1. Introduction
Telogen effluvium (TE) is a non-scarring form of alopecia which may occur in either acute or chronic forms, both characterized by diffuse hair shedding [
1]. The acute mani-festation, known as acute telogen effluvium (aTE) typically lasts less than six months and is triggered by various intrinsic factors including stress, hormonal changes, nutritional deficiencies, medical conditions, as well as extrinsic factors such as diet, drugs, and pol-lution [
2,
3]. Clinically, aTE occurs with a sudden and marked hair loss occurring 2-3 months after the triggering event, due to the premature transition of hair follicles from the anagen (growth) phase to the telogen (resting) phase [
4].
The incidence of TE in the general population is difficult to establish, as many cases remain subclinical and therefore were unreported or undiagnosed [
5]. Although TE can affect both sexes, it shows a significantly higher incidence in women primarily due to hormonally driven events such as those occurring during the postpartum and postmeno-pausal periods. Additionally, women tend to exhibit a heightened psychosocial sensitivity to hair loss, often experiencing greater emotional distress compared to men [
6]. Recent epidemiological analyses estimated the global prevalence of TE at 5.41% (95% CI: 2.73–11.22%), with regional variability and higher rates particularly among women. These findings further underscore TE as one of the most prevalent causes of diffuse hair loss worldwide [
7].
Although aTE is medically considered as a benign and self-limiting condition due to its transient nature, it can nevertheless result in significant psychological distress, reduced self-esteem, and impaired quality of life [
8,
9]. Medical treatments developed for more per-sistent forms of hair loss, such as Minoxidil and Finasteride for androgenetic alopecia, are often adapted for the management of aTE, but often prove to be not suitable due to their side effects, limited compliance, and off-label use [
10].
In recent years, clinical and preclinical interest in safe, non-invasive, and well-tolerated alternatives has increased, particularly in the field of nutraceuticals [
11,
12]. Nutrient-based interventions such as food supplements rich in vitamins, minerals, and plant-derived extracts offer several advantages, including good safety profiles, ease of administration, and higher patient adherence. Moreover, their multifunctional action, in-cluding antioxidants, anti-inflammatory and metabolic support, makes them particularly promising in conditions like aTE, where multiple systemic factors may converge to affect hair follicle cycling [
13]. Underlying causes, such as hormonal or nutritional deficiencies (e.g., low levels of iron, zinc, or vitamin D) or metabolic and inflammatory imbalances, can be more appropriately targeted leading to an improvement in hair growth and health [
5].
This study aimed to assess the efficacy and safety of a commercially available food supplement (Hair’InsideTM; Activ’Inside, Beychac-et-Caillau, France) formulated to sup-port hair growth and health. The product formula combined green tea extract, bamboo ex-tract and selenium, three bioactive ingredients selected for their synergistic potential to target the underlying biological mechanisms associated with aTE.
Several studies have highlighted the beneficial effects of the above-mentioned ingre-dients on physiological processes involved in hair growth and integrity. Flavanol mono-mers, including catechin, epicatechin, or epigallocatechin gallate (EGCG), found in plant food and beverages such as green tea, cocoa or grape, are known to enhance microcircula-tion and endothelial function, thereby promoting dermal papilla cell proliferation and prolonging the anagen phase [
14,
15,
16,
17,
18]. Silicon, contributes to collagen synthesis, which enhances hair tensile strength and elasticity [
19,
20,
21]. Selenium plays a pivotal antioxidant and anti-inflammatory role essential for hair integrity, and its deficiency has been associ-ated with increased hair fragility and shedding [
22,
23].
This study adopted a dual approach: an in vitro assessment of the biological effects of Hair’InsideTM on human hair follicle dermal papilla cells (HFDPC), focusing on markers of cell proliferation and hair cycle regulation, and a double-blind, randomized, place-bo-controlled clinical trial evaluating the safety and efficacy of its oral intake on hair growth and health of 66 female subjects with aTE over an 84-day treatment period.
Although dietary supplements are increasingly used in the management of TE, their clinical effectiveness remains a matter of debate, primarily due to limited high-quality evidence [
24]. In this context, the present study addresses this knowledge gap by demonstrating the use of this nutraceutical formula as a safe and effective strategy for the management of aTE with a science-backed approach.
2. Materials and Methods
2.1. In Vitro Evaluation on Human Dermal Papilla Cells Growth
Cell Culture, Experimental Conditions and Proliferation Assay
Primary Human Follicle Dermal Papilla Cells (HFDPCs) were obtained from PromoCell (C-12071; Batch. 494Z005). Cells were seeded in 96 well plate (Corning® Falcon®) at 30,000 cells per well and cultured in Follicle Dermal Papilla Cell Growth Medium (C-39620, PromoCell, Heidelberg, Germany) supplemented with 4% Fetal Calf Serum, 0.4% Bovine Pituitary Extract, 1 ng/ml Basic Fibroblast Growth Factor and 5 μg/ml recombinant human Insulin, at 37 °C in a humidified atmosphere with 5% CO₂. For the treatment, Hair’InsideTM was solubilized in DMSO and diluted in the cell culture medium to a final concentration of 608.8 µg/L. Cells were treated in six biological replicates per condition for 48 hours. Control cells were maintained under identical conditions without the addition of the product. After the incubation period, cells were fixed and stained with DAPI (4′,6-diamidino-2-phenylindole) to visualize nuclei. Images were captured using epifluorescence microscopy, and nuclei were quantified using ImageJ software (version 1.53k).
2.2. Clinical Study
2.2.1. Trial Design and Ethics
A multicentric, randomized, double-blind, placebo-controlled, parallel-group clinical trial was conducted at Complife Italia S.r.l. facilities in Garbagnate Milanese (Milano) and San Martino Siccomario (Pavia) between September 2024 and January 2025. The intervention period lasted 84 days consisting of a baseline visit (D0) and two follow-up visits after 42 (D42) and 84 (D84) days of product use. The trial included 66 healthy female subjects randomly assigned in a 1:1 ratio to receive either the active supplement (HI) or a placebo (PL), for a final sample of 33 subjects per group. All study procedures were conducted in accordance with the ethical principles outlined in the World Medical Association’s (WMA) Declaration of Helsinki and its amendments. Study protocol, informed consent form and data privacy documentation were approved by the “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche” (ref. no. 2024/07 by 16 July 2024). The trial was registered in ClinicalTrials.gov (NCT06545552,
https://clinicaltrials.gov/study/NCT06545552) and submitted on 7 August 2024. Prior to the initiation of any study-related procedures, all subjects were fully informed about the nature, aims, and potential risks of the study, and provided written informed consent.
