Submitted:
10 August 2025
Posted:
11 August 2025
You are already at the latest version
Abstract
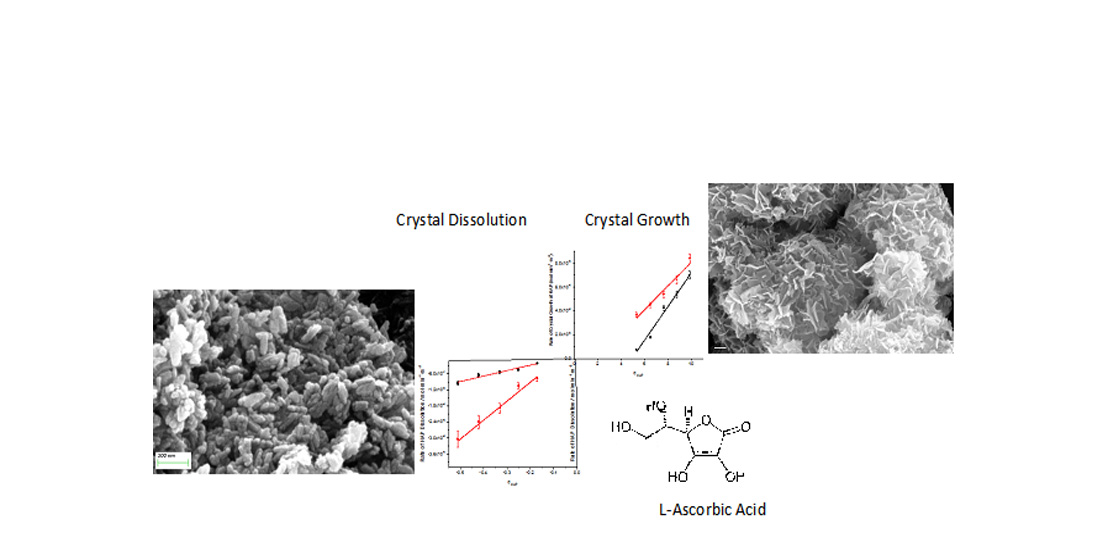
Keywords:
1. Introduction
2. Materials and Methods
2.1. Solutions
2.2. Preparation of HAP seed crystals
2.3. Crystallization of HAP from supersaturated solutions
2.3.1. Crystal growth at constant solution supersaturation
2.3.2. Dissolution of HAP crystals at constant solution undersaturation
2.3.3. Adsorption measurements
2.3.4. X-Ray Photoelectron spectroscopy
2.3.5. Zeta potential measurements
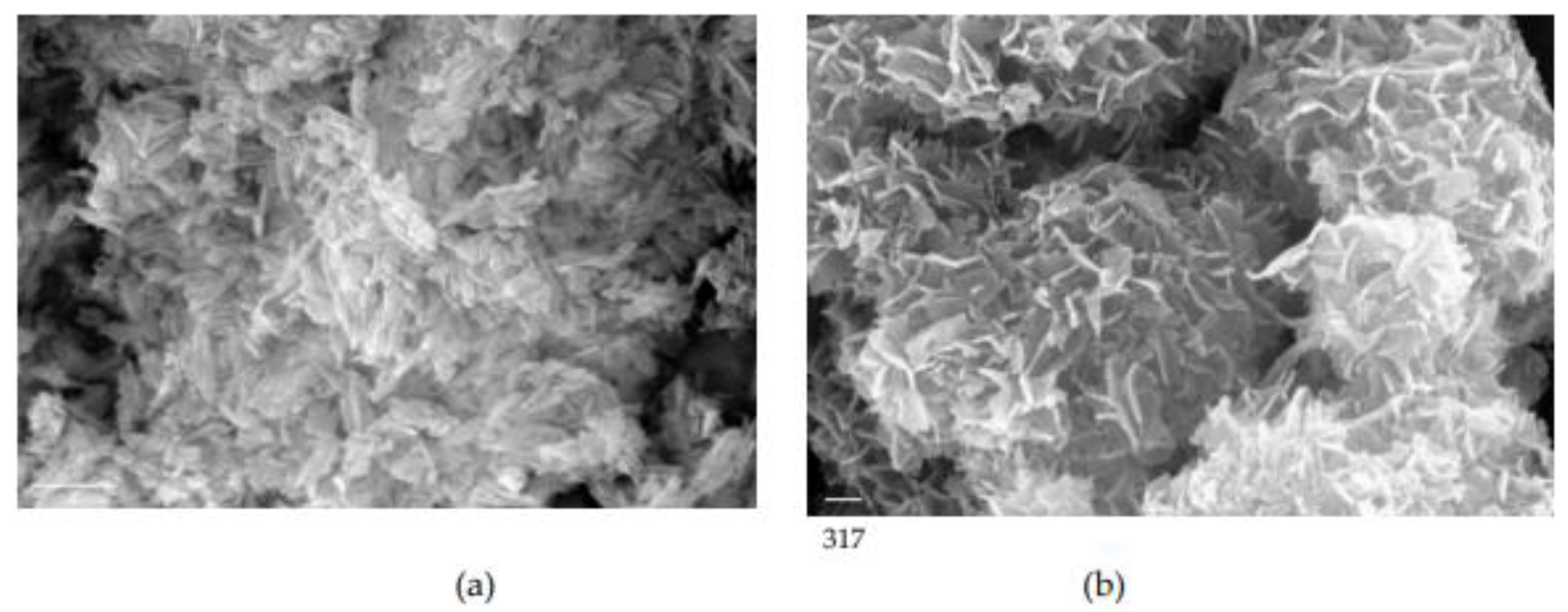
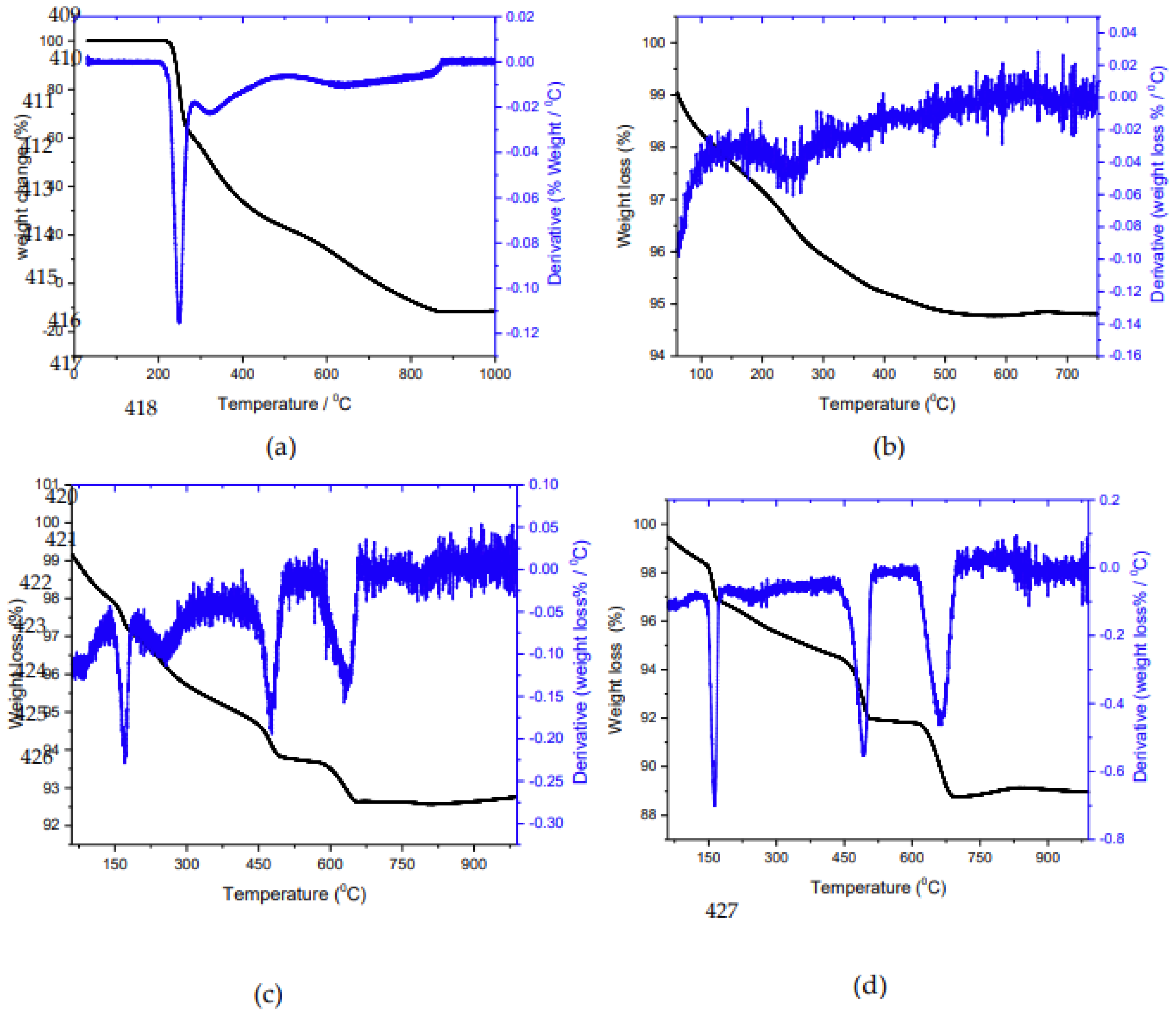
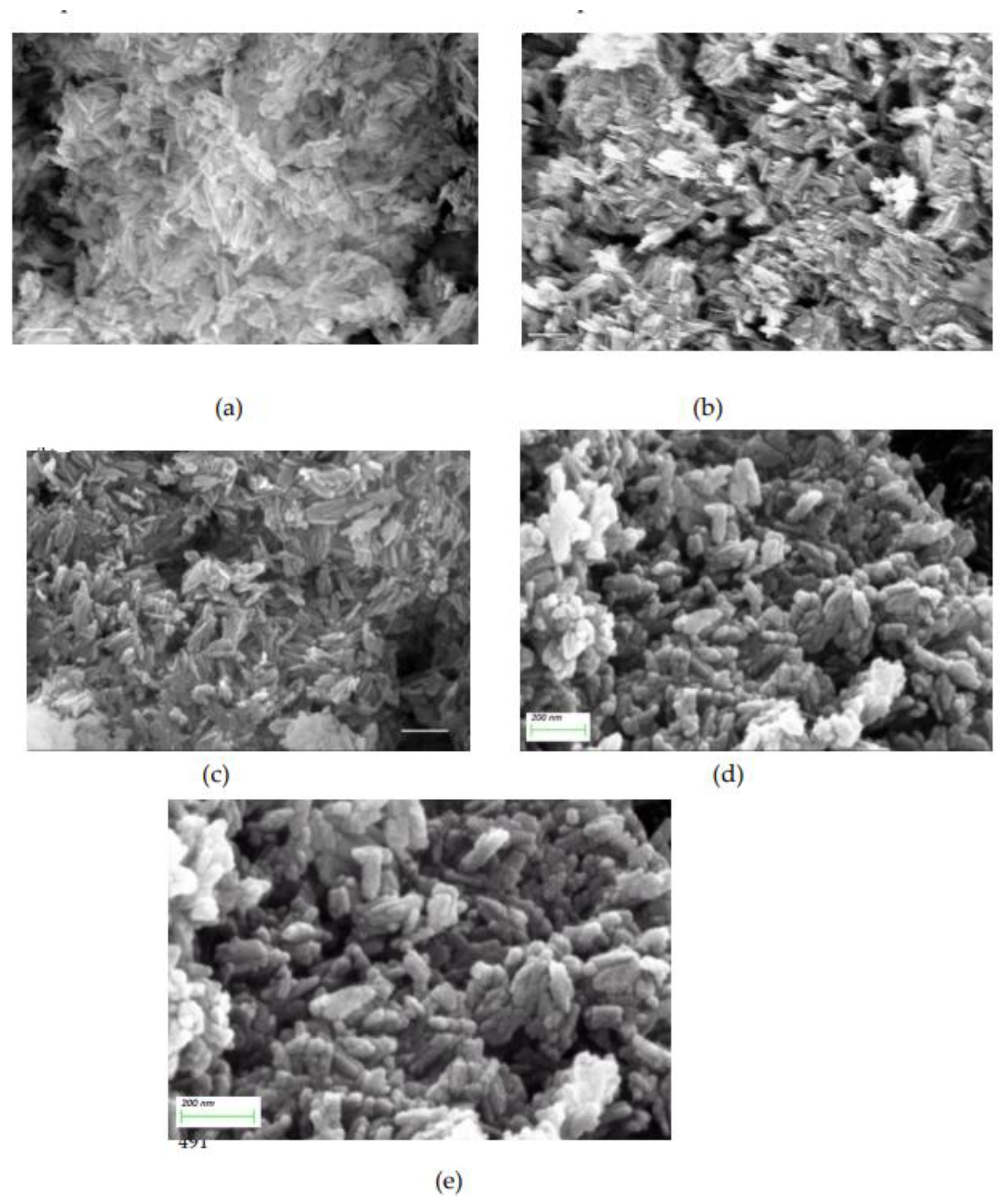
3. Results
3.1. Crystal growth of HAP
3.1.1. Kinetics measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
Abbreviations
| HAP | Hydroxyapatite (Ca5(PO4)3OH |
| AA | Ascorbic Acid (C9H12O6) |
| XPS | X-Ray Photoelectron Sectroscopy |
| TG | Thermo Gravimetric |
| DTG | Differential Thermogravinmetric |
| SEM | Scanning Electron Microscopy |
References
- D'Alessandro CC, Komninou MA, Badria AF, Korossis S, Koutsoukos P, Mavrilas D. Calcification Assessment of Bioprosthetic Heart Valve Tissues Using an Improved In Vitro Model. IEEE Trans Biomed Eng. 2020 Sep;67(9):2453-2461. [CrossRef] [PubMed]
- Wen Shuyu , Zhou Ying , Yim Wai Yen , Wang Shijie , Xu Li , Shi Jiawei , Qiao Weihua , Dong Nianguo. Mechanisms and Drug Therapies of Bioprosthetic Heart Valve Calcification, Frontiers in Pharmacology 13 – 2022 https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2022.909801. [CrossRef]
- D'Alessandro, C. , Komninou, M. & ,Badria, A., Korossis, S., Koutsoukos, P., Mavrilas, D. (2020). Calcification Assessment of Bioprosthetic Heart Valve Tissues Using an Improved In Vitro Model. IEEE Transactions on Biomedical Engineering. PP. 1-1. [CrossRef]
- Onnis,C. , Virmani, R., Kawai,K., Nardi,V., Lerman, A., Cademartiri, F., Scicolone, R., Boi, A., Congiu, T., Faa, G., Libby, P. , Saba, L. Coronary Artery Calcification: Current Concepts and Clinical Implications, Circulation, 149, (3), 2024; 251-266. [CrossRef]
- Onea, H.-L.; Olinic, M.; Lazar, F.-L.; Homorodean, C.; Ober, M.C.; Spinu, M.; Achim, A.; Tataru, D.A.; Olinic, D.M. A Review Paper on Optical Coherence Tomography Evaluation of Coronary Calcification Pattern: Is It Relevant Today? J. Cardiovasc. Dev. Dis. 2024, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Apple D.J., Werner L., Escobar-Gomez M., Pandey S.K. Deposits on the Optical Surfaces of Hydroview Intraocular Lenses. J. Cataract. Refract. Surg. 2000;26:796–797. [CrossRef]
