Submitted:
22 September 2025
Posted:
24 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
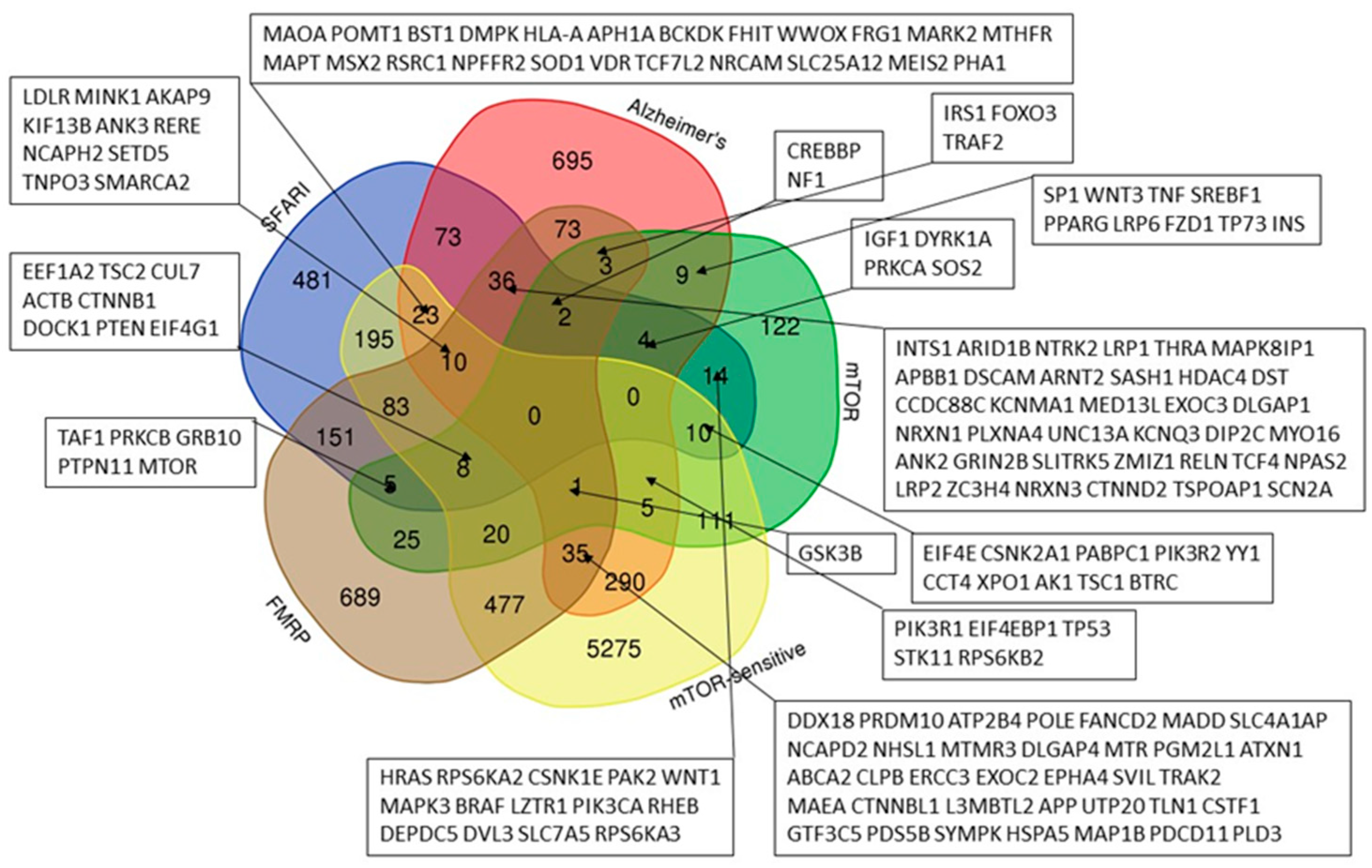
2.1. SFARI Gene Database and AD Genes Comparative Gene-Set and Pathway Analysis
2.2. Analysis of Evolutionary Characteristics of Gene Sets
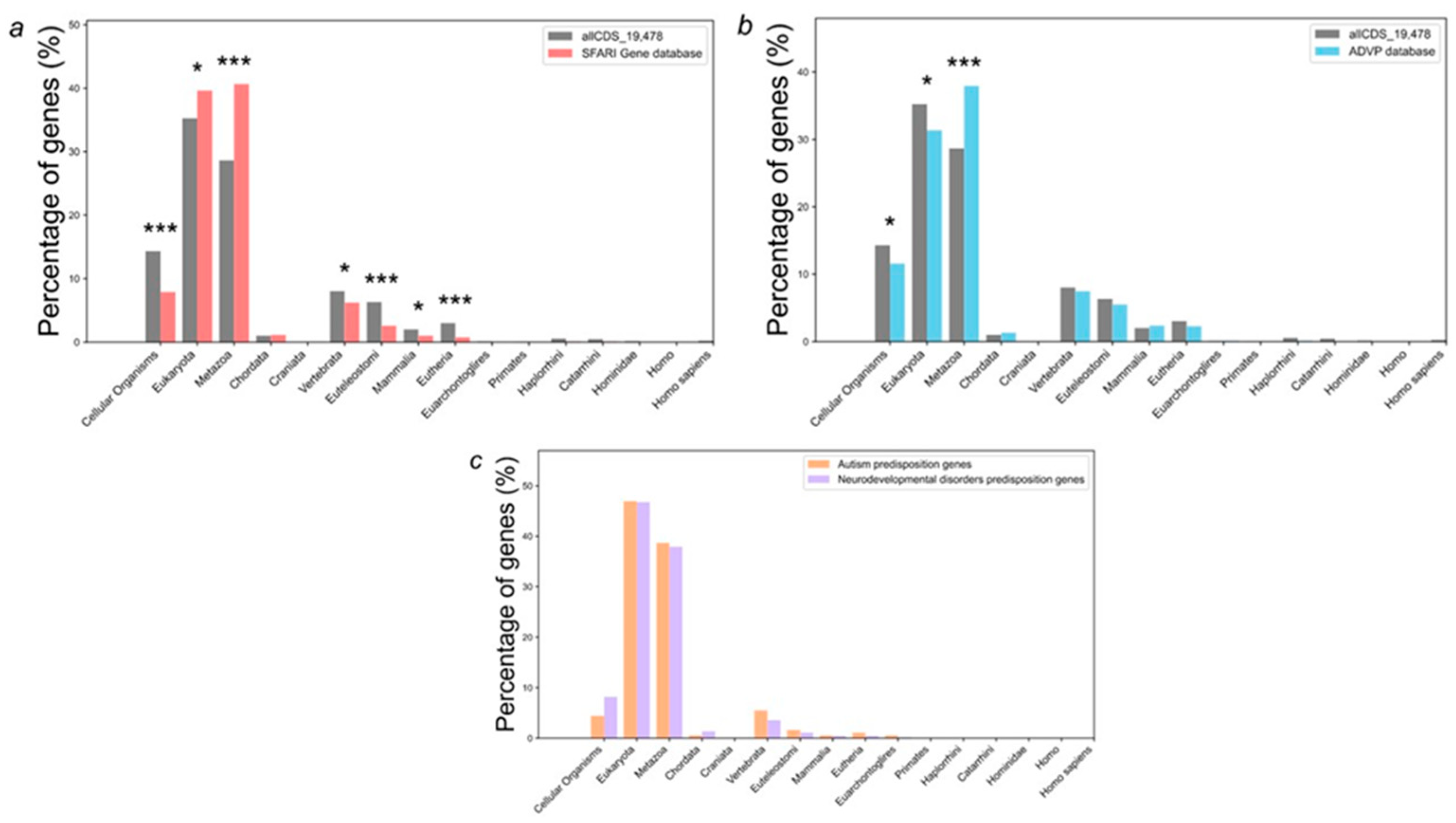
2.2.1. Phylostratigraphic Age of Genes (PAI-Based Analysis)
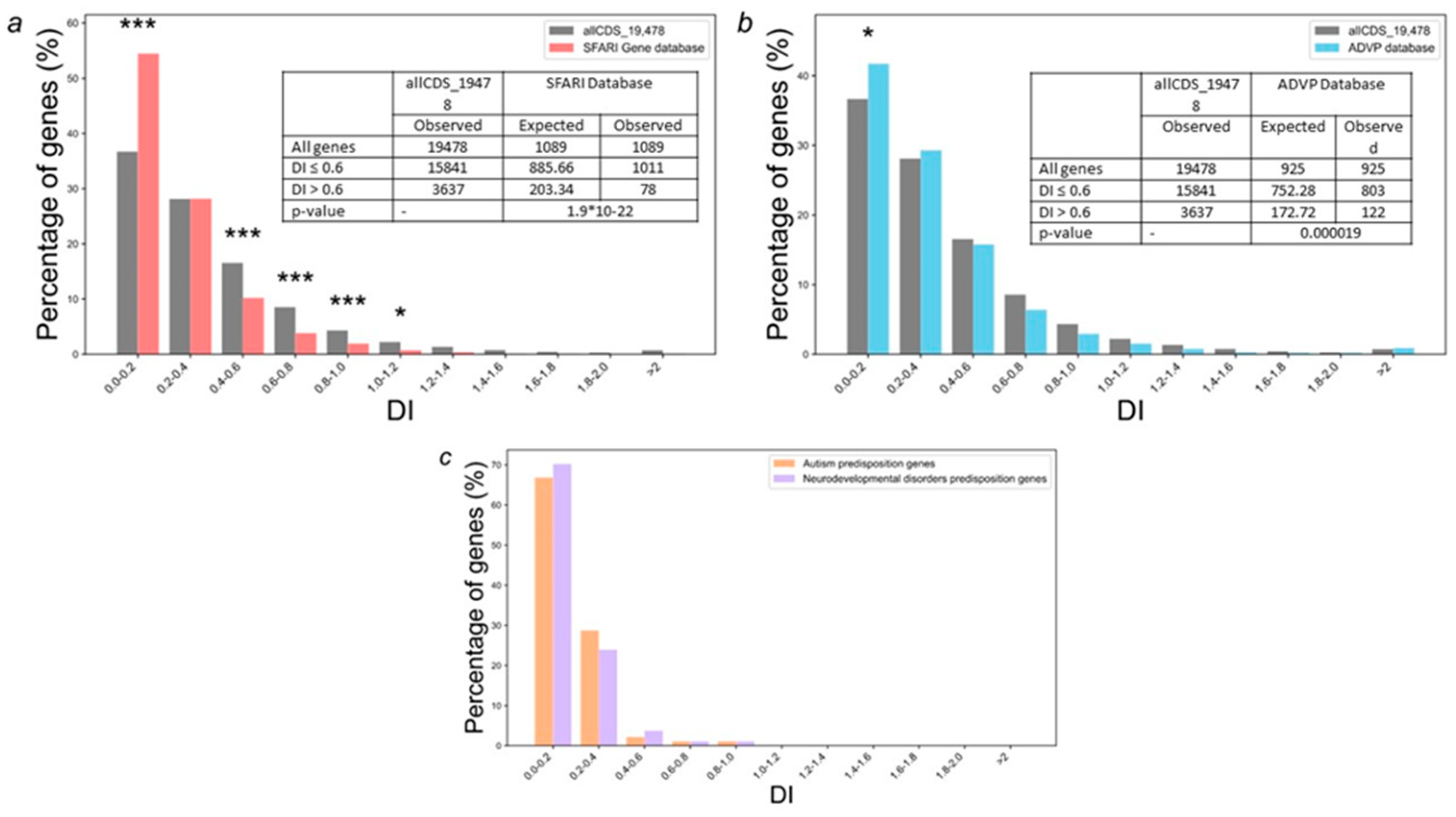
2.2.2. Evolutionary Variability of Genes (DI-Based Analysis)
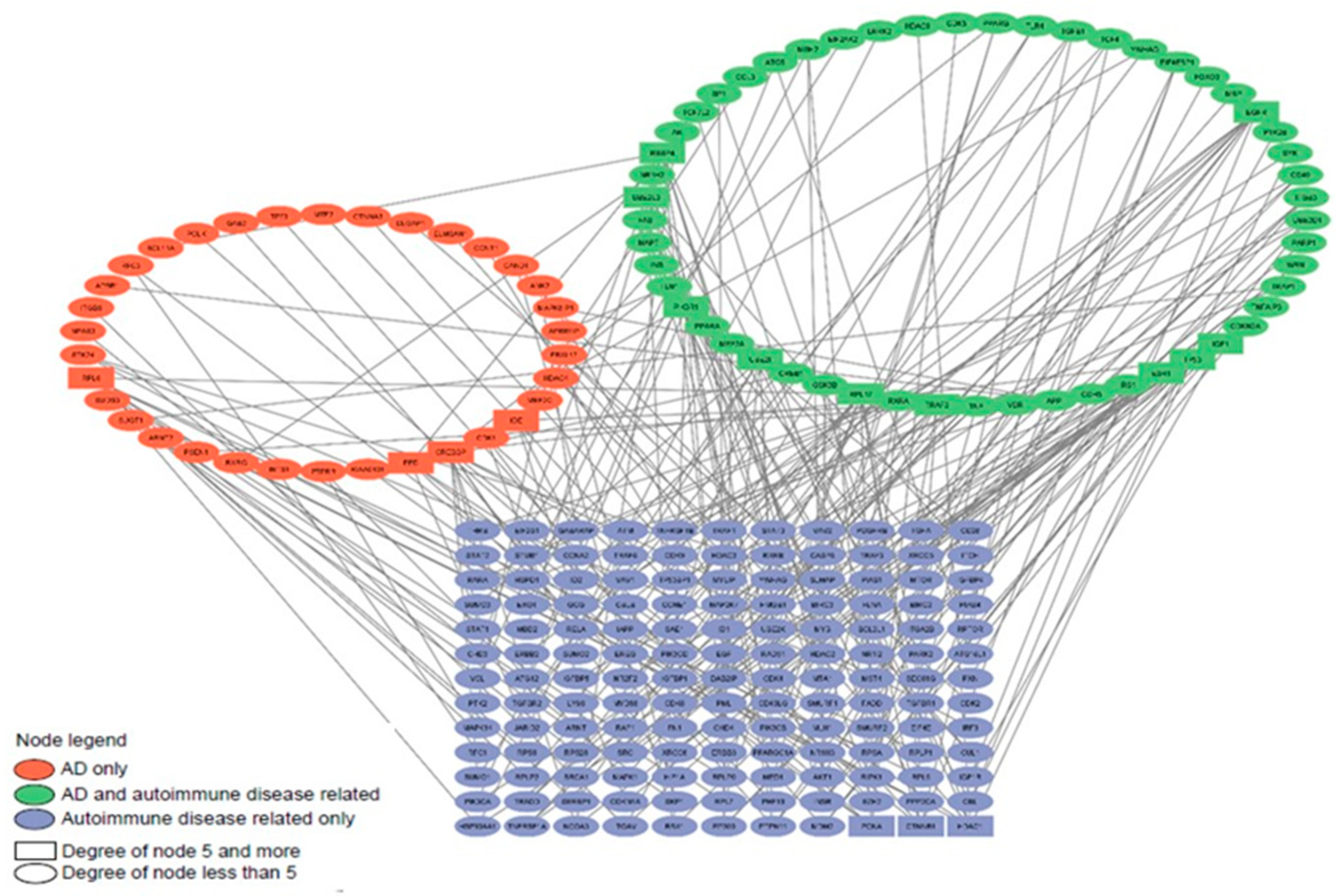
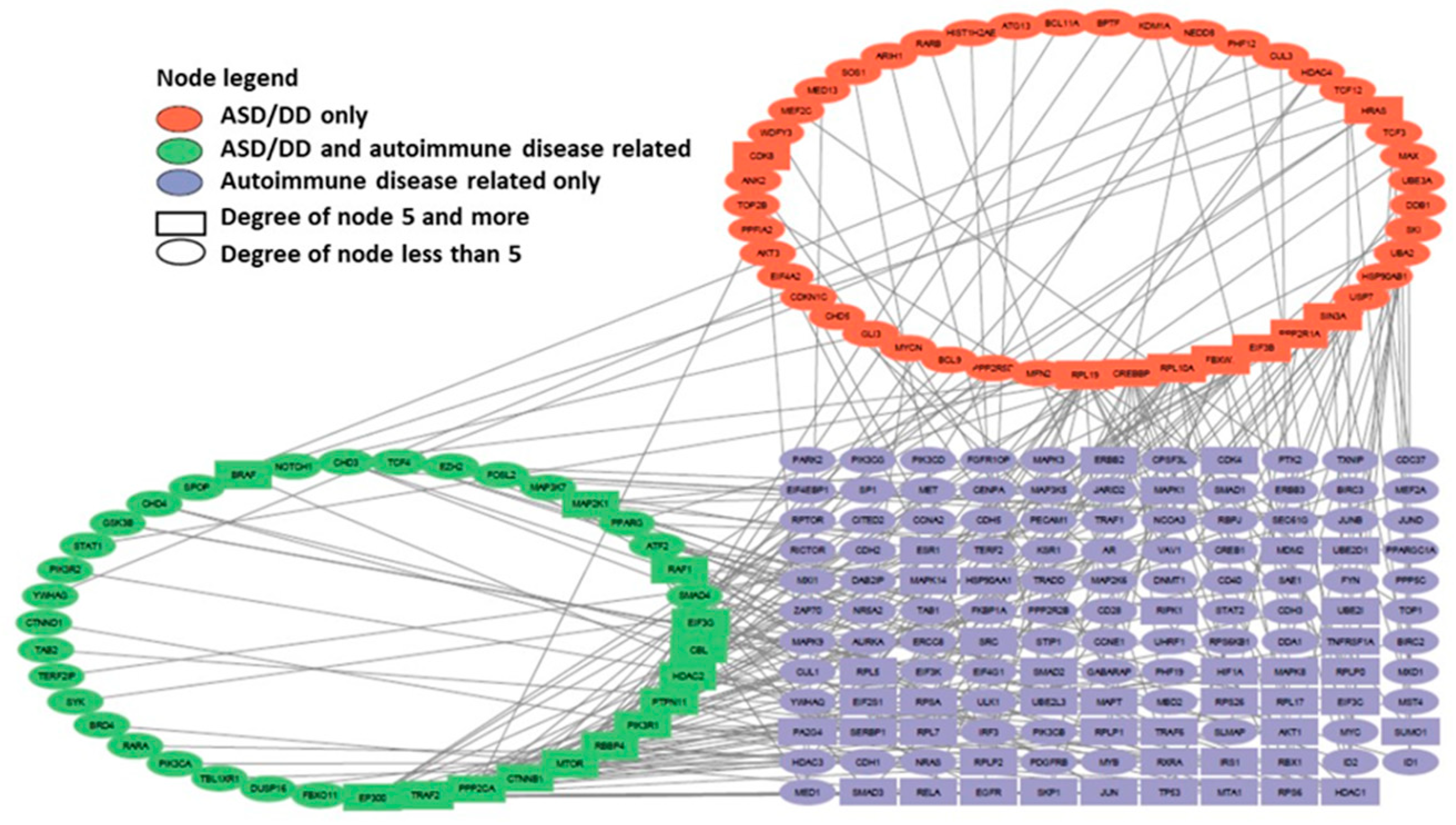
