Submitted:
09 September 2025
Posted:
10 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Methods
3. The Standard of Care: Dietary Management and Follow-Up in CeD
3.1. Dietary Management
3.2. The nutritional Status at Diagnosis of CeD in Adults
3.3. Identification of Those Patients at Risk of Non-Adherence to GFD
3.4. Principles of the Follow-Up of CeD in Adults
3.4.1. Importance and Goals of Long-Term Follow-Up
3.4.2. Benefits of Structured Follow-Up
3.4.3. Models of Follow-Up Care
- Primary care management: In countries with high CeD prevalence, general practitioners (GPs) can manage long-term follow-up, though this requires adequate training and access to specialist consultation [51].
3.4.4. Follow-Up Strategies
- A.
- Fixed-Interval Approach
- B.
- Tailored(Individualized)Approach
- Symptom status and nutritional deficiencies: These patients may require frequent follow-up visits or additional testing, while, asymptomatic individuals may need fewer visits.
- Serological response and mucosal healing: A positive IgA anti-tissue transglutaminase (IgA anti-TG2) result suggests poor dietary adherence, while a negative result does not confirm strict adherence or absence of gluten exposure. Persistently positive antibody levels predict some degree of gluten intake, though the sensitivity for detecting transgressions is low (52–57%) [59,60]. CeD-specific antibodies decline within months of starting a GFD but are not accurate markers of villous atrophy [47,48,61]. In patients with IgA deficiency, IgG anti-TG2 levels often fail to normalize despite strict diet adherence [62,63].
- Objective evaluation of GFD adherence: Adherence to a GFD in patients with CeD can be evaluated using validated dietary adherence questionnaires or detection of gluten immunogenic peptides (GIPs) in urine or feces [64,65]. Fecal GIPs can detect gluten exposure for up to 4 days, whereas urinary GIPs typically reflect intake within 4 to 48 hours [66]. Studies have demonstrated that up to 71% of asymptomatic patients with CeD may test positive for GIPs, indicating ongoing gluten exposure despite the absence of clinical symptoms [67]. Although standardized protocols for GIP testing have not yet been established, the method is a valuable adjunct to conventional monitoring. It provides objective, real-time assessment of dietary adherence and may be particularly useful in cases of diagnostic uncertainty, ongoing symptoms, or in patients at high risk of non-compliance [7].
- Age and comorbidities: Older adults or those with other health conditions may require more integrated care, while younger patients without comorbidities may benefit from less frequent visits focused on education and prevention.
- Coexistence of autoimmune conditions, such as type 1 diabetes mellitus or thyroid disorders, requires a more integrated management approach including consultation with endocrinologists.
- Individual preferences regarding the frequency of visits and desired level of support should be considered.
3.5. Follow-Up Duodenal Biopsy
- Persistent or worsening symptoms and/or biochemical or laboratory evidence of malabsorption.
- Development of new "red flag" symptoms that raise suspicion for complications such as RCD or malignancy.
- Seronegative CeD at diagnosis, where biopsies are essential for confirming the diagnosis and monitoring the response to a GFD.
- Risk-stratified assessment: A biopsy may be reasonable after 1–2 years on a GFD for adults diagnosed after the age of 45 or those with initially severe presentations (e.g., ulcerative jejunitis, severe villous atrophy) to assess for mucosal healing.
- Patient request for reassurance regarding mucosal healing and disease control.
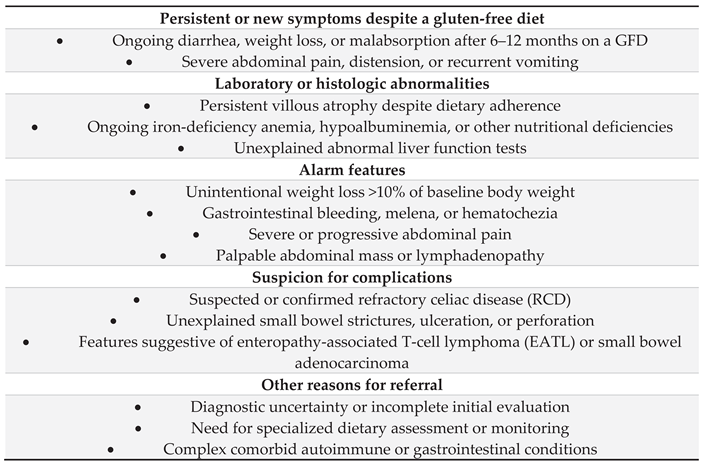
4. The Non-Responsive CeD Patient
4.1. Subdivisions of Non-Responsive Celiac Disease
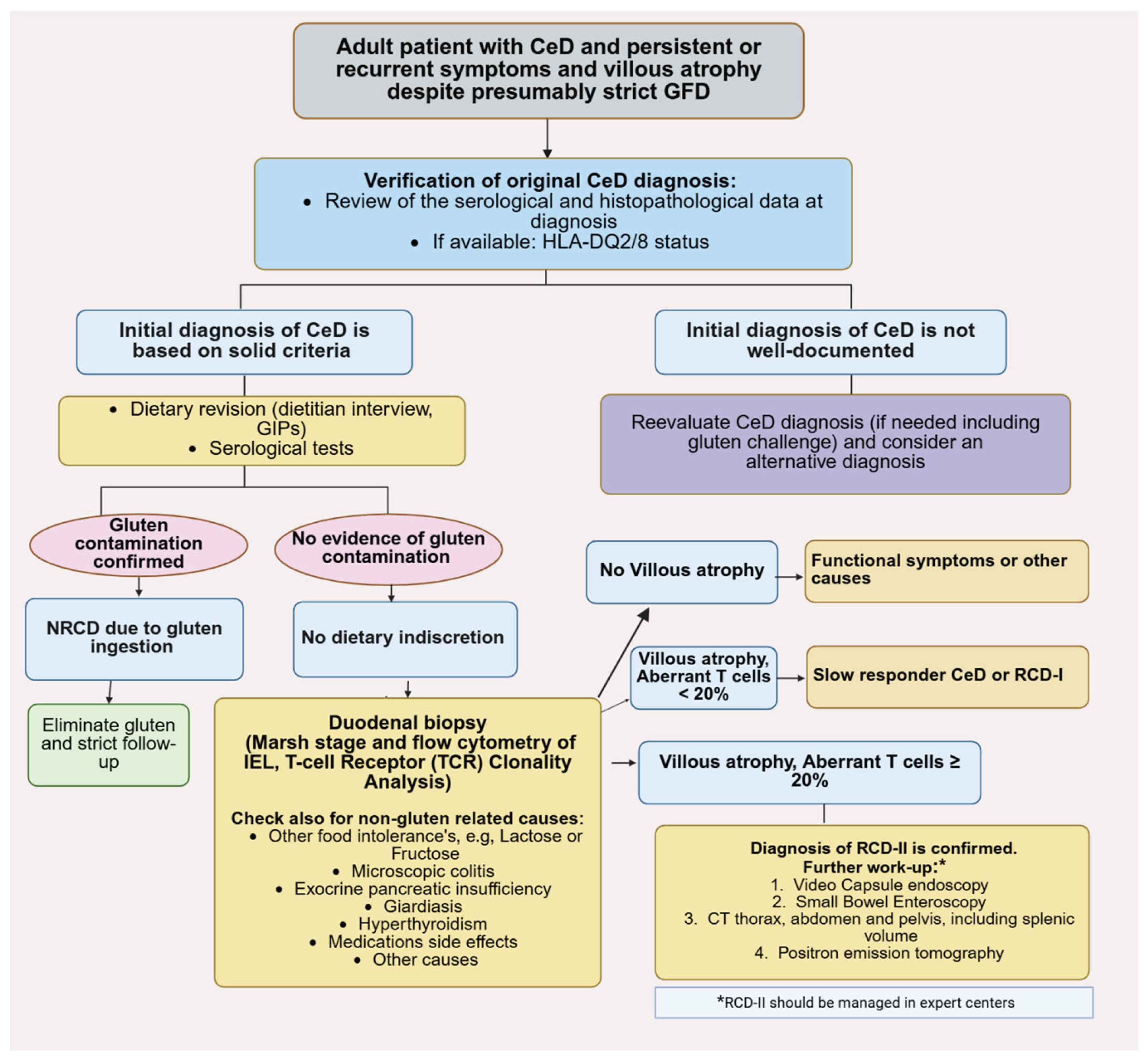
- RCD-I: Characterized by a normal, polyclonal IEL population. It is often difficult to distinguish clinically from slow-responsive CeD.
