1. Introduction
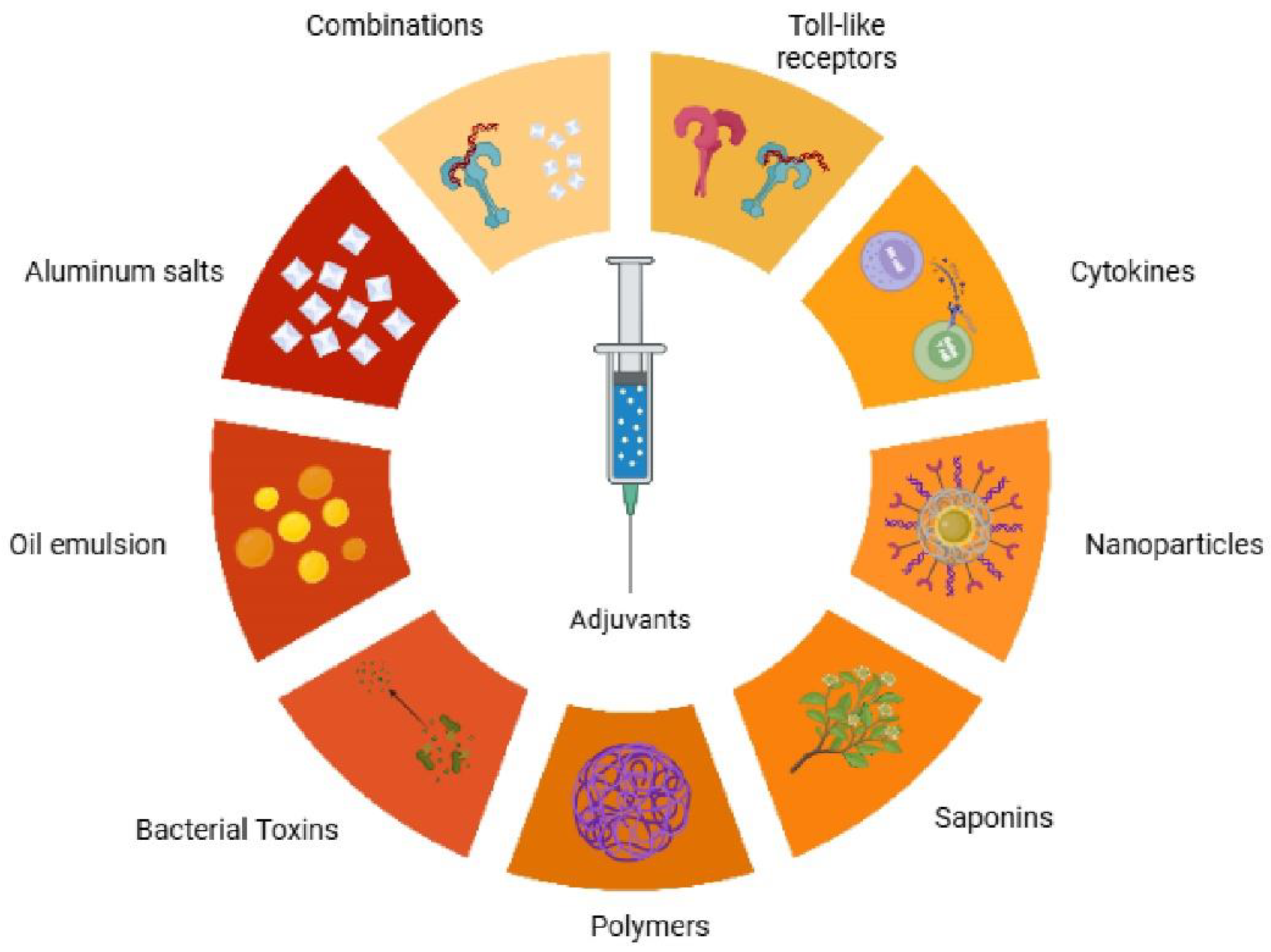
Adjuvants represent a cornerstone in the advancement of modern immunotherapy and vaccine development, serving as critical agents that profoundly enhance immune responses to therapeutic targets. Their fundamental role involves potentiating antigen recognition, activating crucial immune cells, and orchestrating the intricate downstream processes of adaptive immunity. There is a range of adjuvant classes, from traditional mineral salts and emulsions to modern immunostimulatory agents, such as Toll-like receptor agonists and nanoparticles (
Fig. 1). These diverse compounds enhance vaccine efficacy through distinct mechanisms of action. However, the evolving landscape of advanced immunotherapies has exposed the limitations of these traditional agents, particularly their narrow spectrum of immune activation. For instance, many conventional adjuvants often fall short in effectively polarizing T helper 1 (Th1) or T helper 17 (Th17) immune responses, which are indispensable for combating intracellular pathogens and tumors, or in inducing robust cross-presentation necessary for cytotoxic T lymphocyte (CTL)-mediated immunity [1,2,3].
This recognition has propelled the field towards a new generation of adjuvants, meticulously developed by leveraging sophisticated advancements in molecular immunology and material science. Emerging agents, including a synthetic nucleotide-based Toll-like receptor 3 (TLR3) ligand ARNAX, CpG oligodeoxynucleotides (ODNs), stimulator of interferon genes (STING) agonists, beta-glucan derivatives, and synthetic poly (I:C), exhibit unique mechanisms of action that enable immune response modulation with enhanced specificity and efficacy [4,5,6,7,8]. These innovations offer tailored solutions for a diverse range of therapeutic contexts, spanning from tumor immunotherapy to prophylactic and therapeutic vaccines against infectious diseases. Beyond merely augmenting the magnitude of immune responses, adjuvants also play a pivotal role in reducing the required antigen doses, thereby improving vaccine cost-effectiveness and broadening accessibility. While adjuvants have been widely employed in billions of vaccine doses, their precise mechanisms of action were historically not well characterized, often referred to as the immunologists' "dirty little secret." Nevertheless, significant progress has been made in elucidating these mechanisms over the past few decades.
The evolution in adjuvant development signifies a fundamental shift from empirical discovery to a more strategic, rational, and targeted design approach. Historically, the inclusion of adjuvants was often serendipitous, without a deep understanding of their underlying biological effects. However, the increasing elucidation of innate immune mechanisms, particularly the specificities of Pattern Recognition Receptor (PRR) signaling, has enabled a deliberate and precise approach to immunomodulation [9]. This paradigm shift enables the fine-tuning of immune responses, moving beyond general immune boosting to specific immune polarization—for example, directing the immune system toward Th1 responses for cellular immunity or Th2 responses for humoral immunity. This precision is crucial for optimizing vaccine efficacy against diverse threats and represents a significant step towards precision immunology in vaccine development. Such a rational design also holds the promise of reducing off-target effects and enhancing the overall safety profiles of vaccine formulations.
The review aims to provide the reader with an overview of adjuvants and delivery systems, while also offering insight into new developments in this area.
2.-. Adjuvants, Immunostimulants and Delivery Systems.
2.1. Classification of Adjuvants: Immunostimulants vs. Delivery Systems
Based on their primary mechanisms of action, vaccine adjuvants are broadly categorized into two principal classes: Immunostimulants and delivery systems. It is essential to recognize that many adjuvants exhibit a combination of these properties, reflecting the intricate interplay within the immune system [10,11].
Immunostimulants are agents designed to activate or enhance the host's immune response directly. They function as critical "danger signal molecules" that directly engage and activate antigen-presenting cells (APCs) by targeting specific PRRs located on their surfaces or within their cytoplasm. This direct engagement triggers a robust innate immune response, a prerequisite for the crucial maturation and activation of APCs [12,13].
Delivery systems, in contrast, primarily facilitate the efficient uptake, transport, and presentation of antigens by APCs. A classic mechanism associated with these systems is the formation of a depot at the site of injection. This depot ensures the slow and sustained release of antigens, providing prolonged exposure to the immune system and thereby promoting the production of high and persistent antibody titers [14].
Beyond these primary classifications, adjuvants often employ a combination of various mechanisms to elicit immune responses. These include the induction of cytokines and chemokines, the recruitment of different immune cells to the injection site, the enhancement of antigen uptake and presentation by APCs, and the promotion of antigen transport to draining lymph nodes. The activation of innate immune responses by adjuvants is instrumental in creating a local immuno-competent environment at the injection site, which is critical for shaping both the quality and quantity of the subsequent adaptive immune response.
While the classification into immunostimulants and delivery systems provides a practical conceptual framework, it often oversimplifies the actual biological activity of adjuvants. In practice, many adjuvants exhibit overlapping and synergistic functions. For instance, a delivery system that efficiently concentrates and presents an antigen, its primary role, inherently enhances the danger signal perceived by APCs, thereby indirectly acting as an immunostimulant. Conversely, an immunostimulant that activates APCs often simultaneously enhances their capacity for antigen uptake and transport to draining lymph nodes. This suggests a complex interplay in which different mechanisms reinforce one another. An optimal adjuvant formulation typically combines both delivery and immunostimulatory properties, ensuring efficient antigen presentation alongside the provision of critical danger signals necessary for robust APC activation and subsequent T cell priming. This integrated understanding is crucial for designing highly effective adjuvants that orchestrate a comprehensive and sustained immune response, rather than merely inducing isolated effects.
2.2. Immunostimulants as Danger Signals: PAMPs, DAMPs, and their mimics
Immunostimulants function by alerting the immune system to the presence of danger, whether from invading pathogens or damaged host cells. This alert is mediated by molecules broadly categorized as Pathogen-Associated Molecular Patterns (PAMPs) and Damage-Associated Molecular Patterns (DAMPs), or their synthetic mimics [13].
2.2.1. Importance of PAMPs
PAMPs are distinct molecular structures derived from microorganisms that are recognized by the innate immune system as indicators of infection. These patterns are evolutionarily conserved across broad classes of pathogens but are either absent or significantly modified in the host, allowing for their specific recognition as foreign [15].
Key examples of PAMPs include:
LPS: A significant component found on the outer cell wall of gram-negative bacteria.
Bacterial DNA: unmethylated CpG dinucleotides, which are prevalent in bacterial DNA but rare and methylated in vertebrate DNA.
Bacterial Lipoproteins: The cysteine-linked diacyl or triacyl lipid portions of bacterial lipoproteins.
Flagellin: The main structural protein that forms the filament of bacterial flagella.
Viral Nucleic Acids: Such as double-stranded RNA (dsRNA), single-stranded RNA (ssRNA), and 5′-triphosphate RNA, which are often produced during viral replication.
Viral Glycoproteins: Including the fusion protein from Respiratory Syncytial Virus (RSV) or components from measles virus, cytomegalovirus, and herpes simplex virus.
2.1.2. Importance of DAMPs
DAMPs are endogenous molecules derived from host cells that are released or exposed in response to cellular stress, damage, or death. Unlike PAMPs, DAMPs induce what are known as sterile inflammatory responses because their origin is host material, not pathogenic infection. These molecules signal "danger" to the immune system, indicating tissue trauma, ischemia, or abnormal cellular processes. Environments where DAMPs are particularly prevalent include conditions such as myocardial infarction, cancer, autoimmune diseases, and atherosclerosis [13,16].
Notable examples of DAMPs include:
Heat Shock Proteins (HSPs): Such as HSPA1A and HSPB1, which are released from cells under various pathological or non-pathological stress conditions. HSPs are known to interact with immune receptors, such as CD91 and CD40, to activate the immune system.
Products from Dead or Dying Cells: This category broadly includes components released from necrotic cells, including tumor cells.
2.3. Synthetic Mimics and Their Relevance
Immunostimulants can also be chemically synthesized small-molecule agonists specifically designed to mimic the structures and activities of natural PAMPs or DAMPs. This innovative approach enables greater control over the elicited immune response and often helps reduce the inherent toxicity associated with administering whole microbial components.
Key examples of such synthetic mimics include:
ODNs: Synthetic DNA sequences containing unmethylated CpG dinucleotides mimicking bacterial DNA [5].
STING agonists: Synthetic molecules that activate the STING pathway, which is a crucial cytosolic sensor for cyclic dinucleotides (bacterial second messengers) or host-derived DNA fragments [6].
Poly (I:C): A synthetic analog of double-stranded RNA, designed to mimic viral dsRNA [8].
Monophosphoryl Lipid A (MPL): A detoxified derivative of LPS, engineered to retain its potent immunostimulatory activity while significantly reducing the toxicity associated with the parent molecule [17].
The development of these synthetic mimics is a cornerstone of modern adjuvant design, enabling more targeted and safer immune activation for specific therapeutic applications.
The concept that immunostimulants act as danger signal molecules reinforces a profound understanding of immune activation, extending beyond mere non-self-recognition to a more fundamental detection of danger. This perspective, articulated in the "Danger Theory," posits that the immune system is primarily activated by endogenous molecules released or activated during tissue stress or damage, as well as by exogenous pathogen-derived patterns. Adjuvants, by acting as or mimicking these danger signals, effectively alert the immune system to the presence of an antigen, even if the antigen itself is purified and inherently non-dangerous, as is often the case in subunit vaccines. This clarifies why purified subunit vaccines, lacking the intrinsic danger signals of live pathogens, frequently require adjuvants to elicit robust immunity. This understanding also highlights the therapeutic potential of harnessing endogenous DAMPs for applications such as cancer immunotherapy, where the immune system needs to be alerted explicitly to damaged or cancerous host cells.
However, the immune system's response to danger signals, while essential for host defense, is a delicate balance. An uncontrolled, misdirected, or prolonged response can lead to detrimental outcomes, including chronic inflammation, tissue damage, or the breakdown of self-tolerance, potentially resulting in autoimmune diseases. The context-dependent nature of PRR responses further underscores this complexity. This critical balance implies that successful adjuvant design is not simply about maximizing immune activation but about achieving a precise level of immunomodulation. The goal is to elicit a protective response without inducing pathology. This imperative drives the development of safer synthetic mimics, such as MPL, as a detoxified LPS derivative, and fosters a deeper understanding of the precise molecular mechanisms that differentiate beneficial from harmful immune activation. Furthermore, the capacity of immature dendritic cells to induce tolerance highlights the sophisticated regulatory mechanisms that prevent unwanted immune activation against self-antigens, underscoring the need for adjuvants to drive dendritic cells toward a fully mature, immunogenic state.
2.4. Pattern Recognition Receptors (PRRs): The Innate Immune Sensors
Pattern Recognition Receptors (PRRs) are germline-encoded proteins that serve as the primary sensors of the innate immune system. They are capable of recognizing conserved molecular patterns found in pathogens (PAMPs) or molecules released from damaged host cells (DAMPs). PRRs are strategically located throughout the cell and in the extracellular space to detect threats in various compartments. They can be found on cellular and endosomal membranes, within the cytosol, and in secreted forms in the bloodstream and interstitial fluids [18,19].
