1. Introduction
The
exposome, first introduced by Wild [
1], refers to the totality of environmental exposures encountered across the lifespan and their biological interactions. This concept complements genomic research by offering a broader framework to understand complex, multifactorial diseases [
2,
3,
4]. Among the diverse external exposures, those related to air quality are particularly critical. In this context, desert dust intrusions—such as those originating from the Sahara—represent episodic yet substantial components of the atmospheric exposome. These events contribute to sharp increases in particulate matter and transport not only mineral particles but also microorganisms, allergens, and toxic compounds, with significant implications for human health [
5,
6].
Desert dust intrusions are large-scale atmospheric phenomena driven by strong winds and arid conditions, capable of lofting fine and coarse particles into the atmosphere and carrying them thousands of kilometers. As a result, air quality can deteriorate far from the emission source, affecting populations across entire regions [
7,
8]. While dust has traditionally been considered a natural element of Earth’s geochemical cycles, the rising frequency and intensity of dust storms—exacerbated by land degradation, desertification, and climate change—have transformed them into an emerging public health challenge [
9].
One of the most relevant consequences of dust exposure is its impact on respiratory health. Fine particulate matter (PM₁₀ and PM₂.₅), abundant during dust events, can penetrate the lower respiratory tract and trigger inflammation, oxidative stress, and bronchial hyperresponsiveness [
10,
11]. Epidemiological studies have consistently linked dust episodes with higher rates of asthma and chronic obstructive pulmonary disease (COPD) exacerbations, emergency department visits, hospital admissions, and mortality, particularly among vulnerable populations [
12,
13,
14]. Other natural hazards such as wildfires and volcanic eruptions also produce airborne pollutants—fine particulates, carbon monoxide, irritant gases—that contribute to respiratory morbidity. In recent years, wildfires have become more frequent and intense, causing episodes of severe air pollution across wide geographic areas [
15,
16]. In addition, desert dust can carry biological material including allergens, bacteria, and fungi, which may further aggravate symptoms in patients with asthma or immunologic susceptibility [
17,
18].
Real-world data increasingly support the notion that these natural airborne pollution events are followed by short-term surges in inhaled medication use, particularly short-acting beta-agonists (SABAs), inhaled corticosteroids (ICS), and combination maintenance therapies such as ICS–LABA and long-acting antimuscarinics [
19,
20]. In regions affected by Saharan dust—such as Southern Europe and the Middle East—dispensing records have shown a rise in rescue and maintenance inhaler use within days of exposure peaks [
21,
22]. Similar trends have been observed during wildfire seasons in North America and Australia, where pharmacy sales of SABAs increase in parallel with acute respiratory distress [
23,
24]. These patterns highlight both the clinical burden of environmental episodes and the utility of inhaler dispensing as a proxy for population-level respiratory impact. Moreover, inhaler utilization trends reveal disparities in access and preparedness: during periods of elevated pollutant exposure, patients with asthma or COPD may rely more heavily on rescue inhalers, especially when access to routine care is disrupted. This raises important questions regarding the adequacy of preventive strategies, the timeliness of public health alerts, and the resilience of healthcare systems during environmental emergencies.
Previous research suggests that the respiratory impact of air pollution may be more accurately captured through patterns of medication use than by symptom reporting or exacerbation counts [
25,
26]. Using Spain’s universal prescription registry, we investigated Saharan dust intrusions (SDI) in the Canary Islands, a setting uniquely suited to this question given its frequent exposure to SDI [
27,
28] and elevated prevalence of asthma and COPD [
29,
30,
31]. The objective was to determine whether SDI are associated with changes in dispensing of SABA reliever therapy and ICS–LABA maintenance therapy.
2. Materials and Methods
2.1. Definition of ICS-LABA and SABA Sales
Sales data (number of units dispensed) of Inhaled Corticosteroids plus Long-Acting Beta-Agonists (ICS-LABA) and Short-Acting Beta-Agonists (SABA) were retrieved from 29 and 31 pharmacies located in the two provinces of the Canary Islands (Spain), Santa Cruz de Tenerife (TF) and Las Palmas de Gran Canaria (GC), respectively. The data timeline was established from June 2017 to May 2022 (60 months of follow-up). The monthly sum of sales for each drug was calculated, resulting in 60 monthly observations for each province. Sales were assigned to the month following dispensing to better reflect the effective consumption period.
2.2. Definition of SDI
Days with SDI were identified considering the daily concentration of Particulate Matter with a diameter ≤ 10 µm (PM
10), retrieved from the
Canary Islands Air Quality Control and Monitoring Network [
32]. Daily mean PM
10 concentrations were calculated from 30 and 21 monitoring stations in TF and GC, respectively, and the timeline included the same period of 60 months defined above. The presence of SDI was declared when the mean PM
10 concentration exceeded 40 µg/m
3, a threshold at which air quality is classified as “regular” according to the criteria defined by the
Canary Islands Air Quality Control and Monitoring Network [
32].
Since different SDI atmospheric scenarios take place in the Canary Islands depending on the season, the effect of seasonality was also considered. During the winter (October 1
st to March 31
st), Saharan dust is mainly carried by low-level continental African trade winds (
Harmattan winds), reaching altitudes of less than 700 meters above sea level and mostly affecting urban populated areas. However, in the summer (April 1
st to September 30
th), dust is mainly transported by the northern branch of the high-altitude Saharan Air Layer, reaching higher altitudes where urban-populated regions are almost absent [
33,
34,
35].
