Submitted:
08 August 2025
Posted:
08 August 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Methodological Development
2.2. Sample Area and Field Survey
- -
- a wetland dominated by Festuca arundinacea (Agrostio-Deschampsietum caesitosae Ujvárosi1941) (Mende, Hungary), and
- -
- a dry grassland dominated by Festuca pseudovina (Achilleo-Festucetum pseudovinae Soó (1933) 1947 corr. Borhidi 1996 (Bösztör, Hungary).
2.3. Determination of the Amount of Grass Production (Hay)
2.4. Statistics
3. Results
3.1. Methodological Results
3.1.1. Interpretation of Amendments on Methodology
- I.
- Class I: grassland producing >6.0 t dry matter × ha-1 × ha-1 × ha-1
- II.
- Class II: grassland producing between 4.5 and 6.0 t dry matter × ha-1
- III.
- Class III: grassland producing between 3.0 and 4.5 t dry matter × ha-1
- IV.
- Class IV grassland producing between 1.5 and 3.0 t dry matter × ha-1
- V.
- Class V grassland producing between 0.0 and 1.5 t dry matter × ha-1
3.1.2. Quality Value of Species (k)
3.2. Additions and Modifications to the Method Used by Balázs (1960)
3.3. Relative Economic Value of Species (kt) - Based on the Method of Balázs (1960)
- I.
- Class: very good high quality grassland, K-value: >4
- II.
- Class: good quality grassland, K-value: 3-4
- III.
- Class: medium quality grassland, K-value: 2-3
- IV.
- Class: poor, poor quality grassland, K-value: 1-2
- V.
- Class: bad, poor quality grassland, K-value: 0-1
3.4. Case Study
3.4.1. Result of the Yield Estimates
Estimation of Feed Quality
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
| Species | k-value | species | k-value |
| Abutilon theophrasti | -1 | Juncus tenageia | 0 |
| Acer campestre | -1 | Juncus tenuis | 0 |
| Acer negundo | -1 | Juniperus communis | -3 |
| Acer platanoides | -1 | Jurinea mollis | -1 |
| Acer pseudo-platanus | -1 | Jurinea glycacantha | -1 |
| Acer tataricum | -1 | Kickxia elatine | 0 |
| Achillea asplenifolia | 2 | Kickxia spuria | 0 |
| Achillea collina | 2 | Knautia arvensis | 1 |
| Achillea crithmifolia | 2 | Knautia dipsacifolia | 1 |
| Achillea distans | 2 | Knautia drymeia | 1 |
| Achillea horanszkyi | 2 | Knautia kitaibelii | 1 |
| Achillea millefolium | 2 | Kochia laniflora | -1 |
| Achillea nobilis | 2 | Kochia prostrata | -1 |
| Achillea ochroleuca | 2 | Kochia scoparia | -1 |
| Achillea pannonica | 2 | Koeleria cristata | 2 |
| Achillea ptarmica | 1 | Koeleria glauca | 2 |
| Achillea setacea | 2 | Koeleria grandis | 2 |
| Achillea tuzsonii | 2 | Koeleria javorkae | 2 |
| Acinos arvensis | 0 | Koeleria majoriflora | 2 |
| Aconitum anthora | -3 | Koeleria pyramidata | 2 |
| Aconitum moldavicum | -3 | Laburnum anagyroides | -3 |
| Aconitum variegatum | -3 | Lactuca perennis | -2 |
| Aconitum vulparia | -3 | Lactuca quercina | -2 |
| Acorus calamus | -1 | Lactuca saligna | -2 |
| Actaea spicata | -2 | Lactuca serriola | -2 |
| Adenophora liliifolia | 0 | Lactuca viminea | -2 |
| Adonis aestivalis | -2 | Lamium album | 0 |
| Adonis flammea | -2 | Lamium amplexicaule | 0 |
| Adonis vernalis | -2 | Lamium maculatum | 0 |
| Adonis x hybrida | -2 | Lamium orvala | 0 |
| Adoxa moschatellina | 0 | Lamium purpureum | 0 |
| Aegilops cylindrica | 0 | Lappula deflexa | -1 |
| Aegopodium podagraria | -1 | Lappula heteracantha | -1 |
| Aethionema saxatile | 0 | Lappula marginata | -1 |
| Aethusa cynapium | -3 | Lappula squarrosa | -1 |
| Agrimonia eupatoria | 2 | Lapsana communis | -1 |
| Agrimonia procera | 1 | Larix decidua | -1 |
| Agropyron cristatum | 5 | Laser trilobum | -2 |
| Agrostemma githago | -3 | Laserpitium latifolium | -2 |
| Agrostis canina | 3 | Laserpitium prutenicum | -2 |
| Agrostis capillaris | 2 | Lathraea squamaria | -1 |
| Agrostis stolonifera | 5 | Lathyrus aphaca | 2 |
| Agrostis vinealis | 3 | Lathyrus hirsutus | 2 |
| Ailanthus altissima | -3 | Lathyrus latifolius | 4 |
| Aira caryophyllea | 0 | Lathyrus linifolius | 3 |
| Aira elegantissima | 0 | Lathyrus niger | 3 |
| Ajuga chamaepitys | 0 | Lathyrus nissolia | 3 |
| Ajuga genevensis | 0 | Lathyrus pallescens | 4 |
| Ajuga laxmannii | 0 | Lathyrus palustris | 4 |
| Ajuga reptans | 0 | Lathyrus pannonicus | 2 |
| Alcea biennis | -2 | Lathyrus pisiformis | 4 |
| Alcea rosea | -2 | Lathyrus pratensis | 4 |
| Alchemilla acutiloba | 2 | Lathyrus sativus | 6 |
| Alchemilla crinita | 2 | Lathyrus sphaericus | 2 |
| Alchemilla glabra | 2 | Lathyrus sylvestris | 2 |
| Alchemilla glaucescens | 2 | Lathyrus tuberosus | 2 |
| Alchemilla hungarica | 2 | Lathyrus venetus | 2 |
| Alchemilla micans | 2 | Lathyrus vernus | 2 |
| Alchemilla monticola | 2 | Lavandula angustifolia | -1 |
| Alchemilla xanthochlora | 2 | Lavatera thuringiaca | -2 |
| Alisma gramineum | -2 | Lavatera trimestris | -2 |
| Alisma lanceolatum | -2 | Leersia oryzoides | 3 |
| Alisma plantago-aquatica | -2 | Legousia speculum-veneris | 1 |
| Alkanna tinctoria | -2 | Lembotropis nigricans | -2 |
| Alliaria petiolata | -2 | Lens culinaris | 3 |
| Allium angulosum | -2 | Leontodon autumnalis | 1 |
| Allium atropurpureum | -2 | Leontodon hispidus | 2 |
| Allium atroviolaceum | -2 | Leontodon incanus | 1 |
| Allium carinatum | -2 | Leontodon saxatilis | 1 |
| Allium flavum | -2 | Leonurus cardiaca | -1 |
| Allium lusitanicum | -2 | Leonurus marrubiastrum | -1 |
| Allium moschatum | -2 | Lepidium campestre | -2 |
| Allium oleraceum | -2 | Lepidium cartilagineum | -2 |
| Allium paniculatum | -2 | Lepidium densiflorum | -2 |
| Allium rotundum | -2 | Lepidium graminifolium | -2 |
| Allium scorodoprasum | -2 | Lepidium perfoliatum | -2 |
| Allium sphaerocephalon | -2 | Lepidium ruderale | -2 |
| Allium suaveolens | -2 | Lepidium virginicum | -2 |
| Allium ursinum | -2 | Leucanthemella serotinum | -2 |
| Allium vineale | -2 | Leucanthemum margaritae | -2 |
| Alnus glutinosa | -1 | Leucanthemum vulgare | 1 |
| Alnus incana | -1 | Leucojum aestivum | -3 |
| Alnus viridis | -1 | Leucojum vernum | -3 |
| Alopecurus aequalis | 3 | Libanotis pyrenaica | 1 |
| Alopecurus geniculatus | 3 | Ligularia sibirica | -2 |
| Alopecurus myosuroides | 3 | Ligustrum vulgare | -3 |
| Alopecurus pratensis | 4 | Lilium bulbiferum | -2 |
| Althaea cannabina | -1 | Lilium martagon | -2 |
| Althaea hirsuta | -1 | Limodorum abortivum | -1 |
| Althaea officinalis | -1 | Limonium gmelini | 1 |
| Alyssum alyssoides | 0 | Limosella aquatica | 0 |
| Alyssum desertorum | 0 | Linaria angustissima | -1 |
| Alyssum montanum | 0 | Linaria arvensis | -1 |
| Alyssum tortuosum | 0 | Linaria genistifolia | -1 |
| Amaranthus albus | 1 | Linaria vulgaris | -1 |
| Amaranthus blitoides | 1 | Linaria x kocianovichii | -1 |
| Amaranthus blitum | 1 | Lindernia procumbens | 0 |
| Amaranthus bouchonii | 1 | Linum austriacum | -1 |
| Amaranthus crispus | 1 | Linum catharticum | -1 |
| Amaranthus deflexus | 1 | Linum dolomiticum | -1 |
| Amaranthus graecizans | 1 | Linum flavum | -1 |
| Amaranthus patulus | 1 | Linum hirsutum | -1 |
| Amaranthus powellii | 1 | Linum perenne | -1 |
| Amaranthus retroflexus | 1 | Linum tenuifolium | -1 |
| Ambrosia artemisiifolia | -2 | Linum trigynum | -1 |
| Amelanchier ovalis | -1 | Linum usitatissimum | -1 |
| Ammannia verticillata | 1 | Liparis loeselii | -3 |
| Amorpha fruticosa | -3 | Listera ovata | -1 |
| Amygdalus communis | -1 | Lithospermum arvense | -1 |
| Amygdalus nana | -2 | Lithospermum officinale | -1 |
| Anacamptis pyramidalis | -1 | Lithospermum purpureo-coeruleum | -1 |
| Anagallis arvensis | 1 | Lolium multiflorum | 5 |
| Anagallis foemina | 1 | Lolium perenne | 6 |
| Anchusa azurea | -1 | Lolium remotum | -1 |
| Anchusa barrelieri | -1 | Lolium temulentum | -1 |
| Anchusa ochroleuca | -1 | Lonicera caprifolium | -2 |
| Anchusa officinalis | -1 | Lonicera nigra | -2 |
| Androsace elongata | 0 | Lonicera xylosteum | -2 |
| Androsace maxima | 0 | Loranthus europaeus | -3 |
| Anemone nemorosa | -2 | Lotus angustissimus | 6 |
| Anemone ranunculoides | -2 | Lotus borbasii | 7 |
| Anemone sylvestris | -2 | Lotus corniculatus | 7 |
| Anemone trifolia | -2 | Lotus tenuis | 6 |
| Anethum graveolens | 1 | Lotus uliginosus | 6 |
| Angelica archangelica | -2 | Ludwigia palustris | -2 |
| Angelica palustris | -2 | Lunaria annua | -1 |
| Angelica sylvestris | -2 | Lunaria rediviva | -1 |
| Antennaria dioica | 1 | Lupinus albus | -2 |
| Anthemis arvensis | -1 | Lupinus angustifolius | -2 |
| Anthemis austriaca | -1 | Lupinus luteus | -2 |
| Anthemis cotula | -1 | Lupinus polyphyllus | -2 |
| Anthemis ruthenica | -1 | Luzula campestris | -1 |
| Anthemis tinctoria | -1 | Luzula divulgata | -1 |
| Anthericum liliago | -2 | Luzula forsteri | -1 |
| Anthericum ramosum | -2 | Luzula luzuloides | -1 |
| Anthoxanthum odoratum | 2 | Luzula multiflora | -1 |
| Anthriscus caucalis | -2 | Luzula pallidula | -1 |
| Anthriscus cerefolium | -2 | Luzula pilosa | -1 |
| Anthriscus nitidus | -2 | Lychnis coronaria | -1 |
| Anthriscus sylvestris | -2 | Lychnis flos-cuculi | 1 |
| Anthyllis vulneraria | 4 | Lychnis viscaria | -1 |
| Apera interrupta | 1 | Lycium barbarum | -3 |
| Apera spica-venti | 1 | Lycium chinense | -3 |
| Aphanes arvensis | 0 | Lycopodium annotinum | -1 |
| Aphanes australis | 0 | Lycopodium clavatum | -1 |
| Apium graveolens | -2 | Lycopsis arvensis | -1 |
| Apium repens | -2 | Lycopus europaeus | -1 |
| Aquilegia vulgaris | -2 | Lycopus exaltatus | -1 |
| Arabidopsis thaliana | 0 | Lysimachia nummularia | 0 |
| Arabis alpina | 1 | Lysimachia punctata | -1 |
| Arabis auriculata | 1 | Lysimachia thyrsiflora | -1 |
| Arabis hirsuta | 1 | Lysimachia vulgaris | -1 |
| Arabis turrita | 1 | Lythrum hyssopifolia | -1 |
| Arctium lappa | -2 | Lythrum linifolium | -1 |
| Arctium minus | -2 | Lythrum salicaria | -2 |
| Arctium nemorosum | -2 | Lythrum thesioides | -1 |
| Arctium tomentosum | -2 | Lythrum tribracteatum | -1 |
| Aremonia agrimonoides | 1 | Lythrum virgatum | -1 |
| Arenaria leptoclados | 0 | Majanthemum bifolium | -1 |
| Arenaria procera | 0 | Malcolmia africana | 1 |
| Arenaria serpyllifolia | 0 | Malus domestica | -1 |
| Aristolochia clematitis | -2 | Malus sylvestris | -1 |
| Armeria elongata | 0 | Malva alcea | 1 |
| Armoracia lapathifolia | -2 | Malva moschata | 1 |
| Armoracia macrocarpa | -2 | Malva neglecta | 1 |
| Arnica montana | 1 | Malva pusilla | 1 |
| Arrhenatherum elatius | 4 | Malva sylvestris | 1 |
| Artemisia abrotanum | -1 | Malva verticillata | 1 |
| Artemisia absinthium | -2 | Marrubium peregrinum | -1 |
| Artemisia alba | -2 | Marrubium vulgare | -1 |
| Artemisia annua | -2 | Marrubium x paniculatum | -1 |
| Artemisia austriaca | -1 | Marsilea quadrifolia | -2 |
| Artemisia campestris | -1 | Matricaria chamomilla | -1 |
| Artemisia pontica | -1 | Matricaria discoidea | -1 |
| Artemisia santonicum | -1 | Matricaria maritima | -1 |
| Artemisia scoparia | -1 | Matricaria tenuifolia | -1 |
| Artemisia vulgaris | -2 | Matteuccia struthiopteris | -2 |
| Arum maculatum | -3 | Medicago arabica | 3 |
| Arum orientale | -3 | Medicago falcata | 6 |
| Aruncus sylvestris | -1 | Medicago lupulina | 5 |
| Asarum europaeum | -1 | Medicago minima | 4 |
| Asclepias syriaca | -3 | Medicago orbicularis | 3 |
| Asparagus officinalis | 1 | Medicago polymorpha | 4 |
| Asperugo procumbens | -1 | Medicago prostrata | 4 |
| Asperula arvensis | 1 | Medicago rigidula | 3 |
| Asperula cynanchica | 1 | Medicago sativa | 7 |
| Asperula orientalis | 1 | Medicago x varia | 7 |
| Asperula taurina | 1 | Melampyrum arvense | -2 |
| Asperula tinctoria | 1 | Melampyrum barbatum | -2 |
| Asphodelus albus | -1 | Melampyrum cristatum | -2 |
| Asplenium adiantum-nigrum | -2 | Melampyrum nemorosum | -2 |
