Submitted:
27 August 2025
Posted:
01 September 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
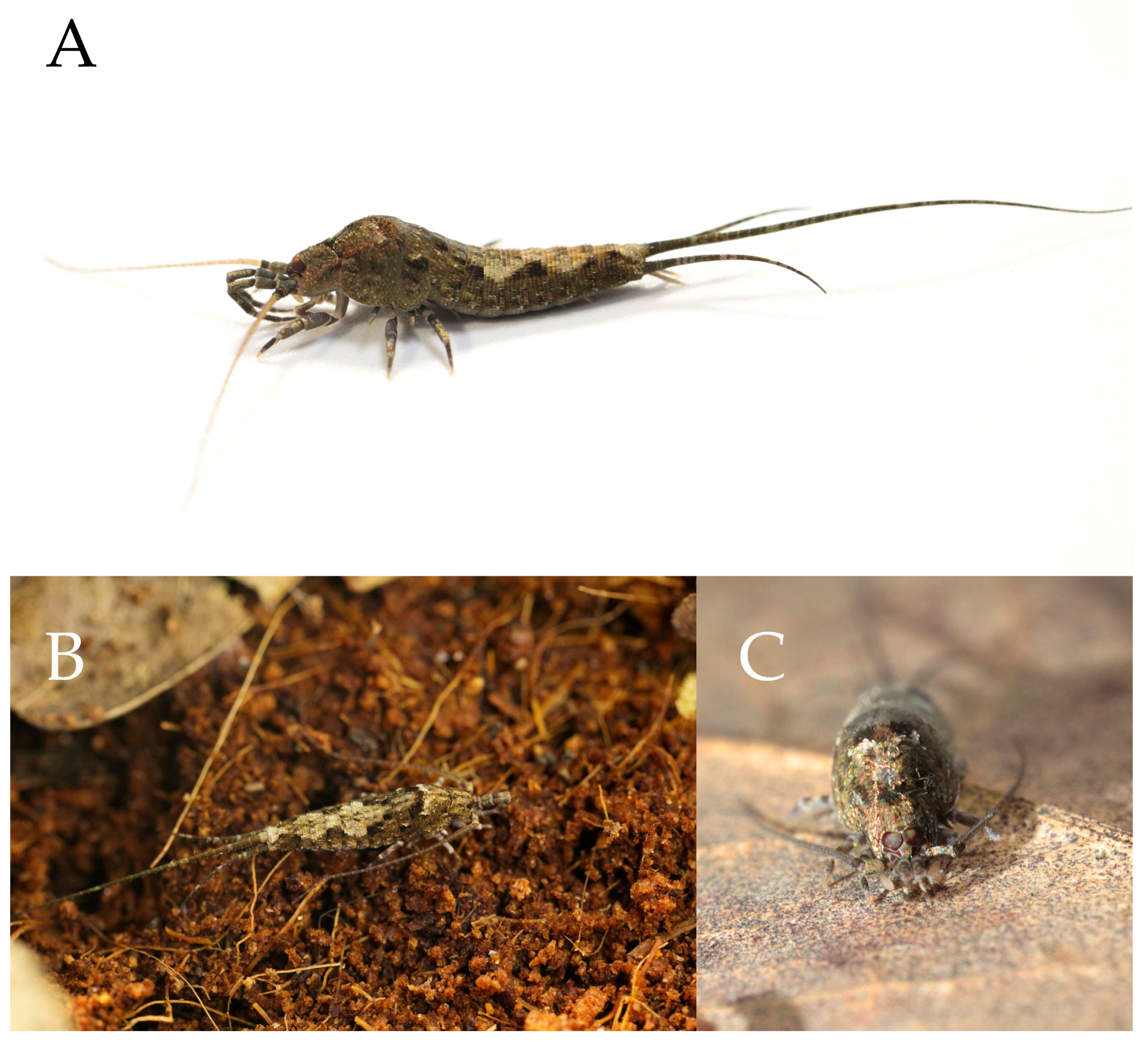
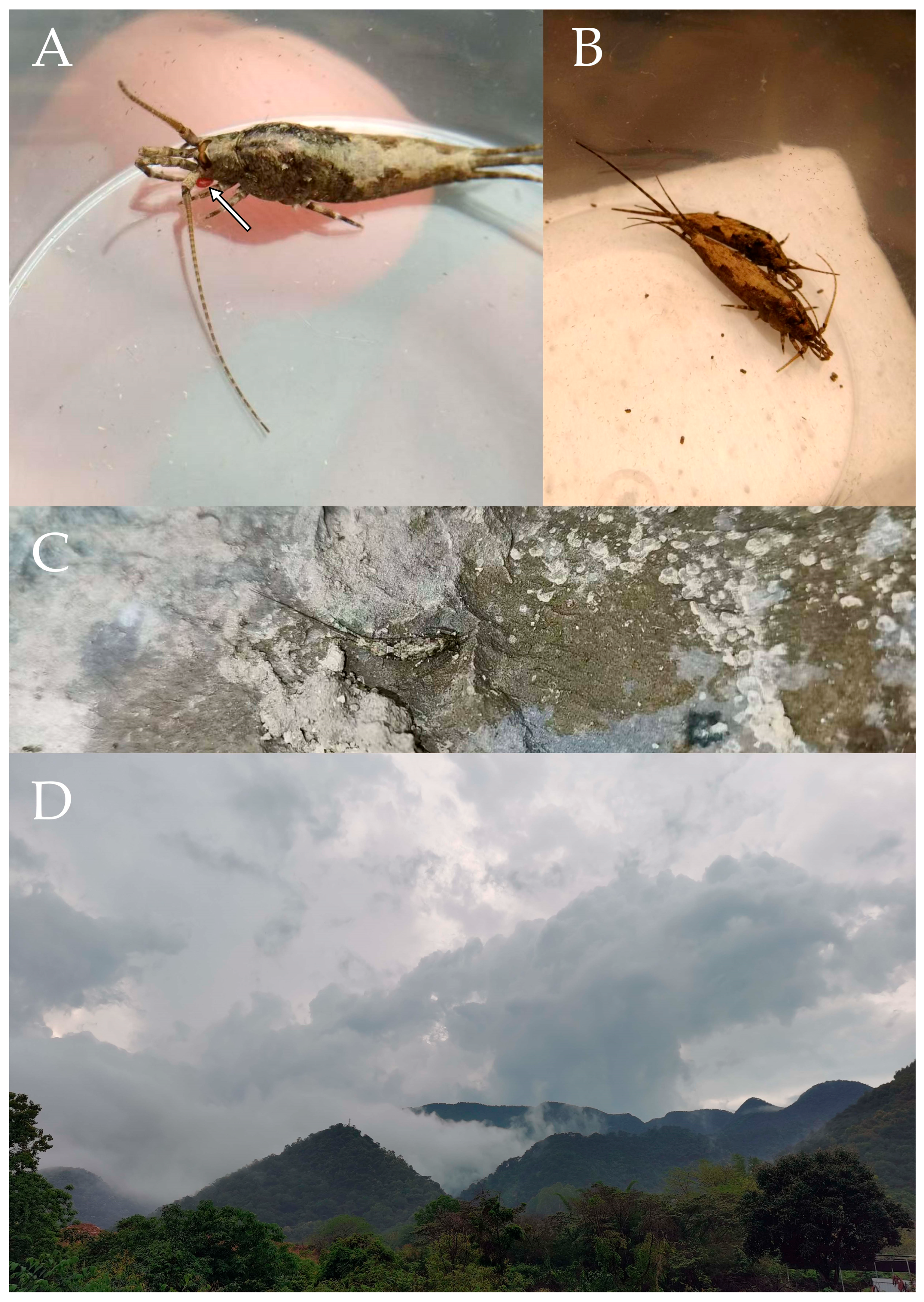
2.1. Specimen Collection and Morphological Examination
2.2. DNA Extraction, PCR, DNA Sequencing and Phylogenetic Tree.
3. Results
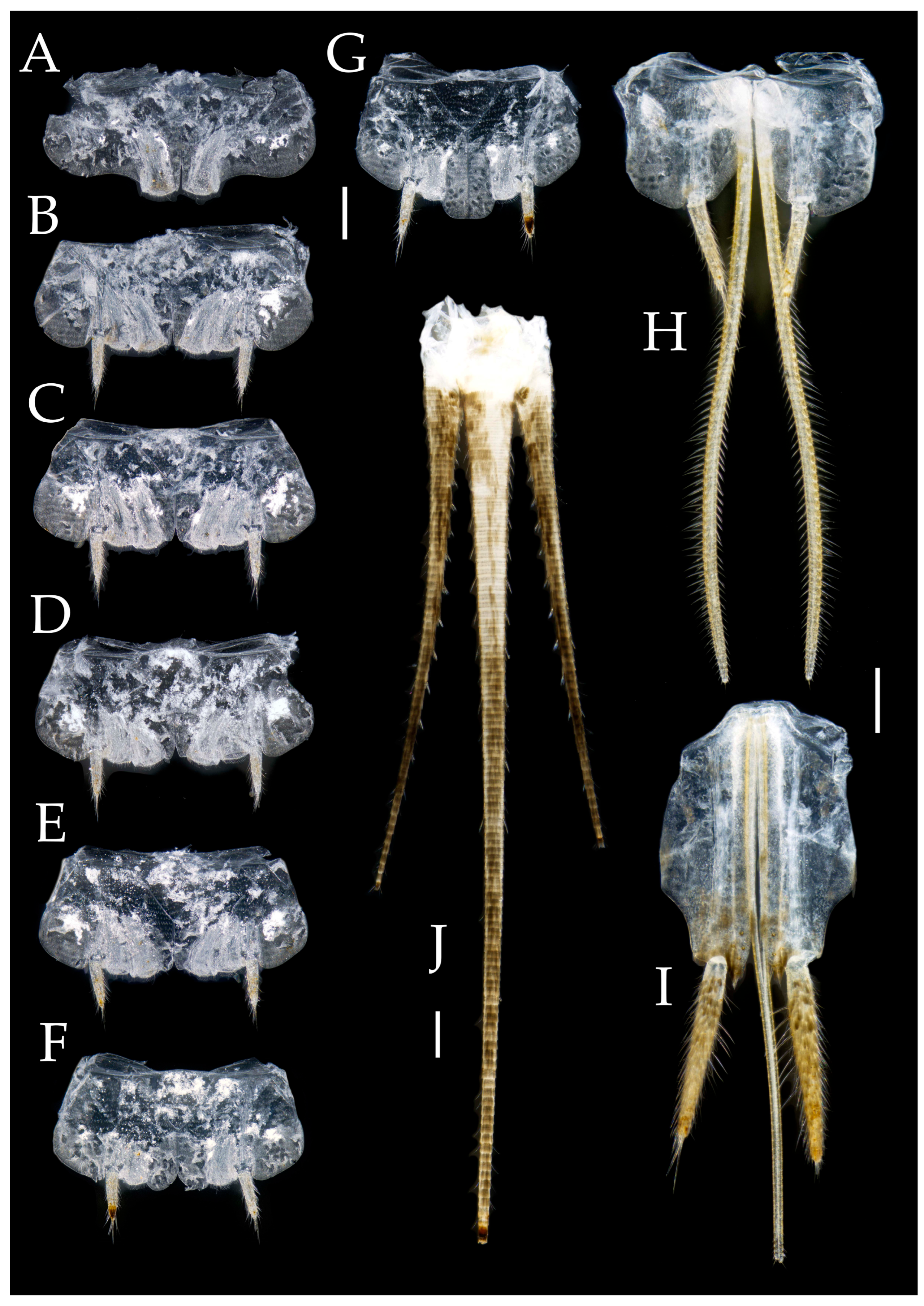
