Introduction
The prevalence of hematophagous mosquitoes is a common feature of summer evenings, prompting defensive reactions driven by the perception of pruritus and the risk of vector-borne diseases like malaria, dengue, and Zika. During hematophagy, a mosquito pierces the skin and inoculates a complex sialome containing a repertoire of pharmacologically active molecules. This salivary cocktail, honed by co-evolution to subvert host hemostasis and immune surveillance, facilitates efficient blood acquisition. Beyond this primary function, these molecules engage with host immune sentinels and orchestrate subsequent innate and adaptive immune cascades. Growing evidence suggests these interactions are not exclusively pathogenic; the mosquito sialome can induce tolerance, modulate inflammation, and contains bioactive peptides with significant therapeutic potential. This manuscript synthesizes recent peer-reviewed findings on the immunological and biochemical utility of the mosquito sialome, focusing on work from the last five years that elucidates molecular mechanisms and emerging clinical applications.
Composition and Pharmacological Effects of the Mosquito Sialome
The mosquito sialome comprises a complex cocktail of proteins and small molecules, refined over millions of years of co-evolution to subvert host hemostatic and immunological barriers. The sialomes of hematophagous arthropods contain compounds with angiogenic, anticoagulant, vasodilatory, and immunomodulatory properties that facilitate hematophagy [
27]. Beyond generic inhibitors, structural and biochemical studies reveal highly specialized molecules with sophisticated mechanisms of action.
D7 proteins are one of the most abundant protein families within the sialome and display remarkable ligand specificity. A long-form D7 from
Culex quinquefasciatus (CxD7L1) binds adenosine diphosphate (ADP) and adenosine triphosphate (ATP) with high affinity, whereas its paralogue CxD7L2 sequesters biogenic amines and eicosanoids. The crystal structure of CxD7L1, solved at 1.97 Å resolution, reveals an ADP-binding pocket with a unique topology not observed in other D7 proteins. Functional assays demonstrated that both CxD7L1 and CxD7L2 inhibit platelet aggregation ex vivo and in vivo, suggesting this function evolved to enhance hematophagy by antagonizing host hemostasis [
14].