2.3. Statistical Analysis
Data analyses were performed using R software (version 4.3.2; R Core Team, 2023) within RStudio (version 2023.09.1+494; Posit Software, PBC). Outliers were identified using the interquartile range (IQR) criteria (datapoints 1.5 × IQR above the third quartile or below the first quartile were excluded). Outliers were identified independently for each analysis group and removed prior to statistical testing. Data normality was assessed using the Kolmogorov-Smirnov test (n > 50). Linear regression models were applied to evaluate the association between ICS-LABA or SABA sales with the presence/absence of SDI, the total number of days with SDI per month, and the monthly average PM10 concentration. Additionally, linear regression models were adjusted for seasonality (winter or summer), defined as described above. Statistical significance was declared for those comparisons with p-value < 0.05, and correlations were evaluated with the Pearson’s coefficient (r).
2.4. Ethical Approval and Consent to Participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of CEIm Hospital Universitario de Canarias, Tenerife, Spain with the reference number P.I.-2017/72 on October 30, 2017. The study was conducted using aggregated pharmaceutical sales records and publicly available air quality data therefore, no identifiable personal information was collected, and all data were anonymized at the pharmacy level. The requirement for informed consent was waived, as the study involved only secondary, non-identifiable data and posed no risk to individuals.
3. Results
3.1. Effect of SDI over SABA and ICS-LABA Sales in the Canary Islands
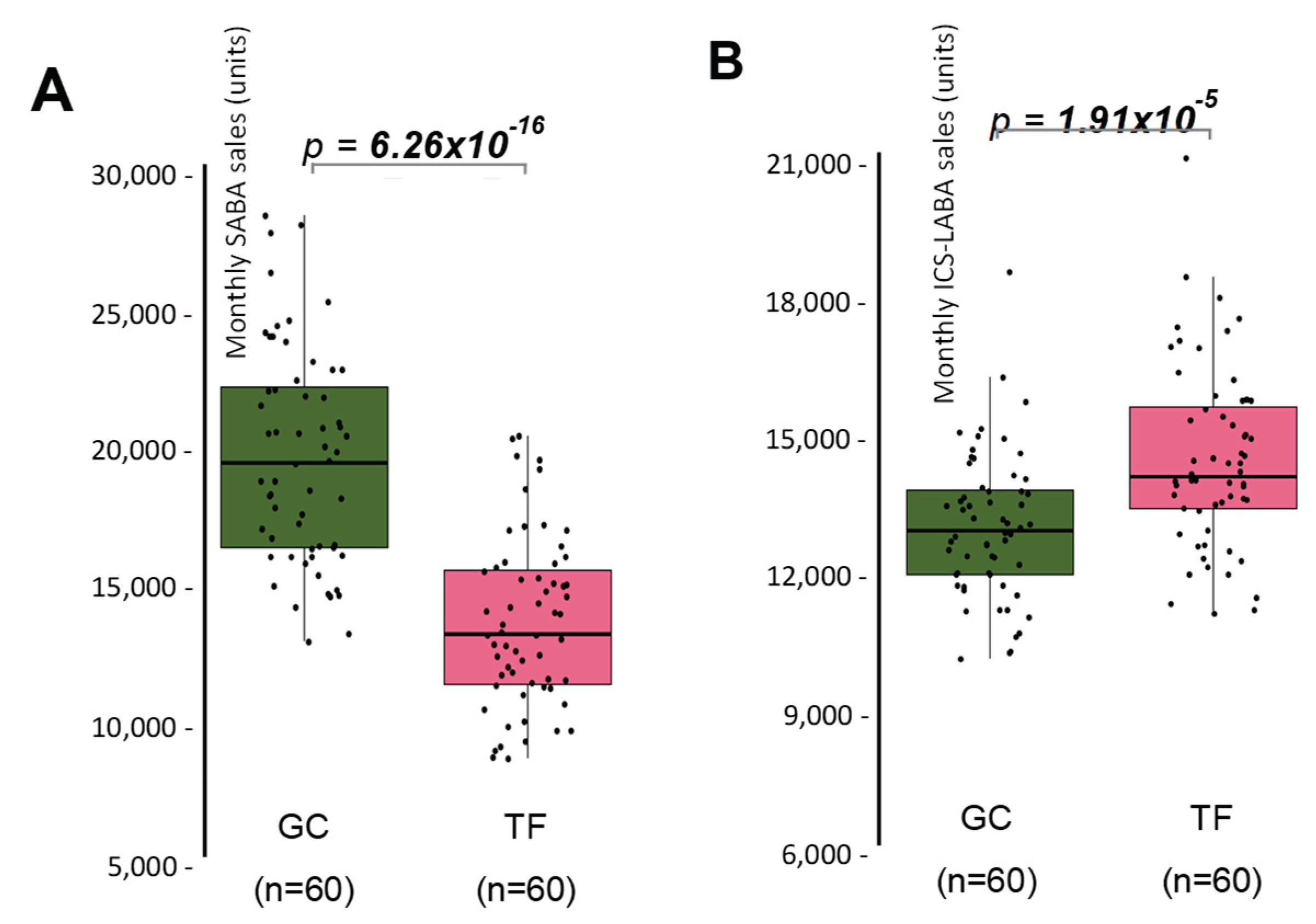
The monthly sales of SABA and ICS-LABA during the 60-months follow-up was studied in the two provinces of the Canary Islands (
Table 1).
Results showed that SABA sales were 14.8% lower in TF, compared with GC (
Figure 1A), while ICS-LABA sales were 10.9% higher (
Figure 1B).