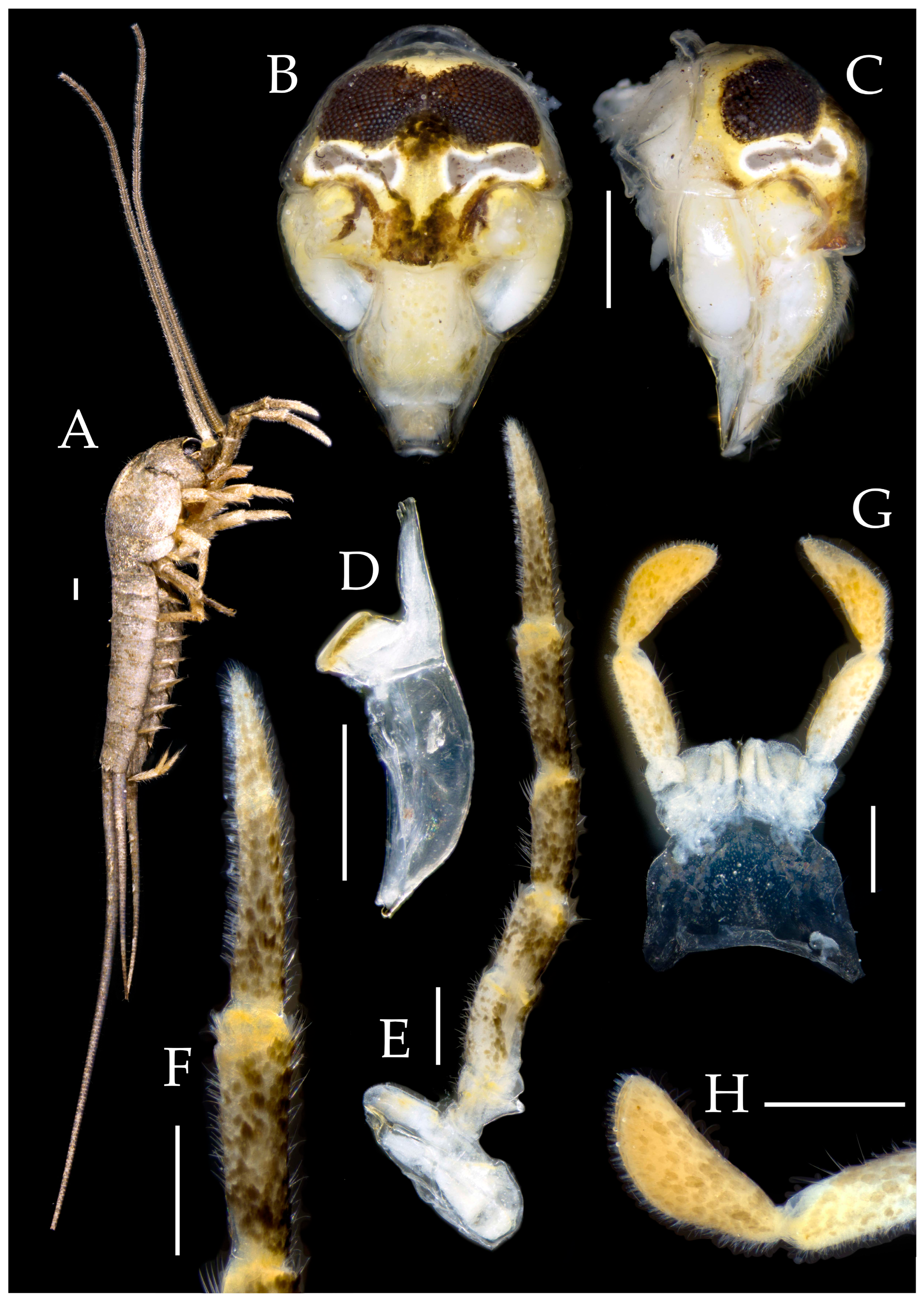
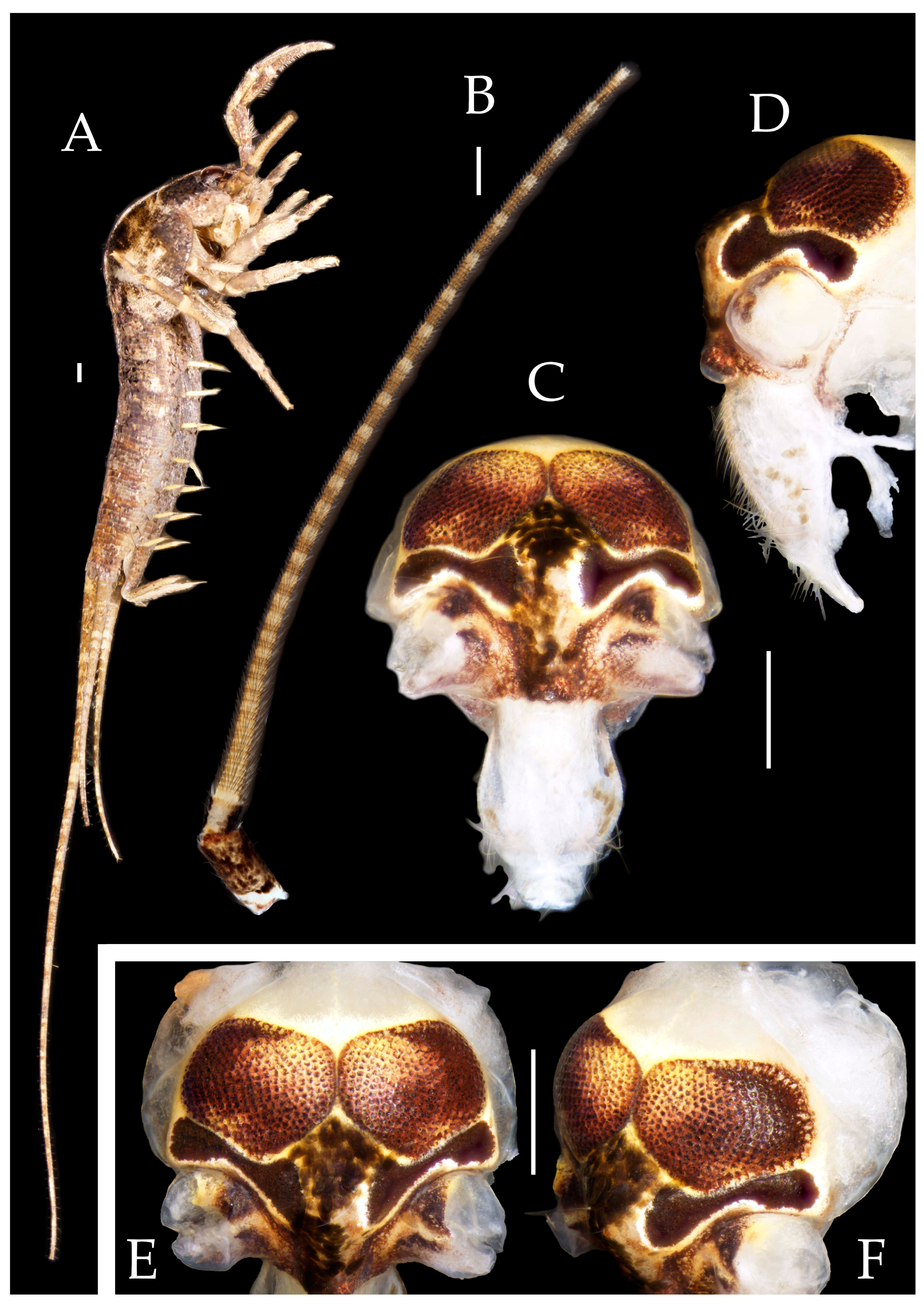
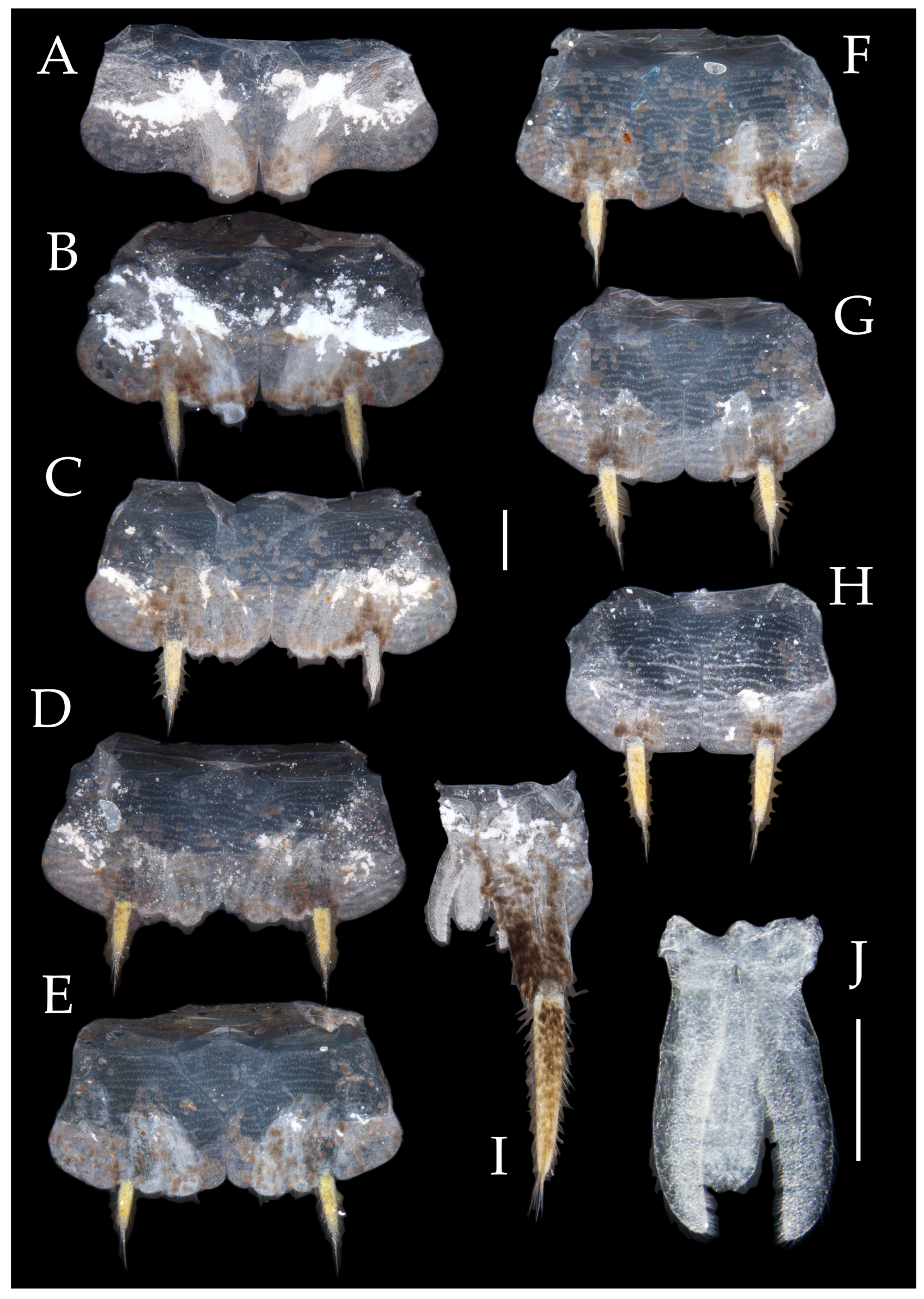
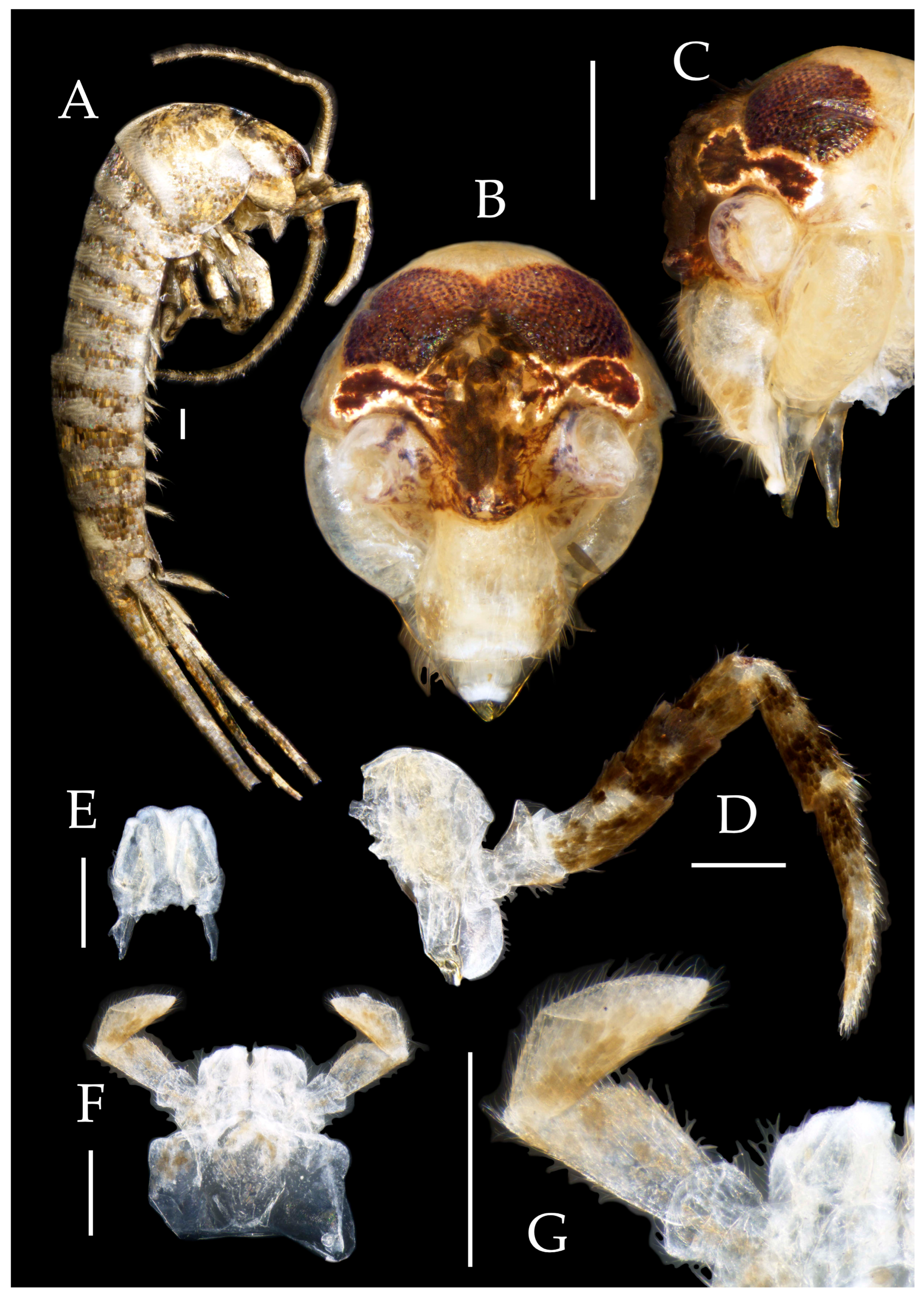
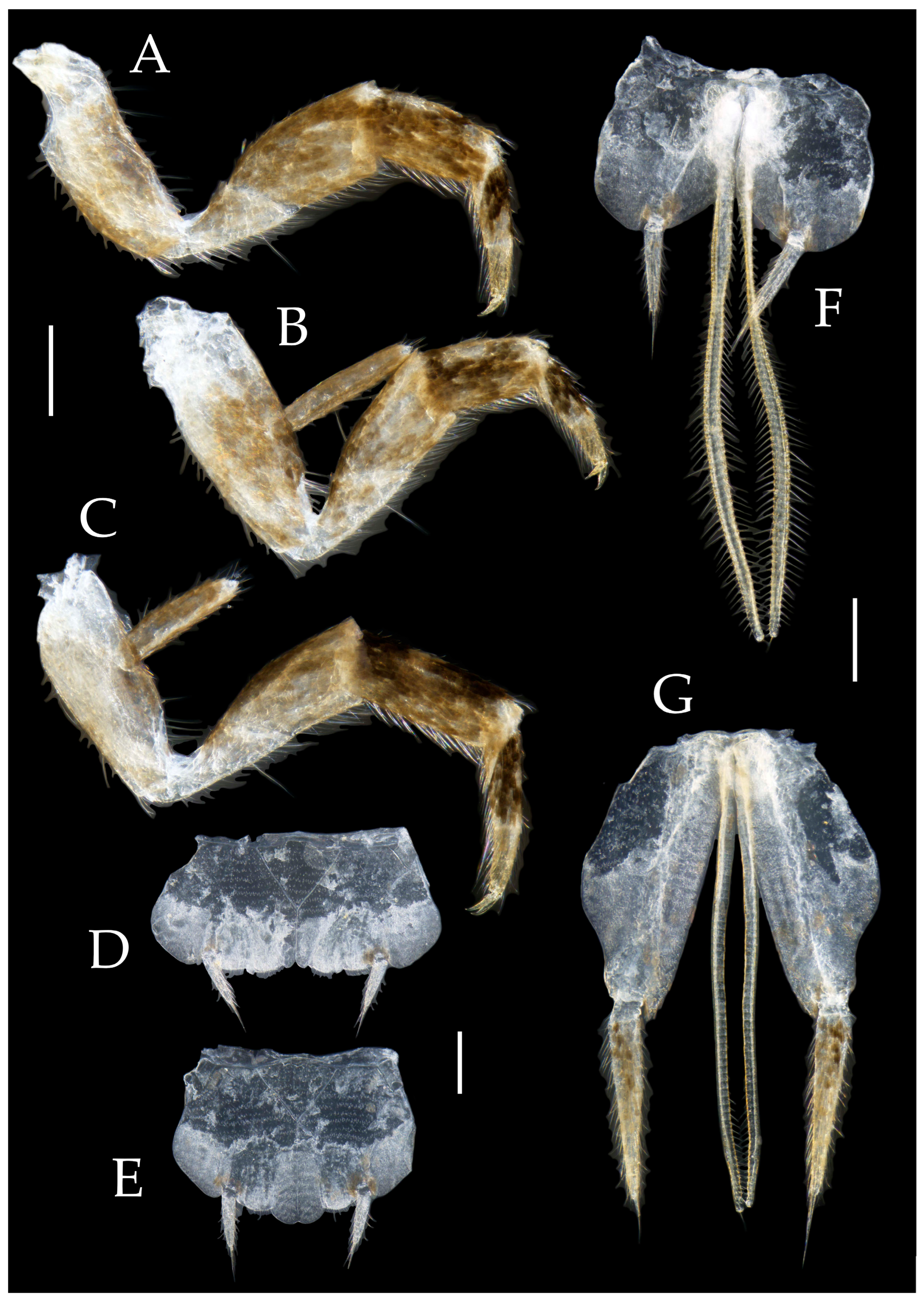
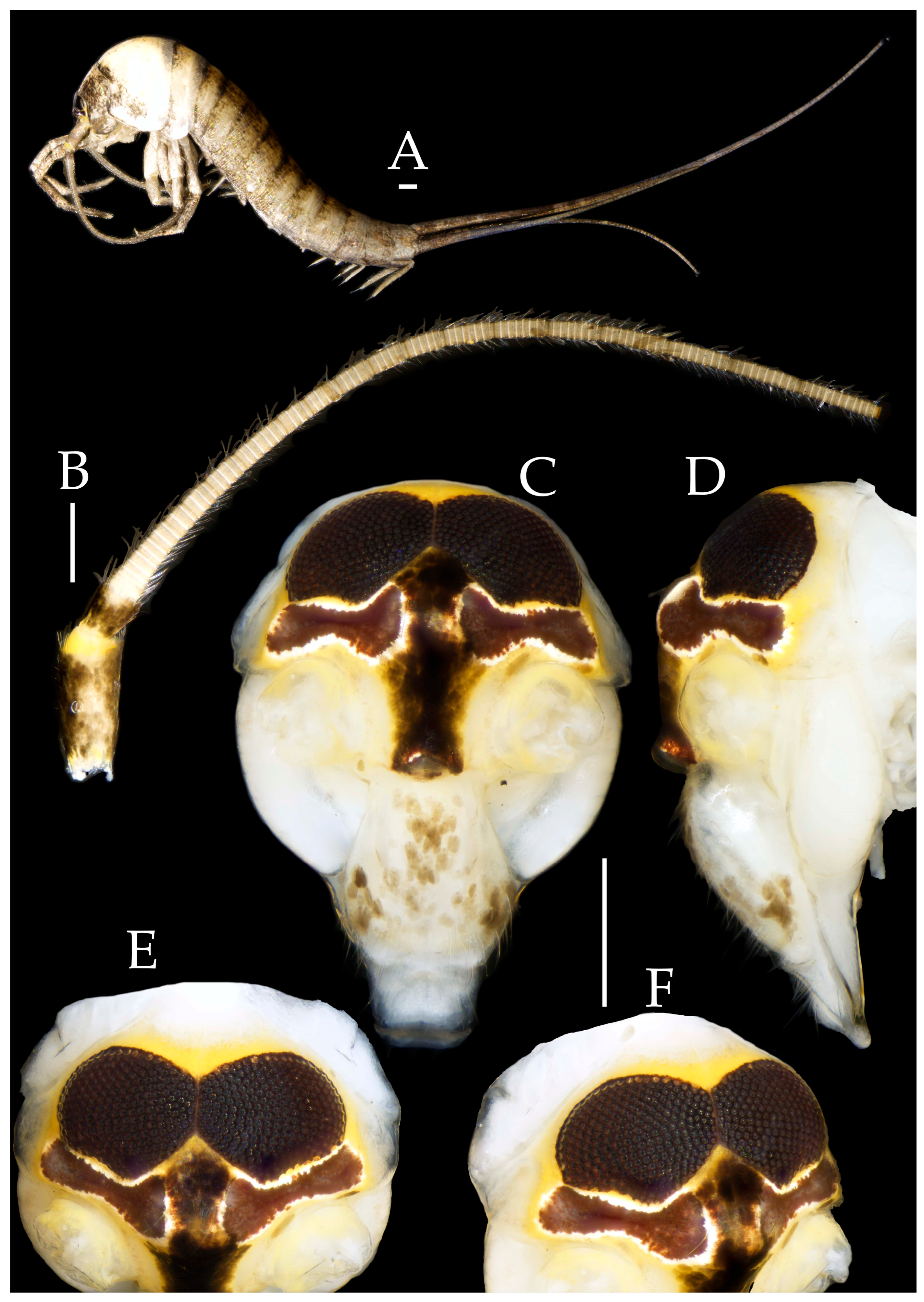
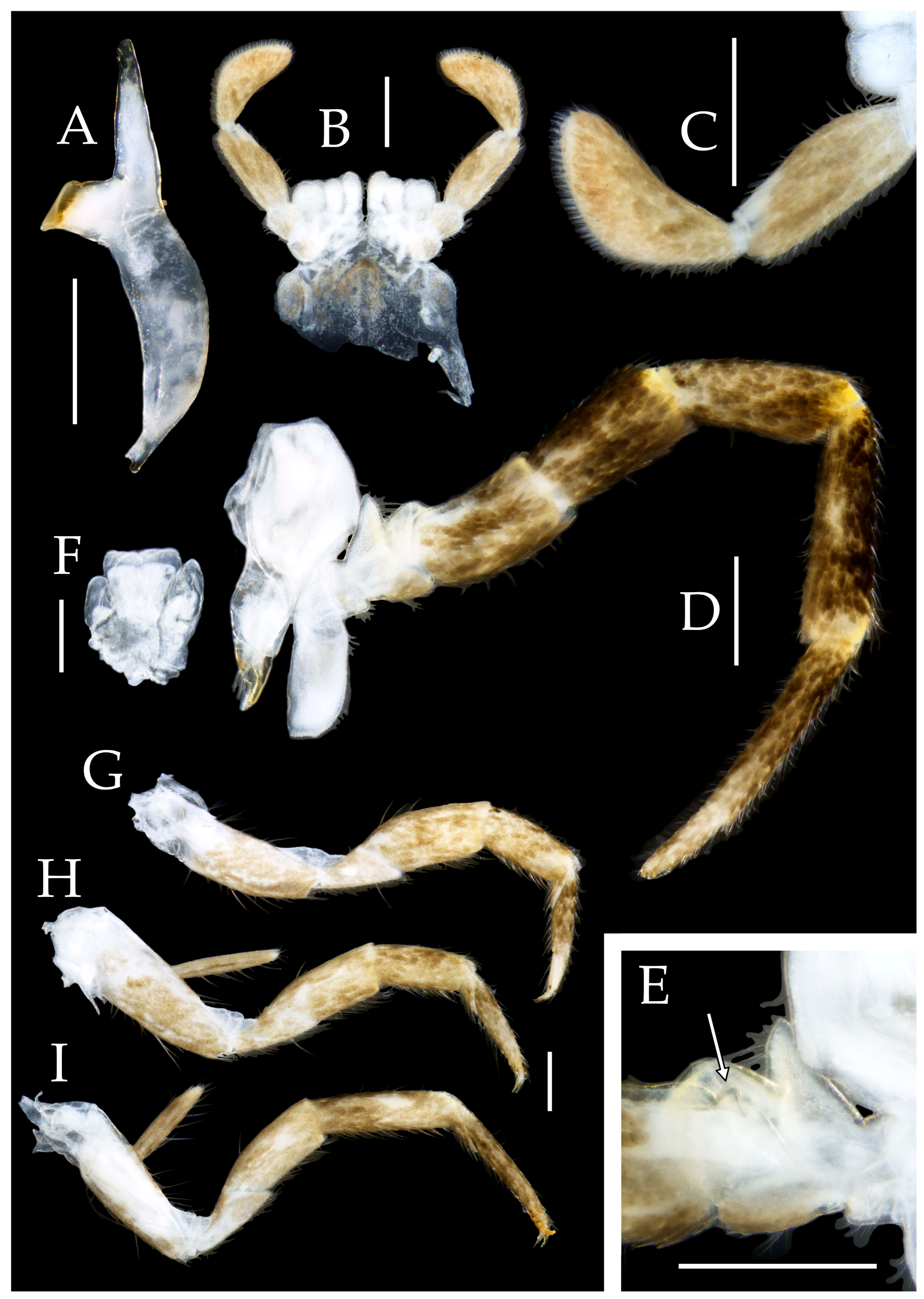
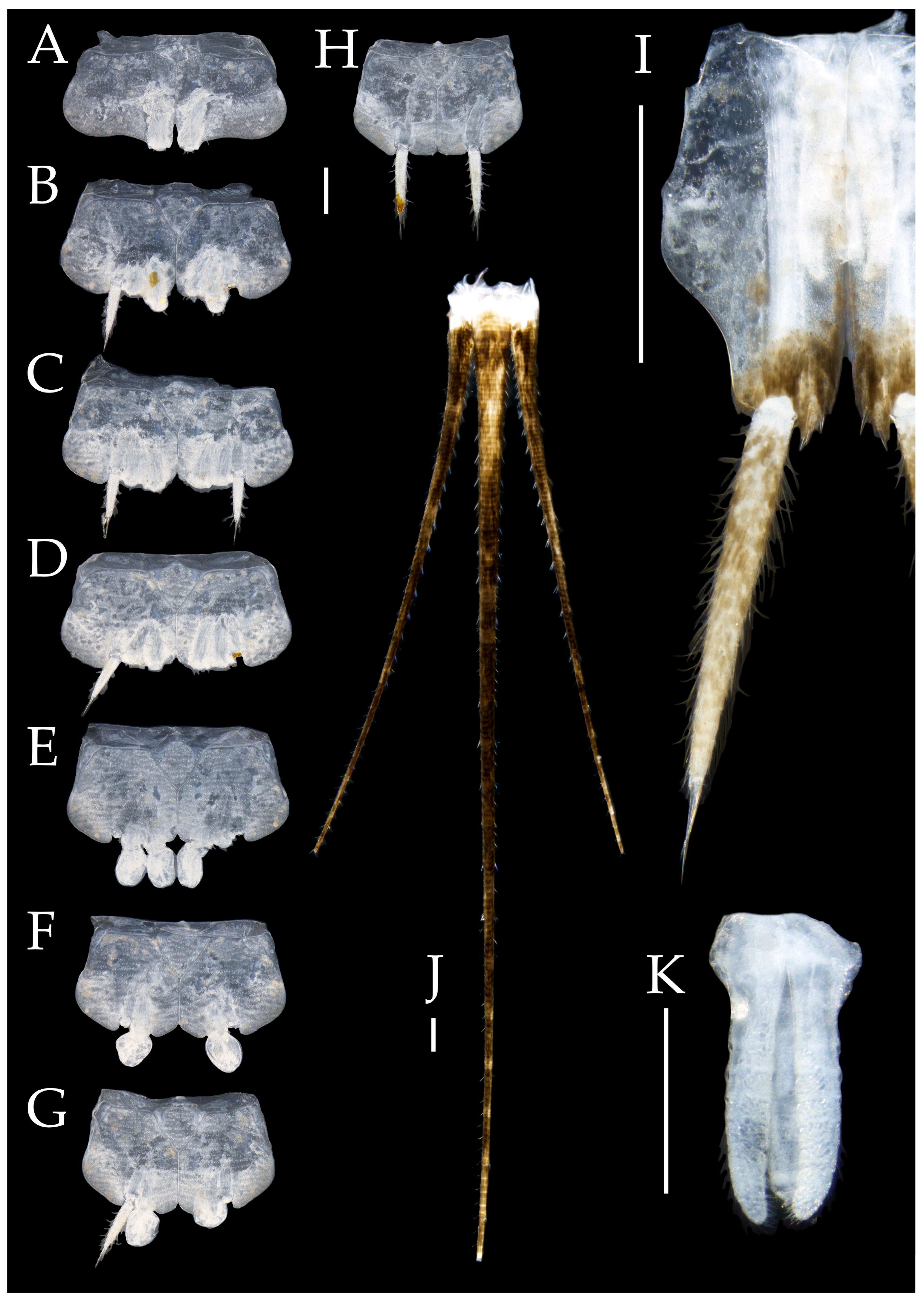
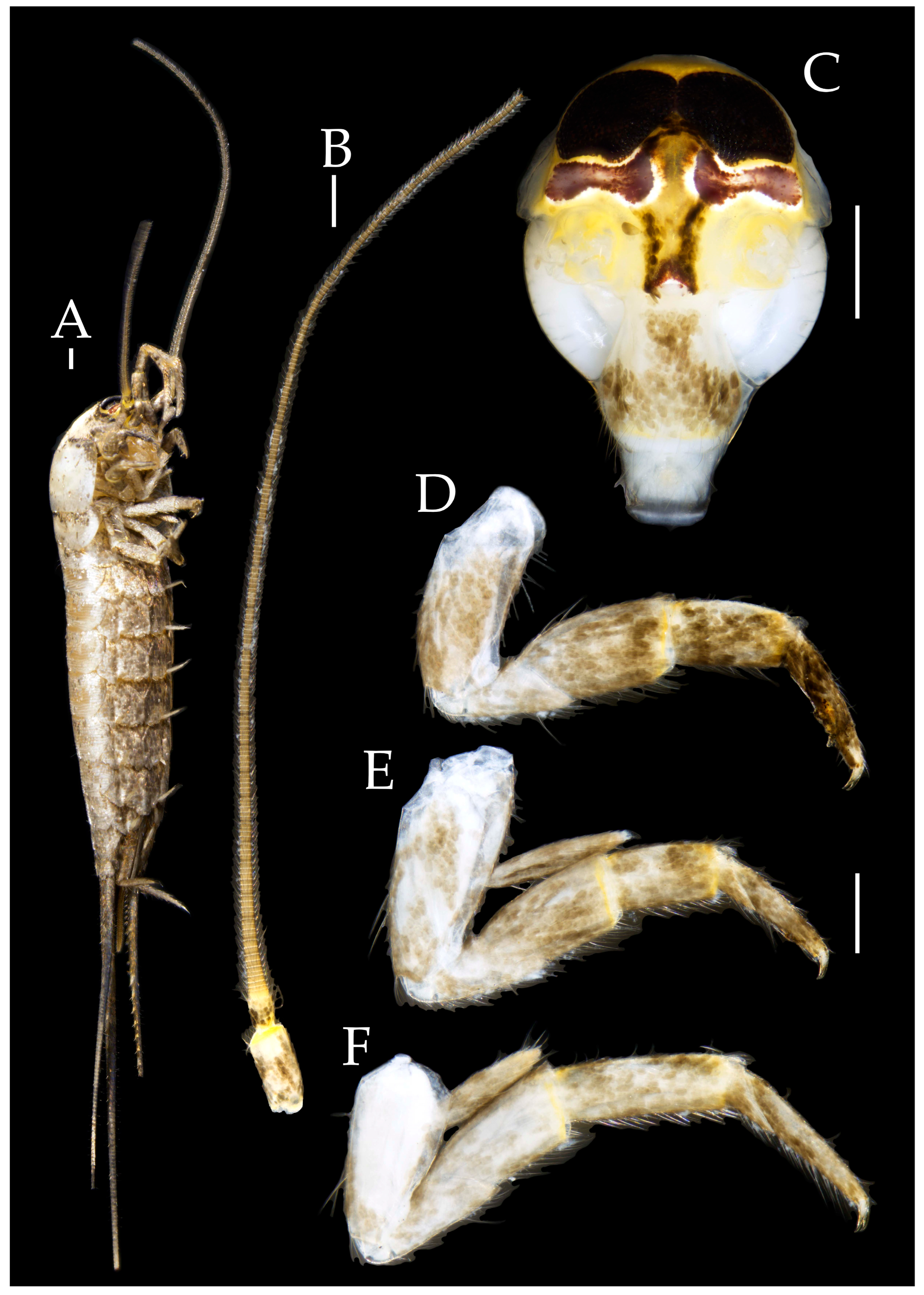
