Submitted:
24 August 2025
Posted:
26 August 2025
You are already at the latest version
Abstract
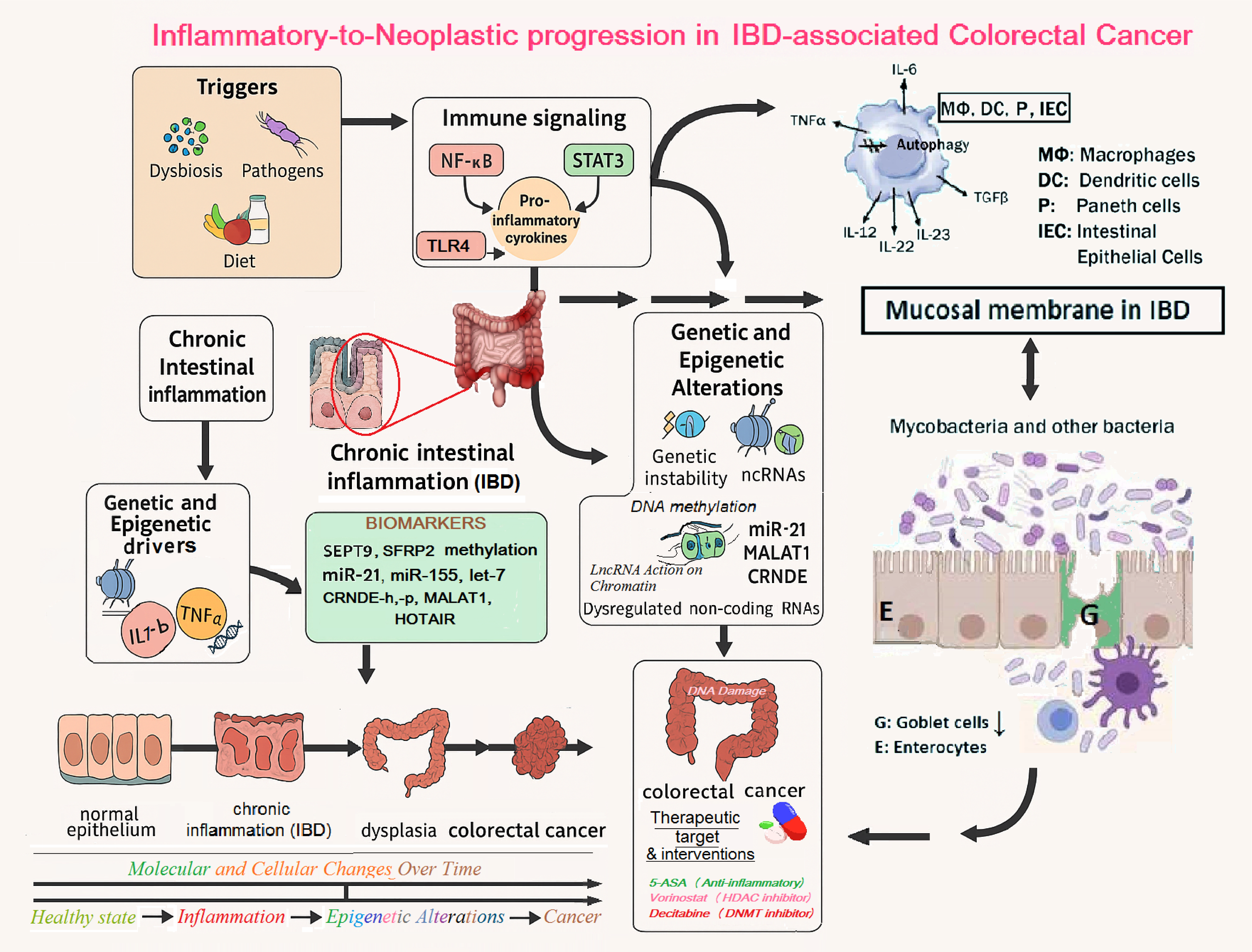
Keywords:
1. Introduction
1.1. The Impact of Chronic Inflammation in Carcinogenesis
1.2. Inflammatory Bowel Disease
2. The Role of Infectious Agents in Inflammation
2.1. The Physiology of Infection: A Brief Overview
2.1.1. Infection Stages
2.1.2. The Immune Response
2.1.3. Factors Influencing the Outcome of the Infection
2.2. Physiological Human-Bacteria Interactions
2.3. Antigens and Subversion of Immune Response
2.4. Bacterial Infection and Immune Dysregulation
2.5. Gastrointestinal Tuberculosis
2.6. Colonic Tuberculosis
2.7. Gastrointestinal Tuberculosis in Animals
3. Animal Models of IBD and Colitis-Associated Colorectal cancer (CAC)
3.1. Murine Models of Gastrointestinal Cancer (GIC) Through Pathogen Infection
3.2. Non-infectious Animal Models for Gastrointestinal Cancer (GIC)
3.2.1. Chemically Induced Models: AOM/DSS
3.2.2. Genetically Engineered Mouse Models (GEMMs)
3.2.3. Xenograft and Patient-Derived Xenograft (PDX) Models
4. Immune Defense in Gastrointestinal (GI) Chronic Inflammation and Carcinogenesis
4.1. Inflammation-Induced Cancers Associated with the GI Tract.
4.2. The Involvement of Bacteria in the Mechanisms of Carcinogenesis
4.3. Chronic Inflammation as a Driver of Colorectal Carcinogenesis
4.4. Effect of bacterial infection on gastrointestinal cancer
4.4.1. Colorectal cancer
5. Overview of Chromatin and Epigenetic Modulations
5.1. Integration of Epigenetic Alterations and Inflammatory Pathways
5.2. Epigenetic Alterations in Inflammation-Associated Pathologies of the GI Tract – an overview
6. Epigenetic Mechanisms Linking Inflammation in IBD to Colorectal Carcinogenesis.
6.1. An Overview of Inflammation-driven Carcinogenic Transition in Humans and Murine Models
7. DNA Metylation and Histone Modifications During Tumorigenesis of the GI
7.1. DNA methylation in IBD, CAC and CRC
7.2. Histone Modifications in Inflammation-related Cancer Progression
7.2.1. Histone Acetylation in CRC
7.2.2. Histone methylation in inflammatory signaling and CRC progression
7.2.3. Histone Phosphorylation in CRC
8. Exploring the Role of Non-coding RNAs in Epigenetic Regulation of IBD and CRC
8.1. MicroRNAs - molecular insights of microRNA dysregulation or aberrant function and its involvement in IBDs and CRC
8.2. MicroRNAs as Biomarkers in GI diseases
8.3. LncRNAs in IBD and CRC- A General Overview
8.3.1. LncRNA as Biomarkers in GI diseases
8.4. Other ncRNAs in GI diseases
8.4.1. PiRNAs
| Name | SNP Expression info | Function | Database (#) | PubMed (PMID) |
|---|---|---|---|---|
| piR-hsa-679 | rs34383331, base change: A>T | May be involved in the development of CRC | piRBase | 25740697 |
| piR-hsa-7400 | rs2070766f, base change: C>G | -“- | piRBase | 25740697 |
| piR-hsa-21417 | rs2070766f, base change: C>G | -“- | piRBase | 25740697 |
| piR-hsa-29786 | rs2070766f, base change: C>G | -“- | piRBase | 25740697 |
| piR-hsa-21517 | rs11776042, base change: T>C | May be involved in the development of CRC | piRBase | 25740697 |
| piR-hsa-29056 | rs9368782, base change: A>G | -“- | piRBase | 25740697 |
| piR-hsa-2363 | rs12483859, base change: A>G | -“- | piRBase | 25740697 |
| piR-hsa-8401 | rs10433310, base change: C>T | -“- | piRBase | 25740697 |
| piR-hsa-3789 | rs12910401, base change:G>A | -“- | piRBase | 25740697 |
| piR-hsa-1245 | up-regulated | It is a novel oncogene and a potential prognostic biomarker in colorectal cancer | piRBase | 29382334 |
| piR-hsa-1282 | up-regulated | It interacts with HSF1 to promote Ser326 phosphorylation and HSF1 activation, enhancing CRC cell proliferation and suppressing cell apoptosis | piRBase | 28618124 |
| piR-hsa-17444 | up-regulated | Formation of PIWIL2/STAT3/phosphorylated-SRC (p-SRC) complex, which activates STAT3 signaling and promotes proliferation, metastasis and chemoresistance of CRC cells | piRBase | 30555542 |
| piR-hsa-1077(*) | up-regulated | Ontology ID: EFO_1001951 | piRNAdb, piRPheno v2.0 | 16751776 |
8.4.2. CircRNAs
| circRNA name | Synonyms |
Predicted interacting RBP (Nοof binding sites) |
Methods | Expression pattern | PubMed ID |
| hsa_circ_0020397 | hsa_circRNA_100722 |
EIF4A3(32); HuR(3); IGF2BP1(2); AGO2(2); SFRS1(1); PTB(1); LIN28A(1); IGF2BP2(1); FUS(1) |
qRT-PCR; dual luciferase reporter assay; in vitro knockdown; in vitro overexpression; western blot; etc. | up-regulated | 28707774 |
| circ-BANP | hsa_circRNA_101902; hsa_circ_0003098 | EIF4A3(12); HuR(1); AGO2(1) | Microarray; RT-PCR; qRT-PCR; in vitro knockdown; ISH; western blot; etc. | up-regulated | 28103507 |
| hsa_circ_0000069 | hsa_circRNA_100213; hsa_circ_001061 | EIF4A3(10); AGO2(9); IGF2BP3(7); PTB(6); IGF2BP2(6); IGF2BP1(4); HuR(2); FMRP(2); SFRS1(1); FXR2(1) |
qRT-PCR; in vitro knockdown; etc. | up-regulated | 28003761 |
| hsa_circ_001569 | N/A | N/A | in vitro knockdown; in vitro overexpression; qRT-PCR; western blot; ; luciferase reporter assay etc. | up-regulated | 27058418 |
| hsa_circ_0001451 | hsa_circ_001988 | EIF4A3(6); HuR(2); LIN28A(1); IGF2BP3(1); IGF2BP2(1); DGCR8(1); AGO2(1) |
RNA-seq; qRT-PCR | down-regulated | 26884878 |
|
circHlPK3 |
circ_0000284, hsa_circ_0000284 | IGF2BP1 (5), HuR (ELAVL1) (8), FUS (4) | RNA-seq; Microarray | up-regulated | 33536039 |
|
circCCDC66 |
circ_0001313 |
HuR (ELAVL1) (6), PTBP1 (7), FUS (5) |
RNA-seq; Microarray; droplet digital PCR | down-regulated | 33536039 |
| circZFR | hsa_circRNA_103809; hsa_circ_0072088 | FMRP(21); EIF4A3(7); AGO2(7); IGF2BP3(6); HuR(6); IGF2BP1(4); AGO1(4); ZC3H7B(1); U2AF65(1); PTB(1); LIN28B(1); IGF2BP2(1) |
Microarray; qRT-PCR | down-regulated | 28349836 |
| circPTK2 | hsa_circRNA_104700; hsa_circ_0005273 | AGO2(5); EIF4A3(2); IGF2BP3(1); IGF2BP2(1); FUS(1) | Microarray; qRT-PCR | down-regulated | 28349836 |
| CDR1as | Cdr1as; ciRS-7; hsa_circRNA_105055; hsa_circ_0001946 |
AGO2(43); FUS(26); IGF2BP1(11); IGF2BP2(10); IGF2BP3(9); AGO1(6); TNRC6(2); TDP43(2) |
qRT-PCR; in vitro overexpression; ; in vivo overexpression; IHC; western blot; etc. | up-regulated | 28174233 |
8.4.3. Functions and Implications of circRNAs
8.4.4. Circular RNA - Protein Interactions: Functional Significance and Binding Site Density
8.5. Inflammation-driven ncRNA Modulation in CRC
| ncRNA Type | Name | Function in CRC | References(PMID) |
| lncRNA | HOTAIR | Recruits PRC2 to silence tumor suppressor genes (e.g., CDKN1A); promotes invasion and metastasis. | 21862635; 28701486 |
| lncRNA | MALAT1 | Enhances β-catenin nuclear translocation; regulates alternative splicing and epithelial-mesenchymal transition (EMT). | 12970751; 34144008 |
| circRNA | circHIPK3 |
Acts as a miR-1207 sponge which is downregulated in CRC; enhanced formin like 2 (FMNL2) in CRC; contributes to chemoresistance and proliferation. |
32046858 |
| circRNA | circCCDC66 | Functions as a ceRNA; sequesters tumor-suppressive miRNAs (e.g., miR-33b); enhances c-MYC and YAP1 pathways. | 28249903 |
| miRNA | miR-21 | Overexpressed in CRC; targets PTEN, PDCD4, suppressing apoptosis; regulated by NF-êB and IL-6 inflammatory stimuli. |
17968323; 20797623; 34771727 |
| miRNA | miR-155 | Induced by NF-κB and STAT3; promotes tumor cell survival and immune evasion by targeting SOCS1 and TP53INP1. |
17242365; 17911593;32702393 |
| mRNA target | PTEN | Tumor suppressor inhibited by miR-21; regulates PI3K/AKT signaling. | 32104279 |
| mRNA target | BCL2 | Anti-apoptotic protein regulated by multiple miRNAs (e.g., miR-15, miR-16); supports resistance to cell death. | 16166262; 28984869 |
9. Diagnostic and Therapeutic Implications: Biomarkers and Epigenetic Drugs
9.1. Translational Epigenetics and RNA-Based Therapeutics in IBD/CRC
9.2. Epigenetic Drugs and Clinical Trials in IBD-Associated CRC
9.3. RNA-Based Therapeutics in Preclinical and Clinical Use
9.4. Diagnostic and Prognostic Utility of Epigenetic and RNA Biomarkers
9.5. Comparative Human-Mouse Evidence
10. Concluding Remarks and Future Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, G.Y.; Nunez, G. Sterile inflammation: sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10(12), 826-837. [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P. Inflammasomes: mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21(7), 677-687. [CrossRef]
- Zhang, S.; Meng, Y.; Zhou, L., Qiu, L.; Wang, H.; Su, D.; Zhang, B.; Chan, K.-M.; Han, J. Targeting epigenetic regulators for inflammation: Mechanisms and intervention therapy. MedComm 2022, 3(4), e173. [CrossRef]
- Tan, S.Y.X., Zhang, J.; Tee, W.W. Epigenetic Regulation of Inflammatory Signaling and Inflammation-Induced Cancer. Front. Cell Dev. Biol. 2022, 10, 931493. PMID: 35757000; PMCID: PMC9213816 . [CrossRef]
- Das, D.; Karthik, N.; Taneja, R. Crosstalk between inflammatory signaling and methylation in cancer. Front. Cell Dev. Biol. 2021, 9, 756458. [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140(6), 883-899. [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420(6917), 860-867. [CrossRef]
- Mishra, S.R.; Mahapatra, K.K.; Behera, B.P.; Bhol, C.S.; Praharaj, P.P.; Panigrahi, D.P.; et al. Inflammasomes in cancer: effect of epigenetic and autophagic modulations. Semin. Cancer Biol. 2022, 83, 399-412. [CrossRef]
- Shi, L.; Wang, L.; Hou, J.; Zhu, B.; Min, Z., Zhang, M.; Song, D.; Cheng, Y.; Wang, X. Targeting roles of inflammatory microenvironment in lung cancer and metastasis. Cancer Metastasis Rev. 2015, 34(2), 319-331. [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454(7203), 436-444. [CrossRef]
- Greten, F.R.; Grivennikov, S.I. Inflammation and cancer: Triggers, mechanisms, and consequences. Immunity 2019, 51(1), 27-41. [CrossRef]
- Vieujean, S.; Caron, B.; Haghnejad, V.; Jouzeau, J.Y.; Netter, P.; Heba, A.C.; Ndiaye, N.C.; Moulin, D.; Barreto, G.; Danese, S.; Peyrin-Biroulet, L. Impact of the exposome on the epigenome in inflammatory bowel disease patients and animal models. Int. J. Mol. Sci. 2022, 23(14). [CrossRef]
- Zeng, Z.; Mukherjee, A.; Zhang, H. From genetics to epigenetics, roles of epigenetics in inflammatory bowel disease. Front. Genet. 2019, 10, 1017. [CrossRef]
- Gerbeth, L.; Glauben, R. Histone Deacetylases in the inflamed intestinal epithelium-Promises of new therapeutic strategies. Front. Med. (Lausanne) 2021, 8, 655956. [CrossRef]
- Jawad, N.; Direkze, N.; Leedham, S.J. Inflammatory bowel disease and colon cancer. Recent Results Cancer Res. 2011, 185, 99-115. [CrossRef]
- Thomson, P.D.; Smith, D.J. Jr. What is infection? Am. J. Surg. 1994, 167(1A), 7S-10S; discussion 10S-11S. PMID: 8109689 . [CrossRef]
- Qerqez, A.N.; Silva, R.P.; Maynard, J.A. Outsmarting pathogens with antibody engineering. Annu. Rev. Chem. Biomol. Eng. 2023 , 14, 217-241. PMID: 36917814; PMCID: PMC10330301. [CrossRef]
- van Seventer, J.M.; Hochberg, N.S. Principles of infectious diseases: transmission, diagnosis, prevention, and control. In International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Academic Press, Elsevier. Cambridge, MA., U.S.A. 2017, 22–39. PMCID: PMC7150340. [CrossRef]
- Burrell, C.J.; Howard, C.R.; .Murphy, F.A. Innate Immunity. In Fenner and White's Medical Virology, 5th ed.; Academic Press, Elsevier, Oxford, U.K. 2017, 57–64. PMCID: PMC7173408 . [CrossRef]
- Schertzer, J.D.; Lam, T.K.T. Peripheral and central regulation of insulin by the intestine and microbiome. Am. J. Physiol. Endocrinol. Metab. 2021, 320(2), E234-E239. [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct. Target Ther. 2021, 6(1), 291. [CrossRef]
- Carretta, M.D.; Quiroga, J.; López, R.; Hidalgo, M.A.; Burgos, R.A. Participation of short-chain fatty acids and their receptors in gut inflammation and colon cancer. Front. Physiol. 2021, 12, 662739. [CrossRef]
- Madison, A.; Kiecolt-Glaser, J.K. Stress, depression, diet, and the gut microbiota: Human-bacteria interactions at the core of psychoneuroimmunology and nutrition. Curr. Opin. Behav. Sci. 2019, 28,105-110. [CrossRef]
- Lloyd-Price, J.; Abu-Ali, G.; Huttenhower, C. The healthy human microbiome. Genome Med. 2016, 8(1), 51. [CrossRef]
- Finlay, B.B.; McFadden, G. Anti-immunology: evasion of the host immune system by bacterial and viral pathogens. Cell. 2006, 124(4), 767-82. [CrossRef]
- Giamarellos-Bourboulis, E.J.; Raftogiannis, M. The immune response to severe bacterial infections: Consequences for therapy. Expert Rev. Anti. Infect. Ther. 2012, 10(3), 369-80. [CrossRef]
- Al-Zanbagi, A.B.; Shariff, M.K. Gastrointestinal tuberculosis: A systematic review of epidemiology, presentation, diagnosis and treatment. Saudi J Gastroenterol. 2021, 27(5), 261-274. [CrossRef]
- Choudhury ,A.; Dhillon, J.; Sekar, A.; Gupta, P.; Singh, H.; Sharma, V. Differentiating gastrointestinal tuberculosis and Crohn's disease- a comprehensive review. BMC Gastroenterol. 2023, 23(1), 246. [CrossRef]
- Eraksoy, H. Gastrointestinal and abdominal tuberculosis. Gastroenterol. Clin. North Am. 2021, 50(2), 341-360. [CrossRef]
- Malikowski T, Mahmood M, Smyrk T, Raffals L, Nehra V. Tuberculosis of the gastrointestinal tract and associated viscera. J. Clin. Tuberc. Other Mycobact. Dis. 2018, 12, 1-8. . Erratum in: J Clin Tuberc Other Mycobact Dis. 2020 Sep 09;21:100177. doi: 10.1016/j.jctube.2020.100177. PMID: 31720391; PMCID: PMC6830173. [CrossRef]
- Gopalaswamy, R.; Dusthackeer, V.N.A.; Kannayan, S.; Subbian, S. Extrapulmonary Tuberculosis—An update on the diagnosis, treatment and drug resistance. J. of Resp. 2021, 1(2), 141–164. [CrossRef]
- Choi, E.; Coyle, W. Gastrointestinal tuberculosis. In Tuberculosis and Nontuberculous Mycobacterial Infections. 7th ed.; Schlossberg, D., Ed.; ASM Press, Wiley. Washington, D.C., U.S.A. 2017, 411–432. [CrossRef]
- Dasgupta, A.; Singh, N.; Bhatia, A. Abdominal tuberculosis: A histopathological study with special reference to intestinal perforation and mesenteric vasculopathy. J. Lab. Physicians 2009, 1(2), 56-61. [CrossRef]
- Maulahela, H.; Simadibrata, M.; Nelwan, E.J.; Rahadiani, N.; Renesteen, E.; Suwarti, S. W.T.; Anggraini, Y.W. Recent advances in the diagnosis of intestinal tuberculosis. BMC Gastroenterol. 2022, 22(1). [CrossRef]
- Chatzicostas, C.; Koutroubakis, I.E.; Tzardi, M.; Roussomoustakaki, M.; Prassopoulos, P.; Kouroumalis, E.A. Colonic tuberculosis mimicking Crohn’s disease: Case report. BMC Gastroenterol. 2002, 2, 10. http://www.biomedcentral.com/1471-230X/2/10.
