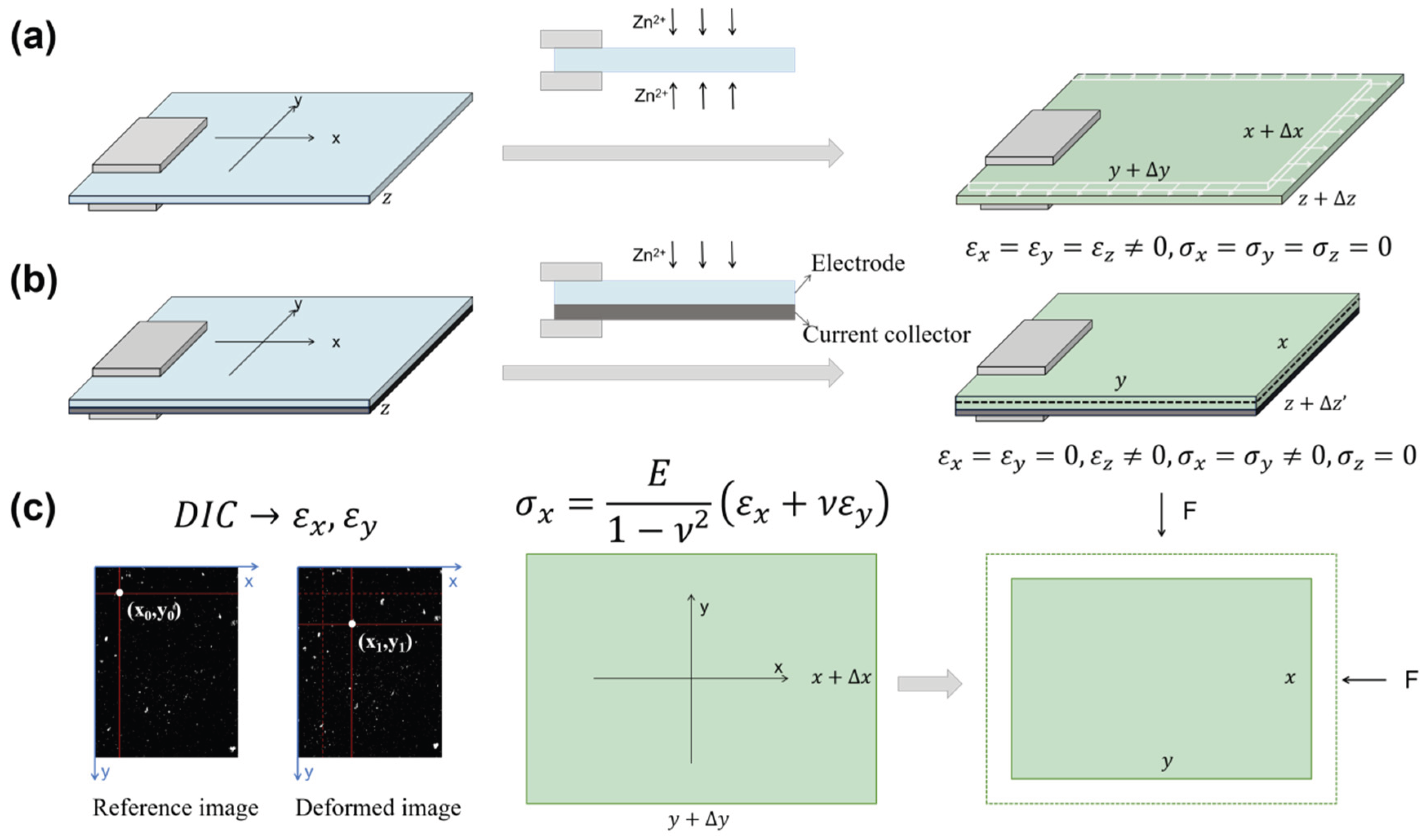
Stress and strain energy evolution during battery cycling critically governs electrode degradation and performance decline. [
1,
2] During electrochemical cycling, ion concentration variations induce dimensional/volume changes in electrodes. These changes generate two distinct stress contributions: (1) diffusion-induced stresses arising from concentration gradients within active particles,[
3,
4] and (2) macroscopic electrode level stresses in film-substrate electrode systems caused by constraints from inactive cell components (e.g., current collectors) and geometric limitations. [
2,
5,
6,
7,
8,
9] These stresses trigger mechanical degradation through electrode fracturing, particle disintegration, and contact loss at interfaces, ultimately causing capacity fade and cell failure. [
6,
10,
11,
12,
13] Meanwhile, the strain energy accumulated during deformation drives crack initiation and propagation as stored energy converts to surface energy during fracture. [
14] Notably, cracks often originate at active/inactive material interfaces, emphasizing the necessity to study DIS and strain energy evolution at electrode levels.
