1. Introduction
Gastrointestinal malignancies represent a significant burden of cancer worldwide, with mesenchymal tumors comprising a distinct subset of these neoplasms. Among mesenchymal tumors, gastrointestinal stromal tumours (GIST) are the most common, accounting for approximately 0.1-3% of all gastrointestinal malignancies [
1]. These tumours arise from the precursors of the interstitial cells of Cajal, which regulate the peristaltic movements of the digestive tract and are known as the “pacemakers of the digestive system”.
The global incidence ranges between 10-15 cases per million per year according to population-based studies [
2]. Although GISTs are most commonly seen in the stomach (55-60%), they can also occur in the small intestine (30-32%), colorectal region (6-7%), oesophagus (2%) and rarely in extraintestinal localisations such as peritoneum, mesentery, omentum and pancreas [
3]. The mean age at diagnosis is 60-70 years and there is no significant difference between genders [
4].
Immunohistochemically, the majority of GISTs show CD117 (80-90%) and CD34 (60-70%) positivity and this feature is of critical importance in diagnosis [
5]. Mutations especially in KIT (CD117) and platelet-derived growth factor receptor alpha (PDGFRA) genes play an important role in GIST pathogenesis. The mutations have resulted in the development of targeted therapies such as tyrosine kinase inhibitors imatinib and sunitinib. However, there remains considerable debate regarding the optimal risk stratification system, with NIH-Fletcher, AFIP-Miettinen, and MSKCC Nomogram offering different approaches to predicting recurrence [
6]. The prognosis of the tumour largely depends on its size, mitotic number and location, and especially large tumours and those with a high mitotic index may have a more aggressive prognosis.
Although the epidemiology, clinical features and treatment approaches of GISTs are increasingly better understood in the literature, comprehensive studies revealing regional differences and reflecting the experiences especially in Turkey are limited [
7]. Controversial issues persist, particularly regarding the management of small GISTs (<2 cm), where some experts advocate for surveillance while others recommend immediate resection [
8]. Although significant advances have been made in treatment with the introduction of tyrosine kinase inhibitors (imatinib), standardised approaches have not yet been clarified, especially regarding the follow-up protocols of high-risk patients and the management of patients with recurrence [
1].
In this study, we aimed to contribute to this gap in the literature by sharing our single centre experience and to evaluate the relationship between the clinical features and surgical outcomes of GISTs in different localisations. Our findings demonstrate that tumor location significantly influences risk classification and that adjuvant imatinib effectively prevents recurrence in high-risk patients, providing important insights for clinical management.
2. Materials and Methods
2.1. Study Design and Population
This retrospective cohort study included 49 patients who underwent surgery for GIST between January 2010 and December 2020 in the Department of General Surgery, Dicle University Faculty of Medicine. Since the number of patients included all patients treated for GIST in our clinic within the specified date range, no sample size calculation was made. Our clinic served patients who met three inclusion criteria: age greater than 18 years and histopathological GIST diagnosis and surgical treatment at our institution. The study excluded patients who lacked clinical or pathological data and received primary treatment outside our center and patients who developed additional primary malignancies.
2.2. Data Collection
The researchers collected information by examining patient files and medical records together with operative notes and pathology reports. The data collection process used a standardized format which included the following variables for recording. The data collection process included demographic information about age and gender together with clinical presentation details and diagnostic procedures. The researchers classified tumors according to their location (stomach, small intestine, colon, pancreas, extraintestinal) and their size (diameter measurement in centimeters) and mitotic index (number of mitotic figures per 50 high-power fields). The researchers performed immunohistochemical marker assessment to examine patients for CD34, CD117, Ki-67, Vimentin, SMA/Desmin. The surgical outcomes consisted of the surgical method and complications as well as follow-up duration and recurrence status. The recorded data received verification from two independent investigators to ensure data reliability.
2.3. Pathological Evaluation
The pathologists performed histopathological examinations through standard procedures. The tumor measurements were recorded as the biggest diameter in centimeters. The number of mitotic figures within 50 high-power fields determined the mitotic index. The risk assessment process utilized Fletcher criteria to classify tumors into low, intermediate and high risk groups according to tumor size and mitotic count. The analysis grouped mitotic counts into two categories which were ≤5 or >10 per 50 high-power fields.
2.4. Immunohistochemical Analysis
The laboratory staff performed immunohistochemical tests using manufacturer-recommended kits according to established protocols. The standard analysis of tumor samples included CD117 and CD34 testing as well as Ki-67 evaluation. The Ki-67 proliferation index evaluation used three distinct categories which spanned from <5% to 5-10% to >10%. The evaluation of Vimentin and SMA/Desmin markers took place only when necessary.
