Submitted:
12 August 2025
Posted:
13 August 2025
You are already at the latest version
Abstract
Keywords:
Introduction
- (i)
- What ecological roles do B. infantis and L. reuteri play across the human lifespan?
- (ii)
- What factors contribute to their depletion in industrialized societies?
- (iii)
- What are the functional and clinical consequences of their loss, particularly regarding immune, metabolic, and neurobehavioral outcomes?
- (iv)
- What is the current evidence base for restoring these keystone species, and how might this inform public health, clinical nutrition, and microbiome-targeted interventions?
Materials & Method
Study Design
- Define the ecological functions of B. infantis and L. reuteri across developmental stages.
- Identify key drivers of their loss in industrialized societies.
- Examine the clinical and mechanistic consequences of their depletion in metabolic, immunologic, and neurobehavioral outcomes.
- Synthesize evidence on therapeutic strategies—including probiotics, prebiotics, and biotherapeutics—targeting their restoration.
Search Strategy
- "gut microbiome", "microbial diversity", "dysbiosis"
- "Bifidobacterium infantis", "Lactobacillus reuteri", "keystone species"
- "short-chain fatty acids", "intestinal permeability", "SCFA production"
- "gut-brain axis", "microbiome restoration", "probiotic", "synbiotic", "prebiotic", "immune modulation"
- "autism spectrum disorder", "depression", "metabolic syndrome", "host genetics"
QualES Eligibility Criteria
- Peer-reviewed human or animal studies
- Published between 2000 and 2024
- Investigated either B. infantis or L. reuteri as a central taxon
-
Explored outcomes in at least one of the following domains:
- o
- Microbial function (e.g., SCFA production, mucosal integrity)
- o
- Immune and metabolic regulation (e.g., insulin sensitivity, cytokine profiles)
- o
- Neurobehavioral modulation (e.g., vagal tone, anxiety, ASD-like behavior)
- o
- Restoration strategies (e.g., probiotic/prebiotic administration, synbiotics, FMT)
- Employed recognized microbiome assessment methods (e.g., 16S rRNA sequencing, metagenomics, metabolomics)
- In vitro studies with no host interaction
- Studies with unclear endpoints or inadequate data
- Sample size <30 for human studies or <10 per group in animal models
- Non-English language, case reports, narrative reviews, and conference abstracts
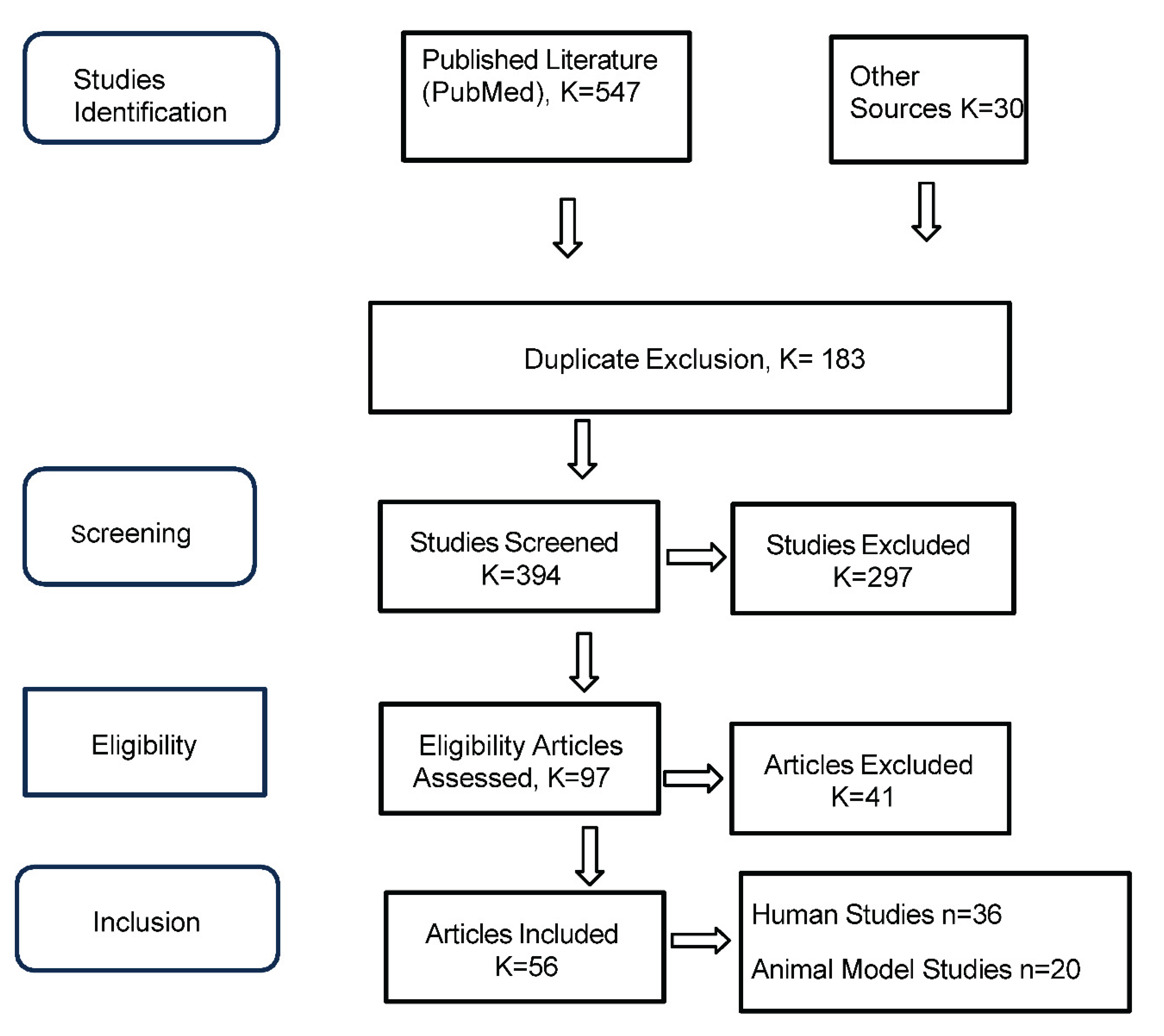
- Title and abstract screening of 394 articles by two independent reviewers
- Full-text review of 97 articles
- 36 human studies
- 20 animal model studies
Data Extraction and Quality Assessment
- Study type and design
- Species/population characteristics
- Intervention details (strain, dose, duration)
- Analytical techniques (e.g., LC-MS, 16S rRNA, behavioral assays)
- Outcomes: metabolic, immune, neurocognitive, microbiome
- Drivers of microbial loss (e.g., antibiotics, birth mode, infant diet)
- Type of restoration strategy (e.g., probiotic-only, diet, combination)
- RCTs: Cochrane Risk of Bias Tool 2.0
- Observational studies: Newcastle-Ottawa Scale
- Animal studies: SYRCLE Risk of Bias tool
- Multi-omics and mechanistic studies: STROBE-Omics checklist
Data Synthesis
- Functional role and ecological significance of B. infantis and L. reuteri
- Drivers of microbial depletion in industrialized contexts
- Consequences of loss on host immune, metabolic, and neurobehavioral health
- Mechanisms of restoration and clinical efficacy of interventions
- Host–microbe coadaptation and microbiome-targeted personalization
Results
-
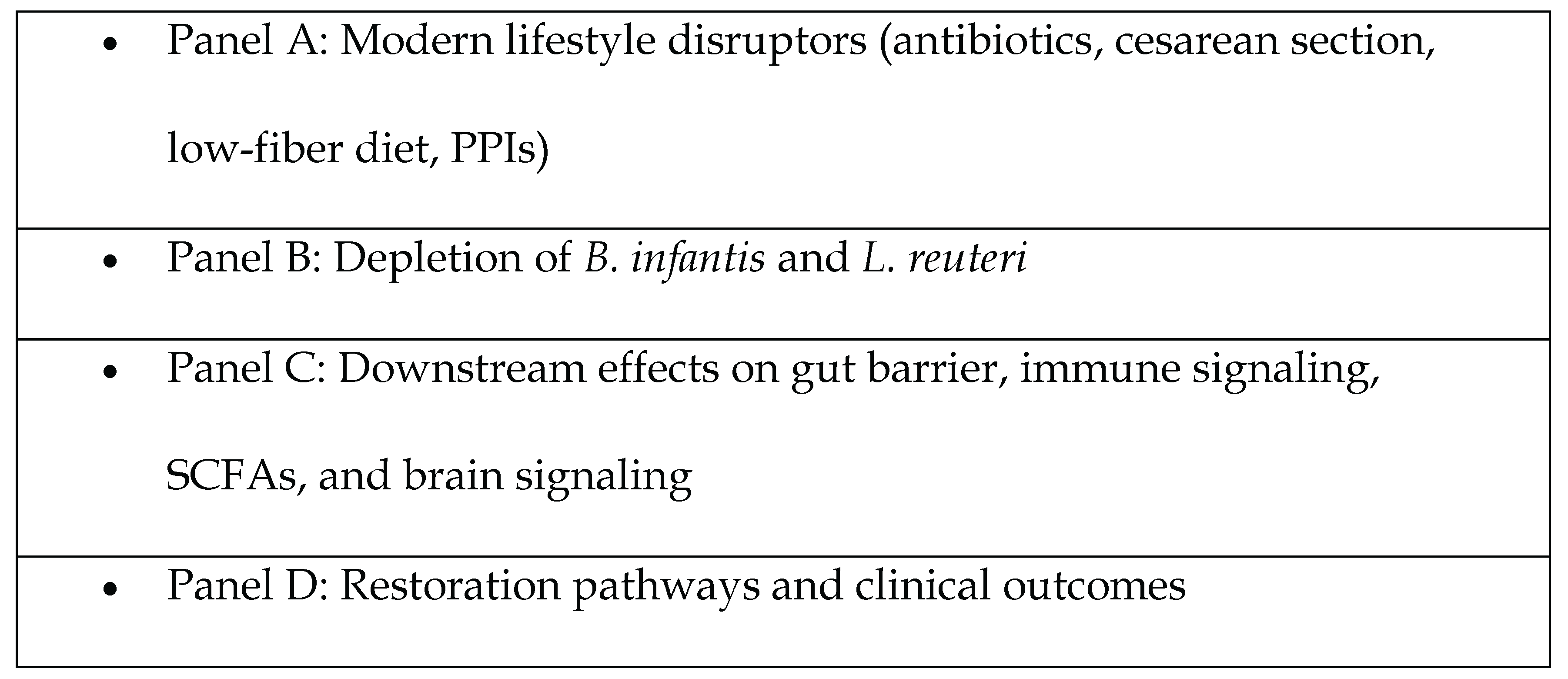
