Introduction
Heart Failure is a complex clinical syndrome that occurs when the heart is unable to pump adequate amounts of blood to meet the body’s metabolic demands. [
1] It remains a leading cause of morbidity and mortality, owing to its life-threatening nature, which leads to frequent hospitalizations and significant decline in quality of life.[
2] Serving as the final common pathway for numerous cardiovascular disorders, it arises from structural or functional impairments that compromise ventricular filling or the ejection of blood. Despite substantial progress in diagnostic capabilities and therapeutic interventions, heart failure continues to remain a significant global health hazard.
Heart failure (HF) affects an estimated 64 million people worldwide, with rising prevalence fueled by ageing population and improved survival following acute cardiac events.[
3] In the United Kingdom, HF accounts for 1–2% of all hospital admissions, and over 900,000 people are currently living with this condition. Despite advances in pharmacological treatments and device-based interventions, the prognosis still remains quite poor. HF continues to carry a 5-year mortality risk of approximately 50%, comparable to many forms of cancers.
The economic implications of Heart Failure are equally alarming. HF management alone contributes to 2% of the NHS annual budget, largely driven by unplanned hospitalisations and readmissions. Alarmingly, up to 25% of patients are readmitted within 30 days of discharge, and nearly 50% within 6 months. These statistics highlight a persistent challenge in achieving timely diagnosis, optimal early intervention, and effective long-term disease management.
The combination of high prevalence, substantial morbidity and mortality, and considerable healthcare costs underscores the urgent need for enhanced strategies in HF prevention, early detection, and comprehensive management. Interventions that focus on patient education, self-care optimisation, multidisciplinary care models, and innovative technologies for remote monitoring may hold promise in reducing readmission rates and improving outcomes.
Many of these adverse outcomes arise not from a lack of effective treatment, but from the delayed recognition of hemodynamic instability that develops quietly in the background. Early, subtle indicators of declining cardiac function are frequently overlooked, allowing the condition to progress until patients present with advanced decompensation. In Heart Failure, elevated left ventricular filling pressures and impaired diastolic compliance often leads to venous congestion and interstitial fluid accumulation. Such changes manifest as weight gain sometimes days before overt clinical symptoms such as dyspnoea or oedema appear. This highlights the critical need for practical and highly sensitive tools capable of identifying deterioration before evident clinical manifestations emerge.
The pathophysiology of heart failure is characterised not merely by mechanical pump dysfunction but also by neurohormonal dysregulation.[
9] Activation of sympathetic nervous system (SNS) and the Renin- Angiotensin Aldosterone System (RAAS) perpetuates vasoconstriction, sodium retention and water accumulation. Daily weight gain is not just a marker of fluid excess but an outward expression of sympathetic overdrive. Repeated cycles of fluid retention, diuretic administration and rebound neurohormonal activation creates a continuous rhythm of hemodynamic instability. Unexplained or persistent weight fluctuations may be conceptualized as proxy for heightened neurohormonal stress, signalling an inadequately suppressed sympathetic drive that accelerates myocardial strain and metabolic exhaustion.[
10]
At the core of HF progression lies cardiac remodelling—a maladaptive cascade of structural, functional and molecular alterations that occur within the myocardium in response to sustained hemodynamic stress. Initially, remodeling may serve as a compensatory mechanism to maintain cardiac output, but over time, it often becomes maladaptive, leading to progressive functional decline. Adverse remodeling is associated with reduced ejection fraction, worsening heart failure symptoms, and higher rates of hospitalization. Understanding and targeting the mechanisms of remodeling is therefore crucial in modern cardiology, not only for treating established heart failure but also for preventing disease progression in at-risk populations. Evidence from landmark studies like the Framingham Heart Study [
4] and the Multi-Ethnic Study of Atherosclerosis (MESA) [
5], has shown that individuals with long-standing hypertension often develop early myocardial changes well before the onset of symptomatic heart failure.
Hemodynamic stress refers to the dynamic load placed on the heart and vascular system due to fluctuations in blood volume, arterial pressure, and cardiac output. In patients with heart failure, even subtle variations in preload and afterload can impose a substantial burden on an already compromised myocardium. Such fluctuations arise from a variety of triggers, including dietary sodium excess, non-adherence to medications, fluid overload, poorly controlled hypertension, or increased sympathetic drive due to physiological and emotional stress. When persistent or recurrent, this stress initiates a cascade of compensatory mechanisms designed to preserve perfusion, most notably the activation of the sympathetic nervous system (SNS) and the renin–angiotensin–aldosterone system (RAAS). Initially, these neurohormonal responses are beneficial, promoting vasoconstriction, fluid retention, and increased heart rate to maintain cardiac output. However, chronic stimulation leads to maladaptive effects—elevated systemic vascular resistance, ventricular wall stress, myocardial oxygen demand, and sodium retention—which further burden the heart and exacerbate fluid congestion.
Over time, the recurring cycle of pressure and volume overload contributes to progressive cardiac remodelling, particularly in structurally vulnerable areas of the myocardium. Moreover, hemodynamic stress extends beyond its mechanical impact, triggering neurohormonal activation and inflammatory pathways that accelerate myocardial fibrosis and dysfunction. Notably, the clinical indications of hemodynamic stress—such as increased weight, subtle oedema, or changes in heart rate and blood pressure—often appear days before overt decompensation. These small but meaningful changes are easily missed without routine monitoring. Thus, in the context of heart failure, hemodynamic stress acts as both a catalyst and a barometer of disease progression, an often silent but potent force that fuels adverse remodelling and worsening outcomes, unless detected early and addressed through proactive surveillance.
Within this pathophysiological framework, daily weight monitoring serves as a simple yet powerful diagnostic signal. It can be used to detect deterioration in patients with heart failure and can become a powerful tool to prevent hospital admission, reducing the overall burden of healthcare facilities worldwide.[
6] Beyond its traditional role in identifying fluid retention, weight tracking can provide real-time insights into subtle hemodynamic changes and early neurohormonal activation. Even a modest increase of 1.5–2.0 kg over 2–3 days may precede overt clinical signs of decompensation. Despite robust support from both NICE and ESC guidelines, adherence to daily weight monitoring remains disappointingly low across both community and inpatient care, limiting its potential to reduce avoidable admissions and ease the strain on healthcare systems worldwide.