2.5. Surgical Procedures
The medical team provided all patients with scheduled surgical procedures. The treatment plan for each patient consisted of individualized surgical approaches that took into account tumor site and dimensions and invasion level. The surgical team performed gastrectomy for stomach tumors through either wedge resection or subtotal procedures. The surgical team treated small intestine and colon tumors by performing segmental resection with anastomosis. The Whipple procedure served as the treatment method for pancreatic tumors. The medical team performed complete tumor removal when the tumor existed outside the intestinal area. The main surgical goal involved obtaining R0 resection status with negative margins while preserving tumor integrity. The treatment plan included simultaneous metastasectomy procedures for patients who had liver metastases. Every surgical procedure at the clinic was performed by an experienced team of surgeons.
2.6. Postoperative Management and Follow-Up
The treatment plan for high-risk patients included imatinib therapy at 400 mg per day for three years following surgery. The follow-up procedures included:
First year: every 3 months
Second year: every 6 months
Subsequent visits took place once a year.
Regular follow-up assessments consisted of physical examinations and abdominopelvic computed tomography tests and endoscopic procedures when the doctor determined their necessity. Surgical re-intervention became possible for patients who experienced recurrence.
2.7. Statistical Analysis
Statistical analysis was performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean ± standard deviation (range) and categorical variables as numbers and percentages. The analysis used the following statistical methods for group comparisons:
The analysis used Chi-square test or Fisher’s exact test for categorical variables.
