1. Introduction
Mesenchymal stem cells (MSC) constitute a multipotent cell-adherent population of fibroblast-like morphology with high renewal capacity. They can differentiate into adipocytes, osteocytes, and chondrocytes, among other cell types [
1]. MSC are positive for CD90, CD105, and CD73 and negative for CD45, CD19, CD14, CD11b, CD34, and DR hu-man leukocyte antigens (HLA-DR) [
2]. In addition, they express the transcription factors of pluripotency, PUO5F1 (previously Oct-4), Sox-2, and Nanog [
3], which indicate the un-differentiated state of the cell. The undifferentiated cells have the potential to become specialized cells, such as muscle cells, blood cells, and brain cells [
4]. MSC possess immuno-regulatory properties [
5] through cell-to-cell contact and cytokine secretion [
6]. The secretome of MSC includes cytokines and growth factors, among them are transforming growth factor beta (TGF β), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), endothelial growth factor (EGF), platelet derived growth factor (PDGF) and Angiopoietin-2 (Ang2) [
7].
Human metapneumovirus (HMPV) is a member of the Pneumoviridae family [
8,
9]. HMPV is a ubiquitous and frequent respiratory pathogen considered one of the leading causes of lower respiratory tract infections in children, the elderly and the immunosuppressed population [
10]. HMPV infects more than 85% of the population by age ten. According to epidemiological reports conducted by the World Health Organization, 5% to 15% of all respiratory tract infections of children under the age of 5 are caused by HMPV, making it the second most common cause of hospitalization of young children after hu-man respiratory syncytial virus (hRSV) [
11,
12]. The HMPV infection starts in the upper respiratory tract but can spread to the lower airways, including the bronchi, bronchioles, and alveoli [
13]. It has a tropism for polarized respiratory epithelial cells, mainly the apical ciliated cells [
14]. A wide variety of cells are susceptible to HMPV infection, including dendritic cells, macrophages and MSC [
15,
16,
17]. MSC are highly susceptible and per-missive to viral infections associated with the expression of diverse cell surface receptors [
17]. This variety of potential viral receptors may facilitate viral entry. Several viruses mediate their entrance using the fusion of the viral envelope with the cellular membrane and exit from the infected cell as a way of budding from the cellular membrane. Both processes imply modifications and redistribution of the components of the cytoskeleton and associated molecules such as annexin V. HMPV infection is not the exception; the infection with this virus modifies the cytoarchitecture of MSC [
17]. The viral infection of MSC also modifies some of their biological properties, like plasticity and the production of soluble mediators such as chemokines and growth factors [
18]. These observations are reported for herpes simplex virus type 1 [
19], which infect MSC and modify their secretome and their ability to differentiate. Thus, the present study is aimed towards analyzing the changes in the phenotype, the differentiation capacity, the secretome and the cytoskeleton of placental MSC (PL-MSC) after infection with HMPV.
2. Materials and Methods
2.1. Cells and Viruses
Human laryngeal epithelial type 2 cells (HEp-2; ATCC CCL23, USA), reported to be contaminated with HeLa cells, were used to isolate HMPV from a clinical sample in our laboratory as described elsewhere [
20] and to multiply the viral isolates. The procedures for propagating the virus and assessing viral infectivity are described in [
21].
2.2. MSC Cultures
The 6 batches of placental MSC (MSC-PL) cultures were obtained from 4 donors characterized by the mesenchymal stem cell laboratory in charge of Dr. Montesinos. [
22]. Research Committees of the Research Division of the Faculty of Medicine of UNAM (FMED/CI/SPLR/004/2016). The MSC cultures were maintained with Dulbecco’s Modified Eagle Medium Nutrient Mixture F12 (DMEM F12;) supplemented with fetal bovine serum (BioWest). Cells were seeded at a 1 × 10
3 cells/cm
2 density and the medium was changed every seven days. When cells reached 80% of confluence, the monolayer was detached (trypsin 0.05%, ethylenediaminetetraacetic acid 0.02%) and reseeded at 1 × 10
3 cells/cm
2 density. This step was repeated until the cells reached R5.
2.3. Characterization of the PL-MSC
The presence (CD105, CD90, CD73 and HLA-ABC) and absence (CD45, and HLA-DR) of PL-MSC markers was evaluated by flow cytometry analysis with monoclonal antibodies coupled with fluorescein isothiocyanate (FITC), phycoerythrin (PE), phycoerythrin-cyanine 7 (PE-Cy7) and allophycocyanin (APC) (BD Biosciences). CD105, CD45, and HLA-DR were coupled to PE, CD90 was coupled to FITC, respectively, HLA-ABC was coupled to PE-Cy7 and CD73 was coupled to APC. Stained cells were evaluated with a FACS CANTO II Flow Cytometer (BD Biosciences) with at least 10,000 events per sample. The data were analyzed with FlowJo 10 (FlowJo LLC.). Isotype controls were run in parallel for each marker. Adipogenic differentiation was induced using an adipogenic medium for 21 days as described in [
23], which contains insulin 10μM and indomethacin 200μM. As follows, adipogenesis was confirmed by Oil Red O Staining (Sigma-Aldrich) that stains the lipids contained in adipocytes. Osteogenic differentiation was induced by using a commercial kit of osteogenic differentiation medium (Osteogenic Differentiation Kit STEMPRO
® Osteogenesis Differentiation Kit, Gibco) supplemented with 0.1 M dexamethasone, 50 µg/mL ascorbic acid, and 10 mM β-glycerol phosphate for 21 days. Osteogenesis was confirmed by Alizarin Red S staining (Sigma-Aldrich), which stains this characteristic enzyme produced by osteoblast. Chondrogenic differentiation was induced with the commercial kit Chondrocyte Differentiation Medium supplemented with 0.1 µM dexamethasone, ascorbate 50 µM, Insulin–Transferrin–Selenium 1 mL/50 mL supplement, sodium pyruvate, proline, and L-glutamine, supplemented with 10 ng/mL TGF-β3 (used for 21 days). Chondrogenesis was confirmed through alcian blue staining (Sigma-Aldrich), which stains mucopolysaccharides generated by chondrocytes.
2.4. HMPV Infection
We previously reported [
17] the kinetics of viral infection of PL-MSC; according to these data, our experiments were done at a multiplicity of infection (MOI) of 1.0 for 48 hours postinfection. After 2 hours of infection at 37°C, the viral input was replaced with fresh DMEM without fetal bovine serum for 2 days at 37 °C in a 5% CO
2 atmosphere. The supernatants were collected at 48 h postinfection and were titrated by TCID50 (Tissue Culture Infectious Dose). The viral titer by TCID50 was calculated according to the Kärber formula [
21]. Crystal violet staining was performed to visualize the syncytia.
