Submitted:
29 August 2025
Posted:
01 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
2.1. Study Participants
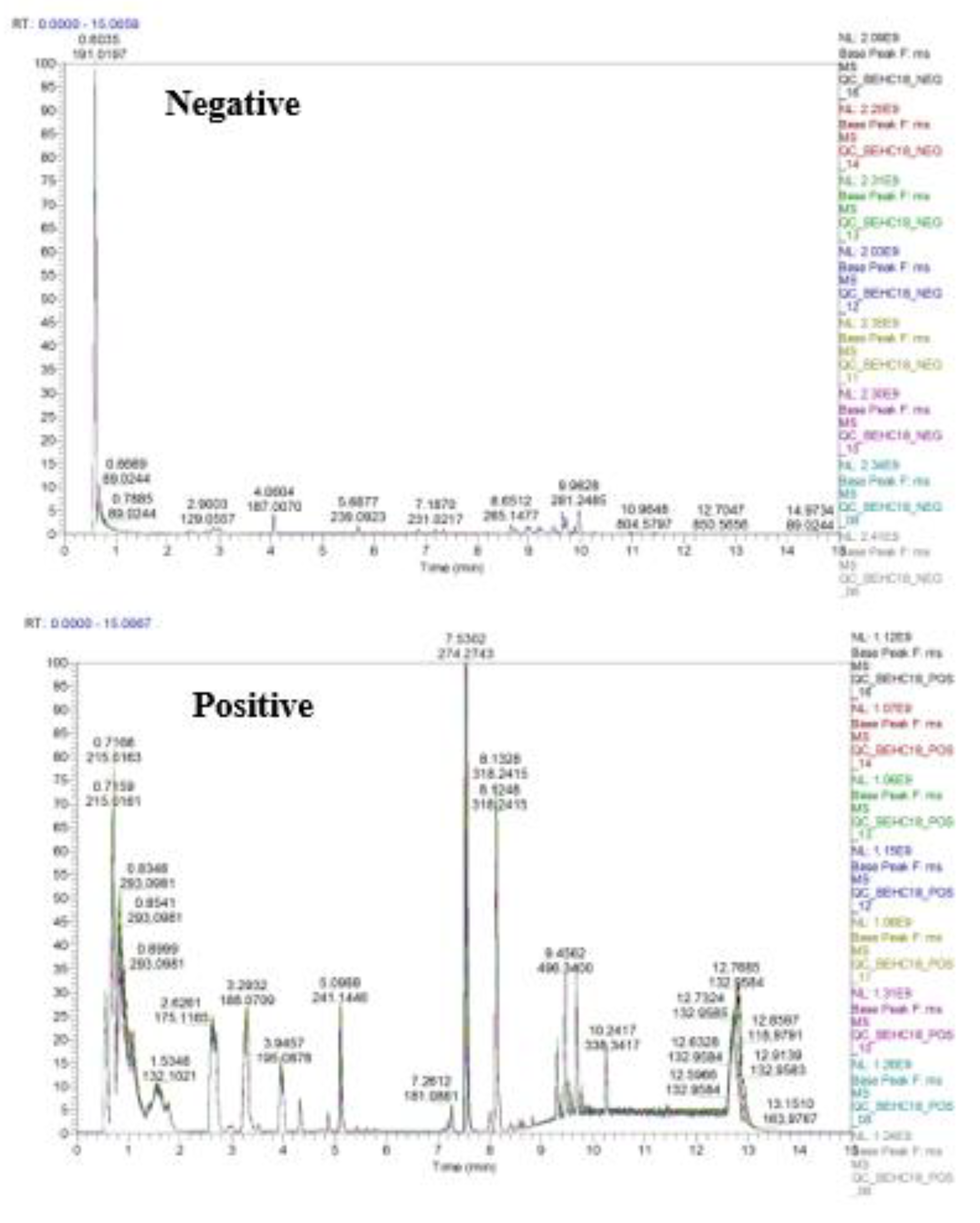
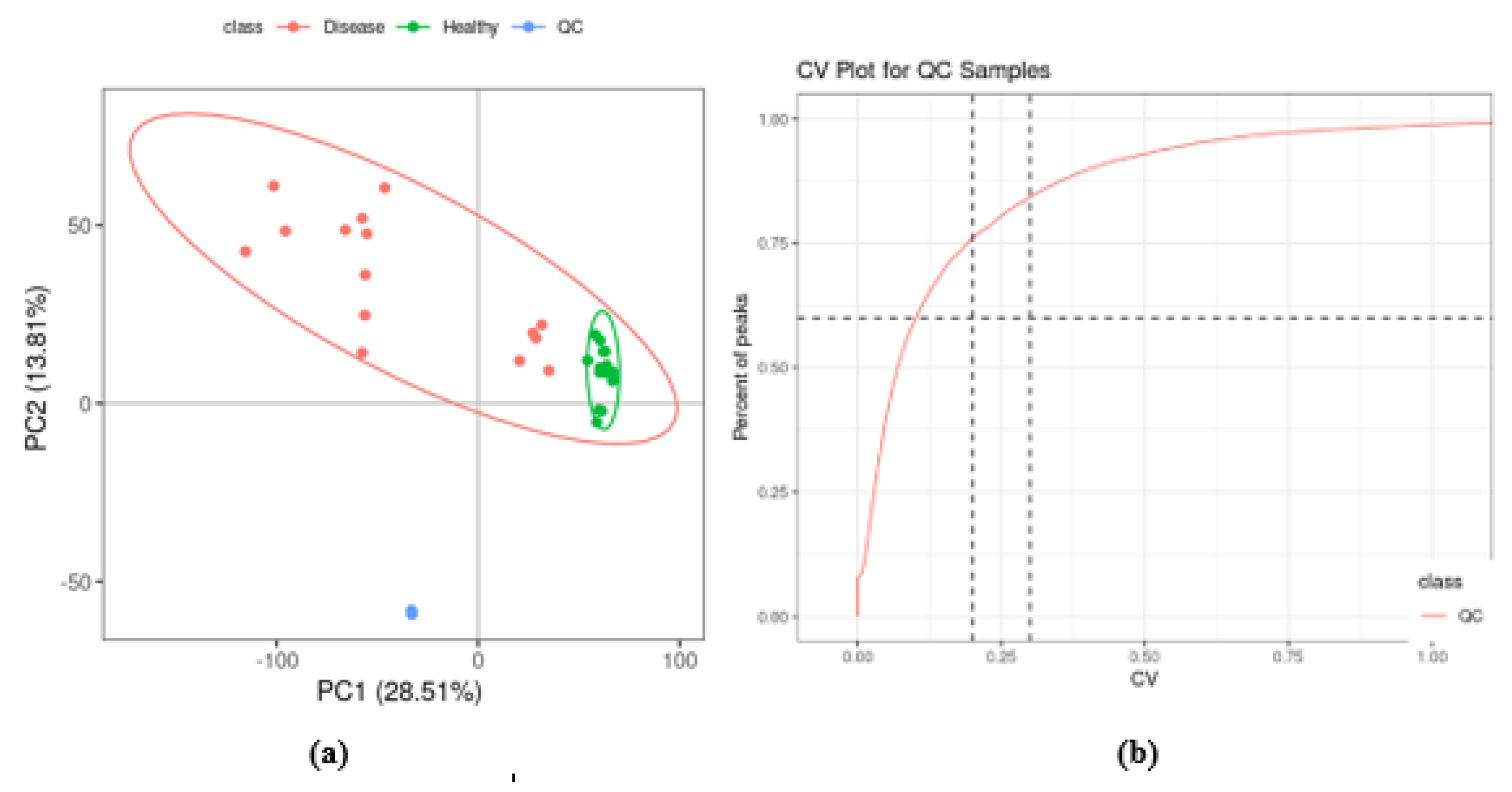
2.2. Quality Control of UPLC-MS Analysis
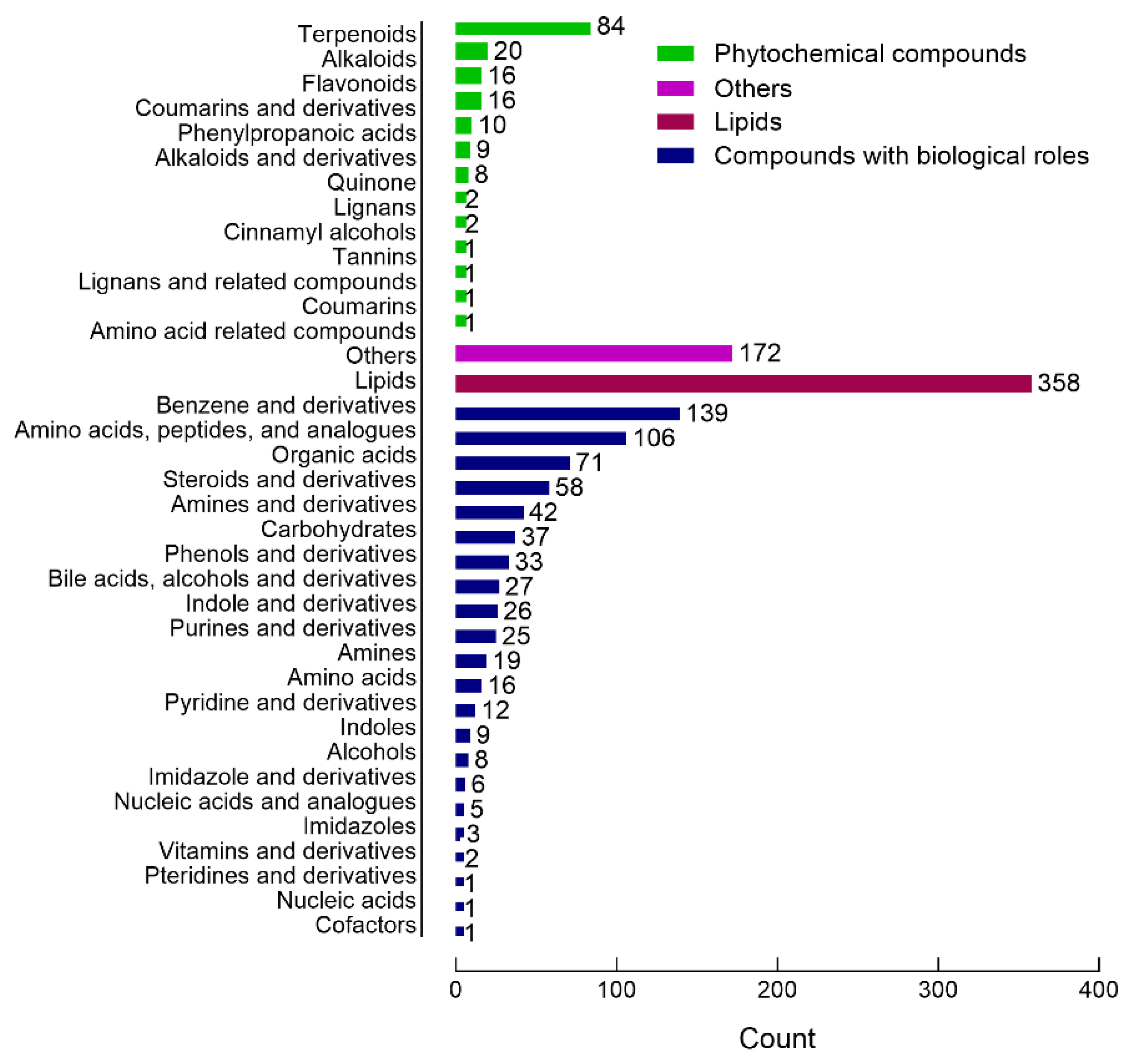
2.3. Metabolite Identification and Classification
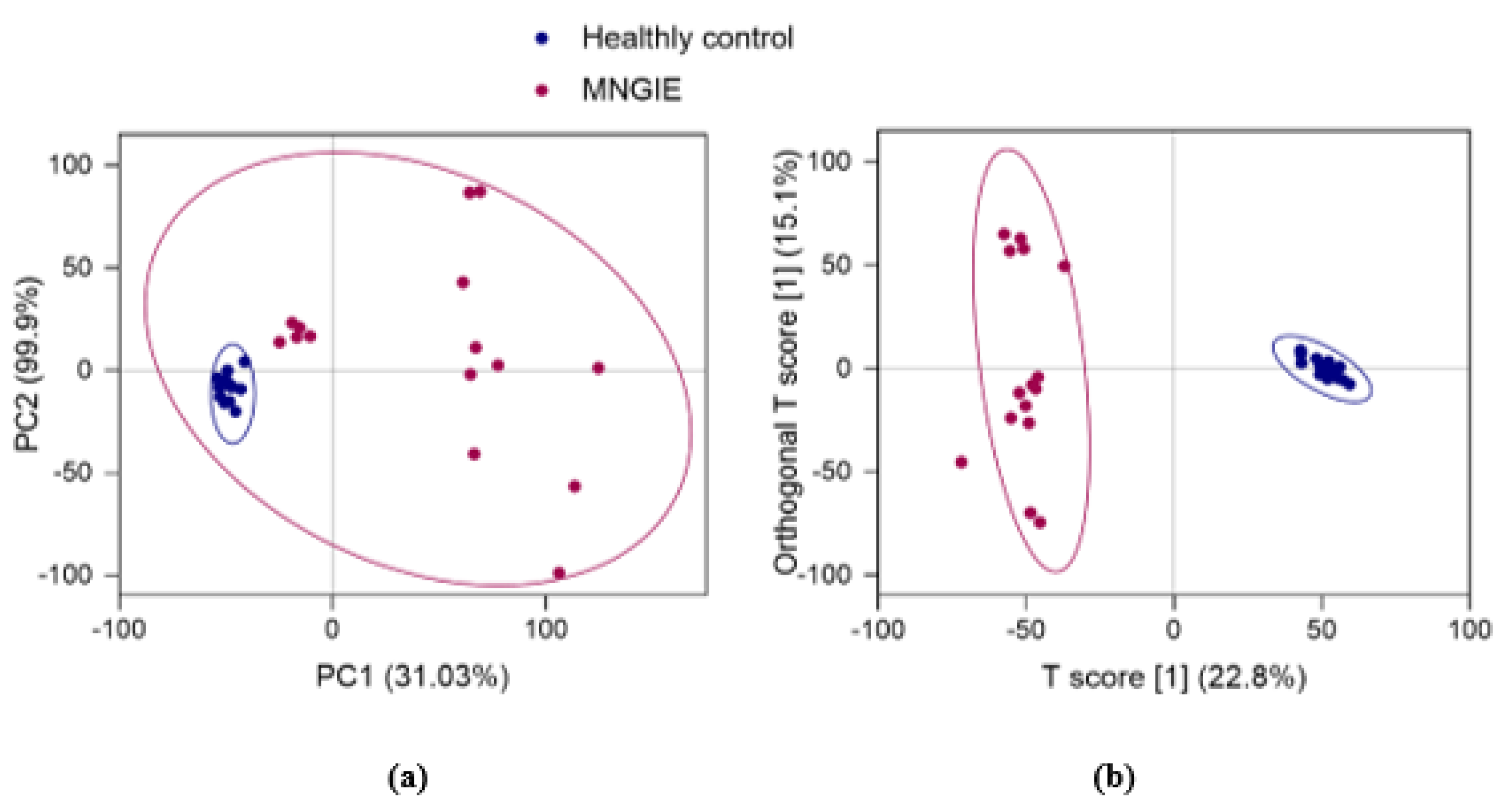
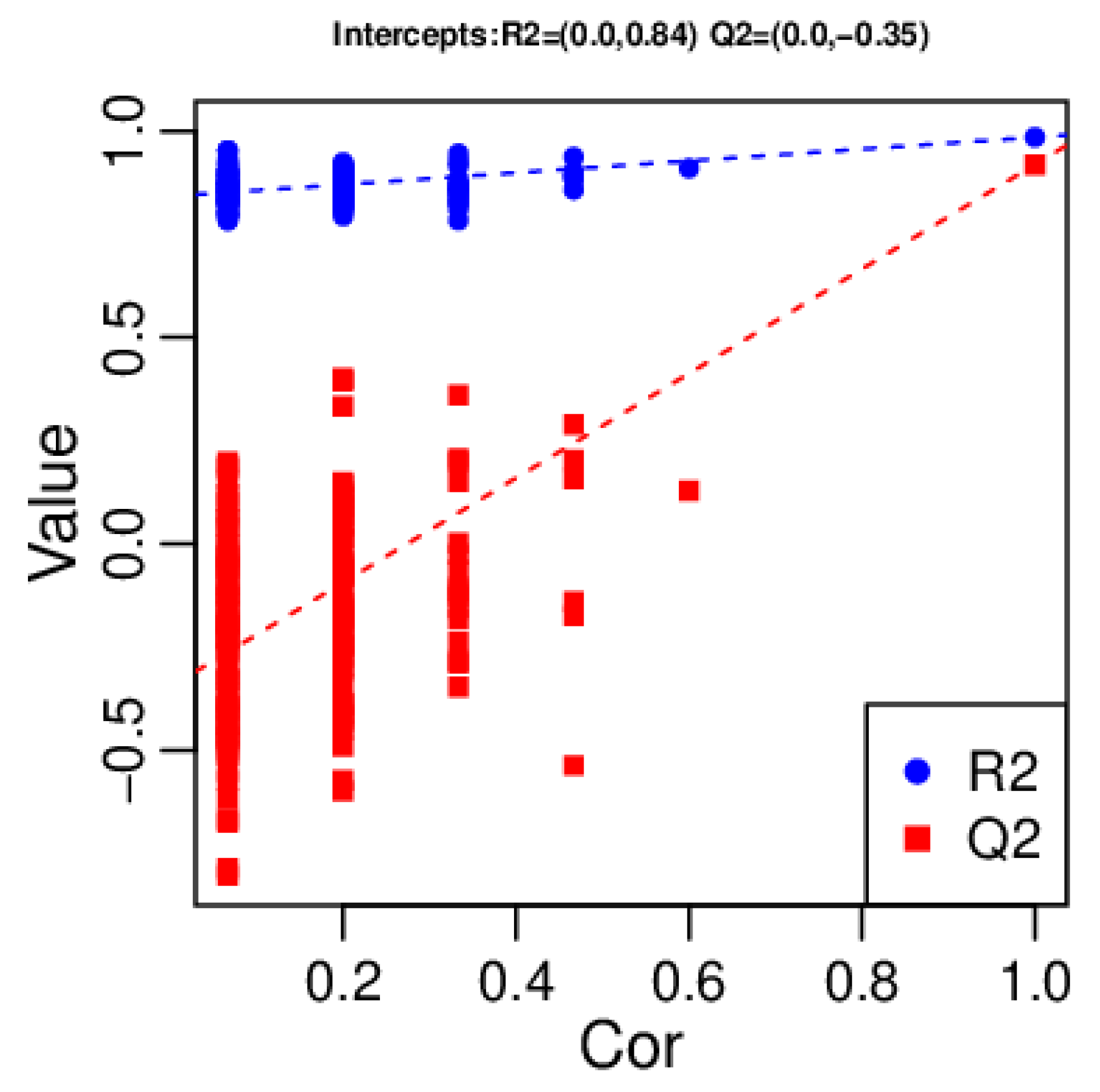
2.4. Multivariate Analysis of Identified Metabolites
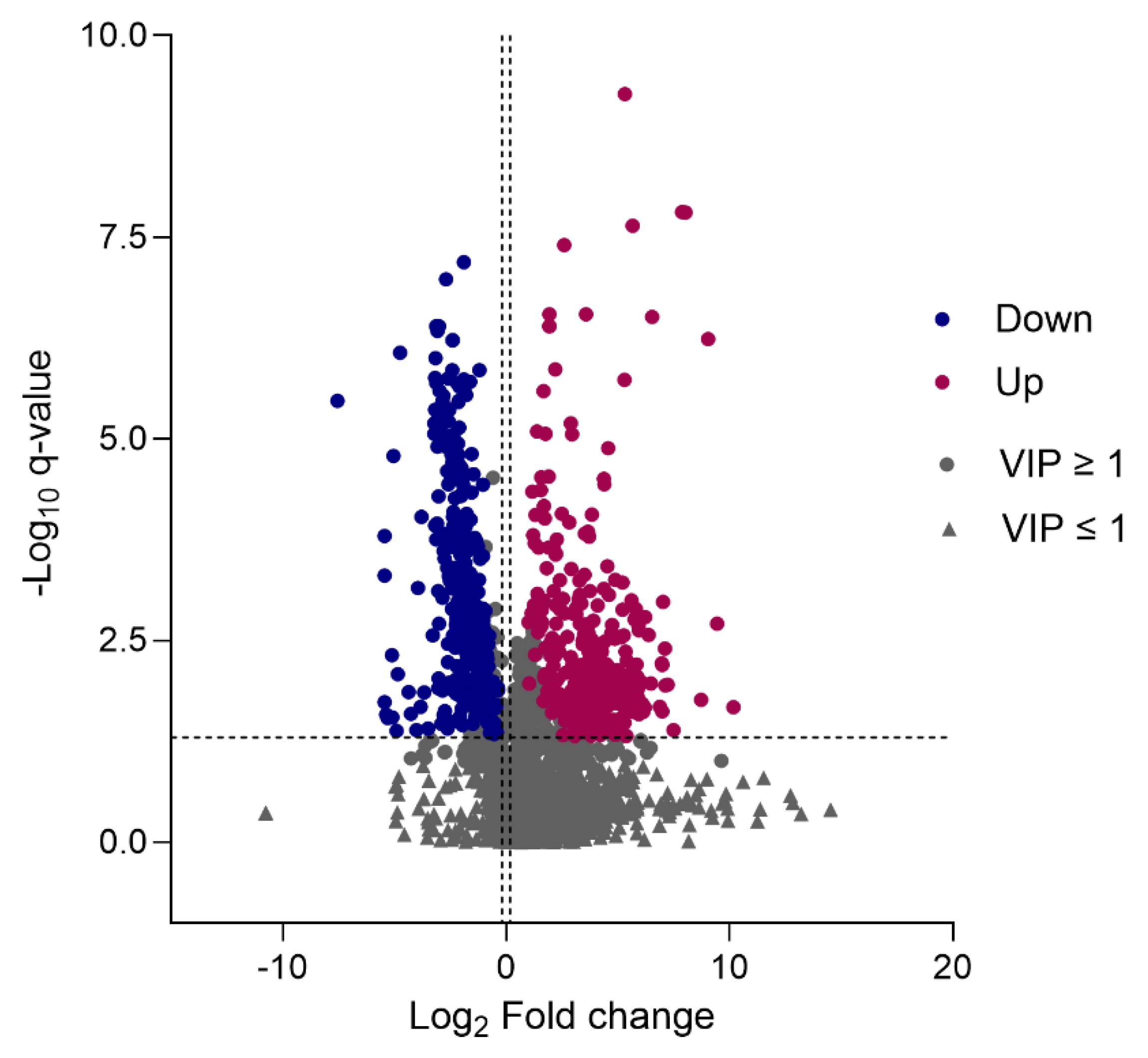
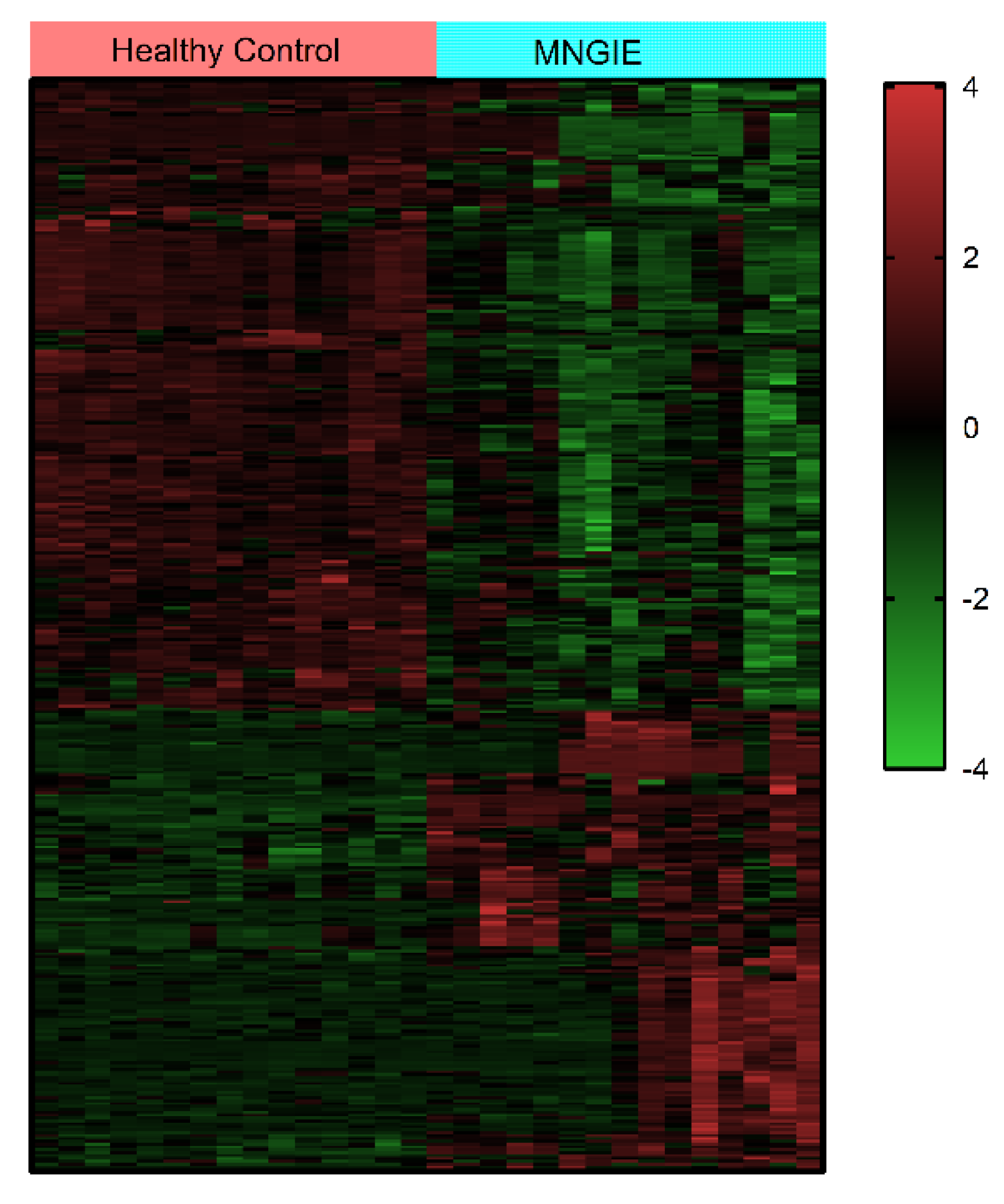
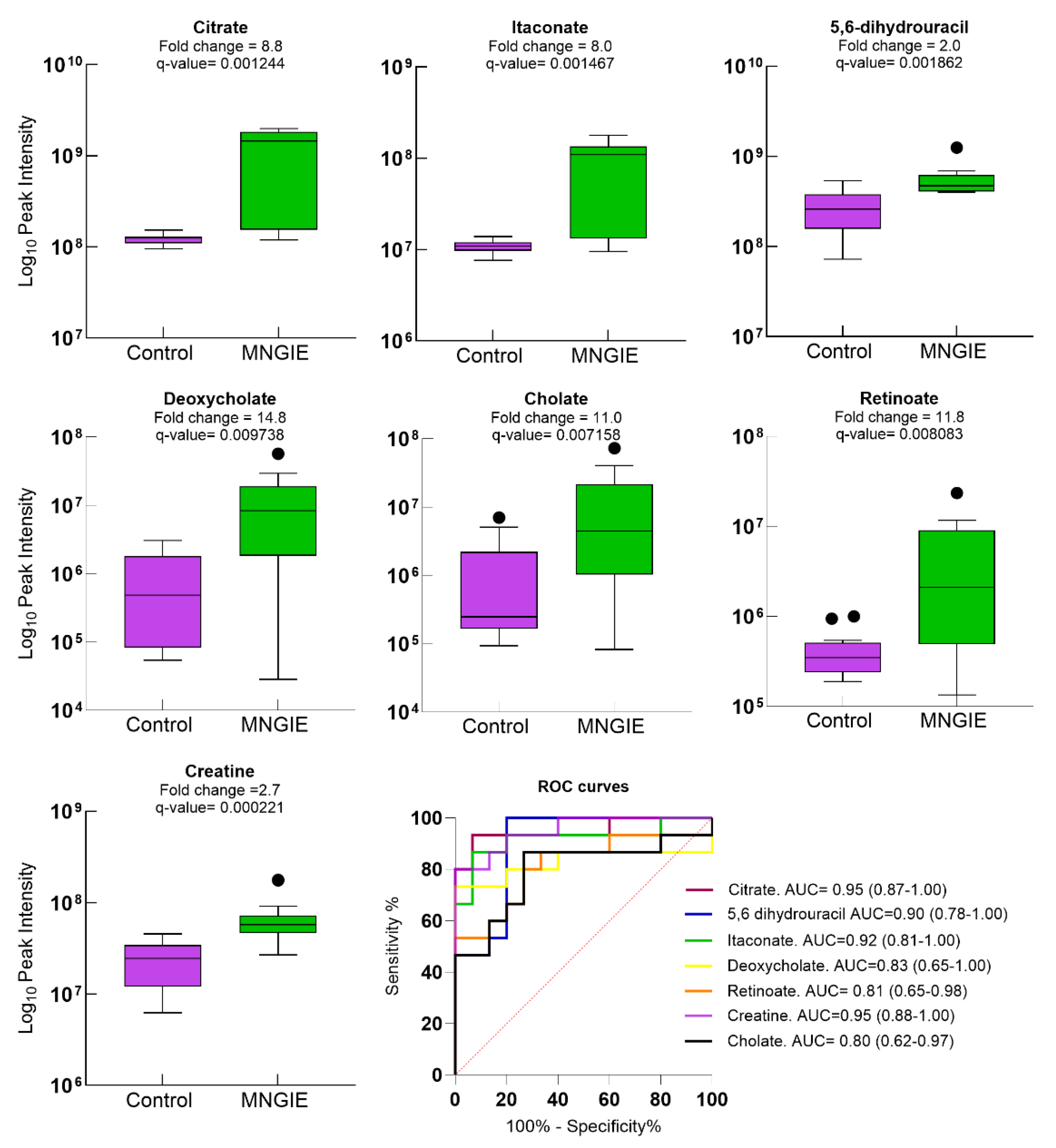
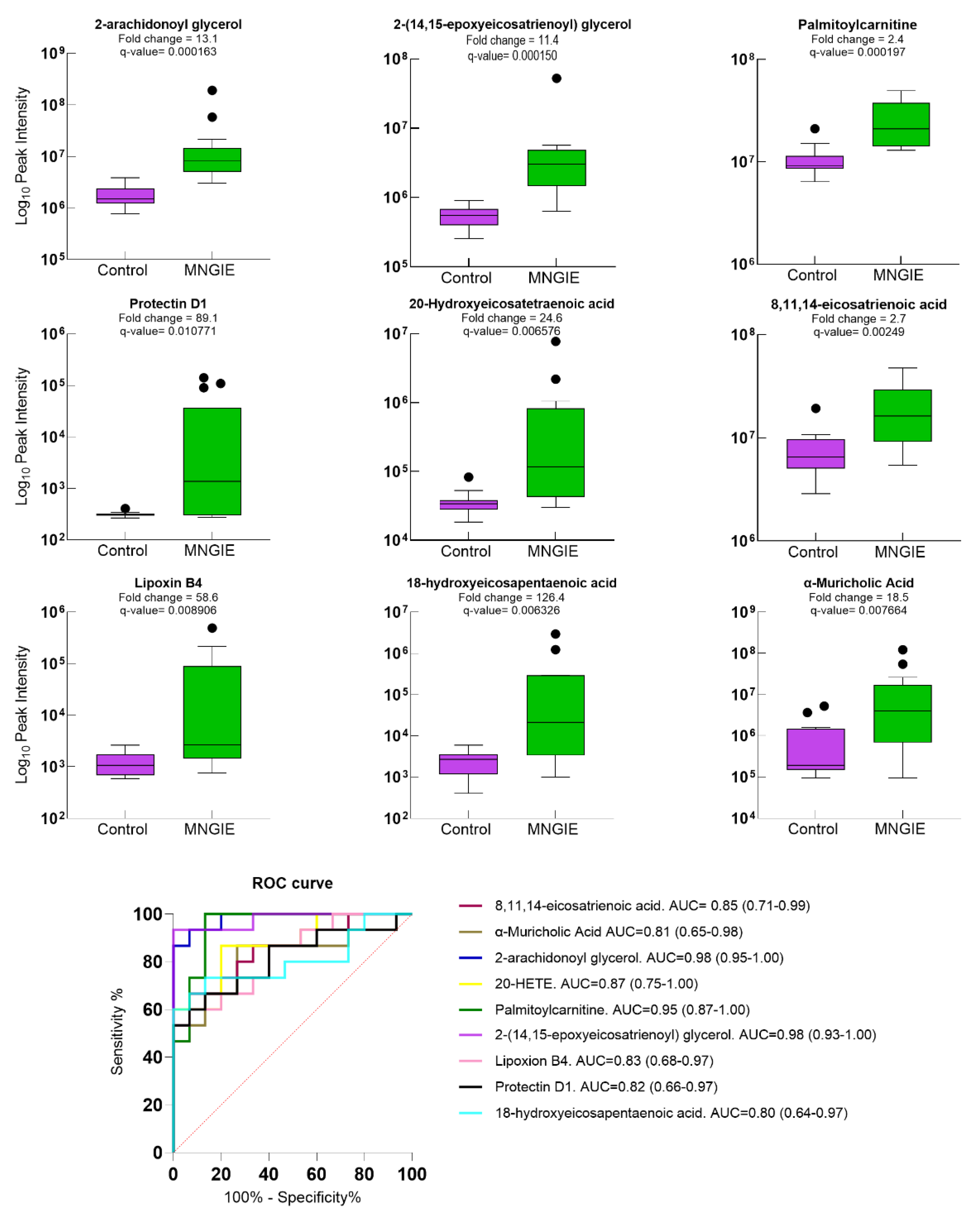
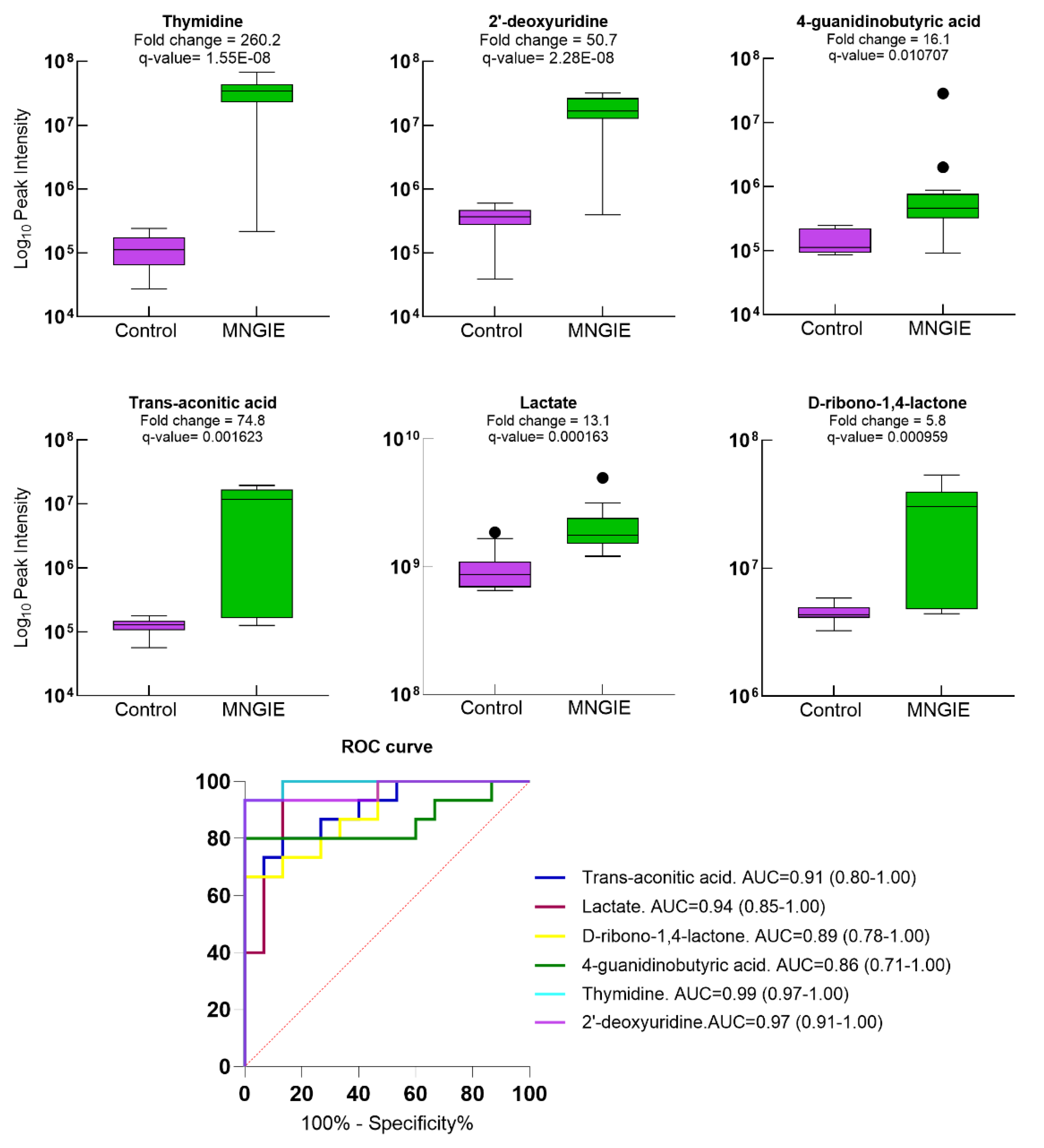
2.5. Differential Metabolite Identification
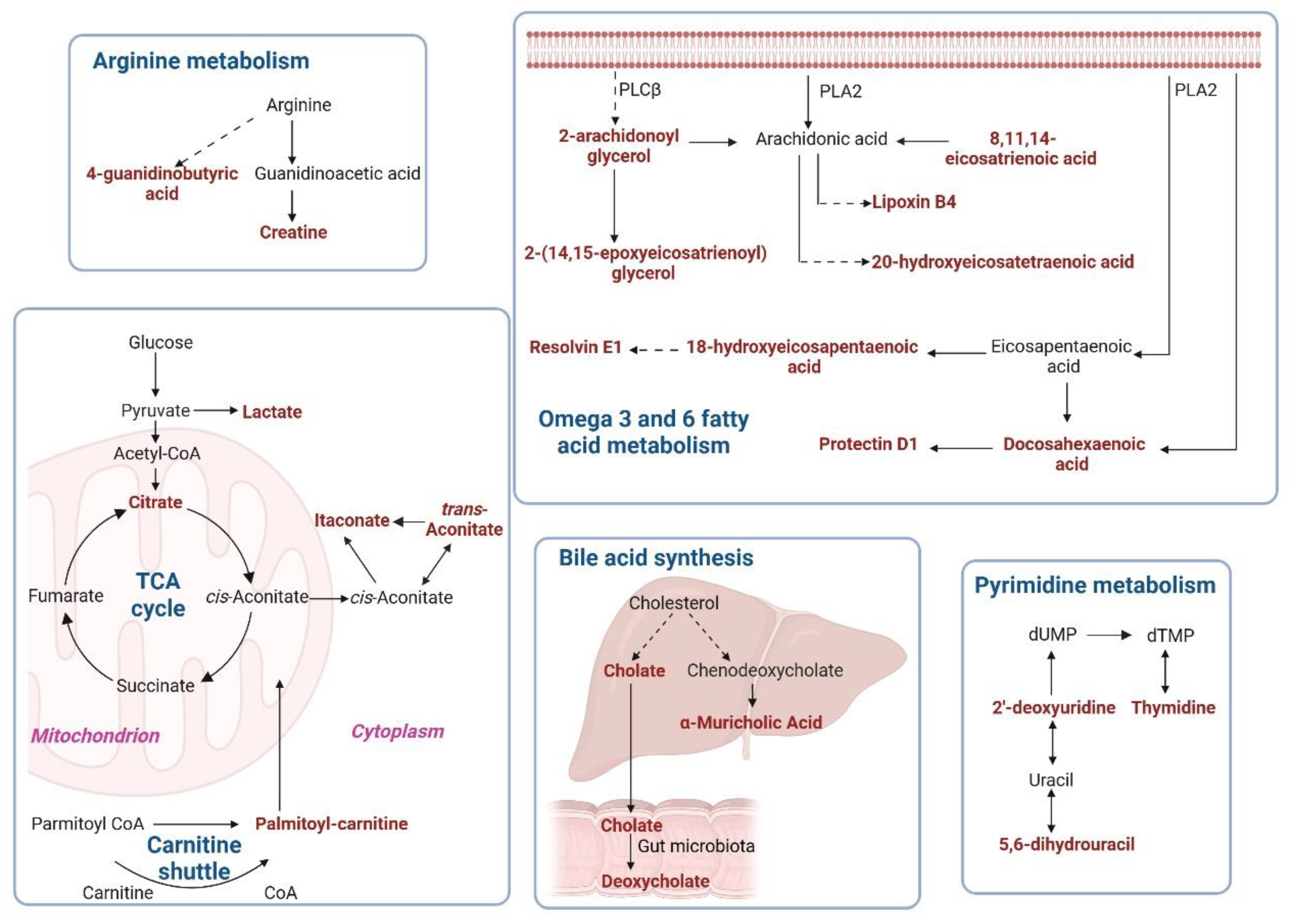
2.6. Metabolic Pathway Enrichment Analysis of Differential Metabolites
2.7. Discriminative Ability of Differential Plasma Metabolites for MNGIE Disease
3. Discussion
