1. Introduction
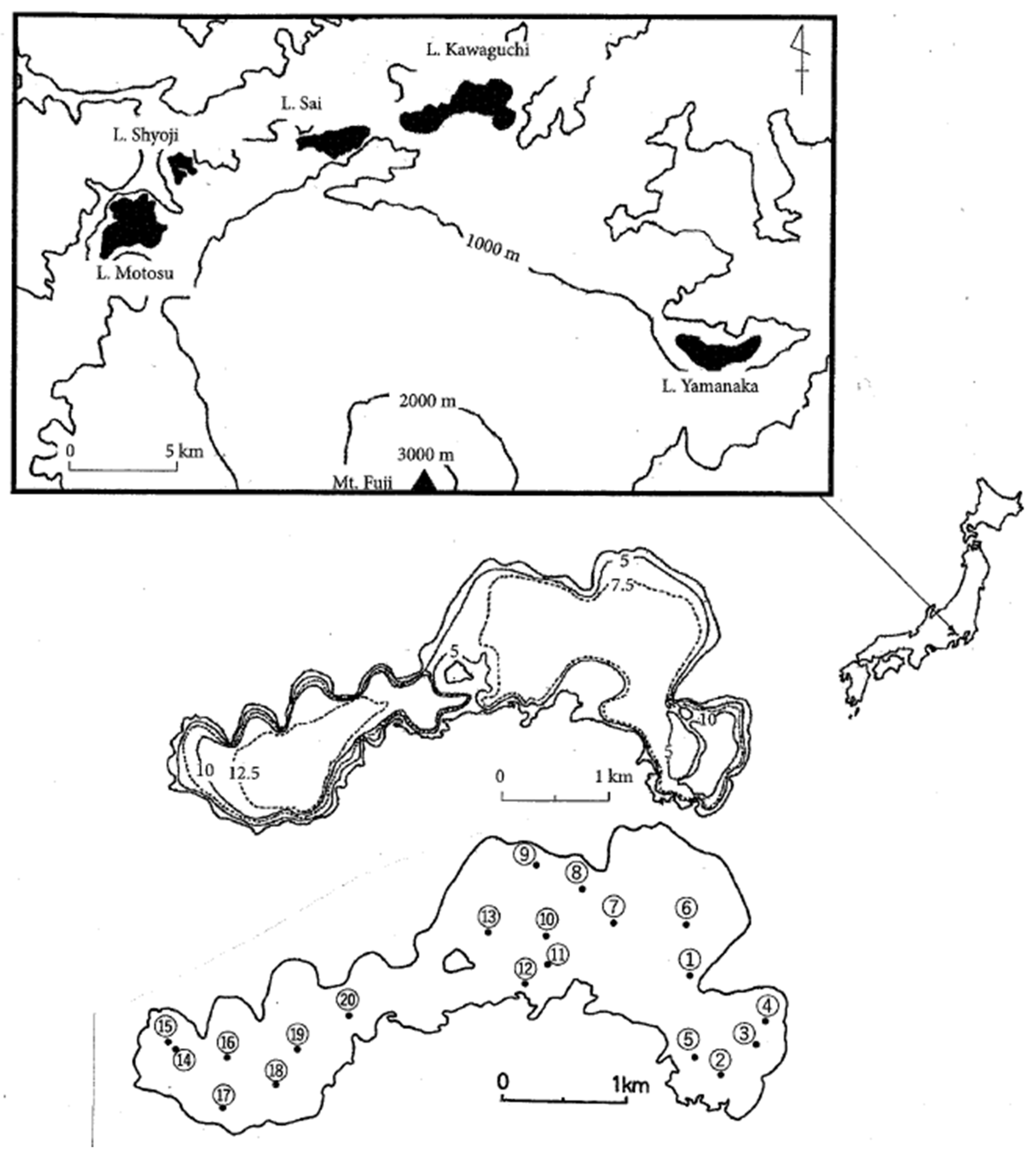
Lake Kawaguchi is located at the base of Mt. Fuji on its northern slope (35°31’N, 138°45’E at the lake center; altitude above sea level 832 m; shore length 17.4 km; surface area 5.96 km
2; maximum depth 16.1 m; mean depth 9.3 m). In 1993, the Yamanashi Prefectural government [
1] reported that transparency in the lake had decreased to about 3.5m as a result of eutrophication. Aizaki et al. [
2], using the modified Carlson’s trophic state index based on transparency, total phosphorus, and chlorophyll-a, ranked Lake Kawaguchi as mesotrophic-eutrophic. Hirabayashi et al. [
3] reported explosive growth and water-bloom formation of
Peridinium bipes in the early summer of 1995, a time when nutrient levels in the water, particularly phosphorus, were very high. Recently, Nakamura et al. [
4] reported an analysis of the transparency, COD, TN, TP, and chlorophyll-a based on the monthly surface water quality observation data from 1974 to 2013 of the Fuji Five Lakes by Yamanashi Prefecture. Although the water quality of Lake Kawaguchi had been growing significantly worse over time, a recovery trend has been seen since 2002.