| Asplenium fontanum | -2 | Melampyrum polyphyllus | -2 |
| Asplenium lepidum | -2 | Melampyrum pratense | -2 |
| Asplenium ruta-muraria | -2 | Melica altissima | 1 |
| Asplenium septentrionale | -2 | Melica ciliata | 1 |
| Asplenium trichomanes | -2 | Melica nutans | 2 |
| Asplenium viride | -2 | Melica picta | 1 |
| Aster amellus | 0 | Melica transsilvanica | 1 |
| Aster lanceolatus | -1 | Melica uniflora | 2 |
| Aster linosyris | 0 | Melilotus albus | 2 |
| Aster novae-angliae | -1 | Melilotus altissimus | 2 |
| Aster novi-belgii | -1 | Melilotus dentatus | 2 |
| Aster oleifolius | -1 | Melilotus officinalis | 2 |
| Aster sedifolius | -1 | Melissa officinalis | -1 |
| Aster tradescantii | -1 | Melittis carpatica | -1 |
| Aster tripolium | 0 | Melittis melissophyllum | -1 |
| Aster x salignus | -1 | Mentha aquatica | -1 |
| Aster x versicolor | -1 | Mentha arvensis | -1 |
| Astragalus asper | 2 | Mentha longifolia | -1 |
| Astragalus austriacus | 2 | Mentha pulegium | -1 |
| Astragalus cicer | 2 | Mentha x carinthiaca | -1 |
| Astragalus contortuplicatus | 2 | Mentha x dalmatica | -1 |
| Astragalus dasyanthus | 2 | Mentha x dumetorum | -1 |
| Astragalus exscapus | 3 | Mentha x gentilis | -1 |
| Astragalus glycyphyllos | 4 | Mentha x verticillata | -1 |
| Astragalus onobrychis | 2 | Menyanthes trifoliata | -3 |
| Astragalus sulcatus | 3 | Mercurialis annua | -2 |
| Astragalus varius | 2 | Mercurialis ovata | -2 |
| Astragalus vesicarius | 2 | Mercurialis perennis | -2 |
| Astrantia major | 1 | Mercurialis x paxii | -2 |
| Asyneuma canescens | 1 | Mespilus germanica | -2 |
| Athyrium filix-femina | -2 | Micropus erectus | 0 |
| Atriplex hortensis | -2 | Microrrhinum minus | 0 |
| Atriplex littoralis | -2 | Milium effusum | 3 |
| Atriplex oblongifolia | -2 | Minuartia fastigiata | 0 |
| Atriplex patula | -2 | Minuartia frutescens | 0 |
| Atriplex prostrata | -2 | Minuartia glomerata | 0 |
| Atriplex rosea | -2 | Minuartia setacea | 0 |
| Atriplex sagittata | -2 | Minuartia verna | 0 |
| Atriplex tatarica | -2 | Minuartia viscosa | 0 |
| Atropa bella-donna | -3 | Misopates orontium | 0 |
| Aurinia saxatilis | 1 | Moehringia muscosa | 0 |
| Avena barbata | 1 | Moehringia trinervia | 0 |
| Avena fatua | 1 | Moenchia mantica | 0 |
| Avena nuda | 1 | Molinia arundinacea | 1 |
| Avena sativa | 3 | Molinia coerulea | 1 |
| Avena sterilis | 1 | Morus alba | -1 |
| Avena strigosa | 1 | Morus nigra | -1 |
| Ballota nigra | -2 | Muscari botryoides | -2 |
| Barbarea stricta | 1 | Muscari comosum | -2 |
| Barbarea verna | 1 | Muscari racemosum | -2 |
| Barbarea vulgaris | 1 | Muscari tenuiflorum | -2 |
| Bassia sedoides | -1 | Myagrum perfoliatum | -1 |
| Beckmannia eruciformis | 4 | Mycelis muralis | -2 |
| Bellis perennis | 2 | Myosotis arvensis | 0 |
| Berberis vulgaris | -3 | Myosotis caespitosa | 0 |
| Berteroa incana | -1 | Myosotis discolor | 0 |
| Berula erecta | -2 | Myosotis nemorosa | 0 |
| Betonica officinalis | 1 | Myosotis palustris | 0 |
| Betula pendula | -1 | Myosotis ramosissima | 0 |
| Betula pubescens | -1 | Myosotis sparsiflora | 0 |
| Bidens cernua | -3 | Myosotis stenophylla | 0 |
| Bidens frondosa | -3 | Myosotis stricta | 0 |
| Bidens tripartita | -3 | Myosotis sylvatica | 0 |
| Bifora radians | -2 | Myosoton aquaticum | 1 |
| Biscutella laevigata | 1 | Myosurus minimus | 0 |
| Blackstonia acuminata | -2 | Narcissus angustifolius | -2 |
| Blechnum spicant | -2 | Narcissus poëticus | -2 |
| Blysmus compressus | 1 | Narcissus pseudonarcissus | -2 |
| Bolboschoenus maritimus | -1 | Nardus stricta | -1 |
| Borago officinalis | 1 | Nasturtium officinale | -2 |
| Bothriochloa ischaemum | 1 | Neottia nidus-avis | -1 |
| Botrychium lunaria | 0 | Nepeta cataria | -1 |
| Botrychium matricariifolium | 0 | Nepeta pannonica | -1 |
| Botrychium multifidum | 0 | Nepeta parviflora | -1 |
| Botrychium virginianum | -2 | Neslea paniculata | 1 |
| Brachypodium pinnatum | 2 | Nicandra physalodes | -3 |
| Brachypodium sylvaticum | 2 | Nicotiana rustica | -3 |
| Brassica elongata | 2 | Nicotiana tabacum | -3 |
| Brassica nigra | 1 | Nigella arvensis | -1 |
| Brassica oleracea | 3 | Nigella damascena | -1 |
| Brassica rapa | 3 | Nonea pulla | 1 |
| Brassica x juncea | 1 | Odontites lutea | -2 |
| Brassica x napus | 2 | Odontites vernus subsp. vernus | -2 |
| Briza media | 3 | Odontites vernus subsp. serotinus | -2 |
| Bromus arvensis | 1 | Oenanthe aquatica | -3 |
| Bromus benekenii | 1 | Oenanthe banatica | -3 |
| Bromus brachystachys | 1 | Oenanthe fistulosa | -3 |
| Bromus carinatus | 1 | Oenanthe silaifolia | -3 |
| Bromus catharticus | 1 | Oenothera biennis | -1 |
| Bromus commutatus | 1 | Oenothera glazioviana | -1 |
| Bromus erectus | 3 | Oenothera rubricaulis | -1 |
| Bromus hordaceus | 0 | Oenothera salicifolia | -1 |
| Bromus inermis | 5 | Oenothera suaveolens | -1 |
| Bromus japonicus | 0 | Oenothera x hoelscheri | -1 |
| Bromus lanceolatus | 1 | Omphalodes scorpioides | 1 |
| Bromus lepidus | 1 | Omphalodes verna | 1 |
| Bromus madritensis | 1 | Onobrychis arenaria | 4 |
| Bromus pannonicus | 3 | Onobrychis viciifolia | 4 |
| Bromus racemosus | 1 | Ononis arvensis | 3 |
| Bromus ramosus | 1 | Ononis pusilla | 1 |
| Bromus reptans | 3 | Ononis spinosa | -3 |
| Bromus rigidus | 1 | Ononis spinosiformis | 3 |
| Bromus secalinus | 0 | Onopordum acanthium | -3 |
| Bromus squarrosus | 0 | Onosma arenarium | -1 |
| Bromus sterilis | 1 | Onosma pseudarenarium | -1 |
| Bromus tectorum | 1 | Onosma tornense | -1 |
| Bryonia alba | -2 | Onosma visianii | -1 |
| Bryonia dioica | -2 | Ophioglossum vulgatum | 0 |
| Bulbocodium versicolor | -2 | Ophrys apifera | -1 |
| Bunias orientalis | -1 | Ophrys fuciflora | -1 |
| Buphthalmum salicifolium | -1 | Ophrys insectifera | -1 |
| Bupleurum affine | 1 | Ophrys scolopax | -1 |
| Bupleurum falcatum | 1 | Ophrys sphecodes | -1 |
| Bupleurum longifolium | 1 | Orchis coriophora | -1 |
| Bupleurum pachnospermum | 1 | Orchis laxiflora | -1 |
| Bupleurum praealtum | 1 | Orchis mascula subsp. signifera | -1 |
| Bupleurum rotundifolium | 1 | Orchis militaris | -1 |
| Bupleurum tenuissimum | 1 | Orchis morio | -1 |
| Butomus umbellatus | -1 | Orchis pallens | -1 |
| Calamagrostis arundinacea | 1 | Orchis purpurea | -1 |
| Calamagrostis canescens | 1 | Orchis simia | -1 |
| Calamagrostis epigeios | 1 | Orchis tridentata | -1 |
| Calamagrostis pseudophragmites | 1 | Orchis ustulata | -1 |
| Calamagrostis purpurea | 1 | Origanum vulgare | 1 |
| Calamagrostis stricta | 1 | Orlaya grandiflora | 1 |
| Calamagrostis varia | 1 | Ornithogalum boucheanum | -2 |
| Calamagrostis villosa | 1 | Ornithogalum comosum | -2 |
| Calamintha einseleana | 1 | Ornithogalum orthophyllum | -2 |
| Calamintha menthifolia | 1 | Ornithogalum pyramidale | -2 |
| Calamintha thymifolia | 1 | Ornithogalum refractum | -2 |
| Caldesia parnassifolia | -2 | Ornithogalum sphaerocarpum | -2 |
| Calepina irregularis | 1 | Ornithogalum umbellatum | -2 |
| Calla palustris | -2 | Ornithogalum x degenianum | -2 |
| Callitriche cophocarpa | 1 | Orobanche alba | -2 |
| Callitriche palustris | 0 | Orobanche alsatica | -2 |
| Calluna vulgaris | -2 | Orobanche arenaria | -2 |
| Caltha palustris | -2 | Orobanche caesia | -2 |
| Calystegia sepium | -2 | Orobanche caryophyllacea | -2 |
| Camelina alyssum | 1 | Orobanche cernua | -2 |
| Camelina microcarpa | 1 | Orobanche coerulescens | -2 |
| Camelina rumelica | 1 | Orobanche elatior | -2 |
| Camelina sativa | 1 | Orobanche flava | -2 |
| Campanula bononiensis | -1 | Orobanche gracilis | -2 |
| Campanula cervicaria | -1 | Orobanche hederae | -2 |
| Campanula glomerata | 1 | Orobanche loricata | -2 |
| Campanula latifolia | -1 | Orobanche lutea | -2 |
| Campanula macrostachya | -1 | Orobanche minor | -2 |
| Campanula moravica | 1 | Orobanche nana | -2 |
| Campanula patula | 1 | Orobanche picridis | -2 |
| Campanula persicifolia | 1 | Orobanche purpurea | -2 |
| Campanula rapunculoides | 1 | Orobanche ramosa | -2 |
| Campanula rapunculus | 1 | Orobanche reticulata | -2 |
| Campanula rotundifolia | 1 | Orobanche teucrii | -2 |
| Campanula sibirica | -1 | Osmunda regalis | -2 |
| Campanula trachelium | 1 | Ostrya carpinifolia | -1 |
| Campanula xylocarpa | 1 | Oxalis acetosella | -2 |
| Camphorosma annua | 1 | Oxalis corniculata | -2 |
| Cannabis sativa | -2 | Oxalis dillenii | -2 |
| Capsella bursa-pastoris | 1 | Oxalis fontana | -2 |
| Capsella rubella | 1 | Oxytropis pilosa | 3 |
| Cardamine amara | -1 | Padus avium | -1 |
| Cardamine bulbifera | -1 | Padus serotina | -1 |
| Cardamine enneaphyllos | -1 | Paeonia officinalis | -3 |
| Cardamine flexuosa | -1 | Panicum capillare | 1 |
| Cardamine glanduligera | -1 | Panicum miliaceum | 1 |
| Cardamine hirsuta | -1 | Panicum philadelphicum | 1 |
| Cardamine impatiens | -1 | Papaver argemone | -2 |
| Cardamine parviflora | -1 | Papaver dubium | -2 |
| Cardamine pratensis | -1 | Papaver hybridum | -2 |
| Cardamine trifolia | -1 | Papaver rhoeas | -2 |
| Cardamine waldsteinii | -1 | Papaver somniferum | -2 |
| Cardaminopsis arenosa | 1 | Parietaria officinalis | -1 |
| Cardaminopsis petraea | 1 | Paris quadrifolia | -3 |
| Cardaria draba | -1 | Parnassia palustris | -1 |
| Carduus acanthoides | -3 | Paronychia cephalotes | 0 |
| Carduus collinus | -3 | Parthenocissus inserta | -2 |
| Carduus crassifolius subsp. glaucus | -2 | Parthenocissus quinquefolia | -2 |
| Carduus crispus | -3 | Parthenocissus tricuspidata | -2 |
| Carduus hamulosus | -3 | Pastinaca sativa | -1 |
| Carduus nutans | -3 | Pedicularis palustris | -3 |
| Carex acuta | -1 | Peplis portula | 1 |
| Carex acutiformis | -1 | Persicaria amphibia | -2 |
| Carex alba | 1 | Persicaria bistorta | -2 |
| Carex appropinquata | -1 | Persicaria dubia | -2 |
| Carex bohemica | 1 | Persicaria hydropiper | -2 |
| Carex brevicollis | 0 | Persicaria lapathifolia | -2 |
| Carex brizoides | -1 | Persicaria maculosa | -2 |
| Carex buekii | -1 | Persicaria minor | -2 |
| Carex buxbaumii | -1 | Petasites albus | -2 |
| Carex caespitosa | 0 | Petasites hybridus | -2 |
| Carex canescens | 0 | Petrorhagia glumacea | 0 |
| Carex caryophyllea | 1 | Petrorhagia prolifera | 0 |
| Carex davalliana | 0 | Petrorhagia saxifraga | 0 |
| Carex diandra | -1 | Peucedanum alsaticum | -1 |
| Carex digitata | 1 | Peucedanum arenarium | -1 |
| Carex distans | 0 | Peucedanum carvifolia | -1 |
| Carex disticha | -1 | Peucedanum cervaria | -1 |
| Carex divisa | 1 | Peucedanum officinale | -1 |
| Carex divulsa | 0 | Peucedanum oreoselinum | -1 |
| Carex echinata | 1 | Peucedanum palustre | -1 |
| Carex elata | -1 | Peucedanum rochelianum | -1 |
| Carex elongata | -1 | Peucedanum verticillare | -1 |
| Carex ericetorum | 1 | Phacelia congesta | 2 |
| Carex flacca | 0 | Phacelia tanacetifolia | 2 |
| Carex flava | 0 | Phalaris arundinacea | 4 |
| Carex fritschii | 0 | Phalaris canariensis | 3 |
| Carex halleriana | 1 | Phaseolus vulgaris | 4 |
| Carex hartmannii | 0 | Phleum bertolonii | 5 |
| Carex hirta | 0 | Phleum paniculatum | 3 |
| Carex hordeistichos | 1 | Phleum phleoides | 3 |
| Carex hostiana | 1 | Phleum pratense | 5 |
| Carex humilis | 1 | Phlomis tuberosa | -1 |
| Carex lasiocarpa | -1 | Pholiurus pannonicus | 1 |
| Carex lepidocarpa | 0 | Phragmites australis | -1 |
| Carex limosa | 1 | Phyllitis scolopendrium | -2 |
| Carex liparicarpos | 1 | Physalis alkekengi | -2 |
| Carex melanostachya | -1 | Physocaulis nodosus | -2 |
| Carex michelii | 1 | Physospermum cornubiense | -2 |
| Carex montana | 1 | Phyteuma orbiculare | 1 |
| Carex nigra | 1 | Phyteuma spicatum | 1 |
| Carex otrubae | -1 | Phytolacca americana | -3 |