- RCD-II: Defined by the presence of an aberrant, clonal IEL population (lacking surface CD3 and CD8, with >20% aberrant cells on flow cytometry). This form is more severe, associated with complications like ulcerative jejunitis and small bowel stenosis, and is considered a pre-lymphomatous state due to its high risk of progression to enteropathy-associated T-cell lymphoma (EATL) [77].
4.2. Systematic Diagnostic Approach to Non-Responsive Celiac Disease
4.3. The Critical Role of the Dietitian in Managing Delayed Responsiveness
4.4. Differential Diagnosis of Persistent Symptoms in Celiac Disease
- An initial misdiagnosis of CeD.
- Other food intolerances, such as lactose or fructose malabsorption.
- Functional gastrointestinal disorders, most notably irritable bowel syndrome (IBS).
- Microscopic colitis (lymphocytic or collagenous colitis).
- Exocrine pancreatic insufficiency (EPI).
- Other autoimmune conditions affecting the gastrointestinal tract.
- Medication side effects or gluten containing medications.
- The development of malignant complications, such as enteropathy-associated T-cell lymphoma (EATL) or small-bowel adenocarcinoma.
4.4.1. Initial Misdiagnosis of Celiac Disease
- This is the most common reason for a diagnostic dilemma. If a patient begins a GFD before undergoing serological and histological testing, the results become unreliable. Celiac‐specific antibodies (IgA anti‐TG2) depend on gluten consumption to be produced. A GFD will cause antibody levels to decline and potentially normalize, leading to false‐negative results. On the other hand, the mucosal histological lesions (villous atrophy, intraepithelial lymphocytosis) will begin to heal on a GFD, making a biopsy inconclusive or normal, even if the patient has CeD.
- A diagnosis might be incorrectly assigned based on incomplete or misinterpreted data, e.g., in cases of isolated borderline positive serology without biopsy confirmation or non-specific histology without positive serology "celiac mimics." Several disorders can cause similar histological changes and/or symptoms, leading to potential misdiagnosis, such as: autoimmune enteropathy, Common Variable Immunodeficiency (CVID), Tropical Sprue, Food llergies (e.g., cow's milk, soy, fish, and Medication-Induced Enteropathy. Certain drugs like Olmesartan, NSIDs, and mycophenolate mofetil can cause severe villous atrophy and symptoms indistinguishable from CeD [88,89,90].
4.4.2. The Role of the Low-FODMAP Diet
4.4.3. Exocrine Pancreatic Insufficiency (EPI)
5. Refractory Celiac Disease (RCD)
5.1. Diagnosis of RCD
- a)
- Clinical Assessment:
- b)
- Endoscopic and Histological Evaluation:
- Ulcerative Jejunitis: The endoscopic finding of mucosal ulcers in the duodenum or jejunum is a highly suspicious feature for RCD-II and possible progression to EATL.
- Video Capsule Endoscopy (VCE): VCE plays a pivotal role in assessing the entire small bowel mucosa. Its primary value in RCD is in evaluating the extent of disease, identifying complications like ulcerative jejunitis, strictures, and suspicious mass lesions suggestive of EATL [109,110,111,112]. A positive VCE study at diagnosis, showing extensive involvement, is a prognostic marker associated with persistent villous atrophy and higher risk of adverse outcomes [113]. VCE is an ideal non-invasive tool to guide subsequent DAE for targeted biopsies [110].
- c)
- Immunophenotyping and T-cell Clonality Analysis:
- Flow Cytometry: This is the gold standard for differentiating RCD-I from RCD-II. It quantifies the population of aberrant IELs. Normal IELs and those in RCD-I are CD3+CD8+. In contrast, RCD-II is defined by a clonal population of >20% aberrant IELs that are cytoplasmic CD3ε+ but lack surface CD3, CD8, and T-cell receptors (TCR). Flow cytometry is superior to immunohistochemistry as it differentiates cytoplasmic from membranous CD3 expression and helps exclude other lymphoproliferative disorders [13,14,115,116,117].
- T-cell Receptor (TCR) Clonality Analysis: This PCR-based analysis involves assessing the TCR gene rearrangements in IELs. RCD-II is characterized by the expansion of a monoclonal or oligoclonal T cell population. This clonal expansion is a hallmark of RCD-II and distinguishes it from RCD-I, which retains a polyclonal T cell population [16,100,114]. In cases lacking TCR-gamma rearrangement, a clonal TCR-delta rearrangement can be identified [8,115]. This analysis can also identify the same malignant clone in extra-intestinal sites (e.g., skin, lung) [116,117,118].
- d)
- Genetic Analysis:
- Germline Mutations: Screening for mutations associated with immune dysregulation (e.g., IL10RA, STAT1) is important to rule out monogenic disorders mimicking RCD, such as autoimmune enteropathy [119].
- Somatic Mutations: Molecular profiling of aberrant IELs in RCD-II and EATL reveals recurrent gain-of-function mutations in the *JAK1-STAT3* pathway (in up to 80-90% of cases). Mutations in genes like *TNFAIP3/A20*, TET2, and KMT2D are also common and may explain resistance to certain therapies, providing potential targets for future treatment [120,121,122].
- e)
- Radiological and Nuclear Medicine Imaging:
- CT/MR Enterography: These techniques are valuable at visualizing mural and extraluminal complications. Key findings suggestive of RCD-II or EATL include cavitating mesenteric lymphadenopathy (necrotic lymph nodes), splenic atrophy, small bowel wall thickening, ulceration, strictures, and mass lesions [123,124,125,126,127,128].
- f)
- Nutritional Assessment:
- f)
- Exclusion of EATL:
5.2. RCD-I versus RCD-IIf
5.3. Management of Refractory Celiac Disease
5.3.1. Treatment of RCD-I
- Nutritional Support: Aggressive nutritional rehabilitation is essential. This includes enteral nutrition (e.g., elemental diets) or, in cases of severe malabsorption, parenteral nutrition to correct deficiencies and reverse catabolism.
- Open-Capsule Budesonide: The administration—opening the capsule and chewing the granules—facilitates early release in the proximal small bowel, targeting the site of inflammation. Evidence from open-label and retrospective studies demonstrates high efficacy, with clinical response in approximately 90% of patients and histological improvement in 83-90% [131,132]. A trial of open-capsule budesonide (9 mg/day divided in 3 times 3 mg) may be given. Patients often require long-term, low-dose maintenance therapy due to a high relapse rate upon withdrawal.
- Systemic Corticosteroids: In patients with severe symptoms or those who cannot tolerate budesonide, a brief course of oral prednisone (0.5–1 mg/kg/day) can be used as a bridge to budesonide therapy.
- Steroid-Sparing Immunosuppressants: For steroid-dependent, refractory, or intolerant patients, the addition of azathioprine or 6-mercaptopurine is a common strategy. Combination therapy with prednisone and azathioprine for 52 weeks has induced clinical and histological remission in 80% of cases [133]. However, due to limited data, this approach requires careful monitoring for adverse effects.
- Treatment Failure: A lack of response to budesonide should prompt a re-evaluation of the original RCD-I diagnosis, including a review of flow cytometry and T-cell clonality to rule out RCD-II.
- Monitoring: Annual follow-up with duodenal biopsies, including histopathology and flow cytometry, is recommended to confirm response and monitor for clonal evolution.
5.3.2. Treatment of RCD-II
- Prerequisites for Treatment: A definitive diagnosis of RCD-II must be established, and EATL must be rigorously excluded using PET-CT, deep enteroscopy, and biopsies before initiating therapy.
- Nutritional Support: As with RCD-I, intensive nutritional support, often including parenteral nutrition, is essential due to severe malabsorption.
- Treatment Strategies: The ultimate goal is to eliminate the aberrant T-cell clone and prevent progression to lymphoma. The following options should be considered sequentially or in combination:
5.4. Prognosis of RCD and EATL
- RCD-I has a generally favorable prognosis, with a 5-year survival rate exceeding 90% with appropriate treatment [16,152].
- RCD-II carries a grave prognosis, with a 5-year survival rate of only 44-58% due to its high risk of transforming into EATL [16,144]. Poor prognostic factors include severe malnutrition, hypoalbuminemia, weight loss, and the development of complications such as ulcerative jejunitis, strictures, or opportunistic infections [16,130,144,145].
