Submitted:
12 August 2025
Posted:
12 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
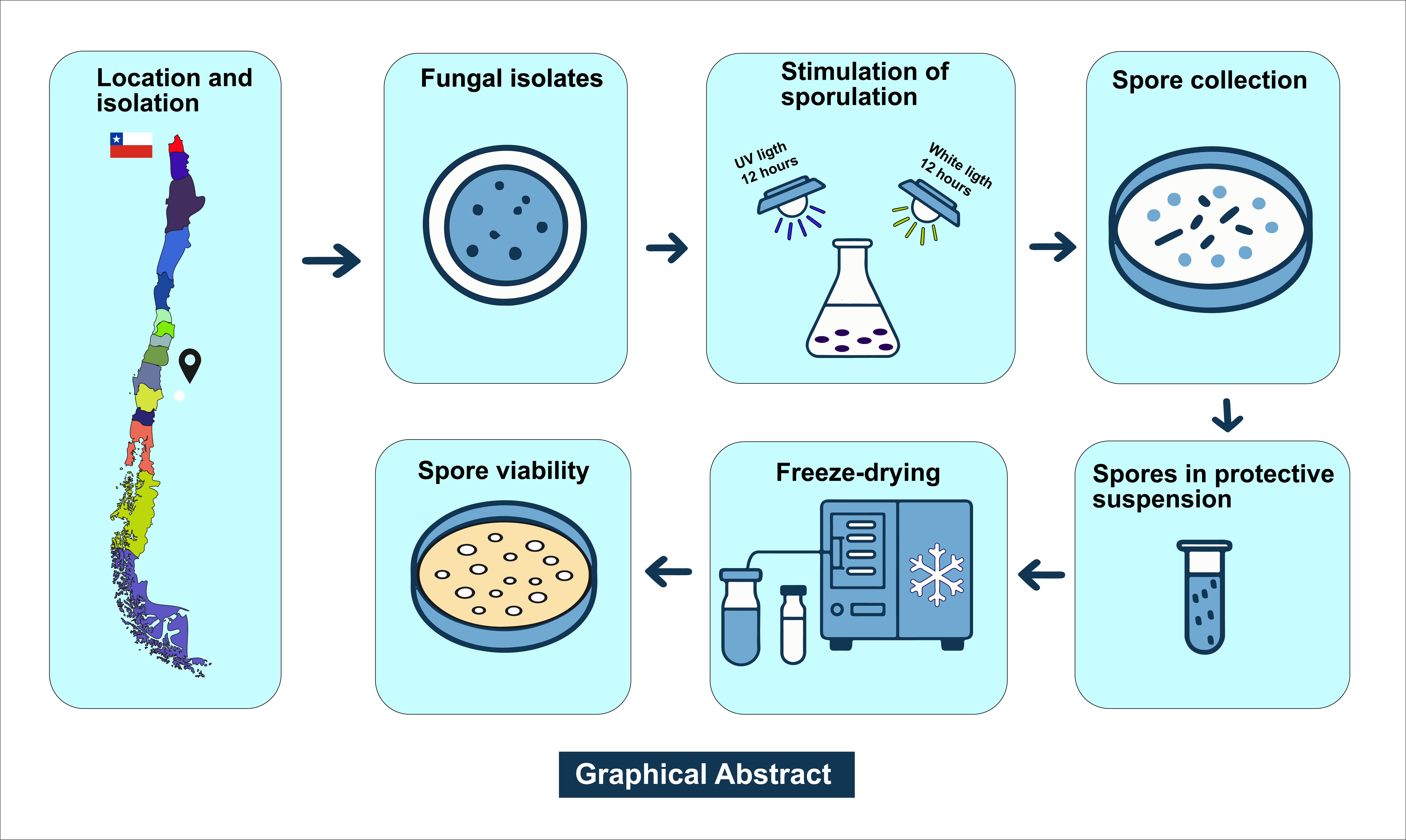
2.1. Study Location
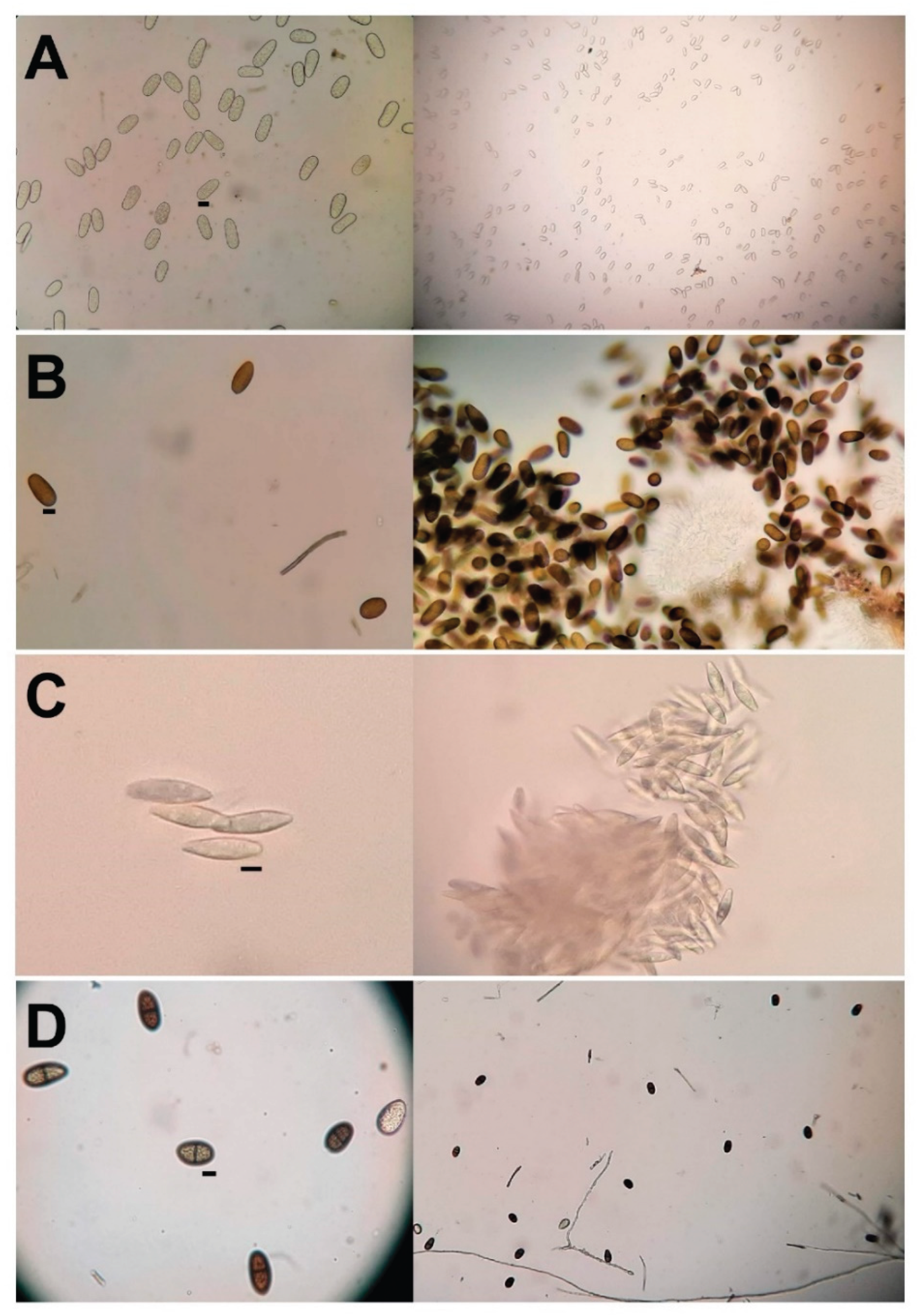
2.2. Isolation and Molecular Identification
2.3. Conidial Production
2.4. Spore Collection, Protective Suspension Media, and Freeze-Drying
2.5. Spore Viability
2.6. Data Availability
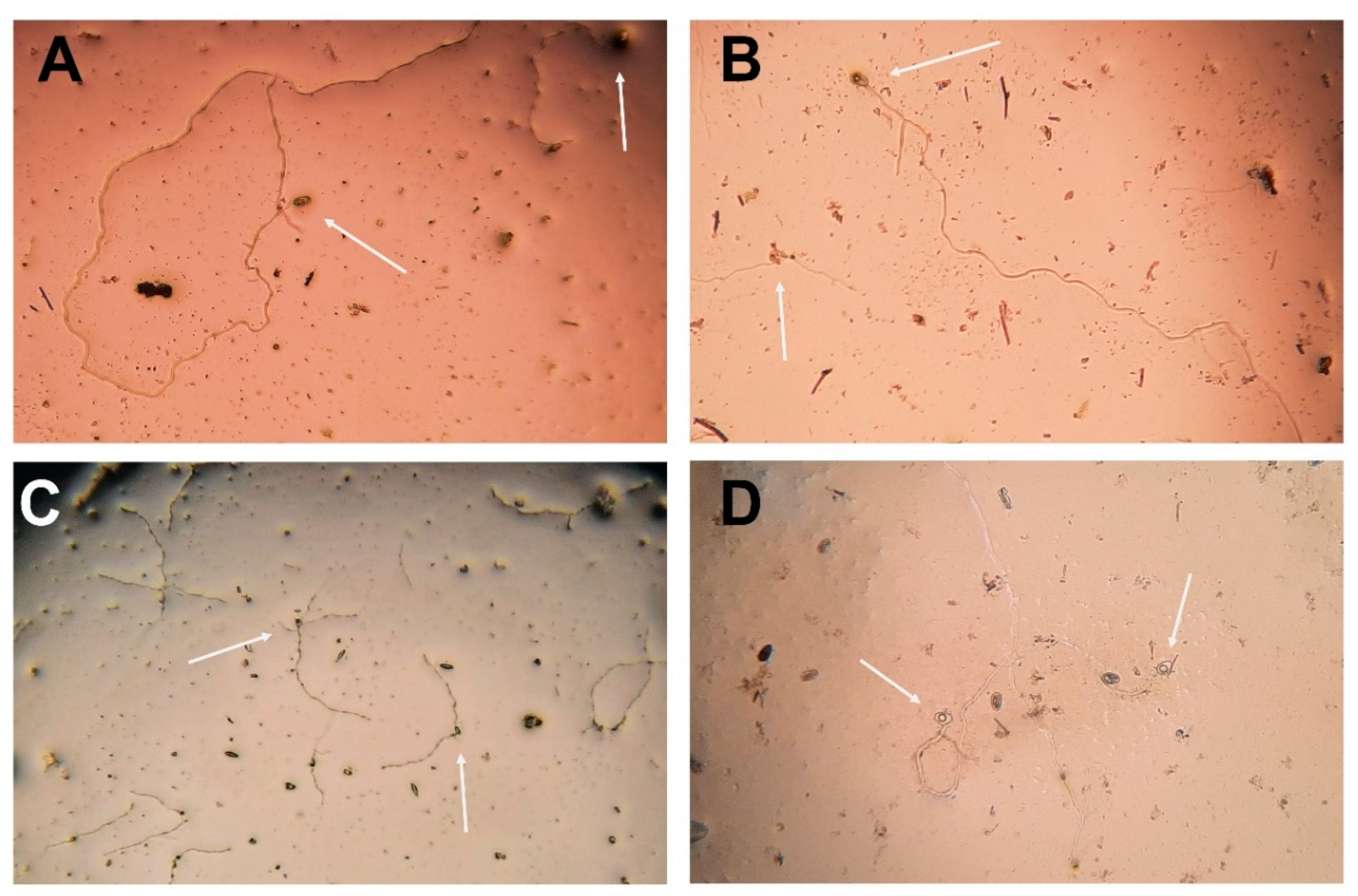
3. Results
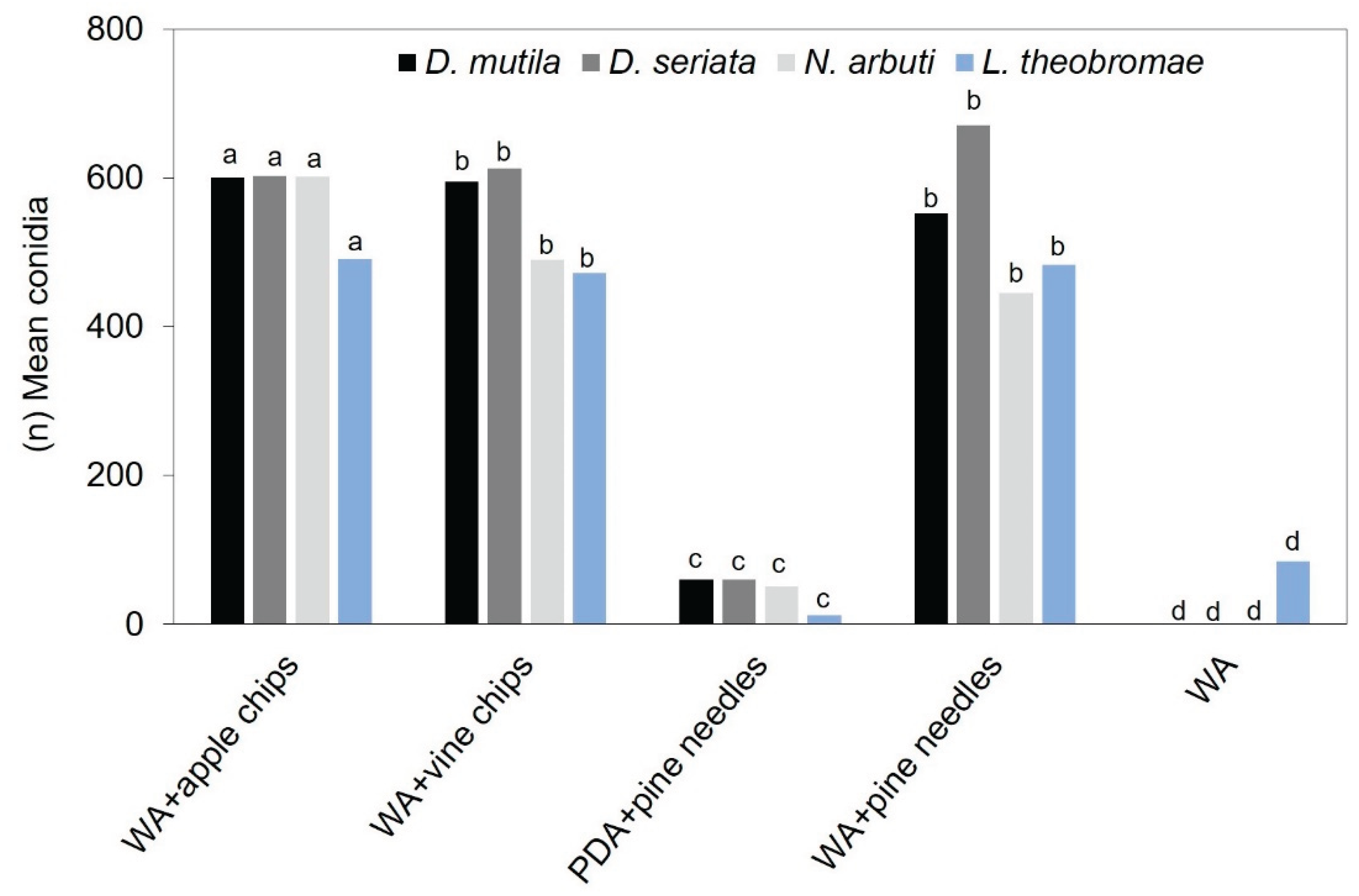
3.1. Conidia Production
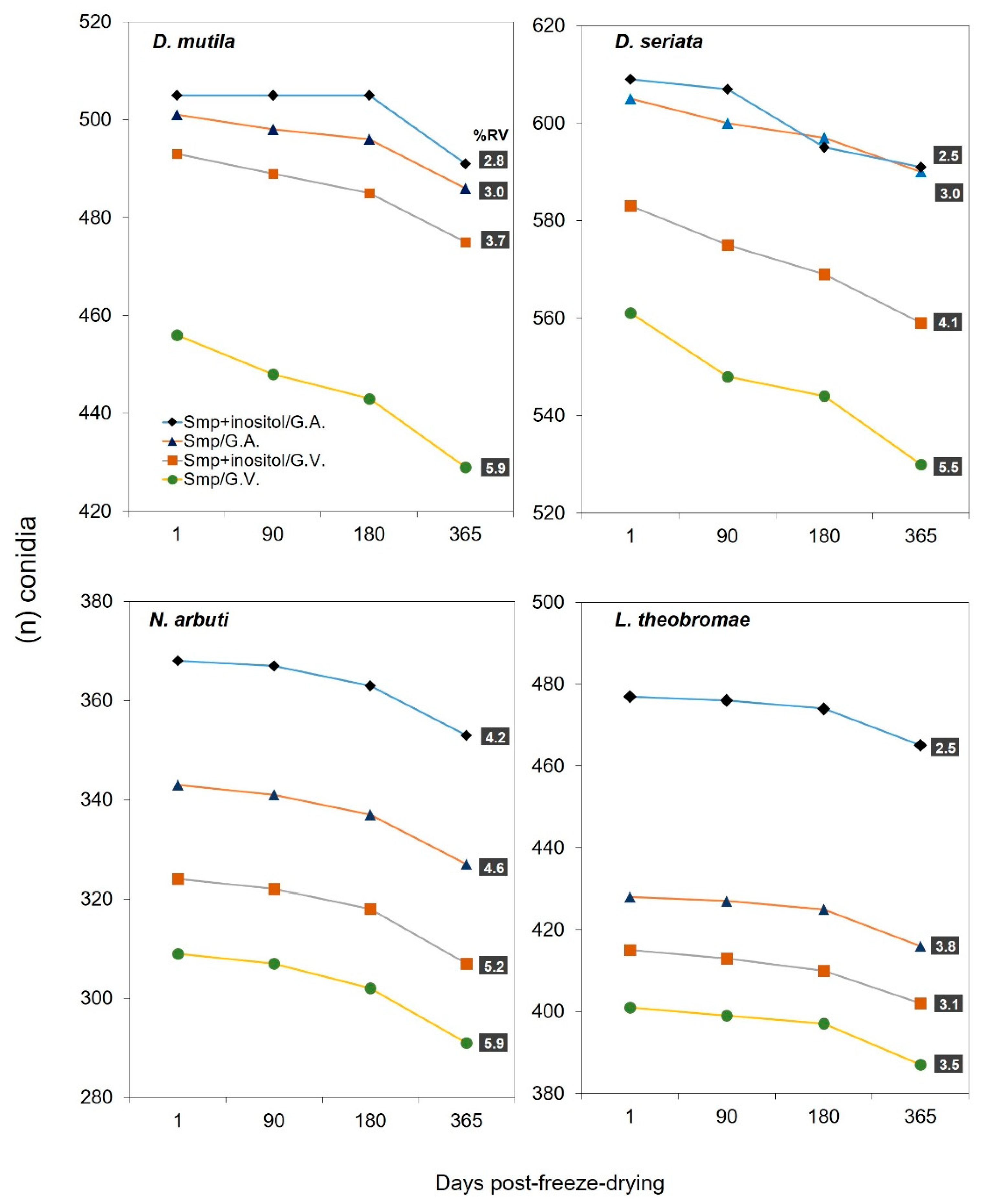
3.2. Lyophilization and Spore Viability
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latorre, B.A.; Toledo, M.V. Occurrence and relative susceptibility of apple cultivars to Botryosphaeria canker in Chile. Plant Dis. 1984, 68, 36–39. [Google Scholar] [CrossRef]
- Brown-Rytlewski, D.E.; McManus, P.S. Virulence of Botryosphaeria dothidea and Botryosphaeria obtusa on apple and management of stem cankers with fungicides. Plant Dis. 2000, 84, 1031–1037. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: diversity, ecology and impact. Fungal Biol. Rev. 2007, 212, 90–106. [Google Scholar] [CrossRef]