- Influence of the exposome: Metabolic profiles are significantly shaped by the exposome, which includes factors such as diet, dietary supplements, medicinal and recreational drugs, personal care products, and occupational exposures. Although known exposome-related metabolites were excluded from our dataset, we cannot entirely rule out the possibility that these exposures influenced the observed metabolic profiles. MNGIE patients typically receive a combination of medications, including analgesics, bowel motility stimulants, anti-emetics, antibiotics, and centrally acting agents tailored to their symptoms. In contrast, healthy controls may be exposed to different confounding factors. Notably, caffeine metabolism emerged as the most significantly downregulated pathway in MNGIE patients. Caffeine is metabolized via demethylation and/or hydroxylation into paraxanthine, theobromine, theophylline, and 1,3,7-trimethyluric acid, all of which were significantly reduced in our patient cohort. This suggests that the observed differences may reflect higher caffeine intake among healthy individuals.
- Sample size constraints: Due to the limited number of MNGIE patients included in this study, we were unable to assess the potential impact of disease progression on the metabolic phenotype. Longitudinal studies are needed to evaluate metabolome dynamics over the course of disease and in response to therapeutic interventions.
- Lack of targeted metabolite validation: The findings from our untargeted metabolomics analysis were not complemented by targeted metabolite quantification. Such validation will be essential to confirm the diagnostic relevance of the 23 identified biomarkers.
- Analytical scope limitations: The chromatography and mass spectrometry parameters used in this study were optimized for non-polar and medium-polar small molecules. Consequently, metabolic disturbances involving highly polar or strongly hydrophilic compounds would not have been captured.
- Annotation challenges: Chemical entities assigned to credibility levels 3 to 5 could not be further analyzed due to current limitations in metabolite classification and annotation databases.
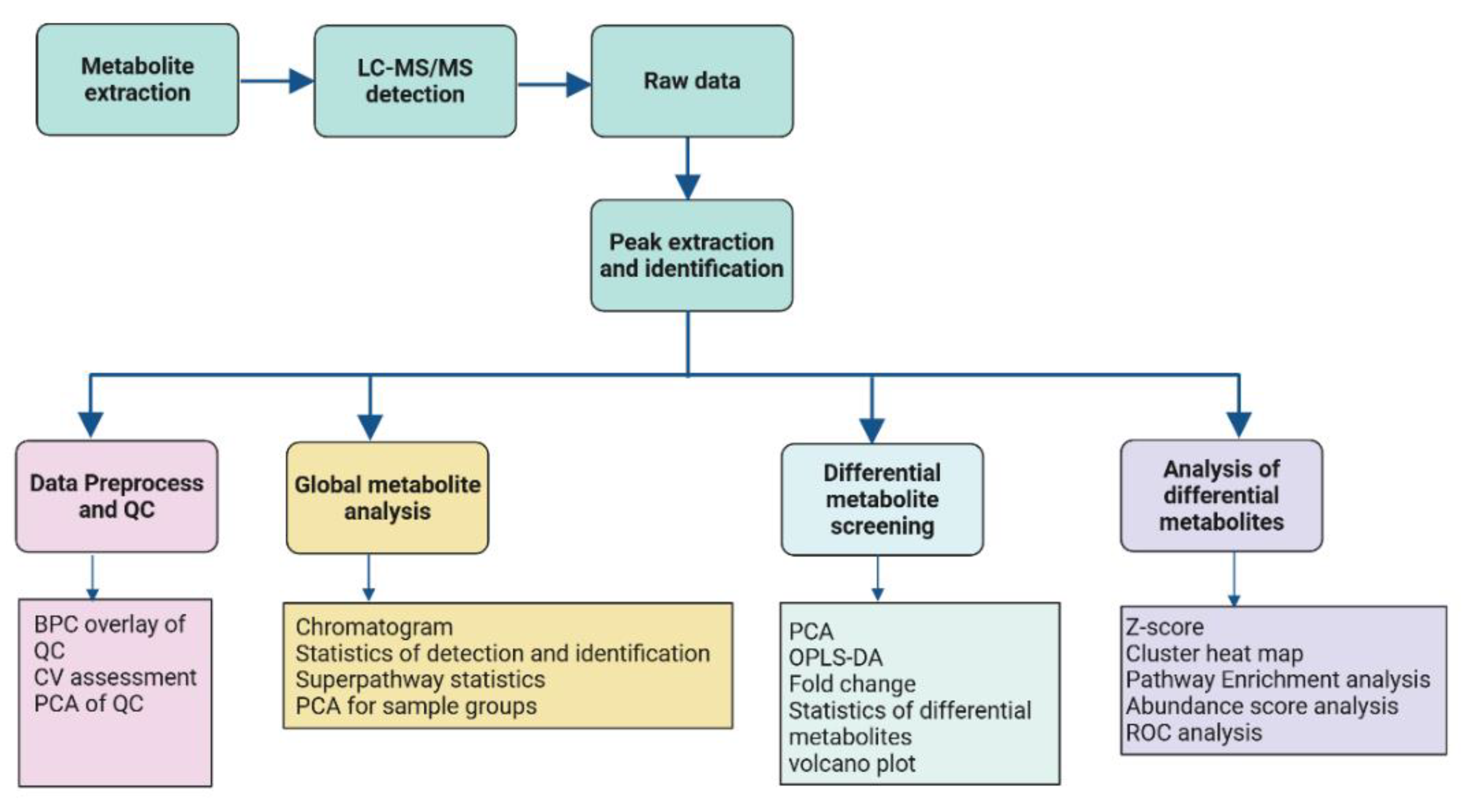
4. Materials and Methods
4.1. Study Participants
