Submitted:
23 September 2025
Posted:
24 September 2025
You are already at the latest version
Abstract
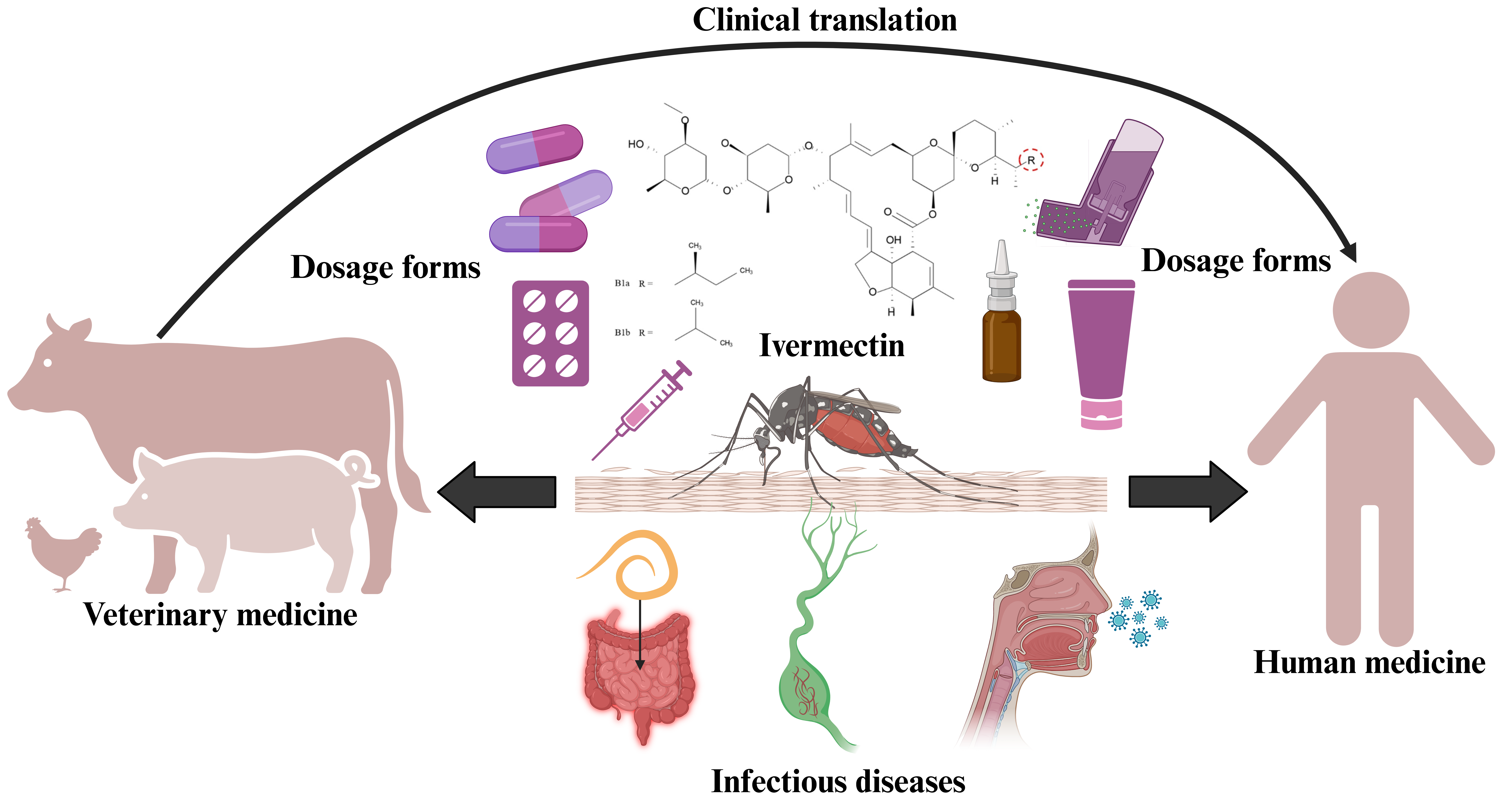
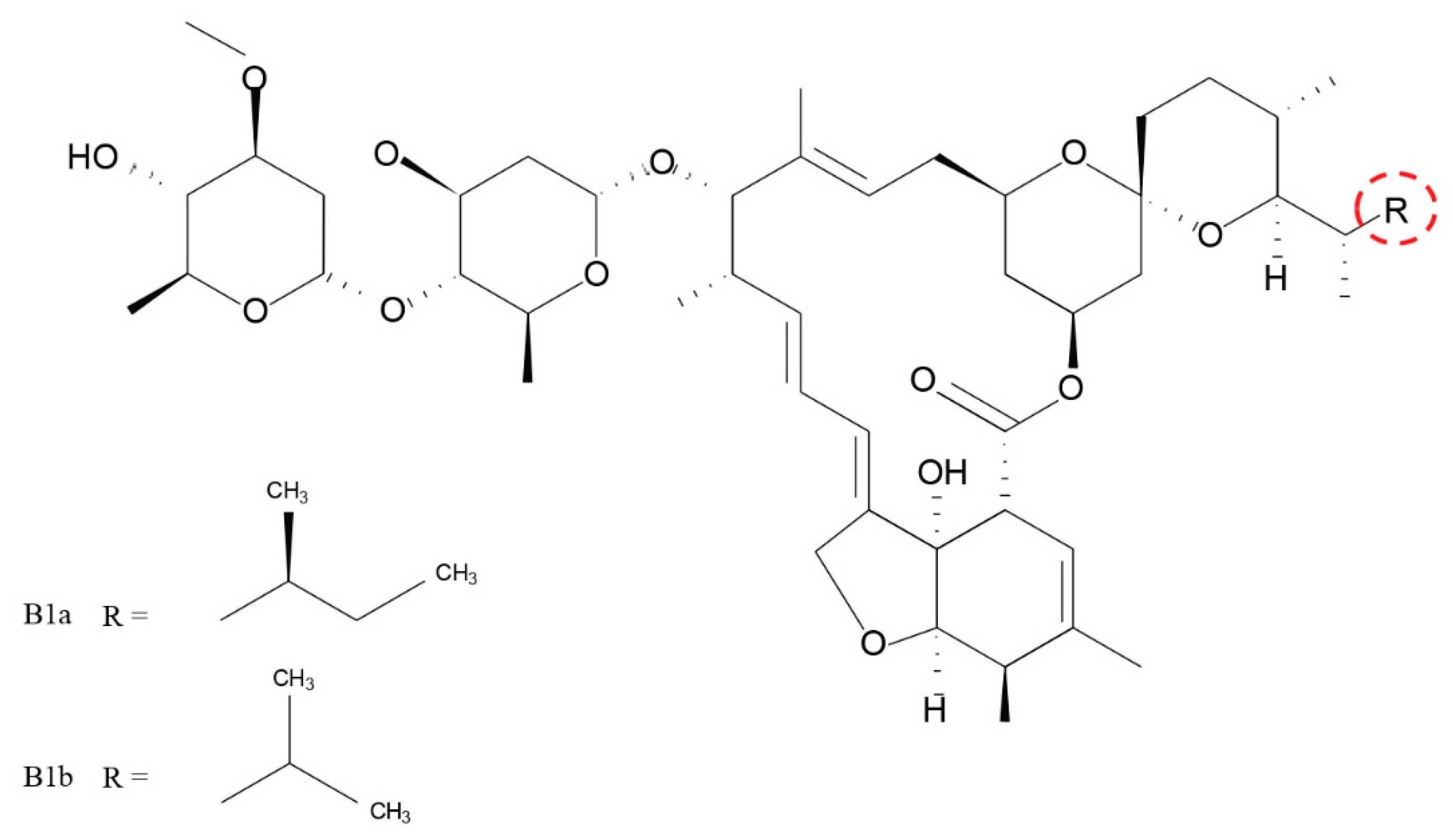
Keywords:
1. Introduction
2. New Targeted Indications
2.1. Antiviral
2.2. Antibacterial
2.3. Anticancer
2.4. Anti-Inflammatory
2.5. Other
- Myiasis
- Trichinosis
- Disease vector control
- African trypanosomiasis
- American trypanosomiasis
- Bedbugs
- Asthma
- Epilepsy
- Neurological diseases
- Metabolically related diseases
- Farnesoid X receptor (FXR)-mediated diseases
3. Ivermectin Toxicity
-
Establishing safe dosage ranges: Toxicity studies have helped determine the maximum tolerated dose and safe dosage ranges for IVM in both humans and animals, lowering the risk of adverse effects. However, IVM has a weak potential for long-term toxicity with a wide margin of safety between therapeutic doses and toxic doses. Despite this, the emphasis on the importance of continued monitoring and research to ensure safe use remains crucial [60]. The acceptable therapeutic dosage ranges for IVM use in humans and animals are summarized as follows:
- Humans: 0.150–0.200 mg/kg (single oral dose every 12 months) or alternatively, 0.050–0.400 mg/kg for any person two years or older in age [62].
-
Animals: [103]
- Small animals: 0.300–0.600 mg/kg (oral dose, once daily, until two respective negative skin scrapings are obtained one month apart)
- Cattle and sheep: 0.200 mg/kg (single dose subcutaneous injection)
- Horses: 0.200 mg/kg (oral dose, repeated as necessary for adequate parasite management)
- Swine: 0.300 mg/kg (subcutaneous injection, repeated two-weekly) or 0.100–0.200 mg/kg (oral dose in feed for seven days)
- Identifying potential drug interactions and enhancing monitoring and management of toxicity: Studies have revealed potential interactions between IVM and other medications, prompting healthcare providers to take precautions and adjust treatment plans accordingly. Drug interactions should be considered prior to administering IVM to animals, since certain interactions increase systemic exposure to IVM, particularly within the CNS of animals, thus increasing the risk of neurotoxicity [106,107]. These drugs include ketoconazole [44,106], itraconazole [106], cyclosporine [106,108], erythromycin [106,109], amiodarone [106], and nifedipine [107,110]. Furthermore, it is known that alcohol, grapefruit, and orange juice metabolically interact with IVM, with IVM exhibiting noticeably higher plasma concentrations when given in conjunction with alcohol and grapefruit juice, while lower plasma concentrations are evident when consumed with orange juice [72]. Research has provided development of strategies for monitoring and managing IVM toxicity, including the use of biomarkers and treatment protocols for overdose or adverse reactions.
-
Developing safety guidelines: Toxicity research has led to the establishment of guidelines for IVM use in vulnerable populations, such as pregnant women [1,14], children, and individuals with liver or kidney disease [60]. The guidelines for IVM use in said individuals are summarized as follows [95,111,112]:
- Pregnancy and breastfeeding: Use should be avoided particularly in the first trimester. However, the normal therapeutic dose (0.050–0.400 mg/kg single dose) [62] may be administered under medical supervision as low concentrations of IVM have been detected in human breast milk. Current manufacturer guidelines advise that treatment during lactation should only be considered when the potential risk of delaying therapy in the mother is deemed greater than the potential risk to the nursing infant [113].
- Children: Approved single oral dosages of 0.200 mg/kg for the treatment of strongyloidiasis and 0.150 mg/kg for the treatment of onchocerciasis for children weighing ≥15 kg [114]. Current reviews of safety data for children under 5 years of age or weighing less than 15 kg, who received IVM at approximate doses of 0.200 mg/kg for various infections, have not revealed any significant safety issues. Regardless, treatment decisions for children in this category should be made in consultation with a qualified healthcare provider [114].
- Patients suffering from porphyria: IVM is typically regarded as safe for individuals diagnosed with porphyria, including the acute subtypes. Nevertheless, its use should be approached with clinical discretion, considering the patient’s comprehensive medical background and present condition. Prior to initiating therapy, it is advisable to seek guidance from a healthcare provider with expertise in the management of porphyria disorders [119].
- Expanding indications: Current uses (parasitic infections, such as onchocerciasis or river blindness [16,18] and research on IVM’s safety profile has enabled its use in new therapeutic applications, for example viral infections [7,54,55] like COVID-19, inflammatory diseases [54,55,74,120], bacterial infections [64,72], cancer treatment [28] and wound healing [9,90,91].
- Optimizing drug delivery via tailored dosage form development: Understanding IVM’s toxicity has led to the development of improved formulations, such as topical and transdermal formulations, which reduce systemic exposure and minimize adverse effects [121].