- Arhan, M.; Köksal, A.Ş.; Özin, Y.; Mesut, Z.; Kılıç, Y.; Tunç, B.; Ülker, A. Colonic tuberculosis or Crohn’s disease ? An important differential diagnosis. Acta Gastroenterol. Belg. 2013, 76(1), 59-61. PMID: 23650785.
- González-Puga, C.; Palomeque-Jiménez, A.; García-Saura, P.L.; Pérez-Cabrera, B.; Jiménez-Ríos, J.A. Colonic tuberculosis mimicking Crohn’s disease: An exceptional cause of massive surgical rectal bleeding. Medecine et Maladies Infectieuses 2015, 45(1–2), 44–46. doi.org/10.1016/j.medmal.2014.11.005.
- Adhikari, G.; Buda, B., Shah, J.K.; Ghimire, B.; Kansaker, P.B.S. Colonic tuberculosis masquerading as ascending colon carcinoma in a patient of FIGO Stage IIB cervical carcinoma following chemo-radiotherapy: A case report. Int. J. Surg. Case Rep. 2022, 93, 106943. [CrossRef]
- Luczynski, P.; Poulin, P.; Romanowski, K. Johston, J.C. Tuberculosis and risk of cancer: A systematic review and meta-analysis. PLO One 2022, 17(12), e0278661. [CrossRef]
- Saidu, A.S.; Okolocha, E.C.; Gamawa, A.A.; Babashani, M.; Bakari, N.A. Occurrence and distribution of bovine tuberculosis (Mycobacterium bovis) in slaughtered cattle in the abattoirs of Bauchi State, Nigeria. Veterinary World, 2015, 8(3), 432–437. [CrossRef]
- Luboya, L.W.; Malangu, M.; Kaleka, M.; Ngulu, N.; Nkokele, B.; Maryabo, K.; Pourrut, X.; Vincent, T.; Gonzalez, J.P. An assessment of caprine tuberculosis prevalence in Lubumbashi slaughterhouse, Democratic Republic of Congo. Tropical Animal Health and Production, 2017, 49(4), 875–878. [CrossRef]
- Engelmann, N.; Ondreka, N.; Michalik, J.; Neiger, R. Intra-abdominal Mycobacterium tuberculosis infection in a dog. J. Vet. Intern. Med. 2014, 28(3), 934-8. https://doi.10.1111/jvim12347.
- Mentula, S.; Karkamo, V.; Skrzypczak, T.; Seppänen, J.; Hyyryläinen, H.L.; Haanperä, M.; Soini, H. Emerging source of infection - Mycobacterium tuberculosis in rescue dogs: a case report. Access Microbiol. 2020, 2(11). [CrossRef]
- Ribeiro, M.G.; Lima, M.C.F.; Franco, M.M.J.; Megid, J.; Soares, L.M.; Machado, L.H.A.; Miyata, M., Pavan, F.R.; Heinemann, M.B.; Souza Filho, A.F.; Lara, G.H.B.; Sanches, O.C.; Sanches, C.D.C.; Listoni, F.J.P.; Paes, A.C. Pre-multidrug-resistant Mycobacterium tuberculosis infection causing fatal enteric disease in a dog from a family with history of human tuberculosis. Transbound. Emerg. Dis. 2017, 64(5), e4-e7. [CrossRef]
- Dhama, K.; Mahendran, M.; Tiwari, R.; Dayal Singh, S.; Kumar, D.; Singh, S.; Sawant, P.M. Tuberculosis in Birds: Insights into the Mycobacterium avium Infections. Vet. Med. Int. 2011, 712369. [CrossRef]
- Bertram, C.A.; Barth, S.A.; Glöckner, B.; Lübke-Becker, A.; Klopfleisch, R. Intestinal Mycobacterium avium Infection in Pet Dwarf Rabbits (Oryctolagus cuniculus). J. Comp. Pathol. 2020, 180, 73-78. [CrossRef]
- Vetere, A.; Bertocchi, M.; Pagano, T.B.; Di Ianni, F.; Nardini G. First case of systemic fatal mycobacteriosis caused by Mycobacterium goodii in a pet Kenyan sand boa (Eryx colubrinus loveridgei). BMC Vet. Res. 2022, 18(1), 291. [CrossRef]
- Lindsay, S.A.; Gray, R. A Novel Presentation of tuberculosis with intestinal perforation in a free-ranging Australian sea lion (Neophoca cinerea). J. Wildl. Dis. 2021, 57(1), 220-224. [CrossRef]
- Elsalem, L.; Jum'ah, A.A.; Alfaqih, M.A.; Aloudat O. The Bacterial Microbiota of Gastrointestinal Cancers: Role in Cancer Pathogenesis and Therapeutic Perspectives. Clin. Exp. Gastroenterol. 2020, 13,151-185. [CrossRef]
- Bundgaard-Nielsen, C.; Baandrup, U.T.; Nielsen L.P.; Sørensen S. The presence of bacteria varies between colorectal adenocarcinomas, precursor lesions and non-malignant tissue. BMC Cancer 2019 19(1), 399. [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68(6), 394-424. . Erratum in: CA Cancer J. Clin. 2020, 70(4), 313. doi: 10.3322/caac.21609. PMID: 30207593. [CrossRef]
- Neufert, C.; Becker, C.; Neurath, M.F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2(8), 1998-2004. [CrossRef]
- Tanaka, T.; Kohno, H.; Suzuki, R.; Yamada, Y.; Sugie, S.; Mori, H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Science, 2003, 94(11), 965–973. [CrossRef]
- Mizoguchi, A.; Takeuchi, T.; Himuro, H.; Okada, T.; Mizoguchi, E. Genetically engineered mouse models for studying inflammatory bowel disease. J. Pathol. 2016, 238(2), 205-19. [CrossRef]
- Ren, J.; Sui, H.; Fang, F.; Li, Q.; Li, B. The application of ApcMin/+ mouse model in colorectal tumor researches. J. Cancer Res. Clin. Oncol. 2019, 145(5), 1111-1122. [CrossRef]
- Moser, A.R.; Pitot, H.C.; Dove, W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science 1990, 247(4940), 322–324. [CrossRef]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; Roman-Roman, S.; Seoane, J.; Trusolino, L.; Villanueva, A. Patient-derived xenograft models: an emerging platform for translational cancer research. Cancer Discovery 2014, 4(9), 998–1013. [CrossRef]
- Balkwill, F.; Mantovani A. Inflammation and cancer: back to Virchow? Lancet 2001, 357(9255), 539-45. [CrossRef]
- Fujiki, H. Gist of Dr. Katsusaburo Yamagiwa's papers entitled "Experimental study on the pathogenesis of epithelial tumors" (I to VI reports). Cancer Sci. 2014, 105(2),143-9. [CrossRef]
- Dvorak, H.F. Tumors: wounds that do not heal-redux. Cancer Immunol. Res. 2015, 3(1), 111. [CrossRef]
- Dvorak, H.F. Tumors: Wounds that do not heal-a historical perspective with a focus on the fundamental roles of increased vascular permeability and clotting. Semin. Thromb. Hemost. 2019, 45(6), 576-592. [CrossRef]
- Vanoli, A.; Parente, P.; Fassan, M.; Mastracci, L.; Grillo, F. Gut inflammation and tumorigenesis: every site has a different tale to tell. Intern. Emerg. Med. 2023, 18(8), 2169-2179. [CrossRef]
- Grady, W. M.; Yu, M.; Markowitz, S. D. Epigenetic alterations in the gastrointestinal tract: current and emerging use for biomarkers of cancer. Gastroenterol. 2021, 160(3), 690-709. [CrossRef]
- Filho, A.M.; Laversanne, M.; Ferlay, J.; Colombet, M.; Piñeros, M.; Znaor, A.; Parkin, D.M.; Soerjomataram, I.; Bray, F. The GLOBOCAN 2022 cancer estimates: Data sources, methods, and a snapshot of the cancer burden worldwide. Int. J. Cancer 2025, 156(7),1336-1346. [CrossRef]
- Chhikara, B.S.; Parang, K. Global Cancer Statistics 2022: the trends projection analysis. Chem. Biol. Lett. 2023, 10(1), 451.
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74(3), 229-263. [CrossRef]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasaq, C.J.; Laversanne, M.;Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020and 2040: incidence and mortality estimates from GLOBOCAN. Gut 2023, 72(2), 338-344. [CrossRef]
- Yang, F.; Li, X.F.; Cheng, L.N.; Li, X.L. Long non-coding RNA CRNDE promotes cell apoptosis by suppressing miR-495 in inflammatory bowel disease. Exp. Cell Res. 2019, 382(2), 111484. [CrossRef]
- Triantaphyllopoulos, K.A. Long non-coding RNAs and their "discrete" contribution to IBD and Johne's disease-what stands out in the current picture? A comprehensive review. Int. J. Mol. Sci. 2023, 24(17), 13566. [CrossRef]
- Vetrano, S.; Borroni, E.M.; Sarukhan, A.; Savino, B.; Bonecchi, R.; Correale, C.; Arena, V.; Fantini, M.; Roncalli, M.; Malesci, A.; Mantovani, A.; Locati, M.; Danese, S. The lymphatic system controls intestinal inflammation and inflammation-associated Colon Cancer through the chemokine decoy receptor D6. Gut, 2010 59(2), 197-206. [CrossRef]
- Pelaseyed,T.; Hansson, G.C. Membrane mucins of the intestine at a glance. J. Cell. Sci. 2020, 133(5), jcs240929. [CrossRef]
- Tang, W.; Liu, J.; Ma, Y.; Wei, Y.; Liu, J.; Wang H. Impairment of intestinal barrier function induced by early weaning via autophagy and apoptosis associated with gut microbiome and metabolites. Front. Immunol. 2021, 12, 804870. [CrossRef]
- Zella, D.; Gallo, R.C. Viruses and bacteria associated with cancer: an overview. Viruses 2021, 13(6), 1039. [CrossRef]
- van Elsland, D.; Neefjes, J. Bacterial infections and cancer. EMBO Rep. 2018, 19(11), e46632. [CrossRef]
- Vogelmann, R.; Amieva, M.R. The role of bacterial pathogens in cancer. Curr. Opin. Microbiol. 2007, 10(1), 76-81. [CrossRef]
- Eaden, J.A.; Abrams, K.R.; Mayberry, J.F. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001, 48(4), 526-35. [CrossRef]
- Jess, T.; Rungoe, C.; Peyrin-Biroulet, L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin. Gastroenterol. Hepatol. 2012, 10(6),639-45. [CrossRef]
- Itzkowitz, S.H.; Yio, X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: The role of inflammation. Am. J. Physiol Gastrointest. Liver Physiol. 2004, 287(1), G7-17. [CrossRef]
- Karin, M.; Greten, F.R. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5(10),749-59. [CrossRef]
- Pikarsky, E.; Porat, R.M.; Stein, I.; Abramovitch, R.; Amit, S.; Kasem, S.; Gutkovich-Pyest, E.; Urieli-Shoval, S.; Galun, E.; Ben-Neriah, Y. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature 2004, 431(7007), 461-6. [CrossRef]
- Bollrath, J.; Phesse, T.J.; von Burstin, V.A.; Putoczki, T.; Bennecke, M.; Bateman, T.; Nebelsiek, T.; Lundgren-May, T.; Canli, O.; Schwitalla, S.; Matthews, V.; Schmid, R.M.; Kirchner, T., Arkan, M.C.; Ernst, M.; Greten, F.R. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009, 15(2), 91-102. [CrossRef]
- Colotta, F.; Allavena, P.; Sica, A.; Garlanda, C.; Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009, 30(7), 1073-81. [CrossRef]
- Rubio, K.; Dobersch, S.; Barreto, G. Functional interactions between scaffold proteins, noncoding RNAs, and genome loci induce liquid-liquid phase separation as organizing principle for 3-dimensional nuclear architecture: implications in cancer. FASEB J. 2019, 33(5), 5814-5822. [CrossRef]
- Zhang, Y. Recent progress in the epigenetics and chromatin field. Cell Res. 2011, 21(3), 373-374. [CrossRef]
- Ozturk, N.; Singh, I.; Mehta, A.; Braun, T.; Barreto, G. HMGA proteins as modulators of chromatin structure during transcriptional activation. Front. Cell Dev. Biol. 2014, 2, 5. [CrossRef]
- Bentley, G.A.; Lewis-Bentley; A.; Finch, J.T.; Podjarny, A.D.; Roth, M. Crystal structure of the nucleosome core particle at 16 A resolution. J. Mol. Biol. 1984, 176(1), 5-75. [CrossRef]
- Richmond, T.J.; Finch, J.T; Rushton, B.; Rhodes, D.; Klug A. Structure of the nucleosome core particle at 7 A resolution. Nature 1984, 311(5986), 532-7. [CrossRef]
- Liang, B.; Wang, Y.; Xu, J.; Shao, Y.; Xing, D. Unlocking the potential of targeting histone-modifying enzymes for treating IBD and CRC. Clin. Epigenetics 2023, 15(1), 146. [CrossRef]
- Singh, I.; Contreras, A.; Cordero, J.; Rubio, K.; Dobersch, S.; Gunther, S.; Jeratsch S, Mehta A, Krüger M, Graumann J, Seeger W, Dobreva G, Braun T, Barreto, G. MiCEE is a ncRNA-protein complex that mediates epigenetic silencing and nucleolar organization.. Nat. Genet. 2018, 50(7), 990-1001. [CrossRef]
- Cedar, H.; Bergman, Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat. Rev. Genet. 2018, 10(5), 295-304. [CrossRef]
- Deaton, A.M.; Bird, A. CpG islands and the regulation of transcription. Genes Dev. 2011, 25(10), 1010-1022. [CrossRef]
- Skinner, M.K. Environmental epigenetics and a unified theory of the molecular aspects of evolution: a neo-lamarckian concept that facilitates neo-darwinian evolution. Genome Biol. Evol. 2015, 7(5), 1296-1302. [CrossRef]
- Kealy, L.; Runting, J.; Thiele, D.; Scheer, S. An emerging maestro of immune regulation: how DOT1L orchestrates the harmonies of the immune system. Front. Immunol. 2024, 15, 1385319. [CrossRef]
- Dobersch, S.; Rubio, K.; Barreto, G. Pioneer Factors and Architectural proteins mediating embryonic expression signatures in cancer. Trends Mol. Med. 2019, 25(4), 287-302. [CrossRef]
- Zhang, M.; Zhao, J.; Lv, Y.; Wang, W.; Feng, C.; Zou, W.; Su, L.; Jiao, J. Histone variants and histone modifications in neurogenesis. Trends Cell Biol. 2020, 30(11), 869-880. [CrossRef]
- Strahl, B.D.; Allis, C.D. The language of covalent modifications. Nature 2000, 403(6765), 41-5. [CrossRef]
- Hake, S.B.; Xiao, A.; Allis, C.D. Linking the epigenetic 'language' of covalent histone modifications to cancer. Br. J. Cancer 2007, 96 Suppl, R31-9. PMID: 17393583.
