1. Introduction
Gout is a common inflammatory disorder characterized by acute flares of joint pain, swelling and redness caused by deposition of Monosodium Urate (MSU) crystals. While hyperuricemia is a prerequisite for the development of gout, only about a third of individuals with elevated urate will develop gout flares.[
1] Furthermore, MSU crystal deposition in joints can also be asymptomatic,[
2] suggesting that additional triggers are required to start the inflammatory response. Important risk factors for gout and hyperuricemia include sex, age, BMI, diet and the conditions are associated with hypertension, chronic kidney disease, diabetes and heart failure.[
1] Genetic factors have been shown to contribute to both hyperuricemia and gout, with most strongly associated polymorphisms located in genes involved in renal urate excretion.[
3]
Inflammation in gout is primarily an innate immune response mediated by Interleukin-1β (IL-1β). The production and release of IL-1β occur through a two-step process: the first step involves transcriptional upregulation of its precursor, pro-IL-1β, while the second requires cleavage of pro-IL-1β into its active form by caspase-1. Several key pathways are known to regulate the expression of pro-IL-1β and other inflammatory cytokines, most notable of which are Nuclear Factor κB (NF-κB) and mitogen-activated protein kinases, both of which are canonically activated by Pattern Recognition Receptors (PRRs) or IL-1 receptors.[
4] The second step required for IL-1β production is the activation of the NLRP3 inflammasome. This multiprotein complex is activated by diverse stimuli – such as extracellular ATP, bacterial toxins, RNA viruses, and other particles - often associated with cellular stress events such as ionic flux changes, mitochondrial dysfunction, lysosomal damage and reactive oxygen species (ROS) accumulation.[
5] Activation of the NLRP3 inflammasome leads to the cleavage of pro-caspase-1 into active caspase-1, which in turn processes pro-IL-1β into its bioactive form, IL-1β, thereby promoting inflammation.
Interferons (IFN) are a group of cytokines that play a key role in the immune response to viral infections. However, a growing body of research has also linked the IFN responses to autoimmune diseases, including Systemic Lupus Erythematosus, Dermatomyositis, Rheumatoid Arthritis, Sjögren syndrome, and Systemic Sclerosis.[
6,
7,
8] IFNs exert complex effects on the immune system, with both pro- and anti-inflammatory roles. Type I IFN expression is regulated by a family of nine transcription factors named Interferon Regulatory Factors (IRF). Among them,
IRF5 is predominantly expressed in bone marrow-derived cells and lymphoid tissues and is recognized for its important proinflammatory role.
IRF5 knockdown was associated with diminished production of inflammatory cytokines TNF and IL-6, macrophage polarization towards anti-inflammatory type M2, and impaired T cell responses. The proinflammatory role of IRF5 was also confirmed in vivo, for example, IRF5 – deficient (IFR5
−/−) mice exhibit significantly reduced joint damage in arthritis models.[
9,
10] IRF5 is activated by PRRs through the NF-κB pathway as well as by DNA damage. Its activation involves post-translational changes, probably including dimerization and nuclear translocation, followed by binding to target DNA sites.[
11] Several mechanisms have been proposed to explain IRF5 function, including synergism with NF-κB at promoters of proinflammatory genes and induction of H3K27 acetylation at multiple enhancer sites. Besides its short-term proinflammatory roles, IRF5 can be involved in regulatory negative feedback mechanisms to control cytokine production. By associating with TRIM28 and histone methyltransferase SETDB1, which can methylate lysine 9 of histone 3 (H3K9me3), the complex can inhibit the expression of TNF.[
12,
13]
A recent genome-wide association study (GWAS) has identified rs4728141 as a lead SNP associated with gout in European population and included
IRF5 as a high priority gene for involvement in gout.[
14] This polymorphism is an intergenic T/C substitution located near the
IRF5 and
KCP genes. Data from the Genotype-Tissue Expression (GTEx) portal indicate that the alternative allele C is associated with increased
IRF5 expression across multiple tissues, alongside a decrease in
TNPO3.[
15] Given these findings, and the previously documented involvement of IRF5 in proinflammatory events, we hypothesize that the rs4728141 C allele increases the IRF5 expression, which in turn enhances the proinflammatory cytokine production, therefore increasing the probability of gout flares. We aim to further investigate the role of this polymorphism in gout and the general innate immune response by examining the cytokine expression and production patterns in human primary peripheral blood mononuclear cells (PBMCs).