6. Discussion
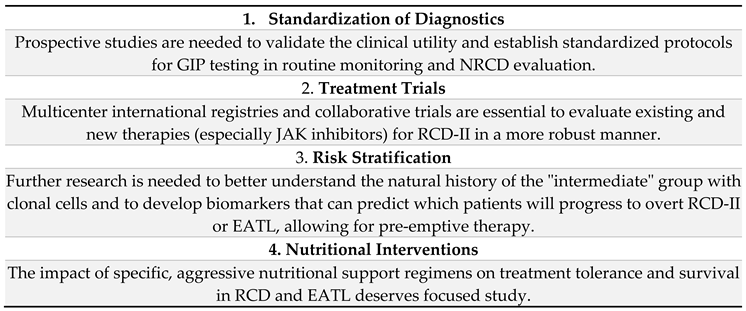
7. Conclusion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.K.; Lo, W.; Memeo, L.; Rotterdam, H.; Green, P.H. Duodenal histology in patients with celiac disease after treatment with a gluten-free diet. Gastrointest. Endosc. 2003, 57, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Saukkonen, J.; Kaukinen, K.; Koivisto, A.-M.; Mäki, M.; Laurila, K.; Sievänen, H.; Collin, P.; Kurppa, K. Clinical Characteristics and the Dietary Response in Celiac Disease Patients Presenting With or Without Anemia. J. Clin. Gastroenterol. 2017, 51, 412–416. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; A Murray, J. Classification and management of refractory coeliac disease. Gut 2010, 59, 547–557. [Google Scholar] [CrossRef]
- Penny, H.A.; Rej, A.; Baggus, E.M.R.; Coleman, S.H.; Ward, R.; Wild, G.; Bouma, G.; Trott, N.; Snowden, J.A.; Wright, J.; et al. Non-Responsive and Refractory Coeliac Disease: Experience from the NHS England National Centre. Nutrients 2022, 14, 2776. [Google Scholar] [CrossRef]
- Dewar, D.H. Celiac disease: Management of persistent symptoms in patients on a gluten-free diet. World J. Gastroenterol. 2012, 18, 1348–56. [Google Scholar] [CrossRef]
- Hall, N.J.; Rubin, G.P.; Charnock, A. Intentional and inadvertent non-adherence in adult coeliac disease. A cross-sectional survey. Appetite 2013, 68, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Coto, L.; Mendia, I.; Sousa, C.; Bai, J.C.; Cebolla, A. Determination of gluten immunogenic peptides for the management of the treatment adherence of celiac disease: A systematic review. World J. Gastroenterol. 2021, 27, 6306–6321. [Google Scholar] [CrossRef]
- Malamut, G.; Afchain, P.; Verkarre, V.; Lecomte, T.; Amiot, A.; Damotte, D.; Bouhnik, Y.; Colombel, J.F.; Delchier, J.C.; Allez, M.; et al. Presentation and Long-Term Follow-up of Refractory Celiac Disease: Comparison of Type I With Type II. Gastroenterology 2009, 136, 81–90. [Google Scholar] [CrossRef]
- Cellier, C.; Patey, N.; Mauvieux, L.; Jabri, B.; Delabesse, E.; Cervoni, J.; Burtin, M.; Guy–Grand, D.; Bouhnik, Y.; Modigliani, R.; et al. Abnormal intestinal intraepithelial lymphocytes in refractory sprue. Gastroenterology 1998, 114, 471–481. [Google Scholar] [CrossRef]
- Green, P.H.; Paski, S.; Ko, C.W.; Rubio-Tapia, A. AGA Clinical Practice Update on Management of Refractory Celiac Disease: Expert Review. Gastroenterology 2022, 163, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment. Pharmacol. Ther. 2014, 39, 488–495. [Google Scholar] [CrossRef]
- Tye-Din, J.A. Review article: Follow-up of coeliac disease. Aliment. Pharmacol. Ther. 2022, 56, S49–S63. [Google Scholar] [CrossRef]
- Branchi, F.; Wiese, J.J.; Heldt, C.; Manna, S.; Dony, V.; Loddenkemper, C.; Bojarski, C.; Siegmund, B.; Schneider, T.; Daum, S.; et al. The combination of clinical parameters and immunophenotyping of intraepithelial lymphocytes allows to assess disease severity in refractory celiac disease. Dig. Liver Dis. 2022, 54, 1649–1656. [Google Scholar] [CrossRef]
- Malamut, G.; Meresse, B.; Cellier, C.; Cerf-Bensussan, N. Refractory celiac disease: from bench to bedside. Semin. Immunopathol. 2012, 34, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Delabie, J.; Holte, H.; Vose, J.M.; Ullrich, F.; Jaffe, E.S.; Savage, K.J.; Connors, J.M.; Rimsza, L.; Harris, N.L.; Müller-Hermelink, K.; et al. Enteropathy-associated T-cell lymphoma: clinical and histological findings from the International Peripheral T-Cell Lymphoma Project. Blood 2011, 118, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Al-Toma, A.; Verbeek, W.H.M.; Hadithi, M.; E von Blomberg, B.M.; Mulder, C.J.J. Survival in refractory coeliac disease and enteropathy-associated T-cell lymphoma: retrospective evaluation of single-centre experience. Gut 2007, 56, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- See, J.A.; Kaukinen, K.; Makharia, G.K.; Gibson, P.R.; Murray, J.A. Practical insights into gluten-free diets. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 580–591. [Google Scholar] [CrossRef]
- Lee, A.R. Review article: Dietary management of coeliac disease. Aliment. Pharmacol. Ther. 2022, 56, S38–S48. [Google Scholar] [CrossRef]
- Thies, F.; Masson, L.F.; Boffetta, P.; Kris-Etherton, P. Oats and bowel disease: a systematic literature review. Br. J. Nutr. 2014, 112, S31–S43. [Google Scholar] [CrossRef]
- Gilissen, L.J.W.J.; Van der Meer, I.M.; Smulders, M.J.M. Why Oats Are Safe and Healthy for Celiac Disease Patients. Med Sci. 2016, 4, 21. [Google Scholar] [CrossRef]
- La Vieille, S.; Pulido, O.M.; Abbott, M.; Koerner, T.B.; Godefroy, S. Celiac Disease and Gluten-Free Oats: A Canadian Position Based on a Literature Review. Can. J. Gastroenterol. Hepatol. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cohen, I.S.; Day, A.S.; Shaoul, R. Gluten in Celiac Disease—More or Less? Rambam Maimonides Med. J. 2019, 10, e0007. [Google Scholar] [CrossRef]
- Rodríguez, J.M.; Estévez, V.; Bascuñán, K.; Ayala, J.; Araya, M. Commercial oats in gluten-free diet: A persistent risk for celiac patients. Front. Nutr. 2022, 9, 986282. [Google Scholar] [CrossRef]
- Wild, D.; Robins, G.G.; Burley, V.J.; Howdle, P.D. Evidence of high sugar intake, and low fibre and mineral intake, in the gluten-free diet. Aliment. Pharmacol. Ther. 2010, 32, 573–581. [Google Scholar] [CrossRef]
- Vincentini, O.; Izzo, M.; Maialetti, F.; Gonnelli, E.; Neuhold, S.; Silano, M. Risk of Cross-Contact for Gluten-Free Pizzas in Shared-Production Restaurants in Relation to Oven Cooking Procedures. J. Food Prot. 2016, 79, 1642–1646. [Google Scholar] [CrossRef]
- Studerus, D.; Hampe, E.I.; Fahrer, D.; Wilhelmi, M.; Vavricka, S.R. Cross-Contamination with Gluten by Using Kitchen Utensils: Fact or Fiction? J. Food Prot. 2018, 81, 1679–1684. [Google Scholar] [CrossRef]
- McDonald, B.D.; Kupfer, S.S. Can We Cross Off Common Kitchen Practices as Causes of Gluten Cross-Contact? Gastroenterology 2020, 158, 51–53. [Google Scholar] [CrossRef] [PubMed]
- Korth, N.; Taylor, S.L.; Clarke, J.L.; Downs, M.L. Gluten Cross-Contact in Restaurant-Scale Pasta Cooking. J. Food Prot. 2021, 84, 2159–2162. [Google Scholar] [CrossRef] [PubMed]
- AOECS - Association of European Coeliac Societies | AOECS. https://www.aoecs.org/. Accessed , 2024. 25 October.
