1. Introduction
Historical Aspects
Marble and gypsum alabaster have coexisted in European sculpture for centuries. Whereas the artistic usage of the first material had been solidly anchored in antique and early medieval tradition, the latter appeared in this role timidly around the 12th century. Gypsum alabaster then soon rivalled marble for economic, technical but also aesthetic and symbolic reasons. While the classical Greek marble deposits were situated beyond reach of European commissioners in the Ottoman empire (1299-1923), the only source of a high-quality white marble were the Tuscan deposits in the wider Carrara area, in Apuan Alps. After a decline during the early medieval period, they enjoyed an almost monopolistic position since the 14th century. [
1,
2].
Gypsum alabaster deposits suitable for sculpture were discovered and used progressively in England (mid 12th cent, Tudbury deposit, [
3]), Spain (early 14th cent. for Beuda albaster, [
4] and France (12th cent, alpine deposits of Notre-Dame-de-Mésage, [
5] and competed regionally, depending on political zones of influence and on fluvial and terrestrial transport networks [
6]. Beyond accessibility, this new material was highly appreciated for its sculptural qualities, its softness making it relatively easy to work in intricate details and its ability to take a high-quality polish [
7]. But each of the materials, marble and alabaster, acquired its own prestige, associated with its specific aesthetic and associated symbolic values. Whereas their parallel history is still to be written, their intimate entanglement led to permanent confusion and, from the 16th century onward, to attempts of clarification and distinction.
The confusion of marble and alabaster may have been unconscious, due to their visual similarity or voluntary, claiming the prestige of one material for the other [
8]. A striking example is the mandate of Margaret of Austria of 1510, concerning the sculptural decoration of the Brou monastery. This text contains one of the shortest shortcuts between both materials “white marble called alabaster” “
marbre blanc qu’on dit albastre”, speaking of the material found in the quarry of Saint-Lothain in her Burgundian possessions [
9]
The same formulation is used in the contract of 1511 between the sculptor Michel Colombe and Margaret: “the aforementioned Jean Lemaire has brought us a piece of alabaster marble from Saint-Lothain” “
ledit Jean Lemaire nous a apporté une pièce de Marbre d’albâtre de Saint-Loutein” ([
10], entry “Lothein”, p. 16). This is also a case of direct competition of both materials, as Michel Colombe specifies that the Saint-Lothain alabaster will be used “without going to look for other marbles in Italy or elsewhere, because the others do not take polish so well and do not keep their whiteness”
“sans aller cuérir austres marbres en Italie ny ailleurs ; car les autres ne se polissent point si bien et ne gardent point leur blancheur”. Carrara marble, promoted by other actors involved in Margaret’s project, was nevertheless imported from Pisa via Genoa and served for specific parts of the funeral monument [
11] showing a clear conscience of their respective physical and visual properties.
The historical confusion worked in both directions, alabaster sculpture claimed to be marble but also in the other way with marble described as alabaster [
12]. One example is found in the contract Charles d’Orléans made in 1409 with the sculptor Jehan de Thoirry for the tomb of his father Louis d’Orléans [
13] where he stipulates the use of “fine alabaster from Pisa”, which could refer to both Carrara marble or, less likely at this time, to Tuscan alabaster. On the contrary, Aldrovandi [
14] speaks in 1648 of “white marble quarried in Volterra”, obviously referring to the Tuscan alabaster quarries. Given the aforementioned visual proximity of the white varieties of both alabaster and marble and the inherited ascriptions, even present-day museum labels need thorough verification with non-destructive methods, preferably simple and inexpensive, which is the object of the present paper.
Mineralogical, Physical and Chemical Aspects
Mineralogically and chemically, marble and gypsum alabaster are clearly distinct: calcite, a calcium carbonate (CaCO3), dominates in white marble; gypsum, a water-bearing calcium sulphate (CaSO4·2H2O), in alabaster. Marble can contain variable proportions of magnesium (dolomite), alabaster can also be the water-free variety of calcium sulphate (anhydrite, CaSO4), yet often partly rehydrated to gypsum.
The terminology inherited from antique authors notably Pliny [
15] (book XXXVI, cap. 8, book XXXVII, cap. 10) and Theophrastus [
16] is further complicated by the fact that “alabaster” in itself is a polysemantic term: “alabastrites”, derived from or closely linked to the Egyptian town of Alabastron, designed in the antique world a banded calcite, geologically a travertine or speleothem. This so-called “oriental alabaster” but also “onyx marble” lost its predominance in late antiquity and gave its name to gypsum alabaster when the latter found increasing used in medieval sculpture. In 1747, Hill [
17], in his notes on his English translation of Theophrastus’ History of Stones (p. 23), summarises nicely the confusion that ensued: “And hence have been a thousand Mistakes in the later Authors […] who have misunderstood Pliny”.
First attempts to distinguish marble and gypsum alabaster were made in early modern times, using physico-chemical criteria, namely the softness of alabaster compared to marble. De Boot [
18] considers the soft gypsum alabaster (
alabastrum) as an “unbaked
alabastrites”, i.e. calcite alabaster, and the latter as “unbaked and imperfect marble”, a concept taken over by later authors till the 17th and 18th century e.g. [
19,
20,
21]. Schröder [
19], liber III, p. 29-30 follows de Boot when he states that « if it becomes so soft that it can be cut with a knife, it is more correctly called gypsum”, a criterion taken up by Aldrovandi [
14] when he notes that “white marble quarried in Volterra is softer, whereas some place it among gypsum”.