2.5. Immunofluorescence Assay
a. Detection of HMPV by commercial anti-N Mabs (Monoclonal antibodies)
The culture medium was removed from the culture slides for the immunofluorescence assay. The monolayers were washed twice with phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde for 8 min. Then, they were washed thrice with PBS and cleaned with cold acetone for 5 min. After this, they were washed once with PBS and blocked with a PBS solution with 2% of Bovine Serum Albumin (BSA) (Sigma-Aldrich) for 1 h at room temperature. 0.05% Triton X-100 (Sigma-Aldrich) in PBS was added for 30 min to permeabilize the cells. The anti-N Mabs were used at 1:300 dilution (anti-mouse IgG NOVUS), and Alexa Fluor 594 were used as a secondary antibody. To visualize cell nuclei, the samples were washed with PBS and incubated with 4’, 6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 30 min in the dark. Last, they were washed and mounted on slides covered with the commercial solution Vectashield® (Vector H-1200) at room temperature.
b. Determination of the distribution of the cytoskeleton proteins and annexin V in HMPV infected mesenchymal stem cultures.
The immunofluorescence assay was done as previously described. For the immuno-detection of annexin V (Merck Millipore) and tubulin (Sigma-Aldrich), the cells were incubated with the corresponding primary antibodies at a dilution of 1:200 overnight at 4 °C in humidity. Then, the layers were washed five times with PBS and incubated with the secondary antibody (anti-mouse IgG conjugated to FITC; Zymed) at a dilution of 1:100 for 1 h in the darkness. The immunodetection of filamentous actin was performed by direct labeling with Alexa Fluor 594 phalloidin (Invitrogen) used at a dilution of 1:100. For the visualization of cell nuclei, the samples were washed with PBS, incubated with 4’,6-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) for 30 min in the dark. Last, they were washed and mounted on slides covered with the commercial solution Vectashield® (Vector H-1200) at room temperature.
All the immunofluorescence-stained images were analyzed with a Nikon TS100 microscope (Nikon Co.) using the microscopy program NIS-Elements Viewer 4.2 (Nikon Co.) and analyzed on Image J. All the assays were performed in parallel with mock-infected cells.
2.6. Differentiation Assay
In the case of the cell differentiation experiments, the cells were fixed with methanol, stained with crystal violet for 3 min for controls, and finally stained with Oil Red O for adipocytes, Alcian blue for chondrocytes, and Alizarin Red S for osteocytes.
2.7. Profile of Growth Factors
PL-MSC were infected with HMPV for 48 h. The control condition was PL-MSC cultures without viral infection, defined as mock-infected. Then, the cell culture supernatants were collected, freeze-dried, and resuspended at 100 µg/mL. A volume of 100 µL was used for the LEGENDplex assay with BioLegend’s LEGENDplex human Growth Factor Panel (13-plex). This assay evaluates the following growth factors: Ang-2, EGF, EPO, FGF-basic, G-CSF, GM-CSF, HGF, M-CSF, PDGF-AA, PDGF-BB, SCF, TGF-α, and VEGF (BioLegend). The data were analyzed using BioLegend’s software Qognit, V. The data were expressed in pg/mL.
2.8. Statistical Analysis
The non-parametric Mann-Whitney test was performed, and the Unpaired t test was used to perform the statistical analysis of the growth factors secreted by the cells infected and non-infected with the GraphPad Prism version 8 program. The statistical tests applied to the cell surface markers and Immunofluorescence assays consisted of an unpaired t-test with a p value < 0.05. Each experiment included at least 7 replicates. Data were analyzed using ImageJ (Schneider et al., 2012) and GraphPad Prism (GraphPad Software, San Diego, CA).
4. Discussion
Our in vitro model aims to study the effect of HMPV infection on different biological activities of PL-MSC and the way in which the viral infection could induce modification in the cytoskeleton organization. First, our model demonstrated the susceptibility and permissiveness of PL-MSC to HMPV, as previously reported [
17]. We could detect by immunofluorescence assay, both the viral presence and the distribution of the cytoskeleton proteins. We also demonstrated that the HMPV infection induces changes in the morphology of the PL-MSC associated with the rearrangement of the cytoskeleton proteins. Regarding these modifications, the morphological changes were due to the differential distribution of cytoskeletal filament proteins. The cytoskeletal filaments are dynamically involved in cell shape and size, contributing to various cellular processes such as cell division, migration, endocytosis, and exocytosis. In the case of some viral infections, the reorganization of the cytoskeleton is essential for the formation of vesicles to release the viral particles from the infected host cells [
26]. Also, this filament modification participates in the viral exit by budding and the passing of viral particles by cell-to-cell fusion.
Moreover, during viral replication, the modification of cytoskeletal filaments trans-ports newly synthesized proteins from the sites of viral replication, called inclusion bodies, to viral assembly sites [
27]. In our study, the cytoskeletal filaments of HMPV-infected PL-MSC were modified. The modifications of actin filaments and microtubules may indicate that the transport of proteins from the inclusion bodies was completed. This has been previously observed in HMPV-infected cells [
28]. It has been shown that modifications in the cytoskeleton filaments, particularly an increase in actin filaments, are associated with the release of viral particles and the syncytia formation (cell-to-cell fusion) [
26,
28]. As de-scribed for hRSV and HMPV, both induce the reorganization of actin filaments to promote virus transmission through cell-to-cell contact [
29].
The HMPV-infected PL-MSC culture exhibited morphological changes, including the formation of cell clusters without inner cytoplasmic boundaries and with multiple nuclei, indicating the formation of syncytia. Under the microscope, syncytia can be observed as large cytoplasmic masses with several nuclei [
30]. It has been documented that HMPV, like other fusogenic viruses, can induce the formation of syncytia to increase the viral production capacity and improve mobility and survival capacities [
31]. It has also been shown that syncytia formation can be induced by the rearrangement of cytoskeletal filaments, particularly actin filaments [
32]. The actin cytoskeleton filaments are essential for the nonlytic egress of several viruses [
33], as is the case with HMPV. In addition, the effect of the virus on cell morphology is at the level of the host cell plasma membrane, the insertion of newly synthesized viral antigens inducing a depolymerization of actin filaments [
34]. Of course, the rearrangement of the cytoskeleton of HMPV-infected MSC has functional implications. For instance, an increase in the migratory capacity of stromal fibroblasts concomitant with increased actin filaments has been documented [
35].
The cytoskeleton-associated protein annexin V participates in various cellular processes, including blood coagulation, inflammation, and responses to cellular stress [
36]. In the studies of influenza virus infection, the concentration of annexin V increases in the cell membranes of infected cells [
37]. At the same time, it has also been observed that annexin V is incorporated into viral particles [
36]. Our data shows that the HMPV infection of PL-MSC induces the expression of annexin V, particularly in cytoplasm. This increase may be related to viral replication since it was only observed in the cytoplasm of infected cells and cell membranes. Without a doubt, the HMPV infection modifies the cell architecture, favoring the annexin V expose since it functions as an associated cytoskeleton molecule.