- Bopp S, Özdemir HB, Aktaş Z, Khoramnia R, Yildirim TM, Schickhardt S, Auffarth GU, Özdek Ş. Clinical Characteristics of Patients with Intraocular Lens Calcification after Pars Plana Vitrectomy. Diagnostics (Basel). 2023 Jun 1;13(11):1943. [CrossRef] [PubMed] [PubMed Central]
- Katsimpris J.M., Theoulakis P.E., Kouzi-Koliakos K., Pavlidou E., Petropoulos I.K., Koliakos G., Vouroutzis N., Konstas A.G. Late Postoperative Opacification of Hydrogel Intraocular Lenses: Analysis of 13 Explanted Lenses. Klin. Monbl. Augenheilkd. 2009;226:264–271. [CrossRef]
- Koutsoukos, P.G.; Natsi, P.D.; Gartaganis, S.P.; Gartaganis, P.S. Biological Mineralization of Hydrophilic Intraocular Lenses. Crystals 2022, 12, 1418. [Google Scholar] [CrossRef]
- Drimtzias EG, Rokidi SG, Gartaganis SP, Koutsoukos PG. Experimental investigation on mechanism of hydrophilic acrylic intraocular lens calcification. Am J Ophthalmol. 2011 Nov;152(5):824-33.e1. [CrossRef] [PubMed]
- E. Bonucci, Biological Calcification, Springer-Verlag, Berlin, Heidelberg, 2007, 592 pp.
- Söhnel O, Grases F. Supersaturation of body fluids, plasma and urine, with respect to biological hydroxyapatite. Urol Res. 2011 Dec;39(6):429-36. [CrossRef] [PubMed]
- Di Costanzo, L.F. Atomic Details of Biomineralization Proteins Inspiring Protein Design and Reengineering for Functional Biominerals. Chemistry 2022, 4, 827–847. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Falini, G.; García-Ruiz, J.M. Biological Crystallization. Crystals 2019, 9, 409. [Google Scholar] [CrossRef]
- Tavafoghi M, Cerruti M. The role of amino acids in hydroxyapatite mineralization. J R Soc Interface. 2016 Oct;13(123):20160462. [CrossRef] [PubMed] [PubMed Central]
- Nancollas, G.H., The involvement of calcium phosphates in biological mineralization and demineralization processes, Pure & Appl. Chern., Vol. 64, No. 11, pp. 1673-1678,1992.
- Hermann Ehrlich , Petros G. Koutsoukos , Konstantinos D. Demadis , Oleg S. Pokrovsky , Principles of demineralization: Modern strategies for the isolation of organic frameworks Part I. Common definitions and history, Micron 39 (2008) 1062–1091. [CrossRef]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: materials for the future? Materials Today 2026, 19, 69–87. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates as Bioceramics: State of the Art. J. Funct. Biomater. 2010, 1, 22–107. [Google Scholar] [CrossRef] [PubMed]