| Carex ovalis | 1 | Picea abies | -1 |
| Carex pairaei | 1 | Picris hieracioides | -1 |
| Carex pallescens | 0 | Pimpinella major | 2 |
| Carex panicea | 1 | Pimpinella saxifraga | 2 |
| Carex paniculata | -1 | Pinguicula alpina | -1 |
| Carex pendula | -1 | Pinguicula vulgaris | -1 |
| Carex pilosa | 0 | Pinus nigra | -1 |
| Carex pilulifera | 1 | Pinus sylvestris | -1 |
| Carex praecox | 1 | Piptatherum miliaceum | 1 |
| Carex pseudocyperus | -1 | Piptatherum virescens | 1 |
| Carex remota | 0 | Pisum elatius | 4 |
| Carex repens | -1 | Pisum sativum | 4 |
| Carex riparia | -1 | Plantago altissima | 2 |
| Carex rostrata | -1 | Plantago indica | 1 |
| Carex secalina | 1 | Plantago argentea | 2 |
| Carex spicata | 0 | Plantago lanceolata | 3 |
| Carex stenophylla | 1 | Plantago major | 2 |
| Carex strigosa | 0 | Plantago maritima | 2 |
| Carex supina | 1 | Plantago maxima | 2 |
| Carex sylvatica | 0 | Plantago media | 2 |
| Carex tomentosa | 1 | Plantago schwarzenbergiana | 2 |
| Carex umbrosa | 0 | Plantago stepposa | 2 |
| Carex vesicaria | -1 | Plantago tenuiflora | 2 |
| Carex viridula | 1 | Platanthera bifolia | -1 |
| Carex vulpina | 0 | Platanthera chlorantha | -1 |
| Carlina acaulis | -3 | Pleurospermum austriacum | -1 |
| Carlina vulgaris | -3 | Poa angustifolia | 5 |
| Carpesium abrotanoides | -1 | Poa annua | 2 |
| Carpesium cernuum | -1 | Poa badensis | 2 |
| Carpinus betulus | -1 | Poa bulbosa | 2 |
| Carpinus orientalis | -1 | Poa compressa | 1 |
| Carthamus lanatus | -3 | Poa humilis | 6 |
| Carum carvi | 2 | Poa nemoralis | 2 |
| Castanea sativa | -1 | Poa palustris | 5 |
| Catabrosa aquatica | 3 | Poa scabra | 2 |
| Caucalis latifolia | -1 | Poa pratensis | 6 |
| Caucalis platycarpos | -1 | Poa remota | 3 |
| Celtis australis | -1 | Poa stiriaca | 3 |
| Celtis occidentalis | -1 | Poa supina | 2 |
| Cenchrus incertus | -3 | Poa trivialis | 5 |
| Centaurea arenaria | 1 | Podospermum canum | 2 |
| Centaurea calcitrapa | -1 | Podospermum laciniatum | 2 |
| Centaurea cyanus | 1 | Polycnemum arvense | -1 |
| Centaurea diffusa | 1 | Polycnemum heuffelii | -1 |
| Centaurea indurata | 1 | Polycnemum majus | -1 |
| Centaurea jacea subsp. angustifolia | 2 | Polycnemum verrucosum | -1 |
| Centaurea jacea subsp. banatica | 2 | Polygala amara | 0 |
| Centaurea jacea subsp. jacea | 2 | Polygala amarella | 0 |
| Centaurea jacea subsp. macroptilon | 1 | Polygala comosa | 0 |
| Centaurea mollis | 2 | Polygala major | 0 |
| Centaurea nigrescens | 2 | Polygala nicaeensis subsp. carniolica | 0 |
| Centaurea pseudophrygia | 1 | Polygala vulgaris | 0 |
| Centaurea salonitana | 1 | Polygonatum latifolium | -2 |
| Centaurea scabiosa subsp. fritschii | 1 | Polygonatum multiflorum | -2 |
| Centaurea scabiosa subsp. scabiosa | 1 | Polygonatum odoratum | -2 |
| Centaurea scabiosa subsp. spinulosa | 1 | Polygonatum verticillatum | -2 |
| Centaurea scabiosa subsp.sadleriana | 1 | Polygonum arenarium | -2 |
| Centaurea solstitialis | -1 | Polygonum arenastrum | -2 |
| Centaurea stenolepis | 2 | Polygonum aviculare | -2 |
| Centaurea stoebe subsp. micranthos | 1 | Polygonum bellardii | -2 |
| Centaurea stoebe subsp. stoebe | 1 | Polygonum graminifolium | -2 |
| Centaurea triumfetti | 2 | Polygonum rurivagum | -2 |
| Centaurium erythraea | 1 | Polypodium interjectum | -2 |
| Centaurium littorale | 1 | Polypodium vulgare | -2 |
| Centaurium pulchellum | 0 | Polystichum aculeatum | -2 |
| Centunculus minimus | 0 | Polystichum braunii | -2 |
| Cephalanthera damasonium | -1 | Polystichum lonchitis | -2 |
| Cephalanthera longifolia | -1 | Polystichum setiferum | -2 |
| Cephalanthera rubra | -1 | Populus alba | -1 |
| Cephalaria pilosa | -2 | Populus deltoides | -1 |
| Cephalaria transsylvanica | -2 | Populus nigra | -1 |
| Cerastium arvense | 1 | Populus simonii | -1 |
| Cerastium brachypetalum | 1 | Populus tremula | -1 |
| Cerastium dubium | 1 | Populus x canadensis | -1 |
| Cerastium fontanum | 1 | Populus x canescens | -1 |
| Cerastium glomeratum | 1 | Portulaca oleracea | 0 |
| Cerastium pumilum | 0 | Potentilla alba | 2 |
| Cerastium semidecandrum | 1 | Potentilla anserina | -1 |
| Cerastium subtetrandrum | 1 | Potentilla arenaria | 1 |
| Cerastium sylvaticum | 1 | Potentilla argentea | 1 |
| Cerasus avium | -1 | Potentilla collina | 2 |
| Cerasus fruticosa | -1 | Potentilla erecta | -1 |
| Cerasus mahaleb | -1 | Potentilla heptaphylla | 1 |
| Cerasus vulgaris | -1 | Potentilla impolita | 2 |
| Ceratophyllum demersum | -2 | Potentilla inclinata | 2 |
| Ceratophyllum submersum | -2 | Potentilla leucopolitana | 2 |
| Cerinthe minor | -1 | Potentilla micrantha | 1 |
| Ceterach javorkaeanum | 0 | Potentilla neumanniana | 1 |
| Ceterach officinarum | 0 | Potentilla palustris | 1 |
| Chaerophyllum aromaticum | -1 | Potentilla patula | 1 |
| Chaerophyllum aureum | -1 | Potentilla pedata | 2 |
| Chaerophyllum bulbosum | -1 | Potentilla pusilla | 1 |
| Chaerophyllum hirsutum | -1 | Potentilla recta | 1 |
| Chaerophyllum temulum | -2 | Potentilla reptans | -1 |
| Chamaecytisus albus | -2 | Potentilla rupestris | 2 |
| Chamaecytisus austriacus | -2 | Potentilla supina | 2 |
| Chamaecytisus ciliatus | -2 | Potentilla thyrsiflora | 2 |
| Chamaecytisus heuffelii | -2 | Potentilla wiemanniana | 2 |
| Chamaecytisus ratisbonensis | -2 | Prenanthes purpurea | 1 |
| Chamaecytisus rochelii | -2 | Primula auricula | -1 |
| Chamaecytisus supinus | -2 | Primula elatior | -1 |
| Chamaecytisus triflorus | -2 | Primula farinosa | -1 |
| Chamaecytisus virescens | -2 | Primula veris | -1 |
| Chelidonium majus | -2 | Primula vulgaris | -1 |
| Chenopodium album subsp. album | -2 | Prunella grandiflora | 1 |
| Chenopodium album subsp. borbasii | -2 | Prunella laciniata | 1 |
| Chenopodium album subsp. pedunculare | -2 | Prunella vulgaris | 1 |
| Chenopodium ambrosioides | -3 | Prunus cerasifera | -1 |
| Chenopodium aristatum | -1 | Prunus domestica | -1 |
| Chenopodium bonus-henricus | -1 | Prunus spinosa | -3 |
| Chenopodium botrys | -1 | Pseudolysimachion incanum | 2 |
| Chenopodium chenipodioides | -1 | Pseudolysimachion longifolium | 2 |
| Chenopodium ficifolium | -1 | Pseudolysimachion orchideum | 2 |
| Chenopodium foliosum | -1 | Pseudolysimachion spicatum | 2 |
| Chenopodium giganteum | -2 | Pseudolysimachion spurium | 2 |
| Chenopodium glaucum | -1 | Pteridium aquilinum | -3 |
| Chenopodium hybridum | -3 | Puccinellia distans | 4 |
| Chenopodium murale | -1 | Puccinellia limosa | 3 |
| Chenopodium opulifolium | -1 | Puccinellia peisonis | 4 |
| Chenopodium polyspermum | -1 | Pulicaria dysenterica | -2 |
| Chenopodium pumilio | -1 | Pulicaria vulgaris | -2 |
| Chenopodium rubrum | -1 | Pulmonaria angustifolia | -1 |
| Chenopodium scheraderianum | -1 | Pulmonaria mollis | -1 |
| Chenopodium strictum subsp. striatiforme | -1 | Pulmonaria obscura | -1 |
| Chenopodium strictum subsp. strictum | -1 | Pulmonaria officinalis | -1 |
| Chenopodium suecicum | -1 | Pulsatilla grandis | -2 |
| Chenopodium urbicum | -2 | Pulsatilla patens | -2 |
| Chenopodium vulvaria | -1 | Pulsatilla pratensis | -2 |
| Chondrilla juncea | -1 | Pyrus communis | -1 |
| Chorispora tenella | 1 | Pyrus magyarica | -1 |
| Chrysopogon gryllus | -1 | Pyrus nivalis | -1 |
| Chrysosplenium alternifolium | -1 | Pyrus pyraster | -1 |
| Cichorium intybus | 3 | Pyrus x austriaca | -1 |
| Cicuta virosa | -3 | Quercus cerris | -1 |
| Circaea alpina | -2 | Quercus dalechampii | -1 |
| Circaea lutetiana | -2 | Quercus frainetto | -1 |
| Circaea x intermedia | -2 | Quercus petraea | -1 |
| Cirsium arvense | -3 | Quercus polycarpa | -1 |
| Cirsium boujartii | -3 | Quercus pubescens | -1 |
| Cirsium brachycephalum | -3 | Quercus robur | -1 |
| Cirsium canum | -3 | Quercus rubra | -1 |
| Cirsium eriophorum | -3 | Quercus virgiliana | -1 |
| Cirsium erisithales | -3 | Radiola linoides | 0 |
| Cirsium furiens | -3 | Ranunculus acris | -2 |
| Cirsium oleraceum | -3 | Ranunculus aquatilis | -3 |
| Cirsium palustre | -3 | Ranunculus arvensis | -2 |
| Cirsium pannonicum | -2 | Ranunculus auricomus | -2 |
| Cirsium rivulare | -3 | Ranunculus baudotii | -3 |
| Cirsium vulgare | -3 | Ranunculus bulbosus | -2 |
| Cladium mariscus | -2 | Ranunculus cassubicus | -2 |
| Cleistogenes serotina | 1 | Ranunculus circinatus | -3 |
| Clematis alpina | -3 | Ranunculus cymbalaria | -2 |
| Clematis integrifolia | -3 | Ranunculus fallax | -2 |
| Clematis recta | -3 | Ranunculus flammula | -3 |
| Clematis vitalba | -3 | Ranunculus fluitans | -3 |
| Clematis viticella | -3 | Ranunculus illyricus | -2 |
| Clinopodium vulgare | 1 | Ranunculus lanuginosus | -2 |
| Cnidium dubium | 2 | Ranunculus lateriflorus | -2 |
| Coeloglossum viride | 0 | Ranunculus lingua | -3 |
| Colchicum arenarium | -3 | Ranunculus parviflorus | -2 |
| Colchicum autumnale | -3 | Ranunculus pedatus | -2 |
| Colchicum hungaricum | -3 | Ranunculus peltatus | -3 |
| Colutea arborescens | -1 | Ranunculus polyanthemos | -2 |
| Commelina communis | 1 | Ranunculus polyphyllus | -2 |
| Conium maculatum | -3 | Ranunculus psilostachys | -2 |
| Conringia austriaca | 1 | Ranunculus repens | -3 |
| Conringia orientalis | 1 | Ranunculus rhipiphyllus | -3 |
| Consolida orientalis | -2 | Ranunculus rionii | -3 |
| Consolida regalis | -2 | Ranunculus sardous | -3 |
| Convallaria majalis | -3 | Ranunculus sceleratus | -3 |
| Convolvulus arvensis | -1 | Ranunculus strigulosus | -2 |
| Convolvulus cantabrica | -1 | Ranunculus trichophyllus | -3 |
| Conyza canadensis | -3 | Raphanus raphanistrum | -2 |
| Corallorhiza trifida | -1 | Raphanus sativus | -2 |
| Coriandrum sativum | -1 | Rapistrum perenne | -2 |
| Corispermum canescens | -1 | Reseda inodora | -1 |
| Corispermum nitidum | -1 | Reseda lutea | -1 |
| Cornus mas | -1 | Reseda luteola | -1 |
| Cornus sanguinea | -1 | Reseda phyteuma | -1 |
| Coronilla coronata | 2 | Rhamnus catharticus | -2 |
| Coronilla vaginalis | 3 | Rhamnus saxatilis | -2 |
| Coronopus didymus | 1 | Rhinanthus alectorolophus | -2 |
| Coronopus squamatus | 1 | Rhinanthus borbasii | -2 |
| Corothamnus procumbens | -1 | Rhinanthus minor | -2 |
| Corydalis cava | -2 | Rhinanthus rumelicus | -2 |
| Corydalis intermedia | -2 | Rhinanthus serotinus | -2 |
| Corydalis pumila | -2 | Rhinanthus wagneri | -2 |
| Corydalis solida | -2 | Ribes alpinum | -1 |
| Corylus avellana | -1 | Ribes aureum | -1 |
| Corylus colurna | -1 | Ribes nigrum | -1 |
| Corynephorus canescens | 2 | Ribes petraeum | -1 |
| Cotinus coggygria | -3 | Ribes rubrum | -1 |
| Cotoneaster integerrimus | -3 | Ribes uva-crispa | -1 |
| Cotoneaster matrensis | -3 | Ricinus communis | -3 |
| Cotoneaster niger | -3 | Robinia pseudo-acacia | -3 |
| Cotoneaster tomentosus | -3 | Rorippa amphibia | -2 |
| Crambe tataria | -1 | Rorippa austriaca | -2 |
| Crataegus calycina | -2 | Rorippa palustris | -2 |
| Crataegus laevigata | -3 | Rorippa sylvestris | -2 |
| Crataegus monogyna | -3 | Rorippa x anceps | -2 |
| Crataegus nigra | -1 | Rorippa x armoracioides | -2 |
| Crepis biennis | -2 | Rorippa x astylis | -2 |
| Crepis capillaris | -1 | Rorippa x hungarica | -2 |
| Crepis nicaeënsis | -1 | Rosa agrestis | -3 |
| Crepis paludosa | -1 | Rosa arvensis | -3 |
| Crepis pannonica | -2 | Rosa caesia | -3 |
| Crepis praemorsa | -1 | Rosa canina | -3 |
| Crepis pulchra | -2 | Rosa corymbifera | -3 |
| Crepis rhoeadifolia | -1 | Rosa dumalis | -3 |
| Crepis setosa | -1 | Rosa elliptica | -3 |
| Crepis taraxicifolia | -1 | Rosa rugosa | -3 |
| Crepis tectorum | -1 | Rosa gallica | -3 |
| Crocus albiflorus | -2 | Rosa gizellae | -3 |
| Crocus heuffelianus | -2 | Rosa glauca | -3 |