3. Discussion
Gout pathogenesis involves complex mechanisms that remain largely unresolved, despite significant research efforts. Genetic factors have been linked to gout susceptibility, with the latest GWAS study to date reporting 377 association with gout.[
14] Since hyperuricemia is the key known factor that leads to gout, many of the consistently top-associated genes, such as
SLC2A9,
SLC22A12 or
ABCG2, are involved in urate transport.[
3] Given the high prevalence of asymptomatic hyperuricemia, loci specific to gout but not hyperuricemia are of high interest. These include genes that are involved in metabolic processes, such as
FADS1, FADS2, as well as inflammatory responses, such as
IL1RN,
IL1R1,
IL6R and others. Enrichment of GWAS loci in immune response gene supports the hypothesis that an inflammatory genetic component may contribute to gout beyond hyperuricemia. Major et al. additionally include a prioritization scheme to identify such genes that may be involved in progression from hyperuricemia to gout based on colocalization with publicly available whole blood or monocyte eQTL data, differential expression in gout, association with white blood cell traits, methylation QTL and previous differential expression studies in monocytes.[
14]
IRF5 was one of the top candidate genes, with rs4728141 as the lead SNP at the gout GWAS identified locus in European population.[
14] Besides gout, genetic variants in
IRF5 were already associated with systemic lupus erythematosus,[
17] rheumatoid arthritis,[
18] Sjogren syndrome,[
19] and other inflammatory diseases.
IRF5 is a transcription factor involved in the regulation of inflammatory cytokines production by interacting with key signaling molecules such as MyD88 and NF-κB subunit p50.[
20,
21] This functional role positions IRF5 as a pivotal mediator of innate immune responses. The genetic variant rs4728141, despite being intergenic, is associated with increased
IRF5 expression. Mechanistically, rs4728141 could change the local chromatin accessibility by modifying the affinity to regulatory proteins, as the polymorphism resides within multiple regulatory motifs reported by RegulomeDB[
22] or by HaploReg.[
23] This DNA region was also shown to be associated with CBX8 in chromatin immunoprecipitation experiments.[
24] CBX8 binds to H3K27me3 and is part of the Polycomb Repressive complex 1, a chromatin-based repressor of gene transcription,[
25,
26] further supporting an epigenetic mechanism in the control of
IRF5 expression by rs4728141. Although IRF5 was not thoroughly investigated in the context of gout, its established proinflammatory role in other autoimmune and inflammatory diseases suggests that heightened IRF5 activity could contribute to increased risk and severity of gout. Interferons have been implicated in the response to urate or MSU crystals. A previous study found type I IFN pathway downregulation on transcriptomic assessment of PBMCs exposed to high concentrations of urate, and this signature included a downregulation of
IRF5 as well as its antagonist
IRF4.[
27] In the current study, we observe that a genotype associated with increased
IRF5 expression correlates with elevated IL-1β production following palmitate ± MSU stimulation. Interestingly, despite urate’s inhibitory effect on
IRF5 and IFN signaling, IL-1β levels remained high, suggesting that urate and palmitate may modulate IL-1β through separate pathways which may not always be mechanistically dependent on IRF5.