The analysis used Student’s t-test or one-way ANOVA for normally distributed continuous variables.
The analysis used Mann-Whitney U test or Kruskal-Wallis test for non-normally distributed data.
The analysis used Log-rank test for survival analysis.
The analysis considered P-values <0.05 as statistically significant. No imputation was required as there were no missing data. The researchers conducted subgroup analyses according to tumor location and risk groups.
2.8. Ethical Approval
This study was approved by the Dicle University Faculty of Medicine Non-Interventional Clinical Research Ethics Committee (approval date: 09.04.2021, approval number: 2021/253) and conducted in accordance with the Declaration of Helsinki. The institutional review board waived individual informed consent for this retrospective study because the research used anonymized data from past cases. The researchers maintained strict confidentiality when handling all patient data.
3. Results
3.1. Demographic and Clinical Characteristics
Of the 49 GIST patients included in our study, 21 were female (42.9%) and 28 were male (57.1%). The mean age of the patients was 58.8±12.3 years and the age range was 28-80 years. When the diagnostic methods were examined, postoperative histological diagnosis was made in the majority of the patients (75.5%), while preoperative biopsy was performed in only 12 patients (24.5%). This difference was statistically significant (χ² = 12.76, p < 0.001). All of our patients were operated under elective conditions, and no condition requiring emergency surgical intervention was observed (
Table 1).
3.2. Tumor Characteristics and Risk Classification
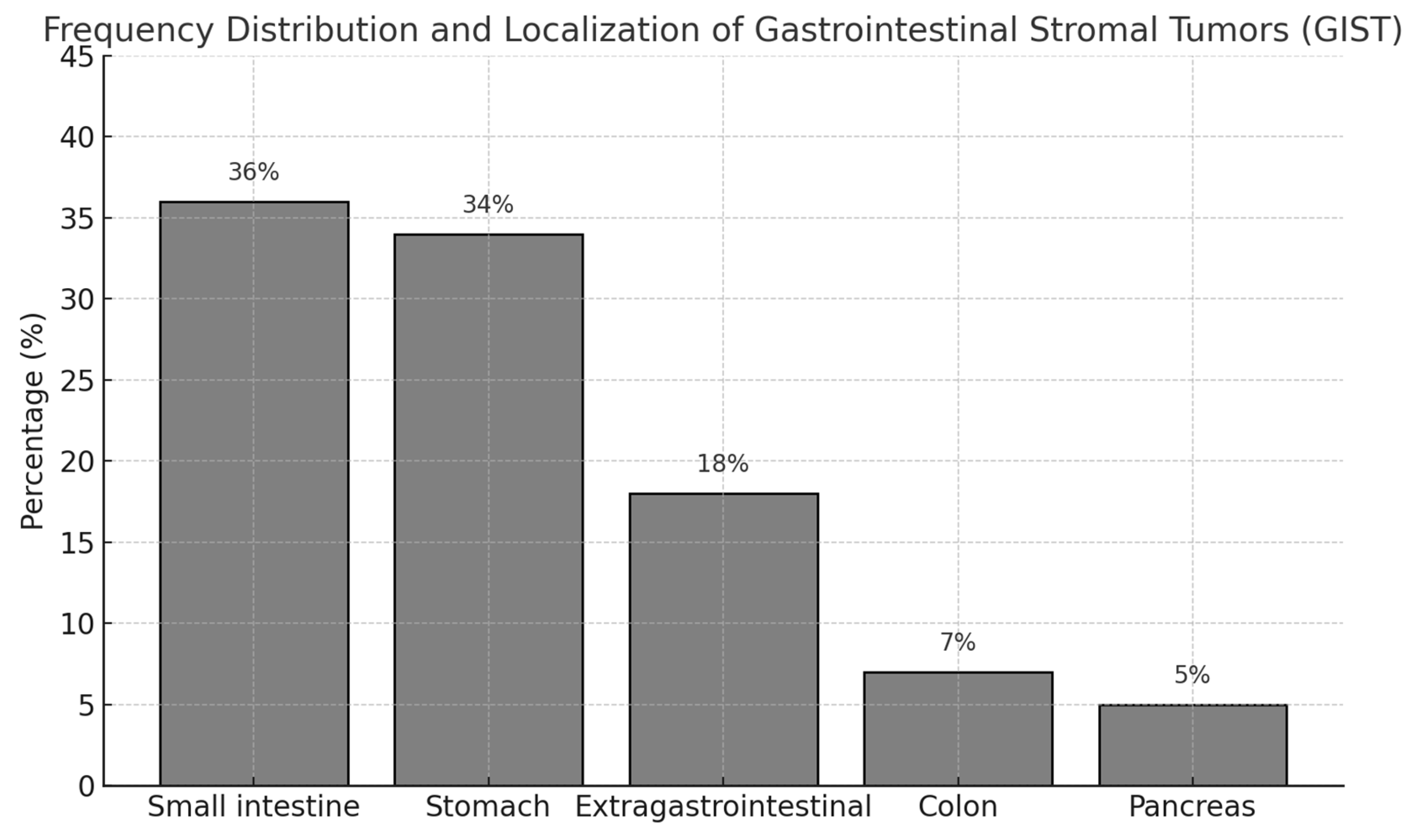