- Spanos, N.; Misirlis, D.Y.; Kanellopoulou, D.G.; Koutsoukos, P.G. Seeded growth of hydroxyapatite in simulated body fluid. J. Mater. Sci. 2006, 41, 1805–1812. [Google Scholar] [CrossRef]
- Levine M, Conry-Cantilena C, Wang Y, Welch RW, Washko PW, Dhariwal KR, Park JB, Lazarev A, Graumlich JF, King J, Cantilena LR. Vitamin C pharmacokinetics in healthy volunteers: evidence for a recommended dietary allowance. Proc Natl Acad Sci U S A. 1996 Apr 16;93(8):3704-9. [CrossRef] [PubMed] [PubMed Central]
- Rose, R.C., and Bode, A.M. (1993) Biology of Free Radical Scavengers: An Evaluation of Ascorbate, FASEB J. 7, 1135–1142. [CrossRef]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989, 86, 6377–81. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A. L. ; Stiner, D . ; Doty , S . B .; Binderman, I. Requirement of Vitamin C for Cartilage Calcification in a Differentiating Chick Limb-Bud Mesenchymal Cell Culture, Bone, 1991,12, 277-282. [CrossRef]
- Ivanov, V.; Ivanova, S.; Niedzwiecki, A.; Rath, M. Vitamin C inhibits the calcification process in human vascular smooth muscle cells, Am J Cardiovasc Dis 2020;10, 108-116.
- Ciceri, P.; Volpi, E.; Brenna, I.; Arnaboldi, L.; Neri, L.; Brancaccio, D.; Cozzolino, M. Combined effects of ascorbic acid and phosphate on rat VSMC osteoblastic differentiation, Nephrol Dial Transplant 2012 27, 122–127. [CrossRef]
- Singh, P. P.; Kiran, R.; Pendse, A. K.; Ghosh, Reeta; and Surana, S. S. Ascorbic Acid is an Abettor in Calcium Urolithiasis: An Experimental Study, Scanning Microscopy 1993, 7 ,Article 28. Available online: https://digitalcommons.usu.edu/microscopy/vol7/iss3/28.
- Bahal, P.; Djemal,S. Dental Erosion from an Excess of Vitamin C. Case Reports in Dentistry 2014, Article ID 485387, 5 pp. [CrossRef]
- Sousa, S.M.G.;, Silva, T.L. Demineralization effect of EDTA, EGTA, CDTA and citric acid on root dentin: a comparative study. Braz Oral Res 2005,19, 188-92. [CrossRef]
- Amin, R.M.; Elfeky, S.A.; Verwanger, T.; Krammer,B. A new biocompatible nanocomposite as a promising constituent of sunscreens, Materials Science and Engineering: C, 2016, 63, 46-51. [CrossRef]
- Muñoz E.M.R. Hydroxyapatite-Based Materials: Synthesis and Characterization, Chapter 4, in Biomedical Engineering – Frontiers and Challenges Fazel-Rezai R. (Ed.) IntechOpen, Rijeka, Croatia, 2011. pp. 75-98.