Yet, in alabaster-working regions like the East Midlands, the differences in their sculptural properties were well-known. In his Chronicles, Holingshed [
22] distinguishes “fine alabaster and hard marble” (p. 31) and states that “If marble will not serve, then have we the finest alabaster that maie elsewhere bee had” (p. 395). He already approaches gypsum plaster (“plaister of paris”, P. 395) and alabaster. Yet, we have to wait mainly till the 18th century for objective criteria to emerge and converge, notably hardness, the possibility to transform gypsum alabaster into plaster, marble into lime, but also the chemical test of acid attack,
“eau forte” in French and
“Scheidewasser” in German reacting with marble, liberating CO
2, but not with gypsum alabaster. Pott [
23] concludes, based on this chemical criterion, that the assumption that alabaster is a species of marble is “completely incorrect”. Some years later Daubenton [
24] even dedicated a whole monography to this problem. Mineralogically, 19th cent. authors like Lucas [
25] clearly distinguish “gypsum alabaster”, identified as a calcium sulphate and “calcareous alabaster” identified as calcium carbonate and related to marble.
The Marble-Alabaster Problem in Modern Collections
In modern literature, art historians did not always attribute high importance to the precise nature of materials till the so-called technical art history opened in the 1990ties the way for transdisciplinary investigations of artworks combining approaches from history, art history and natural sciences (e.g. [
26,
27]. This led to the development of rather sophisticated geochemical methods to identify the provenance of marble [
28,
29,
30] and recently of alabaster [
6,
31,
32] used for European sculpture. Yet, a straightforward, cost-effective and, last but not least, non-invasive test to simply distinguish both materials is still lacking and sought-for, notably by museum curators and restorers. Indeed, erroneous attributions even in the most prominent museum collections are frequent [
33], often inherited from archival documents or a simple examination by the naked eye [
34].
Portable to handheld (< 1000 grams) spectrophotometric instruments encompassing a large range of frequencies, from visible-to-near infrared (VNIR) to X-ray fluorescence (XRF) spectroscopy. They are completed by portable Raman and LIBS and provide a high potential for art material identification and provenancing. An approach to the marble-alabaster problem using portable XRF was recently developed and tested [
34] but this technique is considered less sensitive for light elements like carbon and sulphur than for heavy elements and needed refinement for reliable application [
35].
Here we present a method based on hand-held near-infrared spectroscopy (NIRS). The recent ultra-miniaturisation of such instruments [
36,
37] led to a broad field of applications, namely in food and biomass analysis e.g. [
38,
39], textile [
40] and pharmaceutical [
41] but also in soil characterization [
42]. The identification of marble and alabaster can rely on numerous studies of the NIR spectra of natural carbonate and sulphate minerals. Notably the mineral phases relevant to our problem, i.e. calcite and dolomite [
43] and the more or less hydrated varieties of calcium sulphate (gypsum, the hemi-hydrate bassanite CaSO
4· ½ H
2O, anhydrite, [
44,
45,
46] have been thoroughly characterised in the NIR range (780 nm to 2500 nm).
2. Materials and Methods
We use a NIR-S-G1 NIR spectrometer (InnoSpectra Corporation, Hsinchu, Taiwan). The light source is a pair of broadband 0.7 W tungsten filament lamps. After collimating and focusing, light is emitted and, after reflection by the material, received through a sapphire window (elliptical measuring area of 7 x 4 mm). The reflected light is dispersed by a fixed grating across a digital micro-mirror device (DMD, Texas Instruments DLP2010NIR). Discrete sections of the spectral range of 900– 1700 nm are directed by the actuated mirrors towards a single-point uncooled InGaAs detector. Typical optical resolution is 10 nm, signal-to-noise ratio 5000:1, wavelength accuracy around ±1 nm [
47].
Its Dimensions of 82.2 x 66 x 43.5 mm and its weight of less than 150 g make this device ultra-portable (
Figure S1, Supplementary Material). Connection to a PC (ISC WinForms SDK GUI v3.9.4 software) via USB or a smartphone (ISC NIRScan v3.8 app) provide versatile use in the working conditions in museums or other collections. The measurements are non-invasive and can even be performed without direct contact between the sapphire window and the object being examined, although the best performance is achieved when the sapphire window is placed directly on the object [
47]. All measurements were conducted in diffuse reflectance mode with the Spectralon® diffuse reflectance standard (SRS-99) as reference signal.
Reference materials: We analyzed the following materials as standards: Carrara marble (polished), white gypsum alabaster from different French deposits with historical importance (Notre-Dame-de-Mésage [
5], Saint-Lothain [
48], Malaucène [
49]), and anhydrite alabaster from Notre-Dame-de-Mésage (polished, raw) and from Fauld mine, Staffordshire, east of Nottingham. In each case, several points on the same sample were measured. We also tested fine-grained porous Turonian tuffeau limestone from the Loire valley [
50].