| Crocus reticulatus | -2 | Rosa hungarica | -3 |
| Crocus sativus | -2 | Rosa inodora | -3 |
| Crocus tommasinianus | -2 | Rosa kmetiana | -3 |
| Cruciata glabra | 1 | Rosa jundzillii | -3 |
| Cruciata laevipes | 1 | Rosa majalis | -3 |
| Cruciata pedemontana | 1 | Rosa micrantha | -3 |
| Crupina vulgaris | 1 | Rosa tomentella | -3 |
| Crypsis aculeata | 0 | Rosa pendulina | -3 |
| Cucubalus baccifer | 2 | Rosa polyacantha | -3 |
| Cuscuta approximata | -2 | Rosa rubiginosa | -3 |
| Cuscuta australis | -2 | Rosa villosa | -3 |
| Cuscuta campestris | -2 | Rosa scabriuscula | -3 |
| Cuscuta epilinum | -2 | Rosa sherardii | -3 |
| Cuscuta epithymum subsp. epithymum | -2 | Rosa spinosissima | -3 |
| Cuscuta epithymum subsp. kotschi | -2 | Rosa subcanina | -3 |
| Cuscuta europaea | -2 | Rosa subcollina | -3 |
| Cuscuta lupuliformis | -2 | Rosa szaboi | -3 |
| Cyclamen purpurascens | -2 | Rosa tomentosa | -3 |
| Cydonia oblonga | -1 | Rosa zagrebiensis | -3 |
| Cymbalaria muralis | 0 | Rosa zalana | -3 |
| Cynodon dactylon | 2 | Rubus caesius | -3 |
| Cynoglossum hungaricum | -2 | Rubus fruticosus agg. | -3 |
| Cynoglossum officinale | -2 | Rubus idaeus | -3 |
| Cynosurus cristatus | 4 | Rubus saxatilis | -3 |
| Cynosurus echinatus | 2 | Rumex acetosa | -1 |
| Cyperus difformis | 1 | Rumex acetosella | -1 |
| Cyperus flavescens | -1 | Rumex aquaticus | -2 |
| Cyperus fuscus | 1 | Rumex confertus | -1 |
| Cyperus glaber | -1 | Rumex conglomeratus | -1 |
| Cyperus glomeratus | -1 | Rumex crispus | -2 |
| Cyperus longus | -1 | Rumex dentatus | -1 |
| Cyperus pannonicus | 0 | Rumex hydrolapathum | -2 |
| Cypripedium calceolus | -2 | Rumex kerneri | -2 |
| Cystopteris fragilis | -2 | Rumex maritimus | -1 |
| Dactylis glomerata | 5 | Rumex obtusifolius | -2 |
| Dactylis polygama | 3 | Rumex palustris | -1 |
| Dactylorhiza fuchsii | -1 | Rumex patientia | -2 |
| Dactylorhiza incarnata | -1 | Rumex pseudonatronatus | -2 |
| Dactylorhiza maculata | -1 | Rumex pulcher | -1 |
| Dactylorhiza majalis | -1 | Rumex sanguineus | -1 |
| Dactylorhiza sambucina | -1 | Rumex stenophyllus | -1 |
| Danthonia alpina | 2 | Rumex thyrsiflorus | -2 |
| Danthonia decumbens | 1 | Ruscus aculeatus | -3 |
| Daphne cneorum | -3 | Ruscus hypoglossum | 1 |
| Daphne laureola | -3 | Sagina apetala subsp. apetala | 0 |
| Daphne mezereum | -3 | Sagina apetala subsp. erecta | 0 |
| Datura stramonium | -3 | Sagina nodosa | 0 |
| Daucus carota | 1 | Sagina procumbens | 0 |
| Deschampsia cespitosa | 1 | Sagina sabuletorum | 0 |
| Deschampsia flexuosa | 1 | Sagina saginoides | 0 |
| Descurainia sophia | -1 | Sagina subulata | 0 |
| Dianthus arenarius | 2 | Sagittaria sagittifolia | -3 |
| Dianthus armeria | 2 | Salicornia prostrata | 1 |
| Dianthus barbatus | 2 | Salix alba | -2 |
| Dianthus carthusianorum | 2 | Salix aurita | -2 |
| Dianthus collinus | 2 | Salix caprea | -2 |
| Dianthus deltoides | 2 | Salix cinerea | -2 |
| Dianthus diutinus | 2 | Salix elaeagnos | -2 |
| Dianthus giganteiformis | 2 | Salix fragilis | -2 |
| Dianthus plumarius subsp praecox | 2 | Salix myrsinifolia | -2 |
| Dianthus plumarius subsp. lumnitzeri | 2 | Salix pentandra | -2 |
| Dianthus plumarius subsp. regis-stephani | 2 | Salix purpurea | -2 |
| Dianthus pontederae | 2 | Salix rosmarinifolia | -2 |
| Dianthus serotinus | 2 | Salix triandra | -2 |
| Dianthus superbus | 2 | Salix viminalis | -2 |
| Dichostylis micheliana | 1 | Salix x multinervis | -2 |
| Dictamnus albus | -2 | Salsola kali | -1 |
| Digitalis ferruginea | -3 | Salsola soda | -1 |
| Digitalis grandiflora | -3 | Salvia aethiopis | 1 |
| Digitalis lanata | -3 | Salvia austriaca | 1 |
| Digitalis purpurea | -3 | Salvia glutinosa | 1 |
| Digitaria ciliaris | 1 | Salvia nemorosa | 2 |
| Digitaria ischaemum | 0 | Salvia nutans | 1 |
| Digitaria sanguinalis | 1 | Salvia officinalis | 1 |
| Diphasium complanatum | -2 | Salvia pratensis | 2 |
| Diphasium issleri | -2 | Salvia sclarea | 1 |
| Diphasium tristachyum | -2 | Salvia verbenaca | 2 |
| Diplotaxis erucoides | 1 | Salvia verticillata | 2 |
| Diplotaxis muralis | 1 | Sambucus ebulus | -3 |
| Diplotaxis tenuifolia | 1 | Sambucus nigra | -3 |
| Dipsacus laciniatus | -3 | Sambucus racemosa | -3 |
| Dipsacus sylvestris | -3 | Samolus valerandi | 1 |
| Doronicum austriacum | 1 | Sanguisorba minor | 2 |
| Doronicum hungaricum | 1 | Sanguisorba officinalis | 2 |
| Doronicum orientale | 1 | Sanicula europaea | 2 |
| Dorycnium germanicum | 0 | Saponaria officinalis | -1 |
| Dorycnium herbaceum | 0 | Sarothamnus scoparius | -3 |
| Draba lasiocarpa | 0 | Satureja hortensis | 1 |
| Draba muralis | 1 | Saxifraga adscendens | 0 |
| Draba nemorosa | 1 | Saxifraga bulbifera | 0 |
| Dracocephalum austriacum | -2 | Saxifraga granulata | 0 |
| Dracocephalum moldavica | -2 | Saxifraga paniculata | 0 |
| Dracocephalum ruyschiana | -2 | Saxifraga tridactylites | 0 |
| Drosera anglica | -2 | Scabiosa canescens | 2 |
| Drosera rotundifolia | -2 | Scabiosa columbaria | 2 |
| Dryopteris carthusiana | -2 | Scabiosa ochroleuca | 2 |
| Dryopteris cristata | -2 | Scabiosa triandra | 2 |
| Dryopteris dilatata | -2 | Scandix pecten-veneris | 1 |
| Dryopteris expansa | -2 | Schoenoplectus lacustris | -2 |
| Dryopteris filix-mas | -2 | Schoenoplectus litoralis | -2 |
| Dryopteris pseudo-mas | -2 | Schoenoplectus mucronatus | -1 |
| Ecballium elaterium | -3 | Schoenoplectus setaceus | -1 |
| Echinochloa crus-galli | 1 | Schoenoplectus supinus | -1 |
| Echinochloa eruciformis | 1 | Schoenoplectus tabernaemontani | -2 |
| Echinochloa occidentalis | 1 | Schoenoplectus triqueter | -2 |
| Echinochloa oryzoides | 1 | Schoenus ferrugineus | -1 |
| Echinochloa phyllopogon | 1 | Schoenus nigricans | -1 |
| Echinocystis lobata | -3 | Scilla autumnalis | 0 |
| Echinops ruthenicus | -3 | Scilla drunensis | 0 |
| Echinops sphaerocephalus | -3 | Scilla kladnii | 0 |
| Echium italicum | -2 | Scilla spetana | 0 |
| Echium maculatum | -2 | Scilla vindobonensis | 0 |
| Echium vulgare | -2 | Scirpoides holoschoenus | -1 |
| Elaeagnus angustifolia | -1 | Scirpus pungens | -1 |
| Elatine alsinastrum | 1 | Scirpus radicans | -1 |
| Elatine hexandra | 1 | Scirpus sylvaticus | -1 |
| Elatine hungarica | 1 | Scleranthus annuus | 0 |
| Elatine hydropiper | 1 | Scleranthus dichotomus | 0 |
| Elatine triandra | 1 | Scleranthus perennis | 0 |
| Eleocharis acicularis | 1 | Scleranthus polycarpos | 0 |
| Eleocharis austriaca | 1 | Scleranthus verticillatus | 0 |
| Eleocharis carniolica | 1 | Sclerochloa dura | 0 |
| Eleocharis mamillata | 1 | Scopolia carniolica | -3 |
| Eleocharis ovata | 1 | Scorzonera austriaca | 1 |
| Eleocharis palustris | 1 | Scorzonera hispanica | 1 |
| Eleocharis quinqueflora | 1 | Scorzonera humilis | 1 |
| Eleocharis uniglumis | 1 | Scorzonera parviflora | 1 |
| Eleusine indica | 1 | Scorzonera purpurea | 1 |
| Elymus caninus | 3 | Scrophularia nodosa | -2 |
| Elymus hispidus | 3 | Scrophularia scopolii | -2 |
| Elymus repens | 3 | Scrophularia umbrosa | -2 |
| Ephedra distachya | -2 | Scrophularia vernalis | -2 |
| Epilobium ciliatum | -1 | Scutellaria altissima | -2 |
| Chamaenerion angustifolium | -2 | Scutellaria columnae | -2 |
| Epilobium collinum | -1 | Scutellaria galericulata | -2 |
| Chamaenerion dodonaei | -1 | Scutellaria hastifolia | -2 |
| Epilobium hirsutum | -2 | Secale sylvestre | 1 |
| Epilobium lanceolatum | -1 | Securigera elegans | 5 |
| Epilobium montanum | -1 | Securigera varia | 5 |
| Epilobium obscurum | -1 | Sedum acre | -2 |
| Epilobium palustre | -1 | Sedum album | -2 |
| Epilobium parviflorum | -2 | Sedum caespitosum | -2 |
| Epilobium roseum | -1 | Sedum hispanicum | -2 |
| Epilobium tetragonum | -1 | Hylotelephium telephium subsp. maxium | -2 |
| Epipactis atrorubens | -2 | Sedum neglectum | -2 |
| Epipactis helleborine | -2 | Sedum reflexum | -2 |
| Epipactis leptochila | -2 | Sedum sartorianum | -2 |
| Epipactis microphylla | -2 | Sedum sexangulare | -2 |
| Epipactis muelleri | -2 | Sedum spurium | -2 |
| Epipactis palustris | -2 | Selaginella helvetica | 0 |
| Epipactis pontica | -2 | Selinum carvifolia | 1 |
| Epipactis purpurata | -2 | Sempervivum marmoreum | 0 |
| Epipactis voethii | -2 | Sempervivum tectorum | 0 |
| Epipactis exilis | -2 | Senecio aquaticus | -2 |
| Epipactis mecsekensis | -2 | Tephroseris aurantiaca | -2 |
| Epipactis albensis | -2 | Senecio doria | -2 |
| Epipactis placentina | -2 | Senecio erraticus | -2 |
| Epipactis muelleri | -2 | Senecio erucifolius | -2 |
| Epipactis nordeniorum | -2 | Senecio sarracenicus | -2 |
| Epipactis tallosii | -2 | Senecio inaeequidens | -2 |
| Epipactis bugacensis | -2 | Tephroseris integrifolia | -2 |
| Epipactis greuterii | -2 | Senecio jacobaea | -2 |
| Epipogium aphyllum | -2 | Senecio germanicus | -2 |
| Equisetum arvense | -2 | Senecio ovatus | -2 |
| Equisetum fluviatile | -2 | Tephroseris longifolia | -2 |
| Equisetum hyemale | -2 | Senecio paludosus | -2 |
| Equisetum palustre | -2 | Senecio rupestris | -2 |
| Equisetum ramosissimum | -2 | Senecio sylvaticus | -2 |
| Equisetum sylvaticum | -2 | Senecio umbrosus | -2 |
| Equisetum telmateia | -2 | Senecio vernalis | -2 |
| Equisetum variegatum | -2 | Senecio viscosus | -2 |
| Equisetum x moorei | -2 | Senecio vulgaris | -2 |
| Eragrostis cilianensis | 1 | Serratula lycopifolia | 2 |
| Eragrostis minor | 1 | Serratula radiata | 2 |
| Eragrostis parviflora | 1 | Serratula tinctoria | 2 |
| Eragrostis pilosa | 2 | Seseli annuum | 2 |
| Eranthis hyemalis | 0 | Seseli hippomarathrum | 2 |
| Erechtites hieraciifolia | -2 | Seseli leucospermum | 2 |
| Erigeron acris | -2 | Seseli osseum | 2 |
| Erigeron annus | -2 | Seseli varium | 2 |
| Eriophorum angustifolium | -1 | Sesleria albicans | 1 |
| Eriophorum gracile | -1 | Sesleria heuflerana | 2 |
| Eriophorum latifolium | -1 | Sesleria hungarica | 1 |
| Eriophorum vaginatum | -1 | Sesleria sadlerana | 1 |
| Erodium ciconium | -1 | Sesleria uliginosa | 1 |
| Erodium cicutarium | -1 | Setaria italica | 1 |
| Erodium neilreichii | -1 | Setaria pumila | 1 |
| Erophila praecox | 0 | Setaria verticillata | 1 |
| Erophila spathulata | 0 | Setaria verticilliformis | 1 |
| Erophila verna | 0 | Setaria viridis | 1 |
| Eruca sativa | -2 | Sherardia arvensis | 1 |
| Erucastrum gallicum | 1 | Sicyos angulatus | -2 |
| Erucastrum nasturtiifolium | 1 | Sideritis montana | 0 |
| Eryngium campestre | -3 | Silaum peucedanoides | -1 |
| Eryngium planum | -3 | Silaum silaus | -1 |
| Erysimum cheiranthoides | -3 | Silene alba | 1 |
| Erysimum crepidifolium | -3 | Silene armeria | 1 |
| Erysimum diffusum | -3 | Silene borysthenica | 1 |
| Erysimum hieracifolium | -3 | Silene conica | 0 |
| Erysimum odoratum | -3 | Silene dichotoma | 1 |
| Erysimum pallidiflorum | -3 | Silene dioica | 1 |
| Erysimum repandum | -3 | Silene gallica | 0 |
| Erythronium dens-canis | -3 | Silene longiflora | 1 |
| Euclidium syriacum | 0 | Silene multiflora | 1 |
| Euonymus europaea | -2 | Silene nemoralis | 1 |
| Euonymus verrucosa | -2 | Silene noctiflora | 1 |
| Eupatorium cannabinum | -2 | Silene nutans | 1 |
| Euphorbia amygdaloides | -2 | Silene otites | 1 |
| Euphorbia angulata | -2 | Silene viridiflora | 1 |
| Euphorbia carpatica | -2 | Silene viscosa | 1 |
| Euphorbia cyparissias | -2 | Silene vulgaris | 1 |
| Euphorbia dulcis | -2 | Sinapis alba | -2 |
| Euphorbia epithymoides | -2 | Sinapis arvensis | -2 |
| Euphorbia esula | -2 | Sisymbrium altissimum | -2 |
| Euphorbia exigua | -2 | Sisymbrium loeselii | -2 |
| Euphorbia falcata | -2 | Sisymbrium officinale | -2 |
| Euphorbia helioscopia | -2 | Sisymbrium orientale | -2 |
| Euphorbia humifusa | -2 | Sisymbrium polymorphum | -2 |