2.2.2. Participants and Compliance with Treatment
Sixty-six healthy female subjects, aged 18 to 52 years, were enrolled in the study following confirmation of eligibility criteria during a screening visit conducted by a board-certified dermatologist or an authorized co-investigator. All participants were of Caucasian ethnicity showing signs of aTE due to fatigue, seasonal change, nutritional deficiency, change or imbalance of normal daily routine or emotional stress lasting less than six months. The inclusion and non-inclusion criteria were assessed through a standardized questionnaire and physical examination. Inclusion criteria comprised good general health, a positive hair pull test (extraction of more than 9 hair out of 50–60 gently pulled strands in three scalp areas) [
25], minimum hair length of 6–7 cm, and the willingness to refrain from any treatments, products, or procedures that could interfere with the evaluation of hair growth, structure, or appearance during the study. Subjects were required to have discontinued any anti-hair loss treatment at least three months prior to study initiation.
Subjects under systemic or topical treatments affecting hair physiology, those with chronic hair disorders (e.g., androgenetic alopecia, alopecia areata), pregnant or breastfeeding women, and individuals participating in other clinical trials within the previous 24 weeks were excluded. Additional exclusion criteria included any clinical condition or treatment that could interfere with the evaluation, or recent interventions such as scalp surgery or hair transplants.
At baseline (D0), each subject received one bottle containing 95 capsules of active food supplement or placebo and instructed to take one capsule per day in the morning with a glass of water. Compliance was monitored through a daily diary, and capsules were counted at each follow-up visit (D42 and D84). Compliance with treatment was considered acceptable within the range of 80% to 120%. Subjects exhibiting adherence below 80% or above 120% were excluded from the intention-to-treat (ITT) analysis due to inadequate adherence to the prescribed treatment protocol. Subjects with suspected or confirmed non-compliance could be withdrawn at the discretion of the principal investigator. Subjects retained the right to withdraw from the study at any time. Any premature discontinuation due to adverse events, serious adverse events, loss to follow-up, or protocol violations, was recorded and classified in the case report forms.
2.2.3. Interventions and Randomization
The investigational product Hair’InsideTM was a commercially available (patent pending FR2404808) food supplement supplied by Activ’Inside (Beychac et Caillau France,) containing 280 mg of a green tea extract (rich in epigallocatechin gallate, EGCG), bamboo extract rich in silica and selenium. The product formula was standardized in total polyphenols ≥ 80.0% (DO 280), EGCG ≥ 30.0% (HPLC-DAD), total silica ≥ 4.0% (Gravimetry/calculation), and selenium ≥ 80.0 ppm (ICP-MS). The PL food supplement was a capsule containing maltodextrin, organoleptically indistinguishable from the active supplement in appearance, taste, and packaging (hard-shell capsules, HPMC green size T0).
Subjects were randomized in a 1:1 ratio into two parallel treatment arms. Randomization was conducted by the on-site study director using a computer-generated allocation list based on an appropriate statistical algorithm (“Efron’s biased coin”), implemented with PASS 11 software (version 11.0.8; PASS, LLC, Kaysville, UT, USA). To ensure blinding, group allocation was concealed from both study investigators and participants. The investigational products were indistinguishable in appearance, packaging, and labeling, differing solely by batch numbers. The treatment regimen remained unchanged throughout the duration of the study.
2.2.4. Outcomes
The primary outcome was the rebalancing effect on hair cycle-related parameters (i.e., anagen and telogen phase) by improving hair growth, assessed through phototricogram technique after 84 days of treatment compared to PL. Secondary outcomes included additional assessments of hair shedding and hair quality parameters in terms of brightness and volume after 42 and 84 days of treatment. Product tolerability and safety were also evaluated during all the study period monitoring any potential adverse effects.
2.2.5. Phototricogram: Hair Growth Assessment
The phototricogram procedure involved clipping a standardized 1.8 cm² area at the mid-vertex region of the scalp, followed by hair dye application and cleansing with an alcohol-based solution. High-resolution images were captured 48 hours later using a dermatoscope (Dermogenius Ultra; DermoScan GmbH, Regensburg, Germany). Hair cycle-related parameters were assessed through phototricogram analysis using the TrichoScan
® software (vers. 4.0.10.102; TrichoScan GmbH, Freiburg, Germany) after 42 and 84 days of daily supplementation [
26]. The following parameters were measured: total hair density (number of total hair per cm²), telogen hair density (number of hair in telogen phase per cm²) and proportion (%), anagen hair density (number of hair in anagen phase per cm²) and proportion (%), anagen/telogen ratio. Additionally, the total number of hair in anagen phase (THA) and the number of hair protected against falling out (HPF) were derived according to the equations outlined below:
where: AN
AF is the number of anagen hairs after product use; AN
BE is the number of anagen hairs before product use; TE
AF is the number of telogen hair after product use; TE
BE is the number of telogen hair before product use; SH is the scalp surface area (580 cm²).
2.2.6. Pull Test: Hair Shedding
A standardized hair pull test was performed at D0, D42, and D84. The procedure involved applying gentle traction to a bundle of approximately 60 hairs from three distinct scalp regions (frontal, temporal, and occipital). The test was considered positive if more than 10 hair were extracted in more than one region, consistent with criteria for telogen effluvium [
27]. Subjects were instructed not to wash their hair for 48 hours prior to the test to prevent result bias.
2.2.7. Hair Brightness
Diffuse reflectance spectrophotometry was employed to objectively measure hair brightness at D0 and D84 by using a CM 700D spectrophotometer/colorimeter (Konica Minolta, Milan, Italy) [
28]. The analysis was performed in five standardized points near the hairline. The average of the five measurements was recorded as the 8° gloss mean value, with higher values indicating increased shine.
2.2.8. Clinical Assessment by Expert Scoring
Hair volume was clinically evaluated through digital macrophotography, taken at D42 and D84 under standardized conditions using a professional digital camera. An expert technician scored hair volume changes by comparing images at follow-up versus baseline using a validated 7-point scale ranging from “greatly decreased” (−3) to “greatly increased” (+3) [
29].
2.2.9. Product Tolerability and Safety
Throughout the study period, the dermatologist assessed overall tolerability based on participant feedback and clinical observation. For each reported reaction, relevant details were recorded, including onset, intensity, location, duration, and frequency, along with potential contributing factors (e.g., medical history, concurrent treatments, external exposures). Participants could report any adverse reactions via a dedicated contact number provided at enrollment. All reported symptoms were evaluated to determine their relation to the investigational product, and adverse events (AEs) were documented accordingly.