These results suggest a greater adherence on rescue medication in GC, while TF showed slightly higher use of maintenance therapy, and possibly better adherence to preventive treatment, pointing to differences in disease management practices, or patient adherence. Therefore, the effect of SDI over SABA and ICS-LABA sales was independently studied in both provinces, comparing the sales that took place in months where PM
10 reached 40 µg/m
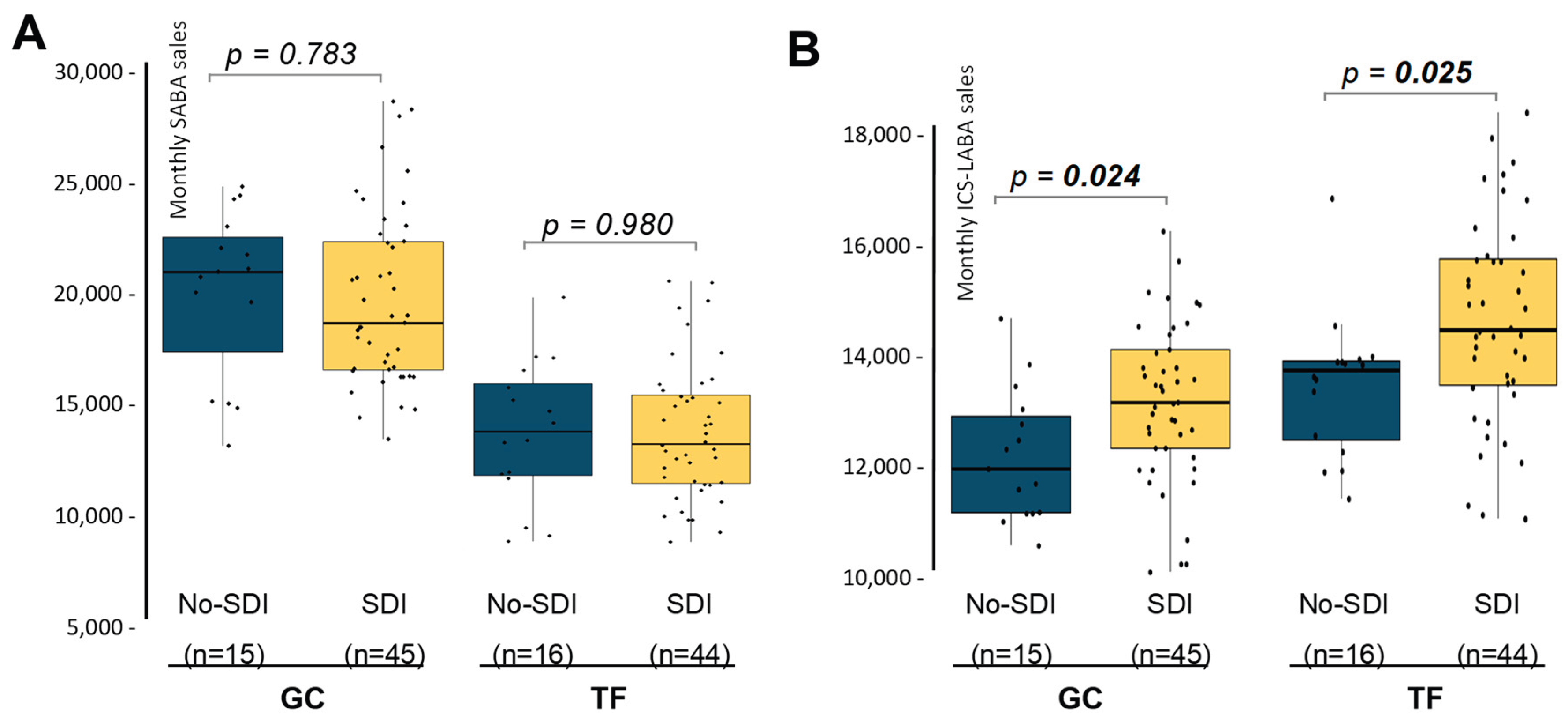
3 in at least one day, with the sales in months with absence of SDI. SABA sales were similar between the two groups, both in TF and GC, meaning that SDI did not affect the dispensation of rescue medication in the two provinces studied (
Figure 2A). Interestingly, months with presence of SDI showed significant higher ICS-LABA sales in the two provinces (
Figure 2B). In the case of GC, the increment reaches 10.2%, while in the province of TF, ICS-LABA sales were increased by 5.7%. These results indicate that SDI exposure is not translated in a higher adherence of rescue medication but is associated with and slightly higher use of maintenance therapy in both provinces.
