Submitted:
02 September 2025
Posted:
02 September 2025
You are already at the latest version
Abstract
Keywords:
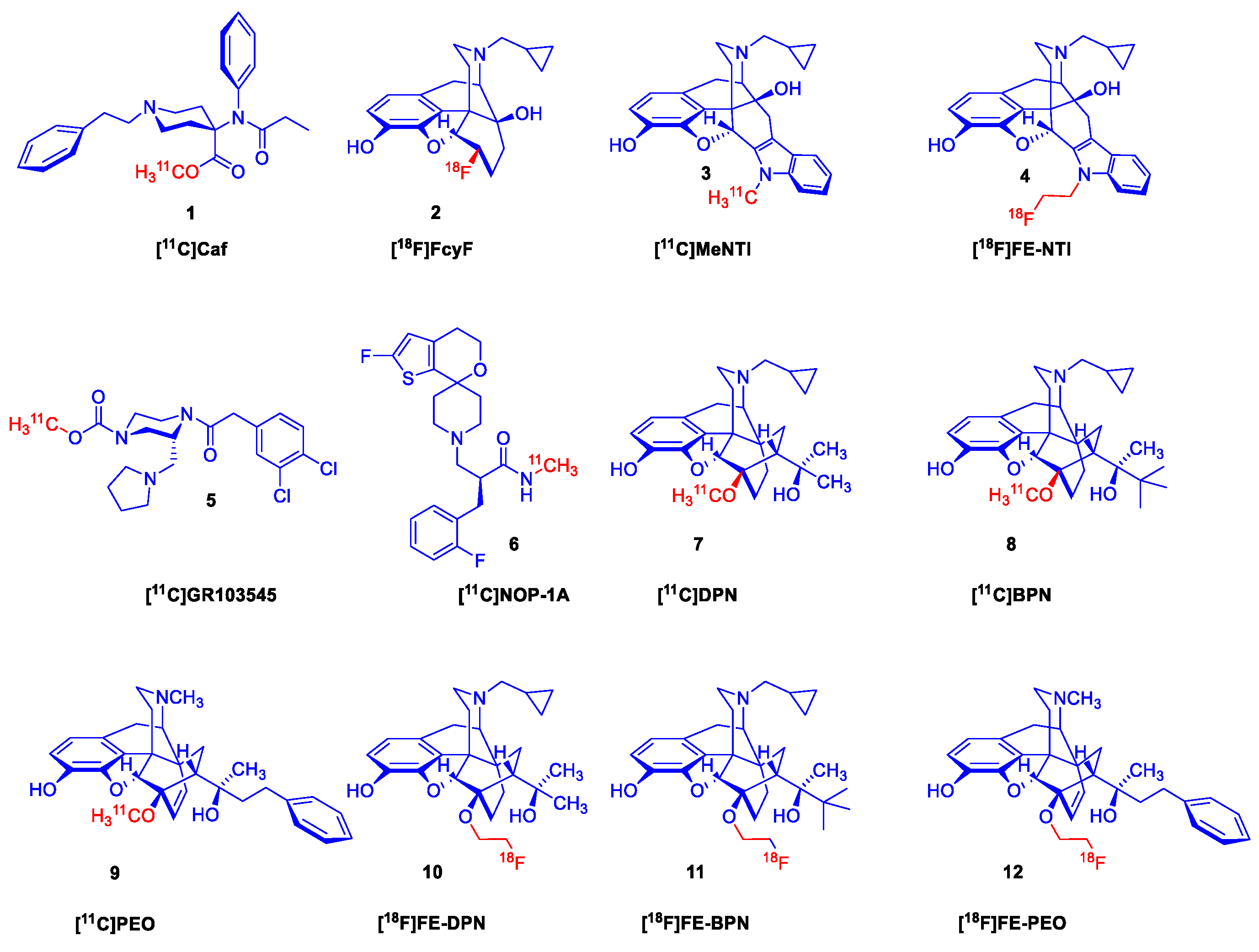
1. Introduction
2. Results and Discussion
2.1. Chemistry
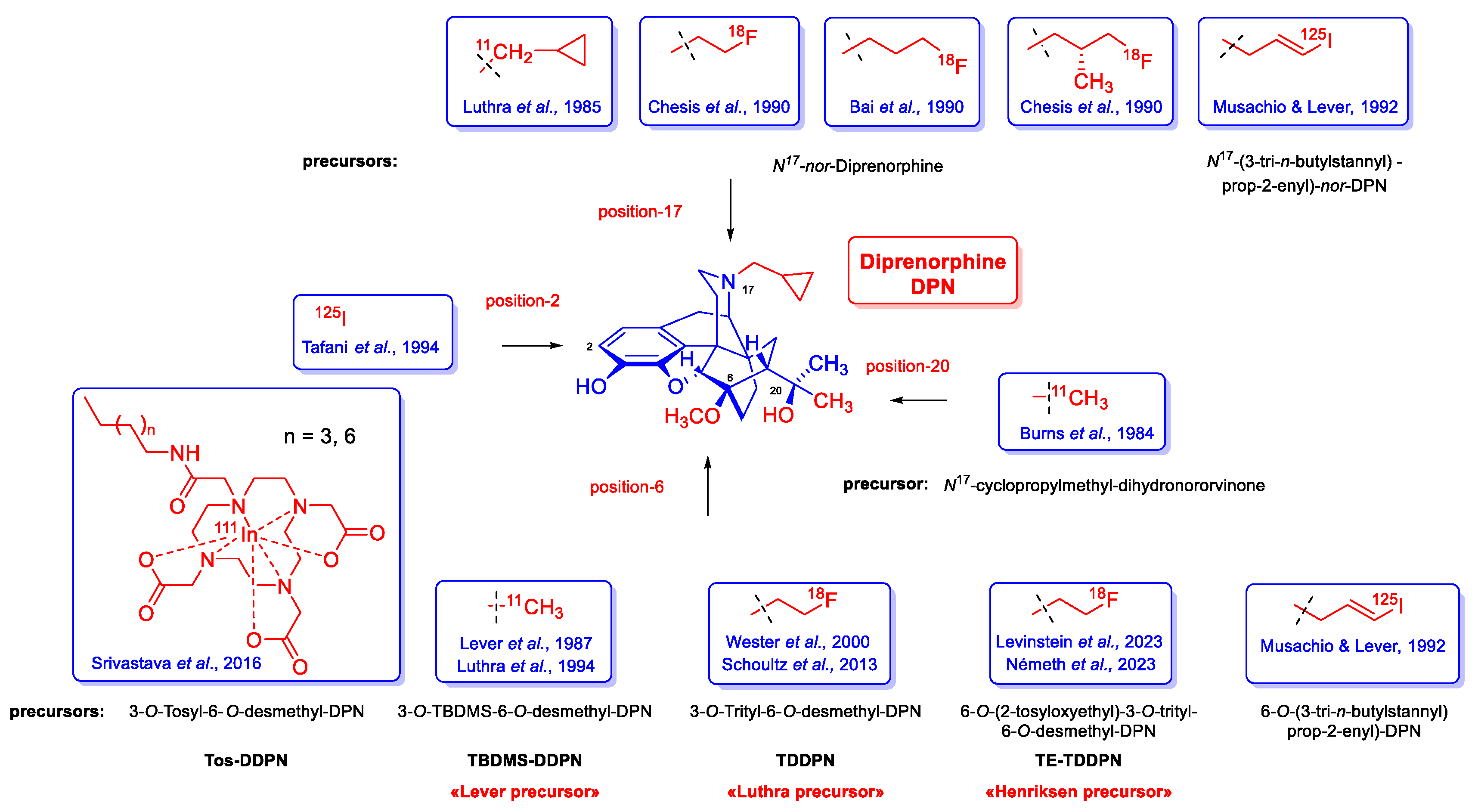
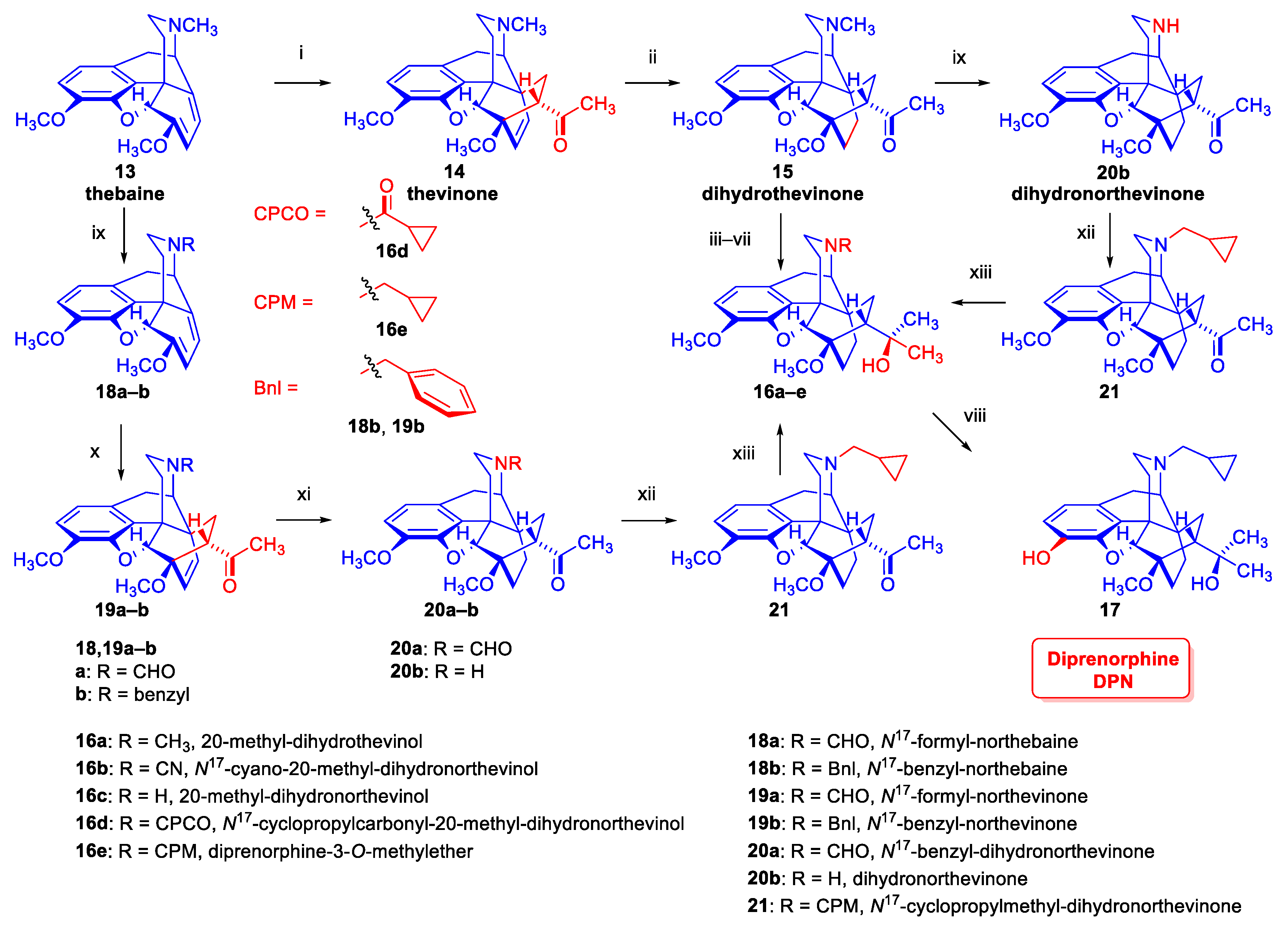
2.1.1. Diprenophine
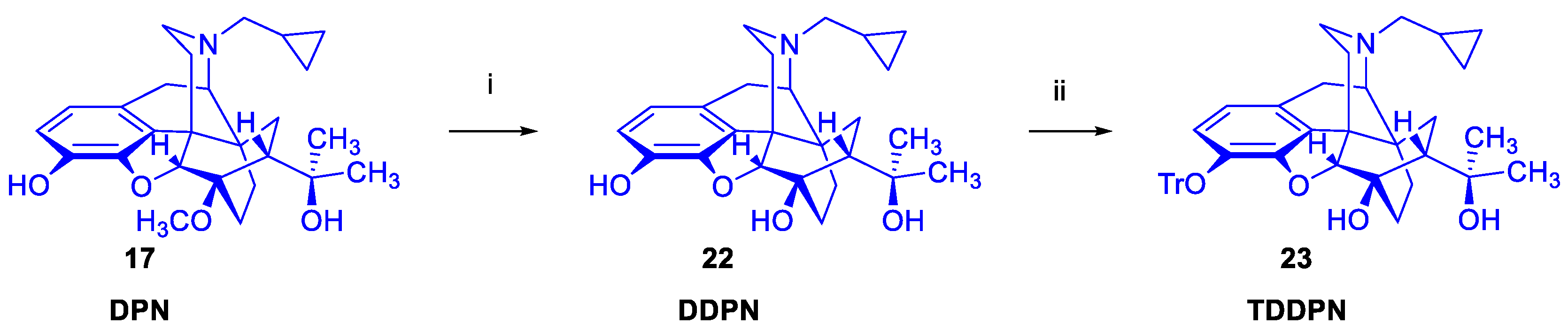
2.1.2. Synthesis of Diprenorphine
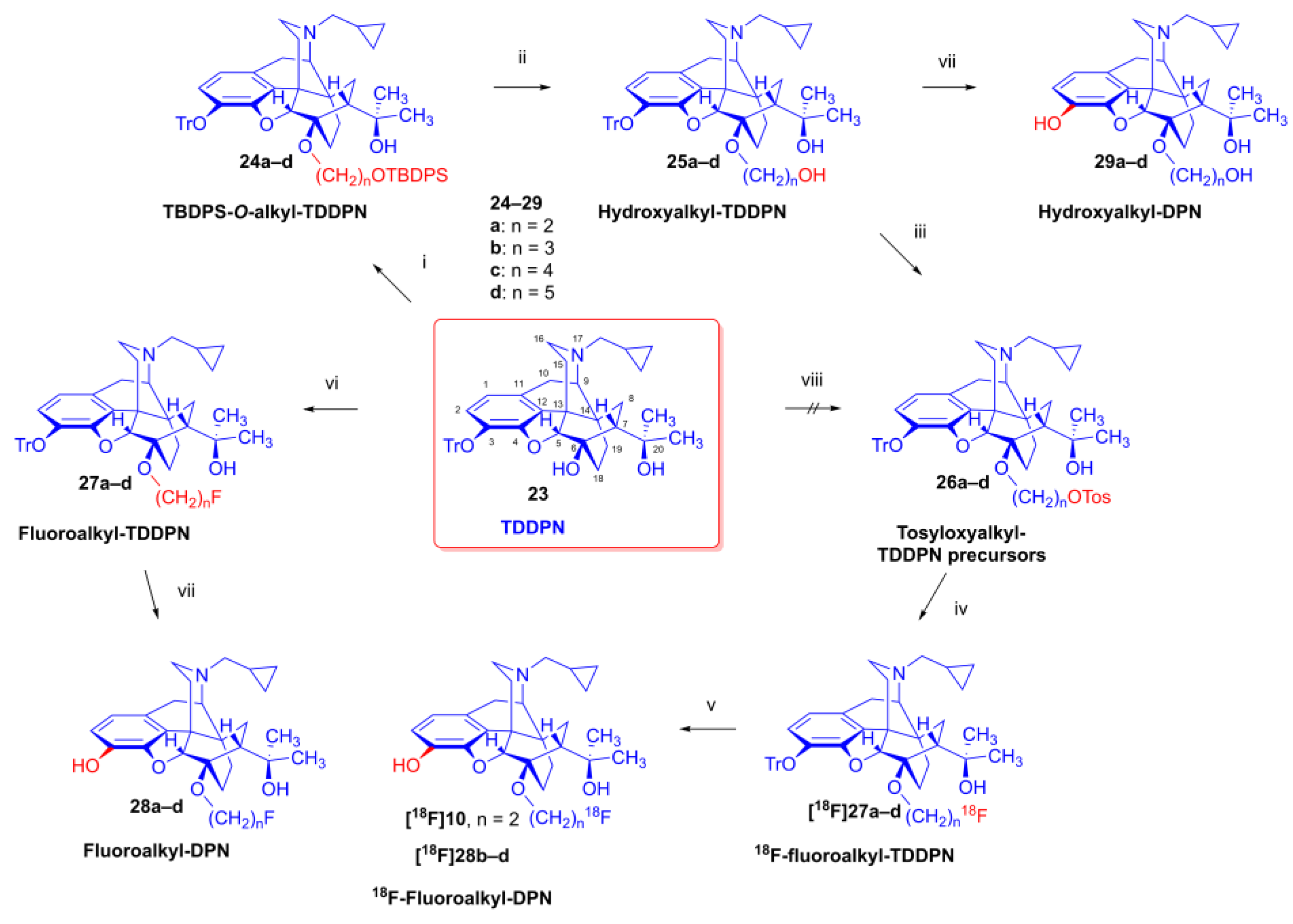
2.1.4. Synthesis of 6-O-Substituted-6-O-desmethyl-diprenorphine derivatives
2.2. In Silico Studies
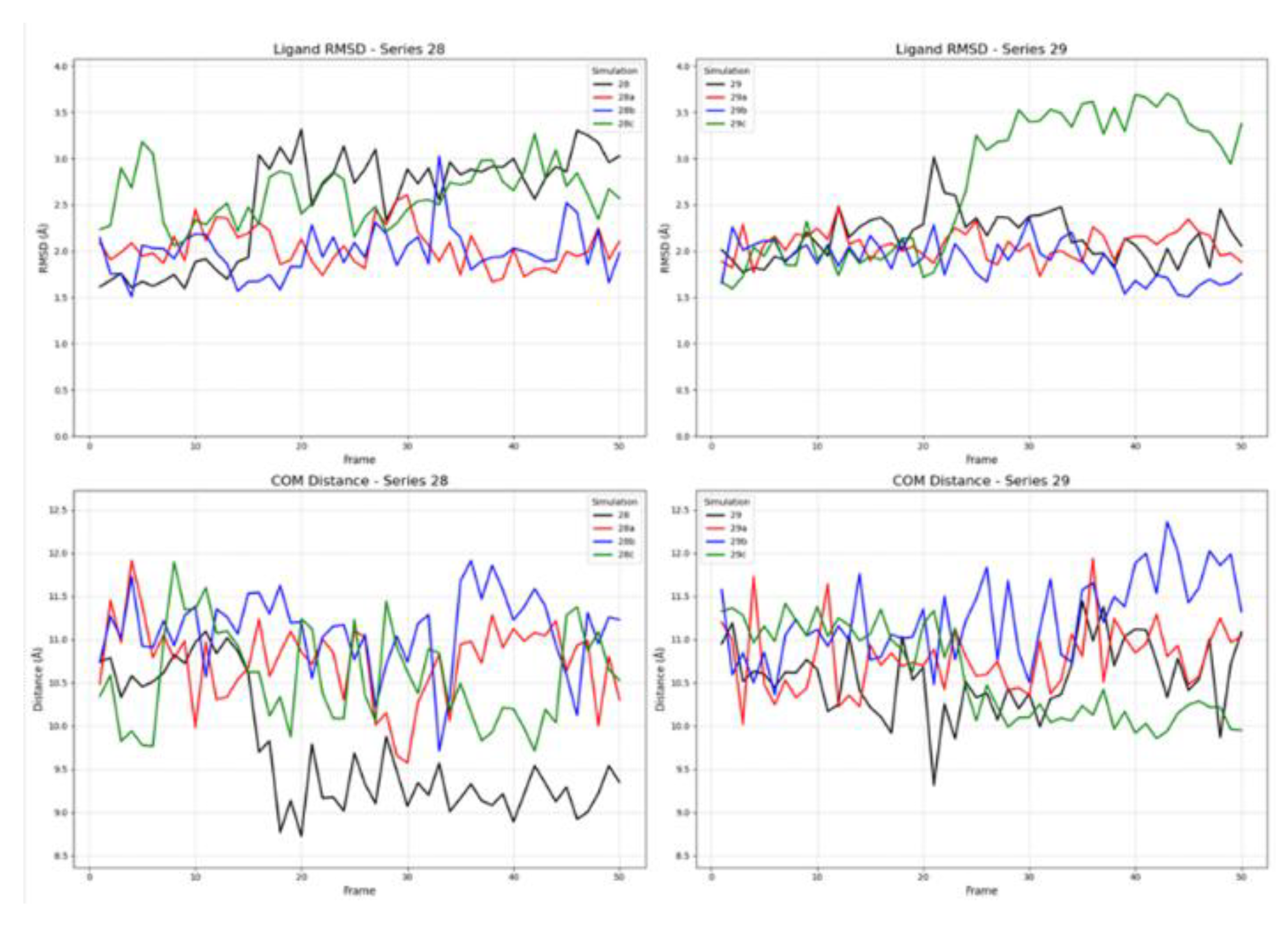
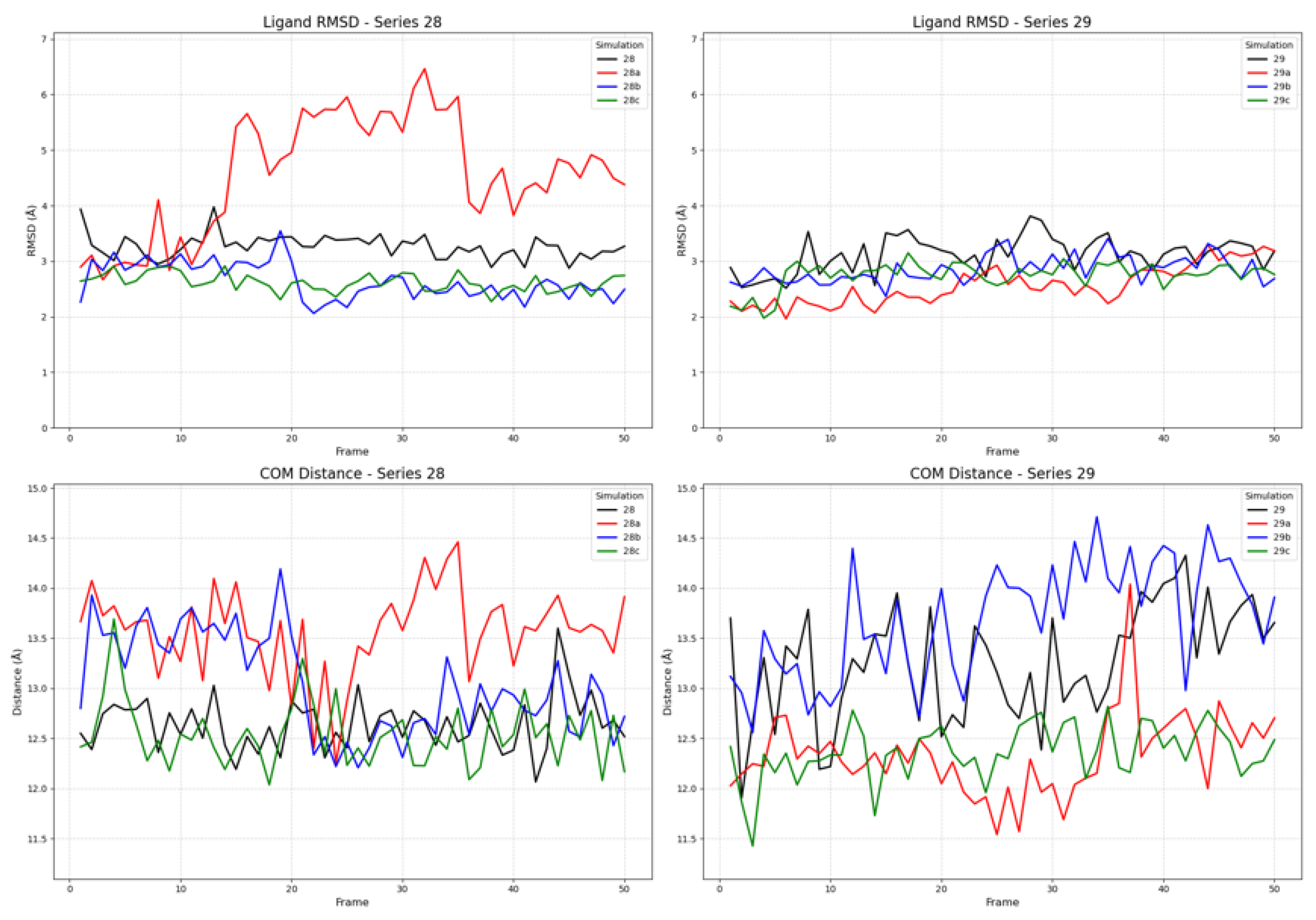
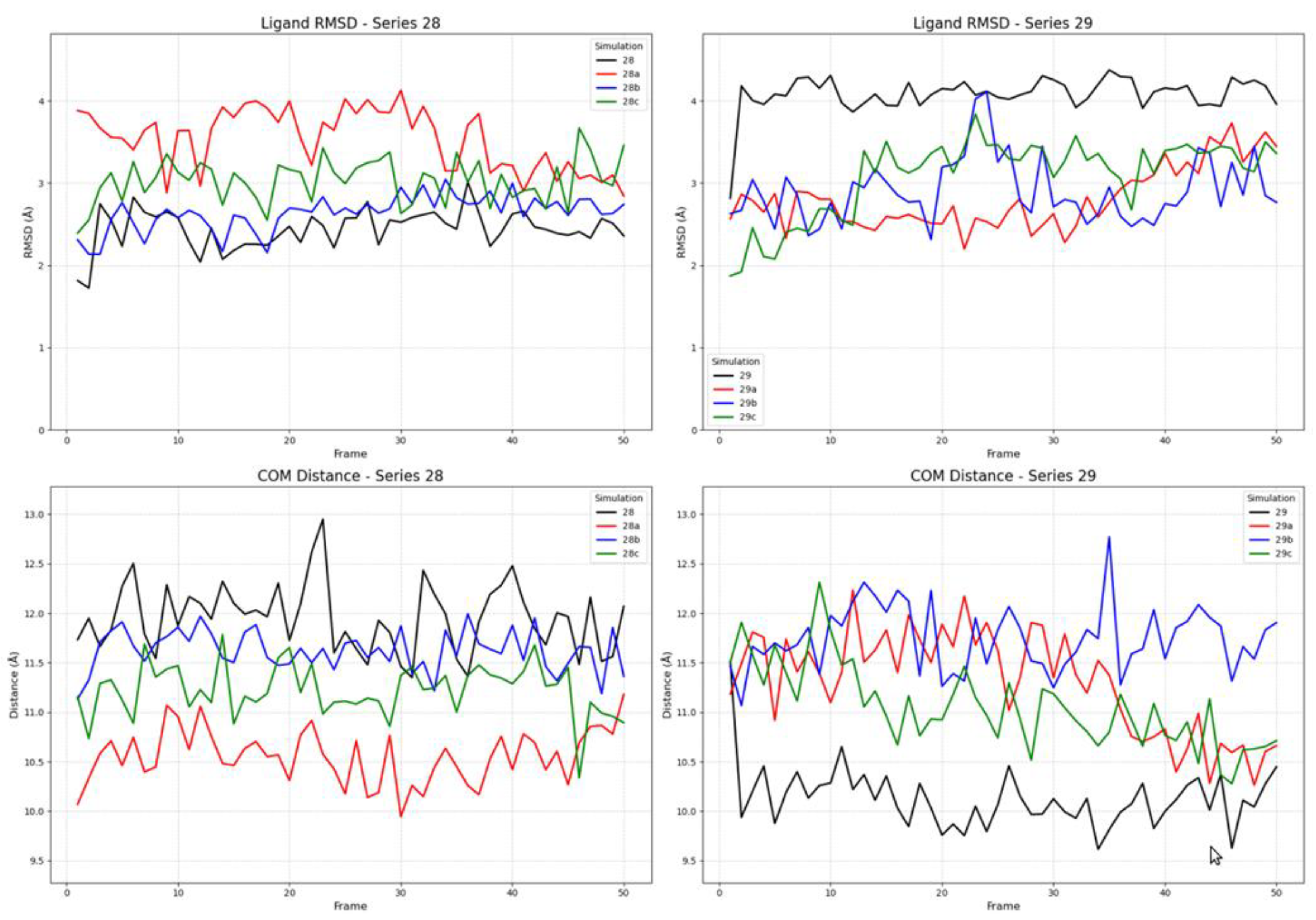
2.2.1. Ligand Binding to the Active Receptor State
2.2.2. Ligand Binding to the Inactive Receptor State
2.2.3. Ligand Binding to the Partial Agonist Receptor State