2.3. Statistical Analysis
2.3.1. In Vitro Data Analysis
Statistical analysis of in vitro data was performed to evaluate the effect of HI on the proliferation of human hair follicle dermal papilla cells (HFDPC). Cell number per surface area was expressed as mean ± standard error of the mean (SEM) across six replicates per condition. Differences between the treated group and the untreated control were assessed using unpaired t-test with Levene's correction. A p-value of < 0.05 was considered statistically significant. Results were expressed as the average number of cells per surface area and as the percentage increase in cell number compared to the control group.
2.3.2. Clinical Sample Size and Statistical Analysis
The sample size of a minimum of 30 subjects per group was determined based on previous clinical study evidences [
25]. Potential dropouts or protocol violations were addressed through the application of a 10% increase, leading to the enrollment of a total of 66 participants.
Descriptive analysis of demographic variables (sex, age, and hair type) was performed by intervention group. Categorical and ordinal data were summarized as percentages, while continuous variables were reported as mean ± standard error of the mean (SEM).
Statistical analysis was carried out on the per-protocol population. Prior to hypothesis testing, the normality of data distribution was assessed using the Shapiro–Wilk test. Intra-group comparisons across time points D0, D42 and D84 were conducted using one-way repeated measures ANOVA (RM-ANOVA), followed by Tukey–Kramer post-hoc test when data were normally distributed. For non-normally distributed data, the Friedman test was applied followed by Tukey–Kramer post-hoc test. These methods were used to analyze hair pull test results and phototricogram data. Intra-group comparisons of hair brightness between D0 and D84 were performed using the paired t-test for normally distributed data, or the Wilcoxon matched-pairs signed rank test for non-normally distributed data. Inter-group comparisons (HI vs. PL) of all parameters were carried out using either unpaired Student’s t-tests (for normally distributed data) or Mann–Whitney U tests (for non-parametric distributions), after 42 and 84 days of supplementation.
All statistical analyses were performed using NCSS 10 software (vers. 10.0.7; NCSS, LLC. Kaysville, UT, USA).
3. Results
3.1. In Vitro Study
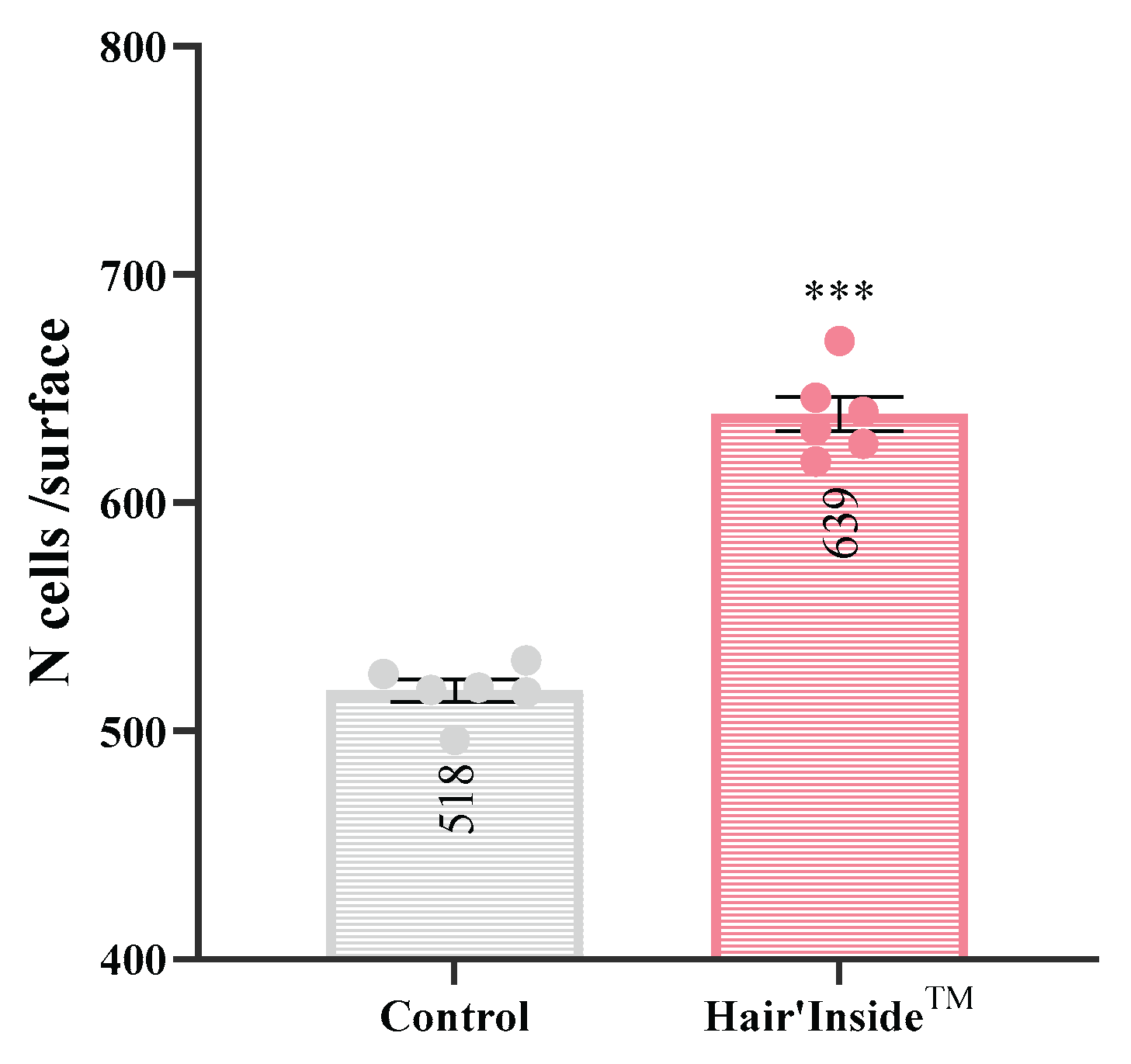
The treatment of human hair follicle dermal papilla cells (HFDPCs) with HI resulted in a statistically significant enhancement of dermal papilla cell growth proliferation compared to the untreated control group (
Figure 1). After 48 hours of treatment, HI significantly stimulated cell proliferation in HFDPC cultures, resulting in a 23.4% higher cell density compared to the placebo-treated control group (639 ± 19 vs. 518 ± 12 cells per unit surface area, respectively;
p < 0.001).