The four major sub-families of PRRs include:
Toll-like Receptors (TLRs): The most extensively studied PRR family, TLRs are transmembrane proteins located on the cell surface (e.g., TLR1, TLR2, TLR4, TLR5, TLR6) or within endosomal compartments (e.g., TLR3, TLR7, TLR8, TLR9). They recognize a wide array of PAMPs, including bacterial lipopeptides (TLR1/2, TLR2/6), LPS (TLR4), flagellin (TLR5), viral single-stranded RNA (TLR7/8), and unmethylated CpG DNA (TLR9) [20].
NOD-like Receptors (NLRs): These are cytoplasmic receptors that detect intracellular PAMPs (e.g., bacterial peptidoglycan fragments like diaminopimelic acid for NOD1) and DAMPs (e.g., uric acid crystals for NLRP3). NLRC4, a specific NLR, is known to recognize flagellin intracellularly [21].
RIG-I-like Receptors (RLRs): These are cytoplasmic RNA helicases (e.g., RIG-I, MDA5) that primarily detect viral nucleic acids, such as double-stranded RNA (dsRNA) and 5′-triphosphate RNA, often produced during viral replication [22].
C-type Lectin Receptors (CLRs): These are transmembrane receptors involved in carbohydrate recognition, frequently found on APCs. An example is Dectin-1, which recognizes β-glucan from fungi [23].
Other PRRs include AIM2-like receptors (AIM2) and the STING pathway, a crucial cytosolic sensor for cyclic dinucleotides, which can be bacterial second messengers or host-derived DNA fragments.
2.5. Mechanisms of PRR Engagement by Immunostimulants
The binding of PAMPs, DAMPs, or their mimics to PRRs induces specific conformational changes in the receptor, which in turn initiates a cascade of intracellular signaling events. These signaling pathways typically involve adaptor molecules, such as MyD88 and TRIF, and lead to the activation of key transcription factors, including NF-κB, mitogen-activated protein kinases (MAPKs), and interferon regulatory factors (IRFs). The outcome of PRR engagement is a broad transcriptional change, resulting in the expression and synthesis of a diverse range of proinflammatory molecules, including cytokines, chemokines, and cell adhesion molecules. This orchestration of the early host response to infection is a prerequisite for the subsequent activation and shaping of adaptive immunity. A critical aspect of PRR signaling is its context-dependency. A single PRR can recognize multiple PAMPs and DAMPs, and simultaneous signaling through different PRRs or in the presence of other cytokines can modulate downstream responses. This intricate network allows the immune system to fine-tune its response based on the nature and location of the perceived threat [10,24].
While innate immunity is often broadly characterized as non-specific compared to the clonal specificity of adaptive immunity, the PRR system demonstrates a remarkable level of specificity in discriminating between distinct molecular signatures. This specificity at the level of PRR recognition is paramount because it directly dictates the activation of particular downstream signaling cascades and the initial cytokine milieu produced by innate immune cells. This inherent specificity forms the fundamental link between the specific type of danger signal (immunostimulant) and the qualitative outcome of the adaptive immune response. Ligand binding to different PRRs leads to the activation of unique intracellular pathways, which in turn result in distinct cytokine profiles, ultimately polarizing T helper responses (e.g., Th1, Th2, Th17) and influencing the generation of CTLs. This precise discrimination forms the basis for the rational design of adjuvants, which aim to tailor vaccine responses to specific pathogens or disease contexts [19].
Furthermore, the subcellular localization of PRRs, for example, on the cell surface for extracellular threats, within endosomes for internalized pathogens, or in the cytosol for intracellular pathogens—and the specific adaptor molecules they recruit (e.g., MyD88 versus TRIF) are critical determinants of the nature and intensity of the immune response. This compartmentalization allows the immune system to interpret the context of the danger signal and mount an appropriate, spatially and functionally relevant defense. For instance, nucleic acid-sensing PRRs are often found in endosomes or the cytosol, reflecting their role in detecting intracellular viral replication. This sophisticated compartmentalization enables the immune system to differentiate between various types of threats (e.g., extracellular bacteria versus intracellular viruses) and tailor the innate response accordingly. For example, PRRs that signal primarily through the TRIF pathway, such as TLR3 (dsRNA) and RLRs (cytosolic RNA), are potent inducers of Type I interferons, which are crucial for antiviral immunity. The differential signaling of MPL versus full LPS via TLR4 is a prime example of how even minor modifications to an immunostimulant can selectively activate specific intracellular pathways, thereby altering the downstream immune outcome (e.g., favoring Type I IFN production) and potentially reducing toxicity. This level of detail in PRR signaling pathways is essential for designing adjuvants that elicit highly specific Th1 (cellular), Th2 (humoral), or CTL responses [19,25].
2.6. APC Maturation and Activation by Immunostimulants
Immunostimulants, by acting as danger signal molecules, are central to driving the maturation and activation of APCs. This process involves a profound transformation in APC phenotype and function, optimizing them for effective T cell priming.
A hallmark of mature APCs, particularly dendritic cells (DCs), is the termination of their high phagocytic and endocytic activity. Immature DCs are highly efficient at antigen uptake. Still, upon receiving maturation stimuli, they downregulate this function, strategically shifting their cellular machinery from antigen capture to the crucial task of antigen presentation [26].
Concomitantly, the ability of APCs to process and present antigens is significantly enhanced during maturation. This involves the efficient processing of internalized antigens into peptides and their subsequent loading onto MHC class I and II molecules. These MHC-peptide complexes, often referred to as Signal 1 for T cell activation, are then transported to the APC surface for display to T lymphocytes. Dendritic cells, being highly specialized APCs, can generate large amounts of MHC-peptide complexes [27,28].
Furthermore, mature APCs express significantly higher levels of co-stimulatory molecules (Signal 2), which are essential for the robust activation of naive T cells. Key co-stimulatory molecules include CD40, CD80 (B7.1), and CD86 (B7.2). Other important co-stimulators encompass CD54/ICAM-1, CD48, CD58/LFA-3, 4-1BB ligand, and BAFF/BlyS. Notably, CD86 is particularly abundant on DCs. It is rapidly upregulated during maturation, clustering with MHC-peptide complexes to form the immunological synapse with T cells, thereby preparing the APC for optimal T cell engagement [27,28].
2.7. Role of Cytokine Secretion in APC Activation
The activation of PRRs by immunostimulants not only induces these profound phenotypic changes but also leads to a dramatic increase in the production and secretion of various inflammatory cytokines and chemokines. These soluble mediators, often referred to as Signal 3 for T cell activation, are critical for shaping the subsequent adaptive immune response [29].
Key Cytokines Produced by Activated APCs include:
Pro-inflammatory cytokines: Such as IL-1, IL-6, IL-12, IL-23, and TNF-α, which contribute to the local inflammatory environment and directly influence T cell differentiation.
T helper-polarizing cytokines: Most notably IL-12, which is crucial for polarizing naive T cells towards a Th1 phenotype. Other important polarizing cytokines include IL-23 (for Th17 differentiation) and IL-15 (for memory T cell sustenance).
Type I Interferons (IFN-α, IFN-β): Produced in response to specific PRR engagements (e.g., TLR3, TLR7/8/9, STING, RLRs), these are vital for antiviral immunity and enhancing antigen presentation on both MHC class I and II molecules.
Immunosuppressive cytokines: Such as IL-10, IL-37, IL-38, TGFβ which can modulate and temper the immune response.
In addition to cytokines, activated APCs, particularly DCs, produce chemokines that recruit other immune cells, including T cells and additional DCs, to the injection site and subsequently to the draining lymph nodes. This orchestrated cellular recruitment is essential for establishing a robust immunocompetent environment [30,31].
Antigen-presenting cell maturation is not a singular event, but a highly complex and multifaceted biological process involving a coordinated and sequential shift in cellular functions. It represents a critical transition from an antigen-capturing sentinel cell to a potent and specialized T cell activator. This involves a strategic trade-off: a reduction in non-specific antigen uptake (phagocytosis) is directly coupled with a dramatic enhancement in the precise presentation of processed antigens via MHC molecules, alongside the upregulation of co-stimulatory signals and the secretion of specific cytokines. The simultaneous and synergistic provision of Signal 1 (MHC-peptide complex), Signal 2 (co-stimulatory molecules), and Signal 3 (cytokines) by mature APCs is critical for the effective activation and differentiation of naive T cells. If any of these "three signals" are suboptimal, incomplete, or absent, the T cell response can be weak, anergic (unresponsive), or even tolerogenic (inducing immune suppression). This underscores the fundamental role of adjuvants in ensuring that APCs receive all necessary cues to undergo complete and appropriate maturation, thereby guaranteeing a robust and productive adaptive immune response [28].
Moreover, the initial activation and maturation of APCs by immunostimulants at the site of antigen encounter (e.g., injection site) is not an isolated cellular event. Instead, it triggers a cascade of local immunological events that establish a dynamic "immuno-competent microenvironment." This environment is characterized by the sustained secretion of cytokines and chemokines, leading to the recruitment of various immune cells, including additional APCs and naive T cells. This localized activity is crucial for efficiently capturing and processing antigens and subsequently transporting antigen-loaded, mature APCs to the draining lymph nodes. This emphasizes that adjuvants do not merely act on individual cells; they orchestrate a profound microenvironmental change that is essential for the efficient initiation of adaptive immunity. The "depot effect" of some adjuvants and the enhanced antigen transport to draining lymph nodes are critical for ensuring prolonged antigen availability and efficient encounter between mature APCs and naive T cells in the secondary lymphoid organs. This complex interplay effectively bridges the innate immune recognition with the subsequent adaptive immune priming, ensuring a coordinated and robust systemic response [32].
2.8. Dendritic Cells: The Master Orchestrators of Adaptive Immunity
DCs are widely recognized as nature's adjuvants due to their unparalleled ability to initiate and shape adaptive immune responses. They are highly specialized in antigen capture, processing, and presentation. They are uniquely equipped to provide the necessary co-stimulatory functions that drive the expansion of both Th1 helper T lymphocytes and cytotoxic T lymphocytes (CTLs). Indeed, antigen-bearing DCs, even in the absence of additional adjuvants, can elicit robust antimicrobial and anti-tumor immunity in experimental models [33,34,35].
Several unique attributes make DCs superior APCs and prime targets for immunostimulant action:
Exceptional Potency: DCs are remarkably potent stimulators of T cell responses. Even small numbers of DCs can mediate strong T cell growth, CTL differentiation, and lymphokine production. They are active at significantly lower ratios (e.g., 1:100) compared to other APCs like B cells and macrophages.
Control of MHC-Restricted Immunity: DCs play a decisive role in controlling the MHC restriction of the immune response, dictating the specificity of T cell recognition in the efferent limb of immunity.
Direct Responsiveness to Danger Signals: DCs directly respond to various danger signals, including DNA and specific CpG oligodeoxynucleotides. This inherent responsiveness means that DNA vaccines, beyond merely encoding specific antigens, actively stimulate DCs to mature and become potent T cell stimulators.
Strategic Distribution and Trafficking: DCs are strategically distributed throughout peripheral tissues and constantly traffic through the lymphatics. They are uniquely designed to capture antigens from any site of deposition efficiently, migrate to regional lymph nodes, and there, efficiently select and activate relevant T cell clones to initiate the immune response. During maturation, DCs reshape their chemokine receptors, notably upregulating CCR7, which is crucial for their homing and function in lymph nodes.
Influence on Immune Quality and Memory: DCs play a crucial role in inducing robust immunity, including the development of Th1-type CD4+ helper T cells and CD8+ cytotoxic T lymphocytes, and rapidly polarizing CD4+ T cell responses towards the Th1 phenotype, which is associated with superior protection and memory in numerous experimental models. They are also capable of boosting the quality of T cell memory.
Bridging Innate and Adaptive Immunity: DCs possess an innate capacity to respond rapidly to microbial stimuli, thereby serving as the primary initiators of the adaptive immune response.
2.8.1. Detailed Mechanisms of DC Antigen Capture, Processing, and Cross-Presentation
The superior antigen-presenting capabilities of DCs are attributed to their sophisticated mechanisms for antigen handling:
Antigen Capture: Immature DCs are highly specialized in capturing antigens through diverse routes, including macropinocytosis (bulk fluid uptake), phagocytosis (uptake of particulates like dead cells or bacteria), and adsorptive or receptor-mediated uptake. This initial capture phase is crucial for gathering antigenic material from the environment.
Processing and MHC-Peptide Formation: Upon receiving a maturation stimulus, DCs undergo intricate internal changes that regulate their endocytic activity, proteolytic machinery, and the formation and transport of MHC-peptide complexes. For instance, they can downregulate cystatin C levels within lysosomal compartments, which enhances the proteolysis of the invariant chain and, consequently, improves the exchange of antigenic peptides with CLIP, facilitating the movement of MHC-peptide complexes to the cell surface.
Unique Endocytic Receptors: DCs possess specialized endocytic receptors like DEC-205, which traffics uniquely through the endocytic system, recycling through MHC II-positive late endosomes or lysosomes. Targeting antigens via DEC-205 can significantly enhance the efficiency of antigen presentation on MHC class II molecules by 10-to 100-fold.
Cross-Presentation: A particularly striking and crucial feature of DCs is their ability to perform "cross-presentation," also known as the exogenous pathway of presentation on MHC class I. This mechanism enables DCs to take up exogenous antigens (e.g., from immune complexes or dead or dying cells), which would typically be presented via MHC class II, and process them for presentation on MHC class I molecules. This enables the activation of CD8+ cytotoxic T lymphocytes (CTLs) against antigens that the DC itself did not synthesize, a critical pathway for immunity against intracellular pathogens and tumors. DCs efficiently present peptides from dying cells on MHC class II.
2.8.2. The Critical Role of DC Maturation as a Control Point for Initiating Immunity
The efficacy of vaccine antigens is profoundly dependent on their presentation by DCs, but critically, DCs must differentiate or mature into potent stimulators of T cell immunity. This maturation process is a central control point for initiating an effective adaptive immune response [36].
Maturation Stimuli: Many microbial products, LPS, double-stranded RNA, and CpG-ODN sequences, are potent stimuli for DC maturation both in vitro and in vivo. Cytokines such as GM-CSF, TNF-α, IL-1β, and PGE2, as well as TNF family members like CD40L, also induce DC maturation [37,38].
