al. High-fiber diet ameliorates gut microbiota, serum metabolism and emotional mood in type 2 diabetes patients. Front. Cell Infect. Microbiol. 2023, 13, 1069954. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Mugo Moses, H. The Impact of Personalized Dietary Interventions on Glycemic Control and Quality of Life in Middle-Aged Adults with Type 2 Diabetes and Co-occurring Depression.
- Radford-Smith, D.E.; Anthony, D.C. Prebiotic and Probiotic Modulation of the Microbiota-Gut-Brain Axis in Depression. Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Asad, A.; Kirk, M.; Zhu, S.; Dong, X.; Gao, M. Effects of Prebiotics and Probiotics on Symptoms of Depression and Anxiety in Clinically Diagnosed Samples: Systematic Review and Meta-analysis of Randomized Controlled Trials. Nutr. Rev. 2025, 83, e1504–e1520. [Google Scholar] [CrossRef]
- Liu, R.T.; Walsh, R.F.L.; Sheehan, A.E. Prebiotics and probiotics for depression and anxiety: A systematic review and meta-analysis of controlled clinical trials. Neurosci. Biobehav. Rev. 2019, 102, 13–23. [Google Scholar] [CrossRef]
- Bankah, A.Z.; Tagoe, T.A.; Darko, E.; Agoha, R.; Ametefe, E.N.; Kukuia, K.K.E.; Adjei, S. Combined Administration of Lactobacillus or Bifidobacterium Offers Enhanced Antidepressant and Anxiolytic Activity in a Dose Dependent Manner. Brain Behav. 2025, 15, e70564. [Google Scholar] [CrossRef]