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011 21(3), 381-95. [CrossRef]
- Mattiroli, F.; Penengo, L. Histone Ubiquitination: An integrative signaling platform in genome stability. Trends Genet. 2021, 37(6), 566-581. [CrossRef]
- Andrews, A. J.; Luger, K. Nucleosome structure(s) and stability: variations on a theme Annu. Rev. Biophys. 2011, 40, 99-117. [CrossRef]
- Kouzarides, T. Chromatin modifcations and their function. Cell 2007, 128(4), 693-705. [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code Science 2001, 93(5532),1074-80. [CrossRef]
- Jones, P. A.; Baylin, S. B. The epigenomics of cancer. Cell 2007, 128(4), 683-692. [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Ting, C.; Zhao, T.C. Histone deacetylases and mechanisms of regulation of gene expression. Crit. Rev. Oncog. 2015, 20(1-2), 35–47. [CrossRef]
- Yang, Z.H.; Dang, Y.-Q.; Ji, G. Role of epigenetics in transformation of inflammation into colorectal cancer. World J. Gastroenterol. 2019, 25(23), 2863-2877. [CrossRef]
- Esteller, M. Cancer epigenomics: DNA methylomes and histone-modification maps. Nat. Rev. Genet. 2007, 8(4), 286-98. [CrossRef]
- Schubeler, D. Function and information content of DNA methylation. Nature 2015, 517(7534), 321-326. [CrossRef]
- Saif, I.; Kasmi, Y.; Allali, K.; Ennaji, M.M. Prediction of DNA methylation in the promoter of gene suppressor tumor. Gene 2018, 651,166-173. [CrossRef]
- Yang, I.V.; Schwartz, D.A. Epigenetic control of gene expression in the lung. Am. J. Respir. Crit. Care Med. 2011, 183(10), 1295-1301. [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes Dev. 2014, 28(8), 812-28. [CrossRef]
- Jin, B.; Robertson, K. D. DNA methyltransferases, DNA damage repair, and cancer. Adv. Exp. Med. Biol. 2013, 754, 3-29. [CrossRef]
- Moore, L.D.; Le, T.; Fan, G. DNA methylation and its basic function. Neuropsychopharmacology, 2013, 38, 23-38. [CrossRef]
- Chakraborty, A.; Viswanathan, P. Methylation-Demethylation Dynamics: Implications of Changes in Acute Kidney Injury. Anal. Cell. Pathol. (Amst) 2018, 2018, 8764384. [CrossRef]
- Szyf, M. DNA methylation and cancer therapy. Drug Resist Updat. 2003, 6(6), 341-53. [CrossRef]
- Greenberg, M.V.C.; Bourc'his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell. Biol. 2019 20(10), 590-607. [CrossRef]
- Nie, L.; Wu, H.J.; Hsu, J.M.; Chang, S.S.; Labaff, A.M.; Li, C.W.; Wang, Y.; Hsu, J.L. Long non-coding RNAs: versatile master regulators of gene expression and crucial players in cancer. Am. J. Transl. Res. 2012, 4(2),127-50. PMID: 22611467; PMCID: PMC3353529.
- Panda, A.C. Circular RNAs Act as miRNA Sponges. Adv. Exp. Med. Biol. 2018, 1087, 67-79. [CrossRef]
- Li, J.; Xu, Q.; Huang, Z.J.; Mao, N.; Lin, Z.T.; Cheng, L.; Sun, B.; Wang, G. CircRNAs: A new target for the diagnosis and treatment of digestive system neoplasms. Cell Death Dis. 2021, 12, 205. [CrossRef]
- Wang,F.; Nazarali, A.J.; Ji, S. Circular RNAs as potential biomarkers for cancer diagnosis and therapy. Am. J. Cancer Res. 2016, 6, 1167–1176. PMID: 27429839; PMCID: PMC4937728.
- Zhou,Z.; Wang, X.; Hu, Q.; Yang, Z. CircZfp609 contributes to cerebral infarction via sponging miR-145a-5p to regulate BACH1. Metab. Brain Dis. 2023, 38, 1971–1981. doi: 10.1007/s11011-023-01208-4. PMID: 37097437.
- Mowel, W.K.; Kotzin, J.J.; McCright, S.J.; Neal, V.D.; Henao-Mejia, J. Control of immune cell homeostasis and function by lncRNAs. Trends Immunol. 2018, 39(1), 55-69. [CrossRef]
- Lei, K.; Bai, H.; Wei, Z; Xie, C.; Wang, J.; Li, J.; Chen, Q. The mechanism and function of circular RNAs in human diseases. Exp. Cell Res. 2018, 368, 147-158. [CrossRef]
- Aalto, A.P.; Pasquinelli, A. E. Small non-coding RNAs mount a silent revolution in gene expression. Curr. Opin. Cell Biol. 2012, 24(3), 333-340. [CrossRef]
- Li, J.; Zhong, Y.; Cai, S.; Zhou, P.; Yao, L. MicroRNA expression profiling in the colorectal normal-adenoma-carcinoma transition. Oncol. Lett. 2019, 18(2), 2013-2018. [CrossRef]
- Peng, Q.; Zhang, X.; Min, M.; Zou, L.; Shen, P.; Zhu, Y. The clinical role of microRNA-21 as a promising biomarker in the diagnosis and prognosis of colorectal cancer: a systematic review and meta-analysis. Oncotarget 2017, 8(27), 44893-44909. [CrossRef]
- Kellermayer, R.; Zilbauer, M. The gut microbiome and the triple environmental hit concept of inflammatory bowel disease pathogenesis. J. Pediatr. Gastroenterol. Nutr. 2020, 71(5), 589-595. [CrossRef]
- Gargalionis, A. N.; Piperi, C.; Adamopoulos, C.; Papavassiliou, A. G. Histone modifications as a pathogenic mechanism of colorectal tumorigenesis. Int. J. Biochem. Cell Biol. 2012, 44(8), 1276-1289. [CrossRef]
- Tamgue, O.; Mezajou, C. F.; Ngongang, N. N.; Kameni, C.; Ngum, J. A.; Simo, U. S. F.; Tatang, F. J.; Akami, M.; Ngono, A. N. Non-Coding RNAs in the Etiology and Control of Major and Neglected Human Tropical Diseases. Front. Immunol. 2021, 12, 703936. [CrossRef]
- Roy, S.; Ghosh, S.; Banerjee, M.; Laha, S.; Bhattacharjee, D.; Sarkar, R.; Ray, S.; Banerjee, A.; Ghosh, R.; Halder, A.; Ghosh, A.; Chatterjee, R.; Datta, S.; Dhali, G. K.; Banerjee, S. A combination of circulating microRNA-375-3p and chemokines CCL11, CXCL12, and G-CSF differentiate Crohn’s disease and intestinal tuberculosis. Sci. Rep. 2021, 11(1), 23303. [CrossRef]
- Yang, F.; Yang, Y.; Chen, L.; Zhang, Z.; Liu, L.; Zhang, C.; Mai, Q.; Chen, Y.; Chen, Z.; Lin, T.; Chen, L.; Guo, H.; Zhou, L.; Shen, H.; Chen, X.; Liu, L.; Zhang, G.; Liao, H.; Zeng, L.; Zeng, G. The gut microbiota mediates protective immunity against tuberculosis via modulation of lncRNA. Gut Microbe 2022, 14(1), 2029997. [CrossRef]
- Zhuo, Q.; Zhang, X.; Zhang, K.; Chen, C.; Huang, Z.; Xu, Y. The gut and lung microbiota in pulmonary tuberculosis: susceptibility, function, and new insights into treatment. Expert Review of Anti-Infective Therapy 2023, 21(12), 1355-1364. [CrossRef]
- Xu, J.; Xu, H.M.; Yang, M.F.; Liang, Y.J.; Peng, Q.Z.; Zhang, Y.; Tian, C.M.; Wang, L.S.; Yao, J.; Nie, Y.Q.; Li. D.F. New insights into the epigenetic regulation of inflammatory bowel disease. Front. Pharmacol. 2022, 13, 813659. [CrossRef]
- Lin, Y.; Qiu, T.; Wei, G.; Que, Y.; Wang, W.; Kong, Y.; Xie, T.; Chen, X. Role of histone post-translational modifications in inflammatory diseases. Front. Immunol. 2022, 13, 852272. [CrossRef]
- Goossens-Beumer, I.J.; Bernard, A.; van Hoesel, A.Q.; Zeestraten, E.C.; Putter, H.; Böhringer, S.; Liefers, G.J.; Morreau, H.; van de Velde, C.J.; Kuppen, P.J. Age-dependent clinical prognostic value of histone modifications in colorectal cancer. Transl. Res. 2015, 165(5), 578-88. [CrossRef]
- Qin, J.; Wen, B.; Liang, Y.; Yu, W.; Li. H. Histone modifications and their role in colorectal cancer. Pathol. Oncol. Res. 2020, 26(4), 2023-2033. [CrossRef]
- Sarvestani, S. K.; Signs, S. A.; Lefebvre, V.; Mack, S.; Ni, Y., Morton, A.; Chan, E.R.; Li, X.; Fox, P.; Ting, A.; Kalady, M.F.; Cruise, M.; Ashburn, J.; Stiene, J.; Lai, W.; Liska, D.; Xiang, S.; Huang, E. H. Cancer-predicting transcriptomic and epigenetic signatures revealed for ulcerative colitis in patient-derived epithelial organoids. Oncotarget 2018 9(47), 28717-28730. [CrossRef]
- Chen, L.; Luo, Z.; Zhao, C.; Li, Q.; Geng, Y.; Xiao, Y.; Chen, M.K.; Li, L.; Chen, Z.X.; Wu, M. Dynamic chromatin states coupling with key transcription factors in colitis-associated colorectal cancer. Adv. Sci. (Weinh) 2022, 9(23), e2200536. [CrossRef]
- Parang, B.; Barrett C.W.; Williams, C.S. AOM/DSS Model of colitis-associated cancer. Methods Mol. Biol. 2016, 1422, 297-307. [CrossRef]
- Xiao, J.; Duan, Q.; Wang, Z.; Yan, W.; Sun, H.; Xue, P.; Fan, X.; Zeng, X.; Chen, J.; Shao, C.; Zhu, F. Phosphorylation of TOPK at Y74, Y272 by Src increases the stability of TOPK and promotes tumorigenesis of colon. Oncotarget 2016, 7(17), 24483-94. [CrossRef]
- Cheung, P., T. K.; Cheung, W.L.; Sassone-Corsi, P.; Denu, J.M.; Allis, C.D. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell 2000, 5(6):905-15. [CrossRef]
- Hu, X.; L. X.; Liu, R.; Ai, N.; Cao, Z.; Li, Y.; Liu, J.; Yu, B.; Liu, K.; Wang, H.; Zhou, C.; Wang, Y.; Han, A.; Ding, F.; Chen, R. Histone cross-talk connects protein phosphatase 1α (PP1α) and histone deacetylase (HDAC) pathways to regulate the functional transition of bromodomain-containing 4 (BRD4) for inducible gene expression. J. Biol. Chem. 2014, 289(33), 23154-23167. [CrossRef]
- Komar, D.; Juszcynski, P. Rebelled epigenome: histone H3S10 phosphorylation and H3S10 kinases in cancer biology and therapy. Clin. Epigenetics 2020, 12(1), 147. [CrossRef]
- Tarcic ,O.; Pateras. I.S.; Cooks, T.; Shema, E.; Kanterman, J.; Ashkenazi, H.; Boocholez, H.; Hubert, A.; Rotkopf, R.; Baniyash, M.; Pikarsky, E.; Gorgoulis, V.G.; Oren. M. RNF20 Links Histone H2B ubiquitylation with inflammation and inflammation-associated cancer. Cell Rep. 2016, 14(6), 1462-1476. [CrossRef]
- Jeusset, L.M.; McManus, K.J. Characterizing and exploiting the many roles of aberrant H2B monoubiquitination in cancer pathogenesis. Seminars Cancer Biol. 2021, 22(4), 1044–1579. [CrossRef]
- Worden, E.J.; Wolberger, C. Activation and regulation of H2B-ubiquitin-dependent histone methyltransferases. Curr. Opin. Struct. Biol. 2020, 59, 98-106. [CrossRef]
- Worden, E.J.; Zhang, X.; Wolberger C. Structural basis for COMPASS recognition of an H2B-ubiquitinated nucleosome. Elife, 2020, 9, e53199. [CrossRef]
- Van Tongelen, A.; Loriot, A.; De Smet, C. Oncogenic roles of DNA hypomethylation through the activation of cancer-germline genes. Cancer Letters 2017, 396, 130-137. [CrossRef]
- Dokun, O. Y.; Florl, A. R.; Seifert, H. H.; Wolff, I.; Schulz, W. A. Relationship of SNCG, S100A4, S100A9 and LCN2 gene expression and DNA methylation in bladder cancer. Int. J. Cancer 2008, 123(12), 2798-2807. [CrossRef]
- Dokun, O. Y.; Florl, A. R.; Seifert, H. H.; Wolff, I.; Schulz, W. A. Relationship of SNCG, S100A4, S100A9 and LCN2 gene expression and DNA methylation in bladder cancer. Int. J. Cancer 2008, 123(12), 2798-2807. [CrossRef]
- Wilson, A.S.; Power, B.E.; Molloy, P.L. DNA hypomethylation and human diseases. BBA, 2007, 1775, 138–162. [CrossRef]
- Porcellini, E.; Laprovitera, N.; Riefolo, M.; Ravaioli, M.; Garajova, I.; Ferracin, M. Epigenetic and epitranscriptomic changes in colorectal cancer: Diagnostic, prognostic, and treatment implications. Cancer Lett. 2018, 419, 84-95. [CrossRef]
- Antelo, M.; Balaguer, F.; Shia, J.; Shen, Y.; Hur, K.; Moreira, L.; Cuatrecasas, M.; Bujanda, L.; Giraldez, M.D.; Takahashi, M.; Cabanne, A.; Barugel, M.E.; Arnold, M.; Roca, E.L.; Andreu, M.; Castellvi-Bel, S.; Llor, X.; Jover, R.; Castells, A.; Boland C.R.; Goel, A. A high degree of LINE-1 hypomethylation is a unique feature of early-onset colorectal cancer PLoS One 2012, 7(9), e45357. [CrossRef]
- Ahn, J.B.; Chung, W.B. Maeda, O.; Shin, S.J.; Kim, H.S.; Chung, H.C.; Kim, N.K.; Issa, J.P. DNA methylation predicts recurrence from resected stage III proximal colon cancer. Cancer 2011, 117(9), 1847-1854. [CrossRef]
- Ogino, S.; Nosho, K.; Kirkner, G.J.; Kawasaki, T.; Chan, A.T.; Schernhammer, E.S.; Giovannucci, E.L.; Fuchs, C.S. A cohort study of tumoral LINE-1 hypomethylation and prognosis in colon cancer. J. Natl. Cancer Inst. 2008, 100(23), 1734-8. [CrossRef]
- Baba, Y.; Huttenhower, C.; Nosho, K.; Tanaka, N.; Shima, K.; Hazra, A.; Schernhammer, E.S.; Hunter, D.J.; Giovannucci, E.L.; Fuchs, C.S.; Ogino. S. Epigenomic diversity of colorectal cancer indicated by LINE-1 methylation in a database of 869 tumors. Mol. Cancer 2010, 9, 125. [CrossRef]
- Swets, M.; Zaalberg, A.; Boot, A.; van Wezel, T.; Frouws, M. A.; Bastiaannet, E.; Gelderblom, H.; van de Velde, C.J.; Kuppen, P.J. Tumor LINE-1 Methylation level in association with survival of patients with stage II colon cancer. Int. J. Mol. Sci. 2016, 18(1). [CrossRef]
- Mima, K.; Nowak, J.A.; Qian, Z.R.; Cao, Y.; Song, M.; Masugi, Y.; Shi, Y.; da Silva, A.; Gu, M.; Li, W.; Hamada, T.; Zhang, X.; Wu, K.; Meyerhardt, J.A.; Baba, H.; Giovannucci, E.L.; Chan, A.T.; Fuchs, C.S.; Ogino, S.; Nishihara, R. Tumor LINE-1 methylation level and colorectal cancer location in relation to patient survival. Oncotarget 2016, 7(34), 55098-55109. [CrossRef]
- Kawakami, K.; Matsunoki, A.; Kaneko, M.; Saito, K.; Watanabe, G.; Minamoto, T. Long interspersed nuclear element-1 hypomethylation is a potential biomarker for the prediction of response to oral fluoropyrimidines in microsatellite stable and CpG island methylator phenotype-negative colorectal cancer. Cancer Sci. 2011, 102(1), 166-174. [CrossRef]
- Robertson, K.D. DNA methylation and human disease. Nat. Rev. Genet. 2005, 6(8), 597-610. [CrossRef]
- Ambrosi, C.; Manzo, M.; Baubec, T. Dynamics and context-dependent roles of dna methylation. J. Mol. Biol. 2017, 429(10), 1459-1475. [CrossRef]
- Kanwal, R.; Gupta, S. Epigenetics and cancer. J. Appl. Physiol. 2010, 109, 598–605. [CrossRef]
- Nimmo, E.R.; Prentergast, J.G.; Aldhous, M.C.; Kennedy, N.A., Henderson, P.; Drummond, H.E.; Ramsahoye, B.H.; Wilson, D.C.; Semple, C.A.; Satsangi, J. Genome-wide methylation profiling in Crohn's disease identifies altered epigenetic regulation of key host defense mechanisms including the Th17 pathway. Inflamm. Bowel Dis. 2012, 18(5), 889-99. [CrossRef]
- Hasler, R.; Feng, Z.; Backdahl, L.; Spehlmann, M.E.; Franke, A.; Teschendorff, A.; Rakyan, V.K.; Down, T.A.; Wilson, G.A.; Feber, A.; Beck, S.; Schreiber, S.; Rosenstiel, P. A functional methylome map of ulcerative colitis. Genome Res. 2012, 22(11), 2130-2137. [CrossRef]
- Cooke, J.; Zhang, H.; Greger, L.; Silva, A.L.; Massey, D.; Dawson, C.; Metz, A.; Ibrahim, A.; Parkes, M. Mucosal genome-wide methylation changes in inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18(11), 2128-2137. doi.org/10.1002/ibd.22942.