4.2. Blood Collection
4.3. Untargeted Metabolomic Profiling
4.3.1. Metabolite Extraction
4.3.2. UPLC-MS Analysis
4.3.3. Metabolite Ion Peak Extraction and Metabolite Identification
4.3.4. Data Preprocessing
4.3.5. Data QC
4.3.6. Classification and Functional Annotation of Detected Metabolites
4.3.7. Statistical Analyses
4.3.8. Bioinformatics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hirano, M.; Silvestri, G.; Blake, D.M.; Lombes, A.; Minetti, C.; Bonilla, E.; Hays, A.P.; Lovelace, R.E.; Butler, I.; Bertorini, T.E.; et al. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Clinical, Biochemical, and Genetic Features of an Autosomal Recessive Mitochondrial Disorder. Neurology 1994, 44, 721–727. [Google Scholar] [CrossRef] [PubMed]
- Nishino, I.; Spinazzola, A.; Hirano, M. Thymidine Phosphorylase Gene Mutations in MNGIE, a Human Mitochondrial Disorder. Science (1979) 1999, 283, 689–692. [Google Scholar] [CrossRef]
- Nishino, I.; Spinazzola, A.; Hirano, M. MNGIE: From Nuclear DNA to Mitochondrial DNA. Neuromuscul Disord 2001, 11, 7–10. [Google Scholar] [CrossRef]