- Phillips, A. J. L.; Lopes, J.; Abdollahzadeh, J.; Bobev, S.; Alves, A. Resolving the Diplodia complex on apple and other Rosaceae hosts. Persoonia. 2012, 29, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T. B.; Aldwinkle, H.S.; Agnello, A.M.; Walgenbach, J.F. Compendium of apple and pear diseases and pest. Second edition. American Phytopathological Society Press, 2014. [Google Scholar]
- Abdollahzadeh, J. Diplodia bulgarica, as a new pathogen and potential threat to the apple industry in Iran. Phytopathol. Mediterr. 2015, 54, 128–132. [Google Scholar]
- Delgado-Cerrone, L.; Mondino-Hintz, P.; Alaniz-Ferro, S. Botryosphaeriaceae species associated with stem canker, die-back and fruit rot on apple in Uruguay. Eur. J. Plant Pathol. 2016, 146, 637–655. [Google Scholar] [CrossRef]
- Havenga, M.; Gatsi, G.M.; Halleen, F.; Spies, C.F.J.; van der Merwe, R.; Mostert, L. Canker and Wood Rot Pathogens Present in Young Apple Trees and Propagation Material in the Western Cape of South Africa. Plant Dis. 2019, 103, 3129–3141. [Google Scholar] [CrossRef]