Some RNA viruses use tubulin microtubules for transport their viral components [
38]. The microtubule network is often hijacked by viruses to transport themselves to inclusion bodies and for facilitate their exit from the cell [
39].
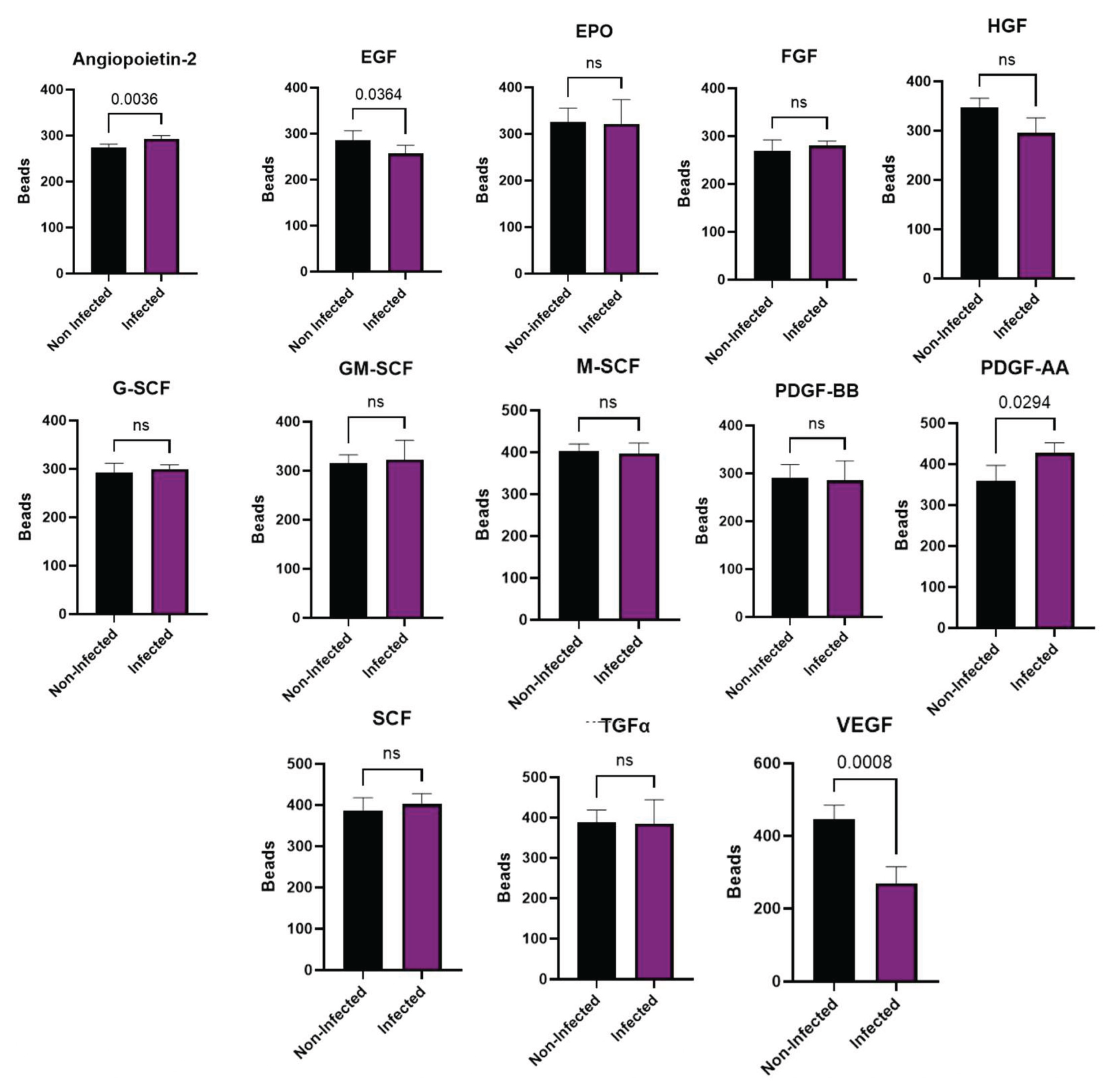
Additionally, the HMPV infection changes the cytokine secretion pattern of PL-MSC without altering their differentiation capacity. Therefore, we analyzed the production of growth factors that participate in tissue repair, the migration of progenitor and stem cells, and the regulation of inflammation. HMPV infection of PL-MSC significantly increased the secretion of Ang-2 and PDGF-AA while significantly decreasing EGF and VEGF secretion without affecting the rest of the tested growth factors. Numerous viruses, including some respiratory viruses, increase VEGF expression and its receptors, which is related to the pathophysiology of viral infections and aggravates the clinical condition of the patients [
40]. VEGF decreases the cytotoxic and antiviral activity of NK and T CD8 cells [
41]. Our results show that the HMPV infection of PL-MSC causes a decrease in VEGF secretion; it might stop the viral infection due to an increase in NK cell cytotoxicity. Further-more, VEGF has been shown to induce tolerance through the polarization of M2 macro-phages [
42]. Thus, a decrease in VEGF secretion by PL-MSC can maintain polarization to M1 macrophages, which may contribute to the limitation of viral infection through the de-creased secretion of VEGF. In the case of EGF secretion by PL-MSC, we observed a de-crease in post-infection. Our results suggest that this factor could decrease EGFR activation, which, in turn, could induce a reduction in HMPV levels as occurs with hRSV [
43]. Although the mechanisms are still poorly understood, it has been demonstrated that inhibition of EGFR during viral infection increases IRF1 and IFN-k and decreases hRSV titers [
44]. An increased EGF secretion or an upregulation of its receptors (EGFR) increases the innate immune response, mucus secretion, the flux of neutrophils, and IL-8 secretion [
45]. Increased EGF secretion and, very significantly, the upregulation of EGFRs induces an antiviral response [
46].
