Introduction
Non-invasive metabolomic analysis of spent blastocyst media (SBM) is a leading new approach to IVF Blastocyst-Embryo competence testing prior to transfer [
1]. Although several developing methods have been reported [
2,
3,
4,
5,
6,
7,
8] the operationalisation of a largely laboratory ‘proof of concept’ omics’ based test to an operational clinical test requires the entire system to be robust to sampling variability, whilst still fulfilling the clinical challenge as accurately as possible.
We have utilised MALDI-ToF mass spectrometry because of its relative ease of use, compared other mass spectrometry methods [
7,
9,
10,
11,
12]. Furthermore, mass spectral intensity data variability, and hence comparability, has been made robust; as has the algorithm that utilises such data to predict probability of viable implantation [
12]. The test is now termed preimplantation metabolic test – Blastocyst competence: PMT-BC.
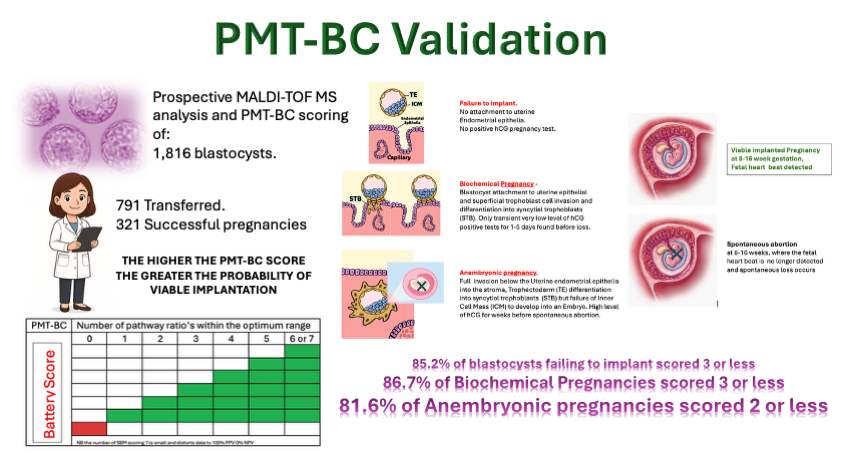
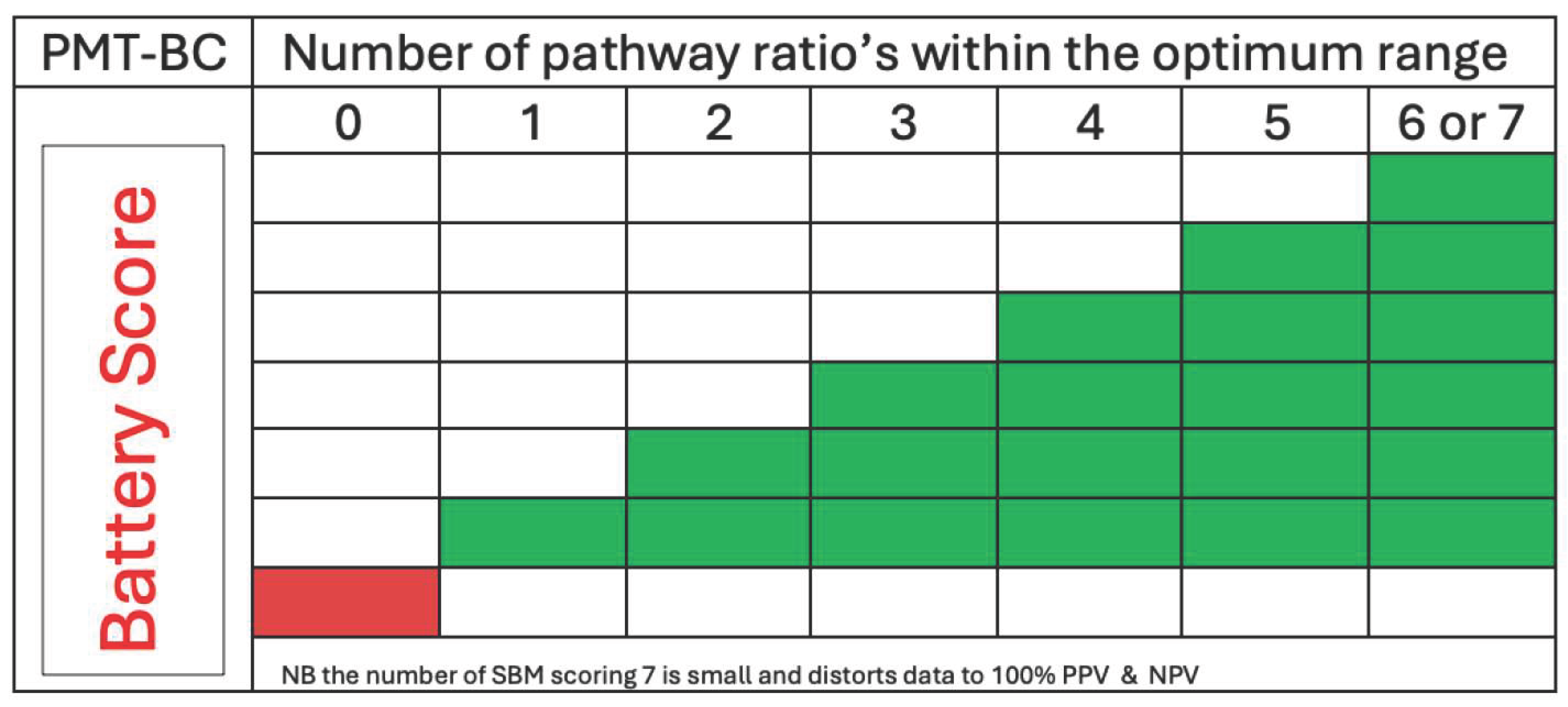
The user focussed PMT-BC SBM metabolomic tests scores 7 metabolic marker pathways and their relative intensity as optimum (green) or non-optimum. The more metabolic marker pathways scored optimum (green) the greater the probability of a viable implantation (see
Figure 1). The importance of any one pathway is not currently weighted.