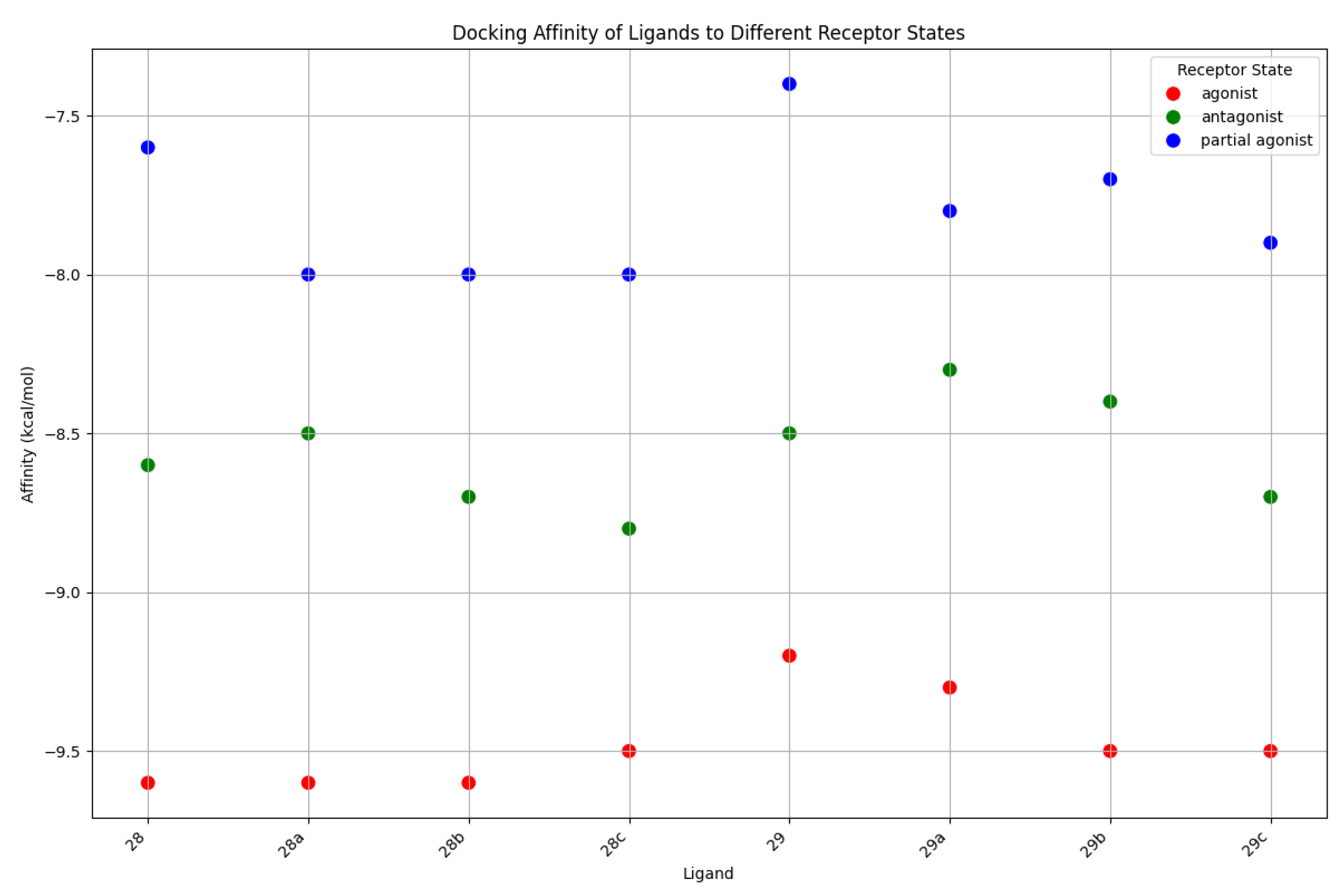
2.2.4. Molecular Docking Reveals a Clear Energetic Preference for the Active State
2.2.5. Conclusion and Implications
3. Materials and Methods
3.1. General Methods
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of 6-O-(tert-Butyldiphenylsilyloxyalkyl)-6-O-desmethyl-3-O-trityl-diprenorphine (24b–d) Derivatives
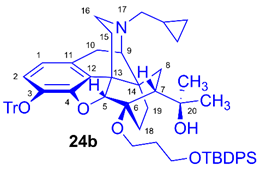
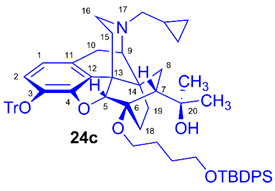
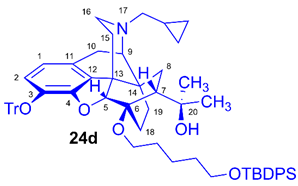
3.2.2. General Procedure for the Synthesis of 6-O-Hydroxyalkyl-6-O-desmethyl-3-O-trityl-diprenorphine (25b–d) Derivatives
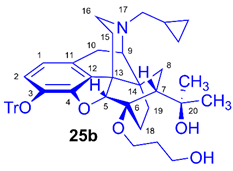
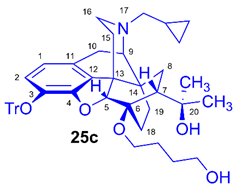
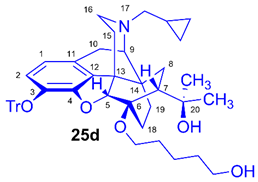
3.2.3. General Procedure for the Synthesis of 6-O-(Tosyloxyalkyl)-6-O-desmethyl-diprenorphine (26b–d) Derivatives