Pharmaceutical agents: Antibiotic exposure, both acute and cumulative, significantly alters microbiome composition and reduces microbial diversity. Perinatal antibiotic exposure triggered an initial suppression of microbial phylogenetic diversity (P < 0.0001 at birth) followed by compensatory hyper-restoration, with richness recovery rates exceeding untreated controls by 12 months [42,80]. Non-steroidal anti-inflammatory drugs (NSAIDs) were associated with:
- Exacerbated mucosal injury when co-administered with PPIs. Endoscopic evaluation revealed significantly higher rates of small bowel mucosal injury in patients receiving nonselective NSAID-PPI combination therapy compared to controls (60-80% vs 16.7%, P = .04) [83]. COX-2 inhibitor/PPI coadministration demonstrated intermediate toxicity (44%) [83]. Lesion burden varied by anatomical site, with the jejunum showing particular vulnerability (P = 0.03 for injury severity) [83]. The observed dose-response relationship (P = 0.02 for erosion count gradient) supports a synergistic damaging mechanism between gastric acid suppression and NSAID-mediated mucosal injury [83].
- Perinatal factors, including cesarean delivery and reduced breastfeeding duration, were linked to early-life microbial deficits [84]. In a cohort of 102 infants, gut microbiota composition at 6 weeks was significantly associated with delivery mode (P < .001; Q < .001) and feeding method (P = .01; Q < .001) [85]. Vaginal delivery (vs cesarean) was linked to increased Bacteroides abundance (P < .001; Q = .02) [85]. Cesarean birth caused greater shifts in microbial profiles than feeding differences (P = .003) [85]. Mixed-fed infants resembled formula-fed peers (P = .002) [85].
- Dietary shifts toward low-fiber and high-processed foods correlated with decreased SCFA-producing taxa [86,87]. Analysis of 64 studies (n = 2099) showed that dietary fiber supplementation significantly increased the relative abundance of Bifidobacterium spp. (SMD = 0.64; 95% CI: 0.42–0.86; P < .00001) and Lactobacillus spp. (SMD = 0.22; 95% CI: 0.03–0.41; P = .02), alongside modest gains in fecal butyrate levels (SMD = 0.24; 95% CI: 0.00–0.47; P = .05), compared to placebo or low-fiber controls [88].
- Environmental pollutants (e.g., glyphosate, emulsifiers, artificial sweeteners) exhibited dose-dependent inhibitory effects on commensal bacteria [89]. Environmental pollutants are known to disrupt the balance of gut microbiota, leading to dysbiosis, and can consequently exert various detrimental effects on overall health [89,90]. Food-borne toxicants and additives disrupt gut microbiota function, compromising intestinal barrier integrity and promoting metabolic disease development [91]. Targeting microbe-toxicant interactions through interventions like fermentable fiber may mitigate these metabolic disruptions [91]. Research consistently demonstrates that these pollutants can specifically inhibit the beneficial functions and composition of the gut microbiota [90,91].
- Gastric acid suppression (proton pump inhibitors, PPIs) was uniquely associated with a reduction in microbial richness [92]. Among 211 PPI users, stool microbiome analysis revealed a significant reduction in Shannon diversity and alterations in approximately 20% of bacterial taxa (FDR < 0.05) [92]. PPI use was associated with increased abundance of oral-origin genera including Rothia (p = 9.8×10⁻³⁸), as well as elevated levels of Enterococcus, Streptococcus, Staphylococcus, and Escherichia coli [92]. Using one-tailed Wilcoxon rank sum tests on 1827 individuals, with a significance threshold of P<.05, findings revealed a significant reduction in gut microbiome diversity among Proton Pump Inhibitor (PPI) users compared to non-users [93]. This indicates a notable impact of PPIs on the gut microbiota [93].
- 2.