- Saito, S.; Kato, J.; Hiraoka, S.; Horii, J.; Suzuki, H.; Higashi, R.; Kaji, E.; Kondo, Y.; Yamamoto, K. DNA methylation of colon mucosa in ulcerative colitis patients: correlation with inflammatory status. Inflamm. Bowel Dis. 2011, 17(9), 1955-1965. [CrossRef]
- Harris, R.A.; Nagy-Szakal, D.; Mir, S.A.; Frank, E.; Szigeti, R.; Kaplan, J.L.; Bronsky, J.; Opekun, A.; Ferry, G.D., Winter, H., Kellermayer, R. DNA methylation-associated colonic mucosal immune and defense responses in treatment-naïve pediatric ulcerative colitis. Epigenetics 2014, 9(8), 1131-7. [CrossRef]
- Koizumi, K.; Alonso, S.; Miyaki, Y.; Okada, S.; Ogura, H.; Shiiya, N.; Konishi, F.; Taya, T.; Perucho, M.; Suzuki, K. Array-based identification of common DNA methylation alterations in ulcerative colitis. Int. J. Oncol. 2012, 40(4), 983-94. [CrossRef]
- Lin, Z.; Hegarty, J.P.; Yu, W.; Cappel, J.A.; Chen, X.; Faber, P.W.; Wang, Y.; Poritz, L.S.; Fan, J.B.; Koltun, W.A. Identification of disease-associated DNA methylation in B cells from Crohn's disease and ulcerative colitis patients. Dig. Dis. Sci. 2012, 57(12), 3145-3153. [CrossRef]
- Foran, E.; Garrity-Park, M.M.; Mureau, C.; Newell, J.; Smyrk, T.C.; Limburg, P.J.; Egan, L.J. Upregulation of DNA methyltransferase-mediated gene silencing, anchorage-independent growth, and migration of colon cancer cells by interleukin-6. Mol. Cancer Res. 2010, 8(4), 471-481. [CrossRef]
- Wang, F.Y.; Arisawa, T.; Tahara, T.; Takahama, K.; Watanabe, M.; Hirata, I.; Nakano, H. Aberrant DNA methylation in ulcerative colitis without neoplasia. Hepatogastroenterol. 2008, 55(81), 62-5.
- Hsieh, C.J.; Klump, B.; Holzmann, K.; Borchard, F.; Gregor, M.; Porschen, R. Hypermethylation of the p16INK4a promoter in colectomy specimens of patients with long-standing and extensive ulcerative colitis. Cancer Res. 1998, 58(17), 942-5.
- Abu-Remaileh, M.; Bender, S.; Raddatz, G.; Ansari, I.; Cohen, D.; Gutekunst, J.; Musch, T.; Linhart, H.; Breiling, A.; Pikarsky, E.; Bergman, Y.; Lyko, F. Chronic inflammation induces a novel epigenetic program that is conserved in intestinal adenomas and in colorectal cancer. Cancer Res. 2015, 75(10), 2120-2130. [CrossRef]
- Sato, F.; Harpaz, N.; Shibata, D.; Xu, Y.; Yin, J.; Mori, Y.; Zou, T.T.; Wang, S.; Desai, K.; Leytin, A.; Selaru, F.M.; Abraham, J.M.; Meltzer, S.J. Hypermethylation of the p14(ARF) gene in ulcerative colitis-associated colorectal carcinogenesis. Cancer Res. 2002, 62(4), 1148-51.
- Gerecke, C.; Scholtka, B.; Lowenstein, Y.; Fait, I.; Gottschalk, U.; Rogoll, D.; Melcher, R.; Kleuser, B. Hypermethylation of ITGA4, TFPI2 and VIMENTIN promoters is increased in inflamed colon tissue: putative risk markers for colitis-associated cancer. J. Cancer Res. Clin. Oncol. 2015, 141(12), 2097-2107. [CrossRef]
- Xie, W.; Schultz, M.D.; Lister, R.; Hou, Z.; Rajagopal, N.; Ray, P.; Whitaker, J.W.; Tian, S.; Hawkins, R.D.; Leung, D.; Yang, H.; Wang, T.; Lee, A.Y.; Swanson, S.A.; Zhang, J.; Zhu, Y.; Kim, A.; Nery, J.R.; Urich, M.A.; Kuan, S.; Yen, C.A.; Klugman, S.; Yu, P.; Suknuntha, K.; Propson, N.E.; Chen, H.; Edsall, L.E.; Wagner, U., Li, Y.; Ye, Z.; Kulkarni, A.; Xuan, Z.; Chung, W.Y.; Chi, N.C.; Antosiewicz-Bourget, J.E.; Slukvin, I.; Stewart, R.; Zhang, M.Q.; Wang, W.; Thomson, J.A.; Ecker, J.R.; Ren, B. Epigenomic analysis of multilineage differentiation of human embryonic stem cells. Cell 2013, 153(5), 1134-48. [CrossRef]
- Issa, J.P. CpG island methylator phenotype in cancer. Nat. Rev. Cancer 2004, 4(12), 988–993. [CrossRef]
- Dong D, Zhang J, Zhang R, Li F, Li Y, Jia Y. Multiprobe Assay for Clinical SEPT9 Methylation Based on the Carbon Dot-Modified Liquid-Exfoliated Graphene Field Effect Transistor with a Potential to Present a Methylation Panorama. ACS Omega 2020, 5(26), 16228-16237. [CrossRef]
- Song, L; Li, Y. SEPT9: A Specific Circulating Biomarker for Colorectal Cancer. Adv. Clin. Chem. 2015, 72, 171-204. [CrossRef]
- Kondo, Y.; Issa, J.P. Epigenetic changes in colorectal cancer. Cancer Metastasis Rev. 2004, 23(1-2), 29-39. [CrossRef]
- Philipp, A.B.; Stieber, P.; Nagel, D.; Neumann, J.; Spelsberg, F.; Jung, A.; Lamerz, R.; Herbst, A.; Kolligs, F.T. Prognostic role of methylated free circulating DNA in colorectal cancer. Int. J. Cancer, 2012, 131(10), 2308-2319. [CrossRef]
- Melotte, V.; Lentjes, M. H.; van den Bosch, S.M.; Hellebrekers, D.M.; de Hoon, J.P.; Wouters, K.A.; Daenen, K.L.; Partouns-Hendriks, I.E.; Stessels, F.; Louwagie, J.; Smits, K.M.; Weijenberg, M.P.; Sanduleanu, S.; Khalid-de Bakker, C.A.; Oort, F.A.; Meijer, G.A.; Jonkers, D.M.; Herman, J.G.; de Bruïne, A.P; van Engeland, M. N-Myc downstream-regulated gene 4 (NDRG4): a candidate tumor suppressor gene and potential biomarker for colorectal cancer. J. Natl. Cancer Inst. 2009, 101(13), 916-927. [CrossRef]
- Loh, K.; Chia, J.A.; Greco, S.; Cozzi, S.J.; Buttenshaw, R.L.; Bond, C.E.; Simms, L.A.; Pike, T.; Young, J.P.; Jass, J.R.; Spring, K.J.; Leggett, B.A; Whitehall, V. L. Bone morphogenic protein 3 inactivation is an early and frequent event in colorectal cancer development. Genes Chromosomes Cancer 2008, 47(6), 449-460. [CrossRef]
- Xing, X.; Cai, W.; Shi, H.; Wang, Y.; Li, M.; Jiao, J.; Chen, M. The prognostic value of CDKN2A hypermethylation in colorectal cancer: a meta-analysis. Br. J. Cancer 2013, 108(12), 2542-2548. [CrossRef]
- Jiang, W.; Wang, P.G.; Zhan, Y.; Zhang, D. Prognostic value of p16 promoter hypermethylation in colorectal cancer: a meta-analysis. Cancer Invest. 2014, 32(2), 43-52. [CrossRef]
- West, N.R.; McCuaig. S.; Franchini, F.; Powrie, F. Emerging cytokine networks in colorectal cancer. Nat. Rev. Immunol. 2015, 15(10), 615-29. [CrossRef]
- Li, Y.; Deuring, J.; Peppelenbosch, M.P.; Kuipers, E.J.; de Haar, C.; van der Woude, C. J. IL-6-induced DNMT1 activity mediates SOCS3 promoter hypermethylation in ulcerative colitis-related colorectal cancer. Carcinogenesis 2012, 33(10), 1889-1896. [CrossRef]
- Clawson, G.A. Histone deacetylase inhibitors as cancer therapeutics. Ann. Transl. Med. 2016, 4(15), 287. PMID: 27568481; PMCID: PMC4980376. [CrossRef]
- Timp, W.; Feinberg, A.P. Cancer as a dysregulated epigenome allowing cellular growth advantage at the expense of the host. Nat. Rev. Cancer 2013, 13(7), 497-510. [CrossRef]
- Fraga, M.F.; Ballestar, E.; Villar-Garea, A.; Boix-Chornet, M.; Espada, J.; Schotta, G.; Bonaldi, T.; Haydon, C.; Ropero, S.; Petrie, K.; Iyer, N.G.; Pérez-Rosado, A.; Calvo, E.; Lopez, J.A.; Cano, A.; Calasanz, M.J.; Colomer, D.; Piris, M.A.; Ahn, N.; Imhof, A.; Caldas, C.; Jenuwein, T.; Esteller, M. Loss of acetylation at Lys16 and trimethylation at Lys20 of histone H4 is a common hallmark of human cancer. Nat. Genet. 2005, 37(4), 391-400. [CrossRef]
- Ashktorab, H.; Belgrave, K.; Hosseinkhah, F.; Brim, H.; Nouraie, M.; Takkikto, M.; Hewitt, S.; Lee, E.L.; Dashwood, R.H.; Smoot D. Global histone H4 acetylation and HDAC2 expression in colon adenoma and carcinoma. Dig. Dis. Sci. 2009, 54(10), 2109-17. [CrossRef]
- Tamagawa, H.; Oshima, T.; Shiozawa, M.; Morinaga, S.; Nakamura, Y.; Yoshihara, M.; Sakuma, Y.; Kameda, Y.; Akaike, M.; Masuda, M.; Imada, T.; Miyagi, Y. The global histone modification pattern correlates with overall survival in metachronous liver metastasis of colorectal cancer. Oncol. Rep. 2012 , 27(3), 637-42. [CrossRef]
- Liu,Y.; Hong, Y.; Zhao, Y.; Ismail, T.M.; Wong, Y.; Eu, K.W. Histone H3 (lys-9) deacetylation is associated with transcriptional silencing of E-cadherin in colorectal cancer cell lines. Cancer Invest. 2008 , 26(6), 575-82. [CrossRef]
- Peláez, I.M.; Kalogeropoulou, M.; Ferraro, A.; Voulgari, A.; Pankotai, T.; Boros, I.; Pintzas, A. Oncogenic RAS alters the global and gene-specific histone modification pattern during epithelial-mesenchymal transition in colorectal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42(6), 911-20. [CrossRef]
- Zuo, X.; Morris, J.S.; Shureiqi, I. Chromatin modification requirements for 15-lipoxygenase-1 transcriptional reactivation in colon cancer cells. J. Biol. Chem. 2008, 283(46), 31341-7. [CrossRef]
- Chen, Y.X.; Fang, J.Y.; Lu, R.; Qiu, D.K. Expression of p21(WAF1) is related to acetylation of histone H3 in total chromatin in human colorectal cancer. World J. Gastroenterol. 2007, 13(15), 2209-13. [CrossRef]
- Li, Q.; Chen, H. Transcriptional silencing of N-Myc downstream-regulated gene 1 (NDRG1) in metastatic colon cancer cell line SW620. Clin. Exp. Metastasis. 2011, 28(2), 127-35. [CrossRef]
- Ishii, M.; Wen, H.; Corsa, C.A.; Liu, T.; Coelho, A.L.; Allen, R.M.; Carson, W.F. 4th; Cavassani, K.A.; Li, X.; Lukacs, N.W.; Hogaboam, C.M.; Dou, Y.; Kunkel, S.L. Epigenetic regulation of the alternatively activated macrophage phenotype. Blood 2009, 114(15), 3244-54. [CrossRef]
- De Santa, F.; Totaro, M.; Prosperini, E.; Notarbartolo, S.; Testa, G.; Natoli. G. The histone H3 lysine-27 demethylase Jmjd3 links inflammation to inhibition of polycomb-mediated gene silencing. Cell 2007, 130(6), 1083-94. [CrossRef]
- Bayarsaihan, D. Epigenetic mechanisms in inflammation. J. Dent. Res. 2011, 90(1), 9-17. [CrossRef]
- Nagarsheth, N.; Peng, D.; Kryczek, I.; Wu, K.; Li, W.; Zhao, E.; Zhao, L.; Wei, S.; Frankel, T.; Vatan, L.; Szeliga, W.; Dou, Y.; Owens, S.; Marquez, V.; Tao, K.; Huang, E.; Wang, G.; Zou W. PRC2 Epigenetically Silences Th1-Type Chemokines to Suppress Effector T-Cell Trafficking in Colon Cancer. . Cancer Res. 2016, 76(2), 275-82. [CrossRef]
- Ghanem, I.; Riveiro, M.; Paradis, V.; Faivre, S.; de Parga, P.M.; Raymond, E. Insights on the CXCL12-CXCR4 axis in hepatocellular carcinoma carcinogenesis. Am. J. Transl. Res. 2014, 6(4), 340-52.
- Liu, H.; Liu, Y.; Liu, W.; Zhang, W.; Xu, J. EZH2-mediated loss of miR-622 determines CXCR4 activation in hepatocellular carcinoma. Nat. Commun. 2015, 6, 8494. [CrossRef]
- Enroth, S.; Alvaro, R.I.; Andersson, R.; Wallerman, O.; Wanders, A.; Påhlman, L.; Komorowski, J.; Wadelius C. Cancer associated epigenetic transitions identified by genome-wide histone methylation binding profiles in human colorectal cancer samples and paired normal mucosa. BMC Cancer 2011, 11, 450. [CrossRef]
- Nguyen, C.T.; Weisenberger, D.J.; Velicescu, M.; Gonzales, F.A.; Lin, J.C.; Liang, G.; Jones, P.A. Histone H3-lysine 9 methylation is associated with aberrant gene silencing in cancer cells and is rapidly reversed by 5-aza-2'-deoxycytidine. Cancer Res. 2002, 62(22), 6456-61. PMID: 12438235.
- An, X.; Lan, X.; Feng, Z.; Li, X.; Su, Q. Histone modification: Biomarkers and potential therapies in colorectal cancer. Ann. Hum. Genet. 2023, 87(6), 274-284. [CrossRef]
- Qiu, F.; Wang, Y.; Chu, X.; Wang, J. ASF1A regulates H4Y72 phosphorylation and promotes autophagy in colon cancer cells via a kinase activity. Artif .Cells Nanomed. Biotechnol. 2019, 47(1), 2754-2763. [CrossRef]
- Ghate, N.B.; Kim, S.; Spiller, E.; Kim, S.; Shin, Y.; Rhie, S.K.; Smbatyan, G., Lenz, H.J.; Mumenthaler, S.M.; An, W. VprBP directs epigenetic gene silencing through histone H2A phosphorylation in colon cancer. Mol. Oncol. 2021, 15(10), 2801-2817. [CrossRef]
- Lee, Y.C.; Yin, T.C.; Chen, Y.T.; Chai, C.Y.; Wang, J.Y.; Liu, M.C.; Lin, Y.C.; Kan, J.Y. High expression of phospho-H2AX predicts a poor prognosis in colorectal cancer. Anticancer Res. 2015, 35(4), 2447-53.