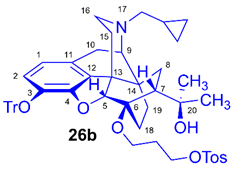
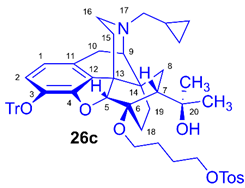
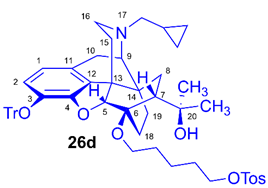
3.2.4. General Procedure for the Preparation of 6-O-Fluoroalkyl-6-O-desmethyl-3-O-trityl-diprenorphine (27b–d) Derivatives
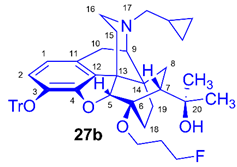
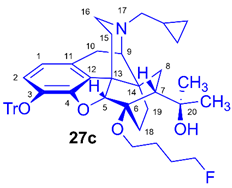
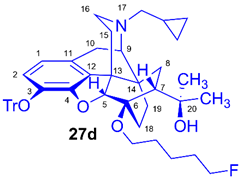
3.2.5. General Procedure for the Preparation of 6-O-Fluoroalkyl-6-O-desmethyl-diprenorphine (28b–d) Derivatives
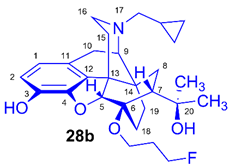
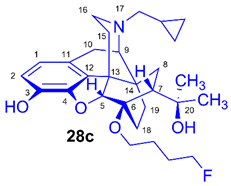
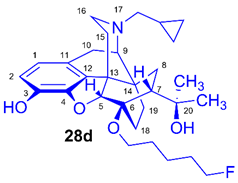
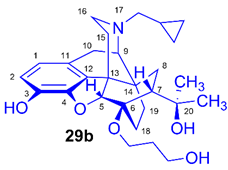
3.2.6. General Procedure for the Preparation of 6-O-(Hydroxyalkyl)-6-O-desmethyl-diprenorphine (29b–d) Derivatives
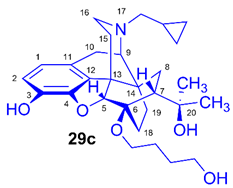