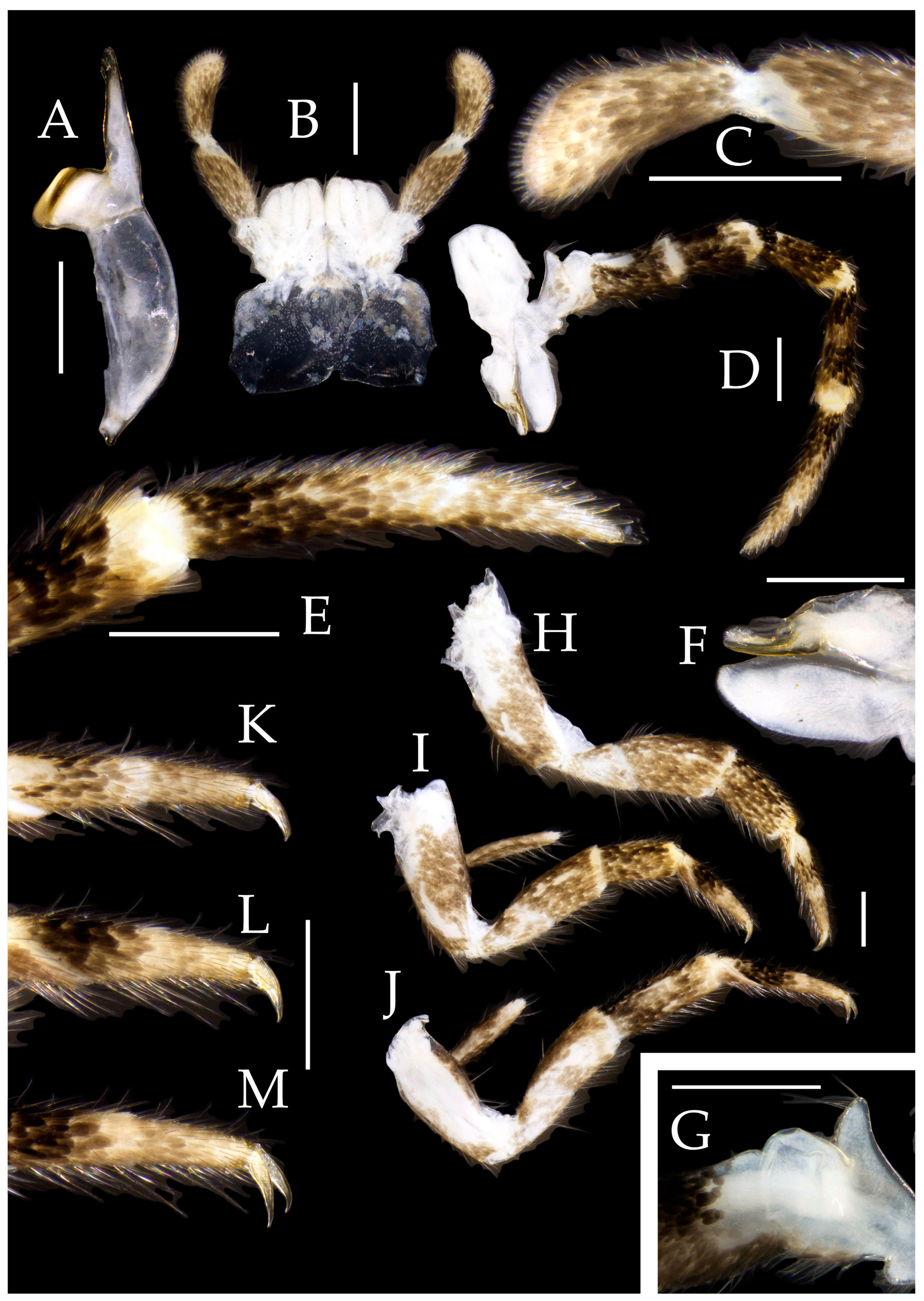
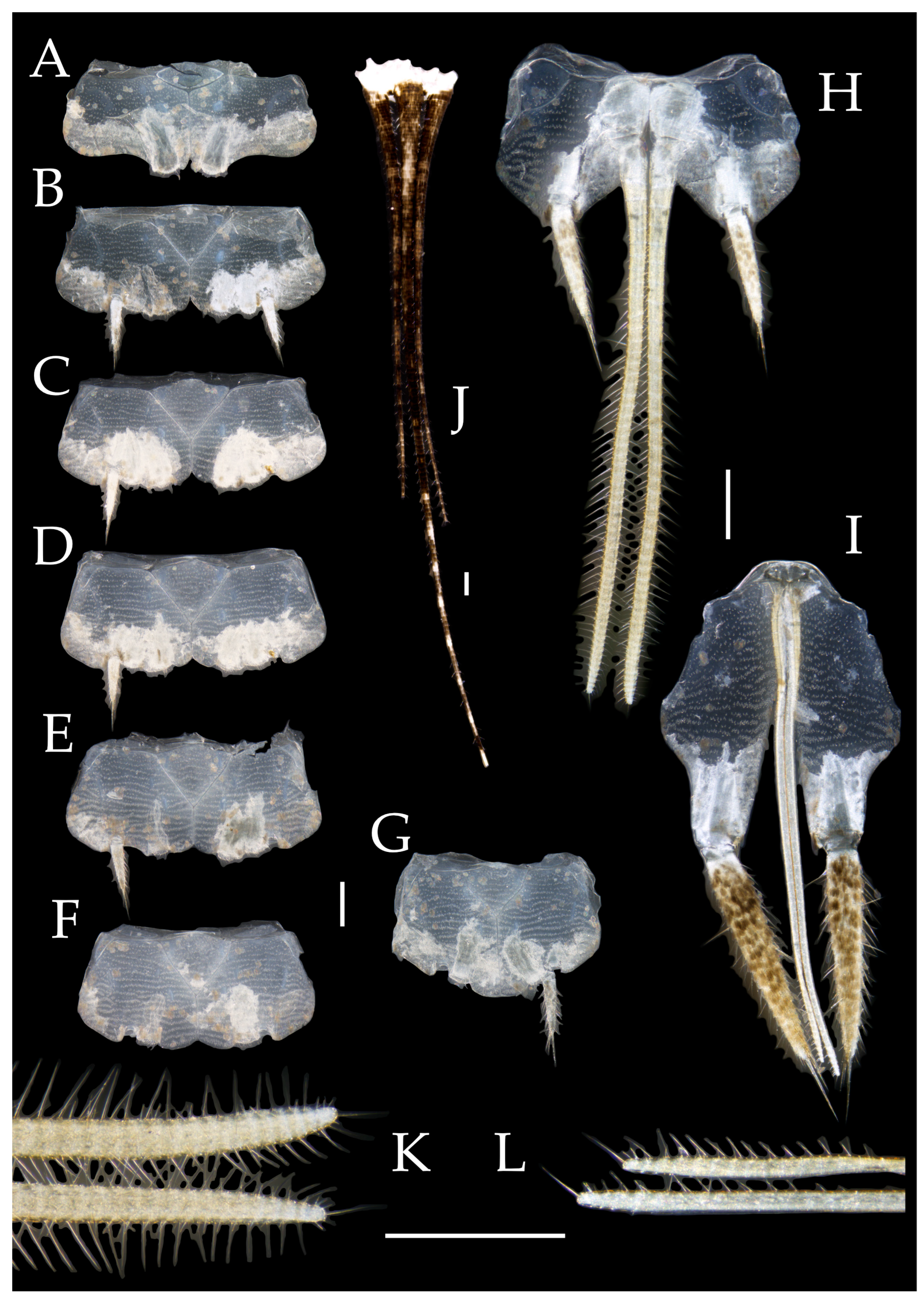
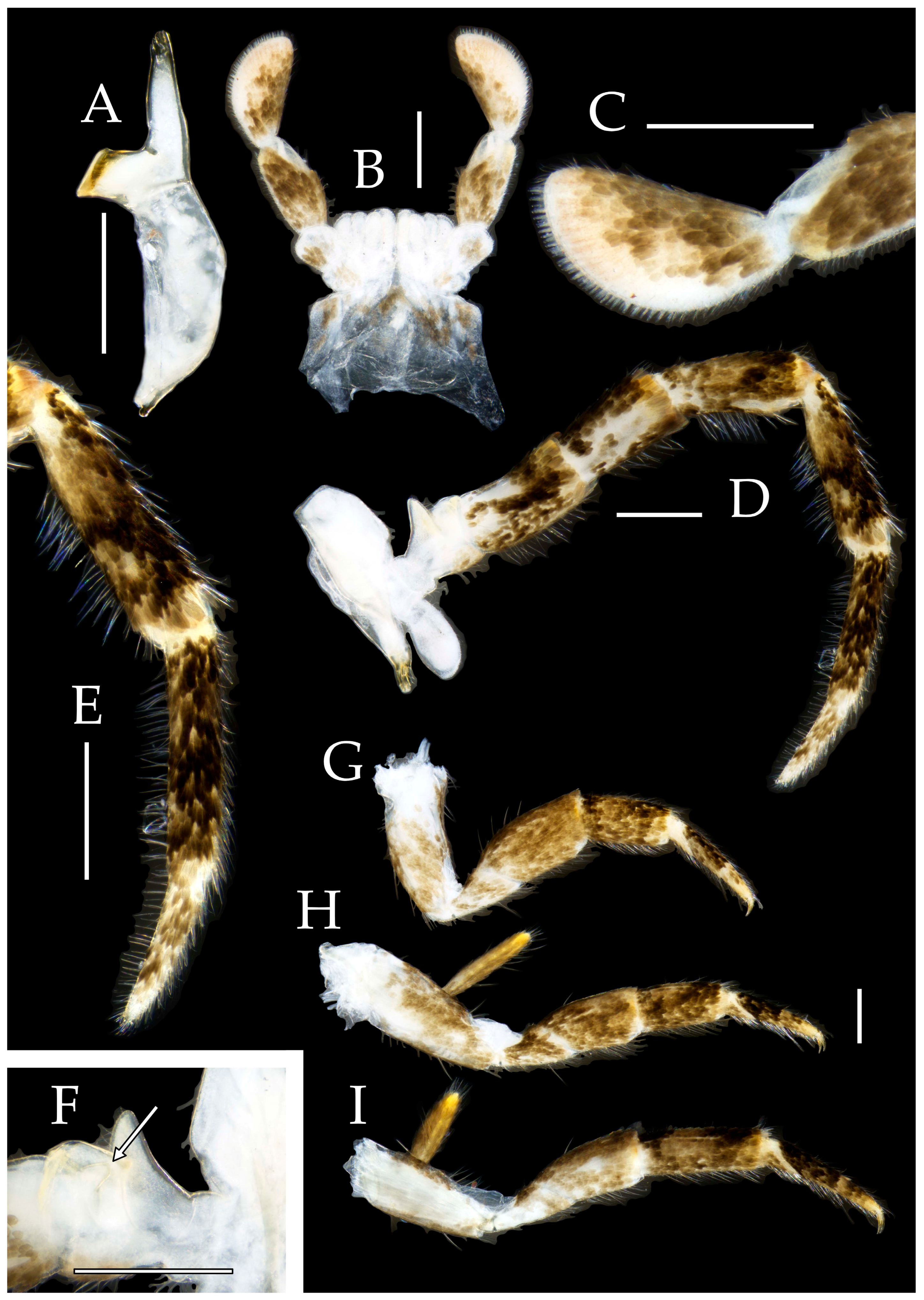
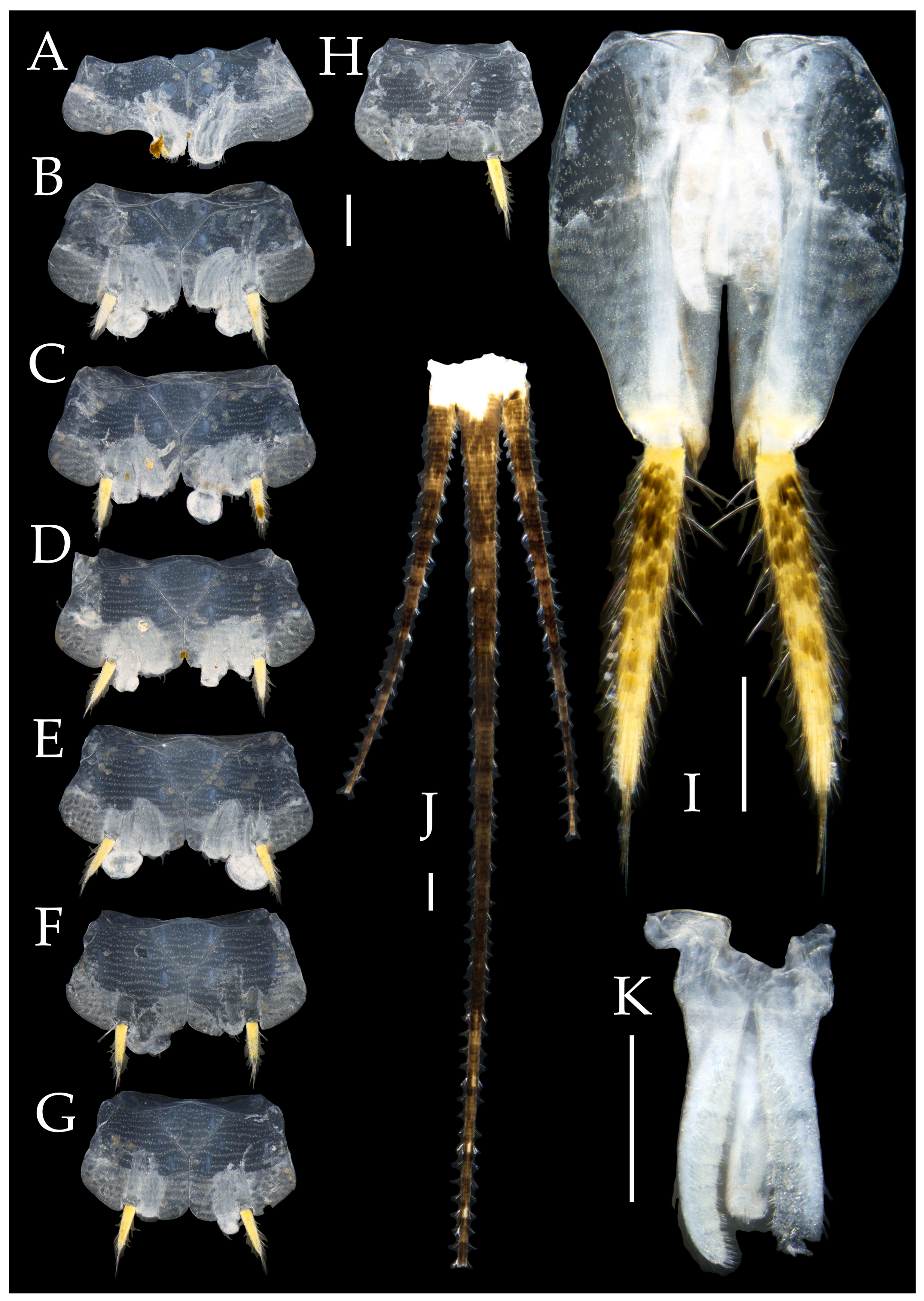
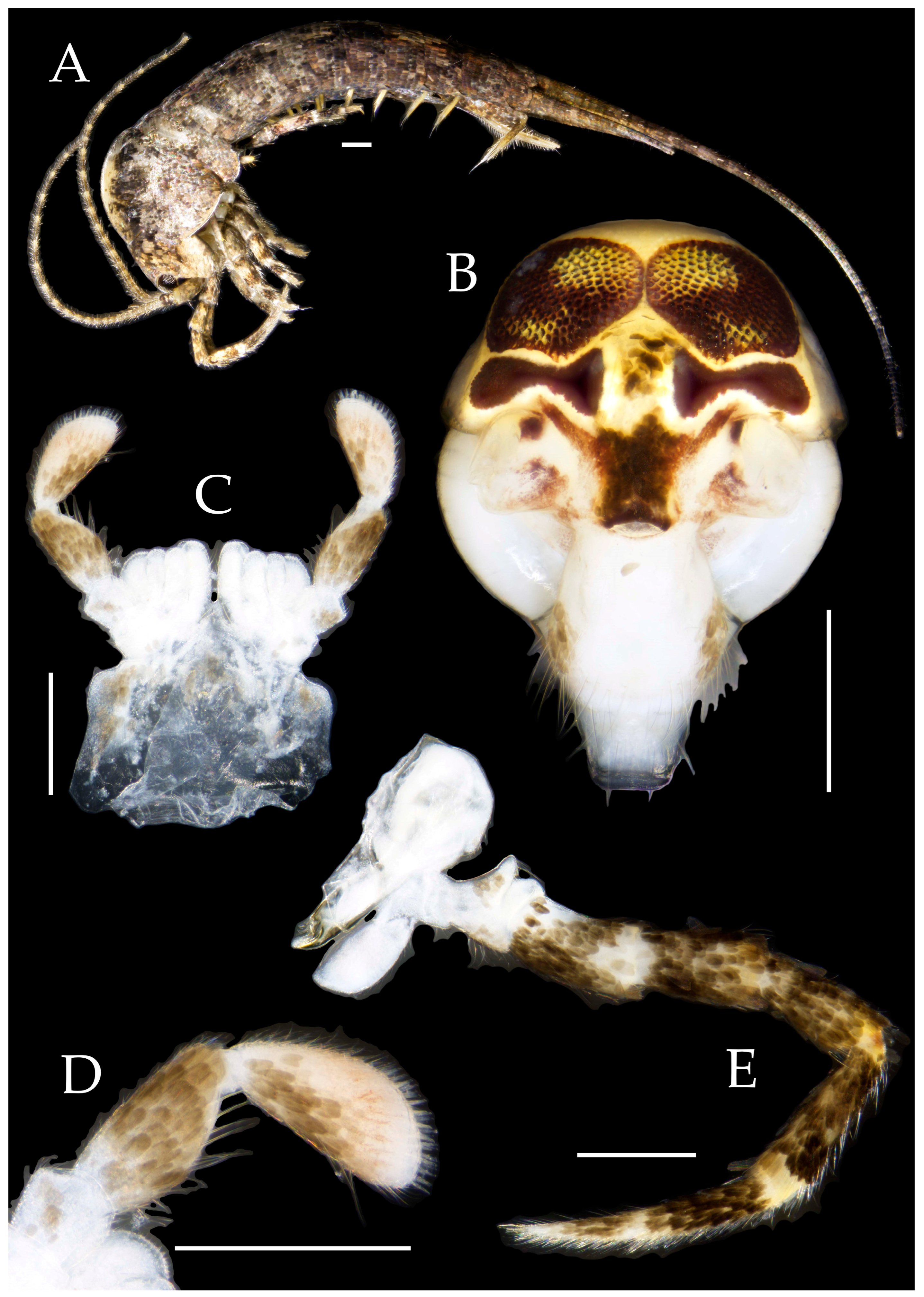
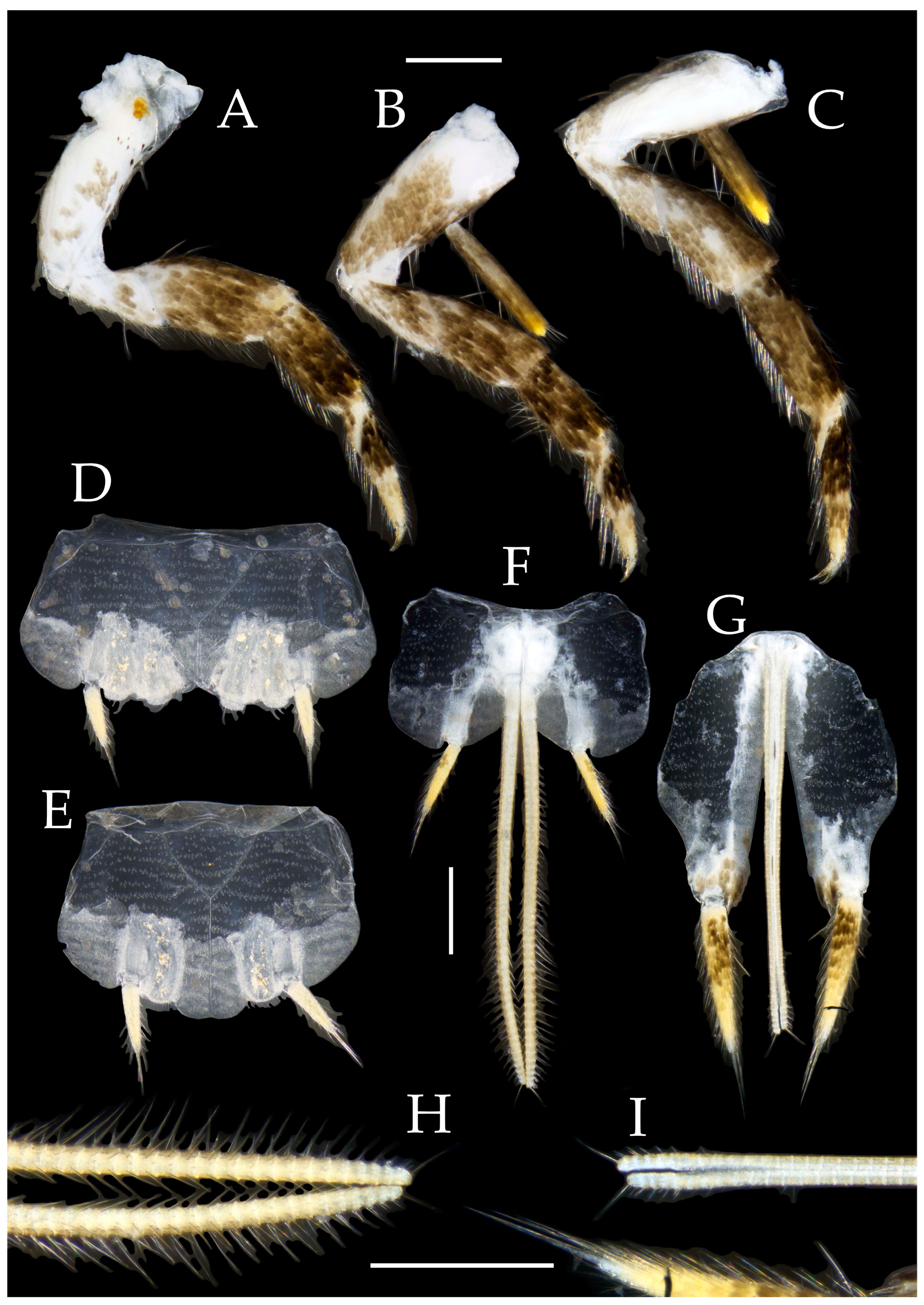
3.1. Taxonomy
3.2. Keys
|
| …………………………………………………………………Pedetontus (Verhoeffilis) Paclt, 1972 |