Consequences of Failed Maturation: If a DNA vaccine (or any antigen delivery strategy) fails to stimulate proper DC maturation, the DCs may instead induce different forms of tolerance and suppress immunity. Immature DCs, particularly in a steady state, are specialized to cross-present antigens from normal cell turnover in a tolerogenic manner, possibly by inducing regulatory T cells or through deletional/anergic tolerance, ensuring the immune system does not react to self-antigens.
Mobilization and Lymph Node Homing: A key phenotypic change during DC maturation is the upregulation of functional CCR7 receptors, which are indispensable for their migration from peripheral tissues to the draining lymph nodes, where T cell priming occurs.
Dendritic cells are not merely superior antigen-presenting cells; they are the central nexus that seamlessly connects the rapid, non-specific recognition of innate immunity with the particular and memory-driven responses of adaptive immunity. Their unique combination of attributes, the ability to capture diverse antigens, undergo precise maturation in response to danger signals, efficiently migrate to lymph nodes, and present antigens with the full complement of co-stimulatory molecules and polarizing cytokines, makes them unparalleled in initiating and shaping robust, specific adaptive immune responses. They effectively act as the decision-makers of the immune system, determining whether an immune response is triggered, its particular type (e.g., Th1 for cellular immunity versus Th2 for humoral immunity), and its overall magnitude and duration. This profound and multifaceted role of DCs signifies that directly targeting DCs with adjuvants or antigens is a highly effective and increasingly utilized strategy in modern vaccine development and immunotherapy. The success of advanced vaccine platforms, such as DNA vaccines, is partly attributed to their ability to stimulate DC maturation. Consequently, a deep understanding of DC physiology and its dynamic states is crucial for enhancing the efficacy and precision of future immunotherapeutic interventions.
Furthermore, DCs exist in distinct functional states, primarily an immature, antigen-capturing state and a mature, antigen-presenting, and T cell-activating state. The transition between these states, profoundly influenced by immunostimulants, represents a critical control point in immune regulation. DCs also play a vital role in inducing immunological tolerance, particularly when they encounter antigens in a steady-state or non-inflammatory context, often in their immature form. This dynamic and dualistic nature of DCs implies that the specific nature, timing, and context of adjuvant delivery are paramount. An adjuvant must not only ensure efficient antigen delivery but also effectively induce the appropriate type of DC maturation to avoid tolerance induction and instead drive a protective immune response. This highlights the intricate complexity of adjuvant design, moving beyond a simplistic goal of merely "boosting" immunity to one that demands precise immunomodulation. The ability of DCs to either enhance or dampen immunity in an antigen-specific way, dependent on their maturation state, underscores the sophisticated regulatory mechanisms that differentiate between "safe" and "dangerous" signals to prevent unwanted immune activation against self-antigens [39].}
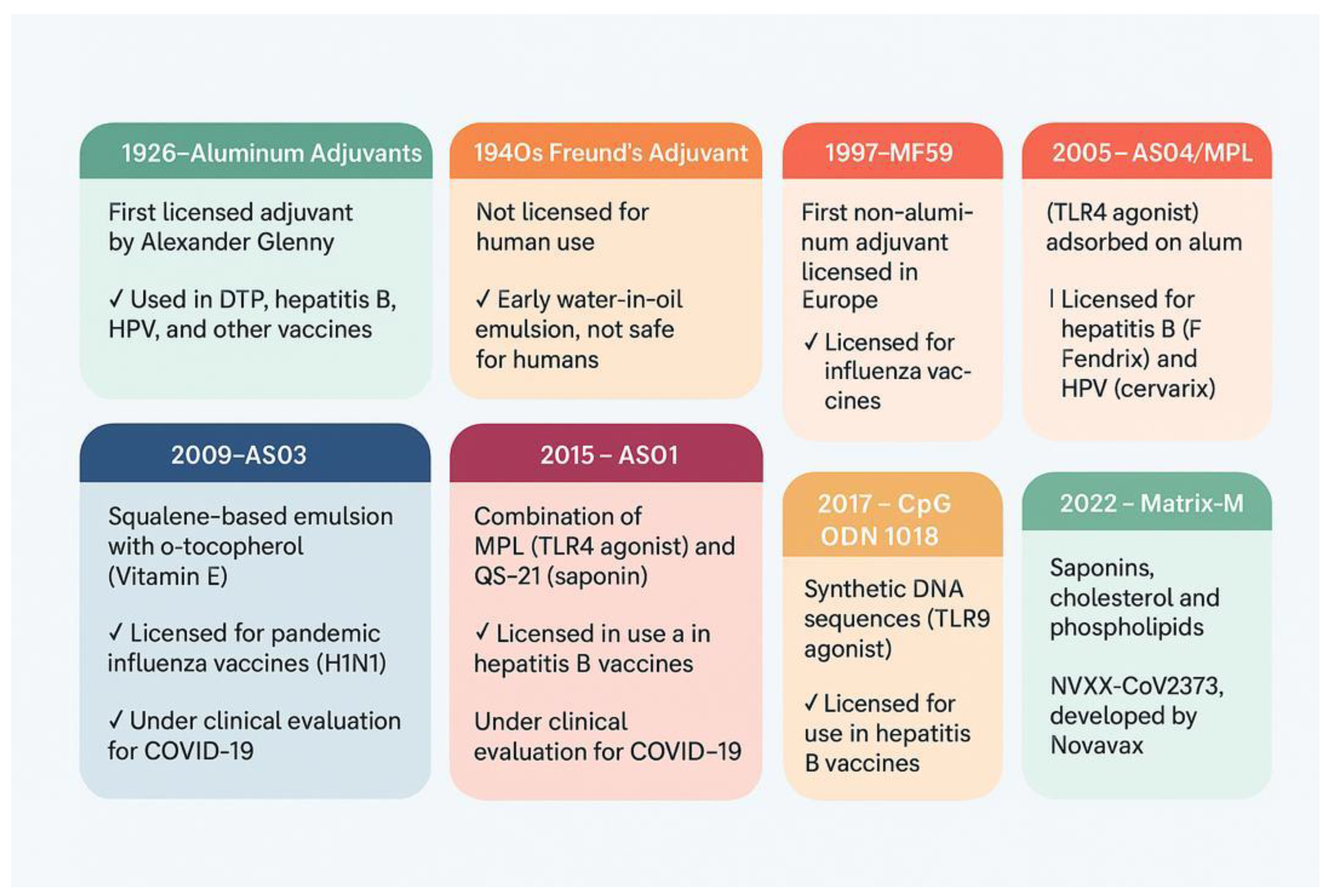
3. Types of Adjuvants and Their Evolution Over Time
3.1. Aluminum Adjuvants
Aluminum salt-based adjuvants—such as alum, alhydrogel, and Adju-Phos—have long been considered the gold standard in clinically approved vaccine adjuvants. Their widespread use in vaccines targeting diseases such as diphtheria, tetanus, pertussis, hepatitis B, and anthrax is attributed mainly to their outstanding safety record and predictable, mild local side effects, typically limited to transient inflammation at the injection site. Their efficacy is primarily linked to their ability to promote a robust humoral response by preferentially stimulating CD4+ T helper lymphocytes towards the Th2 phenotype, enhancing B lymphocyte activation and subsequent antibody production [40].
While the precise mechanisms by which aluminum adjuvants enhance immune responses are not yet fully elucidated, several well-studied models provide insights into their activity. They are understood to boost the production of IgG1 and IgE antibodies by fostering Th2 cell responses. Two main functions have been identified: they serve as delivery systems that bind strongly to antigens, allowing for the gradual release of these antigens, which increases their availability and improves presentation. Second, they serve as immunostimulants by inducing the formation of DAMPs. These DAMPs activate pattern recognition receptors (PRRs) in innate immune pathways, leading to the production of cytokines, such as IL-1β, and promoting Th2-type responses. Recent studies suggest that host DNA or uric acid, released from cell death at the injection site due to the aluminum adjuvant, acts as an endogenous danger signal, further activating innate immune mechanisms. Although some researchers identify NLRP3 as the main PRR engaged by aluminum adjuvants, this view is not universally accepted.
The enduring success of aluminum adjuvants, despite their incompletely understood mechanisms and known limitations, presents a notable paradox in the field of vaccinology. They have been the gold standard for decades, demonstrating an outstanding safety record and widespread success. Yet, their precise mechanisms are not yet fully understood, and they exhibit limitations, such as a restricted ability to induce robust cellular immune responses. This highlights a gap between practical efficacy and theoretical understanding in the field. While empirical success has driven their extensive use, the imperative for next-generation vaccines necessitates a deeper mechanistic understanding to overcome these limitations and design more targeted, broader-spectrum immune responses, as well as robust cellular immunity against pathogens such as certain viruses or cancers. The ongoing research into nanoaluminum variants directly responds to this need [40,41,42,43,44].
3.2. Oil-in-Water Emulsion Adjuvants
Oil-in-water emulsions, such as MF59 and AS03, create a depot effect at the injection site, promoting antigen uptake and stimulating innate immune responses.
3.2.1. MF59
MF59 is an oil-in-water emulsion composed of 4.3% squalene dispersed in a citric acid buffer and stabilized by the nonionic surfactants Tween 80 (0.5%) and Span 85 (0.5%).1 Squalene, a naturally produced component of the human steroid hormone synthesis pathway, is sourced from purified shark liver for vaccine use, while the surfactants are plant-derived. All components of MF59 are biodegradable, natural derivatives known for their safety and tolerability, with oil droplets averaging approximately 160 nm in size. Initially developed as an antigen delivery buffer, MF59 was later discovered to possess surprisingly potent adjuvant properties [45].
The seasonal influenza vaccine, adjuvanted with MF59, received its first license in 1997 for use in elderly individuals and has since been approved for use in over 30 countries, including the United States [46]. Beyond seasonal influenza, MF59-adjuvanted H1N1 pandemic influenza vaccines have been administered to diverse populations, including pregnant women and young children, with nearly 100 million doses distributed.MF59 enhances the immunogenicity of influenza vaccines by increasing levels of hemagglutination-inhibiting (HAI) antibodies and promoting the development of memory T and B lymphocytes against antigenically drifted influenza viruses, thereby improving both pandemic and seasonal vaccine efficacy [47]. Notably, MF59 is recognized for its ability to compensate for insufficient CD4+ T lymphocyte help by enhancing adaptive immunity through a CD4-independent mechanism. This unique feature may explain its proven efficacy across diverse populations, from young children to the elderly and immunocompromised patients. Ongoing studies are expected to expand MF59’s application to various vaccine antigens and platforms while deepening the understanding of its underlying mechanisms.
3.2.2. AS03
AS03 is a squalene-based oil-in-water emulsion, similar to MF59, but uniquely enhanced with α-tocopherol (vitamin E) to further boost immune responses. Licensed for use with pandemic influenza vaccines, AS03 is also under clinical evaluation in several trials of recombinant spike protein COVID-19 vaccines. It significantly amplifies the magnitude and diversity of antibody and CD4+ T cell responses, resulting in superior protection compared to non-adjuvanted vaccines [48].
Animal studies have shown that AS03 triggers a transient surge in cytokine production at the injection site and in the draining lymph nodes. Incorporating α-tocopherol influences the expression of specific chemokines and cytokines, including CCL2, CCL3, IL-6, G-CSF, and CXCL1. This modulation enhances the uptake of antigens by monocytes and promotes the recruitment of granulocytes. Additional investigation has noted that AS03 can temporarily downregulate genes involved in lipid metabolism in draining lymph nodes, a change associated with alterations in lipid composition, endoplasmic reticulum morphology, and activation of the unfolded protein response. Research involving human subjects suggests that elevated levels of IL-6 and IP-10 (CXCL10) observed within 24 hours following vaccination are associated with subsequent robust antibody responses [49,50]. Given that both AS03 and MF59 are squalene-based adjuvants, they likely share common innate activation pathways, though the precise roles of pathways such as those involving IRE1α and RIPK3 remain to be clarified.
The distinct immunomodulatory effects of MF59 and AS03 illustrate the concept of targeted immunomodulation through specific adjuvant components. MF59’s efficacy across diverse populations is linked to its CD4-independent mechanism, suggesting it can bypass specific immunological requirements. AS03's enhanced effects are attributed to α-tocopherol, which modulates particular chemokines and cytokines and enhances antigen uptake by monocytes [49]. This demonstrates that specific components within an adjuvant formulation are not merely generic immunostimulants but can precisely modulate distinct arms of the immune response or overcome specific immunological challenges, such as insufficient CD4+ T cell help. This approach suggests a future characterized by highly specialized adjuvants, wherein components are meticulously selected for their capacity to finely modulate the immune response, achieving specific outcomes such as robust humoral, cellular, or mucosal immunity. Additionally, these adjuvants can be tailored to function effectively within particular populations, such as older adults or individuals with compromised immune systems.
3.3. Other AS0 Family Adjuvants
3.3.1. AS04
AS04 is formulated by using 3-O-desacyl-4-monophosphoryl lipid A (MPL), a detoxified variant of lipopolysaccharide (LPS) from Salmonella minnesota, which is adsorbed onto aluminum salts. Preclinical studies in mice have demonstrated that even when combined with alum, MPL preserves its full immunostimulatory capacity via TLR4 activation. The resultant adjuvant effect is driven by a combination of TLR4-mediated signaling in innate immune cells and the intrinsic immunomodulatory action of alum. When added to hepatitis B and human papillomavirus (HPV) vaccines that use recombinant antigens, AS04 elicits higher antibody titers than formulations with alum alone, underscoring the benefit of including the TLR4 agonist MPL. For instance, this combination in HPV-16/18 vaccines translates into near-perfect, long-lasting efficacy (up to nine years’ post-immunization). However, more research is needed to fully understand how AS04 broadens the immune response against a broader range of HPV strains [51,52]
3.3.2. AS01
AS01 has been successfully incorporated into licensed vaccines, such as Shingrix for varicella zoster, which is approved for individuals aged 50 and older and boasts an efficacy of approximately 97% [53,54]. This adjuvant uniquely combines two immunostimulatory components: MPL, the same TLR4 ligand used in AS04, and QS-21, a purified triterpene glycoside extracted from Quillaja saponaria. While QS-21 alone is a potent enhancer of antibody and cellular immune responses, its standalone use in humans has raised concerns about tolerability. To overcome this, AS01 formulates MPL and QS-21 together within liposomes in the presence of cholesterol, which helps reduce the reactogenicity of QS-21.
