2. From the Angle of Structures
The influence of neurotransmitters and neuromodulators can be discussed from two viewpoints. What substances occur in any particular structure, and in which structures occurs any partcular substance? This main section takes the first viewpoint.
2.1. The Soup
“Nociceptors: the sensors of the pain pathway” (Dubin and Patapoutian 2010). It sounds deceivingly simple: Everything starts with nociceptors and leads up to pain? Indeed, nociception and acute pain start with the activation of nociceptors initiated by an injury to body tissue. However,
right at this peripheral level, the complexity, too, starts with the sensitization or other modulation, as best illustrated by an inflammation (
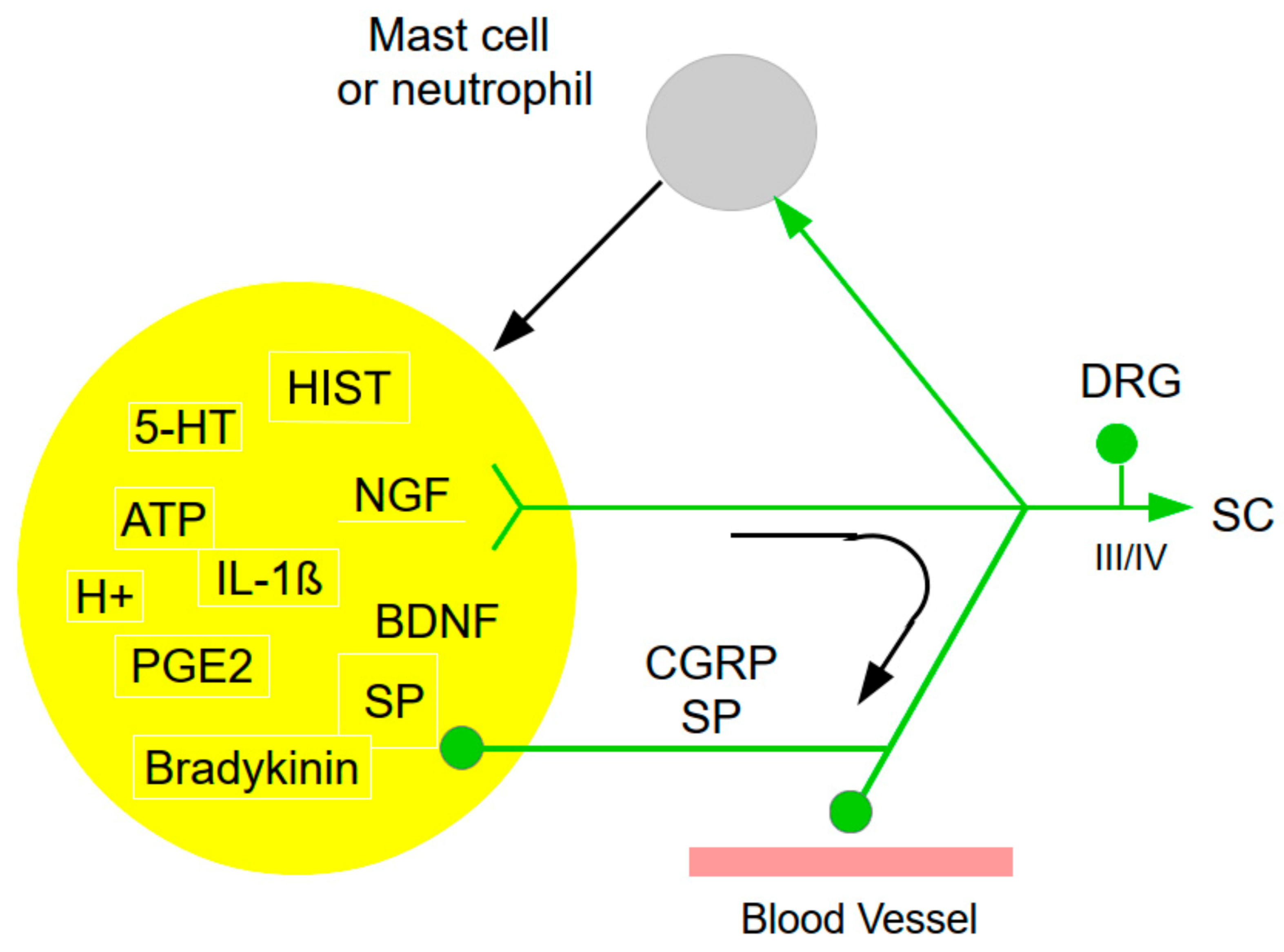
Figure 1). Inflammation induces a complex, self-reinforcing sequence of events. As to nociceptor activation and sensitization, there is a close reciprocal cross-talk between the immune system, in particular mast cells, but also other cell types, with nociceptors. In response to injury, nociceptors release various
inflammatory and vaso-active neuropeptides from their terminals (e.g., substance P, SP) that potently activate and recruit immune cells (e.g., mast cells). Iinfiltrated immune cells in turn release plenty of mediators that further promote sensitization of nociceptors by producing cytokines, chemokines, lipid mediators and growth factors,
thus promoting a vicious cycle of mast cell and nociceptor activation, thus promoting neurogenic inflammation and pain/pruritus (Gupta and Harvima 2018; Liu et al. 2021). This ensemle of agents has been dubbed ‘inflammatory soup’ containing a
“A plethora of painful molecules” (Lewin et al. 2004), and their interactions form a complex network.
More specifically, activation of resident mast cells leads to the release of pro-inflammatory chemokines, cytokines, histamine (HIST), proteases, growth factors, lipid mediators, neuropeptides, and reactive oxygen and reactive nitrogen species (ROS/RNS). Inflammatory agents so far identified also include protons (H+), prostaglandins, SP, bradykinin (BK), serotonin (5-HT), IL-1, and other endogenous chemicals (Costigan et al. 2009; Luo et al. 2015; Nicol and Vasko 2007; Pezet and McMahon 2006). Some agents induce local degenerative processes, sensitize nociceptors, recruit silent nociceptors, and lead to expression of new receptors and ion channels (Finnerup et al. 2021). Many inflammatory chemicals that excite nociceptors activate intracellular signal transduction pathways and modulate sensory receptor channels and voltage-gated ion channels. Moreover, heat can render cutaneous group III (Aδ) mechanical nociceptors sensitive to heat. Pro-inflammatory influences also spread from the peripheral injury site to the dorsal roots and spinal cord (Moalem and Tracey 2006).
2.2. Sensory Afferents
2.2.1. Nociceptors
Nociceptive primary afferents, despite their morphologically simple appearance, are quite complex. A large assembly of neuromodulators influences nociceptor sensitivity. These agents include corticotropin-releasing hormone (CRH), vasopressin (AVP), oxytocin (OXT), dopamine (DA), noradrenaline (NA), 5-HT, bradykinin (BK), prostaglandin E2 (PGE2), HIST, endocannabinoids, opioids, nerve growth factor (NGF), protons (H+), potassium ions (K+), and others. They contain numerous ligands, e.g., SP, growth factors, hormones such as somatostatin (STT), and neurotransmitters, e.g., glutamate, adenosine, adenosine-trisphosphate (ATP). They express dozens of receptors, along with voltage-gated and ligand-gated ion channels that contribute to the detection of mechanical, chemical, thermal and/or microbial stimuli, resulting in action potential generation, regulation of discharge patterns, and release of ligand/neurotransmitters that mediate complex interactions between nociceptors (Bourinet et al. 2014; Carlton 2014; Devesa and Ferrer-Montiel 2014; Dubin and Patapoutian 2010; Levine et al. 1993; Sexton et al. 2014; Woolf and Ma 2007). In the rat, various neuropeptides may be co-expressed in the dorsal root ganglion (DRG) in varying proportions. For example, GAL-immuno-reactive (GAL-IR) cells are present in the lumbar DRG, and may contain CGRP-, SP- and STT-immuno-reactivity (Yoon et al. 2003).
Nociceptors can be directly or indirectly sensitized, meaning that repeated noxious stimulation or tissue damage elicits prolonged increases in nociceptor afferent excitability, increases in spontaneous activity, decreased threshold for activation, increased and proloned discharge in response to a supra-threhold stimulus. This sensitization contributes to hyperalgesia. Endogenous peptides released at the site of injury or inflammation can sensitize nociceptors directly, including interleukin-1 (IL-1) or NGF; non-peptide substances include PGE2, prostaglandin I2 (PGI2), leukotriene B4 (LTB4), adenosine, and 5-HT (Levine et al. 1993).
SP is associated with multiple processes: hematopoiesis, wound healing, micro-vasculature permeability, leukocyte trafficking, cell survival and neurogenic inflammation, but possibly also with tumorigenesis and metastasis. Its receptor neurokinin-1 receptor (NK1R) is found in the nervous system and in peripheral tissues and is involved in cellular responses such as pain transmission, endocrine and paracrine secretion, vasodilation, and modulation of cell proliferation (Garcia-Recio and Gascón 2015). SP plays a crucial role in pain modulation. Elevated SP concentrations are linked to heightened pain sensitivity (Humes et al. 2024). SP can modulate a variety of ion channels resulting in an increase or decrease of neuronal excitability. SP can enhance the N-methyl-D-aspartate (NMDA) channel function leading to greater pain sensitivity. Conversely, during inflammatory processes, inflammatory cells and peripheral nerve terminals release SP, which, in turn, modulates a variety of ion channels rendering sensitization of sensory neurons in an autocrine or paracrine manner. In the peripheral nervous system (PNS), SP mainly exists in the small sensory nociceptors. Release of SP can act on NK1R via differential intracellular mechanisms to potentiate the channel activities of vanilloid transient receptor potential channel vanilloid 1 (TRPV1), Nav1.8, and l- and N-type Ca2+ channels in a subset of small-diameter DRG neurons, thereby resulting in hyperalgesia. SP could also decease the activity of low-threshold K+ channel (kv4) in capsaicin-sensitive DRG neurons and thus sensitize the nociceptors (Chang et al. 2019). Tachykinins, co-localized with CGRP in sensory afferents, are involved in viscero-sensitive responses. The role of tachykinins and CGRP was investigated in both nociceptive and viscero-motor responses to inflammation. In inflammation, neurokinin receptors (NK1R and NK2R) mediate the gastric emptying inhibition and visceral pain, respectively. These responses involve a release of CGRP (Julia and Buéno 1997; Levine et al. 1993).
STT [somatotropin release-inhibiting factor (SRIF)] is widely distributed in the body and exerts a variety of hormonal and neural actions. SRIF is important in nociceptive processing because it is localized in a subset of small-diameter DRG cells, activation of SRIF receptors results in inhibition of both nociceptive behaviors in animals and acute and chronic pain in humans, SRIF inhibits dorsal horn (DH) neuronal activity, and SRIF reduces responses of joint mechano-receptors to noxious rotation of the knee joint. Cutaneous nociceptors are under the tonic inhibitory control of SRIF. In a dose-dependent manner, intra-plantar injection of the SRIF receptor antagonist cyclo-somatostatin (c-SOM) resulted in nociceptive behaviors in normal animals and enhancement of nociceptive behaviors in formalin-injected animals. Intra-plantar injection of SRIF antiserum resulted in nociceptive behaviors. Electrophysiological recordings using an in-vitro glabrous skin-nerve preparation showed increased nociceptor activity in response to c-SOM. Parallel behavioral and electrophysiological studies using the opioid antagonist naloxone showed that endogenous opioids do not maintain a tonic inhibitory control over peripheral nociceptors, nor does opioid receptor antagonism influence peripheral SRIF effects on nociceptors. Hence, SRIF receptors maintain a tonic inhibitory control over peripheral nociceptors, and this may contribute to mechanisms that control the excitability of these terminals (Carlton et al. 2001).
HIST can modulate nociceptor sesnsitivity by several mechanisms. In the skin, H3Rs occur on certain group II (Aβ) fibers, and on keratinocytes and Merkel cells, as well as on deep dermal, peptidergic group III (Aδ) fibers terminating on deep dermal blood vessels. Activation of H3Rs on the latter in the skin, heart, lung, and dura mater reduces SP and CGRP release, leading to anti-inflammatory (but not anti-nociceptive) actions. By contrast, activation of H3Rs on the spinal terminals of these sensory fibers reduces nociceptive responses to low-intensity mechanical stimuli and inflammatory stimuli such as formalin (Hough and Rice 2011).
It has been argued that cannabinoid modulation of pain occurs through inhibition at the level of the DRG, thus inhibiting ascending nociceptive signals. Another hypothesis propounds that modulation occurs through activity at the level of the brainstem, inhibiting pain signals through descending suppression of nociceptive signals. However, these effects need not be exclusive. Cannabinoid receptor1 (CB1R) modulates nociceptive processing at the level of the peripheral nervous system, specifically in nociceptors in the DRG. CB1Rs are typically expressed on the terminals of sensory afferent fibers and are found on a large majority of nociceptive neurons in the DRG. These neurons send CB1R out to the peripheral nerve terminals in response to noxious stimuli, further suggesting a mediating role of endogenous cannabinoids and their receptors on nociception. Intra-thecal- injection of anandamide, an endogenous cannabinoid ligand, inhibited group III (Aδ) and group IV (C) fiber neuronal responses to inflammatory pain. Anandamide blocked acute pain (Milligan et al. 2019).
Opioid binding sites are sythesized in the DRG and then transported into the periphery and central terminals of sensory neurons. Local injection of opioids into inflamed tissue reduces the activity of primary afferents, antagonized by naloxone. μ-Receptors appear to exert the most potent analgesia (Levine et al. 1993).
NGF receptor activation and downstream signaling alter nociception through direct sensitization of nociceptors at the site of injury and changes in gene expression in the DGR that collectively increase nociceptive signaling from the periphery to the CNS. NGF is active in both peripheral and central sensitization and has complex multi-functional roles in the modulation of nociceptive processing through effects on the release of inflammatory mediators, nociceptive ion channel/receptor activity, nociceptive gene expression, and local neuronal sprouting effects (Barker et al. 2020; Finnerup et al. 2021; Mizumura and Murase 2015; Nicol and Vasko 2007; Pezet and McMahon 2006), and regulates the expression of brain-derived neurotrophic factor (BDNF).
During inflammation, injury or certain diseases, inflammatory, immune and Schwann cells release NGF that binds to tropomyosin-related receptor kinase A (TrkA), which in turn directly and rapidly activates and/or sensitizes nociceptors. NGF and its receptor TrkA are retrogradely transported to the DRG, resulting in increased synthesis of neuropeptides (e.g.,: SP, BDNF), receptors, ion channels, and anterograde transport of certain neurotransmitters, receptors and ion channels from the DRG to the periphery tissue and spinal cord. During inflammatory injury, NGF is released from mast cells, but also from other recruited cells. Binding of NGF to TrkA on mast cells causes release of inflammatory mediators, such as HIST, 5-HT, and protons (H+) as well as NGF. Binding of NGF to TrkA on the peptidergic fiber terminal activates intracellular signaling pathways, which results in either increased expression or modulation at the membrane surface of a number of receptors, including, bradykinin receptors (B2R), ion channels, including TRPV1, acid-sensing ion channels (ASIC) 2/3, voltage-gated Na+ (Nav) or Ca2+ (Cav) ion channels, delayed rectifier K+ currents and putative mechano-transducers (Mantyh et al. 2011).
BDNF is synthesized by and released from central terminals of nociceptive afferents and increases the excitability of DH neurons. It is markedly up-regulated in inflammatory conditions in an NGF-dependent fashion, and may play a role as a sensitizing modulator in inflammatory pain states by acting on postsynaptic tropomyosin-related receptor kinase B (TrkB) receptors (Merighi et al. 2008; Pezet and McMahon 2006). Application of BDNF to the adult rat isolated DH with dorsal root attached preparation inhibited the electrically evoked release of SP from sensory neurons. This effect was dose-dependent and reversed by the tyrosine kinase inhibitor, K-252a. BDNF-induced inhibition of SP release was blocked by the γ-amino-butyric acid B (GABAB) receptor (GABABR) antagonist CGP 55485 but not by naloxone. Acute application of BDNF significantly increased K+-stimulated release of GABA in the DH isolated in vitro and this effect was blocked by K-252a. Intra-thecal injection of BDNF into the rat lumbar spinal cord induced a short-lasting increase in hindpaw threshold to noxious thermal stimulation that was blocked by CGP 55485. This suggests that exogenous BDNF can indirectly modulate primary sensory neuron synaptic efficacy via facilitation of the release of GABA from DH interneurons (Pezet et al. 2002). BDNF acts specifically as a central modulator, via binding to post-synaptic TrkB receptors, whereupon the BDNF-TrkB complex switches on intracellular protein kinases leading to phosphorylation of NMDARs and facilitated opening. This increases the probability of central sensitization and facilitated transmission through the DH synapse and via third-order neurons to the sensory cortex in the brain (Mantyh et al. 2011).
In the rat DRG, there is a high level of expression of M2 mRNA, and much lower levels of M3 and M4 mRNA were also detected. All three of these sub-types are preferentially localized in medium- and small-sized DRG neurons. These findings suggest the possible involvement of the M2, M3, and M4 sub-types in the modulation of nociceptive transduction (Pan et al. 2007).
2.2.2. Proprioceptors
Acidosis in inflamed tissues is a major risk factor in the development of chronic musculo-skeletal pain. Not surprisingly, nociceptors express pro-nociceptive proton (H+)-sensing ion channels ASICs, transient receptor potential (TRP) channels, and two-pore K+ (K2P) channels), which are involved in pain associated with tissue acidosis. Strangely, ASICs are also expressed in non-nociceptors such as proprioceptors. Although non-nociceptive cutaneous afferents could contribute to pain hypersensitivity in chronic pain states, whether non-nociceptive muscle afferents are also involved in pain hypersensitivity of deep tissues is unclear (Lee and Chen 2023). In mice, genetic deletion of ASIC3 in proprioceptors, but not in nociceptors, abolished acid-induced chronic hyperalgesia. Chemo-optogenetically activating proprioceptors resulted in hyperalgesic priming that favored chronic pain induced by acidosis. In humans, intra-muscular acidification induced acid perception but not pain (Lee et al. 2025). Functional interactions between nociceptors and proprioceptors also occur in the spinal cord (below). Equivalent data as for muscle spindle afferents don’t appear to exist for Golgi tendon organ (GTO) afferents.
2.3. Spinal Presynapic Inhibition (PSI)
“In search of lost presynaptic inhibition”
Pablo Rudomin (2009)
Before even reaching DH neurons, the effects of sensory afferents are modulated by PSI, which acts by decreasing synaptic transmission from presynaptic terminals of sensory afferents to spinal neurons (Comitato and Bardoni 2021; Guo and HU 2014; Hochman et al. 2010; Lu et al. 2018;
Quevedo 2009; Rudomin 2009; Rudomin and Schmidt 1999; Zimmerman et al.
2019; Figure 2). Stimulation of primary sensory afferents generates depolarization of sensory nerve terminals (primary afferent depolarization: PAD), which is electrotonically conducted into the dorsal root and can here be measured as dorsal-root potential (Hochman et al. 2010; Quevedo 2009).
DH inhibitory interneurons are activated by primary sensory nerve fibers and by fiber tracts descending from supraspinal areas. Group II (Aβ), group III (Aδ) and group IV (C) fibers contact dendrites of DH inhibitory neurons. Glycinergic or mixed GABAergic/glycinergic neurons are preferentially targeted by thickly myelinated low-threshold fibers, whereas purely GABAergic neurons are preferentially contacted by thinly myelinated and un-myelinated fibers. This differential innervation is also reflected in the somewhat different distribution of GABAergic and glycinergic cells with glycinergic neurons being concentrated more in the deeper DH layers. GABAergic and glycinergic inhibitory postsynaptic currents (IPSCs) could be evoked by innocuous mechanical stimulation, and the majority of GABAergic superficial DH neurons received mono- and polysynaptic excitatory input from group IV and group III afferent nerve fibers. The presence of group IV fiber input in GABAergic neurons does not necessarily imply that these neurons are excited by noxious stimuli. Rather, the group IV fibers that excite islet cells are different from typical nociceptive group IV fibers. These cells might correspond to a particular sub-class of group IV fibers with a low activation threshold, which suggest that these fibers convey pleasant touch sensations (Zeilhofer et al. 2012).
Some gross functional input-output patterns in the cat hindlimb are as follows. Group Ia afferents from primary muscle spindle endings of both flexor and extensor muscles are inhibited presynaptically by group Ia and group Ib afferents from Golgi tendon organs (GTOs) in flexor nerves, while group Ib afferents are inhibited only by group Ib inputs from both flexor and extensor muscles and from joint and large cutaneous afferents but may also be inhibited by these cutaneous afferents. PAD of group II afferents is elicited by activation of group II, cutaneous, joint and pudendal afferents. PAD of low-threshold cutaneous afferents is evoked by activation of low-threshold cutaneous, group Ib, group II afferents and high-threshold muscle afferents. High-threshold cutaneous (groups III/IV) afferents are depolarized by noxious stimulation (Quevedo 2009: Rudomin and Schmidt 1999).
The PSI from and to group III/IV afferents is still not fully explored. First, there could be PSI elicited by MS afferents onto nociceptive afferents. In the cat, a group of superficial DH neurons that responded to noxious pinch of the gastrocnemius muscles (GSs) responded to bradykinin injections into GS with three types of responses: excitatory, inhibitory and mixed. The majority of the neurons with excitatory and mixed responses to bradykinin were also influenced by stretches of the GS applied directly after the bradykinin injection. In these neurons, the GS stretch usually counteracted the bradykinin-induced response, i.e., shortening and reducing bradykinin-induced excitation and re-exciting the cells after bradykinin-induced inhibition. At least the inhibitory effect could hypothetically have been effected by PSI from muscle stretch receptors onto nociceptive afferent terminals (
Figure 2, lower left; Björklund et al. 2004). Conversely, afferent group III/IV input, probably excited by GS muscle-fatigue, enhanced PSI elicited from antagonist muscle nerves (Kalezic et al. 2004). In addition to the classic PSI in which low-threshold cutaneous afferents evoke a GABA
A-receptor-dependent form of PSI that inhibits similar afferent sub-types, another mechanism involves small-diameter afferents, which predominantly evoke an NMDAR-dependent form of PSI that inhibits large-diameter fibers. Behaviorally, loss of either GABA
A receptors (GABA
ARs) or NMDARs in primary afferents leads to tactile hypersensitivity across skin types, and loss of GABA
ARs, but not NMDARs, leads to impaired texture discrimination (Zimmerman et al. 2019). In rodents, non-GABA interneurons have been described that could be involved in pre- and postsynaptic inhibition of small-diameter nociceptive afferents. This population of deep layer DH (dDH) inhibitory interneurons express the receptor tyrosine kinase Ret neonatally. The early RET+ dDH neurons receive excitatory as well as polysynaptic inhibitory inputs from touch- and/or pain-sensing afferents. Conversely, they negatively regulate DH pain and touch pathways through both pre- and postsynaptic inhibition. Specific ablation of early RET+ dDH neurons increases basal and chronic pain, whereas their acute activation reduces basal pain perception and relieves inflammatory and neuropathic pain (Cui et al. 2016).
PSI is also subject to influences from spinally descending pathways, which modulate its strength and probably distribution in a task- and context-dependent way. The synaptic efficacy of spindle group Ia and II, GTO group Ib and cutaneous afferents is differentially altered by signals descending in cortico-spinal, rubro-spinal, reticulo-spinal and vestibulo-spinal tracts, as well as by raphé-spinal 5-HT and LC-spinal NA systems (Quevedo 2009; Rudomin 2009; Rudomin and Schmidt 1999). Moreover, supraspinal sites send Adr, NA and 5-HT fibers to the DH (Figure 4). Both NA and 5-HT have specific effects on defined DH neuron populations. In addition to inhibiting excitatory neurons and terminals, NA and 5-HT fibers excite GABAergic and glycinergic interneurons. In addition to 5-HT and NA fibers, a great number of descending GABAergic and glycinergic fibers innervate the DH. A direct inhibitory innervation (i.e., via monosynaptic connections) of DH neurons from the RVM has been demonstrated using in vivo patch-clamp recordings and was confirmed by morphological evidence. The glycinergic innervation is also evident in reporter mice expressing EGFP in glycinergic neurons. In the spinal cord, descending GABAergic and glycinergic projections mainly target presumed excitatory neurons (Zeilhofer et al. 2012).
Based on dorsal root potential (DRP) latency measurements, it had originally been thought that PAD of low-threshold sensory afferents would be mediated by minimally tri-snaptic pathways with pharmacologically identified GABAergic interneurons forming last-order axo-axonic synapses onto afferent terminals. However, it has been argued that there is still no decisive evidence of this organization. This would leave open the possibility of the existence of PAD generated by more direct pathways with a more complex pharmacology than exclusively GABA and GABAARs.
Morphological, physiological and pharmacological data argue for a participation of GABA in PAD. PSI is exerted either in form of rather simple axo-axonic synapses mainly in the case of group II (Aβ) fiber terminals, or in form of complex synaptic arrangements called synaptic gluomeruli. These glomeruli are located in the superficial DH and comprise interneuron axon terminals and postsynaptic dendrites that surround the central primary afferent fiber terminal. The vast majority of glomeruli contain peripheral axons that originate from GABAergic interneurons while the dendrites postsynaptic to the central axon belong to glutamatergic excitatory neurons (Zeilhofer et al. 2012).
Activation of GABAAR on primary sensory neurons induces PAD rather than hyperpolarization. Under certain conditions, glutamate and K+ also contribute to PAD but the GABAergic (bicuculline-sensitive) component usually dominates. PAD inhibits rather than facilitates transmitter release from the primary afferent terminal. Different explanations have been proposed for this phenomenon. PAD may lead to the inactivation of voltage-gated Ca2+ channels on primary afferent terminals and may thus reduce presynaptic Ca2+ influx and transmitter release. Alternatively, it may interfere with action potential propagation into the terminal through either voltage-dependent inactivation of Na+ channels or through activation of a shunting conductance (Zeilhofer et al. 2012).
In the case of most primary nociceptor terminals, especially for peptidergic nociceptors, morphological evidence for a relevant GABAergic innervation is much weaker. Whether or not PSI by GABAergic interneurons is relevant for nociceptive transmission is controversial. Physiological experiments have demonstrated the presence of dorsal root reflexes in capsaicin-sensitive primary afferent axons and the blockade by intra-thecal bicuculline of peripheral flare responses, which depend on the release of CGRP from the peripheral terminals of peptidergic nociceptors (Zeilhofer et al. 2012).
A classic role in PSI has played GABA in regulating nociceptive signal strength and separating nociception from touch signals. Intra-thecal application of bicuculline and strychnine (antagonists of GABAA and glycine receptors, respectively) increased responses to noxious stimuli. Presynaptic GABA receptors located on sensory afferent terminals are involved in gating both tactile and noxious stimuli in the DH. GABA receptors of the A and B type are expressed on both nociceptive and non-nociceptive sensory afferents, where axo-axonic synapses exist. GABAARs are ligand-gated ion channels, most commonly formed by 2α, 2β, and 1γ sub-units. Group IV fibers express the α2, α3, and α5 sub-units, while α1, α2, α3, and α5 are present on myelinated A fiber terminals. The sub-unit β3 is the dominant β sub-unit expressed in DRG neurons of both A and C type (Comitato and Bardoni 2021).
In addition to GABAA, activation of GABAB receptors (GABABRs), expressed on nociceptive and non-nociceptive primary afferent terminals, also contributes to presynaptic inhibition, exerting analgesic and anti-hyperalgesic effects. The GABAB1 isoforms 1a and 1b, together with the sub-unit GABAB2, occur in small and large DRG neurons and in the spinal cord, both on primary afferent terminals and on DH neurons. Endogenous or exogenous activation of GABABRs in superficial DH causes both pre- and postsynaptic effects. In rats, activation of presynaptic GABABRs inhibited pinch- and touch-evoked synaptic responses in vivo and decreased glutamate and peptide release from group III-IV primary afferents and DH neurons. The inhibitory effect of GABABRs on transmitter release is due to the concurrent inhibition of presynaptic Ca2+ channels and release machinery downstream of Ca2+ entry into the nerve terminals. The block of GABABRs increased the first excitatory postsynaptic current (EPSC) in a train of four stimuli, recorded from lamina III–IV neurons. This suggests that, differently from GABAARs, which require the release of GABA through synaptic activation, GABABRs are tonically activated, confirming the finding of a previous study performed in lamina II (Comitato and Bardoni 2021).
When occurring in nociceptor terminals, PAD and PSI should reduce pain. In fact, part of the anti-hyperalgesic action of intra-thecally injected diazepam occurs through an enhancement of as demonstrated in experiments using the sns-α2-deficient mice. However, in nociceptors, PAD cannot only cause PSI, but may under certain conditions also give rise to so-called dorsal root reflexes. These are action potentials elicited in primary sensory fiber terminals by stimulation of a second afferent fiber via an interconnected GABAergic interneuron. They occur when PAD reaches the threshold of action potentials. These action potentials may then propagate both in an orthodromic (central) and antidromic (centrifugal) direction. The centrally propagating action potential is thought to reinforce pain sensation, while the peripheral action potential, in case of peptidergic nociceptors, contributes to neurogenic inflammation, vasodilatation and plasma extravasation through the release of CGRP and SP (Zeilhofer et al. 2012).
However, the circumstances are a bit more complicated than instigated by the tri-synaptic GABAergic circuit. (i) Primary sensory afferents may co-release substances acting on receptors with GABAA pharmacology. (ii) Apart from GABAARs, primary sensory afferents contain more receptors that can contzribute to terminal depolarization. Activation of high-threshold afferents evokes DRP, by substances including AMPA/kainate and NMDA receptors. Activation of presynaptic α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (AMPARs) depresses exitatory transmission in DH neurons. Capsaicin depresses excitatory transmission elicited by group IV afferents. (iIi) PSI may be not related to PAD. Activation of GABAB receptors inhibits Ca2+ channels without presynaptic depolarization and produces a longer-lasing component of inhibition of monosynaptic EPSPs. Activation of adenosine and cannabinoid receptors inhibits transmiitter release by inhibiting Ca2+ channels. (iv) GABA receptors on presynaptic sensory terminals contain bicuculline/picrotoxin-sensitive glycine, nicotinic ACh receptor (nAChR) or 5-HT receptor sub-units. (v) DH lamina III, projection area of group II and III cutaneous afferents, contains cholinergic (ACh) interneurons that receive inputs from myelinated and un-myelinated cutaneous afferents, which thus could feedback onto the same afferents. (vi) Many sensory afferents co-release ACh, taurine or ß-alanine that might activate GABAARs (Hochmann et al. 2010; Quevedo 2009).
In the absence of a direct innervation by axo-axonic synapses of the majority of primary nociceptors, GABA could still act as a volume transmitter. In this case, GABAARs along the intraspinal segment of the primary afferent axon could be activated by ambient GABA to cause voltage-dependent inactivation of Na+ channels or activation of a shunting conductance. Both would prevent the invasion of the presynaptic terminal by axonal action potentials. Direct experimental proof for either of these possibilities is lacking, in part due to the intrinsic difficulties associated with recording from spinal primary afferent axon terminals (Zeilhofer et al. 2012).
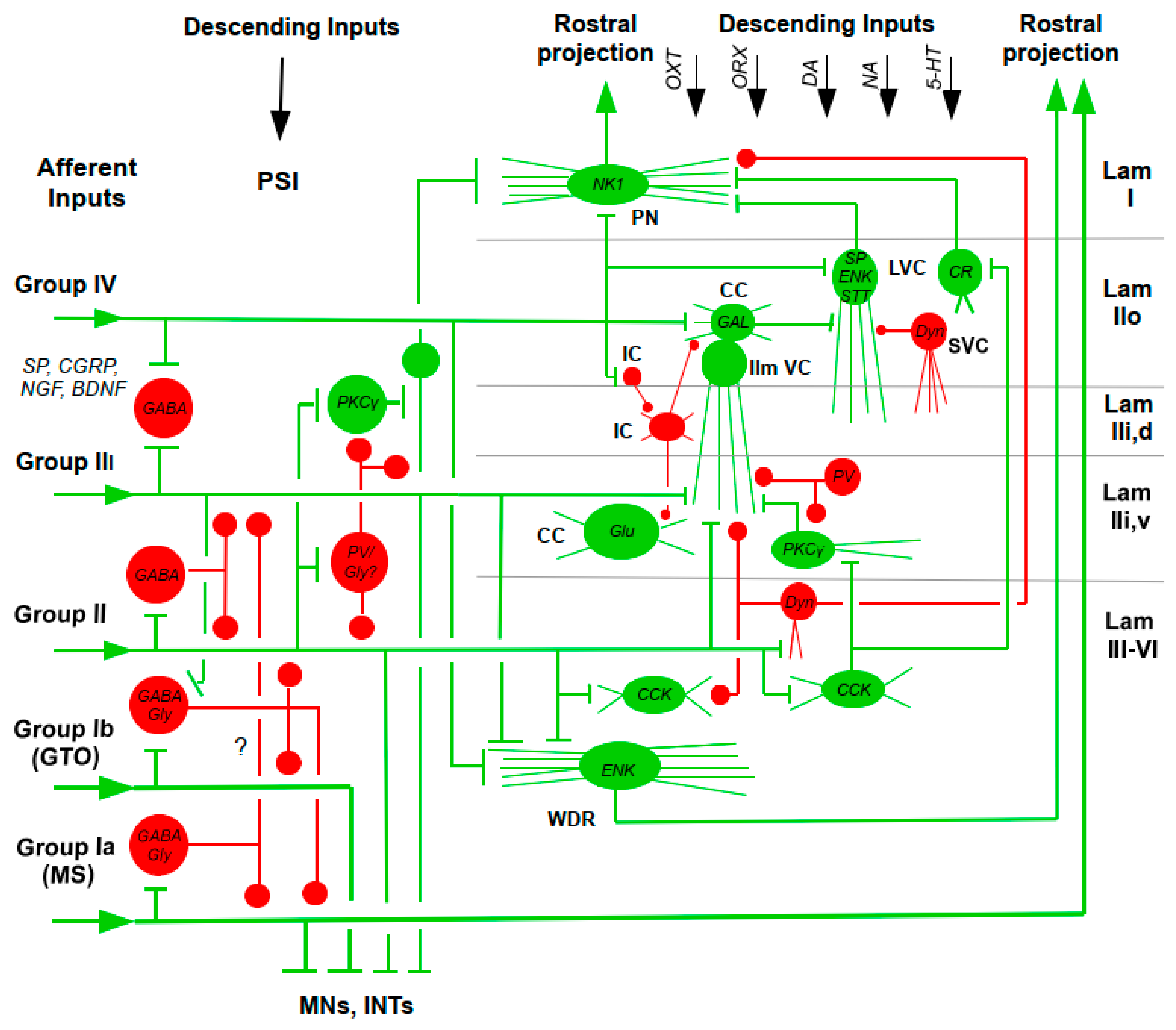
Although the network scheme depicted in
Figure 2 is immensely complex, it is incomplete. Currently, much work is being done to complete it. This work is mostly performed in rodents and continuously adds new details, in terms of circuit motifs and neuromodulators.
In the context of the role of PSI in pain, the emhpasis has been on the interaction between cutaneous non-nociceptive group II afferents and nociceptive group III and IV afferents (e.g., Comitato and Bardoni 2021; Guo and Hu 2014; Hochman et al. 2010; Lu et al. 2018; Zimmerman et al. 2019).
The network scheme in
Figure 2 is a step forward by integrating effects of group Ia and Ib afferents from muscle spindles (MSs) and Golgi tendon organs (GTOs), respectively. The immediate problem arising is that group II afferents must be split into a sub-group arising from the skin and a sub-group originating in skeletal muscles, the latter group containing a sub-sub-group from muscle spindles and the rest. To our knowledge, not all the new possibilities of interaction with PSI have been explored. This would indeed be worthwhile studying because cutaneous pain is not separated from muscle pain nor from movement.
The most serious challenge is of course to understand the operation of this network under natural conditions. Quite a few attempts have been made in humans (e.g., Nielsen 2016) and animals (e.g., Côté et al. 2018) to monitor PSI opeation during rest, stance, (fictive) locomotion and voluntary movements (Dibaj and Windhorst 2024a; Windhorst 2007, 2021; Windhorst and Dibaj 2023). The power of PSI changes in these different conditions, the addional influence of pain remaining under-studied, although pain does of course have an effect on movement. An example is that, in the (resting) cat, afferent group III/IV input, probably excited by gastrocnemius muscle-fatigue, enhanced PSI elicited from antagonist muscle nerves (Kalezic et al. 2004). Finally, however, it will be impossible to interpret the role of the network in
Figure 2 in the context of natural behaviors.
2.4. Spinal Cord
The intensely investigated spinal DH networks exhibit a picture of mind-boggling complexity (
Figure 2). DH neurons receive sensory information from multi-modal primary afferents that innervate the skin and deeper tissues of the body and that respond to specific types of noxious and non-noxious stimuli. Small-diameter afferents contact a large variety of excitatory and inhibitory interneurons that provide for complex signal processing at spinal levels, and also connect with a minority of projection neurons that send axons rostrally. Moreover, descending fiber systems impinge on spinal neurons (Dibaj et al. 2024; Nadrigny et al. 2017).
As can be expected from a structure intimately involved in the modulation of nociceptive transmission, the spinal DH contains a plethora of neurotransmitters/neuromodulator, conveyed by the input fibers or expressed by the intrinsic spinal neurons themselves: CCK, CRH, STT, CGRP, GAL, [tachykinins: SP, neurokinin-A (NK-A), neurokinin-B (NK-B)], NT, neuropeptide Y (NPY),
thyrotropin-releasing hormone (TRH); vasoactive intestinal polypeptide (VIP), catecholamines (DA, NA, adrenaline), 5-HT, opioids (ENKs, ß-endorphin, Dyn), [neurotrophins: NGF, BDNF, glial-derived neurotrophic factor (GDNF)]; ACh, excitatory (glutamate) or inhibitory (GABA, glycine) amino acids; purines; nitric oxide (NO); TRP channels; capsaicin; and nociceptin, and others (Blumenkopf 1988; Fürst 1999; Merighi 2018, 2024; Merighi et al. 2008; Todd and Spike 1993; West et al. 2015; Zeilhofer et al. 2021). A limited collection of these modulators in shown in the simplified scheme of
Figure 2.
WDRs have been investigated in view of their inputs from cutaneous mechano-receptors and nociceptors. It should be noted that some cutaneous mechano-receptors respond to skin stretch, thus allowíng their afferents to react to joint position and/or movements (Edin and Abbs 1991). This establishes a link between motor and nociceptive systems.
Corticosteroids have been used as a supplementary treatment in acute inflammatory pain conditions, but there appears to be a more direct role that steroids play in the generation and clinical management of chronic pain. The end-product of the hypothalamic-pituitary-adrenal (HPA) axis, cortisone, modulates nociceptive transmission at spinal level. In laminae and II, noxious stimulation releases SP and CGRP, and with their expression co-exists a high density of glucocorticoid receptors (GRs). Termination of treatment with cortisone after four weeks leads to loss of an anti-nociceptive effect (McEwen and Kalia 2010). SVC: small vertical cell; WDR: wide-dynamic range neuron (Data from Merighi 2018; Todd and Spike 1993; West et al. 2015; Zeilhofer et al. 2021).
In rats, DA inhibited the nociceptive group III- and group IV-fiber synaptic inputs to lamina I projection neurons via presynaptic actions. Similar inhibitory effects of DA on EPSCs occurred in rats subjected to complete Freund’s adjuvant (CFA) to induce peripheral inflammation (Lu et al. 2018). Regulation of the threshold of synaptic plasticity may determine the proneness to sensitization and hyper-responsiveness to noxious inputs. Increasing the endogenous DA levels in the DH by using re-uptake inhibitor GBR 12935 induced hyper-DA transmission. Conditioning low-frequency (1 Hz) stimulation of the sciatic nerve induced long-term potentiation (LTP) of group IV-fiber-evoked potentials in DH neurons. The magnitude of LTP was attenuated by blockade of either DA D1-like receptors or an NMDAR sub-unit (Buesa et al. 2016). In the spinal cord and striatum, anti-nociception of DA is mainly mediated by D2-like receptors, while in the nucleus accumbens (Nac) and peri-aqueductal gray (PAG), both D1- and D2-like receptors are involved as analgesic targets (Wang et al. 2021).
In the DH, NA suppresses nociceptive signal transmission via several mechanisms, including inhibitory action by α2A-adrenoceptors on central terminals of primary nociceptors (PSI), by direct α2-adrenergic action on nociceptive relay neurons (postsynaptic inhibition), and by α1-adrenoceptor-mediated activation of inhibitory interneurons. Furthermore, α2C-adrenoceptors on axon terminals of excitatory interneurons possibly contribute to spinal control of pain (Pertovaara 2006; Yoshimura and Furue 2006). The end-product of the HPA, cortisone, modulates nociceptive transmission at spinal level. In laminae and II, nociceptive stimulation releases SP and CGRP, and with their expression co-exists a high density of GRs. Termination of treatment with cortisone after four weeks leads to loss of an anti-nociceptive effect (McEwen and Kalia 2010).
The median nucleus raphé magnus (NRM) neurons project preferentially into the deep laminae V-VI of the DH, whereas the lateral nucleus paragiganto-cellularis (PG) neurons send projections exclusively into the superficial laminae I-II. These differential projection patterns support the notion that 5-HT neurons might be differently implicated in pain modulations depending on their location in the NRM vs. PG. The majority of 5-HT fibers form non-synaptic varicosities in the vicinity of DH neurons and astrocytes, suggesting that most 5-HT transmission occurs through volume transmission (Bardoni 2019).