- Drimtzias EG, Rokidi SG, Gartaganis SP, Koutsoukos PG. Experimental investigation on mechanism of hydrophilic acrylic intraocular lens calcification. Am J Ophthalmol. 2011,152, pp:824-33.e1. [CrossRef]
- Kinsey V.E. Comparative chemistry of aqueous humor in posterior and anterior chambers of rabbit eye. Its physiologic significance. A.M.A. Arch. Opthalm.1953, 50,pp.401-417. [CrossRef]
- Christoffersen, J.; Christoffersen, M.R.; Kibalczyc, W.; Andersen, F.A. A contribution to the understanding of the formation of calcium phosphates. J.Crystal Growth 1989, 94, 767–777. [Google Scholar] [CrossRef]
- Ding H.; Pan H; Xu X.; Tang R. Towards a Detailed Understanding of Magnesium ions on Hydroxyapatite Crystallization Inhibition, Cryst. Growth Des. 2014, 14 , 763-769. [CrossRef]
- Bell, L.C.; Mika, H.; Kruger, B.J. Synthetic hydroxyapatite-solubility product and stoichiometry of dissolution, Arch. Oral Biol. 1978, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Henneman, Z.J.; Nancollas, G.H. Constant composition kinetics study of carbonatedapatite dissolution. J. Crystal Growth 2003, 249, 614–624. [Google Scholar] [CrossRef]
- Chauhan N.;Singh Y. L-histidine controls the hydroxyapatite mineralization with plate-like morphology: Effect of concentration and media. Mater Sci Eng C Mater Biol Appl. 2021 Jan;120:111669. [CrossRef] [PubMed]
- Takallu, S.; Mirzaei, E.; Azadi, A.; Karimizade, A.; Tavakol, S. Plate-shape carbonated hydroxyapatite/collagen nanocomposite hydrogel via in situ mineralization of hydroxyapatite concurrent with gelation of collagen at pH = 7.4 and 37°C. J Biomed Mater Res B Appl Biomater. 2019, 107, 1920–1929. [Google Scholar] [CrossRef] [PubMed]
- Koutsoukos, P.G.; Nancollas, G.H. The morphology of hydroxyapatite crystals grown in aqueous solutions at 37°C. J. Crystal Growth, 1981, 55, 369–375. [Google Scholar] [CrossRef]