The prediction mathematics has to allow for uncertainty, as failure to implant and abort a viable blastocyst occurs in 20-30% of transfers due to endometrial receptivity issues. Thus, the prediction is based on cumulative probability [
12].
The initial SBM mass spectral pattern analysis algorithm was developed on a set of 120 PGT-A confirmed Euploid, day5 hatched IVF Blastocyst-Embryo SBM and tested against 440 SBM. The refined Bayesian PMT-BC algorithm was tested on a set of 385
non PGT-A tested, day 5/6 hatched Blastocyst Embryo SBM [
11,
12].
Here we validated the test on a set of 1,840 SBM, regardless of known assessment/selection variables such as aneuploidy, Gardner/morphometric scoring, and maternal age. In addition, day 5, 6 or 7 culture, along with hatching, were not controlled for during selection. Thus, the PMT-BC test was exposed to a large and variable cohort, of Blastocyst-Embryo SBM, in order to validate and establish how robust is the PMT-BC’s ability to resolve viable from non-viable IVF blastocyst-embryo’s for single embryo transfers is?
Sample Cohorts and Analysis
One thousand eight hundred and sixteen, pre outcome MALDI-ToF MS analysed SBM mass spectra collected from a single IVF clinic (VCRM, Reston, Virginia ,USA) were selected from our database and subjected to metabolomic scoring of the BMT-BC data analytic system [
12].
The only inclusion criteria was that the IVF Blastocyst-Embryo SBM samples had to be collected at Day5/6or7 of culture. No selection was made based on maternal age, PGT-A result, Gardner Grade and whether transferred or not. An undefined number of unhatched were included, as the process of “Hatching” from day5 was presumed, but not confirmed.
The samples (and spectra), after BMT-BC scoring, were then sorted according to the following criteria:
Transferred with result known, or not transferred/no data.
Each grouping were examined with respect to their PMT-BC scoring distributions.
Results
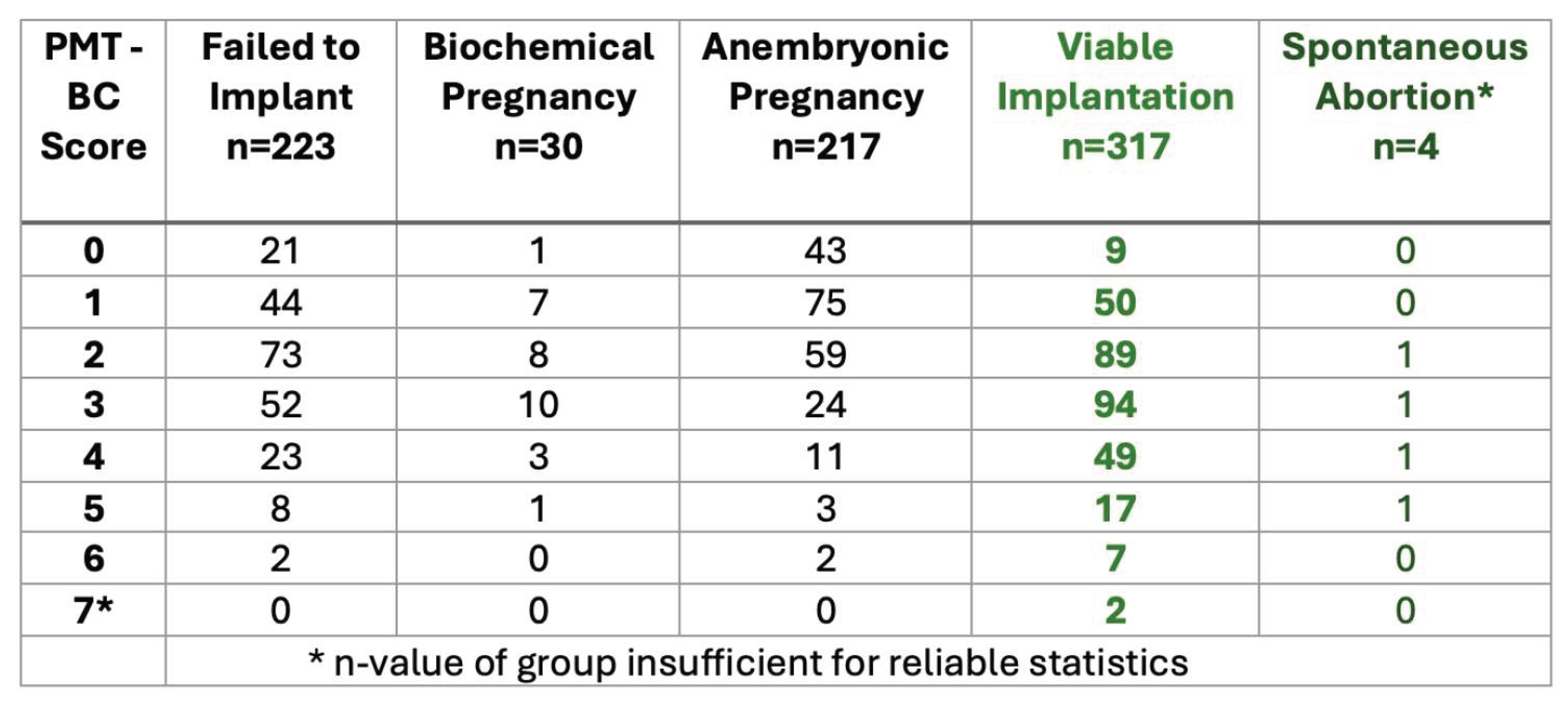
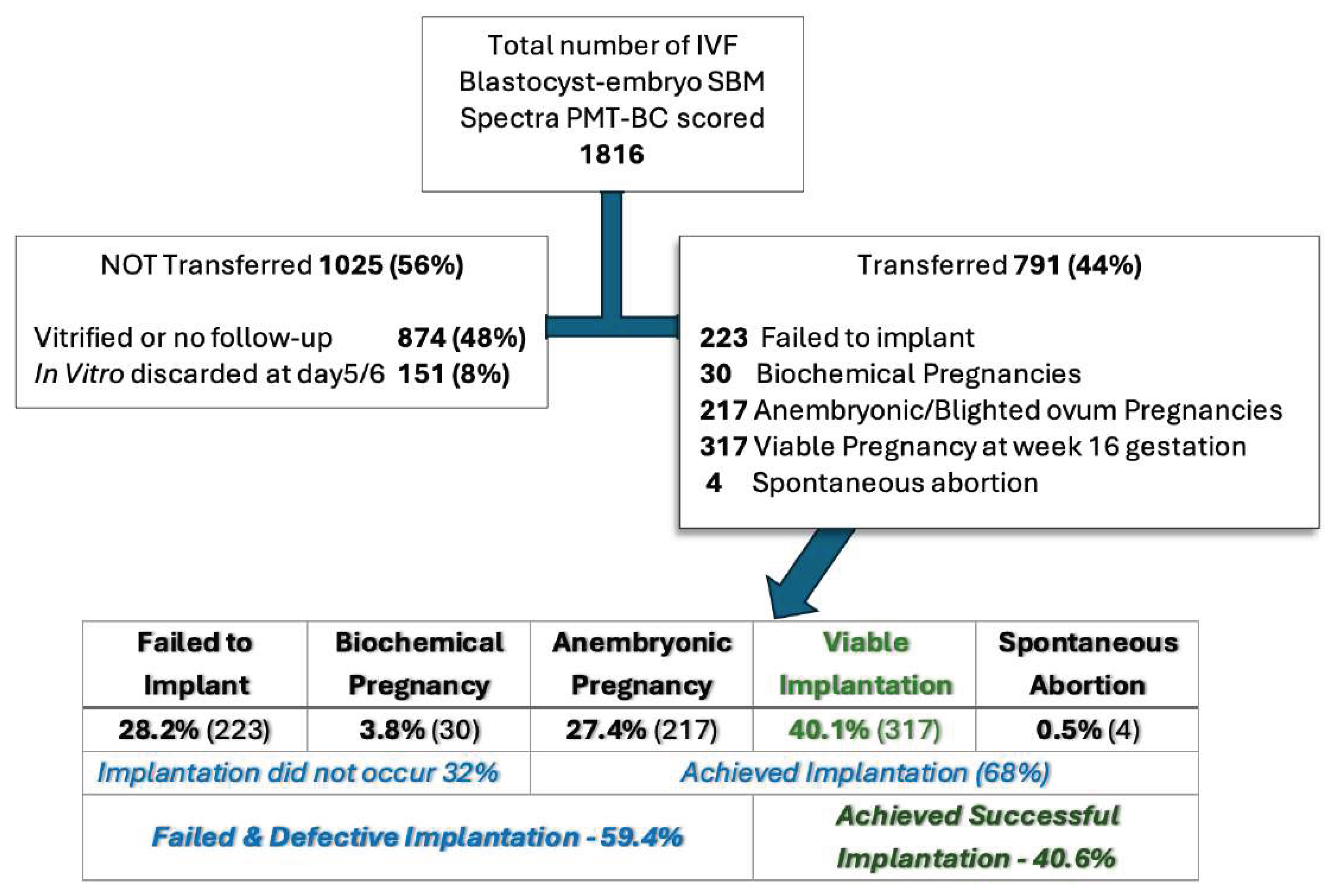
The 1,816 blastocysts, prospectively SBM analysed and PMT-BC scored, were then subdivided and grouped as shown in
Figure 3 :
The IVF clinical aim of a preimplantation IVF Blastocyst-embryo selection test is to increase the rate of successful Implantations.
Analysis the PMT-BC metabolomic scoring for transferred found in each group.
