1. Introduction
Two types of uterine cancer are reported depending on the origin of the malignant tumor [
1]. They are those originating in the endometrium of the uterine body and those originating in the uterine body [
2]. Malignant tumors of the endometrium are composed of malignant cells with various morphologies [
3]. According to the World Health Organization (WHO) classification of gynecological tumors, malignant tumors arising from the endometrium of the uterine body include endometrial cancer, endometrial stromal sarcoma, uterine carcinosarcoma, and uterine adenocarcinoma [
4]. According to the WHO classification of gynecological tumors, tumors arising from within the uterine body include uterine mesenchymal tumors such as uterine leiomyoma and uterine leiomyosarcoma [
5].
Cervical cancer that develops in the cervix can be classified into two types based on differences in cell morphology [
6]. According to the WHO classification of gynecological tumors, two types exists: squamous cell carcinoma, which develops from squamous epithelial cells of the mucosal tissue at the entrance of the cervix, and adenocarcinoma, which develops from columnar epithelial cells of the glandular tissue near the body of the uterus [
7]. The number of individuals with cervical cancer increases from their late 20s onwards, whereas the number of individuals with endometrial cancer increases after the age of 40 years [
8]. In many cases, the location and size of a tumor in the body are determined from the results of imaging tests such as computed tomography (CT) imaging or contrast-enhanced magnetic resonance imaging (MRI); however, these imaging tests rarely lead to an accurate diagnosis of the tumor [
9].
In this patient, the tumor, which was thought to have developed from the uterine body to the cervix, grew outside the uterine body within the pelvis and reached the Douglas pouch. Therefore, the mass did not appear to originate from the uterine or cervical endometrium. Therefore, our medical staff considered the possibility that the patient may have developed a uterine mesenchymal tumor originating from the smooth muscle layer of the uterus. Furthermore, contrast-enhanced MRI revealed that the tumor tended to grow rapidly. Since she was in her 50s when the disease first appeared, we considered the possibility that the tumor was a uterine leiomyosarcoma.
Therefore, we performed radical hysterectomy and bilateral salpingo-oophorectomy to remove the uterus, fallopian tubes, and ovaries. Molecular pathological analysis was performed on the uterine tissue, fallopian tubes, and ovaries removed during surgical treatment. High caveolin 1 expression has been observed in mesenchymal tumors, including uterine leiomyomas and leiomyosarcomas [
10]; however, no caveolin 1 expression has been observed in tumor tissues excised from patients. The concentration of soluble interleukin 2 receptor (sIL-2R) in the serum collected from the patient was 4650 (U/mL), which was high, raising the suspicion of a tumor in hematopoietic cells [
11]. Immunohistochemical staining (IHC) showed that the excised tissue was positive for myeloid cells surface markers (MPO, CD68, CD117, and CD43) [
12]. These results revealed the development of myeloid sarcoma, a malignant tumor primarily caused by bone marrow cells. Myeloid sarcoma is a type of acute myeloid leukemia (AML) blood cancer [
13]. It often occurs simultaneously with AML or as a sign of AML recurrence after treatment. However, AML was not observed in this case.
In this case, we observed a tumor originating from the smooth muscle layer of the uterine body or cervix that grew into the Douglas pouch. Furthermore, contrast-enhanced MRI suggested the possibility of developing a malignant uterine mesenchymal tumor, that is, a uterine leiomyosarcoma. However, IHC results from the tumor tissue removed during surgical treatment confirmed the development of myeloid sarcoma. The patient had no history of AML or treatment, and the tumor extending from the uterine body or cervix to the Douglas pouch was diagnosed as myeloid sarcoma.
2. Materials and Methods
2.1. Enhaced-MRI Image Examination
To determine the presence, size, and location of the mass of the patient, contrast-enhanced MRI was performed to localize the mass using MRI equipment (Vantage Centurian: Vantage Galan 3T MRT-3020, Canon Medical Systems, Inc., Ohtawara, Tochigi, Japan).
2.2. Laparoscopic Surgery
Pyloric gastrectomy and reconstruction of the remnant stomach and duodenum were performed via laparoscopic surgery using a laparoscope (ENDOEYE FLEX 3D, Olympus Corporation, Shinjuku, Tokyo, Japan) and a surgical device (HICURA, Olympus Corporation), respectively, for surgical treatment of the gastric corpus area where the gastric tumor was detected.
2.3. Histopathological Examination
A surgical pathologist performed histopathological analysis of the sections from formalin-fixed paraffin-embedded resected tissue to assess the gross and histopathological characteristics of the specimens [
14].
Using the standard procedure, hematoxylin and eosin staining analyses were performed [
14].