3. Results and Discussion
3.1. Raw Materials
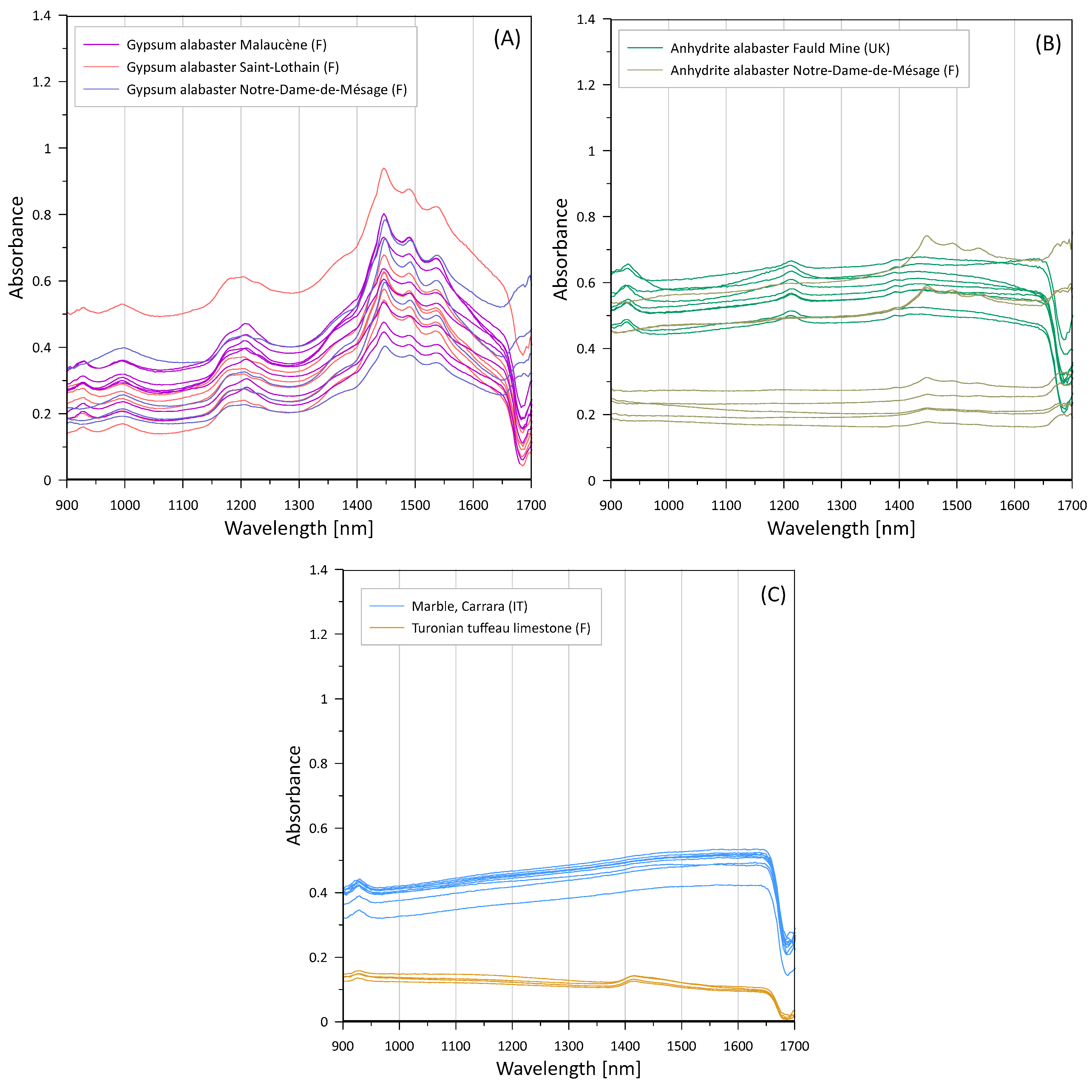
In the investigated spectral range, gypsum alabaster (
Figure 1A) shows as most prominent feature a characteristic triplet between 1440 and 1540 nm [
45,
46,
47]. Other absorption bands characteristic for gypsum are observed near 1000 and 1200 nm [
48,
49,
50,
51]. All these features are related to structural water combinational and overtone modes (H-O-H bending, O-H stretching, [
51]) and are characteristic for hydrated Ca-sulphates in the observed spectral range [
44,
45].
Anhydrite, being per definition anhydrous, its spectra should be featureless in the investigated wavelengths. However, the anhydrite alabasters shows weak absorption bands, around 1200 nm for Fauld mine and in the zone of the gypsum triplet >1400 nm for Notre-Dame-de-Mésage (
Figure 1A). This has been observed previously for other natural anhydrites [
45,
49,
52] and attributed to partial hydration. It is well known that anhydrite in contact with groundwater progressively transforms into gypsum so pure anhydrite in Fauld mine is mainly restricted to depths below around 30 m. [
53,
54]. Its exhumation and rapid, cold hydration under periglacial conditions produced, by the way, the searched-for alabaster-grade gypsum in the wider Nottingham area [
54].
Marble, as pure calcite with few accessory minerals is not expected to exhibit any features in our observation range, CO
3-related overtones starting at >1700 nm. Indeed, the Carrara marble spectrum is flat over the range of approx. 950 to 1650 nm (
Figure 1C).
The similarity of the marble and anhydrite spectra might, in cases of pure, water-free anhydrite, lead to an ambiguity of material identification. However, the use of anhydrite alabaster for sculpture is clearly minor with respect to gypsum. Only 12 of the 340 analysed artworks from the 12th to the 17th century (
Figure S2, Supplementary Material) analysed so far in our studies by standard ion chromatography show sulphate contents >68 wgt%, approaching the theoretical value of 71% for anhydrite, whereas a large majority is situated around the theoretical value of 56% for gypsum. Values <56 % indicate impurities in gypsum alabaster, values between the theoretical gypsum and anhydrite are mixtures of both or partially hydrated anhydrite. In all those cases, we can expect the typical spectral features related to the structural water in gypsum so that we would not expect ambiguities between anhydrite alabaster and marble.
Limestone frequently contains accessory minerals such as clay minerals and other silicates, which is the case of the analysed fine-grained tuffeau limestone from the French Loire Valley. The observed weak feature close to 1400 nm (
Figure 1C) can be related to OH-bearing phyllosilicates as kaolinite [
55].