- Wolf, R.L.; Lebwohl, B.; Lee, A.R.; Zybert, P.; Reilly, N.R.; Cadenhead, J.; Amengual, C.; Green, P.H. Hypervigilance to a Gluten-Free Diet and Decreased Quality of Life in Teenagers and Adults with Celiac Disease. Dig. Dis. Sci. 2018, 63, 1438–1448. [Google Scholar] [CrossRef]
- Satherley, R.; Higgs, S.; Howard, R. Disordered eating patterns in coeliac disease: a framework analysis. J. Hum. Nutr. Diet. 2017, 30, 724–736. [Google Scholar] [CrossRef]
- Trott, N.; A Raju, S.; Rej, A.; Hoffman, O.; Holland, W.; Bebb, J.R.; Seamark, L.; Williams, M.; Batlle, C.C.; Jeanes, Y.M.; et al. Long-term follow-up in patients with coeliac disease in the pandemic-era: a view from Sheffield the NHS England national centre for adult coeliac disease. . 2023, 16, 158–166. [Google Scholar]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Kochan, Z.; Małgorzewicz, S. Expanded Role of a Dietitian in Monitoring a Gluten-Free Diet in Patients with Celiac Disease: Implications for Clinical Practice. Nutrients 2021, 13, 1859. [Google Scholar] [CrossRef]
- Gładyś, K.; Dardzińska, J.; Guzek, M.; Adrych, K.; Małgorzewicz, S. Celiac Dietary Adherence Test and Standardized Dietician Evaluation in Assessment of Adherence to a Gluten-Free Diet in Patients with Celiac Disease. Nutrients 2020, 12, 2300. [Google Scholar] [CrossRef]
- Rej, A.; Buckle, R.L.; Shaw, C.C.; Trott, N.; Urwin, H.; McGough, N.; Aziz, I.; Sanders, D.S. National survey evaluating the provision of gastroenterology dietetic services in England. Front. Gastroenterol. 2020, 12, 380–384. [Google Scholar] [CrossRef]
- Jeanes, Y.M.; Kallos, S.; Muhammad, H.; Reeves, S. Who gets an annual review for coeliac disease? Patients with lower health literacy and lower dietary adherence consider them important. J. Hum. Nutr. Diet. 2024, 37, 1022–1031. [Google Scholar] [CrossRef] [PubMed]
- Venkateswaran, N.; Claxton, B.; Locke, D.; Baragona, A.; Lehman, E.B.; Dalessio, S.; Clarke, K. Referral for Dietary Intervention in Celiac Disease Is Low among Gastroenterologists and Primary Care Providers. Dig. Dis. 2022, 41, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Escobar, P.; Vázquez-Polo, M.; van der Hofstadt, M.; Nuñez, C.; Montoro-Huguet, M.A.; Churruca, I.; Simón, E. Knowledge Gaps in Gluten-Free Diet Awareness among Patients and Healthcare Professionals: A Call for Enhanced Nutritional Education. Nutrients 2024, 16, 2512. [Google Scholar] [CrossRef] [PubMed]
- Hallert, C.; Svensson, M.; Tholstrup, J.; Hultberg, B. Clinical trial: B vitamins improve health in patients with coeliac disease living on a gluten-free diet. Aliment. Pharmacol. Ther. 2009, 29, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Hall, N.J.; Rubin, G.; Charnock, A. Systematic review: adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2009, 30, 315–330. [Google Scholar] [CrossRef]
- Paarlahti, P.; Kurppa, K.; Ukkola, A.; Collin, P.; Huhtala, H.; Mäki, M.; Kaukinen, K. Predictors of persistent symptoms and reduced quality of life in treated coeliac disease patients: a large cross-sectional study. BMC Gastroenterol. 2013, 13, 75–75. [Google Scholar] [CrossRef]
- Villafuerte-Galvez, J.; Vanga, R.R.; Dennis, M.; Hansen, J.; Leffler, D.A.; Kelly, C.P.; Mukherjee, R. Factors governing long-term adherence to a gluten-free diet in adult patients with coeliac disease. Aliment. Pharmacol. Ther. 2015, 42, 753–760. [Google Scholar] [CrossRef]
- Hopkins, S.; Soon, J.M. Nutritional quality, cost and availability of gluten-free food in England. Br. Food J. 2019, 121, 2867–2882. [Google Scholar] [CrossRef]
- Schiepatti, A.; Maimaris, S.; Nicolardi, M.L.; Alimenti, E.; Vernero, M.; Costetti, M.; Costa, S.; Biagi, F. Determinants and Trends of Adherence to a Gluten-Free Diet in Adult Celiac Patients on a Long-term Follow-up (2000–2020). Clin. Gastroenterol. Hepatol. 2022, 20, e741–e749. [Google Scholar] [CrossRef]
- Lebwohl, B.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Predictors of persistent villous atrophy in coeliac disease: a population-based study. Aliment. Pharmacol. Ther. 2014, 39, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Wahab, P.J.; Meijer, J.W.; Mulder, C.J. Histologic Follow-up of People With Celiac Disease on a Gluten-Free Diet. Am. J. Clin. Pathol. 2002, 118, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Leffler, D.A.; Al-Toma, A.; Mulder, C.J.; Elli, L.; Gan, G.B.; Patil, P.; Atsawarungruangkit, A.; Kuijpers, K.C.; Del Gobbo, A.; et al. Clinical Data Do Not Reliably Predict Duodenal Histology at Follow-up in Celiac Disease. Am. J. Surg. Pathol. 2023, 48, 212–220. [Google Scholar] [CrossRef]
- Dickey, W.; Hughes, D.F.; McMillan, S.A. Disappearance of endomysial antibodies in treated celiac disease does not indicate histological recovery. Am. J. Gastroenterol. 2000, 95, 712–714. [Google Scholar] [CrossRef] [PubMed]
- Mandile, R.; Maglio, M.; Mosca, C.; Marano, A.; Discepolo, V.; Troncone, R.; Auricchio, R. Mucosal Healing in Celiac Disease: Villous Architecture and Immunohistochemical Features in Children on a Long-Term Gluten Free Diet. Nutrients 2022, 14, 3696. [Google Scholar] [CrossRef]
- Hære, P.; Høie, O.; Schulz, T.; Schönhardt, I.; Raki, M.; Lundin, K.E.A. Long-term mucosal recovery and healing in celiac disease is the rule – not the exception. Scand. J. Gastroenterol. 2016, 51, 1439–1446. [Google Scholar] [CrossRef]
- Lexner, J.; Hjortswang, H.; Ekesbo, R.; Sjöberg, K. Well-being and dietary adherence in patients with coeliac disease depending on follow-up. Scand. J. Gastroenterol. 2021, 56, 382–390. [Google Scholar] [CrossRef]
- Mulder, C.J.J.; Elli, L.; Lebwohl, B.; Makharia, G.K.; Rostami, K.; Rubio-Tapia, A.; Schumann, M.; Tye-Din, J.; Zeitz, J.; Al-Toma, A. Follow-Up of Celiac Disease in Adults: “When, What, Who, and Where”. Nutrients 2023, 15, 2048. [Google Scholar] [CrossRef]
- Hughey, J.J.; Ray, B.K.; Lee, A.R.; Voorhees, K.N.; Kelly, C.P.; Schuppan, D. Self-reported dietary adherence, disease-specific symptoms, and quality of life are associated with healthcare provider follow-up in celiac disease. BMC Gastroenterol. 2017, 17, 156–156. [Google Scholar] [CrossRef]
- Costas-Batlle, C.; Trott, N.; Jeanes, Y.; Seamark, L.; Gardiner, C. A dietitian-led coeliac service helps to identify and reduce involuntary gluten ingestion with subsequent reduction in the frequency of repeat endoscopies. J. Hum. Nutr. Diet. 2023, 36, 1751–1759. [Google Scholar] [CrossRef]
- Rej, A.; Trott, N.; Kurien, M.; Branchi, F.; Richman, E.; Subramanian, S.; Sanders, D.S. Is Peer Support in Group Clinics as Effective as Traditional Individual Appointments? The First Study in Patients With Celiac Disease. Clin. Transl. Gastroenterol. 2020, 11, e00121. [Google Scholar] [CrossRef] [PubMed]
- Vriezinga, S.; Borghorst, A.; Marle, E.v.D.A.-V.; Benninga, M.; George, E.; Hendriks, D.; Hopman, E.; de Meij, T.; Jong, A.v.d.M.-D.; Putter, H.; et al. E-Healthcare for Celiac Disease—A Multicenter Randomized Controlled Trial. J. Pediatr. 2018, 195, 154–160.e7. [Google Scholar] [CrossRef]
- Perez-Junkera, G.; Vázquez-Polo, M.; Eizagirre, F.J.; Benjumea, L.; Tutau, C.; Esteban, B.; Miranda, J.; Larretxi, I.; Navarro, V.; Churruca, I.; et al. Application of a Platform for Gluten-Free Diet Evaluation and Dietary Advice: From Theory to Practice. Sensors 2022, 22, 732. [Google Scholar] [CrossRef] [PubMed]
- Pekki, H.; Kaukinen, K.; Ilus, T.; Mäki, M.; Huhtala, H.; Laurila, K.; Kurppa, K. Long-term follow-up in adults with coeliac disease: Predictors and effect on health outcomes. Dig. Liver Dis. 2018, 50, 1189–1194. [Google Scholar] [CrossRef]
- Nachman, F.; Sugai, E.; Vázquez, H.; González, A.; Andrenacci, P.; Niveloni, S.; Mazure, R.; Smecuol, E.; Moreno, M.L.; Hwang, H.J.; et al. Serological tests for celiac disease as indicators of long-term compliance with the gluten-free diet. Eur. J. Gastroenterol. Hepatol. 2011, 23, 473–80. [Google Scholar] [CrossRef]
- Vahedi K, Mascart F, Mary JY et al. Reliability of antitransglutaminase antibodies as predictors of gluten-free diet compliance in adult celiac disease. Am J Gastroenterol. 2003;98:1079–87.