In addition, daily weight tracking plays a critical role in protecting patients from hemodynamic stress by preventing sudden increases in preload and afterload that strain the cardiac chambers. Sustained hemodynamic stability allows the myocardium to operate under more favorable loading conditions, promoting reverse or halted remodelling and preserving ventricular compliance. This proactive approach not only prevents acute exacerbations but also interrupts the cycle of neurohormonal activation, inflammation, and myocardial fibrosis that often accompanies recurrent decompensation. If a few numbers on a scale can predict survival, why aren’t we listening?
By enabling early recognition of fluid retention, daily weight monitoring acts as an accessible and powerful strategy to prevent worsening cardiac remodeling in patients with heart failure. Even small increases in body weight can indicate subtle shifts in fluid balance, often preceding overt symptoms of decompensation. By identifying these changes early, clinicians can initiate timely interventions such as diuretic adjustment, dietary modifications, or closer follow-up, reducing the mechanical and metabolic stress on the myocardium. Over time, limiting recurrent episodes of volume overload helps to minimize the chronic pressure and volume stresses that drive structural and functional deterioration of the heart. Thus, daily weight monitoring acts as a low-cost, patient-engaged strategy that directly links symptom surveillance to the physiological protection of the heart.
This retrospective audit investigates whether the absence of daily weight monitoring among hospitalised HF patients is associated with worsened clinical outcomes, including ICU admissions, readmissions, and mortality. It further examines whether this overlooked parameter may serve as an early, non-invasive marker of hemodynamic stress and neurohormonal imbalance, potentially foreshadowing segmental cardiac remodelling. Through this study we wish to see if the humble bathroom scale can be the missing stethoscope in heart failure care.
Materials and Methods
Study Design and Study Setting
This was a retrospective Audit conducted at Mid-Yorkshire NHS Trust, Wakefield, United Kingdom between November 2023 to July 2024.
Data Collection
The Audit was performed in 2 cycles between November 2023 to July 2024. Cycle 1 was conducted between 1st November 2023 to 15th January 2024. Cycle 2 was conducted between 1st April 2024 and 1st July 2024. The data was sourced from hospital records and supplied by the Information Management Team of the Mid-Yorkshire NHS Trust.
Sample Size Estimation Statement
Universal sampling technique was used for both the cycles.
Inclusion Criteria
All patients with a confirmative diagnosis of Congestive Cardiac Failure on Diuretic Therapy were a part of this retrospective Audit.
Exclusion Criteria
All patients with a confirmed diagnosis of hypertensive heart and renal disease accompanied by both congestive cardiac failure and renal failure, as well as those with hypertensive heart disease without congestive heart failure, were excluded from the study.
Audit Procedure
According to the NICE Clinical Guidelines [
8], it is recommended to closely monitor a patient’s renal function, weight, and urine output during diuretic therapy. Following this guideline, we assessed whether the weight of patients admitted to the hospital during the first cycle was documented. We also assessed the outcomes of patients for whom the weights were not measured on daily basis. The outcomes involved ICU Admissions. readmissions and mortality. Based on our findings, we implemented targeted interventions to improve compliance. First, we introduced “Daily Weight” as a dropdown option in Med Chart within E-Meds (Electronic Prescription Chart at Mid- Yorkshire NHS Trust) allowing it to be prescribed for all patients with heart failure, thereby prompting nursing staff to record weights during medication rounds. Second, to raise awareness among both medical and nursing staff as well as doctors, we created posters highlighting the importance of daily weights in heart failure and circulated an informative reminder email to all doctors. After implementing these measures for 2 months, we repeated the data collection from the same unit and analysed the results to assess the impact of our interventions.
Statistical Analysis
The data was accurately entered from the Hospital Records in Microsoft Excel Version 13 and was sent for statistical analysis using the IBM SPSS Version 21. Associations between different outcome variables were measured by Pearson’s Chi Square Test and Likelihood Ratio. All the tests were applied keeping confidence interval at 95% and p < 0.05 was considered to be statistically significant.