Real-time observation techniques, such as in situ X-ray or electron-based microscopy, reveal atomic-scale deformation mechanisms but lack direct stress/strain quantification in practical porous composite electrodes composed of active particles, binders, and conductive agents. [
15,
16,
17] Such investigations were made possible by the use of digital image correlation (DIC) or laser-based techniques employing Stoney’s equation combined with chemo-mechanical modeling. [
9,
18,
19,
20,
21] Although theoretical studies have addressed DIS and strain energy at particle scales, [
14,
22,
23,
24,
25] electrode-level investigations remain limited. Bridging this gap is essential to mitigate fracture-related degradation and optimize next-generation batteries through stress-engineered electrode designs.
In this work, a theoretical framework was developed for estimating stress and strain energy generation during ion insertion/extraction in practical battery electrodes with current collector substrates. To simplify the analysis, we assumed a homogeneous ion concentration distribution within the electrode. The electrode’s porous structure ensures that ion diffusion predominantly occurs across the ligaments of the porous network rather than through the bulk thickness, thereby minimizing significant global concentration gradients. We further adopted a plane stress assumption and treated the deformation as purely elastic. Under plane stress and linear elasticity assumptions, the in-plane stress components
and
are expressed as:
where
and
are effective Young’s modulus and effective Poisson ratio of the composite electrode, respectively.
and
are the strain components along the x- and y-directions. The shear stress
is given by:
where
is effective shear modulus of the composite electrode, and
is shear strain. For isotropic battery electrodes (
and
),
and
simplify to:
In freestanding electrodes (
Figure 1a), ion insertion induces free volumetric expansion, generating isotropic tensile strains (
) across all directions. This strain can be experimentally measured by the Digital-Image-Correlation (DIC)-based approach. In practical electrodes where the active layer is bonded to current collectors (
Figure 1b), however, this expansion is mechanically constrained by the substrate (e.g., Cu foil) and battery casing, resulting in bilayer stresses in film-substrate electrodes. Without casing constraints, this bilayer would bend, which is the principle exploited in traditional laser curvature methods for stress measurement. Under such conditions, total strain (
) comprises both bending strain and chemical strain (
).
Scenario I. Film-Substrate Bilayer Electrodes under Cycling with a Constant Effective Young’s Modulus and No Bending Deformation
The present study first examines film-substrate bilayer electrodes constrained within coin/pouch cells where the casing suppresses bending deformation. Consequently, the total strain equates solely to chemical strain from ion insertion. Due to the thin-film-substrate geometry of the electrode, we assume uniform stress distribution along the thickness direction. If the bilayer electrode’s initial pre-cycling state was used as reference, the in-plane strains (x, y directions), , are approximately zero due to rigid current collector constraints, while the through-thickness strain (z-direction), , develops freely as the soft separator imposes negligible constraint, especially in pouch cells. This represents a reasonably valid approximation for systems with small volume change. It is also worth noting that local in-plane strains may not be strictly zero due to inhomogeneous deformation, which would affect the calculation of the spatial stress/strain energy distributions. For systems with small overall volume change, we consider in-plane strains negligible compared to freestanding electrode strains and thus exclude them from the current model.
To model these systems, we define the freestanding electrode at a given ion concentration as the reference state. The practical electrode (with identical ion content) is then conceptualized as the freestanding electrode compressed biaxially until its in-plane dimensions match those of the constrained system (
Figure 1c). Assuming
, the stress generated during ion insertion can be estimated by Equation (1.3), provided
is measured as a function of ion concentration and stress-concentration coupling effects are neglected. For cases where
, the effective strain (
) must substitute
in Equation (1.3), necessitating experimental determination of
.