Notably, PL-MSC infected with HMPV had a significant increase in the secretion of Ang-2 and PDGF-AA compared to mock-infected PL-MSC. Other viruses, such as hepatitis B, have been shown to promote the secretion of Ang-2 [
47]. In an in vitro wound model of endothelial cells, the factors VEGF and angiopoietin-1 induced rapid wound closure. In contrast, the addition of PDGF and Ang-2 to the culture induced a decrease in wound closure [
48,
49]. This is central to maintaining the integrity of blood vessels. Effective angiogenesis requires stabilizing the newly formed vessels, including the formation of tight junctions between endothelial cells, and the lumen in the new blood vessel. This stabilization is induced by angiopoietin 1 [
50]. In contrast, Ang-2, in the absence or decrease of VEGF, as is the case in our data, opposes angiopoietin-1 by inducing destabilization of newly formed vessels, with the death of endothelial cells and pericytes and an increase in the rupture of tight junctions between endothelial cells [
51,
52]. The destabilization of blood vessels can lead to an increase in edema of the surrounding tissue, which is particularly important in viral infections [
44]. Our results suggest that HMPV infected PL-MSC in lung tissue could increase damage, mainly due to vascular destabilization and edema, exacerbating the HMPV lung infection. This could be further exacerbated considering that, in our experiments, PL-MSC continued to produce viral particles ten days after the infection, probably acting as a transport or reservoir of the infection. Once the infectious process decreases, lung stem cells can promote lung tissue repair, as has been demonstrated with hRSV [
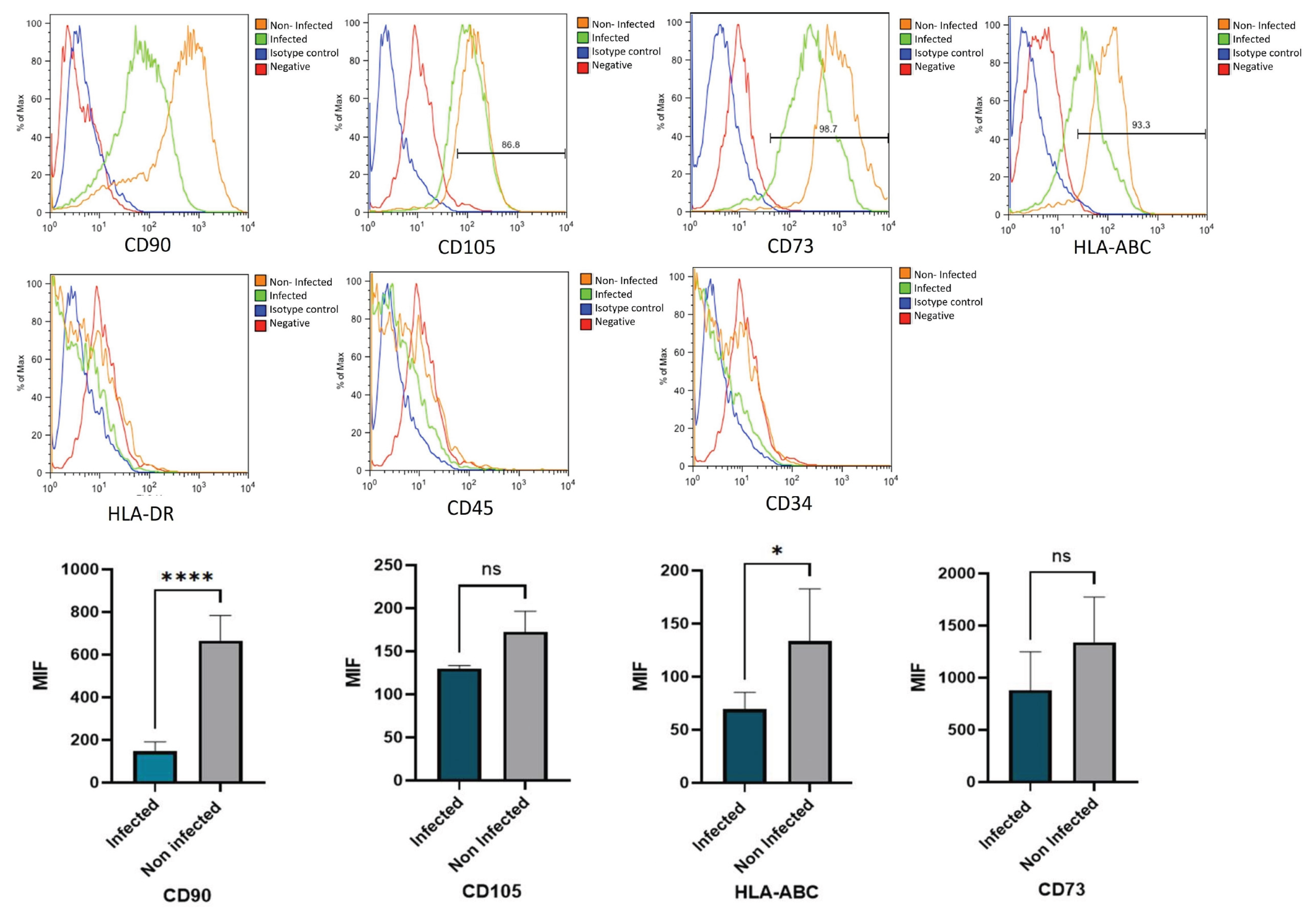
30]. This can also be proposed in the case of HMPV infection, since our study, we observed that the expression of stem cell markers varied after HMPV infection. Specifically, CD105 and CD73 antigens decreased in infected cells (
Figure 4), suggesting that the potentiality of PL-MSC may decrease after infection. Exposure to cytokine-related stimuli or stress factors, such as hypoxia, can induce variations in the expression of these markers during stem cell differentiation [
53].
Interestingly, our results showed that the differentiation capacity of PL-MSC following HMPV infection was not modified, in contrast to previous studies reporting that viral infections of MSC affect their ability to proliferate and differentiate into other cell types. For instance, the Chikungunya virus reduces the ability of MSC to differentiate into osteoblasts [
54], also the Zika virus reduces the ability of MSC to differentiate into osteoblasts [
55].
Figure 1.
Characterization of placental mesenchymal stem cells (PL-MSC). Briefly, the first criterion for the characterization is morphology, (A) Representative phase-contrast photomicrograph showing a spindle-shaped morphology in the culture that demonstrates adherence to plastic (magnification 20×). The second criterion is the detection of the cell-surface molecule characteristics of PL-MSC. (B) The cell-surface markers were analyzed using flow cytometry. Positive markers: CD90, CD105, CD73 and HLA-ABC. Negative markers: HLA-DR, CD45 and CD34. Isotype controls were run in parallel for each marker; and the third criterion is the differentiation process, (C) Adipogenic differentiation was indicated by accumulation of neutral lipid vacuoles that were stained with Oil Red O (ADIPO; magnification 20×); Osteogenic differentiation was indicated by Alizarin Red S which binds to the calcium deposits (OSTEO; magnification 20×) and Chondrogenic differentiation was indicated by chondrogenic matrix colored with Alcian blue (CHONDRO; magnification 20×).
Figure 1.
Characterization of placental mesenchymal stem cells (PL-MSC). Briefly, the first criterion for the characterization is morphology, (A) Representative phase-contrast photomicrograph showing a spindle-shaped morphology in the culture that demonstrates adherence to plastic (magnification 20×). The second criterion is the detection of the cell-surface molecule characteristics of PL-MSC. (B) The cell-surface markers were analyzed using flow cytometry. Positive markers: CD90, CD105, CD73 and HLA-ABC. Negative markers: HLA-DR, CD45 and CD34. Isotype controls were run in parallel for each marker; and the third criterion is the differentiation process, (C) Adipogenic differentiation was indicated by accumulation of neutral lipid vacuoles that were stained with Oil Red O (ADIPO; magnification 20×); Osteogenic differentiation was indicated by Alizarin Red S which binds to the calcium deposits (OSTEO; magnification 20×) and Chondrogenic differentiation was indicated by chondrogenic matrix colored with Alcian blue (CHONDRO; magnification 20×).