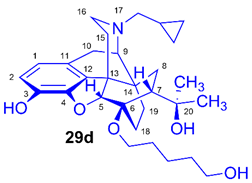
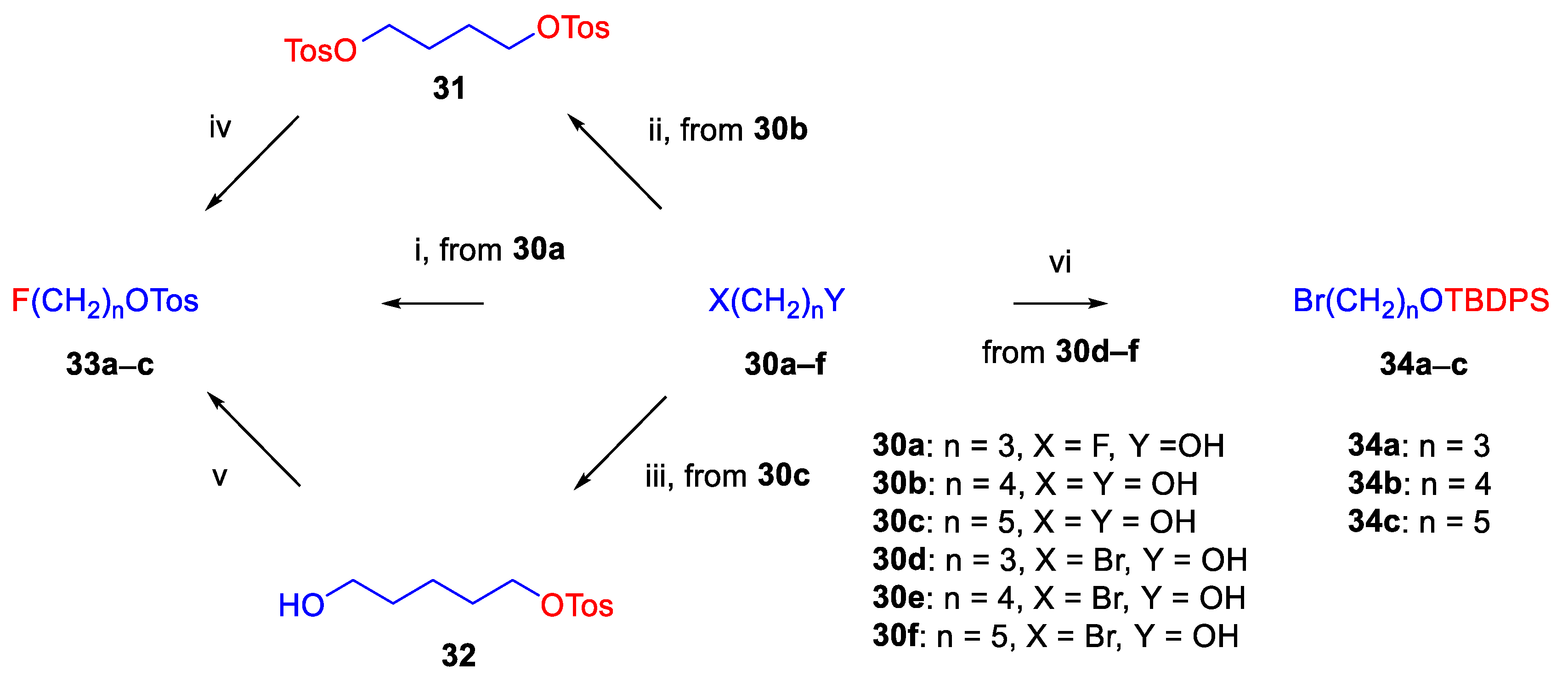
3.2.7. Sythesis of Fluoroalkyl-Tosylates (33a–c, Figure 6)
3.2.8. General Procedure for Preparation of tert-Butyldiphenylsilyl (TBDPS) Protected bromoalkanols (34a–c, Figure 6)
3.3. Computational Part
3.3.1. Ligand and Receptor Preparation for Docking
3.3.2. Molecular Docking Protocol
3.3.3. System Preparation for Molecular Dynamics
3.3.4. Molecular Dynamics Simulation Protocol
Summary
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Presley, C. C.; Lindsley, C. W. , Dark classics in chemical neuroscience: Opium, a historical perspective. ACS Chem. Neurosci. 2018, 9, 2503–2518. [Google Scholar] [CrossRef]
- Bernáth, J. , Poppy, The Genus Papaver. Harwood Academic Publishers: Amsterdam, The Netherlands, 1998.