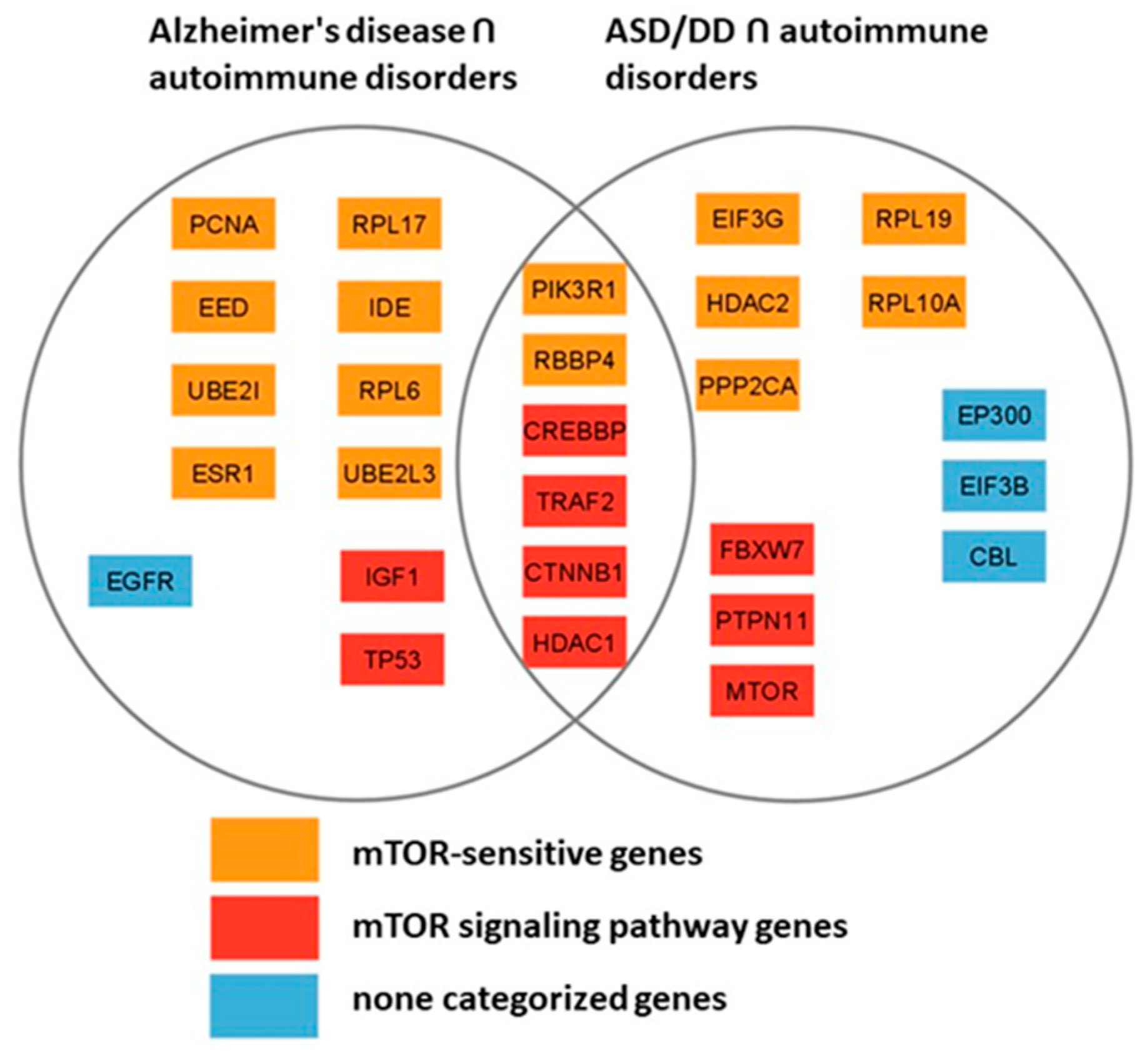
2.3. Comparative Network Analysis of Genes Predisposing to Autism and Alzheimer's Disease with Genes of Autoimmune Diseases
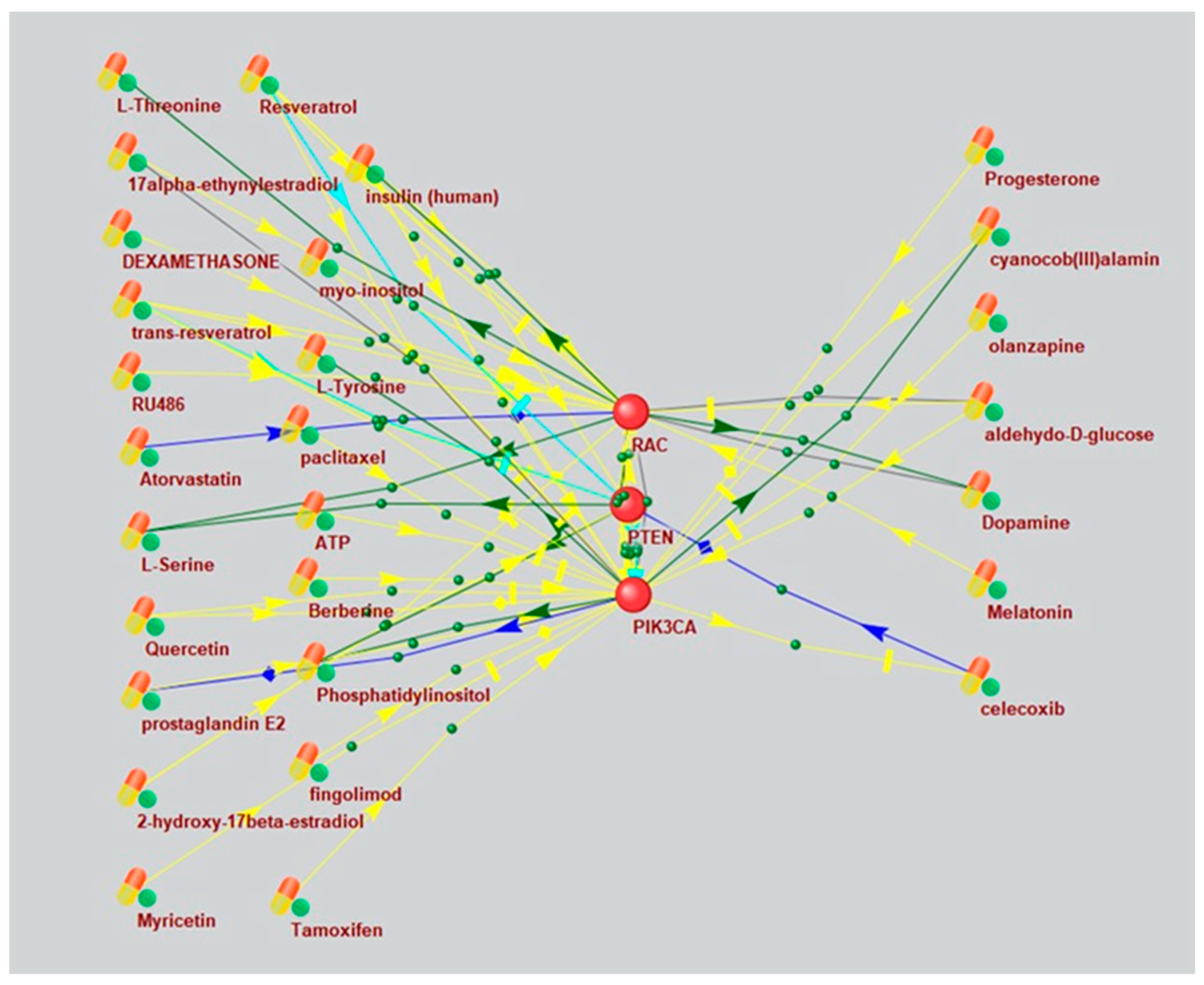
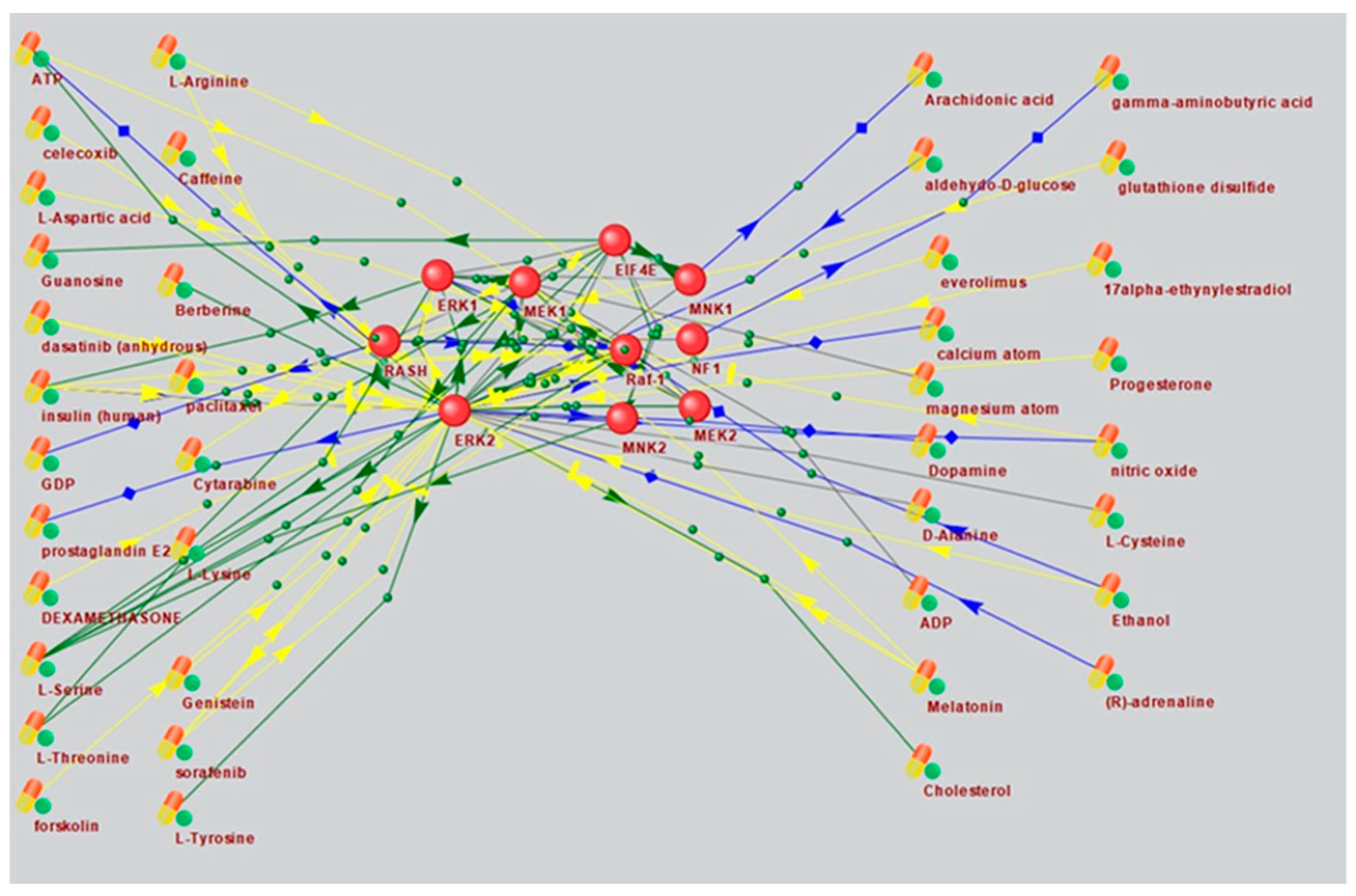
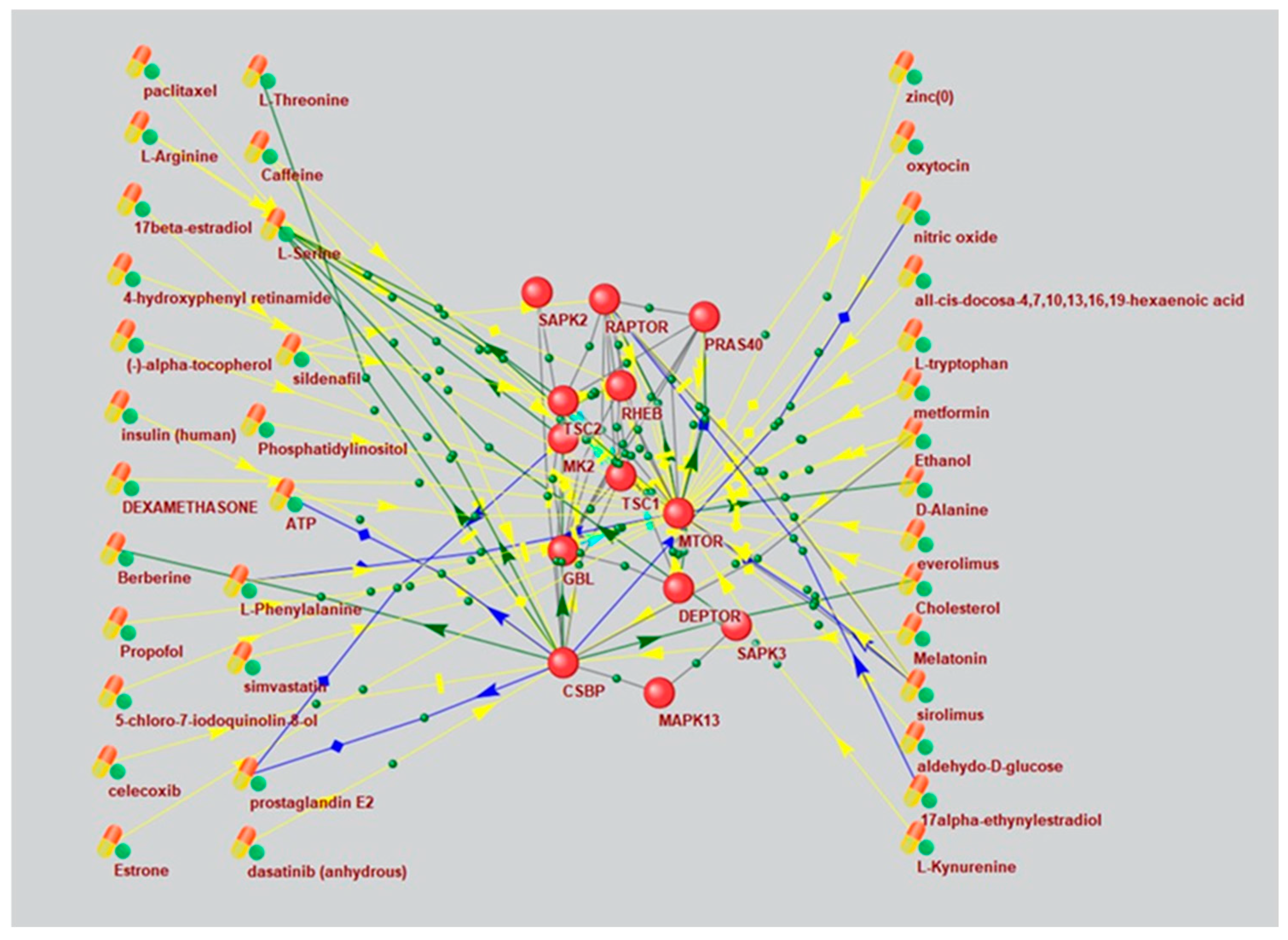
2.4. The Associative Network Analysis of the Main Elements of the mTOR Pathway and Substances Regulating Their Activity Using for ASD and AD Treatment
3. Discussion
4. Materials and Methods
4.1. Extracting Genes from Diverse Data Sources and Gene-Set Analysis