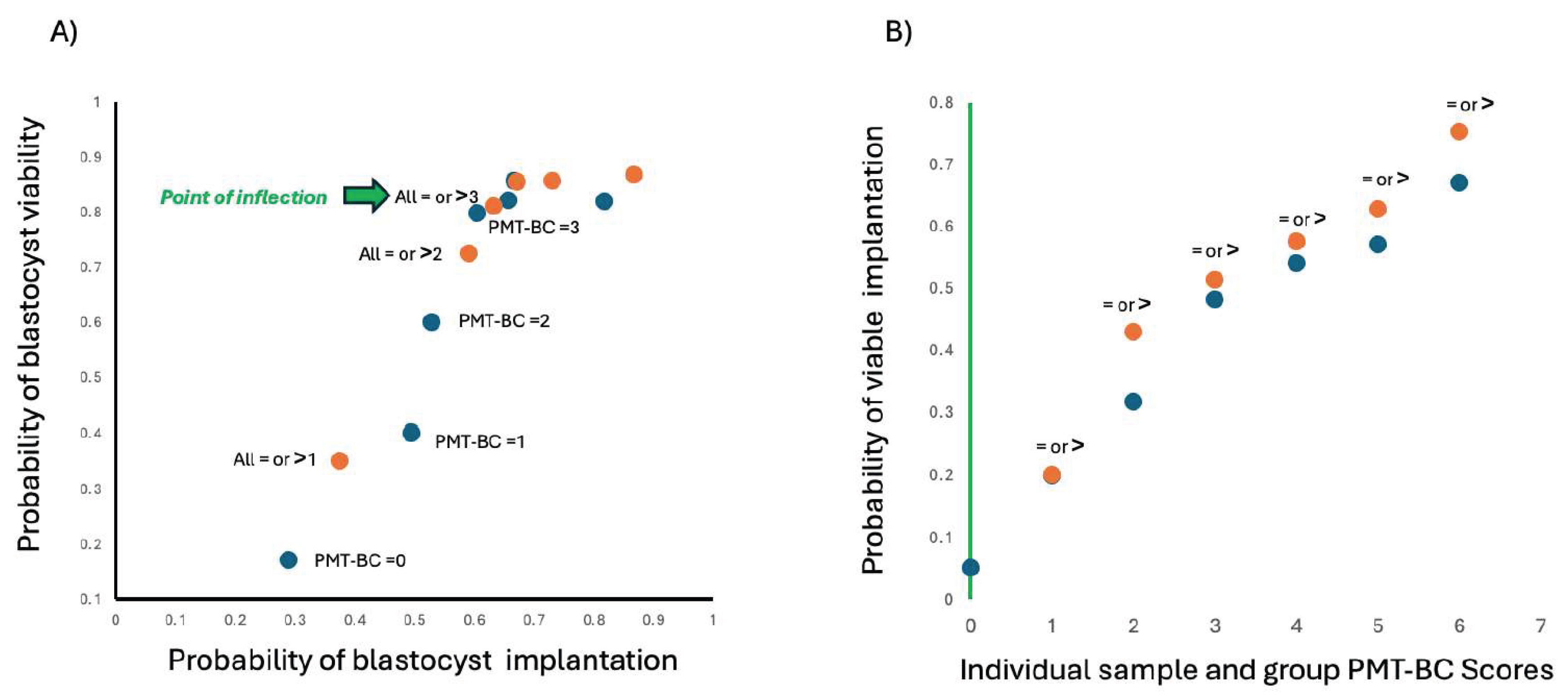
From the score distribution, 50.9% of all failed implantation IVF Blastocyst-embryo and 81.6% of all anembryonic pregnancies scored less than PMT-BC 3. Conversely only 13.9% of all failed implantation IVF Blastocyst-embryos 7.2% of all anembryonic pregnancies scored greater than PMT-BC 3.
Ploting implantation probability versus viability probability (1-probability of an anembryonic pregnancy) at each PMT-BC demonstrates which PMT-BC is the optimum cut off (see
Figure 4A); and enable calculation of probability of a viable implantation at each PMT-BC individual score or cut-off (
Figure 4B).
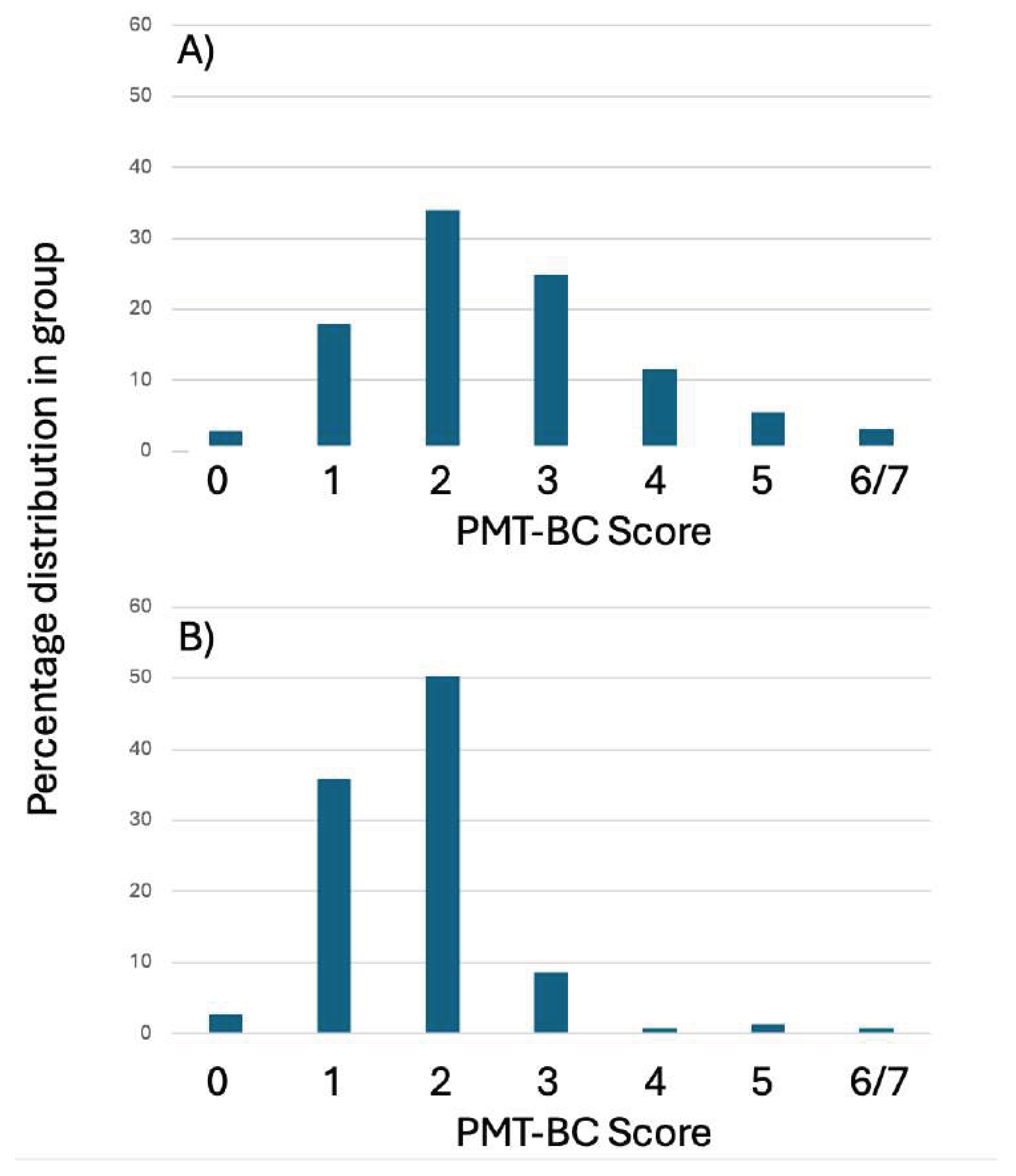
Of the 1025 SBM prospective analysed spectra from non-transferred blastocyst, 151 were discarded by the embryologist and not vitrified. The PMT-BC score profile of these 151 was found to be heavily biased to being PMT-BC <3 (see
