1. Introduction
Mitral valve prolapse (MVP) is the most frequent valvular abnormality in developed countries, with a prevalence estimated at 2–3% in the general population [
1]. It is defined echocardiographically as the systolic billowing of one or both mitral valve leaflets at least 2 mm beyond the plane of the mitral annulus into the left atrium, best visualized in the parasternal long−axis view [
2,
3]. Although most individuals with MVP experience a benign clinical course, the disorder has attracted enduring attention because of its potential complications, including mitral regurgitation (MR), infective endocarditis, atrial and ventricular arrhythmias, and, in selected cases, sudden cardiac death (SCD) [4−8]. In particular, a subgroup characterized by bileaflet or myxomatous prolapse, mitral annular disjunction (MAD), and complex ventricular arrhythmias (VAs) has recently been recognized as a distinct arrhythmogenic phenotype with heightened risk of adverse outcomes [
9,
10].
The relevance of MVP extends beyond the general population to athletic cohorts. Regular participation in competitive sports subjects the cardiovascular system to sustained hemodynamic load and adrenergic stimulation, which may amplify the arrhythmic substrate in susceptible individuals. Although MVP is often detected incidentally during pre−participation cardiovascular screening, its identification in an athlete carries important clinical and medico−legal implications, raising questions regarding eligibility for competition, need for further diagnostic testing, and intensity of follow−up [
11,
12]. Concern stems primarily from the potential contribution of MVP to exercise−related SCD, which remains a leading cause of mortality in young athletes [
10,
13].
Accurate assessment of MVP prevalence in athletes is therefore essential to inform clinical practice. However, estimates have historically been inconsistent, largely due to methodological heterogeneity. Earlier echocardiographic investigations, particularly those performed prior to 1999, frequently applied less specific diagnostic criteria based on M−mode or apical four−chamber imaging, leading to overestimation of prevalence [14−21]. More recent criteria [
2,
3], which account for the saddle−shaped nonplanarity of the mitral annulus [
22,
23], define MVP more strictly as leaflet displacement >2 mm beyond the annulus in the parasternal long−axis view. Adoption of these standards has reduced false positive diagnoses and led to substantially lower prevalence estimates. In a recent systematic review of echocardiographic studies in heterogeneous populations, the pooled prevalence of MVP decreased from 7.8% in studies conducted before 1999 to 2.2% in those performed thereafter [
24].
Despite these advances, the true burden of MVP in athletes remains uncertain. Studies have reported variable rates across sports disciplines, competitive levels, and geographic regions. The lack of uniform diagnostic definitions, differences in study populations, and the absence of pooled analyses have limited the interpretability of available data. Moreover, while MVP is generally considered compatible with athletic participation, its association with VAs and SCD in selected cases underscores the need for precise epidemiological characterization in this setting [
10,
13,
25].
For these reasons, a systematic review of the literature on MVP prevalence among athletes is warranted. By synthesizing evidence from echocardiographic studies performed in sporting populations and contextualizing them within the evolution of diagnostic criteria, the present work aims to provide a reliable estimate of MVP prevalence in athletes. This information is crucial for clinicians involved in pre−participation evaluation, for sports cardiology specialists assessing eligibility, and for researchers seeking to clarify the arrhythmogenic potential of MVP in athletic cohorts.
2. Materials and Methods
This systematic review was performed according to the PRISMA guidelines [
26], and was registered in INPLASY database (INPLASY202590064).
2.1. Search Strategy
We conducted a comprehensive literature search to identify studies evaluating the prevalence of MVP among athletes. The search was performed in PubMed, Scopus, and EMBASE databases from inception through August 2025, without language restrictions. The search terms included combinations of “mitral valve prolapse,” “athletes,” “sports,” “echocardiography,” and “pre−participation screening.” Boolean operators and Medical Subject Headings (MeSH) were applied to maximize sensitivity. In addition, the reference lists of relevant systematic reviews and included studies were screened manually to identify additional eligible reports.
2.2. Eligibility criteria
Studies were considered eligible if they met the following criteria: (1) original research published in peer−reviewed journals; (2) population of competitive or recreational athletes undergoing cardiovascular evaluation; (3) MVP diagnosis based on echocardiography or, in autopsy studies, pathological examination; and (4) report of MVP prevalence or raw numbers allowing calculation. No restriction was applied on athlete age, sex, type of sport, or competitive level. We excluded case reports, editorials, narrative reviews, abstracts without sufficient data, and studies in non−athletic populations. When duplicate publications from the same cohort were identified, the most comprehensive or recent report was included.
2.3. Study Selection and Data Extraction
Two investigators independently screened titles and abstracts retrieved from the search to exclude irrelevant records. Full texts were subsequently assessed for eligibility. Disagreements were resolved by consensus with a third reviewer. Data extraction was performed independently by three experienced cardiologists (A.S., M.B., and G.L.N.) in August 2025, using a standardized data collection form. For each study, the following information was collected: author, year of publication, country, population characteristics (type of sport, sample size, age, sex distribution), study design, diagnostic criteria for MVP, number and prevalence of MVP cases, and presence of arrhythmic complications, including VAs and supraventricular arrhythmias (SVAs). When available, follow−up information and clinical outcomes were also recorded. Extracted data were cross−checked for consistency and compiled in a summary table.
2.4. Risk of Bias Assessment
The methodological quality of the included studies was assessed using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross− Sectional Studies [
27]. This instrument evaluates 14 domains, including clarity of the research question, population definition, participation rate, exposure and outcome measures, length of follow−up (if applicable), and adequacy of statistical analyses. Each study was independently evaluated by the three cardiologists who extracted the data, and judgments were categorized as good, fair, or poor quality according to NIH guidance.
2.5. Statistical Analysis
Prevalence estimates from each study were calculated as the number of MVP cases divided by the total sample size. Exact binomial 95% confidence intervals were computed using the Wilson score method. For pooled analyses, proportions were transformed using the Freeman–Tukey double arcsine method to stabilize variance. A random−effects model (DerSimonian–Laird) was applied to account for between−study heterogeneity. Both an overall pooled prevalence and a sensitivity analysis restricted to unselected, contemporary athlete cohorts were performed. Results are displayed as a forest plot, with individual study estimates and corresponding 95% confidence intervals, alongside pooled estimates. Statistical analyses were conducted using Comprehensive Meta−Analysis version 3.0 (Biostat, Englewood, NJ, USA).