- Genes implicated in autism susceptibility (from SFARI Gene database released 20.10.2022[22]) – 1095 genes;
- Autism predisposition genes (41588_2022_1104_MOESM3_ESM.xlsx [20]) – 185 genes;
- Neurodevelopmental disorders predisposition genes (NDD) (41588_2022_1104_MOESM3_ESM.xlsx [20]) – 664 genes;
- Genes predisposing to Alzheimer's disease (13195_2017_252_MOESM2_ESM.doc [55]) – 430 genes;
- Alzheimer's disease predisposition genes (from the ADVP database [21]) – 956 genes;
- mTOR-sensitive genes (mTOR-sensitive 5UTR.xlsx [59]) – 6543 genes;
- Genes associated with autoimmune diseases (from the GAAD database [60]) – 4186 genes.
- All protein-coding genes of the human genome for which PAI and DI values were calculated [24] – 19478 genes.
4.2. Phylostratigraphic Analysis and Divergence Analysis
4.3. Network Construction
Supplementary Materials
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| AD | Alzheimer's disease |
| SFARI | Simon’s Foundation Autism Research Initiative |
| mTOR | mechanistic target of rapamycin |
| FMRP | fragile X mental retardation protein |
| PAI | Phylostratigraphic Age Index |
| DI | Divergence Index |
| NDD | neurodevelopmental disorders |
References
- Hyman, S.L.; Levy, S.E.; Myers, S.M.; COUNCIL ON CHILDREN WITH DISABILITIES, SECTION ON DEVELOPMENTAL AND BEHAVIORAL PEDIATRICS. Identification, Evaluation, and Management of Children With Autism Spectrum Disorder. Pediatrics 2020, 145, e20193447. doi: 10.1542/peds.2019-3447. [CrossRef]
- Soysal, P.; Tan, S.G. The prevalence and co-incidence of geriatric syndromes in older patients with early-stage Alzheimer’s disease and dementia with Lewy bodies. Aging Clin. Exp. Res. 2021, 33, 2599–2603.