The reflectance and overall quality of the NIR spectrum of gypsum alabaster depends on several parameters, on the translucency of the investigated alabaster, the surface roughness and the distance of the sapphire window from the surface. Reflectance is high for white, opaque, fine- to cryptocrystalline varieties, whereas translucent alabaster as can be found in certain Spanish and Italian deposits (e.g. Gelsa, Volterra) produce weaker spectra. This is illustrated in
Figure 3 showing the difference between spectra obtained on translucent veins and white fine-crystalline parts of a slab of Gelsa alabaster. The amplitude of the peaks for the veins is less and the spectra are noisier, even if the characteristic features of gypsum alabaster are preserved. Two families of spectra, as observed for Gelsa alabaster, may be a distinctive feature for specific deposits. Partial anhydritisation of primary gypsum [
56] or partial rehydration of anhydrite to alabastrine gypsum could also lead to the occurrence of two types of spectra within the same material.
As to be expected, absorbance and noise increase with distance from the surface (
Figure S3, Supplementary Material). Whereas the gypsum spectra are recognisable at all measured distances (2, 4, 6 mm), absorbance increases by a factor of 2.5 at 4 mm and of 6 at 6 mm and the spectra at 6 mm are significantly noisier than the spectra at 0-4 mm.
The spectra obtained on polished and raw (uncut, unpolished) alabaster are very similar in most cases, except some outliers with higher or lower absorbance and noise for the raw material (
Figure S4, Supplementary Material).
3.2. Case Study 1: Saint Catherine
The Saint Catherine of Alexandria, Onze-Lieve-Vrouwkerk, Kortrijk, NL, is one of the most prominent and well-documented European sculptures of the 15th century (
Figure 4). Commissioned by Louis de Male, Count of Flanders for his tomb in the family chapel in the Onze-Lieve-Vrouwkerk, Kortrijk, the statue is the only surviving element of this funeral chapel [
57]. It was executed in 1374 by André Beauneveu, sculptor of renown probably born in Valenciennes in present-day France [
58], p. 347. Ten years earlier, he had been in charge of the effigy of Charles V, King of France for the Cathedral of St Denis. The fortune of the Saint Catherine was eventful, including temporary burial to escape the protestant iconoclasm in the mid-16th century leading to important damage and replacements in 1866, notably of the crown, the sword, the wheel and part of the fingertips [
57]. The state before this restoration is documented in early photographic documents [
59],
Figure 5). Both painter and stone sculptor, Beauneveu worked different materials, limestone, marble and alabaster and a document of 1386, recording the receipt by the dean and chapter of Notre Dame at Kortrijk of a statue of Saint Catherine, refers to the material as alabaster (“une ymage de pierre d’alebastre de Sainte Catherine” [
13,
34]. Even though, the probably first attribution of this artwork to Beauneveu, by Van de Putte [
60], mentions a “beautiful statue of Saint Catherine, executed in white marble” and Dehaisnes [
61], who took up this attribution claims to have “studied with care the statue in white marble”. Casier and Bergmans [
62] in their catalogue of the 1913 exhibition “L’art ancien dans les Flandres (région de de l’Escaut)” refer to the Saint Catherine as made from “Italian Alabaster”. First attempts to clarify this confusion through portable XRF have been undertaken [
34,
35].
The NIR spectra (
Figure 4) provide a rather detailed cartography of the materials used and reveal a complex restauration history. The main body, with face (
Figure 4G), backs of the hands (B), mantle folds (E), foot (F)and also the figure of the trodden-down king (M) are executed in marble. The crown (A) as well as the complete wheel (H) together with at least the index and middle finger of the left hand (I) are made of alabaster. For both replaced pieces, some of the spectra are noisy with a low reflectance (high absorbance) which can be attributed to differences in translucency of the alabaster used and lacking reflectance in the more transparent parts. Such features with translucent veins or globally increased translucency are characteristic for some Spanish and Italian alabasters (
Figure 3). Replacement of parts of the marble mantle folds with alabaster have been observed in two spots on the left (D) and right side (L) of the statue. The sword pommel (C) is made of alabaster whereas the other parts of the sword hilt are partly of marble and partly of alabaster (B, C). This is insofar surprising as only the grip of the sword hilt was preserved in the early 19th century as shown on photographs taken before restauration ([
13],
Figure 5A). The complex subsequent restorations can be followed on historical yet undated photographs. Whereas
Figure 5A seems to show the unrestored aspect, adding the sword’s crossguard together with the lacking right little finger was obviously the first restauration step (
Figure 5B), before any other replacement. According to our analyses, the material used for this crossguard was marble, except its right extremity. Sword pommel as well as crown and wheel are still missing on this image and were added in the subsequent step (
Figure 5C). Interestingly the wheel is broken, which corresponds, as has been pointed out by Nash [
13], to the standard iconography of Saint Catherine, whereas the complete wheel of the final version is unusual. In a last restauration step (
Figure 5D), sword and wheel have been completed as well as the crown with one previously lacking prong having been added, which is close to the present-day shape of the statue (
Figure 5E). All these later additions were made using alabaster. In this context it is important to stress that the history of restorations of the figure of Saint Catherine with various kinds of alabaster and marble provides a perfect record of confusions resulting from the inability to differentiate between these two materials in the past.