The 5-HT pathways descending to the DH exert inhibitory or facilitatory influences on the spinal processing of nociceptive information, depending on acute or chronic pain states and the type of receptor acted upon. To date, more than a dozen types of 5-HT receptors (5-HTRs) have been identified. Most of them are expressed on nociceptors and/or DH neurons in the rodent and human spinal cord. The roles of the different receptor sub-types in pain neurotransmission are not completely known. Due to this diversity, 5-HT can exert both pro-nociceptive and analgesic effects by activating specific types of 5-HTRs, in different pain conditions. Group III and group IV nociceptive fibers express several 5-HTRs on their presynaptic terminals. Activation of the presynaptic 5-HT1A, 5-HT1B, 5-HT1D and 5-HT7 receptors tends to be anti-nociceptive, producing a decrease of glutamate release. The 5-HT2A and 5-HT3 receptor tend to promote nociception. The ionotropic 5-HT3Rs are involved in PAD as a sign of PSI, and their effect on glutamate release could be variable. It has also been reported that 5-HTRs play an active role in mediating synaptic plasticity the DH (Bardoni 2019; Cortes-Altamirano et al. 2018; Ossipov et al. 2014). Equally varied and ill-understood are the pain-modulatory effects of 5-HT1, 5-HT2, 5-HT3 and 5-HT7 receptors above the spinal cord, this modulation depending on the type and distribution of the receptors (Cortes-Altamirano et al. 2018).
Primary nociceptive afferents use glutamate as their principal fast neurotransmitter. However, peptides have an influential role in both mediating and modulating sensory transmission. SP is concentrated in laminae I and II, and one of its receptors, NK1R, is present in the same laminae and the medial half of laminae III-X.
Noxious stimuli elicit the release of SP and neurokinin A (NKA). In the DH, SP, acting on NK1R, and NKA, acting on NK2 receptors, excite nociceptive DH neurons, this release causing a late slow depolarization in DH second-order neurons. A sub-population of DH neurons is however inhibited by SP, this effect probably being indirect via excitation of an interposed inhibitory interneuron. This anti-nociceptive effect may be due to an SP-induced release of opioid peptides from DH interneurons. In vivo, SP potentiated NMDA-induced responses of spino-thalamic tract (STTr) cells, and in vitro, SP potentiated an inward glutamate-gated current, which contributes to the wind-up property, i.e., repeated activation of primary nociceptive afferents results in a progressive increase of firing of DH nociceptive neurons to each stimulus (Levine et al. 1993).
CGRP is a neuromodulator, having limited effects on its own, but strongly potentiating the effects of other substances, in particular SP. Unlike SP, CGRP has limited distribution in the spinal cord, CGRP terminals being concentrated in DH laminae I and II and in the reticulated area of lamina V. CGRP terminals make direct connections with second-order STTr neurons. Noxious thermal, mechanical or electrical stimulation elicited the release of CGRP in the superficial DH. In vivo, iontophoretically administered CGRP produced slow, long-lasting excitation of DH cells. In vitro, CGRP produced a slow depolarization in DH cells, probably by increasing the Ca2+ conductance. Like SP, CGRP can regulate the release of amino acids. In vivo in rats, concentrations of CGRP that alone had little effect strongly potentiated the excitatory action of SP or of noxious stimulation (Levine et al. 1993).
Different acute pain states and itch transmitted via the TRPV1 population may have differential effects becoming apparent when either ablating Trpv1-Cre-expressing neurons or inducing vesicular glutamate transporter 2 (VGLUT2) deficiency in Trpv1-Cre-expressing neurons. Furthermore, in Vglut2-deficient mice, pharmacological inhibition of SP or CGRP signaling was used to evaluate the contribution of SP or CGRP to these sensory modulations, with or without the presence of VGLUT2-mediated glutamatergic transmission in Trpv1-Cre neurons. Together with c-fos analyses, these data showed that glutamate via VGLUT2 in the Trpv1-Cre population together with SP mediate acute cold pain, whereas glutamate together with CGRP mediate noxious heat. Moreover, glutamate together with both SP and CGRP mediated tissue-injury-associated pain. Furthermore, itch, regulated by the VGLUT2-mediated transmission via the Trpv1-Cre population, depended on CGRP and gastrin-releasing peptide receptor (GRPR) transmission because pharmacological blockade of the CGRP or GRPR pathway, or genetic ablation of Grpr, led to a drastically attenuated itch. Hence, different neurotransmitters combined can cooperate with each other to transmit or regulate various acute sensations, including itch (Rogoz et al. 2014).
In some species, CCK is co-localized with SP and/or CGRP. CCK immuno-reactivity also occurs in DH neurons and terminals of descending axons. In rats, CCK iontophoresis excited DH neuons, but weakly and inconsistently. However, CCK antagonized and CCK antagonists potentiated opioid suppression of group IV (C) fiber activation of DH cells (Levine et al. 1993).
In the spinal DH, STT-containing neurons are predominantly localized in laminae I, II and III. About 13% of lamina I and 15% of lamina II neurons express STTR2a receptors. This provides the cellular and molecular basis for the role of STT in the modulation of pain transmission (Pan et al. 2007; Rosen and Schulkin 2022). Pharmacological studies supported either a facilitatory or an inhibitory role for STT in nociception (Levine et al. 1993).
Intra-thecal injection of STT could increase the nociceptive threshold. Intra-plantar injection of the STT analogue octreotide reduced formalin-induced nociceptive behaviors and the responses of group IV (C) fibers to noxious stimulation. Intra-plantar injection of SCR007, a selective non-peptide SSTR2 agonist, significantly increased the nociceptive threshold. STT has been effective in the treatment of patients with certain pain conditions, including cluster headache, headache associated with pituitary tumors, and postoperative pain. Spinal administration STT or octreotide reduced pain in patients with terminal cancer (Pan et al. 2007).
In anesthetized cats, the effects of STT were investigated on nociceptive responses of rostrally projecting DH neurons to different kinds of noxious stimuli (i.e., heat, mechanical and cold stimuli) and to group III and group IV fiber activation of the sciatic nerve. Iontophoretically applied STT suppressed the responses of DH neurons to noxious heat and mechanical stimuli as well as to group IV-fiber activation (Pan et al. 2007). In vitro experiments showed that the STT-induced inhibition of DH cells went along with a hyperpolarization (Levine et al. 1993).
STT also suppressed glutamate-evoked activities of DH neurons. The effects of STT were blocked by the STT receptor antagonist cyclo-STT. This suggests that STT has a dual effect on the activities of DH neurons: facilitation and inhibition, depending on the modality of pain signaled through them and its action site (Jung et al. 2008).
However, STT has also been reported to enhance the responses of DH neurons to noxious cold stimuli and group III-fiber activation (Jung et al. 2008).
Pain information processing in the spinal cord has been postulated to rely on nociceptive transmission (WDR) neurons receiving inputs from nociceptors and group II (Aβ) mechano-receptors, with group II inputs gated through feed-forward activation of spinal inhibitory neurons. Intersectional genetic manipulations were used to identify these critical components of pain transduction. Marking and ablating six populations of spinal excitatory and inhibitory neurons, associated with behavioral and electrophysiological analysis, showed that excitatory neurons expressing STT include WDR-type cells, whose ablation caused loss of mechanical pain. Cells marked by the expression of dynorphin (Dyn) represent inhibitory interneurons, which are necessary to gate group II fibers from activating STT neurons to evoke pain. Hence, peripheral mechanical nociceptors and group II mechano-receptors, together with spinal STT excitatory and Dyn inhibitory neurons, form a micro-circuit that transmits and gates mechanical pain (Duan et al. 2014).
Itch-eliciting stimuli are detected by sensory neurons that innervate the skin. The neuronal pathways for spinal itch neurotransmission was investigated, particularly the contribution of STT. In the periphery, STT was exclusively co-expressed with the neuropeptide natriuretic polypeptide B (Nppb+) in DRG neurons. STT potentiated itch by inhibiting inhibitory Dyn neurons. Elimination of STT from primary afferents and/or from spinal interneurons demonstrated differential involvement of the peptide released from these sources in itch and pain. This defined the neural circuit underlying STT-induced itch and characterized a contrasting anti-nociceptive role for the peptide (Huang et al. 2018).
In many nociceptive, capsaicin-sensitive group IV afferents, GAL co-localizes with SP and CGRP. In thermal nociception, intra-thecal GAL application had an anti-nociceptive effect, although, in mechanical tests at higher doses, it lowered the threshold for vocalization. However, low doses of intra-thecal GAL increased the excitability of the flexion reflex, while at higher doses, it produced a long depression of thermal nociceptive reflexes. The higher doses also blocked the facilitatory effect of SP, CGRP or of the conditioning stimulation of group IV fibers. Thus, like SP, GAL seems to have both pro- and anti-nociceptive effects (Levine et al. 1993).
The endocannabinoid (EC) system consists of two main receptors: cannabinoid type 1 (CB1) receptors exist in both the CNS and periphery, whereas the cannabinoid type 2 (CB2) receptor occurs principally in the immune system and to a lesser extent in the CNS. The EC family consists of two classes of ligands; the N-acyl ethanolamines, such as N-arachidonoyl ethanolamide or anandamide (AEA), and the monoacylglycerols, such as 2-arachidonoyl glycerol. Much work has studied the role of EC in nociceptive processing and the potential of targeting the EC system to produce analgesia. Cannabinoid receptors and ligands are present at almost every level of the pain pathway from peripheral sites, such as peripheral nerves and immune cells, to central integration sites such as the spinal cord, and higher brain regions such as the PAG and the rostral ventro-lateral medulla (RVL) associated with descending control of pain. The EC has been shown to induce analgesia in preclinical models of acute nociception and chronic pain states (Burston and Woodhams 2014).
It has been argued that cannabinoid modulation of pain occurs through inhibition at the level of the DRG, thus inhibiting ascending nociceptive signals. Another hypothesis propounds that modulation occurs through activity at the level of the brainstem, inhibiting pain signals through descending suppression of nociceptive signals. However, these effects need not be exclusive (Milligan et al. 2019).
Many glutamatergic sensory afferents and DH GABAergic interneurons express CB1. It is purported that eCBs are produced after stimulation of glutamatergic nociceptive, small- and medium-diameter group C fibers and activate CB1 receptors expressed on inhibitory interneurons within the DH. This reduces GABAergic signaling and increases nociceptor excitability leading to maladaptive nociception. A small population of astrocytes in the spinal cord express CB1 receptors and activation of these astrocytic receptors leads to transient Ca2+ currents that stimulate the production of 2-arachidonoylglycerol (2-AG), a potent endogenous CB1 agonist. This would suggest that CB1 receptors expressed in the DH are responsible for mediating the effects of chronic, neuropathic pain (Milligan et al. 2019).
The DH is a major target for opioids such as analgesic drugs, and the effects of exogenous (mainly morphine) and endogenous opioids on the release of neuropeptides, in particular SP and CGRP, and the inhibition of DH neurons. Opioids directly applied to the spinal cord suppress behavioral responses to noxious stimuli in animals and produce deep anti-nociception in humans. The central terminals of nociceptive afferents contain μ-, δ- and κ-opioid binding sites. Opioids have a direct effect on primary afferents; in particular, they inhibit K+-induced SP release. However, the effects may depend on receptor type. For example, in rat DH slices, selective δ-ligands reduced capsaicin-induced SP release, but μ-selective ligands had no such effect. Opioids also diminish K+- and capsaicin-induced release of CGRP (Levine et al. 1993).
Complex modulations by opioids occurred in the in vitro (from tissue slices) and in vivo rat preparation whose intra-thecal space was perfused with an artificial cerebro-spinal fluid (CSF) release of SP and CGRP, depending on the opioid receptor (μ, δ, κ, and their sub-types) stimulated by these compounds. The inhibition by δ agonists of SP release from primary afferent fibers, and that by the concomitant stimulation of μ receptors of the release of CGRP are probably involved in the analgesic action of specific opioids and morphine at the level of the spinal cord. The negative modulation (through presynaptic opioid auto-receptors) by δ and μ agonists of the spinal release of met-enkephalin (M-ENK), and the complex inhibitory/excitatory influence of δ, μ and κ receptor ligands on the release of CCK within the DH very likely also contribute to the anti-nociceptive action of these drugs and morphine (Bourgoin et al. 1994).
Cellular interactions between μ- and δ-opioid receptors, including heteromerization, are thought to regulate opioid analgesia. μ- and δ-opioid receptor co-expression is limited to small populations of excitatory interneurons and projection neurons and unexpectedly predominates in ventral horn (VH) motor circuits. Similarly, μ- and δ-opioid receptor co-expression is rare in cortical brain regions, AMY, and parabrachial nucleus (PBN) processing nociceptive information. In the discrete μ- and δ-opioid receptor co-expressing nociceptive neurons, the two receptors internalize and function independently. Conditional knockout experiments revealed that δ-opioid receptors selectively regulate mechanical pain by controlling the excitability of STT-positive DH interneurons. This illuminates the functional organization of δ-opioid receptors and μ- opioid receptor in CNS pain circuits (Wang et al. 2018).
In spinal cord slices, presynaptically acting M-ENK can inhibit glutaminergic input to DH cells in lamina I (Levine et al. 1993).
NGF has a role in both acute, transient nociceptive responses, and in longer-term, chronic pain. NGF belongs to a family of small glycoproteins that also include neurotrophin 3 (NT-3), neurotrophin 4/5 (NT-4/5) and BNDF (below). It is crucial for survival of nociceptive neurons during development, but also plays an important role in nociceptive functions in adults and in the development and modulation of persistent pain. NGF binds to TrkA, whereupon the NGF-TrkA complex is internalized and transported from peripheral terminals to sensory cell bodies in the DRG (Mantyh et al. 2011).
NGF is active in both peripheral and central sensitization and has complex multi-functional roles in the modulation of nociceptive processing through effects on the release of inflammatory mediators, nociceptive ion channel and receptor activity, nociceptive gene expression, and local neuronal sprouting effects (Barker et al. 2020; Boyce and Mendell 2014; Finnerup et al. 2021; Mantyh et al. 2011; Mizumura and Murase 2015; Nicol and Vasko 2007; Pezet and McMahon 2006; Yang and Chang 2019).
Longer-term (days) post-translational effects of NGF-TrkA binding and transport to the DRG include an increase in the concentration of peptides (e.g., SP, CGRP, and BDNF) in DH terminals of peptidergic (TrkA+) primary afferent neurons. Release of these peptides, in addition to glutamate acting on AMPARs, on subsequent stimulation of peptidergic (TrkA+) primary afferent neurons, and binding to their respective receptors (SP to NK1; CGRP to CGRP-R, BDNF to TrkB) may cause strong depolarization of the post-synaptic second-order projection neuron, changes in transcriptional activity in the second order projection neuron (e.g., increased expression of c-fos), and ultimately removal of the Mg2+ block of the glutamatergic NMDAR. BDNF acts specifically as a central modulator, via binding to post-synaptic TrkB receptors, whereupon the BDNF-TrkB complex switches on intracellular protein kinases leading to phosphorylation of NMDARs and facilitated opening. This increases the probability of central sensitization and facilitated transmission through the dorsal horn synapse and via third-order neurons to the sensory cortex in the brain (Mantyh et al. 2011).
NGF is important in inflammatory pain as exemplified by the expression and/or release of NGF by certain inflammatory cells, including eosinophils, lymphocytes, macrophages and mast cells (Kaur et al. 2017; Luo et al. 2015), as a consequence of injury. NGF is also up-regulated in experimental models of inflammation, including those induced by carrageenan, formalin, and CFA. Cutaneous administration of NGF to rodents and to humans causes hyperalgesia within one or three hours, respectively, suggesting that NGF leads to a relatively rapid sensitization of cutaneous nociceptors. These rapid effects in the rat are thought to be mediated primarily through NGF binding with TrkA expressed on mast cells, causing de-granulation and release of a variety of algogenic mediators, such as hydrogen ions (H+), HIST, prostaglandin E2, 5-HT, and bradykinin, as well as additional NGF (Mantyh et al. 2011). NGF plays a central role in initiating and sustaining heat and mechanical hyperalgesia following inflammation. Secreted proforms of nerve NGF (proNT) have biological functions distinct from the processed mature factors raising the possibility that these pro-neurotrophins may have distinct function in painful conditions. ProNTs engage a novel receptor system that may function with or independently of the classic Trk system in regulating inflammatory or neuropathic pain (Lewin and Nykjaer 2014).
NGF may also generate and maintain hypersensitivity by inducing aberrant sprouting and/or neuroma formation in response to tissue and/or nerve injury. Local administration of NGF to normal peripheral nerves can also induce nerve sprouting of peptidergic (TrkA+) nociceptors (Mantyh et al. 2011).
IL-6 is another neurotrophic factor and an important mediator in pain processing. Following nerve injury, IL-6 is released and its concentrations are increased. IL-6 is involved in the development of pain and CNS sensitization. It promotes and mediates various inflammatory pain conditions (Yang and Chang 2019).
BDNF plays important roles in proper growth, neuronal differentiation, development, survival, neuroprotection, neurodegeneration, development and plasticity of glutamatergic and GABAergic synapses, synaptic plasticity, and the control of mood disorders (Merighi 2024). Mature neurotrophin binding to the high-affinity receptor, TrkB receptor, increases cell survival and differentiation, dendritic spine complexity, re-sculptering of neuronal networks, and LTP. Deployment of TrkB receptors significantly increases at synaptic sites following neuronal activity (Phillips 2017; Pitsillou et al. 2020). The precise role of BDNF in pain transmission is still somewhat controversial, though, because evidence has been presented of pro-nociceptive as well as anti-nociceptive and anti-inflammatory activities (Cappoli et al. 2020).
BDNF is thought to intervene in the modulation of pain. BDNF has a widespread distribution and functions in pain pathways. Under basal conditions, BDNF is synthesized by various types of neurons and glia within pain pathways. Noxious stimuli can trigger the production and release of BDNF by these cells and/or up-regulate (Merighi 2024).
Data from BDNF-LacZ reporter mouse showed that primary afferent-derived BDNF contributes minimally to the processing of pain and itch. BDNF was expressed primarily by myelinated primary afferents and had limited overlap with the major peptidergic and non-peptidergic sub-classes of nociceptors and pruritoceptors. There was also an extensive neuronal, but not glial, expression in the DH. BDNF deletion in adult mice altered few itch or acute and chronic pain behaviors, beyond sexually dimorphic phenotypes in the tail immersion, HIST, and formalin tests (Dembo et al. 2018).
In the CNS, ACh acts as a neurotransmitter and neuromodulator upon release from groups of ACh projection and interneurons in both brain and spinal cord. Two primary types of receptors respond to ACh. Neuronal nAChRs are ligand-gated cation channels, which are widely expressed in the CNS (Naser and Kuner 2018).
In the DH, ACh interneurons are a sparse population of cells that, although they represent the main source of ACh in the DH, contact a large number of neurons. ACh receptor (AChRs) regulate nociceptive transmission via pre- and post-synaptic mechanisms. Elevation of spinal ACh concentrations induced analgesia whereas locally decreasing ACh concentrations or activity (via receptor blockade) strengthened nociceptive sensitivity, inducing hyperalgesia and allodynia. In rats exists a tonic ACh inhibition of spinal nociceptive transmission. In rodents and humans, directly activating muscarinic ACh receptors (mAChRs) reduced pain, and conversely, inhibition of spinal mAChRs induced nociceptive hyper-sensitivity. nAChRs have also been implicated in spinal modulation of pain. intra-thecally administered ACh-esterase inhibitors, such as neostigmine, reduce inflammatory hyper-sensitivity, which is sensitive to muscarinic antagonists (Naser and Kuner 2018).
In the rat, noxious mechanical stimulation of the skin induced neuron double-labeling for fos and for each NK1 and GABAB receptors largely in lamina I. The proportions of fos-positive cells immuno-stained for NK1 or GABAB receptors were higher in lamina I than in the remaining spinal laminae. More fos-positive cells were immuno-reactive (IR) for GABAB receptors than for NK1 in all DH laminae. Co-localization of NK1 and GABAB receptors occurred only in lamina I and was higher in neurons expressing fos. As to the morphological lamina I cell class, NK1-positive cells belonged mainly to the fusiform type while similar proportions of fusiform, pyramidal and flattened NK1 neurons expressed GABAB receptors. No differences occurred between those cell types as to the degree of nociceptive activation. This suggests that the co-localization of NK1 and GABAB receptors is a common feature of fusiform, pyramidal and flattened neurons in lamina I (Castro et al. 2004).
Spinal neurons that receive inputs from primary afferent fibers and whose axons project supraspinally to the medulla oblongata may represent a pathway through which nociceptive and non-nociceptive peripheral stimuli may modulate cardio-respiratory reflexes. Expression of the NK1R is believed to be an indicator of lamina I cells that receive nociceptive inputs from SP releasing afferents, and similarly, somatostatin (STT2A) receptor expression may be a marker for neurons receiving STT inputs. In rat spinal neurons, NK1 and STT2A receptors in lamina I were localized mainly to separate populations of retrogradely labeled cells with fusiform, flattened and pyramidal morphologies. With visceral stimulation, many retrogradely labeled cells expressing c-fos were immuno-reactive for the NK1R, and a smaller population was STT2A positive. In contrast, with cutaneous stimulation, only NK1-positive retrogradely labeled cells showed c-fos expression. Hence, lamina I neurons receiving noxious cutaneous and visceral stimuli via NK1R activation project to NTS and may thus be involved in coordinating nociceptive and cardio-respiratory responses. Moreover, a sub-population of projection neurons that respond to visceral stimuli may receive STT inputs of peripheral, local or supraspinal origins (Gamboa-Esteves et al. 2004).
Nociceptive primary afferents release glutamate, activating postsynaptic glutamate receptors on spinal DH neurons. Glutamate receptors, both ionotropic and metabotropic, are also expressed on presynaptic terminals, where they regulate neurotransmitter release. The rodent DH contains presynaptic glutamate ionotropic (AMPA, NMDA, kainate) and metabotropic receptors (Bardoni 2013).
Kainate receptors are expressed in nociceptive pathways, including the DRG, spinal cord, THAL and cortex. Functional kainate receptors are located postsynaptically or are located presynaptically, where they modulate excitatory or inhibitory neurotransmission. Kainate receptors can regulate nociceptive responses (Wu et al. 2007).
There is an interaction between midazolam, a benzodiazepine-GABAAR agonist, and two glutamate receptor antagonists on acute thermal nociception. Sprague-Dawley rats were implanted with chronic lumbar intra-thecal catheters and were monitored for their tail-withdrawal response to an acute heat stimulus after intra-thecal administration of saline, midazolam, AP-5, or YM872, where AP-5 is an NMDAR antagonist and YM872 is an AMPAR antagonist. Motor disturbance and behavioral changes occurred. Dose-dependent increases in the tail-flick latency (TFL) occurred with midazolam, AP-5, and YM872. When combining midazolam with AP-5 or YM872, a potent synergy in analgesia occurred with decreased behavioral changes and motor disturbance (Nishiyama et al.1999).
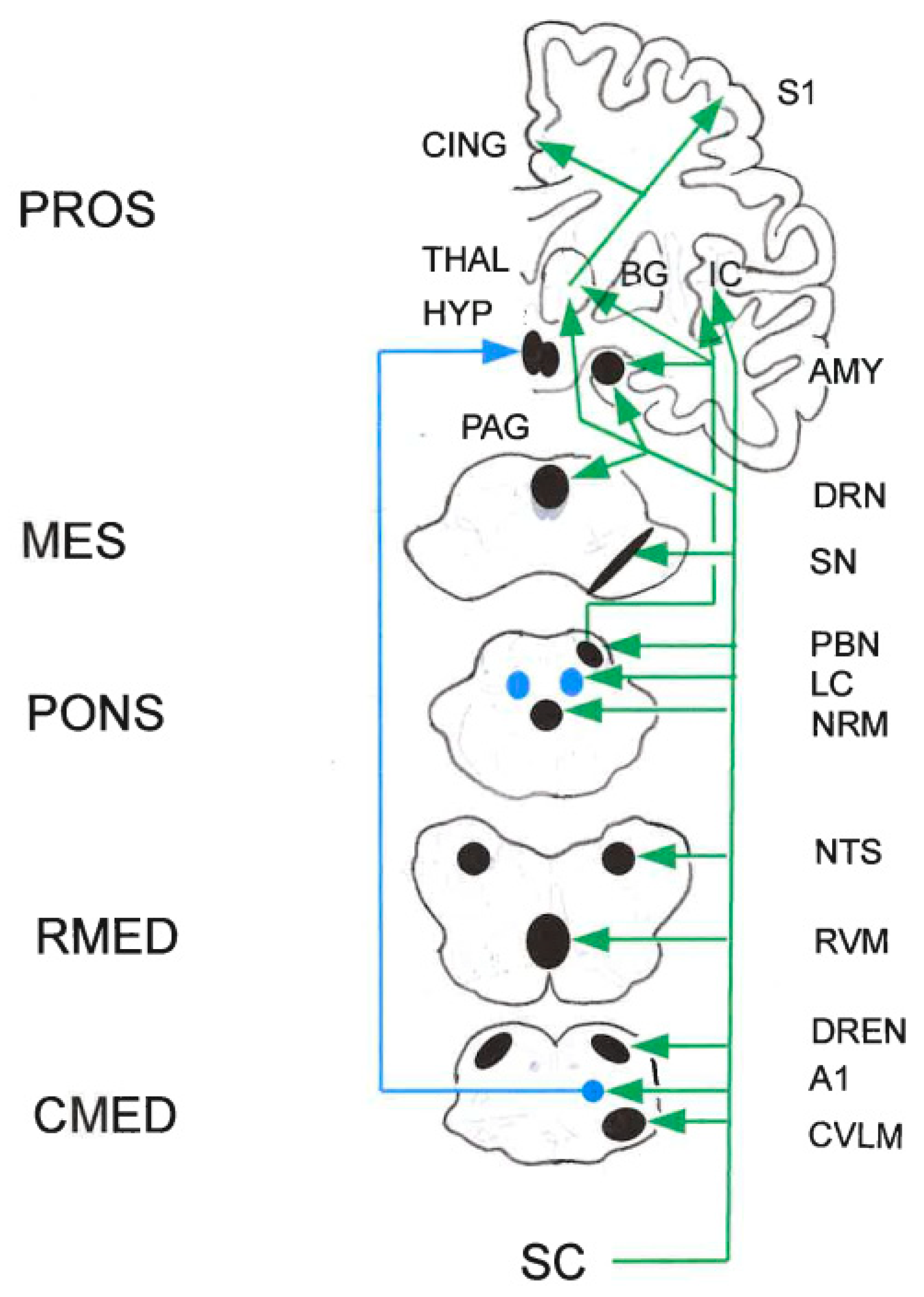
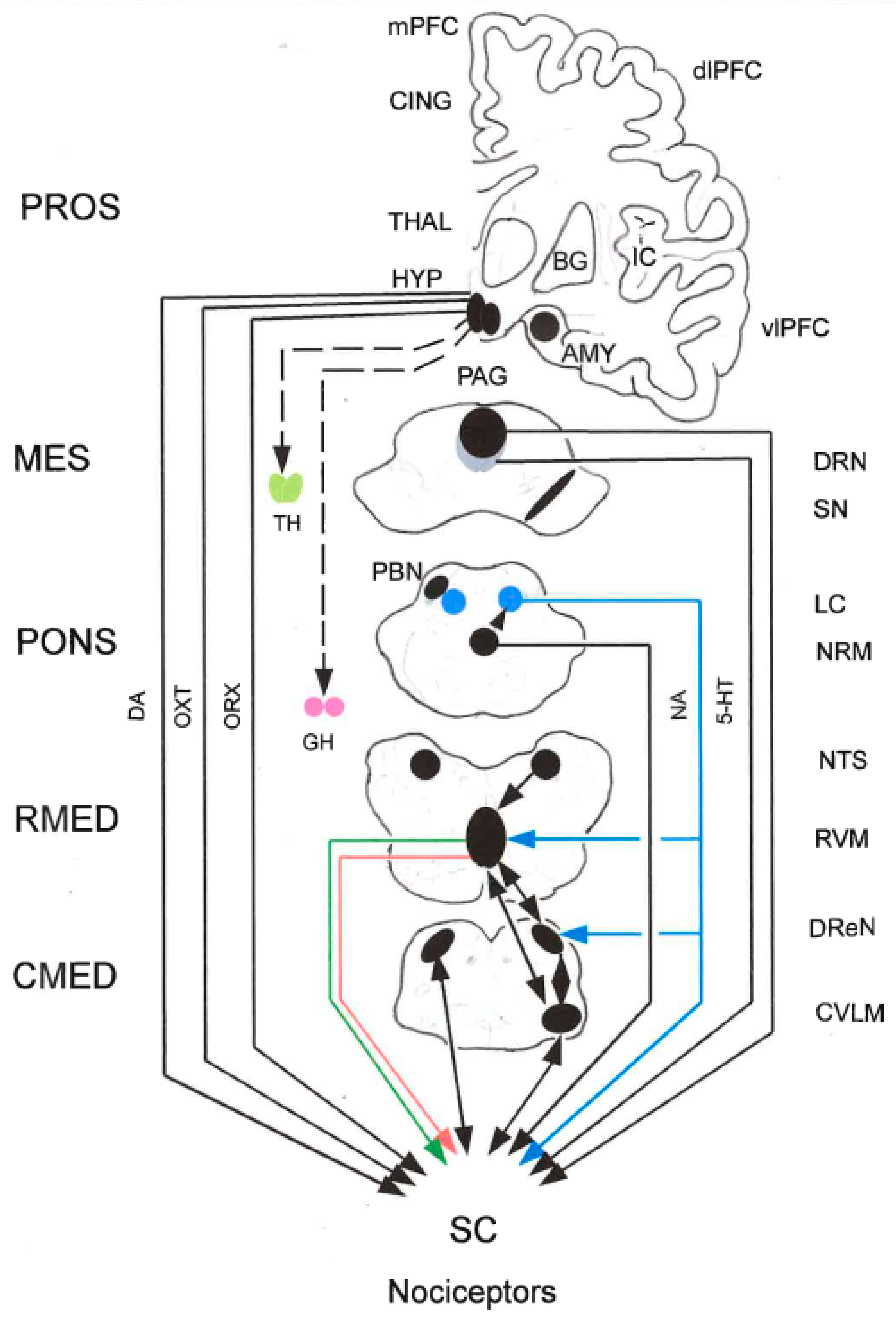
Important nociceptive structures ascending from the spinal cord up to the cerebral cortex are illustrated in
Figure 3. Grosso modo, these structures are also involved in pain modulation (
Figure 4).
2.5. The PAG-Triad Connection
Several brain areas modulate spinal pain transmission through direct projections to the DH. The descending modulation is exerted by neurotransmitters acting both at spinally projecting neurons and at interneurons that target the projection neurons.
In the rat, μ-opioid, GABAB, and NK1 receptors occur in spinally projecting neurons of major medullary pain control areas: RVM, NTS, dorsal reticular nucleus (DReN), ventral reticular nucleus (VReN), and lateral-most part of the caudal ventro-lateral medulla (CVLM). The retrograde tracer cholera toxin sub-unit B was injected into the spinal DH. The RVM contained the majority of double-labeled neurons followed by the dorsal raphé nucleus (DRN). In general, high percentages of μ-opioid- and NK1-expressing neurons were retrogradely labeled, whereas GABAB receptors were mainly expressed in neurons that were not labeled from the cord. Hence, μ-opioid and NK1 receptors play an important role in direct and indirect control of descending modulation. The co-localization of μ-opioid and GABAB in DReN neurons suggests that the pro-nociceptive effects of this nucleus may be controlled by local opoidergic and GABAergic inhibition of the pro-nociception increased during chronic pain (Pinto et al. 2008a,b).
5-HT RVM neurons modulate the activity of RVM neurons. The RVM also contains opioid-sensitive neurons as the activity of OFF-cells is enhanced by μ-opioid agonists whereas the opposite occurs with ON-cells. The local neurochemical control also involves GABA-mediated inhibition, which is triggered by opioids. Local CCK receptors (CCK2) provide further possibilities of fine-tuning the control within the RVM. The endovanilloid system is remotely activated from the PAG since agonists of the TRPV1 injected into the PAG induce glutamate-mediated activation of the activity of OFF-cells. The endovanilloid system in the RVM seems to be inactivated in non-noxious conditions or during acute pain. The system is only activated in the RVM in situations of chronic pain, namely during neuropathic conditions (Martins and Tavares 2017).
Dynamic shifts in the balance between pain-inhibiting and pain-facilitating outflows from the brainstem play a role in setting the gain of nociceptive processing as dictated by behavioral priorities, but are also likely to contribute to pathological pain states (Heinricher et al. 2009).
2.5.1. Peri-Aqueductal Gray (PAG)
Top-down afferents to the PAG arise from various cortical and sub-cortical brain regions, including the anterior cingulate cortex (ACC) and AMY. Changes in connectivity between the ACC and the PAG are prominent in fMRI studies in chronic pain patients. In addition, lesions of the ACC are generally agreed to reduce nociception in human patients. The PFC/ACC-AMY-PAG projections from the PAG to the ventral tegmental area (VTA) have been implicated in avoidancebehaviors in rodents. Both glutamatergic and GABAergic projections from PAG impinge on both DA and GABAergic neurons in VTA. Although DA neurons comprise the majority of the neurons within the VTA and DA in the PAG modulates pain thresholds, the VTA sends primarily GABAergic inputs to PAG (Bagley and Ingram 2020).
The PAG integrates information from cortical and sub-cortical areas to modulate many different behaviors, including defensive responses to pain, threat and stress, as well as cardio-vascular control, and control of respiration, lactation and feeding. Stimulation of the ventro-lateral PAG elicits analgesia in humans and anti-nociception in rats, which is sensitive to naloxone. In rats, the behavioral anti-nociception produced by opioids is mediated by activation of PAG output neurons projecting to the RVM (Bagley and Ingram 2020).
ORX participates in pain modulation. Orexin-1 and orexin-2 receptors (Ox1r and Ox2r) occur at high density in the ventro-lateral PAG (vlPAG). Chemical stimulation of the lateral hypothalamus (lHYP) with carbachol induces anti-nociception in the tail-flick test, a model of acute pain, and Ox1r-mediated anti-nociception in the vlPAG is modulated by the activity of vlPAG cannabinoid CB1 receptors. In the current study, TCS OX2 29, an Ox2r antagonist (5, 15, 50, 150, and 500 nmol/L), was microinjected into the vlPAG 5 min before the administration of carbachol (125 nmol/L). It has been shown that the anti-nociceptive effect of ORX is partially mediated by activation of vlPAG Ox2 receptors. It seems that Ox2 and CB1 receptors act through different pathways and Ox2r-mediated anti-nociception does not depend on CB1 receptor activity (Esmaeili et al. 2017).
A sub-population of DA neurons in the PAG/DRN are important modulators of anti-nociception. It was hypothesized that PAG DA neurons contribute to the analgesic effect of D-amphetamine via a mechanism that involves descending modulation via the RVM. Male C57BL/6 mice showed increased c-fos expression in PAG DA neurons and a significant increase in paw-withdrawal latency to thermal stimulation after receiving a systemic injection of D-amphetamine. Targeted micro-infusion of D-amphetamine, L-DOPA, or the selective D2 agonist quinpirole into the PAG produced analgesia, while a D1 agonist had no effect. Inhibition of D2 receptors in the PAG by eticlopride prevented the systemic D-amphetamine analgesic effect. D-amphetamine and PAG D2 receptor-mediated analgesia were inhibited by intra-RVM injection of lidocaine or the GABAAR agonist muscimol, indicating a PAG-RVM signaling pathway in this model of analgesia. Hence, D-amphetamine analgesia is partially mediated by descending inhibition and D2 receptors in the PAG are responsible for this effect via modulating neurons that project to the RVM (Ferrari et al. 2021).—Micro-injection of cumulative doses of morphine into the ventral PAG (vPAG) caused anti-nociception that was dose-dependently inhibited by a DA receptor antagonist, which had no effect on nociception when administered alone. Injection of the DA receptor agonist (-) apomorphine into the vPAG caused a robust anti-nociception that was inhibited by the D2 antagonist eticlopride but not the D1 antagonist SCH-23390. The effects of DA on GABAA-mediated evoked eIPSCs were measured in PAG slices. Administration of M-ENK inhibited peak eIPSCs by 20-50%. DA inhibited eIPSCs by approximately 20-25%. These data indicate that PAG DA has a direct anti-nociceptive effect in addition to modulating the anti-nociceptive effect of morphine (Meyer et al. 2009). In the PAG and NAc, both D1- and D2-like receptors are involved as analgesic targets, while in the striatum and spinal cord, anti-nociception of DA is mainly mediated by D2-like receptors (Wang et al. 2021).
The lateral HYP (lHYP) has been implicated as part of the descending pain modulatory system. The lHYP modifies nociception in the spinal DH partly through connections with the PAG. To determine whether lHYP-induced anti-nociception mediated by the PAG depends on NK1R, behavioral experiments were conducted in which the cholinergic agonist carbachol was micro-injected into the lHYP of lightly anesthetized female Sprague-Dawley rats- and anti-nociception was obtained on the tail flick or foot withdrawal tests. A specific NK1R antagonist was micro-injected in the PAG, which abolished the lHYP-induced anti-nociception. This supports the hypothesis that anti-nociception produced by activating neurons in the lHYP is mediated in part by the subsequent activation of neurons in the PAG by NK1Rs (Holden et al. 2009).
CGRP receptors are widely distributed in the CNS. In male rats, the effects of intra-cerebro-ventricular (ICV) injection of CGRP was investigated on pain behavioral responses and on levels of monoamines in the PAG during the formalin test. ICV injection of CGRP led to a significant pain reduction in acute, middle and chronic phases of the formalin test. Dialysate concentrations of DA, NA, 5-HT and HIAA in the PGA area showed an increase in acute phase, middle phase and beginning of the chronic phase of the formalin test. Hence, CGRP significantly reduced pain by increased concentrations of monoamines and their metabolites in dialysates from PAG when injected ICV to rats (Rahimi et al. 2018).
The PAG is a critical component of the endocannabinoid system (ECS) since it is densely packed with CB1 receptors. In part, cannabinoids and opiates inhibit pain by activating the PAG. In response to noxious stimuli, the PAG released endogenous anandamide. In rats, electrical PAG stimulation induced analgesic effects after intra-dermal formalin injection, which were associated with increased anandamide release in the PAG. These analgesic effects were attenuated after intra-PAG injection of the CB1R antagonist, S141716, suggesting a critical role of CB1R in this brain region for pain modulation. An important role of PAG eCBs in chronic pain inhibition was also suggested by the fact that, whereas early-phase algesia in the hindpaw after formalin injection was not affected by an exogenous cannabinoid ligand, HU-210, injection directly into the dorsal PAG, late-phase algesia was significantly reduced. Administration of HU210 significantly attenuated formalin-evoked increases in c-fos expression in the caudal lateral PAG (Milligan et al. 2019).
If the anti-nociceptive mechanisms are distinct, cross-tolerance between cannabinoids and opioids should not develop. In male Sprague-Dawley rats, this hypothesis was tested by measuring the anti-nociceptive effect of micro-injecting morphine into the vlPAG of rats pretreated with the cannabinoid HU-210 for two days. The rats were injected twice a day for two days with vehicle, morphine, HU-210, or morphine combined with HU-210 into the vlPAG. Repeated injections of morphine caused a rightward shift in the morphine dose-response curve on Day 3 (i.e., tolerance developed). No tolerance was evident in rats pretreated with morphine combined with HU-210. In rats pretreated with HU-210 alone, morphine anti-nociception was enhanced. This enhancement was blocked by pretreating rats with the cannabinoid receptor antagonist AM-251, and it also disappeared when rats were tested one week later. Acute micro-injection of HU-210 into the PAG antagonized morphine anti-nociception, suggesting that HU-210-induced enhancement of morphine anti-nociception is a compensatory response. Hence, there was cross-tolerance between morphine and HU-210. Cannabinoid pretreatment enhanced the anti-nociceptive effect of micro-injecting morphine into the vlPAG (Wilson et al. 2008).
Noxious stimulation increased the release of opioid peptides in the vlPAG. Endogenous opioids, including L-ENK and M-ENK, and β-endorphin, are widely expressed in the brain. Endogenous opioids contribute to the control of the descending pain modulatory system through the activation of δ-, μ-, κ- receptors, and nociceptin-opioid peptide (NOP) receptors. By order of preference, the endogenous opioids, such as ENKs, β-endorphins, and Dyn, bind to δ-, μ-, κ- receptors, respectively. Opioids are involved in both descending inhibition and descending facilitation from PAG pathways relayed in the RVM and LC, respectively. In the PAG-LC circuit, μ-opioid receptors inhibit a sub-type of glutamate neurons, which project to NA LC neurons. Opioids are also involved in the mediation of descending inhibition from the LC through μ-opioid receptor-mediated inhibition of GABAergic neurons that disinhibit NA neurons projecting to the spinal cord (Tavares et al. 2021).