Recent structural studies have further illuminated the molecular basis of D7 protein function. Biochemical characterization of the
Aedes aegypti D7 protein AeD7L2 reveals it binds the thromboxane A
2 analog U-46619 with nanomolar affinity (
) and reverses vasoconstriction and platelet aggregation, while its paralogue AeD7L1 lacks this activity [
15]. In
Aedes albopictus, the D7 long form AlboD7L1 binds a spectrum of host hemostasis agonists—including biogenic amines, leukotrienes, and U-46619—and inhibits platelet aggregation and leukocyte recruitment, reinforcing the role of D7 proteins as potent anti-hemostatic and anti-inflammatory factors [
16].
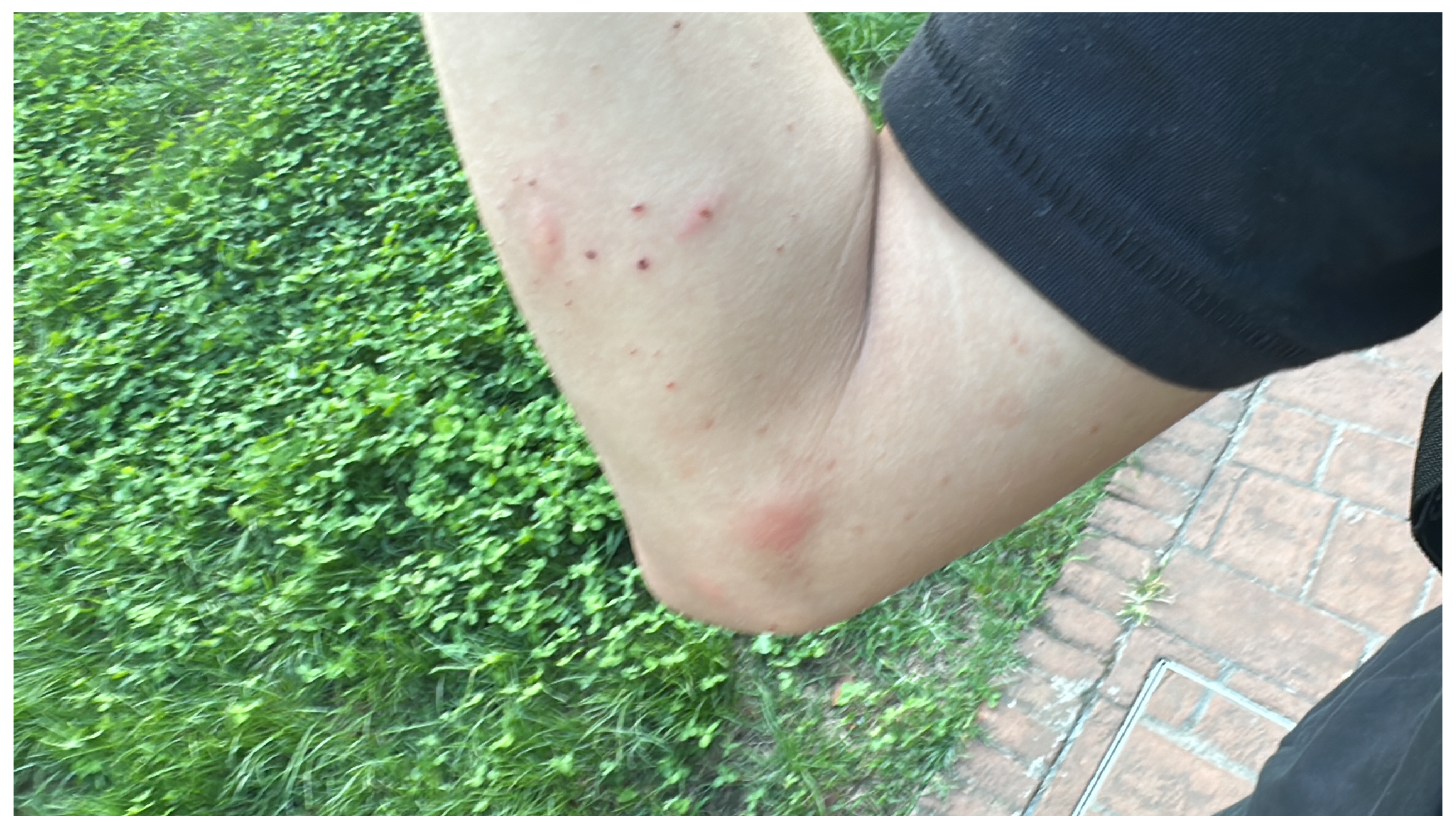
Figure 1.
Cutaneous manifestations on the primary author’s arm, displaying erythematous wheals and papules from recent mosquito bites (September 2025). These acute inflammatory responses, mediated by host recognition of salivary antigens, are paradoxically induced by a sialome that also constitutes a rich reservoir of therapeutic biomolecules.
Figure 1.
Cutaneous manifestations on the primary author’s arm, displaying erythematous wheals and papules from recent mosquito bites (September 2025). These acute inflammatory responses, mediated by host recognition of salivary antigens, are paradoxically induced by a sialome that also constitutes a rich reservoir of therapeutic biomolecules.
In addition to D7 proteins, the sialome contains numerous factors that modulate host physiology. Apyrases, soluble 5’-nucleotidases that hydrolyze ATP and ADP, prevent platelet activation and are upregulated post-blood meal [
11]. Pala et al. (2024) demonstrated that anopheline apyrase also interacts with human tissue plasminogen activator (tPA) to enhance plasmin generation, reducing fibrin formation and facilitating
Plasmodium transmission, highlighting it as a viable vaccine target [
13].
Other enzymes, such as adenosine deaminase and purine hydrolase, reduce vasopermeability and pruritus [
11]. The sialome also contains endonucleases, esterases, and protease inhibitors (e.g., Aegyptin) that disrupt coagulation cascades and extracellular matrix integrity [
12]. Vasodilatory peptides like sialokinin stimulate nitric oxide-mediated endothelial permeability [
19], while peroxidases from
Anopheles species promote vasodilation by degrading hemostatically active biogenic amines [
11].