Following an early study by Terao [
5], a number of researchers have examined the biota of Lake Kawaguchi. However, its macrobenthic fauna has attracted less attention. In a review, Lindegaard [
6] stated that researchers have used indicators including species composition and the relative abundance and distribution pattern of benthic macroinvertebrate communities, particularly chironomid fauna in the profundal zone, to assess the trophic state and pollution of lakes. Lake Kawaguchi has been changing biotically and environmentally since the mid-1990s, and this has affected the bottom fauna, especially the dominant chironomids. We examined the horizontal distribution of benthic macroinvertebrates in Lake Kawaguchi and compared chironomid fauna and density in the this and past studies, and here discuss changes in the chironomid community as they relate to the trophic status of the lake.
2. Materials and Methods
2.1. Study Site
Lake Kawaguchi was formed when lava flows from Mt. Fuji and other volcanoes dammed streams flowing down from northern mountain ranges. These ranges have many porous volcanic deposits so that most of the runoff water flows underground, but there are surface streams as well. The lake is partly surrounded by cultivated land on its eastern shore, and some towns and villages on the northeastern and southeastern shores. Ice covers the lake from January to February, and there is a persistent thermocline in summer [
3].
2.2. Methods
Chironomid larvae are most efficiently sampled from late autumn to early spring, when almost all stay near the sediment surface. The larvae of
P. akamusi burrow deep into the lake bottom sediments, ca. more than 50cm, to aestivate during the summer [
7,
8]. We conducted a multi-point sampling survey on March 7, 2025, using a standard Ekman-Birge grab (15×15 cm). Samples were taken at each of 20 locations (7.2-13.3m depth) in a ca. 800×800m grid (
Figure 1). A Global Positioning System (GPS) was used to record the sampling sites. The sediment was sieved through a Surber net (NGG 38; 560 µm mesh size), after which benthic macroinvertebrates were separated out and counted in the laboratory. The 560 µm-mesh sieve did not capture first and second instar larvae of small chironomids. To identify the obtained chironomids, we soaked some of the larvae in a 10% KOH solution, mounted them on slides with gum-chloral solution, and examined them under a microscope at 100, 200 and 400x magnification. Identification was done to the generic level using the keys of Cranston [
9] and Wiederholm [
10]. To identify them to the species level, the keys of Orendt and Spies [
11] and Sasa [
12] were used.
Samples of bottom sediment for use in organic matter analysis were collected with a core sampler (three cm inner diameter). The upper 3-cm layer of mud in each core was oven-dried at 110°C for two days. Then, to determine the value of loss on ignition (IL), it was ignited in a muffle furnace at 550°C for two hours. The dissolved oxygen concentrations (DO) in the water at the mud-water interface were also measures using the core sampler. The water near the mud surface in the core sampler (which remained above the sediment in the core sampler when it was pulled from the water) was siphoned carefully into a glass bottle. The dissolved oxygen concentration was measured with Wikler’s method with azide modification. A thermistor thermometer was used to measure the water temperature (WT) in the bottom sediment samples.