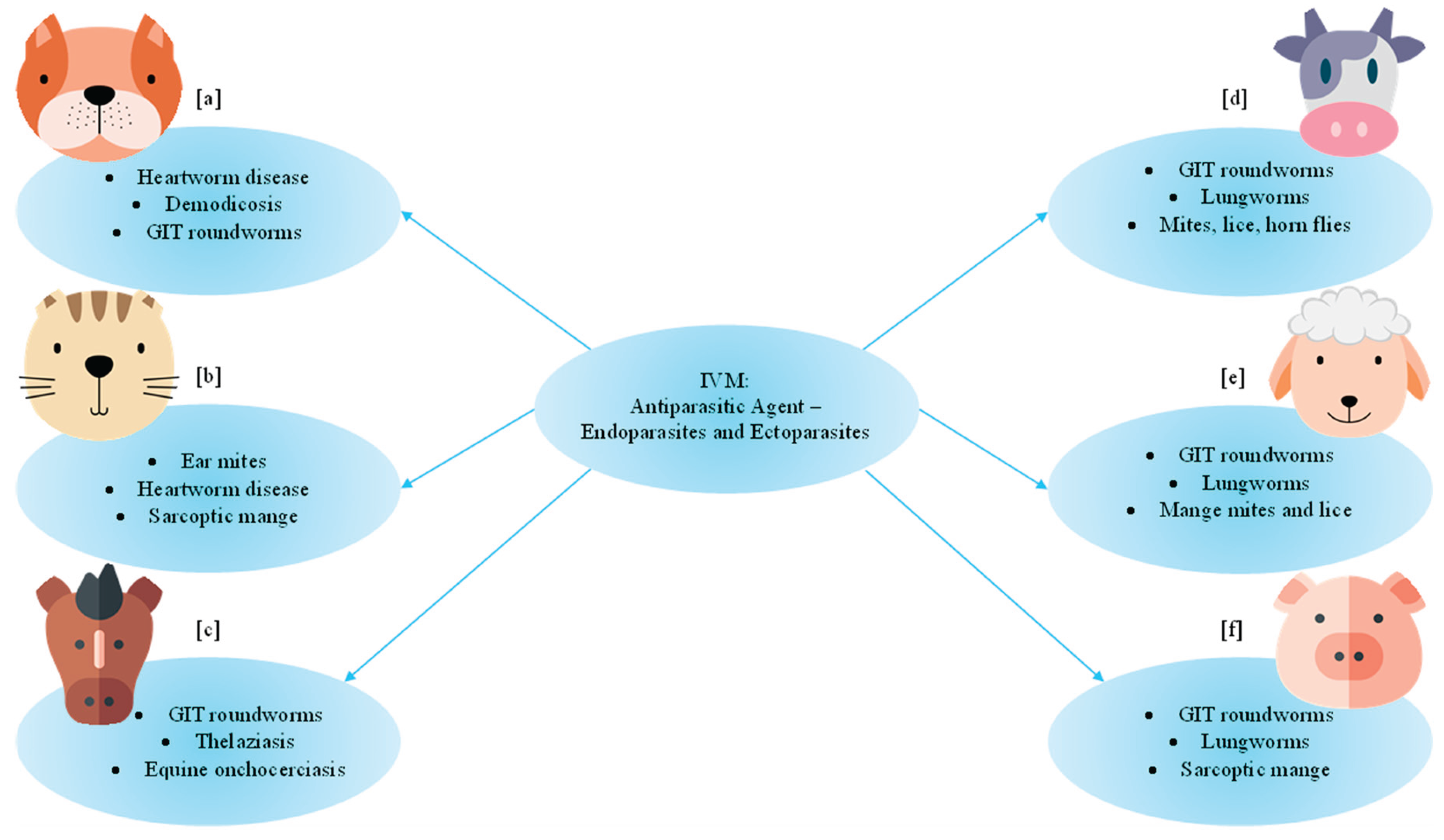
4. Veterinary Uses
5. In Vivo Investigations and Applications
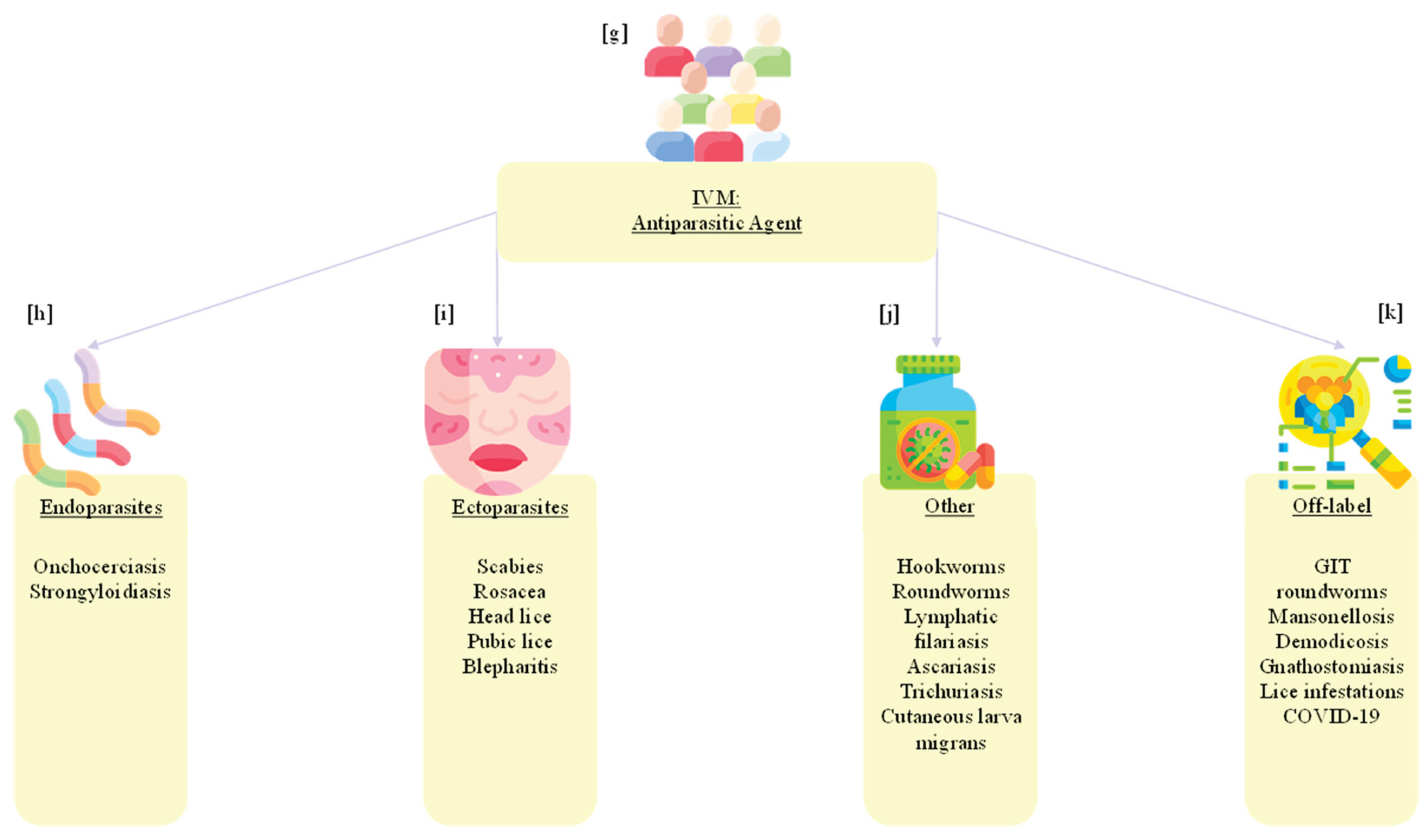
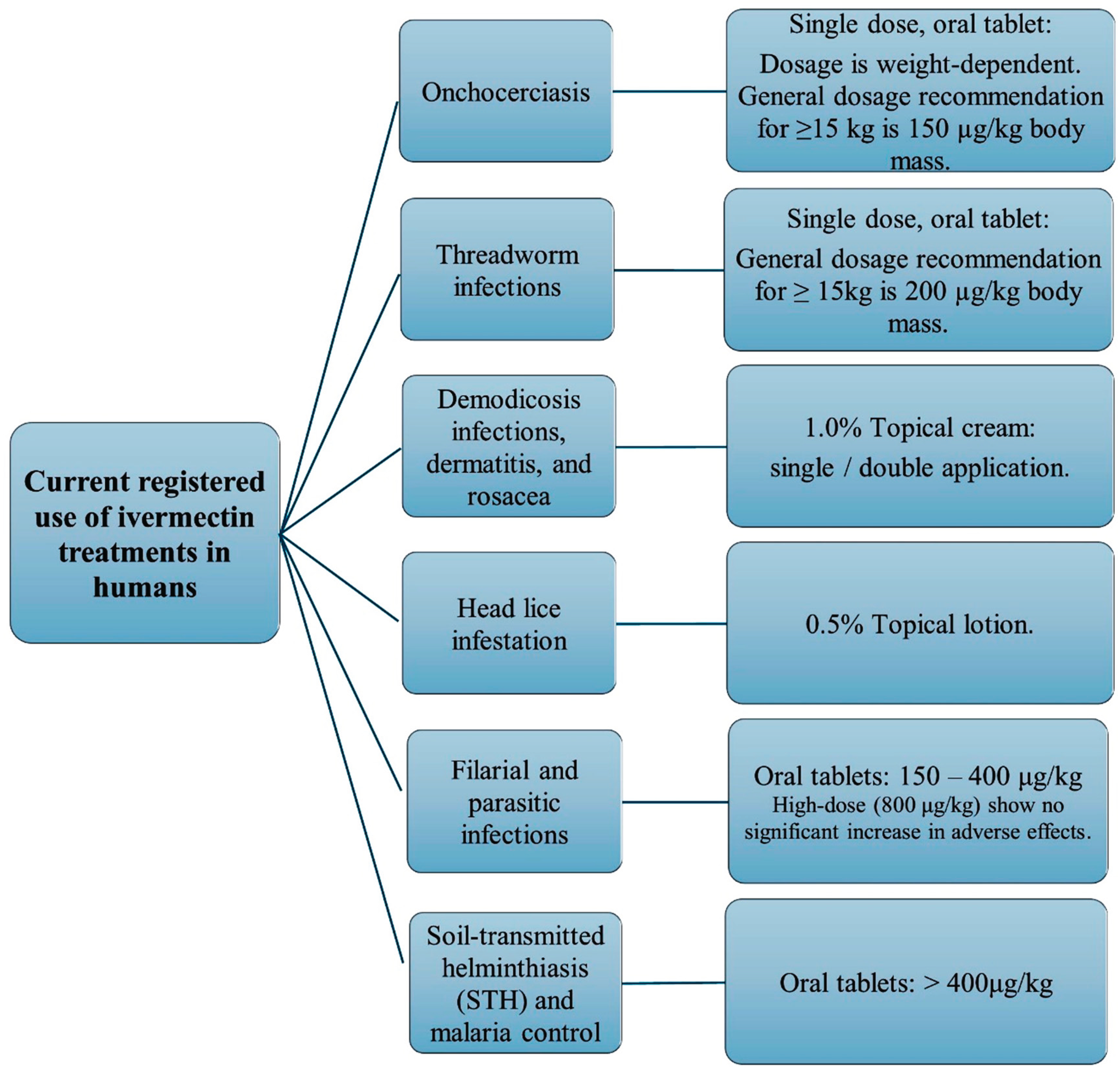
6. Human Uses
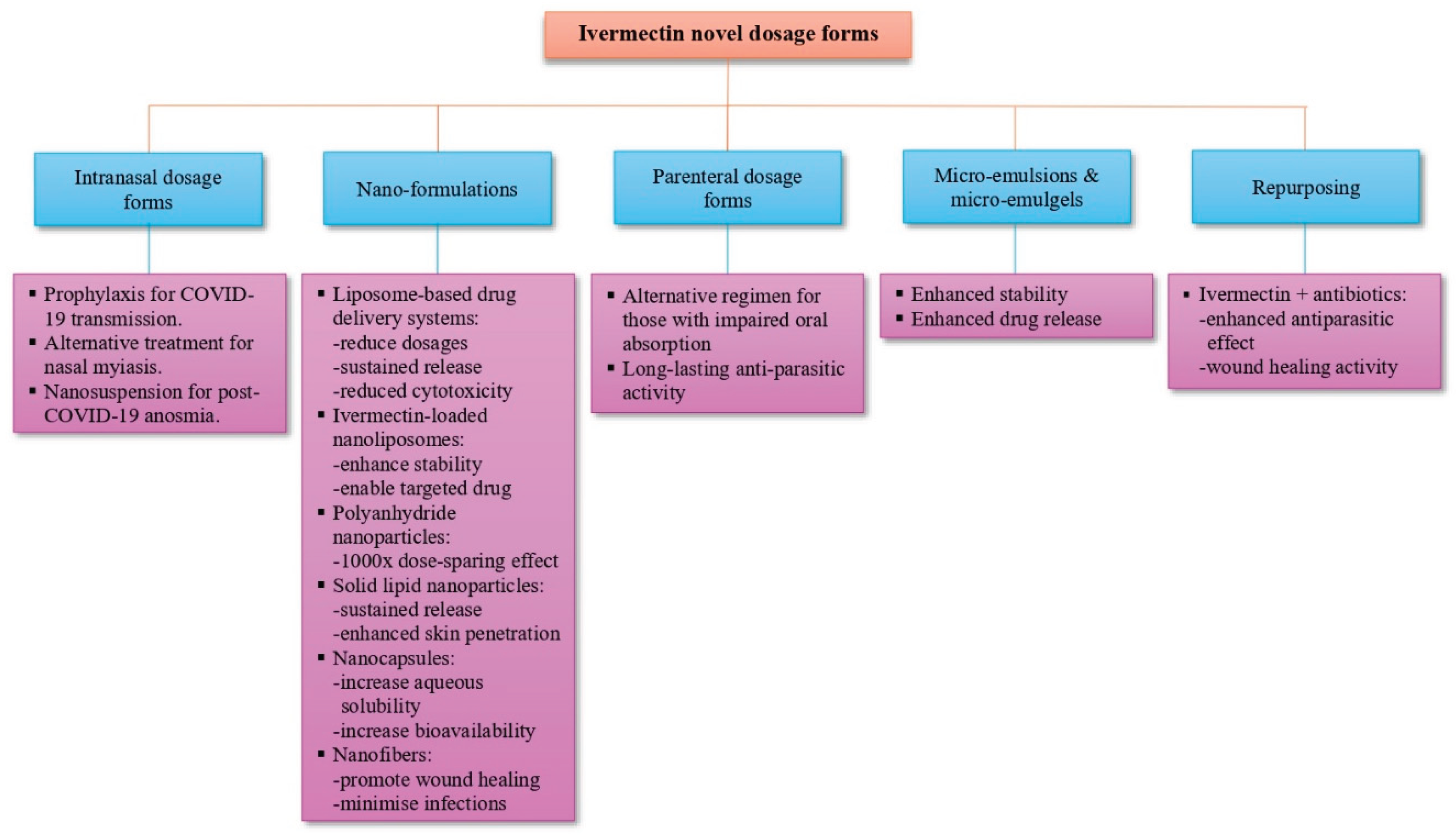
7. Novel Dosage Form Development
7.1. Liquid-Based Dosage Forms
7.2. Solid Oral Dosage Forms
7.3. Powder Dosage Forms
7.4. Semi-Solid Dosage Forms
7.5. Nanoformulations and Nanostructured Carriers
7.6. Lipid-Based Formulations
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Disclaimer/Funding
Abbreviations
| IVM | Ivermectin |
| COVID-19 | Coronavirus disease of 2019 |
| FDA | United States Food and Drug Administration |
| WHO | World Health Organization |
| NIH | National Institutes of Health |
| GIT | Gastrointestinal tract |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| ACE2 | Angiotensin-converting enzyme 2 |
| TMPRSS2 | Transmembrane protease, serine 2 |
| ROS | Reactive oxygen species |
| 3CLpro | 3-Chymotrypsin-like Protease |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| Vero/hSLAM cells | Vero cells strongly expressing human signaling lymphocyte activation molecules |
| HDA | Host-directed agent |
| RNA | Ribonucleic acid |
| IMP | Importin |
| HIV-1 | Human immunodeficiency virus type 1 |
| HAdV | Human adenovirus |
| BoAHV-1 | Varicellovirus bovinealpha 1 |
| MDBK | Madin-Darby Bovine Kidney |
| BT | Bovine turbinate |
| STD | Sexually transmitted disease |
| MIC | Minimum inhibitory concentration |
| DNA | Deoxyribonucleic acid |
| M. ulcerans | Mycobacterium ulcerans |
| S. aureus | Staphylococcus aureus |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| CSCs | Cancer stem-like cells |
| WNT | Wingless signaling |
| TCF | T-cell factor |
| mTOR | Mammalian target of rapamycin |
| PAK1 | p21-activated kinase 1 |
| SID | Surface-induced dissociation |
| MDR | Multi-drug resistance |
| Bax | Bcl-2 associated X protein |
| HIF | Hypoxia-inducible factor |
| JNK | c-Jun N-terminal kinase |
| ERK 1/2 | Extracellular signal-regulated kinase 1 and 2 |
| NO | Nitric oxide |
| PGE2 | Prostaglandin E2 |
| NOS | Nitric oxide synthase |
| COX2 | Cyclooxygenase-2 |
| TGF-β1 | Transforming growth factor-beta 1 |
| VEGF | Vascular endothelial growth factor |
| FXR | Farnesoid X receptor |
| LD50 | Lethal dose 50% |
| w/w | Weight per weight |
| ABCB1 | ATP Binding Cassette Subfamily B Member 1 |
| P-gp | P-glycoprotein |
| ATP | Adenosine triphosphate |
| CNS | Central nervous system |
| ADME | Absorption, distribution, metabolism, and excretion |
| SLNs | Solid lipid nanoparticles |
| Hb | Hemoglobin |
| TLC | Total leucocyte count |
| DLC | Differential leucocyte count |
| BUN | Blood urea nitrogen |
| ALT | Alanine transaminase |
| AST | Aspartate transferase |
| Cmax | Peak plasma concentration |
| t½ | Elimination half-life |
| IV | Intravenous |
| Tmax | Duration/time to reach Cmax |
| SAHPRA | South African Health Product Regulatory Authority |
| HPLC | High-performance liquid chromatography |
| AUC | Analytical peak area |
| ODT(s) | Oral disintegrating tablet(s) |
| EC50 | Half maximal effective concentration |
| ED | Epidermis-dermis |
| SCE | Stratum corneum-epidermis |
| CYP | Cytochrome P |
| PNPs | Polymeric nanoparticles |
| NLCs | Nanostructured lipid carriers |
| SNEDDS | Self-nano-emulsifying drug delivery systems |
| MSNs | Mesoporous silica nanoparticles |
| IVM-MCM | Ivermectin mesoporous silica particles |
| IVM-NC | Ivermectin poly(ε-caprolactone) nanocapsules |
| PEDV | Porcine epidemic diarrhea virus |
| C6 | Coumarin 6 |
| SEDDS | Self-emulsifying drug delivery systems |
References
- Sulik, M.; Antoszczak, M.; Huczyński, A.; Steverding, D. Antiparasitic activity of ivermectin: Four decades of research into a “wonder drug”. Eur. J. Med. Chem. 2023, 261, 115838. [Google Scholar] [CrossRef]
- Nathan, C. Cooperative development of antimicrobials: Looking back to look ahead. Nat. Rev. Microbiol. 2015, 13, 651–657. [Google Scholar] [CrossRef]
- Chen, I.S.; Kubo, Y. Ivermectin and its target molecules: Shared and unique modulation mechanisms of ion channels and receptors by ivermectin. J Physiol 2018, 596, 1833–1845. [Google Scholar] [CrossRef]
- Lumaret, J.P.; Errouissi, F.; Floate, K.; Rombke, J.; Wardhaugh, K. A review on the toxicity and non-target effects of macrocyclic lactones in terrestrial and aquatic environments. Curr. Pharm. Biotechnol. 2012, 13, 1004–1060. [Google Scholar] [CrossRef] [PubMed]
- Chabala, J.C.; Mrozik, H.; Tolman, R.L.; Eskola, P.; Lusi, A.; Peterson, L.H.; Woods, M.F.; Fisher, M.H.; Campbell, W.C. Ivermectin, a new broad-spectrum antiparasitic agent. J Med Chem 1980, 23, 1134–1136. [Google Scholar] [CrossRef] [PubMed]
- Crump, A. Ivermectin: Enigmatic multifaceted “wonder” drug continues to surprise and exceed expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef]
- Rizzo, E. Ivermectin, antiviral properties and COVID-19: A possible new mechanism of action. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2020, 393, 1153–1156. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, A.; Kigure, A.; Anata, T.; Hirota, T. Mechanism for transport of ivermectin to the stratum corneum in rats. Drug Metab. Pharmacokinet. 2015, 30, 385–390. [Google Scholar] [CrossRef]
- Sia, D.K.; Mensah, K.B.; Opoku-Agyemang, T.; Folitse, R.D.; Darko, D.O. Mechanisms of ivermectin-induced wound healing. BMC Vet. Res. 2020, 16, 397. [Google Scholar] [CrossRef]
- Burg, R.W.; Miller, B.M.; Baker, E.E.; Birnbaum, J.; Currie, S.A.; Hartman, R.; Kong, Y.L.; Monaghan, R.L.; Olson, G.; Putter, I.; Tunac, J.B. Avermectins, new family of potent anthelmintic agents: Producing organism and fermentation. Antimicrob Agents Chemother 1979, 15, 361–367. [Google Scholar] [CrossRef]
- Campbell, W.C. History of avermectin and ivermectin, with notes on the history of other macrocyclic lactone antiparasitic agents. Curr Pharm Biotechnol 2012, 13, 853–865. [Google Scholar] [CrossRef]
- Hotson, I.K. The avermectins: A new family of antiparasitic agents. J. S. Afr. Vet. Assoc. 1982, 53, 87–90. [Google Scholar]
- Htay, M.N.N.; Swed, S.; Elsayed, M.G.; Arafat, S.Y.; Marthoenis, M.; Marzo, R.R.; El-Abasiri, R.A.A.; Naing, Z.Y.; San, L.P.P.; Thantry, A.D.K.; Kyaw, T.M. Knowledge and awareness of neglected tropical diseases and control strategies among healthcare students in five Asian countries: A cross-sectional study. Clin.Epidemiol. Glob. Health 2024, 27, 101576. [Google Scholar] [CrossRef]
- Navarro, M.; Camprubí, D.; Requena-Méndez, A.; Buonfrate, D.; Giorli, G.; Kamgno, J.; Gardon, J.; Boussinesq, M.; Muñoz, J.; Krolewiecki, A. Safety of high-dose ivermectin: A systematic review and meta-analysis. J. Antimicrob. Chemother. 2020, 75, 827–834. [Google Scholar] [CrossRef]