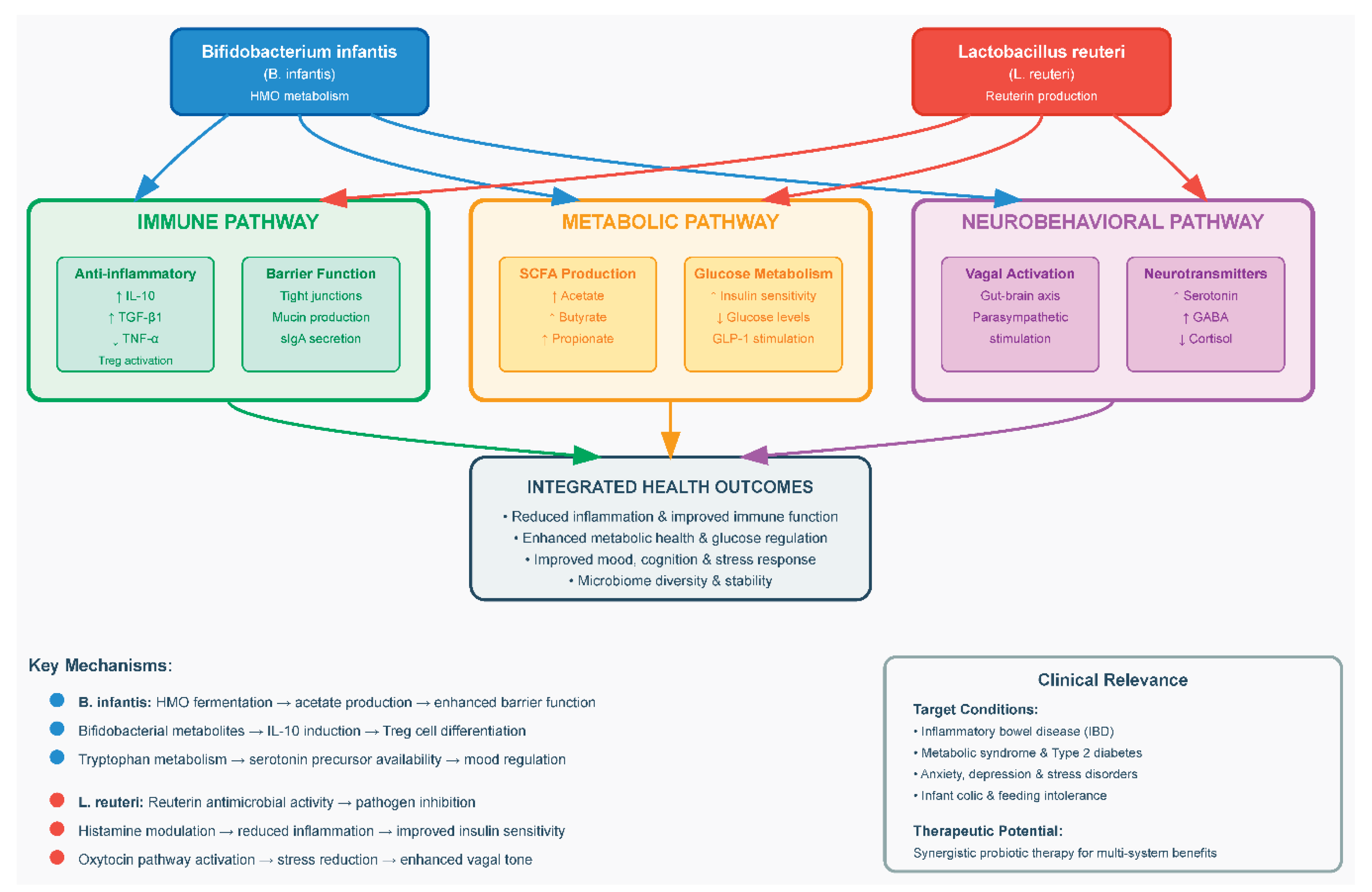
- Metabolic and Immune Outcomes After Keystone Restoration
- 3.
- Neurobehavioral and Gut-Brain Axis Effects
- 4.
- Efficacy of Restoration Strategies
Discussion
Mechanistic Insights
Clinical and Public Health Implications
QualES Limitations
Conclusion and Future Directions
Microbiomes Implication in Disease Causation, Health Outcomes and Future Directions
- Personalized Interventions: Development of diagnostic tools using microbiome sequencing, SCFA profiling, or immune biomarkers will allow precision targeting. Trials should stratify patients based on baseline microbial or immunologic markers to assess differential responses to probiotic therapy.
- Longitudinal and Intergenerational Studies: Extended follow-up is needed to determine the durability of benefits and their effects across generations. For example, supplementing pregnant women with B. infantis could be studied for its impact on neonatal microbiota and early immune development.
- Next-Generation Therapeutics: Engineered strains or microbial consortia could be designed to deliver targeted metabolites or immunomodulators. For instance, a modified B. infantis that overproduces acetate, or L. reuteri strains that optimize oxytocin signaling, could enhance therapeutic precision.
- Mechanistic Human Studies: Future trials should integrate multi-omics with host physiology data. Measuring changes in host gene expression, epigenetics, inflammatory markers, neuroimaging, and vagal tone alongside microbiome dynamics will clarify causal pathways.
- Population-Level Research: Real-world interventions—such as fiber subsidies, fermented food promotion, or early-life microbial seeding—should be evaluated for their capacity to restore keystone taxa and reduce chronic disease incidence.
- Regulatory and Safety Frameworks: As probiotics become clinical tools, robust long-term safety monitoring is essential. Registries and pharmacovigilance systems can track rare adverse events or ecological risks. Regulatory agencies should also evolve to include microbiome endpoints in risk-benefit assessments.
Supplementary Materials
References
- Guinane, C.M.; Cotter, P.D. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther. Adv. Gastroenterol. 2013, 6, 295–308. [Google Scholar] [CrossRef]
- Rook, G.; Bäckhed, F.; Levin, B.R.; McFall-Ngai, M.J.; McLean, A.R. Evolution, human-microbe interactions, and life history plasticity. Lancet 2017, 390, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Hills, Ronald D., Benjamin A. Pontefract, Hillary R. Mishcon, Cody A. Black, Steven C. Sutton, and Cory R. Theberge. "Gut microbiome: profound implications for diet and disease." Nutrients 11, no. 7 (2019): 1613.
- Shelton, C.D.; Byndloss, M.X.; Richardson, A.R. Gut Epithelial Metabolism as a Key Driver of Intestinal Dysbiosis Associated with Noncommunicable Diseases. Infect. Immun. 2020, 88, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Sonnenburg, E.D.; Sonnenburg, J.L. The ancestral and industrialized gut microbiota and implications for human health. Nat. Rev. Microbiol. 2019, 17, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Borody, T.; Herkes, G.; McLachlan, C.; Kiat, H. Neurodegenerative and Neurodevelopmental Diseases and the Gut-Brain Axis: The Potential of Therapeutic Targeting of the Microbiome. Int. J. Mol. Sci. 2023, 24, 9577. [Google Scholar] [CrossRef]
- Tett, A.; Pasolli, E.; Masetti, G.; Ercolini, D.; Segata, N. Prevotella diversity, niches and interactions with the human host. Nat. Rev. Microbiol. 2021, 19, 585–599. [Google Scholar] [CrossRef]
- Duncan, S.H.; Richardson, A.J.; Kaul, P.; Holmes, R.P.; Allison, M.J.; Stewart, C.S. Oxalobacter formigenes and Its Potential Role in Human Health. Appl. Environ. Microbiol. 2002, 68, 3841–3847. [Google Scholar] [CrossRef]
- Camara, A.; Konate, S.; Alou, M.T.; Kodio, A.; Togo, A.H.; Cortaredona, S.; Henrissat, B.; Thera, M.A.; Doumbo, O.K.; Raoult, D.; et al. Clinical evidence of the role of Methanobrevibacter smithii in severe acute malnutrition. Sci. Rep. 2021, 11, 5426. [Google Scholar] [CrossRef]
- Belkacemi, Souad, Maryam Tidjani Alou, Matthieu Million, Anthony Levasseur, Saber Khelaifia, And Didier Raoult. "Prevalence of Treponema species in the Gut Microbiome is Linked to Bifidobacterium sp. and Bacteroides sp." (2020).