|
| Pedetontus sensu stricto Silvestri, 1911 |
| Key to known species of Pedetontus in China |
|
|
|
|
|
| P. (Verhoeffilis) silvestrii Mendes, 1991 |
|
|
| P. (V.) formosanus Silvestri 1943 |
|
| VII)………………………………………………………………………………………………………5 |
|
|
|
|
|
| ………………………………………………………………………………………………………………… |
| ……………………………………………………………………P. (V.) zhoui Yu, Zhang & Zhang 2010 |
|
|
|
|
| P. (V.) uraiensis Uchida 1965 |
|
|
|
|
|
|
|
|
|
|
| ……………………………………………………………………………………………………………… |
| ……………………………………P. (V.) shenzhenensis Shen, Yang, Ji & Zhang sp. n. |
|
|
|
| P. (V.) jinxiuensis Shen, Yang, Ji & Zhang sp. n. |
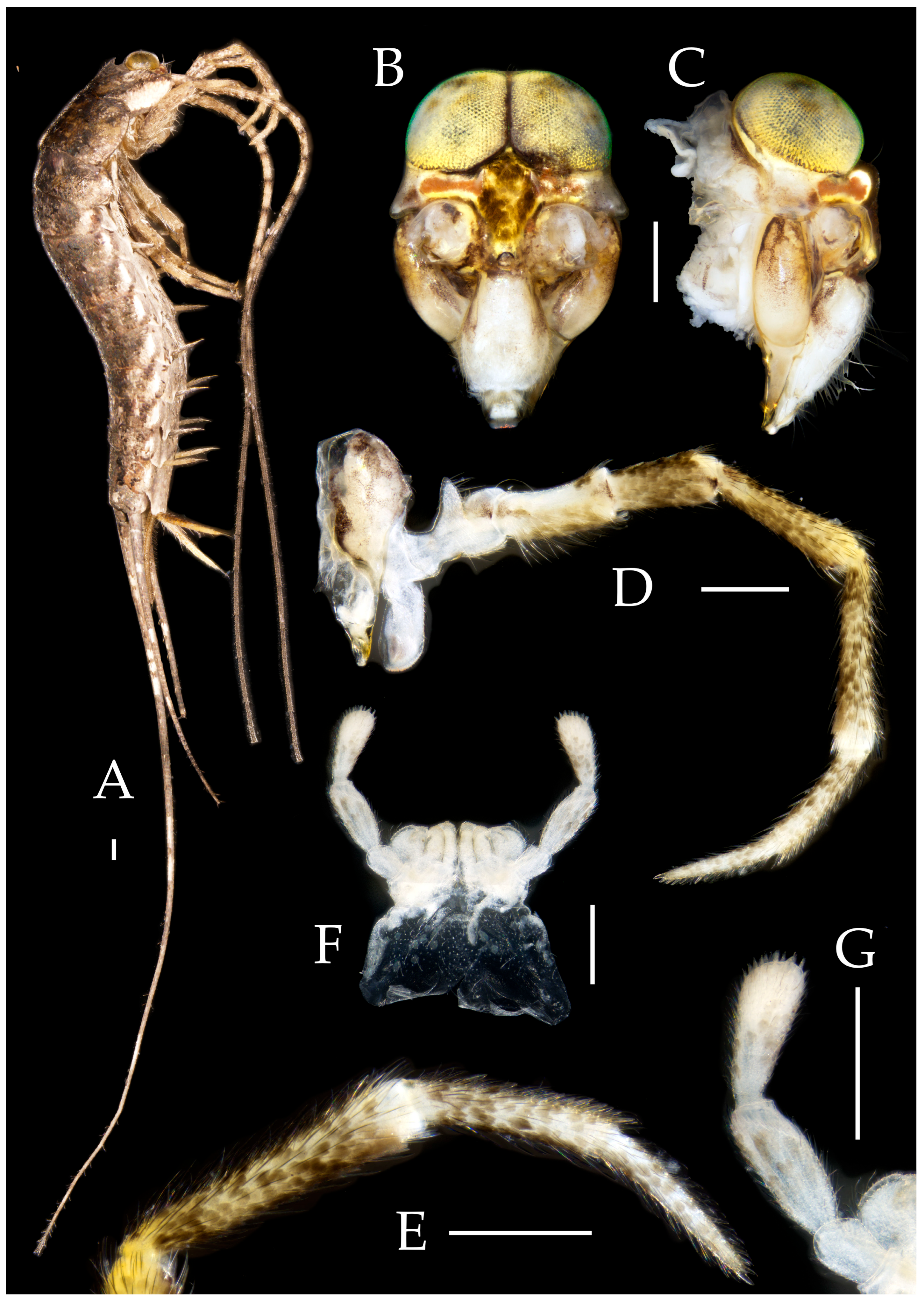
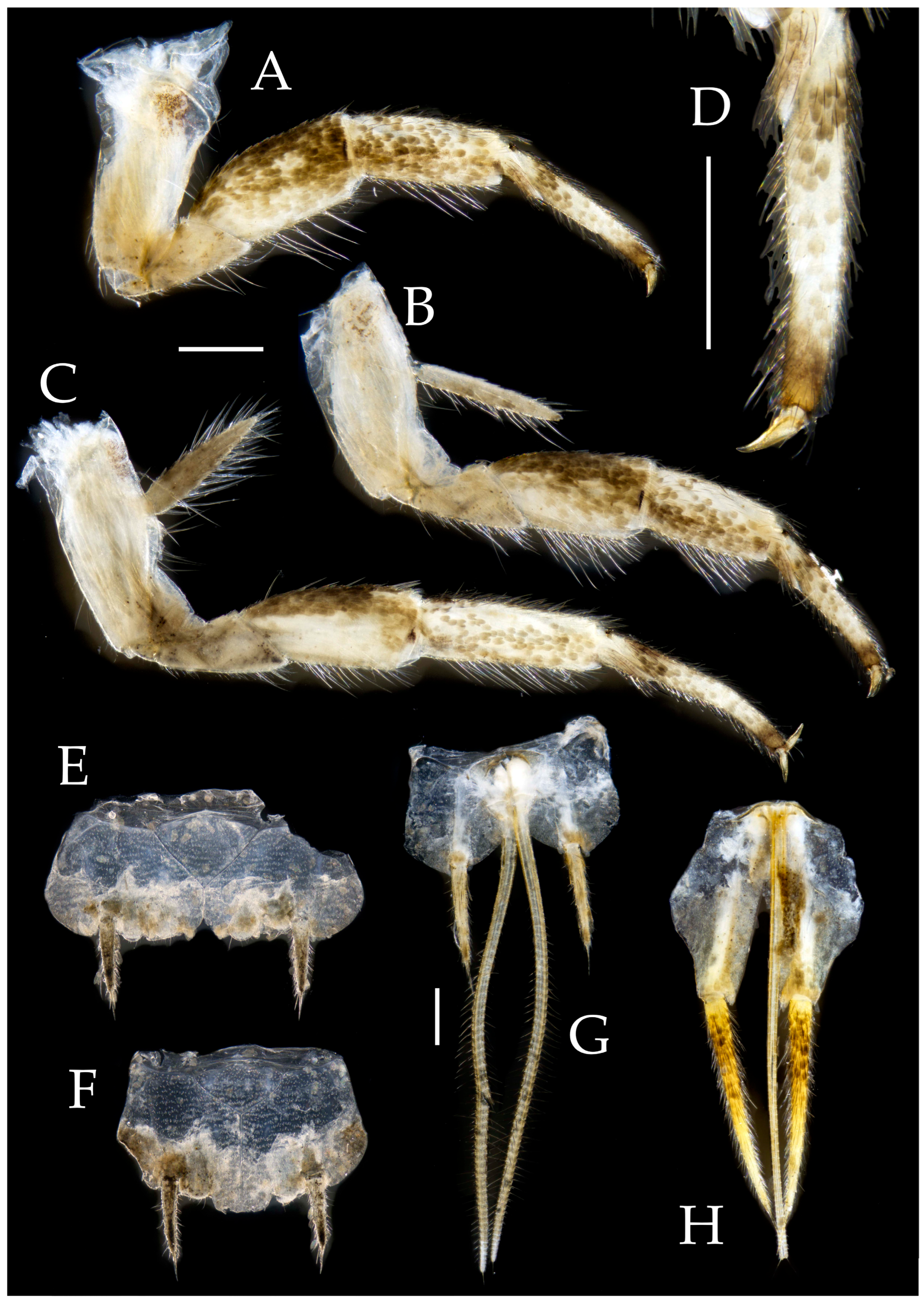