- Fimbo, A.M.; Mnkugwe, R.H.; Mlugu, E.M.; Kunambi, P.P.; Malishee, A.; Minzi, O.M.; Kamuhabwa, A.A.; Aklillu, E. Efficacy of ivermectin and albendazole combination in suppressing transmission of lymphatic filariasis following mass administration in Tanzania: A prospective cohort study. Infect. Dis. Poverty 2024, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Hu, X.; Wang, Y.; Yao, X.; Zhang, W.; Yu, C.; Cheng, F.; Li, J.; Fang, Q. Ivermectin, a potential anticancer drug derived from an antiparasitic drug. Pharmacol. Res. 2021, 163, 105207. [Google Scholar] [CrossRef]
- Ashour, D.S. Ivermectin: From theory to clinical application. Int J Antimicrob Agents 2019, 54, 134–142. [Google Scholar] [CrossRef]
- De Melo, G.D.; Lazarini, F.; Larrous, F.; Feige, L.; Kornobis, E.; Levallois, S.; Marchio, A.; Kergoat, L.; Hardy, D.; Cokelaer, T.; Pineau, P. Attenuation of clinical and immunological outcomes during SARS-CoV-2 infection by ivermectin. EMBO Mol. Med. 2021, 13, e14122. [Google Scholar] [CrossRef]
- Tiberti, N.; Buonfrate, D.; Carbone, C.; Piro, G.; Bisoffi, Z.; Piubelli, C. Systemic profile of immune factors in an elderly Italian population affected by chronic strongyloidiasis. Parasites Vectors 2020, 13, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Jin, C.; Sang, H.; Liu, W.; Wang, J. Ivermectin protects against experimental autoimmune encephalomyelitis in mice by modulating the Th17/Treg balance involved in the IL-2/STAT5 pathway. Inflammation 2023, 46, 1626–1638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Xu, W.; Cheng, J.; Zhang, C.; Gao, J.; Li, Z.; Tao, L.; Zhang, Y. Immunotoxicity induced by Ivermectin is associated with NF-κB signaling pathway on macrophages. Chemosphere 2022, 289, 133087. [Google Scholar] [CrossRef] [PubMed]
- Coles, G.C. Anthelmintic resistance–looking to the future: A UK perspective. Res. Vet. Sci. 2005, 78, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Erez, M.S.; Kozan, E. Anthelmintic resistance in farm animals. Kocatepe Vet. J. 2018, 11, 322–330. [Google Scholar] [CrossRef]
- Fissiha, W.; Kinde, M.Z. Anthelmintic resistance and its mechanism: A review. Infect. Drug Resist. 2021, 14, 5403–5410. [Google Scholar] [CrossRef]
- Furnival-Adams, J.; Kiuru, C.; Sagna, A.B.; Mouline, K.; Maia, M.; Chaccour, C. Ivermectin resistance mechanisms in ectoparasites: A scoping review. Parasitol. Res. 2024, 123, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Gryseels, B. Drug resistance in human helminths: Current situation and lessons from livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef]
- Kornele, M.L.; McLean, M.J.; O’Brien, A.E.; Phillippi-Taylor, A.M. Antiparasitic resistance and grazing livestock in the United States. J. Am. Vet. Med. Assoc. 2014, 244, 1020–1022. [Google Scholar] [CrossRef] [PubMed]
- Laing, R.; Gillan, V.; Devaney, E. Ivermectin - old drug, new tricks? Trends Parasitol. 2017, 33, 463–472. [Google Scholar] [CrossRef]
- Mohammedsalih, K.M.; Ibrahim, A.I.; Juma, F.R.; Abdalmalaik, A.A.; Bashar, A.; Coles, G.; von Samson-Himmelstjerna, G.; Krücken, J. First evaluation and detection of ivermectin resistance in gastrointestinal nematodes of sheep and goats in South Darfur, Sudan. PLoS ONE 2024, 19, e0301554. [Google Scholar] [CrossRef]
- Mphahlele, M.; Molefe, N.; Tsotetsi-Khambule, A.; Oriel, T. Anthelmintic resistance in livestock. In Helminthiasis; Okwa, O.O., Ed.; InTechOpen, 2019. [Google Scholar] [CrossRef]
- Prichard, R.K. Anthelmintic resistance. Vet. Parasitol. 1994, 54, 259–268. [Google Scholar] [CrossRef]
- Prichard, R.K. Ivermectin resistance and overview of the Consortium for Anthelmintic Resistance SNPs’. Expert Opin. Drug Discov. 2007, 2, S41–S52. [Google Scholar] [CrossRef]
- Waller, P.J. Anthelmintic resistance. Vet. Parasitol. 1997, 72, 391–412. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, J.C. Drug resistance in parasites: A review of mechanisms, drivers, and mitigation strategies. Microbes Infect. Dis. 2024, 6, 6152–6160. [Google Scholar] [CrossRef]
- White, N.J.; Pongtavornpinyo, W.; Maude, R.J.; Saralamba, S.; Aguas, R.; Stepniewska, K.; Lee, S.J.; Dondorp, A.M.; White, L.J.; Day, N.P. Hyperparasitaemia and low dosing are an important source of anti-malarial drug resistance. Malar. J. 2009, 8, 1–18. [Google Scholar] [CrossRef]
- Ruan, X.; Gao, X.; Gao, Y.; Peng, L.; Ji, H.; Guo, D.; Jiang, S. Preparation and in vitro release kinetics of ivermectin sustained-release bolus optimized by response surface methodology. PeerJ. 2018, 6, e5418. [Google Scholar] [CrossRef]
- Gnesotto, L.; Cutrone, M.; Mazzatenta, C.; Bassi, A.; Piccolo, V.; Sechi, A. Topical Ivermectin for Permethrin-Resistant Scabies: A Useful Application. Dermatol. Pract. Concept 2024, 14, e2024029. [Google Scholar] [CrossRef]
- Algorta, J.; Krolewiecki, A.; Pinto, F.; Gold, S.; Muñoz, J. Pharmacokinetic characterization and comparative bioavailability of an innovative orodispersible fixed-dose combination of ivermectin and albendazole: A single dose, open label, sequence randomized, crossover clinical trial in healthy volunteers. Front Pharmacol 2022, 13, 914886. [Google Scholar] [CrossRef]
- Algorta, J.; Kepha, S.; Krolewiecki, A.; Li, H.; Giang, J.; Fleitas, P.; Mwandawiro, C.; Muñoz, J.; STOP Consortium. Population Pharmacokinetics and Exposure–Response Analysis of a Fixed-Dose Combination of Ivermectin and Albendazole in Children, Adolescents, and Adults. Clin Pharmacol Ther 2025, 117, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Monedero, I.; Caminero, J.A. Evidence for promoting fixed-dose combination drugs in tuberculosis treatment and control: A review [Unresolved issues]. Int. J. Tuberc. Lung Dis. 2011, 15, 433–439. [Google Scholar] [CrossRef]
- Konopka, J.K.; Chatterjee, P.; LaMontagne, C.; Brown, J. Environmental impacts of mass drug administration programs: Exposures, risks, and mitigation of antimicrobial resistance. Infect. Dis. Poverty 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Mancini, L.; Lacchetti, I.; Chiudioni, F.; Cristiano, W.; Kevin, D.D.; Marcheggiani, S.; Carere, M.; Bindi, L.; Borrello, S. Need for a sustainable use of medicinal products: Environmental impacts of ivermectin. Ann. Ist. Super. Sanita 2020, 56, 492–496. [Google Scholar] [CrossRef]
- Awad, H.; Rawas-Qalaji, M.; El Hosary, R.; Jagal, J.; Ahmed, I.S. Formulation and optimization of ivermectin nanocrystals for enhanced topical delivery. Int J Pharm: X 2023, 6, 100210. [Google Scholar] [CrossRef] [PubMed]
- Chaccour, C.; Barrio, Á.I.; Royo, A.G.G.; Urbistondo, D.M.; Slater, H.; Hammann, F.; Del Pozo, J.L. Screening for an ivermectin slow-release formulation suitable for malaria vector control. Malar J 2015, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Lee, S.H.; Chia, V.D.; Chow, P.S.; Macbeath, C.; Liu, Y.; Shlieout, G. Development of microemulsion based topical ivermectin formulations: Pre-formulation and formulation studies. Colloids Surf. B. Biointerfaces 2020, 189, 110823. [Google Scholar] [CrossRef] [PubMed]
- Hennessy, D.R. Modifying the formulation or delivery mechanism to increase the activity of anthelmintic compounds. Vet. Parasitol. 1997, 72, 367–390. [Google Scholar] [CrossRef]