MPL primarily activates the innate immune system through TLR4, predominantly via TRIF-dependent signaling [55]. Concurrently, studies in mice have shown that QS-21 triggers caspase-1 activation in subcapsular sinus macrophages of the draining lymph nodes; however, it’s in vivo adjuvant effect does not appear to rely on the NLRP3 pathway. When packaged in liposomes, QS-21 is internalized through cholesterol-dependent endocytosis, leading to lysosomal destabilization and subsequent activation of the SYK kinase [56]. The synergy arising from these combined pathways is central to the potent adjuvant effect of AS01, leading to augmented polyfunctional CD4+ T cell responses (such as those secreting IL-2, IFNγ, and TNF) and the production of highly functional antibodies. This dual activation is especially noteworthy for its ability to counteract immunosenescence in older populations. Despite extensive insights from preclinical work, further investigation is necessary to fully elucidate the mechanisms of AS01 in humans.
The development of AS04 and AS01 exemplifies the power of multi-component, rationally designed adjuvant systems. AS04 combines MPL, a microbial product and TLR4 agonist, with aluminum salts, demonstrating improved outcomes over alum alone. AS01 further advances this concept by combining MPL and QS-21, a saponin, within a liposomal delivery system. The text explicitly states that AS01's "synergy arising from these combined pathways is central to the potent adjuvant effect" and that these systems are "rationally designed to maximize immunological potency while ensuring safety and tolerability." This represents a clear progression beyond single-component adjuvants. The trend is towards sophisticated, multi-component adjuvant systems where different immunostimulants and delivery vehicles are combined to achieve a synergistic effect, targeting multiple innate immune pathways and overcoming individual component limitations, such as QS-21 toxicity. This approach marks a significant leap in rational vaccine design, moving toward highly optimized and targeted immune responses, particularly for challenging populations such as older individuals.
3.4. CpG Oligodeoxynucleotides (ODNs)
ODNs mimic bacterial DNA and trigger immune activation by binding to TLR9, which is predominantly expressed in various immune cells, including B cells, plasmacytoid dendritic cells, and macrophages. This binding initiates a cascade of signaling events that produce proinflammatory cytokines (IL-6, TNF-α, and IL-12) and the maturation of antigen-presenting cells. CpG ODNs typically elicit a Th1-biased immune response, which is often desired for combating intracellular pathogens or cancer [57,58,59].
Despite their potent immunostimulatory properties, the clinical application of free CpG ODNs is hampered by several limitations, including rapid nucleosome degradation, poor cellular uptake, and nonspecific distribution in the body. To overcome these challenges, a wide range of nanomaterial-based delivery systems is being explored to improve the stability, targeting, and controlled release of CpG ODNs. While significant progress has been made in enhancing immunoactivation with nanomaterial-based platforms for CpG delivery, several challenges remain. Key issues include the need to elucidate the precise molecular mechanisms governing the enhanced immunostimulatory effects observed with various nanocarriers, ensure long-term biocompatibility and safety while achieving controlled payload release in complex biological environments, and bridge the gap between promising preclinical results and successful clinical translation by developing scalable, stable formulations under physiologically relevant conditions. The convergence of nanotechnology with immunotherapy, through platforms based on DNA nanostructures, inorganic nanoparticles, polymeric systems, metal–organic frameworks, lipid-based carriers, and biologically inspired vehicles, offers promising avenues to overcome the inherent limitations of free CpG ODNs. These advances are setting the stage for the next generation of vaccines and Immunotherapeutics, particularly in cancer treatment and personalized medicine.
Developing nanomaterial-based delivery systems for CpG ODNs illustrates how nanotechnology provides solutions to the pharmacokinetic limitations of potent immunostimulants. CpG ODNs possess potent immunostimulatory properties, but their clinical application is hampered by several limitations, including rapid degradation by nucleases, poor cellular uptake, and nonspecific distribution. The proposed solution involves a "wide range of nanomaterial-based delivery systems that aim to improve the stability, targeting, and controlled release of CpG ODNs. This direct cause-and-effect relationship highlights a broader trend: highly effective biological molecules often suffer from poor pharmacokinetics, limiting their therapeutic potential. Nanotechnology emerges as a critical enabler, transforming promising but unstable compounds into viable therapeutic agents by providing protection, targeted delivery, and controlled release. This principle extends beyond CpG ODNs to other sensitive biomolecules, demonstrating the transformative impact of nanotechnology on drug development [60].
3.5. Matrix-M™
Matrix-M™ is a saponin-based adjuvant formulated for modern subunit vaccines. It consists of two purified fractions of Quillaja saponaria, Molina saponins, combined with cholesterol and phospholipids to form ~40-nm open cage-like nanoparticles. Importantly, Matrix-M is provided as a defined blend (approximately 85% Matrix-A and 15% Matrix-C particles) that balances a highly potent QS–21–rich fraction with less reactogenic saponins, thereby maximizing immunostimulant while improving tolerability. This design confers potent adjuvant activity, enabling substantial antigen dose-sparing, and remains physically stable (in solution at 2–8°C for years) [61].
Mechanistically, intramuscular injection of Matrix-M–adjuvanted vaccine triggers rapid innate immune activation. The adjuvant induces the transient recruitment of neutrophils, monocytes, and other antigen-presenting cells at the injection site, which take up the co-delivered antigen and traffic it to the draining lymph nodes. In these lymphoid tissues, the Matrix-M–conditioned environment enhances antigen presentation, as dendritic cells upregulate MHC and co-stimulatory molecules, and present processed antigen peptides to both CD4+ and CD8+ lymphocytes. Within APCs, Matrix-M saponins are released in acidic lysosomes, causing membrane permeabilization that allows the antigen to escape into the cytosol (facilitating cross-presentation on MHC-I to CD8+ T lymphocytes). Matrix-M also activates innate signaling pathways such as the NLRP3 inflammasome, leading to secretion of pro-inflammatory cytokines (IL-1β, IL-18) that further drive T-cell activation and Th1-type immunity. These early events set the stage for an amplified adaptive response: Matrix-M–adjuvanted vaccines induce markedly higher titers of high-affinity antibodies with broader epitope recognition, as well as robust germinal center formation and memory B-cell development. The T-cell profile is skewed toward Th1 (IFN-γ–producing) helper responses, with an enhanced generation of T follicular helper and cytotoxic CD8+ memory T lymphocytes. Indeed, in preclinical models, Matrix-M-formulated antigens (e.g., SARS-CoV-2 spike, influenza hemagglutinin, Ebola glycoprotein) elicited multifunctional CD4+ and CD8+ T lymphocytes, potent neutralizing antibodies, and long-lived immunity superior to unadjuvanted formulations [62]. Notably, the dose-sparing effect of Matrix-M reflects its ability to focus immune activation in the draining lymph node. Studies show that Matrix-M injection results in enlarged, cell-rich lymph nodes, suggesting that the adjuvant “sets the stage” for optimal adaptive responses, even at low antigen doses.
These immunological features translate into enhanced clinical vaccine efficacy. For example, the NVX-CoV2373 recombinant SARS-CoV-2 spike nanoparticle vaccine (Novavax) is ineffective without an adjuvant; when formulated with Matrix-M, it induces neutralizing antibody titers exceeding those in convalescent sera and achieves approximately 90–96% efficacy against symptomatic COVID-19 in Phase 3 trials [63]. In those trials, Matrix-M augmented both humoral and cellular immunity: Matrix-M–adjuvanted NVX-CoV2373 elicited significantly higher antibody levels and more IFN–γ–biased CD4+ T-cell responses than the antigen alone. Similarly, the R21 malaria vaccine (a circumsporozoite protein VLP) was combined with Matrix-M to improve efficacy. Clinical studies of R21/Matrix-M demonstrated very high anti-CSP antibody titers and ~82% sterile protection in a human challenge study, as well as efficacy of about 74–77% against Plasmodium falciparum infection in African children following three doses. For seasonal influenza, Novavax’s Matrix-M–adjuvanted recombinant hemagglutinin nanoparticle vaccine (qNIV) elicited broader immunity than standard vaccines: in preclinical ferrets, trivalent HA nanoparticles with Matrix-M generated higher hemagglutination-inhibition and microneutralization titers against homologous. They antigenically drifted A(H3N2) and A(H1N1) strains. In a human Phase 2 trial, Matrix-M–adjuvanted quadrivalent HA nanoparticles produced significantly higher and broader HAI antibody responses and much stronger multifunctional (IFN-γ/TNF-α/IL-2 secreting) CD4+ T-cell responses to both vaccine-homologous and drifted A(H3N2) strains than either unadjuvanted HA nanoparticles or high-dose conventional influenza vaccine. In aggregate, these findings demonstrate that Matrix-M enhances protective immune breadth and potency for diverse vaccine targets (COVID-19, malaria, influenza, and other pathogens) compared to unadjuvanted or traditional formulations [64,65]. The following is a breakdown of the different types of adjuvants that have been developed and are currently in use (
Fig. 2).
4. Drug Delivery Systems (DDS)
Drug delivery systems (DDS) represent a relatively new technological paradigm for formulating and storing drug molecules in various forms for targeted delivery. These systems are designed to expedite the arrival of therapeutic molecules to specific body systems, thereby maximizing their efficacy while minimizing their accumulation in unintended areas. DDS has significantly improved patient outcomes across various diseases by enhancing pharmacological control within systemic circulation, leading to the concept of controlled release. Recent advancements in pharmacology and pharmacokinetics have underscored the importance of drug release kinetics in determining therapeutic efficacy.
Over the past few decades, extensive research has led to the development of diverse DDS utilizing advanced systems for more controlled, selective, and simplified release. Each DDS possesses unique physical, chemical, and morphological characteristics that dictate its release rate and mechanism, influencing its affinity and pharmacological efficacy. The evolution of controlled-release drug formulations began in the 1950s with the advent of sustained-release technology, which enabled drug delivery for up to 12 hours, with an initial dose followed by gradual release. By the 1980s, oral and transdermal formulations providing therapeutic durations of up to 24 hours for small molecules became highly relevant. Since then, DDSs have garnered considerable attention due to their significant advantages over conventional drugs, offering predetermined release rates over specific periods, unaffected by physiological conditions, and capable of lasting from days to years [66].
A pivotal development in this field, particularly for vaccine delivery, was the timely emergence of lipid nanoparticle (LNP) formulations for COVID-19 vaccines in 2020 [67]. This breakthrough was the culmination of extensive work in nanomedicine, including the development of formulations designed to escape endosomes and the creation of albumin nanoparticles. This represents a fundamental reorientation of vaccine research and development, where the delivery mechanism has become as, if not more, critical than the antigen itself, particularly for novel nucleic acid vaccines. This shift implies that the primary bottleneck in vaccine development has moved from antigen discovery to creating effective and safe delivery platforms. Future breakthroughs will heavily depend on innovations in materials science, nanotechnology, and bioengineering to develop sophisticated carriers that optimize antigen presentation and immune response.
Recent advancements have shifted vaccine development from traditional approaches toward innovative, technology-driven platforms that prioritize speed, specificity, and enhanced delivery. A notable trend is the rise of nucleic acid–based vaccines, especially those utilizing mRNA. Emerging mRNA vaccines have demonstrated remarkably rapid development timelines and adaptability during global health emergencies. This approach significantly benefits from the use of LNP delivery systems, which protect the mRNA from degradation, enhance cellular uptake, and facilitate robust antigen expression. Further refinements in codon optimization and synthetic biology tools, such as CRISPR-Cas9, have improved the scalability of mRNA design and production, transforming the methodology of vaccine development [67,68,69].
At the same time, there is a strong focus on next-generation delivery systems that integrate nanotechnology with novel administration routes. Polymer-based carriers, such as polymersomes, are being engineered for enhanced stability and targeted delivery, while cell-penetrating peptides (CPPs) are being fine-tuned to facilitate the efficient transport of antigens into cells [70,71]. Beyond these platforms, alternative administration strategies, including microneedle patches, intranasal sprays, and transdermal routes, aim to elicit systemic and mucosal immunity, reducing reliance on traditional intravenous or intramuscular injections. These innovative vaccine platforms are specifically engineered to overcome challenges such as maintaining cold-chain logistics and addressing constraints faced by resource-limited countries.
Furthermore, the industry is witnessing a push toward personalized and therapeutic vaccines that leverage genomic and proteomic insights to tailor immunizations for specific diseases, including various cancers and chronic infections. This customized approach aligns with broader trends in precision medicine, where vaccine formulations can be adapted to individual patient profiles. Digital innovations are also making an impact, with remote patient monitoring, digital health applications, and blockchain technologies being integrated into vaccine rollouts to enhance real-time tracking of immune responses, monitor adverse effects, and improve global vaccine distribution logistics. Collectively, these trends reflect a convergence of biotechnology, nanotechnology, and digital innovation. By combining advanced adjuvant strategies with novel delivery vehicles, the industry is positioned to systematically address emerging pathogens and customize vaccines for improved efficacy and safety across diverse populations. Moreover, advanced DDS offers multifaceted benefits that extend beyond mere delivery. They protect antigens, enhance cellular uptake, facilitate endosomal escape, and even mimic adjuvant-like behavior by activating antigen-presenting cells. These systems also address practical challenges, such as cold-chain logistics, and enable alternative administration routes [72,73]. This holistic approach means that a single DDS platform can solve multiple problems in vaccine development, making them indispensable for rapid response and global deployment.
5. Nanoparticle-Based Systems
Nanotechnology, defined as the manipulation of matter at a scale of 1 to 100 nanometers, has experienced exponential growth over the past four decades. Its impact has been transformative across various fields, from medicine to the food industry, and its global economic influence is projected to be substantial. This field has enabled significant advancements, including extending the shelf life of food, enhancing the intracellular delivery of hydrophobic drugs, and increasing the efficacy of specific therapies, such as anticancer agents. The nature of nanoparticles presents an inherent duality: the same properties that make them revolutionary also carry potential risks. The ability to manipulate matter at this scale offers unprecedented benefits, but simultaneously introduces concerns about potentially toxic biological effects and the possible consequences of continuous exposure. This intrinsic relationship between promise and peril underscores the critical need for a balanced approach in their research and development, where safety must be a primary consideration from the initial stages of design and application [74].
5.1. Key Industrial Applications of Nanotechnology
The versatility of nanotechnology is validated by its cross-sector adoption, demonstrating its ability to enhance properties that conventional materials cannot match. Nanotechnology has profoundly influenced disease treatment in nanomedicine, primarily through the development of advanced drug delivery systems (DDSs). Nanoparticles act as carriers targeting specific cells or tissues, increasing drug efficacy and reducing side effects, and can be designed for controlled release. They are also used as contrast agents in medical imaging (iron oxide for magnetic resonance imaging, silica for computed tomography), offering advantages over radiation-based techniques [75,76,77].