3. Results
3.1. Study Selection
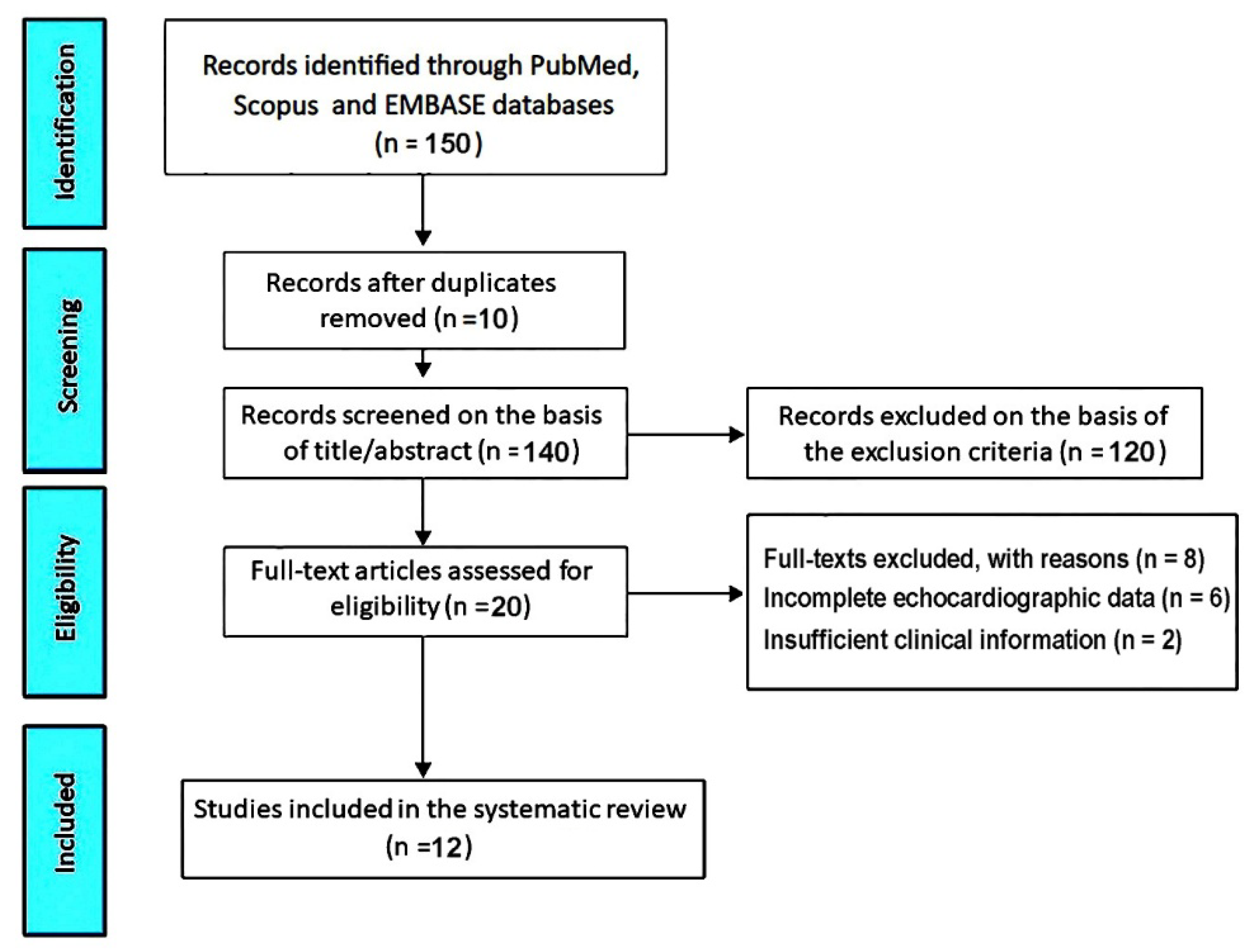
The initial database search retrieved 150 records. After removal of 10 duplicates, 140 titles and abstracts were screened. Of these, 120 records were excluded because they were clearly irrelevant, did not involve athletes, or did not evaluate MVP. Twenty full−text articles were assessed for eligibility. Among these, 8 studies were excluded: six due to incomplete or non−standardized echocardiographic data and two because of insufficient clinical information. Ultimately, 12 studies [28−39] met all inclusion criteria and were included in the present systematic review (
Figure 1).
Clinical characteristics, diagnostic criteria, and main findings of the studies included in the systematic review are summarized in
Table 1.
This systematic review included twelve studies published between 1987 and 2024, evaluating MVP prevalence in athletes and young sporting populations. Together, these studies enrolled a total of 19,463 participants, with the majority being male athletes, though the proportion of males varied across cohorts (range: 51–100%). Specifically, male representation was 71% in U.S. intercollegiate athletes [
28], 83% in U.S. football players [
29], 67% in young U.S. athletes undergoing echocardiography [
31], 100% in italian youth soccer players [
32], 100% in elite african footballers screened in Switzerland/Gabon [
33], 73% in italian athletes with VAs [
34], 76% in italian paralympic athletes [
35], 90% in turkish multisport youth athletes [
36], 100% in canadian draft−eligible hockey players [
37], 67% in a large italian registry of competitive athletes [
38], and 51% in the U.S. sudden cardiac death registry [
39]. One large U.S. cohort (N=2,997) did not specify sex distribution [
30].
The studies originated from multiple countries, including the USA (five studies), Italy (four studies), Switzerland (one study), Turkey (one study), and Canada (one study).
The mean age of athletes spanned a wide spectrum across studies. The youngest cohort was composed of pre−adolescent soccer players in Italy, with a mean age of 11 years [
32], whereas the oldest were italian paralympic athletes, with a mean age of 35 years [
35]. Across the broader cohorts, mean ages ranged from 13 years in turkish youth multisport athletes [
36] to 30 years in a large Italian registry of competitive athletes [
38]. Other studies reported mean ages in the late teens, including U.S. intercollegiate athletes (19.3 years) [
28], American football players (19 years) [
29], young U.S. athletes undergoing echocardiography (17.5 years) [
31], elite Swiss footballers (18.6 years) [
33], canadian draft− eligible hockey players (18 years) [
37], and U.S. sudden cardiac death cases (22 years) [
39]. Some cohorts had unspecified ages [
30].
3.2. Clinical Data
Overall, the pooled mean age across studies clustered in late adolescence and early adulthood (approximately 17–22 years), reflecting the predominant focus on screening competitive youth and young adult athletic cohorts. However, the inclusion of both very young pre−adolescents and older paralympic/elite athletes highlights the broad applicability of MVP screening across diverse athletic populations worldwide.