Results
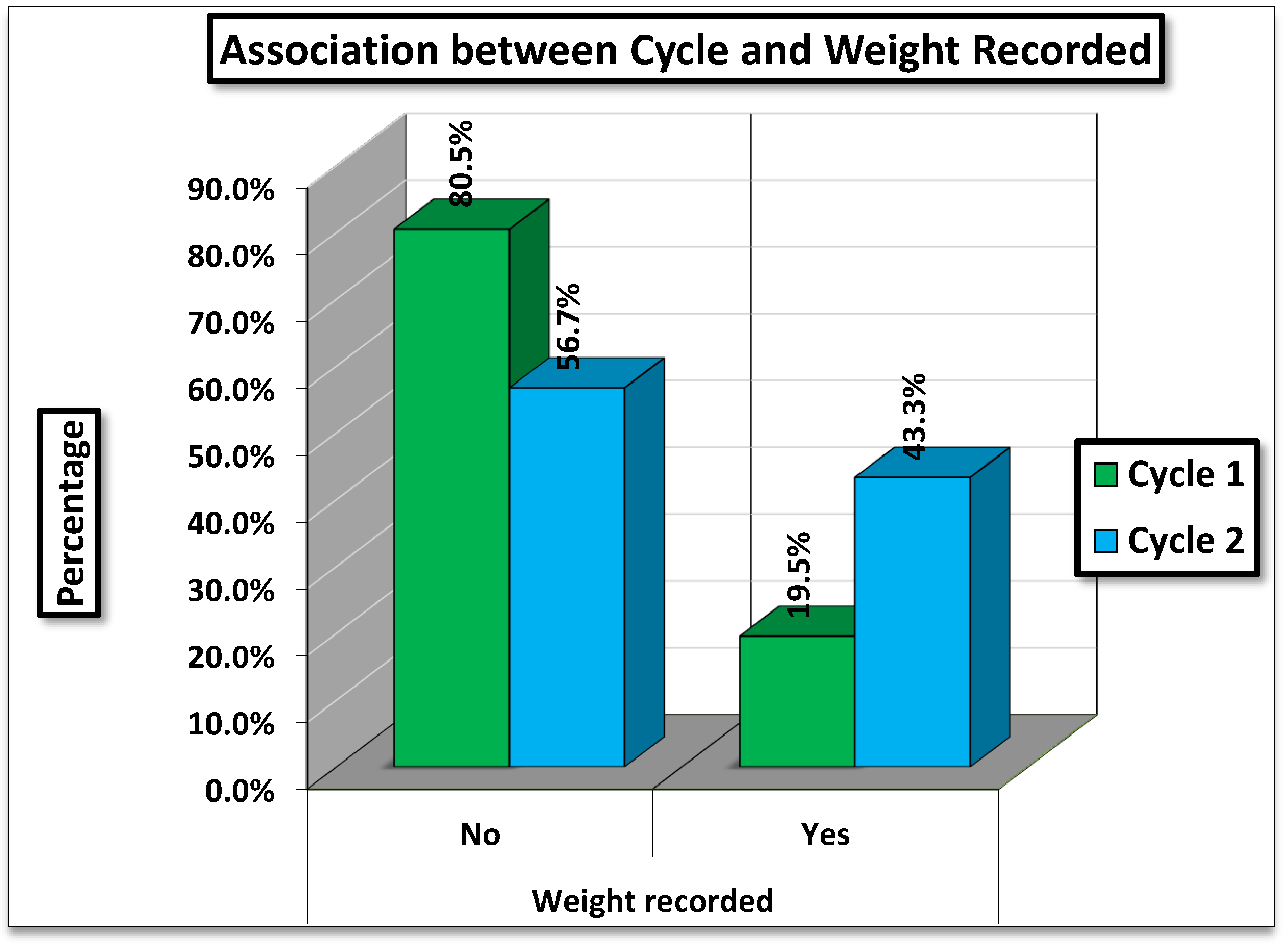
Table 1 presents the comparison of weight recording between two cycles: one before the intervention (Cycle 1) and one after the intervention (Cycle 2).
Cycle 1 (Nov 2023 to Jan 2024): Daily weight charting was done for only 19.5% of patients, with the majority (80.5%) having no daily weight charting.
Cycle 2 (Apr- Jul 2024): Weight Charting improved substantially, being documented in 43.3% of visits, while 56.7% had no daily weight charting.
Statistical Analysis
The difference in weight recording between Cycle 1 and Cycle 2 is highly statistically significant (p < 0.001). This indicates a marked and meaningful improvement in documentation practices between the two observation periods.
Figure 1.
Association between Cycle and Weight Recorded.
Figure 1.
Association between Cycle and Weight Recorded.
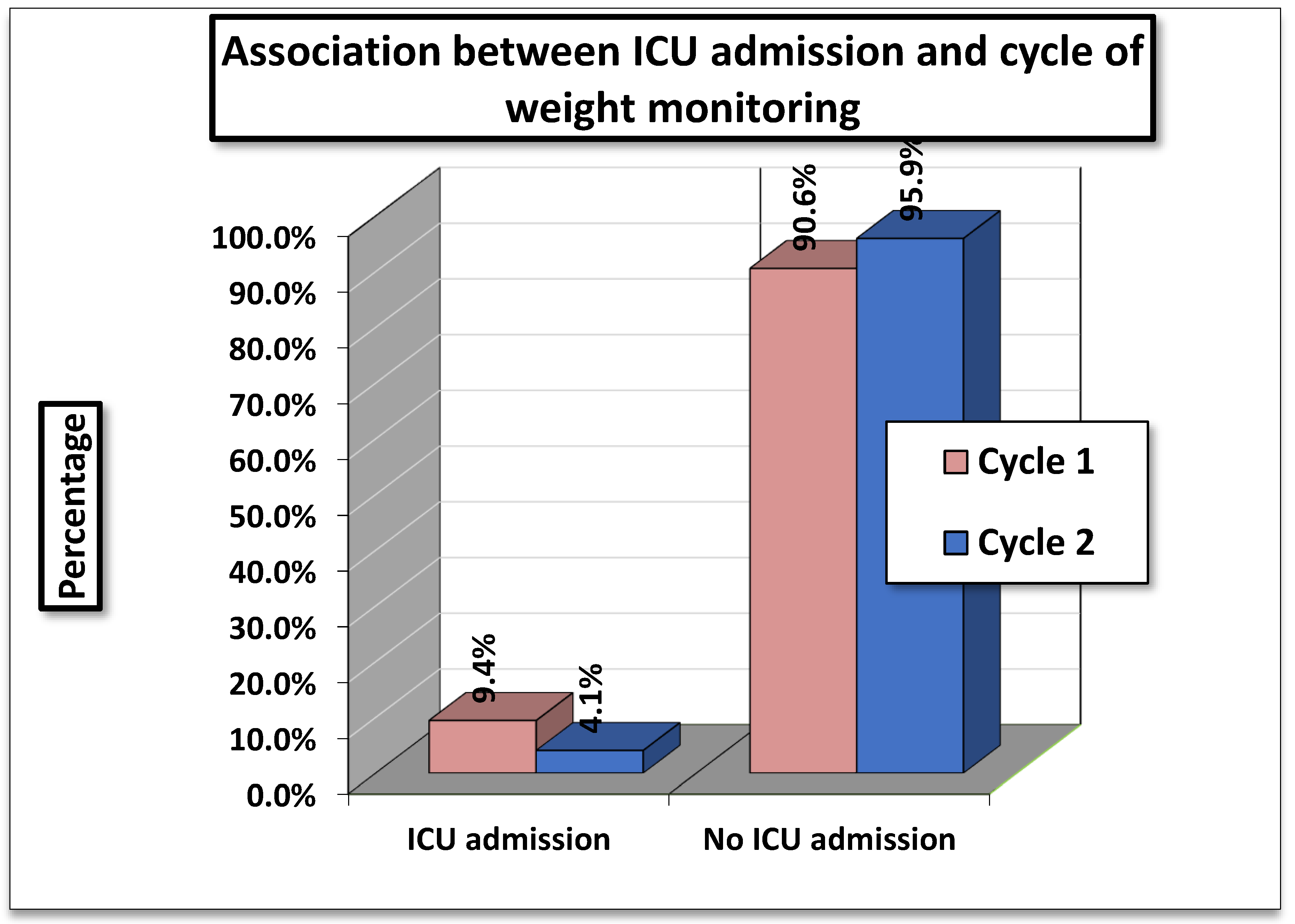
Table 2 shows the association between ICU Admissions and Cycle of Weight Monitoring. In Cycle 1 (01/11/2023-15/01/2024), 9.4 % (49/519) of patients required ICU admission compared to 4.1 % (20/492) in Cycle 2 (01/04/2024- 01/07/2024).