- Qin, J.; Wen, B.; Liang, Y.; Yu, W.; Li, H. Histone modifications and their role in colorectal cancer. Pathol. Oncol. Res. 2020, 26(4), 2023-2033. [CrossRef]
- Yu, D.; Li, Z.; Gan, M.; Zhang, H.; Yin, X.; Tang, S.; Wan, L.; Tian, Y.; Zhang, S.; Zhu, Y.; Lai, M.; Zhang, D. Decreased expression of dual specificity phosphatase 22 in colorectal cancer and its potential prognostic relevance for stage IV CRC patients. Tumour Biol. 2015, 36(11), 8531-5. [CrossRef]
- Chen, T.; Li, J.; Xu, M.; Zhao, Q.; Hou, Y.; Yao, L.; Zhong, Y.; Chou, P.C.; Zhang, W.; Zhou, P.; Jiang, Y. PKCε phosphorylates MIIP and promotes colorectal cancer metastasis through inhibition of RelA deacetylation. Nat. Commun. 2017, 8(1), 1-11. [CrossRef]
- Bao, Z.; Yang, Z.; Huang, Z.; Zhou, Y.; Cui, Q.; Dong, D. LncRNADisease 2.0: An updated database of long non-coding RNA-associated diseases. Nucleic Acids Res. 2019, 47, D1034– D1037. [CrossRef]
- Cheng, L.; Wang, P.; Tian, R.; Wang, S.; Guo, Q.; Luo, M.; Zhou, W.; Liu, G; Jiang, H.; Jiang, Q. LncRNA2Target v2.0: a comprehensive database for target genes of lncRNAs in human and mouse. Nucleic Acids Res. 2019, 47(D1), D140-D144. [CrossRef]
- Ghoussaini, M.; Mountjoy, E.; Carmona, M.; Peat, G.; Schmidt, E.M.; Hercules, A.; Fumis, L.; Miranda, A.; Carvalho-Silva, D.; Buniello, A.; et al. Open Targets Genetics: Systematic identification of trait-associated genes using large-scale genetics and functional genomics. Nucleic Acids Res. 2021, 49(D1), D1311–D1320. [CrossRef]
- Luck, K; Kim, D.K.; Lambourne, L.; Spirohn, K.; Begg, B.E.; Bian, W.; Brignall, R.; Cafarelli, T.; Campos-Laborie, F.J.; Charloteaux, B.; et al. A reference map of the human binary protein interactome. Nature 2020, 580(7803), 402–408. [CrossRef]
- Volders, P.J.; Anckaert, J.; Verheggen, K.; Nuytens, J.; Martens, L.; Mestdagh, P.; Vandesompele, J. LNCipedia 5: Towards a reference set of human long non-coding RNAs. Nucleic Acids Res. 2019, 47(D1), D135–D139. [CrossRef]
- Yang, M.; Lu, H.; Liu, J.; Wu, S.; Kim, P.; Zhou, X. lncRNAfunc: A knowledgebase of lncRNA function in human cancer. Nucleic Acids Res. 2022, 50(D1), D1295–D1306. [CrossRef]
- Zhao, L.; Wang, J.; Li, Y.; Song, T.; Wu, Y.; Fang, S.; Bu, D.; Li, H.; Sun, L.; Pei, D.; et al. NONCODEV6: an updated database dedicated to long non-coding RNA annotation in both animals and plants. Nucleic Acids Res. 2021, 49(D1), D165–D171. [CrossRef]
- Zhou, B.; Ji, B.; Liu, K.; Hu, G.; Wang, F.; Chen, Q.; Yu, R.; Huang, P.; Ren, J.; Guo, C.; et al. EVLncRNAs 2.0: An updated database of manually curated functional long non-coding RNAs validated by low-throughput experiments. Nucleic Acids Res. 2021, 49(D1), D86–D91. [CrossRef]
- Lin, L.; Zhou, G.; Chen, P.; Wang, Y.; Han, J.; Chen, M.; He, Y.; Zhang, S. Which long noncoding RNAs and circular RNAs contribute to inflammatory bowel disease? [Cell Death Dis. 2020, 11(6), 456. [CrossRef]
- Cummins, J.M.; He, Y.; Leary, R.J.; Pagliarini, R.; Diaz, L.A.; Jr.; Sjoblom, T.; Barad, O.; Bentwich, Z.; Szafranska, A.E.; Labourier, E.; Raymond, C.K.; Roberts, B.S.; Juhl. H.; Kinzler, K.W.; Vogelstein, B,;, Velculescu, V.E. The colorectal microRNAome. Proc. Natl. Acad. Sci. U. S. A. 2006, 103(10), 3687-3692. [CrossRef]
- Lanza, G.; Ferracin, M.; Gafa, R.; Veronese, A.; Spizzo, R.; Pichiorri, F.; Liu, C.G.; Calin, G,A,; Croce, C.M,; Negrini, M. mRNA/microRNA gene expression profile in microsatellite unstable colorectal cancer. Mol. Cancer 2007, 6, 54. [CrossRef]
- Garajova, I.; Ferracin, M.; Porcellini, E.; Palloni, A.; Abbati, F.; Biasco, G.; Brandi, G. Non-coding RNAs as predictive biomarkers to current treatment in metastatic colorectal cancer. Int. J. Mol. Sci. 2017, 18(7), 1547. [CrossRef]
- Ragusa, M.; Barbagallo, C.; Statello, L.; Condorelli, A.G.; Battaglia, R.; Tamburello, L.; Barbagallo, D.; Di Pietro, C.; Purrello, M. Non-coding landscapes of colorectal cancer. World J. Gastroenterol. 2015, 21(41), 11709-11739. [CrossRef]
- Lujambio, A.; Ropero, S.; Ballestar, E.; Fraga, M.F.; Cerrato, C.; Setien, F.; Casado, S.; Suarez-Gauthier, A.; Sanchez-Cespedes, M.; Git, A.; Spiteri, I.; Das, P.P.; Caldas, C.; Miska, E.; Esteller, M. Genetic unmasking of an epigenetically silenced microRNA in human cancer cells. Cancer Res. 2007, 67(4), 1424-1429. [CrossRef]
- Balaguer, F.; Link, A.; Lozano, J.J.; Cuatrecasas, M.; Nagasaka, T.; Boland, C.R.; Goel, A. Epigenetic silencing of miR-137 is an early event in colorectal carcinogenesis. Cancer Res. 2010, 70(16), 6609-6618. [CrossRef]
- Davalos, V.; Moutinho, C.; Villanueva, A.; Boque, R.; Silva, P.; Carneiro, F.; Esteller, M. Dynamic epigenetic regulation of the microRNA-200 family mediates epithelial and mesenchymal transitions in human tumorigenesis. Oncogene 2012, 31(16), 2062-2074. [CrossRef]
- Bandres, E.; Agirre, X.; Bitarte, N.; Ramirez, N.; Zarate, R.; Roman-Gomez, J.; Prosper, F.; Garcia-Foncillas, J. Epigenetic regulation of microRNA expression in colorectal cancer. Int. J. Cancer 2009, 125(11), 2737-2743. [CrossRef]
- Coskun, M.; Bjerrum, J.T.; Seidelin, J.B.; Troelsen, J.T.; Olsen, J.; Nielsen, O.H. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J. Gastroenterol. 2013, 19(27), 4289-4299. [CrossRef]
- Baud, V.; Karin, M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat. Rev. Drug Discov. 2005, 8(1), 33-40. [CrossRef]
- Fan, Y.; Mao, R.; Yang, J. NF-κB and STAT3 signaling pathways collaboratively link inflammation to cancer. Protein Cell 2013, 4(3), 176-85. [CrossRef]
- Slattery, M.L.; Mullany, L.E.; Sakoda, L.; Samowitz, W.S.; Wolff, R.K.; Stevens, J.R.; Herrick, J.S. The NF-kappaB signalling pathway in colorectal cancer: associations between dysregulated gene and miRNA expression. J. Cancer Res. Clin. Oncol. 2018, 144(2), 269-283. [CrossRef]
- Iliopoulos, D.; Jaeger, S.A.; Hirsch, H.A.; Bulyk, M.L.; Struhl, K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol. Cell 2010, 39(4), 493-506. [CrossRef]
- Wang, H.; Nie, L.; Wu, L.; Liu, Q.; Guo, X. NR2F2 inhibits Smad7 expression and promotes TGF-beta-dependent epithelial-mesenchymal transition of CRC via transactivation of miR-21. Biochem. Biophys. Res. Commun. 2017, 485(1), 181-188. [CrossRef]
- Wang, N.; He, X.; Zhou, R.; Jia, G.; Qiao, Q. STAT3 induces colorectal carcinoma progression through a novel miR-572-MOAP-1 pathway. Onco. Targets Ther. 2018, 11, 3475-3484. [CrossRef]
- Wang, R.; Jaw, J.J.; Stutzman, N.C.; Zou, Z.; Sun, P.D. Natural killer cell-produced IFN-γ and TNF-α induce target cell cytolysis through up-regulation of ICAM-1. J. Leukoc. Biol. 2012, 91(2), 299-309. [CrossRef]
- Zhang, L.L.; Zhang, L.F.; Shi, Y.B. MiR-24 inhibited the killing effect of natural killer cells to colorectal cancer cells by downregulating Paxillin. Biomed. Pharmacother. 2018, 101, 257-263. [CrossRef]
- Alamdari-Palangi, V.; Vahedi, F.; Shabaninejad, Z.; Dokeneheifard, S.; Movehedpour, A.; Taheri-Anganeh, M.; Savardashtaki, A. MicroRNA in inflammatory bowel disease at a glance. Eur. J. Gastroenterol. Hepatol. 2021, 32(2), 140-148. doi.org/10.1097/MEG.0000000000001815.
- Correia, C.N.; Nalpas, N.C.; McLoughlin, K.E.; Browne, J.A.; Gordon, S.V.; MacHugh, D.; Shaughnessy, R.G. Circulating microRNAs as Potential Biomarkers of Infectious Disease. Front. Immunol. 2017, 8, 118. [CrossRef]
- Rashid, H.; Hossain, B.; Siddiqua, T.; Kabir, M.; Noor, Z.; Ahmed, M.; Haque, R. Fecal microRNAs as potential biomarkers for screening and diagnosis of intestinal diseases. Front. Mol. Biosci. 2020, 7, 181. [CrossRef]
- Thorlacius-Ussing, G.; Schnack Nielsen, B.; Andersen, V.; Holmstrom, K.; Pedersen, A.E. Expression and localization of mir-21 and mir-126 in mucosal tissue from patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23(5), 739-752. doi.org/10.1097/MIB.0000000000001086.
- Feng, Y.H; Tsao, C.J. Emerging role of microRNA-21 in cancer. Biomed. Rep. 2016, 5(4), 395-402. [CrossRef]
- Shi, C.; Liang, Y.; Yang, J.; Xia, Y.; Chen, H.; Han, H.; Yang, Y.; Wu ,W.; Gao, R.; Qin, H. MicroRNA-21 knockout improve the survival rate in DSS induced fatal colitis through protecting against inflammation and tissue injury. PLoS One 2013, 8(6), e66814. [CrossRef]
- Wang, L.G.; Gu, J. Serum microRNA-29a is a promising novel marker for early detection of colorectal liver metastasis. Cancer Epidemiol. 2012, 36(1), e61-67. [CrossRef]
- Zhu, Y.; Xu, A.; Li, J.; Fu, J.; Wang, G.; Yang, Y.; Sun, J. Fecal miR-29a and miR-224 as the noninvasive biomarkers for colorectal cancer. Cancer Biomark. 2016, 16(2), 259-264. [CrossRef]
- Iborra, M.; Bernuzzi, F.; Correale, C.; Vetrano, S.; Fiorino, G.; Beltran, B.; Marabita, F.; Locati, M.; Spinelli, A.; Nos, P.; Invernizzi, P.; Danese, S. Identification of serum and tissue micro-RNA expression profiles in different stages of inflammatory bowel disease. Clin. Exp. Immunol. 2013, 173(2), 250-258. [CrossRef]
- Chen, P.; Li, Y.; Li, L.; Yu, Q.; Chao, K.; Zhou, G.; Qiu, Y.; Feng, R.; Huang, S.; He, Y.; Chen, B.; Chen, M.; Zeng, Z.; Zhang, S. Circulating microRNA146b-5p is superior to C-reactive protein as a novel biomarker for monitoring inflammatory bowel disease. Aliment. Pharmacol. Ther. 2019, 49(6), 733-743. [CrossRef]
- Sun, C.M.; Wu, J.; Zhang, H.; Shi, G.; Chen, Z.T. Circulating miR-125a but not miR-125b is decreased in active disease status and negatively correlates with disease severity as well as inflammatory cytokines in patients with Crohn's disease. World J. Gastroenterol. 2017, 23(44), 7888-7898. [CrossRef]
- Schaefer, J.S.; Attumi, T.; Opekun, A.R.; Abraham, B.; Hou, J.; Shelby, H.; Graham, D.Y.; Streckfus. C.; Klein, J. R. MicroRNA signatures differentiate Crohn's disease from ulcerative colitis. BMC Immunol. 2015, 16, 5. [CrossRef]
- Zahm, A.M.; Thayu, M.; Hand, N.J.; Horner, A.; Leonard, M.B.; Friedman, J.R. Circulating microRNA is a biomarker of pediatric Crohn disease. J. Pediatr. Gastroenterol. Nutr. 2011, 53(1), 26-33. [CrossRef]
- Zahm, A.M.; Hand, N.J.; Tsoucas, D.M.; Le Guen, C.L.; Baldassano, R.N.; Friedman, J.R. Rectal microRNAs are perturbed in pediatric inflammatory bowel disease of the colon. J. Crohns Colitis 2014, 8(9), 1108-1117. [CrossRef]
- Heier, C.R.; Fiorillo, A.A.; Chaisson, E.; Gordish-Dressman, H.; Hathout, Y.; Damsker, J. M.; Conklin, L.S. Identification of pathway-specific serum biomarkers of response to glucocorticoid and infliximab treatment in children with inflammatory bowel disease. Clin. Transl. Gastroenterol. 2016, 7(9), e192. [CrossRef]
- Batra, S.K.; Heier, C.R.; Diaz-Calderon, L.; Tully, C.B.; Fiorillo, A.A.; van den Anker, J.; Conklin, L.S. Serum miRNAs are pharmacodynamic biomarkers associated with therapeutic response in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2020, 26(10), 1597-1606. [CrossRef]
- Kumar, M.; Murugesan, S.; Ibrahim, N.; Elawad, M.; Al Khodor, S. Predictive biomarkers for anti-TNF alpha therapy in IBD patients. J. Transl. Med. 2024, 22(1), 284. [CrossRef]
- Shi, T.; Xie, Y.; Fu, Y.; Zhou, Q.; Ma, Z.; Ma, J.; Chen, J. The signaling axis of microRNA-31/interleukin-25 regulates Th1/Th17-mediated inflammation response in colitis. Mucosal. Immunol. 2017, 10(4), 983-995. [CrossRef]
- Wang, H.; Chao, K.; Ng, S.C.; Bai, A.H.; Yu, Q.; Yu, J.; Zhang, S. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016, 17, 58. [CrossRef]
- Jin, X.; Chen, D.; Zheng, R.H.; Zhang, H.; Chen, Y.P.; Xiang, Z. MiRNA-133a-UCP2 pathway regulates inflammatory bowel disease progress by influencing inflammation, oxidative stress and energy metabolism. World J. Gastroenterol. 2017, 23(1), 76-86. [CrossRef]
- Schonauen, K.; Le, N.; von Arnim, U.; Schulz, C.; Malfertheiner, P.; Link, A. Circulating and fecal microRNAs as biomarkers for inflammatory bowel diseases. Inflamm. Bowel Dis. 2018, 24(7), 1547-1557. [CrossRef]
- Phua, L.C.; Chue, X.P.; Koh, P.K.; Cheah, P.Y.; Chan, E.C.; Ho, H.K. Global fecal microRNA profiling in the identification of biomarkers for colorectal cancer screening among Asians. Oncol. Rep. 2014, 32(1), 97-104. [CrossRef]
- Duran-Sanchon, S.; Moreno, L.; Auge, J.M.; Serra-Burriel, M.; Cuatrecasas, M.; Moreira, L.; Martín, A.; Serradesanferm, A.; Pozo, À.; Costa, R.; Lacy, A.; Pellisé, M.; Lozano, J.J.; Gironella M.; Castells, A. Identification and validation of microrna profiles in fecal samples for detection of colorectal cancer. Gastroenterol. 2020, 158(4), 947-957 e944. [CrossRef]
- Duran-Sanchon, S.; Moreno, L.; Gomez-Matas, J.; Auge, J.M.; Serra-Burriel, M.; Cuatrecasas, M.; Moreira, L.; Serradesanferm, A.; Pozo, À.; Grau, J.; Pellisé, M.; Gironella, M.; Castells, A. Fecal microRNA-based algorithm increases effectiveness of fecal immunochemical test-based screening for colorectal cancer. Clin. Gastroenterol. Hepatol. 2021, 19(2), 323-330 e321. [CrossRef]
- Ma, Y.; Yang, Y.; Wang, F.; Moyer, M.P.; Wei, Q.; Zhang, P.; Yang, Z.; Liu, W.; Zhang, H.; Chen, N.; Wang, H.; Wang, H.; Qin, H. Long non-coding RNA CCAL regulates colorectal cancer progression by activating Wnt/β-catenin signalling pathway via suppression of activator protein 2α. Gut 2016, 65(9), 1494-504. [CrossRef]
- Buda, A.; Jepson, M.A.; Pignatelli, M. Regulatory function of trefoil peptides (TFF) on intestinal cell junctional complexes. Cell Commun. Adhes. 2012, 19(5-6), 63-68. [CrossRef]
- Bian, Z.; Zhang, J.; Li, M.; Feng, Y.; Wang, X.; Yao, S.; Jin, G.; Du, J.; Han, W.; Yin, Y.; Huang, S.; Fei, B.; Zou, J.; Huang, Z. LncRNA-FEZF1-AS1 promotes tumor proliferation and metastasis in colorectal cancer by regulating PKM2 signaling. Clin. Cancer Res. 2018, 24(19), 4808-4819. [CrossRef]
- Xue, J.; Liao, L.; Yin, F.; Kuang, H.; Zhou, X.; Wang, Y. LncRNA AB073614 induces epithelial- mesenchymal transition of colorectal cancer cells via regulating the JAK/STAT3 pathway. Cancer Biomark. 2018, 21(4), 849-858. [CrossRef]
- Li, C.Y.; Liang, G.Y.; Yao, W.Z.; Sui, J.; Shen, X.; Zhang, Y.Q.; Peng, H.; Hong, W.W.; Ye, Y.C.; Zhang, Z.Y.; Zhang, W.H.; Yin, L.H.; Pu, Y.P. Integrated analysis of long non-coding RNA competing interactions reveals the potential role in progression of human gastric cancer. Int. J. Oncol. 2016, 48(5), 1965-76. [CrossRef]
- Kogo, R.; Shimamura, T.; Mimori, K.; Kawahara, K.; Imoto, S.; Sudo, T.; Tanaka, F.; Shibata, K.; Suzuki, A.; Komune, S.; Miyano, S.; Mori, M. Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin modification and is associated with poor prognosis in colorectal cancers. Cancer Res. 2011, 71(20), :6320-6. . Erratum in: Cancer Res. 2012 Feb 15;72(4), 1039. PMID: 21862635. [CrossRef]
- Svoboda, M.; Slyskova, J.; Schneiderova, M.; Makovicky, P.; Bielik, L.; Levy, M.; Lipska, L.; Hemmelova, B.; Kala, Z.; Protivankova, M.; Vycital, O.; Liska, V.; Schwarzova, L.; Vodickova, L.; Vodicka, P. HOTAIR long non-coding RNA is a negative prognostic factor not only in primary tumors, but also in the blood of colorectal cancer patients. Carcinogenesis 2014, 35(7), 1510-5.PMID: 24583926. [CrossRef]
- Zhang, Y.; Bu, D.; Huo, P.; Wang, Z.; Rong, H.; Li, Y.; Liu, J.; Ye, M.; Wu, Y.; Jiang, Z.; Liao, Q.; Zhao, Y. ncFANs v2.0: an integrative platform for functional annotation of non-coding RNAs. Nucleic Acids Res. 2021, 49(W1), W459-W468. [CrossRef]
- Zeng, L.; Zhao, K.; Liu, J.; Liu, M.; Cai, Z.; Sun, T.; Li Z, Liu R. Long noncoding RNA GAS5 acts as a competitive endogenous RNA to regulate GSK-3β and PTEN expression by sponging miR-23b-3p in Alzheimer's disease. Neural. Regen. Res. 2024, 21(1), 392-405. [CrossRef]
- Zhi, H.; Li, X.; Wang, P.; Gao, Y.; Gao, B.; Zhou, D.; Zhang, Y.; Guo, M.; Yue, M.; Shen, W.; Ning, S.; Jin, L.; Li, X. Lnc2Meth: a manually curated database of regulatory relationships between long non-coding RNAs and DNA methylation associated with human disease. Nucleic Acids Res. 2018, 46(D1), D133-D138. [CrossRef]
- Li, Z.; Li, Z.; Zhang, Y.; Zhou, L.; Xu, Q.; Li, L.; Zeng, L.; Xue, J.; Niu, H.; Zhong, J.; Yu, Q.; Li, D.; Gui, M.; Huang, Y.; Tu, S.; Zhang, Z.; Song, C.Q.; Wu, J.; Shen, E.Z. Mammalian PIWI-piRNA-target complexes reveal features for broad and efficient target silencing. Nat. Struct. Mol. Biol. 2024, 31(8), 1222-1231. [CrossRef]
- Kumegawa, K.; Maruyama, R.; Yamamoto, E.; Ashida, M.; Kitajima, H.; Tsuyada, A.; Niinuma, T.; Kai, M.; Yamano, H.O.; Sugai, T.; Tokino, T., Shinomura, Y.; Imai, K.; Suzuki, H. A genomic screen for long noncoding RNA genes epigenetically silenced by aberrant DNA methylation in colorectal cancer. Sci. Rep. 2016, 6, 26699. [CrossRef]
- Diaz-Lagares, A.; Crujeiras, A.B.; Lopez-Serra, P.; Soler, M.; Setien, F.; Goyal, A.; Sandoval, J.; Hashimoto, Y.; Martinez-Cardús, A.; Gomez, A.; Heyn, H.; Moutinho, C.; Espada, J.; Vidal, A.; Paúles, M.; Galán, M.; Sala, N.; Akiyama, Y.; Martínez-Iniesta, M.; Farré, L.; Villanueva, A.; Gross, M.; Diederichs, S.; Guil, S.; Esteller M. Epigenetic inactivation of the p53-induced long noncoding RNA TP53 target 1 in human cancer. Proc. Natl. Acad. Sci. U. S. A. 2016, 113(47), E7535-E7544. [CrossRef]
- Hibi, K.; Nakamura, H.; Hirai, A.; Fujikake, Y.; Kasai, Y.; Akiyama, S.; Ito, K.; Takagi, H. Loss of H19 imprinting in esophageal cancer. Cancer Res. 1996, 56(3), 480-2. PMID: 8564957.