Immunological Tolerance from Repeated Exposure
Individuals with chronic exposure to mosquito bites often report that the pruritic response attenuates over time, a phenomenon with an increasingly well-defined molecular basis. Immunologically, initial exposures induce a delayed T
2-biased reaction with robust IgE responses, whereas chronic exposure drives an immunoglobulin class-switching event toward IgG subclasses—particularly IgG4—which are associated with immunological tolerance [
23]. A study in a dengue-endemic region confirmed that salivary proteins elicit IgG4 antibodies correlated with tolerance induction [
3]. This isotype shift likely attenuates mast cell degranulation and histamine release, accounting for the diminished wheal-and-flare cutaneous reactions observed in highly exposed populations.
Recent work has identified specific molecular mechanisms underlying this tolerance. The salivary protein LTRIN binds to the lymphotoxin-
receptor (LT
R) with high affinity, blocking NF-
B signaling and suppressing pro-inflammatory cytokine production [
21]. This interaction suggests that the sialome actively orchestrates an immunotolerant microenvironment through specific receptor-mediated mechanisms, rather than through mere antigenic desensitization. While this tolerance limits local inflammation, it reflects a form of immunological conditioning that tempers host responses without compromising systemic protective functions.
Saliva-Induced Immunity and Vaccine Paradigms
As mosquitoes co-inoculate their sialome with pathogens, host immunity targeting salivary proteins can significantly modulate pathogen virulence and disease pathogenesis. Recent clinical trials have validated this paradigm in humans. Manning et al. (2020) reported a successful Phase I trial of AGS-v, a mosquito saliva peptide-based vaccine, demonstrating its safety and immunogenicity [
18]. The next-generation vaccine, AGS-v PLUS, showed enhanced efficacy with significantly greater fold-changes in anti-peptide IgG and interferon-
responses [
10].
A key mechanism involves sialokinin, a salivary peptide from
Aedes aegypti. Humanized mice bitten by sialokinin-knockout mosquitoes exhibited a T
1-skewed immune profile, whereas exposure to wild-type saliva induced a T
2-biased response [
1]. Hastings et al. (2022) further showed that sialokinin facilitates viral dissemination via induced vascular permeability, providing a mechanistic rationale for targeting this molecule in vaccine strategies [
19].
Apyrase exemplifies how a biochemical function can precipitate a significant immunological outcome. By degrading ADP, apyrase prevents platelet aggregation and facilitates efficient hematophagy [
11]. Its differential expression across mosquito genera underscores its role in the co-evolutionary arms race with mammalian hosts [
13].
The protective effects of anti-sialome immunity have been demonstrated in multiple systems. Vogt et al. (2018) showed that the mosquito sialome alone has profound modulatory effects on the human immune proteome [
20]. Early work by Donovan et al. showed that mice presensitized to uninfected mosquito bites developed a T
1-biased response that conferred protection against
Plasmodium challenge, a protection lost in IFN-
-deficient animals [
9]. This indicates that immunological priming with the vector sialome can condition the host to eliminate parasites via nitric oxide-mediated effector mechanisms. Meta-analyses and successful human trials of AGS-v vaccines further validate the broad applicability of this vaccination strategy [
10,
18,
24].
Therapeutic Potential of Sialome-Derived Molecules
The mosquito sialome is not merely a source of irritants but represents a rich repository of bioactive peptides with significant biomedical applications. Research on mosquito antimicrobial peptides identified five cecropins (Aeae Cec1–Cec5) with potent anti-inflammatory activity [
2]. These peptides suppressed the expression of inducible nitric-oxide synthase, TNF-
, IL-1
, and IL-6 in macrophages. In a murine model of endotoxin shock, administration of these cecropins reduced systemic and pulmonary inflammatory cytokines, mitigating lung damage. Aeae Cec5 emerged as a promising lead compound for anti-endotoxemic therapies. Subsequent work has shown that cecropins from
Anopheles mosquitoes possess antimalarial activity against drug-resistant
Plasmodium strains [
22].