- Martino, I.; Agustí-Brisach, C.; Nari, L.; Gullino, M.L.; Guarnaccia, V. Characterization and pathogenicity of fungal species associated with dieback of apple trees in Northern Italy. Plant Dis. 2024, 108, 311–331. [Google Scholar] [CrossRef]
- Díaz, G.A.; Valdez, A.; Halleen, F.; Ferrada, E.; Lolas, M.; Latorre, B.A. Characterization and pathogenicity of Diplodia, Lasiodiplodia, and Neofusicoccum species causing Botryosphaeria canker and dieback of apple trees in central Chile. Plant Dis. 2022, 106, 925–937. [Google Scholar] [CrossRef]
- Marsh, P.B.; Taylor, E.E.; Bassler, L.M. A guide to the literature on certain effects of light on fungi: reproduction, morphology, pigmentation, and phototropic phenomena. CABI. 1959, 251–312. [Google Scholar]
- Dahlberg, K.R.; Etten, J.L.V. Physiology and biochemistry of fungal sporulation. Annu. Rev. Phytopatho. 1982, 1982. 20, 281–301. [Google Scholar] [CrossRef]
- Betina, V. Photoinduced conidiation in Trichoderma viride. Folia Microbiol. 1995, 40, 219–224. [Google Scholar] [CrossRef]
- Xu, L.L.; Li, F.; Xie, H.Y.; Liu, X.Z. A novel method for promoting conidial production by a nematophagous fungus, Pochonia chlamydosporia AS6.8. World J. Microbiol. Biotechnol. 2009, 25, 1989–1994. [Google Scholar] [CrossRef]