When the localization of the tumors was evaluated, the most common localization was small intestine (36%). This was followed by stomach (34%), extraintestinal localization (18%), colon (7%) and pancreas (5%). This distribution was statistically significant (χ² = 21.47, p < 0.001). In terms of tumor size, the tumor diameter was less than 5 cm in 19 patients (38.8%), between 5-10 cm in 12 patients (24.5%), and 10 cm or larger in 18 patients (36.7%). There was no statistically significant difference between tumor size distributions (χ² = 1.88, p = 0.391). When the number of mitoses in 50 high-power fields (HPF) were analyzed microscopically, 38 (77.6%) patients had 5 or less mitoses and 11 (22.4%) patients had more than 10 mitoses. The difference between mitotic number distributions was statistically significant (χ² = 8.90, p = 0.012). According to the risk classification using Fletcher criteria, the majority of the patients (52%) were in the low risk group, 13 patients (26%) were in the intermediate risk group and 11 patients (22%) were in the high risk group (χ² = 7.96, p = 0.019) (
Table 2,
Figure 1).
3.3. Endoscopic and Pathological Findings
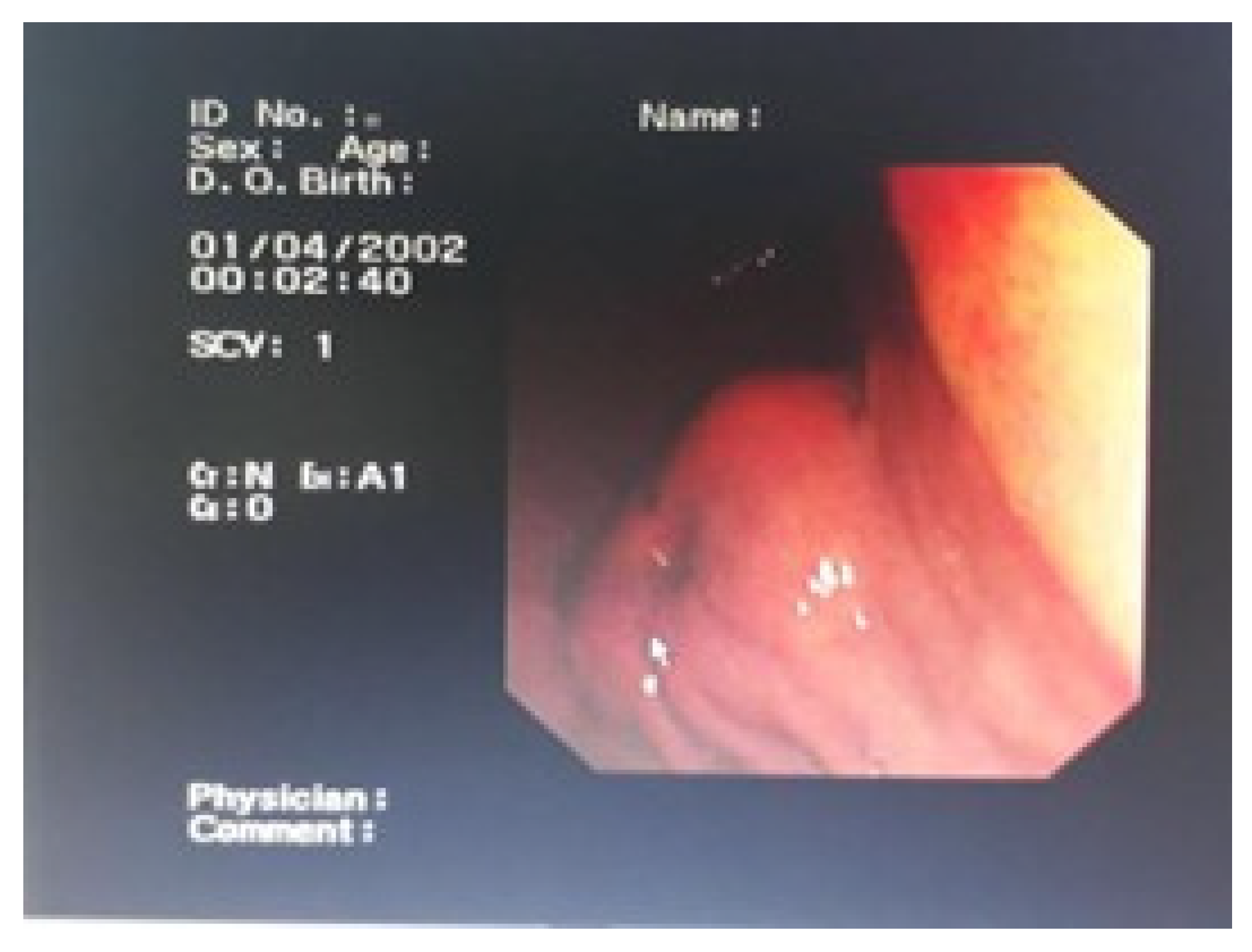
Endoscopic examination revealed a submucosal, well-circumscribed mass lesion in the antrum of the stomach (
Figure 2). This endoscopic appearance represents the typical presentation of our gastric GIST cases and similar endoscopic findings were observed in all gastric GIST cases.
3.4. Immunohistochemical Analysis
In immunohistochemical analyses, CD34, CD117, Vimentin and SMA/Desmin were positive in all tumors. When the Ki-67 proliferation index was analyzed, it was found to be below 5% in the majority of patients (71.4%), between 5-10% in 8.2% and above 10% in 20.4%. These differences in Ki-67 index were statistically significant (χ² = 37.10, p < 0.001) (
Table 3).
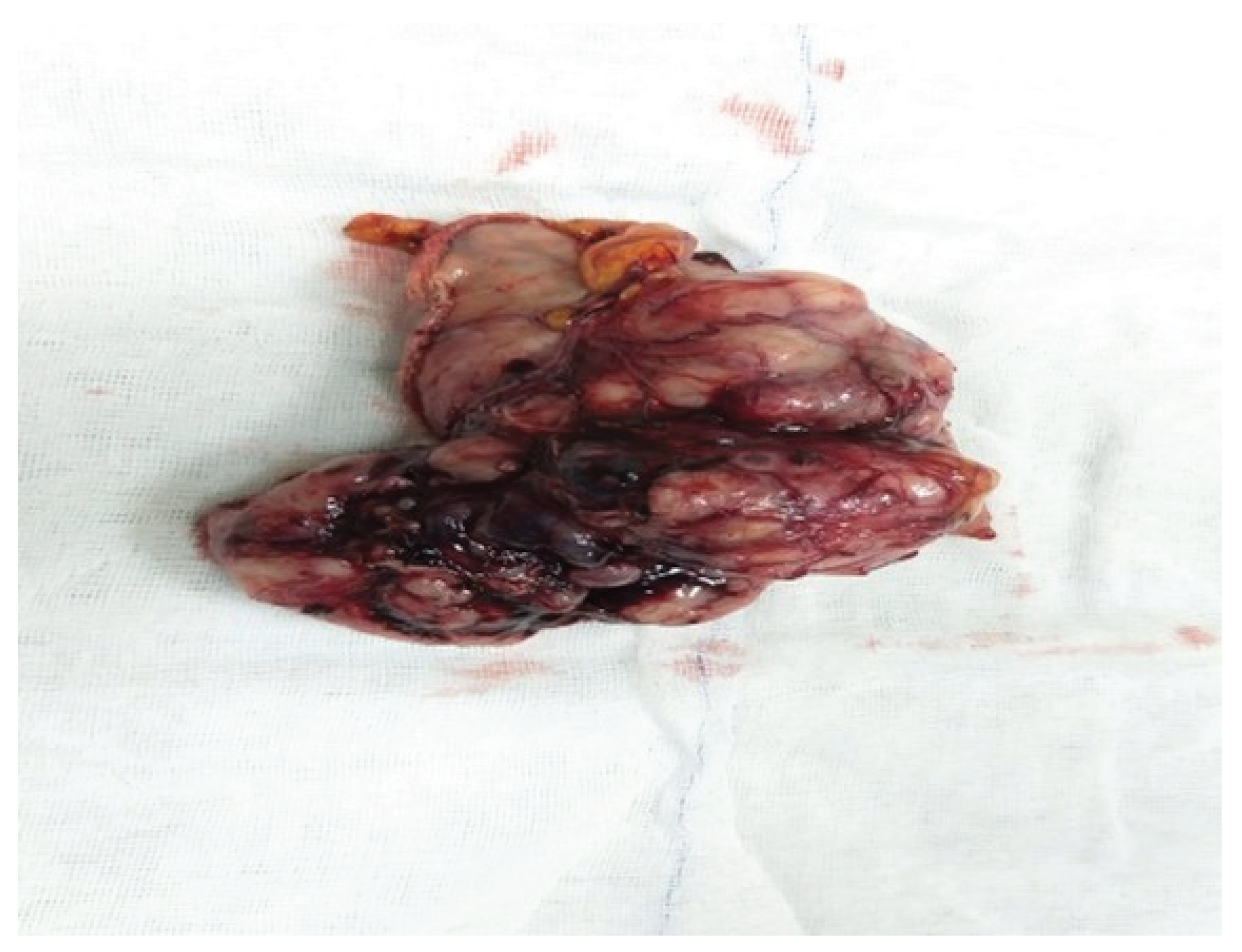
Macroscopic examination of the surgical specimen obtained after wedge resection for gastric GIST revealed a well-circumscribed, solid, submucosal mass with occasional hemorrhagic areas (
Figure 3). This macroscopic appearance showed similar features in other GIST cases in our study.