- Brownstein, M. J. , A brief history of opiates, opioid peptides, and opioid receptors. Proc. Natl. Acad. Sci. 1993, 90, 5391–5393. [Google Scholar] [CrossRef]
- Schiff, P. L. , Opium and its alkaloids. Am. J. Pharm. Educat. 2002, 66, 188–196. [Google Scholar]
- Carod-Artal, F. J. , Psychoactive plants in ancient Greece. Neurosci. Hist. 2013, 1, 28–38. [Google Scholar]
- Molina-Venegas, R.; Verano, R. , The quest for Homer’s moly: exploring the potential of an early ethnobotanical complex. J. Ethnobiol. Ethnomed. 2024, 20, 1–11. [Google Scholar]
- Chovanec, Z.; Rafferty, S.; Swiny, S. , Opium for the Masses: An experimental archaeological approach in determining the antiquity of the opium poppy. Ethnoarchaeology 2012, 4, 5–36. [Google Scholar] [CrossRef]
- Sinclair, A., High times in ancient Egypt. In Alternative Egyptology: Critical essays on the relation between academic and alternative interpretations of ancient Egypt, van den Bercken, B. J. L., Ed. Sidestone Press: Leiden, 2024; pp 76–94.
- Devereaux, A. L.; Mercer, S. L.; Cunningham, C. W. , Dark classics in chemical neuroscience: morphine. ACS Chem. Neurosci. 2018, 9, 2395–2407. [Google Scholar] [CrossRef]
- Schmitz, R. , Friedrich Wilhelm Sertürner and the discovery of morphine. Pharmacy in History 1985, 27, 61–74. [Google Scholar]
- Knutsen, H. K.; Alexander, J; Barregård, L.; Bignami, M.; Brüschweiler, B.; Ceccatelli, S.; Cottrill, B.; Dinovi, M.; Edler, L.; Grasl-Kraupp, B.; Hogstrand, C.; Hoogenboom, L.R.; Nebbia, C. S.; Oswald, I. P.; Petersen, A.; Rose, M.; Roudot, A.-C.; Schwerdtle, T.; Vollmer, G.; Wallace, H.; Benford, D.; Calò, G.; Dahan, A.; Dusemund, B.; Mulder, P.; Németh-Zámboriné, É.; Arcella, D.; Baert, K.; Cascio, C.; Levorato, S.; Schutte, M.; Vleminckx, C. Update of the Scientific Opinion on opium alkaloids in poppy seeds. EFSA Journal 2018, 16, 5, 5243, 119 pp. [CrossRef]
- Borsodi, A.; Bruchas, M.; Caló, G.; Chavkin, C.; Christie, M. J.; Civelli, O.; Connor, M.; Cox, B. M.; Devi, L. A.; Evans, C.; Höllt, V.; Henderson, G.; Husbands, S.; Kelly, E.; Kieffer, B.; Kitchen, I.; Kreek, M. J.; Liu-Chen, L.-Y.; Massot, D.; Meunier, J.-C.; Portoghese, P. S.; Schulz, S.; Shippenberg, T. S.; Simon, E. J.; Toll, L.; Traynor, J. R.; Ueda, H.; Wong, Y. H.; Zaveri, N.; Zimmer, A., Opioid receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. In IUPHAR/BPS GtoPdb CITE 2019, 4. [CrossRef]
- Benyhe, S.; Zádor, F.; Ötvös, F. , Biochemistry of opioid (morphine) receptors: binding, structure and molecular modelling. Acta Biol. Szeged 2015, 59 Suppl 1, 17–37. [Google Scholar]
- Lamberts, J. T.; Traynor, J. R. , Opioid receptor interacting proteins and the control of opioid signaling. Curr. Pharm. Design 2013, 19, 7333–7347. [Google Scholar] [CrossRef] [PubMed]
- Lever, J. R. , PET and SPECT imaging of the opioid system: Receptors, radioligands and avenues for drug discovery and development. Curr. Pharm. Design 2007, 13, 33–49. [Google Scholar] [CrossRef]
- Henriksen, G.; Willoch, F. , Imaging of opioid receptors in the central nervous system. Brain 2008, 131, 1171–1196. [Google Scholar] [CrossRef]
- Dannals, R. F. , Positron emission tomography radioligands for the opioid system J. Label. Compd. Radiopharm. 2013, 56, 187–195. [Google Scholar] [CrossRef]
- Cumming, P.; Marton, J.; Lilius, T. O.; Olberg, D. E.; Rominger, A. , A Survey of Molecular Imaging of Opioid Receptors. Molecules 2019, 24, 4190. [Google Scholar] [CrossRef]
- Marton, J.; Fekete, A.; Cumming, P.; Hosztafi, S.; Mikecz, P.; Henriksen, G. , Diels–Alder adducts of morphinan-6, 8-dienes and their transformations. Molecules 2022, 27, 2863. [Google Scholar] [CrossRef]
- Dannals, R. F.; Ravert, H. T.; Frost, J. J.; Wilson, A. A.; Burns, H. D.; Wagner, H. N. , Radiosynthesis of an opiate receptor binding radiotracer: [11C]carfentanil. Int. J. Appl. Radiat. Isot. 1985, 36, 303–306. [Google Scholar] [CrossRef]
- Pert, C. B.; Danks, J. A.; Channing, M. A.; Eckelman, W. C.; Larson, S. M.; Bennett, J. M.; Burke, T. R.; Rice, K. C. , S-[18F]Acetylcyclofoxy: a useful probe for the visualization of opiate receptors in living animals. FEBS Letters 1984, 177, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Lever, J. R.; Kinter, C. M.; Ravert, H. T.; Musachio, J. L.; Mathews, W. B.; Dannals, R. F. , Synthesis of N1′-([11C]methyl)naltrindole ([11C]MeNTI): A radioligand for positron emission tomographic studies of delta opioid receptors. J. Label. Compd. Radiopharm. 1995, 36, 137–145. [Google Scholar] [CrossRef]
- Mathews, W. B.; Kinter, C. M.; Palma, J.; Daniels, R. V.; Ravert, H. T.; Dannals, R. F.; Lever, J. R. , Synthesis of N1′-([18F]fluoroethyl)naltrindole ([18F]FEtNTI): a radioligand for positron emission tomographic studies of delta opioid receptors. J. Label. Compd. Radiopharm. 1999, 42, 43–54. [Google Scholar] [CrossRef]
- Ravert, H. T.; Mathews, W. B.; Musachio, J. L.; Scheffel, U.; Finley, P.; Dannals, R. F. , [11C]-methyl 4-[(3, 4-dichlorophenyl) acetyl]-3-[(1-pyrrolidinyl) methyl]-1- piperazinecarboxylate ([11C] GR89696): synthesis and in vivo binding to kappa opiate receptors. Nucl. Med. Biol. 1999, 26, 737–741. [Google Scholar] [CrossRef]
- Pike, V. W.; Rash, K. S.; Chen, Z.; Pedregal, C.; Statnick, M. A.; Kimura, Y.; Hong, J.; Zoghbi, S. S.; Fujita, M.; Toledo, M. A.; Diaz, N.; Gackenheimer, S. L.; Tauscher, J. T.; Barth, V. N.; Innis, R. B. Synthesis and evaluation of radioligands for imaging brain nociceptin/orphanin FQ peptide (NOP) receptors with positron emission tomography. J. Med. Chem. 2011, 54, 2687–2700. [Google Scholar] [CrossRef]
- Burns, H. D.; Lever, J. R.; Dannals, R. F.; Frost, J. J.; Wilson, A. A.; Ravert, H. T.; Subramanian, B.; Zeyman, S. E.; Langstrom, B.; Wagner Jr., H. N., Synthesis of ligands for imaging opiate receptors by positron emission tomography: carbon-11 labelled diprenorphine. J. Labell. Compds. Radiopharm. 1984, 22, 1167–1169. [Google Scholar]
- Tafani, J.-A. M.; Francés, B.; Coulais, Y.; Méjan-Galzi, A.; Goeldner, M.; Hirth, C.; Guiraud, R.; Zajac, J.-M. , In vivo binding of [125I]NH2-carfentanil to mu opioid receptors in mouse brain. Nucl. Med. Biol. 1994, 21, 231–238. [Google Scholar] [CrossRef]
- Luthra, S. K.; Pike, V. W.; Brady, F. , The preparation of carbon-11 labelled diprenorphine: a new radioligand for the study of the opiate receptor system in vivo. J. Chem. Soc., Chem. Commun. 1985; 1423–1425. [Google Scholar]
- Chesis, P. L.; Hwang, D.-R.; Welch, M. J. , N-(3-[18F]Fluoropropyl)-N-nordiprenorphine: Synthesis and characterization of a new agent for imaging opioid receptors with positron emission tomography. J. Med. Chem. 1990, 33, 1482–1490. [Google Scholar] [CrossRef]