- Velho, M.C.; Funk, N.L.; Deon, M.; Benvenutti, E.V.; Buchner, S.; Hinrichs, R.; Pilger, D.A.; Beck, R.C.R. Ivermectin-Loaded Mesoporous Silica and Polymeric Nanocapsules: Impact on Drug Loading, In Vitro Solubility Enhancement, and Release Performance. Pharmaceutics 2024, 16, 325. [Google Scholar] [CrossRef]
- Jermain, B.; Hanafin, P.O.; Cao, Y.; Lifschitz, A.; Lanusse, C.; Rao, G.G. Development of a minimal physiologically-based pharmacokinetic model to simulate lung exposure in humans following oral administration of ivermectin for COVID-19 drug repurposing. J. Pharm. Sci. 2020, 109, 3574–3578. [Google Scholar] [CrossRef]
- Roy, S.; Dhaneshwar, S.; Bhasin, B. Drug repurposing: An emerging tool for drug reuse, recycling and discovery. Curr. Drug Res. Rev. 2021, 13, 101–119. [Google Scholar] [CrossRef]
- Su, C.; Saha, T.; Sinha, S.; Hird, C.P.; Smith, S.X.; Quiñones-Mateu, M.E.; Das, S.C. Inhalable spray-dried dry powders combining ivermectin and niclosamide to inhibit SARS-CoV-2 infection in vitro. Int. J. Pharm. 2025, 671, 125302. [Google Scholar] [CrossRef]
- Wehbe, Z.; Wehbe, M.; Iratni, R.; Pintus, G.; Zaraket, H.; Yassine, H.M.; Eid, A.H. Repurposing ivermectin for COVID-19: Molecular aspects and therapeutic possibilities. Front. Immunol. 2021, 12, 663586. [Google Scholar] [CrossRef]
- Wentzel, C.; Gernandt, N.; Gerber, M.; Van Der Kooy, F. Quantification of ivermectin in veterinary products consumed off-label as a treatment for COVID-19. Die Pharmazie 2024, 79, 216–219. [Google Scholar] [CrossRef] [PubMed]
- Porubcin, S.; Rovnakova, A.; Zahornacky, O.; Jarcuska, P. Intravenous veterinary ivermectin in a COVID-19 patient causing neurotoxicity. IDCases 2022, 27, e01446. [Google Scholar] [CrossRef] [PubMed]
- Heidary, F.; Gharebaghi, R. Ivermectin: A systematic review from antiviral effects to COVID-19 complementary regimen. J. Antibiot. 2020, 73, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Castillejos-López, M.; Torres-Espíndola, L.M.; Huerta-Cruz, J.C.; Flores-Soto, E.; Romero-Martinez, B.S.; Velázquez-Cruz, R.; Higuera-Iglesias, A.; Camarena, Á.; Torres-Soria, A.K.; Salinas-Lara, C.; Fernández-Plata, R. Ivermectin: A controversial focal point during the COVID-19 pandemic. Life 2022, 12, 1384. [Google Scholar] [CrossRef]
- Low, Z.Y.; Yip, A.J.W.; Lal, S.K. Repositioning Ivermectin for Covid-19 treatment: Molecular mechanisms of action against SARS-CoV-2 replication. Biochim. Biophys. Acta Mol. Basis Dis. 2022, 1868, 166294. [Google Scholar] [CrossRef]
- Zaidi, A.K.; Dehgani-Mobaraki, P. The mechanisms of action of ivermectin against SARS-CoV-2—An extensive review. J. Antibiot. 2022, 75, 60–71. [Google Scholar] [CrossRef]
- Barati, N.; Motavallihaghi, S.; Nikfar, B.; Chaichian, S.; Momtazi-Borojeni, A.A. Potential therapeutic effects of ivermectin in COVID-19. Exp Biol Med 2022, 247, 1388–1396. [Google Scholar] [CrossRef]
- Mody, V.; Ho, J.; Wills, S.; Mawri, A.; Lawson, L.; Ebert, M.C.; Fortin, G.M.; Rayalam, S.; Taval, S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun. Biol. 2021, 4, 93. [Google Scholar] [CrossRef]
- Zaheer, T.; Pal, K.; Abbas, R.Z.; Torres, M.D.P. COVID-19 and Ivermectin: Potential threats associated with human use. J. Mol. Struct. 2021, 1243, 1–5. [Google Scholar] [CrossRef]
- Martin, R.J.; Robertson, A.P.; Choudhary, S. Ivermectin: An anthelmintic, an insecticide, and much more. Trends Parasitol. 2021, 37, 48–64. [Google Scholar] [CrossRef]
- Ceballos, L.; Alvarez, L.; Lifschitz, A.; Lanusse, C. Ivermectin systemic availability in adult volunteers treated with different oral pharmaceutical formulations. Biomed Pharmacother 2023, 160, 114391. [Google Scholar] [CrossRef]
- Mastrangelo, E.; Pezzullo, M.; De Burghgraeve, T.; Kaptein, S.; Pastorino, B.; Dallmeier, K.; de Lamballerie, X.; Neyts, J.; Hanson, A.M.; Frick, D.N.; Bolognesi, M. Ivermectin is a potent inhibitor of flavivirus replication specifically targeting NS3 helicase activity: New prospects for an old drug. J. Antimicrob. Chemother. 2012, 67, 1884–1894. [Google Scholar] [CrossRef]
- Bray, M.; Rayner, C.; Noël, F.; Jans, D.; Wagstaff, K. Ivermectin and COVID-19: A report in Antiviral Research, widespread interest, an FDA warning, two letters to the editor and the authors’ responses. Antivir Res 2020, 178, 104805. [Google Scholar] [CrossRef]
- Kaur, B.; Blavo, C.; Parmar, M.S. Ivermectin: A Multifaceted Drug With a Potential Beyond Antiparasitic Therapy. Cureus 2024, 16. [Google Scholar] [CrossRef] [PubMed]
- Pérez, S.; Miró, M.V.; Verna, A.; Altamiranda, E.G.; Barcos, O.; Lanusse, C.; Lifschitz, A. Ivermectin antiviral activity against Varicellovirus bovine alpha 1: Assessment of intracellular drug accumulation in virus-infected cells. Arch. Microbiol. 2024, 206, 78. [Google Scholar] [CrossRef]
- Ashraf, S.; Chaudhry, U.; Raza, A.; Ghosh, D.; Zhao, X. In vitro activity of ivermectin against Staphylococcus aureus clinical isolates. Antimicrob Resist Infect Control 2018, 7, 27. [Google Scholar] [CrossRef]
- Bazzano, M.; Di Salvo, A.; Diaferia, M.; Veronesi, F.; Galarini, R.; Paoletti, F.; Tesei, B.; McLean, A.; Veneziano, V.; Laus, F. Anthelmintic efficacy and pharmacokinetics of ivermectin paste after oral administration in mules infected by cyathostomins. Animals 2020, 10, 934. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alqahtani, A.; Ilesanmi, O.B.; Saati, A.A.; El-Mleeh, A.; Hetta, H.F.; Magdy Beshbishy, A. Avermectin derivatives, pharmacokinetics, therapeutic and toxic dosages, mechanism of action, and their biological effects. Pharmaceuticals 2020, 13, 196. [Google Scholar] [CrossRef]
- Piras, C.; Gugliandolo, E.; Castagna, F.; Palma, E.; Britti, D. Ivermectin (IVM) possible side activities and implications in antimicrobial resistance and animal welfare: The authors’ perspective. Vet. Sci. 2022, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Omansen, T.F.; Porter, J.L.; Johnson, P.D.R.; van der Werf, T.S.; Stienstra, Y.; Stinear, T.P. In-vitro Activity of Avermectins against Mycobacterium ulcerans. PLoS Negl. Trop. Dis. 2015, 9, e0003549. [Google Scholar] [CrossRef] [PubMed]
- Stetkevich, S.A.; Anzelc, M.J.; Burkhart, C.G. Intranasal ivermectin spray, the sunscreen to COVID-19. Open Dermatol. J. 2022, 16, e187437222205190. [Google Scholar] [CrossRef]
- Tan, X.; Xie, H.; Zhang, B.; Zhou, J.; Dou, Z.; Wang, X.; Wang, N. A novel ivermectin-derived compound D4 and its antimicrobial/biofilm properties against MRSA. Antibiotics 2021, 10, 1–14. [Google Scholar] [CrossRef]
- Sharun, K.; Shyamkumar, T.S.; Aneesha, V.A.; Dhama, K.; Pawde, A.M.; Pal, A. Current therapeutic applications and pharmacokinetic modulations of ivermectin. Vet. World 2019, 12, 1204–1211. [Google Scholar] [CrossRef]
- Juarez, M.; Schcolnik-Cabrera, A.; Dueñas-Gonzalez, A. The multitargeted drug ivermectin: From an antiparasitic agent to a repositioned cancer drug. Am. J. Cancer Res. 2018, 8, 317–331. [Google Scholar]
- Sharmeen, S.; Skrtic, M.; Sukhai, M.A.; Hurren, R.; Gronda, M.; Wang, X.; Fonseca, S.B.; Sun, H.; Wood, T.E.; Ward, R.; Minden, M.D. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood 2010, 116, 3593–3603. [Google Scholar] [CrossRef] [PubMed]