ENK-containing terminals occur throughout the PAG but are densest in the vlPAG, and are apposed to GABA and non-GABA-containing dendrites, as well as PAG output neurons that project to the RVM. A portion of the PAG-RVM projection neurons express μ-opioid receptors and δ-opioid receptors, indicating that endogenous opioids directly inhibit some PAG-RVM output neuron. Some of the ENK-containing neurons in the PAG send projections to the AMY and the NAc, indicating that opioid release in these areas may help to coordinate the response to pain in higher structures. β-Endorphin-containing fibers from the hypothalamic arcuate nucleus (HYP ARC) project strongly to the PAG. Stimulation of the HYP ARC increases the release of β-endorphin in the PAG, but stimulation of the PAG predominately increases release of M-ENK. Both M-ENK and β-endorphin are full agonists at μ-opioid receptors. Endomorphin 2-containing neurons from the HYP project to PAG and RVM. This peptide is a partial agonist at μ-opioid receptors in the PAG. β-Endorphin release in the PAG is associated with stress-induced analgesia as well as peripheral injury. Similar increases in endomorphin 2 concentrations occurred following neuropathic pain. Stimulation of the AMY induced release of the κ-opioid receptors agonist Dyn in the PAG, but Dyn did not elicit analgesia when micro-injected into the PAG. Thus, the endogenous opioid system responds to painful situations by activating opioid receptors in the PAG (Bagley and Ingram 2020).
Opioid-triggered analgesia was mediated by vlPAG to RVM projections, while non-opioid-triggered analgesia could be elicited by projections of the lateral PAG (lPAG) and the dorso-lateral PAG (dlPAG) to RVM (Peng et al. 2023). Stimulation of the vlPAG produced opioid-mediated analgesia, as well as freezing and quiescent behaviors, whereas stimulation of the lateral PAG column and more dorsal columns produced escape behaviors such as jumping and flight responses (Bouchet and Ingram 2020; Mills et al. 2021). μ-Opioid, δ-opioid, κ-opioid, and nociception/orphanin receptors (NOPRs) are prevalent in the PAG region. NOPRs can presynaptically inhibit GABAergic and glutamatergic neurons in the vlPAG and postsynaptically inhibit PAG-RVM projections, leading to hyperalgesia and reverse opioid-induced analgesia (Peng et al. 2023).—Given the dense expression of μ-opioid receptors and the role of DA in pain, the recently characterized DA neurons in the vPAG/DRN are a potentially crucial site for the anti-nociceptive actions of opioids. In a mouse line, μ-opioid receptor activation led to a decrease in inhibitory inputs onto the vPAG/DRN DA neurons. These neurons also expressed the vesicular glutamate type 2 transporter and co-released DA and glutamate in a major downstream projection structure—the bed nucleus of the stria terminalis (BNST). Hence, vPAG/DRN DA neurons likely play a role in opiate anti-nociception, potentially via the activation of downstream structures through DA and glutamate release (Li et al.2016).
The descending pain modulatory circuit is sexually dimorphic. Male rats have significantly higher concentrations of the μ-opiod receptors in the vlPAG than cycling females, and selective lesions of μ-opiod receptors disrupt morphine analgesia in males, but not females (Bagley and Ingram 2020).
NO concentrations in brain nuclei, such as the hippocampus (HIPP) and brainstem, are involved in morphine analgesia, but the relationship between the dorsal HIPP and the dlPAG needs clarification. In Wistar rats, morphine administered intra-peritoneally ten minutes before formalin injection into the left hind paw reduced inflammatory pain in the early and late stages of the rat formalin test. High levels of NO in dlPAG may regulate the pain process in downward synaptic interactions (Hashemi et al. 2022).
Data suggest that BDNF plays an important role in descending pain modulation, likely through the PAG-RVM pathway. In the four sub-regions of PAG, the distributions of BDNF mRNA and protein differ. Both neurons and astrocytes expressed BDNF, but not microglia. In the formalin-pain model, there were more BDNF-containing neurons projecting to RVM being activated in the vlPAG than other PAG sub-regions. BDNF-containing projection neurons expressed the TrkB in addition to 5-HT, NT, SP, CGRP, NO synthase (NOS), and parvalbumin. It is speculated that BDNF released from vlPAG projection neurons might participate in the descending pain modulation through enhancing the presynaptic release of other neuroactive substances in the RVM (Yin et al. 2014).
Glutamate plays an important role in pain modulation via the PAG-RVM. In the PAG, eight sub-types of glutamate metabotropic receptors (mGluR1-8) exert different effects on nociception modulation, of which hyperalgesia was elicited by activating mGluR1 and mGluR5 (Group I), while activation of mGluR2, mGluR3 (Group II), and mGluR4, 6, 7, 8 (Group III) entailed analgesia. After administration of mGluR8 agonists (S)-3,4-dicarboxyphenylglycine (DCPG) in PAG, glutamate transmission increased, then RVM ON-cell firings were reduced and OFF-cell activities were enhanced, which generated anti-nociception. However, intra-PAG micro-injection of AMN082, a selective mGluR7 agonist, increased RVM ON-cell activity while suppressing OFF-cell activity, which produced hyperalgesia (Peng et al. 2023). Administration of glutamate into the PAG produces analgesia. Thus, transmission through AMPARs is required for the intact PAG-RVM descending pathway (Doan et al. 2015).
GABA also plays an important role in pain modulation via the PAG-RVM pathway with a pattern similar to that of glutamate. There is an inhibitory projection from PAG to inhibitory RVM reticulo-spinal neurons. However, there were also PAG projections to the RVM that did not contain GAD67 immuno-reactivity. Both GAD67- and non-GAD67-immuno-reactive PAG neurons project to RVM ON, OFF, and Neutral cells in the RVM. These inputs include a GAD67-immuno-reactive projection to a GAD67-immuno-reactive ON-cells and non-GAD67 projections to GAD67-immuno-reactive OFF-cells. This pattern is consistent with PAG neurons producing anti-nociception by direct excitation of RVM OFF cells and inhibition of ON cells (Morgan et al. 2008).
Under normal conditions, PAG output neurons to the RVM are inhibited by GABA. Removal of this inhibition results in activation of the descending pain modulatory circuit and analgesia. This hypothesis has been supported by showing that GABAAR antagonists increase the firing of about three quarters of the PAG neurons and that injection of GABAAR antagonists or glutamate agonists into the PAG elicit anti-nociception. Inhibition of GABA release is the primary driver of PAG neuron excitability when compared with opioid-induced hyperpolarization. Nonetheless, in the rat, micro-injections of opioids into the PAG elicit anti-nociception through activation of μ-opioid receptors, not κ-opioid receptors (Bagley and Ingram 2020).
GABAB receptors mediate both presynaptic and postsynaptic effects in PAG. Activation of GABAB receptors yields analgesia in some PAG sub-divisions. With whole-cell patch-clamp recordings on acute rat PAG slices, the responses of presynaptic and postsynaptic GABAB receptors were monitored. The GABAB agonist, baclofen, exhibited less efficacy and potency at GABAB postsynaptic versus presynaptic receptors. This sensitivity bias may contribute to synapse homeostasis (Chen et al. 2017).
In the vlPAG, GABAB receptors play important roles in pain modulation. Using whole-cell recordings on acute PAG slices from adult rats, ambient GABA were shown to exert a tonic inhibition on presynaptic terminals by binding GABAB receptors. Extracellular GABA accumulated by nipecotic acid, which blocks GABA transporters, strengthened GABAB receptor-mediated PSI on both excitatory and inhibitory synapses. Hence, PAG neurons experience GABAB receptor-mediated inhibition determined by GABA transporters (Li et al. 2017a).
Acute de-sensitization is defined as rapid attenuation of receptor-mediated signaling. Multiple inhibitory G-protein-coupled receptors (GPCRs), including GABAB receptors, resist acute de-sensitization in the presynaptic but not postsynaptic compartments of certain neurons in mammal brains. With whole-cell voltage-clamp recordings on acute PAG slices from adult rats, GABAB receptors resisted acute de-sensitization to prolonged administration of baclofen (GABAB receptor agonist) in both presynaptic and postsynaptic compartments. The de-sensitization resistance of postsynaptic GABAB receptors was independent of presynaptic alteration and vice versa. The GABAB receptor-mediated inhibition at inhibitory presynaptic terminals also showed no de-sensitization. This suggests that GABAB receptor-mediated inhibition remains functional in both postsynaptic and presynaptic compartments to sustained agonist administration in rat PAG neurons (Liu et al. 2013).
2.5.2. Rostral Ventro-Medial MEDULLA (RVM)
RVM neurons respond to nociceptive input through an ascending relay from the PBN. The PAG sends dense projections to the RVM, which provides the predominant output from the descending pain modulatory PAG-RVM pathway to the spinal cord. In the rat, PAG-to-RVM projections are both glutamatergic and GABAergic. The GABAergic projections impinge primarily on GABAergic RVM neurons projecting to the spinal cord (Bagley and Ingram 2020).
RVM function is modulated by several neuromodulators including DA, NA, 5-HT, SP, CCK, NT, eCBs, endogenous opioids, and neurotransmitters including ACh, glutamate and GABA.
The contribution of PAG DA neurons (DAergic) to pain modulation via the vlPAG-RVM axis is little known. There may be DA neurons in the vlPAG, but no direct DA projections from the PAG to RVM. DA may however activate the DA receptor 2 (D2R), which was expressed in PAG GABA neuron. Injection of the D2R agonist quinpirole but not DA receptor 1 (D1R) agonists into the vlPAG modulated PAG-RVM projections, increased the threshold for the paw-withdrawal response and induced protective reactions to pain. D2R activation blocked μ-opioid-receptor-induced inhibition of GABA neurons and reduced presynaptic GABA neurotransmission, leading to a decrease in inhibitory input to vlPAG DA neurons and anti-nociception. In contrast, the administration of D-amphetamine to vlPAG inhibited RVM ON-cells by PAG GABA neurons, although increased PAG glutamatergic projections to RVM OFF-cells were not involved in anti-nociception effects. It has been speculated that the analgesic effects of PAG DA neurons are mediated by indirect modulation of the RVM, mainly by interfering with the opioid- and GABA-mediated descending pathway of PAG-RVM (Peng et al. 2023).
The localization of catecholaminergic neuronal cell bodies, which project to the RVM, can be determined with tracing methods. A retrograde neuronal tracer injected into the center of the RVM, the NRM labeled NA neurons in LC (A6), A1, A5, A7 regions, as well as nucleus subcoeruleus and adrenaline neurons in the C1 region were double-labeled, and the ratio of their co-existence was higher in A1, A5, A7 and C1 than in LC and nucleus subcoeruleus. No DA neurons in the midbrain and forebrain were double-labeled. Thus, the RVM is innervated by the ventral groups of lateral tegmental NA and adrenaline neurons in the brainstem (Tanaka et al. 1996).—In the barbiturate-anesthetized rat, the role of NA in regulating the activity of putative nociceptive modulatory neurons in the RVM was assessed by the effects of micro-injection of alpha-adrenergic receptor-selective agents on the nociceptive threshold (as measured by the tail-flick withdrawal response on noxious heat). The data demonstrated that activation of α2-adrenergic receptors in the RVM produced hypoalgesia. However, when antagonists selective for either the α1- or α2-adrenergic receptor were micro-injected alone into the RVM, there was no change in the nociceptive threshold. Hence, the α2-adrenergic receptor has a postsynaptic location and barbiturate anaesthesia suppresses a tonically active or noxious stimulus-activated NA input to the RVM present in the awake animal (Haws et al. 1990).
5-HT is an evolutionary ancient biogenic amine. It is common in the CNS, PNS and the immune system. In general, 5-HT is involved in almost every physiological function, including pain transmission (analgesia), embryogenesis, gastro-intestinal motility, peripheral and central vascular tone, endocrine and circadian rhythms, regulation of sleep rhythm and body temperature, appetite, feeding, cognition, arousal, anxiety, mood, aggressiveness, social interactions, sexual and reproductive activity, impulsive/compulsive behavior, behavioral flexibility, learning and memory, processing of expected and received rewards, and motor tone and motor functions. 5-HT is important for regulating the signaling of nociception and itch, both by peripheral and central mechanisms (Haleem 2019; Olivier 2015; Ploski and Vaidya 2021). 5-HT cells can inhibit or facilitate nociception due to the existence of many sub-types of 5-HT receptors with opposing effects (De Felice and Ossipov 2016). Selective activation of 5-HT cells in mice induced persistent pain sensitization (Cai et al. 2014). Hence, the facilitatory effects appear to predominate over inhibition (Bannister and Dickenson 2016).
Only a small amount of the body’s total 5-HT is synthesized in the CNS. 5-HT neurons display a substantial molecular and physiological heterogeneity, with different clusters co-releasing glutamate, GABA and neuropeptides, and differing in their axonal projection patterns and behavioral states that they modulate. The functional responses to 5-HT are mediated via seven different types of receptors which are further divided into at least 15 sub-types. All the types and sub-types of 5-HT receptors, excluding 5-HT3, are G-protein coupled receptors. Accumulating evidence suggests that activation of the 5-HT1A receptor sub-type can modulate processing and control of signals associated with pain (Haleem 2019; Ploski and Aidya (2021).
RVM 5-HT neurons receive direct excitatory inputs from the S1. Moreover, nociceptive neurons located in spinal laminae V-VIII project back to the NRM, but not the NRD, thus establishing a spino-NRM-spinal loop for regulating the strength of nociceptive processing (Cortes-Altamirano et al. 2018; Kuner and Kuner 2021).
The role of RVM 5-HT neurons in nociception is controversial. For example, in vivo recordings of 5-HT neurons have shown that they are predominantly neural cells, but pharmacological blockade of 5-HT signaling between the RVM and spinal cord has also shown that they can have both pro- and anti-nociceptive effects (Nguyen et al. 2023).
There are two populations of 5-HT neurons in the RVM, those involved in local modulation and those giving rise to spinal projections. As to the local 5-HT neurons, direct micro-injection of 5-HT increased the release of 5-HT in the NRM, modulating ON- and OFF-cells in RVM through 5-HT1R and 5-HT2R, which ultimately decreased TFL, and exerted an inhibitory influence on pain modulation. RVM 5-HT projections to the spinal DH arise from the NRM and terminate densely in the superficial DH laminae I and II and in the deeper laminae IV-VI. Stimulation, of RVM led to increased 5-HT release in the spinal cord, contributing to bi-directional effects on nociceptive modulation. Micro-injection of NT into the RVM resulted in reduced ON-cell discharge and facilitated OFF-cell activation, thus inducing anti-nociceptive effects. Injection of EM-2 into the RVM activated μ-opioid receptors in spinal projection of 5-HTergic neurons and increased descending 5-HTergic facilitatory influences. In sum, evidence suggests that two types of RVM 5-HT neurons can modulate spinal pain transmission in direct and indirect ways, which may be responsible for the bi-directional effects on modulating pain in physiological and pathophysiological conditions. In addition, bi-directional effects in nociception also depend on the activation of various sub-types of the 5-HT receptor (5-HT1R-7R). Generally, 5-HT1AR, 5-HT2AR, and 5-HT4R generate bi-directional effects, while 5-HT1B/DR and 5-HT2CR are primarily anti-nociceptive. By contrast, 5-HT2BR is activated by facilitating CCK2R in RVM ON-cells and induces hyperalgesia. In healthy conditions, spinal 5-HT7Rs only have anti-nociceptive effects (Peng et al. 2023).
5-HT injected into the RVM produces analgesia that is blocked by 5-HT2 receptor antagonists. An important modulator of 5-HT activity is the 5-HT transporter (SERT) which reduces 5-HT signaling through re-uptake into the presynaptic terminal. In the activity-induced muscle pain model, females show widespread pain and increased SERT expression in the RVM, while males show localized pain and no changes in SERT expression. Testosterone protects from the development of widespread pain, and females have widespread pain in the activity-induced pain model. It was hypothesized that testosterone modulates 5-HT signaling to enhance analgesia in female mice with widespread pain. Testosterone reduced the enhanced SERT protein expression and increased 5-HT2A receptor mRNA expression in the RVM normally occurring in the activity-induced pain model in females, but not males. Inhibition of SERT in the RVM was analgesic in both female and male mice. This analgesia was blocked by co-administration of 5-HT2A antagonist. In situ hybridization demonstrated co-expression of SERT, 5-HT2A receptor, and androgen receptor mRNA in cells within the RVM in female mice. Ativation of androgen receptors using dihydrotestosterone reduced hyperalgesia in female mice. These data show expression of androgen receptors in the RVM in female mice, that activation of androgen receptors reduces nociceptive behaviors, and endogenous testosterone modulates SERT and 5-HT2 receptor expression (Plumb et al. 2025).
The 5-HT, NA and DA systems functionally interact with each other in complex manners. For example, DA inputs up-regulate 5-HT and down-regulate NA activity. 5-HT systems exert negative influences on NA and DA systems through 5-HT2A and 5-HT2C receptor-mediated mechanisms, respectively. On the other hand, while increased NA tends to depress DA activity, it can exert complex positive and negative influences on 5-HT neurotransmission, mediated through α1- and α2-adrenergic receptors, respectively. Finally, glutamate and GABA tend to have bi-directional regulatory influences on monoamines (Hamon and Blier 2013; Maletic and Raison 2009).
The RVM is a crucial site for the supraspinal anti-nociceptive actions of opioids. Spinally projecting 5-HT RVM neurons express μ-opioid receptors. Although 5-HT neurons comprise a minority of RVM neurons, they appear to be selectively apposed by an endogenous ligand of μ-opioid receptors, EM-2. Neurons containing EM-2 exist primarily in the dorso-medial HYP (DMH) and project to the RVM, and EM-2 participates in HYP stimulation-induced analgesia (Gu and Wessendorf 2007).
In RVM ON-cells, neurokinin-1 receptors (NK1Rs) of SP are co-expressed with NMDARs. Activation of NK1Rs through micro-injection of the NK1R agonist SP or capsaicin improved ON-cell responses evoked by NMDARs but not those of OFF-cells and promoted hyperalgesia (Peng et al. 2023).
In rats, ON- and OFF-type neurons were identified using noxious heat or mechanical stimuli applied to the tail, and the effect of SP NK1Rs in the RVM was determined using extracellular single-unit recording combined with micro-iontophoresis. Responses evoked by iontophoretic application of NMDA were determined before and after intra-plantar injection of capsaicin or iontophoretic application of SP. In OFF-cells, capsaicin produced an extended pause in ongoing activity but did not alter the subsequent spontaneous discharge rate or NMDA-evoked responses. By contrast, spontaneous discharge rates of ON-cells increased after capsaicin, and their responses to NMDA increased >100% above control values. The increased responses to NMDA after capsaicin were attenuated by iontophoretic application of a selective NK1R antagonist. Similarly to capsaicin, iontophoretic application of a selective NK1R agonist increased the spontaneous discharge rate and NMDA-evoked responses of ON-cells by >100% of control values. A subset of neurons in the RVM had labeled NK1Rs, and nearly all of these neurons were immuno-reactive for the NMDAR1 sub-unit of the NMDAR. Hence, activation of NK1Rs in the RVM enhanced responses of ON-cells evoked by NMDA. The activation of NK1Rs in the RVM and the ensuing sensitization of ON-cells may contribute to the development of central sensitization and hyperalgesia after tissue injury and inflammation (Budai et al. 2007).—RVM ON-cells were activated by noxious algesic and pruritic stimuli and were pro-nociceptive. Many RVM-spinal projection neurons expressed NK1Rs, and ON-cells were excited by local administration of SP. It has been hypothesized that NK1R-expressing RVM ON-cells exert an inhibitory effect on itch opposite to their pro-nociceptive action. Intra-medullary micro-injection of SP significantly potentiated RVM ON-cells and reduced pruritogen-evoked scratching while producing mild mechanical sensitization. Chemogenetic activation of NK1R-expressing RVM neurons also reduced acute pruritogen-evoked scratching. Optotagging experiments confirmed RVM NK1R-expressing neurons to be ON-cells. Hence, NK1R-expressing ON-cells in RVM play a significant role in the modulation of pruriceptive transmission (Follansbee et al. 2022).
In mice, SP modulated the anti-nociceptive action of intra-thecal morphine injections in paw-licking/biting response evoked by subcutaneous injection of capsaicin into the plantar surface of the hindpaw. The intra-thecal injection of morphine inhibited capsaicin-induced paw licking/biting in a dose-dependent manner. SP (25 and 50 pmol) injected intra-thecally alone did not alter capsaicin-induced nociception, whereas SP at a higher dose of 100 pmol significantly reduced the capsaicin response. Combination treatment with SP (50 pmol) and morphine at a sub-threshold dose enhanced the anti-nociceptive effect of morphine. This suggested that morphine-induced anti-nociception may be enhanced through SP (Komatsu et al. 2009).
Micro-injection of CCK exerted no direct effects on ON- or OFF-cells. Only at high doses (30 ng/200 nL), CCK selectively activated ON-cells and produced behavioral hyperalgesia, while CCK at low doses (10 ng/200 nL) attenuated opioid activation of OFF-cells and blocked opioid-mediated analgesia. The anti-opioid and pro-nociceptive effects induced by CCK are thus mediated by OFF- and ON-cells separately (Peng et al. 2023). Stress-induced hyperalgesia (SIH) caused by a prolonged stress stimulus is accompanied by increases in the concentrations of CCK receptors in the RVM. In rats, social defeat stress outcomes, including chronic hyperalgesia and anxiety-like behaviors, were attenuated by intra-RVM injection of a CCK antagonist (Pagliusi and Gomes 2023). Micro-injection of CCK at low doses into the RVM attenuated opioid activation of OFF-cells and blocked opioid-mediated analgesia without exerting direct effects on ON- or OFF-cells. Only administration of CCK at higher doses selectively activated ON-cells and produced behavioral hyperalgesia (Peng et al. 2023).
In awake rats, micro-injection of NT into the RVM produced a dose-dependent inhibition of the viscero-motor response (VMR) to noxious colo-rectal distension (CRD) that lasted 30 to 120 minutes. General motor function was unaffected after intra-RVM injection of NT. Intra-RVM injection of lesser doses of NT enhanced the VMR to noxious CRD that had a short duration (18-30 minutes) and produced a leftward shift of the stimulus-response function to graded CRD without a change in the slope of the function. Additionally, intra-RVM injection of the NT-receptor antagonist SR48692 in naive animals produced dose-dependent inhibition of VMR to noxious CRD, whereas a lesser dose enhanced the VMR Hence, endogenous NT in the RVM modulates VMR to noxious CRD via a prominent interaction with NT receptors that mediate facilitatory influences and a lesser interaction with NT receptors that mediate masked inhibitory influences (Urban et al. 1999).—Micro-injection of NT or the selective NT receptor sub-type 1 (NTR1) agonist PD149163 into the RVM produced dose-dependent anti-nociception, and could be partially blocked by intra-thecal yohimbine, an α-adrenoceptor antagonist, and by methysergide, a 5-HT receptor antagonist. Selective activation of NTR2 in the RVM also produced anti-nociception. Data indicated that activation of NTR1 in the RVM produced anti-nociception through spinal release of NA and 5-HT, and that activation of NTR2 in the RVM produced anti-nociception mediated by spinal release of NA (Buhler et al. 2008).—In lightly anesthetized rats, focal infusion of NT within the RVM were used to activate ON-cells selectively. In awake animals, NT produced a dose-related, bi-directional effect on nociception when applied within the RVM, with hyperalgesia at low doses, and analgesia at higher doses. A combination of single-cell recording and behavioral testing showed that ON-cells are activated selectively by low-dose NT, and that the activation of ON-cells by NT resulted in enhanced nociceptive responding, as measured by the paw-withdrawal reflex. Furthermore, higher NT doses recruited OFF-cells in addition to ON-cells, producing behavioral anti-nociception. Selective activation of ON-cells is thus sufficient to produce hyperalgesia, confirming the role of these neurons in facilitating nociception. Activation of ON-cells likely contributes to enhanced sensitivity to noxious stimulation or reduced sensitivity to analgesic drugs in a variety of conditions (Neubert et al. 2004).
Cannabinoid receptors consist of cannabinoid receptor 1 and 2 (CB1R, CB2R), among which CB1R is expressed in approximately one-third of PAG neurons and is co-expressed with μ-opioid receptors. Activation of PAG CB1R decreases GABA release and activates mGlu5R, leading to the inhibition of RVM ON-cells and disinhibition of OFF-cells, ultimately resulting in analgesia in both normal and neuropathic pain situations (de Novellis et al. 2005; Rea et al. 2007; Ossipov et al. 2010).—In rats, PAG cannabinoid or group I mGluRs are the involved in the formalin-induced changes on the RVM ON- and OFF-cell activities. Subcutaneous injection of formalin into the hind paw produced a transient decrease (4-6 minutes) followed by a longer increase (25-35 minutes) in tail-flick latencies. Formalin also increased the basal activity in RVM ON-cells and decreased it in OFF-cells. Intra-PAG micro-injection of a cannabinoid receptor agonist prevented the formalin-induced changes in RVM cell activities. Higher doses increased the tail-flick latencies, delayed the tail-flick-related onset to ON-cell burst, and decreased the duration of OFF-cell pause. These effects were prevented by a CB1 cannabinoid receptor antagonist, or by a selective mGlu5 glutamate receptor antagonist. Hence, subcutaneous injection of formalin may modify RVM neuronal activities, which is prevented by PAG cannabinoid receptor stimulation (de Novellis et al. 2005). The RVM endocannabinoid and opioid systems also have a critical role in acute SIA. The RVM is enriched with the CB1R, which produces analgesia when activated. Stress enhances glutamatergic signaling into the PAG, leading to local endocannabinoid production and signaling to an analgesic state. As to the ECS, a potentiation of the neurotransmission mediated by endocannabinoids within the RVM leads to an enhancement in SIA, which depends on the activation of local CB1 receptors (Pagliusi and Gomes 2023).
Cannabinoids have been associated with a variety of pathologies, such as chronic pain, brain injury, glaucoma, asthma, cancer and AIDS-associated effects. Motor disorders are also of concern because the control of movement is one of the more relevant physiological roles of the eCB transmission in the brain. Furthermore, Parkinson’s disease (PD) and Huntington’s chorea have a direct relationship with eCBs and their receptors with neurons that degenerate in those disorders. Finally, other neurological pathologies, such as Alzheimer’s disease or multiple sclerosis (MS) present a strong alteration in the control of movement (Fernández-Ruiz et al. 2002).
The RVM is thought to be the main site for opioid analgesia. Opioid receptors are abundantly expressed in the RVM, including the μ-opioid receptor, κ-opioid receptor, δ-opioid receptor, and nociception/orphanin FQ receptors, with especially high expression μ-opioid receptor on ON-cells. Activation of these receptors results in pre- and postsynaptic actions in the RVM in vitro, supporting the in vivo electrophysiological evidence showing direct inhibition of RVM ON-cells and indirect activation of OFF-cells. Dense ENK-containing fibers impinge on RVM ON-cells providing an anatomical substrate for the modulation of ON-cells by endogenous opioid peptides. Opioids in the RVM elicit anti-nociception via reducing the pause in RVM OFF-cells. ON-cell firing is correlated with hyperalgesia and systemic morphine reduces ON-cell firing consistent with the inhibition of ON-cell firing by iontophoresis of μ-opioid receptor agonists directly onto ON-cells in vivo (Bagley and Ingram 2020).
Local application of morphine in the RVM itself is sufficient to produce anti-nociception. As with systemic administration, iontophoretic application of morphine depressed the activity of ON-cells. By contrast, no reliable changes in OFF-cell firing were produced by iontophoretic administration of morphine. NEUTRAL cells were not affected. Hence, direct opioid responsiveness in the RVM was limited to the ON-cell, implying that the anti-nociceptive effect exerted by systemically administered morphine involves at least two components within the RVM: a direct inhibition of ON-cells, and an indirect activation of OFF-cells (Heinricher et al. 1992).
In the PAG-RVM system, μ-opioid-receptor- and δ-opioid-receptor—mediated inhibition of GABAergic neurons disinhibits glutamatergic (Glu) neurons projecting to the RVM. The administration of opioids in the RVM produces anti-nociception through direct inhibition of pro-nociceptive μ-opioid receptor-expressing ON-cells and indirect activation (i.e., disinhibition) of anti-nociceptive OFF-cells. Neurons co-expressing GABA and pre-pro-ENK functionally correspond to OFF-cells and directly project onto nociceptor terminals in the DH to inhibit nociceptive transmission. Other GABAergic RVM neurons express μ-receptors and project to pre-pro-ENK DH interneurons, facilitating the transmission of nociceptive information. Genetic approaches confirmed that μ-opioid receptors activate the PAG-RVM descending pathway via suppression of the inhibitory influence of local GABAergic interneurons (Tavares et al. 2021).—Opioids, but not eCBs, directly hyperpolarized RVM ON-cells. RVM OFF-cells paused firing in response to a nociceptive stimulus and just prior to the behavioral withdrawal from the stimulus. This pause response was reduced by opioids and eCBs, which prolonged the latency to withdraw from the stimulus, i.e., anti-nociception. The drugs elicited firing of OFF-cells by reducing GABAergic inputs to the cells. If OFF-cells discharged and did not pause, the behavioral output was analgesia, regardless of the activity of ON-cells (Bouchet and Ingram 2020). Activation of descending κ-opioid receptor neurons in the RVM, an exclusively GABAergic population, robustly inhibited nociceptive and pruriceptive behaviors, consistent with data indicating that κ-opioid receptor neurons correspond to OFF-cells (Nguyen et al. 2023).—One of the main sources of endogenous opioid release into the RVM is the HYP. Whereas RVM ON-cells express µ-opioid receptors, OFF-cells express κ-opioid receptors. In addition to its effects through the ECS, the RVM also enhances SIA via the endogenous opioid system. SIA was found to be mediated by RVM µ-opioid receptors. Chronic stress can increase the neurotransmission mediated by κ-opioid receptors during the switch from SIA to SIH. Systemic κ-opioid antagonism attenuated analgesia induced by social defeat stress, an effect probably mediated by spinal cord neurons expressing κ-opioid receptors. µ-Opioid receptor agonists inhibit ON-cells (pro-nociceptive) and activate OFF-cells (pro-analgesia). Since the activation of OFF-cells is mediated by PSI of GABAergic terminals, the ablation of µ-opioid receptor-expressing neurons in the RVM would negatively affect the pro-nociceptive pathway. Selective ablation of the RVM µ-opioid receptor-expressing neurons attenuated SIH. This may indicate that these receptors are involved in SIA and SIH by acting differentially through ON- or OFF-cells in the RVM (Pagliusi and Gomes 2023).
ACh signaling contributes to descending modulation of spinal nociceptive processing. By employing viral tracers in ChAT-Cre mice, direct projections of ACh neurons in the RVM to DH lamina III have been suggested. There is also evidence indicating that nicotinic ACh signaling in the brainstem nuclei stimulates descending inhibitory pathways and mediates anti-nociceptive effects (Naser and Kuner 2018).
The effects of GABA and glutamate on RVM ON- and OFF-cells are important, since their dysfunction may represent a specific marker of ON- or OFF-cells. Approximately two-thirds of RVM ON-cells contain express GABA. Administration of the GABAAR antagonist bicuculline methiodide caused analgesic effects, although ON-cells did not show a consistent change in activity (Peng et al.2023).
In barbiturate-anesthetized rats, the activity of a characterized RVM neuron and paw withdrawals to heat (plantar surface) were recorded. Following three baseline trials, mustard oil was applied to the skin above the knee. Cell activity and paw-withdrawal latencies were monitored for an additional 45 minutes. Mustard oil produced an increase in ON-cell firing associated with a substantial decrease in ipsilateral paw-withdrawal latency. Blocking ON-cell activation using local infusion of the NMDAR antagonist AP5 into the RVM prevented hyperalgesia. Secondary thermal hyperalgesia following mustard oil was also associated with a significant decrease in the firing of OFF-cells. Depression of off-cell firing was unaffected by AP5 micro-injection. The firing of NEUTRAL cell was unchanged following mustard oil and also unaffected by AP5 infusion in the RVM (Xu et al. 2007).—Descending facilitatory circuitry that involves the RVM exerts a significant role in the development of anti-nociceptive tolerance and hyperalgesia following chronic morphine treatment. Ketamine, an NMDAR antagonist, attenuates opioid anti-nociceptive tolerance. In male rats under light pentobarbital anesthesia, parallel recordings of RVM cell firing and limb withdrawal response were performed following sustained systemic treatment with morphine or oxycodone at equi-analgesic doses. On the sixth treatment day, ongoing activity and the response to noxious heat and pinch were determined in pro-nociceptive RVM ON-cells and anti-nociceptive OFF-cells. Chronic oxycodone induced anti-nociceptive tolerance both in limb withdrawal and RVM cell activity. Chronic morphine induced anti-nociceptive tolerance in limb withdrawal accompanied by pro-nociceptive heat response changes in RVM ON- and OFF-cells. A behaviorally sub-anti-nociceptive dose of acute ketamine reversed anti-nociceptive tolerance both to morphine and oxycodone in limb withdrawal and reversed the chronic morphine-induced pro-nociceptive discharge changes in RVM cells. Hence, NMDAR-dependent descending pro-nociceptive circuitry involving the RVM has an important role in behavioral anti-nociceptive tolerance to morphine but not oxycodone (Viisanen et al. 2020). Repeated intramuscular injections of glutamat increased the response of ON-cells to glutamate by altering the activity of the NMDAR. By micro-injection of the NMDAR antagonist AP5 into the RVM, only ON-cell activities were inhibited, while activated OFF-cells and NEUTRAL cells were not affected, exerting a block of hyperalgesia. In models of neuropathic and inflammatory pain, the injection of low-dose glutamate into the RVM facilitated nociception (Peng et al. 2023).
The effects of GABA and glutamate on RVM ON- and OFF-cells are important, since their dysfunction may represent a specific marker of ON- or OFF-cells. Approximately two-thirds of RVM ON-cells contain express GABA. Administration of the GABAAR antagonist bicuculline methiodide caused analgesic effects, although ON-cells did not show a consistent change in activity (Peng et al.2023).
Data indicate a co-expression of μ-opioid receptor and GABA in ON-cells. Activation of μ-opioid receptor or δ-opioid receptor produced a concentration-dependent decrease of GABA overflow in the RVM, reduced inhibitory GABAergic activity, and directly hyperpolarized ON-cells. OFF-cells are also GABA neurons. Activating GABAB receptors at low doses facilitated OFF-cells, while these were inhibited at high doses (Peng et al. 2023). Thus, there appear to be more descending pain-modulating patways. In vivo opto- or chemogenetic manipulations and trans-synaptic tracing of genetically identified RVM and DH neurons revealed an RVM-spinal cord-primary afferent circuit controlling pain thresholds. RVM GABA neurons facilitated mechanical pain by inhibiting DH ENK/GABAergic interneurons. These interneurons gated sensory inputs and controlled pain through temporally coordinated ENK- and GABA-mediated PSI of somatosensory neurons. This descending disynaptic inhibitory circuit facilitated mechanical pain, and is engaged during stress (François et al. 2017). This may provide a potential mechanism for ON-cell-induced pain facilitation (Peng et al. 2023). RVM neurons containing the NK1R, which are also GABAergic, were ON-cells that facilitated nociceptive responses but suppressed pruritogen-induced scratching behavior (Nguyen et al. 2023).
2.5.3. Caudal Ventro-Lateral Medulla (CVLM)
The CVLM is an important component of the supraspinal pain modulatory system. Relevant to the functions of the CVLM in pain modulation appear to be NA and angiotensin II. Administration of NA or the α2-adrenoreceptor agonist clonidine into the CVLM inhibited local neurons and produced hyperalgesia. Angiotensin II injected into the CVLM induced hyperalgesia which was mediated by local angiotensin type 1 receptors (AT1 receptors). By a selective manipulation of the projections from the NA A5 cell group to the CVLMlat, it was proposed that CVLMlat neurons expressing AT1 receptors activate NA A5 neurons, which will inhibit nociceptive transmission at the spinal cord. Another important neurochemical control system in the CVLMlat is mediated by opioids. In the VLM, μ-opioid receptors are expressed mainly by CVLMlat neurons that do not project to the spinal cord and overexpression of opioids in the CVLM induces anti-nociceptive effects (decreased behavioral nociceptive responses) and lower nociceptive spinal neuronal activation (Martins and Tavares 2017).
Pain modulation from the CVLM is partially relayed by spinally projecting noradrenergic neurons of the pontine A5 cell group, which leave collateral fibers at the CVLM. The injection of angiotensin II (Ang II) into the CVLM induced hyperalgesia mediated by angiotensin type 1 (AT1) receptors, expressed by CVLM neurons that do not project to the spinal cord. The effects were evaluated by lesioning the NA pontine A5 cell group by the retrograde transport of the selective toxin anti-DA beta-hydroxylase-saporin (anti-DBH-SAP) from the CVLM in pain behavioral responses elicited by Ang II injection into the CVLM. The injection of anti-DBH-SAP induced neurodegeneration restricted to the NA A5 cell group and confirmed by the decrease in the number of NA neurons only in the A5 group. Pain behavioral evaluation using the formalin test showed that Ang II injection into the CVLM induced hyperalgesia, which was partially prevented by lesion of the NA A5 cell group with anti-DBH-SAP. Immuno-staining of AT(1) receptors in CVLM neurons, retrogradely labelled from the NA A5 cell group, showed that CVLM neurons that project to the A5 cells express AT(1) receptors, indicating that Ang II can directly modulate the CVLM-A5 connection. Hence, Ang II-induced hyperalgesia elicited from the CVLM is mediated by an indirect pathway relayed at the pontine NA A5 group (Marques-Lopes et al. 2010).
The 5-HT system also has a dual action depending on the targeted spinal receptor, with an exacerbated activity of the excitatory 5-hydroxytryptamine 3 (5-HT3) receptors in neuropathic pain models (Tavares et al. 2021). The 5-HT1A receptors are implicated in the central mechanisms of visceral pain. Data suggest organic inflammation-triggered neuroplastic changes in the brain 5-HT circuitry, whereby the contribution of 5-HT1A receptors to supraspinal control of visceral pain in normal and post-inflammatory conditions can be assumed. In male Wistar rats, micro-electrode recordings from the CVLM neuron responses to CRD and electromyography (EMG) recording of CRD-evoked viscero-motor reactions were evaluated post-colitis changes in the effects of 5-HT1A agonist buspirone on supraspinal visceral nociceptive transmission. In rats recovered from colitis, the CRD-induced CVLM neuronal excitation and viscero-motor reactions were increased compared with those in healthy animals, revealing post-inflammatory intestinal hypersensitivity. Intravenous buspirone dose-dependently suppressed CVLM excitatory neuron responses to noxious CRD in healthy rats, but caused dose-independent increases in the already enhanced nociceptive activation of CVLM neurons in post-colitis animals. These data indicate a shift from anti- to pro-nociceptive contributions of 5-HT1A-dependent mechanisms to supraspinal transmission of visceral nociception in intestinal hypersensitivity conditions (Lyubashina et al. 2023).
An important neurochemical control system in the CVLMlat is mediated by opioids. In the CVLM, μ-opioid receptors are expressed mainly by CVLMlat neurons that do not project to the spinal cord and overexpression of opioids in the CVLM induces anti-nociceptive effects (decreased behavioral nociceptive responses) and lower nociceptive spinal neuronal activation (Martins and Tavares 2017).
2.5.4. Dorsal Reticular Nucleus (DReN)
Pain facilitation from the DReN is modulated by several neurotransmitters such as NA, opioid peptides, glutamate and GABA (Martins and Tavares 2017).
NA has a dual action in descending pain control. It exerts anti-nociception due to inhibitory effects on the spinal cord. The NA system may induce pro-nociception by directly acting on brainstem pain modulatory circuits, namely, at the LC and medullary DReN (Tavares et al. 2021). NA release in the DReN, measured by in vivo micro-dialysis, increased during the formalin test. The reduction of NA release in the DReN by genetic manipulation of DReN-NA afferents significantly attenuated pain behavior in the formalin test while increasing local extracellular concentrations of NA, by inhibiting its recapture, produced the opposite effect (Martins et al. 2013). The genetic manipulation of DReN-NA afferents was performed by a viral vector derived from the Herpes Simplex virus type 1 (HSV-1) which is retrogradely transported from the DReN to its NA afferents, namely the LC and NA A5 cell group, where it selectively reduces NA synthesis The pain facilitatory actions of NA in the DReN are mediated through activation of α1-adrenoreceptors (Martins et al. 2010).
In the DReN, opioids are an important local modulatory system that can directly and indirectly modulate the spinal-DReN-spinal reverberative pathway. Opioids act through direct inhibition of DReN spinally projecting neurons that express μ-opioid receptors and through disinhibition of ENK interneurons that receive input from GABAergic interneurons that express μ-opioid receptors. These GABAergic interneurons are also presynaptically inhibited by δ-opioid receptor-expressing fibers. Local over-expression of opioid peptides, namely ENK, inhibited DReN pain facilitation. The activation of μ-opioid receptors in the DReN plays a fundamental inhibitory role in the DReN (Tavares et al. 2021). δ-Opioid receptors may indirectly modulate the activity of non-projecting DReN neurons, whereas neurons expressing μ-opioid receptors project to the spinal DH or act as interneurons, the latter of which co-expressing GABAB receptors. In mono-arthritic rats, the expression of μ-opioid receptors decreased in the DReN whereas the concentrations of endogenous ENK remained unaltered (Pinto et al. 2008a). Opioids inhibit DReN pain facilitation. DReN neurons expressing μ-opioid receptors project to the spinal DH or act as interneurons, the latter of which co-expressing GABAB receptors. Opioids also act in the DReN through additional inhibitory mechanisms, likely by disinhibiting ENK interneurons. Opioidergic neurons in the DReN are modulated by GABAergic cells thereby controlling the descending facilitation of pain transmission. The DReN exhibits plastic changes during chronic inflammatory pain, with decreases of opioid receptor expression, which may account for increased descending facilitation during chronic pain (Pinto et al. 2008a). Opioids responsible for the anti-hyperalgesic action are mostly released from local interneurons but also from DReN afferent sources namely the RVM, the NA A5 cell group and the HYP (Martins and Tavares 2017). During neuropathic pain, the opioidergic modulation of brainstem pain control areas is altered, with the release of enhanced local opioids along with reduced expression and de-sensitization of μ-opioid receptors. In the DReR, the installation of neuropathic pain increases the levels of ENKs and induces de-sensitization of μ-opioid receptors, which may enhance descending facilitation from the DReN and impact the efficacy of exogenous opioids (Tavares et al. 2021).