Under a plane-stress state, the strain energy density (u
e) is given by:
For isotropic conditions (
and
), this simplifies to:
The total strain energy (U) is then calculated by integrating
over the electrode volume
V:
where
is the electrode volume and is a function of ion concentration.
The volumetric strain (
) during the “compression” of the freestanding electrode is expressed as:
Under plane stress (
), Hooke’s law yields
. Then, we have:
The electrode volume and its differential form are given by:
And
Substituting Equation (1.10) into Equation (1.6) gives the total strain energy U as follows:
The boundary condition
at
necessitates
, yielding:
By experimentally measuring
as a function of ion concentration and determining
and
, both stress and strain energy generated during cycling of the battery electrode can be quantified using Equation (1.3) and Equation (1.12).
To determine the effective Young’s modulus (
) and Poisson’s ratio (
) of the composite electrode, we can assume
remains constant throughout the analysis, and
is bounded using micromechanical homogenization frameworks. The actual electrode is a composite comprising active materials, conductive additive (Super P), and binder PVDF. We assume that the modulus of the three components is independent of the ion concentration. For the idealized non-porous binary composite comprising conductive additive Super P and binder PVDF, the lower bound of the effective bulk modulus (
Km,l) and effective shear modulus (
Gm,l) can be calculated using the iso-stress (Reuss series) homogenization framework as follows:[
26]
The upper bound of the effective bulk modulus (
Km,u) and effective shear modulus (
Gm,u) can be calculated using the iso-strain (Voigt parallel) homogenization framework as follows:[
27]
where
and
are the volume fractions of Super P and PVDF in the non-porous binary composite.
and
denote the bulk moduli of Super P and PVDF, respectively, while
and
represent their corresponding shear moduli.
and
are the volume of the Super P and PVDF, respectively.
To account for the inherent porosity of practical electrodes, the effective bulk modulus (
Kpm) requires porosity-dependent corrections:[
28,
29]
where
, and
, representing the densities of the fully dense and porous Super P/PVDF binary composite matrix.
and
are the global volume fractions of Super P and PVDF within the fully dense matrix.
and
are the global volume fractions of Super P and PVDF within the porous --matrix.
corresponds to Poisson’s ratio of the porous matrix. Substituting
and
in Equation (1.15) with
,
or
,
yields the lower bound and upper bound of the effective bulk modulus (
Kpm) of porous Super P/PVDF binary composite matrix.
The effective bulk modulus
Ke of the whole porous electrode is given by:[
28]
where
,
,
,
,
, and
.
is the volumetric fraction of active material within the porous composite electrode.
and
are bulk modulus and Poisson ratio of the active material. Then, the effective Young’s modulus of the whole porous electrode can be calculated by:
Using the above theoretical framework and in situ DIC-measured chemical strain data, the stress and strain energy in practical battery electrodes under operational cycling conditions can be calculated for a film-substrate bilayer electrode with a constant effective Young’s modulus and without bending deformation.
Scenario II. Cycling of a Film-Substrate Bilayer Electrode under Bending with Concentration-Dependent Modulus
During cycling, volumetric changes in the electrode film are constrained by the substrate, resulting in a strain mismatch between the two layers. This mismatch generates stress, leading to bending deformation of the bilayer electrode and consequent changes in curvature if there is no constraints from the battery cases along the thickness direction.