- Hirano, M.; Nishigaki, Y.; Martí, R. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): A Disease of Two Genomes. Neurologist 2004, 10, 8–17. [Google Scholar] [CrossRef]
- Nishigaki, Y.; Martí, R.; Copeland, W.C.; Hirano, M. Site-Specific Somatic Mitochondrial DNA Point Mutations in Patients with Thymidine Phosphorylase Deficiency. Journal of Clinical Investigation 2003, 111, 1913–1921. [Google Scholar] [CrossRef]
- Garone, C.; Tadesse, S.; Hirano, M. Clinical and Genetic Spectrum of Mitochondrial Neurogastrointestinal Encephalomyopathy. Brain 2011, 134, 3326–3332. [Google Scholar] [CrossRef]
- Pacitti, D.; Levene, M.; Garone, C.; Nirmalananthan, N.; Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Into the Fourth Decade, What We Have Learned So Far. Front Genet 2018, 9. [Google Scholar] [CrossRef]
- Hirano, M. Mitochondrial Neurogastrointestinal Encephalopathy Disease. 1993. [Google Scholar]
- Debouverie M; Wagner M; Ducrocq X; Grignon Y; Mousson B; Weber M MNGIE Syndrome in 2 Siblings. Rev Neurol (Paris) 1997, 153, 547–553.