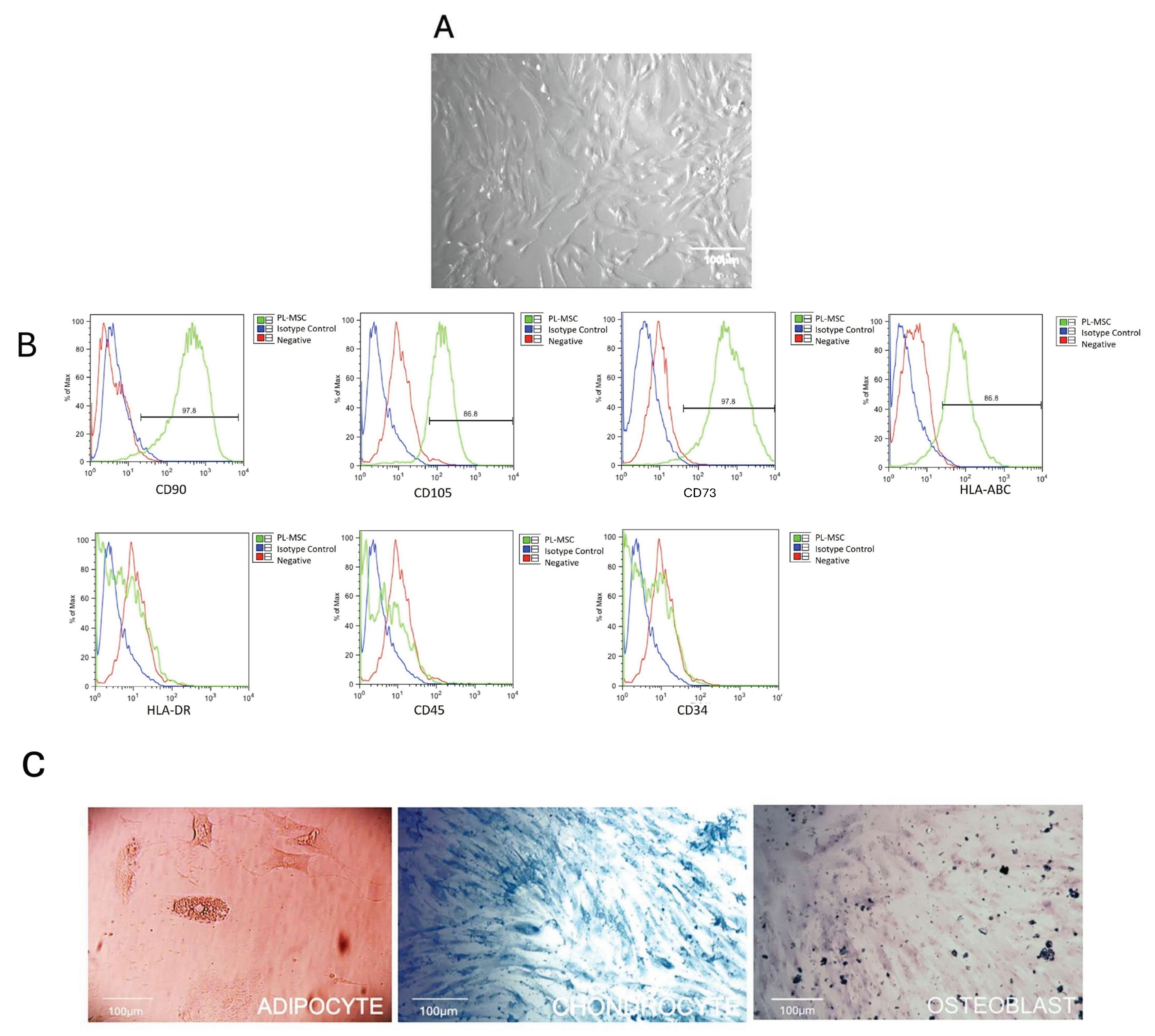
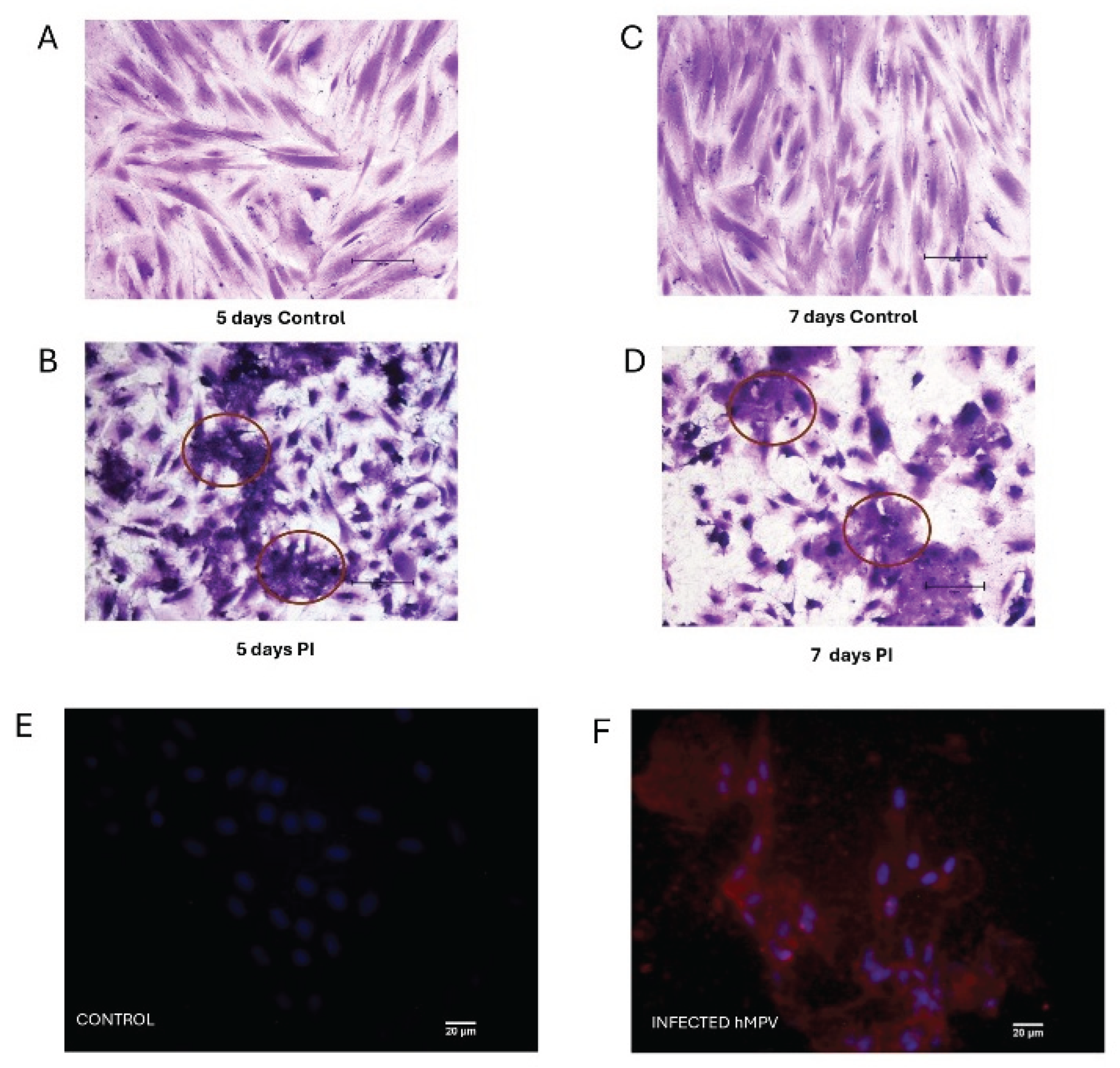
Figure 2.
