1. Introduction
Eosinophils are granulocytic innate immune cells that come from the bone marrow. In healthy individuals, they constitute about 3–5% of all circulating leukocytes [
1]. Eosinophils, classically linked to allergy responses and antiparasitic defense, are now considered multifunctional cells that may play a role in tissue remodeling and anticancer immunity [
2]. From an ultrastructural viewpoint, these innate immune cells display bilobed nuclei with dense peripheral heterochromatin and centrally located euchromatin [
3]. Each eosinophil possesses approximately 200 specific granules [
4] that contain four distinct cationic proteins, such as major basic protein, eosinophil cationic protein, eosinophil peroxidase, and eosinophil-derived neurotoxin, as well as various cytokines, chemokines, and growth factors [
3,
5,
6,
7]. Their effector activities include the production of reactive oxygen species through respiratory burst, the synthesis of lipid mediators, and the regulated release of both pre-formed and newly synthesized mediators through degranulation [
3].
Transmission electron microscopy (TEM) is still an important tool for studying eosinophils, despite its inherent limitation of examining relatively small tissue areas. TEM provides detailed information about important aspects of eosinophil biology, such as the structure of granules, the various mechanisms of degranulation, and intercellular interactions [
3,
8,
9,
10].
Degranulation may proceed through several pathways: classical exocytosis, compound exocytosis, piecemeal degranulation, and cytolytic release of intact granules [
3]. Recent research has identified extracellular trap cell death (ETosis) as an advanced process of eosinophil cytolysis [
11]. More specifically, it is a type of programmed cell death that entails the discharge of cellular contents, such as specific granules and nuclear chromatin, in the form of extracellular traps [
12,
13,
14]. Most of the research on ETosis has been done on eosinophil-related disorders in vitro and in vivo [
15,
16]. However, very little is known about its occurrence and functional importance in cancer.
Lowe et al. [
17] created the term tumor-associated tissue eosinophilia (TATE) to refer to an elevated eosinophil density in the peritumoral and intratumoral inflammatory infiltrate. The role of TATE remains controversial, since it has been characterized as either prognostically favorable or unfavorable [
18,
19,
20]. Furthermore, little is known about the precise processes by which eosinophils inhibit the growth of human tumors and the direct evidence of their anticancer impact. This narrative review synthesizes key studies regarding the prognostic value of TATE, and analyzes the reasons of their controversial significance in the literature. In addition, we conducted a retrospective evaluation of our ultrastructural institutional experience with human gastric cancers characterized by TATE [
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
32]. This review detailed the mutual membrane contact between eosinophils and tumor cells and the alterations that occurred during the interaction. The objective of this study is to clarify the potential antitumoral function of activated eosinophils and to suggest future research directions in the field of oncology.
2. Eosinophils in Tumor Biology: Recruitment, Prognostic Role, and Viral Context
2.1. Tumor-Associated Tissue Eosinophilia (TATE) and Tumor-Associated Blood Eosinophilia in Solid Tumors
The terms "Tumor-associated tissue eosinophilia" (TATE) and "Tumor-associated blood eosinophilia" (TABE) were introduced by Lowe et al. [
17] to draw attention to the potential clinical and biological function of eosinophils in patients with neoplasms. TATE and TABE may manifest concurrently or independently across various tumor histological types and anatomical locations [
17]. TABE is defined by the presence of an increased number of eosinophils in the peripheral blood exceeding the upper limit of normal, 3%–5% of peripheral blood leukocytes, with a corresponding absolute eosinophil count of 0.35–0.5 × 109/L, which varies slightly according to the measuring laboratory [
33].
2.2. Mechanisms of Eosinophil Recruitment in Solid Tumors
Cormier et al. [
34] used a mouse model of melanoma to study the mechanisms that regulate eosinophil infiltration in tumor sites. Histological analysis of these experimental tumors revealed that they had necrotic regions surrounded by living tumor tissue, with fibrous capsules separating the tumor from the surrounding host tissue. Eosinophil infiltration constituted an early event in tumor development, showing a preferential accumulation in both the fibrous capsule and necrotic areas. These findings suggest that zones undergoing necrosis and tissue remodeling primarily attract eosinophils. Immunohistochemical staining for major basic protein demonstrated diffuse extracellular matrix deposition in the necrotic areas, indicating that eosinophil degranulation was localized to these regions and absent from the capsule. Although eosinophil degranulation is primarily observed in necrotic tumor areas, this localization does not necessarily reflect a passive response to tissue damage. Instead, eosinophils might play an active role in harming tumor cells by releasing toxic proteins, which could help continue or increase the tissue damage. Experimental findings of Cormier et al. [
34] served as a foundation for LIAR (Local Immunity and Remodeling) hypothesis, proposed by Lee et al. [
35]. The LIAR hypothesis suggested that cell death, high mitotic activity, and possibly cancer stem cell activity in high-grade solid tumors represent potential foci for eosinophil recruitment. Accordingly, dying tumor cells release damage-associated molecular patterns, such as high-mobility group box 1 and interleukin-33, which promote eosinophilic infiltration. At the same time, proliferating tumor cells secrete factors that enhance eosinophil survival and differentiation, thereby sustaining local eosinophil presence [
35]. Lee et al. [
35] noted that TATE demonstrates significant variability, both between different types of tumors and within the same tumor throughout time. Their observations suggest that necrosis and proliferation, although potentially significant, are insufficient alone to account for eosinophil recruitment [
19]. Additional factors, particularly tumor-derived eosinophil-specific chemotactic signals, must be considered. Among these, eotaxins, especially CCL11, play a crucial role in selectively attracting eosinophils to inflammatory and neoplastic sites by binding to CCR3 on eosinophils [
36,
37,
38]. In summary, the mechanism that regulate TATE are still not fully comprehended; the process is multifaceted and reliant on a variable combination of tumor hypoxia, cytokines, adhesion molecules, and chemokines in the tumor microenvironment.
2.3. Prognostic Significance of TATE in Human Malignancies
A growing body of evidence suggests that TATE is a favorable prognostic marker in several solid malignancies including gastric [
39,
40], colorectal [
41,
42,
43,
44], nasopharyngeal [
45], oral cavity and lip [
46], oral tongue [
47], esophageal [
48], and laryngeal carcinomas [
49], as well as in melanoma [
50,
51], small cell esophageal carcinoma [
52], and breast cancer [
53]. A meta-analysis by Hu et al. [
54] confirmed that TATE is a favorable prognostic marker in solid tumors. The study also demonstrated an intriguing negative correlation between TATE and critical indicators of tumor aggressiveness, including lymph node metastasis, advanced staging, and lymphovascular invasion [
54].
On the other hand, several authors showed that eosinophils may exert pro-tumorigenic effects in specific settings. In cervical squamous cell carcinoma, TATE has been associated with poor prognosis [
55,
56]. In a recent study on HPV-positive anal squamous cell carcinoma, elevated TABE levels were associated with reduced disease-free survival [
57]. Similarly, in HPV-positive oropharyngeal carcinoma, higher TABE levels correlated with poorer outcomes [
57]. Interestingly, in HPV-negative oropharyngeal tumors, increased eosinophil counts were instead linked to improved prognosis [
57]. It is noteworthy that these studies evaluated TABE rather than histologically assessed TATE, thereby limiting direct comparisons. In hematologic malignancies, TATE and/or TABE are frequently observed, particularly in Hodgkin lymphoma [
58,
59,
60] and adult T-cell leukemia/lymphoma (ATLL) [
61].
It is important to recognize that all these neoplasms share a viral genesis. HPV is implicated in the vast majority of anal (88%) [
62], cervical (over 95%, [
63], vaginal (60%) [
64]), vulvar (approximately 70% [
65], penile (over 50%) [
66] squamous cell carcinomas. In HPV-associated tumors, viral oncoproteins skew the immune response toward a Th2 phenotype and stimulate the production of immunosuppressive and pro-angiogenic factors such as TGF-β, MMPs, and VEGF. Eosinophils, in this context, are recruited into the tumor stroma and may amplify these signals, thereby supporting tumor growth and neovascularization [
67]. Approximately 40% of Hodgkin’s lymphoma cases are associated with Epstein–Barr virus (EBV) [
68]. In EBV-positive Hodgkin lymphoma, latent viral proteins such as LMP1 activate signaling cascades including NF-κB and STAT3, leading to the release of eosinophil-attracting chemokines (e.g., CCL5, CCL28) and cytokines (e.g., IL-5, TGF-β) [
69,
70]. Eotaxin has been found to be expressed at a greater rate in Hodgkin lymphoma, and the amount of eotaxin in tissue has been shown to correlate with the extent of tissue eosinophilia. The result is a Th2-dominant microenvironment marked by immunosuppression, fibrosis, and inflammation that supports tumor persistence and expansion. [
69,
70]. Similar mechanisms have been described in ATLL, a mature T cell neoplasm causally associated with human T-cell lymphotrophic virus type 1 (HTLV1), where TABE is an independent unfavorable prognostic marker [
61].
The apparent contradiction in prognostic role of TATE and/or TABE likely reflects differences in the mechanisms of eosinophil recruitment within the tumor microenvironment. In virus-associated cancers, such as HPV-related carcinomas (cervical, anal and oropharyngeal squamous cell carcinomas), EBV-positive Hodgkin lymphoma, and HTLV-1 linked ATLL, eosinophilic infiltration is co-opted by the tumor to promote immune evasion, angiogenesis, and fibrosis, ultimately correlating with a poorer prognosis. Conversely, in tumors of non-viral etiology, eosinophil recruitment may be evoked by ischemic tumor injury, cell proliferation as well as by chemokines and cytokines and is associated with direct cytotoxic activity against tumor cells, contributing to a more favorable clinical outcome.
2.4. Dual Role of Eosinophils in Viral and Non-Viral Tumors
The role of eosinophils in virus-associated neoplasms is outlined in
Table 1. Marked eosinophilic infiltration has been associated in some reports with reduced disease-free survival in classical Hodgkin lymphoma [
58,
59]. However, the evaluation of viral status, specifically EBV, was not conducted in these studies. Given these limitations, Hodgkin lymphoma was not included in
Table 1, which focuses on tumors with both confirmed viral etiology and more consistent evidence of an association between eosinophilia and adverse prognosis. Although one study on cervical squamous cell carcinoma did not check HPV status [
55], the elevated prevalence of HPV infection in this tumor type supports the hypothesis of a likely viral etiology in that case as well. Future studies should include histological evaluation of eosinophilic infiltration and standardized viral testing to gain a better understanding of the prognostic impact of TATE in virus-driven neoplasms.
3. ETosis: A Distinct Mechanism of Eosinophil Degranulation
3.1. ETosis: Brief Overview
Eosinophils utilize four main degranulation pathways to release their granule contents: (1) Classical exocytosis, where individual granules fuse directly with the plasma membrane to discharge their mediators; (2) Compound exocytosis, involving prior granule–granule fusion followed by fusion with the plasma membrane to form large release channels; (3) Piecemeal degranulation, a selective and gradual process whereby granule-derived proteins are transported via eosinophil sombrero vesicles to the extracellular space, in the absence of granule fusion. In this process, the membranes of the specific granules remain intact, giving them the appearance of emptied containers; (4) Cytolysis, a lytic form of degranulation culminating in plasma membrane rupture and extracellular deposition of intact free eosinophil granules (FEGs) [
3].