2.3.1. Immunohistochemistry
Staining for caveolin-1, cyclin B, cyclin E1, LMP2/β1i, Ki-67, desmin, and myogenin was performed on serial tumor sections obtained from patients with uterine mesenchymal tumors (Supplementary Material 1). Monoclonal antibodies against cyclin E1 (clone CCNE1/2460) were purchased from Abcam (Cambridge Biomedical Campus, Cambridge, UK), and the monoclonal antibodies against Ki-67 (clone MIB-1) were purchased from Dako Denmark A/S (DK-2600 Glostrup, Denmark). Monoclonal antibodies against desmin (clone RM234) and myogenin (clone MGN185) were purchased from GeneTex, Inc. (Irvine, CA, USA). Monoclonal antibodies against caveolin-1 (clone sc-53564), cyclin B1 (clone sc-245), and LMP2/b1i (clone sc-373689) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). These antibodies have been used to diagnose mesenchymal tumors in humans. Anti-MPO (clone sc-390109), anti CD68 (clone sc-20060), anti CD117/c-kit (clone sc-365504), and anti CD43 (clone sc-21774) antibodies were purchased from Santa Cruz Biotechnology Inc. These antibodies have been used previously to diagnose myeloid sarcoma. All immunohistochemistry experiments were performed using the avidin–biotin complex method, as previously described (15,16). Briefly, a representative 5 mm tissue section was cut from a paraffin-embedded radical hysterectomy specimen obtained from each patient with a uterine mesenchymal tumor. The sections were first incubated with a biotinylated secondary antibody (Dako, DK-2600 Glostrup, Denmark) and then with a streptavidin complex (Dako). The reaction was developed using 3,39′-diaminobenzidine tetrahydrochloride hydrate (DAB), and the slides were counterstained with hematoxylin. Normal myometrial portions of the specimens were used as positive controls. Tissue sections incubated with normal rabbit immunoglobulin G instead of the primary antibody were used as the negative controls. Brown DAB staining reveals the expression of cyclin E and Ki-67. Normal rabbit or mouse antisera were used as negative controls for primary antibodies. The DAB-stained tissues were scanned using a digital microscope (BZ-X800; Keyence Corporation, Osaka, Japan). Black dots indicate cyclin E and Ki-67 expression levels. Normal rabbit or mouse antisera were used as negative controls for primary antibodies.
2.4. Pathogenic Variants of Patient’s Tumor and Human Uterine Leiomyosarcomas
Pathogenic variants of the patient’s tumor and human uterine leiomyosarcomas were identified using the FoundationOne® CDx tissue examination (Foundation Medicine, Inc., Cambridge, MA, USA) with patient tissues resected via surgical treatment.
2.5. Standard Treatment for Acute Myeloid Leukemia (AML): Treatment of Patients with Myeloid Sarcoma with CP X-351 (Daunorubicin/Cytarabine Encapsulated Liposomes)
CPX-351 is a liposomal formulation of cytarabine and daunorubicin used for AML [
17]. The standard therapy for AML is a combination of cytarabine and daunorubicin. The standard doses of these drugs are 100–200 mg/m
2 for consecutive 7 days, and 45 mg/m
2 for three injections in older adult patients.
2.5. Ethics Approval and Consent to Participate
Shinshu University approved the experiments (Approval No. M192). All experiments using human tissues were conducted at the National Hospital Organization, Kyoto Medical Center (approval no. NHO H31-02), in accordance with the institutional guidelines issued on August 17, 2019, by the Central Ethics Review Board of the National Hospital Organization Headquarters (Tokyo, Japan) and Shinshu University (Nagano, Japan). The authors attended educational lectures on medical ethics in 2020 and 2021, supervised by the Japanese government (completion numbers AP0000151756, AP0000151757, AP0000151769, and AP000351128). Consent for participation in this clinical study was obtained from all the patients. After being briefed on the clinical study and agreeing to the clinical research objectives, the participants signed consent forms. The authors attended seminars on the ethics of experimental research using small animals on July 2, 2020, and July 20, 2021. The code number for ethical approval of experiments with small animals was KMC R02-0702.
3. Results
A woman in her 50s with a history of endometrial hyperplasia visited the gynecology department of a nearby clinic for outpatient treatment due to irregular bleeding and menstrual irregularities. However, during follow-up at our clinic, she experienced genital discomfort; therefore, a contrast-enhanced MRI was performed. Contrast-enhanced MRI revealed a cervical mass. Consequently, in December 2021, she was referred by her doctor to our gynecology department at a general hospital for further examination and treatment of the cervical mass. Examination at the outpatient clinic revealed a prominent mass on her cervical lip. Macroscopic examination of the cervix revealed no abnormalities in the uterine mucosa. Therefore, contrast-enhanced CT and MRI scans were performed. The results of imaging tests indicated that her illness was most likely due to a submucosal stromal lesion, such as a uterine sarcoma. We performed a cervical biopsy, and the pathological results were suggested uterine sarcoma. Therefore, after discussion at a medical conference, the decision was made to surgically remove the tumor. In January 2022, modified radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy were performed. Surgical pathology was performed on surgically removed tissues.