- Bai, L.-Q.; Teng, R.-R.; Shiue, C.-Y.; Wolf, A. P.; Dewey, S. L.; Holland, M. J.; Simon, E. J. , No-carrier-added (NCA) N-(3-[18F]fluoropropyl-N-norbuprenorphine and N-(3-[18F]fluoropropyl-N-nordiprenorphine - Synthesis distribution in mice and rats, and tomographic studies in baboon. Int. J. Radiat. Appl. Instr. Part B, Nucl. Med.Chem. 1990, 17, 217–227. [Google Scholar] [CrossRef]
- Lever, J. R.; Dannals, R. F.; Wilson, A. A.; Ravert, H. T.; Wagner Jr., H. N. , Synthesis of carbon-11 labeled diprenorphine: A radioligand for positron emission tomographic studies of opiate receptors Tetrahedron Lett. 1987, 28, 4015–4018. [Google Scholar]
- Musachio, J. L.; Lever, J. R. , Vinylstannylated alkylating agents as prosthetic groups for radioiodination of small molecules: Design, synthesis and application to iodoallyl analogues of spiperone and diprenorphine. Bioconjugate Chem. 1992, 3, 167–175. [Google Scholar] [CrossRef]
- Luthra, S. K.; Brady, F.; Turton, D. R.; Brown, D. J.; Dowsett, K.; Waters, S. L.; Jones, A. K. P.; Matthews, R. W.; Crowder, J. C. , Automated radiosyntheses of [6-O-methyl-11C]diprenorphine and [6-O-methyl-11C]buprenorphine from 3-O-trityl protected precursors. Appl. Radiat. Isot. 1994, 45, 857–873. [Google Scholar] [CrossRef]
- Marton, J.; Schoultz, B. W.; Hjørnevik, T.; Drzezga, A.; Yousefi, B. H.; Wester, H.-J.; Willoch, F.; Henriksen, G. , Synthesis and evaluation of a full-agonist orvinol for PET-Imaging of opioid receptors: [11C]PEO. J. Med. Chem. 2009, 52, 5586–5589. [Google Scholar] [CrossRef] [PubMed]
- Wester, H.-J.; Willoch, F.; Tölle, T. R.; Munz, F.; Herz, M.; Øye, I.; Schadrack, J.; Schwaiger, M.; Bartenstein, P. , 6-O-(2-[18F]Fluoroethyl-6-O-desmethyl diprenorphine ([18F]DPN): Synthesis, biologic evaluation, and comparison with [11C]DPN in humans J. Nucl. Med. 2000, 41, 1279–1286. [Google Scholar]
- Schoultz, B. W.; Reed, B. J.; Marton, J.; Willoch, F.; Henriksen, G. , A fully automated radiosynthesis of [18F]fluoroethyl-diprenorphine on a single module by use of SPE cartridges for preparation of high quality 2-[18F]fluoroethyl tosylate. Molecules 2013, 18, 7271–7278. [Google Scholar] [CrossRef]
- Schoultz, B. W.; Hjørnevik, T.; Reed, B. J.; Marton, J.; Coello, C. S.; Willoch, F.; Henriksen, G. , Synthesis and evaluation of three structurally related 18F-labeled orvinols of different intrinsic activities: 6-O-[18F]Fluoroethyl-diprenorphine ([18F]FDPN), 6-O-[18F]fluoroethyl-buprenorphine ([18F]FBPN), and 6-O-[18F]fluoroethyl-phenethyl-orvinol ([18F]FPEO). J. Med. Chem. 2014, 57, 5464–5469. [Google Scholar]
- Marton, J.; Cumming, P.; Bauer, B.; Henriksen, G. , A new precursor for the radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) by nucleophilic radiofluorination. Lett. Org. Chem. 2021, 18, 344–352. [Google Scholar] [CrossRef]
- Levinstein, M. R.; Ventriglia, E. N.; Gomez, J. L.; Budinich, R. C.; Marton, J.; Henriksen, G.; Holt, D. P.; Dannals, R. F.; Pomper, M. G.; Zarate, C. A.; Jr, B., J.; Michaelides, M. , 6-O-(2-[18F]Fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) preferentially binds to mu opioid receptors in vivo. Mol. Imag. Biol. 2023, 25, 2, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Németh, E.; Gyuricza, B.; Forgács, V.; Cumming, P.; Henriksen, G.; Marton, J.; Bauer, B.; Mikecz, P.; Fekete, A. , Optimization of a nucleophilic two-step radiosynthesis of 6-O-(2-[18F]fluoroethyl)-6-O-desmethyl-diprenorphine ([18F]FE-DPN) for PET imaging of brain opioid receptors. Int. J. Mol. Sci. 2023, 24, 13152. [Google Scholar] [CrossRef]
- Bentley, K. W., The morphine alkaloids. In The Alkaloids: Chemistry and Physiology, Manske, R. H. F., Ed. Academic Press Inc.: New York, London 1971, Vol. 13.
- Linders, J. T. M.; Maat, L. , Face selectivity of the Diels-Alder reaction of thebaine-like morphinandienes, A computational approach (Chemistry of opium alkaloids, Part XXXI). Bull. Soc. Chim. Belg. 1989, 98, 265–276. [Google Scholar] [CrossRef]
- Maat, L. Novel thebainelike morphinan-dienes and their Diels-Alder adducts. In National Institute on Drug Abuse, Research Monograph Series, Drugs of Abuse: Chemistry, Pharmacology, Immunology, and AIDS, Pham, P. T. K.; Rice, K. C., Eds. 1990; pp 35–49.
- Lewis, J. W.; Husbands, S. M. , The Orvinols and Related Opioids - High Affinity Ligands with Diverse Efficacy Profiles Curr. Pharm. Design 2004, 10, 717–732. [Google Scholar]
- Husbands, S. H. Buprenorphine and related orvinols. In Research and Development of Opioid-Related Ligands, 2013; pp 127–144.
- Sandermann, W. , Diën-Anlagerungsverbindunden des Thebains. Ber. Dtsch. Chem. Ges. 1938, 71, 648–650. [Google Scholar] [CrossRef]
- Schöpf, C.; von Gottberg, K.; Petri, W. , Über Thebain-maleinsäureanhydrid, Thebainchinon, Thebainhydrochinon und dessen Säreumlagerungsprodukt, das Flavothebaon. Justus Liebigs Ann. Chem. 1938, 536, 216–257. [Google Scholar] [CrossRef]
- Li, Z.; Ye, R.; He, Q.; Lu, J.; Sun, Y.; Sun, X.; Tang, S.; Hu, S.; Chai, J.; Kong, L.; Liu, X.; Chen, J.; Fang, Y.; Lan, Y.; Xie, Q.; Liu, J.; Shao, L.; Fu, W.; Wang, Y.; Li, W. , Discovery of an ortho-substituted N-cyclopropylmethyl-7α-phenyl-6,14-endoethano-tetrahydronorthebaine derivative as a selective and potent kappa opioid receptor agonist with subsided sedative effect. J. Med. Chem. 2024. [Google Scholar] [CrossRef]
- Sandulenko, I. V.; Ambartsumyan, A. A.; Moiseev, S. K. , Fluorinated and [18F]fluorinated morphinan based opioid ligands. Org. Biomol. Chem. 2020, 18, 5533–5557. [Google Scholar] [CrossRef] [PubMed]
- Marton, J.; Henriksen, G. , Design and synthesis of an 18F-labeled version of phenylethyl orvinol ([18F]FE-PEO) for PET-imaging of opioid receptors. Molecules 2012, 17, 11554–11569. [Google Scholar] [CrossRef] [PubMed]
- Raynor, K.; Kong, H.; Chen, Y.; Yasuda, K.; Yu, L.; Bell, G. I.; Reisine, T. , Pharmacological characterization of the cloned kappa-, delta-, and mu-opioid receptors. Mol. Pharmacol. 1994, 45, 330–334. [Google Scholar] [CrossRef]
- Bentley, K. W.; Hardy, D. G. , Novel analgesics and molecular rearrangements in the morphine-thebaine group I. Ketones derived from 6,14-endo-ethenotetrahydrtothebaine J. Am. Chem. Soc. 1967, 89, 3267–3273. [Google Scholar]
- Bentley, K. W.; Hardy, D. G.; Meek, B. , Novel analgesics and molecular rearrangements in the morphine-thebaine group. II. Alcohols derived from 6,14-endo-etheno- and 6,14-endo-ethanotetrahydrothebaine. J. Am. Chem. Soc. 1967, 89, 3273–3280. [Google Scholar] [CrossRef]
- Bentley, K. W.; Hardy, D. G. , Novel analgesics and molecular rearrangements in the morphine-thebaine group. III. Alcohols of the 6,14-endo-ethenotetrahydrooripavine series and derived analogs of N-allylnormorphine and -norcodeine. J Am Chem Soc. 1967, 89, 3281–3292. [Google Scholar] [CrossRef]
- Marton, J.; Szabó, Z.; Hosztafi, S. , Herstellung von 6,14 Ethenomorphinan- Derivaten. Liebigs Ann. Chem. 1993, 8, 915–919. [Google Scholar] [CrossRef]