| Euphorbia lucida | -2 | Sisymbrium strictissimum | -2 |
| Euphorbia maculata | -2 | Sium latifolium | -2 |
| Euphorbia nutans | -2 | Sium sisarum | -2 |
| Euphorbia palustris | -2 | Smyrnium perfoliatum | -1 |
| Euphorbia glareosa | -2 | Solanum alatum | -3 |
| Euphorbia peplus | -2 | Solanum dulcamara | -3 |
| Euphorbia platyphyllos | -2 | Solanum villosum | -3 |
| Euphorbia salicifolia | -2 | Solanum nigrum | -3 |
| Euphorbia segetalis | -2 | Solanum rostratum | -3 |
| Euphorbia seguierana | -2 | Solidago canadensis | -2 |
| Euphorbia stricta | -2 | Solidago gigantea | -2 |
| Euphorbia taurinensis | -2 | Solidago virgaurea | -1 |
| Euphorbia verrucosa | -2 | Sonchus arvensis | -1 |
| Euphorbia villosa | -2 | Sonchus asper | -1 |
| Euphorbia virgata | -2 | Sonchus oleraceus | -1 |
| Euphrasia kerneri | -1 | Sonchus palustris | -2 |
| Euphrasia rostkoviana | -1 | Sorbus aria | -1 |
| Euphrasia stricta | -1 | Sorbus aucuparia | -1 |
| Euphrasia tatarica | -1 | Sorbus austriaca | -1 |
| Fagopyrum esculentum | -1 | Sorbus domestica | -1 |
| Fagus sylvatica | -2 | Sorbus graeca | -1 |
| Falcaria vulgaris | -1 | Sorbus torminalis | -1 |
| Fallopia convolvulus | -1 | Sorbus x danubialis | -1 |
| Fallopia dumetorum | -1 | Sorghum bicolor | 3 |
| Fallopia japonica | -3 | Sorghum halepense | 3 |
| Fallopia sachalinensis | -3 | Sorghum sudanense | 3 |
| Fallopia x bohemica | -3 | Sparganium emersum | -2 |
| Ferula sadlerana | 1 | Sparganium erectum | -2 |
| Festuca altissima | 3 | Sparganium minimum | -2 |
| Festuca amethystina | 2 | Spergula arvensis | 0 |
| Festuca arundinacea | 4 | Spergula pentandra | 0 |
| Festuca dalmatica | 3 | Spergularia marina | 0 |
| Festuca drymeia | 2 | Spergularia maritima | 0 |
| Festuca filiformis | 2 | Spergularia rubra | 0 |
| Festuca gigantea | 3 | Spiraea crenata | -1 |
| Festuca heterophylla | 2 | Spiraea media | -1 |
| Festuca nigrescens | 3 | Spiraea salicifolia | -1 |
| Festuca ovina | 2 | Spiranthes aestivalis | -1 |
| Festuca pallens | 2 | Spiranthes spiralis | -1 |
| Festuca pannonica | 2 | Stachys alpina | -1 |
| Festuca pratensis | 6 | Stachys annua | 1 |
| Festuca pseudodalmatica | 3 | Stachys byzantina | -2 |
| Festuca pseudovaginata | 3 | Stachys germanica | -1 |
| Festuca pseudovina | 3 | Stachys palustris | 1 |
| Festuca rubra | 4 | Stachys recta | 1 |
| Festuca rupicola | 3 | Stachys sylvatica | -1 |
| Festuca tenuifolia | 3 | Staphylea pinnata | -1 |
| Festuca vaginata | 2 | Stellaria graminea | 1 |
| Festuca valesiaca | 3 | Stellaria holostea | 1 |
| Festuca vojtkoi | 2 | Stellaria media | 1 |
| Festuca x stricta | 2 | Stellaria nemorum | 1 |
| Festuca x wagneri | 3 | Stellaria palustris | 1 |
| Ficaria verna | 1 | Stellaria uliginosa | 1 |
| Filago arvensis | 0 | Sternbergia colchiciflora | 0 |
| Filago minima | 0 | Stipa borysthenica | -2 |
| Filago vulgaris | 0 | Stipa bromoides | -2 |
| Filipendula ulmaria | 1 | Stipa capillata | -2 |
| Filipendula vulgaris | 1 | Stipa crassiculmis | -2 |
| Foeniculum vulgare | -1 | Stipa dasyphylla | -2 |
| Fragaria moschata | 2 | Stipa eriocaulis | -1 |
| Fragaria vesca | 2 | Stipa pennata | -2 |
| Fragaria viridis | 2 | Stipa pulcherrima | -2 |
| Frangula alnus | -1 | Stipa tirsa | -1 |
| Fraxinus angustifolia | -1 | Stratiotes aloides | -3 |
| Fraxinus excelsior | -1 | Suaeda maritima | 1 |
| Fraxinus ornus | -1 | Suaeda pannonica | 1 |
| Fraxinus pennsylvanica | -1 | Succisa pratensis | 2 |
| Fritillaria meleagris | -2 | Succisella inflexa | 2 |
| Fumana ericoides | 0 | Symphytum officinale | -1 |
| Fumana procumbens | 0 | Symphytum tuberosum | -1 |
| Fumaria officinalis | -1 | Syrenia cana | -3 |
| Fumaria parviflora | -1 | Syringa vulgaris | -3 |
| Fumaria rostellata | -1 | Taeniatherum asperum | 1 |
| Fumaria schleicheri | -1 | Tamarix gallica | -2 |
| Fumaria vaillantii | -1 | Tamarix ramosissima | -2 |
| Gagea bohemica | 0 | Tamarix tetrandra | -2 |
| Gagea lutea | 0 | Tamus communis | -1 |
| Gagea minima | 0 | Tanacetum corymbosum | -2 |
| Gagea pratensis | 0 | Tanacetum parthenium | -2 |
| Gagea pusilla | 0 | Tanacetum vulgare | -2 |
| Gagea spathacea | 0 | Taraxacum bessarabicum | 1 |
| Gagea szovitsii | 0 | Taraxacum laevigatum | 1 |
| Gagea villosa | 0 | Taraxacum officinale | 3 |
| Galanthus nivalis | -3 | Taraxacum palustre | 2 |
| Galega officinalis | 2 | Taraxacum serotinum | 2 |
| Galeobdolon luteum | -2 | Taxus baccata | -3 |
| Galeopsis bifida | -2 | Teesdalia nudicaulis | 1 |
| Galeopsis ladanum | -2 | Telekia speciosa | -2 |
| Galeopsis pubescens | -2 | Tetragonolobus maritimus | 4 |
| Galeopsis segetum | -2 | Teucrium botrys | 1 |
| Galeopsis speciosa | -2 | Teucrium chamaedrys | 1 |
| Galeopsis tetrahit | -2 | Teucrium montanum | 1 |
| Galinsoga ciliata | -1 | Teucrium scordium | 1 |
| Galinsoga parviflora | -1 | Teucrium scorodonia | 1 |
| Galium abaujense | -1 | Thalictrum aquilegiifolium | -2 |
| Galium album | -1 | Thalictrum flavum | -2 |
| Galium aparine | -1 | Thalictrum foetidum | -2 |
| Galium austriacum | -1 | Thalictrum lucidum | -2 |
| Galium boreale | -1 | Thalictrum minus | -2 |
| Galium divaricatum | 1 | Thalictrum simplex | -2 |
| Galium elongatum | -1 | Thelypteris palustris | -2 |
| Galium glaucum | -1 | Thesium arvense | 0 |
| Galium humifusum | -1 | Thesium bavarum | 0 |
| Galium lucidum | -1 | Thesium dollineri | 0 |
| Galium mollugo | -1 | Thesium linophyllon | 0 |
| Galium odoratum | -1 | Thladiantha dubia | -2 |
| Galium palustre | -1 | Thlaspi alliaceum | 1 |
| Galium parisiense | 1 | Thlaspi arvense | 1 |
| Galium pumilum | 1 | Thlaspi coerulescens | 1 |
| Galium rivale | -1 | Thlaspi goesingense | 1 |
| Galium rotundifolium | 1 | Thlaspi jankae | 1 |
| Galium rubioides | -1 | Thlaspi kovatsii | 1 |
| Galium schultesii | -1 | Thlaspi montanum | 1 |
| Galium spurium | 1 | Thlaspi perfoliatum | 1 |
| Galium sylvaticum | -1 | Thymelaea passerina | 0 |
| Galium tenuissimum | 1 | Thymus caespitosus | -1 |
| Galium tricornutum | 1 | Thymus glabrescens | -1 |
| Galium uliginosum | -1 | Thymus pannonicus | -1 |
| Galium verum | -1 | Thymus praecox | -1 |
| Gaudinia fragilis | 2 | Thymus pulegioides | -1 |
| Genista germanica | -2 | Thymus serpyllum | -1 |
| Genista ovata | -2 | Thymus vulgaris | -1 |
| Genista pilosa | -2 | Tilia cordata | -1 |
| Genista pilosa | -2 | Tilia platyphyllos | -1 |
| Genista tinctoria | -2 | Tilia tomentosa | -1 |
| Genistella sagittalis | -1 | Tofieldia calyculata | 1 |
| Gentiana asclepiadea | 1 | Tordylium maximum | 1 |
| Gentiana cruciata | 1 | Torilis arvensis | -1 |
| Gentiana pneumonanthe | 1 | Torilis japonica | -1 |
| Gentianella austriaca | 0 | Torilis ucranica | -1 |
| Gentianella amarella subsp. livonica | 0 | Tragopogon dubius | 2 |
| Gentianopsis ciliata | 0 | Tragopogon floccosus | 2 |
| Geranium bohemicum | 1 | Tragopogon orientalis | 2 |
| Geranium columbinum | 0 | Tragus racemosus | -1 |
| Geranium dissectum | 0 | Traunsteinera globosa | -1 |
| Geranium divaricatum | 1 | Tribulus terrestris | -3 |
| Geranium lucidum | 0 | Trifolium alpestre | 4 |
| Geranium molle | 0 | Trifolium angulatum | 4 |
| Geranium palustre | 1 | Trifolium arvense | 1 |
| Geranium phaeum | 1 | Trifolium aureum | 5 |
| Geranium pratense | 1 | Trifolium campestre | 5 |
| Geranium pusillum | 0 | Trifolium diffusum | 4 |
| Geranium pyrenaicum | 1 | Trifolium dubium | 5 |
| Geranium robertianum | 1 | Trifolium fragiferum | 6 |
| Geranium rotundifolium | 0 | Trifolium hybridum | 7 |
| Geranium sanguineum | 1 | Trifolium incarnatum | 4 |
| Geranium sibiricum | 1 | Trifolium medium | 4 |
| Geranium sylvaticum | 1 | Trifolium micranthum | 5 |
| Geum aleppicum | 1 | Trifolium montanum | 4 |
| Geum urbanum | 1 | Trifolium ochroleucon | 4 |
| Gladiolus byzantinus | -2 | Trifolium ornithopodioides | 3 |
| Gladiolus imbricatus | -2 | Trifolium pallidum | 4 |
| Gladiolus palustris | -2 | Trifolium pannonicum | 5 |
| Glaucium corniculatum | -2 | Trifolium patens | 5 |
| Glaucium flavum | -2 | Trifolium pratense | 7 |
| Glaux maritima | 0 | Trifolium repens | 7 |
| Glechoma hederacea | -2 | Trifolium resupinatum | 5 |
| Glechoma hirsuta | -2 | Trifolium retusum | 4 |
| Gleditsia triacanthos | -3 | Trifolium rubens | 5 |
| Globularia cordifolia | 1 | Trifolium striatum | 5 |
| Globularia punctata | 1 | Trifolium strictum | 5 |
| Glyceria declinata | -1 | Trifolium subterraneum | 2 |
| Glyceria fluitans | -1 | Trifolium vesiculosum | 4 |
| Glyceria maxima | -1 | Triglochin maritimum | -1 |
| Glyceria nemoralis | -1 | Triglochin palustre | -1 |
| Glyceria notata | -1 | Trigonella caerulea | 3 |
| Glycyrrhiza echinata | 2 | Trigonella foenum-graecum | 3 |
| Glycyrrhiza glabra | 2 | Trigonella gladiata | 3 |
| Gnaphalium luteo-album | 0 | Trigonella monspeliaca | 3 |
| Gnaphalium sylvaticum | 0 | Trigonella procumbens | 3 |
| Gnaphalium uliginosum | 0 | Trinia glauca | -1 |
| Goodyera repens | 0 | Trinia ramosissima | -1 |
| Gratiola officinalis | -2 | Trisetum flavescens | 4 |
| Gymnadenia conopsea | -1 | Trollius europaeus | -2 |
| Gymnadenia odoratissima | -1 | Tulipa sylvestris | -2 |
| Gymnocarpium dryopteris | -2 | Turritis glabra | 1 |
| Gymnocarpium robertianum | -2 | Tussilago farfara | -2 |
| Gypsophila fastigiata | 1 | Typha angustifolia | -1 |
| Gypsophila muralis | 1 | Typha latifolia | -1 |
| Gypsophila paniculata | 1 | Typha laxmannii | -1 |
| Haynaldia villosa | 2 | Typha minima | -1 |
| Hedera helix | -3 | Typha shuttleworthii | -1 |
| Heleochloa alopecuroides | 1 | Ulmus glabra | -1 |
| Heleochloa schoenoides | 0 | Ulmus laevis | -1 |
| Helianthemum canum | 0 | Ulmus minor | -1 |
| Helianthemum nummularium | 0 | Ulmus procera | -1 |
| Helianthemum ovatum | 0 | Urtica dioica | 1 |
| Helianthus annuus | -2 | Urtica kioviensis | 1 |
| Helianthus decapetalus | -2 | Urtica pilulifera | 1 |
| Helianthus rigidus | -2 | Urtica urens | 1 |
| Helianthus tuberosus | -2 | Vaccaria hispanica | 2 |
| Helichrysum arenarium | -1 | Vaccinium myrtillus | -1 |
| Helictotrichon adsurgens | 2 | Vaccinium oxycoccos | -1 |
| Helictotrichon compressum | 2 | Vaccinium vitis-idaea | -1 |
| Helictotrichon pratense | 2 | Valeriana dioica | -1 |
| Helictotrichon pubescens | 1 | Valeriana excelsa | -1 |
| Heliotropium europaeum | -2 | Valeriana officinalis | -1 |
| Heliotropium supinum | -2 | Valeriana stolonifera | -1 |
| Helleborus dumetorum | -3 | Valeriana tripteris | -1 |
| Helleborus odorus | -3 | Valerianella carinata | 1 |
| Helleborus purpurascens | -3 | Valerianella coronata | 1 |
| Helleborus viridis | -3 | Valerianella dentata | 1 |
| Helminthia echioides | -1 | Valerianella locusta | 1 |
| Hemerocallis fulva | -3 | Valerianella pumila | 1 |
| Hemerocallis lilio-asphodelus | -3 | Valerianella rimosa | 1 |
| Hepatica nobilis | -1 | Ventenata dubia | 1 |
| Heracleum mantegazzianum | -3 | Veratrum album | -3 |
| Heracleum sphondylium | -3 | Veratrum nigrum | -3 |
| Herniaria glabra | 0 | Verbascum austriacum | -1 |
| Herniaria hirsuta | 0 | Verbascum blattaria | -2 |
| Herniaria incana | 0 | Verbascum densiflorum | -2 |
| Hesperis matronalis | -1 | Verbascum lychnitis | -1 |
| Hesperis sylvestris | -1 | Verbascum nigrum | -1 |
| Hesperis tristis | 1 | Verbascum phlomoides | -2 |
| Hibiscus trionum | 1 | Verbascum phoeniceum | 0 |
| Hieracium pilosella | 1 | Verbascum pulverulentum | -1 |
| Hieracium aurantiacum | 1 | Verbascum speciosum | -2 |
| Hieracium bauhinii | 1 | Verbascum thapsus | -2 |
| Hieracium bifidum | 1 | Verbena officinalis | 1 |
| Hieracium bupleuroides | 1 | Verbena supina | 1 |
| Hieracium caespitosum | 1 | Veronica acinifolia | 0 |