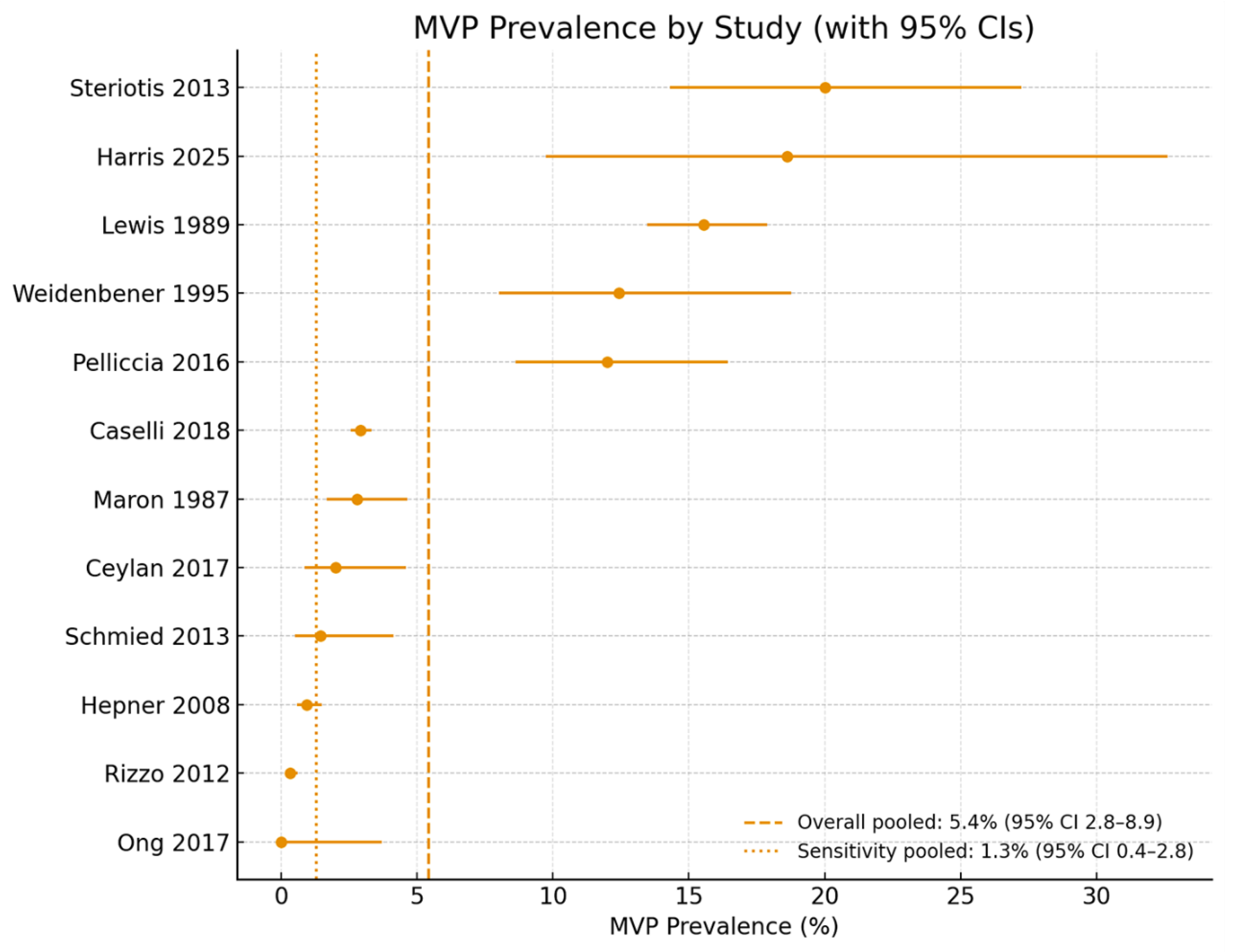
The pooled prevalence of MVP across all included studies was 2.4% (407 cases among 19,463 athletes). Substantial variation was observed across individual cohorts, with estimates ranging from as low as 0.2% in canadian elite hockey players [
37] to as high as 20% in an italian referral cohort of athletes with VAs [
34]. Excluding this highly selected group, most general athletic populations demonstrated prevalence rates between 0.3% and 3%, consistent with estimates in the general population.
Early north american studies [
28,
29] reported higher prevalence (11–15.6%), likely reflecting broader echocardiographic criteria and greater reliance on M−mode displacement. In contrast, more recent European cohorts employing standardized parasternal long−axis criteria [
33,
38] reported lower rates of 1–3%. These discrepancies highlight the impact of evolving diagnostic standards and differences in study populations.
When all studies were combined using a random−effects model, the overall pooled prevalence was 5.4% (95% CI 2.8–8.9%). This estimate was disproportionately influenced by older studies with permissive criteria and by arrhythmia−focused cohorts. A sensitivity analysis restricted to contemporary, unselected athletic populations [
31,
32,
33,
36,
37,
38] yielded a pooled prevalence of 1.3% (95% CI 0.4–2.8%), closely matching community−based estimates.
A forest plot illustrating prevalence by study, along with pooled and sensitivity estimates, is presented in
Figure 2.
From a clinical perspective, most athletes with MVP were asymptomatic at the time of assessment. Palpitations, exertional intolerance, or chest discomfort were occasionally reported but were generally nonspecific and not predictive of structural abnormalities. Physical examination yielded variable findings: systolic clicks or murmurs were sometimes present but lacked sufficient sensitivity or specificity to guide diagnosis, underscoring the superiority of echocardiography in confirming MVP. Importantly, the Harris registry [
39] provided a contrasting autopsy−based perspective, identifying MVP as an under−recognized cause of sudden cardiac death in young athletes. Pathological features associated with these fatal cases included bileaflet prolapse, myxomatous leaflet degeneration, and localized myocardial fibrosis, particularly of the posterolateral left ventricular wall. These findings highlight that while MVP is generally a benign incidental finding in athletes, a small subset with high−risk morphologic variants may carry an arrhythmogenic potential that remains clinically silent during life.
3.3. ECG and Arrhythmia Data
Electrocardiographic data and arrhythmia outcomes were variably reported, with greater emphasis on VAs than SVAs.
Ventricular arrhythmias were most comprehensively assessed in two studies. Steriotis et al. [
34] reported that MVP was present in 20% of athletes referred specifically for VAs, confirming a potential causal association. Caselli et al. [
38] identified VAs in 0.8% of athletes with MVP in their large competitive cohort, often in association with MR or MAD. Other studies noted isolated cases of VAs, including Pelliccia et al. [
35] in paralympic athletes and Ceylan et al. [
36] in pediatric athletes. Schmied et al. [
33], in contrast, reported no VAs among elite African footballers with MVP.
Supraventricular arrhythmias were less common but still observed. Caselli et al. [
38] reported one case of atrial fibrillation requiring hospitalization during long−term follow−up. Ceylan et al. [
36] detected a single case of supraventricular tachycardia by Holter monitoring in a pediatric athlete. Pelliccia et al. [
35] documented four cases of SVAs in paralympic athletes, corresponding to 1.5% of that cohort. All other studies did not report SVAs, indicating that these events were sporadic and uncommon.
Overall, arrhythmia prevalence was low in unselected athletic populations, but certain subgroups—particularly those with bileaflet prolapse, MR, or MAD—appeared more vulnerable to rhythm disturbances. The Harris registry [
39] confirmed the arrhythmogenic potential of MVP, documenting frequent myocardial fibrosis in autopsied sudden death cases, most often in the posterolateral left ventricle.
3.4. Echocardiographic Data
Echocardiography emerged as the cornerstone diagnostic modality in virtually all prospective investigations, given its non−invasive nature, widespread availability, and capacity to provide direct visualization of mitral valve morphology and motion. The prevailing diagnostic definition was systolic displacement of one or both mitral leaflets ≥2 mm beyond the annular plane in the parasternal long−axis view, consistent with modern echocardiographic standards [
32,
35,
38]. Earlier investigations, particularly those from the 1980s, frequently incorporated M−mode criteria, with prolapse defined as posterior displacement ≥3 mm [
28,
29]. This methodological difference likely accounts for the higher prevalence estimates reported in those cohorts, as M−mode has a greater propensity to overestimate leaflet excursion. In contrast, Schmied et al. [
33] applied more stringent echocardiographic thresholds, requiring both systolic displacement and leaflet thickening >5 mm, which reduced the observed prevalence to 1.4%.