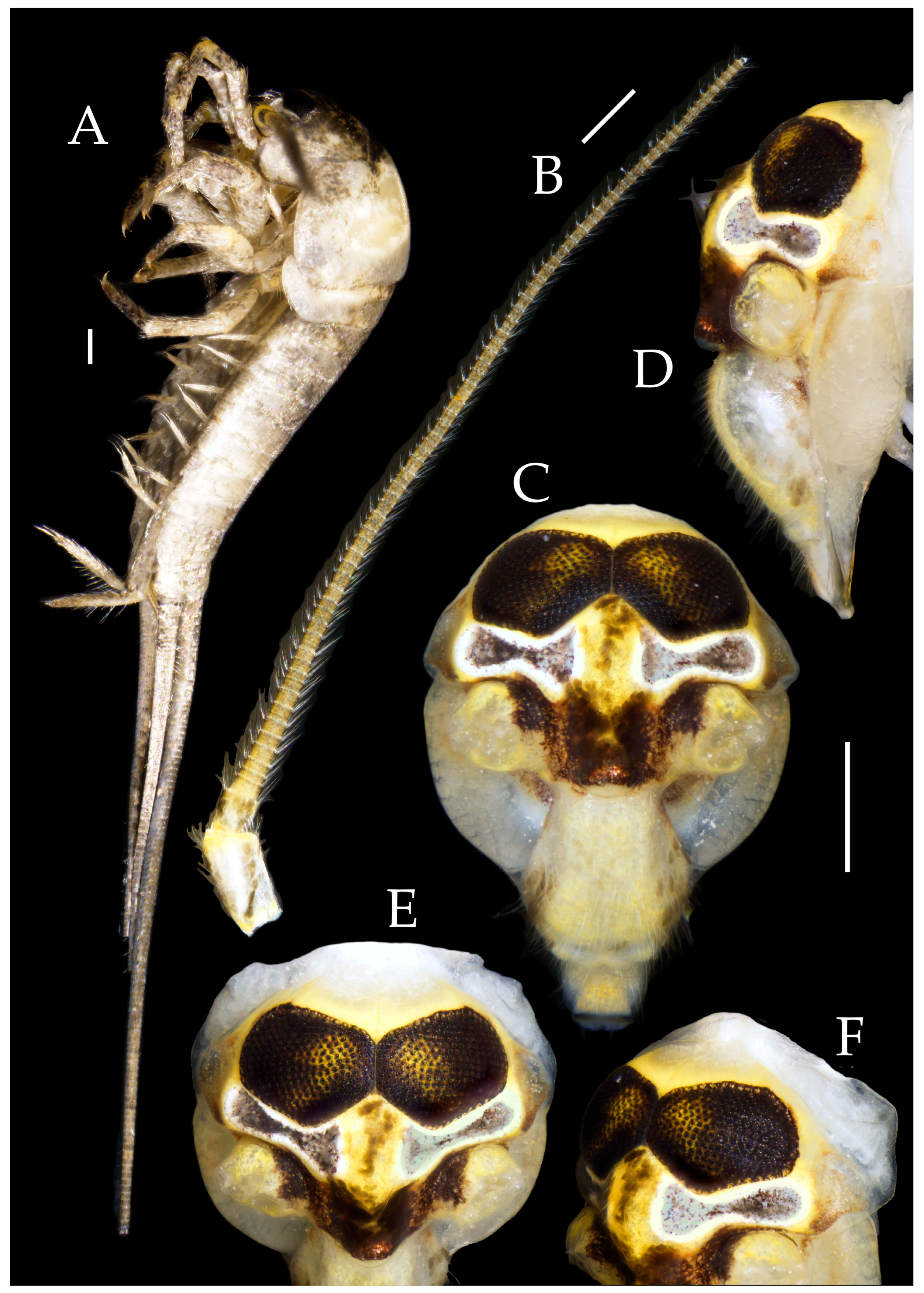
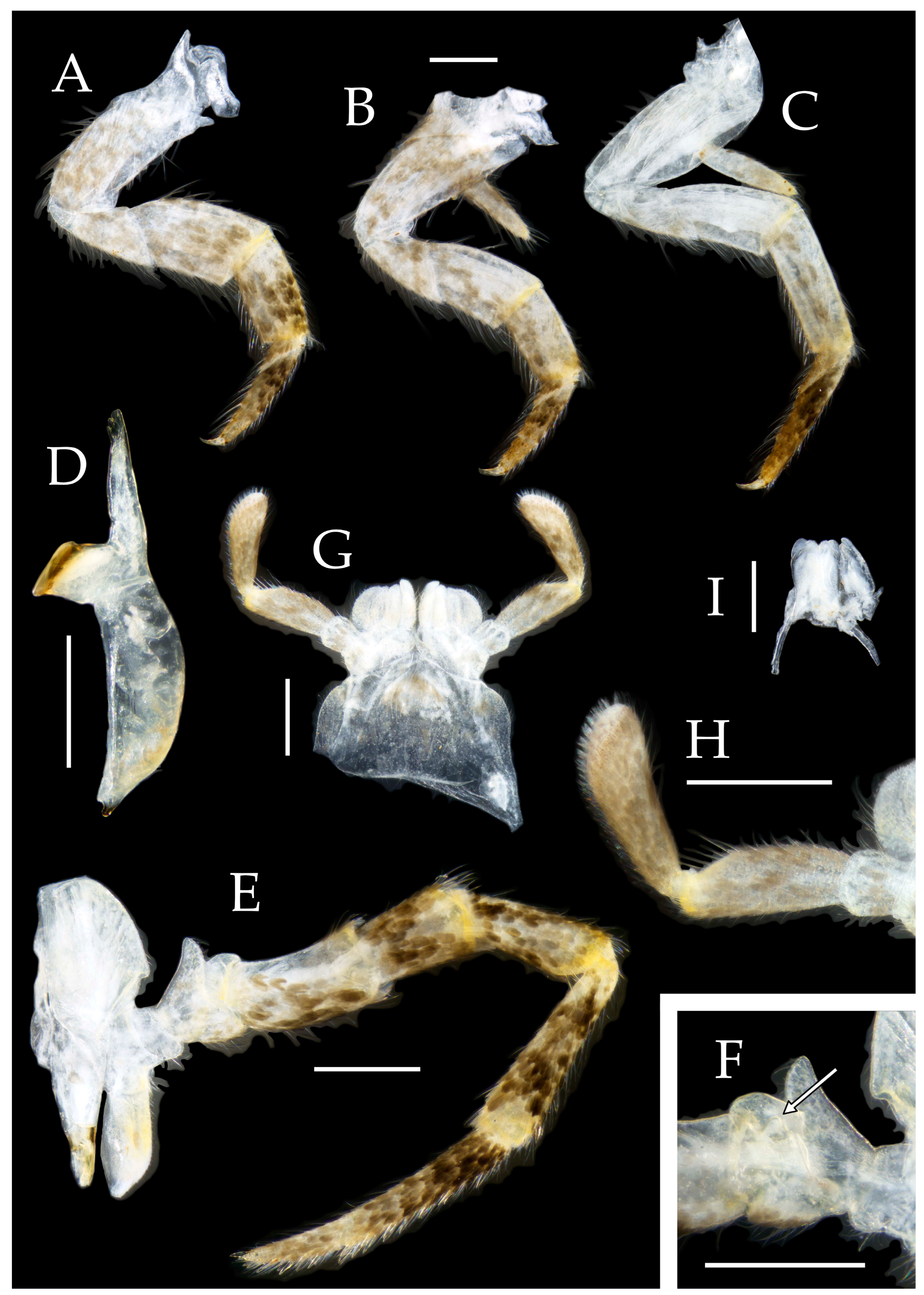
3.3. Description
3.4. Phylogenetic Tree of Machilidae Based on COX1
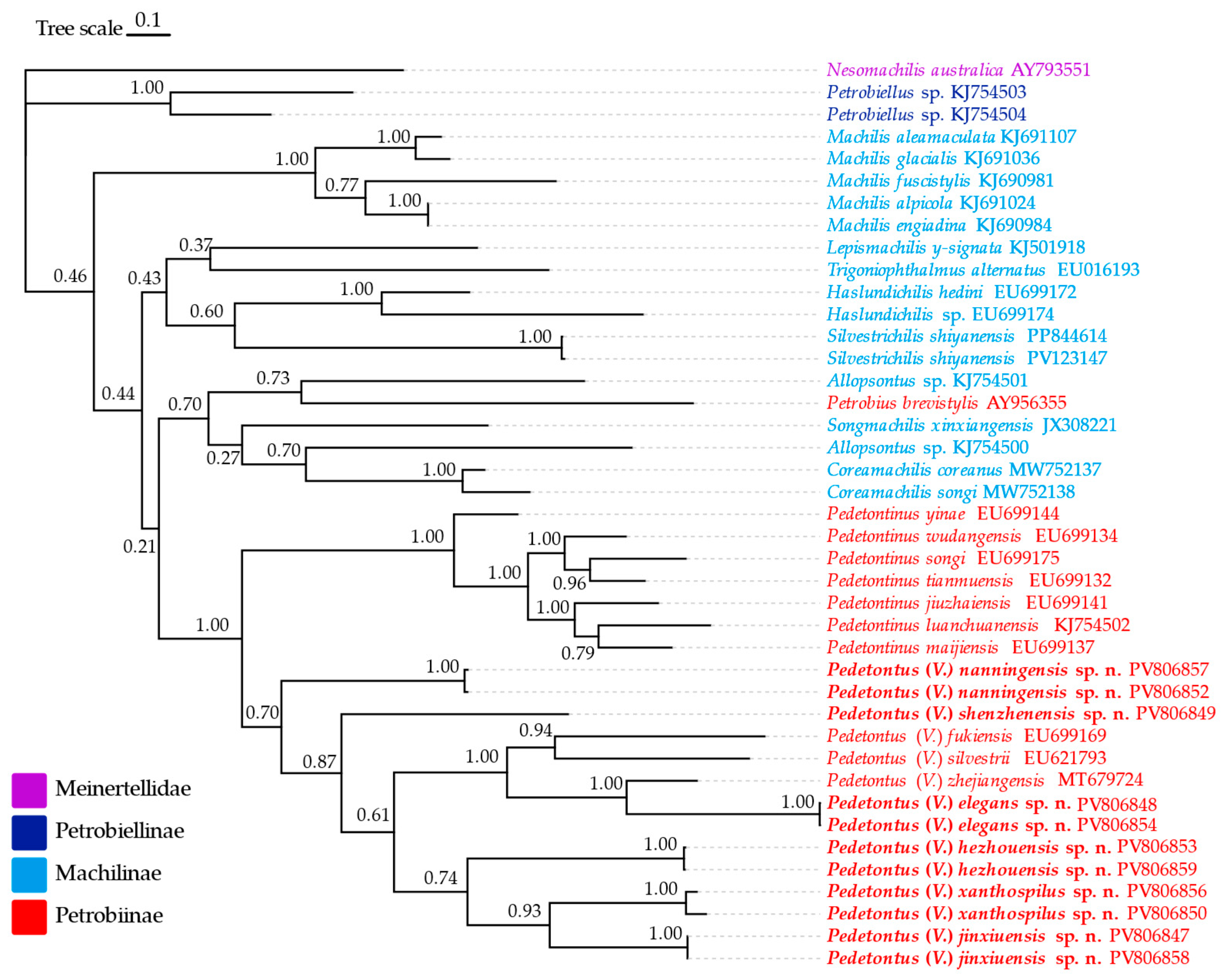
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bach de Roca, C.; Baltanas, R.M.; Gaju Ricart, M. Orden Microcoryphia. Ibero Diversidad Entomológica 2015, 38, 1–12.