In 2004, Brinkmann et al. [
71] discovered a new type of neutrophil cell death. This type of cell death is marked by the release of extracellular traps composed of decondensed chromatin and proteins from neutrophils, including histones, myeloperoxidase, and elastase [
71]. This distinctive process, known as neutrophil ETosis, is mechanistically and morphologically distinct from apoptosis. Apoptosis is a tightly regulated, non-inflammatory form of programmed cell death marked by cytoplasmic shrinkage and well-defined nuclear changes such as chromatin condensation (pyknosis) and DNA fragmentation, whereas nuclear envelope remains structurally intact, although the nucleolus becomes unrecognizable [
72,
73,
74]. In contrast, neutrophil ETosis involves early disintegration of the nuclear envelope and extensive chromatin decondensation that spills into the cytoplasm [
71]. The plasma membrane then breaks and chromatin nets, full of antimicrobial components, are released in the extracellular space [
71]. Unlike apoptosis, neutrophil ETosis does not cause nuclear fragmentation or the production of apoptotic bodies. Instead, it releases extremely pro-inflammatory DNA–protein complexes into the area outside the cells [
71].
Although ETosis was originally described in neutrophils, subsequent studies have demonstrated that this form of cell death also occurs in other immune cells, including eosinophils, mast cells, plasmacytoid dendritic cells, basophils, monocytes, and macrophages [
75]. In 2008, Yousefi et al. [
76] reported that viable eosinophils can actively expel extracellular DNA traps of mitochondrial origin. In contrast to this report, Ueki et al. [
11] demonstrated the discharge of similar DNA traps from cytolytic eosinophils. Expelled DNA was associated with histones, indicating a nuclear rather than mitochondrial origin [
11]. As human eosinophils contain few mitochondria and mitochondrial DNA lacks histone organization, current evidence suggests that eosinophil ETosis is of nuclear rather than mitochondrial origin [
77]. These findings have stimulated growing interest in the role of eosinophil ETosis in various non-neoplastic eosinophilic disorders [
78].
3.2. ETosis in Non-Neoplastic Eosinophilic Disorders
Neves et al. [
15] used TEM to examine tissue samples from patients with eosinophilic disorders such as eosinophilic chronic rhinosinusitis, ulcerative colitis, and hypereosinophilic syndrome. Their study revealed that eosinophil ETosis occurs at two different stages: an early phase and late phase [
15]. In the initial stages of ETosis, the nuclear lobes undergo chromatin decondensation, which eliminates the distinction between euchromatin and heterochromatin. Delobulation and rounding phenomena occur concurrently. In particular, nuclear lobes coalesce into a single rounded mass. As chromatin condenses and expands, the nuclear envelope progressively disintegrates, permitting its contents to pass into the cytoplasm. Concurrently, some cytoplasmic granules are extruded via a budding mechanism, still partially enveloped by remnants of the plasma membrane. In the advanced phase, rupture of the plasma membrane results in the abrupt release of decondensed chromatin into the extracellular space, where it forms extracellular traps, accompanied by the discharge of morphologically preserved FEGs, Charcot-Leyden crystals, and intact eosinophil sombrero vesicles [
15,
79].
Although eosinophil ETosis shares with neutrophil ETosis its proinflammatory nature and the formation of web-like chromatin structures, it differs markedly in granule dynamics: while neutrophils release enzymes such as elastase and myeloperoxidase due to granule disintegration [
71], eosinophil traps are typically associated with intact granules [
15,
79]. The key morphological and functional differences among apoptosis, neutrophil ETosis, and eosinophil ETosis are summarized in
Table 2.
3.3. Our Ultrastructural Observations of Eosinophil ETosis in Gastric Carcinoma
Neves et al. [
15] showed that in non-neoplastic eosinophil-associated disorders, not all cytolytic eosinophils had characteristics of ETosis, suggesting that this process may be limited to a distinct subset of activated eosinophils. Our ultrastructural investigations in gastric malignancies with TATE [
31] confirmed and extended the processes of eosinophil ETosis described in non-neoplastic eosinophil-associated disorders. In particular, in three cases of gastric cancers we observed cytolytic eosinophils without ETosis. These eosinophils exhibited focal plasma membrane disruption, with release of FEGs, that retain their biphasic structure- dense core and surrounding matrix - and are scattered among collagen fibers near tumor cells. The nucleus maintained its bilobed morphology, with incomplete heterochromatin decondensation, and the nuclear envelope appeared mostly preserved or only mildly dilated (
Figure 1).
Cytoplasmic chromatin diffusion, Charcot-Leyden crystals, and extracellular trap formation were not observed [
31]. In four cases of gastric cancers, eosinophils exhibited ultrastructural features compatible with the distinct phases of ETosis, in line with the established dichotomy of intracellular and extracellular stages. Building on this paradigm, we suggested a more accurate three-stage ultrastructural classification that includes the spectrum of morphological changes that occur during ETosis: early, moderate, and advanced [
31].
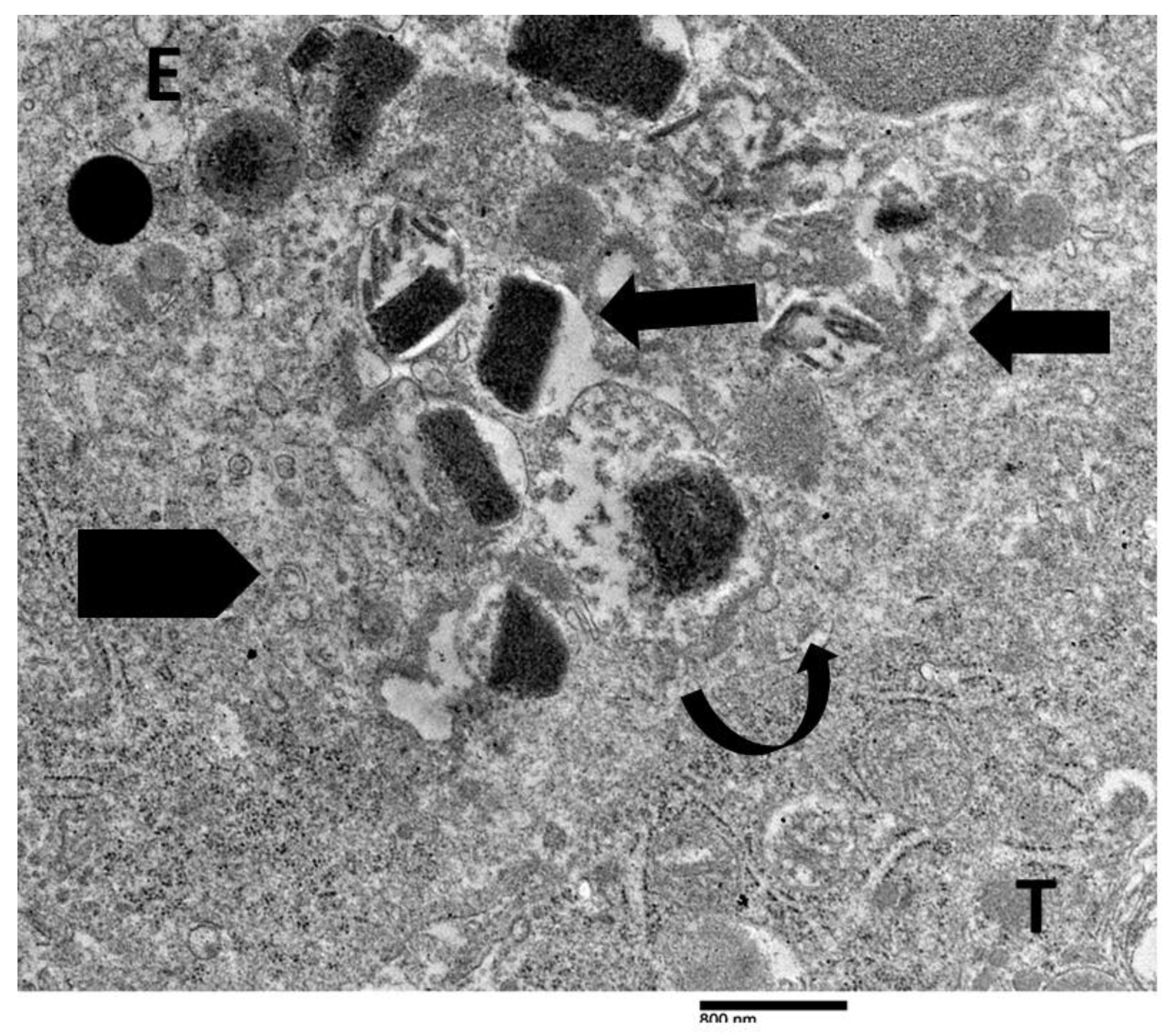
Figure 2 illustrates the early phase of ETosis, corresponding to the initial intracellular stage. The eosinophil displays rounded or oval nuclear lobes with considerable chromatin decondensation during the early stages of ETosis. The nuclear envelope is largely intact, although early signs of perinuclear space expansion are seen. Focal discontinuities in the plasma membrane are observed, together with the release of FEGs.
Figure 3 shows the intermediate stage of ETosis, which is characterized by partial or whole nuclear envelope breakdown. An important ultrastructural characteristic of this stage is the change in nuclear morphology: whereas early-stage ETotic eosinophils retain rounded or oval nuclear lobes, the intermediate stage is characterized by a loss of nuclear symmetry, with lobes acquiring more elongated configurations and irregular, jagged contours, which reflect ongoing nuclear envelope breakdown and chromatin redistribution in the cytoplasm. The cytoplasm is either devoid of specific granules or contained a limited quantity.
Figure 4 illustrates the advanced stage of ETosis, which corresponds to the extracellular stage. This phase is characterized by complete rupture of the plasma membrane and massive release of decondensed chromatin into the extracellular space, forming extracellular traps. These chromatin networks often entangle FEGs and may extend toward or come into direct contact with neighboring tumor cells. Charcot-Leyden crystals may be also identified in proximity of ETotic eosinophils [
31].
It is important to emphasize that eosinophil cytolysis, with or without ETosis, leads to the extracellular release of intact granules [
15,
31]. These granules, capable of ligand-induced secretion, operate as independent secretory organelles and can enhance the effector actions of eosinophils in the tumor microenvironment [
80,
81]. The extracellular deposition of FEGs may be regarded as a "minefield" with potential anticancer activity [
26,
30]. Although TATE is frequently focal and rarely characterized by dense infiltration, the cytotoxic potential of eosinophils may be markedly enhanced through the release of FEGs via cytolytic mechanisms [
26,
30].