Morphology of the infected PL-MSC. To visualize the changes in cellular morphology, the mesenchymal stem cell cultures were fixed with methanol (5 minutes), stained with crystal violet (3 minutes) and washed with a jet of water (magnification 20×). (A) control PL-MSC at 5 days of culture; (B) infected PL-MSC at 5 days post-infection; (C) control PL-MSC at 7 days of culture; (D) infected PL-MSC ay 7 days post-infection. The morphological changes or cytopathic effect (syncytia) were circularized to facilitate the identification of the syncytia in each point of the HMPV infection. (E) Immunofluorescence staining for control cells, the nucleus of each cell was stained in blue by DAPI. (F) The HMPV infected cells were labeled red by anti-N commercial monoclonal antibody and Alexa Fluor 594 as a secondary antibody and the nucleus stained in blue by DAPI.
Figure 2.
Morphology of the infected PL-MSC. To visualize the changes in cellular morphology, the mesenchymal stem cell cultures were fixed with methanol (5 minutes), stained with crystal violet (3 minutes) and washed with a jet of water (magnification 20×). (A) control PL-MSC at 5 days of culture; (B) infected PL-MSC at 5 days post-infection; (C) control PL-MSC at 7 days of culture; (D) infected PL-MSC ay 7 days post-infection. The morphological changes or cytopathic effect (syncytia) were circularized to facilitate the identification of the syncytia in each point of the HMPV infection. (E) Immunofluorescence staining for control cells, the nucleus of each cell was stained in blue by DAPI. (F) The HMPV infected cells were labeled red by anti-N commercial monoclonal antibody and Alexa Fluor 594 as a secondary antibody and the nucleus stained in blue by DAPI.
Figure 3.
Changes in the cytoskeletal organization and annexin V localization in PL-MSC. The changes in the pattern of distribution of the cytoskeleton proteins and annexin V as an accessory cytoskeleton protein were studied by immunofluorescence. PL-MSC cultures (magnification 20×) were stained in red with Alexa Fluor 594 phalloidin to detect the actin filaments (A) control; (B) HMPV infected cells. Tubulin filaments were in green because this protein was detected by a specific primary antibody and revealed by a secondary antibody coupled to FITC. (C) Control; (D) HMPV infected cells. The changes in annexin V were detected by a primary antibody coupled to FITC, thus the cells were stained in green. (E) Control; (F) HMPV infected cells. A mounting medium with DAPI was used for nuclear counterstaining, therefore the nucleus was stained in blue. In order to observe the changes in the actin filaments, an amplification of the section in the box was performed. (G and H). (I) Bar graphs show the mean fluorescence intensity for each marker, under control conditions and after infection with HMPV. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
Figure 3.
Changes in the cytoskeletal organization and annexin V localization in PL-MSC. The changes in the pattern of distribution of the cytoskeleton proteins and annexin V as an accessory cytoskeleton protein were studied by immunofluorescence. PL-MSC cultures (magnification 20×) were stained in red with Alexa Fluor 594 phalloidin to detect the actin filaments (A) control; (B) HMPV infected cells. Tubulin filaments were in green because this protein was detected by a specific primary antibody and revealed by a secondary antibody coupled to FITC. (C) Control; (D) HMPV infected cells. The changes in annexin V were detected by a primary antibody coupled to FITC, thus the cells were stained in green. (E) Control; (F) HMPV infected cells. A mounting medium with DAPI was used for nuclear counterstaining, therefore the nucleus was stained in blue. In order to observe the changes in the actin filaments, an amplification of the section in the box was performed. (G and H). (I) Bar graphs show the mean fluorescence intensity for each marker, under control conditions and after infection with HMPV. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
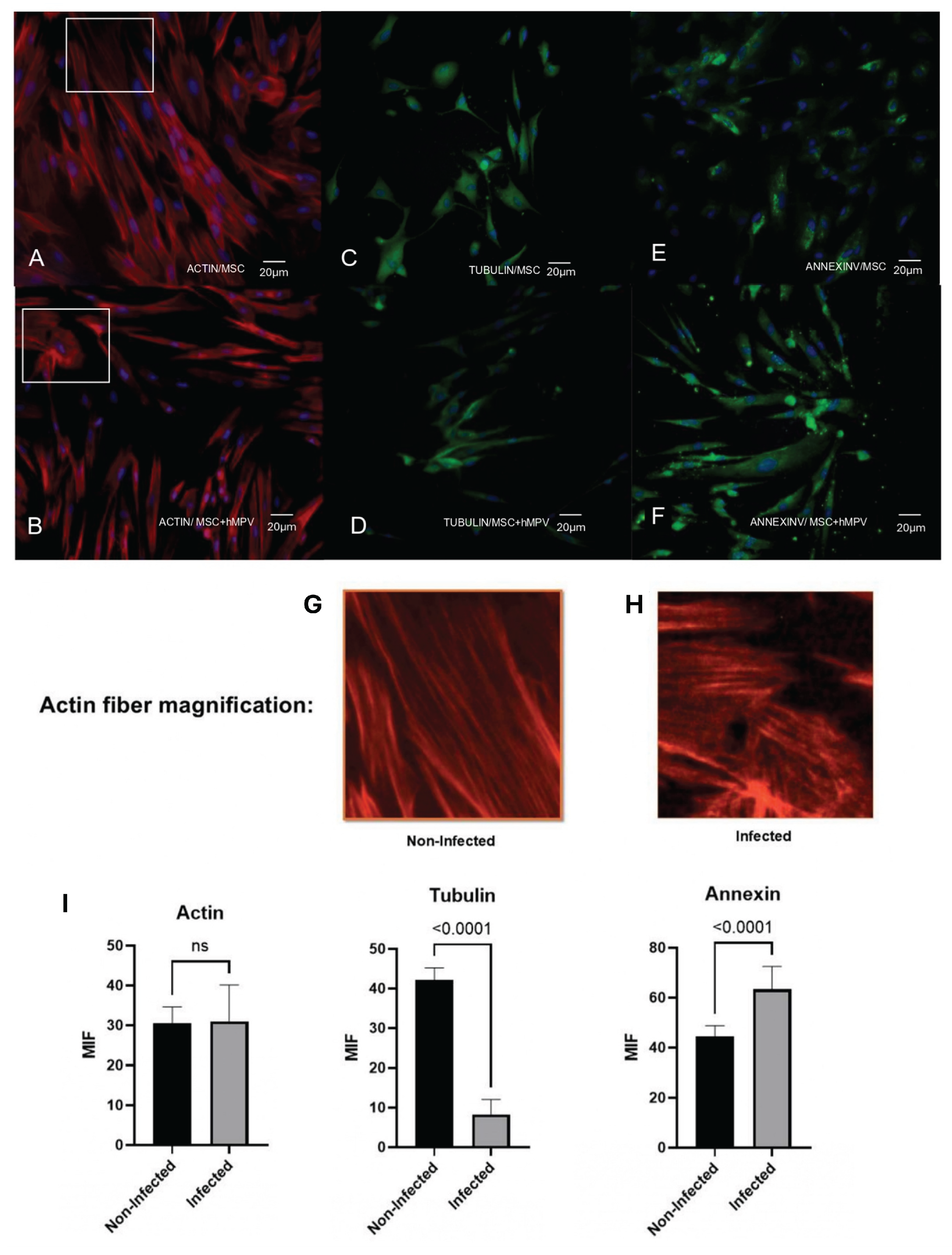
Figure 4.