- Tomás-Pejó, E.; González-Fernández, C.; Greses, S.; Kennes, C.; Otero-Logilde, N.; Veiga, M.C.; Bolzonella, D.; Müller, B.; Passoth, V. Production of short-chain fatty acids (SCFAs) as chemicals or substrates for microbes to obtain biochemicals. Biotechnol. Biofuels Bioprod. 2023, 16, 96. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Zhang, Dan, Yong-Ping Jian, Yu-Ning Zhang, Yao Li, Li-Ting Gu, Hui-Hui Sun, Ming-Di Liu, Hong-Lan Zhou, Yi-Shu Wang, and Zhi-Xiang Xu. "Short-chain fatty acids in diseases." Cell Communication and Signaling 21, no. 1 (2023): 212.
- Ikeda, Takako, Akari Nishida, Mayu Yamano, and Ikuo Kimura. "Short-chain fatty acid receptors and gut microbiota as therapeutic targets in metabolic, immune, and neurological diseases." Pharmacology & therapeutics 239 (2022): 108273.
- Banerjee, S.; Schlaeppi, K.; Van Der Heijden, M.G.A. Keystone taxa as drivers of microbiome structure and functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Brushett, S.; Sinha, T.; Reijneveld, S.A.; de Kroon, M.L.A.; Zhernakova, A. The Effects of Urbanization on the Infant Gut Microbiota and Health Outcomes. Front. Pediatr. 2020, 8, 408. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Cao, Q.; Cheng, Y.; Zhao, D.; Wang, Z.; Yang, H.; Wu, Q.; You, L.; Wang, Y.; Lin, Y.; et al. Chronic stress promotes colitis by disturbing the gut microbiota and triggering immune system response. Proc. Natl. Acad. Sci. 2018, 115, E2960–E2969. [Google Scholar] [CrossRef] [PubMed]
- Abjani, F.; Madhavan, P.; Chong, P.P.; Chinna, K.; Rhodes, C.A.; Lim, Y.A.L. Urbanisation and its associated factors affecting human gut microbiota: where are we heading to? Ann. Hum. Biol. 2023, 50, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Li, Mingjie, Jiuhong Ding, Catherine Stanton, R. Paul Ross, Jianxin Zhao, Bo Yang, and Wei Chen. "Bifidobacterium longum subsp. infantis FJSYZ1M3 ameliorates DSS-induced colitis by maintaining the intestinal barrier, regulating inflammatory cytokines, and modifying gut microbiota." Food & Function 14, no. 1 (2023): 354-368.
- Wang, Luanfeng, Bo Ren, Shufeng Wu, Haizhao Song, Ling Xiong, Fang Wang, and Xinchun Shen. "Current research progress, opportunities, and challenges of limosillactobacillus reuteri-based probiotic dietary strategies." Critical Reviews in Food Science and Nutrition (2024): 1-21.
- Ağagündüz, D.; Bingöl, F.G.; Çelik, E.; Cemali, Ö.; Özenir, Ç.; Özoğul, F.; Capasso, R. Recent developments in the probiotics as live biotherapeutic products (LBPs) as modulators of gut brain axis related neurological conditions. J. Transl. Med. 2022, 20, 460. [Google Scholar] [CrossRef]
- Satya, Shalni, Sakshi Sharma, Garima Choudhary, and Garima Kaushik. "Advances in Environmental Microbiology: A Multi-omic Perspective." In Microbial Omics in Environment and Health, pp. 175-204. Singapore: Springer Nature Singapore, 2024.
- Seneviratne, C.J.; Suriyanarayanan, T.; Widyarman, A.S.; Lee, L.S.; Lau, M.; Ching, J.; Delaney, C.; Ramage, G. Multi-omics tools for studying microbial biofilms: current perspectives and future directions. Crit. Rev. Microbiol. 2020, 46, 759–778. [Google Scholar] [CrossRef]
- Li, N.; Cai, Q.; Miao, Q.; Song, Z.; Fang, Y.; Hu, B. High-Throughput Metagenomics for Identification of Pathogens in the Clinical Settings. Small Methods 2020, 5, 2000792. [Google Scholar] [CrossRef]
- Xiong, W.; Abraham, P.E.; Li, Z.; Pan, C.; Hettich, R.L. Microbial metaproteomics for characterizing the range of metabolic functions and activities of human gut microbiota. Proteomics 2015, 15, 3424–3438. [Google Scholar] [CrossRef]
- Malesza, I.J.; Malesza, M.; Walkowiak, J.; Mussin, N.; Walkowiak, D.; Aringazina, R.; Bartkowiak-Wieczorek, J.; Mądry, E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells 2021, 10, 3164. [Google Scholar] [CrossRef]
- Martinez, K.B.; Leone, V.; Chang, E.B. Western diets, gut dysbiosis, and metabolic diseases: Are they linked? Gut Microbes 2017, 8, 130–142. [Google Scholar] [CrossRef]
- Sonnenburg, J.L.; Sonnenburg, E.D. Vulnerability of the industrialized microbiota. Science 2019, 366, eaaw9255. [Google Scholar] [CrossRef]
- Lange, Kathleen, Martin Buerger, Andreas Stallmach, and Tony Bruns. "Effects of antibiotics on gut microbiota." Digestive diseases 34, no. 3 (2016): 260-268.
- Cusumano, Gaia, Giancarlo Angeles Flores, Roberto Venanzoni, and Paola Angelini. "The Impact of Antibiotic Therapy on Intestinal Microbiota: Dysbiosis, Antibiotic Resistance, and Restoration Strategies." Antibiotics 14, no. 4 (2025): 371.