4. Eosinophil-Mediated Tumor Cytotoxicity and Immune Synapse Formation
4.1. Eosinophil-Mediated Tumor Cytotoxicity: From In Vitro Mechanisms to In Vivo Evidence of Immune Synapse Formation
A landmark study by Legrand et al. [
82] was the first to demonstrate that human eosinophils can kill colorectal cancer cells through contact-dependent processes and the release of soluble cytotoxic mediators. The authors used the Colo-205 cell line to show that eosinophils may induce rapid tumor cell apoptosis and to a lesser extent necrosis through cell–cell interactions mediated by the integrin complex CD11a/CD18. Eosinophils released a combination of effector molecules when they attached to tumor cells. The substances included eosinophil cationic protein, eosinophil-derived neurotoxic, TNF-α, and, importantly, granzyme A. The identification of granzyme A as part of the eosinophil cytotoxic repertoire is particularly important, as it mediates tumor cell death through a caspase-independent pathway and acts synergistically with eosinophil cationic protein and TNF-α [
82]. Gatault et al. [
83] subsequently revealed that IL-18 was a critical factor in the improvement of eosinophil–tumor cell interactions. IL-18 was produced by eosinophils when they came into contact with tumor cells and was essential for cytotoxic activity. Neutralization of IL-18 markedly reduced eosinophil-mediated Colo-205 apoptosis and inhibited eosinophil-tumor cell adhesion [
83]. Following the in vitro demonstrations of eosinophil-mediated tumor cytotoxicity by Legrand et al. [
82] and Gatault et al. [
83], Andreone et al. [
84] extended this line of research to in vivo models, offering crucial mechanistic insights into eosinophil function within the tumor microenvironment. In a melanoma-bearing mouse model, they showed that interleukin-33, an alarmin released by tumor cells in response to stressors such as hypoxia, promotes eosinophil recruitment, activation, and antitumor activity. IL-33-activated eosinophils established stable immune synapses with tumor cells and underwent polarized degranulation. The authors used TEM and confocal microscopy to demonstrate that granule convergence and focal release of cytotoxic mediator, such as eosinophil cationic protein, eosinophil peroxidase, and granzyme B, occurred at the eosinophil–tumor interface. The results provided direct morphological evidence of contact-dependent eosinophil cytotoxicity in vivo, culminating in tumor cell apoptosis [
84].
4.2. Ultrastructural Evidence of Eosinophil-Mediated Tumor Cell Injury in Gastric Carcinomas
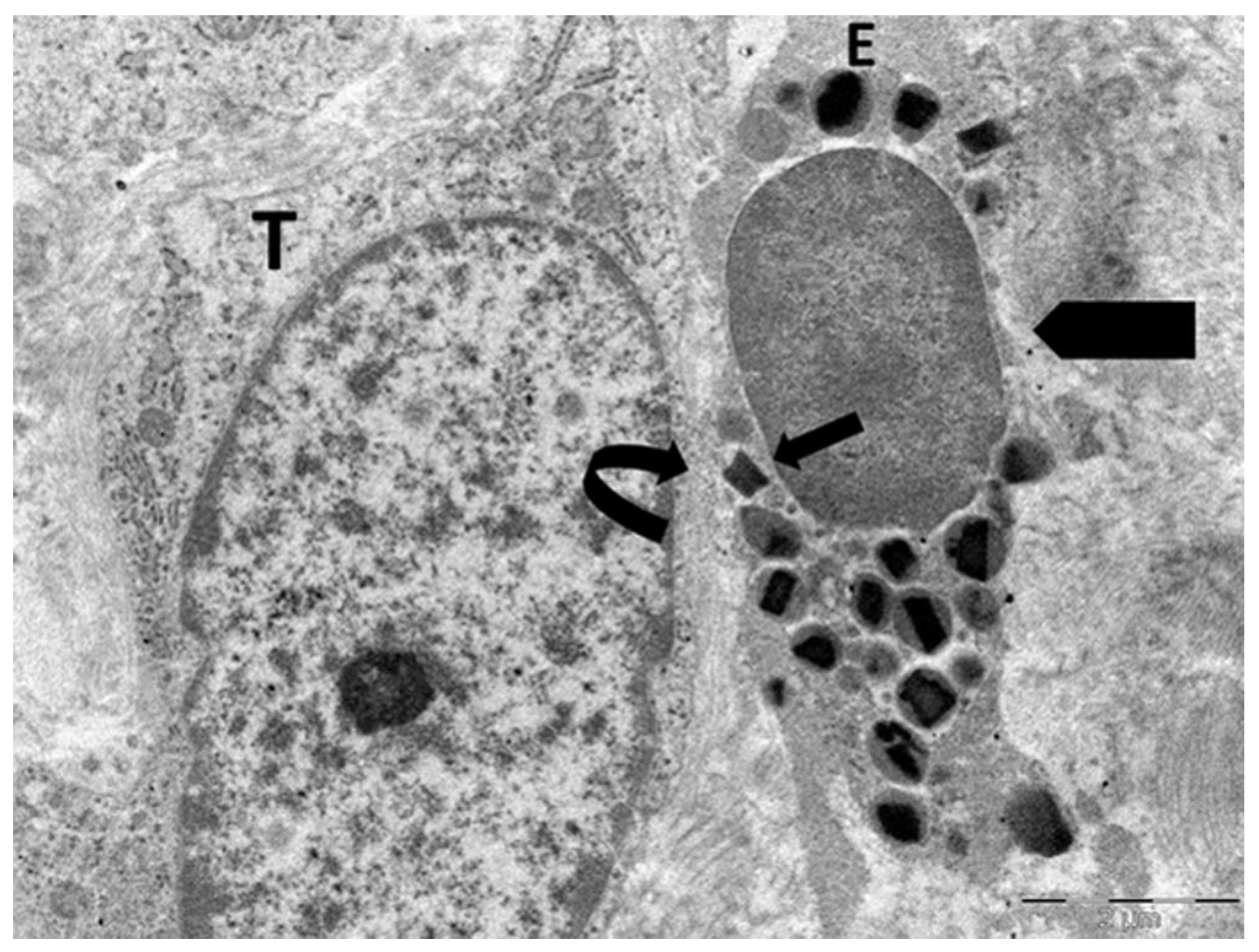
In our previous ultrastructural investigations of gastric cancer specimens with elevated TATE, we focused on the interaction between eosinophils and tumor cells [
29,
30]. Eosinophils in close proximity to tumor cells in a single case of poorly differentiated tubular adenocarcinoma showed compound exocytosis. (
Figure 5) [
29]. TEM revealed that fused specific granules and eosinophil sombrero vesicles were polarized toward the eosinophil–tumor cell interface, indicating a spatially targeted, regulated secretory response (
Figure 5).
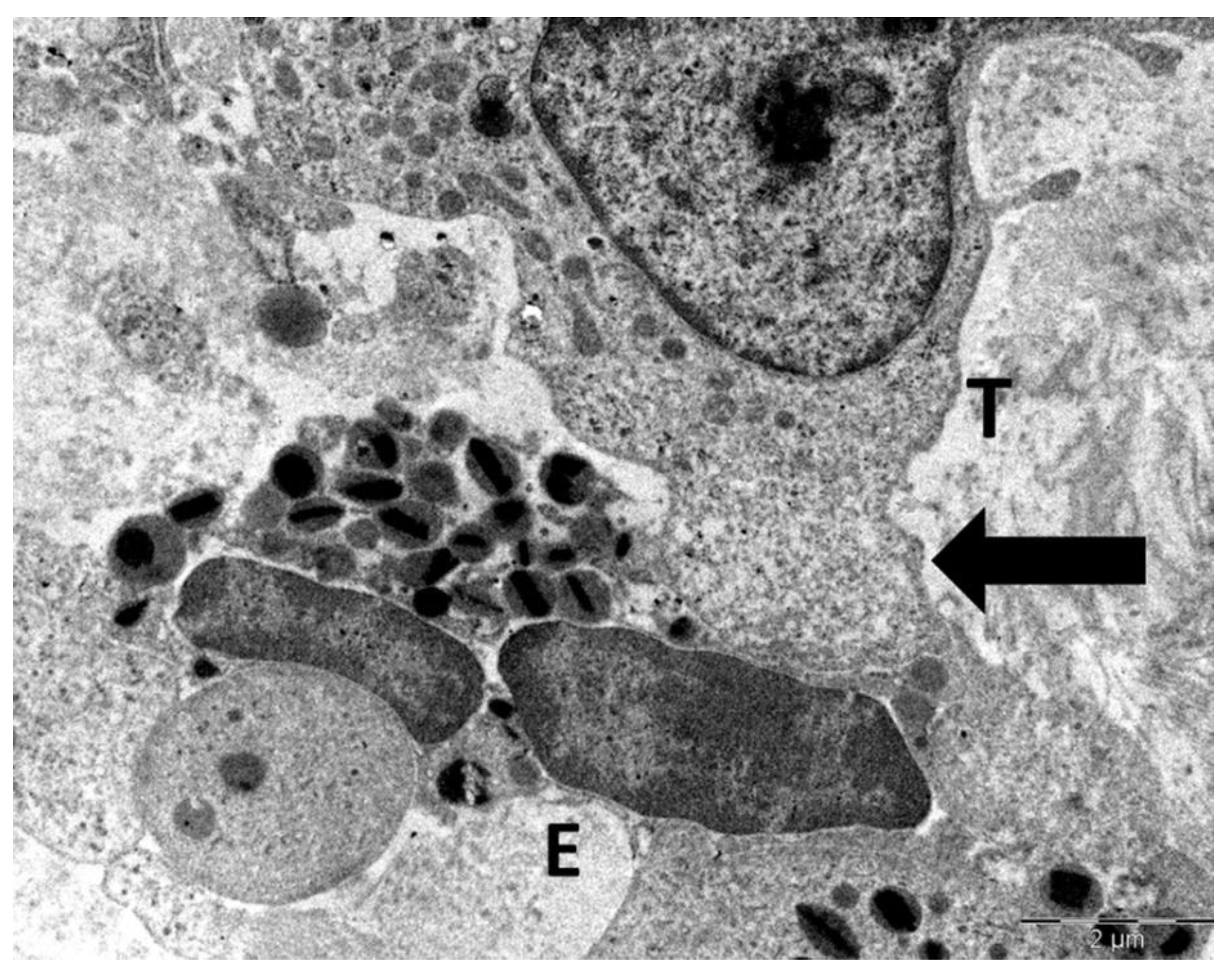
It is important to note that no evidence of eosinophil cytolysis was observed in this case. On the other hand, ETosis seemed to be the main eosinophilic degranulation mechanism in two cases of poorly cohesive gastric carcinoma (not otherwise specified) (
Figure 6,
Figure 7 and
Figure 8) [
30].