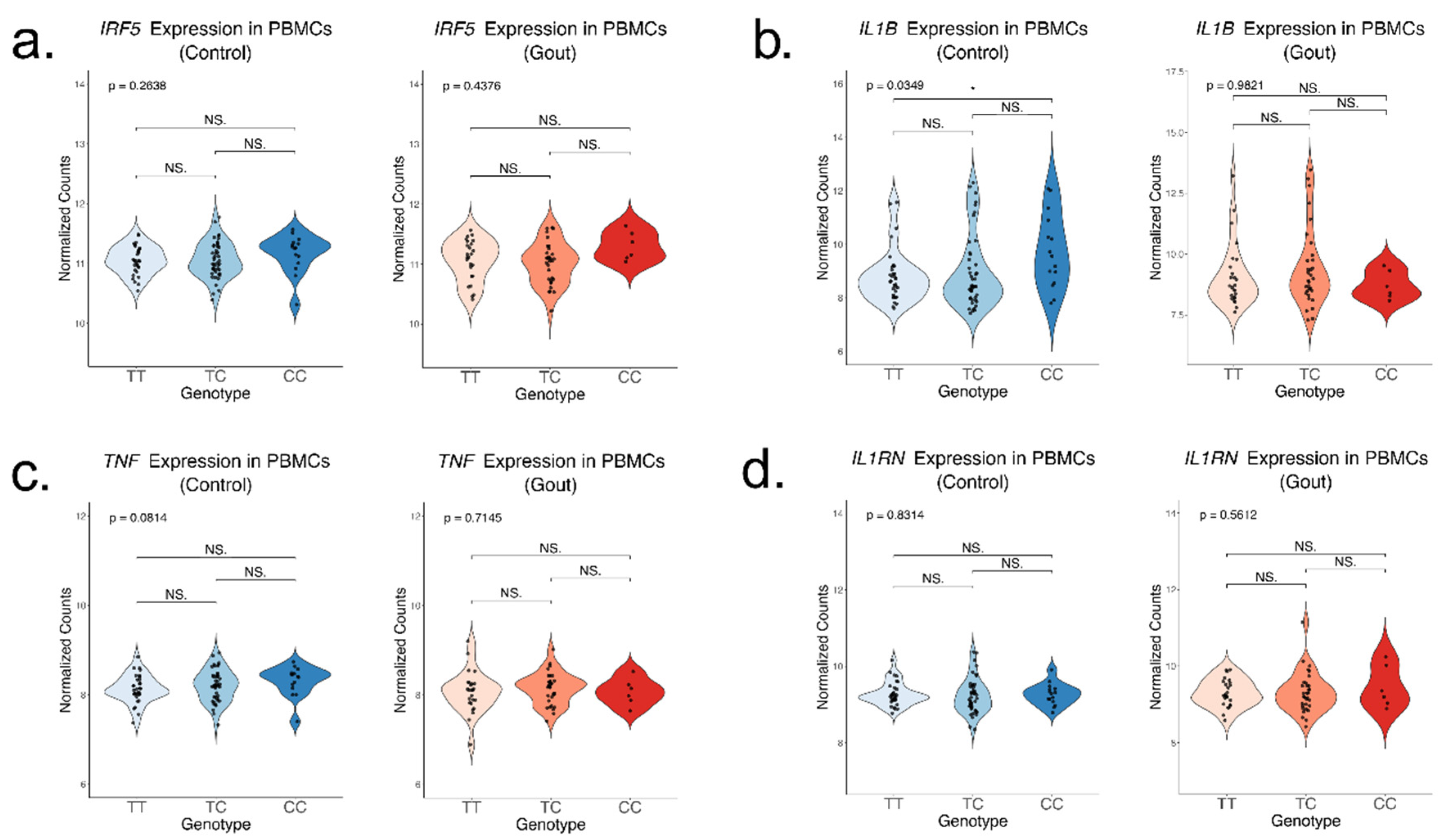
Consistent with prior expectations based on the functional role of IRF5, we observed a more potent inflammatory response associated with the rs4728141 risk allele C represented by increased IL1B expression in unstimulated PBMCs, however this effect was observed only in the normouricemic control group. This is likely due to the increased heterogeneity of gout patients, as many variables such as current active gout flares, BMI, current serum urate level, anti-inflammatory medication and others can significantly alter the systemic immune response. Variability in cell proportions can additionally induce changes in bulk RNA-seq data.