3.1. Contrast-Enhanced MRI Imaging Results
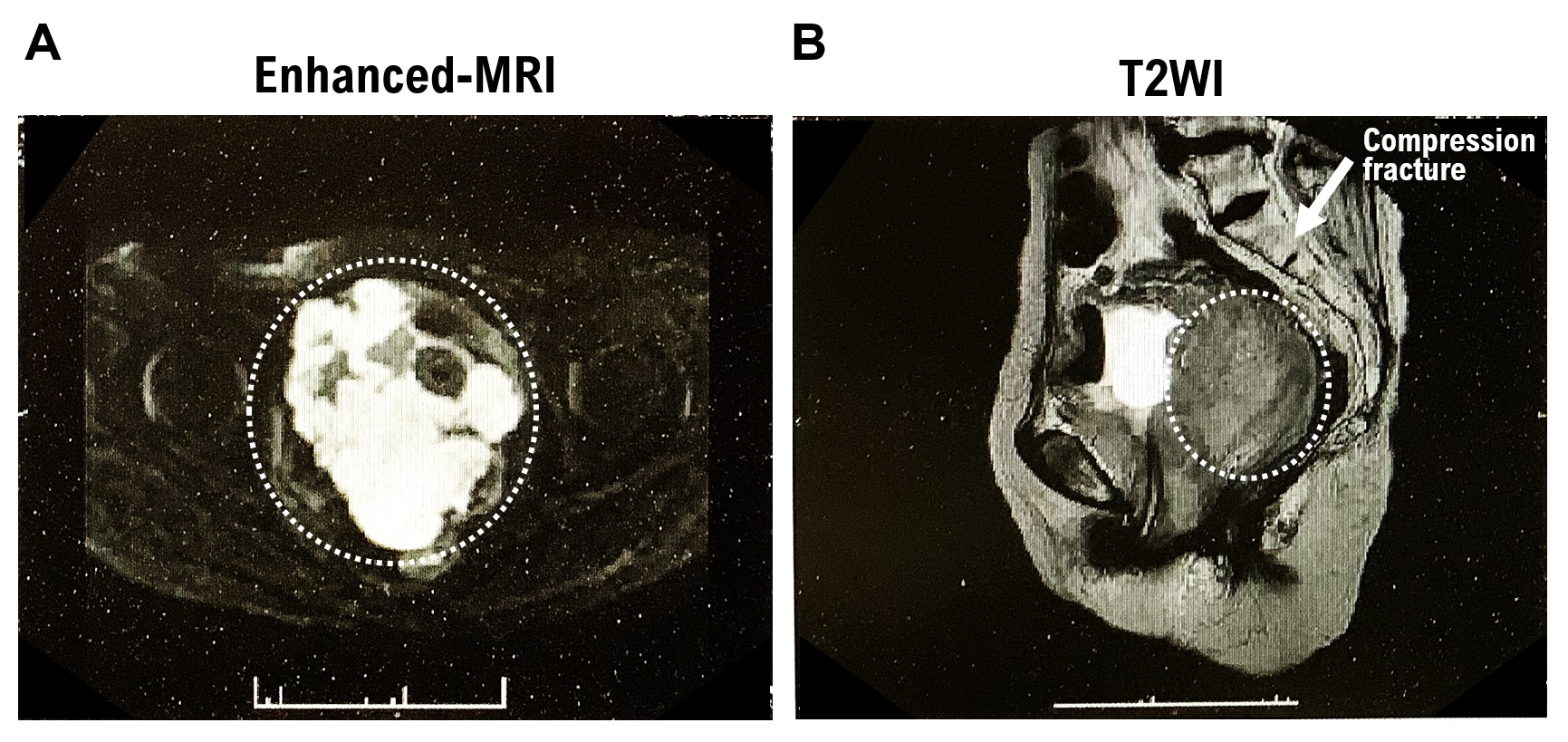
A tumor was observed occupying the area from the urethra to the bladder wall and from the vagina to the uterine vagina. Contrast-enhanced MRI T2W1 imaging results showed a homogeneous, moderate signal (
Supplementary Figure S1A). In addition, chronic compression fractures of the thoracic and lumbar spines were observed. Contrast-enhanced MRI (DW1) revealed severe diffusion restriction (
Supplementary Figure S1B). Based on the extent of the lesion and signal, the mass was considered to be either a uterine leiomyosarcoma or malignant lymphoma. The rectum was compressed by the mass. However, no evidence of tumor invasion was observed in the rectal wall. Bilateral hydronephrosis and hydroureter formation occurred due to tumor progression. Left ureteral stones, left kidney stones, and left kidney atrophy were observed. Lymphadenopathy was not observed.