- Sturm, H.; Machida, R. Archaeognatha. In Handbook of Zoology; Walter de Gruyter: Berlin, New York, 2001; Vol. 4.
- Yanoviak, S.P.; Kaspari, M.; Dudley, R. Gliding Hexapods and the Origins of Insect Aerial Behaviour. Biol. Lett. 2009, 5, 510–512. [CrossRef]
- Grimaldi, D.A.; Engel, M.S. Evolution of the Insects; Repr.; Cambridge Univ. Press: Cambridge, 2006; ISBN 978-0-521-82149-0.
- Mendes, L.F. Taxonomy of Zygentoma and Microcoryphia: Historical Overview, Present Status and Goals for the New Millennium. Pedobiologia 2002, 46, 225–233.
- Kaplin, V.G. A New Species of the Bristletail Genus Pedetontus Silv. (Thysanura, Machilidae) from Thailand. Entmol. Rev. 2013, 93, 887–892. [CrossRef]
- Mendes, L.F. An Annotated List of Generic and Specific Names of Machilidae (Microcoryphia, Insecta) with Identification Keys for the Genera and Geographical Notes; Ministerio do Planeamento e da Administração do Território, Secretaria de Estado da Ciência e Tecnologia, Instituto de Investigação Científica Tropical: Lisbon, 1990; Vol. 155;.
- Silvestri, F. Quelques Formes Nouvelles de La Famille Des Machilides. Annales des Sciences Naturelles (Zool)(9) 1907, 6, 361–370.
- Silvestri, F. Note sui Machilidae. III. Descrizione di un nuovo genere e di sei nuove specie. Redia (Firenze) 1906, 3, 325–335.
- Silvestri, F. Machilidae (Thysanura). Entomologische Ergebnisse der schwedischen Kamtchatka-Expedition 1920-1922. Arkiv för Zoologi 1925, 17, 1–5.
- Silvestri, F. Contributo Alla Conoscenza Dei Machilidae Dell’America Settentrionale. Bolletino del Laboratorio de Zoologia Generale e Agraria della R. Scuola Superiore d’Agricoltura in Portici 1911, 5, 324–350.
- Silvestri, F. Descripzione Di Alcuni Machilidae (Thysanura) Della Cina. Notes d’Entomologie Chinoise 1936, 3, 103–115.
- Silvestri, F. Contributto Alla Conoscenza Dei Machilidae (Insecta, Thysanura) Del Giappone. Bolletino del Laboratorio di Zoologia generale i agraria di Portici 1943, 32, 283–306.
- Sturm, H. Possibilities and Problems of Morphological Taxonomy Shown by North American Representatives of the Subgenus Pedetontus s. str. and Petridiobius Canadensis (Archaeognatha, Machilidae, Petrobiinae). Deutsche Entomologische Zeitschrift 2001, 48, 3–21. [CrossRef]
- Allen, R.T. Pedetontus Gershneri, a New Species of Machilidae from the Interior Highlands of North America (Insecta: Microcoryphia). Entomological News 1995, 106, 195–198.
- Kaplin, V.G. New species of bristletails (Microcoryphia, Machilidae) from the Kuril Islands and Primorsky Kari. Txonomy of Insects of Far East 1980, 3–9.
- Lee, B.H.; Choe, G.H. Two New Species of Microcoryphia (Insecta) from Korea. The Korean journal of systematic zoology 1992, 8, 19–34.
- Machida, R. A New Species of the Genus Pedetontus from Japan (Insecta, Thysanura). Annotationes zoologicae Japonenses 1980, 53, 220–225.
- Mendes, L.F. Sur Quelques Microcoryphia de l’Asie Orientale: Notes et Descriptions (Insecta; Apterygota). Nouvelle revue d’entomologie 1981, 11, 15–28.
- Mendes, L. New Contribution towards the Knowledge of the Northern Korean Thysanurans (Microcoryphia and Zygentoma: Insecta). Garcia Orta 1991, 18, 67–78.
- Uchida, H. Two Species of Pedetontus from Amami-o-Sima (Thysanura: Machilidae). The entomological society of Japan 1960, 28, 247–250.
- Uchida, H. Pedetontus from Formosa ( Thysanura: Machilidae). Special Bulletin of Lepidopterological Society of Japan 1965, 1, 249–252.
- Wygodzinsky, P.W. Survey of the Microcoryphia (Insecta) of the Northeastern United States and Adjacent Provinces of Canada.
- Xue, L.Z.; Yin, W.Y. Two new species of bristletail from the Tianmu Mountain (Microcoryphia, Machilidae). Contributions from Shanghai Institute of Entomology 1991, 10, 77–86.
- Yu, D.N.; Zhang, W.W.; Zhang, J.Y. Two New Species of the Genus Pedetontus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sinica 2010, 35, 444–450.
- Kaplin, V.G. НОВЫЕ ВИДЫ ЩЕТИНОХВОСТОК РОДА PEDETONTUS SILVESTRI, 1911 (MICROCORYPHIA, MACHILIDAE) C ДАЛЬНЕГО ВОСТОКА. ЗООЛОГИЧЕСКИЙ ЖУРНАЛ 2025, 104, 36–46. [CrossRef]
- Zhang, J.Y.; Song, D.X.; Zhou, K.Y. The Complete Mitochondrial Genome of the Bristletail Pedetontus Silvestrii (Archaeognatha: Machilidae) and an Examination of Mitochondrial Gene Variability within Four Bristletails. Annals of the Entomological Society of America 2008, 101, 1131–1136. [CrossRef]
- Chen, H.Y.; Yu, D.N.; Zhang, J.Y. A new species of the genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sinica 2011, 036, 36–39.
- Deng, K.Z.; Zhang, J.Y.; Yu, D.N. A New Species of the Genus Haslundichilis (Microcoryphia, Machilidae) from China and Redescription of Haslundichilis Hedini (Silvestri). Acta Zootaxonomica Sinica 2011, 36, 882–887.
- Huang, F.S.; Song, Z.S.; Liang, A.P. A New Bristletail Species of the Genus Allopsontus Silvestri (Microcoryphia: Machilidae) from Shaanxi, China. Oriental Insects 2006, 40, 267–272. [CrossRef]
- Kaplin, V.G. Description of a New Species of the Bristletail Genus Pedetontinus Silv. (Thysanura, Machilidae) from China with a Review of Species of This Genus. Entmol. Rev. 2015, 95, 73–86. [CrossRef]
- Kaplin, V.G. A New Species of the Genus Allopsontus Silv. (Microcoryphia, Machilidae) from Northwestern China. Entomological Review 2016, 96, 126–131. [CrossRef]
- Kaplin, V.G. A New Species of Bristletails of the Genus Silvestrichilis Wygodzinsky, 1950 (Archaeognatha: Machilidae) from South China. Far East. entomol. 2019, 23–28. [CrossRef]
- Li, P.; Yu, D.N.; Zhang, J.Y. A New Species of the Genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sinica 2012, 37, 740–746.
- Mendes, L.F.; Gaju-Ricart, M.; Bach de Roca, C.; Molero-Baltanás, R. On Some Silvestri Species of Machilidae (Microcoryphia, Insecta) Which Types Are in the Muséum National d’Histoire Naturelle, Paris. Pedobiologia 2000, 44, 268–284. [CrossRef]
- Shen, C.Y.; Wang, L.Y.; Wu, H.Y.; Zhang, J.Y. A New Species of Silvestrichilis Wygodzinsky, 1950 (Insecta: Microcoryphia) from Wudang Mountain, Hubei, China, with the Description of Both Sexes. Zootaxa 2025, 5621, 437–452. [CrossRef]
- Silvestri, F. Schwedisch-chinesiche wissenschaftliche Expedition nach den nordwestlichen Provinzen Chinas (38. Thysanura, Machilidae). Arkiv for Zoologi 1934, 27, 1–7.
- Song, Z.S.; Huang, F.S.; Liang, A.P. Machilontus (s. str.) Medogensis Song & Huang, Sp. n. from Tibet, the Northernmost Record of the Genus Machilontus Silvestri, 1912 and the First Record of the Family Meinertellidae (Insecta: Microcoryphia: Machiloidea) in China. Zootaxa 2011, 2822, 61–68. [CrossRef]
- Zhang, J.Y. A new record genus and species of Machilinae from China (Microcoryphia: Machilidae). Jiangxi Plant Protection 2010, 33, 57–59.
- Zhang, J.Y.; Li, T. A New Bristletail Species of the Genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sinica 2009, 34, 203–206.
- Zhang, J.Y.; Song, D.X.; Zhou, K.Y. A new species of the genus Pedetontinus (Microcoryphia, Machilidae) from China. Acta Zootaxonomica Sinica 2005, 30, 549–554.