- Marton, J.; Hosztafi, S.; Berényi, S.; Simon, C.; Makleit, S. , Herstellung von 6,14-Ethenomorphinan- Derivaten. Monatsh. Chem. 1994, 125, 1229–1239. [Google Scholar] [CrossRef]
- Hosztafi, S.; Timár, T.; Makleit, S. , Morfin alkaloidok N-demetilezése III. Magy. Kém. Foly. 1985, 91, 126–130. [Google Scholar]
- Fleischhacker, W.; Richter, B. , 14,17-Ethano-norcodeinones. Monatsh. Chem. 1992, 123, 837–848. [Google Scholar] [CrossRef]
- Kopcho, J. J.; Schaeffer, J. C. , Selective O-demethylation of 7.alpha.-(aminomethyl)6,14-endo-ethenotetrahydrothebaine. J. Org. Chem. 1986, 51, 1620–1622. [Google Scholar] [CrossRef]
- Szűcs, E.; Marton, J.; Szabó, Z.; Hosztafi, S.; Kékesi, G.; Tuboly, G.; Bánki, L.; Horváth, G.; Szabó, P. T.; Tömböly, C.; Varga, Z. K.; Benyhe, S.; Ötvös, F. , Synthesis, biochemical, pharmacological characterization and in silico profile modelling of highly potent opioid orvinol and thevinol derivatives. Eur. J. Med. Chem. 2020, 191, 112145. [Google Scholar] [CrossRef] [PubMed]
- Czakó, B.; Marton, J.; Berényi, S.; Gach, K.; Fichna, J.; Storr, M.; Tóth, G.; Sipos, A.; Janecka, A. , Synthesis and opioid activity of novel 6-substituted-6-demethoxy-ethenomorphinans. Bioorg. Med. Chem. 2010, 18, 3535–3542. [Google Scholar] [CrossRef]
- Srivastava, S.; Fergason-Cantrell, E. A.; Nahas, R. I.; Lever, J. R. , Synthesis and opioid receptor binding of indium (III) and [111In]-labeled macrocyclic conjugates of diprenorphine: novel ligands designed for imaging studies of peripheral opioid receptors. Tetrahedron Lett. 2016, 72, 6127–6135. [Google Scholar] [CrossRef]
- Goswami, R.; Ponde, D. E.; Kung, M.-P.; Hou, C.; Kilbourn, M. R.; Kung, H. F. , Fluoroalkyl derivatives of dihydrotetrabenazine as positron emission tomography imaging agents targeting vesicular monoamine transporters. Nucl. Med. Biol. 2006, 33, 685–694. [Google Scholar] [CrossRef]
- van Wieringen, J.-P.; Shalgunov, V.; Janssen, H. M.; Fransen, M.; Janssen, A. G. M.; Michel, M. C.; Booij, J.; Elsinga, P. H. , Synthesis and characterization of a novel series of agonist compounds as potential radiopharmaceuticals for imaging dopamine D2/3 receptors in their high-affinity state. J. Med. Chem. 2014, 57, 391–410. [Google Scholar] [CrossRef]
- Galante, E.; Okamura, T.; Sander, K.; Kikuchi, T.; Okada, M.; Zhang, M.-R.; Robson, M.; Badar, A.; Lythgoe, M.; Koepp, M.; Arstad, E. , Development of purine-derived 18F-labeled pro-drug tracers for imaging of MRP1 activity with PET. J. Med. Chem. 2014, 57, 1023–1032. [Google Scholar] [CrossRef]
- Fulmor, W.; Lancaster, J. E.; Morton, G. O.; Brown, J. J.; Howell, C. F.; Nora, C. T.; Hardy, J. A. , Nuclear magnetic resonance studies in the 6, 14-endo-ethenotetrahydrothebaine Series. J. Am. Chem. Soc. 1967, 89, 3322–3330. [Google Scholar] [CrossRef] [PubMed]
- Uff, B. C.; Mallard, A. S.; Davis, J. A.; Henson, R. , NMR spectra and stereochemistry of some 7-substituted 6,14-bridged thebaine derivatives. Magn. Reson. Chem. 1985, 23, 454–459. [Google Scholar] [CrossRef]
- Mazza, S. M.; Erickson, R. H.; Blake, P. R.; Lever, J. R. , Two-dimensional homonuclear and heteronuclear correlation NMR studies of diprenorphine: A prototypic 6α, 14α-endoethanotetrahydrothebaine. Magn. Reson. Chem. 1990, 28, 675–681. [Google Scholar] [CrossRef]
- Marton, J.; Sipos, A.; Henriksen, G.; Cumming, P.; Berényi, S.; Schmitt, B. M.; Szabó, Z. , NMR Analysis of a series of 6,14-ethenomorphinan derivatives as PET precursors and reference substances. ChemistrySelect 2021, 6, 5994–6005. [Google Scholar] [CrossRef]
- Biri, B.; Kalmár, J.; Nagy, L.; Sipos, A.; Zsuga, M.; Kéki, S. Energy-dependent collision-induced dissociation study of buprenorphine and its synthestic precursors. Rapid. Commun. Mass Spectrom. 2011, 25, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M.; Kuriyan, J. Molecular dynamics and protein function. PNAS 2002, 99, 2799–2804. [Google Scholar] [CrossRef]
- Dror, R. O.; Dirks, R. M.; Grossman, J. P.; Xu, H.; Shaw, D. E. Biomolecular simulation: A computational microscope for molecular biology. Annu. Rev. Biophys. 2012, 41, 429–452. [Google Scholar] [CrossRef]
- Kenakin, T. P. Principles of drug-receptor interaction. in Goodman & Gilman’s The Pharmacological Basis of Therapeutics. (eds. Brunton, L.L., Hilal-Dandan, R. & Knollmann, B.C.), McGraw-Hill Education, 2018.
- O’Boyle, N. M.; Banck, M.; James, C. A.; Morley, C.; Vandermeersch, T.; Hutchison, G. R. Open Babel: An open chemical toolbox. J. Cheminformatics, 2011, 3, 1–33. [Google Scholar] [CrossRef]
- Halgren, T. A. Merck molecular force field. I. Basis, form, scope, parameterization, and performance of MMFF94. J. Comput Chem. 1996, 17, 490–519. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A. J. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Eastman, P; Swails, J.; Chodera, J. D.; McGibbon, R. T.; Zhao, Y.; Beauchamp, K. A., et al. OpenMM 7: Rapid development of high performance algorithms for molecular dynamics. PLOS Comput. Biol. 2017, 13, 7: e1005659.
- Case, D. A.; Duke, R. E.; Walker, R. C.; et al. AMBER 2022 Reference Manual. University of California, San Francisco, United States – California, 2022.
- Wang, J.; Wolf, R. M.; Caldwell, J. W.; Kollman, P. A.; Case, D. A. Development and testing of a general AMBER force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Jakalian, A.; Bush, B. L.; Jack, D. B.; Bayly, C. I. Fast, efficient generation of high-quality atomic charges. AM1-BCC model: I. Method. J. Comput. Chem. 2000, 21, 132–146. [Google Scholar] [CrossRef]
- Tian, C.; Kasavajhala, K.; Belfon, K. A. A.; Raguette, L.; et al. ff19SB: Amino-acid-specific protein backbone parameters trained against quantum mechanics energy surfaces in solution. J. Chem. Theory Comput. 2020, 16, 528–552. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W. L.; Chandrasekhar, J.; Madura, J. D.; Impey, R. W.; Klein, M. L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Loncharich, R. J.; Brooks, B. R.; Pastor, R. W. An impulse implementation of Langevin dynamics in a prime power method. Biopolymers 1992, 32, 523–535. [Google Scholar]
- Ryckaert, J. P.; Ciccotti, G.; Berendsen, H. J. Numerical integration of the cartesian equations of motion of a system with constraints: molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
| Position | TP-TDDPN (26b) | TB-TDDPN (26c) | TPe-TDDPN (26d) | |||
| δ13C | δ1H(m, J in Hz) | δ13C | δ1H(m, J in Hz | δ13C | δ1H(m, J in Hz) | |
| 1 | 123.2 | 6.20 (d, 8.4) | 122.9 | 6.18 (d, 8.0) | 122.8 | 6.18 (d, 8.4) |
| 2 | 118.0 | 6.49 (d, 8.4) | 117.9 | 6.46 (d, 8.0) | 117.8 | 6.46 (d, 8.4) |
| 3 | 137.2 | - | 137.2 | - | 137.2 | - |
| 4 | 151.2 | - | 151.2 | - | 151.2 | - |
| 5β | 96.2 | 3.99 (d, 2.3) | 96.5 | 4.05 (d, 1.7) | 96.6 | 4.08 (d, 1.7) |
| 6 | 80.8 | - | 80.5 | - | 80.3 | - |