Studies of D7 proteins reveal a broad spectrum of pharmacological utility. The
Aedes aegypti D7 protein AeD7L2 antagonizes vasoconstriction and platelet aggregation by binding a thromboxane A
2 analog with nanomolar affinity [
15]. The
Aedes albopictus D7 protein AlboD7L1 binds multiple hemostasis agonists and inhibits both platelet aggregation and leukocyte recruitment [
16].
Another salivary protein, the D7 long protein of
Aedes aegypti, demonstrates intrinsic antiviral activity. Originally characterized as a scavenger of biogenic amines, recombinant D7 was shown to directly bind dengue virions and inhibit infection in vitro and in vivo, suggesting it could be a scaffold for novel arboviral therapeutics [
4].
The sialome also contains molecules that can ameliorate xenobiotic-induced hepatotoxicity. In a murine model of acetaminophen overdose, exposure to
Ae. aegypti bites significantly reduced serum transaminases and hepatic necrosis by downregulating pro-inflammatory cytokines and immune cell infiltration [
5].
The synthetic peptide syn-AeMOPE-1 suppressed nitric oxide production and NF-
B expression in macrophages and ameliorated experimental colitis in mice, demonstrating systemic anti-inflammatory potential [
6]. Furthermore, investigations into the atraumatic skin penetration by mosquitoes revealed that salivary components inhibit TRPV1 and TRPA1 pain channels, suggesting salivary factors possess intrinsic antinociceptive properties that could inspire novel analgesics [
7]. Consistent with this,
Aedes aegypti salivary gland extract exerts potent anti-pruriceptive activity by attenuating TRPA1-mediated calcium influx in sensory neurons [
8].
Cardiovascular Applications and Anticoagulant Properties
The anticoagulant properties of the mosquito sialome have garnered significant pharmaceutical interest for bioprospecting. Anophelin, a thrombin inhibitor from
Anopheles mosquitoes, exhibits a unique binding mechanism and exquisite potency (Ki = 5.87 ± 1.46 pM), making it one of the most powerful natural anticoagulants known [
25]. Structural studies have provided scaffolds for the rational design of novel anticoagulants with improved safety profiles.
Alboserpin, from
Aedes albopictus, displays a pleiotropic mechanism of action, inhibiting Factor Xa signaling while also exerting significant anti-inflammatory effects (
) [
26]. This positions alboserpin as a template for therapies targeting both thrombosis and inflammation in cardiovascular disease.
Regulatory Frameworks and Ethical Considerations
Research on mosquitoes and the development of sialome-derived vaccines are not conducted in a regulatory vacuum but are shaped by public health and legal frameworks. In Europe, the ECDC’s guidelines on invasive mosquito surveillance are critical for mitigating the risk of autochthonous transmission of diseases like dengue and chikungunya [
28]. This guidance influences the infrastructure for scientific trials and the approval of interventions like the release of genetically modified mosquitoes. Researchers must navigate complex regulations, such as the EU Directive 2010/63/EU on animal experimentation. The successful progression of AGS-v vaccines through Phase I trials demonstrates that clear regulatory pathways exist for advancing sialome-derived therapeutics [
10,
18]. Ethical considerations, including community engagement and ecological impact assessment, are integral to this process.
Discussion and Conclusions
Mosquito bites are correctly associated with significant morbidity and pathogenic vectoring. Yet within the mosquito sialome lies a functional paradox: the same molecules that facilitate hematophagy and pathogen transmission can also condition the host immune system and provide novel scaffolds for drug discovery. The transition from basic research to clinical application, exemplified by the AGS-v vaccine trials, marks a pivotal moment in translating insights from evolutionary biology into tangible medical innovation.
Repeated exposure induces immunological tolerance, while structural studies of salivary proteins like sialokinin have opened avenues for vaccines designed to abrogate saliva-enhanced viral transmission [
1,
19]. At the biochemical level, sialome-derived peptides like Aeae Cec5 show potent anti-inflammatory effects, and D7 proteins can directly neutralize virions [
2,
4].