- Dickey, W.; Hughes, D. Disappointing sensitivity of endoscopic markers for villous atrophy in a high-risk population: implications for celiac disease diagnosis during routine endoscopy. Am. J. Gastroenterol. 2001, 96, 2126–2128. [Google Scholar] [CrossRef] [PubMed]
- Korponay-Szabo, I.R.; Dahlbom, I.; Laurila, K.; Koskinen, S.; Woolley, N.; Partanen, J.; Kovács, J.B.; Mäki, M.; Hansson, T. Elevation of IgG antibodies against tissue transglutaminase as a diagnostic tool for coeliac disease in selective IgA deficiency. Gut 2003, 52, 1567–1571. [Google Scholar] [CrossRef]
- Cataldo, F.; Lio, D.; Marino, V.; Picarelli, A.; Ventura, A.; Corazza, G.R. IgG1 antiendomysium and IgG antitissue transglutaminase (anti-tTG) antibodies in coeliac patients with selective IgA deficiency. Gut 2000, 47, 366–369. [Google Scholar] [CrossRef]
- Leffler, D.A.; Dennis, M.; Edwards George, J.B.; Jamma, S.; Magge, S.; Cook, E.F.; Schuppan, D.; Kelly, C.P. A Simple Validated Gluten-Free Diet Adherence Survey for Adults With Celiac Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 530–536.e2. [Google Scholar] [CrossRef]
- Elli, L.; Leffler, D.; Cellier, C.; Lebwohl, B.; Ciacci, C.; Schumann, M.; Lundin, K.E.A.; Zammit, S.C.; Sidhu, R.; Roncoroni, L.; et al. Guidelines for best practices in monitoring established coeliac disease in adult patients. Nat. Rev. Gastroenterol. Hepatol. 2023, 21, 198–215. [Google Scholar] [CrossRef]
- Lombardo, V.; Scricciolo, A.; Costantino, A.; Elli, L.; Legnani, G.; Cebolla, Á.; Doneda, L.; Mascaretti, F.; Vecchi, M.; Roncoroni, L. Evaluation of a Single Determination of Gluten Immunogenic Peptides in Urine from Unaware Celiac Patients to Monitor Gluten-Free Diet Adherence. Nutrients 2023, 15, 1259. [Google Scholar] [CrossRef]
- Porcelli, B.; Ferretti, F.; Cinci, F.; Biviano, I.; Santini, A.; Grande, E.; Quagliarella, F.; Terzuoli, L.; Bacarelli, M.R.; Bizzaro, N.; et al. Fecal gluten immunogenic peptides as indicators of dietary compliance in celiac patients. Minerva Gastroenterol. E Dietol. 2020, 66, 201–207. [Google Scholar] [CrossRef]
- Ciccone, A.; Gabrieli, D.; Cardinale, R.; Di Ruscio, M.; Vernia, F.; Stefanelli, G.; Necozione, S.; Melideo, D.; Viscido, A.; Frieri, G.; et al. Metabolic Alterations in Celiac Disease Occurring after Following a Gluten-Free Diet. Digestion 2019, 100, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, N.; Agarwal, A.; Alarouri, H.; Dwarakanathan, V.; Dang, S.; Ahuja, V.; Makharia, G.K. Patients with Celiac Disease Have High Prevalence of Fatty Liver and Metabolic Syndrome. Dig. Dis. Sci. 2024, 69, 3029–3042. [Google Scholar] [CrossRef] [PubMed]
- Kaukinen, K.; Peräaho, M.; Lindfors, K.; Partanen, J.; Woolley, N.; Pikkarainen, P.; Karvonen, A.; Laasanen, T.; Sievänen, H.; Mäki, M.; et al. Persistent small bowel mucosal villous atrophy without symptoms in coeliac disease. Aliment. Pharmacol. Ther. 2007, 25, 1237–1245. [Google Scholar] [CrossRef]
- Sharkey, L.M.; Corbett, G.; Currie, E.; Lee, J.; Sweeney, N.; Woodward, J.M. Optimising delivery of care in coeliac disease – comparison of the benefits of repeat biopsy and serological follow-up. Aliment. Pharmacol. Ther. 2013, 38, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Lebwohl, B.; Granath, F.; Ekbom, A.; Montgomery, S.M.; Murray, J.A.; Rubio-Tapia, A.; Green, P.H.R.; Ludvigsson, J.F. Mucosal healing and mortality in coeliac disease. Aliment. Pharmacol. Ther. 2012, 37, 332–339. [Google Scholar] [CrossRef]
- Hutchinson, J.M.; West, N.P.; Robins, G.G.; Howdle, P.D. Long-term histological follow-up of people with coeliac disease in a UK teaching hospital. Qjm: Int. J. Med. 2010, 103, 511–517. [Google Scholar] [CrossRef]
- Pekki, H.; Kurppa, K.; Mäki, M.; Huhtala, H.; Laurila, K.; Ilus, T.; Kaukinen, K. Performing routine follow-up biopsy 1 year after diagnosis does not affect long-term outcomes in coeliac disease. Aliment. Pharmacol. Ther. 2017, 45, 1459–1468. [Google Scholar] [CrossRef]
- Al-Toma, A.; Volta, U.; Auricchio, R.; Castillejo, G.; Sanders, D.S.; Cellier, C.; Mulder, C.J.; Lundin, K.E.A. European Society for the Study of Coeliac Disease (ESsCD) guideline for coeliac disease and other gluten-related disorders. United Eur. Gastroenterol. J. 2019, 7, 583–613. [Google Scholar] [CrossRef]
- Rubio-Tapia, A.; Hill, I.D.; Semrad, C.; Kelly, C.P.; Greer, K.B.; Limketkai, B.N.; Lebwohl, B. American College of Gastroenterology Guidelines Update: Diagnosis and Management of Celiac Disease. Am. J. Gastroenterol. 2022, 118, 59–76. [Google Scholar] [CrossRef] [PubMed]
- van de Water, J.M.; Nijeboer, P.; de Baaij, L.R.; Zegers, J.; Bouma, G.; Visser, O.J.; van der Peet, D.L.; Mulder, C.J.; Meijerink, W.J. Surgery in (pre)malignant celiac disease. World J. Gastroenterol. 2015, 21, 12403–9. [Google Scholar] [CrossRef]
- Hussein, S.; Gindin, T.; Lagana, S.M.; Arguelles-Grande, C.; Krishnareddy, S.; Alobeid, B.; Lewis, S.K.; Mansukhani, M.M.; Green, P.H.R.; Bhagat, G. Clonal T cell receptor gene rearrangements in coeliac disease: implications for diagnosing refractory coeliac disease. J. Clin. Pathol. 2018, 71, 825–831. [Google Scholar] [CrossRef]
- García-Hoz, C.; Crespo, L.; Lopez, N.; De Andrés, A.; León, R.R.; Santón, A.; Garriga, M.; Butz, E.; León, F.; Ariño, G.R. The Intracellular Intensity of CD3 on Aberrant Intraepithelial Lymphocytes Is a Prognostic Factor of the Progression to Overt Lymphoma in Refractory Celiac Disease Type II (Pre-Enteropathy-Associated T Cell Lymphoma). Dig. Dis. 2020, 38, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Schiepatti, A.; Maimaris, S.; A Raju, S.; Green, O.L.; Mantica, G.; Therrien, A.; Flores-Marin, D.; Linden, J.; Fernández-Bañares, F.; Esteve, M.; et al. Persistent villous atrophy predicts development of complications and mortality in adult patients with coeliac disease: a multicentre longitudinal cohort study and development of a score to identify high-risk patients. Gut 2023, 72, 2095–2102. [Google Scholar] [CrossRef]