3.2. Effect of SDI Frequency and Intensity over SABA and ICS-LABA Sales
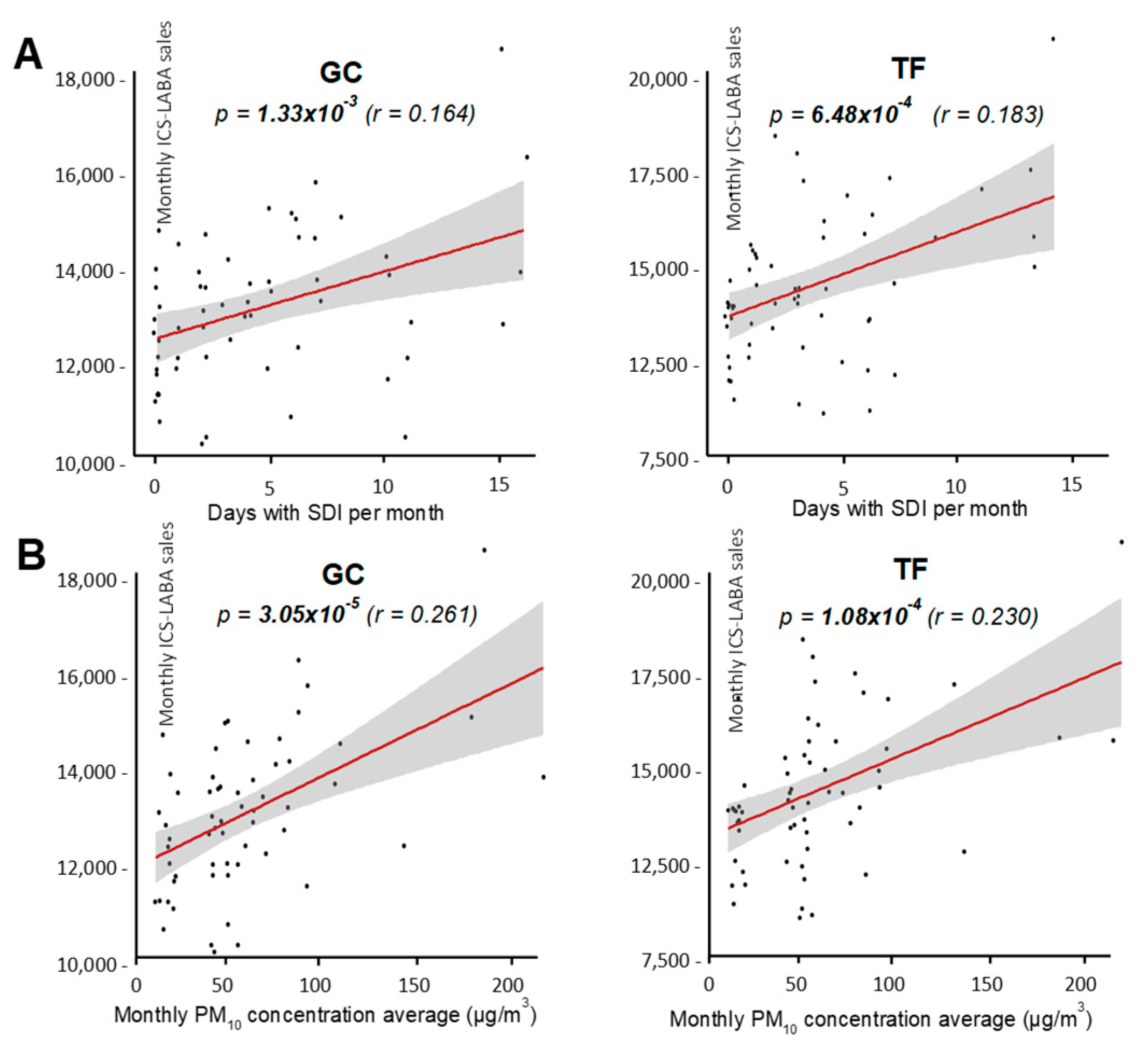
First, the effect of SDI frequency was evaluated by testing the associations between the number of days with SDI per month and the drugs sales, using linear regression models. No associations were found in the case of SABA (not shown), while ICS-LABA sales showed a significant association with the number of days with SDI per month, both in GC and TF (
Figure 3A). To investigate the effect of SDI intensity, associations between the monthly average of PM
10 concentration and the drug sales were also evaluated. Again, SABA sales showed no associations with PM
10 average concentration (not shown), while significant associations were confirmed for ICS-LABA in both provinces (
Figure 3B). Nevertheless, although associations were significantly supported, linear correlations were certainly low for both provinces, when evaluated by the Pearson’s
r coefficient (
Figure 3).
Overall, these results support a significant but weak correlation between the frequency and intensity of SDI and ICS-LABA sales, but not for SABA. This tendency was observed in both provinces independently, suggesting that the more the population is exposed to SDI, the greater the demand for maintenance therapy, but not of rescue medication.
3.3. Effect of SDI Seasonality over SABA and ICS-LABA Sales
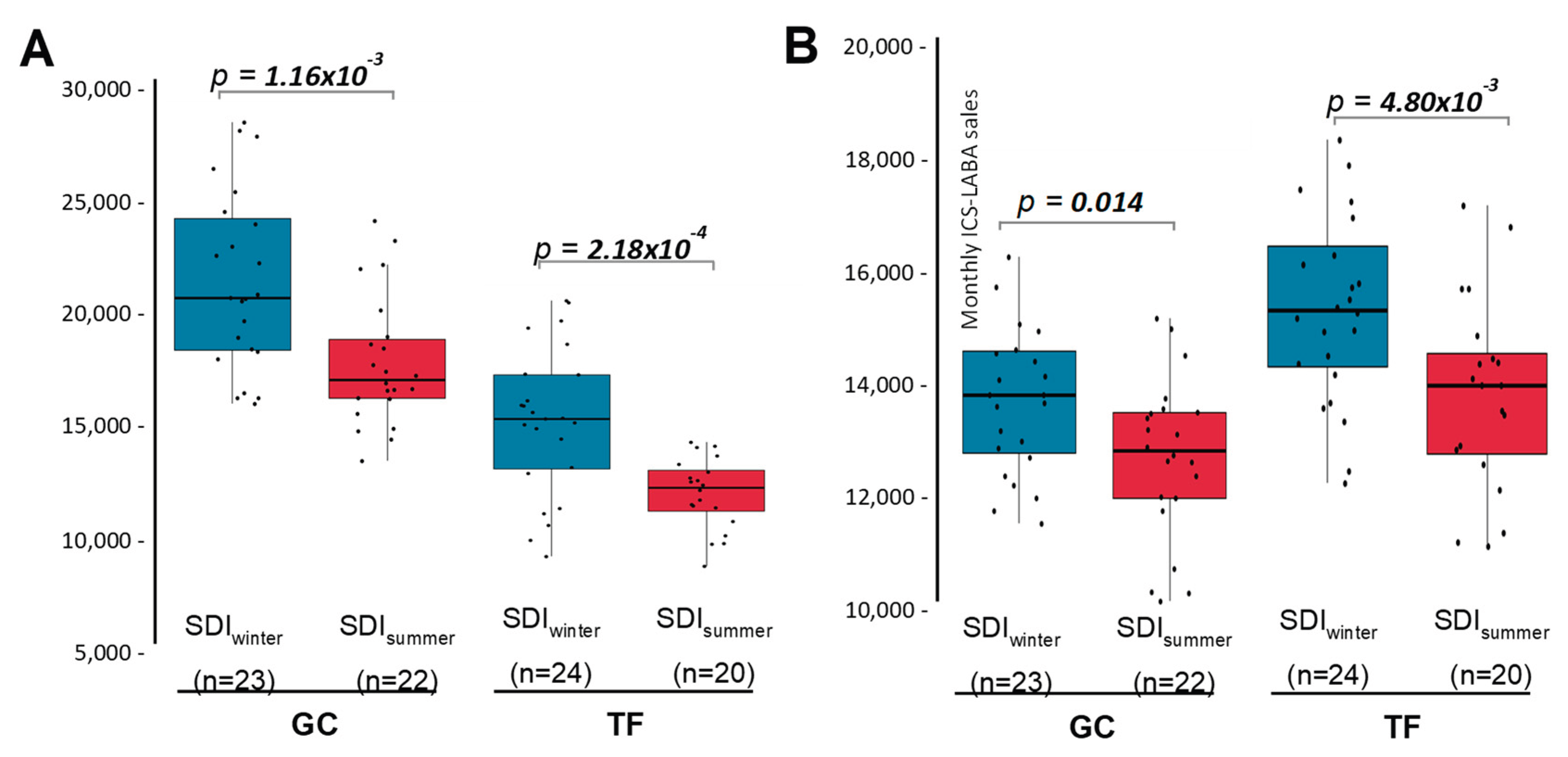
Seasonality strongly modulates SDI in the Canary Islands. In winter, SDI mostly affects populated areas, while in summer it reaches more elevated areas, where urban centres are absent. Therefore, drug sales were compared only for the months with SDI presence, between the winter and summer seasons (
Figure 4). Results showed that ICS-LABA sales were significantly higher during winter months with SDI presence than in summer months with SDI (
Figure 4A). This effect was observed in both provinces independently, reaching 9.6% and 14.3% in GC and TF, respectively (
Figure 4A). Moreover, when the comparison between seasons were repeated, but including those months without SDI only, no differences were observed (
pGC = 0.078;
pTF = 0.257). These results indicate that the increase of ICS-LABA sales detected in the Canary Islands as result of SDI is mostly associated to the events that take place during the winter.
When restricted to months with SDI, SABA sales remained significantly higher in winter compared with summer in both provinces (18.8% in GC and 18.9% in TF;
Figure 4B). Comparable seasonal differences were also observed during months without SDI (pGC = 0.035; pTF = 0.039). These findings indicate that the increase in SABA sales is attributable to seasonality and not to the presence of SDI.
Finally, based on these results, linear regression models showed in
Figure 2 were adjusted to include seasonality as a covariate. Findings showed that the increase in ICS-LABA sales during SDI remained significant even after adjustment by season in both provinces (
pGC = 0.022,
pTF = 0.027). This analysis showed an increase of 1,014.0 ± 431.6 and 1,181.7 ± 520.1 units of ICS-LABA in those months with SDI presence for GC and TF, respectively.
4. Discussion