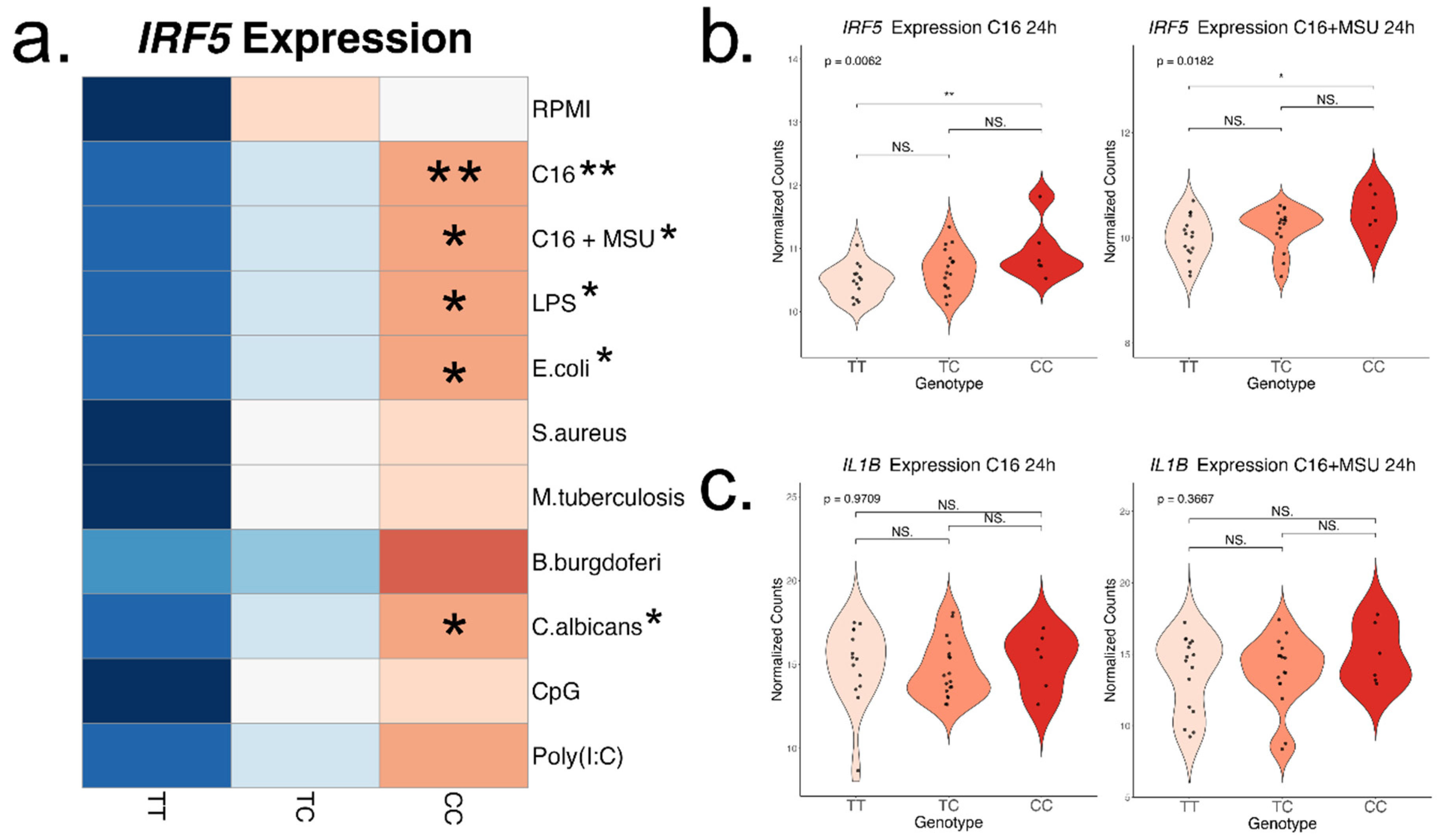
When PBMCs were stimulated with TLR4 ligands for 24h, we were able to confirm the upregulation of
IRF5 expression associated with the rs4728141 risk allele in most experiments. The trend was consistent across all stimuli, suggesting that increasing the sample size would likely reveal the effect in all experiments. The risk allele was not accompanied by increased expression of cytokines such as
IL1B,
IL6 or
TNF. An important limitation of this study is the lack of data on short-term exposure, which can be one of the main reasons why transcriptomic changes are not observed in these experiments as well as why IL-1β production is only increased in the stimulations with palmitate. The peak expression and production of IL-1β in response to LPS is usually observed at 4 hours,[
28] unlike palmitate that is at 24 hours.[
29,
30] Furthermore, even though we obtained high quality metrics, the imputation process can add some additional errors unlike the conventional genotyping methods.