Figure 5).
Discussion
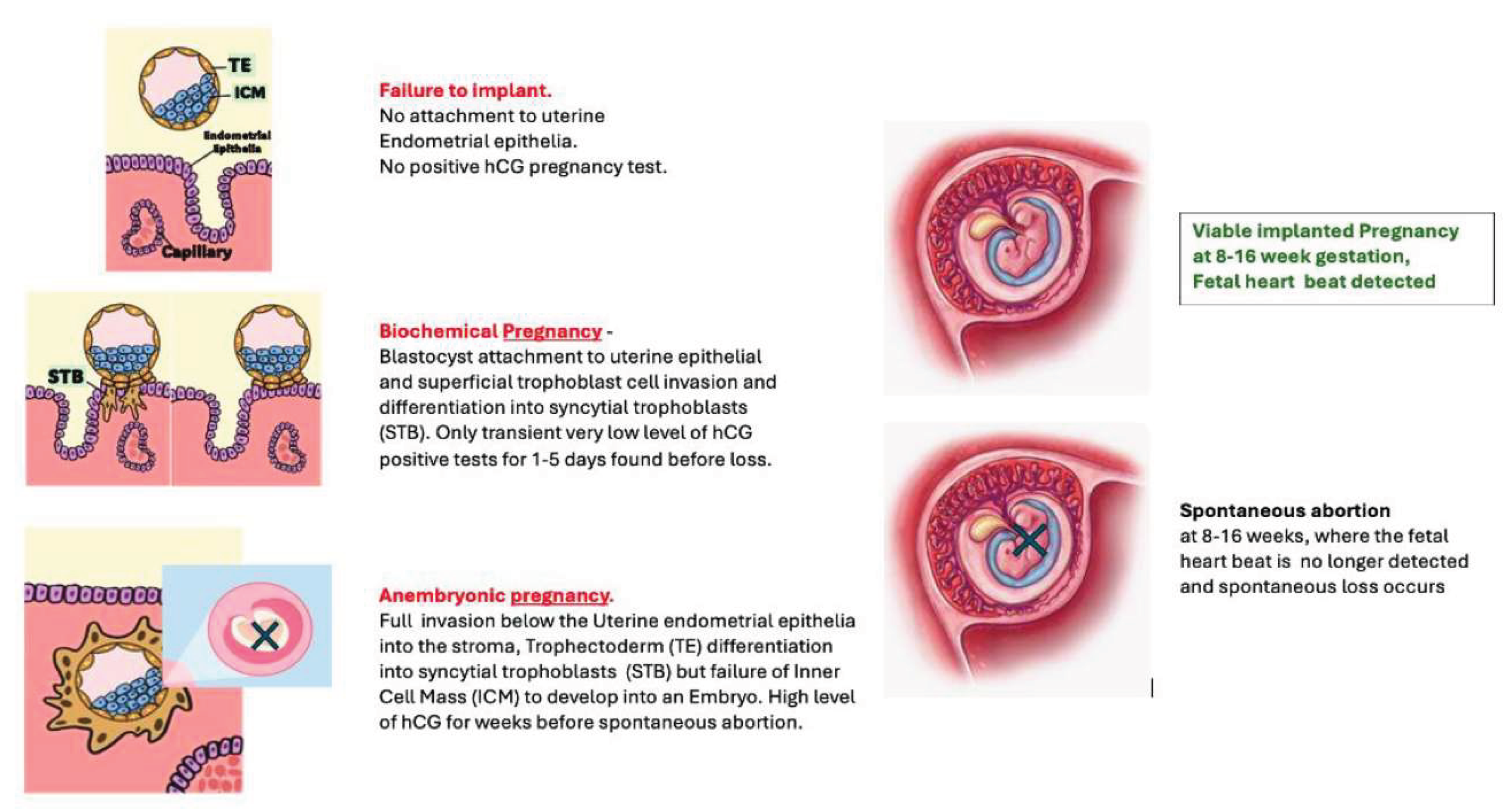
Blastocyst implantation competence is the ability to successfully implant below the uterine endometria and induce a stromal decidual reaction [
13]. And the definition of a successful implanted IVF embryo is one in which an embryo develops from the inner cell mass (ICM) of a successfully implanted blastocyst [
14]. However, implantation alone does not directly correlate with IVF success: Subsequent very early losses can be due to successful implantation, but the embryo fails to develop at all - Anembryonic pregnancy (Blighted ovum) [
15]. This is clinically and functionally distinct from spontaneous abortions due to subsequent arrest of embryo development, cessation of fetal heart beats and consequentially loss. In this respect there is an early stage overlap of blastocyst competence with embryonic competence [
16,
17].