- Sbravati, F.; Cosentino, A.; Lenzi, J.; Fiorentino, M.; Ambrosi, F.; Salerno, A.; Di Biase, A.; Righi, B.; Brusa, S.; Valin, P.S.; et al. Antitissue transglutaminase antibodies’ normalization after starting a gluten-free diet in a large population of celiac children-a real-life experience. Dig. Liver Dis. 2022, 54, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Silvester, J.A.; Comino, I.; Kelly, C.P.; Sousa, C.; Duerksen, D.R.; Bernstein, C.N.; Cebolla, A.; Dominguez, M.R.; Graff, L.A.; Green, K.H.; et al. Most Patients With Celiac Disease on Gluten-Free Diets Consume Measurable Amounts of Gluten. Gastroenterology 2020, 158, 1497–1499.e1. [Google Scholar] [CrossRef]
- Fernández-Bañares, F.; Beltrán, B.; Salas, A.; Comino, I.; Ballester-Clau, R.; Ferrer, C.; Molina-Infante, J.; Rosinach, M.; Modolell, I.; Rodríguez-Moranta, F.; et al. Persistent Villous Atrophy in De Novo Adult Patients With Celiac Disease and Strict Control of Gluten-Free Diet Adherence: A Multicenter Prospective Study (CADER Study). Am. J. Gastroenterol. 2021, 116, 1036–1043. [Google Scholar] [CrossRef] [PubMed]
- Biagi, F.; Vattiato, C.; Agazzi, S.; Balduzzi, D.; Schiepatti, A.; Gobbi, P.; Corazza, G.R. A second duodenal biopsy is necessary in the follow-up of adult coeliac patients. Ann. Med. 2014, 46, 430–433. [Google Scholar] [CrossRef]
- Costa, A.F.; Sugai, E.; de la Paz Temprano, M.; Niveloni, S.I.; Vázquez, H.; Moreno, M.L.; Domínguez-Flores, M.R.; Muñoz-Suano, A.; Smecuol, E.; Stefanolo, J.P.; et al. Gluten immunogenic peptide excretion detects dietary transgressions in treated celiac disease patients. World J. Gastroenterol. 2019, 25, 1409–1420. [Google Scholar] [CrossRef]
- van Gils, T.; Nijeboer, P.; Overbeek, L.I.; Hauptmann, M.; Castelijn, D.A.; Bouma, G.; Mulder, C.J.; E van Leeuwen, F.; de Jong, D. Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up. United Eur. Gastroenterol. J. 2018, 6, 1485–1495. [Google Scholar] [CrossRef]
- Al-Toma, A.; Verbeek, W.; Mulder, C.J.J. Update on the management of refractory coeliac disease. J. Gastrointestin. Liver Dis. 2007, 16, 57–63. [Google Scholar] [CrossRef]
- Sanford, M.L.; Nagel, A.K. A Review of Current Evidence of Olmesartan Medoxomil Mimicking Symptoms of Celiac Disease. J. Pharm. Pr. 2014, 28, 189–192. [Google Scholar] [CrossRef] [PubMed]
- DeGaetani, M.; A Tennyson, C.; Lebwohl, B.; Lewis, S.K.; Abu Daya, H.; Arguelles-Grande, C.; Bhagat, G.; Green, P.H.R. Villous Atrophy and Negative Celiac Serology: A Diagnostic and Therapeutic Dilemma. Am. J. Gastroenterol. 2013, 108, 647–653. [Google Scholar] [CrossRef]
- Schiepatti, A.; Rej, A.; Maimaris, S.; Cross, S.S.; Porta, P.; Aziz, I.; Key, T.; Goodwin, J.; Therrien, A.; Yoosuf, S.; et al. Clinical classification and long-term outcomes of seronegative coeliac disease: a 20-year multicentre follow-up study. Aliment. Pharmacol. Ther. 2021, 54, 1278–1289. [Google Scholar] [CrossRef] [PubMed]
- Rispo, A.; Guarino, A.D.; Siniscalchi, M.; Imperatore, N.; Santonicola, A.; Ricciolino, S.; de Sire, R.; Toro, B.; Cantisani, N.M.; Ciacci, C. “The crackers challenge”: A reassuring low-dose gluten challenge in adults on gluten-free diet without proper diagnosis of coeliac disease. Dig. Liver Dis. 2024, 56, 1517–1521. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.C.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPS Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef]
- Haghbin, H.; Hasan, F.; Gangwani, M.K.; Zakirkhodjaev, N.; Lee-Smith, W.; Beran, A.; Kamal, F.; Hart, B.; Aziz, M. Efficacy of Dietary Interventions for Irritable Bowel Syndrome: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2024, 13, 7531. [Google Scholar] [CrossRef]
- Trott, N.; Rej, A.; Coleman, S.H.; Sanders, D.S. Adult celiac disease with persistent IBS-type symptoms: a pilot study of an adjuvant FODMAP diet., 14, 304–310.
- van Megen, F.; Skodje, G.I.; Lergenmuller, S.; Zühlke, S.; Aabakken, L.; Veierød, M.B.; Henriksen, C.; Lundin, K.E. A Low FODMAP Diet Reduces Symptoms in Treated Celiac Patients With Ongoing Symptoms–A Randomized Controlled Trial. Clin. Gastroenterol. Hepatol. 2022, 20, 2258–2266.e3. [Google Scholar] [CrossRef] [PubMed]
- Lusetti, F.; Schiepatti, A.; Scalvini, D.; Maimaris, S.; Biagi, F. Efficacy of a Low-FODMAP Diet for Coeliac Patients with Persistent IBS-like Symptoms despite a Gluten-Free Diet: A Systematic Review. Nutrients 2024, 16, 1094. [Google Scholar] [CrossRef]
- Jiang, C.; Barkin, J.A.; Barkin, J.S. Exocrine Pancreatic Insufficiency Is Common in Celiac Disease: A Systematic Review and Meta-Analysis. Dig. Dis. Sci. 2023, 68, 3421–3427. [Google Scholar] [CrossRef]
- Alkhayyat, M.; Saleh, M.A.; Abureesh, M.; Khoudari, G.; Qapaja, T.; Mansoor, E.; Simons-Linares, C.R.; Vargo, J.; Stevens, T.; Rubio-Tapia, A.; et al. The Risk of Acute and Chronic Pancreatitis in Celiac Disease. Dig. Dis. Sci. 2020, 66, 2691–2699. [Google Scholar] [CrossRef]
- van Wanrooij, R.L.J.; Bouma, G.; Bontkes, H.J.; Neefjes-Borst, A.; van Grieken, N.C.; E von Blomberg, B.M.; Mulder, C.J.J. Outcome of Referrals for Non-Responsive Celiac Disease in a Tertiary Center: Low Incidence of Refractory Celiac Disease in the Netherlands. Clin. Transl. Gastroenterol. 2017, 8, e218. [Google Scholar] [CrossRef]
- Cellier, C.; Delabesse, E.; Helmer, C.; Patey, N.; Matuchansky, C.; Jabri, B.; Macintyre, E.; Cerf-Bensussan, N.; Brousse, N. Refractory sprue, coeliac disease, and enteropathy-associated T-cell lymphoma. Lancet 2000, 356, 203–208. [Google Scholar] [CrossRef]