Flow cytometry analysis of PL-MSC surface markers. Representative histograms show the per-centage of positive markers: CD90, CD105, CD73 and HLA ABC under control conditions and after infection with HMPV. Isotype controls are shown in blue. Negative markers: HLA-DR, CD45 and CD34 and isotype controls (blue) were run in parallel. The bar graphs represent the mean fluorescence intensity (MFI) for the positive markers, with and without HMPV infection. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
Figure 4.
Flow cytometry analysis of PL-MSC surface markers. Representative histograms show the per-centage of positive markers: CD90, CD105, CD73 and HLA ABC under control conditions and after infection with HMPV. Isotype controls are shown in blue. Negative markers: HLA-DR, CD45 and CD34 and isotype controls (blue) were run in parallel. The bar graphs represent the mean fluorescence intensity (MFI) for the positive markers, with and without HMPV infection. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
Figure 5.
Capacity of differentiation of PL-MSC. The PL-MSC were differentiated in the three main lineages (Adipocytes, Chondrocytes and Osteocytes), for this process the cells were cultured with differential mediums for 10 days. Briefly, the PL-MSC were cultured with adipogenic medium (insulin and indomethacin); chondrogenic medium (dexamethasone, ascorbic acid, and β-glycerol) and osteogenic medium (dexamethasone, ascorbate, insulin–transferrin–selenium supplement, sodium pyruvate). PL-MSC cultures (magnification 20×) were stained in red with Alexa Fluor 594 phalloidin to detect the actin filaments. Tubulin filaments were in green because this protein was detected by a specific primary antibody and revealed by a secondary antibody coupled to FITC. Annexin V was detected by a primary antibody coupled to FITC, thus the cells were stained in green. Nuclear counter-staining was performed with DAPI (blue). (A) Adipocytes; (B) Chondrocytes; (C) Osteocytes. Bar graphs show the mean fluorescence intensity for each marker, under control conditions and after infection with HMPV. Statistical analysis was performed using an unpaired Mann-Whitney test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
Figure 5.