- Dandekar, M.P.; Palepu, M.S.K.; Satti, S.; Jaiswal, Y.; Singh, A.A.; Dash, S.P.; Gajula, S.N.R.; Sonti, R. Multi-strain Probiotic Formulation Reverses Maternal Separation and Chronic Unpredictable Mild Stress-Generated Anxiety- and Depression-like Phenotypes by Modulating Gut Microbiome-Brain Activity in Rats. ACS Chem. Neurosci. 2022, 13, 1948–1965. [Google Scholar] [CrossRef]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Fond, G.B.; Lagier, J.C.; Honore, S.; Lancon, C.; Korchia, T.; Sunhary De Verville, P.L.; Llorca, P.M.; Auquier, P.; Guedj, E.; Boyer, L. Microbiota-Orientated Treatments for Major Depression and Schizophrenia. Nutrients 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Chang, K.T.; Chang, F. Just a gut feeling: Faecal microbiota transplant for treatment of depression - A mini-review. J. Psychopharmacol. 2024, 38, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Das, P.; Lv, X.; Shi, M.; Aa, J.; Wang, K.; Duan, L.; Gilbert, J.A.; Nie, Y.; Wu, X.L. Effects of ‘Healthy’ Fecal Microbiota Transplantation against the Deterioration of Depression in Fawn-Hooded Rats. mSystems 2022, 7, e0021822. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Qiao, Y.; Xie, R.; Lin, L.; Jiang, J.; Wang, C.; Li, G. Fecal microbiota transplantation ameliorates stress-induced depression-like behaviors associated with the inhibition of glial and NLRP3 inflammasome in rat brain. Journal of Psychiatric Research 2021, 137, 147–157. [Google Scholar] [CrossRef]
- Rao, J.; Xie, R.; Lin, L.; Jiang, J.; Du, L.; Zeng, X.; Li, G.; Wang, C.; Qiao, Y. Fecal microbiota transplantation ameliorates gut microbiota imbalance and intestinal barrier damage in rats with stress-induced depressive-like behavior. Eur. J. Neurosci. 2021, 53, 3598–3611. [Google Scholar] [CrossRef]