Glutamate plays an important role in the pro-nociceptive actions of the DReN during the formalin test since the blockade of AMPA/KA, NMDA and mGlu1 glutamate receptors by the local administration of the respective antagonists significantly reduced formalin-induced pain behavior which was accompanied by a reduction of c-fos expression at both the superficial and deep dorsal laminae (Ambriz-Tututi et al. 2013). The tonic activity of glutamate at the DReN likely results from the sustained peripheral afferent input, induced by formalin injection, leading to increased activation of spino-DReN-spinal reverberative circuits (Martins ad Tavares 2017).
GABA is involved in the mediation of pro-nociception from the DReN. GABA release in the DReN, measured by in vivo microdialysis, increased during the formalin test and it increased DReN pain facilitation through activation of GABAB receptors. Indeed, GABAB receptors knock-down at the DReN, mediated by lentiviral vectors, or the pharmacological blockade, via the local administration of a GABAB antagonist, significantly attenuated formalin-induced pain behavior while the local administration of a GABAB agonist induced the opposite. The effect of GABA is likely due to disinhibition of the DReN spinally projecting neurons since a large proportion of GABAB receptors are expressed by local opioidergic neurons inhibiting DReN spinally projecting neurons. GABA might be released from local interneurons but also from IC, somatosensory and motor cortices, which represent the most important afferent pathways to the DReN and they are GABAergic (Martins ad Tavares 2017).
A 30-year-old man arrived at the emergency department following a high-speed motor vehicle collision with severe, burning mid-thoracic pain (T6–T8 dermatomes, rated 9/10 in intensity). The pain was unrelieved by non-opioid analgesics and showed poor response to morphine administration. Accompanying autonomic signs, including diaphoresis and tachycardia was observed, without obvious spinal cord injury on initial CT scan. Neurological exam was largely intact, except for mild allodynia and hyperalgesia to light touch and pinprick in the mid-thoracic region. Reflexes were preserved. MRI spine revealed no cord compression but subtle edema in the dorsal horn of the spinal cord at the level T7, suggestive of transient contusion. CSF analysis ruled out infection. Functional MRI revealed altered functional connectivity between the ACC and vlPAG. The patient’s ineffective response to μ-opioid analgesia suggest overactivation of descending facilitation, likely triggered by initial peripheral injury and spinal DH sensitization. Trial of low-dose ketamine infusion was initiated to reduce central sensitization and interrupt NMDA-mediated glutamatergic transmission within the PAG and spinal DH. Within 12 hours, pain was reduced to 4/10. This case illustrates how disruption of the PAG-RVM-DH triad and its neurochemical modulators—opioids, glutamate, GABA, and monoamines—can precipitate a refractory acute pain syndrome. Functional imaging and mechanistic insights allowed for a tailored therapeutic approach that restored the balance between descending inhibition and facilitation. After 48 hours, pain was manageable (2/10), and the patient was discharged on a tapering course of Pregabalin.
2.6. Nucleus Tractus Solitarii (NTS)
The NTS contains a great diversity of neuroactive substances. Indeed, most of the substances identified within the CNS have also been detected in the NTS and may act, at this level, as classical transmitters and/or neuromodulators (Jean 1991).
The caudal NTS is involved in pain control and the cough reflex. There are similarities between the characteristics of central processing of nociceptive and cough-related inputs. GAL receptors have been found in the NTS and play a role in the inhibitory control of the cough reflex at the level of the caudal NT (Mutolo et al. 2014).
The NTS contains POMC neurons, one of the two major sources of β-endorphin in the brain. In behaving mice, optogenetic and chemogenetic activation of NTS POMC neurons produced sustained thermal analgesia that could be blocked by naloxone. It also produced analgesia in an inflammatory pain model (carrageenan) but not in a neuropathic pain model (tibial nerve transection). Inhibiting NTS POMC neurons did not produce any effect on basal nociception but inhibited (stress-induced analgesia: SIA), unlike inhibition of HYP ARC POMC neurons. This indicates that NTS POMC neurons play a role in the generation of endogenous endorphinergic analgesia and can regulate cardio-respiratory function (Patra et al. 2023).
Decreasing the expression of NMDAR in the NTS using gene transfer to target receptor sub-units and evaluating long-term effects showed that the NR1 NMDAR sub-unit is critical in the regulation of tonic cardio-vascular and nociceptive control (Marques-Lopes et al. 2012).
2.7. Parabrachial Nucleus (PBN)
In the rat, formalin, injected into the plantar skin of one hindpaw, induced expression of c-fos in the ipsilateral spinal DH and the contralateral PBN. Two medetomidine doses administered 12 minutes before formalin strongly suppressed the expression of c-fos in the DH; and in the PBN. Atipamezole produced a significant attenuation in the spinal cord and a complete reversal in PBN of the medetomidine-induced suppression (Pertovaara et al. 1993).
The nocioceptive information carried by nociceptive neurons of the PBN to neurons of the lateral division of the central amydala (CeA-L) is considered to contribute to the affective components of pain and is required for the formation of conditioned-fear memories (Delaney and Crane 2016). Single-fiber inputs from the PBN onto the CeA-L neurons form supra-threshold glutamatergic synapses with multiple release sites. This synapse is potently inhibited by NA, acting at presynaptic α-2 receptors. This is mechanism of presynaptic modulation where the output of a large multiple-release-site synapse is effectively regulated by endogenously released NA (Delaney et al. 2007).
The PBN is critically involved in aversive processes, and chronic pain is associated with amplified activity of PBN neurons in rodent models of neuropathic pain. In mice, catecholaminergic input from the caudal NTS (cNTS) caused amplification of PBN activity and their sensory afferents. Noxious mechanical and thermal stimuli activated cNTS neurons. These stimuli also produced prolonged NA transients in PBN that far outlast the noxious stimuli. Similar NA transients could be evoked by focal electrical stimulation of cNTS, a region that contains the NA A2 cell group that projects densely on PBN. In vitro, optical stimulation of cNTS terminals depolarized PBN neurons and caused a prolonged increase the frequency of excitatory synaptic activity. A dual-opsin approach showed that sensory afferents from the caudal spinal trigeminal nucleus (SpV) were potentiated by cNTS terminal activation. This suggests that A2 neurons of the cNTS generate long-lasting NA transients in PBN, which increase excitability and potentiate responses of PBN neurons to sensory inputs (Ji et al. 2023).
Endomorphin-2 (EM2) has been localized to many CNS regions, including those that regulate anti-nociception, autonomic function, and reward. Colocalization or shared distribution (overlap) of two neurotransmitters, or a transmitter and its cognate receptor, may imply an interaction of these elements in the regulation of functions mediated in that region. EM2 was co-localized with SP and CGRP in the NTS and with SP, CGRP and MOR in the PBN (Greenwell et al. 2007).
Opioids can induce complex neuro-adaptations, including in synaptic transmission.—In male and female Sprague Dawley rats, the use of patch-clamp recordings in acute brainstem slices demonstrated a concentration-dependent, bimodal effect of opioids on excitatory synaptic transmission. While a lower concentration of DAMGO, a selective agonist of the μ-opioid receptor, induced long-term depression (LTD) of synaptic strength (low-DAMGO LTD), abrupt termination of a higher concentration induced a high-DAMGO LTP in a sub-population of cells. LTD involved a mGluR-dependent mechanism. In contrast, LTP required astrocytes and NMDAR activation. Selective optogenetic activation of spinal and PAG inputs to the lateral parabrachial nucleus (lPBN) revealed that, while LTD was expressed at all PBN synapses; LTP was restricted to spino-parabrachial synapses. Thus, there exists opioid-induced long-term plasticity in the PBN that potentially modulates some adverse effects of opioids (Mussetto et al. 2023).
Excitatory transmission between PBN axon terminals and CeA-L neurons can be inhibited by a number of presynaptic receptors, including α2-adrenoceptors (above) and GABAB receptors. Activation of presynaptic GABAB receptors reduced this excitatory transmission by inhibiting N-type Ca2+ channels (Delaney and Crane 2016).
2.8. Hypothalamic and Midbrain Dopamine (DA) Neurons
DA is a neurotransmitter, synthesized in both CNS and the periphery. DA can bind to different G protein-coupled receptors (GPCRs) of five different sub-types: D1, D2, D3, D4, and D5. DA may act both as an inhibitory and excitatory neurotransmitter in presynaptic neurons expressing D1-like receptors, depending on the downstream opening of K+ or Na+ channels. DA receptors are widely expressed in the CNS, but are also found peripherally in blood vessels, kidneys, heart, retina, and adrenals controlling catecholamine release and the renin–angiotensin system. In the brain, D1 and D2 are the most abundantly expressed DA receptors (D1 being the highest), and the two are rarely co-expressed in the same cells (Klein et al. 2018).
2.8.1. Hypothalamic Dopamine (DA) Cell Cluster
The dorsal posterior HYP contains a DA cluster called A11 cell group. These neurons, approximately 300 in rats and 130 in mice, project to the neocortex, which might be related to changes in the perception of ascending sensory information; 5-HT DRN, promoting cardio-vascular and sympathetic activity. They also send descending projections as the source of spinal DA. The terminals are most concentrated in the superficial sensory-related DH and inter-medio-lateral nucleus. The loss of A11 neurons causes a disinhibition of sensory inputs and favors the occurrence of abnormal visceral or muscular sensations. The spinal cord of rats, cats, monkeys, and humans express DA receptors D1, D2, and D3. DA and D2 agonists can depress the monosynaptic reflex amplitude, dependent on D3 receptors, since this effect was absent in D3 knockout mice. Hence, A11 modulatory neurons could hypothetically inhibit spinal somatosensory and sympathetic autonomic circuits (Klein et al. 2018).
2.8.2. Midbrain Dopamine (DA) System
The midbrain DA system, including VTA, substantia nigra pars compacta (SNc), retrorubral field (RRF), is diverse, with complex neurochemical, connectional, and physiological diversity. In primates, a dorsal tier of the DA neurons receives inputs from the ventral (limbic) striatum and the AMY and project widely throughout the cortex. A more ventrally located DA group receives inputs from the limbic and association areas of the striatum and project widely throughout the striatum including sensory-motor areas. These projections enable the DA system to affect a wide range of behaviors (Haber and Fudge 1997). In humans, projections of DA neurons from the SNc to the dorsal striatum, known as the nigro-striatal pathway, control movement and motor skill learning. The VTA DA-expressing neurons influence neural systems subserving important adaptive functions such as arousal and locomotion, control of intended movements, motivation, reward, maternal and reproductive behaviors,.re-inforcement, learning and state of mind, as in, e.g., wanting and willingness to exert effort. The midbrain DA system is a structure that also contains locally and distantly projecting neurons that utilize as transmitters, either co-expressed with DA or separately, GABA, glutamate, CCK and NT and possibly yet unknown compounds (Haber and Fudge 1997; Yetnikoff et al. 2014). In the VTA, the different kinds of neurons interact via intrinsic connections and have differentiated external inputs and outputs (Morales and Margolis 2017).
The meso-limbic DA system comprises neurons in the VTA and SNc, which send DA projections to the NAc. This system was originally described to mediate pleasure and goal-directed movement associated with rewarding stimuli. However, DA, although crucial for reward processing, drives not the hedonic experience of reward (‘liking’) but rather the instrumental behavior of reward-driven actions (‘wanting’). Phasic DA acts as an incentive salience signal underlying reinforcement learning. Moreover, aversive stimuli, such as pain, also stimulate DA. Recent evidence suggests that DA neurons in the VTA and SNc are heterogeneous populations tuned to either (or both) aversive or rewarding stimuli (Taylor et al. 2016). The meso-limbic DA system plays a central role in motivated behaviors, including various types of reward and pleasure. Many DA neurons may release multiple neurotransmitters, and the physiological role of the co-release of these transmitters has been revealed incrementally. Indeed, the meso-limbic DA system and small molecules released in the NAc could contribute to pain modulation (Watanabe and Narita 2018). In the NAc core, DA binds preferentially to D2 receptors. Micro-dialysis supported the hypothesis that pain alleviation is modulated by changes in DA levels in the NAc. In pain processing, DA pathways are used between NAc, VTA and medial PFC (mPFC) (Harris and Peng 2020; Mitsi and Zachariou 2016).
DA neurotransmission has an important role in modulating pain perception and natural analgesia within supraspinal regions. The analgesic effects of DA receptors, particularly D1 and D2 receptors are different in different CNS regions, including the ACC, IC, striatum, NAc, THAL, PAG and spinal cord. These regions express a high density of DA receptors, well suited for pain modulation. D2-like receptors may exert a higher analgesic potency, but D1-like receptors act in different manners across several mechanisms in the mentioned regions. In the striatum and spinal cord, anti-nociception of DA is mainly mediated by D2-like receptors, while in the NAc and PAG, both D1- and D2-like receptors are involved as analgesic targets. D2-like receptor agonists can act as adjuvants of μ-opioid receptor agonists to potentiate analgesic effects and provide a better approach to pain relief (Wang et al. 2021; Wood 2008). Decreased levels of DA likely contribute to the painful symptoms that frequently occur in PD. In addition, abnormalities in DA neurotransmission have been revealed in painful clinical conditions, including burning mouth syndrome, fibromyalgia (FM) and restless legs syndrome. A role for DA in chronic regional pain syndrome and painful diabetic neuropathy has also been suggested (Wood 2008).—Conditioned pain modulation (CPM) is a psychophysical paradigm based on endogenous descending inhibitory pain modulation. In healthy subjects, CPM was assessed by subtracting the response to a phasic painful heat stimulus administered simultaneously with a conditioning cold pain stimulus from the response to the same heat stimulus administered alone. CPM was applied prior to and 25 minutes following a subcutaneous injection of either apomorphine (a DA agonist) or a placebo. CPM following apomorphine administration increased by 27.3% and by only 4% following placebo administration. This suggest that DA pathways both participate in and enhance pain modulation, represented by CPM (Treister et al. 2013).—DA neurons in the SN projecting to the striatum form the nigro-striatal pathway, which participates in the control of motor function and learning capabilities. This pathway controls procedural aspects of movements and motivated behaviors, since it projects to more dorsal basal ganglia (BG) areas where behavioral and cognitive habits are learned and stored. The nigro-striatal DA system is also involved in central pain modulation, in which inhibition is modulated by D2 receptors, without the involvement of D1-like receptors (Klein et al. 2018)
The analgesic effects of DA receptors, particularly D1 and D2 receptors are different in different regions of the CNS, including the striatum, NAc, PAG and spinal cord. These regions express a high density of DA receptors. Thus are well suited for pain modulation. D2-like receptors may exert a higher analgesic potency, but D1-like receptors act in different manners across several mechanisms in the mentioned regions. In the spinal cord and striatum, anti-nociception of DA is mainly mediated by D2-like receptors, while in the NAc and PAG, both D1- and D2-like receptors are involved as analgesic targets. D2-like receptor agonists can act as adjuvants of μ-opioid receptor agonists to potentiate analgesic effects and provide a better approach to pain relief (Wang et al. 2021).
HYP DA located in the posterior region of the HYP (paraventricular nucleus: PVN) are produced by A11 neurons, which project to all levels of the spinal cord and provide the main source of spinal DA. DA receptors are expressed in primary nociceptors and spinal neurons located in different DH laminae, suggesting that DA can modulate pain signals by acting at both presynaptic and postsynaptic targets, and may thus influence the excitability of primary nociceptors and synaptic transmission to DH neuron. DA appears to exert both anti-nociceptive effects mediated by D2-like receptors and pro-nociceptive effects mediated by D1-like receptors (Puopolo 2019).
An example of DA actions on spinal nociceptive reflexes is the following. In high spinal cats, noxious radiant heat induced reflex facilitation, with an early component being mediated by group III fibers and a late component by group IV fibers. After injection of L-DOPA, the onset of reflex facilitation induced by noxious radiant heat was delayed by 4 to 10 s, i.e., the early component was blocked, while the late component persisted. Presumably, therefore, DA preferentially blocks the transmission in nociceptive reflex pathways from group III fibers (Schomburg et al. 2011a, 2011b, 2012, 2013, 2015).
In the rat, immediately after foot-shock termination, extracellular DA concentrations were increased in the NAc shell but remained unaltered in the NAc core. Such activation, especially in the ventral striatum and NAc, also occurred after the application of acute noxious (thermal) stimulus. In rodents, voltammetry showed changes in NAc DA release upon termination of a noxious stimulus (tail-pinch). DA release in the NAc was promoted by noxious tail stimulation and local VTA micro-injection of capsaicin. On the other hand, non-DA neurons in the VTA of anesthetized rats were excited by aversive stimuli, including pain. Moreover, fMRI studies in both humans and rodents showed that the offset of a noxious stimulus resulted in increased activation of the meso-limbic DA system (Mitsi and Zachariou 2016).
Pain relief has a rewarding effect engaging activation of midbrain DA neurons, release of DA in the NAc, and opioid signaling in the ACC (Harris and Peng 2020; Kuner and Kuner 2021; Mitsi and Zachariou 2016; Navratilova et al. 2024). Anticipation and anxiety of pain that enhance pain experience, activate brain regions including the PFC, entorhinal cortex (EC), anterior IC (aIC), AMY, ventral brainstem areas and PAG (Neugebauer et al. 2009; Tracey and Mantyh 2007). The cortico-limbic system is rich in opioids and opioid receptors. There is preclinical evidence for their pain-modulatory effects in different regions of this highly interactive system, and potentially opposing functions of different opioid receptors. ACC’s and AMY’s role in μ-opioid-dependent analgesia is established, and μ-opioid actions in the meso-limbic system appear to be similar but remain to be determined in mPFC regions other than ACC. κ-opioid signaling generally serves opposing functions whereas δ-opioid signaling in the ACC has similar, if not synergistic effects, to μ-opioid (Neugebauer et al. 2023).
In naive, sham, or spinal nerve ligated (SNL) rats, morphine was micro-injected into three regions of the ACC or into the RVM, and pain behaviors were evaluated. In naive animals, the tail-flick response was inhibited by morphine in the RVM, but not ACC. Within the RVM, opioids inhibited nociceptive transmission reflected in both withdrawal thresholds and affective pain behaviors. Activation of μ-opioid receptors within specific rostral ACC circuits, however, selectively modulated affective dimensions of ongoing pain without altering withdrawal behaviors. This suggests that ACC and RVM opioid circuits differentially modulate sensory and affective qualities of pain, allowing for optimal behaviors that promote escape and survival (Gomtsian et al. 2018).
It has commonly been suggested that DA is anti-nociceptive by virtue of its D2 receptors. Some work in humans supports this notion by showing increased affective pain ratings after dietary DA depletion and increased conditioned pain modulation with D2-receptor activation. However, on a variety of pain tests, no effects of DA manipulations have been reported. Observations suggest that the common feature is a motivational-emotional component of the pain tests. In rodent studies, tonic pain assays, such as the formalin or writhing test, frequently reveal decreases in pain behavior with D2-receptor activation than brief phasic pain stimuli, such as tail flick, hot plate, or paw pressure. In rats with ongoing post-surgical pain, blocking DA release prevented conditioned place preference (CPP) associated with peripheral analgesia. In humans, DA manipulations have merely been shown to affect the affective component of pain or strong behaviorally relevant stimuli such as immersion of the hand in ice water. Moreover, striatal DA release positively correlated with the magnitude of perceived pain, which strongly contradicts direct anti-nociceptive effects of DA release. Hence, DA release is no longer equated with pleasure or reward. Instead, DA neurons are considered a heterogeneous population of neurons that respond to both appetitive and aversive stimuli to mediate motivated behavior. Release of DA after an acute painful stimulus may act as a salience cue and be critical for approach or avoidance behavior (Taylor et al. 2016).
A 59-year-old man presented with recurrent, activity-induced episodes of yawning and fatigue, specifically triggered by mild exertion of his right leg (e.g., pushing a bicycle). These symptoms emerged following an L4-5 disc herniation but were not directly related to radicular pain anymore. Neurological, endocrine, and cardiopulmonary evaluations were unremarkable. Provocation testing with apomorphine (a DA receptor agonist) replicated symptoms, while subcutaneous and oral morphine (µ-opioid receptor agonist) effectively relieved them, suggesting dysregulated dopaminergic signaling—likely involving hypothalamic D3 receptor pathways—alongside potential autonomic dysfunction. He was successfully treated with a low-dose oral morphine regimen prior to physical exertion, preventing the yawning-fatigue attacks (Dibaj et al. 2020). In a follow-up study, the same patient underwent a structured graded activity program. Over several months, the intensity and duration of physical exertion were incrementally increased. Eventually, the patient was able to perform the same physical activities without needing opioids and remained symptom-free, indicating successful desensitization (Dibaj et al. 2021; Dibaj and Windhorst 2024b). This outcome suggests that graded physical therapy can modulate the underlying neurophysiological circuits, offering a non-pharmacologic approach to managing similar dopaminergic fatigue syndromes.
2.9. Brainstem Noradrenergic (NA) Cell Groups
NA typically regulates sleep patterns, focus, and alertness, while adrenaline controls the adrenal glands, sleep, alertness, and the fight-or-flight response. As a major monoamine neurotransmitter, DA has essential roles regulating motoneurons (MNs), spatial memory function, motivation, arousal, reward and pleasure, as well as in lactation, sexual behavior, and nausea (Klein et al. 2018).
In addition to the axis from PAG via RVM to the DH and SpV axis, other brainstem regions are able to significantly modulate incoming peripheral nociceptive signals. One source are brainstem NA neurons. The LC releases NA that can have both inhibitory and facilitating effects on neurotransmission throughout the CNS via the activation of α1 and α2 adrenoreceptors (Mills et al. 2021). NA is mainly emitted by peripheral sympathetic nerve fibers and central brainstem cell groups A1-A7.
Multiple separate and distinct descending inhibitory systems are capable of modulating spinal nociceptive transmission. Brainstem sites previously considered to be primarily involved in cardio-vascular function and autonomic regulation (e.g., NTS; LC/subcoeruleus; A5 cell group; lateral reticular nucleus) play a role in the modulation of spinal nociceptive transmission. From these brainstem sites, spinal monoamines (NA and 5-HT) mediate stimulation-produced descending inhibition of nociceptive transmission. The LC/subcoeruleus, PBN, the Kölliker-Fuse nucleus and the A5 cell group are possible sources of the spinal NA innervation involved in the centrifugal modulation of spinal nociceptive transmission. There is evidence suggesting that the LC/subcoeruleus plays a significant role in a functionally important descending inhibitory NA system. Focal electrical stimulation in the LC produced anti-nociception and increased the spinal content of NA metabolites. The inhibition of the nociceptive withdrawal tail-flick reflex (TFR) produced by electrical stimulation in the LC/subcoeruleus is mediated by postsynaptic α2-adrenoceptors in the lumbar spinal cord. Similarly, electrical or chemical stimulation of the LC/subcoeruleus inhibited noxious-evoked DH neuronal activity (Jones 1991).
Brainsten NA cell groups exert pain control via actions on the DH. The LC is the largest NA cell group in the brain and is involved in the descending modulation of pain, mainly through direct spinal cord projections and indirect effects on RVM activity via NA projections. NA exerts intrinsic control of pain through action on α1- and α2-adrenoceptors. In the spinal DH, NA released from descending pathways originating in the pontine A5-A7 cell groups attenuates pain by inhibitory action on α2A-adrenoceptors on central terminals of primary afferent nociceptors (PSI), by direct α2-adrenergic action on spinal pain-relay neurons (postsynaptic inhibition), and by α2-adrenergic activation of inhibitory interneurons. Moreover, α2C-adrenoceptors on axon terminals of excitatory interneurons might contribute to spinal control of pain (Pertovaara 2013).
2.10. Hypothalamus (HYP)
The HYP is a diencephalic complex structure in the basal forebrain (BFB), consisting of several nuclei (Takayanagi and Onaka 2021). The HYP comprises thousands of distinct cell types that form redundant yet functionally discrete circuits (Fong et al. 2023).
The HYP is involved in multiple functions serving homeostasis, which is defined as the maintenance of the internal environment that includes physiological variables such as heart rate, blood pressure, body temperature and blood sugar concentration within a certain narrow ranges. Specific functions include stress responses, control of arousal, regulation of sleep/wake cycles, regulation of body temperature and metabolism, feeding behavior, and reproductive behavior etc. (Takayanagi and Onaka 2021).
Nociceptive inputs reach the HYP via several pathways. Some of the substances produced in the HYP are involved in pain modulation. Neurons of the HYP PVN release CRH, which modulates stress response by acting on various brain regions. The HYP OXT, AVP, ORX and DA are important modulators of pain and emotional processing (Donadon et al. 2018; Jurek and Neumann 2018; Kuner and Kuner 2021;
Figure 3). The STTr sends collaterals to HYP, also indirectly via the noradrenergic A1 cell group (
Figure 3; Kuner and Kuner 2021). The HYP receives converging nociceptive and visceral inputs from the spinal DHs and trigeminal nuclei (Benarroch 2006), and direct and indirect (via PBN and NA A1 cells) nociceptive inputs from the STTr (Kuner and Kuner 2021). In mice, formalin injection induced significantly increased expression of
fos in the PVN, among which OXT-containing neurons are one neuronal phenotype. Under inflammatory pain, neurons in the lPBN may play essential roles in transmitting noxious information to the PVH (Ren et al. 2024).—The different HYP nuclei receive and emit differentiated inputs and outputs. By and large, in addition to nociceptive inputs, the HYP receives direct or indirect inputs from somatic and visceral sensory receptors of different kinds, as well as from the HIPP formation, gyrus cinguli, piriform cortex, orbito-frontal cortex (OFC), mammilary body, septum, AMY,
THAL, from retinal, olfactory and auditory fibers, from the brainstem RF, PAG, raphé nuclei, LC and NTS (Brodal 1981).
In part, the efferent HYP projections are reciprocal to the afferent inputs. Among ‘ascending’ connections, the mamillary tract to the anterior THAL nucleus is the most massive. Other efferents target the septum, HIPP, pulvinar, AMY, vlPAG, pretectal area, superior colliculi, midbrain RF, raphé nuclei, LC, NTS, dorsal motor nucleus of the vagus, pre-ganglionic visceral nuclei, inter-medio-lateral cell column (IML) of the spinal cord (Brodal 1981). There is a descending HYP-DH DA system. In the adult albino rat, cells in the PVN project to autonomic centers in the brainstem or in the spinal cord of the adult albino rat. Both OXT- and AVP-stained cells in the PVH project to the spinal cord and (or) to the dorsal vagal complex (Sawchenko and Swanson 1982).
Immuno-histochemical analysis of hypophysiotropic and other neuropeptides reveals unique and striking neural perikarya, axons, and terminals containing specific peptide immuno-reactivity. In some species, SP, angiotensin II, CCK-like immuno-reactivity, and ENKs occur in nerve terminals in the external layer of the median eminence. All of these neuropeptides occur within HYP neuronal structures, and most also occur, at least to a limited extent, in deep nuclei of the telencephalon, the brainstem and spinal cord, whereas there are few in neurons of the cerebral cortex. These include SOM, VIP, and cholecystokinin-like immunoreactivity (Elde and Hökfelt 1979).
Various types of acute/chronic nociceptive stimuli cause neuro-endocrine responses such as activation of the HPA axis, as well as of OXT, AVP, ORX, and HYP DA systems. AVP, not CRH, predominantly activates the HPA axis in chronic multiple-arthritis. It would therefore be valuable to simultaneously evaluate the effects of acute mono-arthritis on the activity of the OXT/AVP system and the HPA axis. In adult male Wistar rats, an acute mono-arthritic model induced by intra-articular injection of carrageenan in a single knee joint was used. Acute mono-arthritis was confirmed by a significant increase in knee diameter in the carrageenan-injected knee and a significant decrease in the mechanical nociceptive threshold in the ipsilateral hind paw. Immuno-histochemical analysis showed that the number of Fos-immuno-reactive cells in the ipsilateral DH lamina I-II was significantly increased, and the percentage of OXT- immuno-reactive and AVP-immuno-reactive neurons expressing Fos-immuno-reactivity on both sides of the supra-optic (SON) and PVN was increased in acute mono-arthritic rats. In situ hybridization histochemistry revealed that concentrations of OXT mRNA and AVP mRNA in the SON and PVN, CRH mRNA in the PVN, and pro-opio-melanocortin mRNA in the anterior pituitary were also significantly increased. Further, plasma OXT, AVP, and corticosterone concentrations were significantly increased. All this suggests that acute mono-arthritis activates ipsilateral nociceptive afferent pathways at the spinal level and causes simultaneous and integrative activation of the OXT/AVP system. In addition, the HPA axis is activated by both AVP and CRH in acute mono-arthritis with a distinct pattern compared to that in chronic multiple-arthritis (Nishimura et al. 2020).
2.10.1. Hypothalamo-Pituitary-Adrenal (HPA) Axis
Paraventricular Nucleus (PVN)
The heterogeneous PVN synthesizes and releases neuropeptides such as CRH, OXT and AVP, thereby influencing a number of functions (Lamotte et al. 2021).
The PVN produces and releases CRH, which modulates stress responses (including to pain) by acting on various brain regions. In the median eminence, CRH is released into the portal bloodstream. CRH stimulates anterior pituitary cells to release adreno-corticotropic hormone (ACTH), which is carried via the general bloodstream to the adrenal cortex where it causes the synthesis and release of glucocorticoids (mainly cortisol in humans and corticosterone in rodents), and where it releases adrenaline, in conjunction with inputs from pre-ganglionic neurons (Cullinan 2009; Holsboer and Ising 2021; Kvetnansky et al. 2009; Stanton et al. 2019). Cortisol can bind to glucocorticoid and mineralocorticoid receptors (GRs and MRs, respectively). These enable both rapid, non-genomic actions of cortisol as well as slow, gene-mediated (genomic) actions (delayed by about 60-90 minutes), and may last for several days to weeks. Perhaps as important as the initiation of this cascade of events is the act of regulation, and shutting the system down when appropriate (i.e., once the stressor is removed, to restore homeostasis in the body). Cortisol provides its own negative feedback to the HPA axis (via GRs), which is mediated at least in part by limbic and frontal structures such as the HIPP, and mPFC and AMY (Oyola and Handa 2017; Timmers et al. 2019).
CRH is a 41-amino-acid neuropeptide involved in neuro-endocrine, autonomic and behavioral stress responses and pain modulation. CRH exerts its biological roles through G-protein coupled CRH1 and CRH2 receptors, which have different pharmacological profiles and distributions in the CNS and periphery. CRH is secreted from parvocellular neuro-endocrine neurons of the HYP PVN in response to stressors (Neugebauer et al. 2020).
In primates and rodents, regions outside the HYP host CRH-containing neurons of varying density, e.g., cerebral cortex, lateral septum, HIPP, BNST, AMY, THAL, HYP, PAG and deep mesencepahlic nucleus (DMN), DA system and inter-peduncular nucleus (IPN), PBN, raphé nuclei, LC, NTS, and others (Kelly and Fudge 2018).
CRH is an integral part of the HPA axis. In the brainstem, CRH acts on the LC to influence NA modulation of pain (Kuner and Kuner 2021). In midbrain DA neurons, CRH is thought to have inhibitory and excitatory roles, the latter inducing a potentiation of synaptic transmission mediated by NMDARs, and resulting in glutamate release and DA activation (Kelly and Fudge 2018). CRH is also expressed in nociceptors and their neighboring components, giving rise to hypotheses for possible pain modulations at this level (Zheng et al. 2020).
In primates and rodents, regions outside the HYP host CRH-containing neurons of varying density, e.g., cerebral cortex, lateral septum, HIPP, BNST, AMY, THAL, HYP, PAG and deep mesencepahlic nucleus (DMN), DA system and inter-peduncular nucleus (IPN), PBN, raphé nuclei, LC, NTS, and others (Kelly and Fudge 2018).
CRH attains particularly high levels in the CeA and BNST, playing a neuro-modulatory role in synaptic functions. CRH-expressing neurons in CeA are GABAergic and co-localize with other neuropeptides such as STT, NT, and Dyn, but not with ENK. CRH mRNA and protein expression in the CeA is increased in neuropathic pain models. CeA neurons containing CRH and/or STT are a source of long-range projections and serve major output functions, but CRH also acts locally to excite neurons in the CeA and baso-lateral amygdala (BLA). In rats subjected to kaolin/carrageenan-induced knee-joint arthritis, a CRH1, but not CRH2, receptor antagonist (NBI27914 and Astressin-2B, respectively) decreased hyper-activity of lateral and capsular (CeLC) neurons, suggesting that CRH1 receptors were activated endogenously. Pharmacological blockade of CRH1 receptors in the BLA also reduced neuronal sensitization of BLA and CeLC neurons in models of arthritis and neuropathic pain, respectively (Neugebauer et al. 2020). CRH administered into the CeA of normal animals increased nocifensive reflexes and vocalizations evoked by innocuous and noxious mechanical stimuli (compression of the knee joint). In anesthetized rats, CRH administration into the CeA at low concentrations increased, but at higher concentrations decreased activity of CeA neurons, suggesting opposing functions of the CRH receptor sub-types. Increased CRH expression in the CeA produced mechanical and visceral hyper-sensitivity. High doses of CRH in the CeA had anti-nociceptive effects on thermal and mechanical sensitivity tests. Descending modulation of spinal nociceptive processing by the CeA-PAG connections has been implicated in acute SIA as well as anti-nociceptive effects of opioids acting locally in the BLA (Kuner and Kuner 2021). of CeA neurons, suggesting opposing functions of the CRH receptor sub-types. Increased CRH expression in the CeA produced mechanical and visceral hyper-sensitivity. High doses of CRH in the CeA had anti-nociceptive effects on thermal and mechanical sensitivity tests. Descending modulation of spinal nociceptive processing by the CeA-PAG connections has been implicated in acute stress-induced analgesia (SIA) as well as anti-nociceptive effects of opioids acting locally in the BLA (Kuner and Kuner 2021). CRH2 receptors, which have different pharmacological profiles and distributions in the CNS and periphery. CRH is secreted from parvocellular neuro-endocrine neurons of the HYP PVN in response to stressors (Neugebauer et al. 2020).
CRH administered into the CeA of normal animals increased nocifensive reflexes and vocalizations evoked by innocuous and noxious mechanical stimuli (compression of the knee joint). In anesthetized rats, CRH administration into the CeA at low concentrations increased, but at higher concentrations decreased activity of CeA neurons, suggesting opposing functions of the CRH receptor sub-types. Increased CRH expression in the CeA produced mechanical and visceral hyper-sensitivity. High doses of CRH in the CeA had anti-nociceptive effects on thermal and mechanical sensitivity tests. Descending modulation of spinal nociceptive processing by the CeA-PAG connections has been implicated in acute SIA as well as anti-nociceptive effects of opioids acting locally in the BLA (Kuner and Kuner 2021). of CeA neurons, suggesting opposing functions of the CRH receptor sub-types. Increased CRH expression in the CeA produced mechanical and visceral hyper-sensitivity. High doses of CRH in the CeA had anti-nociceptive effects on thermal and mechanical sensitivity tests. Descending modulation of spinal nociceptive processing by the CeA-PAG connections has been implicated in acute SIA as well as anti-nociceptive effects of opioids acting locally in the BLA (Kuner and Kuner 2021).
Corticosteroids have been used as a supplementary treatment in acute inflammatory pain conditions, but there appears to be a more direct role that steroids play in the generation and clinical management of chronic pain. The end-product of the HPA, cortisone, modulates nociceptive transmission at spinal level. In laminae I and II, nociceptive stimulation releases SP and CGRP, and with their expression co-exists a high density of GRs. Termination of treatment with cortisone after four weeks leads to loss of an anti-nociceptive effect (McEwen and Kalia 2010).
Oxytocin (OXT) Neurons
Generally, OXT has peripheral and central functions in breastfeeding, childbirth and maternal behavior, as well as in general health, adaptation, development, reproduction, and social behavior. Endogenous OXT and stimulation of the OXT receptors (OXTRs) support anti-inflammation and healing, stress-coping, resilience, and patterns of growth. OXT also influences the autonomic nervous system (ANS) and the immune system. The effects of OXT are context-dependent, sexually dimorphic, and altered by experience. OXTRs are epigenetically tuned by experience, especially in early life (Carter et al. 2020). OXT promotes multiple aspects of socio-emotional and socio-sexual behaviors, improves learning and memory abilities, modulates feeding, grooming, and drug-seeking behavior, as well as the activity of stress and pain systems (Jurek and Neumann 2018). OXT also modulates various sensory modalities such as olfaction, touch and vision, as well as pain perception and anticipation, and social behavior and emotions. However, there are strong species-dependent differences in the way OXT does so, which can be explained by differences in OXTR expression in brain regions processing sensory information (Althammer et al. 2021; Poisbeau et al. 2018). OXTRs are G-protein-coupled receptors able to excite and inhibit neurons due to different mechanisms (Poisbeau et al. 2018). The two OXTRs are distributed widely within the brain. Brain OXTRs are species-specific and often occur at low concentrations (Jurek and Neumann 2018).
The regulatory sites of OXT are the cerebral cortex, including ACC and IC, the AMY, NAc, PAG, RVM, spinal cord, DRG neurons, and primary afferent fibers. Peripherally, OXTRs are expressed in the terminal nerve endings of group III (Aδ) and group IV (C) fibers and were able to inhibit nociceptive firing. Local peripheral OXT blocked the first sensorial activity of group III recorded (González-Hernández et al. 2017).
The parvocellular cells in particular project to the RVM, NTS, dorsal vagal complex, nucleus phrenicus and the spinal IML, possibly contributing to autonomic functions such as cardio-vascular responses, respiration and gastric motility (Poisbeau et al. 2018; Yang et al. 2022).
OXT is an important modulator of pain and emotional processing. Analgesic OXT effects occur in multiple non-human species including rodents, dogs, cats, and rabbits.particularly in a social context. OXT exerts analgesic effects at target sites within ascending and descending pain pathways, from the spinal cord to limbic and cortical brain regions, which play a major role in the cognitive and emotional processing of pain (Boll et al. 2018; Yang et al. 2022). OXTRs are broadly expressed in CNS and also on peripheral sensory neurons, where they may bind and desensitize the TRPV1 receptor, a prominent sensor of heat, protons, and diverse algogens, thereby indicating analgesic actions for OXT (Kuner and Kuner 2021).
OXTRs are expressed in terminal afferent nerve endings and are able to inhibit nociceptive neuronal firing. Local peripheral OXT blocked the first sensorial activity of group III (Aδ) and group IV (C) fibers recorded in spinal cord neurons. OXTR is expressed in the sciatic nerve. Immuno-fluorescence of primary afferent fibers suggested that OXTRs could be located in nociceptive-specific terminals of the skin (González-Hernández et al. 2017). In freshly isolated DRG neurons of rats, the modulatory effect of OXT on ATP-activated currents (IATP) were determined using whole cell clamp technique. In most of the neurons, extracellular application of OXT suppressed IATP while there was no modulatory effect in the rest no modulatory effect. This OXT-induced inhibition of IATP showed no voltage dependence, and could be blocked by a specific OXTR antagonist. Intracellular application of a chelator of Ca2+ ions could reverse the inhibitory effect of extracellular OXT, while inclusion of an inhibitor of CaMKII in the recording pipette did not affect this effect. This suggested that OXT inhibition on ATP-activated currents was mediated by OXTRs in the membrane of DRG neurons (Yang et al. 2002; Zheng et al. 2021).
While OXTRs in the spinal DH participate in a selective inhibition of the neuronal activity mediated by group III (Aδ) and group IV (C) fibers but not group II (Aβ) fibers (González-Hernández et al. 2017), OXT neurons in the PVN send direct axonal projections to the superficial and central laminae of the spinal DH (
Figure 3). Parvocellular axons project to spinal regions processing somatosensory, visceral and nociceptive signals, and to sympathetic and parasympathetic areas, providing the anatomical basis for modulation of nociceptic and autonomic signals (Poisbeau et al. 2018).
In rodents, OXY administered systemically or at CNS sites such as the spinal intra-thecal space, caudate nucleus of the BG, NAc, AMY and PAG produced analgesia. Thus, rats were less sensitive to electrical, thermal, chemical and mechanical pain stimuli, and had less pain following acute stress, acute inflammation and neuropathic injury. In identified STT neurons, PVN stimulation decreased activity of WDR neurons to noxious but not innocuous stimuli. Intra-thecal OXTR antagonists and bicuculline, a GABAAR antagonist, eliminated the WDR responses to PVN stimulation, indicating that the DH response to PVN stimulation was due to OXY and that activation of the GABAergic systems was necessary in the process. It has been hypothesized that descending PVN neurons synapse on GABAergic DH interneurons, which would then form inhibitory synapses on the afferent terminals of group III and IV fibers, but not group II fibers. Some effects appear to involve opioids.—Human data are more limited but suggest that OXY is able to modulate somatosensory transmission, particularly pain perception, and that endogenous OXT concentrations either are significantly lower among patients with chronic pain compared with healthy controls, or associated with enhanced pain sensitivity. Exogenous administration of OXY, too, seems to decrease pain sensitivity (Goodin et al. 2015; Poisbeau et al. 2018).