Across the pooled populations, MVP identified by echocardiography was generally mild in severity and not associated with clinically significant mitral regurgitation in most athletes [
31,
34,
38]. Morphologic variants such as leaflet thickening, redundancy, or MAD were described in selected cohorts but not consistently quantified [
37,
39]. The clinical relevance of these features remains debated, although accumulating evidence suggests they may contribute to arrhythmic risk in certain subgroups [
39]. Importantly, echocardiography frequently identified MVP in athletes with normal physical examination and surface ECG findings [
32,
36], reinforcing its utility as a sensitive diagnostic modality within preparticipation cardiovascular screening programs. Collectively, these findings highlight that while MVP in athletes is typically benign and of limited hemodynamic consequence, its detection relies almost exclusively on imaging, underscoring echocardiography’s central role in both epidemiologic surveillance and individual risk stratification.
3.5. Follow−up Data
Among all included studies, only Caselli et al. [
38] provided systematic long−term follow−up. Over a mean of 8 ± 2 years, the prognosis of MVP in athletes was generally benign. No cases of sudden cardiac death occurred, though a small subset required mitral valve surgery for progressive regurgitation, and one athlete developed atrial fibrillation necessitating hospitalization. Risk stratification identified arrhythmias, MR, and MAD as predictors of adverse outcomes.
The Harris registry [
39] added complementary retrospective evidence, revealing that MVP may underlie 1.8% of sudden cardiac deaths in young athletes. Pathological analysis highlighted bileaflet prolapse and ventricular fibrosis as potential substrates for malignant arrhythmias. While not a prospective follow−up study, these findings underscore the importance of recognizing high−risk MVP phenotypes.
3.6. Risk of Bias Assessment
The 12 included studies were evaluated using the NIH Quality Assessment Tool for Observational Cohort and Cross−Sectional Studies. The Cohen’s Kappa coefficient for the agreement between the reviewers in the RoB assessment indicated substantial agreement, κ = 0.81. Eleven studies scored 5/14 and were rated
fair quality, while one [
38] scored 8/14 and was judged
good quality (Table 2).
All studies clearly reported their objectives and defined their populations. However, participation rates, sample size calculations, blinding, and adjustment for confounding were not described. Most investigations were cross−sectional pre−participation screenings, lacking temporal sequence or repeated assessments, with the exception of Caselli et al. [
38], which included longitudinal follow−up. Outcome measures—MVP diagnosis by echocardiography—were consistently well defined, supporting internal validity.
4. Discussion
4.1. Summary of Findings
Overall, the twelve studies included in this systematic review show that MVP is uncommon but consistently present in athletic populations. Across all cohorts, 407 cases were identified among 19,463 athletes, corresponding to a crude pooled prevalence of 2.4%. Individual study estimates varied widely, ranging from as low as 0.2% in canadian elite hockey players to as high as 20% in an italian referral cohort enriched for VAs. When all studies were combined in a random−effects meta−analysis, the overall prevalence was 5.4% (95% CI 2.8–8.9%), although this figure was strongly influenced by older investigations using permissive echocardiographic definitions and selected high−risk populations. In contrast, a sensitivity analysis restricted to contemporary, unselected athletic cohorts yielded a pooled prevalence of 1.3% (95% CI 0.4–2.8%), closely matching general population estimates.
This heterogeneity is largely attributable to methodological differences across decades. Early north american studies, conducted before the widespread adoption of standardized two−dimensional echocardiography, often relied on M−mode displacement or apical four−chamber views, which are now recognized to overestimate leaflet excursion. As a result, reported prevalence figures reached 11–16% in those cohorts. More recent european studies employing parasternal long−axis criteria, aligned with current diagnostic recommendations, consistently reported lower prevalence rates between 0.3% and 3%. These temporal and methodological shifts underscore the importance of diagnostic standardization in interpreting epidemiological trends and in comparing athletes with the general population.
Beyond prevalence, several studies provided insight into the clinical profile of MVP in athletes. Most individuals were asymptomatic at the time of screening, and physical examination alone proved insufficient to reliably detect MVP. Arrhythmic manifestations were generally infrequent, but VAs were observed more often than SVAs, particularly in association with bileaflet prolapse, leaflet redundancy, or moderate−to−severe MR. These associations mirror the emerging recognition of an arrhythmogenic MVP phenotype, in which structural features such as MAD or localized myocardial fibrosis may provide the substrate for malignant VAs.
Longitudinal data remain limited. Only one large prospective cohort provided systematic follow−up, confirming that the natural history of MVP in athletes is usually benign, with most individuals remaining asymptomatic and free from progression to severe regurgitation or sustained arrhythmias. Nevertheless, a minority of athletes did experience adverse outcomes, including atrial fibrillation, mitral valve surgery, or incident VAs during follow−up. Taken together, these findings suggest that while MVP should not generally preclude athletic participation, careful attention to high−risk morphologic variants is warranted to guide individualized surveillance and, where appropriate, sport eligibility decisions.
4.2. Comparison Between MVP Prevalence in Athletes and in the General Population
When comparing the present findings with data from the general population, it becomes evident that the prevalence of MVP in athletes does not substantially differ from that reported in unselected cohorts. Large−scale epidemiological studies based on modern echocardiographic criteria consistently demonstrate a prevalence of approximately 2–3% in community−based samples [
1,
2]. Earlier population studies, particularly those relying on M−mode imaging and less specific definitions, reported much higher rates—up to 5–7% or more [14−21]. The discrepancy has since been attributed to methodological limitations, including overdiagnosis due to annular nonplanarity and reliance on apical four−chamber views. Our systematic review aligns with these observations, showing that athletic cohorts evaluated after 1999, using stricter criteria, demonstrate prevalence estimates within the same range as the general population.
This similarity is important, as it suggests that athletic training itself does not predispose to MVP development. Instead, the presence of MVP among athletes reflects the background prevalence of the condition in the general population. Of note, recent reviews and meta−analyses have emphasized that, although MVP is common, the majority of cases carry a benign prognosis, and only a minority evolve toward significant MR, arrhythmias, or adverse outcomes [
40,
41]. This is consistent with the data observed in athletes, in whom follow−up confirmed a predominantly favorable course.
The potential arrhythmic burden of MVP deserves particular consideration. Population−based studies indicate that VAs are observed in 20–30% of MVP cases, though often in low density and without clinical significance [
42]. Supraventricular arrhythmias, most commonly atrial fibrillation, are generally associated with long−standing MR or left atrial dilation [
43,
44]. Within athletic populations, the distribution of arrhythmias appears similar, with VAs more common than SVAs, and typically linked to concomitant structural abnormalities [
45]. Thus, current evidence does not suggest that athletes with MVP are at uniquely elevated arrhythmic risk compared with non−athletes, though heightened vigilance is warranted in the small subset with bileaflet prolapse, MAD, or myocardial fibrosis [
10,
13,
46,
47].