- Tian, F.; Tang, Z.; Song, G.; Pan, Y.; He, B.; Bao, Q.; Wang S. Loss of imprinting of IGF2 correlates with hypomethylation of the H19 differentially methylated region in the tumor tissue of colorectal cancer patients. Mol. Med. Rep. 2012, 5(6), 1536-40. [CrossRef]
- Hidaka, H.; Higashimoto, K.; Aoki, S.; Mishima, H.; Hayashida, C.; Maeda, T.; Koga, Y.; Yatsuki, H.; Joh, K.; Noshiro, H.; Iwakiri, R.; Kawaguchi, A.; Yoshiura, K.I.; Fujimoto, K.; Soejima H. Comprehensive methylation analysis of imprinting-associated differentially methylated regions in colorectal cancer. Clin. Epigenetics 2018, 10(1), 150. [CrossRef]
- Yarani, R.; Mirza, A.H.; Kaur, S.; Pociot, F. The emerging role of lncRNAs in inflammatory bowel disease. Exp. Mol. Med. 2018, 50(12), 1-14. [CrossRef]
- Ding, G.; Ming, Y.; Zhang, Y. LncRNA Mirt2 is downregulated in ulcerative colitis and regulates IL-22 expression and apoptosis in colonic epithelial cells. Gastroenterol. Res. Pract. 2019, 2019, 8154692. [CrossRef]
- Li, F.; Liu, H.; Fu, J.; Fan, L.; Lu, S.; Zhang, H.; Liu, Z. Knockdown of long non-coding RNA NEAT1 relieves inflammation of ulcerative colitis by regulating the miR-603/FGF9 pathway. Exp. Ther. Med. 2022, 23(2), 131. [CrossRef]
- Nie, J.; Zhao, Q. Lnc-ITSN1-2, derived from RNA sequencing, correlates with increased disease risk, activity and promotes CD4(+) T cell activation, proliferation and Th1/Th17 cell differentiation by serving as a ceRNA for IL-23R via sponging mir-125a in inflammatory bowel disease. Front. Immunol. 2020, 11, 852. [CrossRef]
- Hur, K.; Kim, S.H.; Kim, J.M. Potential implications of long noncoding RNAs in autoimmune diseases. Immune Netw. 2019, 19(1), e4. [CrossRef]
- Elamir, A.; Shaker, O.; Kamal, M.; Khalefa, A.; Abdelwahed, M.; Abd El Reheem, F.; Ahmed, T.; Hassan, E.; Ayoub, S. Expression profile of serum LncRNA THRIL and MiR-125b in inflammatory bowel disease. PLoS One 2022, 17(10), e0275267. [CrossRef]
- Tian, Y.; Cui, L.; Lin, C.; Wang, Y.; Liu, Z.; Miao, X. LncRNA CDKN2B-AS1 relieved inflammation of ulcerative colitis via sponging miR-16 and miR-195. Int. Immunopharmacol. 2020, 88, 106970. [CrossRef]
- Ge, Q.; Dong, Y.; Lin, G.; Cao, Y. Long noncoding RNA antisense noncoding RNA in the INK4 locus correlates with risk, severity, inflammation and infliximab efficacy in Crohn's disease. Am. J. Med. Sci. 2019, 357(2), 134-142. [CrossRef]
- Chen, S.W.; Wang, P.Y.; Liu, Y.C.; Sun, L.; Zhu, J.; Zuo, S.; Ma, J.; Li, T.Y.; Zhang, J.L.; Chen, G.W.; Wang, X.; Zhu, Q.R.; Zheng, Y.W.; Chen, Z.Y.; Yao, Z.H.; Pan, Y.S. Effect of long noncoding RNA H19 overexpression on intestinal barrier function and its potential role in the pathogenesis of ulcerative colitis. Inflamm. Bowel Dis. 2016, 22(11), 2582-2592. [CrossRef]
- Liu, R.; Tang, A.; Wang, X.; Chen, X.; Zhao, L.; Xiao, Z.; Shen, S. Inhibition of lncRNA NEAT1 suppresses the inflammatory response in IBD by modulating the intestinal epithelial barrier and by exosome-mediated polarization of macrophages. Int. J. Mol. Med. 2018, 42(5), 2903-2913. [CrossRef]
- Lucafo, M.; Di Silvestre, A.; Romano, M.; Avian, A.; Antonelli, R.; Martelossi, S.; Naviglio, S.; Tommasini, A.; Stocco, G.; Ventura, A.; Decorti, G.; De Iudicibus, S. Role of the long non-coding RNA growth arrest-specific 5 in glucocorticoid response in children with inflammatory bowel disease. Basic Clin. Pharmacol. Toxicol. 2018, 122(1), 87-93. [CrossRef]
- Nemati Bajestan, M.; Piroozkhah, M.; Chaleshi, V.; Ghiasi, N.E.; Jamshidi, N.; Mirfakhraie, R.; Balaii, H.; Shahrokh, S.; Asadzadeh Aghdaei, H.; Salehi, Z.; Nazemalhosseini Mojarad, E. Expression analysis of long noncoding RNA-MALAT1 and interleukin-6 in inflammatory bowel disease patients. Iran. J. Allergy Asthma Immunol. 2023, 22(5), 482-494. [CrossRef]
- Tian, W.; Du, Y.; Ma, Y.; Gu, L.; Zhou, J.; Deng, D. MALAT1-miR663a negative feedback loop in colon cancer cell functions through direct miRNA-lncRNA binding. Cell Death Dis. 2018, 9(9), 857. [CrossRef]
- Wang, S.; Hou, Y.; Chen, W.; Wang, J.; Xie, W.; Zhang, X.; Zeng, L. KIF9-AS1, LINC01272 and DIO3OS lncRNAs as novel biomarkers for inflammatory bowel disease. Mol. Med. Rep. 2018, 17(2), 2195-2202. [CrossRef]
- Cao, L.; Tan, Q.; Zhu, R.; Ye, L.; Shi, G.; Yuan, Z. LncRNA MIR4435-2HG suppression regulates macrophage M1/M2 polarization and reduces intestinal inflammation in mice with ulcerative colitis. Cytokine 2023, 170, 156338. [CrossRef]
- Yang, Y.; Xiong, Z.; Li, W.; Lin, Y.; Huang, W.; Zhang, S. FHIP1A-DT is a potential novel diagnostic, prognostic, and therapeutic biomarker of colorectal cancer: A pan-cancer analysis. Biochem. Biophys. Res. Commun. 2023, 679, 191-204. [CrossRef]
- Guo, K.; Yao, J.; Yu, Q.; Li, Z.; Huang, H.; Cheng, J.; Zhu., Y. The expression pattern of long non coding PVT1 in tumor tissues and in extracellular vesicles of colorectal cancer correlates with cancer progression. Tumour Biol. 2017, 39(4), 1010428317699122. PMID: 28381186. [CrossRef]
- Mani, S.R.; Juliano, C.E. Untangling the Web: The diverse functions of the PIWI/piRNA pathway. Mol. Reprod. Dev. 2013, 80(8), 632-664. [CrossRef]
- Gangaraju, V.K.; Lin, H. MicroRNAs: Key regulators of stem cells. Nat. Rev. Mol. Cell Biol. 2009, 10, 116–125. [CrossRef]
- Stefani, G.; Slack, F.J. Small non-coding RNAs in animal development. Nat. Rev. Mol. Cell Biol. 2008, 9, 219–230. [CrossRef]
- Saxe, J.P.; Lin, H. Small noncoding RNAs in the germline. Cold Spring Harb. Perspect. Biol. 2011, 3, a002717. [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [CrossRef]
- Aravin, A.; Gaidatzis, D.; Pfeffer, S.; Lagos-Quintana, M.; Landgraf, P.; Iovino, N.; Morris, P.; Brownstein, M.J.; Kuramochi-Miyagawa, S.; Nakano, T.; Chien, M.; Russo, J.J.; Ju, J.; Sheridan, R.; Sander, C.; Zavolan, M.; Tuschl, T. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 2006, 442, 203–207. [CrossRef]
- Watanabe, T.; Lin, H. Posttranscriptional regulation of gene expression by Piwi proteins and piRNAs. Mol. Cell 2014, 56, 18– 27. [CrossRef]
- Moyano, M.; Stefani, G. piRNA involvement in genome stability and human cancer. J. Hematol. Oncol. 2015, 8, 38. [CrossRef]
- Rouget, C.; Papin, C.; Boureux, A.; Meunier, A.; Franco, B.; Robine, N.; Lai, E.; Pelisson, A.; Simonelig, M. Maternal mRNA deadenylation and decay by the piRNA pathway in the early Drosophila embryo. Nature 2010, 467, 1128–1132. [CrossRef]
- Aravin, A.; Sachidanandam, R.; Bourc’his, D.; Schaefer, C.; Pezic, D.; Toth, K.; Bestor, T.; Hannon, G. A piRNA pathway primed by individual transposons is linked to de novo DNA methylation in mice. Mol. Cell 2008, 31(6), 785-799. [CrossRef]
- Watanabe, T.; Tomizawa, S.; Mitsuya, K.; Totoki, Y.; Yamamoto, Y.; Kuramochi-Miyagawa, S.; Iida, N.; Hoki, Y.; Murphy, P.; Toyoda, A.; Gotoh, K.; Hiura, H.; Arima, T.; Fujiyama, A.; Sado, T.; Shibata, T.; Nakano, T.; Lin, H.; Ichiyanagi, K.; Soloway, P.; Sasaki, H. Role for piRNAs and noncoding RNA in de novo DNA methylation of the imprinted mouse Rasgrf1 locus. Science 2011, 332(6031), 848-852. [CrossRef]
- Yamanaka, S.; Siomi, M.C; Siomi, H. piRNA clusters and open chromatin structure. Mobile DNA 2014, 5, 22. [CrossRef]
- Siomi, M.C.; Sato, K.; Pezic, D.; Aravin, A.A. PIWI-interacting small RNAs: the vanguard of genome defence. Nat. Rev. Mol. Cell Biol. 2011, 12, 246–258. [CrossRef]
- Roovers, E.F.; Rosenkranz, D.; Mahdipour, M.; Han, C.T.; He, N.; Chuva de Sousa Lopes, S.M.; van der Westerlaken, L.A.; Zischler, H.; Butter, F.; Roelen, B.A.; Ketting, R.F. Piwi Proteins and piRNAs in Mammalian Oocytes and Early Embryos. Cell Rep. 2015, 10(12), 2069-82. [CrossRef]
- Aravin, A.; Hannon, G.; Brennecke, J.; The Piwi-piRNA pathway provides an adaptive defense in the transposon arms race. Science 2007, 318(5851), 761-764. [CrossRef]
- Ghildiyal, M.; Zamore, P.D. Small silencing RNAs: an expanding universe. Nat. Rev. Genet. 2009, 10, 94–108. [CrossRef]
- Muhammad, A.; Waheed, R.; Khan, N.A.; Jiang, H.; Song, X. piRDisease v1.0: a manually curated database for piRNA associated diseases. Database (Oxford) 2019, 2019, baz052. [CrossRef]
- Ghosh, B.; Sarkar, A.; Mondal, S.; Bhattacharya, N.; Khatua, S.; Ghosh, Z. piRNAQuest V.2: an updated resource for searching through the piRNAome of multiple species. RNA Biol. 2022, 19(1), 12-25. [CrossRef]
- Zhang, W.; Wu, S.; Zhang, H.; Guan, W.; Zeng, B., Wei, Y.; Chi-Fung Chan, G.; Li, W. piRPheno: a manually curated database to prioritize and analyze human disease related piRNAs. bioRxiv (The preprint server for biology). 2020. [CrossRef]
- Zhang, P.; Si, X.; Skogerbø, G.; Wang, J.; Cui, D.; Li, Y.; Sun, X.; Liu, L.; Sun, B.; Chen, R.; He, S.; Huang, D.W. piRBase: a web resource assisting piRNA functional study. Database (Oxford) 2014, 2014, bau110. [CrossRef]
- Wang, J.; Zhang, P.; Lu, Y.; Li, Y. Zheng, Y.; Kan, Y.; Chen, R.; He, S. piRBase: a comprehensive database of piRNA sequences. Nucleic Acids Res. 2019, 47(D1), D175-D180. [CrossRef]
- Greene, J.; Baird, A.M.; Brady, L.; Lim, M.; Gray, S.G.; McDermott, R.; Finn, S.P. Circular RNAs: biogenesis, function and role in human diseases. Front. Mol. Biosci. 2017, 4, 38. [CrossRef]