| Hieracium cymosum | 1 | Veronica agrestis | 0 |
| Hieracium echioides | 1 | Veronica anagallis-aquatica | 2 |
| Hieracium lachenalii | 1 | Veronica anagalloides | 1 |
| Hieracium lactucella | 1 | Veronica arvensis | 0 |
| Hieracium laevigatum | 1 | Veronica austriaca | 1 |
| Hieracium macranthum | 1 | Veronica beccabunga | 1 |
| Hieracium murorum | 1 | Veronica catenata | 1 |
| Hieracium piloselloides | 1 | Veronica chamaedrys | 1 |
| Hieracium racemosum | 1 | Veronica dillenii | 0 |
| Hieracium sabaudum | 1 | Veronica hederifolia | 0 |
| Hieracium schmidtii | 1 | Veronica montana | 1 |
| Hieracium staticifolium | 1 | Veronica officinalis | 1 |
| Hieracium umbellatum | 1 | Veronica opaca | 0 |
| Hierochloë australis | 1 | Veronica peregrina | 0 |
| Hierochloë repens | 1 | Veronica persica | 0 |
| Himantoglossum adriaticum | -1 | Veronica polita | 0 |
| Himantoglossum caprinum | -1 | Veronica praecox | 0 |
| Hippocrepis comosa | 2 | Veronica prostrata | 0 |
| Hippocrepis emerus | 2 | Veronica scardica | 1 |
| Hippophaë rhamnoides | -3 | Veronica scutellata | 1 |
| Holcus lanatus | 2 | Veronica serpyllifolia | 1 |
| Holcus mollis | 1 | Veronica teucrium | 1 |
| Holosteum umbellatum | 1 | Veronica triphyllos | 0 |
| Hordelymus europaeus | 1 | Veronica verna | 0 |
| Hordeum hystrix | 1 | Viburnum lantana | -2 |
| Hordeum marinum | 1 | Viburnum opulus | -2 |
| Hordeum murinum | 1 | Vicia angustifolia | 3 |
| Hornungia petraea | 0 | Vicia articulata | 3 |
| Hottonia palustris | -2 | Vicia biennis | 4 |
| Humulus lupulus | -1 | Vicia cassubica | 4 |
| Humulus scandens | -1 | Vicia cracca | 3 |
| Huperzia selago | 0 | Vicia dumetorum | 4 |
| Hydrocharis morsus-ranae | -2 | Vicia ervilia | 3 |
| Hydrocotyle vulgaris | -2 | Vicia faba | 3 |
| Hyoscyamus niger | -3 | Vicia grandiflora | 3 |
| Hypericum barbatum | 1 | Vicia hirsuta | 3 |
| Hypericum elegans | 1 | Vicia lathyroides | 3 |
| Hypericum hirsutum | 1 | Vicia lutea | 3 |
| Hypericum humifusum | 1 | Vicia narbonensis | 3 |
| Hypericum maculatum | 1 | Vicia oroboides | 4 |
| Hypericum montanum | 1 | Vicia pannonica | 3 |
| Hypericum perforatum | 1 | Vicia peregrina | 3 |
| Hypericum tetrapterum | 1 | Vicia pisiformis | 4 |
| Hypochoeris maculata | 1 | Vicia sativa | 4 |
| Hypochoeris radicata | 1 | Vicia sepium | 4 |
| Hyssopus officinalis | 1 | Vicia sparsiflora | 4 |
| Impatiens balfouri | -2 | Vicia sylvatica | 4 |
| Impatiens glandulifera | -2 | Vicia tenuifolia | 3 |
| Impatiens noli-tangere | -2 | Vicia tenuissima | 3 |
| Impatiens parviflora | -2 | Vicia tetrasperma | 3 |
| Inula britannica | 1 | Vicia villosa | 4 |
| Inula conyza | 2 | Vinca herbacea | -2 |
| Inula ensifolia | 2 | Vinca major | -2 |
| Inula germanica | -1 | Vinca minor | -2 |
| Inula helenium | 2 | Vincetoxicum hirundinaria | -3 |
| Inula hirta | -1 | Vincetoxicum pannonicum | -3 |
| Inula oculus-christi | -1 | Viola alba | 1 |
| Inula salicina | 2 | Viola ambigua | 1 |
| Inula spiraeifolia | 2 | Viola arvensis | 0 |
| Ipomoea purpurea | -1 | Viola biflora | 1 |
| Iris aphylla | -2 | Viola canina | 1 |
| Iris arenaria | -2 | Viola collina | 1 |
| Iris germanica | -2 | Viola cyanea | 1 |
| Iris graminea | -2 | Viola elatior | 1 |
| Iris pseudacorus | -3 | Viola hirta | 1 |
| Iris pumila | -2 | Viola kitaibeliana | 0 |
| Iris sibirica | -2 | Viola mirabilis | 1 |
| Iris spuria | -2 | Viola montana | 1 |
| Iris variegata | -2 | Viola odorata | 1 |
| Isatis tinctoria | -1 | Viola palustris | 1 |
| Isopyrum thalictroides | -2 | Viola pumila | 1 |
| Jasione montana | 1 | Viola riviniana | 1 |
| Jovibarba hirta | 0 | Viola rupestris | 0 |
| Jovibarba sobolifera | 0 | Viola stagnina | 1 |
| Juglans nigra | -1 | Viola suavis | 1 |
| Juglans regia | -1 | Viola sylvestris | 1 |
| Juncus alpinus | 0 | Viola tricolor | 1 |
| Juncus articulatus | 0 | Viscum album | -2 |
| Juncus atratus | -1 | Vitis rupestris | -1 |
| Juncus bufonius | 0 | Vitis sylvestris | -1 |
| Juncus bulbosus | 0 | Vitis vinifera | -1 |
| Juncus capitatus | 0 | Vitis vulpina | -1 |
| Juncus compressus | 0 | Vulpia bromoides | 1 |
| Juncus conglomeratus | -1 | Vulpia myuros | 1 |
| Juncus effusus | -1 | Waldsteinia geoides | 1 |
| Juncus gerardii | -1 | Xanthium italicum | -3 |
| Juncus inflexus | -1 | Xanthium spinosum | -3 |
| Juncus maritimus | -2 | Xanthium strumarium | -3 |
| Juncus sphaerocarpus | 0 | Xeranthemum annuum | -1 |
| Juncus subnodulosus | -1 | Xeranthemum cylindraceum | -1 |
References
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. Mowing: A Meta-Analysis of Biodiversity Benefits for Grassland Management. Agriculture, Ecosystems & Environment 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Wesche, K.; Ambarlı, D.; Kamp, J.; Török, P.; Treiber, J.; Dengler, J. The Palaearctic Steppe Biome: A New Synthesis. Biodivers Conserv 2016, 25, 2197–2231. [Google Scholar] [CrossRef]
- Mucina, L.; Bültmann, H.; Dierßen, K.; Theurillat, J.-P.; Raus, T.; Čarni, A.; Šumberová, K.; Willner, W.; Dengler, J.; García, R.G.; et al. Vegetation of Europe: Hierarchical Floristic Classification System of Vascular Plant, Bryophyte, Lichen, and Algal Communities. Applied Vegetation Science 2016, 19, 3–264. [Google Scholar] [CrossRef]
- Calvin, K.; Dasgupta, D.; Krinner, G.; Mukherji, A.; Thorne, P.W.; Trisos, C.; Romero, J.; Aldunce, P.; Barrett, K.; Blanco, G.; et al. IPCC, 2023: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change [Core Writing Team, H. Lee and J. Romero (Eds.)]. IPCC, Geneva, Switzerland.; First.; Intergovernmental Panel on Climate Change (IPCC), 2023.
- Bartha, S.; Campetella, G.; Ruprecht, E.; Kun, A.; Házi, J.; Horváth, A.; Virágh, K.; Molnár, Zs. Will Interannual Variability in Sand Grassland Communities Increase with Climate Change? COMMUNITY ECOLOGY 2008, 9, 13–21. [Google Scholar] [CrossRef]
- Bátori Zoltán; Lengyel Attila; Maróti Miklós; Körmöczi László; Tölgyesi Csaba; Bíró András; Tóth Miklós; Kincses Zoltán; Cseh Viktória; Erdős László Microclimate-Vegetation Relationships in Natural Habitat Islands: Species Preservation and Conservation Perspectives. IDŐJÁRÁS / QUARTERLY JOURNAL OF THE HUNGARIAN METEOROLOGICAL SERVICE 2014, 118, 257–281.
- Bartholy, J.; Pongracz, R.; Torma, C.; Pieczka, I.; Kardos, P.; Hunyady, A. Analysis of Regional Climate Change Modelling Experiments for the Carpathian Basin. International Journal of Global Warming 2009, 1, 238–252. [Google Scholar] [CrossRef]
- Bartholy, J.; Pongrácz, R.; Pieczka, I. How the Climate Will Change in This Century? Hungarian Geographical Bulletin 2014, 63, 55–67. [Google Scholar] [CrossRef]
- Tölgyesi, C.; Körmöczi, L. Structural Changes of a Pannonian Grassland Plant Community in Relation to the Decrease of Water Availability. 2012. [CrossRef]
- Fiala, K.; Blanka, V.; Ladányi, Z.; Szilassi, P.; Benyhe, B.; Dolinaj, D.; Pálfai, I. Drought Severity and Its Effect on Agricultural Production in the Hungarian-Serbian Cross-Border Area. Journal of Environmental Geography 2014, 7, 43–51. [Google Scholar] [CrossRef]
- Mezősi, G.; Bata, T.; Meyer, B.C.; Blanka, V.; Ladányi, Z. Climate Change Impacts on Environmental Hazards on the Great Hungarian Plain, Carpathian Basin. Int J Disaster Risk Sci 2014, 5, 136–146. [Google Scholar] [CrossRef]
- Mezősi Gábor; Blanka Viktória; Zsuzsanna, L.; Bata Teodóra; Urdea Petru; Anna, F.; Meyer Burghard C Expected Mid- and Long-Term Changes in Drought Hazard for the South-Eastern Carpathian Basin. Carpathian Journal of Earth and Environmental Sciences 2016, 11, 355–366.
- Ábrahám, L.; Kun, A.; Tóth, L.; Vörös, J.; Varga, A. Natura 2000 Species and Habitats in Hungary. [Natura 2000 Fajok És Élőhelyek Magyarországon.]; Haraszthy, L., Ed.; Pro Vértes Közalapítvány: Csákvár, 2014; ISBN 978-963-08-8853-0. [Google Scholar]
- Vadász, Cs.; Máté, A.; Kun, R.; Vadász-Besnyői, V. Quantifying the Diversifying Potential of Conservation Management Systems: An Evidence-Based Conceptual Model for Managing Species-Rich Grasslands. Agriculture, Ecosystems & Environment 2016, 234, 134–141. [Google Scholar] [CrossRef]
- Zlinszky, A.; Deák, B.; Kania, A.; Schroiff, A.; Pfeifer, N. Mapping Natura 2000 Habitat Conservation Status in a Pannonic Salt Steppe with Airborne Laser Scanning. Remote Sensing 2015, 7, 2991–3019. [Google Scholar] [CrossRef]
- Huber, R.; Le’Clec’h, S.; Buchmann, N.; Finger, R. Economic Value of Three Grassland Ecosystem Services When Managed at the Regional and Farm Scale. Sci Rep 2022, 12, 4194. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Z.; Wu, J. Grassland Ecosystem Services: A Systematic Review of Research Advances and Future Directions. Landscape Ecol 2020, 35, 793–814. [Google Scholar] [CrossRef]
- Bengtsson, J.; Bullock, J.M.; Egoh, B.; Everson, C.; Everson, T.; O’Connor, T.; O’Farrell, P.J.; Smith, H.G.; Lindborg, R. Grasslands—More Important for Ecosystem Services than You Might Think. Ecosphere 2019, 10, e02582. [Google Scholar] [CrossRef]
- Boval, M.; Dixon, R.M. The Importance of Grasslands for Animal Production and Other Functions: A Review on Management and Methodological Progress in the Tropics. Animal 2012, 6, 748–762. [Google Scholar] [CrossRef]
- Moog, D.; Poschlod, P.; Kahmen, S.; Schreiber, K.-F. Comparison of Species Composition between Different Grassland Management Treatments after 25 Years. Applied Vegetation Science 2002, 5, 99–106. [Google Scholar] [CrossRef]
- Hoekstra, N.J.; De Long, J.R.; Jansma, A.P.; Iepema, G.; Manhoudt, A.; van Eekeren, N. Differences in Grassland Sward Biodiversity and Management Regime Lead to Mixed Effects on Ecosystem Services. European Journal of Agronomy 2023, 149, 126886. [Google Scholar] [CrossRef]
- Frank, D.A.; Wallen, R.L.; White, P.J. Ungulate Control of Grassland Production: Grazing Intensity and Ungulate Species Composition in Yellowstone Park. Ecosphere 2016, 7, e01603. [Google Scholar] [CrossRef]
- Sun, J.; Wang, X.; Cheng, G.; Wu, J.; Hong, J.; Niu, S. Effects of Grazing Regimes on Plant Traits and Soil Nutrients in an Alpine Steppe, Northern Tibetan Plateau. PLOS ONE 2014, 9, e108821. [Google Scholar] [CrossRef]
- Alves, F.G.S.; Gomes ,Ellen C.; Faria ,Ana F. G.; Pompeu ,Roberto C. F. F.; Coutinho ,Danielle N.; and Cândido, M.J.D. The Effects of Grazing Frequency and Intensity on Herbage and Structural Characteristics of Irrigated ‘Tifton 85’ Bermudagrass Grazed by Sheep. New Zealand Journal of Agricultural Research 0, 1–12. [CrossRef]
- Blaix, C.; Chabrerie, O.; Alard, D.; Catterou, M.; Diquelou, S.; Dutoit, T.; Lacoux, J.; Loucougaray, G.; Michelot-Antalik, A.; Pacé, M.; et al. Forage Nutritive Value Shows Synergies with Plant Diversity in a Wide Range of Semi-Natural Grassland Habitats. Agriculture, Ecosystems & Environment 2023, 347, 108369. [Google Scholar] [CrossRef]
- Humbert, J.-Y.; Pellet, J.; Buri, P.; Arlettaz, R. Does Delaying the First Mowing Date Benefit Biodiversity in Meadowland? Environmental Evidence 2012, 1, 9. [Google Scholar] [CrossRef]
- Stolz, C.; Deppe, U.; Kämmer, G.; Kuhwald, M.; Nass, D.; Sönnichsen, L. The Development of Nutrient Contents on a New Conservation Area in the Far North of Germany Concerning Different Types of Use. A Proposal for a Sustainable Development in Nature Conservation Practice. Polish Journal of Soil Science 2018, 51, 133. [Google Scholar] [CrossRef]
- Klapp, E.; Boecker, P.; König, F.; Stählin, A. Wertzahlen der Grünlandpflanzen. [Feed and ecological values of grassland species]. Das Grünland 1953, 2, 38–40. [Google Scholar]
- Balázs F. A gyepek botanikai és gazdasági értékelése. [Botanical and economic evaluation of grasslands]. A Keszthelyi Mezőgazdasági Akadémia Kiadványai. 1960, 8., 3–23.