3.2. Blood Test Results
When a malignant tumor develops in the digestive organs, the serum concentrations of the tumor markers Carcinoembryonic Antigen (CEA) and Carbohydrate Antigen 19-9 (CA19-9) increase [
18]. When a malignant tumor develops in gynecological organs (ovaries, fallopian tubes, or uterus), the serum concentration of the tumor marker Carbohydrate Antigen 125 (CA125) increases [
19]. However, no diagnostic markers malignant uterine mesenchymal tumors (i.e., uterine leiomyosarcoma) were identified (
Supplementary Table S1). Blood test results revealed normal levels of the tumor markers, CEA, CA19-9, and CA125. Therefore, the occurrence of malignant tumors in the digestive or female organs (ovaries, fallopian tubes, and uterus) was excluded. In contrast, the serum sIL-2R concentration was 4650 (U/mL), which was high (normal value is 212 or higher but less than 613) (
Supplementary Table S1). Diseases that may cause high levels of sIL-2R include malignant lymphoma, lymphocytic leukemia, Adult T-cell leukemia (ATL), hemophagocytic syndrome, interstitial pneumonia, and collagen diseases such as rheumatoid arthritis.
3.3. Surgical Pathology Results and Cancer Genome Profiling (CGP)
1. At the time of initial onset, myeloid sarcoma is often accompanied by malignant myeloid tumors such as AML. However, AML was not detected in this patient. The image shows the macroscopic appearance of a tumor in the cervical tissue. Subendometrial tumors are circled in white dotted lines. The tumor has grown from the uterine body to the cervix (Supplementary Figure S1C,D).
2. Metastatic lesions of the myeloid sarcoma were observed in the pelvic lymph nodes.
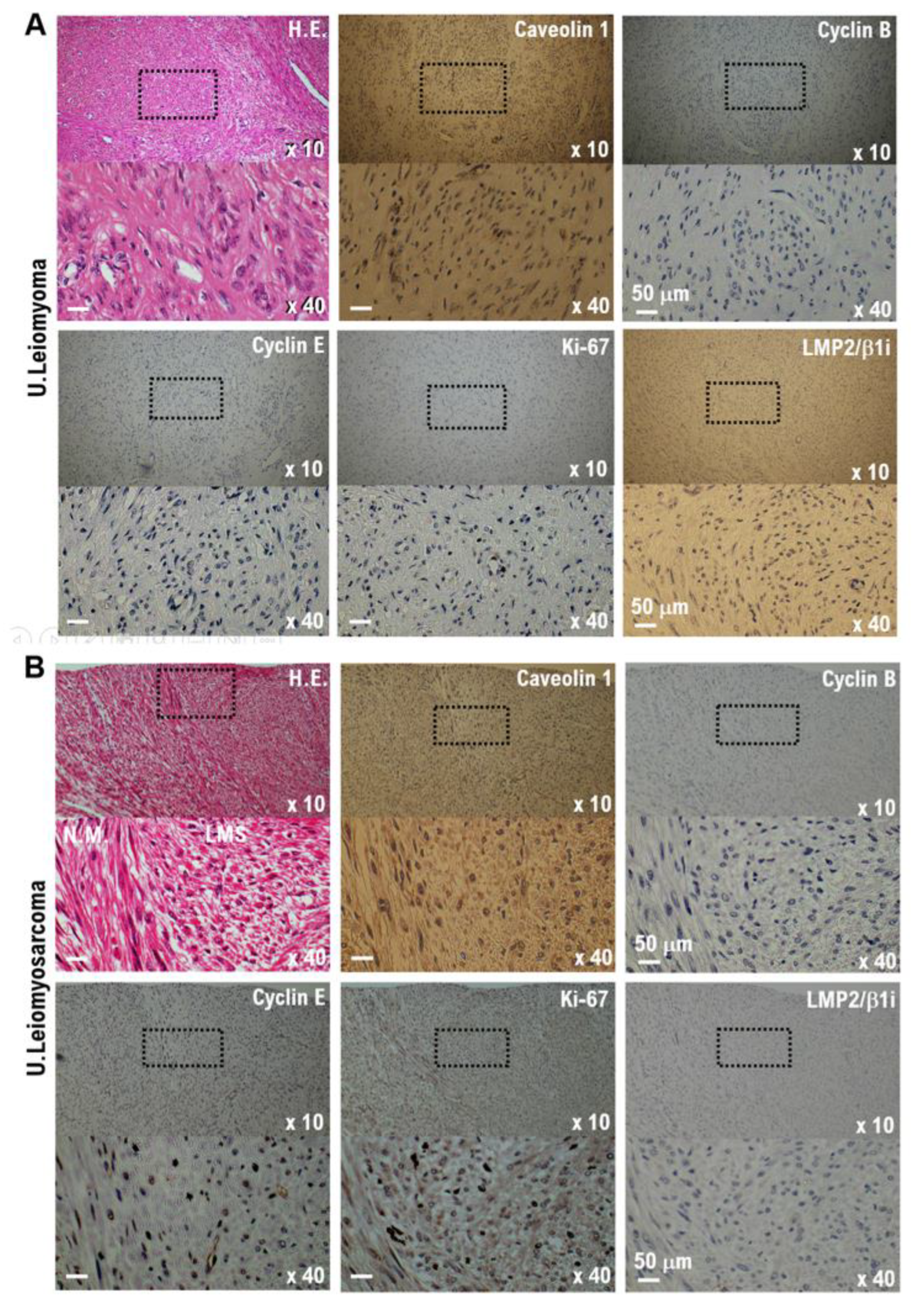
3. The surgical pathological diagnosis performed in this case did not reveal any findings suggesting the development of benign tumors (leiomyoma) or malignant uterine mesenchymal tumors, such as uterine leiomyosarcoma (
Figure 1A,B, Supplementary Table S2).
4. In uterine mesenchymal tumors (uterine leiomyosarcoma), cavellion 1 expression is strong, but the expression of LMP2/β1i is extremely weak or undetectable [
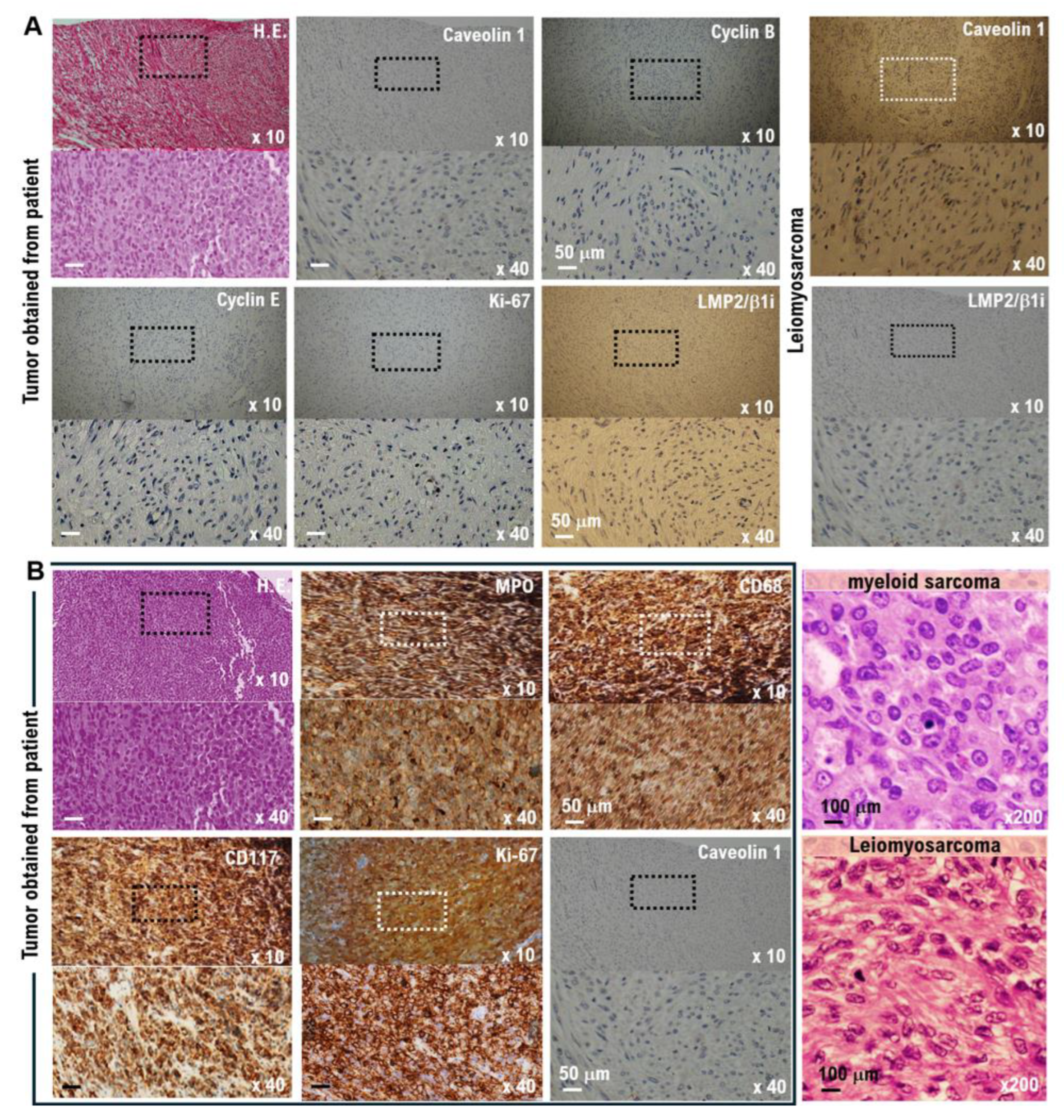
20] (Figure 1B, Supplementary Table S2). However, in tumor tissues obtained from patients following surgical treatment, the expression of cavellion 1 was significantly weak, whereas the expression of LMP2/β1i was strong. Based on these results, the patient did not develop a uterine mesenchymal tumor (
Figure 2A,B).
5. Analysis of cell surface markers: Myeloid cell surface markers (myeloperoxidase (MPO), Cluster Designation 68 (CD68), CD117, and CD43) were all positive (Figure 2B). Based on the results of this pathological test, the tumor was diagnosed as a myeloid sarcoma.
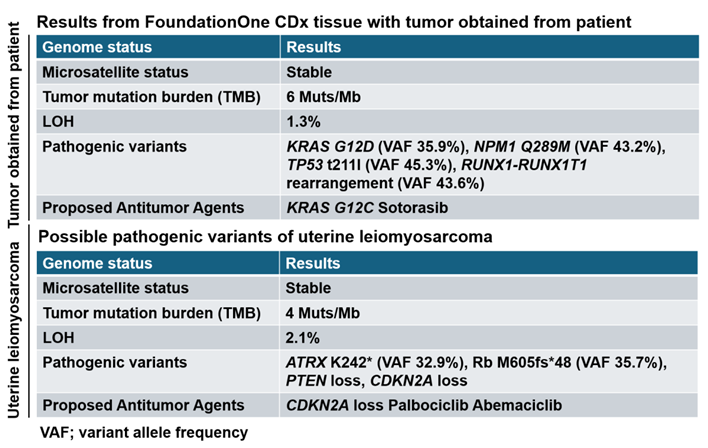
6. Genetic analysis has shown that most patient of AML has nucleophosmin 1 (NPM1) and c-KIT/ CD117 mutations. Based on the results obtained from FoundationOne CDx, NPM1 Q289M was detected as pathogenic variant (
Table 1). The results of cancer gene panel testing FoundationOne CDx using tumor tissue obtained from patients indicate a pathogenic variant specific to myeloid sarcoma but differ from the pathogenic variant specific to uterine leiomyosarcoma (Table 1). The results of cancer gene panel testing FoundationOne CDx using tumor tissue obtained from patients indicate CPG of myeloid sarcoma (Table 1).
Based on the surgical pathological diagnosis of the surgically removed tissue, the patient was diagnosed with myeloid sarcoma and underwent induction therapy with CPX-351 (daunorubicin + cytarabine liposome) and maintenance therapy (high-dose cytarabine), which are the standard treatments for AML. Subsequent contrast-enhanced MRI showed no progression of the malignant tumor, and the patient remained in remission with standard treatment regimen. Subsequently, the patient was not administered antitumor drugs and was monitored.
4. Discussion
A woman in her 50s with a history of endometrial hyperplasia visited the gynecology department of a nearby clinic for outpatient treatment of abnormal bleeding and irregular menstrual cycles. However, during follow-up at this clinic, she reported genital discomfort; therefore, contrast-enhanced MRI was performed. Contrast-enhanced MRI suggested a submucosal uterine sarcoma of the cervix. However, blood tests revealed high serum sIL-2R concentrations. The patient underwent a modified radical hysterectomy, bilateral salpingo-oophorectomy, and pelvic lymphadenectomy. Furthermore, the surgical pathological diagnosis of tumor tissues removed by surgical treatment revealed tissues with characteristics of myeloid sarcoma.
In this case, AML did not develop; however, clinical evidence of myeloid sarcoma recurrence was noted. The patient underwent induction therapy with CPX-351 (daunorubicin + cytarabine liposome) and maintenance therapy (high-dose cytarabine), which are standard treatments for AML [
21]. The patient experienced lower abdominal pain, lower back pain, and discomfort in the genital area; therefore, she visited our gynecology outpatient clinic. During an outpatient visit, a hard lesion extending from the cervix to the vaginal entrance was noted. Contrast-enhanced CT and MRI revealed a suspected myeloid sarcoma recurrence, and pathological examination of a tumor biopsy confirmed myeloid sarcoma recurrence. Therefore, the patient was administered standard treatment, induction therapy with CPX-351 (daunorubicin + cytarabine liposomes), and maintenance therapy (high-dose cytarabine). Four months after the start of treatment, contrast-enhanced CT and MRI showed that the tumor had shrunk, confirming the effectiveness of standard treatment with CPX-351 and maintenance therapy.
As mentioned earlier, many studies recommend chemotherapy before the onset of AML, and our medical team is currently treating a patient with primary cervical myeloid sarcoma. This study showed a tendency to prevent AML onset. In this case, chemotherapy may have prevented the development of AML. Primary myeloid sarcoma of the cervix is extremely rare, but primary myeloid sarcoma may develop into AML and has a poor prognosis. Therefore, careful follow-up is necessary, even if AML does not develop.
Conclusions
In cancer treatment, contrast-enhanced MRI is useful for identifying the size and location of tumor masses. However, contrast-enhanced MRI does not lead to the diagnosis of tumor masses. Therefore, early blood tests and tumor biopsy results are important for differential diagnosis and early treatment decisions.
Supplementary Materials
The following supporting information can be downloaded at: Preprints.org.
Institutional Review Board (IRB) Approval and Consent to Participate
This research on human cancer genome information derived from results of cancer genome panels was conducted at the Kyoto University, its affiliated hospitals, and the National Hospital Organization Kyoto Medical Center in accordance with institutional guidelines (IRB approval no. 50-201504, NHOKMC-2023-2, and H31-cancer-2). All patients were briefed on the clinical study and agreed to take part in the present study by providing informed consent for participation. Our clinical research complied with the Helsinki Statement. Ethics committee name: IRB of the National Hospital Organization Headquarters (approval code: H31-cancer-2; approval date: November 09, 2019, and June 17, 2013). Ethics committee name: IRB of Kyoto University (approval code: R34005; approval date: August 01, 2022).
Ethical compliance with human study
This study involves research with human participants and was approved by the institutional ethics committee(s) and IRBs. This manuscript contains personal and/or medical information and a case report/case history about an identifiable individual; therefore, it has been sufficiently anonymized in line with our anonymization policy. The authors obtained direct consent from the patient.
The authors attended research ethics education through the Education for Research Ethics and Integrity (APRIN e-learning program (eAPRIN)) agency. The completion numbers for the authors are AP0000151756, AP0000151757, AP0000151769, and AP000351128. This study involves research with animal materials and was approved by the institutional ethics committee(s) and IRBs (Ethics Committee for research with animals in National Hospital Organization Headquarter; Meguro, Tokyo, Japan). Ethics committee name: IRB of the National Hospital Organization Headquarters (approval code: H31-1-2; approval date: November 09, 2019, and June 17, 2023, approved code: R07-1-2).
ARRIVE checklist documentation
We never use live animals, the protocol of our research does not involve any live animals.
Conflicts of Interest
Authors have nothing to disclose.
Author Contributions
TH, K.A and Ik were involved in the study design, data collection, data review and interpretation, and manuscript writing. TH, K.A. and IK were involved in the literature search, study design, data collection, data interpretation, and manuscript writing. TH, K.A. and IK were involved in data collection and interpretation. TH, and IK were involved in data collection and interpretation and manuscript writing. TH, and IK were involved in the study conception and design, data analysis and interpretation, and manuscript writing. TH and IK were the medical leads for AstraZeneca, and they participated in the data collection and evaluation and manuscript writing and editing. TH and IK were the lead physicians and were involved in the study design and conduct, data analysis and interpretation, and manuscript review.
Funding
This clinical research was performed using research funding from the following: Japan Society for Promoting Science for TH (grant no. 19K09840), START-program Japan Science and Technology Agency (JST) for TH (grant no. STSC20001), National Hospital Organization Multicenter Clinical Study for TH (grant no. 2019-Cancer in general-02), and Japan Agency for Medical Research and Development (AMED) (grant no. 22ym0126802j0001), Tokyo, Japan. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data Availability Statement
Data are available on various websites and have also been made publicly available. More information can be found in the first paragraph of the Results section. The transparency document associated with this article can be found in the online version at
https://kyoto.hosp.go.jp/html/guide/medicalinfo/ clinical research/expand/gan.html (accessed on 15 March 2025).
Informed Consent Statement
This research includes clinical/human materials, therefore Informed consent is required.
Acknowledgments
The authors want to thank Dr. Kohji Moriyoshi at The pathology division, National Hospital Organization, Kyoto Medical Center. The authors also want to acknowledge all medical staff for clinical research at Kyoto University School of Medicine and the National Hospital Organization Kyoto Medical Center. We also appreciate Dr. Keita Idegami, Chugai Pharma Manufacturing Co., Ltd. (Kitaku, Tokyo, Japan) and Sysmex Corporation. (Kobe, Hyogo, Japan) for providing medical imformation.
References
- WHO Classification of rumors of the uterine corpus. Female Genital Tumors. WHO Classificationof rumors 5th Edition Edited by WHO classification of tumours editorial board. 6.
- WHO Classification of rumors of the uterine corpus. Female Genital Tumors. WHO Classificationof rumors 5th Edition Edited by WHO classification of tumours editorial board. 7-8.
- WHO Classificationof rumors of the uterine corpus. Female Genital Tumors. WHO Classificationof rumors 5th Edition Edited by WHO classification of tumours editorial board. 130-137.
- Tokinaga A, Furuya M, Niino H, Udaka N, Asai-Sato M, Sekido H, Miyagi E. Colonic low-grade endometrial stromal sarcoma and orthotopic endometrial stromal tumor with limited infiltration sharing the JAZF1-SUZ12 gene fusion. Pathol Int. 2014 Apr;64(4):178-82. [CrossRef]
- Uterine leionmyoma. WHO Classification of rumors of the uterine corpus. Female Genital Tumors. WHO Classification of rumors 5th Edition Edited by WHO classification of tumours editorial board. 272-282.
- Uterine leiomyosarcoma. WHO Classification of rumors of the uterine corpus. Female Genital Tumors. WHO Classificationof rumors 5th Edition Edited by WHO classification of tumours editorial board. 283-285.
- Sponchiado M, Liao YS, Reznikov LR. Identification of cholinergic cells with chemosensory traits in the porcine uterus. Cell Tissue Res. 2022 Apr;388(1):33-47. Epub 2022 Jan 27. [CrossRef]
- Söylemez MS, Kemah B, Söylemez UPO, Kılıç B, Ozkan K. Endometrial adenocarcinoma recurrence presenting with tibial metastasis: Report of a case. Int J Surg Case Rep. 2017;36:15-17. [CrossRef]
- Yan PF, Yan L, Zhang Z, Salim A, Wang L, Hu TT, Zhao HY. Accuracy of conventional MRI for preoperative diagnosis of intracranial tumors: A retrospective cohort study of 762 cases. Int J Surg. 2016 Dec;36(Pt A):109-117. [CrossRef]
- Hayashi T, Ichimura T, Yaegashi N, Shiozawa T, Konishi I. Expression of CAVEOLIN 1 in uterine mesenchymal tumors: No relationship between malignancy and CAVEOLIN 1 expression. Biochem Biophys Res Commun. 2015 Aug 7;463(4):982-7. [CrossRef]
- Maes M, Stevens W, Scharpé S, Bosmans E, De Meyer F, D’Hondt P, Peeters D, Thompson P, Cosyns P, De Clerck L. Seasonal variation in peripheral blood leukocyte subsets and in serum interleukin-6, and soluble interleukin-2 and -6 receptor concentrations in normal volunteers. Experientia. 1994 Sep 15;50(9):821-9. [CrossRef]
- Liu Y, Zhang Y, Xie J, Bao W, Xie B, Zhou J, Zeng Q. Correlation analysis between LINC00324 and immunophenotype in peripheral blood leukocytes in patients wit h acute myeloid leukemia. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2019 Sep;35(9):832-837.
- Lapadat R, Tower RL 2nd, Tam W, Orazi A, Gheorghe G. Pure Erythroid Leukemia Mimicking Ewing Sarcoma/Primitive Neuroectodermal Tumor in an Infant. Pediatr Blood Cancer. 2016 May;63(5):935-7. [CrossRef]
- Tanabe Y, Hayashi T, Okada M, Aburatani H, Tonegawa S, Abiko K, Konishi I. POTENTIAL DIAGNOSTIC BIOMARKERS FOR HUMAN MESENCHYMAL TUMORS, ESPECIALLY LMP2/Β1I AND CYCLIN E1/MIB1 DIFFERENTIAL EXPRESSION: PRUM-IBIO STUDY. Georgian Med News. 2024 May;(350):42-48.
- Hayashi T, Sano K, Okada M, Ura T, Konishi I.Hereditary Gastric Cancer Is Linked With Hereditary Breast and Ovarian Cancer. World J Oncol. 2024 Aug;15(4):722-730. [CrossRef]
- Hayashi T, Sano K, Okada M, Muto M, Konishi I. Efficacy and Tolerability of Olaparib Plus Paclitaxel in Patients with Gastric Cancer Associated with Hereditary Breast and Ovarian Cancer. Curr Oncol. 2024 Oct 29;31(11):6723-6734. [CrossRef]
- Alzahrani AM, Alnuhait MA, Alqahtani T. The Clinical Safety and Efficacy of Cytarabine and Daunorubicin Liposome (CPX-351) in Acute Myeloid Leukemia Patients: A Systematic Review. Cancer Rep (Hoboken). 2025 May;8(5):e70199. [CrossRef]
- Romanowicz A, Łukaszewicz-Zając M, Choromańska B, Pączek S, Razak Hady H, Myśliwiec P, Jamiołkowski J, Stępniewski P, Kozłowski L, Mroczko B. A Disintegrin and Metalloprotease 15 (ADAM15) as a Potential Predictor of Distant Metastasis in Colorectal Cancer (CRC). J Clin Med. 2025 Jul 17;14(14):5082. [CrossRef]
- Abul Rub F, Moursy N, Alhedeithy N, Mohamed J, Ifthikar Z, Elahi MA, Mir TA, Rehman MU, Tariq S, Alabudahash M, Chinnappan R, Yaqinuddin A. Modern Emerging Biosensing Methodologies for the Early Diagnosis and Screening of Ovarian Cancer. Biosensors (Basel). 2025 Mar 21;15(4):203. [CrossRef]
- Matsumoto Y, Hayashi T, Amano Y, Abiko K, Konishi I. DEVELOPMENT OF ENDOSALPINGIOSIS IN PATIENTS WITH A HISTORY OF BREAST CANCER. Georgian Med News. 2024 Oct;(355):72-76.
- Nishikawa S, Hayashi T, Amano Y, Yaegashi N, Abiko K, Konishi I. Characteristic of Concurrent Uterine Lipoleiomyoma and Hemangioma by Algorithm of Candidate Biomarkers for Uterine Mesenchymal Tumor. Diagnostics (Basel). 2022 Oct 12;12(10):2468. [CrossRef]
- Mavridou K, Gavriil S, Papoudou-Bai A, Gaitanis G, Piperidou A, Bassukas ID. Isolated cutaneous myeloid sarcoma preceding acute myeloid leukemia: a case report and literature review. Dermatol Reports . 2025 Feb 6;17(1):10013. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).