These examples collectively illustrate two distinct modalities of eosinophil-mediated anticancer activity: (1) a contact-dependent, polarized exocytotic degranulation and (2) a contact-dependent, ETosis. Even though the patterns of eosinophil degranulation were different, similar ultrastructural features of tumor cell damage were noted in both cases. Importantly, tumor cells exhibited a range of ultrastructural changes that suggested progressive damage [
29,
30]. These changes could be various stages of eosinophil-induced cytotoxicity. Early damage was characterized by localized discontinuities in the tumor cell membrane at the site of contact with the eosinophils (
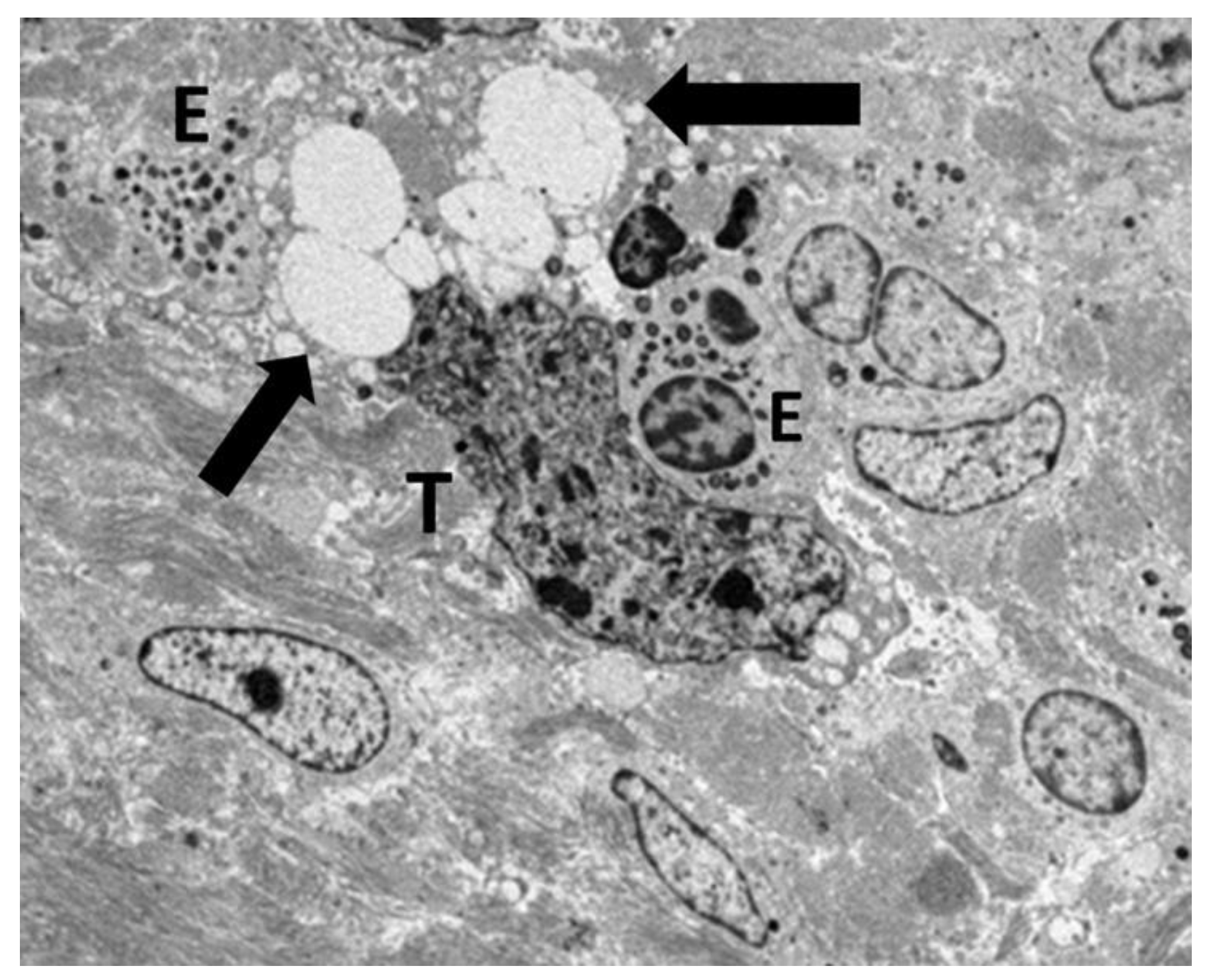
Figure 5 and
Figure 6).
Figure 7 illustrates a scenario where cytoplasmic microvacuoles of the tumor cell occurred close to the eosinophil contact site. The most severe damage, as depicted in
Figure 8, includes mitochondrial swelling, numerous large cytoplasmic vacuoles, and dilation of the nuclear envelope. These ultrastructural results support a model in which both eosinophil ETosis or exocytotic degranulation contribute to kill tumor cell through a non-apoptotic, colloid-osmotic mechanism (
Figure 7 and
Figure 8). The process progresses from eosinophil infiltration and direct cell-cell contact to membrane damage and cytoplasmic vacuolation in tumor cells [
29,
30].
Several important characteristics of the murine melanoma model described by Andreone et al. [
84] are shared by our observations, such as the polarization of eosinophilic granules and the formation of synapse-like junctions between eosinophils and tumor cells. Nevertheless, the nature of tumor cell death is a critical distinction: our results indicate that necrosis is the predominant consequence, in contrast to the apoptosis induced by eosinophils reported by Andreone et al. [
84].
Our ultrastructural findings, which include polarization of specific granules, synapse-like contacts, and localized membrane disruption, collectively provide strong support for a model of contact-dependent eosinophil-mediated tumor cell necrosis in vivo in gastric carcinomas. These ultrastructural data emphasize the potential relevance of eosinophils as effector cells in the tumor microenvironment and necessitate further investigation into their role in antitumor immunity.
5. Limitations
This review was performed as a narrative synthesis of the literature rather than a systematic review with meta-analysis. The quantitative significance of the reported associations between TATE and prognosis should be interpreted with caution, as no pooled statistical estimates were calculated. There is also the possibility of publication bias, as studies that report positive or confirmatory results are more likely to be published than those that present null or unclear data. This may alter the dominant perception of TATE as a generally favorable prognostic marker. However, the integration of original ultrastructural data from gastric carcinoma cases constitutes a distinctive strength of our review. The gold standard to describe eosinophil-specific granules and degranulation patterns is still TEM because it provides a high-resolution view of ETosis and eosinophil degranulation, thereby offering important morphological insights. Future investigations that integrate ultrastructural, immunophenotypic, and functional assessments in larger cohorts are essential for confirming the prognostic role of eosinophils in the tumor microenvironment.
6. Conclusions and Future Directions
Eosinophils have been mainly studied through a dichotomous lens, either as pro-tumorigenic or anti-tumorigenic effectors. This has led to in the prevailing view that their role in cancer is inherently contradictory. However, based on our recent findings, we propose an alternative interpretive paradigm in which the functional orientation of eosinophils in tumor immunology may be strongly influenced by the presence or absence of oncogenic viral infections, particularly HPV. TATE and/or TABE has been associated with poorer clinical outcomes in HPV-related malignancies, including cervical, anal, and oropharyngeal squamous cell carcinomas. HPV can lead eosinophils to develop a phenotype which promotes tumor growth, which may contribute to the progression of the disease. The predictive significance of TATE in Hodgkin lymphomas is still debated. However, further studies are needed to elucidate the potential effects of EBV infection on prognostic significance of TATE. Our review indicates that TATE is consistently associated to favorable clinical outcomes in non–virus-related cancers. The results underscore the necessity of considering viral context in future research aimed at elucidating the immunological functions of eosinophils in the tumor microenvironment.
Our ultrastructural analysis revealed close contact between eosinophils and tumor cells in gastric cancers exhibiting TATE. These contacts were associated with focal disruption of the tumor cell plasma membrane, followed by a series of cytopathic changes, including mitochondrial swelling, cytoplasmic vacuolization, and nuclear envelope dilation, indicative of a non-apoptotic colloid-osmotic tumor cell death. The interaction between eosinophils and tumor cells is analogous to synapse-polarized eosinophil degranulation as described by Andreone et al. in their experimental studies [
84]. Nevertheless, this interpretation is exclusively based on morphological evidence. Additional research employing immunoelectron microscopy or molecular markers of synaptic architecture is required to ascertain if the interactions between eosinophils and tumor cells represent true functional immune synapses.
Eosinophil ETosis has been identified as a mechanism that contributes to tumor control in gastric carcinomas through the release of DNA traps and cytotoxic granule contents. The observation of eosinophil ETosis in tumor tissue provides important morphological information about the immune microenvironment of the tumor, highlighting the need for more research into its potential role in interaction between tumors and immune system.