- Rajan, K.B.; Weuve, J.; Barnes, L.L.; McAninch, E.A.; Wilson, R.S.; Evans, D.A. Population estimate of people with clinical Alzheimer’s disease and mild cognitive impairment in the United States (2020-2060). Alzheimers. Dement. 2021, 17, 1966–1975. doi: 10.1002/alz.12362. [CrossRef]
- Mencer, S.; Kartawy, M.; Lendenfeld, F.; Soluh, H.; Tripathi, M.K.; Khaliulin, I.; Amal, H. Proteomics of autism and Alzheimer's mouse models reveal common alterations in mTOR signaling pathway. Transl Psychiatry 2021, 11, 480. doi: 10.1038/s41398-021-01578-2. [CrossRef]
- Hughes, H.K.; Mills Ko, E.; Rose, D.; Ashwood, P. Immune Dysfunction and Autoimmunity as Pathological Mechanisms in Autism Spectrum Disorders. Front. Cell. Neurosci. 2018, 12, 405. doi: 10.3389/fncel.2018.00405. [CrossRef]
- Ashwood, P.; van deWater, J. Is autism an autoimmune disease? Autoimmun. Rev. 2004, 3, 557–562.
- Edmiston, E.; Ashwood, P.; van de Water, J. Autoimmunity, Autoantibodies, and Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 383–390.
- DiStasio, M.M.; Nagakura, I.; Nadler, M.J.; Anderson, M.P. T lymphocytes and cytotoxic astrocyte blebs correlate across autism brains. Ann. Neurol. 2019, 86, 885–898.
- Chen, X.; Holtzman, D.M. Emerging roles of innate and adaptive immunity in Alzheimer's disease. Immunity. 2022, 55, 2236-2254. doi: 10.1016/j.immuni.2022.10.016. [CrossRef]
- Meier-Stephenson, F.S.; Meier-Stephenson, V.C.; Carter, M.D. et al. Alzheimer’s disease as an autoimmune disorder of innate immunity endogenously modulated by tryptophan metabolites. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12283. doi: 10.1002/trc2.12283. [CrossRef]
- Shuid, A.N.; Jayusman, P.A.; Shuid, N.; Ismail, J.; Kamal, Nor N.; Naina Mohamed, I. Update on Atypicalities of Central Nervous System in Autism Spectrum Disorder. Brain Sci. 2020, 10, 309. doi: 10.3390/brainsci10050309. [CrossRef]
- Delgoffe, G.M.; Pollizzi, K.N.; Waickman, A.T.; Heikamp, E.; Meyers, D.J.; Horton, M.R.; Xiao, B.; Worley, P.F.; Powell, J.D. The kinase mTOR regulates the differentiation of helper T cells through the selective activation of signaling by mTORC1 and mTORC2. Nat. Immunol. 2011, 12, 295-303. doi: 10.1038/ni.2005. [CrossRef]
- Liu, Y.; Zhang, D.T.; Liu, X.G. mTOR signaling in T cell immunity and autoimmunity. Int. Rev. Immunol. 2015, 34, 50-66. doi: 10.3109/08830185.2014.933957. [CrossRef]
- Ortiz-González, X.R. Mitochondrial Dysfunction: A Common Denominator in Neurodevelopmental Disorders? Dev. Neurosci. 2021, 43, 222-229. doi: 10.1159/000517870. [CrossRef]
- Lenzi, P.; Ferese, R.; Biagioni, F.; Fulceri, F.; Busceti, C.L.; Falleni, A.; Gambardella, S.; Frati, A.; Fornai, F. Rapamycin Ameliorates Defects in Mitochondrial Fission and Mitophagy in Glioblastoma Cells. Int. J. Mol. Sci. 2021, 22, 5379. doi: 10.3390/ijms22105379. [CrossRef]
- Thellung, S.; Corsaro, A.; Nizzari, M.; Barbieri, F.; Florio, T. Autophagy Activator Drugs: A New Opportunity in Neuroprotection from Misfolded Protein Toxicity. Int. J. Mol. Sci. 2019, 20, 901. doi: 10.3390/ijms20040901. [CrossRef]
- Richardson, A.; Galvan, V.; Lin, A.L.; Oddo, S. How longevity research can lead to therapies for Alzheimer's disease: The rapamycin story. Exp. Gerontol. 2015, 68, 51-58. doi: 10.1016/j.exger.2014.12.002. [CrossRef]
- Caccamo, A.; Majumder, S.; Richardson, A.; Strong, R.; Oddo, S. Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-beta, and Tau: effects on cognitive impairments. J. Biol. Chem. 2010, 285, 13107-20. doi: 10.1074/jbc.M110.100420. [CrossRef]
- Narayanan, U.; Nalavadi, V.; Nakamoto, M.; Thomas, G.; Ceman, S.; Bassell, G.J.; Warren, S.T. S6K1 phosphorylates and regulates fragile X mental retardation protein (FMRP) with the neuronal protein synthesis-dependent mammalian target of rapamycin (mTOR) signaling cascade. J. Biol. Chem. 2008, 283, 18478-82. doi: 10.1074/jbc.C800055200. [CrossRef]
- Fu, J.M.; Satterstrom, F.K.; Peng, M. et al. Rare coding variation provides insight into the genetic architecture and phenotypic context of autism. Nat. Genet. 2022, 54, 1320-1331. doi: 10.1038/s41588-022-01104-0. [CrossRef]
- Kuksa, P.P.; Liu, C.L.; Fu, W. et al. Alzheimer's Disease Variant Portal: A Catalog of Genetic Findings for Alzheimer's Disease. J Alzheimers Dis. 2022, 86, 461-477. doi: 10.3233/JAD-215055. [CrossRef]
- Abrahams, B.S.; Arking, D.E.; Campbell, D.B.; Meord, H.C.; Morrow, E.M.; Weiss, L.A.; Menashe, I.; Wadkins, T.; Banerjee-Basu, S.; Packer, A. SFARI Gene 2.0: a community-driven knowledgebase for the autism spectrum disorders (ASDs). Mol. Autism 2013, 4, 36. doi: 10.1186/2040-2392-4-36. [CrossRef]
- Caron, E.; Ghosh, S.; Matsuoka, Y.; Ashton-Beaucage, D.; Therrien, M.; Lemieux, S.; Perreault, C.; Roux, P.P.; Kitano, H. A comprehensive map of the mTOR signaling network. Mol. Syst. Biol. 2010, 6, 453.