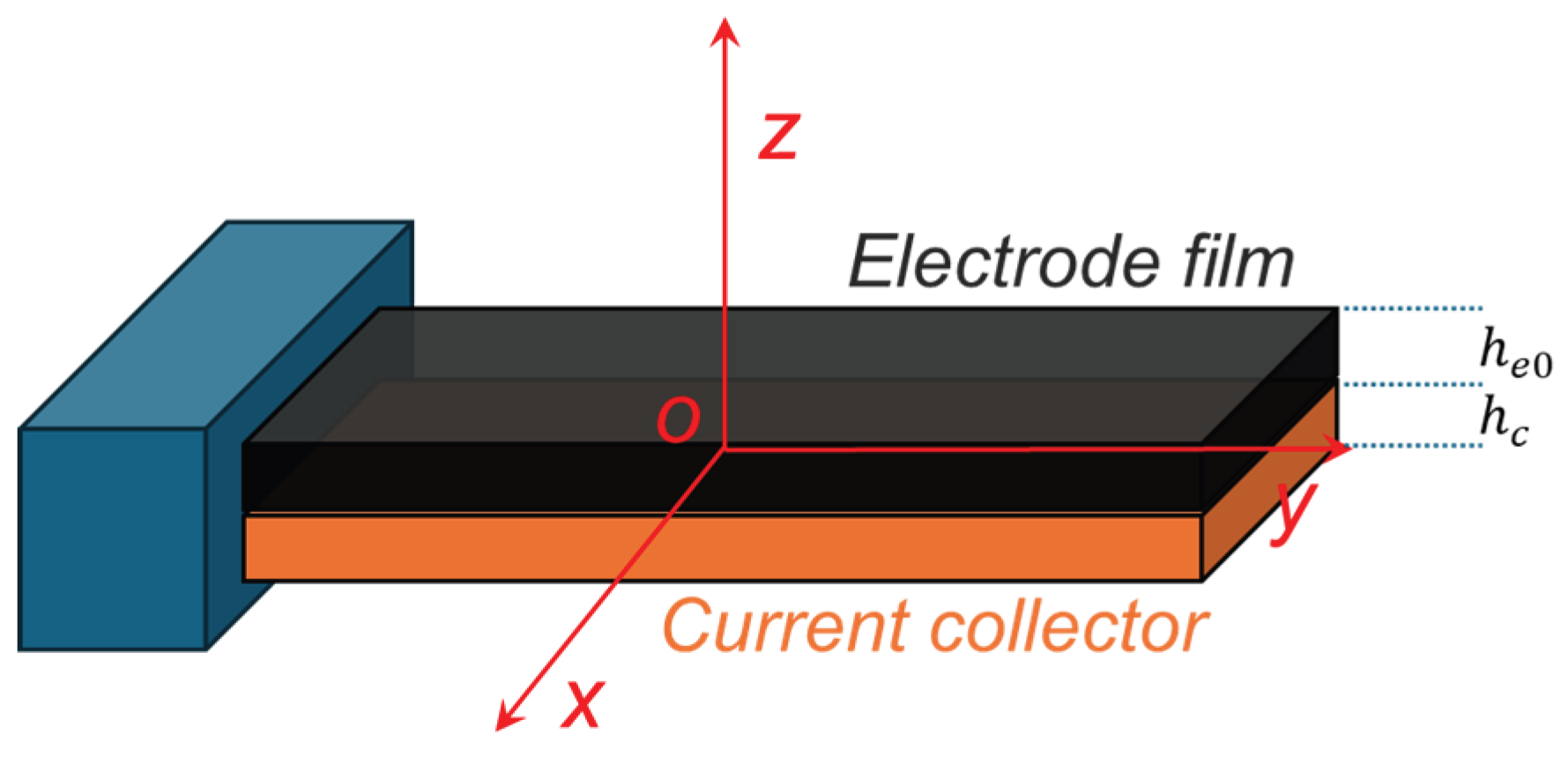
To determine the stress and effective Young’s modulus of the electrode film during cycling, we developed a mechanical model of the bilayer structure comprising the active electrode and current collector. This model represents the first coupled framework that integrates the evolution of active electrode thickness during cycling with in situ curvature measurements and chemical strain data. Similar to Scenario I, the model is founded on the assumptions of isotropic linear elasticity and an equi-biaxial stress state, and uniform distribution of ion concentration within the electrode. The active layer is perfectly bonded to the current collector, ensuring strain continuity across the interface. The interface between the current collector and the active layer is defined as the midplane of the system. A Cartesian coordinate system is established with its origin
ο located at the centroid of this midplane. The z-axis is normal to the electrode plane (along the thickness direction), while the x- and y-axes lie within the midplane, extending along the lateral and transverse directions, as illustrated in
Figure 2.