- Schwartz, D.J.; Langdon, A.E.; Dantas, G. Understanding the impact of antibiotic perturbation on the human microbiome. Genome Med. 2020, 12, 1–12. [Google Scholar] [CrossRef]
- Jakobsson, H.E.; Jernberg, C.; Andersson, A.F.; Sjölund-Karlsson, M.; Jansson, J.K.; Engstrand, L. Short-Term Antibiotic Treatment Has Differing Long-Term Impacts on the Human Throat and Gut Microbiome. PLOS ONE 2010, 5, e9836. [Google Scholar] [CrossRef] [PubMed]
- Marlicz, W.; Łoniewski, I.; Grimes, D.S.; Quigley, E.M. Nonsteroidal Anti-inflammatory Drugs, Proton Pump Inhibitors, and Gastrointestinal Injury: Contrasting Interactions in the Stomach and Small Intestine. Mayo Clin. Proc. 2014, 89, 1699–1709. [Google Scholar] [CrossRef]
- Wallace, J.L.; Syer, S.; Denou, E.; de Palma, G.; Vong, L.; McKnight, W.; Jury, J.; Bolla, M.; Bercik, P.; Collins, S.M.; et al. Proton Pump Inhibitors Exacerbate NSAID-Induced Small Intestinal Injury by Inducing Dysbiosis. Gastroenterology 2011, 141, 1314–1322.e5. [Google Scholar] [CrossRef] [PubMed]
- Le Bastard, Q.; Al-Ghalith, G.A.; Grégoire, M.; Chapelet, G.; Javaudin, F.; Dailly, E.; Batard, E.; Knights, D.; Montassier, E. Systematic review: human gut dysbiosis induced by non-antibiotic prescription medications. Aliment. Pharmacol. Ther. 2017, 47, 332–345. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.L.Y.; Petersen, C.; Turvey, S.E. Reduce, reinforce, and replenish: safeguarding the early-life microbiota to reduce intergenerational health disparities. Front. Public Heal. 2024, 12, 1455503. [Google Scholar] [CrossRef]
- Puigbò, P.; Leino, L.I.; Rainio, M.J.; Saikkonen, K.; Saloniemi, I.; Helander, M. Does Glyphosate Affect the Human Microbiota? Life 2022, 12, 707. [Google Scholar] [CrossRef]
- Li, P.; Qu, R.; Li, M.; Sheng, P.; Jin, L.; Huang, X.; Xu, Z.Z. Impacts of food additives on gut microbiota and host health. Food Res. Int. 2024, 196, 114998. [Google Scholar] [CrossRef]
- Laudisi, F.; Stolfi, C.; Monteleone, G. Impact of Food Additives on Gut Homeostasis. Nutrients 2019, 11, 2334. [Google Scholar] [CrossRef]
- Inan-Eroglu, E.; Ayaz, A. Effects of food additives on gut microbiota: friend or foe? Nutr. Food Sci. 2019, 49, 955–964. [Google Scholar] [CrossRef]
- Brockway, M. The role of antibiotic exposure and the effects of breastmilk and human milk feeding on the developing infant gut microbiome. Front. Public Heal. 2024, 12, 1408246. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Chung, J.; Battaglia, T.; Henderson, N.; Jay, M.; Li, H.; Lieber, A.D.; Wu, F.; Perez-Perez, G.I.; Chen, Y.; et al. Antibiotics, birth mode, and diet shape microbiome maturation during early life. Sci. Transl. Med. 2016, 8, 343ra82. [Google Scholar] [CrossRef] [PubMed]
- Thompson, Amanda L. "Developmental origins of obesity: early feeding environments, infant growth, and the intestinal microbiome." American Journal of Human Biology 24, no. 3 (2012): 350-360.
- Sartor, R.B. Genetics and Environmental Interactions Shape the Intestinal Microbiome to Promote Inflammatory Bowel Disease Versus Mucosal Homeostasis. Gastroenterology 2010, 139, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska, K.; Witkiewicz, W. Correlations of Host Genetics and Gut Microbiome Composition. Front. Microbiol. 2016, 7, 1357. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of Intestinal Mucins in Innate Host Defense Mechanisms against Pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Janssens, S.; Beyaert, R. Role of Toll-Like Receptors in Pathogen Recognition. Clin. Microbiol. Rev. 2003, 16, 637–646. [Google Scholar] [CrossRef]
- Bauer, Stefan, Thomas Müller, and Svetlana Hamm. "Pattern recognition by Toll-like receptors." Target Pattern Recognition in Innate Immunity (2009): 15-34.
- Bonder, M.J.; Kurilshikov, A.; Tigchelaar, E.F.; Mujagic, Z.; Imhann, F.; Vila, A.V.; Deelen, P.; Vatanen, T.; Schirmer, M.; Smeekens, S.P.; et al. The effect of host genetics on the gut microbiome. Nat. Genet. 2016, 48, 1407–1412. [Google Scholar] [CrossRef]
- Cuomo, Paola, Rosanna Capparelli, Marco Alifano, Antonio Iannelli, and Domenico Iannelli. "Gut microbiota host-gene interaction." International Journal of Molecular Sciences 23, no. 22 (2022): 13717.
- Marwaha, K.; Cain, R.; Asmis, K.; Czaplinski, K.; Holland, N.; Mayer, D.C.G.; Chacon, J. Exploring the complex relationship between psychosocial stress and the gut microbiome: implications for inflammation and immune modulation. J. Appl. Physiol. 2025, 138, 518–535. [Google Scholar] [CrossRef]
- Zhu, X.; Han, Y.; Du, J.; Liu, R.; Jin, K.; Yi, W. Microbiota-gut-brain axis and the central nervous system. Oncotarget 2017, 8, 53829–53838. [Google Scholar] [CrossRef]
- Montagnani, M.; Bottalico, L.; Potenza, M.A.; Charitos, I.A.; Topi, S.; Colella, M.; Santacroce, L. The Crosstalk between Gut Microbiota and Nervous System: A Bidirectional Interaction between Microorganisms and Metabolome. Int. J. Mol. Sci. 2023, 24, 10322. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, Daniela, Marjorie K. De la Fuente, Glauben Landskron, María Julieta González, Rodrigo Quera, Gerard Dijkstra, Hermie JM Harmsen, Klaas Nico Faber, and Marcela A. Hermoso. "Short chain fatty acids (SCFAs)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases." Frontiers in immunology 10 (2019): 277.
- Shin, Y.; Han, S.; Kwon, J.; Ju, S.; Choi, T.G.; Kang, I.; Kim, S.S. Roles of Short-Chain Fatty Acids in Inflammatory Bowel Disease. Nutrients 2023, 15, 4466. [Google Scholar] [CrossRef]
- Sarkar, A.; Harty, S.; Johnson, K.V.; Moeller, A.H.; Carmody, R.N.; Lehto, S.M.; Erdman, S.E.; Dunbar, R.I.M.; Burnet, P.W.J. The role of the microbiome in the neurobiology of social behaviour. Biol. Rev. 2020, 95, 1131–1166. [Google Scholar] [CrossRef] [PubMed]
- Severino, A.; Tohumcu, E.; Tamai, L.; Dargenio, P.; Porcari, S.; Rondinella, D.; Venturini, I.; Maida, M.; Gasbarrini, A.; Cammarota, G.; et al. The microbiome-driven impact of western diet in the development of noncommunicable chronic disorders. Best Pr. Res. Clin. Gastroenterol. 2024, 72, 101923. [Google Scholar] [CrossRef]
- Lloyd-Price, Jason, Galeb Abu-Ali, and Curtis Huttenhower. "The healthy human microbiome." Genome medicine 8 (2016): 1-11.
- Safarchi, Azadeh, Ghanyah Al-Qadami, Cuong D. Tran, and Michael Conlon. "Understanding dysbiosis and resilience in the human gut microbiome: biomarkers, interventions, and challenges." Frontiers in Microbiology 16 (2025): 1559521.