- Barış, Z.; Eminoğlu, T.; Dalgıç, B.; Tümer, L.; Hasanoğlu, A. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Case Report with a New Mutation. Eur J Pediatr 2010, 169, 1375–1378. [Google Scholar] [CrossRef] [PubMed]
- Cardaioli, E.; Da Pozzo, P.; Malfatti, E.; Battisti, C.; Gallus, G.N.; Gaudiano, C.; MacUcci, M.; Malandrini, A.; Margollicci, M.; Rubegni, A.; et al. A Second MNGIE Patient without Typical Mitochondrial Skeletal Muscle Involvement. Neurological Sciences 2010, 31, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.A.; Schlesser, P.; Smeets, H.J.M.; Francois, B.; Bierau, J. Biochemical Abnormalities in a Patient with Thymidine Phosphorylase Deficiency with Fatal Outcome. J Inherit Metab Dis 2010, 33. [Google Scholar] [CrossRef] [PubMed]
- Vondráčková, A.; Veselá, K.; Kratochvílová, H.; Kučerová Vidrová, V.; Vinšová, K.; Stránecký, V.; Honzík, T.; Hansíková, H.; Zeman, J.; Tesařová, M. Large Copy Number Variations in Combination with Point Mutations in the TYMP and SCO2 Genes Found in Two Patients with Mitochondrial Disorders. European Journal of Human Genetics 2014, 22, 431–434. [Google Scholar] [CrossRef]
- Peedikayil, M.C.; Kagevi, E.I.; Abufarhaneh, E.; Alsayed, M.D.; Alzahrani, H.A. Mitochondrial Neurogastrointestinal Encephalomyopathy Treated with Stem Cell Transplantation: A Case Report and Review of Literature. Hematology/ Oncology and Stem Cell Therapy 2015, 8, 85–90. [Google Scholar] [CrossRef]
- Du, J.; Zhang, C.; Liu, F.; Liu, X.; Wang, D.; Zhao, D.; Shui, G.; Zhao, Y.; Yan, C. Distinctive Metabolic Remodeling in TYMP Deficiency beyond Mitochondrial Dysfunction. J Mol Med 2023, 101, 1237–1253. [Google Scholar] [CrossRef]
- Hakimi, A.A.; Reznik, E.; Lee, C.-H.; Creighton, C.J.; Brannon, A.R.; Luna, A.; Aksoy, B.A.; Liu, E.M.; Shen, R.; Lee, W.; et al. An Integrated Metabolic Atlas of Clear Cell Renal Cell Carcinoma. Cancer Cell 2016, 29, 104–116. [Google Scholar] [CrossRef]
- Buzkova, J.; Nikkanen, J.; Ahola, S.; Hakonen, A.H.; Sevastianova, K.; Hovinen, T.; Yki-Järvinen, H.; Pietiläinen, K.H.; Lönnqvist, T.; Velagapudi, V.; et al. Metabolomes of Mitochondrial Diseases and Inclusion Body Myositis Patients: Treatment Targets and Biomarkers. EMBO Mol Med 2018, 10. [Google Scholar] [CrossRef]
- Sharma, R.; Reinstadler, B.; Engelstad, K.; Skinner, O.S.; Stackowitz, E.; Haller, R.G.; Clish, C.B.; Pierce, K.; Walker, M.A.; Fryer, R.; et al. Circulating Markers of NADH-Reductive Stress Correlate with Mitochondrial Disease Severity. J Clin Invest 2021, 131. [Google Scholar] [CrossRef]
- Shaham, O.; Slate, N.G.; Goldberger, O.; Xu, Q.; Ramanathan, A.; Souza, A.L.; Clish, C.B.; Sims, K.B.; Mootha, V.K. A Plasma Signature of Human Mitochondrial Disease Revealed through Metabolic Profiling of Spent Media from Cultured Muscle Cells. Proc Natl Acad Sci U S A 2010, 107, 1571–1575. [Google Scholar] [CrossRef] [PubMed]
- Maresca, A.; Del Dotto, V.; Romagnoli, M.; La Morgia, C.; Di Vito, L.; Capristo, M.; Valentino, M.L.; Carelli, V. ; ER-MITO Study Group Expanding and Validating the Biomarkers for Mitochondrial Diseases. J Mol Med (Berl) 2020, 98, 1467–1478. [Google Scholar] [CrossRef]
- Barisic, N.; Bernert, G.; Ipsiroglu, O.; Stromberger, C.; Müller, T.; Gruber, S.; Prayer, D.; Moser, E.; Bittner, R.E.; Stöckler-Ipsiroglu, S. Effects of Oral Creatine Supplementation in a Patient with MELAS Phenotype and Associated Nephropathy. Neuropediatrics 2002, 33, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Borchert, A.; Wilichowski, E.; Hanefeld, F. Supplementation with Creatine Monohydrate in Children with Mitochondrial Encephalomyopathies. Muscle Nerve 1999, 22, 1299–1300. [Google Scholar] [CrossRef]
- Tarnopolsky MA; Roy BD; MacDonald JR A Randomized, Controlled Trial of Creatine Monohydrate in Patients with Mitochondrial Cytopathies. Muscle Nerve 1997, 20, 1502–1509. [CrossRef]
- Komura, K.; Hobbiebrunken, E.; Wilichowski, E.K.G.; Hanefeld, F.A. Effectiveness of Creatine Monohydrate in Mitochondrial Encephalomyopathies. Pediatr Neurol 2003, 28, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Klopstock, T.; Querner, V.; Schmidt, F.; Gekeler, F.; Walter, M.; Hartard, M.; Henning, M.; Gasser, T.; Pongratz, D.; Straube, A.; et al. A Placebo-Controlled Crossover Trial of Creatine in Mitochondrial Diseases. Neurology 2000, 55, 1748–1751. [Google Scholar] [CrossRef] [PubMed]
- Kornblum, C.; Schröder, R.; Müller, K.; Vorgerd, M.; Eggers, J.; Bogdanow, M.; Papassotiropoulos, A.; Fabian, K.; Klockgether, T.; Zange, J. Creatine Has No Beneficial Effect on Skeletal Muscle Energy Metabolism in Patients with Single Mitochondrial DNA Deletions: A Placebo-Controlled, Double-Blind 31P-MRS Crossover Study. Eur J Neurol 2005, 12, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Farr, C. V; Xiao, Y.; El-Kasaby, A.; Schupp, M.; Hotka, M.; di Mauro, G.; Clarke, A.; Pastor Fernandez, M.; Sandtner, W.; Stockner, T.; et al. Probing the Chemical Space of Guanidino-Carboxylic Acids to Identify the First Blockers of the Creatine-Transporter-1. Mol Pharmacol 2024, 106, 319–333. [Google Scholar] [CrossRef]
- Hirano, M.; Carelli, V.; De Giorgio, R.; Pironi, L.; Accarino, A.; Cenacchi, G.; D’Alessandro, R.; Filosto, M.; Martí, R.; Nonino, F.; et al. Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE): Position Paper on Diagnosis, Prognosis, and Treatment by the MNGIE International Network. J Inherit Metab Dis 2021, 44, 376–387. [Google Scholar] [CrossRef]
- Patel, J.; Agasti, A.; Vashishtha, C.; Samarth, A.; Goyal, R.; Oak, P.J.; Sawant, P. An Interesting Case of Intestinal Pseudo-Obstruction: MNGIE. Trop Gastroenterol 2011, 32, 138–141. [Google Scholar] [CrossRef]
- Li, Y.; Chvatal-Medina, M.; Trillos-Almanza, M.C.; Bourgonje, A.R.; Connelly, M.A.; Moshage, H.; Bakker, S.J.L.; de Meijer, V.E.; Blokzijl, H.; Dullaart, R.P.F.; et al. Circulating Citrate Is Reversibly Elevated in Patients with End-Stage Liver Disease: Association with All-Cause Mortality. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Tong, W.; Hannou, S.A.; Wang, Y.; Astapova, I.; Sargsyan, A.; Monn, R.; Thiriveedi, V.; Li, D.; McCann, J.R.; Rawls, J.F.; et al. The Intestine Is a Major Contributor to Circulating Succinate in Mice. FASEB J 2022, 36, e22546. [Google Scholar] [CrossRef]