Although desert dust is an inherent component of the Earth’s climate system, its intensification in recent decades—fueled by desertification, land degradation, and climate change—has emerged as a major public health concern [
36]. The Sahara, which alone accounts for more than half of global dust emissions, is the predominant source, with recurrent plumes that often reach Southern Europe and particularly affect the Canary Islands [
37,
38].
Desert dust intrusions expose the respiratory epithelium to a heterogeneous mixture of mineral particles, metals, organic compounds, and microbial agents that compromise barrier integrity and trigger inflammatory cascades. Experimental models show that dust particles induce oxidative stress and activate the NLRP3 inflammasome, promoting release of IL-1β and epithelial alarmins such as IL-33 and TSLP [
39,
40]. These responses amplify type-2 and neutrophilic inflammation, enhance bronchial hyperresponsiveness, and impair mucociliary clearance, thereby worsening airway obstruction in both asthma and COPD [
41,
42]. Repeated or sustained epithelial injury can lower the threshold for symptoms such as wheezing, cough, and dyspnea, prompting greater reliance on inhaler therapies. Epidemiological studies linking dust exposure to exacerbations and healthcare use are consistent with these mechanistic insights and support the interpretation of inhaler utilization as a clinical marker of dust-related exposure burden within the exposome framework [
43,
44].
In this study, SDI in the Canary Islands were associated with a measurable increase in ICS–LABA dispensations but not in SABA use. This association persisted after adjustment for seasonal variation, indicating that dust episodes exert an independent effect on maintenance therapy utilization. By contrast, the winter increase in SABA sales was attributable to seasonal trends rather than SDI exposure. These results align with previous evidence linking natural air pollution events to heightened medication demand yet the differentiation between reliever and maintenance therapy is notable [
45,
46,
47], While SABA use primarily reflects acute symptom relief, the rise in ICS–LABA sales suggests escalation of preventive therapy, whether initiated by patients or reinforced by clinicians. The modest but significant correlation between ICS–LABA sales and both frequency and intensity of SDI further supports this interpretation.
The absence of a parallel rise in SABA use invites two complementary explanations. First, SDI events in the Canary Islands have consistently been associated with increased morbidity from asthma and COPD, including higher admissions and exacerbations, often with a temporal lag suggestive of subacute inflammation rather than immediate bronchospasm [
48]. This delayed effect may encourage greater reliance on maintenance therapy to stabilize symptom burden. Second, the growing adoption of Maintenance and Reliever Therapy (MART/SMART), in which ICS–formoterol serves as both controller and reliever, could shift symptom-driven medication demand into ICS–LABA dispensations rather than SABA, in line with current guideline recommendations [
49,
50]. Together, these mechanisms suggest that SDI exposure increases both the need for intensified anti-inflammatory therapy and the demand for symptom relief covered under ICS–LABA prescriptions, while leaving SABA utilization largely unaffected.