- Musat, N.; Halm, H.; Winterholler, B.; Hoppe, P.; Peduzzi, S.; Hillion, F.; Horreard, F.; Amann, R.; Jørgensen, B.B.; Kuypers, M.M.M. A single-cell view on the ecophysiology of anaerobic phototrophic bacteria. Proc. Natl. Acad. Sci. 2008, 105, 17861–17866. [Google Scholar] [CrossRef] [PubMed]
- DeGruttola, A.K.; Low, D.; Mizoguchi, A.; Mizoguchi, E. Current Understanding of Dysbiosis in Disease in Human and Animal Models. Inflamm. Bowel Dis. 2016, 22, 1137–1150. [Google Scholar] [CrossRef]
- Mu, Q.; Tavella, V.J.; Luo, X.M. Role of Lactobacillus reuteri in Human Health and Diseases. Front. Microbiol. 2018, 9, 757. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Guo, Z.; Zhang, P.; Li, B.; Wang, Z. Gut microbiota: A key role for human milk oligosaccharides in regulating host health early in life. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13431. [Google Scholar] [CrossRef]
- Luo, Z.; Chen, A.; Xie, A.; Liu, X.; Jiang, S.; Yu, R. Limosilactobacillus reuteri in immunomodulation: molecular mechanisms and potential applications. Front. Immunol. 2023, 14, 1228754. [Google Scholar] [CrossRef]
- Weber, Katherine T., Bernard J. Varian, and Susan E. Erdman. "The gut microbiome and sociability." Frontiers in Neuroscience 18 (2024): 1372274.
- Freedberg, D.E.; Lebwohl, B.; Abrams, J.A. The Impact of Proton Pump Inhibitors on the Human Gastrointestinal Microbiome. Clin. Lab. Med. 2014, 34, 771–785. [Google Scholar] [CrossRef]
- Beasley, D.E.; Koltz, A.M.; Lambert, J.E.; Fierer, N.; Dunn, R.R.; Li, X. The Evolution of Stomach Acidity and Its Relevance to the Human Microbiome. PLOS ONE 2015, 10, e0134116. [Google Scholar] [CrossRef]
- Walsh, J.; Griffin, B.T.; Clarke, G.; Hyland, N.P. Drug–gut microbiota interactions: implications for neuropharmacology. Br. J. Pharmacol. 2018, 175, 4415–4429. [Google Scholar] [CrossRef]
- Tasnim, N.; Abulizi, N.; Pither, J.; Hart, M.M.; Gibson, D.L. Linking the Gut Microbial Ecosystem with the Environment: Does Gut Health Depend on Where We Live? Front. Microbiol. 2017, 8, 1935–1935. [Google Scholar] [CrossRef]
- Camp, J.G.; Kanther, M.; Semova, I.; Rawls, J.F. Patterns and Scales in Gastrointestinal Microbial Ecology. Gastroenterology 2009, 136, 1989–2002. [Google Scholar] [CrossRef] [PubMed]
- Ramos Meyers, Guilherme, Hanen Samouda, and Torsten Bohn. "Short chain fatty acid metabolism in relation to gut microbiota and genetic variability." Nutrients 14, no. 24 (2022): 5361.
- Dargenio, V.N.; Cristofori, F.; Brindicci, V.F.; Schettini, F.; Dargenio, C.; Castellaneta, S.P.; Iannone, A.; Francavilla, R. Impact of Bifidobacterium longum Subspecies infantis on Pediatric Gut Health and Nutrition: Current Evidence and Future Directions. Nutrients 2024, 16, 3510. [Google Scholar] [CrossRef] [PubMed]
- Tudela, H.; Claus, S.P.; Saleh, M. Next Generation Microbiome Research: Identification of Keystone Species in the Metabolic Regulation of Host-Gut Microbiota Interplay. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, P.; Araújo, J.R.; Di Santo, J.P. A Cross-Talk Between Microbiota-Derived Short-Chain Fatty Acids and the Host Mucosal Immune System Regulates Intestinal Homeostasis and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2018, 24, 558–572. [Google Scholar] [CrossRef] [PubMed]
- Nogal, A.; Valdes, A.M.; Menni, C. The role of short-chain fatty acids in the interplay between gut microbiota and diet in cardio-metabolic health. Gut Microbes 2021, 13, 1897212. [Google Scholar] [CrossRef]
- Sanna, Serena, Natalie R. van Zuydam, Anubha Mahajan, Alexander Kurilshikov, Arnau Vich Vila, Urmo Võsa, Zlatan Mujagic et al. "Causal relationships among the gut microbiome, short-chain fatty acids and metabolic diseases." Nature genetics 51, no. 4 (2019): 600-605.
- Arora, T.; Tremaroli, V. Therapeutic Potential of Butyrate for Treatment of Type 2 Diabetes. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Rinninella, Emanuele, Pauline Raoul, Marco Cintoni, Francesco Franceschi, Giacinto Abele Donato Miggiano, Antonio Gasbarrini, and Maria Cristina Mele. "What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases." Microorganisms 7, no. 1 (2019): 14.
- Bibbo, Stefano, Gianluca Ianiro, Valentina Giorgio, Franco Scaldaferri, Luca Masucci, Antonio Gasbarrini, and G. J. E. R. M. P. S. Cammarota. "The role of diet on gut microbiota composition." European Review for Medical & Pharmacological Sciences 20, no. 22 (2016).
- Morreale, C.; Giaroni, C.; Baj, A.; Folgori, L.; Barcellini, L.; Dhami, A.; Agosti, M.; Bresesti, I. Effects of Perinatal Antibiotic Exposure and Neonatal Gut Microbiota. Antibiotics 2023, 12, 258. [Google Scholar] [CrossRef]
- Rogers, M.A.M.; Aronoff, D.M. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin. Microbiol. Infect. 2016, 22, 178.e1. [Google Scholar] [CrossRef]
- Maseda, Damian, and Emanuela Ricciotti. "NSAID–gut microbiota interactions." Frontiers in pharmacology 11 (2020): 558924.
- Washio, Ema, Motohiro Esaki, Yuji Maehata, Masashi Miyazaki, Hiroyuki Kobayashi, Hideki Ishikawa, Takanari Kitazono, and Takayuki Matsumoto. "Proton pump inhibitors increase incidence of nonsteroidal anti-inflammatory drug–induced small bowel injury: a randomized, placebo-controlled trial." Clinical Gastroenterology and Hepatology 14, no. 6 (2016): 809-815.
- Jeong, S. Factors influencing development of the infant microbiota: from prenatal period to early infancy. Clin. Exp. Pediatr. 2021, 65, 438–447. [Google Scholar] [CrossRef]
- Madan, J.C.; Hoen, A.G.; Lundgren, S.N.; Farzan, S.F.; Cottingham, K.L.; Morrison, H.G.; Sogin, M.L.; Li, H.; Moore, J.H.; Karagas, M.R. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016, 170, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Villalba Gimenez, Jazmin. "Gut Microbiome Changes in Urbanizing Brazil and Their Implications for Metabolic Health." (2025).