3.2. Case Study 2: Funeral Monument of Bishop Julius Echter, Würzburg Cathedral (Bavaria, Germany)
The funeral monument for the Bishop Julius Echter von Mespelbrunn (1545-1617) in the St Kilian Cathedral in Würzburg was commissioned 1617/1618 by his successor bishop Johann Gottfried von Aschhausen. From the very beginning of its scholarly investigation the monument was described as made of white marble [
63] (p. 74). Ascribed initially to Michael Kern, it was discarded from the oeuvre of this south-German sculptor active in Forchtenberg on the basis of material argumentation. Gradmann [
64] meant namely (p. 113-114) that “the red marble used for the monument does not appear in the works of the Forchtenberg workshop”. Bruhns [
65], who ascribed (p. 370-372) the tomb to Nikolaus Lenkhart († 1632), interpreted the choice of the material as a reference to the local tradition of bishopric tombs executed in red Salzburg (Adnet) or Upper Bavarian “marble”. Indeed, the fact that monuments of Echter’s two predecessors (Rudolf II. von Scherenberg and Lorenz von Bibra), eminent works by Tilman Riemenschneider (1499 and c. 1520 respectively) were carved in this material made this argument fully convincing. The NIRS spectra obtained on the red “marble” of the funeral monument of Lorenz von Bibra, confirm limestone as sculpting material. Also the most recent publication [
66] names the materials of the Julius Echter memorial “red marble, grey and red sandstone, slate, and alabaster”.
Thus, the predominance of alabaster, revealed by our NIRS study, came as a surprise. Indeed, the only measured elements not made from alabaster are the column shafts and the supporting cornice (
Figure 6) as well as the inscription plate of black slate. Spectra of the column shafts and cornice show the same weak feature near 1400 µm as the limestone reference sample (
Figure 1B). The slate shows very low reflectance, and the spectra are featureless over the whole measured NIR range.
The types of alabaster used show a great diversity of colours and textures. Whereas the base (
Figure 6G, I, M) as well as the bases of the columns (
Figure 6D) features a beige, strongly banded alabaster, decorative elements in the central part, such as the coats-of-arms and the cartouche with its putto (
Figure 6F), are very fine-grained and homogenous. The most spectacular and unexpected facies is the red alabaster of the Bishop’s figure (mantle and sword tested,
Figure 6, J, K), so far considered as marble, recalling indeed certain coloured limestones like the Adnet “marble” [
67]. Other elements made of red alabaster are the frieze separating the base and the central part (
Figure 6E), as well as the pilasters behind the columns (
Figure 6A). Isotope analyses are currently underway to determine the provenance of these different types of alabaster.
5. Conclusions and Outlook
The easily recognisable NIR spectrum of gypsum alabaster strongly contrasting with the featureless spectrum of marble over the range of 900-1700 nm allows for a simple and straightforward differentiation of both materials used for artwork by comparison with to reference spectra obtained on raw materials. Distinction of marble and anhydrite alabaster is less evident but appears possible due to the frequent partial hydration of natural anhydrites, leading to weak but characteristic features in the 1200 nm and 1400-1500 nm ranges. The tested ultra-portable near-infrared spectroscopy (NIRS) device is particularly adapted to the needs of museum conservators and restorers for a non-destructive, efficient, rapid, and cost-effective method for the identification of gypsum alabaster but also other materials, e.g. amber [
68]. Compared to most other portable instruments (XRF, LIBS, FTIR…) the tested device is more than an order of magnitude smaller, lighter and cheaper. It can also be used without any restrictions related to radioactive sources (XRF) and radiation (X-ray, laser…). Its limitations lie in the somewhat restricted spectral coverage, compared to the total range of NIRS (780-2500 nm), cutting off some specific features, notably of carbonates. Yet, it covers the absorption bands of OH-bearing materials like our main target, gypsum alabaster but also clay minerals and metal hydroxides [
47].
The developed technique is also promising with respect to differentiation between different kinds of alabaster. Providing a systematic comparison of raw alabasters, some features in the NIR spectra might allow for distinction of provenances: translucency of some Spanish and Italian deposits, partial anhydritisation or hydration, the presence of iron hydroxides in English alabaster (Fe and Mn lead to specific bands in diverse mineral phases [
47], even if the overall iron contents of Nottingham alabaster are low [
69]). This technique allows for rapid identification of complex, lithologically composite sculptures as illustrated by the Echter funeral monument and also of restorations with stones different from the original material as for the Kortrijk Saint Catherine of Alexandria.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Measurement with the NIR-S-G1 on the sword hilt of the Saint Catherine of Alexandria, Onze-Lieve-Vrouwkerk, Kortrijk, NL; : Histogram of sulphate contents (weight content) obtained by liquid chromatography on European alabaster artworks from the 12th to the 17th century (unpublished results, n=340); Figure S3: NIR spectra of polished alabaster from Notre-Dame-de-Mésage at a distance between the sapphire window and the stone surface of 0, 2, 4, 6 mm; Figure S4: Comparison between polished and rough (uncut, unpolished) alabaster from Notre-Dame-de-Mésage measured for different points on a decimetric sample.
Author Contributions
Conceptualization, A.L. and W.K.; methodology, O.R. and W.K.; investigation, O.R. and W.K.; writing—original draft preparation, W.K.; writing—review and editing, W.K., A.L., and O.R.; project administration, W.K. and A.L.; funding acquisition, W.K. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the Franco-German FRAL program (ANR project ANR-21-FRAL-0014-01 and DFG project 469987104) in the framework of the Materi-A-Net project (https://materi-a-net.uni-koeln.de/en/the-project/).
Data Availability Statement
All raw data have been submitted to the Earth System Data Repository (
https://www.easydata.earth/) and will be soon available (doi attribution underway).