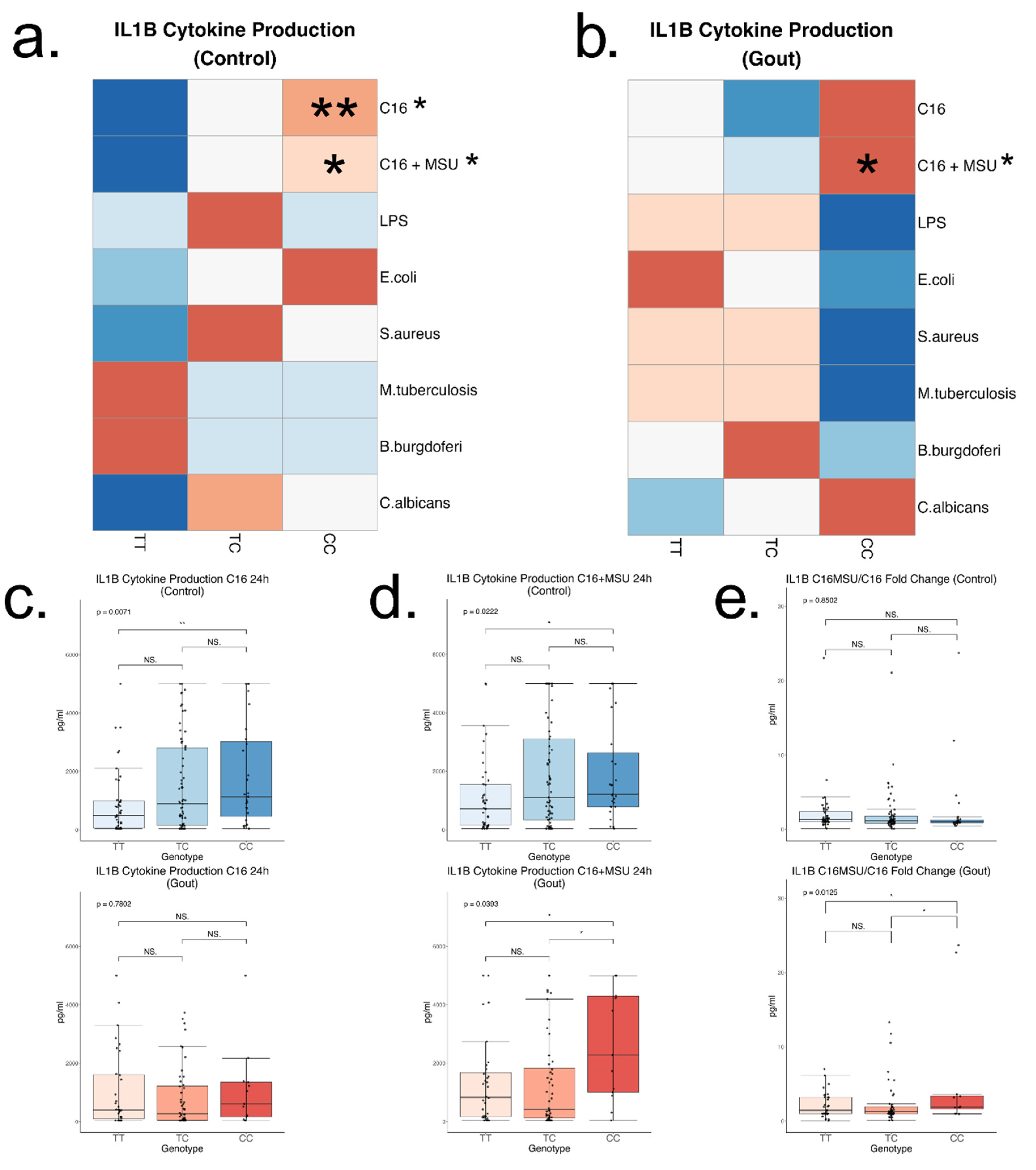
Interestingly, this study shows the association between the rs4728141 C allele and increased IL-1β cytokine production when PBMCs interacted with palmitate in presence or absence of MSU crystals. This effect seemed to be specific to these stimulations, as no changes in cytokine production were observed in any other stimulation with PRR ligands used in this study. The IRF5 SNP is associated with significantly enhanced synergistic cytokine production in response to palmitate and MSU crystals, particularly in the gout subgroup, strongly indicating that this variant modulates cytokine production specifically in gout-relevant context.