- Rivera, K.; Gonzalez, L.; Bravo, L.; Manjarres, L.; Andia, M.E. The Gut–Heart Axis: Molecular Perspectives and Implications for Myocardial Infarction. Int. J. Mol. Sci. 2024, 25, 12465. [Google Scholar] [CrossRef] [PubMed]
- So, D.; Whelan, K.; Rossi, M.; Morrison, M.; Holtmann, G.; Kelly, J.T.; Shanahan, E.R.; Staudacher, H.M.; Campbell, K.L. Dietary fiber intervention on gut microbiota composition in healthy adults: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2018, 107, 965–983. [Google Scholar] [CrossRef] [PubMed]
- Tu, P.; Chi, L.; Bodnar, W.; Zhang, Z.; Gao, B.; Bian, X.; Stewart, J.; Fry, R.; Lu, K. Gut Microbiome Toxicity: Connecting the Environment and Gut Microbiome-Associated Diseases. Toxics 2020, 8, 19. [Google Scholar] [CrossRef]
- Jin, Y.; Wu, S.; Zeng, Z.; Fu, Z. Effects of environmental pollutants on gut microbiota. Environ. Pollut. 2017, 222, 1–9. [Google Scholar] [CrossRef]
- Barra, N.G.; Fang, H.; Bhatwa, A.; Schmidt, A.M.; Syed, S.A.; Steinberg, G.R.; Morrison, K.M.; Surette, M.G.; Wade, M.G.; Holloway, A.C.; et al. Food supply toxicants and additives alter the gut microbiota and risk of metabolic disease. Am. J. Physiol. Metab. 2025, 328, E337–E353. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vila, A.V.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Rodenas, C.L.G.; Lepage, M.; Ngom-Bru, C.; Fotiou, A.; Papagaroufalis, K.; Berger, B. Effect of Formula Containing Lactobacillus reuteri DSM 17938 on Fecal Microbiota of Infants Born by Cesarean-Section. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Alam, M. D. , Mirza Islam, Ziaul MD, M. D. Tayab, Hossain Sahid Kamrul Alam, M. D. Kamrul, Salauddin Mahmood, and A. T. M. Haque. "Role of probiotic Lactobacillus reuteri in improving Gut Health and Immunity in infants and toddlers: a review." International Journal of Nutrition Sciences 7, no. 2 (2022): 75-80.
- Henrick, B.; Chew, S.; Mitchell, R.; Contreras, L.; Casaburi, G.; Frese, S.; Smilowitz, J.; Underwood, M. Restoring Bifidobacterium Infantis EVC001 to the Infant Gut Microbiome Significantly Reduces Intestinal Inflammation (OR12-01-19). Curr. Dev. Nutr. 2019, 3, nzz049–OR12. [Google Scholar] [CrossRef]
- Wong, Chyn Boon, Huidong Huang, Yibing Ning, and Jinzhong Xiao. "Probiotics in the new era of human milk oligosaccharides (HMOs): HMO utilization and beneficial effects of Bifidobacterium longum subsp. infantis M-63 on infant health." Microorganisms 12, no. 5 (2024): 1014.
- Gavzy, S.J.; Kensiski, A.; Lee, Z.L.; Mongodin, E.F.; Ma, B.; Bromberg, J.S. Bifidobacterium mechanisms of immune modulation and tolerance. Gut Microbes 2023, 15, 2291164. [Google Scholar] [CrossRef]
- Donald, K.; Finlay, B.B. Early-life interactions between the microbiota and immune system: impact on immune system development and atopic disease. Nat. Rev. Immunol. 2023, 23, 735–748. [Google Scholar] [CrossRef]
- Groeger, D.; O’mAhony, L.; Murphy, E.F.; Bourke, J.F.; Dinan, T.G.; Kiely, B.; Shanahan, F.; Quigley, E.M. Bifidobacterium infantis35624 modulates host inflammatory processes beyond the gut. Gut Microbes 2013, 4, 325–339. [Google Scholar] [CrossRef] [PubMed]
- Asemi, Zatollah, Zohreh Zare, Hossein Shakeri, Sima-sadat Sabihi, and Ahmad Esmaillzadeh. "Effect of multispecies probiotic supplements on metabolic profiles, hs-CRP, and oxidative stress in patients with type 2 diabetes." Annals of nutrition and metabolism 63, no. 1-2 (2013): 1-9.
- Sheil, B.; MacSharry, J.; O'Callaghan, L.; O'Riordan, A.; Waters, A.; Morgan, J.; Collins, J.K.; O'Mahony, L.; Shanahan, F. Role of interleukin (IL-10) in probiotic-mediated immune modulation: an assessment in wild-type and IL-10 knock-out mice. Clin. Exp. Immunol. 2006, 144, 273–280. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, D.; Xie, Y.; Yao, X.; Li, Y. Bifidobacterium infantis Induces Protective Colonic PD-L1 and Foxp3 Regulatory T Cells in an Acute Murine Experimental Model of Inflammatory Bowel Disease. Gut Liver 2019, 13, 430–439. [Google Scholar] [CrossRef]
- Zuo, Li, Kai-Tao Yuan, Li Yu, Qing-Hong Meng, Peter Chee-Keung Chung, and Ding-Hua Yang. "Bifidobacterium infantis attenuates colitis by regulating T cell subset responses." World Journal of Gastroenterology: WJG 20, no. 48 (2014): 18316.
- O'MAhony, C.; Scully, P.; O'MAhony, D.; Murphy, S.; O'BRien, F.; Lyons, A.; Sherlock, G.; MacSharry, J.; Kiely, B.; Shanahan, F.; et al. Commensal-Induced Regulatory T Cells Mediate Protection against Pathogen-Stimulated NF-κB Activation. PLOS Pathog. 2008, 4, e1000112. [Google Scholar] [CrossRef]
- Xie, R.; Jiang, P.; Lin, L.; Jiang, J.; Yu, B.; Rao, J.; Liu, H.; Wei, W.; Qiao, Y. Oral treatment with Lactobacillus reuteri attenuates depressive-like behaviors and serotonin metabolism alterations induced by chronic social defeat stress. J. Psychiatr. Res. 2020, 122, 70–78. [Google Scholar] [CrossRef]
- Wang, C.; Chen, W.; Jiang, Y.; Xiao, X.; Zou, Q.; Liang, J.; Zhao, Y.; Wang, Q.; Yuan, T.; Guo, R.; et al. A synbiotic formulation of Lactobacillus reuteri and inulin alleviates ASD-like behaviors in a mouse model: the mediating role of the gut–brain axis. Food Funct. 2023, 15, 387–400. [Google Scholar] [CrossRef]
- Desbonnet, L.; Garrett, L.; Clarke, G.; Kiely, B.; Cryan, J.F.; Dinan, T.G. Effects of the probiotic Bifidobacterium infantis in the maternal separation model of depression. Neuroscience 2010, 170, 1179–1188. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Prebiotic and Probiotic Modulation of the Microbiota–Gut–Brain Axis in Depression. Nutrients 2023, 15, 1880. [Google Scholar] [CrossRef] [PubMed]
- McLean, P.G.; Bergonzelli, G.E.; Collins, S.M.; Bercik, P. Targeting the microbiota–gut–brain axis to modulate behavior: Which bacterial strain will translate best to humans? Proc. Natl. Acad. Sci. 2012, 109, E174–E174. [Google Scholar] [CrossRef]
- Wang, H.; Lee, I.-S.; Braun, C.; Enck, P. Effect of Probiotics on Central Nervous System Functions in Animals and Humans: A Systematic Review. J. Neurogastroenterol. Motil. 2016, 22, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Oh, J.S. Interaction of the Vagus Nerve and Serotonin in the Gut–Brain Axis. Int. J. Mol. Sci. 2025, 26, 1160. [Google Scholar] [CrossRef]
- Park, J.C.; Im, S.-H. The gut-immune-brain axis in neurodevelopment and neurological disorders. Microbiome Res. Rep. 2022, 1, 23. [Google Scholar] [CrossRef]
- Sun, Yang, Xiujuan Li, Xiulian Li, Lun Liu, and Song Wei. "Lactobacillus yogurts display antidepressant-like effects in CUMS mice via inhibition of NF-κB pathway, activating CREB-BDNF pathway and regulating gut-brain axis." (2024).