- Melotti, A.; Mas, C.; Kuciak, M.; Lorente-Trigos, A.; Borges, I.; Ruiz i Altaba, A. The river blindness drug I vermectin and related macrocyclic lactones inhibit WNT-TCF pathway responses in human cancer. EMBO Mol. Med. 2014, 6, 1263–1278. [Google Scholar] [CrossRef]
- Diao, H.; Cheng, N.; Zhao, Y.; Xu, H.; Dong, H.; Thamm, D.H.; Zhang, D.; Lin, D. Ivermectin inhibits canine mammary tumor growth by regulating cell cycle progression and WNT signaling. BMC Vet. Res. 2019, 15, 276. [Google Scholar] [CrossRef]
- Dou, Q.; Chen, H.N.; Wang, K.; Yuan, K.; Lei, Y.; Li, K.; Lan, J.; Chen, Y.; Huang, Z.; Xie, N.; Zhang, L. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Cancer Res. 2016, 76, 4457–4469. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, K.; Cheng, L.; Zhu, H.; Xu, T. Progress in understanding the molecular mechanisms underlying the antitumour effects of ivermectin. Drug Des. Devel. Ther. 2020, 14, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Qin, T.; Zhu, Z.; Hong, F.; Xu, Y.; Zhang, X.; Xu, X.; Ma, A. Ivermectin Augments the In Vitro and In Vivo Efficacy of Cisplatin in Epithelial Ovarian Cancer by Suppressing Akt/mTOR Signaling. Am. J. Med. Sci. 2020, 359, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Gallardo, F.; Teiti, I.; Rochaix, P.; Demilly, E.; Jullien, D.; Mariamé, B.; Tilkin-Mariamé, A.F. Macrocyclic lactones block melanoma growth, metastases development and potentiate activity of anti–BRAF V600 inhibitors. Clin. Skin Cancer 2016, 1, 4–14. [Google Scholar] [CrossRef]
- Gallardo, F.; Mariamé, B.; Gence, R.; Tilkin-Mariamé, A.F. Macrocyclic lactones inhibit nasopharyngeal carcinoma cells proliferation through PAK1 inhibition and reduce in vivo tumor growth. Drug Des. Devel. Ther. 2018, 12, 2805–2814. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, H.S.; Kim, S.L.; Lee, D.S. The PAK1-Stat3 Signaling Pathway Activates IL-6 Gene Transcription and Human Breast Cancer Stem Cell Formation. Cancers 2019, 11, 1527. [Google Scholar] [CrossRef]
- Yin, J.; Park, G.; Lee, J.E.; Choi, E.Y.; Park, J.Y.; Kim, T.H.; Park, N.; Jin, X.; Jung, J.E.; Shin, D.; Hong, J.H. DEAD-box RNA helicase DDX23 modulates glioma malignancy via elevating miR-21 biogenesis. Brain 2015, 138, 2553–2570. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Petrie, K.; Leibovitch, B.A.; Zeng, L.; Mezei, M.; Howell, L.; Gil, V.; Christova, R.; Bansal, N.; Yang, S.; Sharma, R. Selective inhibition of SIN3 corepressor with avermectins as a novel therapeutic strategy in triple-negative breast cancer. Mol. Cancer Ther. 2015, 14, 1824–1836. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Wan, H.; Hu, J. Antibiotic ivermectin selectively induces apoptosis in chronic myeloid leukemia through inducing mitochondrial dysfunction and oxidative stress. Biochem. Biophys. Res. Commun. 2018, 497, 241–247. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Barroso-Arranda, J.; McCarty, M. Ivermectin may be a clinically useful anti-inflammatory agent for late-stage COVID-19. Open Heart 2020, 7, e001350. [Google Scholar] [CrossRef]
- Dourmishev, A.L.; Dourmishev, L.A.; Schwartz, R.A. Ivermectin: Pharmacology and application in dermatology. Int. J. Dermatol. 2005, 44, 981–988. [Google Scholar] [CrossRef]
- Cairns, D.M.; Giordano, J.E.; Conte, S.; Levin, M.; Kaplan, D.L. Ivermectin promotes peripheral nerve regeneration during wound healing. ACS Omega 2018, 3, 12392–12402. [Google Scholar] [CrossRef]
- Tian, S.; Zheng, Y.; Xiao, S.; Luo, P.; Sun, R.; Liu, J.; Xia, Z. Ivermectin inhibits cell proliferation and the expression levels of type I collagen, α-SMA and CCN2 in hypertrophic scar fibroblasts. Mol. Med. Rep. 2021, 24, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kalangadan, N.; Mary, A.S.; Mani, K.; Nath, B.; Kondapalli, J.; Soni, S.; Raghavan, V.S.; Parsanathan, R.; Kannan, M.; Jenkins, D.; Gorthi, S.S. Repurposing ivermectin and ciprofloxacin in nanofibers for enhanced wound healing and infection control against MDR wound pathogens. J. Drug Deliv. Sci. Technol. 2023, 90, 105166. [Google Scholar] [CrossRef]
- Green, J.A.; Stockton, R.A.; Johnson, C.; Jacobson, B.S. 5-Lipoxygenase and cyclooxygenase regulate wound closure in NIH/3T3 fibroblast monolayers. Am. J. Physiol. Cell Physiol. 2004, 287, C373–C383. [Google Scholar] [CrossRef]
- Morris-Schaffer, K.; McCoy, M.J. A review of the LD50 and its current role in hazard communication. ACS Chem. Health Saf. 2020, 28, 25–33. [Google Scholar] [CrossRef]
- Guzzo, C.A.; Furtek, C.I.; Porras, A.G.; Chen, C.; Tipping, R.; Clineschmidt, C.M.; Sciberras, D.G.; Hsieh, J.Y.K.; Lasseter, K.C. Safety, tolerability, and pharmacokinetics of escalating high doses of ivermectin in healthy adult subjects. J. Clin. Pharmacol. 2002, 42, 1122–1133. [Google Scholar] [CrossRef]
- Mueller, R.S.; Rosenkrantz, W.; Bensignor, E.; Karaś-Tęcza, J.; Paterson, T.; Shipstone, M.A. Diagnosis and treatment of demodicosis in dogs and cats: Clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 2020, 31, 4–e2. [Google Scholar] [CrossRef] [PubMed]
- Fobi, G.; Gardon, J.; Kamgno, J.; Aimard-Favennec, L.; Lafleur, C.; Gardon-Wendel, N.; Duke, B.O.; Boussinesq, M. A randomized, double-blind, controlled trial of the effects of ivermectin at normal and high doses, given annually or three-monthly, against Onchocerca volvulus: Ophthalmological results. Trans. R. Soc. Trop. Med. Hyg. 2005, 99, 279–289. [Google Scholar] [CrossRef]
- Burnham, G.M. Adverse reactions to ivermectin treatment for onchocerciasis. Results of a placebo-controlled, double-blind trial in Malawi. Trans R Soc Trop Med Hyg 1993, 87, 313–317. [Google Scholar] [CrossRef]
- Chandler, R.E. Serious neurological adverse events after ivermectin—Do they occur beyond the indication of onchocerciasis? Am J Trop Med Hyg 2017, 98, 382. [Google Scholar] [CrossRef]
- Kipp, W.; Bamhuhiiga, J.; Rubaale, T.; Kabagambe, G. Adverse reactions to the ivermectin treatment of onchocerciasis patients: Does infection with the human immunodeficiency virus play a role? Ann. Trop. Med. Parasitol. 2005, 99, 395–402. [Google Scholar] [CrossRef]
- Zea-Flores, R.; Richards Jr, F.O.; González-Peralta, C.; Ramirez, J.C.; Zea-Flores, G.; Collins, R.C.; Cupp, E. Adverse reactions after community treatment of onchocerciasis with ivermectin in Guatemala. Trans. R. Soc. Trop. Med. Hyg. 1992, 86, 663–666. [Google Scholar] [CrossRef]
- Budge, P.J.; Herbert, C.; Andersen, B.J.; Weil, G.J. Adverse events following single dose treatment of lymphatic filariasis: Observations from a review of the literature. PLoS Negl Trop Dis 2018, 12, e0006454. [Google Scholar] [CrossRef]
- Shipstone, M. Antiparasitic Drugs for Integumentary Disease in Animals. MSD Veterinary Manual. Available online: https://www.msdvetmanual.com/pharmacology/systemic-pharmacotherapeutics-of-the-integumentary-system/antiparasitic-drugs-for-integumentary-disease-in-animals#Ivermectin_v3331036 (accessed on 17 April 2025).