In the spinal DH of rats, OXT exerts a selective inhibition of the nociceptive group III and IV fiber activity, but not the activity of proprioceptive fibers, i.e., group II fibers. This inhibition could be implemented by a direct presynaptic mechanism or be mediated by GABA interneurons. Ultrastructural analyses of the DH tissue showed that: (i) OXT and OXTR occurred in asymmetrical synapses; (ii) OXTRs were expressed by GABA interneurons (near unmyelinated fibers), CGRP fibers and glial cells; (iii) OXT was present in supraspinally descending projection fibers (Martínez-Lorenzana et al. 2021).
In rats, the use of whole-cell patch-clamp technique and spinal cord slices showed that OXT had no effect on glutamatergic excitatory transmission while producing a membrane depolarization, and enhancement of GABAergic and glycinergic spontaneous inhibitory transmission (Kumamoto 2019).
Following noxious stimulation, OXT released in the blood stream from the SON in turn released β-endorphin, L-ENKs and M-ENKs, but not Dyn in the PAG, thereby activating opioid-dependent descending inhibition. The caudate nucleus has also been implicated in the anti-nociceptive role of endogenous OXT. Direct activation of the SON increased the concentration of OXT in the caudate nucleus. OXT axons ascending from the parvocellular PVN part innervate limbic and cortical brain regions, particularly the CeA, and thereby mediate OXT effects on emotional processing, including the emotional components of pain. OXT effects on acute pain were associated with increased activity in the ventral striatum and decreased activation of the aIC. Moreover, OXT modulated the accuracy of pain anticipation, which correlated with decreased activation of the posterior insular cortex (pIC) (Kuner and Kuner 2021).
Neurons in the PFC can provide top-down regulation of sensory-affective experiences such as pain, while bottom-up modulation of sensory coding in the PFC, such as OXT signaling from the HYP regulates nociceptive coding in the PFC, remains little known. In vivo time-lapse endoscopic Ca2+ imaging in freely behaving rats showed that OXT selectively enhanced population activity in the PL PFC in response to nociceptive inputs. This population response resulted from the reduction of evoked GABA inhibition and manifested as elevated functional connectivity involving pain-responsive neurons. Direct inputs from OXT-releasing neurons in the HYP PVN are crucial to maintaining this prefrontal nociceptive response. Activation of the PL PFC by OXT or direct optogenetic stimulation of OXT PVN projections reduced acute and chronic pain (Liu et al. 2023).
Central OXT and DA play important roles in nociception at the spinal level as well as supraspinal structures, e.g., ACC, IC, AMY, NAc, and HYP. The interaction between OXT and DA systems may be important in motivational behaviors, such as maternal and sexual behaviors, pair bonding, and salience. It has been proposed that an OXT-DA interaction could be present in nociception (Gamal-Eltrabily and Manzano-García 2018).
In Wistar rats subjected to long-term secondary mechanical allodynia and hyperalgesia induced by formalin, lesion of the NRM reduced the PVN-induced anti-nociception, suggesting a functional interaction between the OXT and the 5-HT system. Intra-thecal application of OXT or 5-HT prevented the formalin-induced sensitization, an effect mimicked by PVN stimulation. Administration of OXT plus 5-HT at ineffective doses, produced anti-nociception. Similar results were obtained with PVN stimulation plus 5-HT. In WDR cell recordings, the PVN-induced anti-nociception was enhanced by intra-thecal 5-HT. This suggested that 5-HT mechanisms at the spinal cord level are partly involved in the OXT-induced anti-nociception (Godínez-Chaparro et al. 2016).
Vasopressin (AVP) Neurons
The nonapeptide AVP acts as a hormone as well as a neurotransmitter or neuromodulator. As a hormone, its target organs include kidney, blood vessels, liver, platelets and anterior pituitary. As a neurotransmitter or neuromodulator, AVP plays a role in autonomic functions, such as cardio-vascular and temperature regulation. It is also involved in complex behavioral and cognitive functions, such as sexual behavior, pair-bond formation and social recognition. At the neuronal level, AVP acts by enhancing membrane excitability and by modulating synaptic transmission. In young rats and mice, AVP exerts a powerful excitatory action on MNs. It acts by generating cationic inward currents and/or by reducing a K+ conductance. In addition, AVP enhances the inhibitory synaptic input to MNs. Thus, AVP may regulate the functioning of neuronal networks involved in motor control (Raggenbass 2008).
In rodents, AVP exhibits analgesic effects when applied by systemic, ICV, intra-thecal, or sub-cutaneous injection. In humans, intra-nasal AVP administration effectively treats headaches and pain after orthopedic surgery (Baba et al. 2022; Mavani et al. 2015). AVP has significant effects on pain perception via the CNS through receptors AVPR1a and AVPR1b located on neuronal cells including those distributed throughout the HYP. AVP can increase the pain threshold to painful stimuli (Mavani et al. 2015).
In rats, the analgesic effects of electrical stimulation of the HYP PVN was determined with chronically implanted electrodes in the parvocellular (PVN-Pc) and magnocellular (PVN-Mg) divisions of the PVN. In AVP-deficient (Brattleboro) and Long-Evans rats, the involvement of AVP and opioid peptides was compared by administering the opiate antagonist naloxone. In lightly anesthetized rats, at least ten days after surgery, the analgesic effects of PVN stimulation were determined using the tail-flick method, and using the hot-plate test in unanesthetized rats. PVN stimulation produced marked analgesia in both tests. Thresholds did not differ significantly between Brattleboro and Long-Evans rats and was not affected by naloxone administration. This indicated that the PVN is part of the brain’s pain inhibitory system, and showed that the analgesia induced by PVN stimulation is not mediated by neither AVP nor opioid peptides (Yirmiya et al. 1990).
As by OXT, nociceptors are modulated by AVP (Zheng et al. 2021). In anesthetized rats, extracellular unitary recordings were performed, measuring the evoked activity mediated by groups II (Aβ), III (Aδ), and IV (C) fibers and post-discharge under sub-cutaneous AVP using formalin and rotarod tests. Selective antagonists to AVP receptors (AVPR1A) or OXT receptors (OXTRs) were used. Additionally, AVP receptors and OXTRs were explored immuno-histochemically in skin tissues. Sub-cutaneous AVP induced anti-nociception and a transitory reduction of the end-tidal CO2. The neuronal activity associated with group III and IV fiber activation was diminished, but no effect occurred on group II fibers. AVP also reduced paw flinches in the formalin test and a transitory locomotor impairment. Hence, sub-cutaneous AVP produced anti-nociception and behavioral analgesia. Both AVPR1a and OXTR participated in those effects. Additionally, sub-cutaneous AVP also produced important systemic effects such as respiratory and locomotor impairment. This may be explained by the demonstration of AVRR1A and OXTR in cutaneous fibers (Manzano-García et al. 2018). AVP may modulate primary afferent activity of nociceptors in the spinal DH (Juif and Poisbeau 2013; Stebbins et al. 1992).
Peripheral hormonal actions of OXT and AVP on nociception and pain responses are not well known, in particular the effects of physiological blood concentrations of OXT and AVP on spinal nociception and on pain responses. In anesthetized male rats in vivo, increasing doses of OXT or AVP were administered intravenously, and the nociceptive processing by spinal cord neurons was analyzed. The action potentials mediated by group IV (C) nociceptive fibers were strongly reduced (anti-nociception) after intravenous injections of low doses of OXT or AVP, whereas an increase (pro-nociception) occurred at higher doses. Anti-nociceptive and pro-nociceptive effects were fully abolished in the presence of the OXTR antagonist and the AVP receptor antagonist type 1A (AVP1A), respectively. This was confirmed by a behavioral model of forced-swim-stress-(FST)-induced analgesia associated with plasmatic release of OXT (and not AVP). SIA was transiently lost after i.v. administration of OXTR antagonist. Hence, blood concentrations of OXT and AVP modulate nociception, windup plasticity and pain responses. The final target structures explaining these effects remains to be identified but are likely to be group IV (C)-type nociceptors (Juif and Poisbeau 2013).
In rats, the effects of intra-thecal AVP on nociception were quantitatively evaluated using four pain tests: tail flick, tail-shock vocalization, hot plate, and formalin, as well as motor effects. Intra-thecal AVP produced a prolonged anti-nociception lasting at least 40 minutes on the tail-flick and formalin tests, and a brief anti-nociception lasting less than 20 minutes on the tail-shock and hot-plate tests. In addition, intra-thecal AVP produced scratching bouts and suppressed hindbody motor function. The motor inhibitory effects of AVP, although severe in some rats, were brief, lasting less than 15 minutes (Thurston et al. 1988). Of importance is the direct connection from HYP to DH. In naive Sprague-Dawley rats, the PVN gives rise to a major AVP projection to the spinal cord, where the spinal cord-projecting AVP neurons are parceled into anatomically distinct cell groups. This provides an anatomical basis for a selective activation of functionally different groups in the PVN as part of a behaviorally adaptive response, including modulation of autonomic activity at the spinal level (Hallbeck and Blomqvist 1999). Strangely, this particular direct projection appears not to modulate spinal nociceptive processing. In contrast to OXT, AVP does not exert anti-nociception in the DH. While both PVN electrical stimulation and topical application of OXT in the vicinity of identified and recorded DH WDR selectively inhibited group III (Aδ) and IV (C) fiber responses, the topical administration of AVP on the same neurons did not affect the nociceptive responses (Rojas-Piloni et al. 2010).
Strangely, while OXT modulates nociceptive transmission at the DH level, AVP does not. In the spinal cord, both PVN electrical stimulation and topical application of OXT in the vicinity of identified and recorded DH WDR selectively inhibited group III and IV afferent responses. The reduction in nociceptive responses caused by PVN stimulation or OXT administration was blocked with a selective OXT antagonist. By contrast, the topical administration of AVP on the same neurons did not affect the nociceptive responses. This suggests that the AVP descending projection does not modulate the anti-nociceptive effects mediated by the PVN on DH neurons. Instead, it is the HYP-spinal OXT projection that regulates nociceptive information (Rojas-Piloni et al. 2010).
In the AMY, AVP can directly excite a sub-population of neurons, whereas OXT can indirectly inhibit these same neurons. In the lateral septum, AVP similarly excites directly a neuronal sub-population, but causes indirect inhibition of virtually all lateral septal neurons. The actions of AVP in the AMY and lateral septum may represent at least part of the neuronal substrate by which AVP influences fear and anxiety-related behavior and social recognition, respectively. Central AVP can modulate cardio-vascular variables by causing excitation of spinal sympathetic pre-ganglionic neurons, by increasing the inhibitory input to cardiac parasympathetic neurons in the nucleus ambiguus, by depressing the excitatory input to PBN neurons, or by inhibiting glutamate release at NTS axon terminals (Raggenbass 2008).
In rats, peripheral injection of AVP did not reduce the pain threshold when the rats were exposed to pain in a graded manner. In contrast, when AVP was applied by an intra-ventricular route, the pain threshold was significantly higher than in control (before AVP application). Brattleboro rats, which are deficient in AVP, showed an exaggerated pain response. However, when AVP was given intra-ventricularly, the pain threshold increased. The analgesic actions of AVP were blocked by a AVPR1a and not a AVPR2 receptor antagonist, suggesting that AVP exerts its anti-nociceptive action exclusively through AVPR1a receptors. Not only intra-cranial but also intra-thecal injections of AVP could cause an anti-nociceptive effect, suggesting that the anti-nociception action of AVP extends to the spinal cord (Mavani et al. 2015).
Orexin (ORX) Neurons
ORX is involved in the modulation of pain transmission, stress regulation, arousal, fear, anxiety, reward, addiction and cognition.
ORX neurons modulate nociceptive responses and pain perception at various sites on spinal and supraspinal levels. Thus, their activation enhanced response thresholds to noxious heat and depressed responses to inflammatory and noxious stimuli, such as formalin; and chemogenetic activation reduced nociceptive sensitivity. In various animal models of inflammatory pain induced by formalin, capsaicin, and carrageenan and in animal models subjected to common peroneal nerve ligation, the injection of exogenous ORX into the spinal cord and supraspinal sites that are associated with the descending pain regulatory circuits could significantly reduce nociceptive responses. ORXs are also involved in endogenous anti-nociception in various types of pain including neuropathic pain, migraine and cluster headache, visceral and orofacial pains. Potent anti-nociceptive effects may be evoked by ORXA acting at ORXA receptors (ORXARs), while ORXB is less or not effective. ORX effects interact with opioidergic mechanisms as well as other neuropeptides, but ORXA-mediated anti-nociception is believed to be opioid-independent. Instead, the analgesic effects of ORXA in the vlPAG could involve endocannabinoid (eCB) signaling. This mechanism of ORX release in the vlPAG from incoming HYP projections has also been postulated to contribute to SIA. ORX neurons have therefore been suggested to be activated as a part of the endogenous pain control system (Kang et al. 2021; Kuner and Kuner 2021; Razavi and Hosseinzadeh 2017). ORXs A and B produced a membrane depolarization and/or enhanced presynaptic spontaneous excitatory transmission. Like OXT, ORXA enhanced both GABAergic and glycinergic transmission, whereas ORXB facilitated glycinergic but not GABAergic transmission (Kumamoto 2019).
In the spinal cord, ORXs are concentrated in the superficial DH laminae and occur in the DRG cells. In the rat formalin test (a model of inflammatory pain) and in the rat hot plate test, intra-thecal injection of ORXA, but not ORXB, decreased the sum of flinches in phases 1 and 2 in the formalin test and increased the hot plate latency. These effects of ORXA were completely antagonized by pre-treatment with a selective ORX1R antagonist. While intra-thecal injection of this antagonist alone had no effect in the formalin test or in the hot plate test, intra-thecal injection of ORXA suppressed the expression of Fos-like immuno-reactivity, induced by formalin injection into the paw, in laminae I-II of L4-5 of the spinal DH. This suggests that the spinal ORXAR is involved in the nociceptive transmission and that the activation of the spinal ORXAR produces analgesic effects in the rat formalin test and in the rat hot plate test (Yamamoto et al. 2002).
In neonatal rats, spinally applied ORXA and ORXB had effects on the primary afferent fiber-evoked nociceptive reflex in the isolated spinal cord. In 0-3 day old rats, single-shock stimulation of a dorsal root (L3-L5) at a strength which can activate group IV (C) fibers induced a slowly depolarizing response (ventral-root potential:VRP) lasting about 30 seconds in the ipsilateral ventral root of the same segment. Bath application of ORXA and ORXB inhibited the slow VRP in a concentration-dependent manner. Bath application of a selective ORXAR antagonist had no effect on the depressant effect of ORXA on slow VRP. Bath application of a selective ORXBR agonist depressed the slow VRP. Both ORXA and ORXB depressed the level of temporal summation of synaptic activity evoked by 20 repetitive stimulations of the dorsal root (Shono and Yamamoto 2008).
Pain and Itch are antagonistically regulated sensations. Pain suppresses itch, while pain inhibition enhances itch. ORX neurons in the lHYP suppress pain while enhancing itch neural processing, as revealed by applying optogenetics to the acute pruritus and pain model. The circuit of ORX neurons from the lHYP to PAG regions served in the contrasting modulation of itch and pain processing. Additionally, by using an atopic dermatitis model, the involvement of ORX neurons in regulating chronic itch processing was confirmed (Kaneko et al. 2024).
Hypothalamic Neurons Producing Other Neuromodulators
CCK is abundant in the DMH and acts in the RVM to enhance nociception. The DMH is the only significant supraspinal source of CCK in the RVM. However, not all neurons projecting from the DMH to the RVM contain CCK. This establishes an anatomical and functional connection between the DMH and RVM by which stress can facilitate pain (Wagner et al. 2013).
The RVM is a crucial site for the supraspinal anti-nociceptive actions of opioids. Spinally projecting 5-HT RVM neurons express μ-opioid receptors. Although 5-HT neurons comprise a minority of RVM neurons, they appear to be selectively apposed by an endogenous ligand of μ-opioid receptors, EM-2. Neurons containing EM-2 exist primarily in the DMH and project to the RVM, and EM-2 participates in HYP stimulation-induced analgesia (Gu and Wessendorf 2007).
2.10.2. Hypothalamo-Pituitary-Gonadal (HPG) Axis
Females typically show heightened responses of the HPA axis to stress. Many neuropsychiatric disorders disproportionately affect females. In the brain, gonadal sex steroids and their neuroactive metabolites play roles in mediating sex differences in HPA axis responses to stress. However, the relationship between neuroactive steroids and stress is complex. Acute stress rapidly increases neuroactive steroid production, which can in turn modulate the HPA-axis activity (Sze and Brunton 2020).
HPA and HPG exhbit a reciprocal relationship, wherein the activation of one affects the function of the other and vice versa. For example, both estrogen and testosterone modulate the response of the HPA axis, whereas activation of the stress axis, especially repeating or chronic activation has an inhibitory effect upon estrogen and testosterone secretion (Toufexis et al. 2014).
Sex differences in pain involve many aspects, including the types and frequency of pain syndromes, the prevalence and severity of pain, pain control and responsiveness to analgesics. Women are at a higher risk than men of several clinical pain conditions, such as migraine, irritable bowel syndrome (IBS), interstitial cystitis and chronic pelvic pain. Distinct sex-related divergence exist in the efficacy of opioid agonists. Lower pain thresholds and decreased anti-nociceptive effects of opioid agonists occur among females as compared to males. Most animal studies have confirmed that opioids produce a greater degree of analgesia in male rodents than in female rodents. Discrepant psychological mechanisms are thought to play a fundamental role, with men and women tending to use different coping methods to manage pain. In addition, between men and women exist differences in the activation of μ-opioid receptors in the CNS. Finally, different circulating levels of gonadal hormones play major roles in mediating the sexual dimorphism of opioid anti-nociception (Xu et al. 2024).
Estrogens, such as estradiol (E2), are synthesized in the ovaries (
Figure 2), but also in the spinal cord and brainstem and act locally in neuronal target organs to influence pain processing. Potential cellular sources of local estrogen may be primary afferent neurons and their central targets in the spinal cord and medulla as well as in the NTS. Estrogens may be detected by the expression of aromatase, the enzyme that catalyzes the conversion of testosterone to estradiol. In an aromatase reporter mouse, immuno-histochemical staining showed that many neurons in DH laminae I and V, in the caudal SpV and in the NTS expressed aromatase (Tran et al. 2017).
The specific role played by estrogen receptors remains elusive. There are several different mechanisms through which the three estrogen receptors (ERs), ERα, ERβ and G protein-coupled estrogen receptor 1 (GPER1) are able to regulate target gene transcription. ERα and ERβ are mostly associated with the direct and indirect genomic signaling pathways that result in target gene expression. Membrane-bound GPER1 is on the other hand responsible for the rapid non-genomic actions of estrogens that activate various protein-kinase cascades (Vrtačnik et al. 2014).
Estrogen receptors α and ß (ERα and ERß) mediate different physiological functions. In wild-type and estrogen receptor ß knockout (ERß KO) mice of both sexes, ERß had effects on acute and persistent pain as well as on endogenous pain-inhibitory mechanisms evoked by hot-plate and formalin tests. In female groups, ovariectomies followed by estrogen and progesterone replacement insured comparable sex hormone concentrations. Nociceptive responses were lower in ERß KO female than in wild-type female mice during the interphase and early tonic phase II of the formalin test but not during acute and late tonic phases. Behavioral and spinal c-fos differences occurred only in females. ERß KO females showed lower c-fos expression in DH laminae I-II and IV-V than did wild-type females. Hence, estrogen, through its actions on ERß, dampens the efficacy of endogenous pain modulation mechanisms during the interphase and/or inflammation process in the early phase II, triggering an increase in spinal nociceptive neuronal activity (Spooner et al. 2007).
Cellular pools of estrogen and other steroid hormones bind to both nuclear and membrane-localized receptors, with varying affinity and response. In the PFC, HIPP, dorsal striatum, and NAc, estrogen binds to membrane-localized receptors to activate second-messenger systems and affect synaptic function. Several central effects of estradiol (E2) are linked to mGlu1/5 receptors. E2 alters neuronal excitability and synaptic plasticity through stimulation of Group 1 receptors in both a sex-dependent and independent manner. Membrane-localized estrogen receptors, ERα and ERβ, are functionally coupled to mGlu1/5 receptors expressed on the cell surface. ER/mGlu complexes are present in both male and female brains, without major sex differences in overall expression (Fabian et al. 2023).
In the intact human brain, positron emission tomography (PET) has enabled the quantification of available mGlu5 receptors, using the allosteric radiotracer [11C]-ABP688 to assess sex differences in mGlu5 receptor availability. A large study found significantly higher rates of [11C]-ABP688 binding potential in men than women across several regions of the PFC, HIPP and striatum. The largest magnitude differences were observed in the OFC and the dorso-lateral PFC (dlPFC), in which binding potentials were 22% and 20% greater in men, respectively. A whole-brain comparison of mGlu5 receptor availability found that [11C]-ABP688 binding potential was 17% greater in men. Hence, the larger availability of mGlu5 receptors in women compared to men appears to conflict with preclinical data, as major sex differences in mGlu5 receptor expression have not been consistently reported in rodents (Fabian et al. 2023).
Progesterone is mainly produced in the corpus luteum and in the placenta and plays important roles in the menstrual cycle, pregnancy and during the development of the embryo. However, progesterone also has multiple non-reproductive functions in the CNS to regulate cognition, mood, inflammation, mitochondrial function, neurogenesis and regeneration, myelination and recovery from traumatic brain injury. Progesterone-regulated neural responses are mediated by an array of progesterone receptors (PR) that include the nuclear PRA and PRB receptors and splice variants of each. These PRs induce regulation of gene expression but also transduce signaling cascades that originate at the cell membrane and ultimately activate transcription factors. PRs are broadly expressed throughout the brain and can be detected in every neural cell type (Diaz Brinton et al. 2008). Progesterone and its metabolite allopregnanolone (also called 3α,5α-tetrahydroprogesterone) are also male hormones, as they are produced in both sexes by the adrenal glands within the nervous system. Increased production of progesterone within the brain may be part of the response of neural cells to injury. Progesterone has trophic effects and is a neuroprotective and pro-myelinating agent (Schumacher et al. 2014).
In the rat, intra-peritoneal injections of high doses of progesterone produced anesthesia (Selye 1941). In rats, spinal GABAAR-dependent activation inhibited the induction of repetitive stimulation-induced spinal-reflex potentiation. Progesterone was capable of producing GABAAR-dependent inhibition of the induction of spinal reflex potentiation by actions through neurosteroid metabolites. The induction of spinal reflex potentiation was attenuated after a short (30 minutes) intra-thecal treatment with the neurosteroids allopregnanolone and 3α,5α-tetrahydrodeoxycorticosterone. Acute intra-thecal administration of the GABAAR antagonist bicuculline reversed the inhibition produced by progesterone and allopregnanolone. This implies that progesterone-mediated effects on GABAAR expression and neural inhibition are regulated by neurosteroids synthesis rather than progesterone receptor activation (Peng et al. 2009).
Allopregnanolone. 3-Alpha reduced neurosteroids, such as allopregnanolone (also called 3α,5α-tetrahydroprogesterone), are remarkable analgesics in various pain states. 3-alpha reduced neurosteroids selectively act as positive allosteric modulators of the inhibitory functions of GABAARs, expressed either at extrasynaptic or synaptic sites. Allopregnanolone exerts analgesic, anxiolytic, anti-depressant, and neuroprotective effects. These effects result from allopregnanolone’s ability to modulate GABAA, glycine, L- and T-type Ca2+ channels, without side effects (Patte-Mensah et al. 2014; Poisbeau et al. 2014).
The actions of androgens such as testosterone and di-hydro-testosterone are mediated via the androgen receptor (AR), a ligand-dependent nuclear transcription factor. It is widely spread in many cells and tissues. The AR thus has many biological actions, i.e., in the development and maintenance of the reproductive, musculo-skeletal, cardio-vascular, immune, neural and haemopoietic systems. Androgens can exert their actions via the AR in a DNA binding-dependent manner to regulate target gene transcription, or in a non-DNA binding-dependent manner to initiate rapid, cellular events such as the phosphorylation of second-messenger signalling cascades. Ligand-independent actions of the AR have also been identified (Davey and Grossmann 2016).
Leydig cells secrete the steroid hormone, testosterone, which is essential for male fertility and reproductive health. Many pain conditions, including temporo-mandibular-joint (TMJ) dysfunction, have a lower prevalence in men than in women. Testosterone could affect the risk of developing TMJ pain and on acute persistent TMJ pain. In the rat, the TMJ formalin test was used as an experimental assay. Intra-TMJ 0.5% formalin induced a significant nociceptive behavior in naive female rats and gonadectomized male rats but not in naive male rats, suggesting that naive male rats have a lower risk for developing TMJ pain. The serum concentration of testosterone but not of estrogen and progesterone significantly decreased in gonadectomized male rats, suggesting that testosterone is the hormone underlying the decreased naive male rat’s risk for developing TMJ pain. The magnitude of the nociceptive behaviors induced by intra-TMJ 1.5% formalin was similar in gonadectomized and naive male rats. Therefore, in contrast to the protective role of testosterone in TMJ pain development, testosterone, at physiological serum concentrations, does not appear to modulate acute persistent TMJ pain induced by the TMJ injection of 1.5% formalin. At a supra-physiological serum concentration, however, testosterone significantly attenuated 1.5% formalin-induced nociception in male rats but not in female rats. This anti-nociceptive effect was not mediated by estrogen derived from testosterone aromatization, because estrogen administration did not affect 1.5% formalin-induced TMJ nociception in gonadectomized male rats (Fischer et al. 2007).
Stress, including pain, increases the secretion of glucocorticoid (corticosterone in rats) that decreases circulating testosterone concentrations, in part through a direct action on its receptors in Leydig cells. Adult male Sprague-Dawley rats orally received vehicle control or 5 or 10 mg/kg DHEA 0.5 hour before being subjected to pain stimulation for one, three, and six hours. The time-course changes of steroidogenic gene expression concentrations were determined after acute pain-induced stress in rats and the possible mechanism of DHEA that prevented it. Plasma corticosterone, luteinizing hormone, and testosterone concentrations were measured, and Leydig cell gene expression levels were determined. Plasma corticosterone concentrations were significantly increased at hour one, three, and six during the pain stimulation, while plasma testosterone concentrations were significantly decreased starting at hour three and six. When five and ten mg/kg DHEA were orally administered to rats half an hour before starting pain stimulation, DHEA prevented pain-mediated decrease in plasma testosterone concentrations without affecting plasma corticosterone concentrations (Zhu et al. 2018).
Androgens, such as testosterone, modulate the meso-cortico-limbic system. Endogenous and exogenous androgens alter behaviors, such as behavioral flexibility, decision-making, and risk taking. ARs are detected in the mPFC, OFC, VTA, and NAc. Similar to DA signaling, there might be optimal levels of androgen signaling within the meso-cortico-limbic system for executive functioning (Tobiansky et al. 2018).
2.10.3. Hypothalamo-Pituitary-Thyroid (HPT) Axis
Thyroid hormones (THs), released from the thyroid gland (
Figure 2), and glucocorticoids are involved in meeting immediate energy demands, thus placing the HPT and HPA axes in a central position. In the HYP, there appears to be a contrasting involvement of distinct TRH cell types, manifested through variability in cellular phenotype and physiology, including rapid responses to energy demands for thermogenesis or physical activity and nutritional status that may be modified according to stress history (Joseph-Bravo et al. 2015).
THs appear to have strong effects on the brain as judged by the effects of even sub-clinical hypo-thyroidism (SCH). In these conditions, gray-matter volume was significantly decreased in the orbital part of inferior frontal, superior frontal, pre- and/or postcentral, inferior occipital, and temporal pole cortex, and regional brain activity was significantly increased as compared to controls (Zhang et al. 2021).
The TH receptors TRα1, TRβ1, and other sub-types, are members of the nuclear receptor superfamily that mediate the action of TH signaling in numerous tissues to regulate important physiological and developmental processes. TRs bind TH response elements in the presence or absence of TH to facilitate the expression of target genes. Although primarily residing in the nucleus, TRα1 and TRβ1 shuttle rapidly between the nucleus and cytoplasm (Anyetei-Anum et al. 2018).
Acute and repeated stress, including pain, can alter TH secretion. Corticosterone has been suggested to play a role in HPT axis regulation. To characterize HPT axis activity after repeated exposure to inescapable foot-shock stress (IFSS), and to examine changes in proposed regulators of the HPT axis, including plasma corticosterone and HYP ARC agouti-related protein (AGRP) mRNA levels, adult male Sprague-Dawley rats were subjected to one daily session of IFSS for 14 days. Plasma corticosterone levels were determined during and after the stress on days 1 and 14. Repeated exposure to IFSS led to a significant decrease in serum concentrations of 3,5,3′-triiodothyronine (T3) and 3,5,3′,5′-tetraiodothyronine (T4). Stress-induced plasma corticosterone levels were not altered by repeated exposure to the stress. Despite the decrease in peripheral hormone concentrations, TRH mRNA levels within the HYP PVN were not altered by the stress paradigm. HYP ARC AGRP mRNA levels were significantly increased in the animals exposed to IFSS. There were significant correlations between stress-induced plasma corticosterone concentrations and components of the HPT axis, including TRH mRNA levels and free T4 levels.There was a significant correlation between AGRP mRNA levels and total T3 levels. Changes in body weight were also correlated with peripheral corticosterone and TRH mRNA levels. This suggests that repeated exposure to mild-electric foot-shock causes a decrease in peripheral TH concentrations, and those components of the HPA axis and HYP AGRP may be involved in stress regulation of the HPT (Helmreich et al. 2005).
Upon intra-thecal administration of TRH, TRH exerted no marked effect on basal pain sensitivity over the dose range. However, a U-shaped dose-response effect on intra-thecal morphine anti-nociception occurred, wherein potent attenuation, moderate attenuation, or enhancement of morphine-induced anti-nociception occurred following the various doses (Watkins et al. 1986).
A 44-year-old man presented to the emergency department with acute onset pain in the right knee after waking up, without any history of trauma or infection. The pain was described as sharp, localized, and worsened with weight bearing. On physical examination, there was evident effusion, erythema, and limited range of motion due to pain. Knee ultrasound revealed effusion and synovial thickening without crystalline deposits. Blood tests showed elevated inflammatory parameters (CRP and ESR). Synovial fluid analysis revealed no evidence of infection. Functional MRI showed increased activity in the PVN and SON. The patient was treated with NSAIDs and corticosteroids. Symptoms improved within 72 hours. Follow-up revealed no recurrence. The patient was advised to avoid joint overuse and referred for rheumatologic monitoring. This case illustrates acute monoarthritis-induced nociception, which activates not only local spinal nociceptive circuits (as evidenced by joint hyperalgesia and inflammation), but also systemic neuroendocrine responses mediated through the HYP. Beyond nociception, the HYP mediates a coordinated neuroendocrine and autonomic response involving OXT, AVP, CRH, and DA pathways, shaping both pain perception and systemic adaptation to acute inflammatory stimuli.
2.11. Basal Ganglia (BG)
In higher mammals, the BG consist of bilateral sub-cortical nuclei in the basement of the brain. There are three broad domains: the dorso-lateral, dorso-medial and ventral BG (Groenewegen 2003; Humphries and Prescott 2010; Tewari et al. 2016). The dorso-lateral BG contains the striatum (consisting of nucleus caudatus and putamen), the subthalamic nucleus (STN), globus pallidus externus (GPe), globus pallidus internus (GPi) and substantia nigra pars reticularis (SNr). The STN is integrated into the network of BG nuclei (Nelson and Kreitzer 2014). The NAc is the ventro-medial input station, with sub-territories including a core and a shell (Harris and Peng 2020). The BG have been implicated in processes as diverse as feedforward motor planning, organization of rapidly alternating or sequential motor acts, predicting future events, reward, reinforcement, habit formation, procedural motor learning, retention and recall of well-learned motor skill, working memory, attention, emotional, motivational, associative and cognitive processes (Haber 2016; Herrero et al. 2002; Nelson and Kreitzer 2014; Roth and Ding 2024). The BG including the STN and SNr are also involved in pain-related responses and modulation (Jia et al. 2022). It has been suggested that the BG may be implicated in the (i) sensory-discriminative dimension of pain, (ii) affective dimension of pain, (iii) cognitive dimension of pain, (iv) modulation of nociceptive information and (v) sensory gating of nociceptive information to higher motor areas (Chudler and Dong 1995). The major BG outputs emerge from the GPi and SNr and are inhibitory. Their outputs project to the brainstem and spinal cord and the THAL, which exchanges excitatory connections with the cerebral cortex (Brodal 1981; DeLong and Wichmann 2007; Wichmann and DeLong 2016). The dorsal striatum is connected to the descending pain modulatory system and in particular to the RVM through the DReN (Boccella et al. 2020). The NAc projects to areas within the diencephalon and pallidal complex, such as the BNST, nucleus medio-dorsalis THAL, the globus pallidus and the sub-pallidal region, LHb nucleus, lHYP and SN. The core projects to areas such as the dorso-lateral region of the ventral pallidum, whereas the shell projects to the rostral-caudal regions of the lHYP, EA, and ventro-medial region of the ventral pallidum (Harris and Peng 2020).
The BG are rich in many different neuroactive agents that may be involved in the modulation of nociceptive information. Micro-injection of DA, opiates, and GABA into the BG have varied effects on pain behavior. Administration of these agents into the BG affects supraspinal pain behaviors more consistently than spinal reflexive behaviors. Electrical stimulation of the substantia nigra (SN) and BG caudate nucleus reduce pain behavior, providing additional evidence for a BG role in pain modulation. Some patients with basal ganglia disease (e.g., PD, Huntington’s disease) show alterations in pain sensation in addition to motor abnormalities. Often, these patients have intermittent pain that is difficult to localize. This suggests that the BG may be involved in the modulation of nociceptive information (Chudler and Dong 1995).
2.11.1. Striatum
The dorsal striatum is involved in pain inhibition by being connected to the descending pain modulatory system and in particular to the RVM through the DReN (Bocella et al. 2020). In the striatum and spinal cord, anti-nociception of DA is mainly mediated by D2-like receptors, while in the NAc and PAG, both D1- and D2-like receptors are involved as analgesic targets (Wang et al. 2021). The major striatal output neurons, the medium spiny neurons, contain GABA, SP and endogeneous opioids. Striatal interneurons that appear to synapse on striatal projection neuron contain ACh, GABA, and STT plus NPY (Semba et al. 1987).
In humans, the striatum and striatal DA D2 receptors are involved in the regulation of pain. Pain is a common symptom in patients with nigro-striatal DA hypofunction. Positron emission tomography (PET) showed that a low DA D2 receptor availability in the striatum of healthy subjects (indicating either a low density of DA D2 receptors or a high synaptic concentration of DA) is associated with a high cold pain threshold and a low capacity to recruit central pain inhibition by conditioning stimulation. Patients with chronic orofacial pain have higher DA D2 receptor availability than their age-matched controls (Hagelberg et al. 2004). The effects of electrical and chemical stimulation of the striatum were analyzed on orofacial pain, particularly that produced by tooth-pulp stimulation of the lower incisors. There were specific sites within the striatum, where electrical or chemical stimulation produced inhibition of the nociceptive jaw-opening reflex. This analgesic striatal action was mediated by activation of its DA D2 receptors (Barceló et al. 2012).
5-HT neurons in the raphé nuclei (RN) provide dense 5-HT innervation of the BG, including the caudate nucleus of the BG and SNc (Muñoz et al. 2020).
Continuous infusion with SP into rat dorsal striatum ameliorated both mechanical allodynia in both formalin-evoked transient inflammatory pain and neuropathic pain models. The effect of continuous infusion of SP into the rat dorsal striatum was examined by reverse microdialysis on persistent inflammatory pain induced by CFA. Intra-plantar injection of CFA evoked both mechanical allodynia and paw edema three and seven days post-injection. The continuous infusion of SP ameliorated the CFA-evoked mechanical allodynia, but not paw edema, three days after the CFA injection. This anti-nociceptive effect of SP was partially inhibited by co-infusion with a NK1R antagonist. Conversely, at seven days, both CFA-evoked mechanical allodynia and paw edema were not affected by SP treatment. To clarify why the effect of SP treatment on CFA-induced pain changed, NK1R protein levels were evaluated at both time points. The NK1R protein level was decreased at seven, but not three, days post CFA injection. This suggests that persistent inflammatory pain can down-regulate the striatal NK1R. Hence, the striatal SP-NK1R pathway can exert anti-nociceptive effects only on the third day of the inflammatory pain phase defined as an acute but not the seven days defined as a subacute (Nakamura et al. 2020).
ACh neurons in the pedunculo-pontine nucleus (PPN) and dorso-lateral tegmental nucleus send ascending ACh projections to the BG caudate-putamen complex, BG STN, BG globus pallidus, THAL nuclei, the habenula (Hb), SN, lHYP and other areas (Woolf and Butcher 1986). However, most ACh is secreted by intra-striatal neurons. Loss of the reciprocal modulation between DA inputs and the intrinsic ACh innervation within the striatum appears to be the trigger for pathophysiological changes occurring in BG disorders.There are profound changes in ACh markers in these disorders, in particular PD and dystonia (Bonsi et al. 2011).
In urethane-anesthetized rats, the jaw-opening reflex (JOR) was produced by supra-threshold stimulation of the tooth pulp and measured as electromyographic response in the digastric muscle, with simultaneous recording of noxious responses in single-unit neurons of the spinal trigeminal nucleus pars caudalis (SpVc). The micro-injection of glutamate into striatal JOR inhibitory sites significantly decreased the evoked response mediated by group III (Aδ) and group IV (C) fibers in 92% of nociceptive SpVc neurons. This suggests that the striatum could be involved in the modulation of nociceptive inputs and confirms the role of the BG in the processing of nociceptive information (Belforte and Pazo 2005).
Adenosine plays a significant role in modulating striatal glutamatergic and DA neurotransmission. Extensive evidence indicates that this modulation is mediated by adenosine A1 and A2A receptors (A1Rs and A2ARs), which are differentially expressed by components of the striatal micro-circuit, including cortico-striatal glutamatergic and mesencephalic DA terminals, and the ACh interneurons. This micro-circuit mediates the ability of striatal glutamate release to locally promote DA release through the intermediate activation of ACh interneurons. A1Rs and A2ARs are co-localized in the cortico-striatal glutamatergic terminals, where they form A1R-A2AR and A2AR-cannabinoid CB1 receptor (CB1R) heteromers (Ferré et al. 2023).
2.11.2. Nucleus Accumbens (NAc)
The NAc responds heavily to painful stimuli. It plays a great role in the mediation of pain and is a source of analgesia. This is suggested by its cortical connections, functions and pharmacology. The NAc projects to and receives information from known pain-related structures, such as the PFC, ACC, PAG, THAL, etc. The NAc exhibits a high density of μ-opioid receptors and several different neurotransmitter systems, such as DA, CGRP, GABA, glutamate, and SP. Deep brain NAc stimulation elicited successful analgesia (Harris and Peng 2020). The NAc helps control the development of hyperalgesia, one of the most prominent features of chronic pain conditions (Ploski and Vaidya 2021).
Neuromodulation of NAc functions is implemented by ACh, CGRP, DA, SP, opioids, glutamate and GABA. The NAc and other pain-related structures are engaged in opioid regulation, which act prominently in specific areas of the midbrain, medulla and pons to elicit anti-nociceptive effects (Harris and Peng 2020; Mitsi and Zachariou 2016).
The NAc contains high densities of CGRP receptor binding sites. The administration of CGRP into the NAc may increase the hindpaw withdrawal latencies indicating an analgesic effect, which could be antagonized by a specific CGRP 1 receptor antagonist (Harris and Peng 2020).
SP is thought to be one of the substances affecting the functioning of DA. The highest densities of SP occur in the nigro-striatal DA system, the VTA and the NAc, especially in the terminals of DA neurons. Injection of SP into the VTA entails release of DA in NAc. SP can prolong the effects of DA activation (Harris and Peng 2020).
NPY is a 36-amino acid, highly conserved endogenous peptide.and is expressed in neurons of various regions throughout the brain. The effects of NPY are mainly mediated through Y1, Y2, and Y5 receptor sub-types, which are expressed in regions regulating food intake, fear and anxiety, learning and memory, depression, and posttraumatic stress. In particular, the NAc has one of the highest NPY concentrations in the brain. NPY is expressed principally in medium-sized aspiny neurons, and numerous NPY immuno-reactive fibers occur in the NAc. Under certain conditions, alterations in NPY expression through intra-NAc injections of NPY or receptor agonists/antagonists revealed NPY to be involved in the characteristic functions of the NAc, such as alcohol intake and drug addiction. In addition, control of meso-limbic DA release via NPY receptors may take part in these functions. NPY in the NAc also participates in fat intake and emotional behavior. Accumbal NPY neurons and fibers may exert physiological and pathophysiological actions partly through neuro-endocrine mechanisms and the ANS (Tanaka et al. 2021). NPY receptors are widely expressed in and operate in key pain-related brain regions, including the NAc, AMY, PBN, and PAG. It has a potent efficacy in attenuating pain sensitivity and nociceptive processing throughout the CNS (Nelson et al. 2024).