- Doll, J.P.K.; Vázquez-Castellanos, J.F.; Schaub, A.C.; Schweinfurth, N.; Kettelhack, C.; Schneider, E.; Yamanbaeva, G.; Mählmann, L.; Brand, S.; Beglinger, C.; et al. Fecal Microbiota Transplantation (FMT) as an Adjunctive Therapy for Depression-Case Report. Front. Psychiatry 2022, 13, 815422. [Google Scholar] [CrossRef]
- Chinna Meyyappan, A.; Forth, E.; Wallace, C.J.K.; Milev, R. Effect of fecal microbiota transplant on symptoms of psychiatric disorders: a systematic review. BMC Psychiatry 2020, 20, 299. [Google Scholar] [CrossRef]
- Cai, T.; Shi, X.; Yuan, L.Z.; Tang, D.; Wang, F. Fecal microbiota transplantation in an elderly patient with mental depression. Int. Psychogeriatr. 2019, 31, 1525–1526. [Google Scholar] [CrossRef]
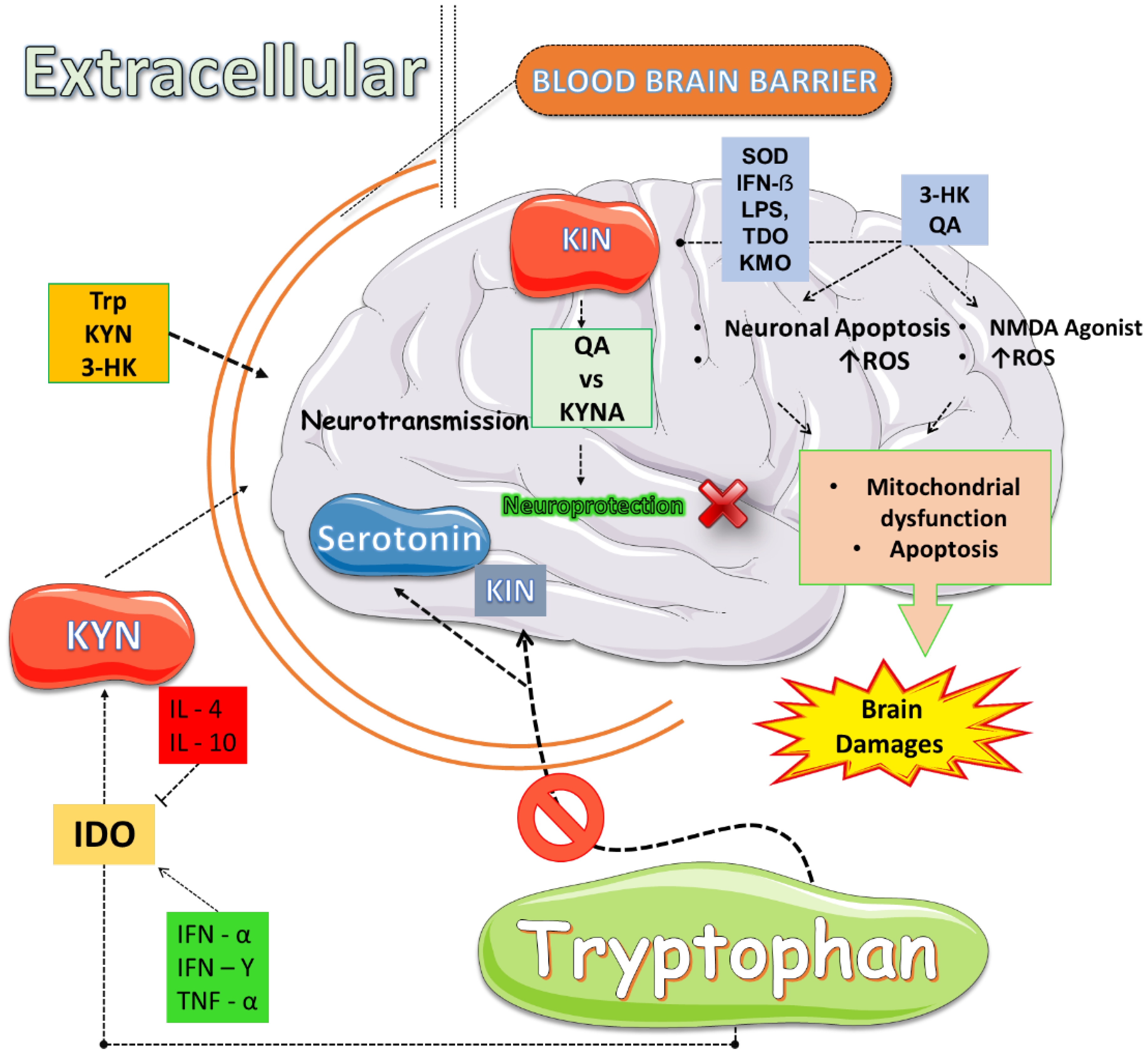
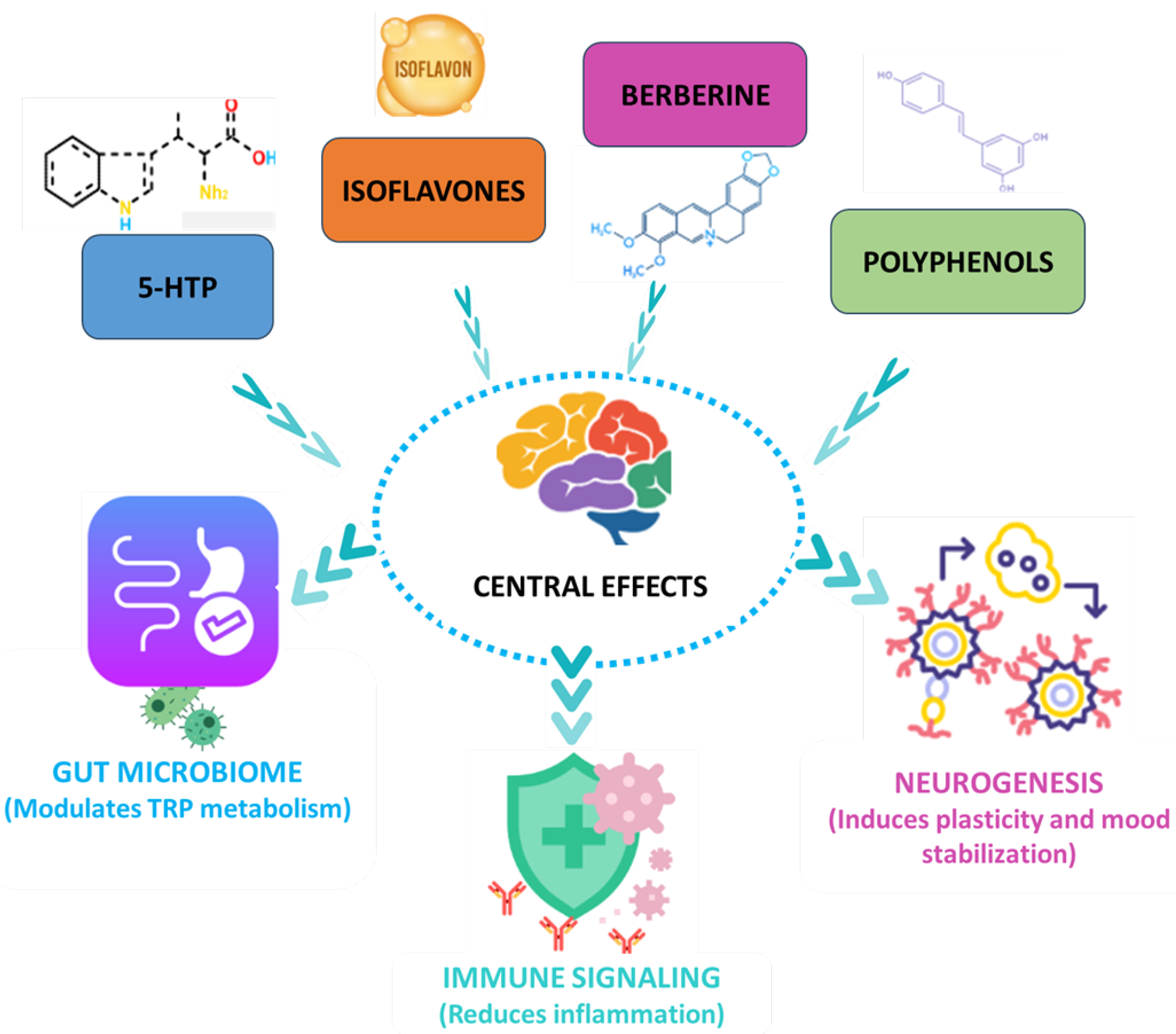
| Phytocompound | Dosage | Population | Duration | Primary Outcomes | Phytocompound |
| L-5-HTP | 50 mg/day | Depression (n=70) | 8 weeks | Comparable efficacy to fluoxetine; mood improvement | L-5-HTP |
| Talbinah (barley + milk + honey) | 25 g/day | Depression (n=36) | 7 weeks | Reduced depressive and anxiety scores | Talbinah (barley + milk + honey) |
| Trp-enriched cereals | 60 mg/day | Elderly (n=35) | 3 weeks | Improved sleep efficiency, reduced anxiety | Trp-enriched cereals |
| Alpha-lactalbumin | Trp-rich pro-tein diet | Recovered de-pressed (n=43) | 4 weeks | Enhanced memory, modest mood effects | Alpha-lactalbumin |
| Egg protein hydroly-sate | Trp-rich sup-plement | High-stress vulnera-ble (n=35) | 2 weeks | Reduced stress-related mood decline | Egg protein hydroly-sate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