Differences among environmental factors and zoobenthos densities were analyzed using Mann-Whitney’s U-test. The Kendal rank correlation test was used to examine correlations between benthic macroinvertebrate densities and environmental variables including depth, IL, WT and DO, using the Nap Ver. 4 statistical package (Igaku-Shoin, Tokyo, Japan).
3. Results
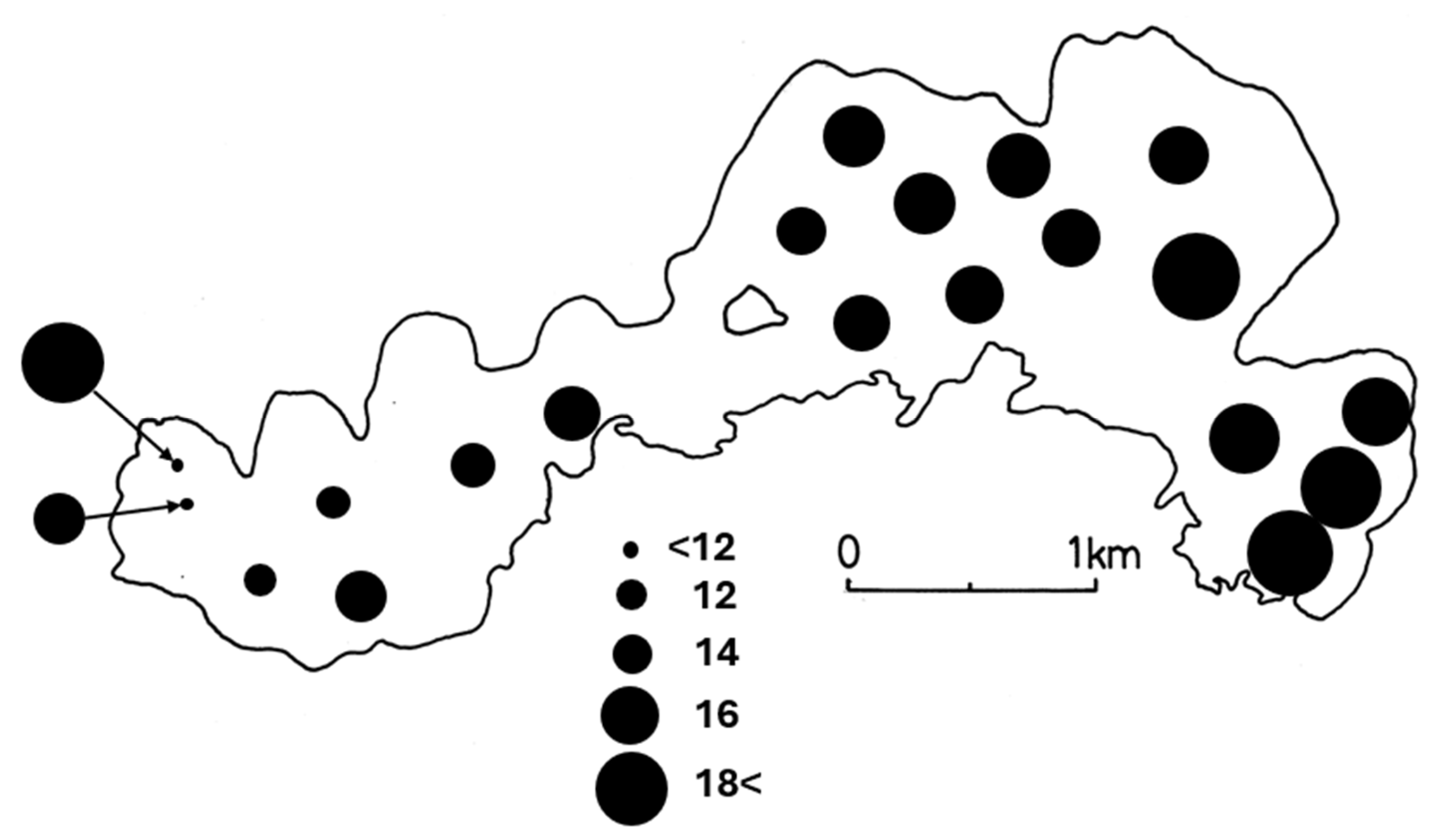
Among environmental factors, the mean values and standard deviations of water depth were 10.0±1.9 m (ranging from 7.2 m to 13.3 m), WT were 5.4±0.4℃, and DO were 9.6±0.6 mg/l, respectively. The differences in DO and WT were small among the sampling sites, indicating that the spring cycle period had begun. The content of organic matter in the sediment’s surface layer (IL) was 16.4±2.0% (ranging from 12.9% to 19.9%) for the entire lake, which was relatively high. The lake basin consists mostly of soft bottoms with more than 15% organic matter content. The inlets in the eastern part (sampling sites 1, 2 and 3) and western part (site 15) of the lake had the highest levels (
Figure 2).