- Piovesan, A.; Antonaros, F.; Vitale, L.; Strippoli, P.; Pelleri, M.C.; Caracausi, M. Human protein-coding genes and gene feature statistics in 2019. BMC Res. Notes 2019, 12, 315. doi: 10.1186/s13104-019-4343-8. [CrossRef]
- Feng, L.; Wang, G.; Song, Q.; Feng, X.; Su, J.; Ji, G.; Li, M. Proteomics revealed an association between ribosome-associated proteins and amyloid beta deposition in Alzheimer's disease. Metab. Brain Dis. 2024, 39, 263-282. doi: 10.1007/s11011-023-01330-3. [CrossRef]
- Liu, P.P.; Han, X.; Li, X.; Dai, S.K.; Xu, Y.J.; Jiao, L.F.; Du, H.Z.; Zhao, L.H.; Li, R.F.; Teng, Z.Q.; Yang, Y.G.; Liu, C.M. An EED/PRC2-H19 Loop Regulates Cerebellar Development. Adv Sci (Weinh) 2025, 12, e2403591. doi: 10.1002/advs.202403591. [CrossRef]
- Liu, J.; Yuan, S.; Niu, X.; Kelleher, R.; Sheridan, H. ESR1 dysfunction triggers neuroinflammation as a critical upstream causative factor of the Alzheimer's disease process. Aging (Albany NY) 2022, 14, 8595-8614. doi: 10.18632/aging.204359. [CrossRef]
- Barral, S.; Reitz, C.; Small, S.A.; Mayeux, R. Genetic variants in a 'cAMP element binding protein' (CREB)-dependent histone acetylation pathway influence memory performance in cognitively healthy elderly individuals. Neurobiol. Aging 2014, 35, 2881.e7-2881.e10. doi: 10.1016/j.neurobiolaging.2014.06.024. [CrossRef]
- Zhu, Y.; Huang, Y.; Tang, T.; Xie, Y. HDAC1 and HDAC2 orchestrate Wnt signaling to regulate neural progenitor transition during brain development. iScience 2024, 27, 110600. doi: 10.1016/j.isci.2024.110600. [CrossRef]
- Lewerissa, E.I.; Nadif Kasri, N.; Linda, K. Epigenetic regulation of autophagy-related genes: Implications for neurodevelopmental disorders. Autophagy 2024, 20, 15-28. doi: 10.1080/15548627.2023.2250217. [CrossRef]
- Xie, P-L.; Zheng, M-Y.; Han, R.; Chen, W-X.; Mao, J-H. Pharmacological mTOR inhibitors in ameliorating Alzheimer’s disease: current review and perspectives. Front. Pharmacol. 2024, 15, 1366061. doi: 10.3389/fphar.2024.1366061. [CrossRef]
- Ehninger, D.; Han, S.; Shilyansky, C.; Zhou, Y.; Li, W.; Kwiatkowski, D.J.; Ramesh, V.; Silva, A.J. Reversalof learning deficits in a Tsc2+/‒ mouse model of tuberoussclerosis. Nat. Med. 2008, 14, 843–848. doi:10.1038/nm1788. [CrossRef]
- Pérez-Cano, L.; Azidane Chenlo, S.; Sabido-Vera, R.; Sirci, F.; Durham, L.; Guney, E. Translating precision medicine for autism spectrum disorder: A pressing need. Drug Discov. Today 2023, 28, 103486. doi: 10.1016/j.drudis.2023.103486. [CrossRef]
- Mustafin, Z.S.; Lashin, S.A.; Matushkin, Yu.G. Phylostratigraphic analysis of gene networks of human diseases. Vavilovskii Zhurnal Genet. Selektsii 2021, 25, 46-56. doi: 10.18699/VJ21.006. [CrossRef]
- Nagy, P.L.; Griesenbeck, J.; Kornberg, R.D.; Cleary, M.L. A trithorax-group complex purified from Saccharomyces cerevisiae is required for methylation of histone H3. Proc. Natl. Acad. Sci. U S A 2002, 99, 90-4. doi: 10.1073/pnas.221596698. [CrossRef]
- Li, S., Qiao, Y., Di, Q., Le, X., Zhang, L., Zhang, X., Zhang, C., Cheng, J., Zong, S., Koide, S. S., Miao, S., Wang, L. Interaction of SH3P13 and DYDC1 protein: a germ cell component that regulates acrosome biogenesis during spermiogenesis. Europ. J. Cell Biol. 2009, 88, 509-520.
- Demenkov, P.S.; Ivanisenko, T.V.; Kolchanov, N.A.; Ivanisenko, V.A. ANDVisio: a new tool for graphic visualization and analysis of literature mined associative gene networks in the ANDSystem. In Silico Biol. 2011, 11, 149-161. doi: 10.3233/ISB-2012-0449. [CrossRef]
- Ivanisenko, V.A.; Saik, O.V.; Ivanisenko, N.V.; Tiys, E.S.; Ivanisenko, T.V.; Demenkov, P.S.; Kolchanov, N.A. ANDSystem: an Associative Network Discovery System for automated literature mining in the field of biology. BMC Syst. Biol. 2015, 9 Suppl 2(Suppl 2), S2. doi: 10.1186/1752-0509-9-S2-S2. [CrossRef]
- Wang, Z.; Cao, B.; Ji, P.; Yao, F. Propofol inhibits tumor angiogenesis through targeting VEGF/VEGFR and mTOR/eIF4E signaling. Biochem Biophys. Res. Commun. 2021, 555, 13-18. doi: 10.1016/j.bbrc.2021.03.094. [CrossRef]
- Bossmann, M.; Ackermann, B.W.; Thome, U.H.; Laube, M. Signaling Cascade Involved in Rapid Stimulation of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) by Dexamethasone. Int. J. Mol. Sci. 2017, 18, 1807. doi: 10.3390/ijms18081807. [CrossRef]
- Morita, M.; Suyama, H.; Igishi, T.; Shigeoka, Y.; Kodani, M.; Hashimoto, K.; Takeda, K.; Sumikawa, T.; Shimizu, E. Dexamethasone inhibits paclitaxel-induced cytotoxic activity through retinoblastoma protein dephosphorylation in non-small cell lung cancer cells. Int. J. Oncol. 2007, 30, 187-192.
- Zhang, P.; He, D.; Song, E.; Jiang, M.; Song, Y. Celecoxib enhances the sensitivity of non-small-cell lung cancer cells to radiation-induced apoptosis through downregulation of the Akt/mTOR signaling pathway and COX-2 expression. PLoS One. 2019, 14, e0223760. doi: 10.1371/journal.pone.0223760. [CrossRef]
- Asadabadi, M.; Mohammadi, M.R.; Ghanizadeh, A.; Modabbernia, A.; Ashrafi, M.; Hassanzadeh, E.; Forghani, S.; Akhondzadeh, S.; Celecoxib as adjunctive treatment to risperidone in children with autistic disorder: a randomized, double-blind, placebo-controlled trial. Psychopharmacology (Berl). 2013, 225, 51-59. doi: 10.1007/s00213-012-2796-8. [CrossRef]
- Lashgari, N.A.; Roudsari, N.M.; Zadeh, S.S.T.; Momtaz, S.; Abbasifard, M.; Reiner, Ž.; Abdolghaffari, A.H.; Sahebkar, A. Statins block mammalian target of rapamycin pathway: a possible novel therapeutic strategy for inflammatory, malignant and neurodegenerative diseases. Inflammopharmacology 2023, 31, 57-75. doi: 10.1007/s10787-022-01077-w. [CrossRef]
- Okubo, S.; Uto, T,; Goto, A.; Tanaka, H.; Nishioku, T.; Yamada, K.; Shoyama, Y. Berberine Induces Apoptotic Cell Death via Activation of Caspase-3 and -8 in HL-60 Human Leukemia Cells: Nuclear Localization and Structure-Activity Relationships. Am. J. Chin. Med. 2017, 45, 1497-1511. doi: 10.1142/S0192415X17500811. [CrossRef]