From a clinical and public health perspective, these findings carry several implications. Inhaler dispensing data represent a valuable real-world indicator of the population impact of environmental exposures, complementing hospital admissions and patient-reported outcomes. The stronger effect observed in winter highlights the role of seasonal atmospheric dynamics in shaping exposure risk and enhances the value of integrating environmental alerts into preventive respiratory care strategies. Moreover, the reliance on maintenance rather than rescue therapy raises important questions regarding adherence, prescribing practices, and whether SDI events prompt sustained adjustments in asthma and COPD management.
Limitations of the present study must be acknowledged. Dispensing data indicate medication supply rather than actual use and may be affected by prescribing practices, stockpiling, or pharmacy availability [
51,
52,
53]. The absence of individual-level clinical outcomes prevents assessing whether increased ICS–LABA use improves control or reduces exacerbations. Dust composition—including allergens, bacteria, and fungi—was also not characterized, despite its potential impact on respiratory outcomes in atopic populations [
54,
55]. Future research should apply advanced time-series models (e.g., distributed lag or autoregressive) to capture delayed and cumulative effects of SDI, and link dispensing patterns with clinical outcomes such as exacerbations, emergency visits, and lung function. Incorporating chemical and biological dust analyses may further clarify exposure–response mechanisms and support targeted preventive strategies in regions recurrently affected by desert dust.
5. Conclusions
This study provides novel evidence that Saharan dust intrusions significantly increase the use of ICS–LABA maintenance therapy in the Canary Islands, independent of seasonal variation. In contrast, SABA utilization patterns reflected only seasonal trends, not dust exposure. These findings highlight important differences in patient and prescriber responses to environmental events and underscore the utility of pharmaceutical dispensing records as a sensitive, real-world proxy for respiratory burden. Public health systems in regions affected by desert dust should anticipate temporary increases in maintenance therapy demand, particularly during winter episodes when populated areas are most exposed. Integrating dust forecasts into early-warning systems and patient education programs may improve disease control, reduce exacerbations, and strengthen healthcare preparedness for future environmental challenges.
Author Contributions
R.G-P. and A.E-E. contributed equally to this work. Conceptualization, R.G-P. and P.P-G.; methodology, R.G-P., P.P-G., A.E-E., and M.G-C; software, A.E-E., and M.G-C.; validation, R.G-P., P.P-G., A.E-E., and M.G-C; investigation, R.G-P., P.P-G., A.E-E., and M.G-C; resources, R.G-P. and M.G-C; data curation, A.E-E., and M.G-C.; writing—original draft preparation, R.G-P., P.P-G., A.E-E., and M.G-C; writing—review and editing, R.G-P., P.P-G., A.E-E., and M.G-C; visualization, A.E-E., and M.G-C.; supervision, R.G-P. and M.G-C; project administration, R.G-P. and P.P-G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee of CEIC Hospital Universitario de Canarias, Tenerife, Spain with the reference number P.I.-2017/72 on October 30, 2017.
Informed Consent Statement
The study was conducted using aggregated pharmaceutical sales records and publicly available air quality data. No identifiable personal information was collected, and all data were anonymized at the pharmacy level. The requirement for informed consent was waived, as the study involved only secondary, non-identifiable data and posed no risk to individuals.
Data Availability Statement
The data that support the findings of this study are available from Servicio Canario de Salud but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of Servicio Canario de Salud.
Acknowledgments
The authors thank Gara Mesa-Ávila, María Cañada-Mancha, and Enrique Benítez-López (GSK Spain) for their support in accessing medication sales data. The authors acknowledge the use of OpenAI’s ChatGPT (GPT-4, OpenAI, San Francisco, CA, USA) for assistance in refining the manuscript text. However, all interpretations, analyses, and conclusions are the responsibility of the authors.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| SDI |
Saharan Dust Intrusions |
| COPD |
Chronic Pulmonary Obstructive Disease |
| SABA |
Short-Acting Beta-Agonists |
| ICS-LABA |
Inhaled Corticosteroid–Long-Acting Beta-Agonist |
References
- Wild, CP. Complementing the genome with an “exposome”: the outstanding challenge of environmental exposure measurement in molecular epidemiology. Cancer Epidemiol Biomarkers Prev. 2005;14(8):1847–50. [CrossRef]
- Wild, CP. The exposome: from concept to utility. Int J Epidemiol. 2012;41(1):24–32. [CrossRef]
- Miller GW, Jones DP. The nature of nurture: refining the definition of the exposome. Toxicol Sci. 2014;137(1):1–2. [CrossRef]
- Vermeulen R, Schymanski EL, Barabási AL, Miller GW. The exposome and health: where chemistry meets biology. Science. 2020;367(6476):392–6. [CrossRef]
- Goudie, AS. Desert dust and human health disorders. Environ Int. 2014;63:101–13. [CrossRef]
- Middleton, NJ. Desert dust hazards: a global review. Aeolian Res. 2017;24:53–63. [CrossRef]
- Prospero JM, Ginoux P, Torres O, Nicholson SE, Gill TE. Environmental characterization of global sources of atmospheric soil dust identified with the Nimbus 7 Total Ozone Mapping Spectrometer (TOMS) absorbing aerosol product. Rev Geophys. 2002;40(1):1002. [CrossRef]
- Querol X, Pey J, Pandolfi M, Alastuey A, Cusack M, Pérez N, et al. African dust contributions to mean ambient PM10 mass-levels across the Mediterranean Basin. Atmos Environ. 2009;43(28):4266–77. [CrossRef]
- Díaz J, Linares C, Carmona R, Russo A. Saharan dust intrusions in southern Europe: impacts on health and associated prevention strategies. Environ Int. 2021;146:106238.