The thickness of the current collector is denoted as
, the initial active electrode thickness as
, and the lithiated electrode thickness as
. Within classical beam theory, the in-plane strain in a bending electrode varies linearly through the thickness direction. The in-plane strains of the active electrode (
and
) and current collector (
and
) can be given by:
is the distance from the midplane, and
is the midplane strain at
.
is the curvature of the electrode.
is the intrinsic chemical strain of the active electrode (
), which exists only in the active electrode portion. Given that the electrode’s thickness is much smaller than its in-plane dimensions, the electrode can be treated as being under a generalized plane stress state during electrochemical processes. Based on the generalized Hooke’s law, the in-plane elastic stresses are derived as follows:
,
,
and
are the in-plane elastic stresses of the active electrode and the current collector, respectively.
and
are the effective Young’s modulus of the active electrode and current collector.
is a constant value, while
varies with the Li concentration.
and
are Poisson’s ratios of the active electrode and the current collector. Due to the absence of mechanical constraint along the z-direction, lithiation leads to thickening of the electrode film in the bilayer structure.
To determine the thickness of the electrode film during cycling, we first consider the freestanding electrode as the reference, with an initial thickness denoted as
. Upon lithiation at a given lithium ion concentration, the freestanding electrode expands freely in all three directions. Under the assumption of isotropy, it develops isotropic chemical strains, denoted as
. Thus, the thickness of the freestanding electrode after expansion can be expressed as
When the electrode film is bonded to the current collector, its expansion is mechanically constrained by the substrate. This restriction induces biaxial stress in the film and causes the electrode to bend. In the following step, we use the freestanding electrode at lithiated state as the reference state. The bilayer electrode (with identical ion content) is then conceptualized as this freestanding electrode subjected to biaxial compression until its in-plane dimensions match those of the constrained system. Due to the Poisson effect, this compression leads to an increase in the thickness of the electrode film. The strain (
) at each point through the thickness of the active electrode film arises primarily from the Poisson effect and can be expressed as:
The average strain through the thickness direction (
) is obtained by integrating over the initial thickness and can be expressed as:
Then, the total thickness of the electrode film after lithiation can be expressed as:
From Equations (2.3), (2.5), and (2.6), we can obtain:
As the system is not subjected to any external forces, it must satisfy the conditions of both force and moment equilibrium:[
30]
Substituting Equation (2.2) into Equations (2.8) and (2.9), we have:
By combining Equations (2.7), (2.10), and (2.11), the electrode thickness, midplane stress in the active electrode (
), and the Young’s modulus of the electrode can be determined as functions of Li concentration during cycling, providing that the electrode undergoes elastic deformation during the entire cycling processes.
Here, we propose the following criterion for elastic deformation during ion insertion and extraction: the strain path during extraction must retrace that of insertion. This requires the absolute strain change rates to be equal at identical ion concentrations. While some residual deformation may occur, it must arise solely from ions trapped within the active material’s lattice.
However, during a battery’s initial cycles, electrolyte decomposition typically causes irreversible side reactions. These reactions form by-products, such as the solid electrolyte interface (SEI), on the active materials, leading to inelastic electrode deformation. To estimate the electrode’s stress and apparent Young’s modulus, we can use the end state of the first cycle as a reference point, assuming most side reactions are complete and subsequent cycling causes elastic deformation.
As discussed above, the midplane is defined as the interface between the current collector and the active layer, with its strain denoted as , which varies during cycling. For a pure Cu foil, the neutral axis lies at the center of its thickness. When the foil is coated with an active film to form a bilayer structure, the neutral axis shifts toward the electrode layer. If the electrode film is sufficiently thick, the neutral axis moves very close to the Cu/electrode interface, allowing the midplane to be approximated as the neutral axis. Then, is negligible compared to the electrode’s chemical strain, , and can thus be omitted.
Thus, Equations (2.1) and (2.7) can be simplified to:
Given the small thickness of both the Cu substrate and the active electrode film, we assume a uniform strain distribution along the z-direction in both layers. Consequently, the average strain in the electrode film and Cu substrate is given by:
Generally, at the end of the first cycle, the electrode will not return to its initial straight state, with the presence of residual stress. We denote the residual stress as
and the apparent Young’s modulus of the electrode film at this state as
. The average stress in the Cu substrate is denoted as
, and is expressed as:
The force balance requires:
Combing Equations (2.15) and (2.16) yields:
Here,
is the thickness of the electrode film after the first cycle, which can be calculated using Equation (13). The
can then be calculated using Equation (2.17).