The statistical tests showed a Pearson Chi- Square value of 14.786 (df=2, p=0.001) and a Likelihood Ratio of 15.688 (p= 0.001). Both results indicate a highly significant association between cycle of weight monitoring and ICU admissions.
Interpretation
The findings suggest that ICU admissions were significantly higher in Cycle 1 compared to Cycle 2. Since, the p- values are <0.01, this difference is unlikely to be due to chance, implying that the cycle of monitoring (or related factors during these periods) had a measurable impact on ICU admission rates.
Figure 2.
Association Between ICU Admission and Cycle of Weight Monitoring.
Figure 2.
Association Between ICU Admission and Cycle of Weight Monitoring.
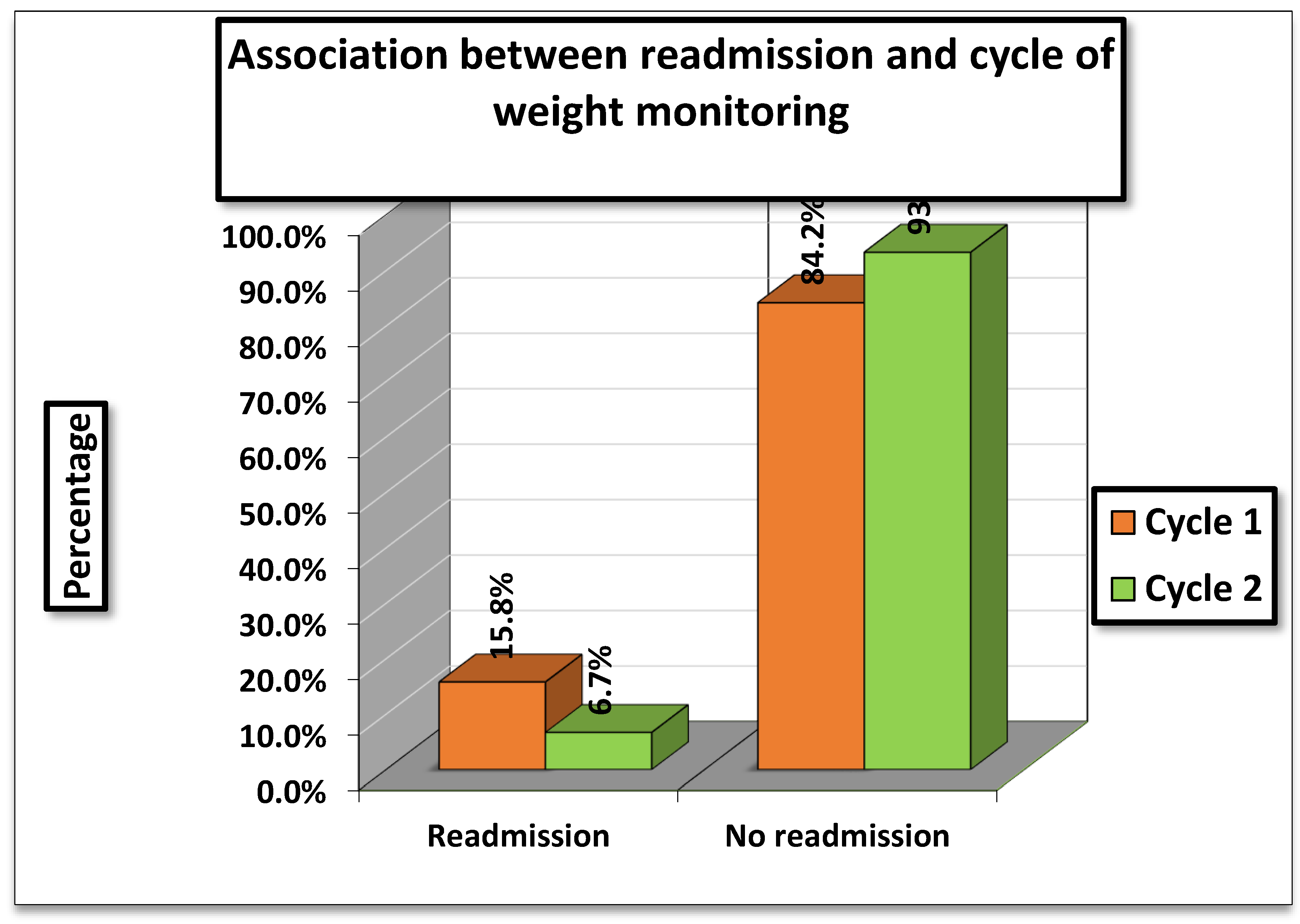
The association between hospital readmissions and cycle of weight monitoring was evaluated. In
Cycle 1 (01/11/2023–15/01/2024), 15.8% (82/519) of patients were readmitted, whereas in
Cycle 2 (01/04/2024–01/07/2024), readmissions were lower at
6.7% (33/492). (
Table 3)
The statistical tests showed a Pearson Chi-square value of 14.786 (df = 2, p = 0.004) and a Likelihood Ratio of 15.688 (p = 0.007). Although the percentages indicate fewer readmissions in Cycle 2, both tests were interpreted as not statistically significant in this table
Interpretation
While readmission rates appeared higher in Cycle 1 compared to V=Cycle 2, the association was not statistically significant, meaning the observed difference could be due to chance. Therefore, no definitive relationship can be established between cycle of weight monitoring and hospital readmission based on the results.