Author Contributions
R.C.: conceptualization, investigation, writing—original draft, writing—review and editing, supervision. V.C. and L.R.: investigation, visualization, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This review was solely based on ultrastructural examinations of tissue samples of stomach cancers that were collected for routine diagnostic procedures between 1998 and 2005. All investigations were performed on anonymized, pre-existing material, with no impact on patient care. According to Italian law, ethics committee approval is required only for interventional clinical trials involving medicinal products for human use. As specified in Articles 6 and 9 of Legislative Decree No. 211/2003, this entirely non-interventional study, which did not involve any medicinal products, was therefore exempt from ethical review.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| TATE |
tumor-associated tissue eosinophilia |
| HPV |
Human papillomavirus |
| HTLV-1 |
Human T-lymphotropic virus type 1 |
| ETosis |
Extracellular trap cell death |
| LIAR |
Local Immunity and Remodeling |
| TEM |
Transmission electron microscopy |
| TABE |
Tumor associated blood eosinophilia |
| EBV |
Epstein–Barr virus |
References
- Blanchard, C.; Rothenberg, M.E. Biology of the eosinophil. Adv. Immunol. 2009, 101, 81–121. [Google Scholar] [CrossRef] [PubMed]
- Long, H.; Liao, W.; Wang, L.; Lu, Q. A Player and Coordinator: The Versatile Roles of Eosinophils in the Immune System. Transfus. Med. Hemother. 2016, 43, 96–108. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Chapter 3 - Eosinophils as secretory cells. In: Melo, R.C.N., Dvorak, A.M., Weller P.F., editors. In Eosinophil Ultrastructure; Academic Press, 2022; pp. 61–105. [Google Scholar]
- Ueki, S.; Ohta, N.; Takeda, M.; Konno, Y.; Hirokawa, M. Eosinophilic Otitis Media: The Aftermath of Eosinophil Extracellular Trap Cell Death. Curr. Allergy Asthma Rep. 2017, 17, 33. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Liu, L.; Xenakis, J.J.; Spencer, L.A. Eosinophil-derived cytokines in health and disease: unraveling novel mechanisms of selective secretion. Allergy. 2013, 68, 274–284. [Google Scholar] [CrossRef]
- Spencer, L.A.; Bonjour, K.; Melo, R.C.N.; Weller, P.F. Eosinophil secretion of granule-derived cytokines. Front. Immunol. 2014, 5, 496. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Weller, P.F. Contemporary understanding of the secretory granules in human eosinophils. J. Leukoc. Biol. 2018, 104, 85–93. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Dvorak, A.M.; Weller, P.F. Contributions of electron microscopy to understand secretion of immune mediators by human eosinophils. Microsc. Microanal. 2010, 16, 653–660. [Google Scholar] [CrossRef]
- Melo, R.C.N.; Silva, T.P. Eosinophil activation during immune responses: an ultrastructural view with an emphasis on viral diseases. J. Leukoc. Biol. 2024, 116, 321–334. [Google Scholar] [CrossRef] [PubMed]
- Melo, R.C.N.; Rothenberg, M.E. Imaging eosinophil secretory granules: From storage containers to active, immune responder organelles. J. Allergy Clin. Immunol. 2025, 155, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Ueki, S.; Melo, R.C.N.; Ghiran, I.; Spencer, L.A.; Dvorak, A.M.; Weller, P.F. Eosinophil extracellular DNA trap cell death mediates lytic release of free secretion-competent eosinophil granules in humans. Blood. 2013, 121, 2074–2083. [Google Scholar] [CrossRef]
- Ueki, S.; Konno, Y.; Takeda, M.; Moritoki, Y.; Hirokawa, M.; Matsuwaki, Y.; Honda, K.; Ohta, N.; Yamamoto, S.; Takagi, Y.; et al. Eosinophil extracellular trap cell death-derived DNA traps: Their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 2016, 137, 258–267. [Google Scholar] [CrossRef]
- Ueki, S.; Tokunaga, T.; Melo, R.C.N.; Saito, H.; Honda, K.; Fukuchi, M.; Konno, Y.; Takeda, M.; Yamamoto, Y.; Hirokawa, M.; et al. Charcot-Leyden crystal formation is closely associated with eosinophil extracellular trap cell death. Blood. 2018, 132, 2183–2187. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, M.; Miyabe, Y.; Furutani, C.; Saga, T.; Moritoki, Y.; Yamada, T.; Weller, P.F.; Ueki, S. How to detect eosinophil ETosis (EETosis) and extracellular traps. Allergol. Int. 2021, 70, 19–29. [Google Scholar] [CrossRef]
- Neves, V.H.; Palazzi, C.; Bonjour, K.; Ueki, S.; Weller, P.F.; Melo, R.C.N. In Vivo ETosis of Human Eosinophils: The Ultrastructural Signature Captured by TEM in Eosinophilic Diseases. Front. Immunol. 2022, 13, 938691. [Google Scholar] [CrossRef]
- Tomizawa, H.; Arima, M.; Miyabe, Y.; Furutani, C.; Kodama, S.; Ito, K.; Watanabe, K.; Hasegawa, R.; Nishiyama, S.; Hizuka, K.; et al. Characteristics and Regulation of Human Eosinophil ETosis In Vitro. Am. J. Respir. Cell Mol. Biol. 2024. [Google Scholar] [CrossRef]
- Lowe, D.; Jorizzo, J.; Hutt, M.S. Tumour-associated eosinophilia: a review. J. Clin. Pathol. 1981, 34, 1343–1348. [Google Scholar] [CrossRef]
- Varricchi, G.; Galdiero, M.R; Loffredo, S.; Lucarini, V.; Marone, G.; Mattei, F.; Marone, G.; Schiavoni, G. Eosinophils: The unsung heroes in cancer? Oncoimmunology. 2017, 7, e1393134. [Google Scholar] [CrossRef] [PubMed]
- Mattei, F.; Andreone, S.; Marone, G.; Gambardella, A.R.; Loffredo, S.; Varricchi, G.; Schiavoni, G. Eosinophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1273, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Sabit, H.; Arneth, B.; Abdel-Ghany, S.; Madyan, E.F.; Ghaleb, A.H.; Selvaraj, P.; Shin, D.M.; Bommireddy, R.; Elhashash, A. Beyond Cancer Cells: How the Tumor Microenvironment Drives Cancer Progression. Cells. 2024, 13, 1666. [Google Scholar] [CrossRef]
- Caruso, R.A.; Giuffrè, G.; Inferrera, C. Minute and small early gastric carcinoma with special reference to eosinophil infiltration. Histol. Histopathol. 1993, 8, 155–166. [Google Scholar] [PubMed]
- Caruso, R.A.; Bersiga, A.; Rigoli, L.; Inferrera, C. Eosinophil-tumor cell interaction in advanced gastric carcinoma: an electron microscopic approach. Anticancer Res. 2002, 22, 3833–3836. [Google Scholar] [PubMed]
- Caruso, R.A.; Ieni, A.; Fedele, F.; Zuccalà, V.; Riccardo, M.; Parisi, E.; Parisi, A. Degranulation patterns of eosinophils in advanced gastric carcinoma: an electron microscopic study. Ultrastruct. Pathol. 2005, 29, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.A.; Fedele, F.; Zuccalà, V.; Fracassi, M.G.; Venuti, A. Mast cell and eosinophil interaction in gastric carcinomas: ultrastructural observations. Anticancer Res. 2007, 27, 391–394. [Google Scholar]
- Caruso, R.A.; Parisi, A.; Quattrocchi, E.; Scardigno, M.; Branca, G.; Parisi, C.; Lucianò, R.; Paparo, D.; Fedele, F. Ultrastructural descriptions of heterotypic aggregation between eosinophils and tumor cells in human gastric carcinomas. Ultrastruct. Pathol. 2011, 35, 145–149. [Google Scholar] [CrossRef]
- Caruso, R.A.; Fedele, F.; Parisi, A.; Paparo, D.; Bonanno, A.; Finocchiaro, G.; Branca, G.; Scardigno, M.; Rigoli, L. Chronic allergic-like inflammation in the tumor stroma of human gastric carcinomas: an ultrastructural study. Ultrastruct. Pathol. 2012, 36, 139–144. [Google Scholar] [CrossRef]
- Ieni, A.; Barresi, V.; Rigoli, L.; Fedele, F.; Tuccari, G.; Caruso, R.A. Morphological and cellular features of innate immune reaction in Helicobacter pylori gastritis: A Brief Review. Int. J. Mol. Sci. 2016, 17, 109. [Google Scholar] [CrossRef]
- Caruso, R.A.; Branca, G.; Fedele, F.; Parisi, A.; Finocchiaro, G.; Ieni, A.; Rigoli, L. Eosinophil-specific granules in tumor cell cytoplasm: Unusual ultrastructural findings in a case of diffuse-type gastric carcinoma. Ultrastruct. Pathol. 2015, 39, 226–230. [Google Scholar] [CrossRef]