- Balázs, F. A gyepek termésbecslése növényszociológia alapján. [Estimating grass yields based on plant sociology.]. Agrártudomány 1949, 1, 26–35. [Google Scholar]
- Briemle, G.; Nitsche, S.; Nitsche, L. Nutzungswertzahlen Für Gefäßpflanzen. [The Utility Values of Vascular Plants. ]. Schriftenreihe für Vegetationskunde 2002, 38, 203–225. [Google Scholar]
- Briemle, G.; Nietsche, S.; Nitsche, L. Grünlandpflanzen Und Ihre Nutzungswertzahlen. [Grassland Species and Their Utility Values.]. Jahrbuch Naturschutz in Hessen 2003, 8, 81–96. [Google Scholar]
- Sala, O.E.; Austin, A.T. Methods of Estimating Aboveground Net Primary Productivity. In Methods in Ecosystem Science; Sala, O.E., Jackson, R.B., Mooney, H.A., Howarth, R.W., Eds.; Springer: New York, NY, 2000; ISBN 978-1-4612-1224-9. [Google Scholar]
- Nagy, G.; Pető, K. A Lábonálló Gyepek Termésének Mérése. [Measuring the Yield of Standing Grasslands.]. Állattanyésztés é s Takarmányozás 2001, 50, 139–154. [Google Scholar]
- Briemle, G. Möglichkeiten Und Grenzen Fer Anwendbarkeit von Wertzahlen Im Grünland. Das wirtschaftseigene Futter 43, 141–164.
- Nitsche, L. Vegetations-Bestanderfassungen Nach Dem Hessischen Biotoppflegesystem Für Magerrasen. [Vegetation Surveys According to the Hessian Biotope Management System for Nutrient-Poor Grasslands.]. Naturschutz und Landschaftsplanung 1993, 25, 17–23. [Google Scholar]
- Briemle, G. Zur Anwendbarkeit Ökologischer Wertzahlen Im Grünland. [On the Applicability of Ecological Values in Grassland.]. Angewandte Botanik 1997, 71, 219–228. [Google Scholar]
- Briemle, G.; Ellenberg, H. Zur Mahdverträglichkeit von Grünlandpflanzen. Möglichkeiten Der Praktischen Anwendung von Ziegerwerten. [On the Mowing Compatibility of Grassland Plants. Practical Applications of Forage and Ecological Values.]. Natur und Landschaft 69, 139–147.
- Scurlock, J.M.O.; Johnson, K.; Olson, R.J. Estimating Net Primary Productivity from Grassland Biomass Dynamics Measurements. Global Change Biology 2002, 8, 736–753. [Google Scholar] [CrossRef]
- Liu, L.; Guan, J.; Zheng, J.; Wang, Y.; Han, W.; Liu, Y. Cumulative Effects of Drought Have an Impact on Net Primary Productivity Stability in Central Asian Grasslands. Journal of Environmental Management 2023, 344, 118734. [Google Scholar] [CrossRef]
- Zhao, F.; Xu, B.; Yang, X.; Jin, Y.; Li, J.; Xia, L.; Chen, S.; Ma, H. Remote Sensing Estimates of Grassland Aboveground Biomass Based on MODIS Net Primary Productivity (NPP): A Case Study in the Xilingol Grassland of Northern China. Remote Sensing 2014, 6, 5368–5386. [Google Scholar] [CrossRef]
- Potočnik Buhvald, A.; Račič, M.; Immitzer, M.; Oštir, K.; Veljanovski, T. Grassland Use Intensity Classification Using Intra-Annual Sentinel-1 and -2 Time Series and Environmental Variables. Remote Sensing 2022, 14, 3387. [Google Scholar] [CrossRef]
- Mutanga, O.; Skidmore, A.K. Hyperspectral Band Depth Analysis for a Better Estimation of Grass Biomass (Cenchrus Ciliaris) Measured under Controlled Laboratory Conditions. International Journal of Applied Earth Observation and Geoinformation 2004, 5, 87–96. [Google Scholar] [CrossRef]
- Jia, W.; Liu, M.; Yang, Y.; He, H.; Zhu, X.; Yang, F.; Yin, C.; Xiang, W. Estimation and Uncertainty Analyses of Grassland Biomass in Northern China: Comparison of Multiple Remote Sensing Data Sources and Modeling Approaches. Ecological Indicators 2016, 60, 1031–1040. [Google Scholar] [CrossRef]
- Schwieder, M.; Buddeberg, M.; Kowalski, K.; Pfoch, K.; Bartsch, J.; Bach, H.; Pickert, J.; Hostert, P. Estimating Grassland Parameters from Sentinel-2: A Model Comparison Study. PFG 2020, 88, 379–390. [Google Scholar] [CrossRef]
- Li, G.; Wang, J.; Wang, Y.; Wei, H.; Ochir, A.; Davaasuren, D.; Chonokhuu, S.; Nasanbat, E. Spatial and Temporal Variations in Grassland Production from 2006 to 2015 in Mongolia Along the China–Mongolia Railway. Sustainability 2019, 11, 2177. [Google Scholar] [CrossRef]
- Reidel, S.; Widmer, S.; Bringuier, O.; Dengler, J. Cover vs. Biomass Sampling in Grassland Vegetation Plots. Tuxenia 2024, 44, 261–275. [Google Scholar] [CrossRef]
- Liang, X.; Li, P.; Wang, J.; Shun Chan, F.K.; Togtokh, C.; Ochir, A.; Davaasuren, D. Research Progress of Desertification and Its Prevention in Mongolia. Sustainability 2021, 13, 6861. [Google Scholar] [CrossRef]
- Yu, B.; Li, Y. A Hybrid Physics-Data-Driven Optimization Model for Grassland Grazing Management. Applied Mathematics and Nonlinear Sciences 2024, 8, 3215–3228. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Zhang, J.; Li, Z.; Wang, L.; Wang, Z.; Wu, Y.; Wang, Y.; Liang, C. Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China. Sustainability 2020, 12, 7169. [Google Scholar] [CrossRef]
- Tatarko, A.R.; Knops, J.M.H. Nitrogen Addition and Ecosystem Functioning: Both Species Abundances and Traits Alter Community Structure and Function. Ecosphere 2018, 9, e02087. [Google Scholar] [CrossRef]
- Jobbágy, E.G.; Sala, O.E. Controls of Grass and Shrub Aboveground Production in the Patagonian Steppe. Ecological Applications 2000, 10, 541–549. [Google Scholar] [CrossRef]
- Mokany, K.; Raison, R.J.; Prokushkin, A.S. Critical Analysis of Root : Shoot Ratios in Terrestrial Biomes. Global Change Biology 2006, 12, 84–96. [Google Scholar] [CrossRef]
- Rayburn, E.B.; Blaser, R.E.; Fontenot, J.P. In Vivo Quality of Tall Fescue as Influenced by Season, Legumes, Age, and Canopy Strata. Agronomy Journal 1980, 72, 872–876. [Google Scholar] [CrossRef]
- Nosalewicz, A.; Siecińska, J.; Kondracka, K.; Nosalewicz, M. The Functioning of Festuca Arundinacea and Lolium Perenne under Drought Is Improved to a Different Extend by the Previous Exposure to Water Deficit. Environmental and Experimental Botany 2018, 156, 271–278. [Google Scholar] [CrossRef]
- Mastalerczuk, G.; Borawska-Jarmułowicz, B.; Darkalt, A. Changes in the Physiological and Morphometric Characteristics and Biomass Distribution of Forage Grasses Growing under Conditions of Drought and Silicon Application. Plants 2023, 12, 16. [Google Scholar] [CrossRef]
- Van den Bossche, T.; De Boever, J.L.; Haesaert, G.; Cougnon, M.; Debevere, S.; Vandaele, L.; Goossens, K. Management Strategies to Improve the Nitrogen Use Efficiency in Grasslands and the Protein Quality in Grass Silages: Lessons from a Practical Study on Dairy Farms in Flanders. Grassland Research 2024, 3, 18–32. [Google Scholar] [CrossRef]
- Bryan, W.B.; Mata, D.J.; Gekara, O.J. Manure and Management Affect Grassland Production and Soil Quality in Organic Lamb Production. Agrosystems, Geosciences & Environment 2019, 2, 190058. [Google Scholar] [CrossRef]
- Chang, S.; Xie, K.; Du, W.; Jia, Q.; Yan, T.; Yang, H.; Hou, F. Effects of Mowing Times on Nutrient Composition and In Vitro Digestibility of Forage in Three Sown Pastures of China Loess Plateau. Animals (Basel) 2022, 12, 2807. [Google Scholar] [CrossRef]
- Zhao, C.; Li, Q.; Cheng, L.; Zhong, R. Effects of Mowing Regimes on Forage Yield and Crude Protein of Leymus Chinensis (Trin.) Tzvel in Songnen Grassland. Grassland Science 2021, 67, 275–284. [Google Scholar] [CrossRef]
- Andueza, D.; Cruz, P.; Farruggia, A.; Baumont, R.; Picard, F.; Michalet-Doreau, B. Nutritive Value of Two Meadows and Relationships with Some Vegetation Traits. Grass and Forage Science 2010, 65, 325–334. [Google Scholar] [CrossRef]
- Andueza, D.; Rodrigues, A.M.; Picard, F.; Rossignol, N.; Baumont, R.; Cecato, U.; Farruggia, A. Relationships between Botanical Composition, Yield and Forage Quality of Permanent Grasslands over the First Growth Cycle. Grass and Forage Science 2016, 71, 366–378. [Google Scholar] [CrossRef]
- Chen, L.; Auh, C.-K.; Dowling, P.; Bell, J.; Chen, F.; Hopkins, A.; Dixon, R.A.; Wang, Z.-Y. Improved Forage Digestibility of Tall Fescue (Festuca Arundinacea) by Transgenic down-Regulation of Cinnamyl Alcohol Dehydrogenase. Plant Biotechnology Journal 2003, 1, 437–449. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.D.; Rayburn, E.B.; Griggs, T.C. Grassland Ecology and Ecosystem Management for Sustainable Livestock Performance. Agronomy 2023, 13, 1380. [Google Scholar] [CrossRef]
- Yang, Y.; Tilman, D.; Furey, G.; Lehman, C. Soil Carbon Sequestration Accelerated by Restoration of Grassland Biodiversity. Nat Commun 2019, 10, 718. [Google Scholar] [CrossRef]
- Liu, D.; Semenchuk, P.; Essl, F.; Lenzner, B.; Moser, D.; Blackburn, T.M.; Cassey, P.; Biancolini, D.; Capinha, C.; Dawson, W.; et al. The Impact of Land Use on Non-Native Species Incidence and Number in Local Assemblages Worldwide. Nat Commun 2023, 14, 2090. [Google Scholar] [CrossRef]
- Anacker, B.L.; Seastedt, T.R.; Halward, T.M.; Lezberg, A.L. Soil Carbon and Plant Richness Relationships Differ among Grassland Types, Disturbance History and Plant Functional Groups. Oecologia 2021, 196, 1153–1166. [Google Scholar] [CrossRef]
- Hayes, F.; Mills, G.; Jones, L.; Abbott, J.; Ashmore, M.; Barnes, J.; Neil Cape, J.; Coyle, M.; Peacock, S.; Rintoul, N.; et al. Consistent Ozone-Induced Decreases in Pasture Forage Quality across Several Grassland Types and Consequences for UK Lamb Production. Science of The Total Environment 2016, 543, 336–346. [Google Scholar] [CrossRef]
- Lumivero XLSTAT Statistical and Data Analysis Solution. Available online: https://www.xlstat.com/ (accessed on 14 January 2025).