Figure 3.
Association between readmission and cycle of weight monitoring.
Figure 3.
Association between readmission and cycle of weight monitoring.
Table 4.
Association between Mortality and Cycle of Weight Monitoring.
Table 4.
Association between Mortality and Cycle of Weight Monitoring.
| Weight recorded |
Mortality |
Total |
| |
Yes |
No |
| Cycle 1 (01/11/2023 to 15/01/2024) |
Count |
33 |
486 |
519 |
| % |
6.358382 |
93.64162 |
100 |
| Cycle 2 (01/04/2024 to 01/07/2024) |
Count |
13 |
479 |
492 |
| % |
2.642276 |
97.35772 |
100 |
| |
Value |
Df |
P-Value |
Interpretation |
| Pearson Chi-Square |
14.786a
|
1 |
.002 |
Significant |
| Likelihood Ratio |
15.688 |
1 |
.001 |
Significant |
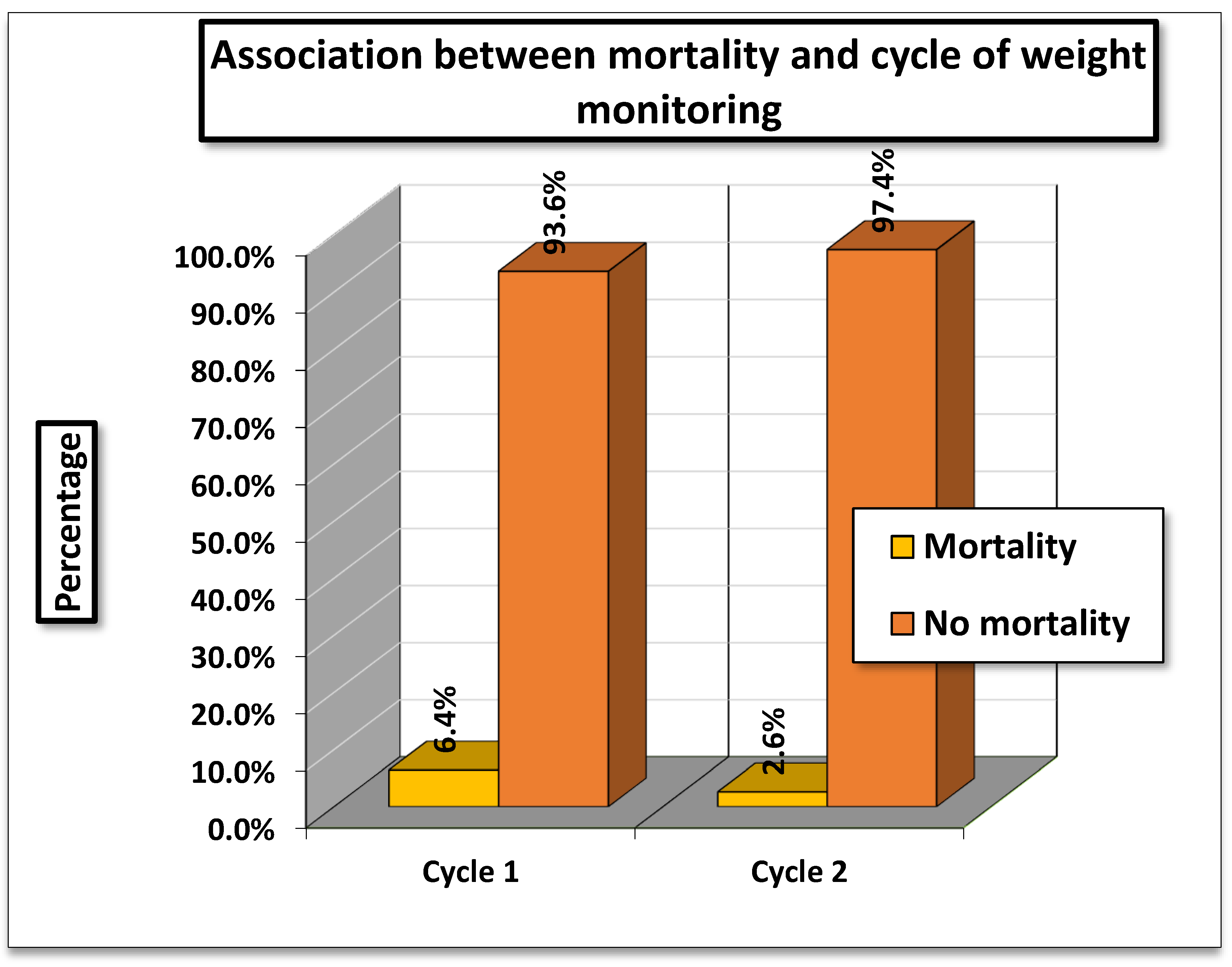
The analysis examined the association between mortality and cycle of weight monitoring. In Cycle 1 (01/11/2023–15/01/2024), mortality was 6.4% (33/519), while in Cycle 2 (01/04/2024–01/07/2024), it was lower at 2.6% (13/492).
The Pearson Chi-square test value was 14.786 (df = 2, p = 0.002), and the Likelihood Ratio was 15.688 (p = 0.001), both indicating a statistically significant association between cycle of monitoring and mortality.