3.2. Participant Characteristics, Tolerability, and Compliance with Treatment
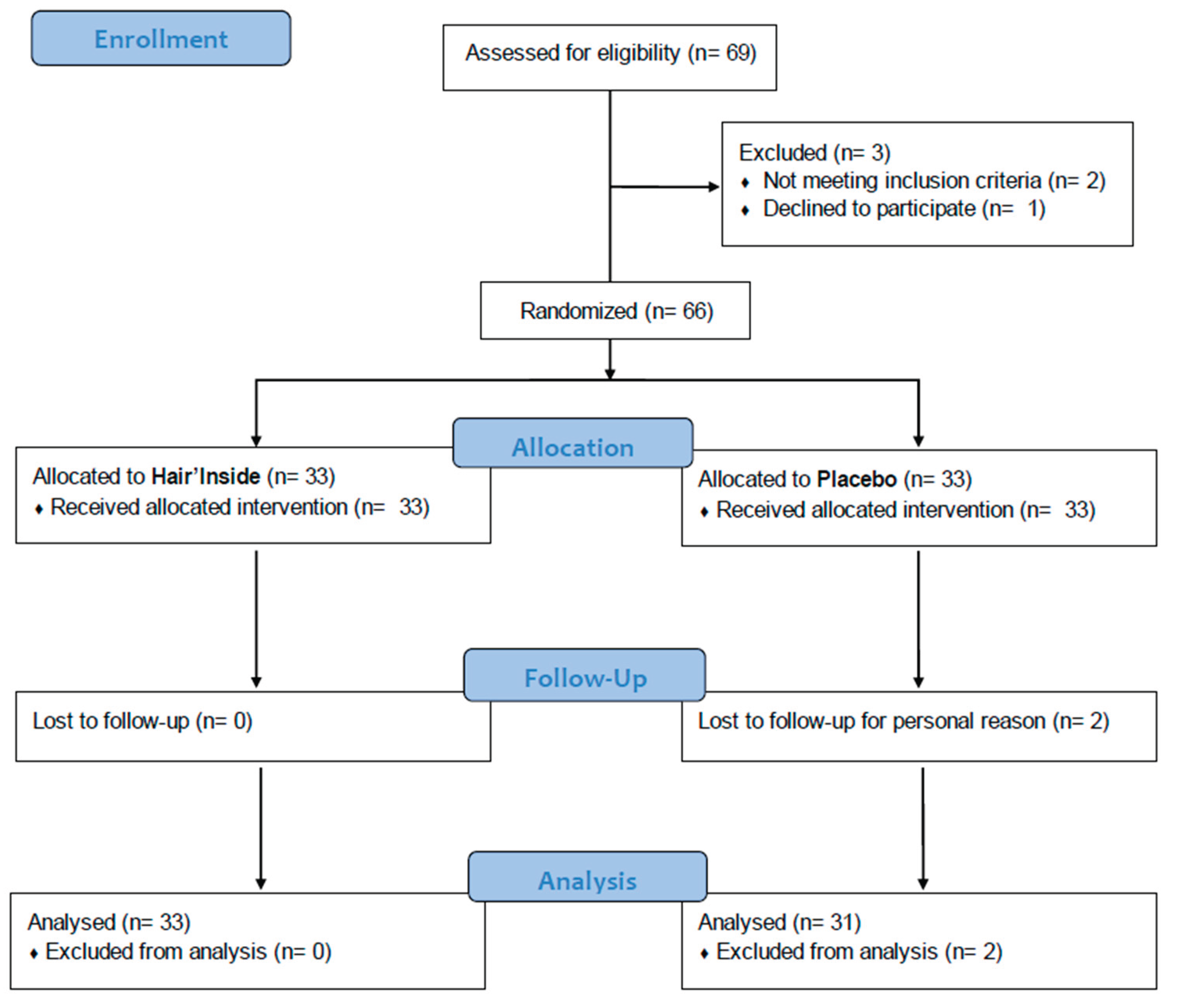
A total of 69 healthy Caucasian female subjects aged between 18 and 52 years with all hair type (normal, oily and dry) were assessed for eligibility. Two of them did not meet the inclusion criteria and 1 declined to participate. A final sample of 66 subjects were enrolled and randomized in the HI or PL group, each composed of 33 subjects (
Figure 2). Two participants, both assigned to the PL group, withdrew from the study after D42 follow up visit, due to personal reasons unrelated to the investigational product. Final population sample resulting in 64 subjects (33 in the active group and 31 in the PL group). There were no significant baseline differences between the groups in terms of demographic characteristics and hair-related parameters (
Table 1).
No adverse events or side effects were reported in either treatment group throughout the duration of the study. The investigational product was well tolerated by all participants, who also demonstrated full compliance with the food supplement protocol.
3.3. Phototricogram Parameters
Supplementation with HI led to progressive and statistically significant (
p < 0.001) improvements in all hair growth parameters assessed by phototricogram, at both D42 and D84 vs baseline (
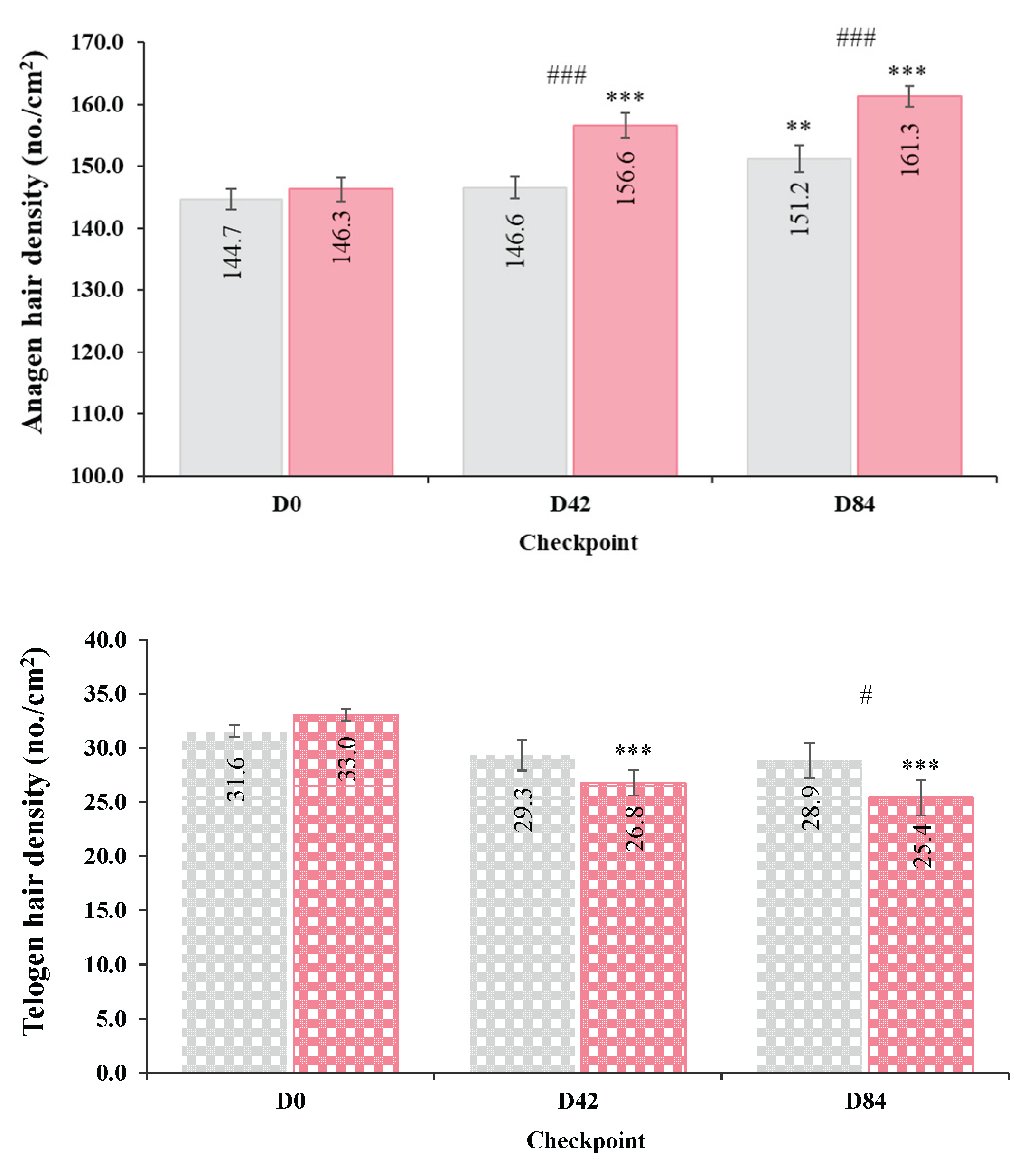
Table 2). Compared to baseline (D0), the active group exhibited significant increases in anagen hair density (+10.3 and +15.0 hair in anagen phase/cm²), along with a concomitant reduction in telogen hair density (-6.3 and -7.6 hair in telogen phase /cm²) (
Figure 3). The total hair density also increased significantly over time. The increase in anagen/telogen ratio mirrors the marked increase in the proportion of hair in the anagen phase and the concomitant decrease in telogen proportion at each timepoints.
Modest but statistically significant improvements in anagen-related parameters were also observed in the PL group at the end of the study including total hair density, anagen hair density and anagen/telogen ratio (p < 0.05). However, no significant changes in telogen hair density and proportion were detected in the PL group and inter-group comparisons demonstrated significantly greater increases in most of the analyzed parameters with HI supplementation. Specifically, the active group showed significantly superior increases in anagen hair density and anagen/telogen ratio at D42 and D84 compared to the PL (p < 0.05). Notably, the reduction in telogen hair density at D84 and in telogen hair proportion already from D42, was significantly greater than in the PL group (p < 0.05).