5.2. Fundamental Physicochemical Properties of Nanoparticles
Nanoparticles possess unique properties that grant them superior reactivity in biological environments compared to larger particles, a fundamental principle in the design of nanomedicine. Size and surface area are critical. Their small size (1-100 nm) provides a high surface-to-volume ratio, enhancing their reactivity and enabling them to easily infiltrate tissues and bodily fluids. Size influences endocytosis, distribution, retention, and elimination. For example, nanoparticles smaller than 200 nm are internalized by clathrin-coated vesicles. Size also determines subcellular localization, such as 2.4 nm AuNP localizing in the nucleus, and their ability to cross biological barriers like the blood-brain barrier (BBB) [78].
Surface chemistry plays a crucial role in determining reactivity and function. Surface modification, such as coating AuNP and DNA with lipid layers, enhances permeability and cellular uptake. PEGylation of liposomes enhances bioavailability by evading immune detection, while conjugation with ligands (e.g., folate, monoclonal antibodies) facilitates the selective targeting of cells, such as cancer cells. Surface chemistry also enables environment-activated drug release, such as in response to acidic pH in tumors [78,79].
The shape of a nanoparticle is vital for its biological function. Spherical nanoparticles are generally more easily endocytosed than rod or tube-shaped ones due to their interaction with the cell membrane. However, long nanorods can offer prolonged bioavailability and greater encapsulation capacity. Shape also influences the endocytosis pathway (clathrin-mediated vs. clathrin-independent). This tunability of physicochemical properties suggests that the future of nanomedicine is geared towards precision design, where nanoparticles are tailored to specific therapeutic challenges [80].
5.3. Nanoparticle-Based Drug Delivery Systems (DSSs) in Disease Treatment
Nanoparticle DSSs address the limitations of conventional drugs, such as low bioavailability and systemic toxicity, enabling targeted and more efficient delivery. Nanoparticles directly address fundamental pharmacological challenges, such as the poor aqueous solubility of 70% of globally synthesized drugs—this positions nanomedicine as a disruptive technology capable of unlocking the therapeutic potential of numerous drug candidates.
Several types of DSSs exist:
5.3.1. Lipid-Based
Micelles (5-50 nm) transport hydrophobic drugs, while liposomes (10 nm-several microns) encapsulate hydrophobic and hydrophilic drugs. Liposomes play a vital role in cancer treatment due to the "enhanced permeability and retention effect" (EPR), which enables their selective accumulation in tumors. PEGylation prolongs their half-life, and ligand conjugation enables active targeting. Lipid nanoparticles (LNPs) are effective for nucleic acid-based therapies (e.g., CRISPR-Cas9) [81].
5.3.2. Polymeric
These materials include PEG, chitosan, PLGA, and PLA. PEG is the most commercially available option and is engineered to release drugs in response to environmental triggers, such as pH levels and reactive oxygen species (ROS), found within the tumor microenvironment [82].
5.3.3. Peptide
Linear or cyclic peptides act as ligands for cell surface receptors, facilitating targeted drug delivery.
5.3.4. Inorganic
These include dendrimers, silica, magnetic, and gold. Dendrimers can encapsulate large amounts of drugs for controlled release, while silica and magnetic nanoparticles can be functionalized for targeted delivery. Gold nanoparticles possess optical properties that enable imaging and photothermal therapy. The paradigm of smart drug delivery, where DSSs respond to stimuli or actively target, represents a fundamental shift towards controlling drug release and localization.
Encapsulation in nanocarriers reduces the systemic toxicity of cytotoxic drugs, such as doxorubicin. Liposomal incorporation of AgNP, for example, has been shown to mitigate inflammation and improve cytotoxicity at lower concentrations, serving as a model for reducing the toxicity of other drugs.
5.4. Nanoparticle Cytotoxicity and Safety Considerations
The widespread use of nanotechnology has raised concerns about health and environmental risks, leading to the development of the field of nanotoxicology. The very properties that make nanoparticles pharmacologically beneficial can also cause their toxicity. This reveals an inherent trade-off between efficacy and safety: optimizing cellular uptake and reactivity can simultaneously increase the risk of unintended cellular damage [83].
Mechanisms of cytotoxicity include infiltration into tissues and cells, where they can disrupt crucial cellular functions. A key mechanism is the rupture of subcellular membranes and the overproduction of ROS, which induces oxidative stress, DNA damage, and cell death.
Physicochemical properties influence toxicity. Cytotoxicity is size-dependent; smaller nanoparticles are more effective at cellular entry, which can increase their toxic impact. Surface modifications can increase cytotoxicity; charged nanoparticles are often more cytotoxic, although PEGylation can decrease cellular uptake and toxicity. A higher aspect ratio (e.g., nano-rods) correlates with increased cytotoxicity due to reduced clearance and increased bioavailability, potentially inducing macrophage cell death and promoting cancer development, similar to asbestos fibers.
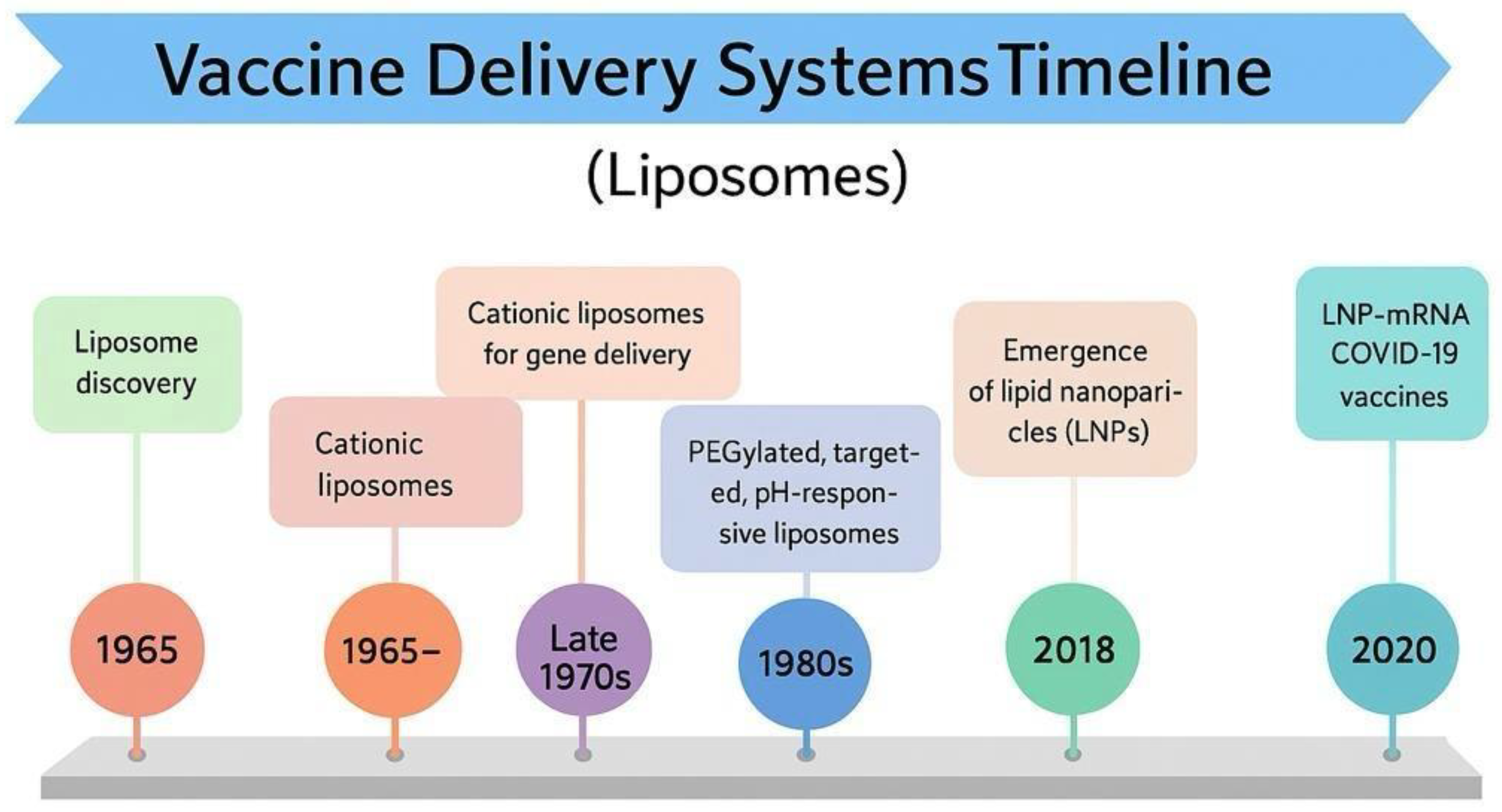
6. Liposomes
Liposomes are engineered, self-assembled phospholipid vesicles that have been extensively explored for drug delivery due to their unique ability to encapsulate hydrophilic and lipophilic molecules. Their bilayer membrane, typically composed of glycerophospholipids and stabilized by cholesterol, protects encapsulated drugs against degradation and offers a controlled release profile. Liposomes have evolved from basic laboratory curiosities into clinically approved products over several decades since their initial characterization (Fig. 3). Their advantageous attributes, including enhanced site targeting through passive accumulation via the enhanced permeability and retention (EPR) effect, as well as the capacity for active targeting by surface modification with ligands, have led to improved therapeutic benefits and reduced toxicity compared to conventional formulations [84].
The clinical translation of liposomal drug products is evidenced by the approval of multiple formulations by regulatory agencies such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA). Notable products, such as Doxil, AmBisome, and Vyxeos, have demonstrated the practical benefits of liposomes in oncology and infectious disease treatment [85]. The design of these products highlights the importance of careful lipid selection and the integration of components, such as polyethylene glycol (PEG) conjugates. PEGylation, for example, creates a hydration layer that reduces uptake by the mononuclear phagocyte system (MPS) and prolongs circulation time. This stealth characteristic is balanced against the potential reduction in cell uptake that may occur with excessively dense or high–molecular–weight coatings.
Manufacturing processes represent another cornerstone in the development of liposomal formulations. Traditional methods, such as the thin-film hydration technique, enable the initial formation of either multilamellar or unilamellar vesicles, which can be downsized through extrusion, high-pressure homogenization, ultrasonication, or a combination of these techniques. More sophisticated approaches, including double emulsification and solvent injection (often employing water-miscible solvents such as ethanol), offer alternative routes for the controlled production of liposomes with narrow size distributions. These processing techniques have a profound impact on the structural properties of liposomes, including lamellarity, particle size, and polydispersity index. For instance, drug-loading efficiency in products such as Doxil is achieved through active loading by establishing a transmembrane ammonium sulfate gradient. This technique forces the cytotoxic drug into the internal aqueous compartment, precipitating in a stabilized complex, thereby reducing systemic exposure and improving tolerability.
A critical theme that emerges across studies is the pivotal role of formulation parameters in determining the in vivo behavior of liposomal products. Particle size and surface characteristics are especially influential; smaller particle sizes (<200 nm) typically result in reduced opsonization and prolonged circulation, while larger vesicles may be more readily taken up by macrophages, which can be beneficial or detrimental depending on the therapeutic objective. Equally important is the phase transition temperature of the lipid bilayer, which governs membrane fluidity and controls the rates and stability of drug release during storage. A well-designed liposomal system must maintain a phase state that minimizes premature drug leakage yet allows for efficient release upon reaching the target tissue [85,86].
Beyond the design and production of liposomal formulations, regulatory considerations are also a key focus. The complexity of liposomal products necessitates a comprehensive understanding of critical quality attributes (CQAs), such as lamellar structure, zeta potential, encapsulation efficiency, and release kinetics. Regulatory guidelines have been developed to address these unique factors over the past years. Agencies emphasize the importance of robust characterization and reproducibility throughout scale-up processes. Variations in lipid source, processing conditions, or even minor changes in formulation can lead to significant alterations in pharmacokinetics or even unexpected toxicities. Therefore, establishing standardized manufacturing processes and implementing stringent quality controls is crucial for ensuring the safety and efficacy of these complex drug delivery systems.
Looking ahead, the field of liposomal drug delivery is poised for further innovation. Current research is expanding the functional landscape of liposomes by integrating active targeting ligands and stimuli-sensitive components that respond to external triggers such as pH, temperature, or enzyme activity. These “smart” liposomal systems promise to release their payloads more precisely at the target site, thereby maximizing therapeutic outcomes while minimizing off-target effects. Although challenges such as batch-to-batch consistency, scalable manufacturing, and in vivo stability remain, the continuing evolution in both design and regulatory science suggests that more sophisticated liposomal products will soon reach clinical use (
Fig.3).
The COVID-19 pandemic has highlighted the crucial need for comprehensive immunization and has prompted a significant transformation in vaccine production, facilitated by advancements in drug delivery technologies. Decades of meticulous research into the encapsulation and targeted delivery of macromolecules have led to the successful development of mRNA vaccines that utilize lipid nanoparticles (LNPs) as their core delivery vehicles. These technologies have enabled the rapid translation of laboratory discoveries into scalable manufacturing processes, revolutionizing the formulation, production, and deployment of vaccines on a global scale. Liposomes, as a well-established and ever-evolving platform with proven clinical effectiveness, demonstrate how a mature technology can drive revolutionary advancements. Their long history and multiple regulatory approvals underscore their foundational strength. This established technology then became the core delivery vehicle for mRNA vaccines during the COVID-19 pandemic, leading to a radical transformation in vaccine production. This progression illustrates that sustained, incremental advancements in a foundational drug delivery technology can suddenly enable revolutionary breakthroughs when combined with other scientific progress, such as in mRNA biology. This highlights the long-term value of investing in fundamental drug delivery research, which can yield unexpected, high-impact applications.