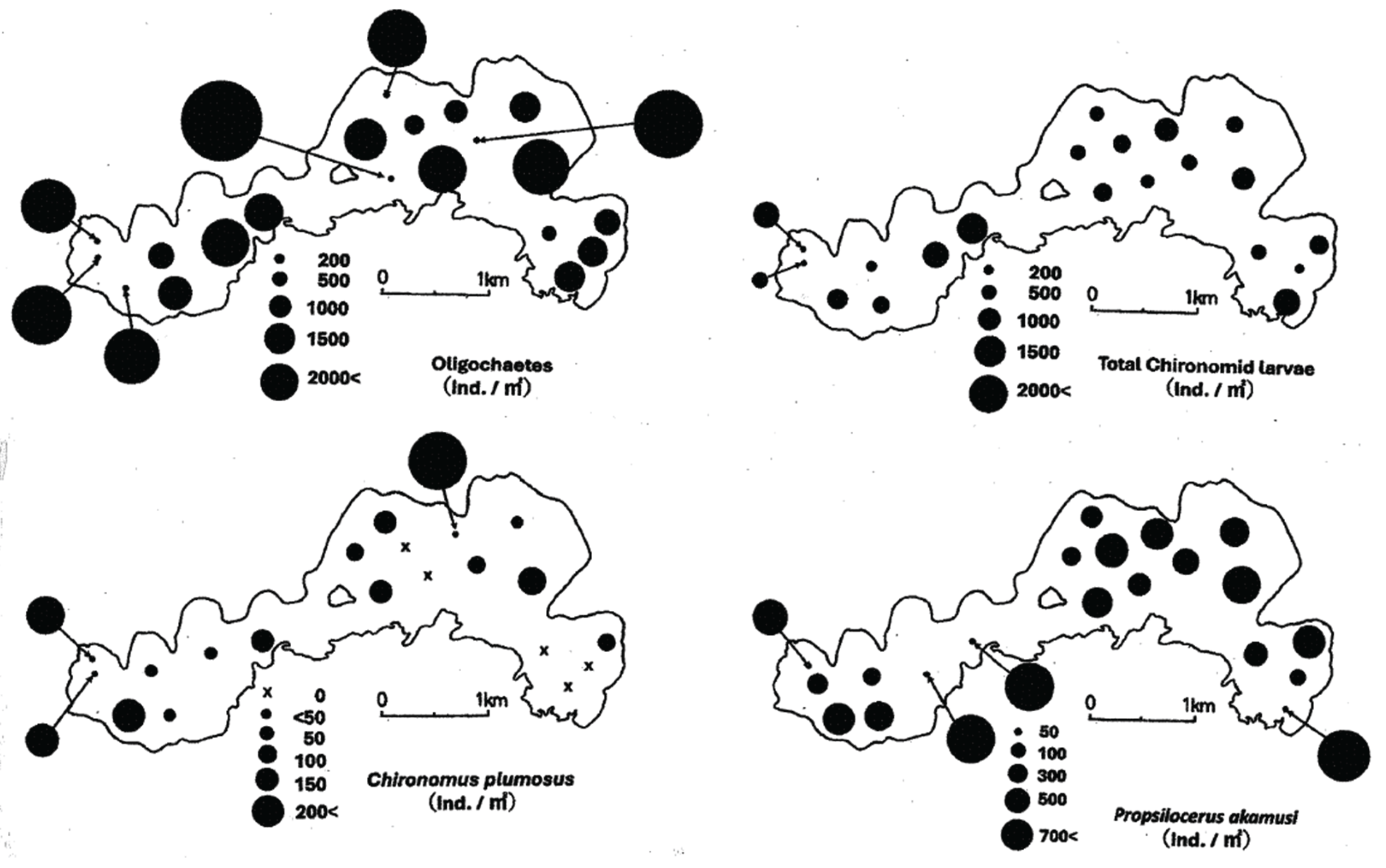
The benthic animals identified in this survey were aquatic oligochaetes, chironomid larvae, shellfish, and others. The mean density of oligochaetes, which is the dominant group, was 2,457±1,247 individuals/m2, followed by that of total chironomid larvae at 816±391 individuals/m2. Chironomidae species belonging to two subfamilies, Chironominae and Orthocladiinae, were identified. The larvae of Propsilocerus akamusi were the most abundant species of the chironomid fauna at 669±358 individuals /m2, followed by Chironomus plumosus at 109±114 individuals/m2. Other chironomids (38±75 individuals/m2) were also captured. Benthic communities were captured at all stations, but each taxa had its own characteristics.
Figure 3 illustrates the bathymetric distribution of the density of oligochaetes and chironomid larvae, which were collected at all sampling sites. Oligochaetes were present at higher densities in the western part of the lake (sampling sites 14, 15 and 17) and at the center of the lake (sites 7, 9 and 12). The densities differed between the sites, with the maximum density of 5200 individuals/m
2 (sampling site 12; 8.4m depth) measured about 250m from the south shore of the lake. Chironomid larvae also inhabited the entire lake bottom, with higher densities in the eastern and western parts of the lake (sampling sites 2 and 20).
P. akamusi had the widest depth distribution (from 7.2 to 13.3 m in depth), followed by
C. plumosus (from 7.4 to 13.3 m in depth) (
Figure 3). The maximum density of
P. akamusi was as high as 1422 individuals/m2 (sampling site 2; 10.0 m depth), measured about 150m from the south-eastern shore of the lake, followed by sites 19 and 20, with the same value of 1289 individuals/m