- Pérez L et al. Coarse particles from Saharan dust and daily mortality. Epidemiology. 2008;19(6):800–7. [CrossRef]
- Schraufnagel DE. The health effects of ultrafine particles. Exp Mol Med. 2020;52(3):311–7. [CrossRef]
- Kanatani KT, Ito I, Al-Delaimy WK, Adachi Y, Mathews WC, Ramsdell JW, et al. Asian Desert Dust and Asthma Study Team. Desert dust exposure is associated with increased risk of asthma hospitalization in children. Am J Respir Crit Care Med. 2010 Dec 15;182(12):1475-81. [CrossRef]
- Mallone S, Stafoggia M, Faustini A, Gobbi GP, Marconi A, Forastiere F. Saharan dust and associations between particulate matter and daily mortality in Rome, Italy. Environ Health Perspect. 2011 Oct;119(10):1409-14. [CrossRef]
- Karanasiou A, Moreno N, Moreno T, Viana M, de Leeuw F, Querol X. Health effects from Sahara dust episodes in Europe: literature review and research gaps. Environ Int. 2012 Oct 15;47:107-14. [CrossRef]
- Reid CE, Brauer M, Johnston FH, Jerrett M, Balmes JR, Elliott CT. Critical Review of Health Impacts of Wildfire Smoke Exposure. Environ Health Perspect. 2016 Sep;124(9):1334-43. [CrossRef]
- Alman BL, Pfister G, Hao H, Stowell J, Hu X, Liu Y, et al. The association of wildfire smoke with respiratory and cardiovascular emergency department visits in Colorado in 2012: a case crossover study. Environ Health. 2016 Jun 4;15(1):64. [CrossRef]
- Kellogg CA, Griffin DW. Aerobiology and global dust transport. Trends Ecol Evol. 2006;21(11):638–44.
- Valavanidis A, Fiotakis K, Vlachogianni T. Airborne particulate matter and human health: toxicological assessment and importance of size and composition of particles for oxidative damage and carcinogenic mechanisms. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2008 Oct-Dec;26(4):339-62. [CrossRef]
- Tornevi A, Olstrup H, Forsberg B. Increase in daily asthma medication sales in association with air pollution levels in Greater Stockholm. Environ Epidemiol. 2023 Jun 22;7(4):e256. [CrossRef]
- Tiotiu AI, Novakova P, Nedeva D, Chong-Neto HJ, Novakova S, Steiropoulos P, et al. Impact of Air Pollution on Asthma Outcomes. Int J Environ Res Public Health. 2020 Aug 27;17(17):6212. [CrossRef]
- Watanabe M, Yamasaki A, Burioka N, Kurai J, Yoneda K, Yoshida A, et al. Correlation between Asian dust storms and worsening asthma in Western Japan. Allergol Int. 2011 Sep;60(3):267-75. [CrossRef]
- Hutchinson JA, Vargo J, Milet M, French NHF, Billmire M, Johnson J, et al. The San Diego 2007 wildfires and Medi-Cal emergency department presentations, inpatient hospitalizations, and outpatient visits: An observational study of smoke exposure periods and a bidirectional case-crossover analysis. PLoS Med. 2018 Jul 10;15(7):e1002601. [CrossRef]
- Henderson SB, Kosatsky T. The public health impact of wildfire smoke exposure. Environ Health Perspect. 2020;128(9):095001.
- Yang CY, Chiu HF. Dust storms and asthma visits in Taiwan. Inhal Toxicol. 2009;21(6–8):567–72.
- Patel M, Pilcher J, Reddel HK, Qi V, Mackey B, Armour C, et al. Metrics of salbutamol use as predictors of future adverse outcomes in asthma. Clin Exp Allergy. 2013;43(10):1144–51.
- Raherison C, Girodet PO. Epidemiology of COPD. Eur Respir Rev. 2009;18(114):213–21.
- Escuela-Escobar A, Perez-Garcia J, Martín-González E, González Martín C, Hernández-Pérez JM, González Pérez R. Impact of Saharan Dust and SERPINA1 Gene Variants on Bacterial/Fungal Balance in Asthma Patients. Int J Mol Sci. 2025 Feb 27;26(5):2158.
- Hernandez Y, Barbosa P, Corral S, Rivas S. An institutional analysis to address climate change adaptation in Tenerife (Canary Islands). Environ Sci Policy. 2018 Nov;89:184-191. [CrossRef] [PubMed] [PubMed Central]
- González-Pérez R, Poza-Guedes P, Pineda F, Galán T, Mederos-Luis E, Abel-Fernández E, et al. Molecular Mapping of Allergen Exposome among Different Atopic Phenotypes. Int J Mol Sci. 2023 Jun 21;24(13):10467. [CrossRef]
- Plaza V, Álvarez F, Calle M, Casanova C, Cosío BG, López-Viña A, et al. Clinical characteristics of patients with COPD in Spain: the EPI-SCAN study. Respir Med. 2011;105(12):1872–83.
- González-Pérez R, Poza-Guedes P, Pineda F, Castillo M, Sánchez-Machín I. Storage Mite Precision Allergy Molecular Diagnosis in the Moderate-to-Severe T2-High Asthma Phenotype. Int J Mol Sci. 2022 Apr 13;23(8):4297. [CrossRef]
- https://www3.gobiernodecanarias.org/medioambiente/calidaddelaire Last accessed on September 14, 2025.