The number of hairs protected against falling out (HPF) improved more in the HI group, with inter-group comparisons showing a significant difference at D84 (p < 0.05). Furthermore, the total number of anagen hairs estimated over the full scalp area (THA) reached significant differences in the HI group at both D42 and D84 (+6002.1±752.3 and +8720.3±890.0, p < 0.001).
Table 2.
Phototricogram results of hair cycle-related parameters. Data are presented as mean ± SEM; asterisks indicate intra-group statistical significance vs. baseline (D0): *p < 0.05, **p < 0.01, ***p < 0.001; Hashes indicate statistical significance between intervention groups at (D42) or (D84): #p < 0.05, ##p < 0.01, ###p < 0.001. HPF: total number of hair protected against falling out, THA: total number of hair in anagen phase
Table 2.
Phototricogram results of hair cycle-related parameters. Data are presented as mean ± SEM; asterisks indicate intra-group statistical significance vs. baseline (D0): *p < 0.05, **p < 0.01, ***p < 0.001; Hashes indicate statistical significance between intervention groups at (D42) or (D84): #p < 0.05, ##p < 0.01, ###p < 0.001. HPF: total number of hair protected against falling out, THA: total number of hair in anagen phase
| |
HI |
PL |
| |
D0 |
D42 |
D84 |
D0 |
D42 |
D84 |
|
Total hair density (no./cm²) |
179.3±1.5 |
183.4±1.4*** (+4.1)##
|
186.7±1.7*** (+7.4) |
176.3±1.6 |
175.9±1.7<break>(-0.4) |
180.0±1.7* (+3.7) |
|
Anagen hair density (no./cm²) |
146.3±1.7 |
156.6±1.8*** (+10.3)###
|
161.3±2.2*** (+15.0)###
|
144.7±1.9 |
146.6±2.1<break>(+1.8) |
151.2±2.1** (+6.4) |
| Anagen hair proportion |
81.5%±0.4 |
85.4%±0.6*** (+3.9%)#
|
86.4%±0.9*** (+4.9%)#
|
82.0%±0.4 |
83.3%±0.8 (+1.3%) |
84.0%±0.9 (+2.0%) |
|
Telogen hair density (no./cm²) |
33.0±0.6 |
26.8±1.2***<break>(-6.3) |
25.4±1.6***<break>(-7.6)#
|
31.6±0.6 |
29.3±1.4 <break>(-2.2) |
28.9±1.6 <break>(-2.7) |
| Telogen hair proportion |
18.5%±0.4 |
14.2%±0.6*** <break>(-4.2%)#
|
13.6%±0.9*** <break>(-4.9%)#
|
18.0%±0.4 |
16.7%±0.8 <break>(-1.3%) |
16.0%±0.9 <break>(-2.0%) |
| Anagen/Telogen ration |
4.5±0.1 |
6.3±0.3*** <break>(+1.8)#
|
7.2±0.5***<break>(+2.7)#
|
4.6±0.1 |
5.5±0.4 <break>(+0.8) |
6.0±0.5** <break>(+1.3) |
|
HPF (no.) |
|
-3631.1±688.2 |
-4428.3±981.0#
|
|
-1293.4±852.5 |
-1562.4±915.0 |
|
THA (no.) |
|
+6002.1±752.3###
|
+8720.3±890.0###
|
|
+1067.0±818.8 |
+3730.8±979.8 |
Figure 3.
Anagen and telogen hair density at each time point (D0, D42, D84) in HI (pink bar) and PL (grey bar) groups. Data are expressed as mean ± SEM. Asterisks indicate statistically significant intra-group differences vs. baseline (D0): ** p < 0.01, *** p < 0.001. Hashes indicate statistical significance between groups (HI vs. PL) at (D42) or (D84): # p < 0.05, ### p < 0.001.