- Spanos, N.; Koutsoukos, P.G. Model studies on the effect of orthophospho-L-serine on biological mineralization. Langmuir 2001, 17, 866–872. [Google Scholar] [CrossRef]
- Lerdkanchanaporn, S.; Dollimore, D.; Alexander, K.S. A thermogravimetric study of ascorbic acid and its excipients in pharmaceutical formulations. Thermochimica Acta 1996, 284, 115–126. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Lv, G.; Zuo, Y.; Mu, Y. Thermal and crystallization studies of nano-hydroxyapatite reinforced polyamide 66 biocomposites. Polymer Degradation and Stability 2006, 91, 1202–1207. [Google Scholar] [CrossRef]
- Wang, L.; Nancollas, G.H. Calcium Orthophosphates: Crystallization and Dissolution. Chem. Rev. 2008, 108, 4628–4669. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.A.; Chia, C.L. The Diffusion Coefficient of Calcium Chloride in Aqueous Solution at 25°C. J. Am. Chem. Soc. 1952, 74, 2776–2777. [Google Scholar] [CrossRef]
- Nancollas, G.H. In vitro studies of calcium phosphate crystallization, in Biomineralization, S.Mann, J.Webb, RJP Williams (Eds). VCJ Cambridge NY, 1989 pp. 166.