The consistency of prevalence rates across athletes and the general population reinforces the concept that MVP is not exercise−induced but rather a pre−existing structural condition occasionally unmasked during sports screening. Nevertheless, because of the implications for eligibility and SCD prevention, its detection in athletes remains clinically relevant.
4.3. Clinical Implications for Screening, Eligibility, and Surveillance in Athletes with MVP
The detection of MVP in athletes has several important clinical implications for screening, eligibility, and longitudinal surveillance. Pre−participation cardiovascular evaluation represents a cornerstone of strategies to prevent sudden cardiac death in young athletes [
48]. While most guidelines recommend history and physical examination as the initial screening tools, echocardiography may be considered in selected individuals with abnormal auscultatory findings, symptoms, or family history of sudden death [
11]. The identification of MVP in such contexts is often incidental and should not, in itself, lead to automatic disqualification from competitive sports. Instead, management should be individualized based on the presence and severity of MR, arrhythmic burden, and structural risk markers such as bileaflet prolapse or MAD [
49].
From an eligibility perspective, international consensus statements emphasize that athletes with mild MVP and no evidence of significant regurgitation or arrhythmias can safely participate in all competitive sports [
12]. Restrictions are generally reserved for those with high−risk features, including moderate−to−severe mitral regurgitation, left ventricular dysfunction, prior syncope of arrhythmic origin, or documented complex VAs during exercise testing [
50,
51]. These recommendations highlight the importance of comprehensive evaluation that extends beyond echocardiographic diagnosis to include ambulatory electrocardiographic monitoring and exercise testing. In selected cases with concerning features, cardiac magnetic resonance imaging may further refine risk stratification by detecting fibrosis of the inferolateral ventricular wall or papillary muscles [
40,
52,
53].
Surveillance strategies should be tailored to the individual risk profile. Athletes with uncomplicated MVP should undergo periodic reassessment with clinical evaluation and echocardiography every few years, while those with regurgitation or arrhythmic manifestations warrant more frequent follow−up [
54]. Importantly, the recognition of arrhythmogenic MVP underscores the need for ongoing vigilance even in young, asymptomatic athletes, as exercise−induced adrenergic stimulation may exacerbate latent electrical instability. Shared decision−making, involving athletes, families, and sports medicine teams, is crucial to balance the low absolute risk of adverse events with the psychological and social benefits of sports participation.
In summary, the clinical approach to MVP in athletes requires a nuanced integration of structural, electrical, and functional data. Universal disqualification is unwarranted; instead, careful stratification enables the majority of athletes with MVP to safely engage in competitive activity, while ensuring timely identification and protection of the minority at increased risk.
4.4. Chest Shape Assessment in Athletes
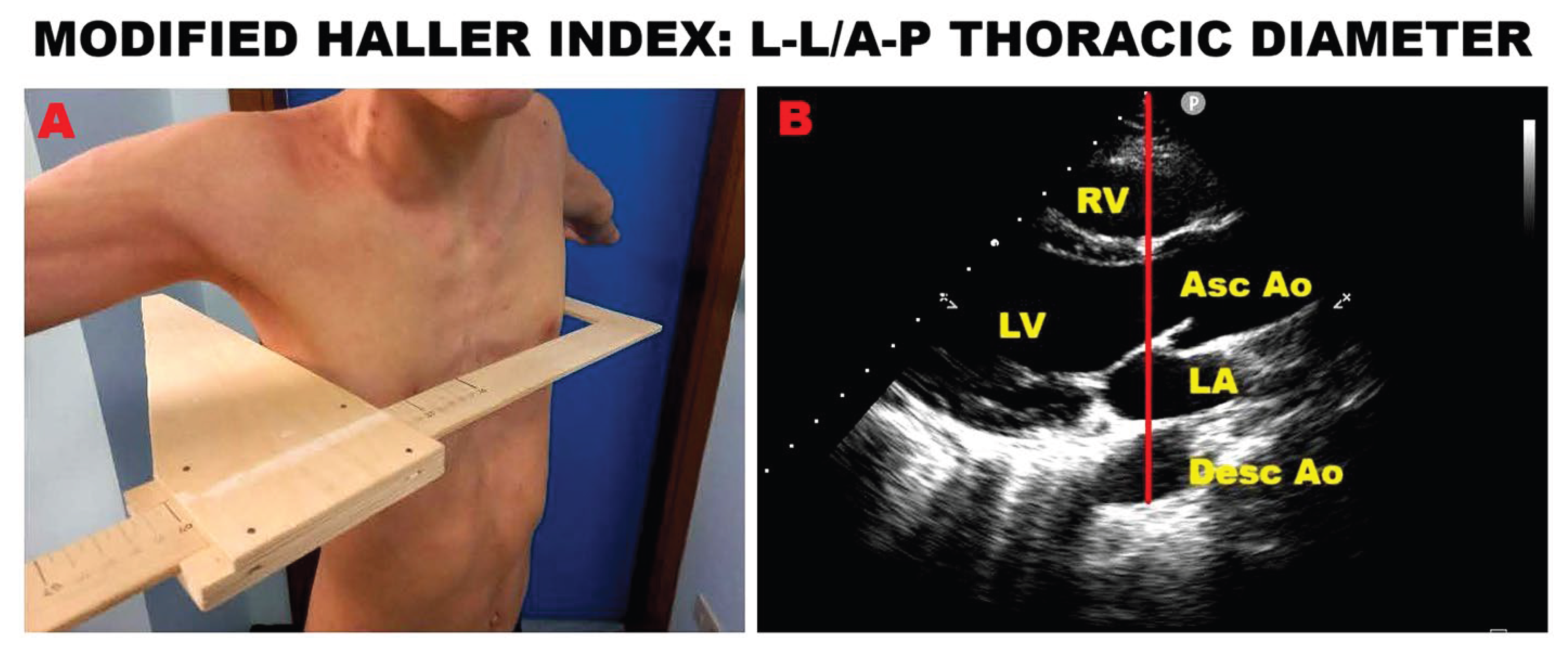
In recent years, our research group developed the modified Haller index (MHI), a novel, noninvasive anthropometric measure of chest shape conformation [
55]. The MHI is calculated by dividing the latero−lateral external thoracic diameter—measured with a rigid ruler and level at the lower third of the sternum—by the antero−posterior (A−P) internal thoracic diameter, obtained from the echocardiographic parasternal long−axis view as the distance between the true apex of the sector and the posterior wall of the descending aorta located just behind the left atrium. In previous studies, we showed that MVP individuals often present with a concave chest wall and/or varying degrees of anterior sternal depression or pectus excavatum (PE) [
56], along with mild reductions in basal longitudinal strain on speckle−tracking echocardiography [
57], non−hemodynamically significant MR during exercise stress echocardiography, and favorable mid− to long−term outcomes [
58]. Importantly, these clinical and imaging features were most commonly observed in MVP patients with an MHI greater than 2.5 and/or an A−P thoracic diameter of ≤13.5 cm.
An example of MHI assessment in a young athlete with PE is shown in
Figure 3.