Capacity of differentiation of PL-MSC. The PL-MSC were differentiated in the three main lineages (Adipocytes, Chondrocytes and Osteocytes), for this process the cells were cultured with differential mediums for 10 days. Briefly, the PL-MSC were cultured with adipogenic medium (insulin and indomethacin); chondrogenic medium (dexamethasone, ascorbic acid, and β-glycerol) and osteogenic medium (dexamethasone, ascorbate, insulin–transferrin–selenium supplement, sodium pyruvate). PL-MSC cultures (magnification 20×) were stained in red with Alexa Fluor 594 phalloidin to detect the actin filaments. Tubulin filaments were in green because this protein was detected by a specific primary antibody and revealed by a secondary antibody coupled to FITC. Annexin V was detected by a primary antibody coupled to FITC, thus the cells were stained in green. Nuclear counter-staining was performed with DAPI (blue). (A) Adipocytes; (B) Chondrocytes; (C) Osteocytes. Bar graphs show the mean fluorescence intensity for each marker, under control conditions and after infection with HMPV. Statistical analysis was performed using an unpaired Mann-Whitney test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
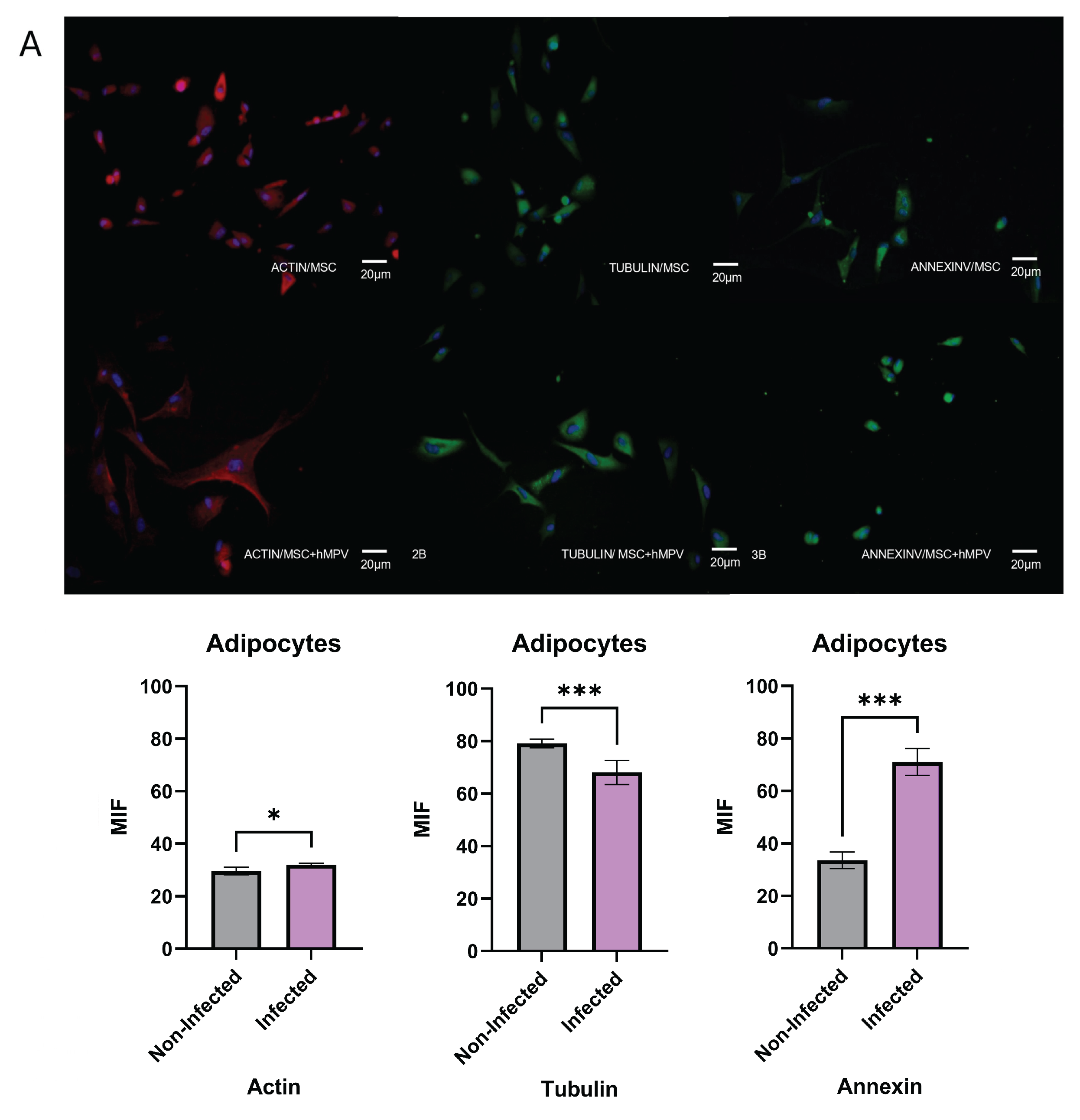
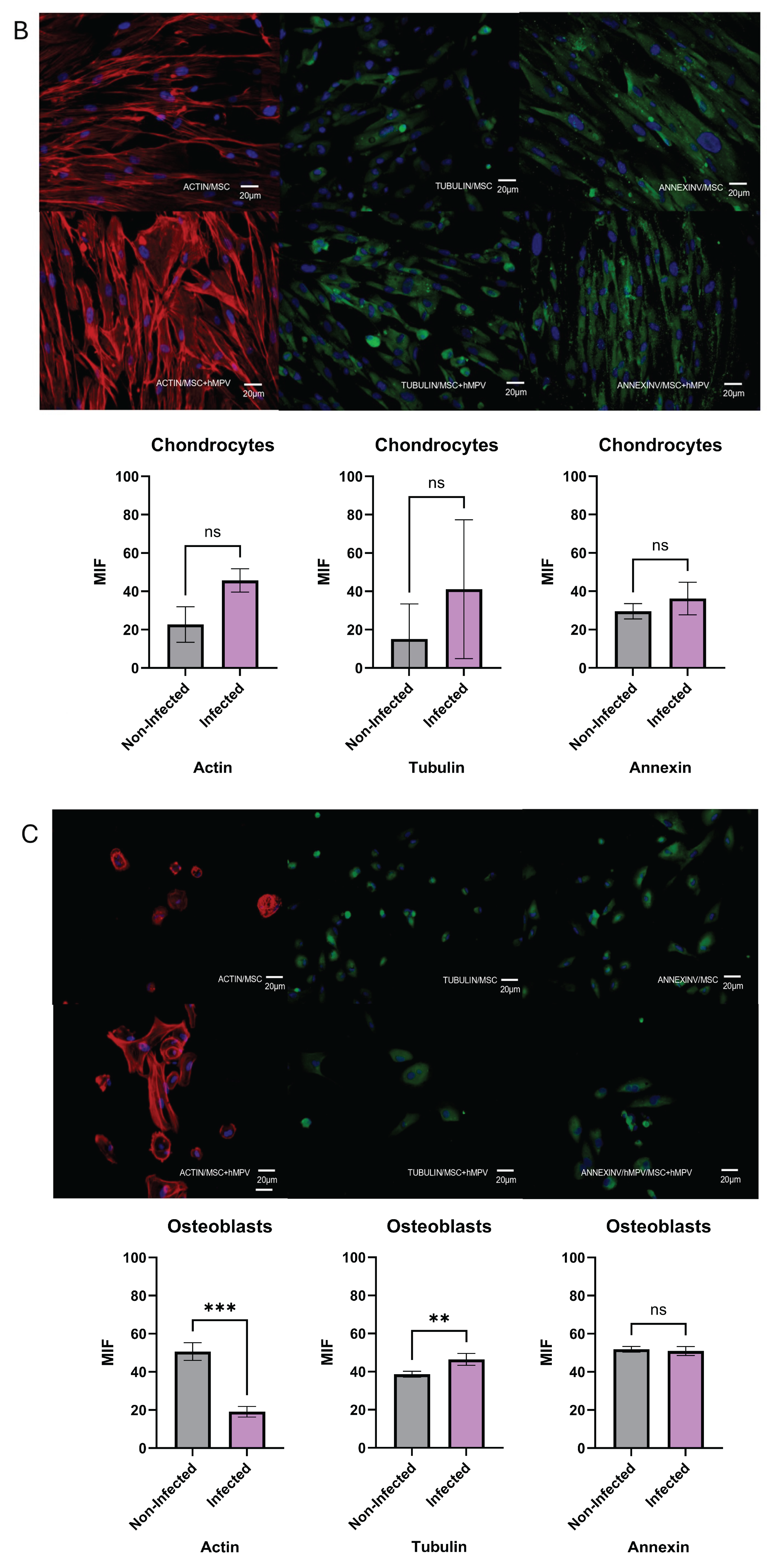
Figure 6.
Secretory profile of PL-MSC. PL-MSC growth factors were analyzed by LEGENDplex. Briefly, the supernatants were collected from the control cells and the HMPV infected cells after 48 hpi. The supernatants under control conditions and after infection with HMPV were quantified with Bio-Legend’s LEGENDplex human Growth Factor Panel (13-plex). Angiopoietin-2 (Ang-2), EGF, EPO, FGF-basic, G-CSF, GM-CSF, HGF, M-CSF, PDGF-AA, PDGF-BB, SCF, TGF-α, and VEGF (Bio-Legend). The data were analyzed using Bio-Legend’s software Qognit V. The data were expressed in pg/mL. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).
Figure 6.
Secretory profile of PL-MSC. PL-MSC growth factors were analyzed by LEGENDplex. Briefly, the supernatants were collected from the control cells and the HMPV infected cells after 48 hpi. The supernatants under control conditions and after infection with HMPV were quantified with Bio-Legend’s LEGENDplex human Growth Factor Panel (13-plex). Angiopoietin-2 (Ang-2), EGF, EPO, FGF-basic, G-CSF, GM-CSF, HGF, M-CSF, PDGF-AA, PDGF-BB, SCF, TGF-α, and VEGF (Bio-Legend). The data were analyzed using Bio-Legend’s software Qognit V. The data were expressed in pg/mL. Statistical analysis was performed using an unpaired t-test. All results were considered significant when p ≤ 0.05, ns (not statistically significant).