Acknowledgments
We thank Marjan Debaene and Sophie Jugie and the other organizers of the Alabaster exhibition in Leuven (14 October 2022 - 26 February 2023) for the access to the Saint Catherine statue. Dr. Wolfgang Schneider Diocesan curator of the Würzburg Cathedral for his kind support of the investigations on the Echter funeral monument. Thanks to Dr. Alicia Muñoz del Pozo and Carmen Morte for providing the Gelsa alabaster sample and to Robert Aillaud for collecting gypsum and anhydrite alabaster from Notre-Dame-de-Mésage.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| DMD |
Digital micro-mirror device |
| FTIR |
Fourier-transform infrared spectroscopy |
| LIBS |
Laser-induced breakdown spectroscopy |
| NIRS |
Near infrared spectroscopy |
| VNIR |
Visible-to-near infrared |
| XRF |
X-ray fluorescence spectroscopy |
References
- Primavori, P. Carrara Marble: a nomination for ‘Global Heritage Stone Resource’ from Italy. Geological Society, London, Special Publications 2015, 407, 137–154. [Google Scholar] [CrossRef]
- Klapisch-Zuber, C. Les maîtres du marbre: Carrare, 1300-1600; Éditions de l’École des hautes études en sciences sociales: 1969; p. 366.
- Hope, W.H.S.J. On the early working of alabaster in England. In Illustrated catalogue of the exhibition of English medieval alabaster work : held in the rooms of the Society of Antiquaries, 26th May to 30th June, 1910; Hope, W.H.S.J., Sir, Eds.; Society of Antiquaries of London: London, 1913. [Google Scholar]
- Espanol, F. L’exploitation des carrières d’albâtre en Catalogne au Moyen Âge. In Proceedings of the Relations, échanges et coopération en Méditerranée,128° congrès national des sociétés historiques et scientifiques, Bastia, 2003., 14–21 April 2003. [Google Scholar]
- Aillaud, R.; Anheim, E. L’albâtre de Notre-Dame de-Mésage. Exploitation, circulation et usages (XIVe-XVIe siècle). Revue de l’Art 2018, 200/2018-2, 31–36. [Google Scholar]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Le Pogam, P.Y.; Cooper, A.H.; Worley, N.; Guerrot, C.; Montech, A.T.; Gallas, A.M.; Aillaud, R. Competing English, Spanish, and French alabaster trade in Europe over five centuries as evidenced by isotope fingerprinting. Proceedings of the National Academy of Sciences 2017, 114, 11856–11860. [Google Scholar] [CrossRef] [PubMed]
- Theiss, H.; Roller, S.; Gonzalez de Quevedo Ibanez, M.; Klopmann, W.; Wille, G. Alabaster. In Handbuch der Oberflächenreinigung, 7 ed.; Eipper, P.-B., Ed.; Verlag Dr. Christian Müller-Straten: München, 2021; Volume 3, pp. 117–207. [Google Scholar]
- Lipińska, A. Alabastrum, id est, corpus hominis: Alabaster in the Low Countries, a cultural history. Netherlands Yearbook for History of Art/Nederlands Kunsthistorisch Jaarboek Online 2013, 62, 84–115. [Google Scholar] [CrossRef]
- Bruchet, M.P.M. Marguerite d’Autriche, Duchesse de Savoie: Ouvrage publié sous les auspices du Comité flamand de France; impr. L. Danel, 93, rue Nationale: 1927.
- Rousset, A.; Moreau, F. Dictionnaire géographique, historique et statistique des communes de la Franche-Comté et des hameaux qui en dépendent, classés par département: département du Jura; Bintot: 1854.
- Tritenne, D. Le marbre de Carrare utilisé à Brou. In Brou, un monument européen à l’aube de la Renaissance = Brou, a European Monument in the Early Renaissance; Paris, 2007; pp. 183–189.
- Jugie, S.; Leroux, L.; Bromblet, P.; Kloppmann, W. L’albâtre et ses sources : incertitudes historiques et ambiguïtés de la documentation levées grâce aux analyses. Techné submitted.
- Nash, S. ‘No Equal in Any Land’. André Beauneveu, Artist to the Courts of France and Flanders. 2007.
- Aldrovandi, U. Musaeum metallicum in libros IV; Bernia, Marcus Antonius: Bologna, 1648. [Google Scholar]
- Pliny the Elder; Eichholz, D. E. Pliny. Natural History, Volume X: Books 36-37. Translated by D. E. Eichholz; Heinemann, W., Ed.; Harvard University Press: Cambridge, MA, 1962. [Google Scholar]
- Caley, E.R.; Richards, J.F. Theophrastus on stones: introduction, Greek text, English translation, and commentary; The Ohio State University Press: Columbus, OH, 1956. [Google Scholar]
- Hill, J. History of Stones. With an English Version and ... Notes, Including the Modern History of the Gems Etc. by John Hill. (etc.); Davis: 1746.
- de Boodt, A. Anselmi Boetii de Boodt,... Gemmarum et lapidum historia... Typis Wechelianis, apud C. Marnium et heredes J. Aubrii: Hanoviae, 1609.
- Schröder, J. Pharmacopoeia medico-chymica sive thesaurus pharmacologicus : quo composita quaeque celebriora, hinc mineralia, vegetabilia & animalia chymico-medice describuntur, atque insuper principia physicae hermetico-hippocraticae candide exhibentur; opus, non minus utile physicis quam medicis, Ed.; Gerlin: Ulmae, 1644. [Google Scholar]
- Plot, R. Natural History of Staffordshire; Oxford, 1686.
- Richelet, P. Albatre, albastre. Dictionnaire françois, 1680. [Google Scholar]
- Holinshed, R.; Harrison, W.; Stanyhurst, R.; Hooker, J.; Thynne, F.; Fleming, A.; Stow, J.; Ellis, H. Holinshed’s Chronicles of England, Scotland and Ireland / [edited 1807 by Sir Henry Ellis]; J. Johnson: London, 1587 [edited 1807].