- Byers, J.P.; Sarver, J.G. Chapter 10: Pharmacokinetic modeling, In: Pharmacology; Hacker, M., Messer, W., Bachmann, K., Eds.; Academic Press: Cambridge, Massachusetts, United States, 2009; pp. 201–277. [Google Scholar] [CrossRef]
- Rowland Yeo, K.; Wesche, D. PBPK modeling of ivermectin—Considerations for the purpose of developing alternative routes to optimize its safety profile. CPT: Pharmacometrics Syst. Pharmacol. 2023, 12, 598–609. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.N.; Torres, S.M.; Plumb, D.C. Section 1: Systemic Drugs. In Canine And Feline Dermatology Drug Handbook; Koch, S.N., Torres, S.M., Plumb, D.C., Eds.; Blackwell Publishing: Hoboken, New Jersey, United States, 2012; pp. 1–218. [Google Scholar]
- Löscher, W. Is the antiparasitic drug ivermectin a suitable candidate for the treatment of epilepsy? Epilepsia 2023, 64, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Rendic, S.P. Metabolism and interactions of Ivermectin with human cytochrome P450 enzymes and drug transporters, possible adverse and toxic effects. Arch. Toxicol. 2021, 95, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Udaykumar, P.; Shetty, B.; Kundapur, A. Drug interactions of ivermectin with a focus on COVID-19 treatment. Muller Journal of Medical Sciences and Research 2021, 12, 42–48. [Google Scholar] [CrossRef]
- Jagodinsky, J.C.; Akgun, U. Characterizing the binding interactions between P-glycoprotein and eight known cardiovascular transport substrates. Pharmacol. Res. Perspect. 2015, 3, e00114. [Google Scholar] [CrossRef]
- González Canga, A.; Sahagún Prieto, A.M.; Diez Liébana, M.J.; Fernández Martínez, N.; Sierra Vega, M.; García Vieitez, J.J. The pharmacokinetics and interactions of ivermectin in humans—A mini-review. AAPS. J. 2008, 10, 42–46. [Google Scholar] [CrossRef]
- Jittamala, P.; Monteiro, W.; Smit, M.R.; Pedrique, B.; Specht, S.; Chaccour, C.J.; Dard, C.; Del Giudice, P.; Khieu, V.; Maruani, A.; Failoc-Rojas, V.E. A systematic review and an individual patient data meta-analysis of ivermectin use in children weighing less than fifteen kilograms: Is it time to reconsider the current contraindication? PLoS Negl. Trop. Dis. 2021, 15, e0009144. [Google Scholar] [CrossRef]
- Wood, N.D.; Smith, D.; Kinrade, S.A.; Sullivan, M.T.; Rayner, C.R.; Wesche, D.; Patel, K.; Rowland-Yeo, K. The use of quantitative clinical pharmacology approaches to support moxidectin dosing recommendations in lactation. PLOS Negl. Trop. Dis. 2024, 18, e0012351. [Google Scholar] [CrossRef]
- Pfarr, K.M.; Krome, A.K.; Al-Obaidi, I.; Batchelor, H.; Vaillant, M.; Hoerauf, A.; Opoku, N.O.; Kuesel, A.C. The pipeline for drugs for control and elimination of neglected tropical diseases: 2. Oral anti-infective drugs and drug combinations for off-label use. Parasit. Vectors 2023, 16, 394. [Google Scholar] [CrossRef]
- Hanafy, A.S.; Abd-Elsalam, S. Challenges in COVID-19 drug treatment in patients with advanced liver diseases: A hepatology perspective. World J. Gastroenterol. 2020, 26, 7272. [Google Scholar] [CrossRef]
- Oscanoa, T.J.; Amado, J.; Romero-Ortuno, R.; Carvajal, A. Hepatic disorders associated with the use of Ivermectin for SARS-CoV-2 infection in adults: A pharmacovigilance study in VigiBase. Gastroenterol. Hepatol. Bed Bench 2022, 15, 426. [Google Scholar] [CrossRef]
- Nunes, L.L.A.; Lima, T.D.M. Use of medicines for covid-19 treatment in patients with loss of kidney function: A narrative review. Braz. J. Nephrol. 2020, 43, 254–262. [Google Scholar] [CrossRef]
- Sonderup, M.W.; Mudini, W.; Spearman, C.W.N. Ivermectin drug-induced liver injury. S. Afr. Med. J. 2023, 113, 1203–1204. [Google Scholar] [CrossRef]
- Badia, B.D.M.L.; de Lima Serrano, P.; Barile, J.P.; Seneor, D.D.; Mendes, P.M.; Cavalheiro, R.B.R.; Peixoto, K.O.; Farias, I.B.; Machado, R.I.L.; de Rezende Pinto, W.B.V.; Oliveira, A.S.B. Practical Recommendations in the Treatment of Acute and Chronic Life-Threatening Infectious Diseases in Patients with Acute Hepatic Porphyria. Metabolites 2025, 15, 99. [Google Scholar] [CrossRef]
- Abou El-Fetouh, M.S.; Elseddawy, N.M.; Abdelsamia, H.M. Insights on the therapeutic use of ivermectin: Mechanism of action and histopathological effects. JAVR 2024, 14, 339–341, https://www.advetresearch.com/index.php/AVR/article/view/1530 (accessed 25 May 2024). [Google Scholar]
- Phatale, V.; Vaiphei, K.K.; Jha, S.; Patil, D.; Agrawal, M.; Alexander, A. Overcoming skin barriers through advanced transdermal drug delivery approaches. J. Control. Release 2022, 351, 361–380. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.; Nava, S.; Miró, V.; Canton, C.; Alvarez, L.; Lanusse, C. Macrocyclic lactones and ectoparasites control in livestock: Efficacy, drug resistance and therapeutic challenges. Int. J. Parasitol. Drugs Drug Resist. 2024, 26, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Suderman, M.T.; Craig, T.M. Efficacy of ivermectin against Dirofilaria immitis microfilariae in naturally infected dogs. Am. J. Vet. Res. 1984, 45, 1031–1032. [Google Scholar] [CrossRef]
- Yazwinski, T.A. Use of febantel or ivermectin for treatment of calves with experimentally induced Bunostomum phlebotomum infection. Am. J. Vet. Res. 1988, 49, 1407–1408. [Google Scholar] [CrossRef] [PubMed]
- Rehbein, S.; Batty, A.F.; Barth, D.; Visser, M.; Timms, B.J.; Barrick, R.A.; Eagleson, S. Efficacy of an ivermectin controlled-release capsule against nematode and arthropod endoparasites in sheep. Vet. Rec. 1998, 142, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Forbes, A.B.; Pitt, S.R.; Baggott, D.G.; Rehbein, S.; Barth, D.; Bridi, A.A.; Carvalho, L.A.; O’Brien, D.J. A review of the use of a controlled-release formulation of ivermectin in the treatment and prophylaxis of Psoroptes ovis infestations in sheep. Vet. Parasitol. 1999, 83, 319–326. [Google Scholar] [CrossRef]
- Alva-Valdes, R.; Wallace, D.H.; Benz, G.W.; Foster, A.G.; Holste, J.E. Efficacy of ivermectin against the mange mite Sarcoptes scabiei var suis in pigs. Am J Vet Res 1984, 45, 2113–2114. [Google Scholar] [CrossRef]
- Bridi, A.A.; Carvalho, L.A.; Cramer, L.G.; Barrick, R.A. Efficacy of a long-acting formulation of ivermectin against Psoroptes ovis (Hering, 1838) on cattle. Vet Parasitol 2001, 97, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Ohba, S.; Toriumi, H.; Takeishi, M.; Noda, R. Efficacy of ivermectin against live mites and eggs of Sarcoptes scabiei in pigs. Jpn. J. Vet. Sci. 1989, 51, 981–985. [Google Scholar] [CrossRef]
- Soll, M.D.; D’Assonville, J.A.; Smith, C.J.Z. Efficacy of topically applied ivermectin against sarcoptic mange (Sarcoptes scabiei var. bovis) of cattle. Parasitol. Res. 1992, 78, 120–122. [Google Scholar] [CrossRef]
- Schulz, J.D.; Coulibaly, J.T.; Schindler, C.; Wimmersberger, D.; Keiser, J. Pharmacokinetics of ascending doses of ivermectin in Trichuris trichiura-infected children aged 2-12 years. J. Antimicrob. Chemother. 2019, 74, 1642–1647. [Google Scholar] [CrossRef]
- Lu, M.; Xiong, D.; Sun, W.; Yu, T.; Hu, Z.; Ding, J.; Cai, Y.; Yang, S.; Pan, B. Sustained release ivermectin-loaded solid lipid dispersion for subcutaneous delivery: In vitro and in vivo evaluation. Drug Deliv. 2017, 24, 622–631. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Dou, D.; Li, X.; Zhang, Q.; Bhutto, Z.A.; Wang, L. Ivermection-loaded solid lipid nanoparticles: Preparation, characterisation, stability and transdermal behaviour. Artif. Cells Nanomed. Biotechnol. 2018, 46, 255–262. [Google Scholar] [CrossRef]
- Steenekamp, E.M.; Liebenberg, W.; Lemmer, H.J.R.; Gerber, M. Formulation and Ex Vivo Evaluation of Ivermectin Within Different Nano-Drug Delivery Vehicles for Transdermal Drug Delivery. Pharmaceutics 2024, 16, 1466. [Google Scholar] [CrossRef]
- Rahnfeld, L.; Luciani, P. Injectable lipid-based depot formulations: Where do we stand? Pharmaceutics 2020, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.J. The efficacy of ivermectin against the eyeworm, Thelazia skrjabini, in experimentally infected cattle. Vet. Parasitol. 1992, 45, 127–131. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Holste, J.E.; Jacobsen, J.A. The efficacy of ivermectin (pour-on) against the eyeworms, Thelazia gulosa and Thelazia skrjabini in naturally infected cattle. Vet. Parasitol. 1994, 55, 263–266. [Google Scholar] [CrossRef]
- MSD Veterinary Manual. Onchocerciasis in Animals. Available online: https://www.msdvetmanual.com/integumentary-system/helminths-of-the-skin/onchocerciasis-in-animals (accessed on 18 August 2023).