- Duong, N.; Bajaj, J.S. The Impact of the Gut Microbiome on Liver Transplantation. Curr Opin Organ Transplant 2021, 26, 587–594. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, F.; Lu, H.; Wang, B.; Chen, Y.; Lei, D.; Wang, Y.; Zhu, B.; Li, L. Characterization of Fecal Microbial Communities in Patients with Liver Cirrhosis. Hepatology 2011, 54, 562–572. [Google Scholar] [CrossRef]
- Peace, C.G.; O’Neill, L.A. The Role of Itaconate in Host Defense and Inflammation. J Clin Invest 2022, 132. [Google Scholar] [CrossRef]
- Yang, W.; Wang, Y.; Tao, K.; Li, R. Metabolite Itaconate in Host Immunoregulation and Defense. Cell Mol Biol Lett 2023, 28, 100. [Google Scholar] [CrossRef]
- Coelho, C. Itaconate or How I Learned to Stop Avoiding the Study of Immunometabolism. PLoS Pathog 2022, 18, e1010361. [Google Scholar] [CrossRef]
- Weiss, J.M.; Palmieri, E.M.; Gonzalez-Cotto, M.; Bettencourt, I.A.; Megill, E.L.; Snyder, N.W.; McVicar, D.W. Itaconic Acid Underpins Hepatocyte Lipid Metabolism in Non-Alcoholic Fatty Liver Disease in Male Mice. Nat Metab 2023, 5, 981–995. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, A.; Botta, E.; Holinstat, M. Eicosanoids in Inflammation in the Blood and the Vessel. Front Pharmacol 2022, 13, 997403. [Google Scholar] [CrossRef] [PubMed]
- Artru, F.; McPhail, M.J.W.; Triantafyllou, E.; Trovato, F.M. Lipids in Liver Failure Syndromes: A Focus on Eicosanoids, Specialized Pro-Resolving Lipid Mediators and Lysophospholipids. Front Immunol 2022, 13, 867261. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, W.; Wang, F.; Wu, D.; Qian, J.; Kang, J.; Li, H.; Ma, E. Nutrition Therapy for Mitochondrial Neurogastrointestinal Encephalopathy with Homozygous Mutation of the TYMP Gene. Clin Nutr Res 2015, 4, 132–136. [Google Scholar] [CrossRef]
- Barisic, A.; Ljubas Kelecic, D.; Vranesic Bender, D.; Karas, I.; Brinar, M.; Miletic, V.; Krznaric, Z. Case Report: A Patient with Mitochondrial Neurogastrointestinal Encephalomyopathy and Chronic Intestinal Failure. Front Nutr 2022, 9, 983873. [Google Scholar] [CrossRef]
- Kasti, A.; Nikolaki, M.; Pyrousis, I.; Synodinou, K.; Oikonomopoulos, N.; Triantafyllou, K. Intensive Nutrition Support May Benefit Patients with a Rare Mitochondrial Disorder. Nutr Clin Pract 2022, 37, 361–365. [Google Scholar] [CrossRef]
- Kartal T; Uzunoğlu S; Kaplan I; Bulut FD; Derya K; Mungan N Nutritional Management in MNGIE Disease: A Case Report. Clin Sci Nutr 2025, 7, 63–67. [CrossRef]
- Al-Shaer, A.E.; Buddenbaum, N.; Shaikh, S.R. Polyunsaturated Fatty Acids, Specialized pro-Resolving Mediators, and Targeting Inflammation Resolution in the Age of Precision Nutrition. Biochim Biophys Acta Mol Cell Biol Lipids 2021, 1866, 158936. [Google Scholar] [CrossRef]
- Borowska, M.; Czarnywojtek, A.; Sawicka-Gutaj, N.; Woliński, K.; Płazińska, M.T.; Mikołajczak, P.; Ruchała, M. The Effects of Cannabinoids on the Endocrine System. Endokrynol Pol 2018, 69, 705–719. [Google Scholar] [CrossRef]
- Westerbacka, J.; Kotronen, A.; Fielding, B.A.; Wahren, J.; Hodson, L.; Perttilä, J.; Seppänen-Laakso, T.; Suortti, T.; Arola, J.; Hultcrantz, R.; et al. Splanchnic Balance of Free Fatty Acids, Endocannabinoids, and Lipids in Subjects with Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 1961–1971.e1. [Google Scholar] [CrossRef] [PubMed]
- Kripps, K.; Nakayuenyongsuk, W.; Shayota, B.J.; Berquist, W.; Gomez-Ospina, N.; Esquivel, C.O.; Concepcion, W.; Sampson, J.B.; Cristin, D.J.; Jackson, W.E.; et al. Successful Liver Transplantation in Mitochondrial Neurogastrointestinal Encephalomyopathy (MNGIE). Mol Genet Metab 2020, 130, 58–64. [Google Scholar] [CrossRef]
- Bax, B.E. Mitochondrial Neurogastrointestinal Encephalomyopathy: Approaches to Diagnosis and Treatment. J Transl Genet Genom 2020, 4, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tuchman, M.; Ramnaraine, M.L.; O’Dea, R.F. Effects of Uridine and Thymidine on the Degradation of 5-Fluorouracil, Uracil, and Thymine by Rat Liver Dihydropyrimidine Dehydrogenase. Cancer Res 1985, 45, 5553–5556. [Google Scholar] [CrossRef]
- Henricks, L.M.; Jacobs, B.A.W.; Meulendijks, D.; Pluim, D.; van den Broek, D.; de Vries, N.; Rosing, H.; Beijnen, J.H.; Huitema, A.D.R.; Guchelaar, H.-J.; et al. Food-Effect Study on Uracil and Dihydrouracil Plasma Levels as Marker for Dihydropyrimidine Dehydrogenase Activity in Human Volunteers. Br J Clin Pharmacol 2018, 84, 2761–2769. [Google Scholar] [CrossRef] [PubMed]
- Šarenac, T.M.; Mikov, M. Bile Acid Synthesis: From Nature to the Chemical Modification and Synthesis and Their Applications as Drugs and Nutrients. Front Pharmacol 2018, 9, 939. [Google Scholar] [CrossRef]
- Dumaswala, R.; Setchell, K.D.; Zimmer-Nechemias, L.; Iida, T.; Goto, J.; Nambara, T. Identification of 3 Alpha,4 Beta,7 Alpha-Trihydroxy-5 Beta-Cholanoic Acid in Human Bile: Reflection of a New Pathway in Bile Acid Metabolism in Humans. J Lipid Res 1989, 30, 847–856. [Google Scholar] [CrossRef]
- Cui, Y.; Xu, B.; Zhang, X.; He, Y.; Shao, Y.; Ding, M. Diagnostic and Therapeutic Profiles of Serum Bile Acids in Women with Intrahepatic Cholestasis of Pregnancy-a Pseudo-Targeted Metabolomics Study. Clin Chim Acta 2018, 483, 135–141. [Google Scholar] [CrossRef]
- Sacquet, E.C.; Gadelle, D.P.; Riottot, M.J.; Raibaud, P.M. Absence of Transformation of Beta-Muricholic Acid by Human Microflora Implanted in the Digestive Tracts of Germfree Male Rats. Appl Environ Microbiol 1984, 47, 1167–1168. [Google Scholar] [CrossRef]
- Sagawa, H.; Tazuma, S.; Kajiyama, G. Protection against Hydrophobic Bile Salt-Induced Cell Membrane Damage by Liposomes and Hydrophilic Bile Salts. Am J Physiol 1993, 264, G835–9. [Google Scholar] [CrossRef]
- Masclee, A.; Tangerman, A.; van Schaik, A.; van der Hoek, E.W.; van Tongeren, J.H. Unconjugated Serum Bile Acids as a Marker of Small Intestinal Bacterial Overgrowth. Eur J Clin Invest 1989, 19, 384–389. [Google Scholar] [CrossRef]