The bioprospecting potential is vast, spanning cardiovascular drugs derived from anticoagulants like anophelin [
25], non-opioid analgesics targeting TRP channels [
7], and immunomodulators for autoimmune diseases [
6,
21]. These examples demonstrate that the study of the mosquito sialome transcends classical entomology, offering profound insights into immune regulation, vaccinology, and pharmacology. Recognizing these benefits highlights the value of basic research in harnessing a vector’s biology for medical innovation. As we continue to explore these compounds, the mosquito—long viewed as one of humanity’s most significant public health adversaries—may paradoxically become an improbable source of therapeutic innovation.
References
- Spencer Clinton, J.L., Vogt, M.B., Kneubehl, A.R., Hibl, B.M., Paust, S., and Rico-Hesse, R. Sialokinin in mosquito saliva shifts human immune responses towards intracellular pathogens. PLOS Neglected Tropical Diseases 17(3), e0011095 (2023).
- Wei, L., Yang, Y., Zhou, Y., et al. Anti-inflammatory activities of Aedes aegypti cecropins and their protection against murine endotoxin shock. Parasites & Vectors 11, 470 (2018).
- Olajiga, O.M., Marin-Lopez, A., Cardenas, J.C., et al. Aedes aegypti anti-salivary proteins IgG levels in a cohort of DENV-like symptoms subjects from a dengue-endemic region in Colombia. Frontiers in Epidemiology 2, 1002857 (2022).
- Conway, M.J., Londono-Renteria, B., Troupin, A., et al. Aedes aegypti D7 saliva protein inhibits dengue virus infection. PLOS Neglected Tropical Diseases 10(9), e0004941 (2016).
- Assis, J.B., Cogliati, B., Esteves, E., Capurro, M.L., Fonseca, D.M., and Sá-Nunes, A. Aedes aegypti mosquito saliva ameliorates acetaminophen-induced liver injury in mice. PLOS One 16(2), e0245788 (2021).
- Lara, P.G., Esteves, E., Sales-Campos, H., et al. AeMOPE-1, a novel salivary peptide from Aedes aegypti, selectively modulates activation of murine macrophages and ameliorates experimental colitis. Frontiers in Immunology 12, 681671 (2021).
- Derouiche, S., Li, T., Sakai, Y., Uta, D., Aoyagi, S., and Tominaga, M. Inhibition of transient receptor potential vanilloid 1 and transient receptor potential ankyrin 1 by mosquito and mouse saliva. Journal of Pharmacological Sciences 148(4), 299–307 (2022).
- Cerqueira, A.R.A., Rodrigues, L., Coavoy-Sánchez, S.A., et al. Aedes aegypti salivary gland extract alleviates acute itching by blocking TRPA1 channels. Frontiers in Physiology 13, 1055706 (2023).
- Donovan, M.J., Messmore, A.S., Scrafford, D.A., Sacks, D.L., Kamhawi, S., and McDowell, M.A. Uninfected mosquito bites confer protection against infection with malaria parasites. Infection and Immunity 75(5), 2523–2530 (2007).
- Friedman-Klabanoff, D.J., Birkhold, M., Short, M.T., et al. Safety and immunogenicity of AGS-v PLUS, a mosquito saliva peptide vaccine against arboviral diseases: a randomized, double-blind, placebo-controlled Phase 1 trial. EBioMedicine 85, 104375 (2022).
- Guerrero, D., Cantaert, T., and Missé, D. Aedes mosquito salivary components and their effect on the immune response to arboviruses. Frontiers in Cellular and Infection Microbiology 10, 407 (2020).
- Wang, Y., Ling, L., Jiang, L., Marin-Lopez, A., et al. Research progress toward arthropod salivary protein vaccine development for vector-borne infectious diseases. PLOS Neglected Tropical Diseases 18(7), e0012618 (2024).
- Pala, Z.R., Alves E Silva, T.L., Minai, M., Crews, B., Patino-Martinez, E., Carmona-Rivera, C., et al. Mosquito salivary apyrase regulates blood meal hemostasis and facilitates malaria parasite transmission. Nature Communications 15, 8194 (2024).