- Crous, P.W.; Slippers, B.; Wingfield, M.J.; Rheeder, J.; Marasas, W.F.; Philips, A.J.; Groenewald, J. Z. Phylogenetic lineages in the Botryosphaeriaceae. Stud. Mycol. 2006, 55, 235–253. [Google Scholar] [CrossRef]
- Miller Jr, O.K. The role of light in the fruiting of Panus fragilis. Can. J. Bot. 1967, 4511, 1939–1943. [Google Scholar] [CrossRef]
- Starostzik, C.; Marwan, W. A photoreceptor with characteristics of phytochrome triggers sporulation in the true slime mould Physarum polycephalum. FEBS Lett. 1995, 370, 146–148. [Google Scholar] [CrossRef]
- Idnurm, A.; Rodríguez-Romero, J.; Corrochano, L.M.; Sanz, C.; Iturriaga, E.A.; Eslava, A.P.; Heitman, J. The Phycomyces madA gene encodes a blue-light photoreceptor for phototropism and other light responses. Proc. Natl. Aca. Sci. 2006, 103, 4546–4551. [Google Scholar] [CrossRef]
- Rakoczy, L. Influence of monochromatic light on the fructification of Physarum nudum. Acta Soc Bot Pol. 1963, 11, 559–562. [Google Scholar]
- Rakoczy, L. Action spectrum in sporulation of slime-mold Physarum nudum Macbr. Acta Soc Bot Pol. 1965, 341, 97–112. [Google Scholar] [CrossRef]