- Alonso-Pérez S, Cuevas E, Querol X, Guerra JC, Pérez C. African dust source regions for observed dust outbreaks over the Subtropical Eastern North Atlantic region, above 25°N. J. Arid Environ.2012;78:100–109. [CrossRef]
- Rodríguez S, Cuevas E, Prospero JM, Alastuey A, Querol X, López-Solano J, García MI, Alonso-Pérez, S. Modulation of Saharan dust export by the North African dipole. Atmospheric Chem. Phys. 2015;15:7471–7486. [CrossRef]
- Tsamalis C, Chédin A, Pelon J, Capelle V. The seasonal vertical distribution of the Saharan Air Layer and its modulation by the wind. Atmospheric Chem. Phys. 2013;13:11235–11257. [CrossRef]
- Domínguez-Rodríguez A, Rodríguez S, Avanzas P, Jiménez-Sosa A, Hernández-Vaquero D, Rodríguez-Esteban M, et al. Impact of Saharan dust exposure on airway inflammation in patients with stable ischemic heart disease. Eur J Prev Cardiol. 2020;27(13):1459-61. [CrossRef]
- Kok JF, Mahowald NM, Evans SN, Scanza RA, Dufresne JL, Albani S, et al. Contribution of the world’s main dust source regions to the global dust cycle. Atmos Chem Phys. 2021;21(15):8169-93.
- Kim D, Mahowald N, Albani S, Chuang PY, Perlwitz JP. Where dust comes from: Global assessment of dust source regions using a multi-model framework. J Geophys Res Atmos. 2024;129(9):e2023JD041377.
- Bredeck G, Dobner J, Stahlmecke B, Wadinga F, Herrmann H, Rossi A, et al. Inhalable Saharan dust induces oxidative stress, NLRP3 inflammasome activation, and inflammatory cytokine release. Environ Int. 2023;173:107732. [CrossRef]
- Caceres L, Abogunloko T, Malchow S, Ehret F, Merz J, Li X, Sol Mitre L, et al. Molecular mechanisms underlying NLRP3 inflammasome activation and IL-1β production in air pollution fine particulate matter (PM2.5)-primed macrophages. Environ Pollut. 2024 Jan 15;341:122997.
- Grytting VS, Refsnes M, Øvrevik J, Halle MS, Schönenberger J, van der Lelij R, et al. Respirable stone particles differ in their ability to induce cytotoxicity and pro-inflammatory responses in cell models of the human airways. Part Fibre Toxicol. 2021 May 6;18(1):18. [CrossRef]
- Bredeck G, Dobner J, Stahlmecke B, Fomba KW, Herrmann H, Rossi A, et al. Saharan dust induces NLRP3-dependent inflammatory cytokines in an alveolar air-liquid interface co-culture model. Part Fibre Toxicol. 2023 Oct 20;20(1):39. [CrossRef]
- Georgakopoulou VE, Papalexis P, Zisi K, Adamou A, Lykouras D, Koulouris NG, et al. Saharan dust and respiratory health: Understanding the impact and implications. Exp Ther Med. 2024;27(3):133.
- Zhang X, Zhao C, Liu L, Liu D, Gong S, An Z, et al. A systematic review of global desert dust and associated health outcomes. Atmosphere. 2016;7(12):158.
- Tornevi A, Forsberg B, Axelsson G, Loibl W, Stockfelt L, Boström SN. Increase in daily asthma medication sales in association with ambient air pollutant levels in an urban population: a panel study. BMJ Open. 2023;13(5):e10403006.
- Yitshak-Sade M, Novack V, Katra I, Gorodischer R, Tal A, Novack L. Non-anthropogenic dust exposure and asthma medication purchase in children. Eur Respir J. 2015 Mar;45(3):652-60. [CrossRef]
- Williams AM, Phaneuf DJ, Barrett MA, Su JG. Short-term impact of PM2.5 on contemporaneous asthma medication use: Behavior and the value of pollution reductions. Proc Natl Acad Sci U S A. 2019 Mar 19;116(12):5246-5253. [CrossRef]
- López-Villarrubia E, Costa Estirado O, Iñiguez Hernández C, Ballester Díez F. Do Saharan Dust Days Carry a Risk of Hospitalization From Respiratory Diseases for Citizens of the Canary Islands (Spain)? Arch Bronconeumol. 2021;57(7):464-70. [CrossRef]
- Beasley R, Kiriakou C, Bateman ED, O’Byrne PM, Reddel HK. The ICS/formoterol reliever therapy regimen in asthma. Eur Respir J. 2023;61(6):2202079.
- Reddel HK, Hancox RJ, Abramson MJ, Papi A, FitzGerald JM, Buhl R, et al. A practical guide to implementing SMART in asthma. J Allergy Clin Immunol Pract. 2022;10(10):2568-79.e3.
- Tanskanen A, Taipale H, Koponen M, Tolppanen AM, Hartikainen S, Ahonen R, et al. From prescription drug purchases to drug use periods – a second generation method (PRE2DUP). BMC Med Inform Decis Mak. 2015;15:21. [CrossRef]
- Galozy A, et al. Pitfalls of medication adherence approximation through dispensing or prescription data: implications for research and practice. Res Social Adm Pharm. 2020;16(7):986-90.
- Fussell JC, Ramirez-Aguilar M, Schraufnagel DE. Mechanisms underlying the health effects of desert sand dust. Environ Sci Pollut Res Int. 2021;28(10):11105-22.
- Perez-Garcia J, González-Carracedo M, Espuela-Ortiz A, Hernández-Pérez JM, González-Pérez R, Sardón-Prado O, et al. The upper-airway microbiome as a biomarker of asthma exacerbations despite inhaled corticosteroid treatment. J Allergy Clin Immunol. 2023 Mar;151(3):706-715. [CrossRef]
- Tischer C, Zock JP, Valkonen M, Doekes G, Guerra S, Heederik D, et al. Predictors of microbial agents in dust and respiratory health in the Ecrhs. BMC Pulm Med. 2015 May 2;15:48.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).