At the very minimum IVF-blastocyst-embryo competence has to exclude biochemical pregnancies. In our metabolomic studies successful implantation metabolic biomarkers overlaps significantly with metabolomic signature of ICM development [
11,
12,
18]. Thus it is difficult to completely resolve solely implantation metabolic biomarkers, from very early ICM development. Therefore, the true clinical picture is that an IVF Blastocyst -Embryo selection test has to address implantation and also ICM development/ organogenesis, as detected by a fetal heartbeat [
19].
The PMT-BC metabolic test has been developed cognisant with all these fundamentals of Embryology/reproductive physiology; and this validation study establishes the PMT-BC score profiles at each IVF failure scenario short of 2nd trimester spontaneous abortion. This is clearly demonstrated in this validation with, PMT-BC test’s ability to predict those blastocysts that, if they do implant, have a high probability of will spontaneous first trimester abortion. i.e., the large group of anembryonic IVF Blastocysts - formerly termed blighted ovum.
In addition, the robustness of the BMT-BC test was examined with these IVF blastocyst-embryos in light of the difficulties possibly encountered by the clinical embryologists when collecting SBM . This included developmental delay in expanded blastocyst formation; so collection on day5, 6 or 7 of In vitro culture, and uncertainty as to whether SBM collection was during the hatching process and not ‘hatched” blastocysts per se. Indeed, undoubtedly a number of unhatched SBM are within the cohort.
In this study group, of the IVF blastocyst-embryo’s selected for transfer by the IVF clinic, the selection standard was Gardner scoring, with supplemental PGT-A testing on some samples. Using this method of predictive selection only 40.6% of those selected for transfer were successful viable blastocyst-embryo implantations.
The validation test set of IVF Blastocyst-embryo samples utilised here, were collected to nullify pre selection bias, such as maternal age, morphometric-visual score, ploidy etc, that would positively bias results [
20]. Hatching is a significant influence in PMT-BC, but we chose to assume hatching had occurred, so as to more closely reflect the degree of uncertainty, inherent in the real life Embryology laboratory, when SBM will be collected/sampled.
As previously described [
12] the lower the BMT-BC score the lower the probability of viable implantation, and scores less than 3 have significantly lower probability of viable implantation. This was confirmed in this validation study.
Furthermore, selection for transfer based on PMT-BC would have increased the viable successful transfer rates by 29-50% if a simple cut off value of PMT-BC 3 or 4 was applied. The impact of the “highest BMT-BC scoring IVF Blastocyst-embryo” based selection for transfer on success rates will be significantly greater.
An additional sub group emerged in the cohort of non-transferred IVF Blastocyst analysed SBM samples. These were 151, embryologist determined, discarded day5/6 blastocysts. This will be the result of extremely poor Gardner-based visual morphology scores, and/or spontaneous disintegration in-vitro. The mass spectra from these discarded blastocysts were also prospectively characterised for their metabolomic PMT-BC scores. Highly significantly, 89.9% of this sub-group had a PMT-BC of less than 3. Thus confirming the negative predictive value (NPV) of poor outcome metabolomic characteristics for PMT-BC scores below 3.
In conclusion, IVF blastocyst-Embryo selection tests, to be of clinical value, have to be non-invasive simple sampling and not severely restricted to a defined age group, or other such criteria, such as patient BMI [
21], or conditional to subgroups such as first pretesting for Euploidy [
22]. SBM analysis for proteomic and metabolomic biomarkers is the most promising approach; and incorporates new high throughput omics’ technology and computing to achieve this aim. However, in addition sampling of SBM criteria has to be as flexible as possible. Indeed some metabolomic test are critically restricted as to the culture media being used - because in their analysis they rely on what metabolites and biomolecules are being consumed rather than secreted [
23,
24]. Such testing are inherently susceptible to huge consistency and reproducibility issues; uncorrected variability in measurement being due entirely to difference in the culture media used. It’s not just a matter of using a consistent culture-media brand formulation, but also inherent batch to batch manufacturing variation that hinders operationalisation of such metabolomic tests [
25,
26]. Focussing on the metabolomic secreteome circumvents these problems [
27,
28].