2 in the center of the lake. The distribution of
P. akamusi was skewed toward the western part of the lake. The maximum density of
C. plumosus was as high as 444 individuals/m
2 (sampling site 8; 9.0 m depth), measured about 250m from the northern shore of the lake. At sampling sites 2, 3, 5, 10 and 11,
C. plumosus was not collected from the bottom samples.
Table 1 presents correlation matrices of the densities of oligochaetes (Oli), total chironomid larvae (T-Ch),
P. akamusi (PA),
C. plumosus (CP), and environmental factors. The
P. akamusi density was positively correlated with total chironomid density.
4. Discussion
Species composition and the relative abundance and distribution pattern of benthic macroinvertebrate communities, particularly in the profundal zone, have been used by many researchers as indicators of the trophic state and pollution of lakes (reviews by Brinkhurst [
13]; Lindegaard [
6]). Chironomid fauna and chaoborids have been used in trophic classifications of Japanese lakes [
14,
15,
16]. According to Iwakuma et al. [
17],
C. plumosus and
P. akamusi are common in eutrophic lakes in Japan. An attempt was made to compare the results of this study with previous Lake Kawaguchi studies [
18,
19,
20,
21] (
Table 2), but the different sampling seasons among the reports, especially between ours and Miyadi [
18] and Kitagawa [
19], makes discussion of the long-term change in chironomid fauna in this lake difficult. On the other hand, our survey and the Hirabayashi et al. [
20,
21] surveys were conducted in the same season and with the same methods. Thus, the results can be simply compared.