- French, K.E. Species Composition Determines Forage Quality and Medicinal Value of High Diversity Grasslands in Lowland England. Agriculture, Ecosystems & Environment 2017, 241, 193–204. [Google Scholar] [CrossRef]
- Bettin, K.; Komainda, M.; Tonn, B.; Isselstein, J. Relationship between Concentrate Feeding Strategy and Grassland Phytodiversity on Dairy Farms. Agriculture, Ecosystems & Environment 2023, 344, 108293. [Google Scholar] [CrossRef]
- Gilhaus, K.; Hölzel, N. Seasonal Variations of Fodder Quality and Availability as Constraints for Stocking Rates in Year-Round Grazing Schemes. Agriculture, Ecosystems & Environment 2016, 234, 5–15. [Google Scholar] [CrossRef]
- Sölter, U.; Hopkins, A.; Sitzia, M.; Goby, J.P.; Greef, J.M. Seasonal Changes in Herbage Mass and Nutritive Value of a Range of Grazed Legume Swards under Mediterranean and Cool Temperate Conditions. Grass and Forage Science 2007, 62, 372–388. [Google Scholar] [CrossRef]
- King, C.; McEniry ,J.; Richardson ,M.; and O’Kiely, P. Yield and Chemical Composition of Five Common Grassland Species in Response to Nitrogen Fertiliser Application and Phenological Growth Stage. Acta Agriculturae Scandinavica, Section B — Soil & Plant Science 2012, 62, 644–658. [CrossRef]
- Chirilă, S.D.; Răileanu, Ștefan; David, L.O.; Covaliov, S.; Doroftei, M. An Analysis of Plant Palatability on Pastures of the Delta: Case Study, Danube Delta Area, Romania. Notulae Botanicae Horti Agrobotanici Cluj-Napoca 2024, 52, 13568–13568. [CrossRef]
- Nakano, T.; Bat-Oyun, T.; Shinoda, M. Responses of Palatable Plants to Climate and Grazing in Semi-Arid Grasslands of Mongolia. Global Ecology and Conservation 2020, 24, e01231. [Google Scholar] [CrossRef]
- Scharenberg, A.; Arrigo, Y.; Gutzwiller, A.; Soliva, C.R.; Wyss, U.; Kreuzer, M.; Dohme, F. Palatability in Sheep and in Vitro Nutritional Value of Dried and Ensiled Sainfoin (Onobrychis Viciifolia) Birdsfoot Trefoil (Lotus Corniculatus), and Chicory (Cichorium Intybus). Arch Anim Nutr 2007, 61, 481–496. [Google Scholar] [CrossRef]
- Sheaffer, C.C.; Wyse, D.L.; Ehlke, N.J. Palatability and Nutritive Value of Native Legumes. Native Plants Journal 2009, 10, 224–231. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for the in Vitro Digestion of Forage Crops. Grass and Forage Science 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Pereña-Ortiz, J.F.; Salvo-Tierra, Á.E.; Sánchez-Mata, D. Application of Phytosociological Information in the Evaluation of the Management of Protected Areas. Plants 2023, 12, 406. [Google Scholar] [CrossRef]
- Flombaum, P.; Sala, O.E. A Non-Destructive and Rapid Method to Estimate Biomass and Aboveground Net Primary Production in Arid Environments. Journal of Arid Environments 2007, 69, 352–358. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, X.; Jiang, M.; Tong, S.; Lu, X. Spatiotemporal Change of Aboveground Biomass and Its Response to Climate Change in Marshes of the Tibetan Plateau. International Journal of Applied Earth Observation and Geoinformation 2021, 102, 102385. [Google Scholar] [CrossRef]
- Wang, W.; Liu, H.; Zhang, J.; Li, Z.; Wang, L.; Wang, Z.; Wu, Y.; Wang, Y.; Liang, C. Effect of Grazing Types on Community-Weighted Mean Functional Traits and Ecosystem Functions on Inner Mongolian Steppe, China. Sustainability 2020, 12, 7169. [Google Scholar] [CrossRef]
- Serba, D.D.; Hejl, R.W.; Burayu, W.; Umeda, K.; Bushman, B.S.; Williams, C.F. Pertinent Water-Saving Management Strategies for Sustainable Turfgrass in the Desert U.S. Southwest. Sustainability 2022, 14, 12722. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, J.; Gu, R.; Liang, Y.; Yan, Y.; Gao, Q.; Yang, J. Influence of Different Mowing Systems on Community Characteristics and the Compensatory Growth of Important Species of the Stipa Grandis Steppe in Inner Mongolia. Sustainability 2016, 8, 1121. [Google Scholar] [CrossRef]
- Koyama, A.; Kubo, D.; Yoshihara, Y.; Jamsran, U.; Okuro, T. Response of Degraded Vegetation to Introduction of Prescribed Burning or Mowing Management in a Mongolian Steppe. Grassland Science 2016, 62, 37–44. [Google Scholar] [CrossRef]
- Socher, S.A.; Prati, D.; Boch, S.; Müller, J.; Klaus, V.H.; Hölzel, N.; Fischer, M. Direct and Productivity-Mediated Indirect Effects of Fertilization, Mowing and Grazing on Grassland Species Richness. Journal of Ecology 2012, 100, 1391–1399. [Google Scholar] [CrossRef]
- Winkler, J.; Malovcová, M.; Adamcová, D.; Ogrodnik, P.; Pasternak, G.; Zumr, D.; Kosmala, M.; Koda, E.; Vaverková, M.D. Significance of Urban Vegetation on Lawns Regarding the Risk of Fire. Sustainability 2021, 13, 11027. [Google Scholar] [CrossRef]
- Binder, S.; Isbell, F.; Polasky, S.; Catford, J.A.; Tilman, D. Grassland Biodiversity Can Pay. Proceedings of the National Academy of Sciences 2018, 115, 3876–3881. [Google Scholar] [CrossRef]
- Uchida, K.; Ushimaru, A. Biodiversity Declines Due to Abandonment and Intensification of Agricultural Lands: Patterns and Mechanisms. Ecological Monographs 2014, 84, 637–658. [Google Scholar] [CrossRef]
- Ran, L.; Wang, X.; Li, S.; Zhou, Y.; Xu, Y.-J.; Chan, C.N.; Fang, N.; Xin, Z.; Shen, H. Integrating Aquatic and Terrestrial Carbon Fluxes to Assess the Net Landscape Carbon Balance of a Highly Erodible Semiarid Catchment. Journal of Geophysical Research: Biogeosciences 2022, 127, e2021JG006765. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, M.; Sun, J.; Qiu, J.; Pei, W.; Zhang, K.; Xu, X.; Liu, D. Dynamic of Grassland Degradation and Its Driving Forces from Climate Variation and Human Activities in Central Asia. Agronomy 2023, 13, 2763. [Google Scholar] [CrossRef]
- Wang, X.; Chen, X.; Xu, J.; Ji, Y.; Du, X.; Gao, J. Precipitation Dominates the Allocation Strategy of Above- and Belowground Biomass in Plants on Macro Scales. Plants 2023, 12, 2843. [Google Scholar] [CrossRef]
- Yang, Y.; Fang, J.; Ma, W.; Wang, W. Relationship between Variability in Aboveground Net Primary Production and Precipitation in Global Grasslands. Geophysical Research Letters 2008, 35. [Google Scholar] [CrossRef]

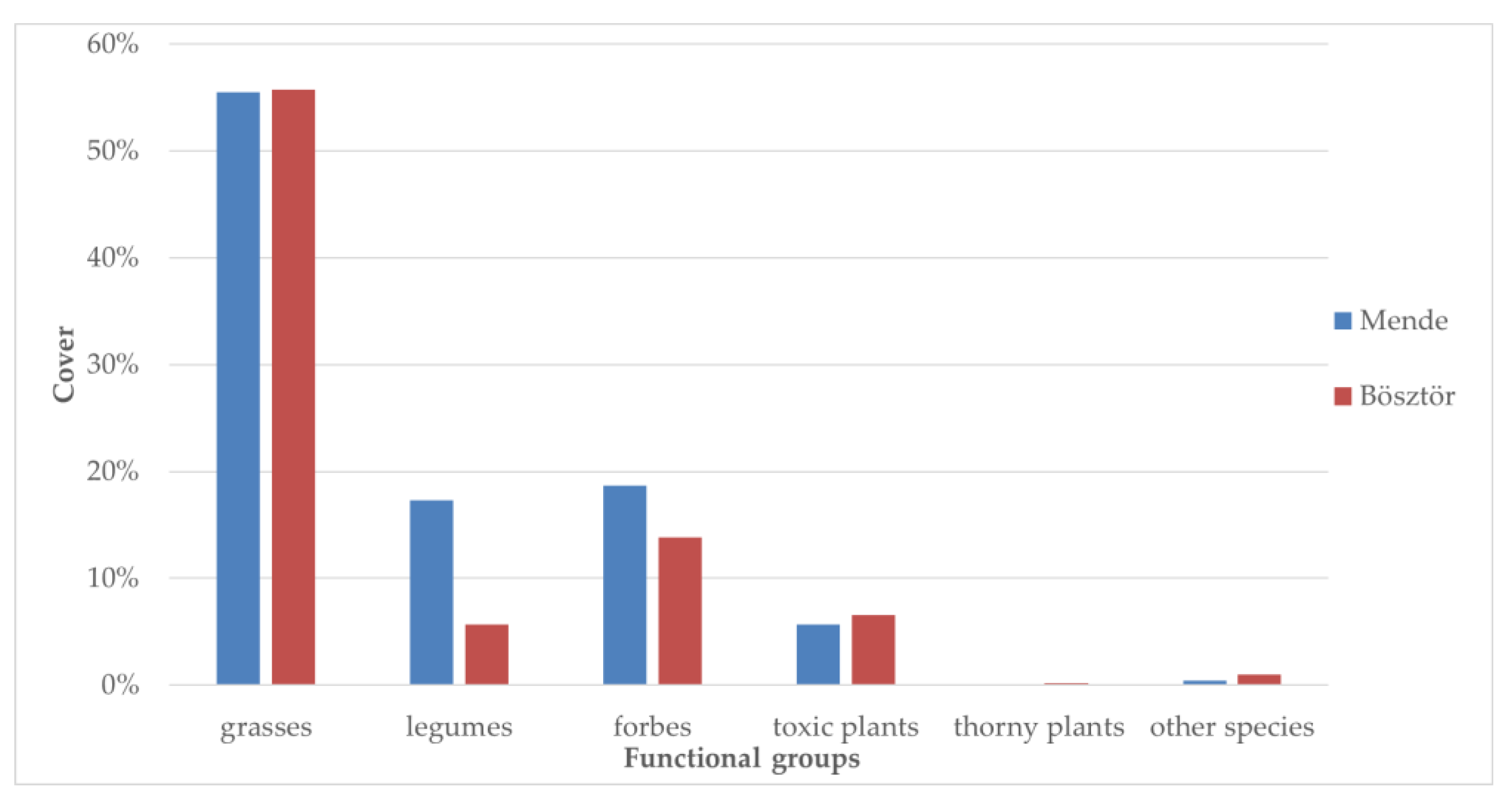
| Species | DB | m(cm) | t | k | kt |
| Agrostis stolonifera | 1.6 | 33 | 52.8 | 5 | 264 |
| Festuca arundinacea | 19 | 48 | 921.6 | 4 | 3686.4 |
| Poa pratensis | 2.9 | 32 | 92.2 | 6 | 553 |
| Trifolium hybridum | 0.3 | 37 | 11.8 | 7 | 82.9 |
| Trifolium pratense | 3.2 | 40 | 128 | 7 | 896 |
| Achillea collina | 1 | 46 | 44.2 | 2 | 88.3 |
| Cichorium intybus | 0.6 | 44 | 28.2 | 3 | 84.5 |
| Daucus carota | 0 | 44 | 1.4 | 1 | 1.4 |
| Pastinaca sativa | 0 | 40 | 1.3 | -1 | -1.3 |
| Plantago lanceolata | 0.1 | 23 | 1.2 | 3 | 3.7 |
| Plantago media | 0.6 | 15 | 9.6 | 2 | 19.2 |
| Ranunculus acris | 0 | 35 | 1.1 | -2 | -2.2 |
| Ranunculus repens | 0.6 | 22 | 14.1 | -2 | -28.2 |
| Rumex acetosa | 0.1 | 50 | 5.3 | -1 | -5.3 |
| Taraxacum officinale | 1.6 | 23 | 36.8 | 3 | 110.4 |
| Σ | 32 | 1349.6 | 5752.7 | ||
| M | 42.3 | ||||
| K | 4.3 | ||||
| P | 57.5 |
| Year | Number of moving | Number of growth | ∑DB1 | T2 | Σkt3 | K4 | P5 | Sz6 | DOM7 | Sz × DOM |
| (t/ha) | (%) | (t/ha) | ||||||||
| 2016 | 2 | 1 | 31.7 | 4655.8 | 19395.2 | 4.2 | 194 | 17.04 | 58.2 | 9.92 |
| 2 | 31.4 | 3641.1 | 13842.1 | 3.8 | 138.4 | 13 | 59.76 | 7.77 | ||
| 3 | 1 | 31.9 | 4217.4 | 17977.2 | 4.3 | 179.8 | 15.27 | 69.49 | 10.61 | |
| 2 | 31.2 | 2869.4 | 11333.5 | 3.9 | 113.3 | 9.92 | 61.19 | 6.07 | ||
| 3 | 31.7 | 3337.5 | 13439.1 | 4 | 134.4 | 11.76 | 61.37 | 7.22 | ||
| 4 | 1 | 31.9 | 2740.3 | 11897.8 | 4.3 | 119 | 9.37 | 72.72 | 6.81 | |
| 2 | 31.8 | 2522.1 | 10534.8 | 4.2 | 105.3 | 8.5 | 69.16 | 5.88 | ||
| 3 | 31.3 | 2628.5 | 9379.1 | 3.6 | 93.8 | 8.95 | 62.8 | 5.62 | ||
| 4 | 31.9 | 3732.1 | 15218 | 4.1 | 152.2 | 13.33 | 65.66 | 8.75 | ||
| 2017 | 2 | 1 | 31.9 | 4883.4 | 21015.1 | 4.3 | 210.2 | 17.94 | 62.79 | 11.26 |
| 2 | 31.9 | 3625.1 | 13990.7 | 3.9 | 139.9 | 12.91 | 64.89 | 8.37 | ||
| 3 | 1 | 31.9 | 3357.4 | 14903.2 | 4.4 | 149 | 11.83 | 75.4 | 8.92 | |
| 2 | 31 | 3874.7 | 15398.2 | 4 | 154 | 13.95 | 63.68 | 8.88 | ||
| 3 | 31.8 | 3338.8 | 13395.8 | 4 | 134 | 11.77 | 66.72 | 7.85 | ||
| 4 | 1 | 32 | 2828.4 | 11676.8 | 4.1 | 116.8 | 9.72 | 77.78 | 7.56 | |
| 2 | 31.7 | 2772.6 | 11722.1 | 4.2 | 117.2 | 9.5 | 67.23 | 6.39 | ||
| 3 | 31.3 | 2080 | 7060.7 | 3.4 | 70.6 | 6.76 | 70.91 | 4.79 | ||
| 4 | 31.8 | 3210.3 | 13085.9 | 4.1 | 130.9 | 11.25 | 68.58 | 7.72 | ||
| 2018 | 2 | 1 | 31.3 | 4121.5 | 18399.7 | 4.5 | 184 | 14.92 | 70.16 | 10.47 |
| 2 | 29.7 | 3349.4 | 13384.6 | 4 | 133.8 | 11.91 | 67.11 | 7.99 | ||
| 3 | 1 | 29 | 2168.2 | 8886.2 | 4.1 | 88.9 | 7.22 | 75.72 | 5.47 | |
| 2 | 31.6 | 3101.3 | 12528 | 4 | 125.3 | 10.83 | 62.2 | 6.73 | ||
| 3 | 32 | 3487 | 13062.8 | 3.7 | 130.6 | 12.35 | 64.65 | 7.98 | ||
| 4 | 1 | 28.1 | 2013.7 | 8678 | 4.3 | 86.8 | 6.65 | 75.33 | 5.01 | |
| 2 | 30.7 | 3754.6 | 15741 | 4.2 | 157.4 | 13.48 | 71.16 | 9.59 | ||
| 3 | 27.3 | 2275.7 | 8867.7 | 3.9 | 88.7 | 7.74 | 70.8 | 5.48 | ||
| 4 | 29.8 | 2903.5 | 11667 | 4 | 116.7 | 10.12 | 70.96 | 7.18 | ||
| 2019 | 2 | 1 | 32 | 5095.4 | 22195.1 | 4.4 | 222 | 18.78 | 55.7 | 10.46 |
| 2 | 31.3 | 4347.2 | 17119.1 | 3.9 | 171.2 | 15.82 | 69.02 | 10.92 | ||
| 3 | 1 | 31.8 | 4151.4 | 16261.4 | 3.9 | 162.6 | 15.02 | 68.38 | 10.27 | |
| 2 | 31.8 | 3372.7 | 12108.5 | 3.6 | 121.1 | 11.9 | 69.73 | 8.3 | ||
| 3 | 31.3 | 4652.2 | 17651.6 | 3.8 | 176.5 | 17.04 | 68.09 | 11.61 | ||
| 4 | 1 | 31 | 3902.3 | 15072.3 | 3.9 | 150.7 | 14.06 | 80.75 | 11.35 | |
| 2 | 30.8 | 2326.7 | 8891.7 | 3.8 | 88.9 | 7.77 | 73.64 | 5.72 | ||
| 3 | 32.1 | 2287.1 | 8372.9 | 3.7 | 83.7 | 7.54 | 74.21 | 5.6 | ||
| 4 | 31.5 | 3734.3 | 14149.3 | 3.8 | 141.5 | 13.36 | 74.03 | 9.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