- Corrochano, L.M. Fungal photoreceptors: sensory molecules for fungal development and behaviour. Photochem. Photobiol. Sci. 2007, 6, 725–736. [Google Scholar] [CrossRef]
- Rodriguez-Romero, J.; Hedtke, M.; Kastner, C.; Müller, S.; Fischer, R. Fungi, hidden in soil or up in the air: light makes a difference. Annu. Rev. Microbiol. 2010, 641, 585–610. [Google Scholar] [CrossRef]
- Ruger-Herreros, C.; Rodríguez-Romero, J.; Fernández-Barranco, R.; Olmedo, M.; Fischer, R.; Corrochano, L.M.; Canovas, D. Regulation of conidiation by light in Aspergillus nidulans. Genetics. 2011, 1884, 809–822. [Google Scholar] [CrossRef] [PubMed]
- Froehlich, A.C.; Liu, Y.; Loros, J.J.; Dunlap, J.C. White Collar-1, a circadian blue light photoreceptor, binding to the frequency promoter. Science. 2002, 297, 815–819. [Google Scholar] [CrossRef]
- He, Q.; Cheng, P.; Yang, Y.; Wang, L.; Gardner, K.H.; Liu, Y. White collar-1, a DNA binding transcription factor and a light sensor. Science. 2002, 297, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Schafmeier, T.; Diernfellner, A.C. Light input and processing in the circadian clock of Neurospora. FEBS Lett. 2011, 585, 1467–1473. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Arreguín, A.; Pérez-Martínez, A.S.; Herrera-Estrella, A. Proteomic analysis of Trichoderma atroviride reveals independent roles for transcription factors BLR-1 and BLR-2 in light and darkness. Eukaryot. Cell. 2012, 111, 30–41. [Google Scholar] [CrossRef]
- Smith, D. Long-term preservation of test strains fungus. Int. Biodeterior. Biodegrad. 1993, 313, 227–230. [Google Scholar]
- Baskarathevan, J.; Jaspers, M.V.; Jones, E.E.; Ridgway, H.J. Evaluation of different storage methods for rapid and costeffective preservation of Botryosphaeria species. N. Z. Plant Prot. 2009, 62, 234–237. [Google Scholar] [CrossRef]
- Smith, D.; Onions, A.H.S. The preservation and maintenance of living fungi, 2nd edition. IMI Technical Handbook 2. CAB International, Wallingford, Oxon, United Kingdom.
- Burdsall Jr, H.H.; Dorworth, E.B. Preserving cultures of wood-decaying Basidiomycotina using sterile distilled water in cryovials. Mycologia. 1994, 86, 275–280. [Google Scholar] [CrossRef]
- Elliott, M.L. Survival, growth and pathogenicity of Gaeumannomyces graminis var. graminis with different methods of long-term storage. Mycologia.
- Bueno, C.J.; Ambrósio, M.M.; Souza, N.L. Storage of soilborne phytopathogenic fungi. Summa Phytopathol. 2006, 32, 42–50. [Google Scholar] [CrossRef]
- Ryan, M.J.; Jeffries, P.; Bridge, P.D.; Smith, D. Developing cryopreservation protocols to secure fungal gene function. Cryo Lett. 2001, 222, 115–124. [Google Scholar]
- Borman, A.M.; Szekely, A.; Campbell, C.K.; Johnson, E.M. Evaluation of the viability of pathogenic filamentous fungi after prolonged storage in sterile water and review of recent published studies on storage methods. Mycopathologia. 2006, 161, 361–368. [Google Scholar] [CrossRef]
- Smith, D.; Onions, A.H. A comparison of some preservation techniques for fungi. Trans. Br. Mycol. Soc. 1983, 813, 535–540. [Google Scholar] [CrossRef]
- Gallo, M.B.; Guimarães, D.O.; Momesso, L.D.; Pupo, M.T. Natural products from endophytic fungi. Microbiol. Biotechnol. 2008, 4, 39–147. [Google Scholar]
- Ward, K.R.; Matejtschuk, P. Lyophilization of Pharmaceuticals and Biologicals. 2019. Springer New York, New York, NY.