From the second cycle onward, we assume that the deformation of the electrodes is elastic. The average stress in the electrode film during these cycles is denoted as
. The force balance in this case requires:
This leads to:
If we further assume the electrode film thickness (
) remains nearly constant after the first cycle, combing Equations (2.17) and (2.19) gives:
Using Equation (2.20), the
can be calculated for the electrodes during the second and subsequent cycles.
We now discuss the apparent Young’s modulus of the electrode film. As previously stated, we treat the deformation from the second cycle onward as elastic. The average strain during these cycles is obtained from Equation (2.14), allowing us to establish a stress-strain relationship. However, since the stress-strain curve may be non-linear due to the composite nature of the electrode, we can employ the Chord Modulus Method to estimate the apparent Young’s modulus, using the residual stress as the origin.
Summary
In conclusion, a theoretical framework was developed to quantify stress and strain energy evolution in practical battery electrodes with current collectors during ion insertion/extraction cycles. The model integrates in situ strain data from freestanding electrodes, measured via DIC, under plane stress conditions and linear elastic assumptions. When the electrode modulus was assumed constant, iso-stress (Reuss) and iso-strain (Voigt) homogenization models were used, along with porosity-dependent corrections, to estimate bounds for the effective Young’s modulus of the composite electrodes. Conversely, when the modulus was treated as concentration-dependent, both the stress and the apparent Young’s modulus could be determined simultaneously by measuring the electrode curvature and the intrinsic chemical strain. This work establishes a foundational methodology for assessing stress evolution and mechanical energy dissipation during practical battery cycling.
References
- M. K. S. Verma, S. Basu, K. S. Hariharan, S. M. Kolake, T. Song, and J. Jeon, A Strain-Diffusion Coupled Electrochemical Model for Lithium-Ion Battery, Journal of The Electrochemical Society 164, A3426 (2017). [CrossRef]
- K. Li, S. Wang, X. Shi, and Y. Huang, An Analysis of the Chemical Stress Field Under Potentiostatic Intermittent Titration Techniques for Interfacial Reaction-Controlled Systems, Acta Mechanica Solida Sinica (2025). [CrossRef]
- A. Verma, A. Singh, and A. Colclasure, On the Impact of Mechanics on Electrochemistry of Lithium-Ion Battery Anodes, JOM 76, 1171 (2024). [CrossRef]
- X. Zhang, W. Shyy, and A. Marie Sastry, Numerical Simulation of Intercalation-Induced Stress in Li-Ion Battery Electrode Particles, Journal of The Electrochemical Society 154, A910 (2007).
- V. A. Sethuraman, N. Van Winkle, D. P. Abraham, A. F. Bower, and P. R. Guduru, Real-time stress measurements in lithium-ion battery negative-electrodes, J Power Sources 206, 334 (2012). [CrossRef]
- A. Mukhopadhyay, A. Tokranov, X. Xiao, and B. W. Sheldon, Stress development due to surface processes in graphite electrodes for Li-ion batteries: A first report, Electrochimica Acta 66, 28 (2012). [CrossRef]
- J. M. Tarascon and M. Armand, Issues and challenges facing rechargeable lithium batteries, Nature 414, 359 (2001). [CrossRef]
- A. Tokranov, B. W. Sheldon, P. Lu, X. Xiao, and A. Mukhopadhyay, The Origin of Stress in the Solid Electrolyte Interphase on Carbon Electrodes for Li Ion Batteries, Journal of The Electrochemical Society 161, A58 (2014). [CrossRef]
- A. Mukhopadhyay, A. Tokranov, K. Sena, X. Xiao, and B. W. Sheldon, Thin film graphite electrodes with low stress generation during Li-intercalation, Carbon 49, 2742 (2011). [CrossRef]
- D. Zane, A. Antonini, and M. Pasquali, A morphological study of SEI film on graphite electrodes, J Power Sources 97-98, 146 (2001). [CrossRef]
- A. Mukhopadhyay and B. W. Sheldon, Deformation and stress in electrode materials for Li-ion batteries, Prog Mater Sci 63, 58 (2014). [CrossRef]
- Y. Zhu and C. Wang, Strain accommodation and potential hysteresis of LiFePO4 cathodes during lithium ion insertion/extraction, J Power Sources 196, 1442 (2011). [CrossRef]
- B. W. Sheldon, S. K. Soni, X. Xiao, and Y. Qi, Stress Contributions to Solution Thermodynamics in Li-Si Alloys, Electrochemical and Solid-State Letters 15, A9 (2011).