- Pott, J.H. Chymische Untersuchungen Welche fürnehmlich von der Lithogeognosia oder Erkäntniß und Bearbeitung der gemeinen einfacheren Steine und Erden Ingleichen von Feuer und Licht handeln; Christian Friedrich Voß: Potsdam, 1746. [Google Scholar]
- Daubenton, L.J.-M. Mémoire sur l’albâtre. Mémoires de mathématiques et de physique tirés des registres de l’Académie royale des sciences 1759, 1754, 237–249. [Google Scholar]
- Lucas, J.-A.-H. Albâtre calcaire, Albâtre gypseux. Nouveau dictionnaire d’histoire naturelle, appliquée aux arts, à l’agriculture, à l’économie rurale et domestique, à la médecine, etc: Aba - Ani, 1816; 283–285. [Google Scholar]
- Bomford, D. Introduction. In Looking Through Paintings. The Study of Painting Techniques and Materials in Support of Art Historical Research, Hermens, E., Ed.; Archetype Publication: London, 1998. [Google Scholar]
- Roberts, J.L. Things. Material Turn, Transnational Turn. American Art 2017, 61, 64–69. [Google Scholar] [CrossRef]
- Attanasio, D.; Brilli, M.; Ogle, N. The isotopic signature of classical marbles; L’Erma di Bretschneider: Rome, 2006. [Google Scholar]
- Antonelli, F.; Lazzarini, L. An updated petrographic and isotopic reference database for white marbles used in antiquity. Rendiconti Lincei 2015, 26, 399–413. [Google Scholar] [CrossRef]
- Craig, H.; Craig, V. Greek Marbles: Determination of Provenance by Isotopic Analysis. Science 1972, 176, 401–403. [Google Scholar] [CrossRef]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Le Pogam, P.-Y.; Montech, A.T.; Guerrot, C. A pan-European art trade in the late middle ages: Isotopic evidence on the master of Rimini enigma. Plos one 2022, 17, e0265242. [Google Scholar] [CrossRef]
- Kloppmann, W.; Leroux, L.; Bromblet, P.; Guerrot, C.; Proust, E.; Cooper, A.H.; Worley, N.; Smeds, S.A.; Bengtsson, H. Tracing Medieval and Renaissance Alabaster Works of Art Back to Quarries: A Multi-Isotope (Sr, S, O) Approach. Archaeometry 2014, 56, 203–219. [Google Scholar] [CrossRef]
- Kloppmann, W.; Le Pogam, P.-Y.; Leroux, L. La sculpture sur albâtre en France du xive au xvie siècle : enjeux, méthodes et résultats d’un programme de recherche. Revue de l’Art 2018, 200/2018-2, 9–20. [Google Scholar]
- de Roy, J.; Fontaine, L.; Patigny, G. alabaster. In Alabaster Sculpture in Europe 1300-1650, Debaene, M., Ed.; Harvey Miller Publishers: London/Turnhout, 2022; pp. 31–37. [Google Scholar]
- de Roy, J.; Fontaine, L.; Patigny, G. New Insights on St Catherine of Alexandria by André Beauneveu. Correct Identification and Provenance Analysis of the Original Sculpture Material and Latest Findings on the Polychromy. In Alabaster as a Material for Medieval and Renaissance Sculpture. 8th Annual Ards Conference; Brepols: 2024, submitted.
- Beć, K.B.; Grabska, J.; Siesler, H.W.; Huck, C.W. Handheld near-infrared spectrometers: Where are we heading? NIR news 2020, 31, 28–35. [Google Scholar] [CrossRef]
- Beć, K.B.; Grabska, J.; Huck, C.W. Principles and Applications of Miniaturized Near-Infrared (NIR) Spectrometers. Chemistry – A European Journal 2021, 27, 1514–1532. [Google Scholar] [CrossRef]
- Singh, H.; Sridhar, A.; Saini, S.S. Ultra-Low-Cost Self-Referencing Multispectral Detector for Non-Destructive Measurement of Fruit Quality. Food Analytical Methods 2020, 13, 1879–1893. [Google Scholar] [CrossRef]
- Wolfrum, E.J.; Payne, C.; Schwartz, A.; Jacobs, J.; Kressin, R.W. A Performance Comparison of Low-Cost Near-Infrared (NIR) Spectrometers to a Conventional Laboratory Spectrometer for Rapid Biomass Compositional Analysis. BioEnergy Research 2020, 13, 1121–1129. [Google Scholar] [CrossRef]
- Cura, K.; Rintala, N.; Kamppuri, T.; Saarimäki, E.; Heikkilä, P. Textile recognition and sorting for recycling at an automated line using near infrared spectroscopy. Recycling 2021, 6, 11. [Google Scholar] [CrossRef]
- Chavan, R.B.; Bhargavi, N.; Lodagekar, A.; Shastri, N.R. Near infra red spectroscopy: a tool for solid state characterization. Drug Discovery Today 2017, 22, 1835–1843. [Google Scholar] [CrossRef]
- Tang, Y.; Jones, E.; Minasny, B. Evaluating low-cost portable near infrared sensors for rapid analysis of soils from South Eastern Australia. Geoderma Regional 2020, 20, e00240. [Google Scholar] [CrossRef]
- Kim, Y.; Caumon, M.-C.; Barres, O.; Sall, A.; Cauzid, J. Identification and composition of carbonate minerals of the calcite structure by Raman and infrared spectroscopies using portable devices. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy 2021, 261, 119980. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, A.Z.; Freemen, J.J. Raman, MIR, and NIR Spectroscopic Study of Calcium Sulfates: Gypsum, Bassanite, and Anhydrite. 2009.