The results of this study show that the mean value of organic matter in the upper sediment layer has increased since Hirabayashi et al. [
20]. Compared to 2006 (19 years ago; [
21]), the density of
C. plumosus was almost the same, whereas there was a decrease of one third compared to 1993 (32 years ago; Hirabayashi et al. 1995). Moreover, comparative analysis revealed a decrease in the percentage of
C. plumosus larvae in the chironomid community since Kitagawa [
19], i.e., 13.3% in the present study. However, the density of
P. akamusi had doubled since 2006, whereas compared to 1993, the density of
P. akamusi was almost the same. The percentage of
P. akamusi larvae in the chironomid community increased over time, from 29.2% (1973) and 50.5% (1993) to 70.7% (2006) and 82.0% (2025). In this study,
P. akamusi larvae were the most abundant chironomid species. The density of oligochaetes had doubled since 2006, but was less than half compared to 1993. No noticeable change has occurred in the percentage of oligochaetes among the benthic macroinvertebrates since 1931, except in 1973, ranging from 70.5% to 81.4%.
Recently, the chironomid community has had an increased percentage of
P. akamusi larvae and a decreased percentage of
C. plumosus. Increased organic matter in the upper sediment layer is also seen. Iwakuma and Yasuno [
8] reported that high temperature and low oxygen concentrations are unfavorable for
C. plumosus larvae. However, mature
P. akamusi larvae can withstand anoxic conditions, especially during the summer, by burrowing deep into the sediment to aestivate [
7]. Nakamura et al. [
4] reported that the water quality of Lake Kawaguchi had been recovering since 2002, but the fact that the percentage of
P. akamusi larvae, which is an indicator species of eutrophication [
17], in the chironomid community has increased from 1993, suggests increased eutrophication of this water body.
5. Conclusions
To summarize, in recent years, the most abundant chironomid species in Lake Kawaguchi has been
P. akamusi larvae, which is an indicator species of eutrophication [
17], and the chironomid community has shown an increased percentage of
P. akamusi and a decreased percentage of
C. plumosus larvae. In addition, the increased levels of organic matter in the upper sediment layer of Lake Kawaguchi suggest ongoing eutrophication. Although previous studies classified this lake as mesotrophic-eutrophic [
1,
2], reconsideration of this classification is warranted given the findings of this study. We suggest that this lake be ranked as a eutrophic lake based on long term investigation of the changes in chironomid fauna.
Author Contributions
Conceptualization, Hirabayashi K.; methodology, Hirabayashi K.; software, Hirabayashi K; validation, Hirabayashi K.; formal analysis, Takeda M.; investigation, Hirabayashi K and Takeda M.; resources, Hirabayashi K; data curation, Hirabayashi K; writing—original draft preparation, Hirabayashi K.; writing—review and editing, Hirabayashi K; visualization, Hirabayashi K. and Takeda M.; supervision, Hirabayashi K.; project administration, Hirabayashi K. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Acknowledgments
Part of this research will be used as basic information for the writing of the Kawaguchiko Town Chronicle, and I would like to thank Mr. Yuki Sugimoto of the Kawaguchiko Town Board of Education for his great help in carrying out the research. I would like to express my deepest apologies.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yamanashi Prefecture. The water quality of the Fuji Five Lakes over 21 years. Yamanashi Prefecture: Kofu, Japan, 1993; pp. 1-138 (In Japanese).
- Aizaki, M.; Otsuki, A.; Fukushima, T.; Kawai, T.; Hosomi, M.; Muraoka, M. Application of modified Carlson’s trophic state index to Japanese lakes and its relationships to other parameters related to trophic state. Res. Rep. Nat. Inst. Environ. Stud. Jpn. 1981, 23, 13-31.
- Hirabayashi, K.; Yoshizawa, K.; Yoshida, N.; Ariizumi, K.; Kazama, F. Long-term dynamics of freshwater red tide in shallow lake in central Japan. Environ. Health Prev. Med. 2007, 12, 33-39.
- Nakamura, S.; Uejima, T.; Watanabe, H.; Matsuyama-Serisawa, K.; Serisawa, Y. Seasonal changes and long-term fluctuations of water quality in the Fuji Five Lakes, Central Japan. Fujisan Kenkyu. 2016, 10, 31-40.