- Martin-Martin, I., Paige, A., Chagas, A.C., Valenzuela-Leon, P.C., Calvo, E., and Ribeiro, J.M.C. ADP binding by the Culex quinquefasciatus mosquito D7 salivary protein enhances blood feeding on mammals. Nature Communications 11, 2911 (2020).
- Martin-Martin, I., Kern, O., Brooks, S., Smith, L.B., Valenzuela-Leon, P.C., Bonilla, B., Ackerman, H., and Calvo, E. Biochemical characterization of AeD7L2 and its physiological relevance in blood feeding in the dengue mosquito vector, Aedes aegypti. FEBS Journal 288(6), 2014–2029 (2021).
- Martin-Martin, I., Smith, L.B., Chagas, A.C., Sá-Nunes, A., Shrivastava, G., Valenzuela-Leon, P.C., and Calvo, E. Aedes albopictus D7 salivary protein prevents host hemostasis and inflammation. JCI Insight 5(11), e137212 (2020).
- Marín-López, A., Raduwan, H., Chen, T.-Y., Utrilla-Trigo, S., Wolfhard, D.P., and Fikrig, E. Mosquito salivary proteins and arbovirus infection: from viral enhancers to potential targets for vaccines. Current Opinion in Virology 60, 101371 (2023).
- Manning, J.E., Oliveira, F., Coutinho-Abreu, I.V., et al. Safety and immunogenicity of a mosquito saliva peptide-based vaccine: a randomised, placebo-controlled, double-blind, phase 1 trial. The Lancet 395(10242), 1998–2007 (2020).
- Hastings, A.K., Uraki, R., Gaitsch, H., et al. Mosquito saliva enhances virus infection through sialokinin-dependent vascular leakage. Proceedings of the National Academy of Sciences 119(26), e2114309119 (2022).
- Vogt, M.B., Lahon, A., Arya, R.P., Kneubehl, A.R., Spencer Clinton, J.L., Paust, S., and Rico-Hesse, R. Mosquito saliva alone has profound effects on the human immune system. PLOS Neglected Tropical Diseases 12(5), e0006439 (2018).
- Jin, L., Zhang, Y., Chen, Q., et al. Recognition of Aedes aegypti mosquito saliva protein LTRIN by the human receptor LTβR for controlling the immune response. Cell Discovery 10(1), 42 (2024).
- Barreto, L.C., Costa, S.M., Pires, R.C., et al. Antimalarial activity of cecropin antimicrobial peptides derived from Anopheles mosquitoes. Scientific Reports 14, 13847 (2024).
- Mellanby, R.H., Peng, Y., and Oka, T. Update on mosquito bite reaction: Itch and hypersensitivity, pathophysiology, prevention, and treatment. Frontiers in Immunology 13, 1024559 (2022).
- Leitner, W.W., Wali, T., and Kincaid, R. Meta-analysis of the effects of insect vector saliva on host immune responses and infection of vector-transmitted pathogens: A focus on leishmaniasis. PLOS Neglected Tropical Diseases 8(10), e3197 (2014).
- Figueiredo, A.C., de Sanctis, D., and Pereira, P.J. Unique thrombin inhibition mechanism by anophelin, an anticoagulant from the malaria vector. Proceedings of the National Academy of Sciences 109(52), E3649–E3658 (2012).
- Chagas, A.C., McPhie, P., San, H., et al. Alboserpin, the main salivary anticoagulant from the disease vector Aedes albopictus, displays anti–FXa-PAR signaling in vitro and in vivo. Journal of Thrombosis and Haemostasis 20(6), 373–383 (2022).
- Kazimírová, M. and Štibrániová, I. Tick salivary compounds: their role in modulation of host defences and pathogen transmission. Frontiers in Cellular and Infection Microbiology 3, 43 (2013).
- European Centre for Disease Prevention and Control. Guidelines for the surveillance of invasive mosquitoes in Europe. Stockholm: ECDC; 2012.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).