Figure 3.
Anagen and telogen hair density at each time point (D0, D42, D84) in HI (pink bar) and PL (grey bar) groups. Data are expressed as mean ± SEM. Asterisks indicate statistically significant intra-group differences vs. baseline (D0): ** p < 0.01, *** p < 0.001. Hashes indicate statistical significance between groups (HI vs. PL) at (D42) or (D84): # p < 0.05, ### p < 0.001.
3.4. Hair Shedding Assessed by Pull Test
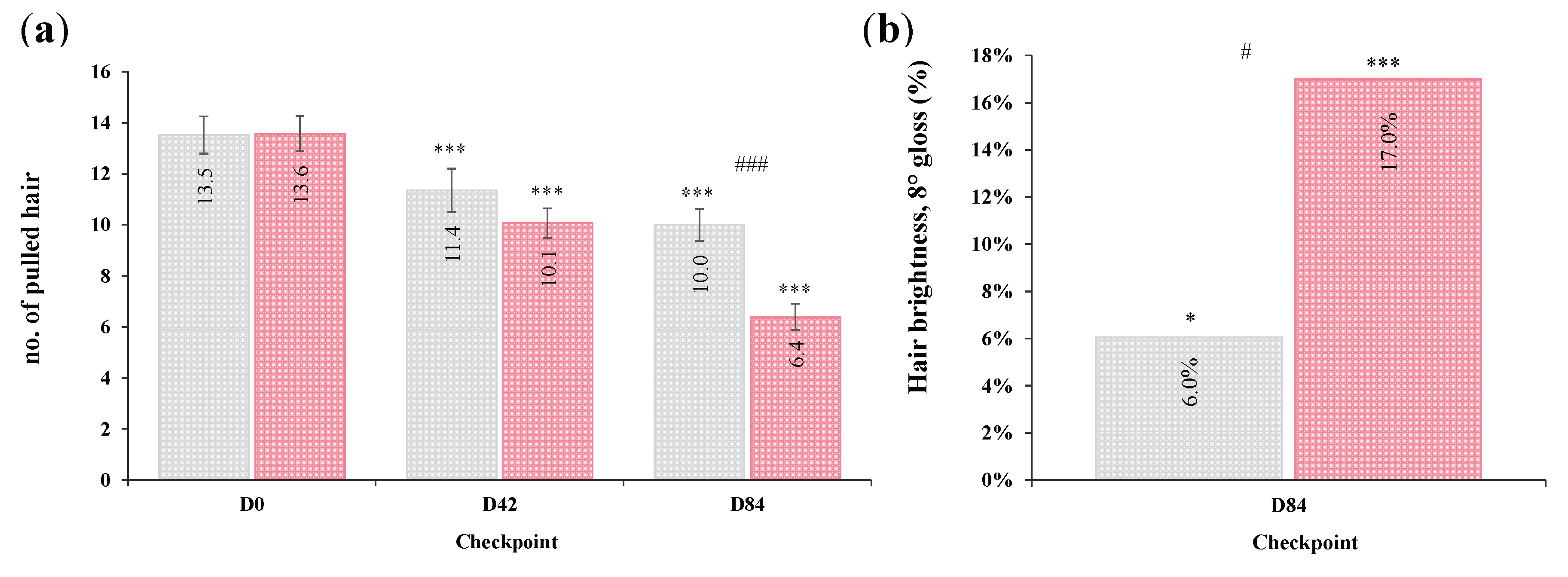
Both groups showed a statistically significant reduction in the number of extracted hair over time (
p < 0.001), with HI group exhibiting a decrease of −24.6% at D42 and −51.3% at D84 and PL group −16.0% and −24.0%, respectively. However, inter-group analysis highlighted a significantly greater reduction of pulled hair in the HI group compared to the PL at D84 (
p < 0.001) (
Figure 4a).
3.5. Hair Brightness
After 84 days of treatment, gloss parameter increased from 3.23 ± 0.2 to 3.70 ± 0.2 in the HI group, corresponding to a +17.0% improvement vs baseline (
p < 0.001). In the PL group, gloss parameter increased from 3.89 ± 0.2 to 4.10 ± 0.2, with a +6.0% (
p < 0.030). Although both groups showed improvements, the inter-group comparison revealed a statistically significant difference between groups variations (
p = 0.022), highlighting a more pronounced enhancement in hair shine following active supplementation (
Figure 4b).
3.6. Hair Volume
Expert scoring evaluation resulted in an improvement of hair volume in 48.5% (+0.5 ± 0.1) of participants treated with HI after 42 days, rising to 69.7% (0.8 ± 0.1) at the end of the study. In contrast, the PL group showed improvement in 38.5% (+0.4 ± 0.1) of subjects and 48.4% (0.6 ± 0.1), respectively. The distribution of scores at both timepoints indicated that most improvements in the active group were progressive over time. Although no statistically significant differences were detected between groups (
p = 0.264 at D42;
p = 0.125 at D84), only the active group consistently exceeded the 50% responder rate, supporting the clinical benefit of the supplement (
Figure 5).
4. Discussion
Hair loss is a common condition influenced by a wide range of physiological, emotional, and environmental factors. Acute telogen effluvium (aTE) is one of the most common diffuse hair loss often triggered by stressors or systemic imbalances that prematurely shift hair follicles from the growth (anagen) to the resting (telogen) phase [
1]. Despite its reversible course, aTE often leads to considerable psychophysical distress, including reduced self-esteem and general discomfort. This emotional burden may, in some cases, further aggravate the condition, prolonging the shedding phase and compromising quality of life [
30]. Therefore, timely interventions that support hair regrowth and help restore hair cycle dynamics are of real clinical and psychosocial value.
The use of nutritional strategies, including food supplement, is increasingly widespread in clinical practice, especially for their favorable safety profile and good tolerability compared to pharmacological approaches [
31]. Targeted-developed formulations to support hair health often contain micronutrients (e.g., biotin, selenium, iron), amino acids (e.g., taurine, methionine, cysteine), and hydrolyzed collagen, which are believed to play a key role in supporting hair follicle metabolism and keratin synthesis [
22]. Among these, the present investigational product (Hair’Inside™) combines green tea extract, bamboo extract and selenium targeting key pathways implicated in acute telogen effluvium. These components may act synergistically to stimulate dermal papilla cell activity, promote collagen synthesis, and reinforce antioxidant defenses, supporting their relevance in hair loss management [
15,
20,
22].