- Wu, W.; Ji, P.; Zhao, F. CircAtlas: an integrated resource of one million highly accurate circular RNAs from 1070 vertebrate transcriptomes. Genome Biol. 2020, 21(1), 101. [CrossRef]
- Hao, S.; Cong, L.; Qu, R.; Liu, R.; Zhang, G.; Li, Y. Emerging roles of circular RNAs in colorectal cancer. Onco. Targets Ther. 2019, 12, 4765-4777. [CrossRef]
- Li, J.; Ni, S.; Zhou, C.; Ye, M. The expression profile and clinical application potential of hsa_circ_0000711 in colorectal cancer. Cancer Manag. Res. 2018, 10, 2777-2784. [CrossRef]
- Zhang, W.; Wang, B.; Lin, Y.; Yang, Y.; Zhang, Z.; Wang, Q.; Zhang, H.; Jiang, K.; Ye, Y.; Wang, S.; Shen, Z. Hsa_circ_0000231 promotes colorectal cancer cell growth through upregulation of CCND2 by IGF2BP3/miR-375 dual pathway. Cancer Cell Int. 2022, 22(1), 27. [CrossRef]
- Sun, S.; Li, C.; Cui, K.; Liu, B.; Zhou, M.; Cao, Y.; Zhang, J.; Bian, Z.; Fei, B.; Huang, Z. Hsa_circ_0062682 promotes serine metabolism and tumor growth in colorectal cancer by regulating the miR-940/PHGDH axis. Front. Cell Dev. Biol. 2021, 9, 770006. [CrossRef]
- Long, F.; Lin, Z.; Li, L.; Ma, M.; Lu, Z.; Jing, L.; Li, X.; Lin, C. Comprehensive landscape and future perspectives of circular RNAs in colorectal cancer. Mol. Cancer 2021, 20(1), 26. [CrossRef]
- Sun, Z.; Chen, C.; Su, Y.; Wang, W.; Yang, S.; Zhou, Q.; Wang, G.; Li, Z.; Song, J.; Zhang, Z.; Yuan, W.; Liu, J. Regulatory mechanisms and clinical perspectives of circRNA in digestive system neoplasms. J. Cancer 2019, 10(13), 2885-2891. [CrossRef]
- Lai, H.; Li, Y.; Zhang, H.; Hu, J.; Liao, J.; Su, Y.; Li, Q.; Chen, B.; Li, C.; Wang, Z.; Li, Y.; Wang, J.; Meng, Z.; Huang, Z.; Huang, S. ExoRBase 2.0: an atlas of mRNA, lncRNA and circRNA in extracellular vesicles from human biofluids. Nucleic Acids Res. 2022, 50(D1), D118-D128. [CrossRef]
- Wen, J.; Liao, J.; Liang, J.; Chen, X.P.; Zhang, B.; Chu, L. Circular RNA HIPK3: a key circular rna in a variety of human cancers. Front. Oncol. 2020, 10, 773. [CrossRef]
- Viralippurath Ashraf, J.; Sasidharan Nair, V.; Saleh, R.; Elkord, E. Role of circular RNAs in colorectal tumor microenvironment. Biomed. Pharmacother. 2021, 137, 111351. [CrossRef]
- Liang, X.; Long, L.; Guan, F.; Xu, Z.; Huang, H. Research status and potential applications of circRNAs affecting colorectal cancer by regulating ferroptosis. Life Sci. 2024, 352, 122870. [CrossRef]
- Camandona, A.; Gagliardi, A.; Licheri, N.; Tarallo, S.; Francescato, G.; Budinska, E.; Carnogurska, M.; Zwinsová, B.; Martinoglio, B.; Franchitti, L.; Gallo, G.; Cutrupi, S.; De Bortoli, M.; Pardini, B.; Naccarati, A.; Ferrero, G. Multiple regulatory events contribute to a widespread circular RNA downregulation in precancer and early stage of colorectal cancer development. Biomark. Res. 2025, 13(1), 30. [CrossRef]
- Mao, J., Lu, Y. Roles of circRNAs in the progression of colorectal cancer: novel strategies for detection and therapy. Cancer Gene Ther. 2024, 31(6), 831-841. [CrossRef]
- Li, L., Wei, C., Xie, Y., Su, Y.; Liu, C.; Qiu, G., Liu, W.; Liang, Y.; Zhao, X.; Huang, D.; Wu, D. Expanded insights into the mechanisms of RNA-binding protein regulation of circRNA generation and function in cancer biology and therapy. Genes Dis. 2024, 12(4), 101383. [CrossRef]
- Hussen, B.M.; Abdullah, S.R.; Mohammed, A.A.; Rasul, M.F.; Hussein, A.M.; Eslami, S.; Glassy, M.C.; Taheri, M. Advanced strategies of targeting circular RNAs as therapeutic approaches in colorectal cancer drug resistance. Pathol. Res. Pract. 2024, 260, 155402. [CrossRef]
- Asangani, I.A.; Rasheed, S.A.; Nikolova, D.A.; Leupold, J.H.; Colburn, N.H.; Post, S.; Allgayer, H. MicroRNA-21 (miR-21) post-transcriptionally downregulates tumor suppressor Pdcd4 and stimulates invasion, intravasation and metastasis in colorectal cancer. Oncogene 2008, 27(15), 2128-36. [CrossRef]
- Lai, C.Y.; Yeh, K.Y.; Liu, B.F.; Chang, T.M.; Chang, C.H.; Liao, Y.F.; Liu, Y.W.; Her, G.M. MicroRNA-21 plays multiple oncometabolic roles in colitis-associated carcinoma and colorectal cancer via the PI3K/AKT, STAT3, and PDCD4/TNF-α signaling pathways in zebrafish. Cancers (Basel) 2021, 13(21), 5565. [CrossRef]
- O'Connell, R.M.; Taganov, K.D.; Boldin, M.P.; Cheng, G.; Baltimore, D. MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 2007, 104(5), 1604-9. [CrossRef]
- Jabłońska, E.; Białopiotrowicz, E.; Szydłowski, M.; Prochorec-Sobieszek, M.; Juszczyński, P.; Szumera-Ciećkiewicz, A. DEPTOR is a microRNA-155 target regulating migration and cytokine production in diffuse large B-cell lymphoma cells. Exp. Hematol. 2020, 88, 56-67.e2. [CrossRef]
- Uthman, Y.A., Ibrahim, K.G., Abubakar, B., Bello, M.B.; Malami, I.; Imam, M.U.; Qusty, N.; Cruz-Martins, N.; Batiha, G.E.; Abubakar, M.B. MALAT1: a promising therapeutic target for the treatment of metastatic colorectal cancer. Biochem. Pharmacol. 2021, 190, 114657. [CrossRef]
- Yan, Y.; Su, M.; Qin, B. CircHIPK3 promotes colorectal cancer cells proliferation and metastasis via modulating of miR-1207-5p/FMNL2 signal. Biochem. Biophys. Res. Commun. 2020, 524(4), 839-846. [CrossRef]
- Hsiao, K.Y.; Lin, Y.C.; Gupta, S.K.; Chang, N.; Yen, L.; Sun, H.S.; Tsai, S.J. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017, 77(9), 2339-2350. [CrossRef]
- Hatley, M.E.; Patrick, D.M.; Garcia, M.R.; Richardson, J.A.; Bassel-Duby, R.; van Rooij, E.; Olson, E.N. Modulation of K-Ras-dependent lung tumorigenesis by microRNA-21. Cancer Cell 2010, 18(3), 282-93. [CrossRef]
- Wang, P.; Zhuang, L.; Zhang, J.; Fan, J.; Luo, J.; Chen, H.; Wang, K.; Liu, L.; Chen, Z.; Meng, Z. The serum miR-21 level serves as a predictor for the chemosensitivity of advanced pancreatic cancer, and miR-21 expression confers chemoresistance by targeting FasL. Mol. Oncol. 2013, 7(3), 334-45. [CrossRef]
- Kong, W.; Yang, H.; He, L.; Zhao, J.J.; Coppola, D.; Dalton, W.S.; Cheng, J.Q. MicroRNA-155 is regulated by the transforming growth factor beta/Smad pathway and contributes to epithelial cell plasticity by targeting RhoA. Mol. Cell. Biol. 2008, 28(22), 6773-84. [CrossRef]
- Wang, P.; Guo, Q.; Qi, Y.; Hao, Y.; Gao, Y.; Zhi, H.; Zhang, Y.; Sun, Y.; Zhang, Y.; Xin, M.; Zhang, Y.; Ning, S.; Li, X. LncACTdb 3.0: an updated database of experimentally supported ceRNA interactions and personalized networks contributing to precision medicine. Nucleic Acids Res. 2022, 50(D1), D183-D189. [CrossRef]
- Pillich, R.T.; Chen, J.; Churas, C.; Fong, D.; Gyori, B.M.; Ideker, T.; Karis, K.; Liu, S.N.; Ono, K.; Pico, A.; Pratt, D. NDEx IQuery: a multi-method network gene set analysis leveraging the network data exchange. Bioinformatics 2023, 39(3), btad118. [CrossRef]
- Persson, E.; Castresana-Aguirre, M.; Buzzao, D.; Guala, D.; Sonnhammer, E.L.L. FunCoup 5: functional association networks in all domains of life, supporting directed links and tissue-specificity. J. Mol. Biol. 2021, 433(11), 166835. [CrossRef]
- Ilango, S.; Paital, B.; Jayachandran, P.; Padma, P.R.; Nirmaladevi, R. Epigenetic alterations in cancer. Front. Biosci. (Landmark Ed) 2020, 25(6), 1058-1109. [CrossRef]
- Li, Y.; Seto, E. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb. Perspect. Med. 2016, 6(10), a026831. [CrossRef]
- Gajjela, B.K.; Zhou, M.M. Bromodomain inhibitors and therapeutic applications. Curr. Opin. Chem. Biol. 2023, 75, 102323. PMID: 37207401; PMCID: PMC10524616. [CrossRef]
- Martínez-Gutierrez, A.; Carbajal-Lopez, B.; Bui, T.M.; Mendoza-Rodriguez, M.; Campos-Parra, A.D.; Calderillo-Ruiz, G.; Cantú-De Leon, D.; Madrigal-Santillán, E.O.; Sumagin, R.; Pérez-Plasencia, C.; Pérez-Yépez, E.A. A microRNA panel that regulates proinflammatory cytokines as diagnostic and prognosis biomarkers in colon cancer. Biochem. Biophys. Rep. 2022, 30,101252. PMID: 35313644; PMCID: PMC8933814. [CrossRef]
- Xie, X.; Liu, P.; Wu, H.; Li, H.; Tang, Y.; Chen, X.; Xu, C.; Liu, X.; Dai, G. MiR-21 antagonist alleviates colitis and angiogenesis via the PTEN/PI3K/AKT pathway in colitis mice induced by TNBS. Ann. Transl. Med. 2022, 10(7), 413. PMID: 35530951; PMCID: PMC9073802 . [CrossRef]
- Carter, J.V.; Galbraith, N.J.; Yang, D.; Burton, J.F.; Walker, S.P.; Galandiuk, S. Blood-based microRNAs as biomarkers for the diagnosis of colorectal cancer: a systematic review and meta-analysis. Br. J. Cancer 2017, 116(6), 762-774. PMID: 28152545; PMCID: PMC5355921 . [CrossRef]
- Kalkusova, K.; Taborska, P.; Stakheev, D.; Smrz, D. The role of miR-155 in antitumor immunity. Cancers (Basel) 2022, 14(21), 5414. PMID: 36358832; PMCID: PMC9659277. [CrossRef]
- Zhang, J.; Wang, C.; Guo, Z.; Da, B.; Zhu, W.; Li, Q. miR-223 improves intestinal inflammation through inhibiting the IL-6/STAT3 signaling pathway in dextran sodium sulfate-induced experimental colitis. Immun. Inflamm. Dis. 2021, 9(1), 319-327. PMID: 33332758; PMCID: PMC7860526 . [CrossRef]
- Zhao, W.; Song, M.; Zhang, J.; Kuerban, M.; Wang, H. Combined identification of long non-coding RNA CCAT1 and HOTAIR in serum as an effective screening for colorectal carcinoma. Int. J. Clin. Exp. Pathol. 2015, 8(11), 14131-40. PMID: 26823726; PMCID: PMC4713512.