| 7β | 48.1 | 1.75 (app t, 10.1) | 48.0 | 1.78 (app t, 9.4) | 48.0 | 1.80 (app t, 9.7) |
| 8α | 32.1 | 0.95 (dd, 13.3, 9.7) | 32.1 | 0.96 (dd, 13.3, 9.7) | 32.1 | 0.97 (dd, 13.1, 9.8) |
| 8β | 2.74 (ddd, 13.4, 12.4, 3.9) | 2.75 (ddd, 13.8, 12.4, 3.9) | 2.76 (ddd, 13.1, 11.9, 3.9) | |||
| 9α | 57.9 | 2.89 (d, 6.1) | 57.9 | 2.89 (d, 6.0) | 57.9 | 2.89 (d, 6.4) |
| 10α | 22.5 | 2.05 (dd, 18.7, 6.1) | 22.5 | 2.04 (dd, 18.8, 6.0) | 22.5 | 2.04 (dd, 18.4, 6.4) |
| 10β | 2.85 (d, 18.7) | 2.84 (d, 18.8) | 2.85 (d, 18.4) | |||
| 11 | 130.7 | - | 130.6 | - | 130.6 | - |
| 12 | 131.9 | - | 131.9 | - | 132.0 | - |
| 13 | 46.8 | - | 46.7 | - | 46.7 | - |
| 14 | 35.7 | - | 35.7 | - | 35.7 | - |
| 15ax | 35.4 | 1.86 (td, 13.2, 5.4) | 35.4 | 1.87 (td, 13.9, 5.6) | 35.4 | 1.89 (td, 13.7, 5.7) |
| 15eq | 1.30–1.41(mb) | 1.42 (dd, 13.1, 2.7) | 1.26–1.62 (md) | |||
| 16ax | 43.5 | 2.14 (td, 13.0, 3.6) | 43.6 | 2.14 (td, 11.5, 3.8) | 43.6 | 2.14 (td, 13.2, 3.8) |
| 16eq | 2.54 (dd, 11.9, 5.0) | 2.54 (dd, 12.0, 5.4) | 2.55 (dd, 12.1, 4.7) | |||
| 18syn | 17.4 | 1.30–1.41 (mb) | 17.6 | 1.32–1.37 (m) | 17.6 | 1.26–1.62 (md) |
| 18anti | 1.49 (m) | 1.40–1.63 (mc) | 1.26–1.62 (md) | |||
| 19syn | 30.3 | 0.43 (m) | 29.7 | 0.41 (m) | 29.8 | 0.42 (tt, 12.7, 5.7) |
| 19anti | 0.88 (td, 12.4, 5.7) | 0.88 (td, 13.6, 5.7) | 0.88 (td, 13.3, 5.9) | |||
| 20 | 74.2 | - | 74.2 | - | 74.2 | - |
| Others | ||||||
| 20-CH3 | 24.8 | 1.13 (s) | 24.9 | 1.14 (s) | 24.8 | 1.14 (s) |
| 20-CH3 | 29.7 | 1.26 (s) | 29.7 | 1.31 (s) | 29.7 | 1.32 (s) |
| 6-O-(CH2)nOTos | ||||||
| Tos-CH3 | 21.6 | 2.41 (s) | 21.6 | 2.42 (s) | 21.6 | 2.43 (s) |
| Tos-3,5 | - | 7.31 (d, 8.1) | - | 7.33 (d, 8.1) | - | 7.33 (d, 8.4) |
| Tos-2,6 | - | 7.78 (d, 8.1) | - | 7.79 (d, 8.1) | - | 7.79 (d, 8.4) |
| Tos-C1 | 133.1 | - | 133.1 | - | 133.2 | - |
| Tos-C2,6 | 129.9 | - | 127.9 | - | 127.9 | - |
| Tos-C3,5 | 129.8 | - | 129.8 | - | 129.8 | - |
| Tos-C4 | 144.6 | - | 144.6 | - | 144.6 | - |
| cPropCH2syn | 3.2 | 0.05 (m) | 3.2 | 0.05 (m) | 3.2 | 0.05 (m) |
| cPropCH2anti | 4.1 | 0.43 (m) | 4.1 | 0.46 (m) | 4.1 | 0.46 (m) |
| cPropCH | 9.3 | 0.75 (m) | 9.3 | 0.75 (m) | 9.3 | 0.75 (m) |
| NCH2 (a) | 59.8 | 2.18 (dd, 12.9, 6.7) | 59.8 | 2.17 (dd, 12.8, 6.7) | 59.8 | 2.18 (dd, 12.6, 6.7) |
| NCH2 (b) | 2.32 (dd, 12.9, 5.6) | 2.32 (dd, 12.8, 5.7) | 2.32 (dd, 12.6, 5.7) | |||
| Trityl (Tr) | ||||||
| Tr (m,p) | - | 7.21–7.24 (m) | - | 7.19–7.24 (m) | - | 7.18–7.22 (m) |
| Tr (o) | - | 7.39–7.42 (m) | - | 7.38–7.42 (m) | - | 7.39–7.44 (m) |
| Ph3CO | 91.5 | - | 91.4 | - | 91.4 | - |
| pCTr | 127.3 | - | 127.3 | - | 127.2 | - |
| mCTr | 127.4 | - | 127.4 | - | 127.3 | - |
| oCTr | 129.4 | - | 129.3 | - | 129.3 | - |
| TrC1 | 144.2 | - | 144.2 | - | 144.2 | - |
| 20-OH | - | 4.83 (br s) | - | 5.07 (br s) | - | 5.18 (br s) |
| 6-O-(CH2)3OTos | 33.4 | 1.79–1.84 (m) | - | - | - | - |
| 6-O-(CH2)3OTos | 60.4, 67.4 | 3.49–4.12 (m) | - | - | - | - |
| 6-O-(CH2)4OTos | - | - | 25.7, 26.7 | 1.40–1.63 (mc) | - | - |
| 6-O-(CH2)4OTos | - | - | 64.0, 70.2 | 3.36–3.99 (m) | - | - |
| 6-O-(CH2)5OTos | - | - | - | - | 64.3, 70.3 | 1.26–1.62 (m) |
| 6-O-(CH2)5OTos | - | - | - | - | 28.6, 30.0 | 3.36–3.98 (m) |
| Position | TP-DPN (28b) | TB-DPN (28c) | TPe-DPN (28d) | |||
| δ 13C | δ 1H (m, J in Hz) | δ 13C | δ 1H (m, J in Hz) | δ 13C | δ 1H (m, J in Hz) | |
| 1 | 119.5 | 6.50 (d, 8.0) | 119.4 | 6.50 (d, 8.0) | 119.4 | 6.49 (d, 8.0) |
| 2 | 116.3 | 6.69 (d, 8.0) | 116.3 | 6.68 (d, 8.0) | 116.3 | 6.68 (d, 8.0) |
| 3 | 137.2 | - | 137.2 | - | 137.2 | - |
| 4 | 145.4 | - | 145.4 | - | 145.5 | - |
| 5β | 97.6 | 4.42 (d, 1.2) | 97.6 | 4.40 (d, 1.3) | 97.7 | 4.40 (d, 1.1) |
| 6 | 80.8 | - | 80.6 | - | 80.5 | - |
| 7β | 48.2 | 1.89–2.05 (me) | 48.1 | 1.94 (m) | 48.0 | 1.93 (app t, 10.0) |
| 8α | 32.3 | 1.02–1.10 (mf) | 32.2 | 1.01–1.09 (mm) | 32.2 | 1.01–1.09 (ms) |
| 8β | 2.87 (ddd, 13.5, 12.0, 4.0) | 2.86 (ddd, 13.7, 12.2, 3.7) | 2.86 (ddd, 13.8, 12.1, 3.7) | |||
| 9α | 58.1 | 3.01 (d, 6.7) | 58.1 | 3.01 (d, 6.5) | 58.1 | 3.00 (d, 6.7) |
| 10α | 22.6 | 1.89–2.05 (me) | 22.6 | 2.03 (dd, 18.4, 6.5) | 22.6 | 2.20 (dd, 18.7, 6.7) |
| 10β | 2.98 (d, 18.7) | 2.97 (d, 18.4) | 2.97 (d, 18.7) | |||
| 11 | 128.3 | - | 128.2 | - | 128.3 | - |
| 12 | 132.3 | - | 132.3 | - | 132.3 | - |
| 13 | 47.3 | - | 47.3 | - | 47.2 | - |
| 14 | 35.9 | - | 35.9 | - | 35.9 | - |
| 15ax | 35.5 | 2.18–2.30 (mg) | 35.3 | 2.17–2.30 (mn) | 35.5 | 2.03 (td, 13.8, 5.7) |
| 15eq | 1.67 (dd, 13.1, 2.7) | 1.66 (dd, 13.3, 2.5) | 1.46–1.79 (mt) | |||
| 16ax | 43.7 | 2.18–2.30 (mg) | 43.7 | 2.17–2.30 (mn) | 43.7 | 2.22–2.30 (m)u |
| 16eq | 2.63 (dd, 12.0, 5.4) | 2.63 (dd, 12.0, 5.0) | 2.63 (dd, 12.1, 5.0) | |||
| 18syn | 17.8 | 1.76–1.79 (mh) | 17.9 | 1.77–1.82 (mo) | 17.7 | 1.46–1.79 (mt) |
| 18anti | 1.76–1.79 (mh) | 1.77–1.82 (mo) | 1.46–1.79 (mt) | |||
| 19syn | 29.9 | 0.74 (m) | 29.7 | 0.73 (m) | 29.9 | 0.73 (m) |
| 19anti | 1.02–1.10 (mf) | 1.01–1.09 (mm) | 1.01–1.09 (ms) | |||
| 20 | 74.4 | - | 74.5 | - | 74.5 | - |
| Others | ||||||
| 20-CH3 | 24.8 | 1.20 (s) | 24.8 | 1.20 (s) | 24.8 | 1.20 (s) |
| 20-CH3 | 29.7 | 1.39 (s) | 29.9 | 1.39 (s) | 29.7 | 1.39 (s) |
| cPropCH2syn | 3.3 | 0.10 (m) | 3.3 | 0.09 (m) | 3.3 | 0.09 (m) |
| cPropCH2anti | 4.2 | 0.49 (m) | 4.1 | 0.49 (m) | 4.1 | 0.49 (m) |
| cPropCH | 9.4 | 0.80 (m) | 9.4 | 0.80 (m) | 9.4 | 0.80 (m) |
| NCH2 (a) | 59.8 | 2.18–2.30 (mg) | 59.8 | 2.17–2.30 (mn) | 59.8 | 2.22–2.30 (m)u |
| NCH2 (b) | 2.37 (dd, 12.6, 5.7) | 2.37 (dd, 12.7, 6.0) | 2.37 (dd, 12.6, 6.0) | |||
| 20-OH | - | 5.13 (br s) | - | 5.30 (br s) | - | 5.34 (br s) |
| 3-OH | 4.94 (br s) | 5.13 (br s) | 5.10 (br s) | |||
| 6-O-CH2CH2CH2F (b,b’) | 31.6 db | 1.89–2.05 (me) | - | - | - | - |
| 6-O-CH2CH2CH2F (a) | 60.8 dc | 3.78 (dt, 9.3, 6.3, 1H) | - | - | - | - |
| 6-O-CH2CH2CH2F (a’) | 4.07 (dt, 9.3, 5.9, 1H) | - | - | - | - | |
| 6-O-CH2CH2CH2F (c,c’) | 81.1 dd | 4.51–4.60 (m, 2H) | - | - | - | - |
| 6-O-CH2CH2CH2CH2F (a,a’) | - | - | 64.2 di | 3.67 (dt, 9.3, 6.1) | - | - |
| - | - | 3.99 (dt, 9.3, 6.1) | - | - | ||
| 6-O-CH2CH2CH2CH2F (b,b’) | - | - | 26.6 dj | 1.77–1.82 (mo) | - | - |
| 6-O-CH2CH2CH2CH2F (c,c’) | - | - | 27.3 dk | 1.77–1.82 (mo) | - | - |
| 6-O-CH2CH2CH2CH2F (d,d’) | - | - | 83.8 d | 4.42–4.51 (m) | - | - |
| 6-O-CH2CH2CH2CH2CH2F (a) | - | - | - | - | 64.5 | 3.63 (dt, 8.9, 6.4) |
| 6-O-CH2CH2CH2CH2CH2F (a’) | - | - | - | - | 3.94 (dt, 8.9, 6.4) | |
| 6-O-CH2CH2CH2CH2CH2F (b,b’) | - | - | - | - | 22.0 dp | 1.46–1.79 (mt) |
| 6-O-CH2CH2CH2CH2CH2F (c,c’) | - | - | - | - | 30.1 dq | 1.46–1.79 (mt) |
| 6-O-CH2CH2CH2CH2CH2F (d,d’) | - | - | - | - | 30.2 | 1.46–1.79 (mt) |
| 6-O-CH2CH2CH2CH2CH2F (e) | - | - | - | - | 83.9 dr | 4.38 (t, 6.0) |
| 6-O-CH2CH2CH2CH2CH2F (e’) | - | - | - | - | 4.48 (t, 6.0) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