- Pironi, L.; Konrad, D.; Brandt, C.; Joly, F.; Wanten, G.; Agostini, F.; Chambrier, C.; Aimasso, U.; Zeraschi, S.; Kelly, D.; et al. Clinical classification of adult patients with chronic intestinal failure due to benign disease: An international multicenter cross-sectional survey. Clin. Nutr. 2018, 37, 728–738. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, D.G.A.M.; Amelio, G.S.; Lava, S.A.G.; Bianchetti, M.G.; Simonetti, G.D.; Agostoni, C.; Fossali, E.F.; Milani, G.P. D-lactic acidosis in humans: systematic literature review. Pediatr. Nephrol. 2017, 33, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Verbeek, W.H.; Schreurs, M.W.; Visser, O.J.; E von Blomberg, B.M.; Al-Toma, A.; Mulder, C.J. Novel approaches in the management of refractory celiac disease. Expert Rev. Clin. Immunol. 2008, 4, 205–219. [Google Scholar] [CrossRef]
- Daum, S.; Cellier, C.; Mulder, C.J. Refractory coeliac disease. Best Pr. Res. Clin. Gastroenterol. 2005, 19, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Pastré, J.; Juvin, K.; Malamut, G.; Derrieux, C.; Cellier, C.; Israël-Biet, D. Phenotypically aberrant clonal T cells in the lungs of patients with type II refractory celiac disease. Blood 2014, 123, 3674–3675. [Google Scholar] [CrossRef]
- Maguire, A.A.; Greenson, J.K.; Lauwers, G.Y.; Ginsburg, R.E.; Williams, G.T.; Brown, I.S.; Riddell, R.H.; O'DOnoghue, D.; Sheahan, K.D. Collagenous Sprue. Am. J. Surg. Pathol. 2009, 33, 1440–1449. [Google Scholar] [CrossRef]
- Kung, V.L.; Liu, T.-C.; Ma, C. Collagenous Enteritis is Unlikely a Form of Aggressive Celiac Disease Despite Sharing HLA-DQ2/DQ8 Genotypes. Am. J. Surg. Pathol. 2018, 42, 545–552. [Google Scholar] [CrossRef]
- Vakiani, E.; Arguelles-Grande, C.; Mansukhani, M.M.; Lewis, S.K.; Rotterdam, H.; Green, P.H.; Bhagat, G. Collagenous sprue is not always associated with dismal outcomes: a clinicopathological study of 19 patients. Mod. Pathol. 2010, 23, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Barret, M.; Malamut, G.; Rahmi, G.; Samaha, E.; Edery, J.; Verkarre, V.; Macintyre, E.; Lenain, E.; Chatellier, G.; Cerf-Bensussan, N.; et al. Diagnostic Yield of Capsule Endoscopy in Refractory Celiac Disease. Am. J. Gastroenterol. 2012, 107, 1546–1553. [Google Scholar] [CrossRef]
- Hadithi, M.; Al-Toma, A.; Oudejans, J.; van Bodegraven, A.A.; Mulder, C.J.; Jacobs, M. The Value of Double-Balloon Enteroscopy in Patients With Refractory Celiac Disease. Am. J. Gastroenterol. 2007, 102, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Heine, G.D.; Al-Toma, A.; Mulder, C.J.J.; Jacobs, M.A.J.M. Milestone in gastrointestinal endoscopy: Double-balloon enteroscopy of the small bowel. Scand. J. Gastroenterol. 2006, 41, 32–38. [Google Scholar] [CrossRef]
- Al-Toma, A.; Beaumont, H.; Koornstra, J.J.; van Boeckel, P.; Hergelink, D.O.; van der Kraan, J.; Inderson, A.; de Ridder, R.; Jacobs, M.A. The performance and safety of motorized spiral enteroscopy, including in patients with surgically altered gastrointestinal anatomy: a multicenter prospective study. Endoscopy 2022, 54, 1034–1042. [Google Scholar] [CrossRef]
- Zammit, S.C. Small bowel capsule endoscopy in refractory celiac disease: A luxury or a necessity? Ann. Gastroenterol. 2021, 34, 188. [Google Scholar] [CrossRef]
- Verbeek, W.H.; Goerres, M.S.; von Blomberg, B.M.E.; Oudejans, J.J.; Scholten, P.E.; Hadithi, M.; Al-Toma, A.; Schreurs, M.W.; Mulder, C.J. Flow cytometric determination of aberrant intra-epithelial lymphocytes predicts T-cell lymphoma development more accurately than T-cell clonality analysis in Refractory Celiac Disease. Clin. Immunol. 2008, 126, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Cheminant, M.; Bruneau, J.; Malamut, G.; Sibon, D.; Guegan, N.; van Gils, T.; Cording, S.; Trinquand, A.; Verkarre, V.; Lhermitte, L.; et al. NKp46 is a diagnostic biomarker and may be a therapeutic target in gastrointestinal T-cell lymphoproliferative diseases: a CELAC study. Gut 2018, 68, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Cellier, C. Refractory Celiac Disease. Gastroenterol. Clin. North Am. 2019, 48, 137–144. [Google Scholar] [CrossRef]
- Malamut, G.; Cellier, C. Refractory coeliac disease. Curr. Opin. Oncol. 2013, 25, 445–451. [Google Scholar] [CrossRef]
- Al-Toma, A.; Visser, O.J.; van Roessel, H.M.; von Blomberg, B.M.E.; Verbeek, W.H.M.; Scholten, P.E.T.; Ossenkoppele, G.J.; Huijgens, P.C.; Mulder, C.J.J. Autologous hematopoietic stem cell transplantation in refractory celiac disease with aberrant T cells. Blood 2006, 109, 2243–2249. [Google Scholar] [CrossRef]
- Charbit-Henrion, F.; Haas, M.; Chaussade, S.; Cellier, C.; Cerf-Bensussan, N.; Malamut, G.; Khater, S.; Khiat, A.; Cording, S.; Parlato, M.; et al. Genetic Diagnosis Guides Treatment of Autoimmune Enteropathy. Clin. Gastroenterol. Hepatol. 2022, 21, 1368–1371.e2. [Google Scholar] [CrossRef]
- Cording, S.; Lhermitte, L.; Malamut, G.; Berrabah, S.; Trinquand, A.; Guegan, N.; Villarese, P.; Kaltenbach, S.; Meresse, B.; Khater, S.; et al. Oncogenetic landscape of lymphomagenesis in coeliac disease. Gut 2021, 71, 497–508. [Google Scholar] [CrossRef]
- Malamut, G.; El Machhour, R.; Montcuquet, N.; Martin-Lannerée, S.; Dusanter-Fourt, I.; Verkarre, V.; Mention, J.-J.; Rahmi, G.; Kiyono, H.; Butz, E.A.; et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease–associated inflammation and lymphomagenesis. J. Clin. Investig. 2010, 120, 2131–2143. [Google Scholar] [CrossRef]
- Cellier, C.; Bouma, G.; van Gils, T.; Khater, S.; Malamut, G.; Crespo, L.; Collin, P.; Green, P.H.R.; E Crowe, S.; Tsuji, W.; et al. Safety and efficacy of AMG 714 in patients with type 2 refractory coeliac disease: a phase 2a, randomised, double-blind, placebo-controlled, parallel-group study. Lancet Gastroenterol. Hepatol. 2019, 4, 960–970. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Li, Z.; Huang, S.-Y.; Shen, X.-D.; Li, X.-H. Cross-sectional imaging: current status and future potential in adult celiac disease. Eur. Radiol. 2023, 34, 1232–1246. [Google Scholar] [CrossRef] [PubMed]
- Mallant M, Hadithi M, Al-Toma A-B, et al. Abdominal computed tomography in refractory coeliac disease and enteropathy associated T-cell lymphoma CLINICAL RESEARCH. World J Gastroenterol. 2007;13(11):1696-1700.