Chest wall conformation, particularly the A−P thoracic diameter, has been increasingly recognized as an important determinant of cardiovascular findings in athletes. A narrow A−P diameter (≤13.5 cm) or concave thoracic shape has been shown to predispose to MVP, most often associated with only trivial MR and benign forms of MAD [
59]. According to the “mechanical theory,” extrinsic sternal compression and altered chest geometry exert abnormal traction on the submitral apparatus, promoting leaflet billowing and subtle annular disjunction without causing major myocardial injury.
Athletes with this chest phenotype frequently demonstrate false−positive results on exercise stress testing and stress echocardiography, with apparent wall motion abnormalities that do not correspond to obstructive coronary artery disease (CAD). Indeed, such individuals typically exhibit a low prevalence of obstructive CAD, and their abnormal exercise findings are better explained by dynamic ventricular dyssynchrony and paradoxical septal motion induced by thoracic compression [
60].
Incorporating noninvasive chest shape assessment, either through the MHI or echocardiographic A−P diameter measurement, into pre−participation evaluations may therefore help differentiate benign structural variants from clinically relevant phenotypes. This strategy can prevent unnecessary sports disqualification while ensuring appropriate surveillance for the small subgroup at risk.
4.5. Limitations of the Included Studies
Several limitations of the included studies should be acknowledged. First, there was considerable heterogeneity across the 12 investigations in terms of population characteristics, competitive level, and type of sport, which complicates direct comparisons and the pooling of prevalence estimates. Most cohorts consisted of young male soccer players or mixed athletic groups from Europe and North America, leaving important populations—such as female athletes, endurance sports, and athletes from non−Western regions—underrepresented. Second, diagnostic definitions of MVP were inconsistent. Early studies often relied on M−mode or apical four−chamber displacement, approaches now recognized to overestimate prevalence. More recent investigations employed standardized parasternal long−axis criteria, yet the coexistence of both methods across the literature introduces variability and limits comparability. Third, three studies had a retrospective design, increasing the risk of selection and information bias, particularly in the assessment of arrhythmic outcomes and follow−up data. Fourth, longitudinal follow−up was largely absent; only one cohort systematically reassessed athletes over time, restricting conclusions on disease progression and prognosis. Finally, only a minority of studies reported participation rates, sample size or power calculations, or statistical adjustment for confounding variables, further weakening the robustness of the evidence base.
5. Conclusions
This systematic review of 12 studies including nearly 20,000 athletes shows that MVP is uncommon but consistently present. Across all cohorts, the pooled prevalence was 2.4%; a random−effects meta−analysis yielded 5.4% (95% CI 2.8–8.9%), driven by older permissive definitions and referral cohorts. Restricting analyses to contemporary, unselected athletes, prevalence was 1.3% (95% CI 0.4–2.8%), similar to the general population. Most cases were mild and hemodynamically insignificant, supporting continued sports participation in the majority. Arrhythmic events were infrequent but clustered in subgroups with high−risk phenotypes such as bileaflet prolapse, leaflet redundancy, or significant regurgitation. These findings reinforce the generally benign prognosis of MVP in athletes, while highlighting the need for individualized surveillance. Future multicenter, prospective studies with standardized imaging and long−term follow−up are required to better define arrhythmic risk.
Author Contributions
Conceptualization, A.S. and G.L.N.; methodology, A.S. and G.L.N.; software, A.S.; validation, G.L.N.; formal analysis, A.S.; investigation, A.S.; resources, A.S.; data curation, A.S. and G.L.N..; writing—original draft preparation, A.S.; writing—review and editing, G.L.N.; visualization, M.L. and M.B.; supervision, M.L. and M.B.; project administration, A.S.; funding acquisition, A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Italian Ministry of Health, Ricerca Corrente IRCCS MultiMedica.
Institutional Review Board Statement
In accordance with the guidelines by the Comitato Etico Territoriale Lombardia 5, ethical review and approval were not required for this retrospective study.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data extracted from included studies will be publicly available on Zenodo (
https://zenodo.org).
Acknowledgments
The authors wish to thank Monica Fumagalli for their graphical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Freed, L.A.; Levy, D.; Levine, R.A.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical outcome of mitral−valve prolapse. N. Engl. J. Med. 1999, 341, 1–7. [Google Scholar] [CrossRef]
- Freed, L.A.; Benjamin, E.J.; Levy, D.; Larson, M.G.; Evans, J.C.; Fuller, D.L.; Lehman, B.; Levine, R.A. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J. Am. Coll. Cardiol. 2002, 40, 1298–1304. [Google Scholar] [CrossRef]
- Parwani, P.; Avierinos, J.F.; Levine, R.A.; Delling, F.N. Mitral Valve Prolapse: Multimodality Imaging and Genetic Insights. Prog. Cardiovasc. Dis. 2017, 60, 361–369. [Google Scholar] [CrossRef]
- Nishimura, R.A.; McGoon, M.D.; Shub, C.; Miller, F.A., Jr.; Ilstrup, D.M.; Tajik, A.J. Echocardiographically documented mitral−valve prolapse. Long−term follow−up of 237 patients. N. Engl. J. Med. 1985, 313, 1305–1309. [Google Scholar] [CrossRef]
- Devereux, R.B.; Hawkins, I.; Kramer−Fox, R.; Lutas, E.M.; Hammond, I.W.; Spitzer, M.C.; Hochreiter, C.; Roberts, R.B.; Belkin, R.N.; Kligfield, P.; et al. Complications of mitral valve prolapse. Disproportionate occurrence in men and older patients. Am. J. Med. 1986, 81, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Düren, D.R.; Becker, A.E.; Dunning, A.J. Long−term follow−up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J. Am. Coll. Cardiol. 1988, 11, 42–47. [Google Scholar] [CrossRef]