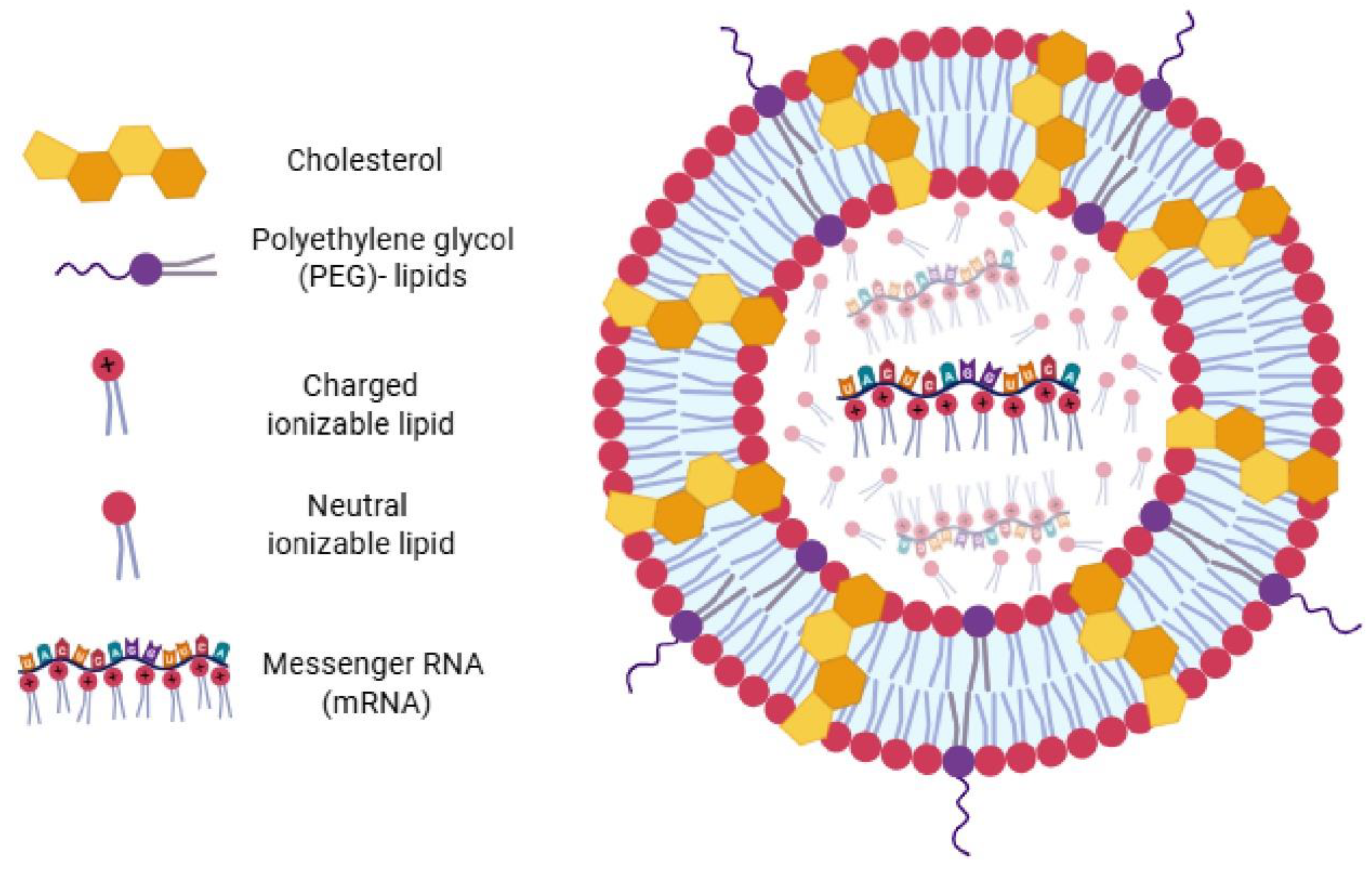
6.1. Lipid Nanoparticles (LNPs) and the mRNA Vaccine Era
The emergence of messenger RNA (mRNA) based vaccines represents one of the most significant breakthroughs in modern vaccinology, profoundly emphasizing the critical importance of delivery systems. Lipid nanoparticles (LNPs) have been the cornerstone of this revolution, serving as indispensable delivery vehicles for mRNA vaccines, particularly during the COVID-19 pandemic [88].
LNPs perform several vital functions: they protect mRNA from enzymatic degradation, dramatically improve its uptake by cells, and facilitate robust antigen expression. Their development is the culmination of extensive work in nanomedicine, including formulations engineered to escape endosomes—a critical step for mRNA release into the cytoplasm. The COVID-19 pandemic served as a powerful catalyst, accelerating research and development in these technologies and enabling the rapid translation of laboratory discoveries to global manufacturing scale (
Fig. 4).
This success has fundamentally reshaped vaccine research and development, where the delivery mechanism has become as, if not more, critical than the antigen itself, especially for nucleic acid vaccines. This shift implies that the primary challenge in vaccine development has moved from discovering antigens to creating effective and safe delivery platforms. Future vaccine innovations will heavily rely on advancements in materials science, nanotechnology, and bioengineering, fostering greater interdisciplinary collaboration. Furthermore, advanced DDS, such as LNPs, offer multifaceted benefits beyond mere delivery; they protect antigens, enhance cellular uptake, facilitate endosomal escape, and even exhibit adjuvant-like behavior by activating antigen-presenting cells [89]. This inherent sophistication in LNP design, combining protection, targeted delivery, and immune co-stimulation, is what makes them so potent and revolutionary for mRNA, reducing or enhancing the need for separate adjuvants.
The evolution of vaccine delivery systems, from the pioneering liposomes to the groundbreaking lipid nanoparticles for mRNA, showcases a remarkable scientific synergy. Foundational research into liposomes laid the groundwork for the design and optimization of LNPs, demonstrating how a mature platform can underpin disruptive innovations. The rapid deployment of mRNA/LNP vaccines during the COVID-19 pandemic was not accidental but the direct result of decades of meticulous research into macromolecule encapsulation and targeted delivery. The pandemic did not create this technology but rather accelerated its validation and scalability, proving the resilience and adaptability of pharmaceutical science. This event not only validated the technology's efficacy but also served as an unprecedented catalyst for its swift clinical translation and global deployment. This progression underscores the pivotal role of delivery systems in modern vaccinology, where the ability to protect, target, and optimize antigen presentation is as crucial as the antigen itself. The ongoing convergence of biotechnology, nanotechnology, and pharmaceutical engineering promises a new era of more effective, safer, and adaptable vaccines to meet global health challenges.
7. ISCOMs and ISCOMATRIX
Immunostimulatory complexes (ISCOMs) are advanced particulate vaccine delivery vehicles that integrate antigens with cholesterol, phospholipids, and saponins to form distinctive, cage-like nanoparticles, typically around 40 nm in size. This uniquely assembled structure encapsulates hydrophobic antigens and functions as a sustained-release system, ensuring prolonged antigen bioavailability and enhanced uptake by antigen-presenting cells [90].
One of the most significant advantages of ISCOMs lies in their dual role as both antigen carriers and inherent adjuvants. By mimicking the size and structure of pathogens, these complexes facilitate efficient targeting by dendritic cells and macrophages, thereby promoting robust activation of both humoral and cellular immune responses. This targeted delivery system bridges innate and adaptive immunity, transforming how antigens are presented to the immune system and ultimately boosting vaccine efficacy [90,91].
ISCOMs have demonstrated versatility across multiple routes of administration, with their efficacy highlighted in both oral and parenteral vaccine formulations. For example, studies integrating segments of the influenza virus or cholera toxin into ISCOMs have demonstrated a significant increase in immunogenicity, accompanied by notable enhancements in serum-neutralizing antibodies. Early research on respiratory syncytial virus vaccines revealed that ISCOM formulations could induce strong T-cell responses, in addition to antibody production, in animal models.
An offshoot of this platform, ISCOMATRIX, employs a similar lipid-based framework but is formulated without a pre-incorporated antigen.1 This flexibility allows the antigen to be mixed in during vaccine preparation, providing additional control over formulation and dosage while still harnessing the powerful delivery and adjuvant properties of the ISCOM system. Compared to traditional carrier systems, both ISCOM and ISCOMATRIX show superior performance in antigen presentation and in stimulating a comprehensive immune response with lower antigen and adjuvant requirements.
Beyond influenza, ISCOM-based delivery systems have been evaluated in a variety of vaccine candidates, including those targeting human papillomavirus (HPV), human immunodeficiency virus (HIV), herpes simplex virus (HSV), hepatitis C virus (HCV), and even in cancer immunotherapy. These studies have consistently demonstrated balanced cellular and humoral responses, along with a favorable safety profile, in both preclinical evaluations and clinical trials. Although some concerns persist regarding the toxicity of specific saponins at high concentrations, more refined molecules, such as QS-21 and QuilA, be safe at effective doses.
The strength of ISCOMs lies in their elegant design as delivery systems that protect and slowly release antigens, while actively enhancing immune stimulation. Their promising performance in preclinical and clinical settings continues to pave the way for next-generation vaccine formulations that are both potent and safe. ISCOMs represent a pinnacle of rational vaccine design, where the physical structure of the delivery vehicle itself is engineered to possess intrinsic adjuvant properties and optimize antigen presentation. This model provides a blueprint for future generations of vaccines that aim for highly potent and balanced immune responses with minimal antigen and adjuvant requirements [90,92].
8. Exosomes
Exosomes are vesicle-like structures, identified in the 1980s, with diameters ranging from 40 to 1000 nm. They mediate intercellular communications through their ability to transfer exogenous substances, such as proteins, mRNAs, microRNAs (miRNAs), and lipids, from a donor cell to recipient cells. These naturally equipped nanotransporters have been explored for drug delivery due to their inherently higher biocompatibility and lower toxicity than synthetic drug transporters [93].
Depending on various conditions, exosomes secreted by immune cells can induce immune stimulation or inhibition. Dendritic cell exosomes, for instance, have been studied in vesicle-based vaccination strategies for cancer and infectious diseases. A potential advantage of these vaccines is their capacity to induce Th1-type responses and cell-mediated immunity, which are crucial for combating viral and bacterial infections. Investigations involving exosomes carrying mycobacterial antigens have demonstrated the induction of a Th1-type reaction against M. tuberculosis in mice. Notably, these mycobacterial antigen-bearing exosomes were superior to protein subunit-based vaccines, which preferentially induced Th2-type responses and antibody-mediated immunity in inhibiting the growth of M. tuberculosis in the mouse lung [95,96].
Similarly, exosomes released from antigen-pulsed dendritic cells have been investigated as a cell-free nanoscale vaccine in cancer immunotherapy. Natural killer (NK) cells have also been utilized to produce exosomes with anti-tumor effects, achieved by encapsulating cellular perforin and granzymes during the extracellular vesicle biogenesis process. Currently, clinical trials are evaluating the efficacy of different exosome therapies for colon and pancreatic cancer (Phase 1) and small cell lung cancer (Phase 2) [97,98]. Despite these promising developments, exosomes still face significant challenges, including the need for stable mass production, efficient isolation and purification, effective surface modification, and optimized storage conditions. Exploring exosomes for drug delivery highlights the promise and unique challenges associated with biologically derived DDS. Exosomes are presented as naturally equipped nanotransporters with higher biocompatibility and lower toxicity than synthetic drug transporters, leveraging natural intercellular communication mechanisms. However, the text immediately points out that exosomes still face many challenges, including the stable production of their mass, isolation and purification, surface modification, and storage conditions. This illustrates that while synthetic DDS offer control and scalability, biologically derived systems, such as exosomes, offer unparalleled biocompatibility and inherent targeting capabilities. The central challenge lies in translating these natural advantages into scalable, consistent, and cost-effective therapeutic platforms. This area represents a crucial frontier for future research, focusing on bridging the gap between biological complexity and pharmaceutical engineering.
9. Drug Delivery Routes
The development of new and more effective therapeutic alternatives is driven by the need to overcome limitations in administration routes, rapid elimination, and the inability to cross biological barriers for various diseases, including multiple sclerosis, inflammatory bowel disease, and pulmonary fibrosis.1 Different strategies employing DDS and nanoparticles have been explored for these conditions. Various delivery routes are currently utilized in DDS, each with advantages and disadvantages. The selection of a drug delivery route constitutes a strategic decision that extends beyond mere convenience. This choice plays a significant role in shaping the type of immune response elicited and impacting the logistical considerations associated with deployment. For instance, the intranasal or oral routes can elicit "strong mucosal immunity in addition to systemic responses," which is "especially valuable when targeting respiratory pathogens." Similarly, microneedle patches offer "minimally invasive" delivery and can reduce the need for "cold-chain storage". This understanding indicates that future vaccine strategies will increasingly consider the optimal delivery route based on the pathogen's entry point, the desired immune effector mechanism (e.g., mucosal for respiratory viruses), and logistical considerations for global distribution, particularly in resource-limited settings. This marks a move beyond the traditional intramuscular injection as the default, towards a more nuanced approach to vaccine administration [99].
9.1. Oral
The oral route of administration is the most acceptable and commonly used for conventional drugs, and it is often preferred for chronic patients. The pathophysiological environment of the gastrointestinal (GI) tract plays a crucial role in drug absorption and bioavailability. Recent developments in biomacromolecule drugs have spurred research into alternative drug delivery methods to prevent degradation within the digestive tract [100]. Promising results have been observed with DDS such as yeast-based β-glucan and mucoadhesive polymeric systems. Chitosans, a mucoadhesive carrier for DDS, have been investigated for various targets within the GI system, including their use in mycosphere bases and even in nanoparticles as a DNA vaccine against diseases caused by Aeromonas hydrophila in fish [101,102]. While nanoparticles, microspheres, and chemical modifications constitute the primary delivery methods for biomacromolecules, there are few documented success stories in humans, indicating ongoing research is essential to develop effective nanotransporters capable of overcoming the transmucosal barrier and facilitating epithelial absorption. |
9.2. Transdermal
Transdermal drug delivery systems offer several advantages over oral and intravenous routes of administration. They are less invasive, bypass first-pass metabolism, are easy to apply and administer without requiring expert personnel, and have the potential to reduce the frequency of administration. As technology and research have advanced, numerous potential application areas have been explored, including transdermal microneedle patches for insulin delivery, and transdermal patches of propranolol, clonidine, and nitroglycerin for cardiovascular diseases, as well as for central nervous system disorders, hormone deficiencies, and contraception. Transdermal microneedle patches have also been developed for vaccine delivery, with promising results demonstrated in mice for the smallpox vaccine. Similarly, transdermal microneedle patches for the influenza virus in mice have shown more efficient clearance of the lung virus and improved cellular recall responses after vaccination [103,104}.
9.3. Subcutaneous
The subcutaneous route is typically used for drugs that are more concentrated and absorbed into the systemic circulation more slowly, primarily through the lymphatic system, distinguishing it from the intravenous route. Immunogenicity via this route has been studied with vaccines. However, ongoing research continues to investigate the immune response triggered by antibodies specifically designed to mimic human proteins, such as human monoclonal antibodies (mAb) and endogenous replacement products that do not contain adjuvants. The subcutaneous route has also proven to be a safe method for administering high-dose biologics, such as using a purified recombinant human form of hyaluronidase, which is both practical and safe. This route has gained prominence for mAb administration over the last decade, with 60% of monoclonal antibodies approved by the FDA in 2017 being administered subcutaneously [105,106].