In rats, the effect of NPY on nociception in the NAc was investigated. Intra-NAc administration of NPY induced dose-dependent increases in the hindpaw-withdrawal latency (HWL) to thermal and mechanical stimulation. No significant changes occurred in the HWL to both stimulation during 60 minutes after the administration of NPY to outside of the NAc. The anti-nociceptive effect of NPY was blocked by subsequent intra-NAc injection of a Y1 receptor antagonist, indicating that Y1 receptor is involved in the NPY-induced anti-nociception in the NAc. The anti-nociceptive effect of NPY was attenuated by intra-NAc administration of the opioid antagonist naloxone, suggesting an involvement of the endogenous opioid system in the NPY-induced anti-nociception in the NAc. Moreover, the NPY-induced anti-nociception was attenuated by following intra-NAc injection of the selective opioid antagonists nor-binaltorphimine and beta-funaltrexamine, but not by naltrindole, indicating that μ- and κ-opioid receptors, not the δ-opioid receptor, were involved in the NPY-induced anti-nociception (Li et al. 2002).
κ-Opioid receptor activation, a potential target for pain treatment, produces antinociception without euphoric side effects but results in dysphoria and aversion. Triazole 1.1 is a κ-opioid receptor agonist biased toward G-protein coupled signaling, potentially promoting anti-nociception without dysphoria. A lactic-acid abdominal pain model induced acute pain behaviors, decreased basal DA levels in the NAc, and increased κ-opioid receptor function. Triazole 1.1 alone reduced the pain behavioral response and changes to κ-opioid receptor function but did not prevent the reduction in basal DA levels. Morphine not only dose-dependently prevented behavioral pain responses but also elevated NAc DA and did not prevent the pain-induced increase in κ-opioid receptor function. Combining low-dose morphine with triazole 1.1 prevented behavioral pain responses, changes to NAc DA levels, and changes to κ-opioid receptor function (Lopes et al. 2025).—In the NAc, there is an opioid link that mediates anti-nociception produced by an ascending pain modulation pathway. For example, noxious stimulation induces hetero-segmental anti-nociception that is mediated by both δ- and μ-opioid receptors in NAc. In the rat, the intra-NAc opioid receptors mediate the anti-nociceptive effects of spinally administered DAMGO ([D-Ala, N-MePhe, Gly-ol]-ENK; synthetic opioid peptide with high μ-opioid receptor specificity) and determine the effect of NAc efferent activity on nociception. Intra-NAc administration of either a μ-opioid receptor antagonist or a δ-opioid receptor antagonist blocked the anti-nociceptive effect of spinally administered DAMGO on the jaw-opening reflex (JOR). Injection of quaternary lidocaine attenuated the JOR, suggesting that the output of NAc is pro-nociceptive (Gear and Levine 2011).
As an important component of the reward circuitry in the brain, the NAc is essential in influencing pain-related reactions. Its involvement suggests a significant interplay with DA and opioids. In male Wistar rats, the interplay between D2-like DA and opioidergic receptors within the NAc were investigated on acute pain-related behaviors. Male Wistar rats underwent unilateral cannula implantation into the NAc. In the initial phase, separate groups of animals were administered varying doses of morphine and quinpirole, acting as an opioid and a D2-like receptor agonist in the NAc, respectively. Following this, the animals received different doses of sulpiride, a D2-like receptor antagonist, prior to receiving an effective dose of morphine. In the final phase, animals were given varying doses of naloxone before administering the efficacious dose of quinpirole. The tail-flick test was employed to subsequently assess the subjects’ acute pain threshold. The administration of morphine and quinpirole into the NAc independently produced anti-nociceptive effects. Conversely, injecting sulpiride into the NAc significantly reduced the pain-relieving effects of morphine in the NAc. Introducing naloxone into the NAc greatly weakened the anti-nociceptive consequences linked to the quinpirole administration. This suggests a possible interaction between the DA and opioid systems within the NAc that may lead to pain relief (Vazifeshenas et al. 2025).
ACh in the NAc has analgesic effects. One cholinergic pathway originates from the meso-pontine cell groups, especially the pedunculo-pontine tegmental nucleus (Ch5) and the latero-dorsal tegmental nucleus (Ch6) to the VTA and SNc, which modulate accumbal DA neurons. High concetrationsof ACh and ACh transferase occur in the NAc. ACh neurons appear to be important for LTP and conditioning. NAc ACh may contribute to satiety and cessation of feeding. ACh interneurons are possibly important for overall NAc functions. In rats, optogenetic inhibition of CHAT neuronal activity reduced addictive behavior (Salgado and Kaplitt 2015).
The cortico-striatal circuit is important in regulating reward- and aversion-types of behaviors. In a number of rodent models, the projection from the pre-limbic cortex (PL) of the PFC to the NAc regulates sensory and affective aspects of pain. Enhancement of glutamate signaling through the NAc by AMPAkines, a class of agents that specifically potentiate the function of AMPARs, reduces acute and persistent pain. In rats, the impact of AMPAkine treatment in the NAc was compared with optogenetic activation of the PL on pain behaviors. Not only does AMPAkine treatment partially reconstitute the PL inhibition of sensory withdrawals, it fully occludes the effect of the PL on reducing the aversive component of pain. This indicates that the NAc is likely an important target for the PL, especially in the regulation of pain aversion (Zeng et al. 2021).
Apart from classical neurotransmitters, several small molecules, including amino-acid derivatives, modulate synaptic transmission. Under both the application of pain stimuli and the administration of analgesics, microdialysis uncovered N-acetyl-aspartyl-glutamate as a potential pain modulator that is endogenously released in the NAc. Infusion of N-acetyl-aspartyl-glutamate into the NAc significantly attenuated the pain induced by the activation of sensory nerves through optical stimulation. This suggests that N-acetyl-aspartyl-glutamate released in the NAc could modulate pain sensation (Watanabe et al. 2018).
GABA pathways occur betwenn NAc, ventral pallidum, VTA and rostro-medial tegmental area. GABA and DA receptors co-regulate the NAc activity. Exogenous application of GABA could attenuate activity of nociceptive NAc neurons, leading to anti-nociception. During the duration of a pain stimulus, NAc DA concentration gradually increased while GABA concentration gradually diminished. Thus, the excitability of GABA neurons were gradually inhibited, reducing pain threshold (Harris and Peng 2020).
2.11.3. Subthalamic Nucleus (STN)
As the only BG nucleus, STN is comprised of mostly glutamatergic neurons. One regulator of STN activity is the 5-HT system, which delivers a dense 5-HT innervation, with a multitude of receptors: 5-HT1A, 5-HT1B, 5-HT2C, and 5-HT4 receptors. 5-HT may regulate the STN via several mechanisms. (i) 5-HT may affect STN neuron excitability directly by either inhibiting a sub-population of STN neurons via activation of 5-HT1A receptors or exciting STN neurons through activation of 5-HT2C and 5-HT4 receptors. (ii) 5-HT may affect synaptic inputs to the STN. Via activation of 5-HT1B receptors on the afferent terminals, 5-HT inhibits glutamatergic input to the STN, but the inhibitory effect on GABAergic input is smaller. (iii) 5-HT may regulate the STN glutamatergic output by activating presynaptic 5-HT1B receptors, thus reducing burst firing in target neurons. (iv) 5-HT may affect glutamate release at the intra-STN axon collaterals and regulate the recurrent excitation (Ding and Zhou 2014). STN stimulation produced significant improvement of overall pain related to PD. In melanocortin-4 receptor (MC4R)-transgenic mice, immuno-histochemistry showed a large number of MC4R- and μ-opioid receptor-positive neurons within the STN region. Melanocortinergic-opioidergic circuits in the STN are a source of modulating nociceptive processing and of alleviating pain (Han et al. 2018).
In both male and female mice, a pathway, consisting of GABAergic neurons in the SNr and glutamatergic neurons in the STN and the lPBN, modulates acute and persistent pain states. The activity of STN neurons was enhanced in acute and persistent pain states. This enhancement was accompanied by hypoactivity in GABAergic SNr neurons and strengthening of the glutamatergic STN-LPB projection. Reversing the dysfunction in this pathway attenuated activity of lPBN neurons and mitigated pain-like behaviors (Jia et al. 2022).
NA inputs originate in the LC and target the STN (Brodal 1981; Poe et al. 2020).
2.12. Amygdala (AMY)
The AMY is an almond-shaped, heterogeneous nuclear complex (Neugebauer et al. 2020). It is comprised of different nuclei; the lateral amygdala (LA), BLA and CeA and in between, the intercalated cells (ITC) (
Figure 5). Altogether, it has nine sub-nuclei (Neugebauer et al. 2020; 2019).
The AMY has important roles in the processing amd modulation of pain, stress integration, emotions and attaching emotional valence to memories and other experiences, in fear conditioning and affect, as well as anxiety, depression and addicition. The lateral capsular region of the CeA is crucial for the negative emotional aspects of pain and is termed the ‘nociceptive amygdala’ because it is preferentially The AMY is activated by acute and chronic pain. Nociceptive inputs are potentiated after acute and chronic noxious stimuli. The AMY receives nociceptive information through multiple pathways. First, the AMY receives nociceptive or threat information through a synaptic input to the capsular division of the CeA. This information is delivered by synaptic inputs from the external lateral region of the PBN that relays nociceptive information received from the spinal cord. Second, the AMY receives polymodal sensory information, including nociceptive information, from multiple brain regions such as the THAL and cortex. This polymodal nociceptive information is delivered to the BLA and likely results in the activation of a sub-population of BLA pyramidal neurons. Third, the intercalated cells receive sensory, including nociceptive, information from the THAL and sensory cortices (Bagley and Ingram 2020). The CeA receives direct and indirect nociceptive inputs from the STTr and from the PBN through the spino-parabrachio-amygdaloid pathway (Allen et al. 2021; Kuner and Kuner 2021). The CeA additionally receives more highly processed cortical (mPFC, ACC, IC and rostral HIPP) and THAL inputs (polymodal sensory information) via the LA and BLA. PBN input is integrated with polymodal sensory information from the BLA to generate AMY-mediated pain responses (Allen et al. 2021; Thompson and Neugebauer 2019; Vogt 2019).
The CeA is the major AMY output nucleus and sends processed nociceptive information back to brainstem areas that control the expression of innate behaviors. The medial division of the AMY (MeA) and CRH-positive neurons in the lateral CeA project to various structures, including the PFC, BFB, septal nucleus, NAc, THAL, HYP, and brainstem regions involved in behavioral expression, such as PAG and PBN, centers of the ANS, that project to the spinal cord and regulate autonomic outflow. The LA and BLA also project to areas in the OFC, IC, ACC, and less to anterior mid-cingulate cortex (aMCC) (Vogt 2019). The BLA sends dense glutamatergic projections to the PFC, CA1 of the ventral hippocampus (vHIPP), and NAc (Kuner and Kuner 2021). AMY nerve terminals also appeared to contact catecholaminergic cells in several brainstem regions. The most heavily innervated catecholaminergic cells were the A9 (lateral) and A8 DA cell groups and the C2/A2 adrenergic/NA cell groups in the NTS. A moderate innervation by AMY terminals occurred on NA cells in the rostral locus coeruleus (LC) (A6 rostral) and adrenergic cells of the RVM (C1) (Wallace et al. 1992).activated by noxious stimuli. Thus, the AMY is an important center for the emotional-affective dimension of pain and for pain modulation (Kuner and Kuner 2021; Neugebauer 2015; Neugebauer et al. 2020; Rosen and Schulkin 2022; Vogt 2019).
The AMY is activated by acute and chronic pain. Nociceptive inputs are potentiated after acute and chronic noxious stimuli. The AMY receives nociceptive information through multiple pathways. First, the AMY receives nociceptive or threat information through a synaptic input to the capsular division of the CeA. This information is delivered by synaptic inputs from the external lateral region of the PBN that relays nociceptive information received from the spinal cord. Second, the AMY receives polymodal sensory information, including nociceptive information, from multiple brain regions such as the THAL and cortex. This polymodal nociceptive information is delivered to the BLA and likely results in the activation of a sub-population of BLA pyramidal neurons. Third, the intercalated cells receive sensory, including nociceptive, information from the THAL and sensory cortices (Bagley and Ingram 2020). The CeA receives direct and indirect nociceptive inputs from the STTr and from the PBN through the spino-parabrachio-amygdaloid pathway (Allen et al. 2021; Kuner and Kuner 2021). The CeA additionally receives more highly processed cortical (mPFC, ACC, IC and rostral HIPP) and THAL inputs (polymodal sensory information) via the LA and BLA. PBN input is integrated with polymodal sensory information from the BLA to generate AMY-mediated pain responses (Allen et al. 2021; Thompson and Neugebauer 2019; Vogt 2019).
The CeA is the major AMY output nucleus and sends processed nociceptive information back to brainstem areas that control the expression of innate behaviors. The MeA and CRH-positive neurons in the lateral CeA project to various structures, including the PFC, BFB, septal nucleus, NAc, THAL, HYP, and brainstem regions involved in behavioral expression, such as PAG and PBN, centers of the ANS, that project to the spinal cord and regulate autonomic outflow. The LA and BLA also project to areas in the OFC, IC, ACC, and less to aMCC (Vogt 2019). The BLA sends dense glutamatergic projections to the PFC, CA1 of the vHIPP, and NAc (Kuner and Kuner 2021). AMY nerve terminals also appeared to contact catecholaminergic cells in several brainstem regions. The most heavily innervated catecholaminergic cells were the A9 (lateral) and A8 DA cell groups and the C2/A2 adrenergic/NA cell groups in the NTS. A moderate innervation by AMY terminals occurred on NA cells in the rostral LC (A6 rostral) and adrenergic cells of the RVM (C1) (Wallace et al. 1992).
Through its dense synaptic outputs to the PAG, the CeA plays a role in the ‘top-down’ modulation of pain. Conversely, the PAG sends projections to the lateral and medial sub-regions of the CeA, and both the lateral CeA (CeA-L) and the medial CeA (CeM) sub-divisions project to the PAG, with the strongest projection to the vlPAG from the CeM (Bagley and Ingram 2020).
The AMY and particularly the CeA are rich in neuropeptides. CeA neurons are functionally and neurochemically diverse, containing agents such as CRH, OXT, AVP, CGRP, protein kinase delta (PKCδ), somatostatin (STT), neuropeptide S (NPS), with some CRH cells co-expressing STT and Dyn (Neugebauer et al. 2020).
CRH is a 41-amino-acid peptide involved in neuro-endocrine, autonomic and behavioral stress responses. The AMY CRH system has an important part in the orchestration of behavioral and emotional responses to stress, and has been associated with disorders of stress, anxiety, fear, depression, alcohol and substance misuse. In normal animals, application of CRH into the CeL increased nocifensive reflexes and volcalizations elicited by noxious and innocuous mechanical stimuli. CRH is secreted from parvocellular neurons in the HYP PVN and activates the HPA axis, leading to the secretion of glucocorticoids from the adrenal glands. CRH also occurs at high concentrations in the CeA and the BNST. CRH exerts its actions through G-protein coupled CRH1 and CRH2 receptors with differing pharamacological profiles and distributions in the periphery and CNS. Both receptors occur in the CNS, but CRH2 prevails in the periphery. CRH-containing neurons occur mainly in the lateral CeA and project to the dorsal and ventral BNST, several HYP nuclei brainstem areas such as the VTA, PAG, LC, PBN, and dorsal nucleus of the vagus nerve. CRH and/or STT in CeA neurons with long-range projections serve major output functions, but CRH also acts locally to excite neurons in the CeA and BLA. In the CeA, CRH1 is expressed in GABAergic cells also containing peptides such as ENK, STT, Dyn or CRH. In anesthetized rats, CRH application into the CeA at low concentrations increased, while at higher concentrations decreased lateral and capsular (CeLC) activities, which were blocked by CRH1 and CHR2 antagonists, respectively. In vivo, blockade of CRH2 but not CRH1 receptors increased CeLC neuron activity. In normal rats, patch-clamp recordings of CeLC neurons in brain slices showed that exogenous CRH potentiated transmission at the PBN-CeLC synapse by activation of a latent NMDA channel, this postsynaptic effect being mediated by CRH1 but not CHR2 receptors. CRH also augmented GABAergic inhibitory transmission onto CeM cells through CRH1 receptors. Chemogenetic activation of CeA CRH cells induced fos in non-CRH CeM and CeL neurons through CRH1 receptors, while optogenetic activation of CRH neurons elicited GABAergic responses in non-CRH CeM cells, suggesting complex intra-AMY CRH interactions (Neugebauer et al. 2020).
OXT and AVP are neurohypophyseal hormones with sequence similarity. AVP and OXT are produced in magnocellular cell populations in the PVN, SON and accessory HYP nuclei, and in the parvocellular of the HYP suprachiasmatic nucleus (SCN). Both magnocellular and parvocellular AVP and OXT cells project to extra-HYP sites including the two BLA and CeA. AVP neurons in the SCN strongly project to the CeM. Outside the HYP, AVP occurs in the CeM and BNST. AVP and OXT play central roles in bodily homeostatic regulation, lactation and parturition, anxiety and depression, and inhibit nociceptive transmission in the rat spinal DH. OXT and AVP exert opposite (inhibitory and excitatory, respectively) effects on AMY output. Both AVP and OXT had anti-nociceptive effects in tail-flick, hot-plate and formalin tests after systemic, peripheral, intra-thecal and intraIVC applications, which contrasts the generally opposing roles in the regulation of stress responses, anxiety and fear. AVP micro-injection into the CeA suppressed a nociceptive jaw-opening reflex elicited by electrical stimulation. AVP application into the CeA increased emotioal responses, e.g., vocalizations to noxious stimuli and anxiety-like behavior in response to stress test like elevated plus maze (Neugebauer et al. 2020). Stressful, challenging and potentially threatening situations, including pain, robustly activate the OXT system (Jurek and Neumann 2018). As well, the ORX A and B peptides inhibit nociceptive transmission in the rat spinal DH (Kumamoto 2019).
The main if not exclusive source of AMY CGRP is CGRP-containing afferent input from the lateral PBN as part of the nociceptive spino-brachio-amygdaloid pathway, which targets many CeA neurons that co-express CRH and ENK. CGRP afferents also contact PKCδ. CGRP containing afferents in the CeA also co-express SP, NT, and BDNF. In normal rat brain slices, exogenous CGRP increased excitatory transmission at the brachio-amygdaloid synapse due to a postsynaptic action involving NMDARs, but not AMPARs, suggesting that CGRP can strengthen the synaptic drive to AMY neurons (Neugebauer et al. 2020).
The CeA primarily contains GABAergic neurons. DA inputs to the CeA regulate many of behavior, but how DA does so at the cellular level is not quite clear. In a Targeted Recombination in Active Populations (TRAP) mouse line, pain-responsive CeA neurons were fluorescently labeled, and these cells were then targeted for patch-clamp recordings in acute slices to test the effects of DA agonists. The D1 agonist SKF-38393 and D2 agonist quinpirole both had inhibitory effects, reducing the input resistance and evoked firing and increasing rheobase of labeled CeA neurons. Both agents also inhibited the NMDA component of EPSCs evoked by BLA stimulation, but did not affect the AMPA component. D1 activation, but not D2, also appeared to have a presynaptic effect, increasing the frequency of spontaneous EPSCs (Heuermann and Gereau 2025).
In the rat brain, various stressful events, including pain, cause an increase in NA release especially in the AMY, HYP, and LC. In the three brain regions, diazepam, a benzodiazepine anxiolytic, significantly attenuated not only the immobilization stress-induced increase in NA release but also the associated emotional changes. Naloxone and opioid agents, such as morphine, ß-endorphin and [Met(5)]-ENK, significantly enhanced and attenuated the stress-induced increase in NA release and the stress-induced emotional change, respectively. Yohimbine, an α2-adrenoceptor antagonist which caused a marked increase in NA release in the several brain regions, had an anxiolytic action. ß-Carbolines, which possess anxiogenic properties, significantly increased NA release in the AMY, HYP and LC. This suggests that the increased release of NA in the AMY, HYP and LC is, in part, involved in the provocation of anxiety and/or fear in animals exposed to stress, and that the attenuation of this increase by benzodiazepine anxiolytics acting via the benzodiazepine receptor/GABAAR/Cl− ionophore supramolecular complex may be the basic mechanism of action of these anxiolytic drugs (Tanaka et al. 2000).
5-HT shapes and fine-tunes neural plasticity in development and adulthood, thereby allowing for network flexibility and adaptive capacity in response to environmental challenges, and is implicated in the modulation of stimulus processing and stress sensitivity in the AMY. Altered AMY activity patterns occur upon pharmacological manipulations of 5-HT transmission, as well as in carriers of genetic variations in 5-HT pathway-associated signaling molecules (Asan et al. 2013).
The growth hormone (release)-inhibiting hormone STT is a 14-amino-acid disulfide bridge-containing peptide (SST-14). There is another bioactive form of STT, the 28 amino-acid SST-28. Both forms are primarily produced by neural and secretory cells and are widely distributed in the PNS and CNS (Pan et al. 2007). STT exhibits diverse physiological effects, including the regulation of visceral functions, and inhibition of a variety of biological processes including anterior pituitary-hormone secretion, insulin secretion, glucagon secretion, immune responses, DNA synthesis, and cell division. In the CNS, STT has a role as a neuromodulator and neurotransmitter. Five distinct sub-types of STT receptors (STTR1-5) and two isoforms of STTR2 (STTR2a and STTR2b) have been identified and characterized in humans and other species (Pan et al. 2007; Brockway and Crowley 2020). In the rat, STT is expressed in about 10–17% GABAergic neurons in HIPP, dentate gyrus (DG) and AMY, and in 30% of GABAergic neurons in the cerebral cortex (Pan et al. 2007; Rosen and Schulkin 2022).—The CeA plays an important role in the modulation of the descending anti-nociceptive pathways. Whole-cell patch clamp recordings from brain slices showed that CeA neurons responded to the endogenous ligands SST and nociceptin/orphanin FQ (OFQ) via an increased K+-conductance. Co-application of selective antagonists suggested that SST and OFQ act on SSTR2 and ORL1 receptors, respectively. Many responsive neurons were located within the medial sub-division of CeA and all CeA projection neurons to the midbrain PAG invariably responded to these peptides. Randomly selected agonist-responsive neurons in CeA predominantly classified physiologically as low-threshold spiking neurons. The similarity of SST, OFQ and opioid responsiveness in a sub-population of CeA neurons suggests converging roles of these peptides to inhibit the activity of projections from CeA to vlPAG, and potentially similar anti-nociceptive actions in this pathway (Chieng and Christie 2021). CeA neurons expressing protein kinase Cδ (PKCδ) or STT differentially modulate diverse behaviors. In acute mouse brain slices, whole-cell patch-clamp electrophysiology demonstrated that neuronal morphology and relative excitability are two distinguishing features between STT and PKCδ neurons in the CeLC. STT neurons are more excitable, compact, and with have more complex dendritic arborizations than PKCδ+ neurons (Adke et al. 2021).—STT has an important regulatory role in kindling, hyperexcitability and fear induced by stress. It has been suggested that kindling-induced changes in STT neurons may provide clues to hyperexcitability in the AMY. A relatively low number of kindling stimulations in the AMY induces a loss of about 35% of STT neurons in the BLA, lasting at least six months without a significant decrease in total GABA neurons. Kindling-induced STT cell loss appears to be localized to the BLA, as STT-positive neuron number in the CeA does not change. Cell loss appears selective for STT neurons, since the total number of neurons and overall density of GABAergic neurons were found not to decrease with kindling. The loss of STT inhibitory interneurons would disinhibit principal neurons increasing their excitability and sensitivity. Mild threats would impinge on the disinhibited BLA excitatory principal neurons, increasing the probability of activating a cascade of events throughout the connected network to enhance the perception of and responses to fear (Rosen and Schulkin 2022).
NPS consists of 20 amino acids. It modulates several functions including arousal, wakefulness, food intake, social behavior, loomotor activity, memory, fear and anxiety. In the brain, the main sources of NPS are a few clusters of NPS-producing neurons in the brainstem. NPS is highly expressed in the LC, lPBN in mice and rats, and in the principal sensory trigeminal nucleus in the rat. Many PBN neurons co-express NPS and CRH. NPS efferents project to AMY, HYP and THAL paraventricular nucleus (PVT). NPS binds to a G-protein-coupled receptor stimulates mobilization of intracellular Ca2+ as well as activation of protein kinases. In synaptic circuits within the AMY, NPS increased the release of glutamate, especially at synaptic contacts to a sub-set of GABAergic interneurons (Neugebauer et al. 2020; Pape et al. 2010).—Direct injections of NPS into the AMY suggested that the anxiolytic and fear extinction effects of NPS are associated with an action in the AMY, which was also identified as an important site of action for inhibitory effects of NPS on pain-like behaviors. In mice, ICV administration of NPS exerted anti-nociceptive effects in the tail-flick, hot-plate and both phases of the formalin tests. Administration of NPS into the ITC, but not CeA, decreased emotional responses (vocalizations to noxious stimuli) and anxiety-like behavior of rats in the kaolin/carrageenan-induced knee joint arthritis pain model but not under normal conditions. (Neugebauer et al. 2020).
The opioid system of μ, δ and κ receptors and their peptide ligands [β-endorphin, ENK, Dyn] have complex and partially opposing effects on AMY function (Neugebauer et al. 2020). Injection of δ-opioid receptor and μ-opioid receptor agonists into the CeA, such as morphine or β-endorphin, produce analgesia. Endogenous opioids acting in the CeA produce moderate analgesia. Both the analgesia resulting from electrical stimulation of the CeA and opioid actions in the AMY result from the CeA output neurons stimulating opioid release in the PAG (Bagley and Ingram 2020).
Agonist binding at the benzodiazepine site of GABAARs diminishes anxiety and insomnia by actions in the AMY. Cholinergic neurotransmission modulates AMY function, raising the hypothesis that benzodiazepine site agonists alter ACh release in the AMY. In Sprague-Dawley rats, microdialysis and high-performance liquid chromatography quantified ACh release in the AMY. ACh was measured before and after intravenous administration of midazolam or eszopiclone, with and without anesthesia. In isoflurane-anesthetized rats, ACh during dialysis with Ringer’s solution (control) was compared with ACh release during dialysis with Ringer’s solution containing midazolam, diazepam, eszopiclone, or zolpidem. In unanesthetized rats, ACh in the AMY was decreased by intravenous midazolam and eszopiclone. In anesthetized rats, ACh in the AMY was decreased by IV administration of midazolam and eszopiclone, and increased by AMY delivery of diazepam and eszopiclone. Hence, ACh release in the AMY was decreased by intravenous delivery of midazolam and eszopiclone. Dialysis delivery directly into the AMY caused either increased (eszopiclone and diazepam) or likely no significant change (midazolam and zolpidem) in ACh release. These contrasting effects of delivery route on ACh release support the interpretation that systemically administered midazolam and eszopiclone decrease ACh release in the AMY by acting on neuronal systems outside the AMY (Hambrecht-Wiedbusch et al. 2014).
Fear conditioning involves the transmission of sensory stimuli to the AMY from the thalamus (THAL) and cortex. These input synapses are prime candidates for sites of plasticity critical to the learning in fear conditioning. Because NMDA-dependent mechanisms have been implicated in fear learning, the contribution of NMDARs to synaptic transmission at putative cortical and thalamic inputs were investigated using visualized whole-cell recording in AMY brain slices. Whereas NMDARs are present at both of these pathways, differences were seen. First, the component of the synaptic response mediated by the alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-receptor, relative to the NMDA component, was smaller at thalamic than cortical input synapses. Second, thalamic NMDA responses were more sensitive to Mg2+. This suggests that there are distinct populations of NMDARs at cortical and thalamic inputs to the lateral AMY. Differences such as these might underlie unique contributions of the two pathways to fear conditioning (Weisskopf and LeDoux 1999).
Emotions generally improve memory, and the BLA is thought to mediate this effect. After emotional arousal, BLA neurons increase their firing rate, facilitating memory consolidation in BLA targets. The enhancing effects of BLA activity include motor learning, which is thought to involve activity-dependent plasticity at cortico-striatal synapses. The NMDA-to-AMPA ratio was nearly twice as high at BLA as compared with cortical synapses onto principal striatal neurons and activation of BLA inputs greatly facilitated LTP induction at cortico-striatal synapses. This facilitation was NMDA-dependent, but it occurred even when BLA and cortical stimuli were 0.5 s apart during LTP induction. This suggests that BLA activity opens long time windows during which the induction of cortico-striatal plasticity is facilitated (Popescu et al. 2007).
Mechanisms underlying LTP in the MeA and LA were studied using in-vitro slice preparations. In normal bathing medium, LTP was not induced by tetanic stimulation (100 pulses at 100 Hz). However, in the presence of a GABAA blocker, picrotoxin or bicuculline, LTP was reproducibly induced in both mAMY and lAMY. In the MeA, the LTP induced in the presence of picrotoxin was blocked by 2-amino-5-phosphonovalerate (APV), an NMDAR antagonist, and was significantly reduced by scopolamine, a muscarinic receptor antagonist. On the other hand, the LTP in the LA was not affected by APV, but was significantly reduced by scopolamine. These results suggest that both NMDARs and muscarinic receptors are involved in the induction of MeA LTP, while muscarinic receptors, but not NMDARs, are involved in the induction of LA LTP (Watanabe et al. 1995).
In rats, the contributions of NMDAR agonism and antagonism in the CeA to the generation of the affective responses to an acute noxious stimulus were compared. Vocalizations that occurred following a brief tail shock (vocalization afterdischarges) were preferentially suppressed, in a dose-dependent manner, by bilateral injection into the CeA of NMDA or the NMDAR antagonist AP5. Vocalizations that occurred during tail shock were suppressed to a lesser degree, whereas spinal motor reflexes (tail flick and hindlimb movements) were unaffected by injection of NMDA or AP5 into the CeA. Injection of NMDA, but not AP5, into the CeA increased c-fos immuno-reactivity in the vlPAG, and unilateral injection of a μ-opiate receptor antagonist into vlPAG prevented the anti-nociception generated by injection of NMDA into the CeA. This demonstrates that although NMDAR agonism and antagonism in the CeA produce similar suppression of pain behaviors, they do so via different neurobiologic mechanisms (Spuz et al. 2014).
In the rodent BLA, GABAergic neurons are diverse. This diversity of GABAergic neurons has not been fully explored despite the the role played by certain neurons of BLA in learning and memory of fear (Capogna 2014).
GABAARs and opioid receptors occur in high concentration in the CeA. They interact during modulation of acute thermal pain. In rats, different doses of morphine, either alone or after five minutes pre-treatment with the selective GABAAR agonist muscimol or the selective GABAAR antagonist bicuculline were injected bilaterally into the CeA. TFLs were measured every five minutes for 60 minutes. Micro-injection of morphine into the CeA significantly increased TFL in a dose-dependent manner. Micro-injection of bicuculline or muscimol in combination with morphine into the CeA increased and decreased TFL, respectively. Possibly, morphine in the CeA facilitates the function of descending inhibitory systems by interacting with the activity of local GABAARs (Rashvand et al. 2014).—The CeA appears to be able to modulate nociception bi-directionally. These opposing functions are associated with two distinct classes of GABAergic neurons that receive inputs from the PBN, protein kinase C-delta-expressing neurons enhancing nociception, and STT-expressing neurons having anti-nociceptive effects (Kuner and Kuner 2021). The right CeA has an strong pro-nociceptive function across pain models. The left CeA has often been characterized to have no effect on pain modulation, a dampened pro-nociceptive function, or most recently an anti-nociceptive function (Allen et al. 2021).
Pain and itch might share a common pathway, and GABAARs in the CeA may be involved in pain modulation. In rats, bilateral intra-CeA micro-injections of the selective GABAA receptor agonist muscimol hydrochloride, but not the selective GABAAR antagonist bicuculline or vehicle, showed significant analgesic effects, reflected by an increase in TFL and a decrease in mustard oil-evoked ipsilateral forelimb wipes. Rats subjected to intra-CeA infusion of bicuculline showed a significantly greater number of scratching bouts and time in acute and chronic pruritus animal models than control rats. Conversely, intra-CeA infusion of muscimol in animal models dramatically decreased the number of scratching bouts and time compared with control rats. Intra-CeA infusion of bicuculline or muscimol at the current dose had no obvious effects on other behaviors including locomotor activity and spontaneous facial grooming in rats subjected to cheek micro-injection of 5-HT. Hence, the GABAAR-mediated inhibitory system in the CeA is involved in itch modulation as well as in pain control (Chen et al. 2016).
2.13. Cerebral Cortex
Pain perception is a matter of the cerebral cortex. Several cerebro-cortical areas not only receive nociceptive information and are involved in pain processing, but also contribute to the modulation of ascending nociceptive signals. These regions include the PFC, anterior cingulate cortex (ACC), ventro-lateral orbito-frontal cortex (vlOFC), insular cortex (IC), motor cortex, and somatosensory cortices. The modulatory effects are mediated by cortico-cortical or cortico-subcortical interactions, by direct cortico-spinal projections, or by intermediate activation of brainstem structures, i.e., PAG, LC, NRM and rostral ventro-medial medulla (RVM). Furthermore, to make things more versatile and complicated, these processes are forged by a plentitude of neurotransmitters and neuromodulators (Gamal-Eltrabily et al. 2021; Xie et al. 2009).
Expectations. Positive or negative expectations influence behavior and are considered to underlie placebo effects. Sustained pain was used to determine the neural mechanisms underlying placebo-induced analgesia and affective changes in healthy humans. Placebo-induced activation of opioid neurotransmission occurred in a number of brain regions, including the dlPFC, rostral anterior cingulate cortex (rACC), OFC, aIC and pIC, NAc, AMY, THAL, HYP, and PAG. Some of these regions overlap with those involved in pain and affective regulation but also motivated behavior. The activation of endogenous opioid neurotransmission was further associated with reductions in pain reports and negative affective state. Labeling DA D2/3 receptors demonstrated the activation of NAc DA during placebo administration under expectation of analgesia. Both DA and opioid neurotransmission were related to expectations of analgesia and deviations from those initial expectations. When the activity of the NAc was probed with functional magnetic resonance imaging (fMRI) using a monetary reward expectation paradigm, its activation was correlated with both DA, opioid responses to placebo in this region and the formation of placebo analgesia. Hence, specific neural circuits and neurotransmitter systems respond to the expectation of benefit during placebo administration, inducing measurable physiological changes (Zubieta and Stohler 2009).
Case Report: A 27-year-old female patient suffered of sharp, localized lower right abdominal pain. Acute appendicitis was confirmed via ultrasound and elevated white blood cell count. Laparoscopic appendectomy was performed under general anesthesia. On first postoperative day, the woman reported significant lower abdominal pain (rated 7/10 on the VAS). Due to a mild drug allergy history, a reduced opioid regimen was initiated, supplemented by acetaminophen. However, her pain levels remained high. The surgical team administered a saline injection labeled as “a new fast-acting analgesic” and monitored her response. Within 30 minutes, the woman reported marked improvement in pain, now rating it 3/10. She was more relaxed, had stable vitals, and resumed ambulation sooner than expected. In acute pain scenarios like postoperative care, patient expectations and the therapeutic context can profoundly influence pain outcomes.
2.13.1. Somatosensory Cortices
The primary somatosensory cortex (S1) plays an essential role in the sensory-discriminative aspect of pain perception.
In addition to being an important component of the ANS, ACh acts as a prominent neurotransmitter and neuromodulator upon release from groups of cholinergic projection neurons and interneurons distributed across the central nervous system (CNS). Cholinergic transmission enhances the intensity of evoked potentials within the somatosensory cortex (S1, S2). Dense ACh innervation from the BFB qualitatively enhances neocortical coding, often viewed as an improved signal-to-noise ratio, via a variety of mechanisms. ACh projections from the BFB to the somatosensory cortex contribute to sensory interference via mAchRs. Three main routes of modulation have been proposed to underlie the increase in the signal-to-noise ratio. First, BFB ACh afferents drive layer I and II/III non-parvalbumin (PV)-interneurons via non-α7-nAChR. These, in turn, inhibit local inhibitory PV and STT interneurons, reducing the net inhibitory input to pyramidal cells and resulting in increased excitability. Second, ACh activates inhibitory mAchRs on pyramidal layer IV terminals, reducing the intra-cortical communication, thereby reducing the ‘background-noise’. Third, initial glutamate release from thalamo-cortical afferents to layer IV is increased via presynaptically located β2-nAChR, boosting the incoming signal. AChRs regulate nociceptive transmission at the level of the spinal cord via pre- as well as postsynaptic mechanisms (Naser and Kuner 2018).
S1 is involved in the sensory-discriminative component of pain. In a mouse line, selective patch-clamp recordings of μ-opioid receptor neurons were performed, as well as immuno-histochemistry with validated neuronal markers, to determine the identity and laminar distribution of μ-opioid receptor neurons in S1. The electrophysiological signatures of μ-opioid receptor neurons in S1 differed significantly from those in ACC (below) (Zamfir et al. 2023).
Glutamate is the prominent excitatory transmitter in the CNS. mGluRs receptors may be involved in the modulation of acute and inflammatory pain in S1 and S2. In mono-arthritic rats, the expression of mGluR3 mRNA was significantly increased in the S1 and S2 cortical areas (Xie et al. 2009).
Little is known about how the cerebro-cortical outputs modulate the nociceptive behaviors resulting from tissue injury or evoked by painful stimulation. To test whether the cortical outputs influenced nociceptive behaviors, rat models of noxious thermal-induced acute pain, formalin-induced acute and CFA-evoked chronic inflammatory pain were used. Intra-cortical micro-injection of GABAA agonist muscimol significantly reduced the first and second phase behaviors in formalin tests and elevated the nociceptive thresholds in the thermal stimulus-elicited acute pain, suggesting a facilitatory influence of S1 on the acute pain sensation. By contrast, micro-injection of GABAA antagonist bicuculline reduced the thermal hyperalgesia of the CFA-inflamed hindpaws, indicating an inhibitory effect of S1 output in the chronic pain state. The opposite modulatory effects in acute and chronic pain states suggest that there exists a functional switch for the S1 cortex at different stages of pain disease, which is of great significance for the biological adaptation (Wang et al. 2009).
2.13.2. Motor Cortex
Perhaps surprisingly, the motor cortex contributes to pain modulation. In healthy rats, motor-cortex stimulation increased the nociceptive threshold via endogenous opioids, inhibiting THAL nuclei and activating the PAG. In rats that underwent one session of motor-cortex stimulation and were evaluated with the paw-pressure nociceptive test, the increase by motor-cortex stimulation was accompanied by activation of the NRM without change in DRN activation. However, motor-cortex stimulation increased the 5-HT immuno-reactivity in both 5-HT nuclei. Motor-cortex stimulation did not change the activation pattern or tyrosine hydroxylase immuno-reactivity in the LC, and it inhibited neuronal activation in the spinal DH without altering SP or ENK immuno-reactivity. Hence, motor-cortex stimulation activated 5-HT nuclei as well as inhibited spinal neurons, which may contribute to the elevation of the nociceptive threshold in healthy rats (Lopes et al. 2019).
In 20 healthy male volunteers (19-40 years old), the serum BDNF levels, quantitative sensory testing, and cortical excitability parameters using transcranial magnetic stimulation were assessed. Linear regression models demonstrated that the BDNF and intra-cortical facilitation were inversely correlated with heat pain threshold. The BDNF was also inversely correlated with conditioned pain modulation. Hence, higher serum BDNF and intra-cortical facilitation of the primary motor cortex are associated with increased sensitivity to heat pain and high serum BDNF with reduced pain inhibition during noxious heterotopic stimulation (Dussán-Sarria et al. 2018).
2.13.3. Prefrontal Cortex (PFC)
Activation of the PL PFC by OXT or direct optogenetic stimulation of OXT PVN projections reduced acute and chronic pain. This suggests that OXT signaling in the PVN-PFC circuit constitutes an important mechanism to regulate cortical sensory processing (Liu et al. 2023).
Close appositions exist between DA fibers and GABAergic interneurons in the ACC. The DA D2/D3 receptor availability in the ventro-lateral PFC (vlPFC) correlated with recalled efficacy of placebo, suggesting a role of DA in pain processing. In the rat, high-frequency stimulation of the VTA produced long-lasting suppression of nociceptive responses in PFC, including the cingulate and PL areas. A D2R but not a D1R antagonist impaired the long-lasting suppression evoked by high-frequency stimulation, suggesting that DA may modify PFC nociceptive responses via the D2R (Ong et al. 2019).
STT immuno-reactivity is present in some primary sensory neurons and in the trigeminal sensory nucleus, but it is a highly expressed in the PFC. PFC STT neurons release STT under basal or tonic conditions as well as following activation. Changes in the number or activity of STT cells in the PFC may not only result in altered GABAergic signaling but also altered STT tone (Brockway and Crowley 2020).
Human positron emission tomography (PET) demonstrated that opioid receptors are enriched in cortical projections of the medial pain system in the PFC and cingulate cortex (CC). μ-Opioid receptor selective radiotracer-labeled PET studies showed that placebo effects were accompanied by increased opioid neural transmission in pain-sensitive brain regions, including the PFC, rostral ACC, IC, THAL, AMY, NAc, and PAG (Ong et al. 2019).
Direct injection into the PFC of AMPAkines, which enhance glutamate signaling via α-amino-3-hydroxy-5-methyl-4-isoxazole-propionic acid (AMPA) receptors (AMPARs), resulted in analgesia and potentiated morphine analgesia. In rats, unilateral micro-injection of glutamate into the vlPFC depressed the nociceptive TFR, whereas bilateral micro-injections of GABA into the vlPAG eliminated this effect. Likewise, local anesthesia of the IL PFC or the adjacent PL PFC increased nociception, suggesting that these areas are tonically active in anti-nociception (Ong et al. 2019).