- Parkhurst, D. L., & Appelo, C. A. J. (2013). Description of input and examples for PHREEQC version 3--A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations: Vol. book 6 (p. 497). U.S. Geological Survey. Available online: https://pubs.usgs.gov/tm/06/a43.
- Martel A.E.; Smith, R.M. Critical Stability Constants. Volume 3: Other Organic Ligands,Plenum Press, New York, 1977, p.264.
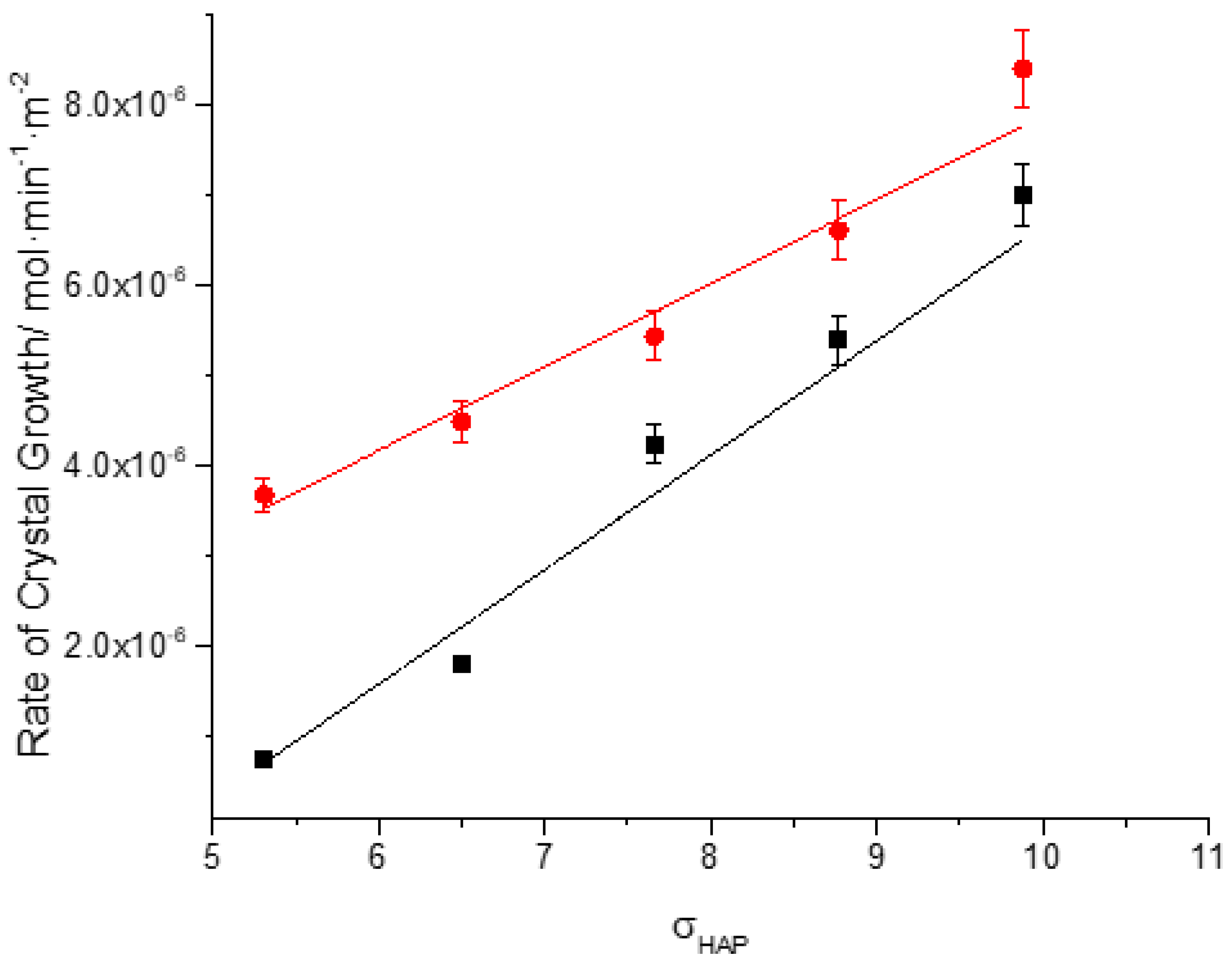
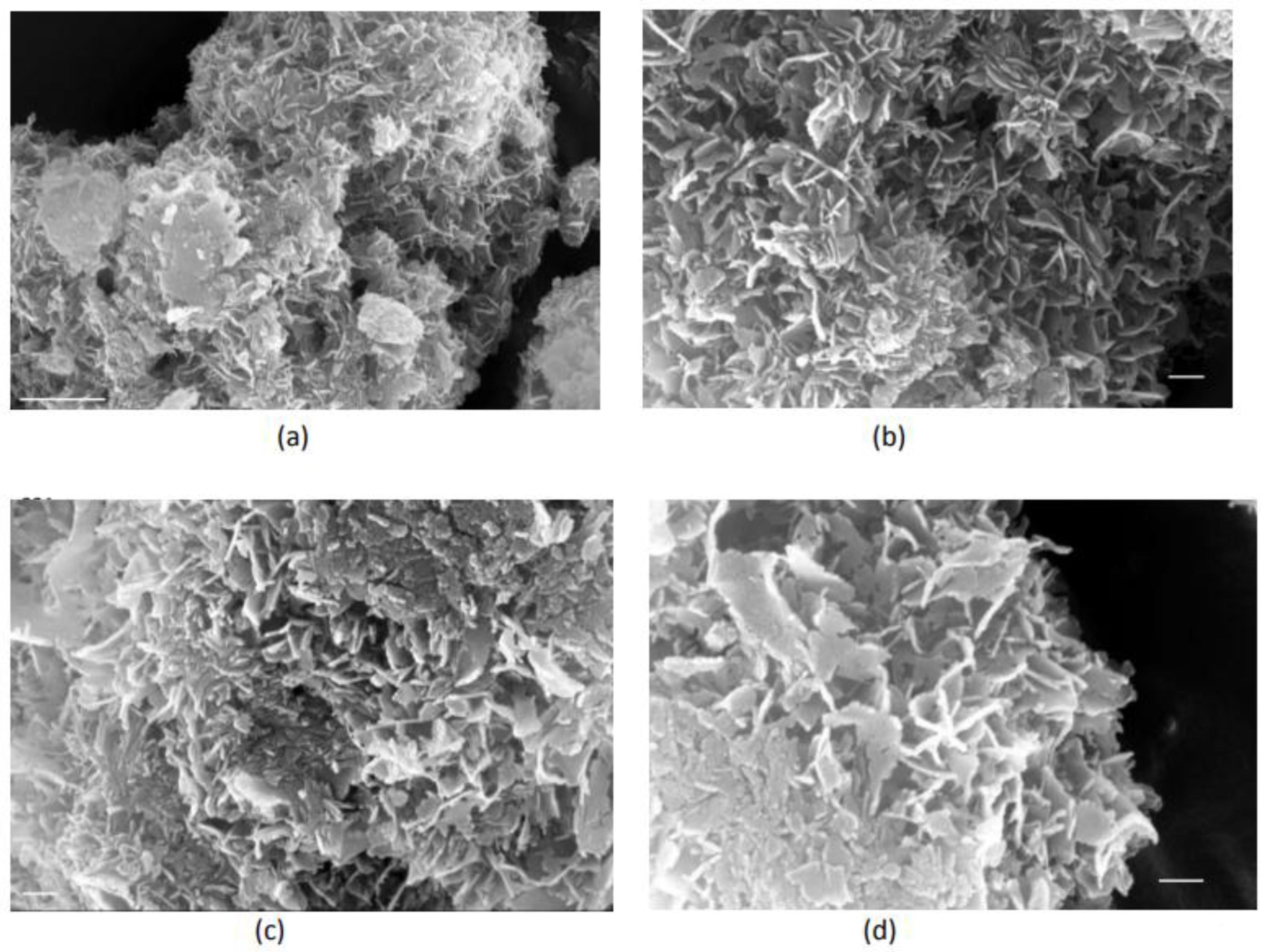
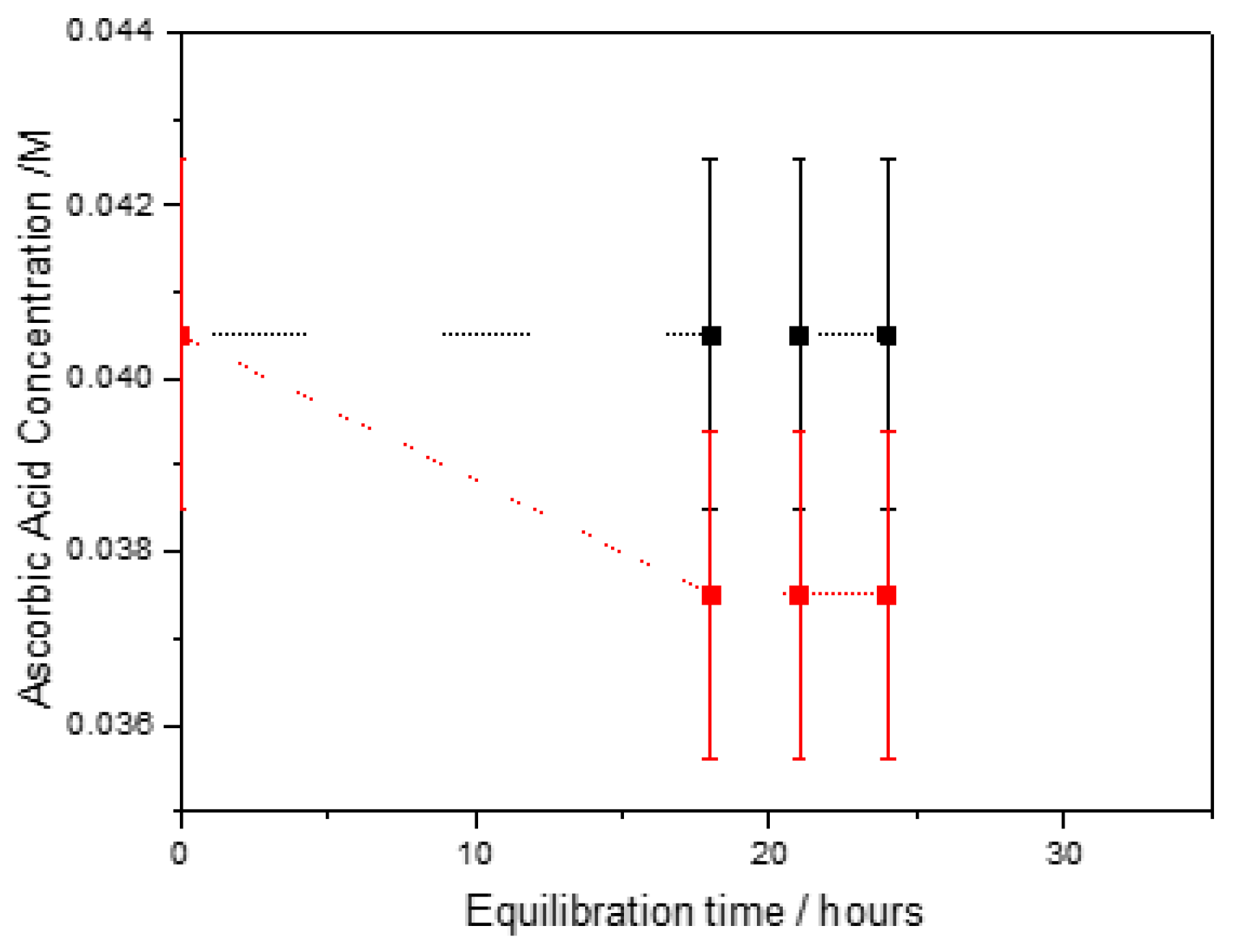
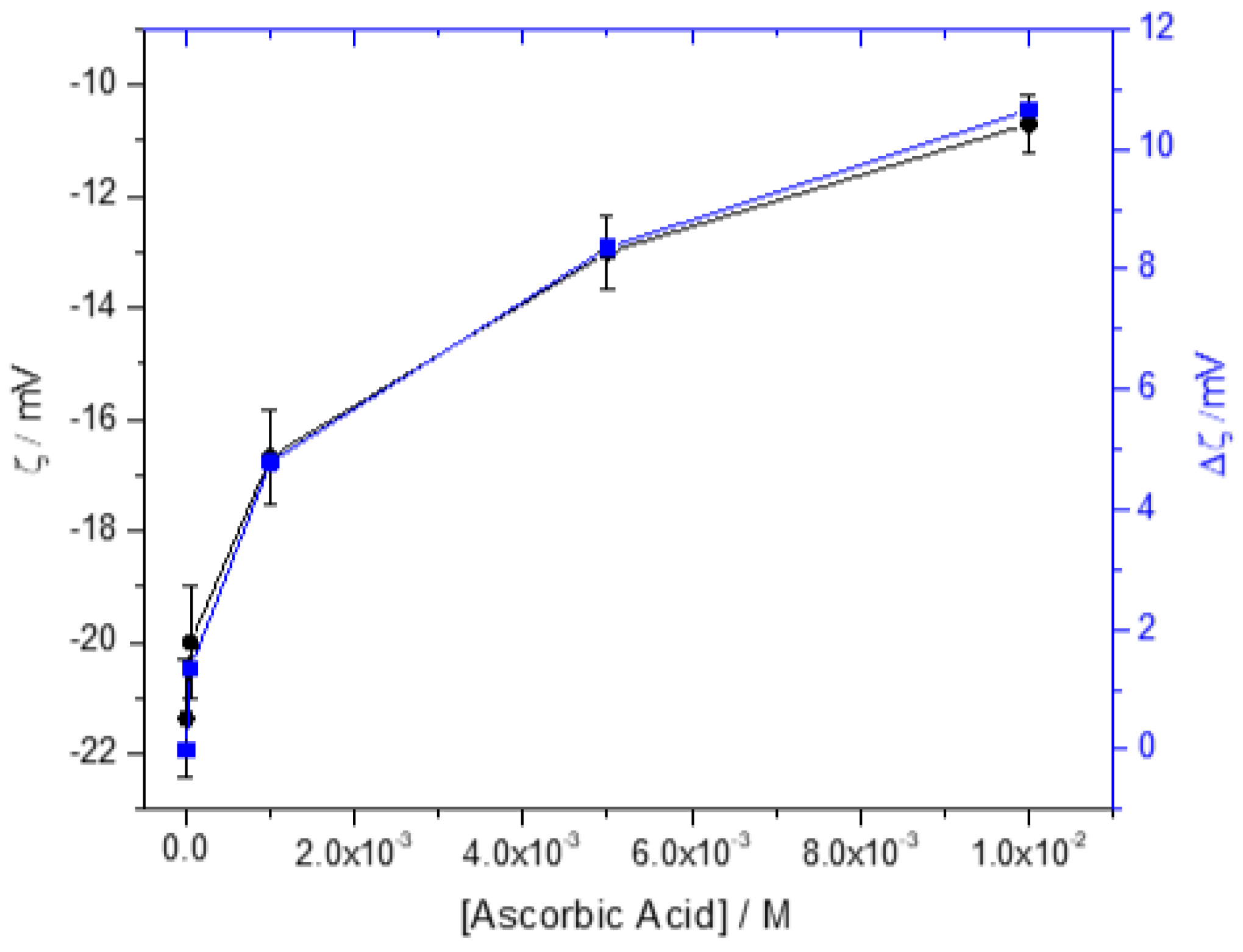
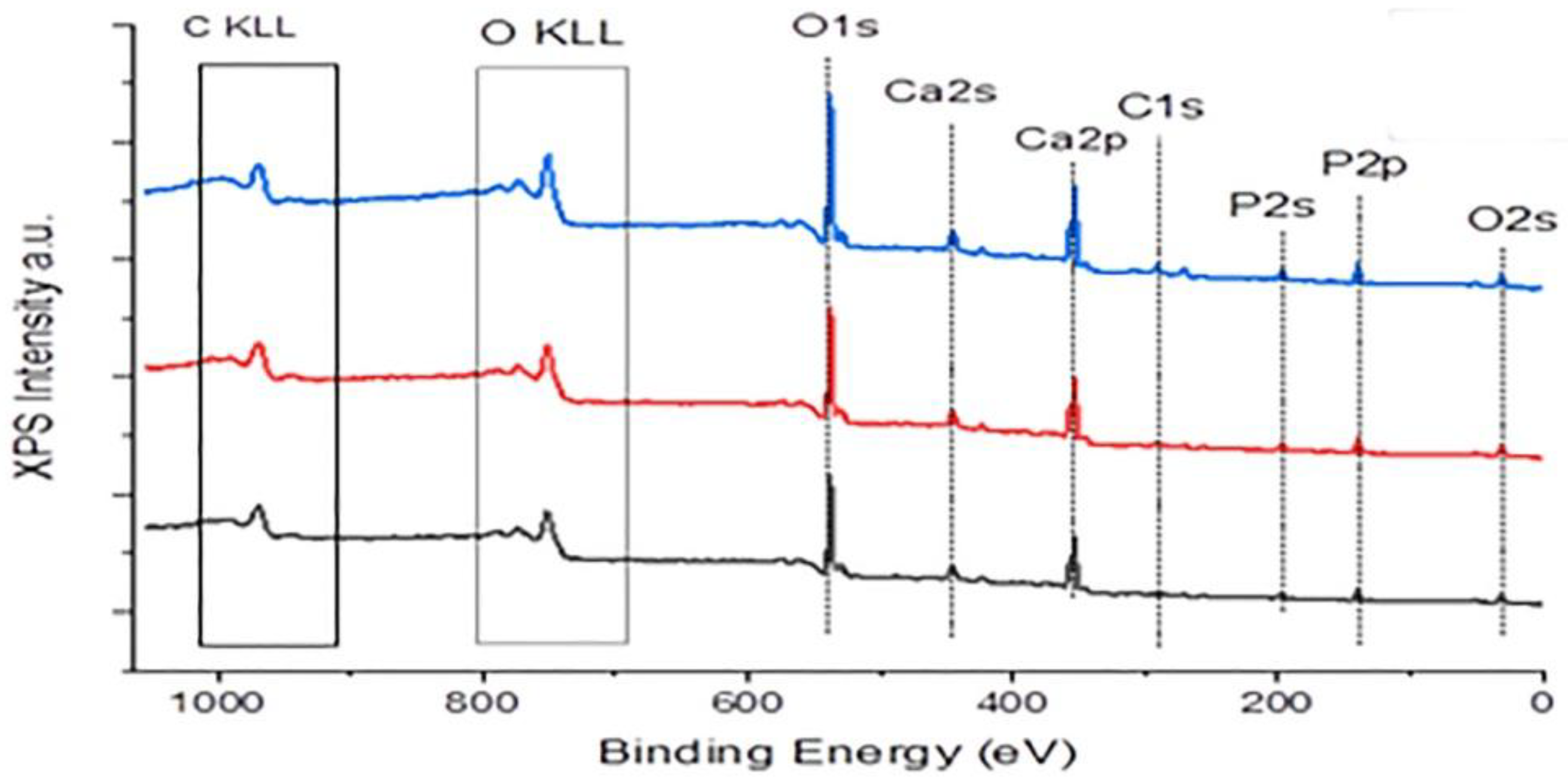
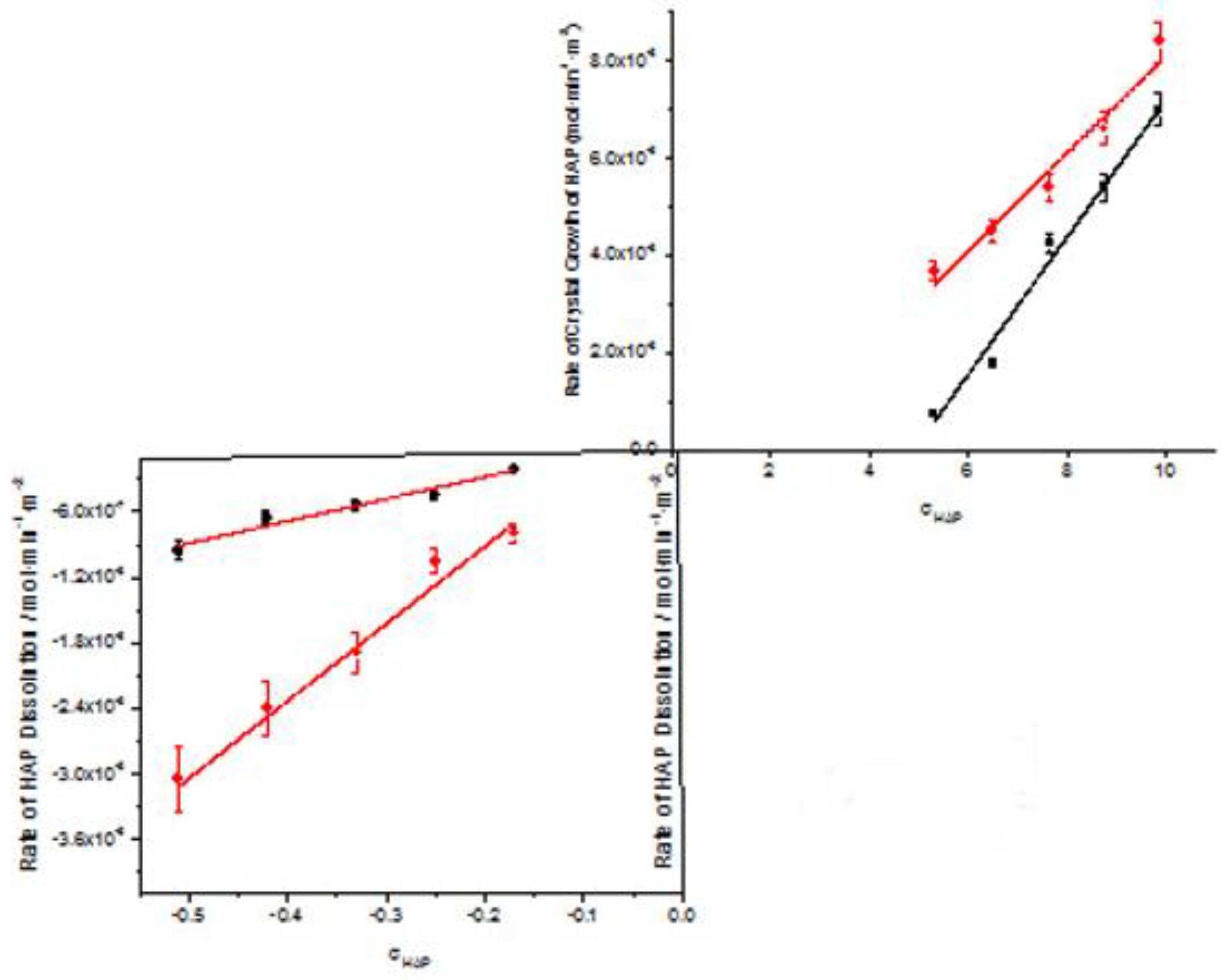
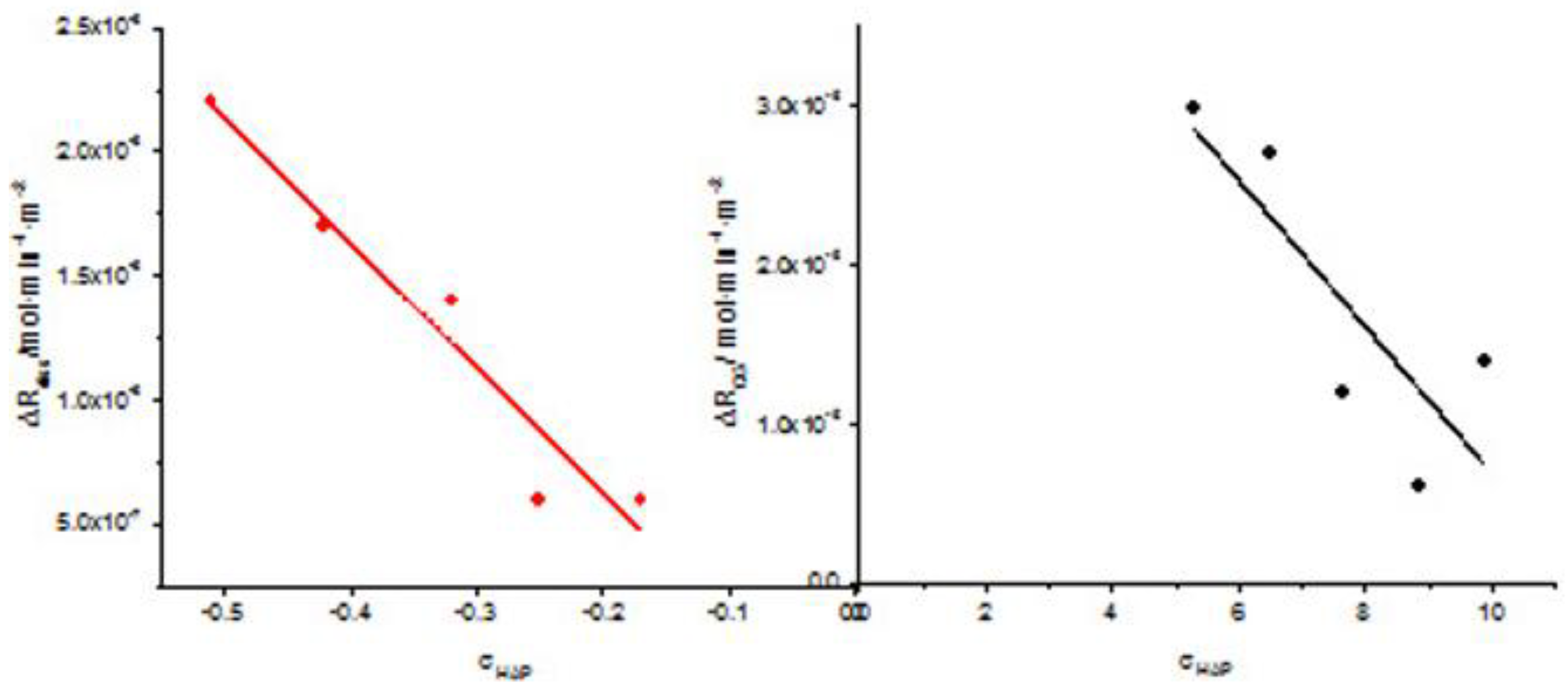
| Total Calcium, Cat/ x10-3M | Relative supersaturation with respect to HAP, σHAP | Rate of HAP crystal growth RCG/ x10-6 mol·min-1· m-2 |
|
| Absence of AA | In Presence of AA | ||
| 0.9 | 5.31 | 0.75 | 3.68 |
| 1.1 | 6.5 | 1.8 | 4.49 |
| 1.3 | 7.66 | 4.24 | 5.44 |