3.5. Surgical Treatment and Outcomes
When the surgical treatments were analyzed, 16 patients underwent small bowel resection+anastomosis and 3 patients underwent liver metastasectomy. In gastric tumors, 11 patients underwent wedge resection and 5 patients underwent subtotal gastrectomy. Total mass excision was performed in 9 patients with extraintestinal tumors, colon resection+anastomosis was performed in 3 patients with colon tumors, and Whipple procedure was performed in 2 patients with pancreatic tumors. When the follow-up periods were evaluated, the mean follow-up period for small bowel tumors was 17 months in patients who underwent liver metastasectomy and 21 months in patients who underwent bowel resection only. In gastric tumors, the mean follow-up time was 24 months in the wedge resection group and 18 months in the subtotal gastrectomy group. The follow-up period was 36 months in the extraintestinal group, 19 months in the colon group and 11 months in the pancreas group. There was no significant difference in survival between the surgical methods in patients who underwent small bowel surgery (Log rank = 2.31, p = 0.128) (
Table 4).
3.6. Complications and Recurrence
In terms of postoperative complications, mortality was observed in 2 patients (4.0%). One patient who underwent wedge resection died on postoperative day 6 due to capillary leak syndrome and the other patient who underwent subtotal gastrectomy died on postoperative day 7 due to myocardial infarction. Mortality rates were calculated as 9.1% and 16.7%, respectively, but there was no statistical difference between the two groups (Fisher exact, p = 0.723). As a morbidity, pancreatic fistula developed in one patient (2.0%) who underwent Whipple procedure, but closed spontaneously in the 2nd week of follow-up (
Table 5).
Recurrence was observed in two patients in long-term follow-up. One patient operated for low-risk extraintestinal GIST recurred at 33 months (11.1%) and one patient with intermediate-risk colonic GIST recurred at 25 months (33.3%). There was no statistically significant difference between the recurrence rates according to tumor localization (χ² = 4.15, p = 0.126). These two patients were re-operated and no complications developed. When recurrence rates were compared according to risk groups, recurrence rates were 4.0% in the low-risk group and 7.7% in the intermediate-risk group, while no recurrence was observed in the high-risk group. All high-risk patients had received adjuvant imatinib treatment for 3 years in the postoperative period. The difference in recurrence rate between the risk groups was statistically significant (χ² = 6.91, p = 0.032) (
Table 5).
3.7. Relationship Between Tumor Localization and Risk Groups
When the relationship between the localization of tumors and risk groups was examined, 52.9% of gastric tumors were in the low risk group, 29.4% in the intermediate risk group and 17.7% in the high risk group. Among small intestinal tumors, 55.6% were in the low risk group, 16.7% in the intermediate risk group and 27.8% in the high risk group. In extraintestinal tumors, all three risk groups were equally distributed (33.3%). There were no high-risk cases among the tumors located in the colon and pancreas. In the colon, 66.7% of the cases were in the low risk group and 33.3% in the intermediate risk group, while 50.0% of the pancreatic tumors were in the low risk group and 50.0% in the intermediate risk group. However, this relationship between localization and risk groups was not statistically significant (χ² = 11.32, p = 0.184) (
Table 6).
4. Discussion
The research evaluated both clinical aspects and pathological features of gastrointestinal stromal tumours together with surgical treatment results. Our research examined the distribution patterns and risk classification and long-term results of GIST patients treated at our center based on their tumor locations. The clinical progression and treatment responses of GISTs exhibit substantial variations according to our research findings. The management of rare mesenchymal tumours can be improved through understanding the relationships between tumour location and risk groups and surgical outcomes.