Here, validation testing of PMT-BC SBM analysis and scoring has shown the PMT-BC assessment process and classification algorithm to be robust and clinically valuable in determining the probability of successful implantation for an individual IVF blastocyst-embryo. This impacts not only clinic strategies of which to transfer, and in what order; but also which to vitrify and freeze thaw for repeat transfers and future family planning. The nature of the scoring also helps in the management of patient/client expectations and emotional well-being during the IVF journey with a high probability of first trimester loss, such as an anembryonic pregnancy.
Morphometric analysis, and genetic testing all have their effect on improving IVF Success. The magnitude of the impact is debatable, as is when to employ the different Blastocyst-Embryo assessment testing; such as only where there is advanced maternal age and repeat miscarriage [
29,
30]. As demonstrated, the PMT-BC test metabolomic assessment is truly orthogonal and significantly additive to morphology and genetic testing in advancing IVF success rates.
Table 1.
Data Matrix of the number of samples from each IVF transfer clinical outcome at specific PMT-BC Scores.
Table 1.
Data Matrix of the number of samples from each IVF transfer clinical outcome at specific PMT-BC Scores.
Ethical Approval
All couples gave consent for the culture media to be used. The study was approved by VCRM’s Institutional Review Board (fshararaVCRMED20230126).
Conflict of Interest Declaration
Dr Ray K Iles is Chief Scientific officer of Embryomic Ltd. and the Inventor of patents EP2976647B1 and EP3198279B1; Dr Sara Nasser is a Medical Advisor to Embryomic Ltd. All other authors declare no conflict of interest.
References
- Tesaik , J. Noninvasive Biomarkers of Human Embryo Developmental Potential PREprints.org 2025. https://www.preprints.org/manuscript/202504.1568/v1.
- Nagy, Z.P.; Sakkas, D.; Behr, B. Symposium: innovative techniques in human embryo viability assessment. Non-invasive assessment of embryo viability by metabolomic profiling of culture media (‘metabolomics’). Reprod Biomed Online. 2008, 17(4):502-507. [CrossRef]
- Vergouw, C.G.; Kieslinger, D.C.; Kostelijk, E.H.; Botros, L.L.; Schats, R.; Hompes, P.G.; Sakkas, D.; Lambalk, C.B. Day 3 embryo selection by metabolomic profiling of culture medium with near-infrared spectroscopy as an adjunct to morphology: a randomized controlled trial. Hum Reprod. 2012, 27(8):2304-2311.
- Rinaudo, P.; Shen, S.; Hua, J.; Qian, S.; Prabhu, U.; Garcia, E.; Cedars, M.; Sukumaran, D.; Szyperski, T.; Andrews, C. (1)H NMR based profiling of spent culture media cannot predict success of implantation for day 3 human embryos. J Assist Reprod Genet. 2012, 29(12):1435-1442.
- Zhao, Q.; Yin, T.; Peng, J.; Zou, Y.; Yang, J.; Shen, A.; Hu, J. Noninvasive metabolomic profiling of human embryo culture media using a simple spectroscopy adjunct to morphology for embryo assessment in in vitro fertilization (IVF). Int J Mol Sci. 2013, 14(4):6556-70.
- Baştu, E.; Parlatan, U.; Başar, G.; Yumru, H.; Bavili, N.; Sağ, F.; Bulgurcuoğlu, S.; Buyru, F. Spectroscopic analysis of embryo culture media for predicting reproductive potential in patients undergoing in vitro fertilization. Turk J Obstet Gynecol. 2017, 14(3):145-150. [CrossRef]
- F. I. Sharara, S.A. Butler, R.J. Pais, R. Zmuidinaite, S. Keshavarez, R.K. Iles “BESST, a Non-Invasive compuational Tool for Embryo selection using mas spectral profiling of embryo culture media. EMJ Repro Health. 2019, 5(1):59-60, https://www.emjreviews.com/reproductive-health/abstract/besst-a-non-invasive-computational-tool-for-embryo-selection-using-mass-spectral-profiling-of-embryo-culture-media/.
- Meng, H.; Huang, S.; Diao, F.; Gao, C.; Zhang, J.; Kong, L.; Gao, Y.; Jiang, C.; Qin, L.; Chen, Y.; Xu, M.; Gao, L.; Liang, B.; Hu, Y. Rapid and non-invasive diagnostic techniques for embryonic developmental potential: a metabolomic analysis based on Raman spectroscopy to identify the pregnancy outcomes of IVF-ET. Front Cell Dev Biol. 2023, 11:1164757.