- Sun, X.; Zhang, Y.; Wang, J.; Wei, L.; Li, H.; Hanley, G.; Zhao, M.; Li, Y.; Yin, D. Beta-arrestin 2 modulates resveratrol-induced apoptosis and regulation of Akt/GSK3ß pathways. Biochim. Biophys. Acta 2010, 1800, 912-918. doi: 10.1016/j.bbagen.2010.04.015. [CrossRef]
- Russo, M.; Milito, A.; Spagnuolo, C.; Carbone, V.; Rosén, A.; Minasi, P.; Lauria, F.; Russo, G.L. CK2 and PI3K are direct molecular targets of quercetin in chronic lymphocytic leukaemia. Oncotarget. 2017, 8, 42571-42587. doi: 10.18632/oncotarget.17246. [CrossRef]
- Lim, J.Y.; Lee, J.Y.; Byun, B.J.; Kim, S.H. Fisetin targets phosphatidylinositol-3-kinase and induces apoptosis of human B lymphoma Raji cells. Toxicol. Rep. 2015, 2, 984-989. doi: 10.1016/j.toxrep.2015.07.004. [CrossRef]
- Marchezan, J.; Deckmann, I.; da Fonseca, G.C.; Margis, R.; Riesgo, R.; Gottfried, C. Resveratrol Treatment of Autism Spectrum Disorder-A Pilot Study. Clin. Neuropharmacol. 2022, 45, 122-127. doi: 10.1097/WNF.0000000000000516. [CrossRef]
- Alvarez-Arellano, L.; Salazar-García, M.; Corona, J.C. Neuroprotective Effects of Quercetin in Pediatric Neurological Diseases. Molecules 2020, 25, 5597. doi: 10.3390/molecules25235597. [CrossRef]
- Song, X.; Tan, L.; Wang, M.; Ren, C.; Guo, C.; Yang, B.; Ren, Y.; Cao, Z.; Li, Y.; Pei, J. Myricetin: A review of the most recent research. Biomed Pharmacother. 2021, 134, 111017. doi: 10.1016/j.biopha.2020.111017. [CrossRef]
- Xiong, D.; Pan, J.; Zhang, Q.; Szabo, E.; Miller, M.S.; Lubet, R.A.; You, M.; Wang, Y. Bronchial airway gene expression signatures in mouse lung squamous cell carcinoma and their modulation by cancer chemopreventive agents. Oncotarget 2017, 8, 18885-18900. doi: 10.18632/oncotarget.13806. [CrossRef]
- Rameh, L.E; York, J.D.; Blind, R.D. Inositol phosphates dynamically enhance stability, solubility, and catalytic activity of mTOR. J. Biol. Chem. 2025, 301, 108095. doi: 10.1016/j.jbc.2024.108095. [CrossRef]
- van Sadelhoff, J.H.J.; Perez Pardo, P.; Wu, J.; Garssen, J.; van Bergenhenegouwen, J.; Hogenkamp, A.; Hartog, A.; Kraneveld, A.D. The Gut-Immune-Brain Axis in Autism Spectrum Disorders; A Focus on Amino Acids. Front. Endocrinol. (Lausanne) 2019, 10, 247. doi: 10.3389/fendo.2019.00247. [CrossRef]
- Hu, Y.S.; Xin, J.; Hu, Y.; Zhang, L.; Wang, J. Analyzing the genes related to Alzheimer's disease via a network and pathway-based approach. Alzheimers Res. Ther. 2017, 9, 29. doi: 10.1186/s13195-017-0252-z. [CrossRef]
- Darnell, J.C.; Van Driesche, S.J.; Zhang, C.; Hung, K.Y.; Mele, A.; Fraser, C.E.; Stone, E.F.; Chen, C.; Fak, J.J.; Chi, S.W.; Licatalosi, D.D.; Richter, J.D.; Darnell, R.B. FMRP stalls ribosomal translocation on mRNAs linked to synaptic function and autism. Cell 2011, 146, 247-261. doi: 10.1016/j.cell.2011.06.013. [CrossRef]
- Jansen, A.; Dieleman, G.C.; Smit, A.B.; Verhage, M.; Verhulst, F.C.; Polderman, T.J.C.; Posthuma, D. Gene-set analysis shows association between FMRP targets and autism spectrum disorder. Eur. J. Hum. Genet. 2017, 25, 863-868. doi: 10.1038/ejhg.2017.55. [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27-30. doi: 10.1093/nar/28.1.27. [CrossRef]
- Gandin, V.; Masvidal, L.; Hulea, L.; Gravel, S.P.; Cargnello, M.; McLaughlan, S.; Cai, Y.; Balanathan, P.; Morita, M.; Rajakumar, A.; Furic, L.; Pollak, M.; Porco, J.A Jr.; St-Pierre, J.; Pelletier, J.; Larsson, O.; Topisirovic, I. nanoCAGE reveals 5' UTR features that define specific modes of translation of functionally related MTOR-sensitive mRNAs. Genome Res. 2016, 26, 636-48. doi: 10.1101/gr.197566.115. [CrossRef]
- Lu, G.; Hao, X.; Chen, W.H.; Mu, S. GAAD: A Gene and Autoimmiune Disease Association Database. Genomics Proteomics Bioinformatics 2018, 16, 252-261. doi: 10.1016/j.gpb.2018.05.001. [CrossRef]
- Trifonova, E.A.; Klimenko, A.I.; Mustafin, Z.S.; Lashin, S.A.; Kochetov A.V. The mTOR Signaling Pathway Activity and Vitamin D Availability Control the Expression of Most Autism Predisposition Genes. Int. J. Mol. Sci. 2019, 20, 6332. doi: 10.3390/ijms20246332. [CrossRef]
- Domazet-Loso, T.; Brajković, J.; Tautz, D. A phylostratigraphy approach to uncover the genomic history of major adaptations in metazoan lineages. Trends Genet. 2007, 23, 533-9. doi: 10.1016/j.tig.2007.08.014. [CrossRef]
- Domazet-Lošo, M.; Široki, T.; Šimičević, K.; Domazet-Lošo, T. Macroevolutionary dynamics of gene family gain and loss along multicellular eukaryotic lineages. Nat. Commun. 2024, 15, 2663. doi: 10.1038/s41467-024-47017-w. [CrossRef]
- Kanehisa, M.; Furumichi, M.; Tanabe, M.; Sato, Y.; Morishima, K. KEGG: new perspectives on genomes, pathways, diseases and drugs. Nucleic Acids Res. 2017, 45, D353-D361. doi: 10.1093/nar/gkw1092. [CrossRef]
- Ivanov, R.A.; Mukhin, A.M.; Kazantsev, F.V.; Mustafin, Z.S.; Afonnikov, D.A.; Matushkin, Y.G.; Lashin, S.A. Orthoweb: A Software Package for Evolutionary Analysis of Gene Networks. Vavilovskii Zhurnal Genet. Selektsii 2025, 28, 874–881, doi: 10.18699/vjgb-24-95. [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; Jensen, L.J.; Mering, C.V. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607-D613. doi: 10.1093/nar/gky1131. [CrossRef]
| Gene set number | Gene sets | PAI | DI |
| 1 | Genes implicated in autism susceptibility (from SFARI Gene database) [22] | 2.86 | 0.24 |
| 2 | Autism predisposition genes [20] | 2.80 | 0.16 |
| 3 | Neurodevelopmental disorders predisposition genes (NDD) [20] | 2.59 | 0.15 |
| 5 | Alzheimer's disease predisposition genes (from the ADVP database) [21] | 3.18 | 0.33 |
| 7 | Genes included in the mTOR signaling network [23] | 2.29 | 0.18 |
| 10 | All protein-coding human genes [24] | 3.29 | 0.38 |
| PAI | Phylostratum |
| 1 | Cellular Organisms |
| 2 | Eukaryota |
| 3 | Metazoa |
| 4 | Chordata |
| 5 | Craniata |
| 6 | Vertebrata |
| 7 | Euteleostomi |
| 8 | Mammalia |
| 9 | Eutheria |
| 10 | Euarchontoglires |
| 11 | Primates |
| 12 | Haplorrhini |
| 13 | Catarrhini |
| 14 | Hominidae |
| 15 | Homo |
| 16 | Homo sapiens |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