Palmitate is a saturated fatty acid made of 16 carbon atoms found in most fats and oils. It is also the most common fatty acid found in human blood.[
31] Conditions that are typically associated with gout such as type 2 diabetes, obesity and dyslipidemia are also associated with elevated concentrations of fatty acids. Palmitate is currently recognized as a TLR4/TLR2 ligand which induces an NF-κB-mediated transcriptional response;[
32] however, some evidence challenged this assertion.[
33] It also activates the NLRP3 inflammasome with the typically associated lysosome destabilization, accumulation of reactive oxygen species and mitochondrial stress.[
34] Palmitate and LPS have also been shown to possess a synergistic proinflammatory effect.[
35,
36] Unlike LPS however, palmitate induced upregulation of IL-1β can last over 24 hours.[
30] Therefore, a potential explanation of our findings is that palmitate stimulations had the best time window to observe the effect of rs4728141 on IL-1β production. Interestingly, there were no SNP-associated changes in
IL1B mRNA. This discrepancy between unchanged
IL1B mRNA and elevated IL-1β protein likely reflects additional layers of regulation, such as translational control or secretion dynamics, which are not directly captured by measuring mRNA alone. IRF5 is a transcription factor known to regulate a broad range of proinflammatory cytokines at the transcriptional level, nevertheless it is plausible that the cytokine responses observed here are controlled by IRF5 through mechanisms beyond mRNA expression changes. IRF5 is involved in metabolic and inflammatory changes specifically in response to palmitate, as its knockdown leads to alterations in tricarboxylic acid cycle and mitochondrial activity.[
37] The immune cell metabolic state is strongly linked to cytokine production through epigenetic regulation[
38] and activation of the NLRP3 inflammasome.[
39] These data suggest a multifaceted role for IRF5 in modulating inflammatory responses.
To conclude, we find that the rs4728141 gout risk allele C is associated with a more potent inflammatory reaction in response to both palmitate and to palmitate/MSU crystals combination by upregulating the IL-1β production. Given the central role that IL-1β plays in gout, this increase in cytokine production adds functional evidence to the genetic association of this variant with gout. The rs4728141 C allele may lower the inflammatory threshold, accelerating the shift from silent hyperuricemia to symptomatic gout after MSU crystal formation. This hypothesis requires further investigation in future studies. Therefore, the rs4728141 C allele holds potential as a genetic polymorphism for identifying those at greater risk of developing gout and could play a role in shaping early intervention strategies for gout management.
4. Materials and Methods
4.1. Participants
The subjects were recruited at the Rheumatology Department of the “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania as part of the HINT Project (Hyperuricemia-induced Inflammation: Targeting the central role of uric acid in rheumatic and cardiovascular diseases, ID P 37 762; MySMIS 103587). Written informed consent was obtained. The study was approved by the Ethical Committee of the „Iuliu Hațieganu” University of Medicine and Pharmacy, Cluj-Napoca (approval no. 425/2016). All study participants in the gout group were included based on ACR/EULAR 2015 classification criteria with a minimum score of 8. The control group is represented by individuals from a similar age group with a serum urate value of less than 7 mg/dl when the cells were obtained. A main set of clinical parameters of participants is presented in Supplementary Table 1.
4.2. PBMC Isolation and Stimulation
Peripheral blood was drawn from the cubital vein into EDTA tubes under sterile conditions. PBMCs were separated by Ficoll-Paque density gradient centrifugation and washed 3 times with phosphate buffer saline solution. The cells were resuspended in culture medium RPMI (Roswell Park Memorial Institute) 1640, supplemented with 50 µg/ml gentamicin, 2 mM L-glutamine and 1 mM pyruvate. PBMC concentration was adjusted to 10^7 PBMCs/ml. 106 freshly isolated unstimulated cells were stored in TRIzol for transcriptomic analysis. Stimulation experiments were performed using 0,5*106 cells per well, in duplicate, in 96-well plates. Cells were incubated at 37 °C 5% CO2 for 24h with the following stimuli: Palmitate (50 μM), Palmitate (50 μM) with Monosodium Urate Crystals (300 μg/ml), LPS (10 ng/ml), HK E.coli (106 CFU/ml), HK S. aureus (106 /ml), M. tuberculosis lysate (5 μg/ml), B. burgdorferi (106 CFU /ml), heat killed(HK) C. albicans (106 CFU /ml), Poly(I:C) (10 μg/ml), CpG (1 μg/ml), and culture medium as negative control. LPS was subjected to ultrapurification before cell culture experiments. Palmitate stock solutions were prepared in ethanol and subsequently conjugated to human albumin (Albuman, approved for intravenous use) prior to cell stimulation. The conjugates were maintained at 37 °C until applying to cells. At the end of each experiment, the supernatants were collected for cytokine measurement and the remaining cells were stored in 50 μl TRIzol Reagent (Invitrogen) at −80 °C.