Interpretation
The findings suggest that mortality rates differed significantly between the two cycles, with higher mortality observed during Cycle 1 compared to Cycle 2. Since, the p values are below 0.05, this difference is unlikely due to chance. Thus, the timing or cycle of weight monitoring appears to be significantly associated with patient mortality.
Figure 4.
Association between mortality and Cycle of Weight Monitoring.
Figure 4.
Association between mortality and Cycle of Weight Monitoring.
Discussion
Daily weight monitoring though simple and widely recommended is frequently underappreciated in routine heart failure care. Beyond reflecting fluid retention, fluctuations in daily weight may serve as an accessible surrogate of hemodynamic stress and neurohormonal activation. Sudden gains often precede overt congestion, while persistent variability may represent underlying sympathetic overdrive driving sodium retention and vascular tone. In this context, daily weights offer an indirect but valuable signal of pathophysiological instability, providing clinicians with a low-cost means to anticipate decompensation.
Moreover, failure to recognize the broader implications of weight changes may obscure the progressive structural remodeling that underpins worsening outcomes in heart failure. Repeated cycles of congestion and relief, reflected in weight oscillations, impose mechanical strain and perpetuate maladaptive remodeling processes such as fibrosis and dilation. Integrating weight monitoring with biomarkers, imaging, or digital health technologies could reposition it as part of a comprehensive surveillance system, bridging clinical practice with real-time physiological insight. Thus, daily weight should not be dismissed as a basic metric but appreciated as a window into the deeper interplay of hemodynamic burden, sympathetic overdrive, and cardiac remodeling.
Considering the long-term benefits of this simple non-invasive technique of effective daily weight monitoring, we conducted a retrospective two-cycle audit: one prior to introducing weight monitoring measures and one after its implementation. The audit evaluated its impact on patient health and its role in reducing complications such as ICU admission, readmission, and mortality.
In cycle 1 of this retrospective audit, weight was documented for only 101 of the 519 patients, whereas the remaining 418 patients did not undergo routine weight monitoring. These findings suggest that routine weight monitoring was omitted for majority of the patients during cycle 1 which underscores a clinical gap in heart failure management. Failure to monitor weight not only delays recognition of worsening heart failure but also indirectly contributes to hemodynamic stress and progressive cardiac remodelling, which accelerates functional decline. Therefore, the lack of consistent monitoring in this cohort highlights a missed opportunity for timely intervention, risk reduction, and long-term preservation of cardiac structure and function.
Following the introduction of daily weight monitoring measures, patient weights were systematically recorded for those admitted with heart failure on diuretic therapy and documented in cycle 2. In this cycle, weights were recorded for 43.3% of patients, a notable improvement from only 19.5% in cycle 1. This improvement underscores the potential benefits of daily weight monitoring practices in patients with heart failure receiving diuretic therapy. By facilitating early detection of progressive congestion and maladaptive cardiac remodeling, weight surveillance offers clinicians an opportunity to intervene before patients enter the recurrent cycle of hemodynamic stress, fluid retention, and associated comorbidities. Such timely identification not only mitigates acute decompensations but also contributes to long-term disease stabilization and improved clinical outcomes.
Jones CD et al., in their randomized trial, reported that adherence to daily weight monitoring was linked to a significant reduction in heart failure-related emergency department visits.[
11] Similarly, Backholer K et al. highlighted that daily weight monitoring is a crucial self-care activity for patients with heart failure.[
12] Conversely, Anker SD et al et al. observed that regular weight monitoring did not improve clinical outcomes in all cases; however, they emphasized that monitoring weight changes—particularly sudden weight gain—remains important, as it is associated with an increased risk of hospitalization.[
13]
In the present audit, the authors also assessed complications such as ICU admissions, hospital readmissions, and mortality during both the cycles (Cycle 1 and Cycle 2) that could arise from the absence of consistent weight monitoring practices. Evaluation of the relationship between weight monitoring and ICU admission risk revealed that 9.44% of patients were admitted to the ICU in cycle 1, compared to only 4.06% in cycle 2. These findings underscore the importance of this simple bathroom scale - daily weight monitoring ,in reducing the risk of ICU admissions among patients with heart failure on diuretic therapy.
Goldberg LR et al., in a multicentre randomized trial, evaluated the role of daily electronic home weight monitoring combined with symptom tracking in patients with heart failure and reported a 56.2% reduction in mortality in the intervention group [
14]. Similarly, Park C et al. demonstrated that interventions such as weight monitoring significantly decreased hospital readmissions and healthcare costs while improving patient outcomes [
15]. In addition, Chaudhry SI et al. observed that weight gain was closely associated with subsequent hospitalization for heart failure, typically beginning at least one week prior to admission.[
16] These evidences highlight the critical importance of routine weight monitoring in predicting and preventing adverse clinical events.
Post-discharge outcomes significantly influence recovery, but readmissions following treatment failure reinforce hemodynamic stress, promote cardiac remodeling, and exacerbate fluid retention, thereby hastening the progression of congestive heart failure. With the dual aim of minimizing overt clinical signs and integrating daily weight monitoring into standard care, the present audit sheds light on patterns of hospital readmission after recovery. In cycle 1, 16% of patients required readmission, while 84% did not. In contrast, following the intervention in cycle 2, only 7% of patients were readmitted, and 93% avoided hospitalization.
These findings indicate that in a disease which is driven by subtle shifts, daily weight may be the most powerful warning sign hiding in plain sight. Daily weight monitoring practices are invaluable not only for detecting subclinical signs of sympathetic overdrive and cardiac remodelling ultimately leading to congestive cardiac failure but also for enabling early, proactive treatment. Incorporation of consistent weight tracking practices can therefore improve patient outcomes reducing hospital readmissions and preventing further complications.