The majority of GIST cases in our study occurred in the small intestine at 36% and stomach at 34% [
9]. The study results showed different results than the gastric predominance which many studies in the literature have reported. Refai (2024) in Saudi Arabia reported that 66% of GIST cases were localised in the stomach [
9]. Similarly, a large population-based study using the SEER database reported that 63% of GISTs were localised in the stomach [
10].
In terms of gender distribution, the rate of male patients in our study (57.1%) was found to be slightly higher than the rate of male patients in the SEER database study (52%), but lower than the male rates reported in Saudi Arabian and European studies (72% and 71.1%) [
9,
11]. The mean age of 58.8±12.3 years in our study is largely compatible with the mean/median values of 60.5 years, 61.8 years and 62-64 years reported in studies in the literature [
9,
11,
12]. Regional differences, genetic factors and referral of cases with rare localisations due to the fact that our centre is a reference centre may explain the difference in the distribution of tumour localisation.
In our study, the tumour diameter was found to be less than 5 cm in 38.8% of the patients and 10 cm or larger in 36.7%. This distribution differs from the data reported in the study of Hashem et al. (2021); in their series, tumour size was ≤5 cm in only 14.7% of patients, while it was reported as >10 cm in 35.3% [
13]. In the study of Xu et al. (2021), the tumour size was reported to be <5 cm in 43.7% of the patients, which is closer to our results [
14].
In the risk classification, the majority of our patients (52%) were in the low risk group, while 44.04% of the patients were classified in the low malignancy potential (very low and low risk) category in the study by Tian and Chen (2024) [
15]. In the study of Hashem et al. (2021), 33.3% of the patients were found in the high risk group according to the AFIP scheme, which is higher than the 22% rate in our study [
13]. The detection of Ki-67 proliferation index below 5% in 71.4% of our patients is compatible with low malignancy potential as stated by Tian and Chen (2024) [
15].
In our study, organ-sparing surgical approaches as wedge resection (68.8%) and subtotal gastrectomy (31.3%) were preferred in gastric GISTs. This approach is consistent with the current approach reported by Cananzi et al. (2022), who considered R0 resection as the gold standard in surgical treatment [
16]. In our series, postoperative mortality rate was 4%, which is higher than the mortality rate (0%) observed in patients who underwent surgery after neoadjuvant imatinib treatment by Vassos et al. (2021) [
17]. In terms of morbidity, only one patient (2%) developed pancreatic fistula in our study, and similarly, pancreatic fistula was reported in one patient in the duodenal GIST study of Vassos et al. (2021) [
18]. The low complication rate in our series indicates the success of the surgical technique.
In patients with advanced GIST, Li et al. (2024) reported that there was no significant difference in overall survival between patients with and without surgery (76.5 months vs. 78.9 months) [
19]. This finding suggests that the role of surgery may be limited in metastatic patients. In our series, liver metastasectomy was performed in 15.8% (3/19) of small bowel tumours, indicating the aggressiveness of the surgical approach.
The low recurrence rate of 4.1% found in our study in long-term follow-up is compatible with some studies in the literature. Jakob et al. (2022) reported similarly low recurrence rates in their study of 350 patients, and local recurrence was observed in only one patient [
20]. Interestingly, it is noteworthy that the patients with recurrence in our study were in the low and intermediate risk groups, while no recurrence was observed in the high risk group. This unexpected finding is supported by the statistically significant difference in the recurrence rate between the risk groups (p=0.032) and may be explained by the efficacy of adjuvant imatinib treatment in high-risk patients, as shown in the study by Blay et al. In our series, adjuvant imatinib treatment was administered to all high-risk patients for 3 years, and the efficacy of longer-term treatment is also reported in the literature. Blay et al. showed that 6 years of imatinib treatment reduced the recurrence rate up to 28% in high-risk localised GIST patients [
21].
In addition, the fact that recurrence developed in a case of extragastrointestinal GIST in our study supports the finding reported by Feng et al. (2021) that extragastrointestinal stromal tumours may have worse disease-free survival [
22]. When the relationship between tumour localisation and risk groups is evaluated, the fact that stomach and small intestine tumours are mostly in the low-risk group, although not mentioned in the study by Stavrou et al. (2025), is in line with the general observations in the literature [
23]. The fact that there were no high-risk cases in tumours located in the colon and pancreas may be due to the limited sample size.
Our study has some limitations. The study results lack general applicability because it used retrospective design and data from a single center. The small number of patients in the study reduced statistical power particularly for GISTs that occur infrequently in different locations. The study benefits from its extended follow-up duration and thorough immunohistochemical assessments and complete risk group classification system. Future research should investigate how molecular markers affect patient outcomes while comparing surgical methods and assessing the success of additional treatments. In addition, the establishment of a multicentre GIST database in our country will strengthen evidence-based approaches in the management of these rare tumours.