- Iles, R.K.; Sharara, F.I.; Zmuidinaite, R.; Abdo, G.; Keshavarz, S.; Butler, S.A. Secretome profile selection of optimal IVF embryos by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J Assist Reprod Genet. 2019, 36(6):1153-1160. [CrossRef]
- Pais, R.J.; Sharara, F.; Zmuidinaite, R.; Butler, S.; Keshavarz, S.; Iles, R. Bioinformatic identification of euploid and aneuploid embryo secretome signatures in IVF culture media based on MALDI-ToF mass spectrometry. J Assist Reprod Genet. 2020, 37(9):2189-2198. [CrossRef]
- Iles, R.K., Zmuidinaite, R., Iles, J.K., Nasser, S. The influence of defining desired outcomes on prediction algorithms: Mass Spectral analysis of Spent blastocyst media in Ai/ML prediction of IVF Embryo implantation potential – Implantation, or Viability, or both? PREprints, 2025. [CrossRef]
- Iles, RK, Zmuidinaite R, Iles JK, Nasser S. The development of a robust and user friendly preimplantation metabolic test of blastocyst competence. PMT-BC: Mass Spectral analysis of Spent blastocyst media in Ai/ML-Bayesian prediction of IVF Embryo implantation potential. PREprints, 2025. [CrossRef]
- van den Brûle, F., Berndt, S., Simon, N., Coulon C, Le Goarant J, Munaut C, Noël A, Frankenne F, Foidart JM. Trophoblast invasion and placentation: molecular mechanisms and regulation. Chem Immunol Allergy. 2005;88:163-180. [CrossRef]
- Ai J, Jin L, Zheng Y, Yang P, Huang B, Dong X. The Morphology of Inner Cell Mass Is the Strongest Predictor of Live Birth After a Frozen-Thawed Single Embryo Transfer. Front Endocrinol (Lausanne). 2021, 12:621221. [CrossRef]
- Macklon NS, Geraedts JP, Fauser BC. Conception to ongoing pregnancy: the ‘black box’ of early pregnancy loss. Hum Reprod Update. 2002, 8(4):333-43. [CrossRef]
- Wilcox, A. J., Baird, D.D., Weinberg C.R. (). Time of implantation of the conceptus and loss of pregnancy. New England Journal of Medicine, 1999, 340:1796–1799. [CrossRef]
- Kurjak, A., Kupesic, S. and Tripalo, A. Ultrasound evaluation of abnormal early pregnancy. In: Donald School Textbook of Ultrasound in Obstetrics & Gynecology, 2004 p.202.
- Chousal JN, Morey R, Srinivasan S, Lee K, Zhang W, Yeo AL, To C, Cho K, Garzo VG, Parast MM, Laurent LC. Molecular profiling of human blastocysts reveals primitive endoderm defects among embryos of decreased implantation potential. Cell Reports. 2024; 43(2):113701. [CrossRef]
- Zegers-Hochschild, F., Adamson, GD., Dyer, S., Racowsky, C., de Mouzon J et al. (2017). The International Glossary on Infertility and Fertility Care,.Fertility and Sterility, 2017, 108(3):393–406. [CrossRef]
- Kragh, M.F., Karstoft, H. Embryo selection with artificial intelligence: how to evaluate and compare methods?. J Assist Reprod Genet 2021, 38:1675–1689. [CrossRef]
- Qiu, J., Li, P., Dong, M. et al. Personalized prediction of live birth prior to the first in vitro fertilization treatment: a machine learning method. J Transl Med 2019, 17:317. [CrossRef]
- Majumdar G, Majumdar A, Verma IC, Upadhyaya KC. Relationship Between Morphology, Euploidy and Implantation Potential of Cleavage and Blastocyst Stage Embryos. J Hum Reprod Sci. 2017, 10(1):49-57.
- Siristatidis C, Dafopoulos K, Papapanou M, Stavros S, Pouliakis A, Eleftheriades A, Sidiropoulou T, Vlahos N. Why Has Metabolomics So Far Not Managed to Efficiently Contribute to the Improvement of Assisted Reproduction Outcomes? The Answer through a Review of the Best Available Current Evidence. Diagnostics (Basel). 2021 11(9):1602. [CrossRef]
- Nami S, Govahi A, Najjar N, Ghasemi S, Rezaei F, Amjadi F, Taheripak G. Metabolomic profiling of embryo culture media in patients with repeated implantation failure during assisted reproductive technology cycles. Clin Exp Reprod Med. 2024, 51(3):260-267. [CrossRef]
- Catherine M Castillo, Joyce Harper, Stephen A Roberts, Helen C O’Neill, Edward D Johnstone, Daniel R Brison, The impact of selected embryo culture conditions on ART treatment cycle outcomes: a UK national study, Human Reproduction Open. 2020, 2020(1):hoz031. [CrossRef]
- Cabello-Pinedo, S., Abdulla, H., Mas, S. et al. Development of a Novel Non-invasive Metabolomics Assay to Predict Implantation Potential of Human Embryos. Reprod. Sci. 2024, 31:2706–2717. [CrossRef]
- de Oliveira Fernandes, G., de Lima, C. B., Fidelis, A. A. G., Milazzotto, M. P., & Dode, M. A. N.). Metabolic signature of spent culture media shows lipid metabolism as a determinant of pregnancy outcomes. Reproduction in Domestic Animals, 2023, 58:117–128. [CrossRef]
- Iles, RK, Zmuidinaite R, Iles JK, Nasser S. The influence of Hatching on blastocyst metabolomic analysis: Mass Spectral analysis of Spent blastocyst media in Ai/ML prediction of IVF Embryo implantation potential– To Hatch or not to Hatch? PREprints.org 2025. [CrossRef]
- Simopoulou, M., Sfakianoudis, K., Maziotis, E. et al. PGT-A: who and when? A systematic review and network meta-analysis of RCTs. J Assist Reprod Genet38, 1939–1957 (2021). [CrossRef]
- Tong, J.; Niu, Y.; Wan, A.; et al. Next-Generation Sequencing (NGS)-Based Preimplantation Genetic Testing for Aneuploidy (PGT-A) of Trophectoderm Biopsy for Recurrent Implantation Failure (RIF) Patients: a Retrospective Study. Reprod. Sci. 2021, 28, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).