- Bishop, J.L.; Lane, M.D.; Dyar, M.D.; King, S.J.; Brown, A.J.; Swayze, G.A. Spectral properties of Ca-sulfates: Gypsum, bassanite, and anhydrite. American Mineralogist 2014, 99, 2105–2115. [Google Scholar] [CrossRef]
- Harrison, T.N. Experimental VNIR reflectance spectroscopy of gypsum dehydration: Investigating the gypsum to bassanite transition. American Mineralogist 2012, 97, 598–609. [Google Scholar] [CrossRef]
- Cloutis, E.; Craig, M.; Kruzelecky, R.; Jamroz, W.; Scott, A.; Hawthorne, F.; Mertzman, S. Spectral reflectance properties of minerals exposed to simulated Mars surface conditions. Icarus 2008, 195, 140–168. [Google Scholar] [CrossRef]
- Hunt, G.R.; Salisbury, J.W.; Lenhoff, C.J. Visible and near-infrared spectra of minerals and rocks: IV. Sulphides and sulphates. Modern Geology 1971, 3, 1–14. [Google Scholar]
- Kokaly, R.F.; Clark, R.N.; Swayze, G.A.; Livo, K.E.; Hoefen, T.M.; Pearson, N.C.; Wise, R.A.; Benzel, W.; Lowers, H.A.; Driscoll, R.L.; et al. USGS Spectral Library Version 7; 1035; Reston, VA, 2017; p. 68.
- Clark, R.N. Reflectance spectra. AGU handbook of physical constants 1995, 178. [Google Scholar]
- Crowley, J.K. Visible and near-infrared (0.4–2.5 μm) reflectance spectra of playa evaporite minerals. Journal of Geophysical Research: Solid Earth 1991, 96, 16231–16240. [Google Scholar] [CrossRef]
- Cloutis, E.A.; Hawthorne, F.C.; Mertzman, S.A.; Krenn, K.; Craig, M.A.; Marcino, D.; Methot, M.; Strong, J.; Mustard, J.F.; Blaney, D.L. Detection and discrimination of sulfate minerals using reflectance spectroscopy. Icarus 2006, 184, 121–157. [Google Scholar] [CrossRef]
- Young, J.A. Alabaster; Derbyshire Museum Service: Nottingham, 1990; p. 68. [Google Scholar]
- Firman, R.J. A geological approach to the history of English Alabaster. Mercian Geologist 1984, 9, 161–178. [Google Scholar]
- Hunt, G.R.; Ashley, R.P. Altered rock spectra in the visible and near infrared; 1979.
- Ortí, F.; Rosell, L.; PlayÀ, E.; M. Salvany, J. Meganodular anhydritization: a new mechanism of gypsum to anhydrite conversion (Palaeogene-Neogene, Ebro Basin, North-east Spain). Sedimentology 2012, 59, 1257–1277. [Google Scholar] [CrossRef]
- Woelk, M. 2 St Catherine of Alexandria. In Alabaster Sculpture in Europe 1300-1650, Debaene, M., Ed.; Harvey Miller Publishers: London/Turnhout, 2022; pp. 40–41. [Google Scholar]
- Le Pogam, P.-Y.; Jugie, S. La sculpture gothique. 1140-1430; Hazan: Paris, 2020. [Google Scholar]
- Photographer unknown. André Beauneveu. Saint Catherine of Alexandria by André Beauneveu - Catholic University of Leuven, Belgium - Public Domain. between 1839 and 1939.
- Van de Putte, F. La chapelle des comtes de Flandre à Courtrai; Beyaert: 1875.
- Dehaisnes, C.C.A. Histoire de l’art dans la Flandre, l’Artois & le Hainaut avant le XVe siècle; L. Quarré: 1886.
- Casier, J.; Bergmans, P. L’art ancien dans les Flandres (région de l’Escaut): mémorial de l’exposition rétrospective organisée à Gand en 1913; G. van Oest & cie: 1914.
- Scharold, C.G. Geschichte und Beschreibung des St. Kilians-Doms zu Würzburg; Thein: 1837.
- Gradmann, G. Michael Kern, Bildhauer. Inaugural-Dissertation zur Erlangung der Doktorwürde, Universität zu Tübingen, Strassburg, 1916.
- Bruhns, L. Würzburger Bildhauer der Renaissance und des werdenden Barock, 1540-1650; Verlag für Praktische Kunstwissenschaft, Dr. F.X. Weizinger: 1923.
- Dombrowski, D. Cat. No 21.7. In Julius Echter: Patron Der Künste. Konturen Eines Fürsten Und Bischofs Der Renaissance, Dombrowski, D., Maier, M., Müller, F., Eds.; De Gruyter: 2017; pp. 385–387.
- Moshammer, B.; Uhlir, C.; Rohatsch, A.; Unterwurzacher, M. Adnet ‘Marble’, Untersberg ‘Marble’and Leitha limestone—best examples expressing Austria’s physical cultural heritage. In Proceedings of the Engineering Geology for Society and Territory-Volume 5: Urban Geology, Sustainable Planning and Landscape Exploitation; 2015; pp. 253–257. [Google Scholar]
- Golloch, A.; Heidbreder, S.; Lühr, C. Identification of amber and imitations by near infrared reflection spectroscopy. Fresenius’ journal of analytical chemistry 1998, 361, 545–546. [Google Scholar] [CrossRef]
- Dubelaar, W. Albast uit Nottingham geologie, winning en verspreiding in de Lage Landen. gea 2009, 42, 72–77. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).