- Britt, D.P.; Preston, J.M. Efficacy of ivermectin against Dictyocaulus arnfieldi in ponies. Vet Rec 1985, 116, 343–345. [Google Scholar] [CrossRef]
- Herd, R.P.; Donham, J.C. Efficacy of ivermectin against Onchocerca cervicalis microfilarial dermatitis in horses. Am. J. Vet. Res. 1983, 44, 1102–1105. [Google Scholar] [CrossRef]
- French, D.D.; Klei, T.M.; Foil, C.S.; Miller, R.I.; Foil, L.D.; Chapman, M.R.; McClure, J.J. Efficacy of ivermectin in paste and injectable formulations against microfilariae of Onchocerca cervicalis and resolution of associated dermatitis in horses. Am. J. Vet. Res. 1988, 49, 1550–1554. [Google Scholar] [CrossRef]
- Schröder, J.; Swan, G.E.; Soll, M.D.; Hotson, I.K. Efficacy of ivermectin against ectoparasites of cattle in South Africa. J. S. Afr. Vet. Assoc 1985, 56, 31–35. [Google Scholar]
- Da Silva, C.F.; Almeida, T.; de Melo Barbosa, R.; Cardoso, J.C.; Morsink, M.; Souto, E.B.; Severino, P. New trends in drug delivery systems for veterinary applications. Pharm. Nanotechnol. 2021, 9, 15–25. [Google Scholar] [CrossRef]
- Alabaster, V. The fall and rise of in vivo pharmacology. Trends Pharmacol Sci 2002, 23, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Workman, P.; Aboagye, E.O.; Balkwill, F.; Balmain, A.; Bruder, G.; Chaplin, D.J.; Double, J.A.; Everitt, J.; Farningham, D.A.H.; Glennie, M.J.; Kelland, L.R. Guidelines for the welfare and use of animals in cancer research. Br. J. Cancer 2010, 102, 1555–1577. [Google Scholar] [CrossRef] [PubMed]
- Madrid, R.R.; Mathews, P.D.; Patta, A.C.; Gonzales-Flores, A.P.; Ramirez, C.A.; Rigoni, V.L.; Tavares-Dias, M.; Mertins, O. Safety of oral administration of high doses of ivermectin by means of biocompatible polyelectrolytes formulation. Heliyon 2021, 7, e05820. [Google Scholar] [CrossRef] [PubMed]
- Archana, N.K.; Gond, V.; Kumar, S.; Singh, S.; Jayachandran, C. Clinico-haematobiochemical profile after repeated subcutaneous administration of ivermectin in goats. J Vet Pharmacol Ther 2013, 12, 79–81. [Google Scholar] [CrossRef]
- Al-Azzam, S.I.; Fleckenstein, L.; Cheng, K.J.; Dzimianski, M.T.; McCall, J.W. Comparison of the pharmacokinetics of moxidectin and ivermectin after oral administration to beagle dogs. Biopharm Drug Dispos 2007, 28, 431–438. [Google Scholar] [CrossRef]
- Sartini, I.; Łebkowska-Wieruszewska, B.; Krupa, M.; Lisowski, A.; Poapolathep, A.; Giorgi, M. Pharmacokinetics of ivermectin after oral and intravenous administration in Biłgorajska geese (Anser anser domesticus). N. Z. Vet. J. 2022, 70, 313–318. [Google Scholar] [CrossRef]
- Shu, E.N.; Okonkwo, P.O. The Pharmacokinetics of Ivermectin in Rabbit. Orient J. Med. 2003, 15, 42–45. [Google Scholar] [CrossRef]
- Vanachayangkul, P.; Im-Erbsin, R.; Tungtaeng, A.; Kodchakorn, C.; Roth, A.; Adams, J.; Chaisatit, C.; Saingam, P.; Sciotti, R.J.; Reichard, G.A.; Nolan, C.K. Safety, pharmacokinetics, and activity of high-dose ivermectin and chloroquine against the liver stage of Plasmodium cynomolgi infection in rhesus macaques. Antimicrob. Agents Chemother. 2020, 64, 10–11. [Google Scholar] [CrossRef] [PubMed]
- Buckley, S.T.; Fischer, S.M.; Fricker, G.; Brandl, M. In vitro models to evaluate the permeability of poorly soluble drug entities: Challenges and perspectives. Eur J Pharm Sci 2012, 45, 235–250. [Google Scholar] [CrossRef]
- Porat, D.; Dahan, A. Active intestinal drug absorption and the solubility-permeability interplay. Int. J. Pharm. 2018, 537, 84–93. [Google Scholar] [CrossRef]
- Van der Merwe, J.; Steenekamp, J.; Steyn, D.; Hamman, J. The role of functional excipients in solid oral dosage forms to overcome poor drug dissolution and bioavailability. Pharmaceutics 2020, 12, 393. [Google Scholar] [CrossRef]
- Ponte, M.; Liebenberg, W.; Gerber, M. Formulation and in vitro skin diffusion of colchicine using different drug delivery vehicles. J. Drug Delivery Sci. Technol. 2023, 88, 10489. [Google Scholar] [CrossRef]
- Steyn, J.D.; Haasbroek-Pheiffer, A.; Pheiffer, W.; Weyers, M.; van Niekerk, S.E.; Hamman, J.H.; van Staden, D. Evaluation of Drug Permeation Enhancement by Using In Vitro and Ex Vivo Models. Pharmaceuticals 2025, 18, 195. [Google Scholar] [CrossRef] [PubMed]
- Medi Media. Ivermectin (systemic): Drug information. Available online: https://medilib.ir/uptodate/show/83315 (accessed on 28 May 2024).
- South African Health Products Regulatory Authority (SAHPRA). SAHPRA registers Soolantra 10mg/g cream – an ivermectin formulation. Available online: https://www.sahpra.org.za/press-releases/sahpra-registers-soolantra-10mg-g-cream-an-ivermectin-formulation/ (accessed on 28 May 2024).
- Errecalde, J.; Lifschitz, A.; Vecchioli, G.; Ceballos, L.; Errecalde, F.; Ballent, M.; Marín, G.; Daniele, M.; Turic, E.; Spitzer, E.; Toneguzzo, F. Safety and pharmacokinetic assessments of a novel ivermectin nasal spray formulation in a pig model. J. Pharm. Sci. 2021, 110, 2501–2507. [Google Scholar] [CrossRef]
- Desu, H.R.; Narang, A.S.; Kumar, V.; Thoma, L.A.; Mahato, R.I. Chapter 10: Liquid dosage forms. In Pharmaceutics, 2nd ed.; Dash, A.K., Singh, S., Eds.; Academic Press: Cambridge, Massachusetts, United States, 2024; pp. 271–318. [Google Scholar] [CrossRef]
- Maggi, L.; Friuli, V.; Perugini, P.; Musitelli, G.; Venco, L. Dosage variability of veterinary drug products, containing furosemide, linked to tablet splitting. Open Vet. J. 2021, 11, 471–482. [Google Scholar] [CrossRef]
- Walsh, J.; Ranmal, S.R.; Ernest, T.B.; Liu, F. Patient acceptability, safety and access: A balancing act for selecting age-appropriate oral dosage forms for paediatric and geriatric populations. Int. J. Pharm. 2018, 536, 547–562. [Google Scholar] [CrossRef]
- Lin, T.Y.; Rodriguez Jr, C.O.; Li, Y. Nanomedicine in veterinary oncology. Vet. J. 2015, 205, 189–197. [Google Scholar] [CrossRef]
- Van Staden, D.; Gerber, M.; Lemmer, H.J.R. The Application of Nano Drug Delivery Systems in Female Upper Genital Tract Disorders. Pharmaceutics 2024, 16, 1475. [Google Scholar] [CrossRef] [PubMed]
- Mahato, R.I.; Narang, A.S. Chapter 18: Pharmaceutical Solutions. In Pharmaceutical Dosage Forms and Drug Delivery: Revised and Expanded, 3rd ed.; Mahato, R.I., Narang, A.S., Eds.; CRC Press: Boca Raton, Florida, United States, 2017; pp. 425–426. [Google Scholar]
- Martinez, M.N.; Papich, M.G.; Fahmy, R. Impact of gastrointestinal differences in veterinary species on the oral drug solubility, in vivo dissolution, and formulation of veterinary therapeutics. ADMET DMPK 2022, 10, 1–25. [Google Scholar] [CrossRef]
- Mestorino, N.; Turic, E.; Pesoa, J.; Echeverría, J.; Errecalde, J.O. Pharmacokinetics in plasma of ivermectin after its oral (solution and tablets) administration to sheep. J. Vet. Pharmacol. Ther. 2003, 26, 307–309. [Google Scholar] [CrossRef] [PubMed]
- Barker, S.A. . Chapter 26: Suspensions, In: Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 407–423. [Google Scholar]
- Murdan, S. Chapter 24: Solutions. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 386–396. [Google Scholar]
- Brako, F.; Boateng, J. Transmucosal drug delivery: Prospects, challenges, advances, and future directions. Expert Opin Drug Deliv 2025, 22, 525–553. [Google Scholar] [CrossRef]
- Alderborn, G.; Frenning, G. Chapter 31: Tablets and compaction. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 501–541. [Google Scholar]
- Jones, B.E. Chapter 35: Hard capsules. In Aulton’s Pharmaceutics: The Design and Manufacture of Medicines, 6th ed.; Aulton, M.E., Taylor, K.M.G., Eds.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021; pp. 586–598. [Google Scholar]
- Muñoz, J.; Ballester, M.R.; Antonijoan, R.M.; Gich, I.; Rodríguez, M.; Colli, E.; Gold, S.; Krolewiecki, A.J. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl. Trop. Dis. 2018, 12, e0006020. [Google Scholar] [CrossRef]
- Sjöholm, E.; Mathiyalagan, R.; Wang, X.; Sandler, N. Compounding tailored veterinary chewable tablets close to the point-of-care by means of 3D printing. Pharmaceutics 2022, 14, 1339. [Google Scholar] [CrossRef]
- Thombre, A.G. Oral delivery of medications to companion animals: Palatability considerations. Adv. Drug Deliv. Rev. 2004, 56, 1399–1413. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.J.; Todd, K.S.; Acre, K.E.; Plue, R.E.; Wallace, D.H.; French, R.A.; Wallig, M.A. Efficacy of ivermectin chewable tablets and two new ivermectin tablet formulations against Dirofilaria immitis larvae in dogs. Am. J. Vet. Res. 1991, 52, 1922–1923. [Google Scholar] [CrossRef]
- Canga, A.G.; Prieto, A.M.S.; Liébana, M.J.D.; Martínez, N.F.; Vega, M.S.; Vieitez, J.J.G. The pharmacokinetics and metabolism of ivermectin in domestic animal species. Vet J 2009, 179, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Gogolewski, R.P.; Allerton, G.R.; Langholff, W.K.; Cramer, L.G.; Eagleson, J.S. An ivermectin tablet for sheep: Efficacy against gastro-intestinal nematodes and a bioavailability comparison with a liquid ivermectin formulation. Vet. Parasitol. 1995, 60, 297–302. [Google Scholar] [CrossRef]
- Al-Obaidi, I.; Krome, A.K.; Wagner, K.G.; Pfarr, K.; Kuesel, A.C.; Batchelor, H.K. Drugs for neglected tropical diseases: Availability of age-appropriate oral formulations for young children. Parasit Vectors 2022, 15, 462. [Google Scholar] [CrossRef] [PubMed]
- Del Moral Sanchez, J.M.; Gonzalez-Alvarez, I.; Cerda-Revert, A.; Gonzalez-Alvarez, M.; Navarro-Ruiz, A.; Amidon, G.L.; Bermejo, M. Biopharmaceutical optimization in neglected diseases for paediatric patients by applying the provisional paediatric biopharmaceutical classification system. Br J Clin Pharmacol 2018, 84, 2231–2241. [Google Scholar] [CrossRef]
- Juan, C.; Rodriguez, D.; Ceballos, L.; Lanusse, C.; Gallo, L.; Gonzalez Vidal, N. Development of ivermectin orally disintegrating tablets using factorial design: In-vitro evaluation and in vivo absorption pattern in rats. J Drug Deliv Sci Technol 2023, 87, 104757. [Google Scholar] [CrossRef]
- Wei, S.; Yue, H.; Li, G.; Sang, N. Particulate matter induces airway epithelial barrier dysfunction in vivo and in vitro: From a more realistic inhalation scenario. Environ. Sci.: Nano 2022, 9, 2665–2677. [Google Scholar] [CrossRef]
- Lam, J.K.W.; Cheung, C.C.K.; Chow, M.Y.T.; Harrop, E.; Lapwood, S.; Barclay, S.I.G.; Wong, I.C.K. Transmucosal drug administration as an alternative route in palliative and end-of-life care during the COVID-19 pandemic. Adv Drug Deliv Rev 2020, 160, 234–243. [Google Scholar] [CrossRef]
- Khadka, P.; Ro, J.; Kim, H.; Kim, I.; Kim, J.T.; Kim, H.; Cho, J.M.; Yun, G.; Lee, J. Pharmaceutical particle technologies: An approach to improve drug solubility, dissolution and bioavailability. Asian J Pharm Sci 2014, 9, 304–316. [Google Scholar] [CrossRef]
- Morton, D.A.V.; Barling, D. Developing Dry Powder Inhaler Formulations. J Aerosol Med Pulm Drug Deliv 2024, 37, 90–99. [Google Scholar] [CrossRef]
- Oldham, J.; Sahota, A.; O’Toole, E.; Cunningham, M. P095 Ten cases of scabies successfully treated with topical ivermectin. Br J Dermatol 2024, 191, i60–i60. [Google Scholar] [CrossRef]