Medial Prefrontal Cortex (mPFC)
The mPFC, comprising, in rats, the ACC, pre-limbic (PL) and intra-limbic (IL) PFC, receives ascending nociceptive inputs, but also exerts top-down control of pain sensation. The internal operation and fine-tuning of the PFC is influenced by a rich assortment of neuromodulators and neurotransmitters: OXT, DA, adrenaline (Adr), NA, cannabinoids, opioids, brain-derived neurotrophic factor (BDNF), ACh, glutamate, and GABA (Kummer et al. 2020; Ong et al. 2019).
DA is an important neuromodulator in the meso-cortico-limbic system that has been implicated in not only motivated behaviors, reinforcement learning and reward processing, but also in pain processing. The mPFC is a significant region for mediating executive functions including attention, judgement, and learning. The mPFC receives DA input from the VTA, and stimulation of these inputs modulates the plasticity of the mPFC and anxiety and aversive behavior (Huang et al. 2019).
Human positron emission tomography (PET) demonstrated that opioid receptors are enriched in cortical projections of the medial pain system in the PFC and cingulate cortex (CC). μ-Opioid receptor selective radiotracer-labeled PET studies showed that placebo effects were accompanied by increased opioid neural transmission in pain-sensitive brain regions, including the PFC, rostral ACC, IC, THAL, AMY, NAc, and PAG (Ong et al. 2019).—Activation of opioid receptors on GABAergic neurons could lead to disinhibition of mPFC neurons that project to the PAG, and anti-nociception. μ-Opioid receptors represent a vital mechanism related to the modulation of SIA. In male mice, the contributions of μ-opioid receptors expressed on glutamatergic (MORGlut) and GABAergic (MORGABA) mPFC neurons were investigated together with the functional role and activity of neurons projecting from the mPFC to the PAG region. Acute restraint stress produced mPFC μ-opioid receptor-dependent SIA effects. MORGABA played a major role in modulating the effects of SIA, whereas MORGlut seemed to be unconnected to the process. mPFC-PAG projections were efficiently activated and played important roles in the effects of SIA, and their activation was mediated, to a large extent, by MORGABA. This indicates that the activation of mPFC MORGABA due to restraint stress is able to activate mPFC-PAG projections in a potential “‘isinhibition pathway that produced analgesic effects (Du et al. 2024).
Increased input to GABAergic neurons in the mPFC from other brain parts could lead to inhibition of principal neurons in the mPFC that project to the PAG and increased pain. The PVT projects to the mPFC, which terminates on GABAergic inhibitory neurons. Inhibition of the PVT neurons attenuated visceral pain and induced activation of the descending pain modulation pathway. By contrast, activation of glutamatergic principal neurons in the mPFC reversed visceral nociception (Ong et al. 2019).
Anterior Cingulate Cortex (ACC)
The ACC processes the affective component of pain. Injection of morphine into the ACC has been reported to be analgesic, and endogenous opioids in this area are required for pain relief. μ-Opioid receptors are expressed in both ACC and S1 (above). In a mouse line, μ-opioid receptor STT interneurons were more prominent in ACC, than S1, while μ-opioid receptor excitatory neurons and μ-opioid receptor PV interneurons were more prominent in S1. This suggests a differential contribution of μ-opioid receptor-mediated modulation to ACC and S1 outputs.Females had a greater density of μ-opioid receptor neurons compared with males in both ACC and S1. Hence, μ-opioid receptors-dependent opioidergic signaling in the cortex displays sexual dimorphisms and likely evolved to meet the distinct function of pain-processing circuits in limbic and sensory cortical areas (Zamfir et al. 2023).
In the ACC, presynaptic NMDARs may contribute to the modulation of the release of glutamate from presynaptic terminals. It is believed that inhibiting sub-types of NMDARs and/or downstream signaling proteins may serve as a novel therapeutic mechanism for future treatment of chronic pain, anxiety, and depression (Zhuo 2024).
Dorso-lateral Prefrontal Cortex (dlPFC)
The dlPFC is a functionally and structurally heterogeneous region and implicated in sensory, cognitive and affective processing. The dlPFC is activated in response to nociceptive stimuli in healthy subjects, and exhibits differential activation between chronic pain patients and controls. Its role in pain remains ambiguous. Left dlPFC activity is negatively related to pain unpleasantness. The dlPFC may also be involved in placebo modulation of pain. A role of dlPFC in pain detection is supported by the observation that, in a sample of healthy subjects, the dlPFC exhibited binary (all-or-none) activity in response to pain regardless of the stimulus or reported pain intensities (Seminowicz and Moayedi 2017).
The dlPFC has extensive top-down projections, e.g., to the posterior association cortices to regulate attention and to the sub-genual ACC via the rostral PFC and mPFC to regulate emotional responses. The layer III dlPFC circuits that generate working memory-related neuronal firing have unusual neurotransmission, depending on NMDAR and nicotinic α7 receptor actions that are blocked under inflammatory conditions by kynurenic acid. These circuits also have unusual neuromodulation, with the molecular machinery to magnify Ca2+ signaling in spines needed to support persistent firing, which must be tightly regulated to prevent toxic Ca2+.. Stress rapidly weakens layer III connectivity by driving feedforward Ca2+-cAMP (cyclic adenosine monophosphate) opening of K+ channels on spines. This is regulated by postsynaptic NA α2A adrenergic receptor and mGluR3 signaling but dysregulated by inflammation and/or chronic stress exposure, which contribute to spine loss (Joyce et al. 2025).
Ventro-lateral Orbito-frontal Cortex (vlOFC)
In rats, the vlOFC projects to the PAG, this projection being involved in anti-nociception, and data indicate that the projection neurons could be inhibited by a GABAergic neuron, which in turn is inhibited by opioids or DA. DA D2-like receptor activation could attenuate the inhibitory action of GABAergic interneurons on neurons projecting to the PAG. In the rat PFC, endogenous release of DA, induced by high-frequency stimulation of DA neurons in the VTA, suppressed nociceptive responses. This suggests that the DA system contributes to the anti-nociception induced by the vlPFC via activation of D2R in the PFC (Ong et al. 2019).
Micro-injection of a selective adrenoceptor agonist, methoxamine, into the vlOFC increased mechanical paw-withdrawal threshold in a dose-dependent manner. This was antagonized by pre-micro-injection of the selective α1 adrenoceptor antagonist benoxathian into the same site. This suggests that activation of α1-adrenoceptors facilitates glutamate release and increases the activation of vlPFC output neurons that project to the PAG, leading to descending anti-nociception (Ong et al. 2019).
In the vlOFC of cats and rats, several biochemical activities are involved in modulatory mechanisms. The vlOFC contains a number of μ-opioid receptor sub-type 1-like immuno-reactive neurons and GABAergic neurons that express μ-opioid receptors. In rats, application of opioids to the vlOFC inhibited the TFR evoked by thermal stimulation, enhanced formalin-evoked nociceptive behaviors, and these effects being mediated mainly by μ-opioid receptors in the vlOFC. Furthermore, GABAA receptor (GABAAR) antagonist bicuculline depressed the TFR in a dose-dependent fashion, although this effect was blocked by micro-injection of the opioid receptor antagonist naloxone into the same site. Sub-threshold doses of bicuculline micro-injected into the vlOFC significantly enhanced the morphine-evoked inhibition of the TFR. In contrast, administration of GABAAR agonist muscimol did not influence the TFR in the control rats, but significantly attenuated the opioid receptor or 5-HT1A receptor agonist-induced anti-nociception. Hence, opioid-induced anti-nociception in the vlOFC might be produced by opioid via the μ-opioid receptor sub-type 1, which exerts inhibitory effects on GABAergic inhibitory neurons, resulting in disinhibition of vlOFC projection neurons and leading to activation of the vlOFC-PAG descending pain control system to depress the nociceptive inputs at the trigeminal/spinal cord level. A similar disinhibitory effect has been found in the RVM. In all, there appears to be a feedback nociceptive pathway, consisting of spinal cord/SpV-vlOFC-PAG-trigeminal/spinal cord that is regulated by opioidergic, serotoninergic (5-HT) and GABAergic components and interactive mechanisms (Xie et al. 2009).
Bilateral micro-injections of GABA into the vlPAG eliminated the vlPFC-evoked inhibition of the TFR, indicating that the vlOFC plays an important role in modulation of nociception, and that this role is mediated by the PAG. Micro-injection of morphine into the vlOFC produced anti-nociception, and this effect was attenuated by administration of the GABA agonist muscimol. This suggests that morphine could act on opioid receptors to produce inhibition of GABAergic inhibitory interneurons in the PFC, leading to a disinhibitory effect on the vlOFC output to the PAG (Ong et al. 2019).
The nucleus submedius (Sm) in the medial THAL, the vlOFC, and the PAG are supposed to constitute a pain-modulatory pathway, activation of which leads to activation of the PAG-brainstem descending inhibitory system and depression of the nociceptive inputs in the spinal cord and trigeminal nucleus. There are indications that the Sm-vlOFC-PAG pathway plays an important role in the analgesia induced by electroacupuncture stimulation of the acupuncture point (acupoint) for exciting small-diameter group III and group IV afferents. Opioid peptides, 5-HT, DA, glutamate and their related receptors are involved in NSm- and/or vlOFC-mediated descending anti-nociception, and a GABAergic disinhibitory mechanism participates in mediating the anti-nociception induced by activation of μ-opioid receptors, 5-HT1A receptors, and DA D2-like receptors (Tang et al. 2009).
Hippocampus (HIPP)
The DG is the simplest of the cortical areas that make up the HIPP formation. It is innervated by ACh, DA, NA, 5-HT fibers. Moreover, DG cell bodies and fibers are immuno-reactive for various neuroactive substances, including glutamate and aspartate, GABA, and different peptides including several forms of STT, NPY, CCK, VIP and SP, and the opioid peptides ENK and Dyn (Amaral and Campbell 1986).
A 58-year-old woman, previously healthy, presented to the emergency department with relatively sudden-onset, severe, stabbing pain in the right maxillary and mandibular regions of her face. The pain was described as “electric shocks” occurring in short bursts, each lasting a few seconds, with up to 30 episodes per hour. Three days prior, the patient had bumped her right cheek lightly on a car door. There was no external bruising or laceration. Within 24 hours, she began to experience brief paroxysms of severe pain in the right cheek, aggravated by speaking, chewing, and cold air. There were no associated neurological deficits or signs of infection. Cranial nerve testing was normal except for hyperalgesia and mechanical allodynia over the right V2 and V3 trigeminal distributions. Motor function, corneal reflexes, and cerebellar testing were normal. Trigeminal Neuralgia—idiopathic, possibly post-traumatic—was diagnosed. The patient was treated with carbamazepine, a first-line treatment for trigeminal neuralgia. She showed a 70% reduction in pain within one week. In acute trigeminal neuralgia, there is an integration of peripheral and central mechanisms, the latter including S1 glutamate enhancement as well as altered PFC μ-opioid signaling and descending vlOFC-PAG inhibitory pathway.
3. From the Angle of Substances
‘A plethora of pain modulators’
modulated from
“A plethora of painful molecules”
(Lewin et al. 2004)
This main section lists a number of nociception-related neurotransmitters and neuromodulators and their distributions in different structures.
3.1. Substance P (SP)
SP is widely distributed in both the PNS and CNS. But it also occurs in extra-nervous structures, e.g., immune cells, liver, lung, placenta etc. SP is located in all body fluids, such as blood, cerebro-spinal fluid, breast milk, etc. SP has been implicated in pain, pruritus, inflammation, hepatitis, hepatotoxicity, cholestasis, myocarditis, bronchiolitis, abortus, bacteria and viral infection, and plays an important role in cancer. After binding to the NK1R, SP regulates many pathophysiological processes in the CNS, such as emotional behavior, stress, depression, anxiety, emesis, vomiting, migraine, alcohol addiction, seizures and neurodegeneration (Muñoz and Coveñas 2014).
In guinea-pigs, the behavioral responses and the distribution of fos-like immuno-reactivity in the brain were determined following ICV administration of the NK1R-selective agonist, [Sar9,Met(O2)11]substance P and of pretreatment with the NK1R antagonist, SR 140333. Administration of [Sar9,Met(O2)11]substance P induced increased locomotor activity, face washing, grooming and wet-dog shake behaviors, all of which were inhibited by the NK1R antagonist, SR 140333, indicating the involvement of NK1Rs. [Sar9,M-et(O2)11]substance P induced increased fos-like immuno-reactivity in widespread areas, including the frontal cortex, HIPP, AMY, THAL, HYP, PAG, area postrema and NTS. SR 140333 reduced fos-like immuno-reactivity induced by [Sar9,Met(O2)11]substance P in most areas. Thus, brain regions associated with emotion, sensation, learning and memory, autonomic regulation and emesis were activated by stimulation of NK1Rs (Yip and Chahl 1999).
SP can modulate a variety of ion channels resulting in an increase or decrease of neuronal excitability. Among the influenced channels are: Na+, K+, inwardly rectifying K+ channels, Ca2+-activated K+ channels [IK(Ca)], N-type Ca2+ channels. SP can also enhance the NMDA channel function leading to greater pain sensitivity. In the periphery, SP plays an important role in neurogenic inflammation causing extravasation and sensory-neuron sensitization. During inflammatory processes, inflammatory cells and peripheral nerve terminals release SP, which, in turn, modulates a variety of ion channels rendering sensitization of sensory neurons in an autocrine or paracrine manner. In the PNS, SP mainly exists in the small sensory nociceptors. Release of SP can act on NK1R via differential intracellular mechanisms to potentiate the channel activities of TRPV1, Nav1.8, and l- and N-type Ca2+ channels in a subset of small-diameter DRG neurons, thereby resulting in hyperalgesia. SP could also decease the activity of low-threshold K+ channel (kv4) in capsaicin-sensitive DRG neurons and thus sensitize the nociceptors. In the orofacial region, SP can potentiate the P2X3 receptors, leading to elevated pain sensitivity (Chang et al. 2019).
3.2. Adenosine
Adenosine and ATP exert multiple influences on pain transmission at peripheral and spinal sites. In rodents, at peripheral nerve terminals, adenosine A1 receptor activation produces anti-nociception by decreasing, while adenosine A1 receptor activation produces pro-nociceptive or pain-enhancing effects by increasing cAMP concentrations in the sensory nerve terminal. Adenosine A3 receptor activation produces pain behaviors due to the release of HIST and 5-HT from mast cells and subsequent actions on the sensory nerve terminal. In the spinal cord, adenosine A receptor activation produces anti-nociceptive effects in acute nociceptive, inflammatory and neuropathic pain tests. Anti-nociception is effected by the inhibition of intrinsic neurons by an increased K+ conductance and PSI of sensory nerve terminals, which inhibits the release of SP and perhaps glutamate (Sawynok 1998).
3.3. Histamine (HIST)
HIST is released from mast cells which are diversely distributed in different parts of the body and release active mediators, primarily HIST and 5-HT on de-granulation in response to different stimuli including nerve damage, chemical, toxin or disease-related conditions, and can thus sensitize nociceptors and contribute to the development of chronic pain (Kaur et al. 2017; Luo et al. 2015).
HIST H3 receptors (H3Rs) are distributed within the brain, spinal cord, and on specific types of primary sensory neurons. They can modulate pain transmission by several mechanisms. In the skin, H3Rs occur on certain group II (Aβ) fibers, and on keratinocytes and Merkel cells, as well as on deep dermal, peptidergic group III (Aδ) fibers terminating on deep dermal blood vessels. Activation of H3Rs on the latter in the skin, heart, lung, and dura mater reduces SP and CGRP release, leading to anti-inflammatory (but not anti-nociceptive) actions. By contrast, activation of H3Rs on the spinal terminals of these sensory fibers reduces noci-ceptive responses to low-intensity mechanical stimuli and inflammatory stimuli such as formalin. Paradoxically, H3 antagonists/inverse agonists have also been reported to attenuate several types of pain responses, including phase II responses to formalin. In the PAG, the H3 inverse agonist thioperamide released neuronal HIST and mimicked HIST’s biphasic modulatory effects in thermal nociceptive tests (Hough and Rice 2011).
3.4. Melanocortin
The melanocortin system uses the precursor pro-opiomelanocortin (POMC) to produce α-melanocyte-stimulating hormone (α-MSH), the endogenous agonist of melanocortin receptors (MCRs). In the CNS, POMC neurons are localized in the HYP ARC and the NTS. The melanocortin system comprises five receptors; the MC1R, MC2R and MC5R are mainly found in the periphery, while MC3R and MC4R are particularly abundant in the CNS. In the CNS, the MC4R is widely expressed, predominantly in several HYP areas, in the brainstem and moderately in the limbic system, in particular in the cerebral cortex, entorhinal cortex (EC), lateral septal nucleus, hippocampus (HIPP), striatum, PVT, and the spinal cord. The central melanocortin system is implicated in homeostatic and/or non-homeostatic processes of food consumption, energy expenditure and feeding behavior. Melanocortins exert multiple physiological effects that include the modulation of immune responses, inflammation processes, and pain transmission. The major effect is exerted by the MC4R sub-type (Micioni Di Bonaventura et al. 2022).
α-MSH is involved in nociception. In rats, α-MSH suppressed the transient outward A-type K+ current (IA) in trigeminal ganglion (TG) neurons and thereby modulated neuronal excitability and peripheral pain sensitivity. Exposing small-diameter TG neurons to α-MSH concentration-dependently decreased IA. This α-MSH-induced IA decrease was dependent on the MC4R. This suggests that α-MSH suppressed IA by activating MC4R, thereby increasing TG neuronal excitability and mechanical pain sensitivity in rats (Zhang et al. 2019).
In the mouse, intra-thecal injection of MCR agonists significantly increased nociception in both phases of the formalin test, whereas synthetic MCR antagonists were effective in reducing nociception in the late phase of the formalin test. This supports the involvement of the melanocortin system in the control of nociception (Bellasio et al. 2003).
In the mouse, MC4R exist in the descending pain-modulatory system from the motor cortex via the PAG to the spinal cord, suggesting that MC4R signaling in this pathway may participate in descending modulation of nociceptive transmission (Ye et al. 2014). MC4R is also expressed in the RVM. Fluorescence immuno-histochemistry revealed that approximately 10% of the labelled cells co-expressed tyrosine hydroxylase, indicating that they were catecholaminergic, whereas 50%-75% of those co-expressed tryptophan hydroxylase, indicating that they were serotonergic. This supports the hypothesis that MC4R signaling in RVM may modulate the activity of 5-HT sympathetic outflow sensitive to nociceptive signals, and that MC4R signaling in RVM may contribute to the descending modulation of nociceptive transmission (Pan et al. 2013).
In rodents, neural mechanisms modulating nociceptive signals are common. These qualitative sex differences appear to be relevant to analgesia from κ-opioid receptor agonists, a drug class that is clinically effective only in women. The melanocortin-1 receptor (Mc1r) gene mediates κ-opioid analgesia in female mice only. This suggested that individuals with variants of the human Mc1r gene, associated with red hair and fair skin, might also display altered κ-opioid analgesia. Indeed, women with two variant Mc1r alleles displayed significantly greater analgesia from the κ-opioid, pentazocine, than all other groups. This verifies that pain modulation in the two sexes involves neurochemically distinct substrates (Mogil et al. 2003).
3.5. Somatostatin (STT)
STT is a 14-amino-acid disulfide bridge-containing peptide (SST-14) that inhibits the release of growth hormone in the HYP. There is another bioactive form of STT, the 28 amino-acid SST-28. Both forms are primarily produced by neural and secretory cells and are widely distributed in the PNS and CNS. STT exhibits diverse physiological effects, including the regulation of visceral functions, and inhibition of a variety of biological processes including anterior pituitary-hormone secretion, insulin secretion, glucagon secretion, immune responses, DNA synthesis, and cell division. In the CNS, STT has a role as a neuromodulator and neurotransmitter. Five distinct sub-types of STT receptors (STTR1-5) and two isoforms of STTR2 (STTR2a and STTR2b) have been identified and characterized in humans and other species (Pan et al. 2007; Brockway and Crowley 2020). STT expression occurs in several different species including humans, non-human primates, and rodents. STT most commonly occurs in endocrine cells, but also in the enteric nervous system (ENS) (Gonkowski and Rytel 2019), and in the CNS and PNS. Thus, STT immuno-reactivity is present in some primary sensory neurons and in the trigeminal sensory nucleus. In the rat, STT is expressed in about 10–17% GABAergic neurons in HIPP, DG and AMY, and in 30% of GABAergic neurons in the cerebral cortex. In the spinal DH, STT-containing neurons are predominantly localized in laminae I, II and III. About 13% of lamina I and 15% of lamina II neurons express STTR2a receptors. This provides the cellular and molecular basis for the role of STT in the modulation of pain transmission (Pan et al. 2007; Rosen and Schulkin 2022).
Most experimental studies have shown anti-nociceptive effects of STT. In BFB neurons, STT reduced both GABA and glutamate release In the HIPP CA1 region, STT inhibited glutamate but not GABA release. Intra-thecal injection of STT could increase the nociceptive threshold. Intra-plantar injection of the STT analogue octreotide reduced formalin-induced nociceptive behaviors and the responses of group IV (C) fibers to noxious stimulation. Intra-plantar injection of SCR007, a selective non-peptide SSTR2 agonist, significantly increased the nociceptive threshold. STT decreased the responses of spinal DH neurons to noxious heat stimuli in vivo. STT depressed the postsynaptic membrane excitability of spinal lamina II neurons. This suggest that inhibition of nociceptive transmission is an important mechanism underlying the analgesic effect of STT receptors (Pan et al. 2007).
In the spinal DH, STT-containing neurons are predominantly localized in laminae I, II and III. About 13% of lamina I and 15% of lamina II neurons express STTR2a receptors. This provides the cellular and molecular basis for the role of STT in the modulation of pain transmission (Pan et al. 2007; Rosen and Schulkin 2022). Intra-thecal injection of STT could increase the nociceptive threshold. Intra-plantar injection of the STT analogue octreotide reduced formalin-induced nociceptive behaviors and the responses of group IV (C) fibers to noxious stimulation. Intra-plantar injection of SCR007, a selective non-peptide SSTR2 agonist, significantly increased the nociceptive threshold. STT has been effective in the treatment of patients with certain pain conditions, including cluster headach, headache associated with pituitary tumors, and postoperative pain. Spinal administration STT or octreotide reduced pain in patients with terminal cancer (Pan et al. 2007).
The CeA plays an important part in the modulation of the descending anti-nociceptive pathways. Whole-cell patch clamp recordings from brain slices showed that CeA neurons responded to the endogenous ligands SST via an increased K+-conductance. Co-application of selective antagonists suggested that SST acts on SSTR2 receptors. Many responsive neurons were apparently located within the medial sub-division of CeA, and all CeA projection neurons to the midbrain PAG invariably responded to STT. The similarity of SST and opioid responsiveness in a sub-population of CeA neurons suggests converging roles of these peptides in inhibiting the activity of projections from CeA to vlPAG (Chieng and Christie 2021).
In the rat, STT is expressed in about 10–17% GABA neurons in HIPP, DG and AMY, and in 30% of GABA neurons in the cerebral cortex (Pan et al. 2007; Rosen and Schulkin 2022).
The CeA contains two major sub-populations of GABAergic neurons that express STT or protein kinase Cδ (PKCδ). In the formalin-induced pain model in mice, optogenetic activation of PKCδ neurons sufficed to induce mechanical hyperalgesia without changing anxiety-like behavior in naïve mice. Conversely, chemogenetic inhibition of PKCδ neurons significantly reduced the mechanical hyperalgesia in the pain model. By contrast, optogenetic inhibition of STT neurons induced mechanical hyperalgesia in naïve mice. Optogenetic activation of STT neurons slightly reduced the mechanical hyperalgesia in the pain model but did not change the mechanical sensitivity in naïve mice. Instead, it induced anxiety-like behavior. This suggests that the PKCδ+ and STT CeA neurons exert different functions in regulating pain-like and anxiety-like behaviors in mice (Chen et al. 2022).
STT is a highly expressed in the PFC. PFC STT neurons release STT under basal or tonic conditions as well as following activation. Changes in the number or activity of STT cells in the PFC may not only result in altered GABA signaling but also altered STT tone (Brockway and Crowley 2020).
In mice with CCI, the use of in vivo two-photon imaging revealed that electro-acupuncture systemically modulated the Ca2+ activity of neural circuits in S1, including the suppression of excitatory pyramidal neurons, potentiation of GABAergic somatostatin-positive interneurons, and suppression of VIP-positive interneurons. Furthermore, electro-acupuncture-mediated alleviation of pain hypersensitivity and cortical modulation depended on the activation of CB1R (Wei et al. 2021).
3.6. Neuropeptide Y (NPY)
NPY is a 36-amino acid, highly conserved endogenous peptide.
NPY has been implicated in the regulation of feeding, food intake, energy homeostasis, cardio-vascular function, blood pressure, reproductive behavior, circadian rhythm, cell proliferation, angiogenesis, emotional regulation, learning and memory (Bowers et al. 2012; Brockway and Crowley 2020; Holsboer and Ising 2021; Kautz et al. 2017; Kormos and Gaszner 2013; Kumamoto 2019; Sabban et al. 2016; Rana et al. 2022). NPY has also been implicated in both pro- and anti-nociceptive effects, depending on the brain region (Holsboer and Ising 2021). In fact, it is abundantly expressed in the spinal DH, forebrain limbic regions and brainstem nuclei processing pain, stress and emotional stimuli, and is important because of its anti-nociceptive effects exerted by inhibiting nociceptive transmission via activation of NPY Y1 receptors in the DH (Nelson et al. 2024). NPY exerts its actions primarily through receptor sub-types Y1, Y2, Y4 and Y5, which have dense and overlapping gene expression in brain regions related to anxiety and depression, including the PFC, HIPP, BNST, AMY, HYP, and LC (Kautz et al. 2017; Nelson and Taylor 2021; Sabban et al. 2016; Schmeltzer et al. 2016).
NPY is also expressed in the superficial laminae of the spinal DH, where it appears to mediate its anti-nociceptive actions via the Y1 and Y2 receptors. Y1 is located in important nodes of pain transmission, including the peptidergic sub-population of primary afferent neurons and a sub-population of small, excitatory, glutamatergic and STT interneurons that are densely expressed in the DH, particularly in superficial lamina I-II. Spinal NPY release and the consequent inhibition of pain facilitatory Y1 interneurons represent an important mechanism of endogenous analgesia. Pharmacological activation of Y1 also inhibits mechanical and HIST-induced itch. Whole cell recordings in the rat spinal-cord slice indicated that bath application of NPY inhibited both presynaptic and postsynaptic components of excitatory neurotransmission in the DH. Voltage-clamp recordings revealed that Y2- but not Y1-selective agonists inhibit the frequency but not amplitude of TTX-resistant miniature excitatory postsynaptic currents (mEPSCs). Likewise, Y2- but not Y1-selective antagonists blocked the ability of NPY itself to inhibit mEPSC frequency (Nelson and Taylor 2021).
Applying NPY or a Y1 receptor agonist directly into the PAG dose-dependently increased hindpaw withdrawal responsivenss to mechanical and thermal stimuli, analgesia in the tail-flick test. NPY application into the PAG dose-dependently reduced mechanical and thermal hyper-sensitivity produced by inflammation and nerve injury (Nelson et al. 2024).
ON- and OFF-cells show abundant Y1 expression. In fact, NPY enhanced the activity of ON- and OFF-cells. In non-injured rodents, the application of NPY directly into the RVM dose-dependently decreased the hindpaw sensitivity to thermal and mechanical stimuli, prevented by co-administration of a Y1 receptor antagonist (Nelson et al. 2024)
Since the PBN is part of the spino-parabrachio-amygdaloid pathway, it might be amenable to anti-nociceptive influences. Indeed, during hunger states, a sub-set of HYP ARC neurons release NPY in the PBN and dampen behavioral symptoms of inflammatory pain (Hökfelt et al. 2018; Nelson et al. 2024).
NPY and its receptors are abundantly expressed in the HYP ARC, which contributes to pain modulation, endogenous opiate release and stress-induced analgesia. Direct NPY infusion into the HYP ARC induced dose-dependent decreases in hindpaw sensitivity to thermal and mechanical stimuli (Nelson et al. 2024).
In the BLA, opposite effects of NPY and CRH similar to those in BNST occurred in stress-related behaviors (Nelson et al. 2024).
Direct NPY infusion into the NAc induced a dose-dependent increase in mechanical and thermal withdrawal thresholds, reversed by co-administration of a Y1 antagonist. Direct infusion of NPY Y1, but not Y2, receptor agonists into the NAc dose-dependently reduced inflammation-induced mechanical and thermal hyper-sensitivity (Nelson et al. 2024).
The BNST has a role in the negative emotional aspects of pain. BNST NPY and CRH have opposing effects on pain-induced aversion. Whereas intra-BNST infusion of CRH induced aversion, NPY infusion suppressed CRH- and formalin-induced pain aversion (Nelson et al. 2024).
3.7. Neuropeptide S (NPS)
NPS is associated with inhibitory ITC neurons. NPS modulates several central functions including arousal, wakefulness, food intake, alcohol and drug addiction, social behavior, locomotor activity, memory processes, fear and anxiety. NPS and its receptor NPSR are mainly expressed in the brain. In mice, ICV administration of NPS evoked anti-nociceptive effects in the tail-flick, hot-plate and both phases of the formalin tests. Systemic application of antagonists for DA D1 (SCH 23390) or D2 (sulpiride) and ICV injection of antagonists for adenosine A1 (DPCPX) or A2A (ZM241385) receptors blocked the effects of NPS. In rats subjected to the kaolin/carrageenan-induced knee-joint arthritis pain, but not under normal conditions, administration of NPS into the ITC, but not CeA, decreased emotional responses (vocalizations to noxious stimuli) and anxiety-like behavior (elevated plus maze). In neuropathic rats (SNL model), aministration of NPS into the BLA attenuated mechanical and thermal hyper-sensitivity. In a rat arthritis pain model (kaolin/carrageenan-induced knee-joint arthritis), electrophysiological recordings showed that NPS administered nasally or stereotaxically into the ITC area inhibited the activity of CeA neurons. In brain slices from arthritic rats, NPS increased the feedforward inhibition of CeA neurons but this effect involved a direct action on ITC cells based on the analysis of mEPSC (Neugebauer et al. 2020).
3.8. Cholecystokinin (CCK)
CCK is a gastrin-like peptide, synthesized as a 115 amino acid pre-pro-hormone and converted into multiple isoforms, and is.released in the gastro-intestinal tract and mammalian brain. It is involved in numerous physiological functions, including nociception, pain modulation, feeding, satiety, gallbladder contraction, temperature regulation, sexual functions, learning, memory, anxiety and panic disorders, and depressive disorders. External CCK application increases ventilation, blood pressure and heart rate. There are two CCK receptors: CCK1R and CCK2R (Bowers et al. 2012; Hebb et al. 2005; LaVigne and Alles 2022; Rana et al. 2022; Roberts 1986). In the rat, CCK is found in high concentrations throughout the CNS, including cerebral cortex, ACC, NAc, AMY, THAL, HYP, SN, PAG, VTA, RVM, and the spinal cord. Upon injury, CCK or CCK2R concentrations increase in the DRG and spinal cord. CCK1Rs increase DA release and decrease opioid analgesia. CCK2Rs have an effect opposite to that of CCK1Rs on DA release and a similar negative effect on opioid-induced analgesia. There is a significant overlap of CCK2R presence in pathways modulating both sensory and affective components of pain processing (LaVigne and Alles 2022).
CCK and Opioids are localized in similar neural pathways in the CNS, from the spinal cord, where they are involved in pain responses, up to the limbic system (e.g., the PFC and HIPP), where they are involved in anxiety and major depression disorder (MDD) as well as learning and memory. CCK and opiods act antagonistically in influencing pain and emotion, cognition and motor actions (Hebb et al. 2005).
3.9. Calcitonin Gene-Related Peptide (CGRP)
CGRP is a 37-amino-acid peptide and exists in two forms, αCGRP and ßCGRP; in some species, βCGRP is not found. The peptides have a range of biological activities. Evidence suggests that CGRP exists in non-nerve cells, such as epithelial cells, endothelial cells, endothelial progenitor cells, T and B-lymphocytes, peripheral blood mononuclear cells, and adipocytes (Hu et al. 2016). Epigenetic regulation of the CGRP gene has been linked to anxiety- and depression like behaviors. The CGRP receptor is a G-protein coupled receptor complex (Hay et al. 2018; Neugebauer et al. 2020; Schou et al. 2017; Yu et al. 2009). CGRP and its receptors are widely distributed in nociceptive pathways in human PNS and CNS. CGRP and CGRP receptors are involved in the transmission and modulation of pain information (Hay et al. 2018; Neugebauer et al. 2020; Schou et al. 2017; Yu et al. 2009).
CGRP plays an important role in neurogenic inflammation, in which sensory nerves peripherally release mediators that promote inflammation. In this case, CGRP causes vasodilatation and promotes fluid exudation from blood vessels. CGRP might have a pro-inflammatory role in PNS by leading to the release of pro-nociceptive substances and by facilitating central nociceptive transmission and contributing to central sensitization. However, the exact mechanisms and involvement of CGRP in nociceptive processing have not been fully elucidated. There is an association between measured CGRP levels and somatic, visceral, neuropathic and inflammatory pain. Increased CGRP levels were reported in plasma, synovial and CSF in subjects with musculo-skeletal pain (Schou et al.2017).
CGRP is an important peptide in the afferent nociceptive pathway from the PBN and mediates excitatory drive of CeA neurons (Neugebauer et al. 2020). In anesthetized rats subjected to arthritis pain (kaolin/carrageenan-induced knee-joint arthritis), pharmacological blockade of CGRP receptors with selective antagonists in the AMY inhibited neuronal activity that was increased six hours after induction of the knee-joint arthritis, while the antagonists had little effects under normal conditions. In brain slices from arthritic rats, CGRP receptor antagonists inhibited synaptic plasticity at the parabrachio-amygdaloid synapse. CGRP receptor blockade also decreased NMDAR-mediated currents and neuronal excitability, without effects in brain slices from normal animals. Importantly, in CGRP knockout mice, potentiation at the parabrachio-amygdaloid synapse in the formalin pain model (six hours post-induction) was significantly attenuate (Neugebauer et al. 2020). In various pain models, hindering the actions of CGRP in the AMY by pharmacological blockade of CGRP1 receptors exerted anti-nociceptive effects. Conversely, intra-amygdaloid injection of CGRP led to pain-related behaviors, such as vocalizations and paw withdrawal in the absence of exogenous noxious stimuli and potentiated excitatory synaptic transmission at PBN-amygdaloid synapses. CGRP enhances NMDAR-mediated excitatory potentials (Kuner and Kuner 2021).
A 32-year-old woman with episodic migraines since age 22 (generally managed with NSAIDs) presented to the Emergency Department with a sudden onset of severe, pulsatile headache over the right frontotemporal region, associated with nausea, photophobia, and phonophobia, lasting for more than 6 hours. The pain intensity was reported as 8/10 on the VAS. Neurological examination revealed no focal deficits and no signs of meningeal irritation. CT Haed was normal without any signs of hemorrhage or infarct. Serum CGRP level was elevated. This case demonstrates an acute migraine attack with likely involvement of CGRP in the pathogenesis. The patient was treated with ubrogepant (a CGRP receptor antagonist), metoclopramide and magnesium. Pain resolution occurred within 45 minutes, and the patient was observed for 4 hours with full recovery. Recognition of CGRP as a key neuromodulator in acute migraine provides both a mechanistic understanding and a targeted treatment approach.
3.10. Galanin (GAL)
GAL has 29 amino acids in animals and 30 in humans. GAL has a number of functions, including nociception, mood regulation, feeding behavior, cardio-vascular and sleep regulation, learning and memory, and some neuro-protective effects in the PNS and on the promotion of neurogenesis (Osório et al. 2017; Kumamoto 2019). GAL binds with high affinity to several receptor sub-types designated as GAL1R, GAL2R and GAL3R, which have different characteristics, distributions and region-specific effects. Central GAL1Rs are expressed mainly in the cerebral cortex, HIPP, AMY, THAL, HYP and medulla oblongata. Central GAL2Rs occur in the piriform cortex, DG, AMY, and HYP nuclei including the mamillary bodies. GAL3Rs occur predominantly in the HYP, but also in cortex, HIPP and AMY (Hökfelt et al. 2018; Kormos and Gaszner 2013; Millón et al. 2017; Rana et al. 2022).
One of the GAL functions most often suggested is pain modulation in the spinal cord, based on its preferential distribution in the dorsal spinal cord. GAL is expressed in a small percentage of sensory neurons of the DRG and the superficial laminae of the spinal DH. It is generally agreed that spinally applied GAL produces a biphasic, dose-dependent effect on spinal nociception through activation of GAL1R (inhibitory) or GAL2R (excitatory) receptors. GAL also appears to have an endogenous inhibitory role, particularly after peripheral nerve injury when the synthesis of GAL is increased in sensory neurons (Xu et al. 2010). GAL shows a differential role in pain, depending on the pain state, site of action, and concentration. Normally, GAL can modulate nociceptive processing through both a pro- and anti-nociceptive action, in a dose-dependent manner (Fonseca-Rodrigues et al. 2022).
In normal rats and rats with neuropathy, hindpaw withdrawal latencies (HWLs) to thermal and mechanical stimulations were increased in a dose-dependent manner after intra-CeA GAL injection. The increased HWLs were significantly attenuated by intra-CeA injection of GAL receptor antagonist M40. In normal rats, intra-CeA administration of the GalR 1 agonist M 617 induced increases in HWLs. This indicates that GAL induced anti-nociception in CeA in normal rats and rats with neuropathy, and there is an up-regulation of GAL 1 receptor (GaALR1) expression in rats with neuropathy (Li et al. 2017b).
Intra-ACC GAL injection increased HWLs in response to thermal and mechanical stimulations in both normal rats and rats with mononeuropathy. The increased HWLs were attenuated by intra-ACC injection of GAL2R antagonist M871, indicating an involvement of GAL2R in nociceptive modulation in ACC. The GAL-induced HWL was higher in rats with mononeuropathy than that in normal rats. This indicated that GAL induced anti-nociception in ACC in both normal rats and rats with mononeuropathy (Zhang et al. 2017).
3.11. Neurotensin (NT)
NT is an endogenous neuropeptide consisting of 13 amino acids with profound opioid-independent analgesic effects. This role is considered to be mediated by both NT sub-type 1 (NTS1) and NT receptor sub-type 2 (NTS2). NT and its receptors are widely distributed in the pain circuits in CNS. NT might therefore modulate pain in many structures of pain pathway, such as spinal cord, RVM and PAG. In fact, intra-thecal NT application or direct NT injection into PAG or RVM or ICV NT injection showed analgesic effects. NT exerted its anti-nociceptive effects in both acute pain and chronic pain models (Feng et al. 2015). NT can produce a profound analgesia or enhance pain responses, depending on the circumstances. This may be due to a dose-dependent recruitment of distinct populations of pain modulatory neurons. NT knockout mice display deficits in both basal nociceptive responses and SIA (Dobner 2006).
In animal models of acute and persistent pain, central NT administration results in a naloxone-insensitive anti-nociceptive response. Both NTS1 and NTS2 receptors were required for different aspects of NT-induced analgesia. NTS2-selective agonists and NTS1/NTS2 mixed compounds differently modulated the early (21-39 min) and late (40-60 min) tonic phase 2 and recruited endogenous pain inhibitory mechanisms integrated at different CNS levels (Roussy et al. 2009).
NTS2 receptors were associated with ascending nociceptive pathways, both at the level of the DRG and of the spinal DH. Spinally administered NTS2-selective agonists induced dose-dependent anti-nociceptive responses in the acute tail-flick test. Furthermore, spinally applied NT and NT69L agonists, which bind to both NTS1 and NTS2 receptors, significantly reduced pain-evoked responses during the inflammatory phase of the formalin test. Pre-treatment with the NTS2-selective analogs JMV-431 and levocabastine was effective in inhibiting the aversive behaviors induced by formalin. Activation of spinal NTS2 receptors reduced formalin-induced c-fos expression in DH neurons. While non-selective drugs suppressed pain-related behaviors activity in both part of phase 2, intra-thecal injection of NTS2-selective agonists was only efficient in reducing pain during the late phase 2 (Roussy et al. 2009).