This study aimed to evaluate the efficacy of HI through preclinical in vitro investigation and a randomized, double-blind, PL-controlled clinical trial. The combination of these two approaches offers a comprehensive understanding of the product's effects on the biological and clinical parameters associated with hair growth and health.
In vitro, HI significantly increased the proliferation of human follicle dermal papilla cells (HFDPCs), with a +23.4% increase in cell density compared to the untreated control (p < 0.001). These results confirm the stimulatory effect of the supplement on cells to the initiation and maintenance of the anagen phase, supporting its proposed mechanism of action. HFDPCs play a critical role in signaling keratinocytes and orchestrating follicle cycling. Active compounds such as epigallocatechin gallate (EGCG), present in the formulation, are known to promote HFDPC proliferation and delay catagen onset [
16]. In literature, EGCG is suggested to stimulate human hair growth cycle through the regulation of sonic hedgehog (Shh) and protein kinase B (AKT) signaling pathways [
15]. It has also been shown to increase the protein levels of PTCH, Smo, and Gli1 in hair follicles [
17]. This supports the consistency of our findings with previous evidence regarding the effects of EGCG. This biological effect translated into measurable and clinically relevant outcomes in the present
in vivo study, where HI significantly improved several key parameters of hair growth and scalp health in women with aTE. After 84 days of supplementation, the density of anagen hair markedly increased, accompanied by a significant reduction in telogen hair, and a notable improvement in the anagen/telogen ratio compared to baseline values. Noticeably, these changes were already evident after 42 days of supplementation, indicating a relatively early onset of action.
While some modest improvements were also observed in the PL group, particularly in anagen-related variables, these were within the expected spontaneous recovery of aTE [
25]. However, the more substantial and statistically significant (
p-value <0.05) changes in the active group in all the above-mentioned parameters and the increase in HPF (hair protected against falling out), suggest a biologically driven acceleration of the hair cycle restoration overall after 84 days of supplementation.
Additional clinical benefits were observed in terms of hair shedding. The hair pull test showed a 51.3% reduction in hair loss at D84 in the active group, significantly superior to the 24.6% reduction observed in the PL group (p < 0.000), leading to the resolution of aTE in the active group at the end of the study. Improvements in hair brightness (+17%) and hair volume (69.7%), assessed instrumentally and by expert scoring, further confirmed the supplement’s action not only on follicular cycling but also on the health and quality of the hair.
From a safety perspective, the supplement was well tolerated, with no serious adverse events reported and a high level of compliance. Tolerability assessments, including self-reporting and clinical monitoring, did not reveal any significant reactions attributable to the product, aligning with previous evidence on the safety of its components.
5. Conclusions
This study demonstrates that Hair’Inside™, a nutraceutical formulation containing green tea extract, bamboo extract, and selenium, promotes hair regrowth and improves key clinical parameters associated with acute telogen effluvium (aTE) in women. The alignment between preclinical and clinical outcomes reinforces the validity of the observed effects. The increase in HFDPC proliferation in vitro mirrors the increased anagen density and reduced telogen burden in vivo, followed by an overall hair quality improvement in terms of hair brightness and volume. This study highlights HI as a biologically grounded, clinically effective, and well-tolerated strategy for managing aTE
Author Contributions
Conceptualization, V.N., C.P., L.P. and C.M.O.; methodology, V.N.; formal analysis, J.L.; investigation, G.R.; resources, V.N. and J.L.; data curation, J.L.; writing—original draft preparation, J.L.; writing—review and editing, J.L. and V.N.; visualization, J.L, C.P.; supervision, V.N.; project administration, V.N.; funding acquisition, V.N.
Funding
This research was funded by Activ’Inside (Beychac-et-Caillau, France). The APC was funded by Activ’Inside (Beychac-et-Caillau, France)”.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Ethics Committee “Comitato Etico Indipendente per le Indagini Cliniche Non Farmacologiche” (ref. no. 2024/07 by 16 July 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patient(s) to publish this paper.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available, since they are the property of the sponsor of the study (Activ’Inside, Beychac-et-Caillau, France).
Acknowledgments
Authors would like to express their gratitude to the Complife Italia staff, who contributed to the study and recruited the subjects, for their professionalism and support during study development.
Conflicts of Interest
This study was funded by Activ’Inside (Beychac & Caillau, Bordeaux, France). C.P., L.P., and C.M.O. are full-time employees of Activ’Inside (Beychac & Caillau, Bordeaux, France). The other authors declare no conflicts of interest. The funders had no role in the design of this study, in the collection, analysis, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
References
- Asghar, F.; Shamim, N.; Farooque, U.; Sheikh, H.; Aqeel, R. Telogen Effluvium: A Review of the Literature. Cureus 2020, 12, e8320. [CrossRef]
- Lin, X.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [CrossRef]
- Trüeb, R.M. The Impact of Oxidative Stress on Hair. Int. J. Cosmet. Sci. 2015, 37 Suppl 2, 25–30. [CrossRef]
- Harrison, S.; Bergfeld, W. Diffuse Hair Loss: Its Triggers and Management. Cleve. Clin. J. Med. 2009, 76, 361–367. [CrossRef]
- Hughes, E.C.; Syed, H.A.; Saleh, D. Telogen Effluvium. In StatPearls; StatPearls Publishing: Treasure Island (FL), 2025.