- Caruso, R.; Irato, E.; Rigoli, L. Eosinophil exocytosis in a poorly differentiated tubular gastric adenocarcinoma: case report. Ultrastruct. Pathol. 2022, 46, 139–146. [Google Scholar] [CrossRef]
- Caruso, R.; Caruso, V.; Rigoli, L. Ultrastructural evidence of eosinophil clustering and ETosis in association with damage to single tumour cells in a case of poorly cohesive NOS gastric carcinoma. Eur. J. Case Rep. Intern Med. 2023, 10, 004016. [Google Scholar] [CrossRef]
- Caruso, R.; Caruso, V.; Rigoli, L. Eosinophil cytolysis with or without ETosis in four cases of human gastric cancer: a comparative ultrastructural study. Explor. Target Antitumor Ther. 2025, 6, 1002309. [Google Scholar] [CrossRef] [PubMed]
- Caruso, R.; Caruso, V.; Rigoli, L. Ultrastructural evidence of interactions between eosinophils and mast cells in gastric cancer: Considerations in AllergoOncology research. Gastrointest. Disord. 2025, 7, 41. [Google Scholar] [CrossRef]
- Shomali, W.; Gotlib, J. World Health Organization-defined eosinophilic disorders: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 129–148. [Google Scholar] [CrossRef] [PubMed]
- Cormier, S.A.; Taranova, A.G.; Bedient, C.; Nguyen, T.; Protheroe, C.; Pero, R.; Dimina, D.; Ochkur, S.I.; O’Neill, K.; Colbert, D.; et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J. Leukoc. Biol. 2006, 79, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Jacobsen, E.A.; McGarry, M.P.; Schleimer, R.; Lee, N.A. Eosinophils in health and disease: the LIAR hypothesis. Clin. Exp. Allergy. 2010, 40, 563–575. [Google Scholar] [CrossRef]
- Kataoka, S.; Konishi, Y.; Nishio, Y.; Fujikawa-Adachi, K.; Tominaga, A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004, 23, 549–560. [Google Scholar] [CrossRef]
- Korbecki, J.; Kojder, K.; Simińska, D.; Bohatyrewicz, R.; Gutowska, I.; Chlubek, D.; Baranowska-Bosiacka, I. CC Chemokines in a Tumor: A Review of Pro-Cancer and Anti-Cancer Properties of the Ligands of Receptors CCR1, CCR2, CCR3, and CCR4. Int. J. Mol. Sci. 2020, 21, 8412. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Oon, C.; Diaz, L.; Sandborg, H.; Stempinski, E.S.; Saoi, M.; Morgan, T.K.; López, C.S.; Cross, J.R.; Sherman, M.H. Autotaxin-lysolipid signaling suppresses a CCL11-eosinophil axis to promote pancreatic cancer progression. Nat. Cancer. 2024, 5, 283–298. [Google Scholar] [CrossRef]
- Iwasaki, K.; Torisu, M.; Fujimura, T. Malignant tumor and eosinophils. I. Prognostic significance in gastric cancer. Cancer. 1986, 58, 1321–1327. [Google Scholar] [CrossRef]
- Cuschieri, A.; Talbot, I.C.; Weeden, S.; MRC Upper. GI Cancer Working. Party. Influence of pathological tumour variables on long-term survival in resectable gastric cancer. Br. J. Cancer. 2002, 86, 674–679. [Google Scholar] [CrossRef]
- Pretlow, T.P.; Keith, E.F; Cryar, A.K.; Bartolucci, A.A.; Pitts, A.M.; Pretlow, T.G.; Kimball, P.M.; Boohaker, E.A. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983, 43, 2997–3000. [Google Scholar]
- Nielsen, H.J.; Hansen, U.; Christensen, I.J.; Reimert, C.M.; Brünner, N.; Moesgaard, F. Independent prognostic value of eosinophil and mast cell infiltration in colorectal cancer tissue. J. Pathol. 1999, 189, 487–495. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Galindo-Gallego, M.; Sanz, J.; Aljama, A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000, 88, 1544–1548. [Google Scholar] [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women's Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Fujii, M.; Yamashita, T.; Ishiguro, R.; Tashiro, M.; Kameyama, K. Significance of epidermal growth factor receptor and tumor associated tissue eosinophilia in the prognosis of patients with nasopharyngeal carcinoma. Auris Nasus Larynx. 2002, 29, 175–181. [Google Scholar] [CrossRef]
- Peurala, E.; Tuominen, M. L. E.; Syrjänen, S.; Rautava, J. Eosinophilia is a favorable prognostic marker for oral cavity and lip squamous cell carcinoma. A.P.M.I.S. 2018, 126, 201–207. [Google Scholar] [CrossRef]
- Caponio, V.C.A.; Togni, L.; Zhurakivska, K.; Santarelli, A.; Arena, C.; Rubini, C.; Lo, M. L.; Troiano, G.; Mascitti, M. Prognostic assessment of different methods for eosinophils detection in oral tongue cancer. J. Oral Pathol. Med. 2022, 51, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Ohashi, Y.; Suzuki, T.; Miyazaki, S.; Moriya, T.; Satomi, S.; Sasano, H. Tumor-associated tissue eosinophilia in human esophageal squamous cell carcinoma. Anticancer Res. 2006, 26, 1419–1424. [Google Scholar] [PubMed]
- Thompson, A.C.; Bradley, P.J.; Griffin, N.R. Tumor-associated tissue eosinophilia and long-term prognosis for carcinoma of the larynx. Am. J. Surg. 1994, 168, 469–471. [Google Scholar] [CrossRef]
- Moreira, A.; Leisgang, W.; Schuler, G.; Heinzerling, L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. 2017, 9, 115–121. [Google Scholar] [CrossRef]
- Brand, C.L; Hunger, R.E.; Seyed, J. S.M. Eosinophilic granulocytes as a potential prognostic marker for cancer progression and therapeutic response in malignant melanoma. Front. Oncol. 2024, 14, 1366081. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ren, H.; Wang, L.; Ning, Z.; Zhuang, Y.; Gan, J.; Chen, S.; Zhou, D.; Zhu, H.; Tan, D.; Zhang, H. Clinical impact of tumor-infiltrating inflammatory cells in primary small cell esophageal carcinoma. Int. J. Mol. Sci. 2014, 15, 9718–9734. [Google Scholar] [CrossRef]
- Ownby, H.E.; Roi, LD.; Isenberg, R.R.; Brennan, M.J. Peripheral lymphocyte and eosinophil counts as indicators of prognosis in primary breast cancer. Cancer. 1983, 52, 126–130. [Google Scholar] [CrossRef]
- Hu, G.; Wang, S.; Zhong, K.; Xu, F.; Huang, L.; Chen, W.; Cheng, P. Tumor-associated tissue eosinophilia predicts favorable clinical outcome in solid tumors: a meta-analysis. BMC Cancer. 2020, 20, 454. [Google Scholar] [CrossRef]
- van Driel, W.J.; Hogendoorn, P.C.; Jansen, F.W.; Zwinderman, A.H.; Trimbos, J.B.; Fleuren, G.J. Tumor-associated eosinophilic infiltrate of cervical cancer is indicative for a less effective immune response. Hum. Pathol. 1996, 27, 904–911. [Google Scholar] [CrossRef]
- Xie, F.; Liu, L.B.; Shang, W.Q.; Chang, K.K.; Meng, Y.H.; Mei, J.; Yu, J.J.; Li, D.J.; Li, M.Q. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015, 364, 106–117. [Google Scholar] [CrossRef]
- Rimini, M.; Franco, P.; Bertolini, F.; Berardino, B.; Giulia, Z.M.; Stefano, V.; Andrikou, K.; Arcadipane, F.; Napolitano, M.; Buno, L.V.; et al. The prognostic role of baseline eosinophils in HPV-related cancers: a multi-institutional analysis of anal SCC and OPC Patients Treated with Radical CT-RT. J. Gastrointest. Cancer. 2023, 54, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Enblad, G.; Sundstrom, C.; Glimelius, B. Infiltration of eosinophils in Hodgkin's disease involved lymph nodes predicts prognosis. Hematol. Oncol. 1993, 11, 187–193. [Google Scholar] [CrossRef]
- von Wasielewski, R.; Seth, S.; Franklin, J.; Fischer, R. H. K.; Hansmann, M.L; Diehl, V.; Georgii, A. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin's disease, allowing for known prognostic factors. Blood. 2000, 95, 1207–1213. [Google Scholar] [CrossRef]
- Danielson, D.T.; Aguilera, N.S.; Auerbach, A. Head and neck classic Hodgkin, T and NK lymphomas with eosinophilia. Head Neck Pathol. 2025, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, A.; Ishida, T.; Inagaki, A.; Ishii, T.; Yano, H.; Komatsu, H.; Iida, S.; Yonekura, K.; Takeuchi, S.; Takatsuka, Y.; Ueda, R. Clinical significance of a blood eosinophilia in adult T-cell leukemia/lymphoma: a blood eosinophilia is a significant unfavorable prognostic factor. Leuk. Res. 2007, 31, 915–920. [Google Scholar] [CrossRef]