3.12. Endocannabinoids (eCBs)
Extracts of the Cannabis sativa plant have been used as analgesics for centuries. Most commonly, cannabis is claimed to relieve chronic pain, stimulate appetite, and acts as anti-emetic, with the underlying mechanisms being little known. Among more than 450 constituents in cannabis, the most abundant cannabinoids are Δ9-tetrahydrocannabinol (THC) and cannabidiol (CBD) (Louis-Gray et al. 2022). eCBs are classified by their source: herbal, endogenous (eBCs, produced by animal cells) or synthetic. eCBs regulate multiple physiological and pathological conditions, e.g., regulation of food intake, immuno-modulation, inflammation, analgesia, cancer, addictive behavior, epilepsy and others (Guindon and Hohmann 2009). Within the nervous system, the components of the ECS comprise the G-protein-coupled cannabinoid receptors CB1R and CB2R, their endogenous ligands anandamide (AEA) and 2-arachidonyl glycerol (2-AG; derived from arachidonic acid)), and their respective major synthetic N-acylphosphatidylethanolamine phospholipase D (NAPE-PLD) and diacyglyerol lipase α (DAGLα), as well as degradative fatty acid amide hydrolase (FAAH) and monoacyglycerol lipase (MAGL) enzymes. The best characterized eBCs to date are AEA and 2-AG eCBs. AEA and 2-AG also exert cannabino-mimetic effects through the CB1Rs and CB2Rs, which are located on presynaptic membranes in the CNS and in peripheral tissues, respectively. The eCBs are synthesized on demand and are eliminated rapidly after their usage by hydrolyzing enzymes. eCBs also bind to certain subsets of TRP channels (Bouchet and Ingram 2020; Finn et al. 2021; Hillard 2014, 2015; Hillard et al. 2016; Huang et al. 2016; Kumamoto 2019; Micale and Drago 2018; Morena et al. 2016; Pan et al. 2007; Tasker et al. 2015; Woodhams et al. 2017).—The above components are expressed almost ubiquitously throughout nociceptive pathways. Thus targeting the ECS via exogenous cannabinoid ligands or enhancement of endogenous signaling can regulate nociceptive signaling at multiple sites, in the periphery, the spinal DH and in supraspinal pain-associated brain regions. Within the rat brain, CB1Rs are present on many neuronal sub-types. Very high densities of CB1Rs are found in the PFC, ACC, HIPP, BG, SN and cerebellum. Moderate to low densities occur in the BFB, primary motor cortex (M1), BNST, AMY, NAc, THAL, HYP (PVN) and brainstem regions such as the PAG and LC. CB1Rs are also present in the spinal DH, specifically on interneurons and axon terminals of descending inputs and peripheral afferents. CB1Rs are epressed on GABAergic, glutamatergic, 5-HT, NA, and DA terminals, but the predominant effects of eCB signaling occur at GABAergic and glutamatergic synapses. Sympathetic nerves express CB1R (Bouchet and Ingram 2020; Finn et al. 2021; Hillard 2014, 2015; Hillard et al. 2016; Huang et al. 2016; Micale and Drago 2018; Morena et al. 2016; Tasker et al. 2015; Woodhams et al. 2017).
Cannabinoids suppress behavioral responses to noxious stimulation and nociceptive processing through activation of cannabinoid CB1R and CB2R sub-types (Guindon and Hohmann 2009). It has been argued that eCB receptors are involved in the supraspinal modulation of pain. Intra-cerebral micro-injections of cannabinoid ligands or positive modulators have proved to be analgesic in different pain models, whereas eCB receptor antagonists or antisense nucleotides towards CB1Rs have facilitated pain. Cannabinoids produce centrally mediated analgesia by activating a descending pathway, which includes the PAG and its projection to downstream RVM neurons, which in turn send inhibitory projections to the spinal DH. A supraspinal regulation of cannabinoids exerts effects on GABA and glutamate release, which inhibit and enhance the anti-nociceptive descending pathway, respectively. Cannabinoid receptor activation expressed on presynaptic GABAergic terminals reduces the probability of neurotransmitter release, thus dis-inhibiting the PAG-RVM-DH anti-nociceptive pathway. Cannabinoids seem to increase glutamate release (maybe as consequence of GABA decrease) and to require glutamate receptor activation to induce anti-nociception. Consequently, the outcome is behavioral analgesia, which is reproduced in several pain conditions, from acute to chronic pain models such as inflammatory and neuropathic pain (Palazzo et al. 2010).
The PAG synthesizes eCBs, which in turn are released into the RVM. The PAG-to-RVM projections mediate eCB-induced analgesia and contribute to SIA. Micro-injection of eCB receptor agonists into the RVM decreased the firing of ON-cells while increasing ongoing OFF-cell activities, thus increasing the rat TFL. CB1R is expressed in approximately one-third of PAG neurons and is co-expressed with μ-opioid receptor. Activation of PAG CB1R decreased GABA release and activated mGlu5R, leading to the inhibition of ON-cells and disinhibition of OFF-cells, ultimately resulting in SIA and analgesia in both normal and neuropathic pain situations. CB2R agonists inhibited presynaptic GABA release and ON-cell activities in the RVM in CFA-treated but not in naïve rats. eCBs activated opioid-insensitive SIA predominantly through the CB1R rather than the CB2R, and inhibiting eCB hydrolysis in RVM can enhance SIA (Peng et al. 2023).
eCBs either directly or indirectly modulate ion-channel function. TRPV1 is an ion channel responsible for mediating several modalities of pain. It is expressed in the peripheral and the central pain pathways. Activation of TRPV1 in sensory neurons mediates nociception in the ascending pain pathway, while activation of TRPV1 in the central descending pain pathway, which involves the PAG and RVM, mediates anti-nociception. Activation of TRPV1 can also cause the release of CGRP and other neuropeptides and neurotransmitters from the peripheral and central nerve terminals, including the vagal nerve innervating the gut and forming central synapses in the NTS (Louis-Gray et al. 2022). Although eCBs are not as efficacious as opioids in reducing acute pain when administered directly into the PAG or RVM, they appear to have increased efficacy in chronic pain states (Bouchet and Ingram 2020).
A 29-year-old woman developed severe acute nociceptive pain following a distal radius fracture after a snowboarding accident (fall on outstretched hand during snowboarding, resulting in a closed, displaced distal radius fracture). Despite standard analgesic regimens (NSAIDs and low-dose opioids), her pain remained uncontrolled until administration of a cannabinoid receptor agonist (a THC:CBD 1:1 oromucosal spray, 2.7 mg THC/2.5 mg CBD). Within 30 minutes, the patient reported significant pain relief. Motor restlessness decreased, and heart rate normalized. The patient was discharged with a multimodal pain plan, relying on NSAIDs and controlled cannabinoid use. The CB1R-mediated inhibition of GABA release in PAG likely disinhibited the descending inhibitory pathway from the PAG to the RVM and DH. This case illustrates the clinical relevance of the endocannabinoid system in acute pain modulation and its interaction with GABAergic and glutamatergic pathways in the descending pain circuitry.
3.13. Endogenous Opioids
There are four major families of endogenous opioid ligands: β-endorphins, ENKs, Dyns, and nociceptin/orphanin. Endogenous opioids are expressed throughout the PNS and CNS, especially along the pain pathways, including DRG and DH neurons (Cesselin 1995; Corder et al. 2018). They regulate many different neuronal circuits and functions, including pain relief (analgesia), modulation of respiration, cardio-vascular, gastro-intestinal, endocrine, autonomic and immune functions, as well as adaptation to environmental and psychic stressors, stress resilience, euphoria induction, perception of reward, learning and memory, and drug abuse. The endogenous opioid receptors include four seven-transmembrane G protein-coupled receptors: μ, κ, δ and ε (Corder et al. 2018). Opioid receptors differ in terms of their distribution and affinity to ligands (Bagley and Ingram 2020; Bowers et al. 2012; Ferdousi and Finn 2018; Hebb et al. 2005; Kumamoto 2019; Le Merrier et al. 2009; Neugebauer et al. 2020; Pan et al. 2007; Shenoy and Lui 2021).
Opioid receptors are found throughout the brain and spinal cord in networks relevant to the modulation of pain. Opioid neuropeptides L-ENK, M-ENK and Dyn can be synthetized and released locally by neurons within the AMY nuclei. These endogenous peptides can also be released from terminals of neurons located in brain regions that innervate the AMY. Three opioid receptors termed μ, δ, and κ and their peptide ligands (β-endorphin, ENK, Dyn) have complex and partially opposing effects on AMY function. ENKs likely activate the μ-opioid and δ-opioid receptors in the AMY promoting physiological effects. In the BLA, opioid analgesics would be expected to inhibit neuronal activity because of hyper-activity of BLA neurons in pain conditions. Brain-slice electrophysiology studies showed that μ-opioid receptor activation hyperpolarized non-pyramidal neurons in the LA. A κ-opioid receptor agonist increased inhibitory synaptic transmission in BLA pyramidal cells from adolescent rats (postnatal day 30–45), but had no effect in adult rats (postnatal day > 60). Conversely, the κ-opioid receptor agonist increased glutamatergic transmission at the adult but not adolescent age. The observed diversity of physiological responses to opioids in distinct populations of BLA neurons may be necessary for encoding of a wide range of behavioral outcomes. ITC cells modulated the flow of information within AMY micro-circuits, could regulate CeA output neurons, and were driven by direct or indirect (prefrontal) cortical influences (Neugebauer et al. 2020).
Although opioid analgesia attenuates the sensory aspects of pain, a major component of the analgesic response involves a blunting of the negative affective component of pain. In animals and humans, many stressors, including those that are non-noxious, produce an analgesia that is cross-tolerant with morphine and is antagonized by naloxone. The diminution of pain could be considered a broader function to counter stress. The ENKs, which signal through the μ-opioid peptide receptor and δ-opioid peptide receptor, have roles in analgesia and SIA, emotional behaviors, anxiety and depression (Valentino and van Bockstaele 2015). An important area for opioid action is the vlPAG. Opioid receptors are expressed on a subset of vlPAG neurons, as well as on both GABAergic and glutamatergic presynaptic terminals that impinge on vlPAG neurons. Micro-injection of opioids into the vlPAG produces analgesia and micro-injection of the opioid receptor antagonist naloxone blocks stimulation-mediated analgesia. Endogenous opioid effects within the vlPAG are complex and likely depend on specific neuronal circuits activated by acute and chronic pain stimuli (McPherson and Ingram 2022). vlPAG projects to the RVM, which projects on to the spinal cord to modulate processing of incoming nociceptive afferents. Stimulation within either the PAG or RVM results in analgesia (Bagley and Ingram 2020).—Some opioid targets may be components of homeostatic systems tending to reduce the effects of opioids. ‘Anti-opioid’ properties have been attributed to various peptides, especially CCK, neuropeptide FF (NPFF) and melanocyte-inhibiting factor (MIF)-related peptides. Paradoxically, some opioid peptides themselves exert anti-opioid effects. These peptidescan oppose some of the acute effects of opioids, and a hyperactivation of anti-opioid peptidergic neurons due to the chronic administration of opioids may be involved in the development of opioid tolerance and/or dependence. In fact, CCK, NPFF and the MIF family of peptides can act as opioid-like as well as anti-opioid peptides. Opioid modulating peptides act through the activation of their own receptors. For example, CCK appears to exert its anti-opioid actions mainly through the activation of CCK-B receptors, whereas its opioid-like effects appear to result from the stimulation of CCK-A receptors (Cesselin 1995).
The NTS contains POMC neurons, one of the two major sources of β-endorphin in the brain. In behaving mice, optogenetic and chemogenetic activation of NTS POMC neurons produced sustained thermal analgesia that could be blocked by naloxone. It also produced analgesia in an inflammatory pain model (carrageenan) but not in a neuropathic pain model (tibial nerve transection). Inhibiting NTS POMC neurons did not produce any effect on basal nociception but inhibited (SIA), unlike inhibition of HYP ARC POMC neurons. This indicates that NTS POMC neurons play a role in the generation of endogenous endorphinergic analgesia and can regulate cardio-respiratory function (Patra et al. 2023).
All four opioid receptors inhibit in N-, P/Q-, and L-type voltage-gated Ca2+ channels, via the Gβγ sub-unit inhibition of the channel. This decreases the presynaptic Ca2+-dependent neurotransmitter release. In DRG neurons, N-type Ca2+ channels along with opioid receptors can be co-internalized following prolonged agonist exposure, which may further reduce neurotransmitter release and the transmission of pain signals to the CNS. Postsynaptically, opioids also cause a Gβγ-mediated activation of G protein-gated inwardly rectifying potassium (GIRK) channels. Mutant mice lacking GIRK channels, or expressing dysfunctional channels, show reduced opioid anti-nociception. Although the acute action of opioids on calcium and potassium channels typically reduces neurotransmission within seconds to minutes, chronic (hours to days) or abruptly interrupted opioid signaling can also facilitate excitatory synaptic plasticity. For example, withdrawal of exogenous opioids can elicit LTP of synaptic transmission between primary afferent DRG nociceptors and second-order spinal cord neurons. This form of spinal LTP is considered a major substrate for opioid-induced hyperalgesia (OIH), a paradoxical decrease in pain threshold following opioid administration, and might contribute to analgesic tolerance. The mechanisms underlying OIH and analgesic tolerance have not been fully resolved, but they require presynaptic μ-opioid receptors in nociceptors and involve the activation of microglia and molecules (Corder et al. 2018).
3.14. Neurotrophins
Neurotrophins are involved in pain modulation (Pezet and McMahon 2006).
3.14.1. Nerve Growth Factor (NGF)
In distinction to BDNF, NGF has a mostly peripheral distribution and action.
3.14.2. Brain-Derived Neurotrophic Factor (BDNF)
Under basal conditions, BDNF is synthesized by various types of neurons and glia within pain pathways. Noxious stimuli can trigger the production and release of BDNF by these cells and/or up-regulate BDNF synthesis and release (Merighi 2024). BDNF is synthetized in both the PNS and CNS by neurons under physiological conditions and by astrocytes following injury, inflammation, or administration of anti-depressants. In the CNS, BDNF concentration is highest in the cortex, HIPP, AMY, BFB, dorsal vagal complex, and midbrain (Colucci-D’Amato et al. 2020; Phillips 2017). In the brain, neurons are considered a significant cellular source of BDNF (Philipps 2017). In contrast to other neurotrophic factors, BDNF can be anterogradely transported to its targets, which explains its messenger role in the modulation of synaptic activity. Thus, neurons in the cerebral cortex, PBN, HIPP, and LC synthesize and transport BDNF anterogradely. As to peripheral neurons, peptidergic small- to medium-sized dark neurons in DRGs produce and transport BDNF to their central terminals in the spinal DH. BDNF plays important roles in neuronal differentiation, development, survival, neuroprotection, neurodegeneration, synaptic plasticity, and the control of mood disorders. Dysregulation of BDNF has been implicated in a range of neurological disorders, including Alzheimer’s disease, PD, Huntington’s disease, and amyotrophic lateral sclerosis, and low BDNF concentrations are associated with anxiety and depression (Merighi 2024). Mature neurotrophin binding to the high-affinity receptor, TrkB receptor, increases cell survival and differentiation, dendritic spine complexity, LTP, and the re-sculptering of networks. Deployment of TrkB receptors significantly increases at synaptic sites following neuronal activity (Phillips 2017; Pitsillou et al. 2020). BDNF is important for the proper growth, development and plasticity of glutamatergic and GABAergic synapses. Through modulation of neuronal differentiation, it influences 5-HT and DA neurotransmission, acting as a paracrine and autocrine factor on both presynaptic and postsynaptic target sites. It is crucial in the transformation of synaptic activity into long-term synaptic memories, influencing dendritic spines and, at least in the HIPP, adult neurogenesis (Colucci-D’Amato et al. 2020; Phillips 2017).
BDNF is thought to be a neurotransmitter that intervenes in the modulation of pain. BDNF has a widespread distribution and functions in pain pathways (Merighi 2024). The precise role of BDNF in pain transmission is still somewhat controversial, though, because evidence has been presented of pro-nociceptive as well as anti-nociceptive and anti-inflammatory activities (Cappoli et al. 2020).
The CeA contains a high concentration of BDNF in terminals, originating from the pontine PBN. Since the spino-parabrachio-amygdaloid neural pathway conveys nociceptive information, a possible involvement of BDNF in supraspinal pain-related processes might occur. In adult floxed-BDNF mice, localized deletion of BDNF in the PBN was achieved using local bilateral injections of adeno-associated viruses. Basal thresholds of thermal and mechanical nociceptive responses were not altered by BDNF loss and no behavioral deficit occurred in anxiety and motor tests. However, BDNF-deleted animals displayed a major decrease in the analgesic effect of morphine. In control mice, intra-CeA injections of the BDNF scavenger TrkB-Fc also decreased morphine-induced analgesia. Finally, the number of c-fos immuno-reactive nuclei after acute morphine injection was decreased by 45% in the extended AMY of BDNF-deleted animals. The absence of BDNF in the PBN thus altered the parabrachio-amygdaloid pathway. Hence, BDNF produced in the PBN modulates the functions of the parabrachio-amygdaloid pathway in opiate analgesia (Sarhan et al. 2013).
Through modulation of neuronal differentiation, it influences 5-HT and DA neurotransmission, acting as a paracrine and autocrine factor on both presynaptic and postsynaptic target sites. It is crucial in the transformation of synaptic activity into long-term synaptic memories, influencing dendritic spines and, at least in the HIPP, adult neurogenesis (Colucci-D’Amato et al. 2020; Phillips 2017).
In rats, the effects of short- and long-term administration of melatonin on central BDNF concentrations were evaluated in acute and chronic inflammatory pain. In experiment 1, all rats were injected with CFA to induce inflammation and were randomly allocated to receiving melatonin or vehicle. Injections were administered one hour after CFA and once daily for two more days (for a total of three days of melatonin administration). In experiment 2, fifteen days after CFA injection, the rats were treated with melatonin or vehicle for eight days. BDNF expression was studied in the PFC, spinal cord, and brainstem. In the first experiment, the BDNF concentrations of the melatonin group were reduced in the PFC and increased in the spinal cord. In experiment 2, BDNF concentrations were similar in both groups for all structures. The PFC presented higher BDNF concentrations than other structures. Hence, the high spinal cord BDNF concentrations and the low PFC BDNF concentrations in rats with acute CFA-induced inflammation following short-term melatonin administration may be related to the pain-modulating and neuro-protective effects of this protein (Laste et al. 2015).
3.15. Neurotransmitters
3.15.1. Acetylcholine (ACh)
ACh plays roles in the CNS, PNS, ANS and at the neuromuscular junction. The widely distributed expression of AChRs in different human organs suggests actions in other biological processes in addition to synaptic transmission (Chen et al. 2019).
In the CNS, ACh acts as a neurotransmitter and neuromodulator upon release from groups of ACh projection and interneurons in both brain and spinal cord. Two primary types of receptors respond to ACh. Neuronal nAChRs are ligand-gated cation channels, which are widely expressed in the CNS (Naser and Kuner 2018). The muscarinic mAChRs are widely expressed throughout the CNS and PNS. In the rat DRG, there is a high level of expression of M2 mRNA, and much lower levels of M3 and M4 mRNA was also detected. All three of these sub-types are preferentially localized in medium- and small-sized DRG neurons. These findings suggest the possible involvement of the M2, M3, and M4 sub-types in the modulation of nociceptive transduction (Pan et al. 2007).
There are four rostro-caudally distinct, yet partially overlapping, populations of ACh projection neurons: Ch1-Ch4. The Ch1 and Ch2 groups project to the HIPP formation, whereas Ch3 innervates the olfactory cortex. The Ch4 population largely corresponds to the ‘nucleus basalis of Meynert’ (NBM) and projects into higher cortical areas. The BFB neurons receive a wide variety of inputs from almost all brain areas. Thus, input from diverse pain-related areas as the IC, the CeA, and midbrain areas such as the PAG or RVM, could also mediate neuroplastic changes in BFB projections. In turn, cortical and sub-cortical brain regions receive ACh inputs from BFB projection neurons (Naser and Kuner 2018). Whereas local ACh neurons form dense networks in a few regions, such as the striatum, the remaining parts of the brain are widely modulated by ACh projections extending to almost all cortical regions, including those implicated in pain processing. A broad activation of the BFB ACh centers leads to a large overall increase in cortical excitation (Kuner and Kuner 2021). ACh inputs to the neocortex, dorsal HIPP and BLA are critical for neuronal function and synaptic plasticity in these brain regions. Synaptic plasticity in the neocortex, dorsal and vHIPP, and BLA has been implicated in fear and extinction memory (Knox 2016).
ACh profoundly modifies the perception of pain. In rodents and humans, directly activating ACh receptors or extending the action of endogenous ACh via pharmacological blockade of ACh-esterase reduces pain. Conversely, inhibition of mAChRs induces nociceptive hyper-sensitivity. ACh modulation influences some of the key regions in nociceptive processing and pain, such as the S1, mPFC, IC, ACC, and descending modulatory systems. In addition to pain modulation, ACh has other modulatory qualities. Peripherally, ACh neurons control the sympathetic and parasympathetic branches of the ANS on the ganglionic level. Additionally, parasympathetic terminals release ACh and thereby mediate the ‘rest and digest’ function in the ANS (Naser and Kuner 2018).—Systemic administration of ACh drugs strongly implicate ACh modulation in analgesia. There may be an effect unspecific for pain in that ACh stimulation broadly enhances sensory processing in nearly all sensory cortices. These would not only directly facilitate processing of noxious stimuli and promote saliency detection in S1, but also contribute indirectly by enhancing attention via modulation of PFC circuits. In the naïve rat ACC, pharmacological M1-mAChR stimulation exerts an analgesic effect by virtue of increasing the frequency and amplitude of GABAA-mediated IPSCs. Bi-directionally modulating the activity of medial-septal ACh neurons showed that their inhibition suppressed pain affect in a model of inflammatory pain, whereas chemogenetic activation of medial septal ACh neurons elicited anti-nociceptive effects. Pro-nociceptive modulation may arise from ACh neurons in latero-dorsal tegmental area, which project to the DA neurons of the VTA. These ACh inputs enhance the responsivity of VTA DA neurons to aversive stimuli. There are central ACh contributions to opioid-induced analgesia as well as to endogenous control of pain, particularly in the AMY circuits as well as the vlPAG and the RVM in the descending inhibitory pathways, supporting a role in anti-nociception (Kuner and Kuner 2021; Naser and Kuner 2018).
One week after nerve injury, ACh modulation of layer V pyramidal neurons is severely impaired, due to a 60% reduction of an m1 ACh receptor-coupled, pirenzepine-sensitive depolarizing current. Reciprocal interactions between ACh and opioidergic modulation likely impact the function and efficacy of both opioids and cholinomimetic drugs (Ong et al. 2019).
A multitude of neurotransmitter systems contributes to the fine-tuning of the local circuitry, of which ACh and GABAergic signaling are emerging as relevant components of affective pain processing within the PFC (Kummer et al. 2020). A portion of inputs from the BLA to mPFC terminate on GABA interneurons, allowing for feedforward inhibition of mPFC output through modulation of mPFC projection neurons. The ITC send GABA projections to CeA projection neurons, allowing for feedforward inhibitory control of AMY output by the mPFC (Thompson and Neugebauer 2019).
ACh also exerts actions in the spinal DH (Kumamoto 2019). In the rat DRG, there is a high level of expression of M2 mRNA, and much lower levels of M3 and M4 mRNA were detected. All three of these sub-types are preferentially localized in medium- and small-sized DRG neurons. These findings suggest the possible involvement of the M2, M3, and M4 sub-types in the modulation of nociceptive transduction (Pan et al. 2007).
3.15.2. Glutamate and Its Receptors
Glutamate is the most abundant excitatory neurotransmitter in the brain. Glutamate and glutamate receptors are located in areas of the brain, spinal cord and periphery, which are involved in pain sensation and transmission. Glutamate interacts with the opioid system, and intra-thecal or systemic co-administration of glutamate receptor antagonists with opioids may enhance analgesia while reducing the development of opioid tolerance and dependence. In the brain, glutamate actions appear to be complex. In some brain areas, activation of glutamate receptors seems to be pro-nociceptive (e.g., in THAL, trigeminal nucleus), while in other brain areas, it seems to be anti-nociceptive (e.g., PAG, ventro-lateral medulla). Application of glutamate, or agonists selective for one of the several types of glutamate receptor, to the spinal cord or periphery induces nociceptive behaviors. Inhibition of glutamate release, or of glutamate receptors, in the spinal cord or periphery attenuates both acute and chronic pain in animal models (Fundytus 2001).
Glutamate has a pivotal role in pain sensation, transmission and modulation. Once released into the synapse, glutamate acts through ionotropic glutamate receptors (iGluRs), which are ligand-gated ion channels triggering fast excitatory neurotransmission, and through mGluRs, which are G-protein-coupled receptors modulating synaptic transmission (Pereira and Goudet 2019).
Nociceptive primary afferents release glutamate, activating postsynaptic glutamate receptors on spinal DH neurons. Glutamate receptors, both ionotropic and metabotropic, are also expressed on presynaptic terminals, where they regulate neurotransmitter release (Bardoni 2013).
Ionotropic Glutamate Receptors
Ionotropic glutamate receptors are classified into three broad sub-types: AMPA, kainate, and NMDA (Pan et al. 2007).
NMDARs function as plasma membrane ionic channels and take part in very tightly controlled cellular processes activating neurogenic and inflammatory pathways. The NR1 sub-unit (new terminology: GluN1) is required for many neuronal and non-neuronal cell functions, including plasticity, survival, and differentiation. Physiologic levels of glutamate agonists and NMDAR activation are required for normal neuronal functions such as neuronal development, learning, and memory. When glutamate receptor agonists are present in excess, binding to NMDARs produces neuronal/CNS/PNS LTP, conditions of acute pain, ongoing severe intractable pain, and potential excitotoxicity and pathology. The GluN1 sub-unit composition and specifically nuclear GluN1 has major physiologic potential in tissue and/or sub-nuclear functioning assignments. The shift of the GluN1 sub-unit from a surface cell membrane to nuclear localization assigns the GluN1 promoter immediate early gene behavior with access to nuclear and potentially nucleolar functions (McNearney and Westlund 2023).
Metabotropic Glutamate Receptors (mGluRs)
mGluRs are broadly distributed throughout the CNS. mGluRs have diverse neuromodulatory actions of glutamate at the levels of synaptic plasticity, neuronal excitability, and gene transcription (Fabian et al. 2023). Eight mGluRs (mGluR1-mGluR8) have been cloned and are classified into three groups based on similarities in their amino-acid sequences, their linkage to second messenger systems, and their pharmacology. Group I mGluRs (mGluRs 1 and 5) generally increase neuronal firing and synaptic transmission. In contrast, stimulation of group II mGluRs (mGluRs 2 and 3) and group III mGluRs (mGluRs 4, 6, 7, and 8) generally reduces neuronal excitability and synaptic transmission. Thus, group I mGluR antagonists and group II and III mGluR agonists generally produce anti-nociceptive effects. All three groups of mGluRs are distributed throughout the CNS. Group II mGluRs (mGluR2/3) are present at the afferent terminals in the spinal superficial DH and DRG. Two sub-types of group III mGluRs, mGluR4 and mGluR7, are located in the DH, particularly laminae I and II. mGluR4 and mGluR7 mRNA also occurs in the DRG (Pan et al. 2007). All mGluR sub-types (except mGlu6 receptor) are expressed within the nociceptive pathways where they modulate pain transmission (Mazzitelli et al. 2018; Pereira and Goudet 2019). Group II mGluR2 and mGluR3 are expressed in peripheral, spinal and supraspinal elements of pain-related neural processing and mainly presynaptically. They typically inhibit the release of neurotransmitters, including glutamate and GABA. Group II mGluRs are linked to pain modulation. In pre-clinical models of acute and chronic pain, group II mGluR agonists have anti-nociceptive/analgesic effects (Mazzitelli et al. 2018).
Postsynaptic metabotropic sub-type 1 (mGlu1) receptors exist in the THAL. In urethane-anesthetized rats, extracellular recordings were obtained in vivo with multi-barrel iontophoretic electrodes from single neurons in the THAL. Responses to iontophoretic applications of the Group I mGlu agonist 3,5-dihydroxy-phenylglycine (DHPG) were selectively potentiated by co-application of the mGlu1 positive allosteric modulator Ro67-4853, whereas they were selectively reduced upon co-application of the mGlu1 receptor orthosteric antagonist LY367385. This indicates that THAL DHPG responses are mediated primarily via mGlu1 receptors. Ro67-4853 also potentiated responses of THAL neurons to noxious thermal stimulation. By contrast, nociceptive responses were reduced by LY367385. Hence, mGlu1 receptors are important in the THAL processing of sensory information, particularly with respect to nociceptive responses (Salt et al. 2014).
A way to counteract glutamatergic hyperactivation, e.g., in PD, is through the activation of group III mGluRs, which are located on presynaptic terminals inhibiting neurotransmitter release. Selective ligands for each group III mGluR, in particular positive and negative allosteric modulators, have allowed elucidating the role of each sub-type. The neuroprotective potential of group III mGluRs in pathological conditions, such as those characterized by elevated glutamate, has been shown. In the dorsal striatum, mGluR7 and mGluR8 are located at glutamatergic cortico-striatal terminals, and their stimulation inhibits pain in pathological conditions such as neuropathic pain. The two receptors in the dorsal striatum have instead a different role in pain control in normal conditions (Bocella et al. 2020).
Despite the ineffective function of CeA under normal conditions, AMY-mediated hyperalgesia in pain-related disorders occurs in CeA through the interactions with mGluR1/5, since CeA contains many nociceptive neurons, and under conditions of chronic pain, the excitability of CeA increased. CeA exerts anti-nociceptive effects by acting on the mGluR8. Under carrageenan-triggered inflammatory pain conditions, intra-CeA micro-injections of mGluR8 agonists increased OFF-cell activities while decreasing ON-cell activities, thus creating anti-nociceptive effects. Hence, the AMY-RVM pathway, particularly CeA-rvm projections, modulate pain through acting at mGluRs (Peng et al. 2023).
There are basal sex differences in mGluR expression and function. Gonadal hormones, in particular estradiol, regulate mGluR signaling. There are sex-specific mechanisms by which mGlu receptors differentially modulate synaptic plasticity and behavior in basal states and models relevant for disease (Fabian et al. 2023).
3.15.3. Glycine and Its Receptors
GABA and glycine mediate fast inhibitory neurotransmission in different CNS areas including the spinal cord. Under healthy conditions, they limit the excitability of spinal terminals of primary sensory nerve fibers and of intrinsic DH neurons through pre- and postsynaptic mechanisms (Zeilhofer et al. 2012).
Glycine is a second fast inhibitory neurotransmitter in the spinal cord, brainstem and a few other selected areas of the CNS including the retina. It activates a plasma membrane Cl− channel that is selectively blocked by strychnine. It distinguishes inhibitory glycine receptors not only from GABA receptors but also from excitatory NMDARs, which also possess a glycine-binding site. At these excitatory receptors, glycine functions as endogenous co-agonists and are required, together with the principal excitatory neurotransmitter L-glutamate, for full channel activation. The sub-unit composition of strychnine-sensitive glycine receptors shows considerably less heterogeneity than that of GABAARs. Unlike GABAARs, the repertoire of sub-units that glycine receptors can draw from is limited to four α sub-units, designated α1-α4, and one β sub-unit. Recent evidence suggests that the β sub-units also participate in the formation of the glycine-binding site and that glycine receptors are composed of two α and three β sub-units. Besides strychnine, picrotoxin, a mixture of picrotin and picrotoxinin, is sometimes used to pharmacologically characterize inhibitory glycine receptors (Zeilhofer et al. 2012).
Glycine receptors show restricted distributions. A high density of glycine receptors is present in both the spinal VH and the DH, in various nuclei of the brain stem, including the trigeminal nucleus, and the cerebellum. In adulthood, most glycine receptors comprise α1/β heteromers. Channel complexes containing the α3 sub-unit occur in the spinal cord and in HIPP. In the spinal cord, α3 sub-units are concentrated in the superficial DH layers where nociceptive primary afferent fibers terminate (Zeilhofer et al. 2012).
3.15.4. GABA and Its Receptors
The inhibitory transmitters GABA and glycine play an important role in modulating spinal pain transmission, both in normal and in pathological situations. There are three types og GABA receptors. In mammals, GABAARs are widely distributed throughout the nervous system and peripheral tissues. GABAB receptors occur in the olfactory bulb, neocortex, HIPP, THAL and cerebellum. GABAC receptors occur mainly in the retina, but are also distributed in the spinal cord, THAL, pituitary gland, and intestine. The rapid inhibition by the GABA is mediated by GABAARs (Huang et al. 2023).
GABAA Receptors (GABAARs)
GABAARs are composed of a repertoire of 19 sub-units. GABAARs are the most diverse family of neurotransmitter receptors in the mammalian nervous system. The majority of these receptors contain two α subunits, two β subunits and one γ subunit. They are activated by GABA released from presynaptic terminals. They mediate phasic inhibition. In the brain, most GABAAR isoforms are composed of α1, β2, and γ2 sub-units. In the spinal cord, α2 and α3 are more abundant than α1 sub-units, and β2 is replaced in the majority of spinal GABAARs by β3. The physiological activator GABA binds to an interface formed by the α and β sub-units, which occurs twice in a typical GABAAR. In addition to the physiological activator GABA, many GABAARs bind endogenous neuromodulators, such as neurosteroids and modulatory drugs, including benzodiazepines, barbiturates, alcohols, and anesthetics. A sub-set of GABAARs, which possess the δ or ε sub-unit instead of the γ sub-unit, are benzodiazepine-insensitive and are exclusively located at extrasynaptic sites. They typically exhibit a higher affinity for GABA than γ2 sub-unit containing receptors and mediate tonic inhibitory currents. These channels exhibit a highly restricted distribution within the CNS. The δ sub-unit is most abundant in the cerebellum but is also found in several forebrain areas including the DG, the neostriatum, and certain cortical layers. The ε sub-unit is found in the HYP, the spinal cord, and several hindbrain areas. Bicuculline is the most commonly used GABAAR antagonist. It blocks all ionotropic GABA receptors, with the exception of those containing ρ su-bunits, but also inhibits certain K+ channels. Gabazine is another GABAAR antagonist, which elicits preferential block of synaptic GABAAR. In the CNS, GABAARs may exert functions beyond inhibitory neurotransmission. Such additional processes include adult HIPP neurogenesis, which is impaired in mice carrying deficits in γ2 subunit containing GABAARs. Evidence for adult neurogenesis in the spinal cord is lacking. Functional GABAARs are also expressed by spinal astrocytes. Astrocytes indirectly participate in sensory processing and contribute to the generation of chronic pain states (Zeilhofer et al. 2012).
GABAergic communication occurs within rodent peripheral sensory ganglia and can modulate transmission of pain-related signals from the peripheral sensory nerves to the CNS. Sensory neurons expressed major proteins necessary for GABA synthesis and release, and sensory neurons released GABA in response to depolarization. In vivo, focal infusion of GABA or GABA re-uptake inhibitor to sensory ganglia dramatically reduced acute peripherally induced nociception and alleviated neuropathic and inflammatory pain. Focal application of GABA receptor antagonists to sensory ganglia triggered or exacerbated peripherally induced nociception. Chemogenetic or optogenetic depolarization of GABAergic neurons in the DRG in vivo reduced acute and chronic peripherally induced nociception. Mechanistically, GABA depolarized the majority of sensory neuron somata, yet produced a net inhibitory effect on the nociceptive transmission due to the filtering effect at nociceptive fiber T-junctions (Du et al. 2017).
Besides, in PSI (below), GABA neurons are important in organizing the operation of the complex DH network. They may contain one or more of the following agents: glycine, ACh, NPY, ENK, NOS or parvalbumin. Parvalbumin-immuno-reactivity was restricted to those GABA-immuno-reactive neurons that also showed glycine-immuno-reactivity and was not co-localized with NPY-immuno-reactivity or NADPH-diaphorase activity. NADPH diaphorase activity was a reliable marker for NOS. Neurons that possess GABA- but not glycine-immuno-reactivity may contain NPY, ENK, ACh or NADPH diaphorase, and all ACh neurons appear to contain NADPH diaphorase. Peptide-immuno-reactivity did not co-exist with NADPH diaphorase. Hence, several phenotypically distinct groups of GABA-immuno-reactive neuron can be identified in DH laminae I-III, and these groups may represent different functional types of inhibitory neuron (Laing et al. 1994).
To localize spinal glycine/GABA neurons, in situ hybridization for identifying spinal neurons that use the transmitter(s) glycine and/or GABA (glycine/GABA neurons) and immuno-histochemistry for c-fos, a marker for neuronal activation, were combined. This procedure was used with acute pain models induced by the injection of capsaicin or formalin. In all models, glycine/GABA neurons were activated as indicated by their expression of c-fos. The pattern of glycine/GABA neuronal activation was different for every model, both anatomically and quantitatively. Morphine application decreased the total number of activated neurons and activated glycine/GABA neurons, showing that morphine did not specifically activate glycine/GABA neurons to achieve nociceptive inhibition (Hossaini et al. 2010).
The expression pattern of the major GABAAR isoforms in the spinal cord has been studied mainly in mice and rats. α2 sub-units are most abundant in the superficial DH and in MNs. The α1 and α5 sub-units are most densely expressed in laminae III-VIII, while the lamina I/II) are largely devoid of these sub-units. The distribution of GABAARs in the human hindbrain and most rostral segments of the cervical spinal cord is mostly consonant with that in rodents. In the adult spinal cord, the α2 sub-unit mRNA was particularly concentrated in VH MNs, while the α3 sub-unit mRNA was expressed to an equal degree in both ventral and DH. The α2 subunit mRNA is strongly expressed in large-diameter DRG neurons and to a lesser degree in small-diameter cells. This correlates well with electrophysiological studies, which showed that large-diameter capsaicin-insensitive DRG neurons exhibit larger GABAergic membrane currents than small-diameter capsaicin-sensitive cells. α2 (and α3) sub-units are expressed on DH axons and/or axon terminals of nociceptive (CGRP- and IB4-positive) and non-nociceptive afferents (i.e., those positive for the VGLUT1). A significant portion of the DH α2 sub-units is still located on intrinsic DH neurons (Zeilhofer et al. 2012).
Itch and pain may in part share common pathways, and GABAARs in the CeA are involved in pain modulation. In rats, bilateral intra-CeA micro-injection of a selective GABAA receptor agonist muscimol hydrochloride, but not a selective GABAA receptor antagonist bicuculline or vehicle, showed significant analgesic effects, reflected by an increase in TFL and a decrease in ipsilateral forelimb wipes evoked by mustard oil. Rats subjected to intra-CeA infusion of bicuculline showed a significantly greater number of scratching bouts and time in acute and chronic pruritus animal models than control rats. Conversely, intra-CeA infusion of muscimol in animal models dramatically decreased the number of scratching bouts and time compared with control rats. In addition, intra-CeA infusion of bicuculline or muscimol had no obvious effects on other behaviors including locomotor activity and spontaneous facial grooming in rats subjected to cheek micro-injection of 5-HT. This has been taken to indicate that the GABAAR-mediated inhibitory system in the CeA is involved in itch modulation as it is in pain control (Chen et al. 2016).
GABAB Receptors (GABABRs)
GABA is the most important inhibitory neurotransmitter. GABA activates three pharmacologically distinct types of receptors: ionotropic GABAA and GABAC receptors and G protein-coupled GABAB receptors. GABA is widely distributed throughout the CNS. In the brain, GABAB receptor binding sites are present in the THAL, AMY, PAG, PBN, and medullary raphé nucleus. GABAB receptors are also present in primary afferent neurons and the spinal cord. GABAB receptor immuno-reactivities are distributed in the DH laminae I-III and in DRG neurons (Pan et al. 2007).
In animal models of acute pain, the GABAB receptor agonist baclofen produced an anti-nociceptive effect upon intra-thecal administration. GABAB receptor agonists exerted effects on ion channels and synaptic transmission. In cultured newborn rat DRG neurons, activation of GABAB receptors reduced high voltage-gated Ca2+ channel activity. In the rat small-diameter trigeminal ganglion neurons, baclofen inhibited neuronal excitability through potentiation of voltage-dependent K+ currents. Activation of presynaptic GABAB receptors likely contributes to the anti-nociceptive effect of baclofen by inhibiting the release of glutamate and neuropeptides from primary afferents. In rat spinal cord slices, baclofen dose-dependently decreased the glutamate release from primary afferent terminals. In the spinal cord, baclofen had a greater effect on group IV (C)-fiber- than on group III (Aδ)-fiber-evoked glutamate release suggesting that GABAB receptors may be preferentially expressed on group IV fibers as opposed to group III-fiber afferent terminals. GABAB receptors are also involved in the inhibitory effect of ACh on spinal glutamatergic synaptic transmission. Blockade of GABAB receptors attenuated the inhibition of ascending DH neurons produced by mAChR agonists and the acetylcholinesterase inhibitor neostigmine. Thus, activation of GABAB receptors contributed to the anti-nociceptive effect of intra-thecally administered mAChR agonists or neostigmine. Increased GABA release after activation of mAChRs could spill over sufficiently to activate presynaptic GABAB receptors on the neighboring glutamatergic terminals to indirectly inhibit glutamate release. In addition to reducing glutamatergic synaptic transmission, GABAB receptor activation attenuated GABA and glycine release in the spinal DH. In the spinal DH neurons, baclofen activated GIRK channels, which are important in maintaining the resting membrane potential duration in lamina II neurons (Pan et al. 2007).
The ventro-basal complex of the THAL (VB) participates in the transmission and modulation of noxious information. GABAB receptors in the VB might be involved in the modulation of neuronal activity in response to chronic noxious input. To investigate the role of VB GABAB receptors in acute inflammatory pain, the formalin test of nociception was performed in rats stereotaxically injected in the VB contralateral to the formalin-injected paw, with saline (controls), baclofen, a specific GABAB receptor agonist or CGP35348, a GABAB receptor antagonist. Control animals exhibited phase 1 (acute pain) and phase 2 (tonic pain) nociception-related activities. A higher dose of baclofen induced a significant decrease of all pain-related behaviors in both phases of the test and had no observable effects on the animals’ motor function, while a lower dose could not reduce the total pain-related activities. Injection of CGP35348 prior to baclofen reduced the anti-nociceptive effect caused by baclofen during phase 2 in the paw-jerks and in total pain-related activities. CGP35348 alone had anti-nociceptive effects in both phases, though less pronounced than baclofen 0.875 microgram in the total pain-related activities during phase 2. This demonstrates that both the blockade and the activation of GABAB receptors in the VB of rats induce anti-nociception in acute and tonic pain (Soares Potes et al. 2006).