5. Conclusions
GISTs represent the most prevalent mesenchymal tumors which occur in the gastrointestinal tract while their clinical behavior and treatment outcomes show significant variability. The majority of GIST cases appeared in the small intestine and stomach and more than half of the patients belonged to the low risk group. The surgical procedures that used organ-preserving methods produced minimal complications and recurrence rates. The follow-up and treatment planning of patients received guidance from immunohistochemical markers and risk classification systems. A multidisciplinary approach with suitable surgical strategy enables successful treatment of GIST patients. The administration of adjuvant imatinib treatment reduces recurrence rates in patients who have high-risk disease.
Author Contributions
Conceptualization, Ömer Başol, İbrahim Öcal, Hüseyin Alakuş, Ali Çalışkan, Hüseyin Bilge, Ulaş Aday and Abdullah Oğuz; Data curation, İbrahim Öcal and Abdullah Oğuz; Formal analysis, İbrahim Öcal and Ali Çalışkan; Funding acquisition, İbrahim Öcal; Investigation, İbrahim Öcal and Abdullah Oğuz; Methodology, Ömer Başol, İbrahim Öcal, Ulaş Aday and Abdullah Oğuz; Project administration, İbrahim Öcal; Resources, İbrahim Öcal; Software, Ömer Başol, İbrahim Öcal, Hüseyin Alakuş, Hüseyin Bilge and Ulaş Aday; Supervision, Ömer Başol and İbrahim Öcal; Validation, Ömer Başol, İbrahim Öcal, Hüseyin Alakuş, Hüseyin Bilge, Ulaş Aday and Abdullah Oğuz; Visualization, İbrahim Öcal; Writing – original draft, Ömer Başol, İbrahim Öcal and Ali Çalışkan; Writing – review & editing, Ömer Başol, İbrahim Öcal and Ali Çalışkan. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Dicle University Faculty of Medicine Non-Interventional Clinical Research Ethics Committee (protocol code 2021/253 and date of approval 09.04.2021).
Informed Consent Statement
Written informed consent has been obtained from the patients to publish this paper.
Data Availability Statement
Data used in this study can be provided on reasonable request.
Acknowledgments
not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GIST |
Gastrointestinal stromal tumor |
| HPF |
High-power field |
| CD117 |
c-KIT protein |
| CD34 |
Cluster of differentiation 34 |
| PDGFRA |
Platelet-derived growth factor receptor alp |
| SMA |
Smooth muscle actin |
| NIH |
National Institutes of Health |
| AFIP |
Armed Forces Institute of Pathology |
| MSKCC |
Memorial Sloan Kettering Cancer Center |
| SD |
Standard deviation |
| IRB |
Institutional Review Board |
References
- El-Menyar, A.; Mekkodathil, A.; Al-Thani, H. Diagnosis and management of gastrointestinal stromal tumors: An up-to-date literature review. J. Cancer Res. Ther. 2017, 13, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Søreide, K.; Sandvik, O.M.; Søreide, J.A.; Giljaca, V.; Jureckova, A.; Bulusu, V.R. Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol. 2016, 40, 39–46. [Google Scholar] [CrossRef]
- Sanchez-Hidalgo, J.M.; Duran-Martinez, M.; Molero-Payan, R.; Rufian-Peña, S.; Arjona-Sanchez, A.; Casado-Adam, A.; Cosano-Alvarez, A.; Briceño-Delgado, J. Gastrointestinal stromal tumors: A multidisciplinary challenge. World J. Gastroenterol. 2018, 24, 1925–1941. [Google Scholar] [CrossRef]
- Parab, T.M.; DeRogatis, M.J.; Boaz, A.M.; Grasso, S.A.; Issack, P.S.; Duarte, D.A.; Urayeneza, O.; Vahdat, S.; Qiao, J.H.; Hinika, G.S. Gastrointestinal stromal tumors: a comprehensive review. J. Gastrointest. Oncol. 2019, 10, 144–154. [Google Scholar] [CrossRef]
- Wu, C.E.; Tzen, C.Y.; Wang, S.Y.; Yeh, C.N. Clinical diagnosis of gastrointestinal stromal tumor (GIST): from the molecular genetic point of view. Cancers 2019, 11, 679. [Google Scholar] [CrossRef]
- Belfiori, G.; Sartelli, M.; Cardinali, L.; Tranà, C.; Bracci, R.; Gesuita, R.; Marmorale, C. Risk stratification systems for surgically treated localized primary gastrointestinal stromal tumors (GIST). Review of literature and comparison of the three prognostic criteria: MSKCC Nomogram, NIH-Fletcher and AFIP-Miettinen. Ann. Ital. Chir. 2015, 86, 219–227. [Google Scholar]