- Gami, B.; Kubba, F.; Ziprin, P. Human papilloma virus and squamous cell carcinoma of the anus. Clin. Med. Insights Oncol. 2014, 8, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Nicolás, I.; Marimon, L.; Barnadas, E.; Saco, A.; Rodríguez-Carunchio, L.; Fusté, P.; Martí, C.; Rodriguez-Trujillo, A.; Torne, A.; Del, P. M.; Ordi, J. HPV-negative tumors of the uterine cervix. Mod. Pathol. 2019, 32, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Saito, M.; Okayama, K.; Okodo, M.; Kurose, N.; Sakamoto, J.; Sasagawa, T. HPV genotyping by molecular mapping of tissue samples in vaginal squamous intraepithelial neoplasia (VaIN) and vaginal squamous cell carcinoma (VaSCC). Cancers. 2021, 13, 3260. [Google Scholar] [CrossRef]
- Li, Z.; Liu, P.; Wang, Z.; Zhang, Z.; Chen, Z.; Chu, R.; Li, G.; Han, Q.; Zhao, Y.; Li, L.; Miao, J.; Kong, B.; Song, K. Prevalence of human papillomavirus DNA and p16INK4a positivity in vulvar cancer and vulvar intraepithelial neoplasia: a systematic review and meta-analysis. Lancet Oncol. 2023, 24, 403–414. [Google Scholar] [CrossRef]
- Mustasam, A.; Parza, K.; Ionescu, F.; Gullapalli, K.; Paravathaneni, M.; Kim, Y.; Sandstrom, R.E.; Al Assaad, M.; Grass, G.D.; Johnstone, P.; et al. The Prognostic role of HPV or p16INK4a status in penile squamous cell carcinoma: A Meta-Analysis. J. Natl. Compr. Canc. Netw. 2025, 23, e247078. [Google Scholar] [CrossRef]
- Xu, L.; Jin, Y.; Qin, X. Comprehensive analysis of significant genes and immune cell infiltration in HPV-related head and neck squamous cell carcinoma. Int. Immunopharmacol. 2020, 87, 106844. [Google Scholar] [CrossRef]
- Jarrett, A.F.; Armstrong, A.A.; Alexander, E. Epidemiology of EBV and Hodgkin's lymphoma. Ann. Oncol. 1996, 7 Suppl 4, 5–10. [Google Scholar] [CrossRef]
- Teruya-Feldstein, J.; Jaffe, E.S.; Burd, P.R.; Kingma, D.W.; Setsuda, J.E.; Tosato, G. Differential chemokine expression in tissues involved by Hodgkin's disease: direct correlation of eotaxin expression and tissue eosinophilia. Blood. 1999, 93, 2463–2470. [Google Scholar] [CrossRef]
- Tsotridou, E.; Hatzipantelis, E. Epstein-Barr Infection, Hodgkin's Lymphoma, and the Immune System: Insights into the Molecular Mechanisms Facilitating Immune Evasion. Cancers. 2025, 17, 1481. [Google Scholar] [CrossRef] [PubMed]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science. 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Nomenclature Committee on Cell Death. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ. 2005, 12, 1463–1467. [Google Scholar] [CrossRef]
- Caruso, R.A.; Branca, G.; Fedele, F.; Irato, E.; Finocchiaro, G.; Parisi, A.; Ieni, A. Mechanisms of coagulative necrosis in malignant epithelial tumors. Oncol. Lett. 2014, 8, 1397–1402. [Google Scholar] [CrossRef]
- Nija, R.J.; Sanju, S.; Sidharthan, N.; Mony, U. Extracellular Trap by blood cells: Clinical implications. Tissue Eng. Regen. Med. 2020, 17, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Yousefi, S.; Gold, J.A.; Andina, N.; Lee, J.J.; Kelly, A.M.; Kozlowski, E.; Schmid, I.; Straumann, A.; Reichenbach, J.; Gleich, G.J.; Simon, H.U. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat. Med. 2008, 14, 949–953. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, M.; Lacy, P.; Ueki, S. Eosinophil Extracellular Traps and Inflammatory Pathologies-Untangling the Web! Front. Immunol. 2018, 9, 2763. [Google Scholar] [CrossRef] [PubMed]
- Arima, M.; Ito, K.; Abe, T.; Oguma, T.; Asano, K.; Mukherjee, M.; Ueki, S. Eosinophilic mucus diseases. Allergol. Int. 2024, 73, 362–374. [Google Scholar] [CrossRef]
- Neves, V.H.; Palazzi, C.; Malta, K.K; Bonjour, K.; Kneip, F.; Dias, F.F.; Neves, J.S.; Weller, P.F.; Melo, R.C.N. Extracellular sombrero vesicles are hallmarks of eosinophilic cytolytic degranulation in tissue sites of human diseases. J. Leukoc. Biol. 2024, 116, 398–408. [Google Scholar] [CrossRef]
- Neves, J.S.; Perez, S.A.; Spencer, L.A.; Melo, R.C.N.; Reynolds, L.; Ghiran, I.; Mahmudi-Azer, S.; Odemuyiwa, S.O.; Dvorak, A.M.; Moqbel, R.; Weller, P.F. Eosinophil granules function extracellularly as receptor-mediated secretory organelles. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 18478–18483. [Google Scholar] [CrossRef]
- Muniz, V.S.; Baptista-Dos-Reis, R.; Neves, J.S. Functional extracellular eosinophil granules: a bomb caught in a trap. Int. Arch. Allergy Immunol. 2013, 162, 276–282. [Google Scholar] [CrossRef]
- Legrand, F.; Driss, V.; Delbeke, M.; Loiseau, S.; Hermann, E.; Dombrowicz, D.; Capron, M. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J. Immunol. 2010, 185, 7443–7451. [Google Scholar] [CrossRef] [PubMed]
- Gatault, S.; Delbeke, M.; Driss, V.; Sarazin, A.; Dendooven, A.; Kahn, J.E.; Lefèvre, G.; Capron, M. IL-18 is involved in eosinophil-mediated tumoricidal activity against a colon carcinoma cell line by upregulating LFA-1 and ICAM-1. J. Immunol. 2015, 195, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Andreone, S.; Spadaro, F.; Buccione, C.; Mancini, J.; Tinari, A.; Sestili, P.; Gambardella, A.R.; Lucarini, V.; Ziccheddu, G.; Parolini, I.; et al. IL-33 promotes CD11b/CD18-mediated adhesion of eosinophils to cancer cells and synapse-polarized degranulation leading to tumor cell killing. Cancers. 2019, 11, 1664. [Google Scholar] [CrossRef] [PubMed]
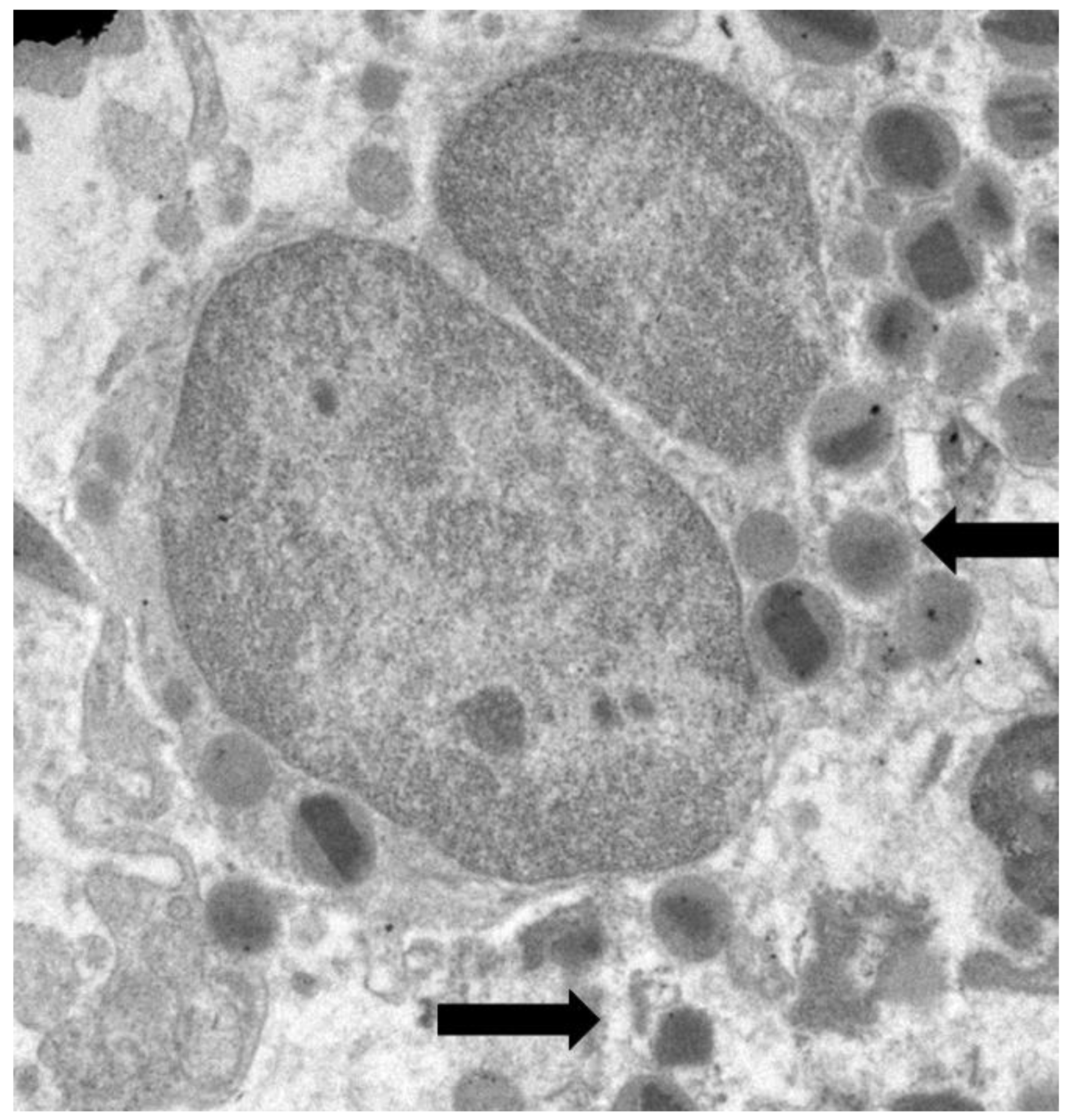
Figure 1.
Electron micrograph of an eosinophil showing ultrastructural signs of cytolysis such as rounded nuclear lobes, chromatin decondensation and the release of FEGs (black arrows).
Figure 1.
Electron micrograph of an eosinophil showing ultrastructural signs of cytolysis such as rounded nuclear lobes, chromatin decondensation and the release of FEGs (black arrows).
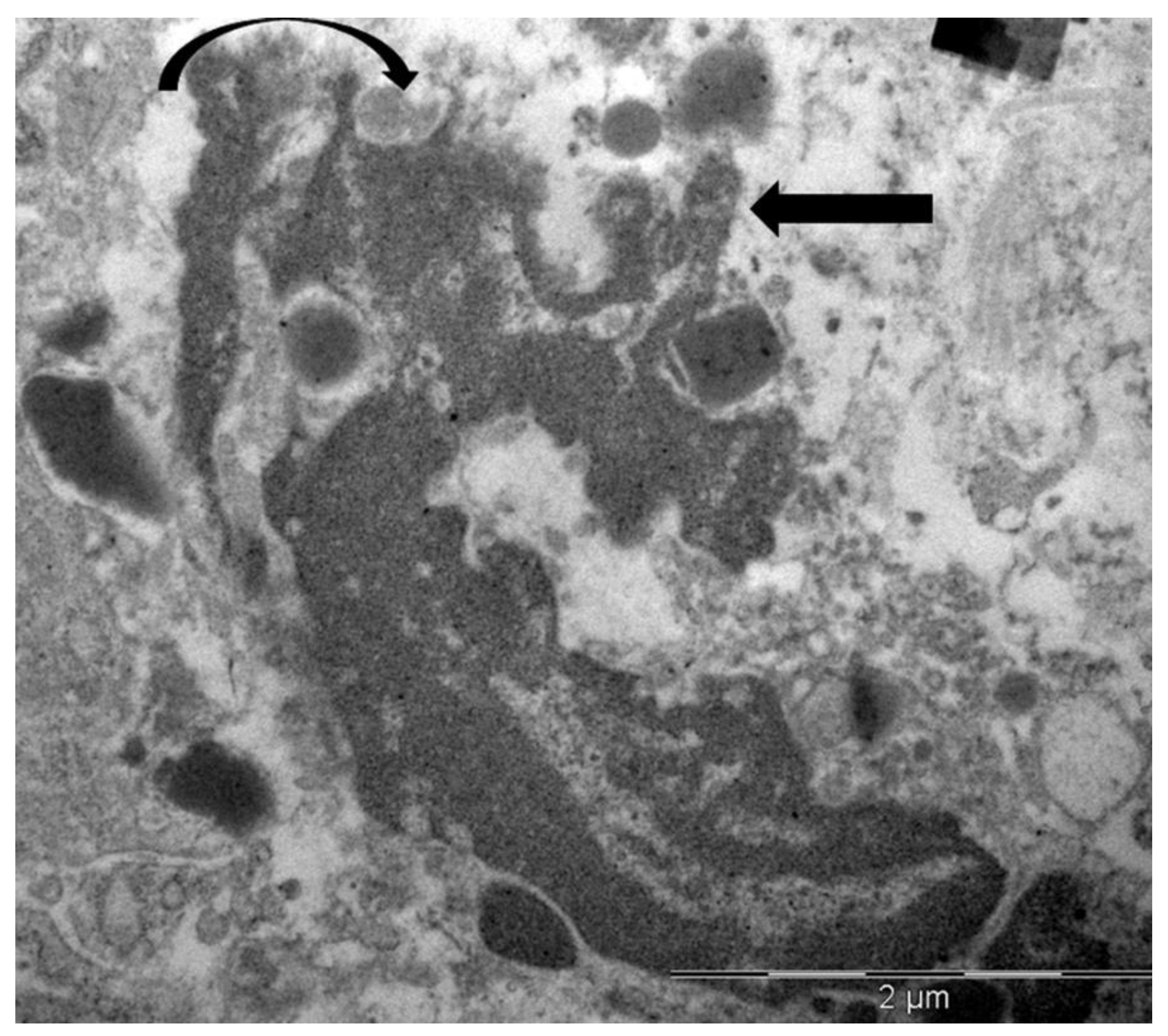
Figure 2.
Electron micrograph of an eosinophil showing ultrastructural signs of early ETosis, including a slightly irregular profile of nuclear lobe, chromatin decondensation, dilation of nuclear envelope (pentagonal arrow), focal disruption of plasma membrane (black arrow), and the release of specific granules (curved arrow).
Figure 2.
Electron micrograph of an eosinophil showing ultrastructural signs of early ETosis, including a slightly irregular profile of nuclear lobe, chromatin decondensation, dilation of nuclear envelope (pentagonal arrow), focal disruption of plasma membrane (black arrow), and the release of specific granules (curved arrow).
Figure 3.
Electron micrograph depicting an intermediate phase of eosinophil ETosis. A few specific granules are present in the eosinophil (curved arrow), while decondensed chromatin appears as a cytoplasmic mass with irregular outlines (black arrows).
Figure 3.
Electron micrograph depicting an intermediate phase of eosinophil ETosis. A few specific granules are present in the eosinophil (curved arrow), while decondensed chromatin appears as a cytoplasmic mass with irregular outlines (black arrows).
Figure 4.
Electron micrograph illustrating an advanced stage of eosinophil ETosis. Extracellular DNA traps appear as decondensed chromatin structures with finger-like projections (curved arrow) or as sinuous, filamentous strands (black arrow), often located near free extracellular granules (FEGs).
Figure 4.
Electron micrograph illustrating an advanced stage of eosinophil ETosis. Extracellular DNA traps appear as decondensed chromatin structures with finger-like projections (curved arrow) or as sinuous, filamentous strands (black arrow), often located near free extracellular granules (FEGs).