- Smirnova, E.; Muthiah, M.D.; Narayan, N.; Siddiqui, M.S.; Puri, P.; Luketic, V.A.; Contos, M.J.; Idowu, M.; Chuang, J.-C.; Billin, A.N.; et al. Metabolic Reprogramming of the Intestinal Microbiome with Functional Bile Acid Changes Underlie the Development of NAFLD. Hepatology 2022, 76, 1811–1824. [Google Scholar] [CrossRef]
- Midzak, A.S.; Chen, H.; Aon, M.A.; Papadopoulos, V.; Zirkin, B.R. ATP Synthesis, Mitochondrial Function, and Steroid Biosynthesis in Rodent Primary and Tumor Leydig Cells. Biol Reprod 2011, 84, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.C.; Noy, N. All-Trans-Retinoic Acid Represses Obesity and Insulin Resistance by Activating Both Peroxisome Proliferation-Activated Receptor Beta/Delta and Retinoic Acid Receptor. Mol Cell Biol 2009, 29, 3286–3296. [Google Scholar] [CrossRef] [PubMed]
- Mercader, J.; Ribot, J.; Murano, I.; Felipe, F.; Cinti, S.; Bonet, M.L.; Palou, A. Remodeling of White Adipose Tissue after Retinoic Acid Administration in Mice. Endocrinology 2006, 147, 5325–5332. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Sriram, S.; Chan, X.H.D.; Ong, W.K.; Yeo, C.R.; Tan, B.; Lee, S.-A.; Kong, K.V.; Hoon, S.; Jiang, H.; et al. Retinoic Acid Mediates Visceral-Specific Adipogenic Defects of Human Adipose-Derived Stem Cells. Diabetes 2016, 65, 1164–1178. [Google Scholar] [CrossRef]
- Gautheron, J.; Lima, L.; Akinci, B.; Zammouri, J.; Auclair, M.; Ucar, S.K.; Ozen, S.; Altay, C.; Bax, B.E.; Nemazanyy, I.; et al. Loss of Thymidine Phosphorylase Activity Disrupts Adipocyte Differentiation and Induces Insulin-Resistant Lipoatrophic Diabetes. BMC Med 2022, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. MetaX: A Flexible and Comprehensive Software for Processing Metabolomics Data. BMC Bioinformatics 2017, 18, 183. [Google Scholar] [CrossRef]
- Di Guida, R.; Engel, J.; Allwood, J.W.; Weber, R.J.M.; Jones, M.R.; Sommer, U.; Viant, M.R.; Dunn, W.B. Non-Targeted UHPLC-MS Metabolomic Data Processing Methods: A Comparative Investigation of Normalisation, Missing Value Imputation, Transformation and Scaling. Metabolomics 2016, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. Procedures for Large-Scale Metabolic Profiling of Serum and Plasma Using Gas Chromatography and Liquid Chromatography Coupled to Mass Spectrometry. Nat Protoc 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
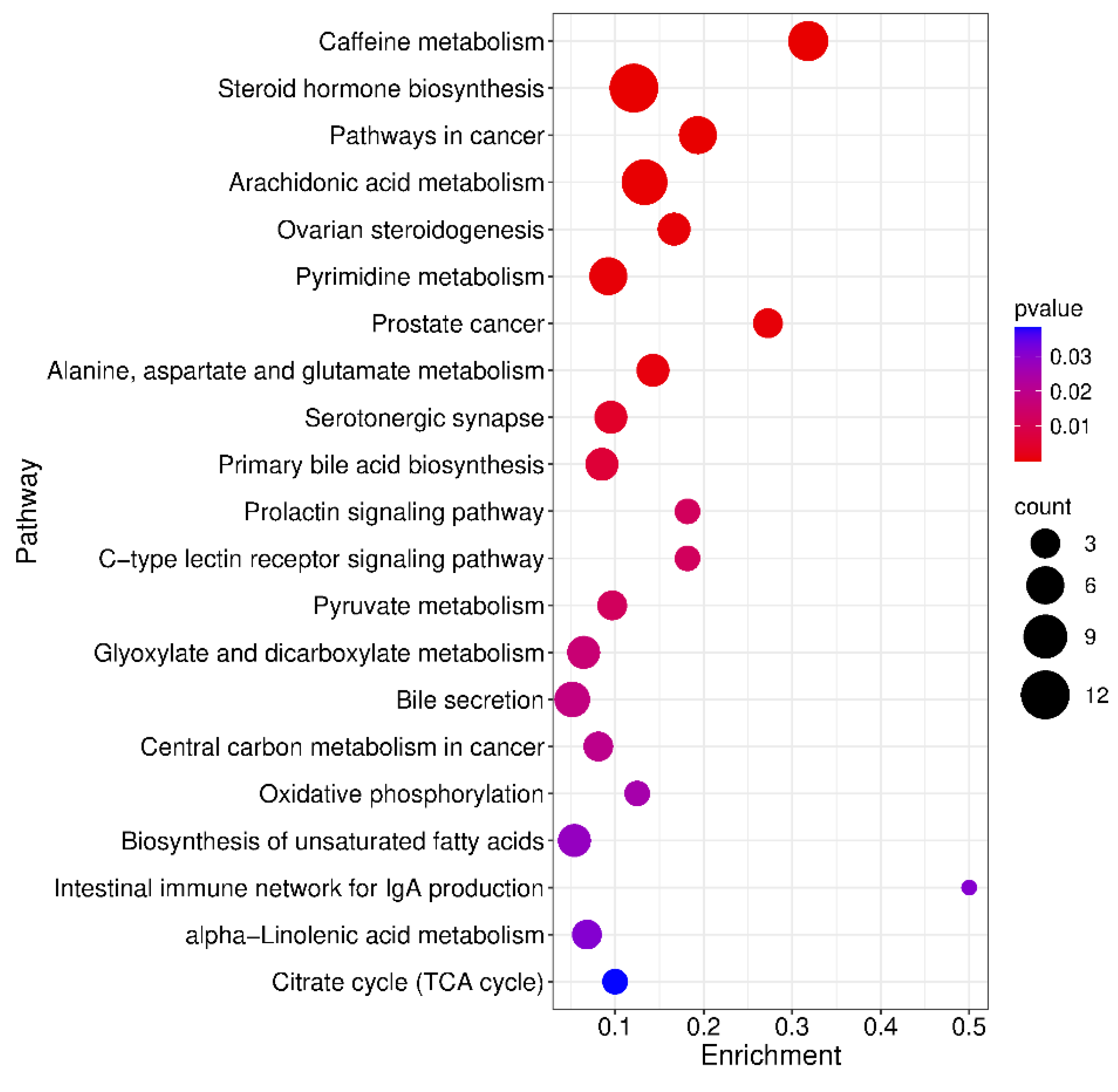
| KEGG Pathway | p value | KEGG Names | KEGG Identification | Differential abundance score and pathway regulation status |
|---|---|---|---|---|
| Caffeine metabolism map 00232 | 2.922E-08 | 7-methylxanthine;3-methylxanthine; Theobromine; Paraxanthine; Theophylline; 1-Methyluric acid;1,3,7-trimethyluric acid | C16353+C16357+C07480+C13747+ C07130+C16359+C16361 | -1 Down |
| Steroid hormone biosynthesis Map 00140 | 4.579E-08 | 5α-pregnan-3,20-dione;17α-hydroxyprogesterone; Androsterone; Androsterone glucuronide; Dehydroepiandrosterone ; Aldosterone; Etiocholanolone; Estrone; 20-oxopregn-5-en-3-yl hydrogen sulfate; Tetrahydrocortisol; Androstenedione | C03681+C01176+C00523+C11135+ C01227+C01780+C04373+C00468+ C18044+C05472+C00280 | -0.8 Down |
| Pathways in cancer map 05200 | 7.263E-06 | Retinoate; Dehydroepiandrosterone (dhea); Prostaglandin e2; Fumaric acid; Androstanolone; Androstenedione | C00777+C01227+C00584+C00122+ C03917+C00280 | 0 Down |
| Arachidonic acid metabolism Map 00590 | 1.679E-4 | Lipoxin b4;11-dehydro thromboxane b2; Thromboxane b2; Prostaglandin e2; Prostaglandin a2; 20-hydroxy-(5z,8z,11z,14z)-eicosatetraenoic acid;15-keto prostaglandin f2α, 11,12-DHET, 15-(S)HPETE, 11,12-EET | C06315+C05964+C05963+C00584+ C05953+C14748+C05960+ C14774 + C05966 +C14770 |
0.8 Up |
| Ovarian steroidogenesis Map 04913 | 4.820E-4 | 17α-hydroxyprogesterone; Dehydroepiandrosterone (dhea); Estrone; Androstenedione, 11,12-EET | C01176+C01227+C00468+C00280+ C14770 | -1 Down |
| Pyrimidine metabolism map 00240 | 5.228E-4 | L-glutamine; 5,6-dihydrouracil; Uracil;2'-deoxyuridine; Thymine; Thymidine | C00064+C00429+C00106+C00526+ C00178+C00214 | 0.7 Up |
| Prostate cancer map 05215 | 5.652E-4 | Dehydroepiandrosterone (dhea); Androstanolone; Androstenedione | C01227+C03917+C00280 | -1 Down |
| Alanine, aspartate and glutamate metabolism map 00250 | 8.844E-4 | Citrate; L-glutamine; Fumaric acid; 2-keto-glutaramic acid | C00158+C00064+C00122+C00940 | 0 Down |
| Serotonergic synapse map 04726 | 4.076E-3 | 11-dehydro thromboxane b2; Thromboxane b2; Prostaglandin e2; Prostaglandin a2, 11,12-EET | C05964+C05963+C00584+C05953+ C14770 | 1 Up |
| Prolactin signaling pathway map04917 | 0.012158 | Estrone; Androstenedione | C00468+C00280 | -1 Down |
| C-type lectin receptor signaling pathway map 04625 | 0.012158 | Prostaglandin e2; Fucose | C00584 + C01019 | 1 Up |
| Pyruvate metabolism map 00620 | 0.012235 | Fumaric acid; S-lactoylglutathione;2-butynedioic acid | C00122 +C03451+ C03248 | 0.3 Up |
| Glyoxylate and dicarboxylate metabolism map 00630 | 0.015951 | Citrate; L-glutamine; 4-hydroxy-2-oxoglutaric acid;3-oxalomalic acid | C00158+C00064+C05946+C01990 | -0.5 Down |
| Bile secretion map 04976 | 0.017976 | Cholate; Deoxycholate; Thromboxane b2; Prostaglandin e2; Bilirubin | C00695+C04483+C05963+C00584+ C00486 | 0.6 Up |
| Central carbon metabolism in cancer map 05230 | 0.019754 | Citrate; L-glutamine; Fumaric acid | C00158+C00064+C00122 | 0.3 Up |
| Oxidative phosphorylation Ma p00190 | 0.025202 | Fumaric acid; Ubiquinol 10 | C00122+C11378 | 0 Down |
| Biosynthesis of unsaturated fatty acids ma p01040 | 0.028497 | Docosahexaenoic acid; Dihomo-gamma-linolenate; Eicosapentaenoate; Nervonic acid | C06429+C03242+C06428+C08323 | 0.8 Up |
| Intestinal immune network for IgA production map 04672 | 0.030961 | Retinoate | C00777 | 1 Up |
| alpha-Linolenic acid metabolism map00592 | 0.031107 | 13(s)-hotre; 12-oxo phytodienoic acid; 9(s)-hotre | C16316+C01226+C16326 | 0.3 Up |
| Citrate cycle (TCA cycle) map 00020 | 0.038311 | Citrate; Fumaric acid | C00158+C00122 | 0.5 Up |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