4.3. Genotyping
Genomic DNA was isolated from whole blood (Promega) and genotyping was performed using Infinium Global Screening Array-24 v1.0 BeadChip. Initial quality control was performed with Illumina GenomeStudio: The SNPs with < 95% call rate were excluded, and all the remaining SNPs were verified and manually re-clustered or removed when clustering was not possible. The data were exported to PLINK format and further filters were applied: minor allele frequency (MAF) < 0.05; Hardy-Weinberg equilibrium test p value < 10−6; samples with mismatches between the reported and estimated genetic sex, heterozygosity rate of ±3 standard deviations and related individuals were excluded. The strands were flipped, and all the data was verified to align to GRCh37 hg19. Imputation was then performed on the Michigan Imputation Server with the HRC (Version r1.1 2016) reference. rs4728141 was imputed with an R2 of 0.85712.
4.4. Transcriptomics
Bulk RNA-Sequencing analysis was performed on the DNBseq platform, outsourced to Beijing Genomics Institute, BGI, Denmark. After assessing the integrity of the RNA with Agilent 2100 Bioanalyzer, the mRNA was enriched with (dT)-attached magnetic beads, followed by reverse transcription to double stranded DNA, 3’ adenylation and adaptor ligation, PCR amplification, denaturation and cyclization. After initial quality control, which included removal of reads with adaptors, low quality reads (unknown bases >10% or QC score < 15 in over 50% of the base pairs) and rRNA mapped reads (SOAPnuke v1.5.2), the reads were then mapped to UniGene database (Bowtie2) and the counts were estimated (RSEM v.1.2.12). In freshly isolated PBMC samples, the average number of reads per sample was 32.59 million, with an average clean read ratio of 98.32% and mapping ratio of 94.07%. For the stimulation experiments the same metrics were at an average of 25.39 million reads, 93.41% clean read ratio and 77.6% mapping ratio. All samples passed the initial quality control.
The reads were normalized with DESeq2 and additional quality control was performed by removing samples with: 1) mismatches between the reported sex and the one estimated by assessing the expression of Y chromosome genes (DDX3Y, USP9Y and UTY); 2) after performing a Principal Component Analysis, the samples with more than 3 Standard Deviations on principal components 1 and 2. 24 samples were removed as outliers due to an unknown technical error and 8 samples due to sex mismatch. The count data was then exported as normalized counts using the DESeq2 vst function. The PBMC samples were analyzed in 2 batches, and a batch correction was additionally applied using the limma package.
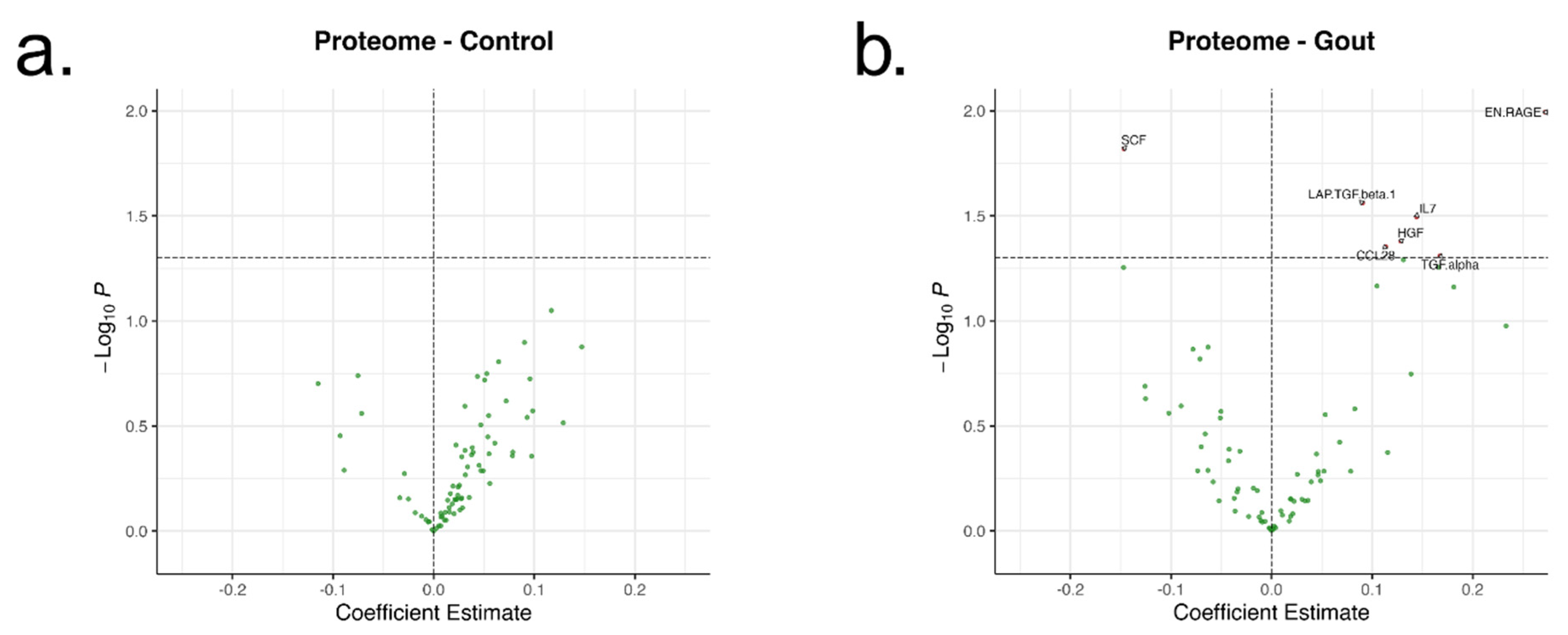
4.5. Proteomics
Serum proteomics data from the donors included in this study were retrieved from Cabău et al. [