- Harrison, S.; Sinclair, R. Telogen Effluvium. Clin. Exp. Dermatol. 2002, 27, 389–385. [CrossRef]
- Jeon, J.J.; Kim, Y.H.; Kang, H.; Ha, M.C.; Jung, S.-W.; Son, H.; Kim, M.H.; Lee, W.-S.; Lee, S. Global Epidemiology of Telogen Effluvium after the COVID-19 Pandemic: A Systematic Review and Modeling Study. JAAD Int. 2025, 18, 24–26. [CrossRef]
- van der Donk, J.; Passchier, J.; Knegt-Junk, C.; van der Wegen-Keijser, M.H.; Nieboer, C.; Stolz, E.; Verhage, F. Psychological Characteristics of Women with Androgenetic Alopecia: A Controlled Study. Br. J. Dermatol. 1991, 125, 248–252. [CrossRef]
- Hunt, N.; McHale, S. The Psychological Impact of Alopecia. BMJ 2005, 331, 951–953. [CrossRef]
- Gupta, A.K.; Talukder, M.; Williams, G. Comparison of Oral Minoxidil, Finasteride, and Dutasteride for Treating Androgenetic Alopecia. J. Dermatol. Treat. 2022, 33, 2946–2962. [CrossRef]
- Ham, S.; Lee, Y.I.; Kim, I.A.; Suk, J.; Jung, I.; Jeong, J.-M.; Lee, J.H. Efficacy and Safety of Persimmon Leaf Formulated with Green Tea and Sophora Fruit Extracts (BLH308) on Hair Growth: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Skin Res. Technol. Off. J. Int. Soc. Bioeng. Skin ISBS Int. Soc. Digit. Imaging Skin ISDIS Int. Soc. Skin Imaging ISSI 2023, 29, e13448. [CrossRef]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Mol. Basel Switz. 2024, 29, 2288. [CrossRef]
- Du, F.; Li, J.; Zhang, S.; Zeng, X.; Nie, J.; Li, Z. Oxidative Stress in Hair Follicle Development and Hair Growth: Signalling Pathways, Intervening Mechanisms and Potential of Natural Antioxidants. J. Cell. Mol. Med. 2024, 28, e18486. [CrossRef]
- Chakrawarti, L.; Agrawal, R.; Dang, S.; Gupta, S.; Gabrani, R. Therapeutic Effects of EGCG: A Patent Review. Expert Opin. Ther. Pat. 2016, 26, 907–916. [CrossRef]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Human Hair Growth Enhancement in vitro by Green Tea Epigallocatechin-3-Gallate (EGCG). Phytomedicine Int. J. Phytother. Phytopharm. 2007, 14, 551–555. [CrossRef]
- Yu, Y.; Zhao, B.; Li, J.; Yang, J.; Bao, Z.; Cai, J.; Chen, Y.; Wu, X. (-)-Epigallocatechin-3-Gallate Promotes the Dermal Papilla Cell Proliferation and Migration through the Induction of VEGFA. Biochim. Biophys. Acta Mol. Cell Res. 2025, 1872, 119902. [CrossRef]
- Wickett, R.R.; Kossmann, E.; Barel, A.; Demeester, N.; Clarys, P.; Vanden Berghe, D.; Calomme, M. Effect of Oral Intake of Choline-Stabilized Orthosilicic Acid on Hair Tensile Strength and Morphology in Women with Fine Hair. Arch. Dermatol. Res. 2007, 299, 499–505. [CrossRef]
- Alañón ME, Castle SM, Serra G, Lévèques A, Poquet L, Actis-Goretta L, Spencer JPE. Acute study of dose-dependent effects of (-)-epicatechin on vascular function in healthy male volunteers: A randomized controlled trial. Clin Nutr. 2020 Mar;39(3):746-754. Epub 2019 Apr 9. PMID: 31014775. [CrossRef]
- Adelman, M.J.; Bedford, L.M.; Potts, G.A. Clinical Efficacy of Popular Oral Hair Growth Supplement Ingredients. Int. J. Dermatol. 2021, 60, 1199–1210. [CrossRef]
- Barel, A.; Calomme, M.; Timchenko, A.; De Paepe, K.; Demeester, N.; Rogiers, V.; Clarys, P.; Vanden Berghe, D. Effect of Oral Intake of Choline-Stabilized Orthosilicic Acid on Skin, Nails and Hair in Women with Photodamaged Skin. Arch. Dermatol. Res. 2005, 297, 147–153. [CrossRef]
- Whanger, P.; Vendeland, S.; Park, Y.C.; Xia, Y. Metabolism of Subtoxic Levels of Selenium in Animals and Humans. Ann. Clin. Lab. Sci. 1996, 26, 99–113.
- Milani, M.; Colombo, F. Efficacy and Tolerability of an Oral Supplement Containing Amino Acids, Iron, Selenium, and Marine Hydrolyzed Collagen in Subjects with Hair Loss (Androgenetic Alopecia, AGA or FAGA or Telogen Effluvium). A Prospective, Randomized, 3-month, Controlled, Assessor-blinded Study. Skin Res. Technol. 2023, 29, e13381. [CrossRef]
- Arias, E.M.; Floriach, N.; Moreno-Arias, G.; Camps, A.; Arias, S.; Trüeb, R.M. Targeted Nutritional Supplementation for Telogen Effluvium: Multicenter Study on Efficacy of a Hydrolyzed Collagen, Vitamin-, and Mineral-Based Induction and Maintenance Treatment. Int. J. Trichology 2022, 14, 49–54. [CrossRef]
- Gassmueller, J.; Rowold, E.; Frase, T.; Hughes-Formella, B. Validation of TrichoScan Technology as a Fully-Automated Tool for Evaluation of Hair Growth Parameters. Eur. J. Dermatol. EJD 2009, 19, 224–231. [CrossRef]
- Nobile, V.; Dudonné, S.; Kern, C.; Cestone, E.; Garcia, C. Recovery Effects of Oral Supplementation with Polar Lipid-Rich Wheat Extracts on Acute Telogen Effluvium: A Randomized Double-Blind Placebo-Controlled Study. Clin. Cosmet. Investig. Dermatol. 2025, 18, 1239–1251. [CrossRef]
- Hoffmann, R. TrichoScan: A Novel Tool for the Analysis of Hair Growth in Vivo. J. Investig. Dermatol. Symp. Proc. 2003, 8, 109–115. [CrossRef]
- McDonald, K.A.; Shelley, A.J.; Colantonio, S.; Beecker, J. Hair Pull Test: Evidence-Based Update and Revision of Guidelines. J. Am. Acad. Dermatol. 2017, 76, 472–477. [CrossRef]
- Jiang, Y.; Xu, Z.; Qiu, Y.; Zheng, X. Comparative Study of Instrumental Measurement and Sensory Evaluation Methods for the Repairing Effect of Mildly Damaged Hair Bundles. Skin Res. Technol. 2023, 29, e13394. [CrossRef]
- Zanzottera, F.; Michelotti, A.; Nobile, V. Efficacy of a Nutritional Supplement, Standardized in Fatty Acids and Phytosterols, on Hair Loss and Hair Health in Both Women and Men. 2017.
- Hadshiew, I.M.; Foitzik, K.; Arck, P.C.; Paus, R. Burden of Hair Loss: Stress and the Underestimated Psychosocial Impact of Telogen Effluvium and Androgenetic Alopecia. J. Invest. Dermatol. 2004, 123, 455–457. [CrossRef]
- García-Navarro, A.; Vasallo-Morillas, M.I.; Navarro-Belmonte, R.; Vilanova, C.; Torrent, D.; Kilasoniya, A.; Moles-Ugeda, I.; Gallego-Herrera, E.; Ramírez-Boscá, A. Randomized Clinical Trial to Evaluate the Effect of Probiotic Intake on Androgenic Alopecia. Nutrients 2024, 16, 2900. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).