- Terao, S. Study on Leptodora in Lake Kawaguchi. Jpn. J. Zool. 1912, 24, 650-651.
- Lindegaard, C. Classification of water-bodies and pollution. In The Chironomidae: Biology and Ecology of Non-Biting Midges; Armitage, P.D., Cranston, P.S., Pinder, L.C.V., Eds.; Chapman & Hall: London, UK, 1995; pp. 385–404.
- Yamagishi, H.; Fukuhara, H. Vertical migration of Spaniotoma akamusi larvae (Diptera: Chironomidae) through the bottom deposits of Lake Suwa. Jpn. J. Ecol. 1972, 22, 226-227.
- Iwakuma, T; Yasuno, M. Chironomid productions in highly eutrophic Lake Kasumigaura. Verh. Internat. Verein. Limnol. 1981, 21, 664-674. [CrossRef]
- Cranston, P.S. A key to the larvae of the British Orthocladiinae (Chironomidae). Freshwater biological association scientific publication 1982, 45, 1-152. [CrossRef]
- Wiederholm, T. Chironomidae of Holarctic region. keys and diagnoses. Part 1. larvae. Entmol. Scand. Suppl. 1983, 19, 1–457.
- Orendt, C.; Spies, M. Chironomus Meigen (Diptera: Chironomidae). Key to the larvae of importance to biological water analysis in Germany and adjacent area. Ehnert and Blankenburg GmbH, Leipzig, Germany, 2012; pp. 1-24.
- Sasa, M. Taxonomical and biological notes on Tokunagayusurika akamusi (Tokunaga), with description of immature stages (Diptera, Chironomidae). Jpn. J. Sani. Zool. 1978, 29, 93-101. [CrossRef]
- Brinkhurst, R.O. Distribution and abundance of tubificid (Oligochaeta) species in Toronto Harbour, Lake Ontario. J. Fish. Res. Bd. Canada, 1970, 27, 1961-1969. [CrossRef]
- Miyadi, D. Studies on the bottom fauna of Japanese lakes. X. Regional characteristics and system of Japanese Lakes based on the bottom fauna. Jpn. J. Zool. 1933, 4, 417-437.
- Kitagawa, N. A classification of Japanese lakes based on hypolimnetic oxygen and benthonic fauna. Jpn. J. Limnol. 1978, 39, 1-8. [CrossRef]
- Yasuno, M.; Iwakuma, T.; Sugaya, Y.; Sasa, M. Zoobenthos of Japanese lakes of different trophic status, with special reference to Chironomidae. Res. Rep. Spec. Res. Proj. Environ. Sci. 1983, B182-R12-17, 21-48.
- Iwakuma, T.; Yasuno, M.; Sugaya, Y.; Sasa, M. Three large species of Chironomidae (Diptera) as biological indicators of lake eutrophication. In Biological Monitoring of Environmental Pollution; Yasuno, M., Whitton, A.B. Eds.; Tokai University Press: Tokyo, Japan, 1988; pp. 101-113.
- Miyadi, D. Studies on the bottom fauna of Japanese lakes. 5. Five Lakes at the north foot of Mt. Hudi and Lake Asi. Jpn. J. Zool. 1932, 4, 81-125.
- Kitagawa, N. Studies on the bottom fauna of The Fuji Five Lakes and Lake Ashino. Rikusui Fueiyouka no Kisotekikenkyu. 1973, 2, 32-37.
- Hirabayashi, K.; Yoshizawa, K.; Horiuchi, M. Horizontal distribution of benthic macroinvertebrates in Lake Kawaguchi, Japan. Rep. Suwa Hydrobiol. 1995, 9, 121-129.
- Hirabayashi, K.; Yoshizawa, K.; Oga, K.; Yoshida, N.; Ariizumi, K.; Kazama, F. Change of chironomid fauna (Diptera: Chironomidae) in eutrophic Lake Kawaguchi, Japan. Bol. Mus. Mun. Funchal, Sup. 2008, 13, 109-117.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).