- Wilcken, D.E.; Hickey, A.J. Lifetime risk for patients with mitral valve prolapse of developing severe valve regurgitation requiring surgery. Circulation 1988, 78, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Marks, A.R.; Choong, C.Y.; Sanfilippo, A.J.; Ferré, M.; Weyman, A.E. Identification of high−risk and low−risk subgroups of patients with mitral−valve prolapse. N. Engl. J. Med. 1989, 320, 1031–1036. [Google Scholar] [CrossRef]
- Essayagh, B.; Sabbag, A.; Antoine, C.; Benfari, G.; Batista, R.; Yang, L.T.; Maalouf, J.; Asirvatham, S.J.; Michelena, H.I.; Enriquez−Sarano, M. Presentation and outcome of arrhythmic mitral valve prolapse. J. Am. Coll. Cardiol. 2020, 76, 637–649. [Google Scholar] [CrossRef]
- Basso, C.; Perazzolo Marra, M.; Rizzo, S.; De Lazzari, M.; Giorgi, B.; Cipriani, A.; Frigo, A.C.; Rigato, I.; Migliore, F.; Pilichou, K.; et al. Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation 2015, 132, 556–566. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Friedman, R.A.; Kligfield, P.; Levine, B.D.; Viskin, S.; Chaitman, B.R.; Okin, P.M.; Saul, J.P.; Salberg, L.; Van Hare, G.F.; et al. Assessment of the 12−lead ECG as a screening test for detection of cardiovascular disease in healthy general populations of young people (12–25 years of age): A scientific statement from the American Heart Association and the American College of Cardiology. J. Am. Coll. Cardiol. 2014, 64, 1479–1514. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2019 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef] [PubMed]
- Vriz, O.; Landi, I.; Eltayeb, A.; Limongelli, G.; Mos, L.; Delise, P.; Bossone, E.; D Andrea, A. Mitral Valve Prolapse and Sudden Cardiac Death in Athletes at High Risk. Curr. Cardiol. Rev. 2023, 19, e201222212066. [Google Scholar] [CrossRef]
- Markiewicz, W.; Stoner, J.; London, E.; Hunt, S.A.; Popp, R.L. Mitral valve prolapse in one hundred presumably healthy young females. Circulation 1976, 53, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Procacci, P.M.; Savran, S.V.; Schreiter, S.L.; Bryson, A.L. Prevalence of clinical mitral−valve prolapse in 1169 young women. N. Engl. J. Med. 1976, 294, 1086–1088. [Google Scholar] [CrossRef]
- McLarin, C.; Arensberg, D.; Felner, J.M.; Schlant, R.C. Echocardiographically determined mitral valve prolapse in male patients. South. Med. J. 1979, 72, 1416–1417. [Google Scholar] [CrossRef] [PubMed]
- Wann, L.S.; Grove, J.R.; Hess, T.R.; Glisch, L.; Ptacin, M.J.; Hughes, C.V.; Gross, C.M. Prevalence of mitral prolapse by two dimensional echocardiography in healthy young women. Br. Heart J. 1983, 49, 334–340. [Google Scholar] [CrossRef]
- Savage, D.D.; Garrison, R.J.; Devereux, R.B.; Castelli, W.P.; Anderson, S.J.; Levy, D.; McNamara, P.M.; Stokes, J., 3rd; Kannel, W.B.; Feinleib, M. Mitral valve prolapse in the general population. 1. Epidemiologic features: the Framingham Study. Am. Heart J. 1983, 106, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Bryhn, M.; Persson, S. The prevalence of mitral valve prolapse in healthy men and women in Sweden. An echocardiographic study. Acta Med. Scand. 1984, 215, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.R.; Sheikh, M.U.; Lee, K.J. Prevalence of mitral valve prolapse in presumably healthy Korean adults. Clin. Cardiol. 1985, 8, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Warth, D.C.; King, M.E.; Cohen, J.M.; Tesoriero, V.L.; Marcus, E.; Weyman, A.E. Prevalence of mitral valve prolapse in normal children. J. Am. Coll. Cardiol. 1985, 5, 1173–1177. [Google Scholar] [CrossRef]
- Levine, R.A.; Stathogiannis, E.; Newell, J.B.; Harrigan, P.; Weyman, A.E. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J. Am. Coll. Cardiol. 1988, 11, 1010–1019. [Google Scholar] [CrossRef]
- Levine, R.A.; Handschumacher, M.D.; Sanfilippo, A.J.; Hagege, A.A.; Harrigan, P.; Marshall, J.E.; Weyman, A.E. Three−dimensional echocardiographic reconstruction of the mitral valve, with implications for the diagnosis of mitral valve prolapse. Circulation 1989, 80, 589–598. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Bruno, A.; Lombardo, M.; Muti, P. Echocardiographic assessment of mitral valve prolapse prevalence before and after the year 1999: A systematic review. J. Clin. Med. 2024, 13, 6160. [Google Scholar] [CrossRef] [PubMed]
- Compagnucci, P.; Selimi, A.; Cipolletta, L.; Volpato, G.; Gasperetti, A.; Valeri, Y.; Parisi, Q.; Curcio, A.; Natale, A.; Dello Russo, A.; et al. Arrhythmic Mitral Valve Prolapse and Sports Activity: Pathophysiology, Risk Stratification, and Sports Eligibility Assessment. J. Clin. Med. 2024, 13, 1350. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta−analyses: the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: what are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.J.; Bodison, S.A.; Wesley, Y.E.; Tucker, E.; Green, K.J. Results of screening a large group of intercollegiate competitive athletes for cardiovascular disease. J. Am. Coll. Cardiol. 1987, 10, 1214–1221. [Google Scholar] [CrossRef]
- Lewis, J.F.; Maron, B.J.; Diggs, J.A.; Spencer, J.E.; Mehrotra, P.P.; Curry, C.L. Preparticipation echocardiographic screening for cardiovascular disease in a large, predominantly black population of collegiate athletes. Am. J. Cardiol. 1989, 64, 1029–1033. [Google Scholar] [CrossRef]
- Weidenbener, E.J.; Krauss, M.D.; Waller, B.F.; Taliercio, C.P. Incorporation of screening echocardiography in the preparticipation exam. Clin. J. Sport Med. 1995, 5, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Hepner, A.D.; Morrell, H.; Greaves, S.; Greaves, J.; Movahed, M.R. Prevalence of mitral valvar prolapse in young athletes. Cardiol. Young 2008, 18, 402–404. [Google Scholar] [CrossRef]
- Rizzo, M.; Spataro, A.; Cecchetelli, C.; Quaranta, F.; Livrieri, S.; Sperandii, F.; Cifra, B.; Borrione, P.; Pigozzi, F. Structural cardiac disease diagnosed by echocardiography in asymptomatic young male soccer players: implications for pre−participation screening. Br. J. Sports Med. 2012, 46, 371–373. [Google Scholar] [CrossRef]
- Schmied, C.; Di Paolo, F.M.; Zerguini, A.Y.; Dvorak, J.; Pelliccia, A. Screening athletes for cardiovascular disease in Africa: a challenging experience. Br. J. Sports Med. 2013, 47, 579–584. [Google Scholar] [CrossRef]
- Steriotis, A.K.; Nava, A.; Rigato, I.; Mazzotti, E.; Daliento, L.; Thiene, G.; Basso, C.; Corrado, D.; Bauce, B. Noninvasive cardiac screening in young athletes with ventricular arrhythmias. Am. J. Cardiol. 2013, 111, 557–562. [Google Scholar] [CrossRef]
- Pelliccia, A.; Quattrini, F.M.; Squeo, M.R.; Caselli, S.; Culasso, F.; Link, M.S.; Spataro, A.; Bernardi, M. Cardiovascular diseases in Paralympic athletes. Br. J. Sports Med. 2016, 50, 1075–1080. [Google Scholar] [CrossRef]