- Nasran, H.S.; Mohd Yusof, H.; Halim, M.; Abdul Rahman, N.A. Optimization of protective agents for the freeze-drying of Paenibacillus polymyxa Kp10 as a potential biofungicide. Molecules. 2020, 2511, 2618. [Google Scholar] [CrossRef] [PubMed]
- Olukotun, G.B.; Adamu, B.B.; Asake, O.J.; Zobeashia, S.S. Preservation of Indigenous Fungal Cultures by Freeze Drying Technique using Skim Milk and Honey as the Protectants. Asian J. Biotechnol. Bioresour. Technol. 2021, 73, 36–42. [Google Scholar] [CrossRef]
- Guerrero-Sanchez, M.; Passot, S.; Campoy, S.; Olivares, M.; Fonseca, F. Effect of protective agents on the storage stability of freeze-dried Ligilactobacillus salivarius CECT5713. Appl. Microbiol. Biotechnol. 2022, 106, 7235–7249. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.S.; Van Ingen, C.W.; Talsma, H.; Van Miltenburg, J.C.; Steffensen, C.L.; Vlug, I.A.; Stalpers, J.A. Freeze-drying of fungi: influence of composition and glass transition temperature of the protectant. Cryobiology. 1995, 32, 60–67. [Google Scholar] [CrossRef]
- Bunse, T.; Steigleder, G.K. The preservation of fungal cultures by lyophilization: Die Konservierung von Pilzkulturen mittels Gefriertrocknung. Mycoses. 1991, 34, 173–176. [Google Scholar] [CrossRef]
- Larena, I.; Melgarejo, P.; De Cal, A. Drying of conidia of Penicillium oxalicum, a biological control agent against Fusarium wilt of tomato. J. Phytopathol. 2003, 151, 600–606. [Google Scholar] [CrossRef]
- Milošević, M.; Medić-Pap, S.; Ignjatov, M.; Milošević, D. Lyophilization as a method for pathogens long term preservation. Zb. Mat. Srp. Prirod. Nauke. 2007, 113, 203–210. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Messner, R.; Gruber, F.; Mach, R.L.; Kubicek-Pranz, E.M. The Trichoderma cellulase regulatory puzzle: from the interior life of a secretory fungus. Enzyme Microb. Technol. 1993, 15, 90–99. [Google Scholar] [CrossRef]
- Hardham, A.R. Cell biology of fungal and Oomycete infection of plants. In Biology of the Fungal Cell. Berlin, Heidelberg: Springer Berlin Heidelberg. 2007, pp. 251-289.
- Leach, C.M. Sporulation of diverse species of fungi under near-ultraviolet radiation. Can. J. Bot. 1962, 401, 151–161. [Google Scholar] [CrossRef]
- Su, Y.Y.; Qi, Y.L.; Cai, L. Induction of sporulation in plant pathogenic fungi. Mycology. 2012, 33, 195–200. [Google Scholar] [CrossRef]
- Cruickshank, I.A.M. Environment and Sporulation in Phytopathogenic Fungi Li. Conidia Formation in Peronospora tabacina Adam as a Function of Temperature. Aust. J. Biol. Sci. 1961, 14, 198–207. [Google Scholar] [CrossRef]
- Zhao, Q.; Shi, Y.; Wang, Y.; Xie, X.; Li, L.; Fan, T.; Li, B. Temperature and Humidity Regulate Sporulation of Corynespora cassiicola That Is Associated with Pathogenicity in Cucumber Cucumis sativus L. Biology. Biology. 2022, 11, 1675. [Google Scholar] [CrossRef] [PubMed]
- Batista, E.; Lopes, A.; Alves, A. What do we know about Botryosphaeriaceae? An overview of a worldwide cured dataset. Forests. 2021, 12, 313. [Google Scholar] [CrossRef]
- Rockinger, U.; Funk, M.; Winter, G. Current approaches of preservation of cells during freeze- drying. J. Pharm. Sci. 2021, 1108, 2873–2893. [Google Scholar] [CrossRef]
- Balla, T. Inositol-lipid binding motifs: signal integrators through protein-lipid and protein-protein interactions. J. Cell Sci. 2005, 118, 2093–2104. [Google Scholar] [CrossRef]
- Crowe, J.H.; Crowe, L.M.; Chapman, D. Preservation of membranes in anhydrobiotic organisms: the role of trehalose. Science. 1984, 223, 701–703. [Google Scholar] [CrossRef]
- Crowe, J.H.; Carpenter, J.F.; Crowe, L.M.; Anchordoguy, T.J. Are freezing and dehydration similar stress vectors? A comparison of modes of interaction of stabilizing solutes with biomolecules. Cryobiology. 1990, 27, 219–231. [Google Scholar] [CrossRef]
- Sinskey, T.J.; Silverman, G.J.; Goldblith, S.A. Influence of Platen Temperatures and Storage Conditions on the Survival of Freeze-dried Salmonella typhimurium. Appl. Microbiol. 1967, 151, 22–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