- Borre, Yuliya E., Rachel D. Moloney, Gerard Clarke, Timothy G. Dinan, and John F. Cryan. "The impact of microbiota on brain and behavior: mechanisms & therapeutic potential." Microbial endocrinology: The microbiota-gut-brain axis in health and disease (2014): 373-403.
- Foster, J.A.; McVey Neufeld, K.-A. Gut–brain axis: How the microbiome influences anxiety and depression. Trends Neurosci. 2013, 36, 305–312. [Google Scholar] [CrossRef]
- Salvatore, S.; Pensabene, L.; Borrelli, O.; Saps, M.; Thapar, N.; Concolino, D.; Staiano, A.; Vandenplas, Y. Mind the gut: probiotics in paediatric neurogastroenterology. Benef. Microbes 2018, 9, 883–898b. [Google Scholar] [CrossRef]
- Dinan, T.G.; Cryan, J.F. Brain-Gut-Microbiota Axis and Mental Health. Psychosom. Med. 2017, 79, 920–926. [Google Scholar] [CrossRef]
- Mobini, R.; Tremaroli, V.; Ståhlman, M.; Karlsson, F.; Levin, M.; Ljungberg, M.; Sohlin, M.; Forslund, H.B.; Perkins, R.; Bäckhed, F.; et al. Metabolic effects of Lactobacillus reuteriDSM 17938 in people with type 2 diabetes: A randomized controlled trial. Diabetes, Obes. Metab. 2016, 19, 579–589. [Google Scholar] [CrossRef]
- Patro-Gołąb, B.; Szajewska, H. Systematic Review with Meta-Analysis: Lactobacillus reuteri DSM 17938 for Treating Acute Gastroenteritis in Children. An Update. Nutrients 2019, 11, 2762. [Google Scholar] [CrossRef] [PubMed]
- Dinleyici, Ener Cagri, PROBAGE Study Group, Yvan Vandenplas, Nazan Dalgic, Sirin Guven, Özge Metin, Makbule Eren et al. "Lactobacillus reuteri DSM 17938 effectively reduces the duration of acute diarrhoea in hospitalised children." Acta Paediatrica 103, no. 7 (2014): e300-e305.
- Wang, G.; Huang, S.; Cai, S.; Yu, H.; Wang, Y.; Zeng, X.; Qiao, S. Lactobacillus reuteri Ameliorates Intestinal Inflammation and Modulates Gut Microbiota and Metabolic Disorders in Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2020, 12, 2298. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Zheng, F.; Stanton, C.; Ross, R.P.; Zhao, J.; Zhang, H.; Chen, W. Lactobacillus reuteri FYNLJ109L1 Attenuating Metabolic Syndrome in Mice via Gut Microbiota Modulation and Alleviating Inflammation. Foods 2021, 10, 2081. [Google Scholar] [CrossRef] [PubMed]
- Marras, L.; Caputo, M.; Bisicchia, S.; Soato, M.; Bertolino, G.; Vaccaro, S.; Inturri, R. The Role of Bifidobacteria in Predictive and Preventive Medicine: A Focus on Eczema and Hypercholesterolemia. Microorganisms 2021, 9, 836. [Google Scholar] [CrossRef]
- Aron-Wisnewsky, Judith, Moritz V. Warmbrunn, Max Nieuwdorp, and Karine Clément. "Metabolism and metabolic disorders and the microbiome: the intestinal microbiota associated with obesity, lipid metabolism, and metabolic health—pathophysiology and therapeutic strategies." Gastroenterology 160, no. 2 (2021): 573-599.
- Fujimori, S.; Gudis, K.; Mitsui, K.; Seo, T.; Yonezawa, M.; Tanaka, S.; Tatsuguchi, A.; Sakamoto, C. A randomized controlled trial on the efficacy of synbiotic versus probiotic or prebiotic treatment to improve the quality of life in patients with ulcerative colitis. Nutrition 2009, 25, 520–525. [Google Scholar] [CrossRef]
- Vangay, P.; Johnson, A.J.; Ward, T.L.; Al-Ghalith, G.A.; Shields-Cutler, R.R.; Hillmann, B.M.; Lucas, S.K.; Beura, L.K.; Thompson, E.A.; Till, L.M.; et al. US Immigration Westernizes the Human Gut Microbiome. Cell 2018, 175, 962–972.e10. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate Improves Insulin Sensitivity and Increases Energy Expenditure in Mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- Sheng, K.; He, S.; Sun, M.; Zhang, G.; Kong, X.; Wang, J.; Wang, Y. Synbiotic supplementation containing Bifidobacterium infantis and xylooligosaccharides alleviates dextran sulfate sodium-induced ulcerative colitis. Food Funct. 2020, 11, 3964–3974. [Google Scholar] [CrossRef]
- Simon, M.-C.; Strassburger, K.; Nowotny, B.; Kolb, H.; Nowotny, P.; Burkart, V.; Zivehe, F.; Hwang, J.-H.; Stehle, P.; Pacini, G.; et al. Intake of Lactobacillus reuteri Improves Incretin and Insulin Secretion in Glucose-Tolerant Humans: A Proof of Concept. Diabetes Care 2015, 38, 1827–1834. [Google Scholar] [CrossRef]
- Urbańska, M.; Gieruszczak-Białek, D.; Szajewska, H. Systematic review with meta-analysis: Lactobacillus reuteri DSM 17938 for diarrhoeal diseases in children. Aliment. Pharmacol. Ther. 2016, 43, 1025–1034. [Google Scholar] [CrossRef]
- Jang, H.-M.; Lee, K.-E.; Kim, D.-H. The Preventive and Curative Effects of Lactobacillus reuteri NK33 and Bifidobacterium adolescentis NK98 on Immobilization Stress-Induced Anxiety/Depression and Colitis in Mice. Nutrients 2019, 11, 819. [Google Scholar] [CrossRef]
- Ahn, S.-I.; Cho, S.; Jeon, E.; Park, M.; Chae, B.; Ditengou, I.C.P.; Choi, N.-J. The Effect of Probiotics on Intestinal Tight Junction Protein Expression in Animal Models: A Meta-Analysis. Appl. Sci. 2022, 12, 4680. [Google Scholar] [CrossRef]
- Dooling, S.W.; Sgritta, M.; Wang, I.-C.; Duque, A.L.R.F.; Costa-Mattioli, M.; Yildirim, S. The Effect of Limosilactobacillus reuteri on Social Behavior Is Independent of the Adaptive Immune System. mSystems 2022, 7, e0035822. [Google Scholar] [CrossRef]
- Shi, Y.-C.; Cai, S.-T.; Tian, Y.-P.; Zhao, H.-J.; Zhang, Y.-B.; Chen, J.; Ren, R.-R.; Luo, X.; Peng, L.-H.; Sun, G.; et al. Effects of Proton Pump Inhibitors on the Gastrointestinal Microbiota in Gastroesophageal Reflux Disease. Genom. Proteom. Bioinform. 2019, 17, 52–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