- Ceylan, Ö.; Meşe, T.; Gürsu, A.H. Using cardiovascular imaging modalities to determine cardiac disorders before starting sports activities. Turk. Kardiyol. Dern. Ars. 2017, 45, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Ong, G.; Connelly, K.A.; Goodman, J.; Leong−Poi, H.; Evangelista, V.; Levitt, K.; Gledhill, N.; Jamnik, V.; Gledhill, S.; Yan, A.T.; et al. Echocardiographic Assessment of Young Male Draft−Eligible Elite Hockey Players Invited to the Medical and Fitness Combine by the National Hockey League. Am. J. Cardiol. 2017, 119, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Caselli, S.; Mango, F.; Clark, J.; Pandian, N.G.; Corrado, D.; Autore, C.; Pelliccia, A. Prevalence and Clinical Outcome of Athletes With Mitral Valve Prolapse. Circulation 2018, 137, 2080–2082. [Google Scholar] [CrossRef]
- Harris, K.M.; Mackey−Bojack, S.; Fisher, G.; Nwaudo, D.; Maron, B.J. Arrhythmogenic Mitral Valve Prolapse Revisited: A Not Uncommon Cause of Youthful Sudden Death in Athletes and Women. Am. J. Med. 2025, 138, 156–160. [Google Scholar] [CrossRef]
- Basso, C.; Iliceto, S.; Thiene, G.; Perazzolo Marra, M. Mitral Valve Prolapse, Ventricular Arrhythmias, and Sudden Death. Circulation 2019, 140, 952–964. [Google Scholar] [CrossRef]
- Battaglia, V.; Santangelo, G.; Bursi, F.; Simeoli, P.; Guazzi, M. Arrhythmogenic Mitral Valve Prolapse and Sudden Cardiac Death: An Update and Current Perspectives. Curr. Probl. Cardiol. 2023, 48, 101724. [Google Scholar] [CrossRef]
- Spartalis, M.; Tzatzaki, E.; Spartalis, E.; Athanasiou, A.; Moris, D.; Damaskos, C.; Garmpis, N.; Voudris, V. Mitral valve prolapse: an underestimated cause of sudden cardiac death—A current review of the literature. J. Thorac. Dis. 2017, 9, 5390–5398. [Google Scholar] [CrossRef] [PubMed]
- Zilberszac, R.; Gleiss, A.; Massetti, M.; Wisser, W.; Binder, T.; Gabriel, H.; Rosenhek, R. Left atrial size predicts outcome in severe but asymptomatic mitral regurgitation. Sci. Rep. 2023, 13, 3892. [Google Scholar] [CrossRef]
- Tastet, L.; Lim, L.J.; Bibby, D.; Hu, G.; Cristin, L.; Rich, A.H.; Jhawar, R.; Fang, Q.; Arya, F.; Delling, F.N. Primary Atriopathy in Mitral Valve Prolapse: Echocardiographic Evidence and Clinical Implications. Circ. Cardiovasc. Imaging 2024, 17, e016319. [Google Scholar] [CrossRef]
- Petek, B.J.; Baggish, A.L. Valvular Heart Disease in Athletes. Curr. Treat. Options Cardiovasc. Med. 2021, 23, 69. [Google Scholar] [CrossRef]
- Cavarretta, E.; Peruzzi, M.; Versaci, F.; Frati, G.; Sciarra, L. How to manage an athlete with mitral valve prolapse. Eur. J. Prev. Cardiol. 2021, 28, 1110–1117. [Google Scholar] [CrossRef]
- Pistelli, L.; Vetta, G.; Parlavecchio, A.; Crea, P.; Parisi, F.; Magnocavallo, M.; Caminiti, R.; Frea, S.; Vairo, A.; Desalvo, P.; et al. Arrhythmic risk profile in mitral valve prolapse: A systematic review and metanalysis of 1715 patients. J. Cardiovasc. Electrophysiol. 2024, 35, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Corrado, D.; Basso, C.; Schiavon, M.; Thiene, G. Screening for hypertrophic cardiomyopathy in young athletes. N. Engl. J. Med. 1998, 339, 364–369. [Google Scholar] [CrossRef]
- Kim, J.H.; Baggish, A.L.; Levine, B.D.; Ackerman, M.J.; Day, S.M.; Dineen, E.H.; Guseh, J.S., II; La Gerche, A.; Lampert, R.; Martinez, M.W.; et al. Clinical Considerations for Competitive Sports Participation for Athletes With Cardiovascular Abnormalities: A Scientific Statement From the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 2025, 85, 1059–1108. [Google Scholar] [CrossRef]
- Chatrath, N.; Papadakis, M. Physical activity and exercise recommendations for patients with valvular heart disease. Heart 2022, 108, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Segreti, A.; Celeski, M.; Monticelli, L.M.; Perillo, A.; Crispino, S.P.; Di Gioia, G.; Cammalleri, V.; Fossati, C.; Mega, S.; Papalia, R.; et al. Mitral and Tricuspid Valve Disease in Athletes. J. Clin. Med. 2023, 12, 3562. [Google Scholar] [CrossRef]
- Han, H.C.; Ha, F.J.; Teh, A.W.; Calafiore, P.; Jones, E.F.; Johns, J.; Koshy, A.N.; O’Donnell, D.; Hare, D.L.; Farouque, O.; et al. Mitral Valve Prolapse and Sudden Cardiac Death: A Systematic Review. J. Am. Heart Assoc. 2018, 7, e010584. [Google Scholar] [CrossRef] [PubMed]
- Cristin, L.; Tastet, L.; Shah, D.J.; Miller, M.A.; Delling, F.N. Multimodality Imaging of Arrhythmic Risk in Mitral Valve Prolapse. Circ. Cardiovasc. Imaging 2025, 18, e017313. [Google Scholar] [CrossRef]
- Oxborough, D.; George, K.; Cooper, R.; Bhatia, R.; Ramcharan, T.; Zaidi, A.; Gati, S.; Prakash, K.; Rakhit, D.; Robinson, S.; et al. Echocardiography in the cardiac assessment of young athletes: a 2025 guideline from the British Society of Echocardiography (endorsed by Cardiac Risk in the Young). Echo Res. Pract. 2025, 12, 7. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Baravelli, M.; Vincenti, A.; Trevisan, R.; Zompatori, M.; Nicolosi, G.L.; Lombardo, M.; Anzà, C. A New modified anthropometric haller index obtained without radiological exposure. Int. J. Cardiovasc. Imaging 2018, 34, 1505–1509. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M. The relationship between mitral valve prolapse and thoracic skeletal abnormalities in clinical practice: a systematic review. J. Cardiovasc. Med. (Hagerstown) 2024, 25, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Lombardo, M.; Gensini, G.F.; Ambrosio, G. Influence of chest conformation on myocardial strain parameters in healthy subjects with mitral valve prolapse. Int. J. Cardiovasc. Imaging 2021, 37, 1009–1022. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Nicolosi, G.L.; Rigamonti, E.; Lombardo, M. Impact of Chest Wall Conformation on the Outcome of Primary Mitral Regurgitation due to Mitral Valve Prolapse. J. Cardiovasc. Echogr. 2022, 32, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sonaglioni, A.; Nicolosi, G.L.; Muti−Schünemann, G.E.U.; Rispoli, G.A.; Lombardo, M.; Muti, P. Does Preliminary Chest Shape Assessment Improve the Prognostic Risk Stratification of Individuals with Mitral Annular Disjunction? A Case Report and Narrative Review. J. Clin. Med. 2025, 14, 2277. [Google Scholar] [CrossRef]
- Sonaglioni, A.; Rigamonti, E.; Nicolosi, G.L.; Lombardo, M. Prognostic value of modified Haller index in patients with suspected coronary artery disease referred for exercise stress echocardiography. J. Cardiovasc. Echogr. 2021, 31, 85–95. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).