Wang XH et al in their randomized trial, focused on daily weight management interventions such as patient education on weight monitoring and coping strategies, and demonstrated a significant reduction in heart failure–related hospitalizations further reinforcing the findings of our audit.[
17] Similarly, Park C et al reported that timely interventions delivered through apps and Bluetooth-enabled devices substantially reduced readmission rates, from 23% to 10%. [
18] In their observational study, Barsuk JH et al evaluated a nurse-driven diuretic dosing protocol in patients with acute decompensated heart failure and found significantly lower odds of 30-day readmission, accompanied by greater weight loss.[
19] Gill GS et al after analysing data from 9,000 elderly patients, showed that weight loss between admission and discharge was associated with markedly lower 30-day heart failure readmission rates, further supporting our results.[
20] Additionally, Gwadry-Sridhar FH et al., in their systematic review and meta-analysis, concluded that patient education on weight maintenance can substantially reduce hospital readmissions, thereby strengthening and validating the findings of our audit.[
21] All of these established researches further reinforce the message of our audit that even modest, low-cost interventions like daily weight monitoring can significantly improve outcomes and prevent hospital readmissions in patients with heart failure on diuretic therapy.
Mortality remains the most devastating consequence in medicine. This study answers a simple yet profound question that we are overlooking this smallest signal with the biggest consequence. In cycle 1, mortality was observed in 33 patients (6.35%) out of 519, whereas in cycle 2 it declined to 13 patients (2.62%) out of 479. These findings emphasize that simple interventions, such as daily weight monitoring, can have a significant long-term impact by reducing mortality associated with delayed diagnosis of conditions like cardiac remodeling and congestive heart failure.
Gill GS et al. emphasized that effective fluid management and decongestion, as reflected through weight monitoring, were associated with lower short-term mortality, consistent with the findings of the present audit [
20]. Konstam MA et al in a secondary analysis of a clinical trial, evaluated week-to-week adherence to weight telemonitoring in heart failure patients and found that each additional day of adherence was linked to an 11% reduction in hospitalization and a 19% reduction in mortality risk the following week, further supporting our results [
22]. On the contrary, Pocock SJ et al in their study of 6,900 heart failure patients reported that a weight loss exceeding 5% over six months possibly due to over-diuresis was associated with at least a 50% increased risk of mortality [
23].
Haynes SC et al in a meta-analysis, demonstrated that even a modest intervention like daily weight tracking could substantially reduce mortality among heart failure patients, echoing our findings [
24]. Finally, Umeh CA et al through a comprehensive meta-analysis of 38 randomized controlled trials and observational studies involving nearly 15,000 patients, confirmed that weight-monitoring interventions significantly reduced mortality, reinforcing the conclusions of our audit [
25]. Together, all these studies reinforce the findings of our audit stating that simple yet structured approaches to weight monitoring practices and patient tracking correlate with meaningful reductions in mortality among hospitalized heart failure patients on diuretic therapy
Our audit highlights that a simple intervention such as daily weight monitoring can serve as a powerful tool for revealing underlying sympathetic overdrive, neurohormonal and hemodynamic stress, and ongoing cardiac remodeling, all of which contribute to fluid retention and ultimately drive the progression to congestive heart failure. Given that no prior studies have provided such detailed analysis across multiple outcomes including ICU admissions, readmissions and mortality, our research represents a pioneering contribution to the realm of Clinical Medicine. These findings may serve as a valuable tool for improving the management of heart failure patients on diuretic therapy, enabling more successful treatment with fewer complications.
Conclusion
This audit challenges us to rethink daily weight not as routine, but as a lifesaving diagnostic tool as daily weight monitoring is capable of reducing ICU admissions, readmissions, mortality, and patient suffering by enabling early detection of hemodynamic stress and fluid retention. Beyond improving immediate outcomes, it also encourages patient participation in self-care and offers clinicians a practical means to intervene before progression to advanced disease. Future research should focus on integrating this low-cost practice with advanced modalities such as segmental heart imaging, creating a comprehensive approach for the early identification of stress-induced cardiac remodeling and personalized heart failure management.
Conflict of Interest: The authors declare no conflicts of interest.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Shreya Manihar conceived and designed the experiments, performed the experiments, authored or reviewed drafts of the article, and approved the final draft. Himanshu Upadhyay conceived and designed the experiments, performed the experiments, and approved the final draft. Meet Manihar conceived and designed the experiments and approved the final draft. Priya Wani prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft. Varun Wani prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft
Funding
This research received no external funding.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to it being a retrospective audit based on retrospective data and did not involve direct contact with humans or animals
Informed Consent Statement
Patient’s Consent was waived off since this is a Retrospective Audit involving no contact with direct patients.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author(s).
Abbreviations
| HF |
Heart Failure |
| SNS |
Sympathetic nervous system |
| RAAS |
Renin- Angiotensin Aldosterone System |
References
- Kemp CD, Conte JV. The pathophysiology of heart failure. Cardiovascular pathology. 2012 Sep 1;21(5):365-71. [CrossRef]
- Tomasoni D, Adamo M, Lombardi CM, Metra M. Highlights in heart failure. ESC heart failure. 2019 Dec;6(6):1105-27. [CrossRef]
- Groenewegen A, Rutten FH, Mosterd A, Hoes AW. Epidemiology of heart failure. European journal of heart failure. 2020 Aug;22(8):1342-56. [CrossRef]
- Mahmood SS, Levy D, Vasan RS, Wang TJ. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. The lancet. 2014 Mar 15;383(9921):999-1008. [CrossRef]
- Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, JacobsJr DR, Kronmal R, Liu K, Nelson JC. Multi-ethnic study of atherosclerosis: objectives and design. American journal of epidemiology. 2002 Nov 1;156(9):871-81. [CrossRef]
- Lyngå P, Persson H, Hägg-Martinell A, Hägglund E, Hagerman I, Langius-Eklöf A, Rosenqvist M. Weight monitoring in patients with severe heart failure (WISH). A randomized controlled trial. European journal of heart failure. 2012 Apr;14(4):438-44. [CrossRef]
- Pinsky MR, Cecconi M, Chew MS, De Backer D, Douglas I, Edwards M, Hamzaoui O, Hernandez G, Martin G, Monnet X, Saugel B. Effective hemodynamic monitoring. Critical Care. 2022 Sep 28;26(1):294.