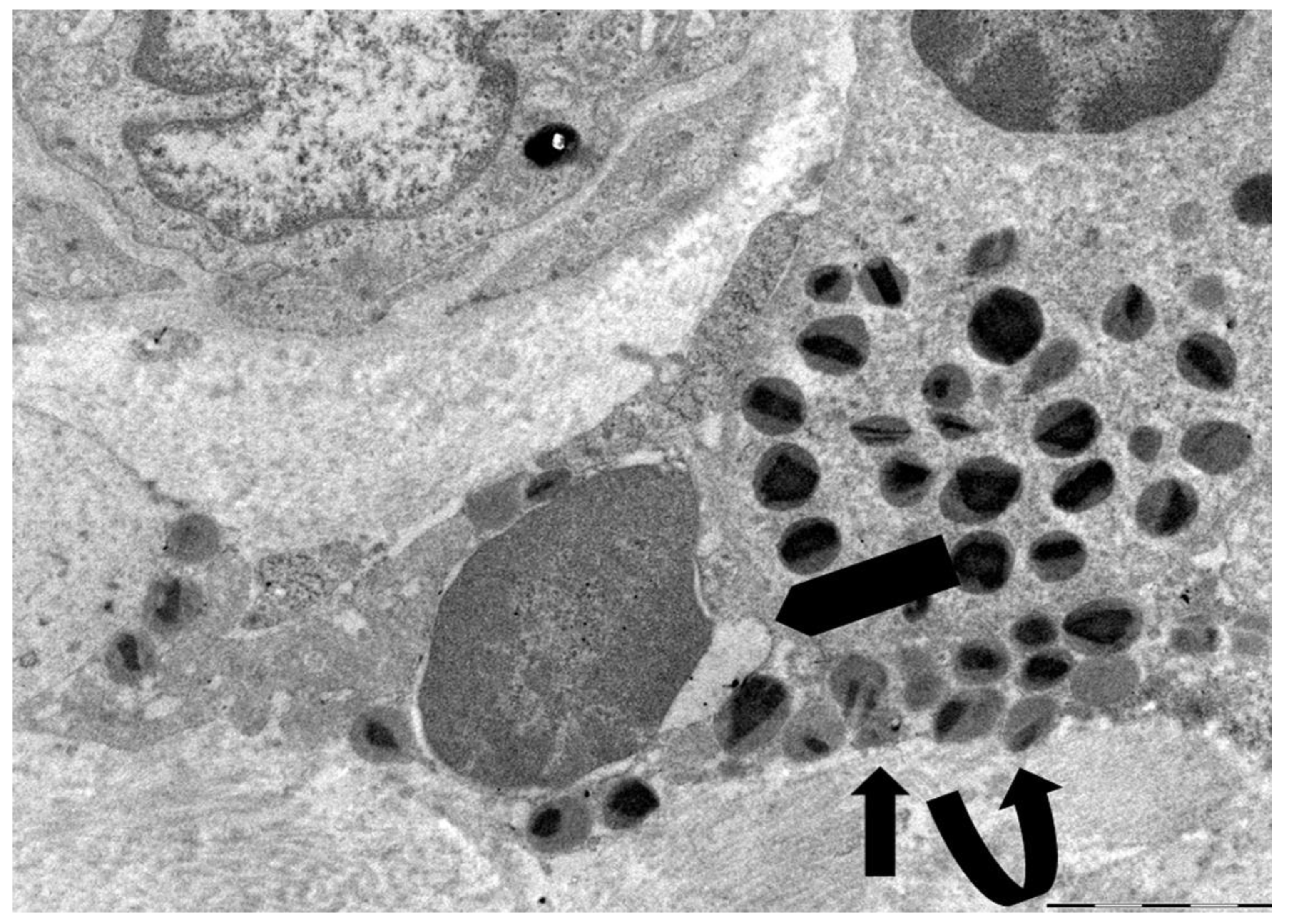
Figure 5.
Ultrastructural image showing the interaction between tumor cell (T) and eosinophil (E). Fused specific granules (black arrows), indicative of compound exocytosis, are observed in the cytoplasm next to the tumor cell. Sparse sombrero vesicles are also observable (pentagonal arrow). The tumor cell and eosinophil exhibit discontinuities of plasma membrane (curved arrows).
Figure 5.
Ultrastructural image showing the interaction between tumor cell (T) and eosinophil (E). Fused specific granules (black arrows), indicative of compound exocytosis, are observed in the cytoplasm next to the tumor cell. Sparse sombrero vesicles are also observable (pentagonal arrow). The tumor cell and eosinophil exhibit discontinuities of plasma membrane (curved arrows).
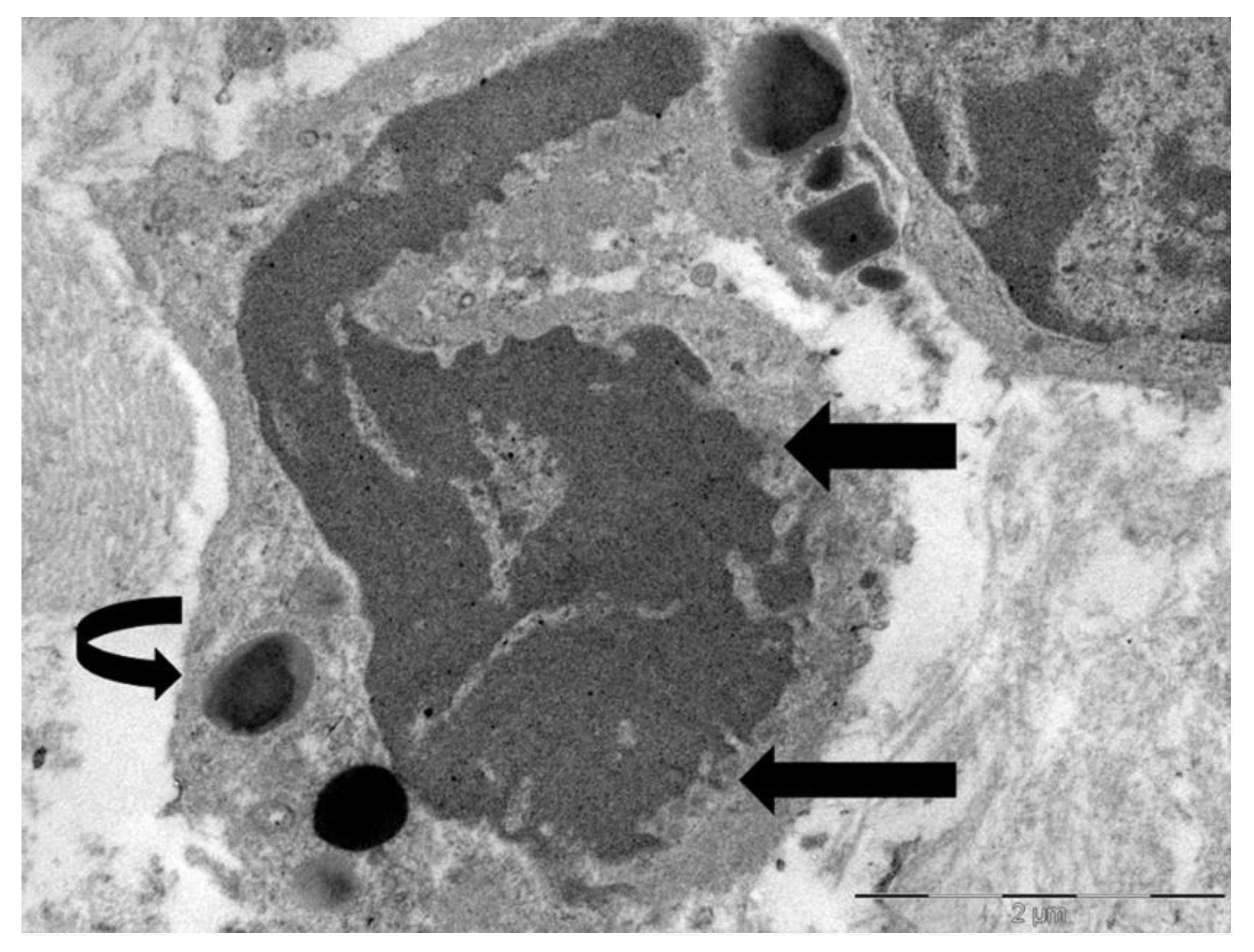
Figure 6.
Electron micrograph showing the interaction between an eosinophil (E) and a tumor cell (T). The eosinophil displays initial ultrastructural signs of ETosis, including arounded nuclear lobe, chromatin decondensation (pentagonal arrow), and the degranulation of specific granules near the tumor cell contact (black arrow). The tumor cell exhibits a discontinuity in the plasma membrane at the site of eosinophil degranulation (curved arrow), suggesting a potential sublethal effect.
Figure 6.
Electron micrograph showing the interaction between an eosinophil (E) and a tumor cell (T). The eosinophil displays initial ultrastructural signs of ETosis, including arounded nuclear lobe, chromatin decondensation (pentagonal arrow), and the degranulation of specific granules near the tumor cell contact (black arrow). The tumor cell exhibits a discontinuity in the plasma membrane at the site of eosinophil degranulation (curved arrow), suggesting a potential sublethal effect.
Figure 7.
Electron micrograph illustrates an eosinophil (E) exhibiting early ETosis. The adjacent tumor cell (T) shows numerous cytoplasmic microvacuoles (arrow) in proximity to the contact area with ETotic eosinophil.
Figure 7.
Electron micrograph illustrates an eosinophil (E) exhibiting early ETosis. The adjacent tumor cell (T) shows numerous cytoplasmic microvacuoles (arrow) in proximity to the contact area with ETotic eosinophil.
Figure 8.
Heterotypic interaction between eosinophils (E) and a tumor cell (T). Numerous large cytoplasmic vacuoles (black arrows) are identified within the tumor cell cytoplasm adjacent to eosinophils. The presence of these vacuoles suggests colloid-osmotic tumor cell death, likely induced by eosinophils.
Figure 8.
Heterotypic interaction between eosinophils (E) and a tumor cell (T). Numerous large cytoplasmic vacuoles (black arrows) are identified within the tumor cell cytoplasm adjacent to eosinophils. The presence of these vacuoles suggests colloid-osmotic tumor cell death, likely induced by eosinophils.
Table 1.
TATE/TABE in Viral and Non-Viral Neoplasms.
Table 1.
TATE/TABE in Viral and Non-Viral Neoplasms.
| Neoplasm |
Virus identification |
Type of Eosinophilia |
Clinical outcome |
References |
| Cervical squamous cell carcinoma |
HPV |
TATE |
TATE increases with cancer progression |
Xie et al., [56] |
| Cervical squamous cell carcinoma |
Not performed |
TATE |
TATE predicts a worse overall survival |
Van Driel et al., [55] |
| Anal squamous cell carcinoma |
HPV |
TABE |
High eosinophil count is a negative independent prognostic factor for disease-free survival |
Rimini et al., [57] |
| Oropharyngeal cancer |
HPV |
TABE |
High eosinophil count is associated to a worse disease-free survival |
Rimini et al., [57] |
| Adult T-cell leukemia/lymphoma |
HTLV-1 |
TABE |
Blood eosinophilia is an independent unfavorable prognostic factor |
Utsunomiya et al., [61] |
Table 2.
Granulocyte Cell Death Modalities: Distinguishing Apoptosis, Neutrophil ETosis, and Eosinophil ETosis.
Table 2.
Granulocyte Cell Death Modalities: Distinguishing Apoptosis, Neutrophil ETosis, and Eosinophil ETosis.
| Feature |
Apoptosis |
Neutrophil ETosis |
Eosinophil ETosis |
| Type of cell death |
Programmed, non-inflammatory |
Programmed, pro-inflammatory |
Programmed, pro-inflammatory |
| Nuclear organization |
Condensation and fragmentation |
Delobulation and decondensation |
Delobulation and decondensation |
| Nuclear envelope integrity |
Preserved until late stages |
Disrupted |
Disrupted |
| Plasma membrane rupture |
No |
Yes |
Yes |
| Cytoplasmic granules |
Intact, not involved |
Disintegrated; enzymes released into nucleus |
Intact; associated with extracellular traps |
| Content of extracellular traps |
Absent |
DNA + elastase, myeloperoxidase, other granule-derived enzymes |
DNA + morphologically intact specific granules |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).