- Liu, J.; Guo, B. RNA-based therapeutics for colorectal cancer: Updates and future directions. Pharmacol. Res. 2020, 152, 104550. PMID: 31866285; PMCID: PMC7027327 . [CrossRef]
- Fitzgerald, K.; Frank-Kamenetsky, M.; Shulga-Morskaya, S.; Liebow, A.; Bettencourt, B.R.; Sutherland, J.E.; Hutabarat, R.M.; Clausen, V.A.; Karsten, V.; Cehelsky, J.; Nochur, S.V.; Kotelianski, V.; Horton, J.; Mant, T.; Chiesa, J.; Ritter, J.; Munisamy, M.; Vaishnaw, A.K.; Gollob, J.A.; Simon, A. Effect of an RNA interference drug on the synthesis of proprotein convertase subtilisin/kexin type 9 (PCSK9) and the concentration of serum LDL cholesterol in healthy volunteers: a randomised, single-blind, placebo-controlled, phase 1 trial. Lancet 2014, 383(9911) 60-68. PMID: 24094767; PMCID: PMC4387547 . [CrossRef]
- Hitchins, M.P.; Vogelaar, I.P.; Brennan, K.; Haraldsdottir, S.; Zhou, N.; Martin, B.; Alvarez, R; Yuan, X.; Kim, S.; Guindi, M.; Hendifar, A.E.; Kalady, M.F.; DeVecchio, J.; Church, J.M.; de la Chapelle, A.; Hampel, H.; Pearlman, R.; Christensen, M.; Snyder, C.; Lanspa, S.J.; Haile, R.W.; Lynch, H.T. Methylated SEPTIN9 plasma test for colorectal cancer detection may be applicable to Lynch syndrome. BMJ Open Gastroenterol. 2019, 6(1), e000299. . PMID: 31275589; PMCID: PMC6577308. [CrossRef]
- Sui, C.; Ma, J.; Chen, Q.; Yang, Y. The variation trends of SFRP2 methylation of tissue, feces, and blood detection in colorectal cancer development. Eur. J. Cancer Prev. 2016, 25(4), 288-98. . PMID: 26258809. [CrossRef]
- Gonneaud, A.; Gagné, J.M.; Turgeon, N.; Asselin, C. The histone deacetylase Hdac1 regulates inflammatory signalling in intestinal epithelial cells. J. Inflamm. (Lond.) 2014, 11(1), 43. . PMID: 25606026; PMCID: PMC4299484. [CrossRef]
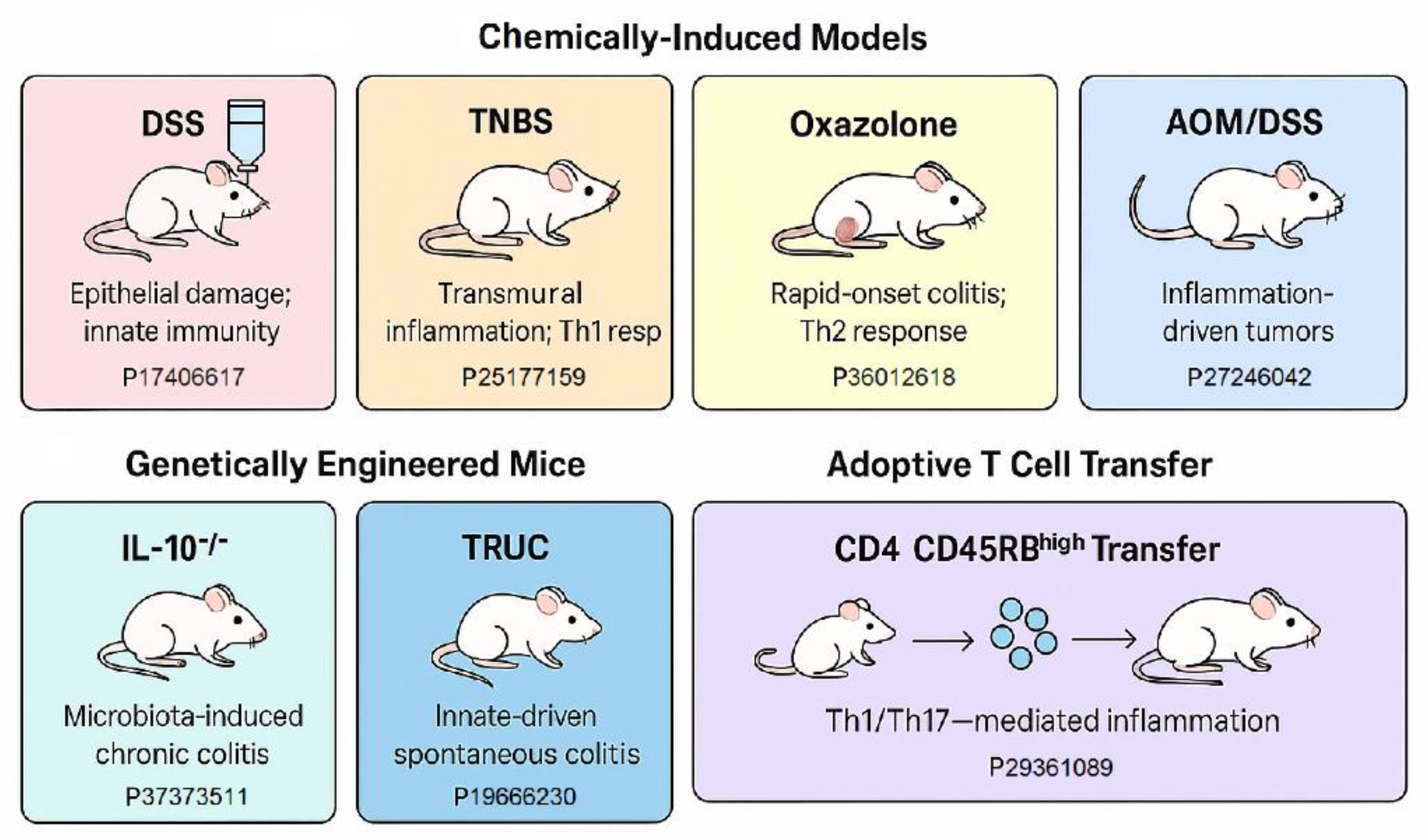
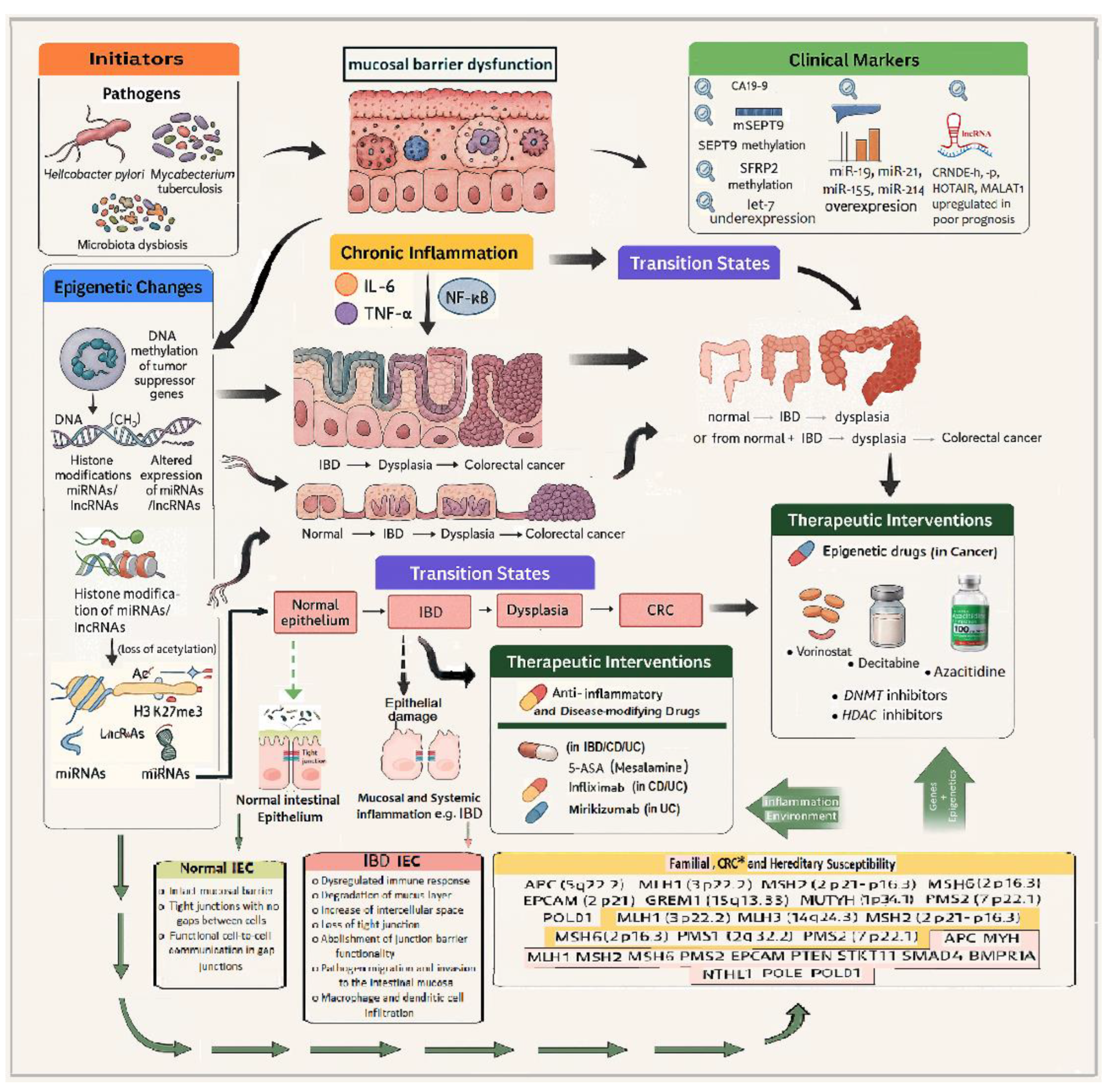
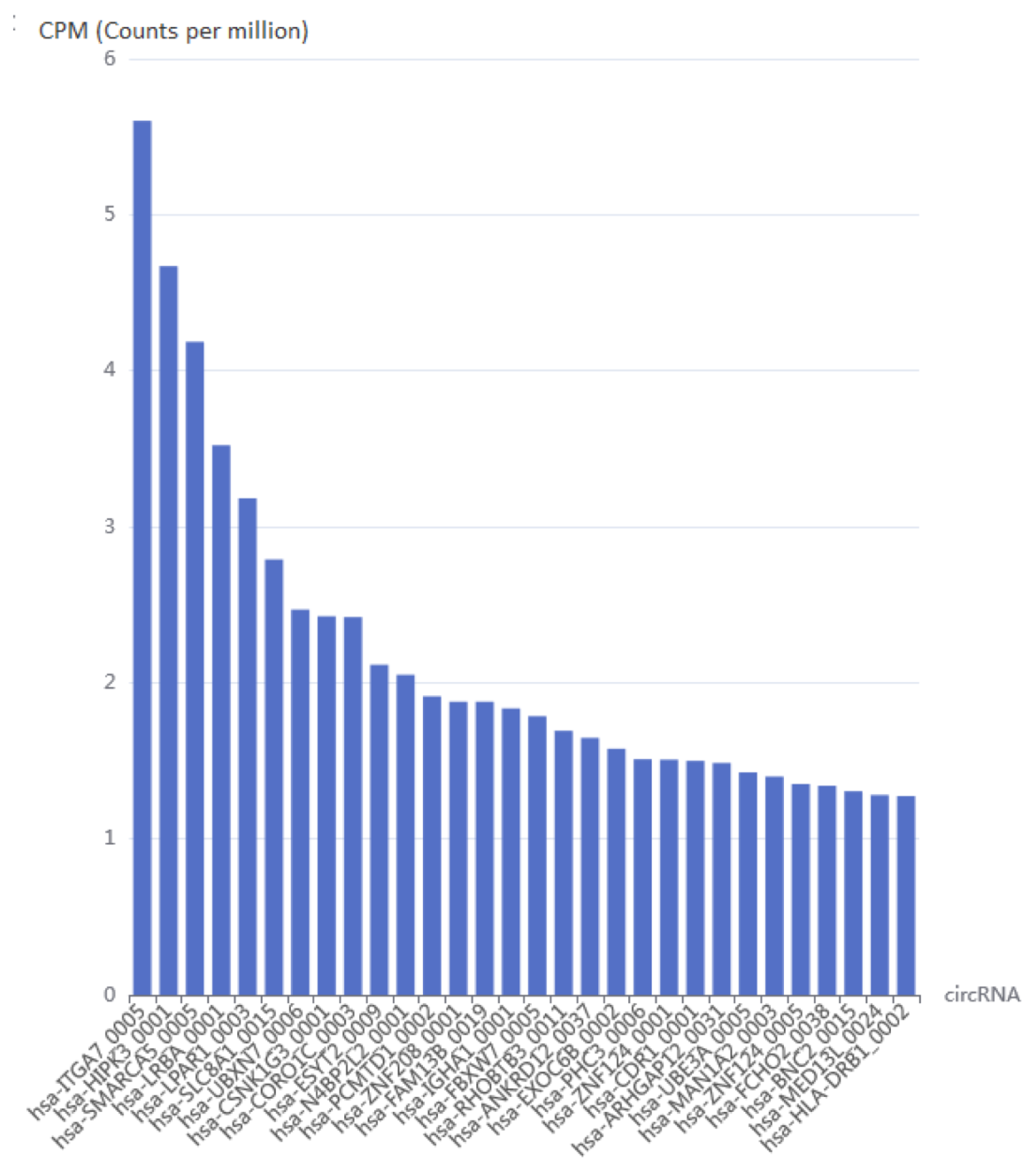
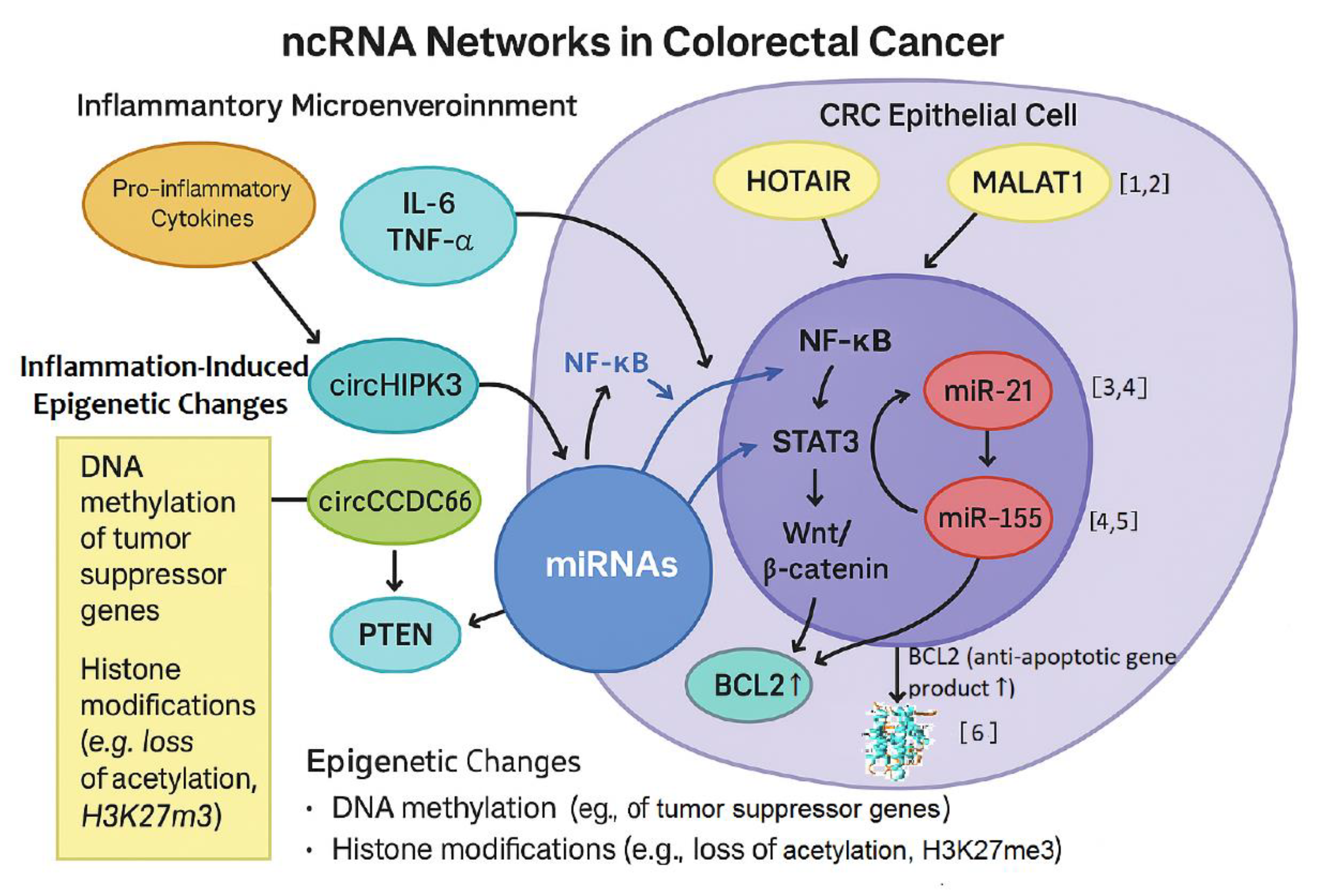
| Model | Method | Immune System Involvement | Advantages | Limitations | Reference (PMID) |
|---|---|---|---|---|---|
| DSS | Oral DSS in drinking water | Innate | Rapid, simple, epithelial injury | No adaptive immune involvement | 17406617 36012618 34440615 |
| AOM/DSS | AOM injection + DSS cycles | Innate + DNA damage | Models CAC pathogenesis | Less suitable for sporadic CRC | 27246042 |
| TNBS | Rectal instillation of TNBS | Adaptive (Th1) | Crohn's-like inflammation | Variability, toxic risk | 25177159 34440615 |
| Oxazolone | Chemical | Adaptive (Th2) | Mimics UC; rapid onset | Short-lived; strain-dependent | 36012618 |
| IL-10⁻/⁻ | Genetic deletion of IL-10 | Adaptive | Spontaneous colitis | Microbiota dependence | 37373511 |
| TRUC (T-bet⁻/⁻ × RAG⁻/⁻) | Double knockout | Innate | Innate immunity-driven model progressing to colonic dysplasia and rectal adenocarcinoma | Complex breeding, microbiota sensitive | 19666230 |
| APCMin/+ | Genetic (multiple intestinal neoplasia) | Sporadic Intestinal Tumors |
FAP model; Wnt pathway activation | Small intestine focus | 30887153 |
| CD4+CD45RBhigh T Cell Transfer to RAG⁻/⁻ Mice |
Adaptive cell transfer | Adaptive | T-cell driven colitis | Requires expertise, chronic model | 29361089 34440615 25989337 |
| Disease Type | Sample Type | Methylated markers | Methylation status | Reference (PMID) |
| IBD | Rectal biopsies | THRAP2, FANCC and GBGT1 | ↑ | 22419656 |
| CD | Blood | WDR8 and ITGB2 | ↑ | 27886173 |
| CD | Rectal biopsies | DOK2 and TNFSF4 | ↓ | 22419656 |
| CD | Blood | VMP1 | ↓ | 27886173 |
| UC | Rectal biopsies | CARD9 and CDH1 | ↑ | 22419656 |
| UC | Blood | WDR8 | ↑ | 27886173 |
| UC | Rectal biopsies | ICAM3, DOK2 and TNFSF4 | ↓ | 22419656 |
| UC | Blood | VMP1 | ↓ | 27886173 |
| UC | Colon biopsies | EBI3 | ↓ | 22419656 |
| CRC | Rectal biopsies | TGFB2, SLIT2, HS3ST2, TMEFF2, | ↑ | 27886173 |
| CRC | Colon biopsies | FOXE1, SYNE1 | ↑ | 22419656 |
| CRC | Colonic mucosa | APC, CDH13, MGMT, RUNX3 and MLH1 | ↑ | 27886173 |
| CRC | Colon biopsies | ITGA4 | ↑ | 34069352 |
| Disease Type | Sample Type |
Histone modifying enzymes/histone markers |
Modification type/ status | Reference (PMID) | |
| IBD | Intestinal tissue | KAT2B | Acetylation ↓ | 26802082 | |
| UC/CAC | Colon Tissue | H3K27ac | Acetylation ↑ | 29983891 | |
| CD | Colonic biopsy | H2Bub1 | Ubiquitination ↓ | 34088983 | |
| IBD | Colon Tissue | HDAC | Acetylation ↑ | 38903915 | |
| IBD | Colon Tissue | HDAC8 | Acetylation ↑ | 36558966 | |
| CRC | Primary cancer tissue | H3K9me2 | Methylation ↑ | 22076537 |
| Disease Type | Sample Type | miRNAs | Gene Expression change | Reference (PMID) | |
| UC | Serum | miR-29a, miR-196b, miR-127-3p | ↑ | 23607522 | |
| CD | Serum | miR-140-3p, miR-127-3p | ↑ | 23607522 | |
| UC | Serum | miR-150 | ↓ | 23607522 | |
| CD | Blood | miRNA-125a | ↓ | 29209130 | |
| UC | Blood | miRNA-19a | ↑ | 25886994 | |
| UC | Blood | miRNA-146a | ↓ | 25886994 | |
| CD | Blood | miR-31 | ↓ | 25886994 | |
| CD | Blood | miR-101 | ↑ | 25886994 | |
| IBD | Serum | miR-146b-5p | ↑ | 30734320 | |
| IBD | Feces | miR-223, miR-1246 | ↑ | 32850969 | |
| IBD | Serum | miR-16, miR-21, and miR-223 | ↑ | 29668922 | |
| IBD | Feces | miR-223, miR-155 | ↑ | 29668922 | |
| IBD | Serum / Feces | miR-21, miR-142-3p, miR-146a and let-7i | ↑ | 24613022 | |
| IBD | IECs | miR-192, miR-194, miR-200b, miR-375 | ↓ | 24613022 | |
| IBD | Serum | miR-192, miR-195, miR-20a, miR-30a, miR-484 and let-7b | ↑ | 21546856 | |
| IBD | Serum | miR-146a, miR-146b, miR-320a, miR-126 and let-7c | ↓ | 32793975 | |
| IBD | Colonic tissue | miR-133a | ↓ | 28104982 | |
| CRC | Feces | miR-135b, miR-223 and miR-451 | ↑ | 24841830 | |
| CRC | Feces | miR-29a, miR-223 and miR-224 | ↓ | 26756616 | |
| CRC | Feces | miR-421, miR130b-3p miR27a-3P | ↑ | 31622624 |
| Disease Type | Sample Type | lncRNAs | Gene Expression change | Reference (PMID) | |
| UC | Plasma | Mirt2 | ↓ | 31687015 | |
| CRC | Blood | HOTAIR | ↑ | 21862635, 24583926 | |
| CRC | COAD tissue | FHIP1A-DT | ↓ | 37703762 | |
| UC | Plasma | IFNG-AS1 | ↑ | 34970354 | |
| UC | Plasma | ITSN1-2 | ↑ | 32547537 | |
| IBD | Serum | THRIL | ↑ | 36206229 | |
| UC | Blood | CDKN2B-AS1 | ↓ | 33182065 | |
| CD | Blood | CDKN2B-AS1 | ↑ | 30665494 | |
| UC | Colonic tissue | H19 | ↑ | 27661667 | |
| IBD | Colonic tissue | CRNDE | ↑ | 31251902 | |
| IBD | Serum | NEAT1 | ↑ | 30132508 | |
| UC | Blood | GAS5 | ↑ | 28722800 | |
| IBD/ CRC | Intestinal tissue | MALAT1 | ↑ | 38085149 | |
| IBD | Plasma/ Tissue | KIF9-AS1 and LINC01272 | ↑ | 29207070 | |
| IBD | Plasma/ Tissue | DIO3OS | ↓ | 29207070 | |
| UC | Human intestinal epithelial Caco2 cells and murine macrophage RAW264.7 cells | MIR4435-2HG | ↑ | 37597495 | |
| CRC | Colon/Rectal biopsies | RVT1 | ↑ | 28381186 |
| Condition | DNA Methylation | Histone Acetylation | Histone Methylation | Non-coding RNAs (miRNAs/lncRNAs) |
References (PMID) |
| Normal Colon | Homeostatic balance of methylation | Balanced H3/H4 acetylation regulating gene expression | Physiological levels of H3K4me3 and H3K27me3 | Normal expression of regulatory miRNAs and lncRNAs | 17447863, 15800552 |
| Inflammatory Bowel Disease (IBD) | ↑ Promoter hypermethylation of anti-inflammatory genes | ↑ H3K27ac | ↑ H3K4me3, ↑ H3K9me3 | ↑ Inflammatory miRNAs (e.g., miR-155), altered lncRNAs | 27886173 |
| Colitis (IBD) | Global hypomethylation; hypermethylation of SOCS3 | ↓ H3/H4 acetylation; HDAC overexpression | ↑ H3K9me3 (heterochromatinization); ↓ H3K27me3 | ↑ miR-155, miR-21; ↓ let-7; ↑ lncRNA HOTAIR | 26573286, 27385797, 20404267 |
| Dysplasia | ↑ CIMP phenotype, ↑ Promoter hypermethyl-ation of MLH1, CDKN2A, APC | Loss of histone acetylation at tumor suppressor loci | ↑ H3K27me3, H3K9me3 by EZH2; ↓ H3K4me3 at differentiation genes | ↑ MALAT1, CRNDE, ↓ MEG3; ↑ oncogenic lncRNAs, miRNAs, ↓ tumor-suppressive circRNAs | 24833482, 29559771 |
| Colorectal Cancer | Global hypomethylation; ↑ Promoter CpG island hypermethylation | Aberrant HDAC recruitment; hypoacetylation at TSGs; ↓ H4K16ac | ↑ H3K9me2/3; ↑ EZH2-mediated H3K27me3; ↓ H3K4me3 | ↑ Oncogenic miRNAs (e.g. miR-21, miR-135b); ↑ lncRNAs NEAT1, PVT1, CCAT1; ↓ lncRNAs like GAS5 | 31483834, 33081851, 31230041 |
| Drug / RNA | Type | Target | Disease Phase | Function/Application Notes | Reference (PMID) |
| OTX015 | BET inhibitor | MYC, NF-κB pathways | Phase I/II clinical trials | Solid tumors, anti-inflammatory profile | 37207401 |
| 5-Azacytidine | DNMT inhibitor | DNA methylation reversal | Approved (hematologic); off-label, investigational in CRC | Reverses methylation silencing | 24583822; 26317465, 33359448 |
| Anti-miR-21 Oligonucleotides | Antisense oligo | miR-21 suppression | Preclinical studies | Targets PDCD4, inhibits tumor invasion | 17968323 |
| miR-92a sponge | miRNA decoy | miR-92a suppression | Preclinical studies | Biomarker and therapeutic target | 28957811; 33620640 |
| Romidepsin | HDAC inhibitor | HDAC1/2 (Histone deacetylation) | Approved (T-cell lymphoma); clinical trials for CRC | Suppresses inflammation and tumor growth | 27599530 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