- Hoffmann, M.; Vogelsang, H.; Kletter, K.; Zettinig, G.; Chott, A.; Raderer, M. 18F-fluoro-deoxy-glucose positron emission tomography (18F-FDG-PET) for assessment of enteropathy-type T cell lymphoma. Gut 2003, 52, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.Y.; Lee, E.; Khong, P.-L.; Tse, E.W.C.; Kwong, Y.-L. Positron emission tomography computed tomography features of monomorphic epitheliotropic intestinal T-cell lymphoma. Hematology 2017, 23, 10–16. [Google Scholar] [CrossRef]
- Van Weyenberg, S.J.; Mulder, C.J.; Van Waesberghe, J.H.T. Small Bowel Imaging in Celiac Disease. Dig. Dis. 2015, 33, 252–259. [Google Scholar] [CrossRef]
- van Gils, T.; Nijeboer, P.; van Waesberghe, J.H.T.; Coupé, V.M.; Janssen, K.; A Zegers, J.; A Nurmohamed, S.; Kraal, G.; Jiskoot, S.C.; Bouma, G.; et al. Splenic volume differentiates complicated and non-complicated celiac disease. United Eur. Gastroenterol. J. 2017, 5, 374–379. [Google Scholar] [CrossRef]
- Hadithi, M.; Mallant, M.; Oudejans, J.; Van Waesberghe, J.-H.T.M.; Mulder, C.J.; I Comans, E.F. 18F-FDG PET versus CT for the detection of enteropathy-associated T-cell lymphoma in refractory celiac disease. . 2006, 47, 1622–7. [Google Scholar]
- Wierdsma, N.J.; Nijeboer, P.; de van der Schueren, M.A.; Berkenpas, M.; van Bodegraven, A.A.; Mulder, C.J. Refractory celiac disease and EATL patients show severe malnutrition and malabsorption at diagnosis. Clin. Nutr. 2016, 35, 685–691. [Google Scholar] [CrossRef] [PubMed]
- Mukewar, S.S.; Sharma, A.; Rubio-Tapia, A.; Wu, T.-T.; Jabri, B.; A Murray, J. Open-Capsule Budesonide for Refractory Celiac Disease. Am. J. Gastroenterol. 2017, 112, 959–967. [Google Scholar] [CrossRef]
- Saitta, D.; Henneken, L.M.; Apputhurai, P.; Mei, S.L.C.Y.; Tye-Din, J.A. Budesonide Induces Favourable Histologic and Symptomatic Recovery in Patients with Non-responsive and Refractory Coeliac Disease When Given in an Open Capsule Format. Dig. Dis. Sci. 2024, 69, 2548–2557. [Google Scholar] [CrossRef] [PubMed]
- Goerres, M.S.; Meijer, J.W.R.; Wahab, P.J.; Kerckhaert, J.A.M.; Groenen, P.J.T.A.; Van Krieken, J.H.J.M.; Mulder, C.J.J. Azathioprine and prednisone combination therapy in refractory coeliac disease. Aliment. Pharmacol. Ther. 2003, 18, 487–494. [Google Scholar] [CrossRef]
- Al–Toma, A.; Goerres, M.S.; Meijer, J.W.; von Blomberg, B.M.E.; Wahab, P.J.; Kerckhaert, J.A.; Mulder, C.J. Cladribine Therapy in Refractory Celiac Disease With Aberrant T Cells. Clin. Gastroenterol. Hepatol. 2006, 4, 1322–1327. [Google Scholar] [CrossRef]
- Tack, G.J.; Verbeek, W.H.M.; Al-Toma, A.; Kuik, D.J.; Schreurs, M.W.J.; Visser, O.; Mulder, C.J.J. Evaluation of Cladribine treatment in refractory celiac disease type II. World J. Gastroenterol. 2011, 17, 506–513. [Google Scholar] [CrossRef]
- Tack, G.J.; Wondergem, M.J.; Al-Toma, A.; Verbeek, W.H.M.; Schmittel, A.; Machado, M.V.; Perri, F.; Ossenkoppele, G.J.; Huijgens, P.C.; Schreurs, M.W.J.; et al. Auto-SCT in refractory celiac disease type II patients unresponsive to cladribine therapy. Bone Marrow Transplant. 2010, 46, 840–846. [Google Scholar] [CrossRef]
- Al-Toma, A.; Koene, H.R. Hematopoietic Stem Cell Transplantation in Refractory Celiac Disease: An Overview with Focus on Infectious Complications. OBM Transplant. 2020, 04, 1–17. [Google Scholar] [CrossRef]
- Al-Toma, A.; Nijeboer, P.; Bouma, G.; Visser, O.; Mulder, C.J. Hematopoietic stem cell transplantation for non-malignant gastrointestinal diseases. World J. Gastroenterol. 2014, 20, 17368–75. [Google Scholar] [CrossRef] [PubMed]
- Grewal, J.K.; Kassardjian, A.; A Weiss, G. Successful novel use of tofacitinib for type II refractory coeliac disease. BMJ Case Rep. 2022, 15, e244692. [Google Scholar] [CrossRef]
- Dieckman, T.; Schumann, M.; Beaumont, H.; Bontkes, H.J.; Koning, F.; Bouma, G.; Byrnes, V.; McCarthy, J.; Neefjes-Borst, A.; Loddenkemper, C.; et al. Enduring Clinical Remission in Refractory Celiac Disease Type II With Tofacitinib: An Open-Label Clinical Study. Clin. Gastroenterol. Hepatol. 2024, 22, 2334–2336. [Google Scholar] [CrossRef]
- Tack, G.J.; van Asseldonk, D.P.; van Wanrooij, R.L.J.; van Bodegraven, A.A.; Mulder, C.J. Tioguanine in the treatment of refractory coeliac disease – a single centre experience. Aliment. Pharmacol. Ther. 2012, 36, 274–281. [Google Scholar] [CrossRef]
- Gillett, H.R.; Arnott, I.D.; McIntyre, M.; Campbell, S.; Dahele, A.; Priest, M.; Jackson, R.; Ghosh, S. Successful infliximab treatment for steroid-refractory celiac disease: A case report. Gastroenterology 2002, 122, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.M.; Moorghen, M.; Probert, C.S. Refractory coeliac disease: remission with infliximab and immunomodulators. Eur. J. Gastroenterol. Hepatol. 2005, 17, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Malamut, G.; Chandesris, O.; Verkarre, V.; Meresse, B.; Callens, C.; Macintyre, E.; Bouhnik, Y.; Gornet, J.-M.; Allez, M.; Jian, R.; et al. Enteropathy associated T cell lymphoma in celiac disease: A large retrospective study. Dig. Liver Dis. 2013, 45, 377–384. [Google Scholar] [CrossRef]
- Al-Toma, A.; Verbeek, W.; Visser, O.; Kuijpers, K.; Oudejans, J.; Kluin-Nelemans, H.; Mulder, C.; Huijgens, P. Disappointing outcome of autologous stem cell transplantation for enteropathy-associated T-cell lymphoma. Dig. Liver Dis. 2007, 39, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Jantunen, E.; Boumendil, A.; Finel, H.; Luan, J.-J.; Johnson, P.; Rambaldi, A.; Haynes, A.; Duchosal, M.A.; Bethge, W.; Biron, P.; et al. Autologous stem cell transplantation for enteropathy-associated T-cell lymphoma: a retrospective study by the EBMT. Blood 2013, 121, 2529–2532. [Google Scholar] [CrossRef]
- Nijeboer, P.; Malamut, G.; Mulder, C.; Cerf-Bensussan, N.; Sibon, D.; Bouma, G.; Cellier, C.; Hermine, O.; Visser, O. Enteropathy-Associated T-Cell Lymphoma: Improving Treatment Strategies. Dig. Dis. 2015, 33, 231–235. [Google Scholar] [CrossRef]
- Rowinski, S.A.; Christensen, E. Epidemiologic and therapeutic aspects of refractory coeliac disease - a systematic review. . 2016, 63. [Google Scholar]
- Therrien, A.; Silvester, J.A.; Leffler, D.A.; Kelly, C.P. Efficacy of Enteric-Release Oral Budesonide in Treatment of Acute Reactions to Gluten in Patients With Celiac Disease. Clin. Gastroenterol. Hepatol. 2020, 18, 254–256. [Google Scholar] [CrossRef] [PubMed]
| Feature | RCD-I | RCD-II |
|---|---|---|
| Malabsorption features | In ~50% of cases | In >80% of cases |
| Key risk factors | Older age, poor GFD adherence | Older age, HLA-DQ2 homozygosity (~60%) |
| CeD serology | Usually negative | Usually negative |
| Hypoalbuminemia | Present in ~50% of cases | Present in almost all cases |
| Endoscopic ulcers/stenosis | Rare | Common |
| Persistent villous atrophy | In all cases | In all cases |
| Defining Diagnostic Method | Flow cytometry: <20% aberrant IELs | Flow cytometry: >20% aberrant IELs & T-cell clonality |
| Immunophenotype (Flow Cytometry) | Normal (sCD3+CD8+) | Aberrant (cCD3ε+, sCD3-, CD8-, TCR-) |
| Immunohistochemistry | Normal IEL phenotype (CD3+CD8+) | Loss of surface CD8 on IELs |
| 5-year survival | >80% | < 50%; High risk of EATL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