| 1.5 | 8.77 | 5.4 | 6.61 |
| 1.7 | 9.88 | 7 | 8.4 |
| % | Binding Energy eV |
HAP | HAP equilibrated 0.05 mM AA | HAP equilibrated 10.0 mM AA |
| C1S | 287 (Ca5(PO4)3OH) | 5.8 | 8.1 | 9.7 |
| Ca2p | 347(Ca5(PO4)3OH) | 21.7 | 21.3 | 20.2 |
| O2S | 530.9(Ca5(PO4)3OH) | 55.7 | 54.2 | 54.5 |
| P2p | 132.9(Ca5(PO4)3OH) | 16.8 | 16.4 | 15.6 |
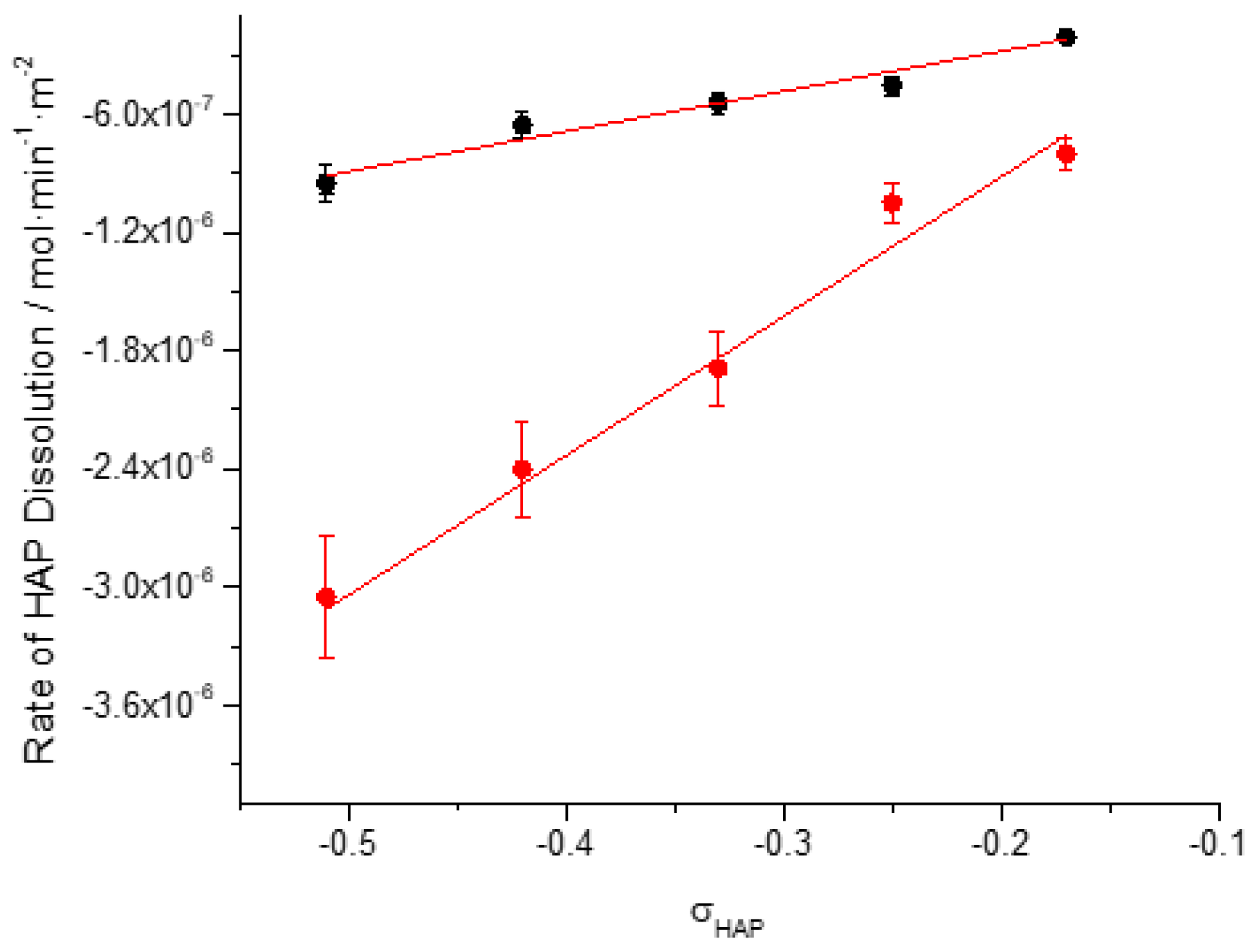
| Cat / 10-3M | Relative undersaturation with respect to HAP, σHAP | Rate of dissolution, Rdiss /x 10-7 |
|
| In the absence of AA | In the presence of AA | ||
| 0.05 | -0.51 | -9.5 | -30,5 |
| 0.06 | -0.42 | -6.6 | -24,0 |
| 0.07 | -0.33 | -5.4 | -18.9 |
| 0.08 | -0.25 | -4.5 | -10.5 |
| 0.09 | -0.17 | -2.1 | -8.0 |
| Equilibrium | Log K |
| H+ + AA2- = HAA- | 11.34 |
| HAA- + H+ = H2AA | 4.03 |
| Ca2+ +HAA- =CaHAA+ | 1.05 |
| Ca2+ + AA2- = CaAA | 1.40 |
| 2Ca2+ + AA2- = Ca2AA2+ | 1.85 |
| 3Ca2+ +4L2- =Ca3AA42- | 10.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