- NICE Clinical guideline [CG187] Acute heart failure: diagnosis and management: 1.3.5.
- Hsu S, Fang JC, Borlaug BA. Hemodynamics for the heart failure clinician: a state-of-the-art review. Journal of cardiac failure. 2022 Jan 1;28(1):133-48. [CrossRef]
- Logeart D, Isnard R, Resche-Rigon M, Seronde MF, de Groote P, Jondeau G, Galinier M, Mulak G, Donal E, Delahaye F, Juilliere Y. Current aspects of the spectrum of acute heart failure syndromes in a real-life setting: the OFICA study. European journal of heart failure. 2013 Apr;15(4):465-76. [CrossRef]
- Jones CD, Holmes GM, Dewalt DA, Erman B, Broucksou K, Hawk V, Cene CW, Wu JR, Pignone M. Is adherence to weight monitoring or weight-based diuretic self-adjustment associated with fewer heart failure-related emergency department visits or hospitalizations?. Journal of cardiac failure. 2012 Jul 1;18(7):576-84. [CrossRef]
- Backholer K, Wong E, Freak-Poli R, Walls HL, Peeters A. Increasing body weight and risk of limitations in activities of daily living: a systematic review and meta-analysis. Obesity reviews. 2012 May;13(5):456-68. [CrossRef]
- Anker SD, Khan MS, Butler J, Ofstad AP, Peil B, Pfarr E, Doehner W, Sattar N, Coats AJ, Filippatos G, Ferreira JP. Weight change and clinical outcomes in heart failure with reduced ejection fraction: insights from EMPEROR-Reduced. European journal of heart failure. 2023 Jan;25(1):117-27. [CrossRef]
- Goldberg LR, Piette JD, Walsh MN, Frank TA, Jaski BE, Smith AL, Rodriguez R, Mancini DM, Hopton LA, Orav EJ, Loh E; WHARF Investigators. Randomized trial of a daily electronic home monitoring system in patients with advanced heart failure: the Weight Monitoring in Heart Failure (WHARF) trial. Am Heart J. 2003 Oct;146(4):705-12. [CrossRef] [PubMed]
- Park C, Otobo E, Ullman J, Rogers J, Fasihuddin F, Garg S, Kakkar S, Goldstein M, Chandrasekhar SV, Pinney S, Atreja A. Impact on Readmission Reduction Among Heart Failure Patients Using Digital Health Monitoring: Feasibility and Adoptability Study. JMIR Med Inform. 2019 Nov 15;7(4):e13353. [CrossRef] [PubMed] [PubMed Central]
- Chaudhry SI, Wang Y, Concato J, Gill TM, Krumholz HM. Patterns of weight change preceding hospitalization for heart failure. Circulation. 2007 Oct 2;116(14):1549-54. [CrossRef]
- Wang XH, Qiu JB, Ju Y, Chen GC, Yang JH, Pang JH, Zhao X. Reduction of heart failure rehospitalization using a weight management education intervention. J Cardiovasc Nurs. 2014 Nov-Dec;29(6):528-34. [CrossRef] [PubMed]
- Park C, Otobo E, Ullman J, Rogers J, Fasihuddin F, Garg S, Kakkar S, Goldstein M, Chandrasekhar SV, Pinney S, Atreja A. Impact on Readmission Reduction Among Heart Failure Patients Using Digital Health Monitoring: Feasibility and Adoptability Study. JMIR Med Inform. 2019 Nov 15;7(4):e13353. [CrossRef] [PubMed] [PubMed Central]
- Barsuk JH, Gordon RA, Cohen ER, Cotts WG, Malkenson D, Yancy CW, Williams MV. A diuretic protocol increases volume removal and reduces readmissions among hospitalized patients with acute decompensated heart failure. Congest Heart Fail. 2013 Mar-Apr;19(2):53-60. [CrossRef] [PubMed]
- Gill GS, Lam PH, Brar V, Patel S, Arundel C, Deedwania P, Faselis C, Allman RM, Zhang S, Morgan CJ, Fonarow GC, Ahmed A. In-Hospital Weight Loss and Outcomes in Patients With Heart Failure. J Card Fail. 2022 Jul;28(7):1116-1124. [CrossRef] [PubMed]
- Gwadry-Sridhar FH, Flintoft V, Lee DS, Lee H, Guyatt GH. A Systematic Review and Meta-analysis of Studies Comparing Readmission Rates and Mortality Rates in Patients With Heart Failure. Arch Intern Med. 2004;164(21):2315–2320. [CrossRef]
- Konstam, MA. Home monitoring should be the central element in an effective program of heart failure disease management. Circulation. 2012 Feb 14;125(6):820-7. [CrossRef]
- Pocock SJ, McMurray JJ, Dobson J, Yusuf S, Granger CB, Michelson EL, Ostergren J, Pfeffer MA, Solomon SD, Anker SD, Swedberg KB. Weight loss and mortality risk in patients with chronic heart failure in the candesartan in heart failure: assessment of reduction in mortality and morbidity (CHARM) programme. Eur Heart J. 2008 Nov;29(21):2641-50. [CrossRef] [PubMed]
- Haynes SC, Tancredi DJ, Tong K, Hoch JS, Ong MK, Ganiats TG, Evangelista LS, Black JT, Auerbach A, Romano PS; Better Effectiveness After Transition–Heart Failure (BEAT-HF) Research Group. Association of Adherence to Weight Telemonitoring With Health Care Use and Death: A Secondary Analysis of a Randomized Clinical Trial. JAMA Netw Open. 2020 Jul 1;3(7):e2010174. [CrossRef] [PubMed] [PubMed Central]
- Umeh CA, Torbela A, Saigal S, Kaur H, Kazourra S, Gupta R, Shah S. Telemonitoring in heart failure patients: Systematic review and meta-analysis of randomized controlled trials. World journal of cardiology. 2022 Dec 26;14(12):640. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).