- McDonnell, M.J.; Punnoose, S.; Viswanath, Y.K.S.; Wadd, N.J.; Dhar, A. Gastrointestinal stromal tumours (GISTs): an insight into clinical practice with review of literature. Frontline Gastroenterol. 2017, 8, 19–25. [Google Scholar] [CrossRef]
- Nishida, T.; Goto, O.; Raut, C.P.; Yahagi, N. Diagnostic and treatment strategy for small gastrointestinal stromal tumors. Cancer 2016, 122, 3110–3118. [Google Scholar] [CrossRef] [PubMed]
- Refai, F. Gastrointestinal stromal tumors: a retrospective study at a tertiary care center in Saudi Arabia in the last decade. Cureus 2024, 16, e64560. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Ullah, A.; Waheed, A.; Karki, N.R.; Adhikari, N.; Vemavarapu, L.; Foley, K.L.; Moeller, K.D.; Wu, M.J.; Kassir, M.F.; et al. Gastrointestinal stromal tumors (GIST): a population-based study using the SEER database, including management and recent advances in targeted therapy. Cancers 2022, 14, 3689. [Google Scholar] [CrossRef]
- Mohammadi, M.; IJzerman, N.S.; Hohenberger, P.; Rutkowski, P.; Jones, R.L.; Martin-Broto, J.; Alvarez, R.; Bauer, S.; Casali, P.G.; Dei Tos, A.P.; et al. Clinicopathological features and treatment outcome of oesophageal gastrointestinal stromal tumour (GIST): a large, retrospective multicenter European study. Eur. J. Surg. Oncol. 2021, 47, 2173–2181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; de la Torre, J.; IJzerman, N.S.; Sutton, T.L.; Zhao, B.; Khan, T.M.; Tsai, S.; Christians, K.K.; Gamblin, T.C.; Heinrich, M.C.; et al. Location of gastrointestinal stromal tumor (GIST) in the stomach predicts tumor mutation profile and drug sensitivity. Clin. Cancer Res. 2021, 27, 5334–5342. [Google Scholar] [CrossRef]
- Hashem, W.B.; El-Nahas, T.H.; Fawzy, M.; Mashhour, S.N.; Zedan, M.H.; Mashhour, K.N. Gastrointestinal stromal tumor: clinicopathological features, management, and comparison of three risk stratification schemes. Res. Oncol. 2021, 17, 73–79. [Google Scholar] [CrossRef]
- Xu, S.J.; Zhang, S.Y.; Dong, L.Y.; Lin, G.S.; Zhou, Y.J. Dynamic survival analysis of gastrointestinal stromal tumors (GISTs): a 10-year follow-up based on conditional survival. BMC Cancer 2021, 21, 1170. [Google Scholar] [CrossRef]
- Tian, J.; Chen, W. Prediction of Ki-67 expression and malignant potential in gastrointestinal stromal tumors: novel models based on CE-CT and serological indicators. BMC Cancer 2024, 24, 1412. [Google Scholar] [CrossRef]
- Cananzi, F.C.M.; Ruspi, L.; Samà, L.; Renne, S.L.; Sicoli, F.; Quagliuolo, V. The gist of surgical margins in GIST: a narrative review. Laparosc. Surg. 2022, 6, 4. [Google Scholar] [CrossRef]
- Vassos, N.; Jakob, J.; Kähler, G.; Reichardt, P.; Marx, A.; Dimitrakopoulou-Strauss, A.; Rathmann, N.; Wardelmann, E.; Hohenberger, P. Preservation of organ function in locally advanced non-metastatic gastrointestinal stromal tumors (GIST) of the stomach by neoadjuvant imatinib therapy. Cancers 2021, 13, 586. [Google Scholar] [CrossRef]
- Vassos, N.; Perrakis, A.; Hohenberger, W.; Croner, R.S. Surgical approaches and oncological outcomes in the management of duodenal gastrointestinal stromal tumors (GIST). J. Clin. Med. 2021, 10, 4459. [Google Scholar] [CrossRef]
- Li, J.; Huang, Z.; Zhou, H.; Li, H.; Zhang, J. Survival outcomes and prognostic factors of advanced gastrointestinal stromal tumors: in the era of multiple tyrosine kinase inhibitors. J. Gastrointest. Oncol. 2024, 15, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Jakob, J.; Salameh, R.; Wichmann, D.; Charalambous, N.; Zygmunt, A.C.; Kreisel, I.; Kähler, G.; Bauer, S.; Hohenberger, P. Needle tract seeding and abdominal recurrence following pre-treatment biopsy of gastrointestinal stromal tumors (GIST): results of a systematic review. BMC Surg. 2022, 22, 202. [Google Scholar] [CrossRef] [PubMed]
- Blay, J.Y.; Schiffler, C.; Bouché, O.; Brahmi, M.; Duffaud, F.; Toulmonde, M.; Llacer, C.; Bompas, E.; Rios, M.; Collard, O.; et al. A randomized study of 6 versus 3 years of adjuvant imatinib in patients with localized GIST at high risk of relapse. