Submitted:
05 August 2025
Posted:
05 August 2025
You are already at the latest version
Abstract
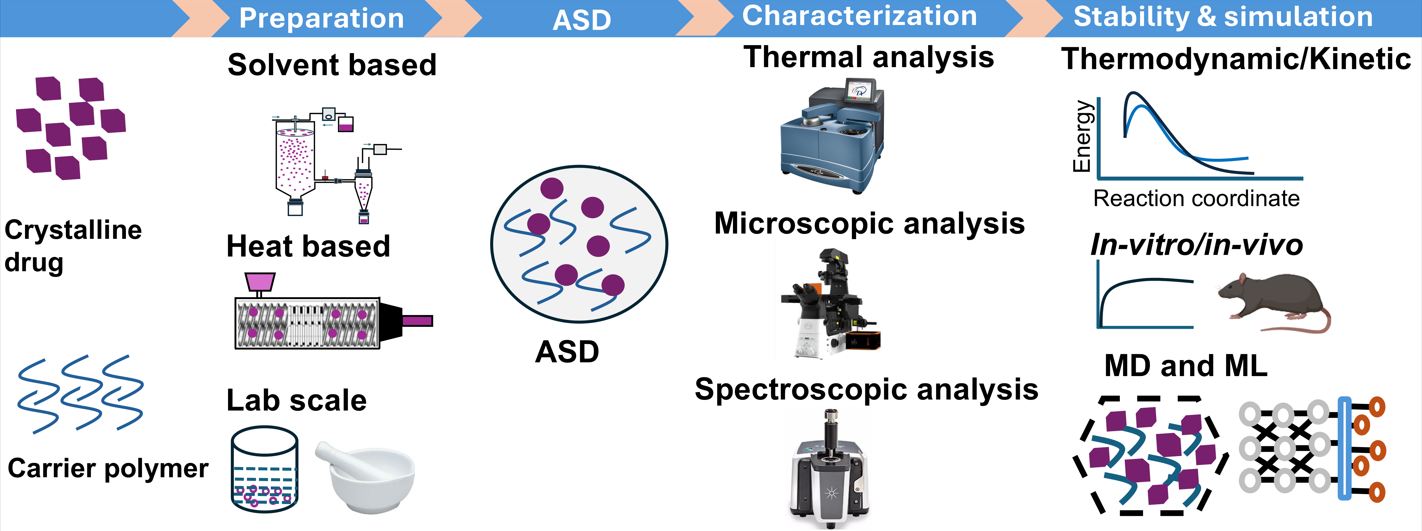
Keywords:
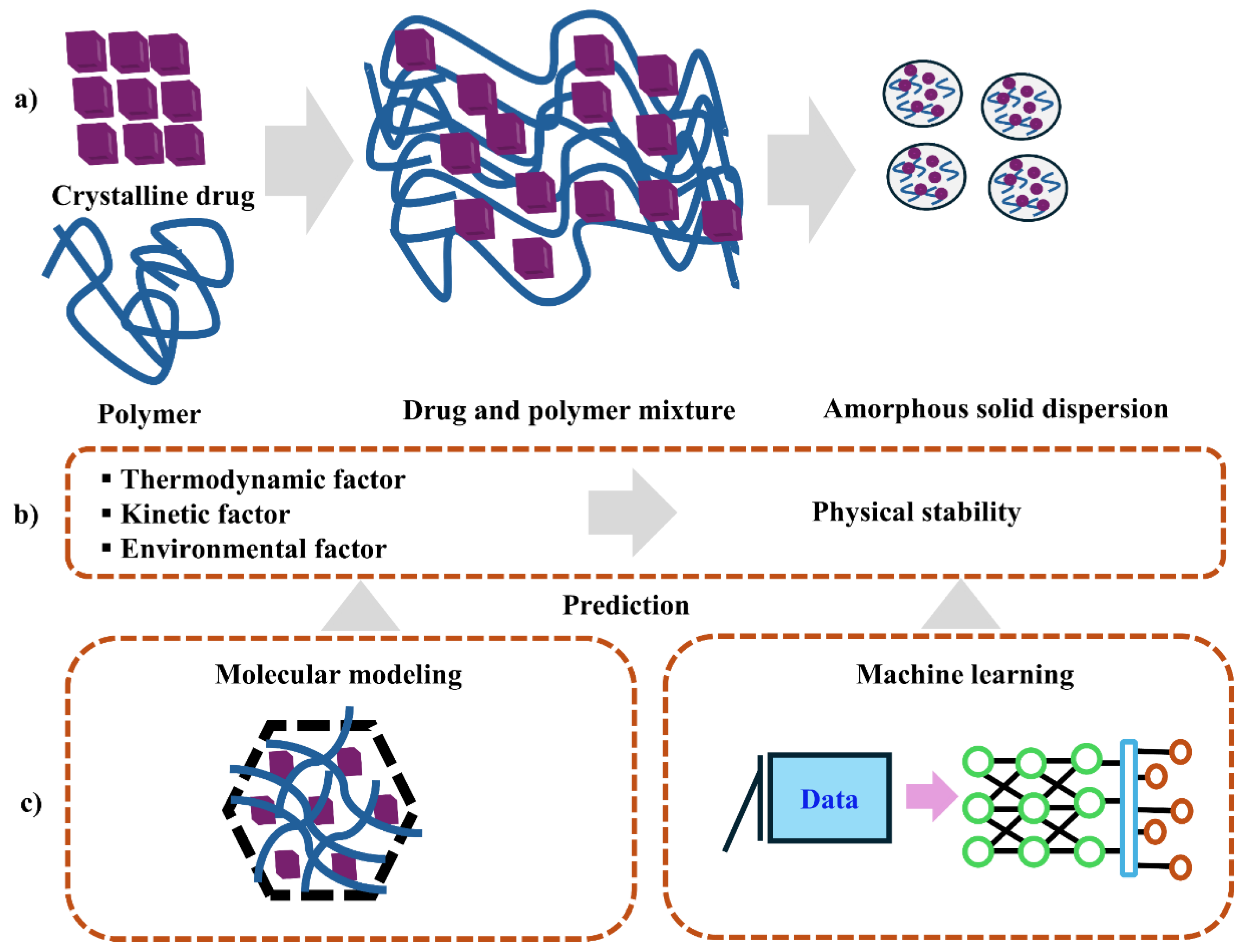
1. Introduction
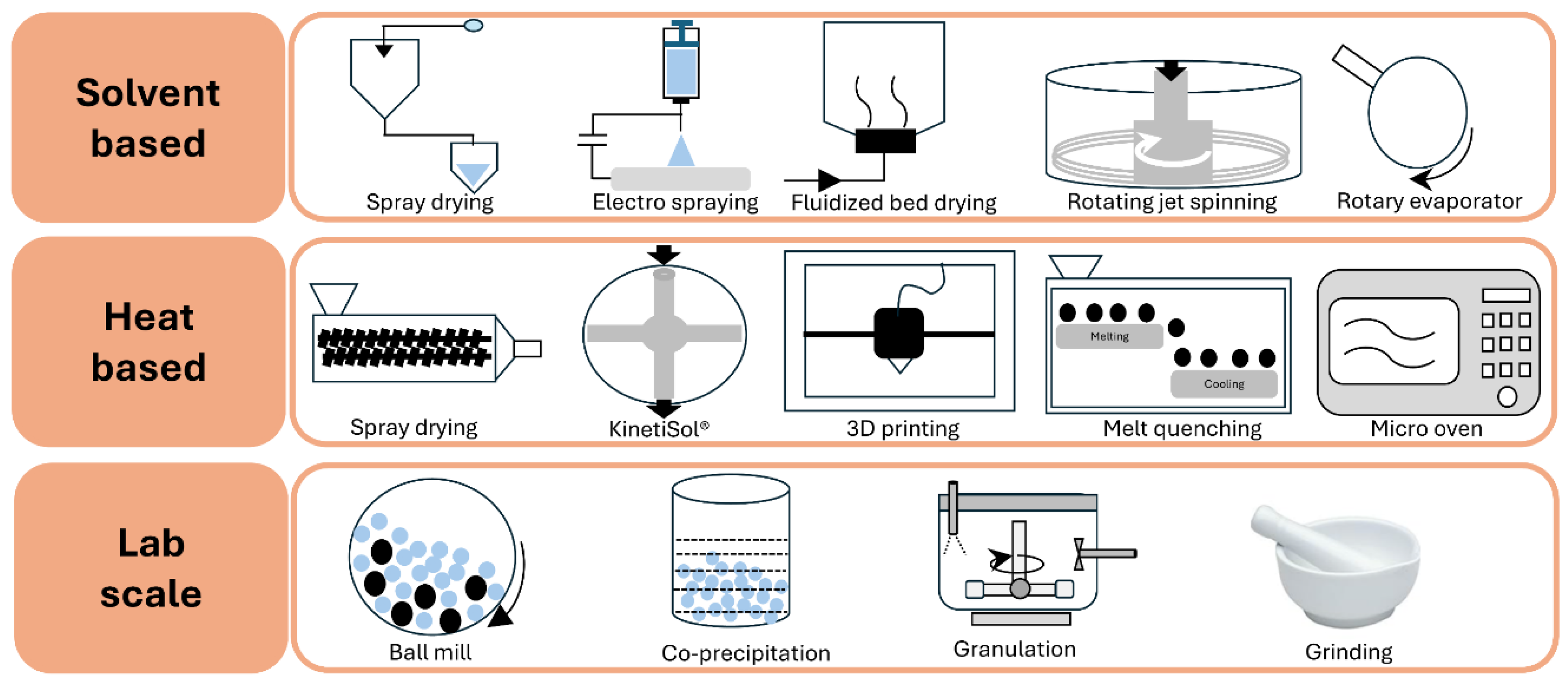
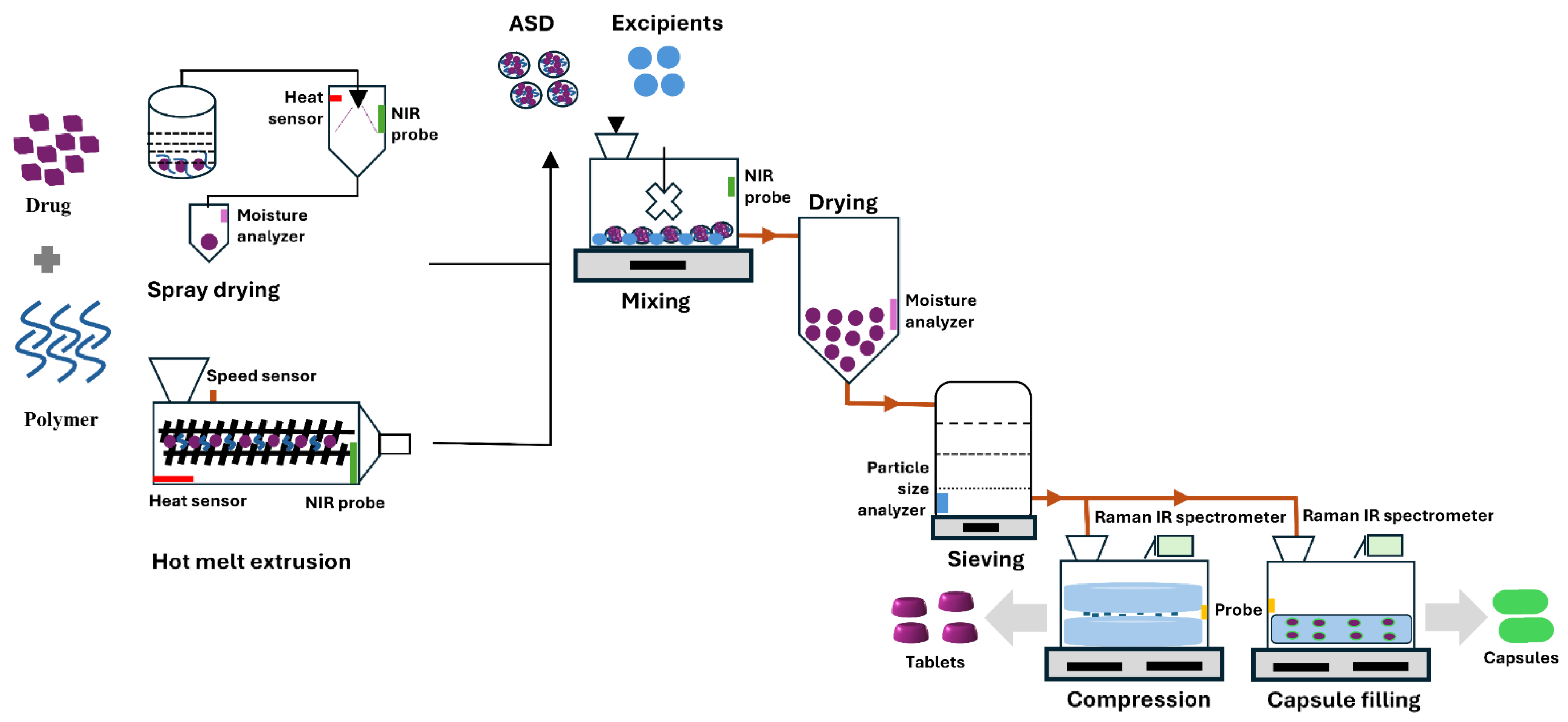
2. Effects of Material and Process Attributes
Quality Design and Process Parameter Tools
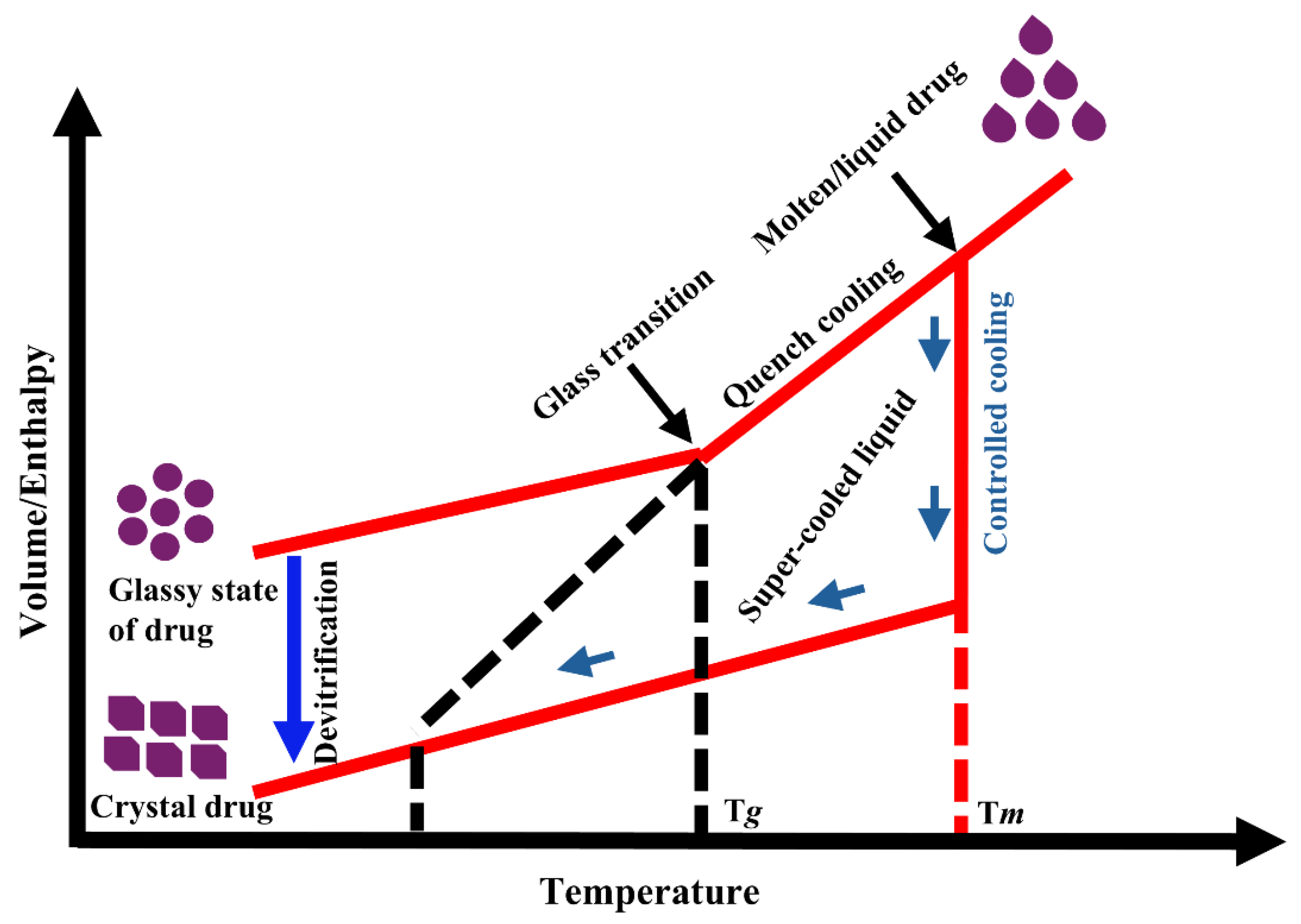
3. Physical Stability of Amorphous Solid Dispersion
3.1. Thermodynamic Factors on Physical Stability
3.1.1. Solubility of Drug in Polymer
3.1.2. Phase Separation
3.1.3. Compatibility of Drug and Polymers
3.1.4. Glass Transition Temperature
3.1.5. Drug–Polymer Interaction
3.2. Kinetic Factors on Physical Stability
3.2.1. Molecular Mobility
3.2.2. Nucleus Formation
3.2.3. Growth of Nucleus
3.3. Environmental Factors on Physical Stability
4. Molecular Simulation and Statistical Methods
4.1. Quantum Mechanics (QM)
4.2. Molecular Mechanics (MM) and Molecular Dynamics (MD)
4.3. Docking Studies of Drug in Polymer Carrier
5. Machine Learning for Better Performance
6. Future Perspectives
7. Conclusions
Author Contributions
Acknowledgments
References
- Zhang, J.; Guo, M.; Luo, M.; Cai, T. Advances in the development of amorphous solid dispersions: The role of polymeric carriers. Asian J. Pharm. Sci. 2023, 18, 100834. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, K. Crystallization tendency of pharmaceutical glasses: Relevance to compound properties, impact of formulation process, and implications for design of amorphous solid dispersions. Pharmaceutics 2019, 11, 202. [Google Scholar] [CrossRef] [PubMed]
- Pandi, P.; Bulusu, R.; Kommineni, N.; Khan, W.; Singh, M. Amorphous solid dispersions: An update for preparation, characterization, mechanism on bioavailability, stability, regulatory considerations and marketed products. Int. J. Pharm. 2020, 586, 119560. [Google Scholar] [CrossRef] [PubMed]
- Arun, G.; Shweta, P.; Upendra, K. Formulation and evaluation of ternary solid dispersion of curcumin. Int. J. Pharm. Pharm. Sci. 2012, 4, 360–365. [Google Scholar]
- Vaka, S.R.K.; Bommana, M.M.; Desai, D.; Djordjevic, J.; Phuapradit, W.; Shah, N. Excipients for amorphous solid dispersions. In Amorphous Solid Dispersions: Theory and Practice; Shah, N., Sandhu, H., Choi, D.S., Chokshi, H., Malick, A.W., Eds.; Springer New York: New York, NY, 2014; pp. 123–161. [Google Scholar]
- Zhang, M.; Li, H.; Lang, B.; O’Donnell, K.; Zhang, H.; Wang, Z.; Dong, Y.; Wu, C.; Williams III, R.O. Formulation and delivery of improved amorphous fenofibrate solid dispersions prepared by thin film freezing. Eur. J. Pharm. Biopharm. 2012, 82, 534–544. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Del. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef]
- Kumari, N.; Ghosh, A. Cocrystallization: Cutting edge tool for physicochemical modulation of active pharmaceutical ingredients. Curr. Pharm. Des. 2020, 26, 4858–4882. [Google Scholar] [CrossRef]
- Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D.; Jamroz, W.; Paluch, M.; Rams-Baron, M.; Jachowicz, R.; Boldyreva, E.; Zhou, D. Why amorphous drugs?; Springer: 2018.
- Zhou, Y.; Wang, J.; Xiao, Y.; Wang, T.; Huang, X. The effects of polymorphism on physicochemical properties and pharmacodynamics of solid drugs. Curr. Pharm. Des. 2018, 24, 2375–2382. [Google Scholar] [CrossRef]
- Jones, W.; Eddleston, M.D. Crystal imperfections in molecular crystals: Physical and chemical consequences. Disord. Pharm. Mater. 2016, 83–102. [Google Scholar]
- Grohganz, H.; Löbmann, K.; Priemel, P.; Jensen, K.T.; Graeser, K.; Strachan, C.; Rades, T. Amorphous drugs and dosage forms. J. Drug Deliv. Sci. Technol. 2013, 23, 403–408. [Google Scholar] [CrossRef]
- Graeser, K.A.; Patterson, J.E.; Zeitler, J.A.; Rades, T. The role of configurational entropy in amorphous systems. Pharmaceutics 2010, 2, 224–244. [Google Scholar] [CrossRef]
- Sleutel, M.; Van Driessche, A.E. Role of clusters in nonclassical nucleation and growth of protein crystals. PNAS 2014, 111, E546–E553. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hu, Y.; Liu, L.; Su, L.; Li, N.; Yu, J.; Tang, B.; Yang, Z. Physical stability of amorphous solid dispersions: A physicochemical perspective with thermodynamic, kinetic and environmental aspects. Pharm. Res. 2018, 35, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Zhang, G.G.; Law, D.; Grant, D.J.; Schmitt, E.A. Thermodynamics, molecular mobility and crystallization kinetics of amorphous griseofulvin. Mol. Pharm. 2008, 5, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Wlodarski, K.; Sawicki, W.; Kozyra, A.; Tajber, L. Physical stability of solid dispersions with respect to thermodynamic solubility of tadalafil in PVP-VA. Eur. J. Pharm. Biopharm. 2015, 96, 237–246. [Google Scholar] [CrossRef]
- Xie, T.; Taylor, L.S. Effect of temperature and moisture on the physical stability of binary and ternary amorphous solid dispersions of celecoxib. J. Pharm. Sci. 2017, 106, 100–110. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H.; Wang, H.; Ouyang, L.; Zhu, M. Amorphous alloys for hydrogen storage. J. Alloys Compd. 2023, 941, 168945. [Google Scholar] [CrossRef]
- Kaushik, R.; Budhwar, V.; Kaushik, D. An overview on recent patents and technologies on solid dispersion. Recent Pat. Drug Deliv. Formul. 2020, 14, 63–74. [Google Scholar] [CrossRef]
- Alshehri, S.; Imam, S.S.; Hussain, A.; Altamimi, M.A.; Alruwaili, N.K.; Alotaibi, F.; Alanazi, A.; Shakeel, F. Potential of solid dispersions to enhance solubility, bioavailability, and therapeutic efficacy of poorly water-soluble drugs: Newer formulation techniques, current marketed scenario and patents. Drug Dmelivery 2020, 27, 1625–1643. [Google Scholar] [CrossRef]
- Moseson, D.E.; Tran, T.B.; Karunakaran, B.; Ambardekar, R.; Hiew, T.N. Trends in amorphous solid dispersion drug products approved by the US Food and Drug Administration between 2012 and 2023. Int. J. Pharm. 2024, 7, 100259. [Google Scholar]
- Shah, H.S.; Chaturvedi, K.; Kuang, S.; Wang, J. Accelerating pre-formulation investigations in early drug product life cycles using predictive methodologies and computational algorithms. Ther. Deliv. 2021, 12, 789–797. [Google Scholar] [CrossRef] [PubMed]
- S’ari, M.; Blade, H.; Cosgrove, S.; Drummond-Brydson, R.; Hondow, N.; Hughes, L.P.; Brown, A. Characterization of amorphous solid dispersions and identification of low levels of crystallinity by transmission electron microscopy. Mol. Pharm. 2021, 18, 1905–1919. [Google Scholar] [CrossRef] [PubMed]
- Santitewagun, S.; Thakkar, R.; Zeitler, J.A.; Maniruzzaman, M. Detecting crystallinity using terahertz spectroscopy in 3D printed amorphous solid dispersions. Mol. Pharm. 2022, 19, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Bhujbal, S.V.; Zemlyanov, D.Y.; Cavallaro, A.; Mangal, S.; Taylor, L.S.; Zhou, Q.T. Qualitative and quantitative characterization of composition heterogeneity on the surface of spray dried amorphous solid dispersion particles by an advanced surface analysis platform with high surface sensitivity and superior spatial resolution. Mol. Pharm. 2018, 15, 2045–2053. [Google Scholar] [CrossRef]
- Hancock, B.C.; Chauhan, H.V. Special topic cluster of articles on”Advancement in the formulation, characterization and performance of amorphous solid dispersions (ASDs)”. J. Pharm. Sci. 2021, 110, 1431. [Google Scholar] [CrossRef]
- WY Lee, T.; A Boersen, N.; Hui, H.; Chow, S.; Wan, K.; HL Chow, A. Delivery of poorly soluble compounds by amorphous solid dispersions. Curr. Pharm. Des. 2014, 20, 303–324. [Google Scholar] [CrossRef]
- Mukesh, S.; Mukherjee, G.; Singh, R.; Steenbuck, N.; Demidova, C.; Joshi, P.; Sangamwar, A.T.; Wade, R.C. Comparative analysis of drug-salt-polymer interactions by experiment and molecular simulation improves biopharmaceutical performance. Commun. Chem. 2023, 6, 201. [Google Scholar] [CrossRef]
- Pinket, W.; Aphibanthammakit, C.; Kasemwong, K.; Puttipipatkhachorn, S. Hydroxypropyl methylcellulose phthalate films reinforced with nanocrystalline cassava starch and intended its applications for colonic drug delivery. J. Drug Deliv. Sci. Technol. 2024, 98, 105908. [Google Scholar] [CrossRef]
- Butreddy, A.; Sarabu, S.; Almutairi, M.; Ajjarapu, S.; Kolimi, P.; Bandari, S.; Repka, M.A. Hot-melt extruded hydroxypropyl methylcellulose acetate succinate based amorphous solid dispersions: Impact of polymeric combinations on supersaturation kinetics and dissolution performance. Int. J. Pharm. 2022, 615, 121471. [Google Scholar] [CrossRef]
- Kallakunta, V.R.; Sarabu, S.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Repka, M.A. Stable amorphous solid dispersions of fenofibrate using hot melt extrusion technology: Effect of formulation and process parameters for a low glass transition temperature drug. J. Drug Deliv. Sci. Technol. 2020, 58, 101395. [Google Scholar] [CrossRef]
- Nair, A.R.; Lakshman, Y.D.; Anand, V.S.K.; Sree, K.N.; Bhat, K.; Dengale, S.J. Overview of extensively employed polymeric carriers in solid dispersion technology. AAPS PharmSciTech 2020, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Kurakula, M.; Rao, G.K. Pharmaceutical assessment of polyvinylpyrrolidone (PVP): As excipient from conventional to controlled delivery systems with a spotlight on COVID-19 inhibition. J. Drug Deliv. Sci. Technol. 2020, 60, 102046. [Google Scholar] [CrossRef] [PubMed]
- Nikam, A.; Sahoo, P.R.; Musale, S.; Pagar, R.R.; Paiva-Santos, A.C.; Giram, P.S. A systematic overview of Eudragit® based copolymer for smart healthcare. Pharmaceutics 2023, 15, 587. [Google Scholar] [CrossRef] [PubMed]
- Parikh, T.; Gupta, S.S.; Meena, A.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-III: Polymethacrylates and polymethacrylic acid based polymers. J. Excip. Food Chem. 2016, 5. [Google Scholar]
- Browne, E.; Worku, Z.A.; Healy, A.M. Physicochemical properties of poly-vinyl polymers and their influence on ketoprofen amorphous solid dispersion performance: A polymer selection case study. Pharmaceutics 2020, 12, 433. [Google Scholar] [CrossRef]
- Terao, K. Poly(acrylic acid) (PAA). In Encyclopedia of polymeric nanomaterials; Kobayashi, S., Müllen, K., Eds.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2021; pp. 1–6. [Google Scholar]
- Long, R.; Long, S.; Zou, L.; Huang, Z.; Huang, Y.; Hu, C.; Li, D.; Li, X. Rheology, crystallization, and enhanced mechanical properties of uniaxially oriented ethylene-octene copolymer/polyolefin elastomer blends. Polymer 2022, 243, 124655. [Google Scholar] [CrossRef]
- Braun, S. Encapsulation of cells (cellular delivery) using sol-gel systems. In Comprehensive Biomaterials; Ducheyne, P., Ed.; Elsevier: Oxford, 2011; pp. 529–543. [Google Scholar]
- Sandhu, H.; Shah, N.; Chokshi, H.; Malick, A.W. Overview of amorphous solid dispersion technologies. Amorphous solid dispersions: theory and practice 2014, 91-122.
- Huang, S.; Williams, R.O. Effects of the preparation process on the properties of amorphous solid dispersions. AAPS PharmSciTech 2018, 19, 1971–1984. [Google Scholar] [CrossRef]
- FDA. Impurities: Guideline for residual solvents Q3 (R8) 2021.
- Demmon, S.; Bhargava, S.; Ciolek, D.; Halley, J.; Jaya, N.; Joubert, M.K.; Koepf, E.; Smith, P.; Trexler-Schmidt, M.; Tsai, P. A cross-industry forum on benchmarking critical quality attribute identification and linkage to process characterization studies. Biologicals 2020, 67, 9–20. [Google Scholar] [CrossRef]
- Yu, L.X.; Amidon, G.; Khan, M.A.; Hoag, S.W.; Polli, J.; Raju, G.; Woodcock, J. Understanding pharmaceutical quality by design. AAPS J. 2014, 16, 771–783. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, J.H.; Kim, M.-S.; Jeong, S.H.; Choi, D.H. Process analytical technology tools for monitoring pharmaceutical unit operations: a control strategy for continuous process verification. Pharmaceutics 2021, 13, 919. [Google Scholar] [CrossRef]
- Panzitta, M.; Calamassi, N.; Sabatini, C.; Grassi, M.; Spagnoli, C.; Vizzini, V.; Ricchiuto, E.; Venturini, A.; Brogi, A.; Font, J.B. Spectrophotometry and pharmaceutical PAT/RTRT: Practical challenges and regulatory landscape from development to product lifecycle. Int. J. Pharm. 2021, 601, 120551. [Google Scholar] [CrossRef]
- Araújo, A.S.; Andrade, D.F.; Babos, D.V.; Pricylla, J.; Castro, J.A.G.; Sperança, M.A.; Gamela, R.R.; Machado, R.C.; Costa, V.C.; Guedes, W.N. Key information related to quality by design (QbD) applications in analytical methods development. Braz. J. Anal. Chem 2021, 8, 14–28. [Google Scholar]
- Puchert, T.; Holzhauer, C.-V.; Menezes, J.; Lochmann, D.; Reich, G. A new PAT/QbD approach for the determination of blend homogeneity: Combination of on-line NIRS analysis with PC scores distance analysis (PC-SDA). Eur. J. Pharm. Biopharm. 2011, 78, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Almeida, J.; Bezerra, M.; Markl, D.; Berghaus, A.; Borman, P.; Schlindwein, W. Development and validation of an in-line API quantification method using a QbD principles based on UV-vis spectroscopy to monitor and optimise continuous hot melt extrusion process. Pharmaceutics 2020, 12, 150. [Google Scholar] [CrossRef] [PubMed]
- Iyer, R.; Petrovska Jovanovska, V.; Berginc, K.; Jaklič, M.; Fabiani, F.; Harlacher, C.; Huzjak, T.; Sanchez-Felix, M.V. Amorphous solid dispersions (ASDs): The influence of material properties, manufacturing processes and analytical technologies in drug product development. Pharmaceutics 2021, 13, 1682. [Google Scholar] [CrossRef]
- Fabijański, M.; Gołofit, T. Influence of processing parameters on mechanical properties and degree of crystallization of polylactide. Materials 2024, 17, 3584. [Google Scholar] [CrossRef]
- Maclean, N.; Khadra, I.; Mann, J.; Williams, H.; Abbott, A.; Mead, H.; Markl, D. Investigating the role of excipients on the physical stability of directly compressed tablets. Int. J. Pharm. 2022, 4, 100106. [Google Scholar] [CrossRef]
- Sihorkar, V.; Dürig, T. Chapter 5 - The role of polymers and excipients in developing amorphous solid dispersions: An industrial perspective. In Drug Delivery Aspects, Shegokar, R., Ed.; Elsevier: 2020; pp. 79–113.
- Pendam, D.; Tomake, P.; Debaje, S.; Guleria, K.; Saha, A.; Thakran, P.; Sangamwar, A.T. Advances in formulation strategies and stability considerations of amorphous solid dispersions. J. Drug Deliv. Sci. Technol. 2025, 106922. [Google Scholar] [CrossRef]
- Bhujbal, S.V.; Mitra, B.; Jain, U.; Gong, Y.; Agrawal, A.; Karki, S.; Taylor, L.S.; Kumar, S.; Zhou, Q.T. Pharmaceutical amorphous solid dispersion: A review of manufacturing strategies. Acta Pharm. Sin. B. 2021, 11, 2505–2536. [Google Scholar] [CrossRef]
- Bookwala, M.; Wildfong, P.L. The implications of drug-polymer interactions on the physical stability of amorphous solid dispersions. Pharm. Res. 2023, 40, 2963–2981. [Google Scholar] [CrossRef]
- Qian, K.; Stella, L.; Jones, D.S.; Andrews, G.P.; Du, H.; Tian, Y. Drug-rich phases induced by amorphous solid dispersion: Arbitrary or intentional goal in oral drug delivery? Pharmaceutics 2021, 13, 889. [Google Scholar] [CrossRef]
- Li, N.; Taylor, L.S. Microstructure formation for improved dissolution performance of lopinavir amorphous solid dispersions. Mol. Pharm. 2019, 16, 1751–1765. [Google Scholar] [CrossRef]
- Hiew, T.N.; Zemlyanov, D.Y.; Taylor, L.S. Balancing solid-state stability and dissolution performance of lumefantrine amorphous solid dispersions: The role of polymer choice and drug-polymer interactions. Mol. Pharm. 2021, 19, 392–413. [Google Scholar] [CrossRef]
- Akbari, F.; Didehban, K.; Farhang, M. Solubility of solid intermediate of pharmaceutical compounds in pure organic solvents using semi-empirical models. Eur. J. Pharm. Sci. 2020, 143, 105209. [Google Scholar] [CrossRef]
- Tian, B.; Wang, X.; Zhang, Y.; Zhang, K.; Zhang, Y.; Tang, X. Theoretical prediction of a phase diagram for solid dispersions. Pharm. Res. 2015, 32, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Jacobs, E.; Jones, D.S.; McCoy, C.P.; Wu, H.; Andrews, G.P. The design and development of high drug loading amorphous solid dispersion for hot-melt extrusion platform. Int. J. Pharm. 2020, 586, 119545. [Google Scholar] [CrossRef] [PubMed]
- Sarpal, K.; Delaney, S.; Zhang, G.G.; Munson, E.J. Phase behavior of amorphous solid dispersions of felodipine: Homogeneity and drug-polymer interactions. Mol. Pharm. 2019, 16, 4836–4851. [Google Scholar] [CrossRef]
- Mansuri, A.; Münzner, P.; Heermant, A.; Hänsch, S.; Feuerbach, T.; Fischer, B.r.; Winck, J.; Vermeer, A.W.; Hoheisel, W.; Böhmer, R. Characterizing phase separation of amorphous solid dispersions containing imidacloprid. Mol. Pharm. 2023, 20, 2080–2093. [Google Scholar] [CrossRef]
- Krummnow, A.; Danzer, A.; Voges, K.; Kyeremateng, S.O.; Degenhardt, M.; Sadowski, G. Kinetics of water-induced amorphous phase separation in amorphous solid dispersions via Raman mapping. Pharmaceutics 2023, 15, 1395. [Google Scholar] [CrossRef]
- Pourhakkak, P.; Taghizadeh, A.; Taghizadeh, M.; Ghaedi, M.; Haghdoust, S. Fundamentals of adsorption technology. In Interface Science and Technology, Ghaedi, M., Ed.; Elsevier: 2021; Volume 33, pp. 1–70.
- Rubinstein, M.; Panyukov, S. Elasticity of polymer networks. Macromolecules 2002, 35, 6670–6686. [Google Scholar] [CrossRef]
- Higgins, J.S.; Cabral, J.T. A thorny problem? Spinodal decomposition in polymer blends. 2020, 53, 4137–4140. [Google Scholar]
- Lin, D.; Huang, Y. A thermal analysis method to predict the complete phase diagram of drug-polymer solid dispersions. Int. J. Pharm. 2010, 399, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Qian, K.; Stella, L.; Liu, F.; Jones, D.S.; Andrews, G.P.; Tian, Y. Kinetic and thermodynamic interplay of polymer-mediated liquid-liquid phase separation for poorly water-soluble drugs. Mol. Pharm. 2024, 21, 2878–2893. [Google Scholar] [CrossRef] [PubMed]
- Mathers, A.; Pechar, M.; Hassouna, F.; Fulem, M. API solubility in semi-crystalline polymer: Kinetic and thermodynamic phase behavior of PVA-based solid dispersions. Int. J. Pharm. 2022, 623, 121855. [Google Scholar] [CrossRef] [PubMed]
- Janssens, S.; Van den Mooter, G. Physical chemistry of solid dispersions. J. Pharm. Pharmacol. 2009, 61, 1571–1586. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. What are the important factors that influence API crystallization in miscible amorphous API–excipient mixtures during long-term storage in the glassy state? Mol. Pharm. 2021, 19, 378–391. [Google Scholar] [CrossRef]
- Alhalaweh, A.; Alzghoul, A.; Mahlin, D.; Bergström, C.A. Physical stability of drugs after storage above and below the glass transition temperature: Relationship to glass-forming ability. Int. J. Pharm. 2015, 495, 312–317. [Google Scholar] [CrossRef]
- Yu, D.; Li, J.; Wang, H.; Pan, H.; Li, T.; Bu, T.; Zhou, W.; Zhang, X. Role of polymers in the physical and chemical stability of amorphous solid dispersion: A case study of carbamazepine. Eur. J. Pharm. Sci. 2022, 169, 106086. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. The role of drug-polymer hydrogen bonding interactions on the molecular mobility and physical stability of nifedipine solid dispersions. Mol. Pharm. 2015, 12, 162–170. [Google Scholar] [CrossRef]
- Rusdin, A.; Mohd Gazzali, A.; Ain Thomas, N.; Megantara, S.; Aulifa, D.L.; Budiman, A.; Muchtaridi, M. Advancing drug delivery paradigms: Polyvinyl pyrolidone (PVP)-based amorphous solid dispersion for enhanced physicochemical properties and therapeutic efficacy. Polymers 2024, 16, 286. [Google Scholar] [CrossRef]
- Frank, D.S.; Matzger, A.J. Effect of polymer hydrophobicity on the stability of amorphous solid dispersions and supersaturated solutions of a hydrophobic pharmaceutical. Mol. Pharm. 2019, 16, 682–688. [Google Scholar] [CrossRef]
- Du, X.; Zhao, Z.; Li, Y.X. Production of soluble pea protein/sodium caseinate co-dispersions using ultrasonication and their acid coagulation properties. Food Hydrocoll. 2023, 139, 108562. [Google Scholar] [CrossRef]
- Zhi, Z.; Yan, L.; Li, H.; Dewettinck, K.; Van der Meeren, P.; Liu, R.; Van Bockstaele, F. A combined approach for modifying pea protein isolate to greatly improve its solubility and emulsifying stability. Food Chem. 2022, 380, 131832. [Google Scholar] [CrossRef]
- Gui, Y.; McCann, E.C.; Yao, X.; Li, Y.; Jones, K.J.; Yu, L. Amorphous drug-polymer salt with high stability under tropical conditions and fast dissolution: the case of clofazimine and poly (acrylic acid). Mol. Pharm. 2021, 18, 1364–1372. [Google Scholar] [CrossRef] [PubMed]
- Que, C.; Qi, Q.; Zemlyanov, D.Y.; Mo, H.; Deac, A.; Zeller, M.; Indulkar, A.S.; Gao, Y.; Zhang, G.G.; Taylor, L.S. Evidence for halogen bonding in amorphous solid dispersions. Cryst. Growth Des. 2020, 20, 3224–3235. [Google Scholar] [CrossRef]
- Lu, X.; Li, M.; Huang, C.; Lowinger, M.B.; Xu, W.; Yu, L.; Byrn, S.R.; Templeton, A.C.; Su, Y. Atomic-level drug substance and polymer interaction in posaconazole amorphous solid dispersion from solid-state NMR. Mol. Pharm. 2020, 17, 2585–2598. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Huang, C.; Lowinger, M.B.; Yang, F.; Xu, W.; Brown, C.D.; Hesk, D.; Koynov, A.; Schenck, L.; Su, Y. Molecular interactions in posaconazole amorphous solid dispersions from two-dimensional solid-state NMR spectroscopy. Mol. Pharm. 2019, 16, 2579–2589. [Google Scholar] [CrossRef]
- Orszulak, L.; Lamrani, T.; Bernat, R.; Tarnacka, M.; Żakowiecki, D.; Jurkiewicz, K.; Zioła, P.; Mrozek-Wilczkiewicz, A.; Zięba, A.; Kamiński, K. The influence of PVP polymer topology on the liquid crystalline order of itraconazole in binary systems. Mol. Pharm. 2024, 21, 3027–3039. [Google Scholar] [CrossRef]
- Mehta, M.; Ragoonanan, V.; McKenna, G.B.; Suryanarayanan, R. Correlation between molecular mobility and physical stability in pharmaceutical glasses. Mol. Pharm. 2016, 13, 1267–1277. [Google Scholar] [CrossRef]
- Quan, P.; Wan, X.; Tian, Q.; Liu, C.; Fang, L. Dicarboxylic acid as a linker to improve the content of amorphous drug in drug-in-polymer film: Effects of molecular mobility, electrical conductivity and intermolecular interactions. J. Controlled Release 2020, 317, 142–153. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mizoguchi, R.; Kawakami, K.; Miyazaki, T. Influence of the crystallization tendencies of pharmaceutical glasses on the applicability of the Adam-Gibbs-Vogel and Vogel-Tammann-Fulcher equations in the prediction of their long-term physical stability. Int. J. Pharm. 2022, 626, 122158. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; Buss, H.G.; McCloskey, B.D. The compensation effect in the Vogel–Tammann–Fulcher (VTF) equation for polymer-based electrolytes. Macromolecules 2017, 50, 3831–3840. [Google Scholar] [CrossRef]
- Sahoo, A.; Kumar, N.K.; Suryanarayanan, R. Crosslinking: An avenue to develop stable amorphous solid dispersion with high drug loading and tailored physical stability. J. Controlled Release 2019, 311, 212–224. [Google Scholar] [CrossRef] [PubMed]
- Kashchiev, D. Nucleation: Basic theory with applications. 1st edition. 2000. [Google Scholar]
- Zhang, J.; Jiang, Q.; Xu, Z.; Yang, Q.; Hao, G.; Liu, M.; Zeng, Z. Recent progress on crystal nucleation of amorphous solid dispersion. Cryst. Growth Des. 2024, 24, 8655–8666. [Google Scholar] [CrossRef]
- Schmelzer, J.W. On the determination of the kinetic pre-factor in classical nucleation theory. J. Non-Cryst. Solids 2010, 356, 2901–2907. [Google Scholar] [CrossRef]
- Tournier, R.F. Crystal growth nucleation and Fermi energy equalization of intrinsic spherical nuclei in glass-forming melts. Sci. Technol. Adv. Mater. 2009, 10, 014607. [Google Scholar] [CrossRef]
- Karthika, S.; Radhakrishnan, T.; Kalaichelvi, P. A review of classical and nonclassical nucleation theories. Cryst. Growth Des. 2016, 16, 6663–6681. [Google Scholar] [CrossRef]
- Trasi, N.S.; Taylor, L.S. Effect of polymers on nucleation and crystal growth of amorphous acetaminophen. CrystEngComm 2012, 14, 5188–5197. [Google Scholar] [CrossRef]
- López Burgos, G.; Hernández Espinell, J.R.; Graciani-Massa, T.; Yao, X.; Borchardt-Setter, K.A.; Yu, L.; López-Mejías, V.; Stelzer, T. Role of heteronucleants in melt crystallization of crystalline solid dispersions. Cryst. Growth Des. 2022, 23, 49–58. [Google Scholar] [CrossRef]
- Kolisnyk, T.; Mohylyuk, V.; Fil, N.; Bickerstaff, E.; Li, S.; Jones, D.S.; Andrews, G.P. High drug-loaded amorphous solid dispersions of a poor glass forming drug: The impact of polymer type and cooling rate on amorphous drug behaviour. Int. J. Pharm. 2025, 670, 125095. [Google Scholar] [CrossRef]
- Tian, Y.; Jones, D.S.; Andrews, G.P. An investigation into the role of polymeric carriers on crystal growth within amorphous solid dispersion systems. Mol. Pharm. 2015, 12, 1180–1192. [Google Scholar] [CrossRef]
- Moseson, D.E.; Corum, I.D.; Lust, A.; Altman, K.J.; Hiew, T.N.; Eren, A.; Nagy, Z.K.; Taylor, L.S. Amorphous solid dispersions containing residual crystallinity: competition between dissolution and matrix crystallization. AAPS J. 2021, 23, 69. [Google Scholar] [CrossRef]
- Ojo, A.T.; Ma, C.; Lee, P.I. Elucidating the effect of crystallization on drug release from amorphous solid dispersions in soluble and insoluble carriers. Int. J. Pharm. 2020, 591, 120005. [Google Scholar] [CrossRef]
- Li, W.; Buckton, G. Using DVS-NIR to assess the water sorption behaviour and stability of a griseofulvin/PVP K30 solid dispersion. Int. J. Pharm. 2015, 495, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Almotairy, A.; Almutairi, M.; Althobaiti, A.; Alyahya, M.; Sarabu, S.; Alzahrani, A.; Zhang, F.; Bandari, S.; Repka, M.A. Effect of pH modifiers on the solubility, dissolution rate, and stability of telmisartan solid dispersions produced by hot-melt extrusion technology. J. Drug Deliv. Sci. Technol. 2021, 65, 102674. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nollenberger, K.; Albers, J.; Craig, D.; Qi, S. Microstructure of an immiscible polymer blend and its stabilization effect on amorphous solid dispersions. Mol. Pharm. 2013, 10, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Nollenberger, K.; Albers, J.; Craig, D.; Qi, S. Molecular indicators of surface and bulk instability of hot melt extruded amorphous solid dispersions. Pharm. Res. 2015, 32, 1210–1228. [Google Scholar] [CrossRef]
- Wegiel, L.A.; Mauer, L.J.; Edgar, K.J.; Taylor, L.S. Crystallization of amorphous solid dispersions of resveratrol during preparation and storage-impact of different polymers. J. Pharm. Sci. 2013, 102, 171–184. [Google Scholar] [CrossRef]
- Yang, F.; Su, Y.; Small, J.; Huang, C.; Martin, G.E.; Farrington, A.M.; DiNunzio, J.; Brown, C.D. Probing the molecular-level interactions in an active pharmaceutical ingredient (API)-polymer dispersion and the resulting impact on drug product formulation. Pharm. Res. 2020, 37, 1–16. [Google Scholar] [CrossRef]
- Telang, C.; Mujumdar, S.; Mathew, M. Improved physical stability of amorphous state through acid base interactions. J. Pharm. Sci. 2009, 98, 2149–2159. [Google Scholar] [CrossRef]
- Mazurek, A.H.; Szeleszczuk, Ł.; Pisklak, D.M. Periodic DFT calculations-review of applications in the pharmaceutical sciences. Pharmaceutics 2020, 12, 415. [Google Scholar] [CrossRef]
- Van Der Kamp, M.W.; Mulholland, A.J. Combined quantum mechanics/molecular mechanics (QM/MM) methods in computational enzymology. Biochemistry 2013, 52, 2708–2728. [Google Scholar] [CrossRef]
- Meng, F.; Trivino, A.; Prasad, D.; Chauhan, H. Investigation and correlation of drug polymer miscibility and molecular interactions by various approaches for the preparation of amorphous solid dispersions. Eur. J. Pharm. Sci. 2015, 71, 12–24. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Morgan, D.J.; Mendham, A.P.; Pang, J.; Snowden, M.J.; Douroumis, D. Drug-polymer intermolecular interactions in hot-melt extruded solid dispersions. Int. J. Pharm. 2013, 443, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Pang, J.; Morgan, D.J.; Douroumis, D. Molecular modeling as a predictive tool for the development of solid dispersions. Mol. Pharm. 2015, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Maniruzzaman, M.; Snowden, M.J.; Bradely, M.S.; Douroumis, D. Studies of intermolecular interactions in solid dispersions using advanced surface chemical analysis. RSC Adv. 2015, 5, 74212–74219. [Google Scholar] [CrossRef]
- Nie, H.; Mo, H.; Zhang, M.; Song, Y.; Fang, K.; Taylor, L.S.; Li, T.; Byrn, S.R. Investigating the interaction pattern and structural elements of a drug-polymer complex at the molecular level. Mol. Pharm. 2015, 12, 2459–2468. [Google Scholar] [CrossRef]
- Wang, B.; Wang, D.; Zhao, S.; Huang, X.; Zhang, J.; Lv, Y.; Liu, X.; Lv, G.; Ma, X. Evaluate the ability of PVP to inhibit crystallization of amorphous solid dispersions by density functional theory and experimental verify. Eur. J. Pharm. Sci. 2017, 96, 45–52. [Google Scholar] [CrossRef]
- Antolović, I.; Vrabec, J.; Klajmon, M. COSMOPharm: Drug–Polymer Compatibility of Pharmaceutical Amorphous Solid Dispersions from COSMO-SAC. Mol. Pharm. 2024, 21, 4395–4415. [Google Scholar] [CrossRef]
- Ma, S.-M.; Zhao, L.; Wang, Y.-L.; Zhu, Y.-L.; Lu, Z.-Y. The coarse-grained models of poly (ethylene oxide) and poly (propylene oxide) homopolymers and poloxamers in big multipole water (BMW) and MARTINI frameworks. PCCP 2020, 22, 15976–15985. [Google Scholar] [CrossRef] [PubMed]
- Mendonsa, N.; Almutairy, B.; Kallakunta, V.R.; Sarabu, S.; Thipsay, P.; Bandari, S.; Repka, M.A. Manufacturing strategies to develop amorphous solid dispersions: An overview. J. Drug Deliv. Sci. Technol. 2020, 55, 101459. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Huang, D.; Zhang, T.; Luo, T. Determining influential descriptors for polymer chain conformation based on empirical force-fields and molecular dynamics simulations. Chem. Phys. Lett. 2018, 704, 49–54. [Google Scholar] [CrossRef]
- Mao, Q.; Feng, M.; Jiang, X.Z.; Ren, Y.; Luo, K.H.; van Duin, A.C. Classical and reactive molecular dynamics: Principles and applications in combustion and energy systems. Prog. Energy Combust. Sci. 2023, 97, 101084. [Google Scholar] [CrossRef]
- He, X.; Man, V.H.; Yang, W.; Lee, T.-S.; Wang, J. A fast and high-quality charge model for the next generation general AMBER force field. J. Chem. Phys. 2020, 153. [Google Scholar] [CrossRef]
- Shelke, R.; Velagacherla, V.; Nayak, U.Y. Recent advances in dual-drug co-amorphous systems. Drug Discov. Today 2024, 29, 103863. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Suryanarayanan, R. Local mobility in amorphous pharmaceuticals-characterization and implications on stability. J. Pharm. Sci. 2009, 98, 2935–2953. [Google Scholar] [CrossRef]
- Kothari, K.; Ragoonanan, V.; Suryanarayanan, R. Influence of molecular mobility on the physical stability of amorphous pharmaceuticals in the supercooled and glassy states. Mol. Pharm. 2014, 11, 3048–3055. [Google Scholar] [CrossRef]
- Bookwala, M.; DeBoyace, K.; Buckner, I.S.; Wildfong, P.L. Predicting density of amorphous solid materials using molecular dynamics simulation. AAPS PharmSciTech 2020, 21, 1–11. [Google Scholar] [CrossRef]
- Xiang, T.-X.; Anderson, B.D. Molecular dynamics simulation of amorphous indomethacin. Mol. Pharm. 2013, 10, 102–114. [Google Scholar] [CrossRef]
- Kasimova, A.O.; Pavan, G.M.; Danani, A.; Mondon, K.; Cristiani, A.; Scapozza, L.; Gurny, R.; Möller, M. Validation of a novel molecular dynamics simulation approach for lipophilic drug incorporation into polymer micelles. J. Phys. Chem. B. 2012, 116, 4338–4345. [Google Scholar] [CrossRef]
- Chen, S.; Li, J.; Wei, L.; Jin, Y.; Khosla, T.; Xiao, J.; Cheng, B.; Duan, H. A molecular modeling study for miscibility of polyimide/polythene mixing systems with/without compatibilizer. J. Polym. Eng. 2018, 38, 891–898. [Google Scholar] [CrossRef]
- Andrews, J.; Handler, R.A.; Blaisten-Barojas, E. Structure, energetics and thermodynamics of PLGA condensed phases from Molecular Dynamics. Polymer 2020, 206, 122903. [Google Scholar] [CrossRef]
- Muljajew, I.; Erlebach, A.; Weber, C.; Buchheim, J.R.; Sierka, M.; Schubert, U.S. A polyesteramide library from dicarboxylic acids and 2, 2′-bis (2-oxazoline): Synthesis, characterization, nanoparticle formulation and molecular dynamics simulations. Polym. Chem. 2020, 11, 112–124. [Google Scholar] [CrossRef]
- Xiang, T.-X.; Anderson, B.D. Molecular dynamics simulation of amorphous hydroxypropyl-methylcellulose acetate succinate (HPMCAS): Polymer model development, water distribution, and plasticization. Mol. Pharm. 2014, 11, 2400–2411. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Goliaei, A.; Tsereteli, L.; Berkowitz, M.L. Properties of poloxamer molecules and poloxamer micelles dissolved in water and next to lipid bilayers: results from computer simulations. J. Phys. Chem. B. 2016, 120, 5823–5830. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Nunes, C.; Jonnalagadda, S. A molecular dynamics approach for predicting the glass transition temperature and plasticization effect in amorphous pharmaceuticals. Mol. Pharm. 2013, 10, 4136–4145. [Google Scholar] [CrossRef]
- Gupta, J.; Nunes, C.; Vyas, S.; Jonnalagadda, S. Prediction of solubility parameters and miscibility of pharmaceutical compounds by molecular dynamics simulations. J. Phys. Chem. B. 2011, 115, 2014–2023. [Google Scholar] [CrossRef]
- Shahzad, Y.; Sohail, S.; Arshad, M.S.; Hussain, T.; Shah, S.N.H. Development of solid dispersions of artemisinin for transdermal delivery. Int. J. Pharm. 2013, 457, 197–205. [Google Scholar] [CrossRef]
- Razmimanesh, F.; Amjad-Iranagh, S.; Modarress, H. Molecular dynamics simulation study of chitosan and gemcitabine as a drug delivery system. J. Mol. Model. 2015, 21, 1–14. [Google Scholar] [CrossRef]
- Gong, C.; Deng, S.; Wu, Q.; Xiang, M.; Wei, X.; Li, L.; Gao, X.; Wang, B.; Sun, L.; Chen, Y. Improving antiangiogenesis and anti-tumor activity of curcumin by biodegradable polymeric micelles. Biomaterials 2013, 34, 1413–1432. [Google Scholar] [CrossRef]
- Fule, R.; Amin, P. Development and evaluation of lafutidine solid dispersion via hot melt extrusion: Investigating drug-polymer miscibility with advanced characterisation. Asian J. Pharm. Sci. 2014, 9, 92–106. [Google Scholar] [CrossRef]
- Fule, R.; Amin, P. Hot melt extruded amorphous solid dispersion of posaconazole with improved bioavailability: investigating drug-polymer miscibility with advanced characterisation. Biomed. Res. Int. 2014, 2014, 146781. [Google Scholar] [CrossRef] [PubMed]
- Aulifa, D.L.; Al Shofwan, A.A.; Megantara, S.; Fakih, T.M.; Budiman, A. Elucidation of molecular interactions between drug-polymer in amorphous solid dispersion by a computational approach using molecular dynamics simulations. Adv. Appl. Bioinform. Chem. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Fule, R.; Meer, T.; Sav, A.; Amin, P. Solubility and dissolution rate enhancement of lumefantrine using hot melt extrusion technology with physicochemical characterisation. J. Pharm. Investig. 2013, 43, 305–321. [Google Scholar] [CrossRef]
- Gangurde, A.B.; Kundaikar, H.S.; Javeer, S.D.; Jaiswar, D.R.; Degani, M.S.; Amin, P.D. Enhanced solubility and dissolution of curcumin by a hydrophilic polymer solid dispersion and its insilico molecular modeling studies. J. Drug Deliv. Sci. Technol. 2015, 29, 226–237. [Google Scholar] [CrossRef]
- Yani, Y.; Kanaujia, P.; Chow, P.S.; Tan, R.B. Effect of API-polymer miscibility and interaction on the stabilization of amorphous solid dispersion: A molecular simulation study. Ind. Eng. Chem. Res. 2017, 56, 12698–12707. [Google Scholar] [CrossRef]
- Macháčková, M.; Tokarský, J.; Čapková, P. A simple molecular modeling method for the characterization of polymeric drug carriers. Eur. J. Pharm. Sci. 2013, 48, 316–322. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Karagianni, A.; Katopodis, K.; Vardaka, E.; Kachrimanis, K. Molecular modelling and simulation of fusion-based amorphous drug dispersions in polymer/plasticizer blends. Eur. J. Pharm. Sci. 2019, 130, 260–268. [Google Scholar] [CrossRef]
- Gupta, K.M.; Chin, X.; Kanaujia, P. Molecular interactions between APIs and enteric polymeric excipients in solid dispersion: insights from molecular simulations and experiments. Pharmaceutics 2023, 15, 1164. [Google Scholar] [CrossRef]
- Xiang, T.-X.; Anderson, B.D. Molecular dynamics simulation of amorphous indomethacin-poly (vinylpyrrolidone) glasses: Solubility and hydrogen bonding interactions. J. Pharm. Sci. 2013, 102, 876–891. [Google Scholar] [CrossRef]
- Xiang, T.-X.; Anderson, B.D. Molecular dynamics simulation of amorphous hydroxypropylmethylcellulose and its mixtures with felodipine and water. J. Pharm. Sci. 2017, 106, 803–816. [Google Scholar] [CrossRef]
- Medarević, D.P.; Kachrimanis, K.; Mitrić, M.; Djuriš, J.; Djurić, Z.; Ibrić, S. Dissolution rate enhancement and physicochemical characterization of carbamazepine-poloxamer solid dispersions. Pharm. Dev. Technol. 2016, 21, 268–276. [Google Scholar] [CrossRef]
- Mosquera-Giraldo, L.I.; Borca, C.H.; Meng, X.; Edgar, K.J.; Slipchenko, L.V.; Taylor, L.S. Mechanistic design of chemically diverse polymers with applications in oral drug delivery. Biomacromolecules 2016, 17, 3659–3671. [Google Scholar] [CrossRef]
- Erlebach, A.; Ott, T.; Otzen, C.; Schubert, S.; Czaplewska, J.; Schubert, U.S.; Sierka, M. Thermodynamic compatibility of actives encapsulated into PEG-PLA nanoparticles: In silico predictions and experimental verification. J. Comput. Chem. 2016, 37, 2220–2227. [Google Scholar] [CrossRef] [PubMed]
- Turpin, E.R.; Taresco, V.; Al-Hachami, W.A.; Booth, J.; Treacher, K.; Tomasi, S.; Alexander, C.; Burley, J.; Laughton, C.A.; Garnett, M.C. In silico screening for solid dispersions: The trouble with solubility parameters and χFH. Mol. Pharm. 2018, 15, 4654–4667. [Google Scholar] [CrossRef] [PubMed]
- Iesavand, H.; Rahmati, M.; Afzali, D.; Modiri, S. Investigation on absorption and release of mercaptopurine anticancer drug from modified polylactic acid as polymer carrier by molecular dynamic simulation. Mater. Sci. Eng. C Biomimetic Supramol. Syst. 2019, 105, 110010. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, P.; Gokarna, V.; Deshpande, V.; Vavia, P. Bioavailability enhancement of olmesartan medoxomil using hot-melt extrusion: In-silico, in-vitro, and in-vivo evaluation. AAPS PharmSciTech 2020, 21, 254. [Google Scholar] [CrossRef]
- Eslami, M.; Nikkhah, S.J.; Hashemianzadeh, S.M.; Sajadi, S.A.S. The compatibility of tacrine molecule with poly (n-butylcyanoacrylate) and chitosan as efficient carriers for drug delivery: A molecular dynamics study. Eur. J. Pharm. Sci. 2016, 82, 79–85. [Google Scholar] [CrossRef]
- Kapourani, A.; Eleftheriadou, K.; Kontogiannopoulos, K.N.; Barmpalexis, P. Evaluation of rivaroxaban amorphous solid dispersions physical stability via molecular mobility studies and molecular simulations. Eur. J. Pharm. Sci. 2021, 157, 105642. [Google Scholar] [CrossRef]
- Hussan, K.S.; Govindaraj, G.; Correia, N.T.; Shinyashiki, N.; Thayyil, M.S.; Babu, T.D. Molecular dynamics and interactions in amorphous solid dispersion of Erlotinib HCl for improved cancer therapy. J. Mol. Struct. 2025, 1336, 142014. [Google Scholar] [CrossRef]
- Mendyk, A.; Szlȩk, J.; Jachowicz, R. A heuristic decision support system for microemulsions formulation development. In Formulation tools for pharmaceutical development; Elsevier: 2013; pp. 39–71.
- Zhang, Z.-h.; Pan, W.-s. Expert system for the development and formulation of push-pull osmotic pump tablets containing poorly water-soluble drugs. In Formulation tools for pharmaceutical development; Elsevier: 2013; pp. 73–108.
- Das, M.K.; Chakraborty, T. ANN in pharmaceutical product and process development. In Artificial neural network for drug design, delivery and disposition; Elsevier: 2016; pp. 277–293.
- Han, R.; Yang, Y.; Li, X.; Ouyang, D. Predicting oral disintegrating tablet formulations by neural network techniques. Asian J. Pharm. Sci. 2018, 13, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Nurzyńska, K.; Booth, J.; Roberts, C.J.; McCabe, J.; Dryden, I.; Fischer, P.M. Long-term amorphous drug stability predictions using easily calculated, predicted, and measured parameters. Mol. Pharm. 2015, 12, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Przybyłek, M.; Jeliński, T.; Cysewski, P. Application of multivariate adaptive regression splines (MARSplines) for predicting hansen solubility parameters based on 1D and 2D molecular descriptors computed from SMILES string. J. Chem. 2019, 2019, 9858371. [Google Scholar] [CrossRef]
- Moore, M.D.; Wildfong, P.L. Informatics calibration of a molecular descriptors database to predict solid dispersion potential of small molecule organic solids. Int. J. Pharm. 2011, 418, 217–226. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Kachrimanis, K.; Georgarakis, E. Solid dispersions in the development of a nimodipine floating tablet formulation and optimization by artificial neural networks and genetic programming. Eur. J. Pharm. Biopharm. 2011, 77, 122–131. [Google Scholar] [CrossRef]
- Barmpalexis, P.; Koutsidis, I.; Karavas, E.; Louka, D.; Papadimitriou, S.A.; Bikiaris, D.N. Development of PVP/PEG mixtures as appropriate carriers for the preparation of drug solid dispersions by melt mixing technique and optimization of dissolution using artificial neural networks. Eur. J. Pharm. Biopharm. 2013, 85, 1219–1231. [Google Scholar] [CrossRef]
- Han, R.; Xiong, H.; Ye, Z.; Yang, Y.; Huang, T.; Jing, Q.; Lu, J.; Pan, H.; Ren, F.; Ouyang, D. Predicting physical stability of solid dispersions by machine learning techniques. J. Controlled Release 2019, 311, 16–25. [Google Scholar] [CrossRef]
- Vojinovic, T.; Potpara, Z.; Vukmirovic, M.; Turkovic, N.; Ibric, S. Artificial neural networks and their application in the optimization of carbamazepine solid dispersions. Acta Pol. Pharm. 2022, 79. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, A.; Ma, X.; Ouyang, D.; Williams III, R.O. The applications of machine learning to predict the forming of chemically stable amorphous solid dispersions prepared by hot-melt extrusion. Int. J. Pharm. 2023, 5, 100164. [Google Scholar] [CrossRef]
- Di Mare, E.J.; Punia, A.; Lamm, M.S.; Rhodes, T.A.; Gormley, A.J. Data-driven design of novel polymer excipients for pharmaceutical amorphous solid dispersions. Bioconj. Chem. 2024, 35, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Hartmanshenn, C.; Scherholz, M.; Androulakis, I.P. Physiologically-based pharmacokinetic models: approaches for enabling personalized medicine. J. Pharmacokinet. Pharmacodyn. 2016, 43, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Khalil, F.; Läer, S. Physiologically based pharmacokinetic modeling: Methodology, applications, and limitations with a focus on its role in pediatric drug development. J. Biomed. Biotechnol. 2011, 2011, 907461. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Thomas, S.; Tsang, Y.C.; Almeida, S.; Ashraf, M.; Fotaki, N.; Heimbach, T.; Patel, N.; Shah, H.; Jiang, X. Advances in physiologically based pharmacokinetic (PBPK) modeling and its regulatory utility to support oral drug product development and harmonization. Pharm. Res. 2025, 1–15. [Google Scholar] [CrossRef]
- Miller, N.A.; Reddy, M.B.; Heikkinen, A.T.; Lukacova, V.; Parrott, N. Physiologically based pharmacokinetic modelling for first-in-human predictions: an updated model building strategy illustrated with challenging industry case studies. Clin. Pharmacokinet. 2019, 58, 727–746. [Google Scholar] [CrossRef]
- Bharti, K.; Deepika, D.; Kumar, M.; Jha, A.; Manjit; Akhilesh; Tiwari, V.; Kumar, V.; Mishra, B. Bharti, K.; Deepika, D.; Kumar, M.; Jha, A.; Manjit; Akhilesh; Tiwari, V.; Kumar, V.; Mishra, B. Development and evaluation of amorphous solid dispersion of riluzole with pbpk model to simulate the pharmacokinetic profile. AAPS PharmSciTech 2023, 24, 219. [Google Scholar] [CrossRef]
- Pakravesh, A.; Mohammadi, A.H.; Richon, D. Performance evaluation of PρT-SAFT, PρT-PC-SAFT, PC-SAFT, and CPA equations of state for predicting density, thermal expansion coefficient, isothermal compressibility, isobaric heat capacity, speed of sound, and saturated vapor pressure of three pure ethylene glycols and their mixtures. Int. J. Thermophys. 2025, 46, 30. [Google Scholar]
- Ascani, M.; Sadowski, G.; Held, C. Simultaneous predictions of chemical and phase equilibria in systems with an esterification reaction using PC-SAFT. Molecules 2023, 28, 1768. [Google Scholar] [CrossRef]
- Dong, J.; Gao, H.; Ouyang, D. PharmSD: A novel AI-based computational platform for solid dispersion formulation design. Int. J. Pharm. 2021, 604, 120705. [Google Scholar] [CrossRef]
- Saha, G. Artificial Intelligence (AI) in formulation development. In Computer Aided Drug Development; 2024; pp. 459–481.
- Agrawal, G.; Tushir, S.; Arora, D.; Sangwan, K. Artificial intelligence in pharmaceutical drug delivery. In Proceedings of the 2024 International conference on computational Intelligence and computing applications (ICCICA); 2024; pp. 406–410. [Google Scholar]
- Xiao, L.; Zhang, Y. AI-driven smart pharmacology. Int. Pharm. 2023, 1, 179–182. [Google Scholar] [CrossRef]
- Au-Yeung, L.; Tseng, C.-Y.; Tam, Y.K.; Tsai, P.A. Machine learning based quantitative structure-dissolution profile relationship. J. Chem. Inf. Model. 2025, 65, 6273–6286. [Google Scholar] [CrossRef]
- Modhave, D.; Vrielynck, S.; Roeleveld, K. Assessing drug product shelf life using the accelerated stability asessment pogram: A case study of a GLPG4399 capsule formulation. Pharmaceutics 2024, 16, 1400. [Google Scholar] [CrossRef]
- Flavier, K.; McLellan, J.; Botoy, T.; Waterman, K.C. Accelerated shelf life modeling of appearance change in drug products using ASAP prime®. Pharm. Dev. Technol. 2022, 27, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Lennard, A.; Zimmermann, B.; Clenet, D.; Molony, M.; Tami, C.; Aviles, C.O.; Moran, A.; Pue-Gilchrist, P.; Flores, E.L. Stability modeling methodologies to enable earlier patient access. J. Pharm. Sci. 2024. [Google Scholar] [CrossRef] [PubMed]
- Waterman, K.C. The application of the accelerated stability assessment program (ASAP) to quality by design (QbD) for drug product stability. AAPS PharmSciTech 2011, 12, 932–937. [Google Scholar] [CrossRef] [PubMed]
- Rack, C. An introduction to the accelerated stability assessment program. Am. Pharm. Rev. 2017, 20, 86–90. [Google Scholar]
- Sarabu, S.; Kallakunta, V.R.; Bandari, S.; Batra, A.; Bi, V.; Durig, T.; Zhang, F.; Repka, M.A. Hypromellose acetate succinate based amorphous solid dispersions via hot melt extrusion: Effect of drug physicochemical properties. Carbohydr. Polym. 2020, 233, 115828. [Google Scholar] [CrossRef]
- Roxin, P.; Karlsson, A.; Singh, S.K. Characterization of cellulose acetate phthalate (CAP). Drug Dev. Ind. Pharm. 1998, 24, 1025–1041. [Google Scholar] [CrossRef]
- Gupta, S.S.; Meena, A.; Parikh, T.; Serajuddin, A.T. Investigation of thermal and viscoelastic properties of polymers relevant to hot melt extrusion-I: Polyvinylpyrrolidone and related polymers. J. Excip. Food Chem. 2016, 5. [Google Scholar]
- Gong, C.; Xie, Y.; Wu, Q.; Wang, Y.; Deng, S.; Xiong, D.; Liu, L.; Xiang, M.; Qian, Z.; Wei, Y. Improving anti-tumor activity with polymeric micelles entrapping paclitaxel in pulmonary carcinoma. Nanoscale 2012, 4, 6004–6017. [Google Scholar] [CrossRef]
- Kapourani, A.; Chatzitheodoridou, M.; Kontogiannopoulos, K.N.; Barmpalexis, P. Experimental, thermodynamic, and molecular modeling evaluation of amorphous simvastatin-poly (vinylpyrrolidone) solid dispersions. Mol. Pharm. 2020, 17, 2703–2720. [Google Scholar] [CrossRef]

| Trade name | Drug(s) | Polymer(s) | Manufacturing method | Dosage form | Company | Year of approval |
|---|---|---|---|---|---|---|
| Cesamet® | Nabilone | PVP | Solvent evaporation | Tablet | Valeant | 1985 |
| Isoptin® | Verapamil | HPC/HPMC | Hot melt extrusion | Tablet | Abbott | 1987 |
| Rezulin® | Troglitazone | HPMC | Hot melt extrusion | Tablet | Pfizer | 1997 |
| Sporanox® | Itraconazole | HPMC | Fluidized bed layering | Capsule | Janssen | 1992 |
| Prograf® | Tacrolimus | HPMC | Solvent evaporation | Capsule | Astella | 1994 |
| NuvaRing® | Etonogestrel and ethyl estradiol | EVA | Hot melt extrusion | Ring | Merck | 2001 |
| Crestor® | Rosuvastatin | HPMC | Spray drying | Tablet | AstraZeneca | 2002 |
| Cymbalta® | Duloxetine | HPMCAS | Not disclosed | Capsule | Eli Lilly | 2004 |
| Kaletra® | Ritonavir/lopinavir | PVP–VA64 | Hot melt extrusion | Tablet | Abbott | 2007 |
| Intelence® | Etravirine | HPMC | Spray drying | Tablet | Janssen | 2008 |
| Samsca® | Tolvaptan | HPC | Spray drying | Tablet | Otsuka | 2009 |
| Zortress® | Everolimus | HPMC | Spray drying | Tablet | Novartis | 2010 |
| Norvir® | Ritonavir | PVP–VA64 | Hot melt extrusion | Tablet | Abbott | 2010 |
| Onmel® | Itraconazole | HPMC | Hot melt extrusion | Tablet | Merz | 2010 |
| Zelboraf® | Vemurafenib | HPMCAS | Solvent/antisolvent precipitation | Tablet | Roche | 2011 |
| Incivek® | Telaprevir | HPMCAS | Spray drying | Tablet | Vertex | 2011 |
| Kalydeco® | Ivacaftor | HPMCAS | Spray drying | Tablet | Vertex | 2012 |
| Noxafil® | Posaconazole | HPMCAS | Hot melt extrusion | Tablet | Merck | 2013 |
| Astagraf XL® | Tacrolimus | HPMC; EC | Wet granulation | Capsule | Astella | 2013 |
| Belsomra® | Suvorexant | PVP–VA64 | Hot melt extrusion | Tablet | Merck | 2014 |
| Harvoni® | Ledipasvir/sofosbuvir | PVP–VA64 | Spray drying | Tablet | Gilead | 2014 |
| Viekira XR™ | Dasabuvir/ombitasvir/paritaprevir/ritonavir | PVP–VA64; HPMC | Hot melt extrusion | Tablet | AbbVie | 2014 |
| Epclusa® | Sofosbuvir/velpatasvir | PVP–VA64 | Spray drying | Tablet | Gilead | 2016 |
| Orkambi® | Lumacaftor/ivacaftor | HPMCAS | Spray drying | Tablet and granule | Vertex | 2016 |
| Venclexta® | Venetoclax | PVP–VA64 | Hot melt extrusion | Tablet | AbbVie | 2016 |
| Zepatier™ | Elbasvir/grazoprevir | PVP–VA64 | Spray drying | Tablet | Merck | 2016 |
| Mavyret™ | Glecaprevir/pibrentasvir | PVP–VA64 | Hot melt extrusion | Tablet | AbbVie | 2017 |
| Vosevi™ | Sofosbuvir/velpatasvir/voxilaprevir | PVP–VA64 | Spray drying | Tablet | Gilead | 2017 |
| Idhifa® | Enasidenib | HPMCAS | Hot melt extrusion | Tablet | Bristol | 2017 |
| Lynparza® | Olaparib | PVP–VA | Hot melt extrusion | Tablet and capsule | AstraZeneca | 2017 |
| Jynarque® | Tolvaptan | HPC | Spray drying | Tablet | Otsuka | 2018 |
| Tibsovo® | Ivosidenib | HPMCAS | Spray drying | Tablet | Servier | 2018 |
| Pifeltro® | Doravirine | HPMCAS | Spray drying | Tablet | Merck | 2018 |
| Delstrigo® | Doravirine/lamivudine/tenofovir disoproxil fumarate | HPMCAS | Spray drying | Tablet | Merck | 2018 |
| Tolsura® | Itraconazole | HPMCP | Spray drying | Capsule | Mayne | 2018 |
| Erleada® | Apalutamide | HPMCAS | Spray drying | Tablet | Janssen | 2018 |
| Orilissa® | Elagolix | HPMCAS | Wet granulation | Tablet | AbbVie | 2018 |
| Symdeko® | Tezacaftor/ivacaftor and ivacaftor | HPMCAS | Spray drying | Tablet | Vertex | 2018 |
| Braftovi® | Encorafenib | PVP–VA64 | Hot melt extrusion | Capsule | Array | 2018 |
| Trikafta® | Elexacaftor/ivacaftor/tezacaftor | HPMCAS | Spray drying | Tablet | Vertex | 2019 |
| Ubrelvy® | Ubrogepant | PVP–VA64 | Hot melt extrusion | Tablet | AbbVie | 2019 |
| Oriahnn® | Elagolix/estradiol/norethindrone acetate | PVP–VA | Hot melt extrusion | Tablet | AbbVie | 2020 |
| Tukysa® | Tucatinib | PVP–VA | Hot melt extrusion | Tablet | Seagen | 2020 |
| Xtandi® | Enzalutamide | HPMCAS | Hot melt extrusion | Tablet | Astella | 2020 |
| Qinlock® | Ripretinib | PVP–VA | Spray drying | Tablet | Deciphera | 2020 |
| Qulipta® | Atogepant | PVP–VA64 | Hot melt extrusion | Tablet | AbbVie | 2021 |
| Welireg® | Belzutifan | HPMCAS | Hot melt extrusion | Tablet | Merck | 2021 |
| Sotyktu® | Deucravacitinib | HPMCAS | Spray drying | Tablet | Bristol | 2022 |
| Sunlenca® | Lenacapavir | PVP–VA | Spray drying | Tablet | Gilead | 2022 |
| Jaypirca® | Pirtobrutinib | HPMCAS | Spray drying | Tablet | Loxo Oncology | 2023 |
| Phyrago® | Dasatinib | Methacrylic acid–ethyl acrylate copolymer | Electro spraying | Tablet | Nanocopoeia | 2023 |
| Paxlovid® | Nirmatrelvir/ritonavir | PVP–VA | Hot melt extrusion | Tablet | Pfizer | 2023 |
| Alvaiz® | Eltrombopag | PVP–VA | Hot melt extrusion | Tablet | Teva | 2023 |
| Category | Polymer type | Polymer subtype | Mol. wt. (g/mol) |
Tg/Tm (°C) |
Degradation temp. (°C) | Moisture retention | Solubility | Key features | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Cellulose derivative | HPMCAS | HPMCAS LG | 144,700 | 119 | 204 | Low | pH 5.5–6.0 | Anionic | [31,32,190] |
| HPMCAS MG | 103,200 | 120 | 190 | Low | pH 6.0–6.5 | Anionic | [31,32,190] | ||
| HPMCAS HG | 75,100 | 122 | 200 | Low | Above pH 6.8 | Anionic | [31,32,190] | ||
| HPMCP | HPMCP 50 | 37,900 | 137 | 160–190 | Low | Below pH 5 | Amphiphilic | [30] | |
| HPMCP 55 | 45,600 | 133 | 150 | Low | below pH 5.5 | Amphiphilic | [30] | ||
| HPMC | HPMC E | 40,000–150,000 | 141 | NA | High | Water | Nonionic | [33] | |
| HPMC F | 40,000–150,000 | 160 | 240 | High | Water | Nonionic | [33] | ||
| HPMC K | 40,000–150,000 | 172 | 260 | High | Water | Nonionic | [33] | ||
| CAP | 2534.12 | 175 | 200 | Low | Below pH 6 | Nonionic | [191] | ||
| Polyvinyl derivatives | PVP | PVP K12 | 2000–3000 | 72 | 196 | High | Water | Amphiphilic | [34,192] |
| PVP K17 | 7000–11,000 | 140 | 217 | High | Water | Amphiphilic | [34,192] | ||
| PVP K25 | 28,000–34,000 | 153 | 166 | High | Water | Amphiphilic | [34,192] | ||
| PVP K30 | 44,000–54,000 | 160 | 171 | High | Water | Amphiphilic | [34,192] | ||
| PVP K90 | 1,000,000–1,500,000 | 177 | 194 | High | Water | Amphiphilic | [34,192] | ||
| PVP/VA | 45,000–70,000 | 115 | 270 | High | Water | Amphiphilic | [34,192] | ||
| Soluplus® | 90,000–140,000 | 72 | 278 | Moderate | Water | Amphiphilic | [34,192] | ||
| Polymethacrylate derivatives | Eudragit® EPO | 47,000 | 48 | 250 | Low | Below pH 5 | Cationic | [35,36] | |
| Eudragit® L100 | 125,000 | 150 | 176 | Low | Above pH 6 | Anionic | [35,36] | ||
| Eudragit® S100 | 125,000 | >150 | 173 | Low | Above pH 7 | Anionic | [35,36] | ||
| Eudragit® L100–55 | 250,000 | 110 | 176 | Low | Above pH 5.5 | Anionic | [35,36] | ||
| Miscellaneous | PVAP | 47,000–61,000 | 46/116 | 150 | Low | Below pH 6 | Nonionic | [37] | |
| PAA | 1800–450,000 | 126 | 200 | Low | Water | Nonionic | [38] | ||
| PEG/POE | 1000–7,000,000 | 55–66 | >200 | Low | Water | Nonionic | [39] | ||
| Lutrol® | 7600–17,400 | 52–57 | >200 | Low | Water | Nonionic | [40] | ||
| Solvent | Boiling point | Solubility in water (g/mL) | Density at 25 °C (g/mL) | Viscosity (at 25 °C, cP) | Dielectric constant | ICH class (limit, ppm) |
|---|---|---|---|---|---|---|
| Acetone | 56.2 | Miscible | 1.049 | 0.295 | 20.7 | Class 3 |
| Butanone | 79.6 | 29 | 0.805 | 0.4 | 18.51 | Class 3 |
| Butyl acetate | 126.1 | 0.68 | 0.882 | 0.685 | 5.07 | Class 3 |
| Chloroform | 61.7 | 0.795 | 1.498 | 0.536 | 4.81 | Class 2 (60) |
| Dichloromethane | 39.6 | 1.32 | 1.326 | 0.413 | 9.08 | Class 2 (600) |
| Dimethyl acetamide | 165 | Miscible | 0.937 | 0.92 | 37.78 | Class 2 (1090) |
| Dimethyl formamide | 153 | Miscible | 0.944 | 0.97 | 36.7 | Class 2 (880) |
| Dimethyl sulfoxide | 189 | 25.3 | 1.092 | 1.987 | 47 | Class 3 |
| Ethanol | 78.5 | Miscible | 0.789 | 1.04 | 24.6 | Class 3 |
| Ethyl acetate | 77 | 8.7 | 0.895 | 0.428 | 6 | Class 3 |
| Glycerin | 290 | Miscible | 1.261 | 954 | 42.5 | - |
| Isopropanol | 82.6 | Miscible | 0.786 | 1.96 | 18.2 | Class 3 |
| Methanol | 64.6 | Miscible | 0.791 | 0.543 | 32.6 | Class 2 (3000) |
| Tetrahydrofuran | 66 | Miscible | 0.889 | 0.48 | 7.52 | Class 2 (720) |
| Water | 100 | - | 0.998 | 1 | 78.5 | - |
| -, not applicable | ||||||
| Drug candidates | Polymer carrier | Simulation | Software | Force field | Summary | Reference |
|---|---|---|---|---|---|---|
| Indomethacin | Eudragit® PEO, glucose, sucrose | Molecular dynamics | Material Studio 4.0 | COMPASS | Eudragit® PEO was miscible, glucose was immiscible, and sucrose had borderline miscibility with indomethacin, as shown by thermal analysis. | [136] |
| Paclitaxel | PEG, PCL, MPEG–PCL | Molecular dynamics | HyperChem | CHARMM 27 | Paclitaxel binds to the PCL segments of MPEG–PCL copolymer, forming a core–shell micelle structure with PEG surrounding the core. | [193] |
| Curcumin | MPEG–PCL | Molecular dynamics | HyperChem | CHARMM 27 | An increased number of hydrophobic binding sites for curcumin indicate enhanced stability and stronger binding between the copolymer and drug. | [139] |
| Artemisinin | PEG, PVP | Molecular dynamics | Material Studio 6.0 | COMPASS | Polymers were miscible with artemisinin, forming stable solid dispersions and suggesting molecular dispersion of the drug within the polymer matrix. | [137] |
| Lumefantrine | Soluplus®, Kollidon® VA64, Plasdone™ S630 | Molecular dynamics | Maestro Schrodinger | GAUSSIAN | Strong interactions occurred between the hydroxyl and carbonyl groups of the polymers and the chlorine and amine groups of lumefantrine, respectively. | [143] |
| Cyclosporin A | L/D–polylactide, chitosan, polyglycolic acid, PEG, cellulose | Molecular docking | Materials Studio | PCFF | Polycellulose and polychitosan exhibited high miscibility, attributed to their larger open surface area for drug interaction. | [146] |
| Indomethacin | PVP | Molecular dynamics | AMBER | AMBER | The solubility of indomethacin increased when dispersed with PVP, compared to pure indomethacin. | [149] |
| Lafutidine | Soluplus®, PEG 400, Lutrol® F127, Lutrol® F68 | Molecular dynamics | Maestro Schrodinger | GAUSSIAN | Strong interactions occurred between the hydroxyl and carbonyl groups of the polymers and the chlorine and amine groups of lafutidine, respectively. | [140] |
| Posaconazole | Soluplus®, PEG 400, Lutrol® F127, Lutrol® F68, TPGS | Molecular dynamics | Maestro Schrodinger | GAUSSIAN | Strong hydrogen bonding between the drug and polymer resulted in the lowest energy and highest binding interaction. | [141] |
| Propranolol HCl, diphenhydramine HCl, paracetamol, ibuprofen, diclofenac sodium, hydrocortisone | Eudragit® L100, Eudragit® EPO, Eudragit® L100–55, Kollidon® VA64 | Quantum mechanical | Gaussian 09 | GAUSS VIEW | The strength of interactions depended on both the donor and acceptor types, as well as the number of hydrogen bonds formed between drug and polymer, as observed by DSC. | [114] |
| Cetirizine HCl, verapamil HCl | Eudragit® L100, Eudragit® L100–55 | Molecular dynamics | Maestro Schrodinger | GAUSSIAN 09 | The strongest interactions were between the amine groups of cetirizine HCl and verapamil HCl and the carboxylate groups of the polymers, indicating higher binding energy and increased stability. | [115] |
| Gemcitabine | Chitosan | Molecular dynamics | Material Studio 4.3 | COMPASS | Maximum drug loading was suggested to result from the strongest interaction between chitosan and gemcitabine. | [138] |
| Carbamazepine | Lutrol® F68 | Molecular dynamics | XenoView | PCFF | Carbamazepine molecules showed a strong tendency to aggregate, which is a critical step in nucleation and crystal formation. | [151] |
| Telaprevir | Cellulose derivatives | Quantum mechanical | HyperChem 8.0.3 | CHARMM | Effective polymers contain carboxylate groups with optimal hydrocarbon chain length, resulting in more favorable solvation free energy. | [152] |
| Tacrine | Chitosan, PBCA | Molecular dynamics | LAMMPS | PCFF | Interaction between tacrine and polymeric nanoparticles increased with the length of the polymer chain. | [157] |
| Indomethacin | PEG, PLA | Molecular dynamics | Material Studio 8.0 | COMPASS | Indomethacin exhibited significant miscibility with both PEG and PLA as carriers, resulting in high encapsulation efficiency. | [153] |
| Clonazepam, ibuprofen, fenofibrate, alprazolam | PVP–VA64, HPMC, Eudragit® EPO | Molecular dynamics | Materials Studio 7.0 | COMPASS | Ibuprofen/PVP–VA64 and ibuprofen/Eudragit® EPO formed strong hydrogen bonds, resulting in stable solid dispersions. | [145] |
| Felodipine | HPMC | Molecular dynamics | AMBER | GLYCAM | Miscibility of HPMC at various concentrations was supported by observation of single Tg values from DSC. | [150] |
| Aspirin, caffeine, carbamazepine, finasteride, flufenamic acid, flutamide, mefenamic acid, salicylamide, theophylline | PVP–VA64, poly (glycerol adipate) and derivatives | Molecular dynamics | GROMACS | CHARMM | Solubility and interaction parameters did not correlate with miscibility; six of nine API–PGA polymers were miscible. | [154] |
| Ibuprofen, carbamazepine | Soluplus®, PEG | Molecular docking | AutoDock Vina | CHARMM | Miscible blends were formed for ibuprofen–Soluplus®/PEG and carbamazepine–Soluplus®/PEG, with the latter showing stronger interactions. | [147] |
| 6–Mercaptopurine | PLA, PEG–modified PLA | Molecular docking | XenoView v.3.7.9.0 | PCFFD | The degree of polymerization was found to be optimal for solubility of 6–mercaptopurine in PLA and PEG polymers. | [155] |
| Olmesartan medoxomil | PVP–VA64, Soluplus® | Molecular dynamics | Maestro Schrodinger | OPLS | Strong hydrogen bonding between the carbonyl residues of pyrrolidone and acetate monomers in PVP–VA64 and the tetrazole and aromatic rings of olmesartan medoxomil inhibited recrystallization. | [156] |
| Simvastatin | PVP | Molecular dynamics | XenoView v.3.7.9.0 | PCFF | Simvastatin contains hydrogen bond donor and acceptor groups, while the PVP chain contains hydrogen bond acceptors, resulting in intermolecular interactions that stabilized the amorphous solid dispersion. | [194] |
| Rivaroxaban | Soluplus® | Molecular dynamics | XenoView | PCFF | Strong specific homo molecular interactions and Soluplus® chain shrinkage led to recrystallization under high relative humidity. | [158] |
| Naproxen, indomethacin | PVP, PVA | Quantum mechanical | COSMO–SAC | GAUSSIAN | Drug solubility in the polymer and thermodynamic compatibility of drug and polymer was investigated. | [118] |
| Ritonavir | Lutrol® | Molecular dynamics | GROMACS | AMBER 99SB-ILDN, AMBER (GAFF) | Strong intermolecular interactions suppressed molecular mobility, supported the amorphous state, and prevented recrystallization. | [142] |
| Erlotinib HCl | PEG, PVP | Molecular dynamics | Material Studio 7 | COMPASS | Erlotinib HCl formed weak hydrogen bonds with PEG and PVP individually, while the composite polymer enhanced molecular interactions through hydrogen bonding. | [159] |
| Year | Target feature | Input feature | Algorithm | Dataset | Reference |
|---|---|---|---|---|---|
| 2011 | Dispersion potential of drug–polymer (miscible dispersion) | Molecular descriptors and 3D structure derived from molecular structure, topology, and atomic properties | LR | Twelve compounds solidified with PVP–VA64 | [166] |
| 2011 | Percentage drug release at 60 min, time to 90 % drug dissolution, floating properties, physical stability | Proportions of drug, polymer, and effervescent agents | ANN/GP | Twenty-five mixture proportions | [167] |
| 2013 | The percentage of Tibolone dissolved in 30 min (Y30min) | Molecular weight of PEG, mixing temperature, drug amount, and total mixing time | ANN | Thirty-six experiments with four independent factors | [168] |
| 2015 | Enhanced dissolution rate | Optimization of ternary solid dispersions of carbamazepine, Soluplus®, and Lutrol® F68 | ANN | Twenty-two using D-optimal mixture experimental design and three for predictive modeling | [151] |
| 2019 | Physical stability of solid dispersions at 3 months and 6 months | Drug loading ratio, polymer molecular weight, drug properties, environmental conditions, preparation method, and temperature | ANN, SVM, RF, DT, Light GBM, kNN, NB, DNN | Fifty drug compounds with ten molecular descriptors | [169] |
| 2020 | Quantification and differentiation of amorphous solid dispersion systems | Crystalline and amorphous drug content of rivaroxaban with Soluplus® | ANN, PLS, PCR | Thirty sample formulations | [158] |
| 2022 | Dissolution percentage after 10 min (Q10) and 30 min (Q30) | Percentages of carbamazepine, Neusilin, and Kollidon® VA64. | GRNN, MLP | Twenty drug compounds | [170] |
| 2023 | Amorphization and chemical stability of ASD via HME | Proportions of drug and polymer, extruder configuration, barrel temperature, screw speed, and feed rate | XGBoost, Light GBM, RF, SVM, SHAP, IG | Forty-nine drug molecules | [171] |
| 2024 | Glass transition temperature determination (Tg) | Hydrophilic backbone methylation, hydrophilic feed fraction, hydrophobic backbone methylation | RF | Fifty unique copolymers with probucol | [172] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