- Zhang, J.Y.; Zhou, K.Y. Descriptions of One New Genus and Six New Species of Machilidae (Insecta: Archaeognatha) from China: Morphological and Molecular Data. Journal of Natural History 2011, 45, 1131–1164. [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA Primers for Amplification of Mitochondrial Cytochrome c Oxidase Subunit I from Diverse Metazoan Invertebrates. Mol. Mar. Biol. Biotechnol. Molecular Marine Biology and Biotechnology 1994, 3, 294–299.
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [CrossRef]
- Guan, J.Y.; Shen, S.Q.; Zhang, Z.Y.; Xu, X.D.; Storey, K.B.; Yu, D.N.; Zhang, J.Y. Comparative Mitogenomes of Two Coreamachilis Species (Microcoryphia: Machilidae) along with Phylogenetic Analyses of Microcoryphia. Insects 2021, 12, 795. [CrossRef]
- Gassner, M.; Dejaco, T.; Schönswetter, P.; Marec, F.; Arthofer, W.; Schlick-Steiner, B.C.; Steiner, F.M. Extensive Variation in Chromosome Number and Genome Size in Sexual and Parthenogenetic Species of the Jumping-Bristletail Genus Machilis (Archaeognatha). Ecology and Evolution 2014, 4, 4093–4105. [CrossRef]
- Shen, S.Q.; Cai, Y.Y.; Xu, K.K.; Chen, Q.P.; Cao, S.S.; Yu, D.N.; Zhang, J.Y. The Complete Mitochondrial Genome of Pedetontus Zhejiangensis (Microcoryphia: Machilidae) and Its Phylogeny. Mitochondrial DNA Part B 2020, 5, 3143–3145. [CrossRef]
- He, K.; Zhang, J.Y.; Deng, K.Z.; Chen, Z. The Complete Mitochondrial Genome of the Bristletail Songmachilis Xinxiangensis (Archaeognatha: Machilidae). Mitochondrial DNA 2013, 24, 99–101. [CrossRef]
- Ma, Y.; He, K.; Yu, P.P.; Yu, D.N.; Cheng, X.F.; Zhang, J.Y. The Complete Mitochondrial Genomes of Three Bristletails (Insecta: Archaeognatha): The Paraphyly of Machilidae and Insights into Archaeognathan Phylogeny. PLoS One 2015, 10, e0117669. [CrossRef]
- Podsiadlowski, L. The Mitochondrial Genome of the Bristletail Petrobius Brevistylis (Archaeognatha: Machilidae). Insect Molecular Biology 2006, 15, 253–258. [CrossRef]
- Carapelli, A.; Liò, P.; Nardi, F.; Van der Wath, E.; Frati, F. Phylogenetic Analysis of Mitochondrial Protein Coding Genes Confirms the Reciprocal Paraphyly of Hexapoda and Crustacea. BMC evolutionary biology 2007, 7, S8. [CrossRef]
- Cameron, S.L.; Miller, K.B.; D’Haese, C.A.; Whiting, M.F.; Barker, S.C. Mitochondrial Genome Data Alone Are Not Enough to Unambiguously Resolve the Relationships of Entognatha, Insecta and Crustacea Sensu Lato (Arthropoda). Cladistics 2004, 20, 534–557. [CrossRef]
- Ronquist, F.; Teslenko, M.; Van Der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian Phylogenetic Inference and Model Choice across a Large Model Space. Systematic biology 2012, 61, 539–542. [CrossRef]
- Xie, J.; Chen, Y.R.; Cai, G.J.; Cai, R.L.; Hu, Z.; Wang, H. Tree Visualization By One Table (tvBOT): A Web Application for Visualizing, Modifying and Annotating Phylogenetic Trees. Nucleic acids research 2023, 51, W587–W592. [CrossRef]
- Kaplin, V.G. On the Fauna of Bristletails of the Genera Petrobius and Trigoniophthalmus (Thysanura, Machilidae) from the Caucasus. Entmol. Rev. 2010, 90, 387–404. [CrossRef]
- Kaplin, V.G. On the Fauna of the Bristletail Family Machilidae (Thysanura) of the Caucasus and Southern Kazakhstan. Entmol. Rev. 2012, 92, 951–965. [CrossRef]

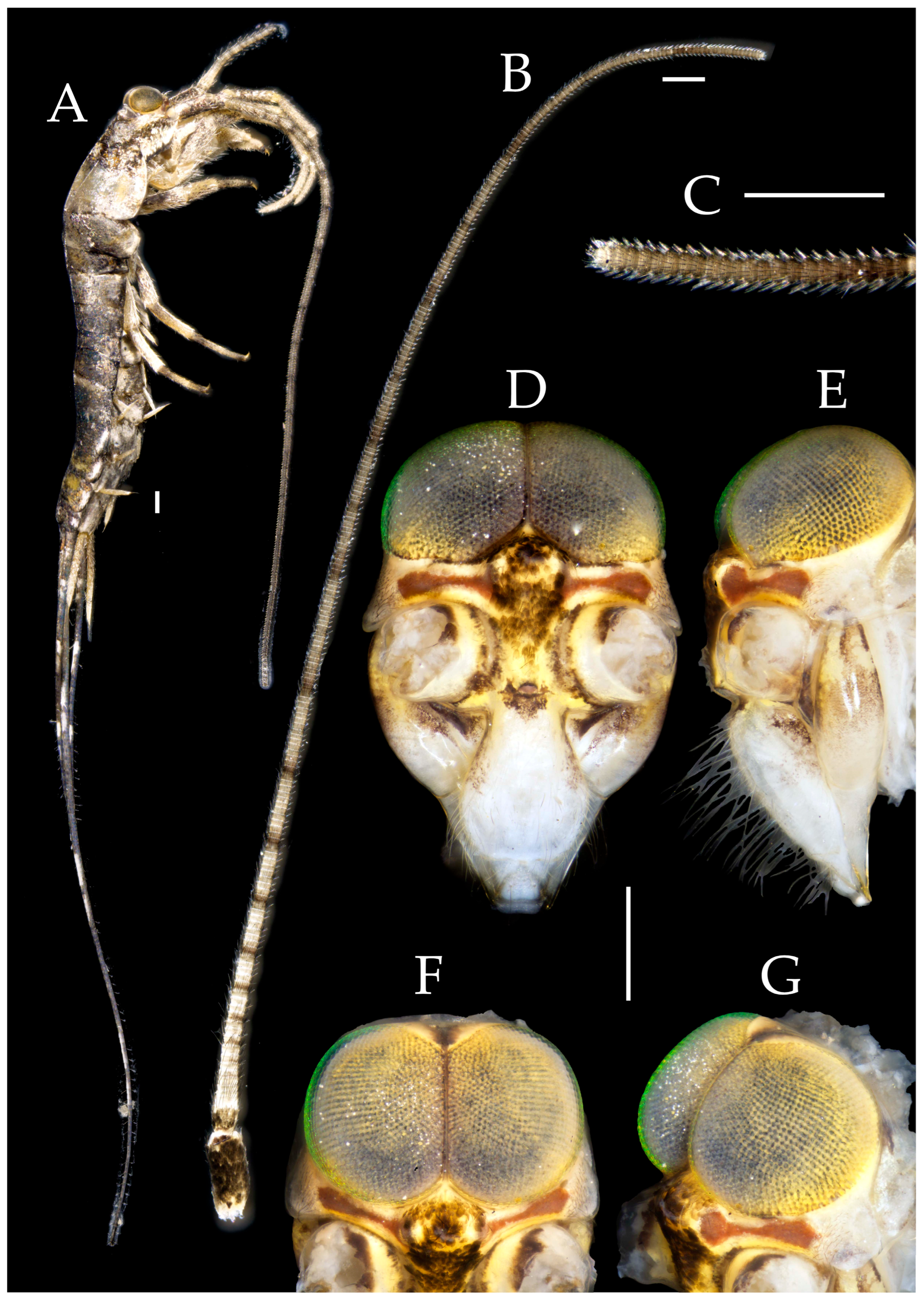
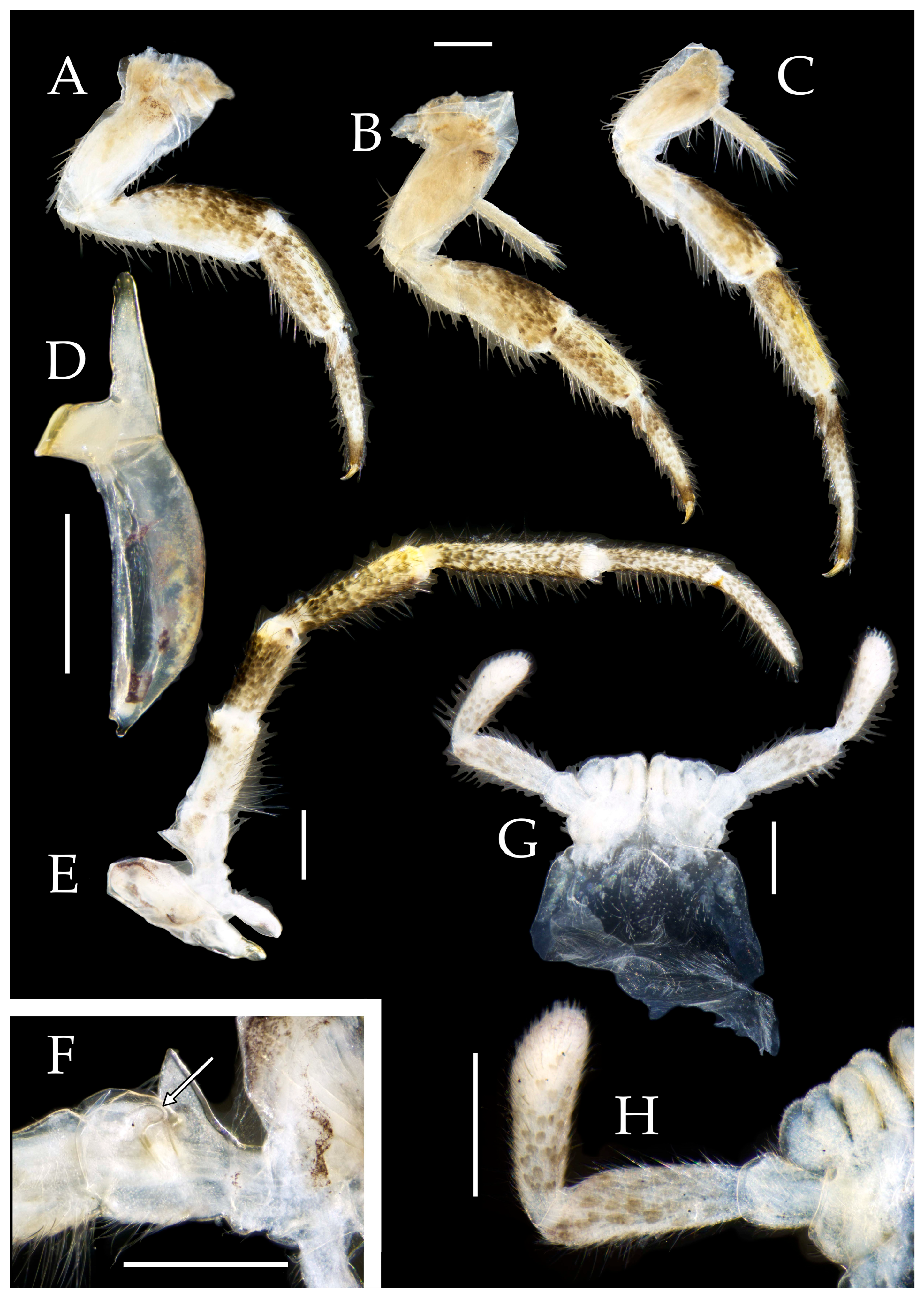
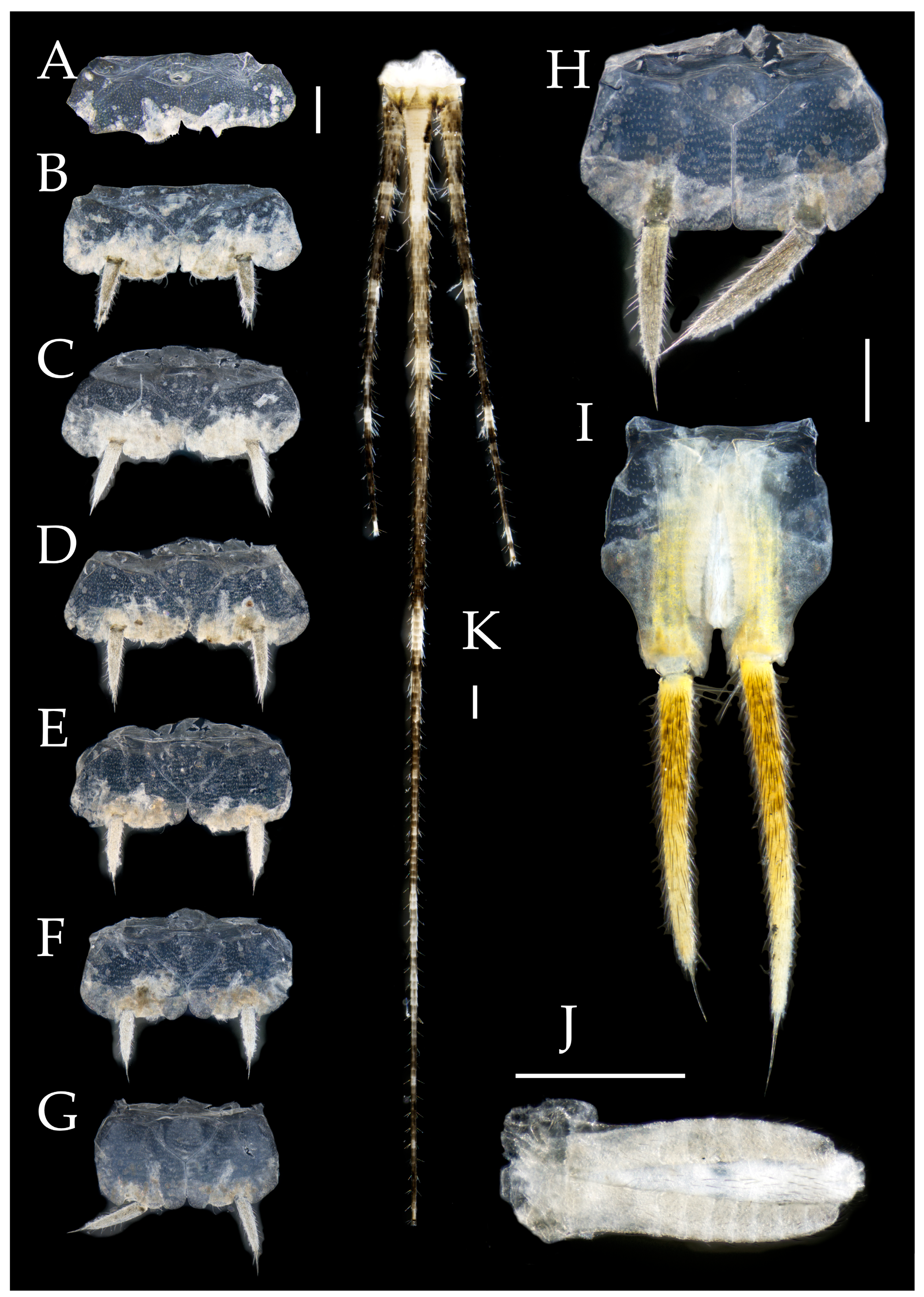







| Ratio | Eye length to width | Eye contact line length to eye length | Ocellus length to width | Distance between inner margins of ocelli to combined width of eyes |
|---|---|---|---|---|
| P.(V.) elegans sp. n. | 1.01–1.09 | 0.59–0.71 | 0.30–0.33 | 0.19–0.22 |
| P.(V.) hezhouensis sp. n. | 0.79–0.84 | 0.32–0.39 | 0.35–0.43 | 0.18 |
| P.(V.) jinxiuensis sp. n. | 0.81–0.85 | 0.20–0.25 | 0.33–0.36 | 0.15–0.16 |
| P.(V.)nanningensissp. n. | 0.82–0.84 | 0.31–0.37 | 0.38–0.40 | 0.10–0.15 |
| P.(V.) shenzhenensissp. n. | 0.84 | 0.41 | 0.40 | 0.19 |
| P.(V.) xanthospilus sp. n. | 0.83–0.84 | 0.27–0.28 | 0.36 | 0.21–0.22 |
| Species | Number of dorsal transparent spines on maxillary palps | Sensory cones on apex of labial palp III | |||
|---|---|---|---|---|---|
| V | VI | VII | |||
| P.(V.) elegans sp. n. | Male | 1–4 | 6–14 | 7–14 | 25–31 |
| Female | 2–3 | 10–16 | 10–14 | 23–26 | |
| P.(V.) hezhouensis sp. n. | Male | 2 | 7 | 6–7 | 50 |
| Female | 4–5 | 11–15 | 11–13 | 35–39 | |
| P.(V.) jinxiuensis sp. n. | Male | 0–1 | 11–12 | 8–9 | 0–43 |
| Female | 4 | 12 | 15 | 0 | |
| P.(V.)nanningensissp. n. | Male | 3 | 12 | 9–11 | 46–50 |
| Female | 6–7 | 16 | 15–16 | 36–40 | |
| P.(V.) shenzhenensissp. n. | Male | N/A | N/A | N/A | N/A |
| Female | 5–6 | 16–17 | 13 | 36–45 | |
| P.(V.) xanthospilus sp. n. | Male | 0–2 | 10 | 12–14 | 55–58 |
| Female | 2–3 | 12–14 | 11–12 | 36–38 | |
| Legs | Number of needle-shaped setae on ventral surfaces | ||
|---|---|---|---|
| Foreleg | Femur | 0 | |
| Tibia | 0 | ||
| Tarsi | Tarsomere I | 2 | |
| Tarsomere II | 5 | ||
| Tarsomere III | 0 | ||
| Midleg | Femur | 0 | |
| Tibia | 0 | ||
| Tarsi | Tarsomere I | 3 | |
| Tarsomere II | 6 | ||
| Tarsomere III | 1 | ||
| Hindleg | Femur | 0 | |
| Tibia | 4 | ||
| Tarsi | Tarsomere I | 3 | |
| Tarsomere II | 10 | ||
| Tarsomere III | |||
| Species | Urosternite I | Urosternite II |
Urosternite III | Urosternite IV | Urosternite V |
Urosternite VI | Urosternite VII | Urosternite VIII (male) |
|---|---|---|---|---|---|---|---|---|
| P. elegans (V.)sp. n. | 129–138 | 78–92 | 88–93 | 92–94 | 86–95 | 74–92 | 99–113 | 123 |
| P. hezhouensis (V.)sp. n. | 129–131 | 78–87 | 78–83 | 82–83 | 81–84 | 91–96 | 89–100 | 91 |
| P. jinxiuensis (V.)sp. n. | 129–141 | 68–76 | 75–77 | 79–82 | 75–84 | 79–95 | 79–97 | 95 |
| P. nanningensis (V.) sp. n. | 123–129 | 67–88 | 68–82 | 78–86 | 83–88 | 79–88 | 80–101 | 84 |
| P. shenzhenensis (V.) sp. n. | 140 | 80 | 73 | 77 | 77 | 77 | 81 | N/A |
| P. xanthospilus (V.)sp. n. | 123–129 | 76–83 | 73–80 | 78–80 | 84–86 | 82 - 83 | 83 - 89 | 80 |
| Ratio | Styli (without supporting spines) length to coxites length of abdominal segment V | Styli (without supporting spines) length to supporting spines length of abdominal segment V | Basal width to length of sternite of abdominal segment V |
|---|---|---|---|
| P. (V.) elegans sp. n. | 0.58–0.64 | 0.40–0.43 | 1.33–1.34 |
| P. (V.)hezhouensis sp. n. | 0.42–0.48 | 0.53–0.58 | 1.08–1.24 |
| P. (V.)jinxiuensis sp. n. | 0.37–0.43 | 0.65–0.71 | 1.18–1.21 |
| P.(V.) nanningensis sp. n. | 0.43 | 0.52 | 1.06–1.36 |
| P.(V.)shenzhenensissp. n. | 0.47 | 0.60 | 1.20 |
| P. (V.)xanthospilus sp. n. | 0.38–0.41 | 0.55–0.68 | 1.19–1.25 |
| Species | Number of segments of anterior gonapophyses | Number of segments of posterior gonapophyses |
|---|---|---|
| P. (V.) elegans sp. n. | 56–59 | 58–63 |
| P. (V.)hezhouensis sp. n. | 60–67 | 53–55 |
| P. (V.)jinxiuensis sp. n. | 60 | 56–58 |
| P.(V.) nanningensis sp. n. | 59 | 50–55 |
| P.(V.)shenzhenensissp. n. | 42–54 | 48–49 |
| P. (V.)xanthospilus sp. n. | 50–51 | 49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