- Paradis, M.; de Jaham, C.; Pagé, N. Topical (pour-on) ivermectin in the treatment of canine scabies. Can Vet J 1997, 38, 379–382. [Google Scholar]
- Smith, M.; Wolffsohn, J.S.; Chiang, J.C.B. Topical ivermectin 1. 0% cream in the treatment of ocular demodicosis. Cont. Lens Anterior Eye 2023, 47, 102099. [Google Scholar] [CrossRef]
- Zargari, O.; Aghazadeh, N.; Moeineddin, F. Clinical applications of topical ivermectin in dermatology. Dermatol. Online J. 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Aucamp, Z.; Liebenberg, W.; Lemmer, H.J.; Gerber, M. Formulation of semi-solid dosage forms intended for transdermal delivery of ivermectin. J Drug Deliv Sci Technol 2024, 101, 106174. [Google Scholar] [CrossRef]
- Nakatsuji, T.; Cheng, J.Y.; Butcher, A.; Shafiq, F.; Osuoji, O.; Gallo, R.L.; Hata, T.R. Topical ivermectin treatment of rosacea changes the bacterial microbiome of the skin. J. Investig. Dermatol. 2025, 145, 1226–1228. [Google Scholar] [CrossRef]
- Victoria, J.; Trujillo, R. Topical ivermectin: A new successful treatment for scabies. Pediatr. Dermatol. 2001, 18, 63–65. [Google Scholar] [CrossRef]
- Sim, S.; Wong, N.K. Nanotechnology and its use in imaging and drug delivery (Review). Biomed Rep 2021, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Morad, R. Coating of Remdesivir and Ivermectin on silver nanoparticles: A density functional theory and molecular dynamics study. Results Surfaces Interfaces 2025, 19, 100540. [Google Scholar] [CrossRef]
- Goharshadi, E.K.; Goharshadi, K.; Moghayedi, M. The use of nanotechnology in the fight against viruses: A critical review. Coord Chem Rev 2022, 464, 214559. [Google Scholar] [CrossRef]
- Babadi, D.; Dadashzadeh, S.; Osouli, M.; Daryabari, M.S.; Haeri, A. Nanoformulation strategies for improving intestinal permeability of drugs: A more precise look at permeability assessment methods and pharmacokinetic properties changes. J Control Release 2020, 321, 669–709. [Google Scholar] [CrossRef]
- Kumari, S.; Goyal, A.; Sönmez Gürer, E.; Algın Yapar, E.; Garg, M.; Sood, M.; Sindhu, R.K. Bioactive loaded novel nano-formulations for targeted drug delivery and their therapeutic potential. Pharmaceutics 2022, 14, 1091. [Google Scholar] [CrossRef]
- Gamboa, G.U.; Palma, S.D.; Lifschitz, A.; Ballent, M.; Lanusse, C.; Passirani, C.; Benoit, J.P.; Allemandi, D.A. Ivermectin-loaded lipid nanocapsules: Toward the development of a new antiparasitic delivery system for veterinary applications. Parasitol. Res. 2016, 115, 1945–1953. [Google Scholar] [CrossRef]
- Xu, X.; Gao, S.; Zuo, Q.; Gong, J.; Song, X.; Liu, Y.; Xiao, J.; Zhai, X.; Sun, H.; Zhang, M.; Gao, X. Enhanced in vitro antiviral activity of ivermectin-loaded nanostructured lipid carriers against porcine epidemic diarrhea virus via improved intracellular delivery. Pharmaceutics 2024, 16, 601. [Google Scholar] [CrossRef]
- Czajkowska-Kośnik, A.; Szekalska, M.; Winnicka, K. Nanostructured lipid carriers: A potential use for skin drug delivery systems. Pharmacol. Rep. 2019, 71, 156–166. [Google Scholar] [CrossRef]
- Dave, K.; Krishna Venuganti, V.V. Dendritic polymers for dermal drug delivery. Ther. Deliv. 2017, 8, 1077–1096. [Google Scholar] [CrossRef]
- Tanner, T.; Marks, R.J.S.R. Delivering drugs by the transdermal route: Review and comment. Skin Res. Tech. 2008, 14, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Mohite, P.; Singh, S.; Pawar, A.; Sangale, A.; Prajapati, B.G. Lipid-based oral formulation in capsules to improve the delivery of poorly water-soluble drugs. Front Drug Deliv. 2023, 3, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Rajput, A.; Pingale, P.; Telange, D.; Chalikwar, S.; Borse, V. Lymphatic transport system to circumvent hepatic metabolism for oral delivery of lipid-based nanocarriers. J Drug Deliv Sci Technol 2021, 66, 102934. [Google Scholar] [CrossRef]
- Yan, S.; Cheng, Y.; Li, L.; Zhong, C.; Chen, C.; Gao, X. Lipid-based formulations: A promising approach for poorly soluble drug delivery via the intestinal lymphatic system. J. Drug Deliv. Sci. Technol. 2023, 87, 104770. [Google Scholar] [CrossRef]
- Basak, R.; Bandyopadhyay, R. Encapsulation of hydrophobic drugs in Pluronic F127 micelles: Effects of drug hydrophobicity, solution temperature, and pH. Langmuir 2013, 29, 4350–4356. [Google Scholar] [CrossRef] [PubMed]
- Cholakova, D.; Vinarov, Z.; Tcholakova, S.; Denkov, N.D. Self-emulsification in chemical and pharmaceutical technologies. Curr. Opin. Colloid Interface Sci. 2022, 59, 101576. [Google Scholar] [CrossRef]
- Patel, V.; Lakkad, H.; Ashara, K. Formulation studies of solid self-emulsifying drug de-livery system of ivermectin. Folia Med.(Plovdiv) 2018, 60, 580–593. [Google Scholar] [CrossRef]
- Bennuru, S.; Nutman, T.B. Lymphatics in Human Lymphatic Filariasis: In Vitro Models of Parasite-Induced Lymphatic Remodeling. Lymphat Res Biol 2009, 7, 215–219. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.R.; Ricci, F.M.; Ottesen, E.A. Ivermectin: Effectiveness in lymphatic filariasis. Parasitol 2000, 121, S133–S146. [Google Scholar] [CrossRef]
- Failoc-Rojas, V.E.; Silva-Díaz, H.; Maguiña, J.L.; Rodriguez-Morales, A.J.; Díaz-Velez, C.; Apolaya-Segura, M.; Valladares-Garrido, M.J. Evidence-based indications for ivermectin in parasitic diseases: An integrated approach to context and challenges in Peru. Parasite Epidemiol Control 2023, 23, e00320. [Google Scholar] [CrossRef]
- Gowtham, S.; Karthikeyan, K. Wonder drug for worms: A review of three decades of ivermectin use in dermatology. Indian J. Dermatol. Venereol. Leprol. 2019, 85, 674–678. [Google Scholar] [CrossRef]
- Dy, J.H.; Juangco, D.N. Identifying the Toxidrome of Ivermectin Toxicity. Cureus 2023, 15, e42603. [Google Scholar] [CrossRef]
- Hoang, R.; Temple, C.; Correia, M.S.; Clemons, J.; Hendrickson, R.G. Characteristics of ivermectin toxicity in patients taking veterinary and human formulations for the prevention and treatment of COVID-19. Clin. Toxicol. 2022, 60, 1350–1355. [Google Scholar] [CrossRef]
- Brown, M.B.; Martin, G.P.; Jones, S.A.; Akomeah, F.K. Dermal and transdermal drug delivery systems: Current and future prospects. Drug Deliv 2006, 13, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.T.; Gerber, M.; Plessis, J.D.; Hamman, J.H. Transdermal drug delivery enhancement by compounds of natural origin. Molecules 2011, 16, 10507–10540. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Essential oils and their constituents as skin penetration enhancer for transdermal drug delivery: A review. J. Pharmacy Pharmacol. 2015, 67, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved topical drug delivery: Role of permeation enhancers and advanced approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; Ghogale, P.P. Polymorphs, salts, and cocrystals: What’s in a name? Cryst Growth Des 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Dhondale, M.R.; Nambiar, A.G.; Singh, M.; Mali, A.R.; Agrawal, A.K.; Shastri, N.R.; Kumar, P.; Kumar, D. Current trends in API co-processing: Spherical crystallization and co-precipitation techniques. J. Pharm. Sci. 2023, 112, 2010–2028. [Google Scholar] [CrossRef]
- Qiu, Y.; Zhang, G. Development of Modified-Release Solid Oral Dosage Forms. In Developing Solid Oral Dosage Forms. In Developing Solid Oral Dosage Forms; Qiu, Y., Chen, Y., Zhang, G.G.Z., Liu, L., Porter, W.R., Eds.; Academic Press: Cambridge, Massachusetts, United States, 2009; pp. 501–517. [Google Scholar]
- Van Staden, D.; du Plessis, J.; Viljoen, J. Development of topical/transdermal self-emulsifying drug delivery systems, not as simple as expected. Sci Pharm 2020, 88, 1–24. [Google Scholar] [CrossRef]
- Albert Lo, P.K.; Fink, D.W.; Williams, J.B.; Blodinger, J. Pharmacokinetic studies of ivermectin: Effects of formulation. Vet Res Commun 1985, 9, 251–268. [Google Scholar] [CrossRef]
- King, T.A. The One Medicine concept: Its emergence from history as a systematic approach to re-integrate human and veterinary medicine. Emerg Top Life Sci 2021, 5, 643–654. [Google Scholar] [CrossRef]
- Lifschitz, A.; Pis, A.; Alvarez, L.; Virkel, G.; Sanchez Bruni, S.F.; Sallovitz, J.M.; Kujanek, R.; Lanusse, C.E. Bioequivalence of ivermectin formulations in pigs and cattle. J. Vet. Pharmacol. Ther. 1999, 22, 27–34. [Google Scholar] [CrossRef] [PubMed]
| Country | Registered IVM trade product(s) |
| United Arab Emirates | Imectin |
| Argentina | Ivertal; Vermectin; Ivermectina Monserrat éclair; Ivercass; Securo; Iver p |
| Austria | Scabioral; Ivergelan |
| Australia | Stromectol |
| Bangladesh | Veratin; Ivacure; Avemac; Iverum; A mectin; Ivactin; Imec; Alice; Parakil |
| Belgium | Ivermectin substipharm |
| Bulgaria | Huvemec |
| Brazil | Iverneo; Revectina; Vermectil; Ivermec; Leverctin; Ivermectina; Plurimec; Iverliv; Uciose |
| Chile | Kaonol |
| China | Hai zheng mai ke ding |
| Czech Republic | Loutol |
| Germany | Ivermectin carefarm; Driponin; Ivermectin Padia; Iveraxiro; Scabioral |
| Dominican Republic | Ivermectina; Ivexterm; Ivermectina mamey; Ivermectina calox |
| Ecuador | Ivermin; Amectin; Ivermectina; Comviral |
| Estonia | Stromectol; Ivermectine arrow lab |
| Egypt | Iverzine; Ivactin; Razimectin |
| Spain | Ivergalen; Ivercare |
| France | Ivermectine Zentiva; Ivermectine arrow; Ivermectine sandoz; Ivermectine cristers; Ivermectine mylan; Ivermectine eg; Ivermectine pierre fabre; Iverscal; Ivermectine biogaran; Stromectol |
| Finland | Scatol; Ivermectin medical valley |
| United Kingdom | Stromectol |
| Greece | Scaball |
| Hong Kong | Stromectol |
| Indonesia | Mectinsanbe; Ivercov |
| India | Ivertero; Afdiver; Covimac; Vermac; Itin; Covidmectin; Ivecop; Mectin; Ivepack; Isco; Ivercid; Vermectin; Iverlin; Iver sol; Ivermectol; Viomectin; Iverpil; Ivernock; Iversure; Ivor; Ivercoast; Ivscab; Scavista; Iverzen; Tough; Iversurge; Vermact; Vimect |
| Italy | Stromectol; Iverscab |
| Japan | Stromectol |
| Kenya | Ivermectol |
| Republic of Korea | Iverin |
| Lebanon | Ivermectine biogaran; Ivermectine; Ivactin; Iverzine; Iver p |
| Lithuania | Stromectol; Scabioral |
| Latvia | Stromectol |
| Mexico | Ivermectina; Stromectol; Ivexterm; Veridex |
| Malaysia | Ivermectol |
| Netherlands | Stromectol; Ivermectine xiromed |
| Norway | Ivermectin medical valley; Stromectol; Driponin; Stromectol specific; Scatol |
| New Zealand | Stromectol |
| Pakistan | Iverest; Iverterm; Norm; Everlite; Iveratan; Ivermite; Mectis; Felvot; Suint |
| Peru | Kaonol |
| Poland | Posela; Ivermectin medical valley |
| Puerto Rico | Stromectol |
| Portugal | Stromectol; Mectizan |
| Paraguay | Vivermet; Ivermectina dutriec; Kaonol; Ivermectina guayaki; Yvermil |
| Sweden | Scatol; Ivermectin medical valley |
| Singapore | Stromectol |
| Slovenia | Stromectol; Scabioral |
| Slovakia | Ivermectin exeltis |
| Sierra Leone | Mectizan |
| Turkey | Ziver |
| Taiwan | Stromectol |
| Uruguay | Ivermectina Athena; Iver 6; Sanifer; Ivermectina |
| Bolivarian Republic of Venezuela | Ivergot; Ivertal; Ivermectina; Iverwell |
| Vietnam | Ivermectin nic |
| South Africa | Soolantra*; Iladek |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