9.4. Intravenous
It is a method that ensures a high concentration of the drug enters the bloodstream quickly and directly, bypassing the gastrointestinal tract, thereby offering high bioavailability and a faster ability to overcome physiological barriers to drug absorption. Several investigations involve new intravenous drugs that utilize viral vectors to express tumor suppressor genes. However, due to systemic immune responses, these are often limited to intratumoral administration or a single intravenous dose. However, short drug duration and a lack of standardization are the main limitations encountered by experts utilizing this pathway [107]
9. Conclusions
Adjuvants remain essential in enhancing the efficacy of vaccines, bridging the gap between antigen recognition and a robust, long-lasting immune response. While aluminum-based adjuvants have traditionally dominated human vaccine formulations due to their exceptional safety profile and ability to elicit strong humoral immunity, their limited capacity to induce cellular immune responses underscores the need for next-generation adjuvants.
Recent advances in immunology have catalyzed the development of novel adjuvants and delivery systems with enhanced immunomodulatory capabilities. Adjuvants can be broadly classified as immunostimulants such as TLR agonists, C-type lectin receptor (CLR) ligands, and cyclic GMP-AMP synthase–stimulator of interferon genes (cGAS–STING) activators and delivery systems that prolong antigen bioavailability, direct antigen presentation to antigen-presenting cells (APCs), and enable cross-presentation to CD8+ T cells.
Noteworthy innovations include squalene-based emulsions, such as MF59 and AS03, which generate local inflammatory environments conducive to effective immune priming. Rationally designed combinations, such as AS04 and AS01, which incorporate TLR agonists with aluminum salts or saponins, demonstrate synergistic effects that enhance both humoral and cellular responses. Matrix-M, a saponin-based nanoparticle system, exemplifies the new wave of delivery platforms that not only recruit and activate antigen-presenting cells (APCs) but also promote high-affinity antibody responses and potent CD4+ and CD8+ T cell immunity, all while maintaining a favorable safety profile.
The interplay between immunostimulants and delivery systems, amplified by advances in nanotechnology, is driving a paradigm shift in vaccinology. These components now function as both passive carriers and active immunomodulators, facilitating targeted and balanced immune activation. Moreover, emerging strategies such as sustained-release hydrogels, microneedle patches, and mucosal delivery routes are being investigated to extend antigen exposure and reduce reactogenicity.
Nevertheless, significant challenges persist. These include elucidating the precise mechanisms of classical adjuvants (such as the debated role of antigen depots in aluminum-based adjuvants), ensuring the safety and tolerability of novel formulations, and translating promising preclinical data into reproducible clinical outcomes.
In conclusion, the integration of advanced adjuvants and delivery systems heralds a new era in vaccine development, one characterized by tailored immunogenic profiles, broad and durable protection, and refined safety profiles. Future research must continue to refine these approaches to address diverse populations and pathogens, ultimately paving the way for next-generation vaccines that meet the complex challenges of modern public health.
Author Contributions
Conceptualization, A.H.G. and J.B.D.S.; investigation, A.H.G. and J.B.D.S.; resources, A.H.G. and J.B.D.S.; writing—original draft preparation, A.H.G.; writing—review and editing, A.H.G. and J.B.D.S.; supervision, A.H.G. and J.B.D.S.; project administration, A.H.G. and J.B.D.S.; funding acquisition, A.H.G. and J.B.D.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Fund for Science, Technology, and Innovation (FONACIT), an entity affiliated with the Ministry of Popular Power for Science and Technology of the Bolivarian Republic of Venezuela (MINCYT). JBDS was partially financed by the National Institute of Virology and Bacteriology [Program EXCELES, ID Project No. LX22NPO5103]—Funded by the European Union—Next Generation EU from the Ministry of Education, Youth, and Sports of the Czech Republic (MEYS).
Acknowledgements
The authors wish to thank Andrea García for figure design.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- Ghattas, M.; Dwivedi, G.; Lavertu, M.; Alameh, M.G. Vaccine Technologies and Platforms for Infectious Diseases: Current Progress, Challenges, and Opportunities. Vaccines (Basel). 2021 Dec 16;9(12):1490. [CrossRef]
- García, A.; De Sanctis, J.B. An overview of adjuvant formulations and delivery systems. APMIS. 2014 Apr;122(4):257-67. [CrossRef]
- Facciolà, A.; Visalli, G.; Laganà, A.; Di Pietro, A. An Overview of Vaccine Adjuvants: Current Evidence and Future Perspectives. Vaccines (Basel). 2022 May 22;10(5):819. [CrossRef]
- Seya, T.; Takeda, Y.; Matsumoto, M. A Toll-like receptor 3 (TLR3) agonist ARNAX for therapeutic immunotherapy. Adv Drug Deliv Rev. 2019 Jul; 147:37-43. [CrossRef]
- Kayraklioglu, N.; Horuluoglu, B.; Klinman, D.M. CpG Oligonucleotides as Vaccine Adjuvants. Methods Mol Biol. 2021; 2197:51-85. [CrossRef] [PubMed]
- Shen, Y.; Huang, W.; Nie, J.; Zhang, L. Progress Update on STING Agonists as Vaccine Adjuvants. Vaccines (Basel). 2025 Mar 31;13(4):371. [CrossRef]
- Angulo, M.; Ramos-Vega, A.; Angulo, C. Trained immunity-based Adjuvated vaccines (TIbAV) approach: β-glucans as example. Vaccine. 2025 May 31; 57:127240. [CrossRef]
- Martins, K.A.; Bavari, S.; Salazar, A.M. Vaccine adjuvant uses of poly-IC and derivatives. Expert Rev Vaccines. 2015 Mar;14(3):447-59. [CrossRef]
- Ong, G.H.; Lian, B.S.X.; Kawasaki, T.; Kawai, T. Exploration of Pattern Recognition Receptor Agonists as Candidate Adjuvants. Front Cell Infect Microbiol. 2021 Oct 6; 11:745016. [CrossRef]
- Zhao, T.; Cai, Y.; Jiang, Y.; He, X.; Wei, Y.; Yu, Y.; Tian, X. Vaccine adjuvants: mechanisms and platforms. Sig Transduct Target Ther 2023; 8, 283. [CrossRef]
- Reed, S.; Orr, M.; Fox, C. Key roles of adjuvants in modern vaccines. Nat Med 2013; 19, 1597–1608. [CrossRef]
- Wang, G.; Wang, Y.; Ma, F. Exploiting bacterial-origin immunostimulants for improved vaccination and immunotherapy: current insights and future directions. Cell Biosci 2024; 14, 24. [CrossRef]
- Lee, S.M.; Kim, P.; You, J.; Kim, EH. Role of Damage-Associated Molecular Pattern/Cell Death Pathways in Vaccine-Induced Immunity. Viruses. 2021 Nov 23;13(12):2340. [CrossRef]
- Donnelly, RF. Vaccine delivery systems. Hum Vaccin Immunother. 2017 Jan 2;13(1):17-18. [CrossRef]
- Yan, Y.; Yao, D.; Li, X. Immunological Mechanism and Clinical Application of PAMP Adjuvants. Recent Pat Anticancer Drug Discov. 2021;16(1):30-43. [CrossRef]
- Jentho, E.; Weis, S. DAMPs and Innate Immune Training. Front Immunol. 2021 Oct 22; 12:699563. [CrossRef]
- De Becker, G.; Moulin, V.; Pajak, B.; Bruck, C.; Francotte, M.; Thiriart, C.; Urbain, J.; Moser, M. The adjuvant monophosphoryl lipid A increases the function of antigen-presenting cells. Int Immunol. 2000 Jun;12(6):807-15. [CrossRef]
- Li, D.; Wu, M. Pattern recognition receptors in health and diseases. Signal Transduct Target Ther. 2021 Aug 4;6(1):291. [CrossRef]
- Carroll, S.L.; Pasare, C.; Barton, G.M. Control of adaptive immunity by pattern recognition receptors. Immunity. 2024 Apr 9;57(4):632-648. [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front Immunol. 2022 Mar 3; 13:812774. [CrossRef]
- Almeida-da-Silva, C.L.C.; Savio, L.E.B.; Coutinho-Silva, R.; Ojcius, DM. The role of NOD-like receptors in innate immunity. Front Immunol. 2023 Mar 15; 14:1122586. [CrossRef]
- Zheng, J.; Shi, W.; Yang, Z.; Chen, J.; Qi, A.; Yang, Y.; Deng, Y.; Yang, D.; Song, N.; Song, B.; Luo, D. RIG-I-like receptors: Molecular mechanism of activation and signaling. Adv Immunol. 2023; 158:1-74. [CrossRef]
- Scur, M.; Parsons, B.D.; Dey, S.; Makrigiannis, AP. The diverse roles of C-type lectin-like receptors in immunity. Front Immunol. 2023 Feb 27; 14:1126043. [CrossRef]
- Sharma, M.; Wagh, P.; Shinde, T.; Trimbake, D.; Tripathy, AS. Exploring the Role of Pattern Recognition Receptors as Immunostimulatory Molecules. Immun Inflamm Dis. 2025 May;13(5):e70150. [CrossRef]
- Chuenchor, W.; Jin, T.; Ravilious, G.; Xiao, T.S. Structures of pattern recognition receptors reveal molecular mechanisms of autoinhibition, ligand recognition and oligomerization. Curr Opin Immunol. 2014 Feb; 26:14-20. [CrossRef]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014 May 16;33(10):1104-16. [CrossRef]
- Blum, J.S.; Wearsch, P.A.; Cresswell, P. Pathways of antigen processing. Annu Rev Immunol. 2013; 31:443-73. [CrossRef]
- Pishesha, N.; Harmand, T.J.; Ploegh, H.L. A guide to antigen processing and presentation. Nat Rev Immunol. 2022 Dec;22(12):751-764. [CrossRef]
- Kaminska, P.; Tempes, A.; Scholz, E.; Malik, A.R. Cytokines on the way to secretion. Cytokine Growth Factor Rev. 2024 Oct;79:52-65. [CrossRef]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018 Aug;285(16):2944-2971. [CrossRef]
- McColl, S.R.. Chemokines and dendritic cells: a crucial alliance. Immunol Cell Biol. 2002 Oct;80(5):489-96. [CrossRef]
- Wang, R.; Lan, C.; Benlagha, K.; Camara, N.O.S.; Miller, H.; Kubo, M.; Heegaard, S.; Lee, P.; Yang, L.; Forsman, H.; Li, X.; Zhai, Z.; Liu, C. The interaction of innate immune and adaptive immune system. MedComm (2020). 2024 Sep 15;5(10):e714. [CrossRef]
- Villar, J.; Segura, E. Decoding the Heterogeneity of Human Dendritic Cell Subsets. Trends Immunol. 2020 Dec;41(12):1062-1071. [CrossRef]
- Ho, N.I.; Huis In 't Veld, L.G.M.; Raaijmakers, T.K.; Adema, G.J. Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front Immunol. 2018 Dec 13; 9:2874. [CrossRef]
- Mellman, I. Dendritic cells: master regulators of the immune response. Cancer Immunol Res. 2013 Sep;1(3):145-9. [CrossRef]
- Lee, K.W.; Yam, J.W.P.; Mao, X. Dendritic Cell Vaccines: A Shift from Conventional Approach to New Generations. Cells. 2023 Aug 25;12(17):2147. [CrossRef]
- Abdi, K.; Singh, N.J.; Matzinger, P. Lipopolysaccharide-activated dendritic cells: "exhausted" or alert and waiting? J Immunol. 2012 Jun 15;188(12):5981-9. [CrossRef]
- Behboudi, S.; Chao, D.; Klenerman, P.; Austyn, J. The effects of DNA containing CpG motif on dendritic cells. Immunology. 2000 Mar;99(3):361-6. [CrossRef]
- Segura E. Human dendritic cell subsets: An updated view of their ontogeny and functional specialization. Eur J Immunol. 2022 Nov;52(11):1759-1767. [CrossRef]
- Marrack, P., McKee, A. & Munks, M. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol 2009; 9, 287–293. [CrossRef]
- Verma, S.K.; Mahajan, P.; Singh, N.K.; Gupta, A.; Aggarwal, R.; Rappuoli, R.; Johri, A.K. New-age vaccine adjuvants, their development, and future perspective. Front Immunol. 2023 Feb 24; 14:1043109. [CrossRef]
- Danielsson, R.; Eriksson, H. Aluminium adjuvants in vaccines - A way to modulate the immune response. Semin Cell Dev Biol. 2021 Jul; 115:3-9. [CrossRef]
- Lan, J.; Feng, D.; He, X.; Zhang, Q.; Zhang, R. Basic Properties and Development Status of Aluminum Adjuvants Used for Vaccines. Vaccines (Basel). 2024 Oct 18;12(10):1187. [CrossRef]
- Laera, D.; HogenEsch, H.; O'Hagan, D.T. Aluminum Adjuvants-'Back to the Future'. Pharmaceutics. 2023 Jul 4;15(7):1884. [CrossRef]
- O'Hagan, D.T.; Ott, G.S.; Nest, G.V.; Rappuoli, R.; Giudice, G.D. The history of MF59(®) adjuvant: a phoenix that arose from the ashes. Expert Rev Vaccines. 2013 Jan;12(1):13-30. [CrossRef]
- O Murchu, E.; Comber, L.; Jordan, K.; Hawkshaw, S.; Marshall, L.; O'Neill, M.; et al. Systematic review of the efficacy, effectiveness and safety of MF59® adjuvanted seasonal influenza vaccines for the prevention of laboratory-confirmed influenza in individuals ≥18 years of age. Rev Med Virol. 2023 May;33(3):e2329. [CrossRef]