16]. Serum samples were collected at patient inclusion and stored at −80 °C. The stored serum samples were thawed on ice, mixed and randomized before plating on 96-well PCR microplate and Olink
® Target 96 Inflammation panel was performed. Proteomic assay, data normalization and quality control were performed at Olink Proteomics, Uppsala, Sweden. Normalized protein expression (NPX) values were examined and additional quality control filters consisted of: over 3 standard deviations from the mean IQR, sample median in distribution plots or deviated in the principal component analysis, samples that were found to be biological duplicates, samples that had missing serum urate data, proteins that had NPX values below the limit of detection in more than or equal to 20% of samples. The panel included 92 proteins and after 19 were dropped due to low NPX values, a total of 73 proteins were examined.
4.6. Cytokine Measurements
The cytokine concentrations were measured by sandwich ELISA with IL-1β, IL-1Ra, IL-6, and TNF kits (R&D Systems). Before performing the assay, the samples were diluted 10-fold (IL-1β, IL-1Ra, TNF) or 20-fold (IL-6). Lowest detection range was 78 pg/ml (IL-1β), 390 pg/ml (IL-1Ra) and 94 pg/ml (IL-6).
Samples with detected IL-1β, IL-6 or TNF in RPMI control wells were removed due to suspicion of contamination.
4.7. Statistical Analysis
The statistical analysis was performed with R base functions and figures were generated with ggplot2. When comparing the three genotypes, a linear regression was performed where the genotypes were given values 0, 1, and 2. Differences between individual groups were assessed by Tukey range test with 0.95 confidence interval.
Author Contributions
Conceptualization, Valentin Nica, Tania Crisan and Leo Joosten; Data curation, Valentin Nica, Orsolya Gaal, Medeea Badii and Georgiana Cabau; Formal analysis, Valentin Nica; Funding acquisition, Tania Crisan and Leo Joosten; Investigation, Valentin Nica, Orsolya Gaal, Medeea Badii, Georgiana Cabau, Andreea Mirea and Tania Crisan; Methodology, Mihai Netea, Tania Crisan and Leo Joosten; Project administration, Radu Popp, Tania Crisan and Leo Joosten; Resources, Ioana Hotea, Cristina Pamfil and Simona Rednic; Supervision, Tania Crisan and Leo Joosten; Visualization, Valentin Nica; Writing – original draft, Valentin Nica; Writing – review & editing, Tania Crisan and Leo Joosten.
Acknowledgements
We thank all the members of the HINT consortium: Leo A. B. Joosten, Ioan V. Pop, Radu A. Popp, Simona Rednic, Cristina Pamfil, Tania O. Crişan, Marius Farcaş, Dragoş H. Marginean, Orsolya I. Gaal, Medeea O. Badii, Ioana Hotea, Loredana Peca, Andreea-Manuela Mirea, Georgiana Cabău, Valentin Nica, Ancuța Straton, Maria Muntiu, Doina Colcear, Mariana S. Pop, Ancuta Rus, Cristina Olovina