- R. Deshpande, Y.-T. Cheng, M. W. Verbrugge, and A. Timmons, Diffusion Induced Stresses and Strain Energy in a Phase-Transforming Spherical Electrode Particle, Journal of The Electrochemical Society 158, A718 (2011).
- S.-C. Chao, Y.-C. Yen, Y.-F. Song, Y.-M. Chen, H.-C. Wu, and N.-L. Wu, A study on the interior microstructures of working Sn particle electrode of Li-ion batteries by in situ X-ray transmission microscopy, Electrochemistry Communications 12, 234 (2010). [CrossRef]
- Y. Tian, A. Timmons, and J. R. Dahn, In Situ AFM Measurements of the Expansion of Nanostructured Sn–Co–C Films Reacting with Lithium, Journal of The Electrochemical Society 156, A187 (2009). [CrossRef]
- L. Y. Beaulieu, K. W. Eberman, R. L. Turner, L. J. Krause, and J. R. Dahn, Colossal Reversible Volume Changes in Lithium Alloys, Electrochemical and Solid-State Letters 4, A137 (2001). [CrossRef]
- E. Chason and B. W. and Sheldon, Monitoring Stress in Thin Films During Processing, Surface Engineering 19, 387 (2003).
- V. A. Sethuraman, M. J. Chon, M. Shimshak, V. Srinivasan, and P. R. Guduru, In situ measurements of stress evolution in silicon thin films during electrochemical lithiation and delithiation, J Power Sources 195, 5062 (2010). [CrossRef]
- V. A. Sethuraman, V. Srinivasan, A. F. Bower, and P. R. Guduru, In Situ Measurements of Stress-Potential Coupling in Lithiated Silicon, Journal of The Electrochemical Society 157, A1253 (2010). [CrossRef]
- V. A. Sethuraman, M. J. Chon, M. Shimshak, N. Van Winkle, and P. R. Guduru, In situ measurement of biaxial modulus of Si anode for Li-ion batteries, Electrochemistry Communications 12, 1614 (2010). [CrossRef]
- Y.-T. Cheng and M. W. Verbrugge, Evolution of stress within a spherical insertion electrode particle under potentiostatic and galvanostatic operation, J Power Sources 190, 453 (2009). [CrossRef]
- Y. Hu, X. Zhao, and S. Zhigang, Averting cracks caused by insertion reaction in lithium–ion batteries, Journal of Materials Research 25, 1007 (2010). [CrossRef]
- H.-Y. Shadow Huang and Y.-X. Wang, Dislocation Based Stress Developments in Lithium-Ion Batteries, Journal of The Electrochemical Society 159, A815 (2012). [CrossRef]
- X. Xiao, P. Liu, M. W. Verbrugge, H. Haftbaradaran, and H. Gao, Improved cycling stability of silicon thin film electrodes through patterning for high energy density lithium batteries, J Power Sources 196, 1409 (2011). [CrossRef]
- A. Reuss, Berechnung der Fließgrenze von Mischkristallen auf Grund der Plastizitätsbedingung für Einkristalle, 9, 49 (1929).
- W. Voigt, Ueber die Beziehung zwischen den beiden Elasticitätsconstanten isotroper Körper, Annalen der Physik 274, 573 (1889).
- E. M. C. Jones, M. N. Silberstein, S. R. White, and N. R. Sottos, In Situ Measurements of Strains in Composite Battery Electrodes during Electrochemical Cycling, Experimental Mechanics 54, 971 (2014). [CrossRef]
- L. J. Gibson and M. F. Ashby, Cellular Solids: Structure and Properties (Cambridge University Press, Cambridge, 1997), 2 edn., Cambridge Solid State Science Series.
- H. Xie, Y. Kang, H. Song, J. Guo, and Q. Zhang, In situ method for stress measurements in film-substrate electrodes during electrochemical processes: key role of softening and stiffening, Acta Mechanica Sinica 36, 1319 (2020). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).