- Knuf, M.; Leroux-Roels, G.; Rümke, H.C.; Abarca, K.; Rivera, L., Lattanzi, M. et al. Safety and immunogenicity of an MF59-adjuvanted A/H1N1 pandemic influenza vaccine in children from three to seventeen years of age. Vaccine. 2015 Jan 1;33(1):174-81. [CrossRef]
- Garçon, N.; Vaughn, D.W.; Didierlaurent, A.M. Development and evaluation of AS03, an Adjuvant System containing α-tocopherol and squalene in an oil-in-water emulsion. Expert Rev Vaccines. 2012 Mar;11(3):349-66. [CrossRef]
- Morel, S.; Didierlaurent, A.; Bourguignon, P.; Delhaye, S.; Baras, B.; Jacob, V.; et al. Adjuvant system AS03, containing α-tocopherol, modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011 Mar 16;29(13):2461-73. [CrossRef]
- Grigoryan, L.; Feng, Y.; Bellusci, L.; Lai, L.; Wali, B.; Ellis, M.; et al. AS03 adjuvant enhances the magnitude, persistence, and clonal breadth of memory B cell responses to a plant-based COVID-19 vaccine in humans. Sci Immunol. 2024 Apr 5;9(94):eadi8039. [CrossRef]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009 Nov;183(10):6186-6197. [CrossRef]
- Romanowski, B.; Schwarz, T.F.; Ferguson, L.M.; Peters, K.; Dionne, M.; Schulze, K.; et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. 2011 Dec;7(12):1374-86. [CrossRef]
- Roman, F.; Burny, W.; Ceregido, M.A.; Laupèze, B.; Temmerman, S.T.; Warter, L.; Coccia, M. Adjuvant system AS01: from mode of action to effective vaccines. Expert Rev Vaccines. 2024 Jan-Dec;23(1):715-729. [CrossRef]
- Syed, Y.Y. Recombinant Zoster Vaccine (Shingrix®): A Review in Herpes Zoster. Drugs Aging. 2018 Dec;35(12):1031-1040. [CrossRef]
- Wang, Y.Q.; Bazin-Lee, H.; Evans, J.T.; Casella, C.R.; Mitchell, T.C. MPL Adjuvant Contains Competitive Antagonists of Human TLR4. Front Immunol. 2020 Oct 16; 11:577823. [CrossRef]
- Lacaille-Dubois, M.A. Updated insights into the mechanism of action and clinical profile of the immunoadjuvant QS-21: A review. Phytomedicine. 2019 Jul; 60:152905. [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev Vaccines. 2011 Apr;10(4):499-511. [CrossRef]
- Scheiermann, J.; Klinman, D.M. Clinical evaluation of CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases and cancer. Vaccine. 2014 nov 12;32(48):6377-89. [CrossRef]
- Shirota, H.; Klinman, DM. Recent progress concerning CpG DNA and its use as a vaccine adjuvant. Expert Rev Vaccines. 2014 Feb;13(2):299-312. [CrossRef]
- Takahashi, H.; Misato, K.; Aoshi, T.; Yamamoto, Y.; Kubota, Y.; Wu, X.; Kuroda, E.; Ishii, K.J.; Yamamoto, H.; Yoshioka, Y. Carbonate Apatite Nanoparticles Act as Potent Vaccine Adjuvant Delivery Vehicles by Enhancing Cytokine Production Induced by Encapsulated Cytosine-Phosphate-Guanine Oligodeoxynucleotides. Front Immunol. 2018 Apr 18;9:783. [CrossRef]
- Stertman, L.; Palm, A.E.; Zarnegar, B.; Carow, B.; Lunderius Andersson, C.; Magnusson, S.E.; Carnrot, C.; Shinde, V.; Smith, G.; Glenn, G.; Fries, L.; Lövgren Bengtsson, K. The Matrix-M™ adjuvant: A critical component of vaccines for the 21st century. Hum Vaccin Immunother. 2023 Dec 31;19(1):2189885. [CrossRef]
- Bengtsson, K.L.; Karlsson, K.H.; Magnusson, S.E.; Reimer, J.M.; Stertman, L. Matrix-M adjuvant: enhancing immune responses by 'setting the stage' for the antigen. Expert Rev Vaccines. 2013 Aug;12(8):821-3. [CrossRef]
- Heath, P.T.; Galiza, E.P.; Baxter, D.N.; Boffito, M.; Browne, D.; Burns, F, et al. Safety and Efficacy of the NVX-CoV2373 Coronavirus Disease 2019 Vaccine at Completion of the Placebo-Controlled Phase of a Randomized Controlled Trial. Clin Infect Dis. 2023 Feb 8;76(3):398-407. [CrossRef]
- Feehan, J.; Plebanski, M.; Apostolopoulos, V. Recent perspectives in clinical development of malaria vaccines. Nat Commun 2025; 16, 3565. [CrossRef]
- Genton, B. R21/Matrix-M™ malaria vaccine: a new tool to achieve WHO's goal to eliminate malaria in 30 countries by 2030? J Travel Med. 2023 Dec 28;30(8):taad140. [CrossRef]
- Park, H.; Otte, A.; Park, K. Evolution of drug delivery systems: From 1950 to 2020 and beyond. J Control Release. 2022 Feb; 342:53-65. [CrossRef]
- Felgner, J.; Hernandez-Davies, J.E.; Strahsburger, E.; Silzel, E.; Nakajima, R.; Jain, A.; Laster, J.; Chiang, J.L.; Tsai, Y.; Felgner, P.L.; Davies, D.H.; Liang, L. Lipid Nanoparticle Development for A Fluvid mRNA Vaccine Targeting Seasonal Influenza and SARS-CoV-2. npj Vaccines 2025; 10, 123, doi.org/10.1038/s41541-025-01153-6.
- Swetha, K.; Kotla, N.G.; Tunki, L.; Jayaraj, A.; Bhargava, S.K.; Hu, H.; Bonam, S.R.; Kurapati, R. Recent Advances in the Lipid Nanoparticle-Mediated Delivery of mRNA Vaccines. Vaccines (Basel). 2023 Mar 14;11(3):658. [CrossRef]
- Yang, F.; Aliyari, S.; Zhu, Z.; Zheng, H.; Cheng, G.; Zhang, S. CRISPR-Cas: a game-changer in vaccine development and the fight against viral infections. Trends Microbiol. 2025 Jun;33(6):650-664. [CrossRef]
- Zhang, W.; Liu, H.; Zhu, B.; Li, W.; Han, X.; Fu, J.; Luo, R.; Wang, H.; Wang, J. Advances in Cytosolic Delivery of Proteins: Approaches, Challenges, and Emerging Technologies. Chem Biodivers. 2025 Jun;22(6):e202401713. [CrossRef]
- Fonseca, M.; Jarak, I.; Victor, F.; Domingues, C.; Veiga, F.; Figueiras, A. Polymersomes as the Next Attractive Generation of Drug Delivery Systems: Definition, Synthesis and Applications. Materials (Basel). 2024 Jan 8;17(2):319. [CrossRef]
- Sinani, G.; Şenel, S. Advances in vaccine adjuvant development and future perspectives. Drug Deliv. 2025 Dec;32(1):2517137. [CrossRef]
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; Melo, K.M.T.B.; Leal, L.R.S.; Espinoza, B.C.F.; Invenção, M.D.C.V.; Pinho, S.S.; Freitas, A.C. Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives. Pharmaceutics. 2025 May 12;17(5):640. [CrossRef]
- Bezbaruah, R.; Chavda, V.P.; Nongrang, L.; Alom, S.; Deka, K.; Kalita, T.; Ali, F.; Bhattacharjee, B.; Vora, L. Nanoparticle-Based Delivery Systems for Vaccines. Vaccines (Basel). 2022 Nov 17;10(11):1946. [CrossRef]
- Cheng, X.; Xie, Q.; Sun, Y. Advances in nanomaterial-based targeted drug delivery systems. Front Bioeng Biotechnol. 2023 Apr 13;11:1177151. [CrossRef]
- Liu, Y.; Liang, Y.; Yuhong, J.; Xin, P.; Han, J.L.; Du, Y.; Yu, X.; Zhu, R.; Zhang, M.; Chen, W.; Ma, Y. Advances in Nanotechnology for Enhancing the Solubility and Bioavailability of Poorly Soluble Drugs. Drug Des Devel Ther. 2024 May 1;18:1469-1495. [CrossRef] [PubMed]
- Kurul, F.; Turkmen, H.; Cetin, A.E.; Topkay, S.D. Nanomedicine: How nanomaterials are transforming drug delivery, bio-imaging, and diagnosis. Next Nanotechnology 2025; 7: 100129. [CrossRef]
- Joseph, T.M.; Kar Mahapatra, D.; Esmaeili, A.; Piszczyk, Ł.; Hasanin, M.S.; Kattali, M.; Haponiuk, J.; Thomas, S. Nanoparticles: Taking a Unique Position in Medicine. Nanomaterials (Basel). 2023 Jan 31;13(3):574. [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater Med. 2020; 1:10-19. [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 2021; 20, 101–124. [CrossRef]
- Gandhi, S.; Shastri, D.H. Lipid-Based Nanoparticles as Drug Delivery System for Modern Therapeutics. Pharm Nanotechnol. 2024 Oct 11. [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int J Mol Sci. 2022 Feb 12;23(4):2034. [CrossRef]
- Qamar, W.; Gulia, S.; Athar, M.; Ahmad, R.; Imam, M.T.; Chandra, P.; Singh, B.P.; Haque, R.; Hassan, M.I.; Rahman, S. An insight into impact of nanomaterials toxicity on human health. Peer J. 2024 Sep 30;12:e17807. [CrossRef]
- Sobol, Ż.; Chiczewski, R.; Wątróbska-Świetlikowska, D. Advances in Liposomal Drug Delivery: Multidirectional Perspectives on Overcoming Biological Barriers. Pharmaceutics. 2025 Jul 5;17(7):885. [CrossRef]
- Bulbake, U.; Doppalapudi, S.; Kommineni, N.; Khan, W. Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 2017 Mar 27;9(2):12. [CrossRef]
- Kulkarni, S.A.; Feng, SS. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm Res 2013; 30, 2512–2522. [CrossRef]
- Wilson, B.; Geetha, K.M. Lipid nanoparticles in the development of mRNA vaccines for COVID-19. J Drug Deliv Sci Technol. 2022 Aug;74:103553. [CrossRef]
- Parvin, N.; Mandal, T.K.; Joo, S.W. The Impact of COVID-19 on RNA Therapeutics: A Surge in Lipid Nanoparticles and Alternative Delivery Systems. Pharmaceutics. 2024 Oct 25;16(11):1366. [CrossRef]
- Mochida, Y.; Uchida, S. mRNA vaccine designs for optimal adjuvanticity and delivery. RNA Biol. 2024 Jan;21(1):1-27. [CrossRef]
- Garcia, A.; Lema, D. An Updated Review of ISCOMSTM and ISCOMATRIXTM Vaccines. Curr Pharm Des. 2016;22(41):6294-6299. [CrossRef] [PubMed]
- Duewell, P.; Kisser, U.; Heckelsmiller, K.; Hoves, S.; Stoitzner, P.; Koernig, S.; Morelli, A.B.; Clausen, B.E.; Dauer, M.; Eigler, A.; Anz, D.; Bourquin, C.; Maraskovsky, E.; Endres, S.; Schnurr, M. ISCOMATRIX adjuvant combines immune activation with antigen delivery to dendritic cells in vivo leading to effective cross-priming of CD8+ T cells. J Immunol. 2011 Jul 1;187(1):55-63. [CrossRef]
- Sun, H.X.; Xie, Y.; Ye, Y.P. ISCOMs and ISCOMATRIX. Vaccine. 2009 Jul 16;27(33):4388-401. [CrossRef]
- Chen, Y.; Qi, W.; Wang, Z.; Niu, F. Exosome Source Matters: A Comprehensive Review from the Perspective of Diverse Cellular Origins. Pharmaceutics. 2025 Jan 22;17(2):147. [CrossRef]
- Lema, D.A.; Burlingham, W.J. Role of exosomes in tumour and transplant immune regulation. Scand J Immunol. 2019 Nov;90(5):e12807. [CrossRef]
- Cheng, Y.; Schorey, J.S. Exosomes carrying mycobacterial antigens can protect mice against Mycobacterium tuberculosis infection. Eur J Immunol. 2013 Dec;43(12):3279-90. [CrossRef]
- Sun, Y.F.; Pi, J.; Xu, J.F. Emerging Role of Exosomes in Tuberculosis: From Immunity Regulations to Vaccine and Immunotherapy. Front Immunol. 2021 Apr 1;12:628973. [CrossRef]
- El-Tanani, M.; Nsairat, H.; Matalka, I.I.; Aljabali, A.A.A.; Mishra, V.; Mishra, Y.; Naikoo, G.A.; Chava, S.R.; Charbe, N.B.; Tambuwala, M.M. Impact of exosome therapy on pancreatic cancer and its progression. Med Oncol. 2023 Jul 5;40(8):225. [CrossRef]
- Zhou, J.; Li, X.L.; Chen, Z.R.; Chng, W.J. Tumor-derived exosomes in colorectal cancer progression and their clinical applications. Oncotarget. 2017 Aug 10;8(59):100781-100790. [CrossRef]
- Bae, Y.H.; Park, K. Advanced drug delivery 2020 and beyond: Perspectives on the future. Adv Drug Deliv Rev. 2020; 158:4-16. [CrossRef]
- Rai, C.I.; Kuo, T.H.; Chen, Y.C. Novel Administration Routes, Delivery Vectors, and Application of Vaccines Based on Biotechnologies: A Review. Vaccines (Basel). 2024 Sep 1;12(9):1002. [CrossRef]
- Kumar, R.; Islam, T, Nurunnabi M. Mucoadhesive carriers for oral drug delivery. J Control Release. 2022 Nov;351:504-559. [CrossRef]
- Thirumalaikumar, E.; Lelin, C.; Sathishkumar, R.; Vimal, S.; Anand, S.B.; Babu, M.M.; Citarasu, T. Oral delivery of pVAX-OMP and pVAX-hly DNA vaccine using chitosan-tripolyphosphate (Cs-TPP) nanoparticles in Rohu, (Labeo rohita) for protection against Aeromonas hydrophila infection. Fish Shellfish Immunol. 2021 Aug;115:189-197. [CrossRef]
- Ita, K. Transdermal delivery of vaccines - Recent progress and critical issues. Biomed Pharmacother. 2016 Oct; 83:1080-1088. [CrossRef]
- Nguyen, H.X. Beyond the Needle: Innovative Microneedle-Based Transdermal Vaccination. Medicines (Basel). 2025 Feb 7;12(1):4. [CrossRef]
- McLennan, D.N.; Porter, C.J.; Charman, S.A. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov Today Technol. 2005 Spring;2(1):89-96. [CrossRef]
- Hamuro, L.; Kijanka, G.; Kinderman, F.; Kropshofer, H.; Bu, D.X.; Zepeda, M.; Jawa, V. Perspectives on Subcutaneous Route of Administration as an Immunogenicity Risk Factor for Therapeutic Proteins. J Pharm Sci. 2017 Oct;106(10):2946-2954. [CrossRef]
- Giuliano, K.K. Intravenous Smart Pumps: Usability Issues, Intravenous Medication Administration Error, and Patient Safety. Crit Care Nurs Clin North Am. 2018 Jun;30(2):215-224. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).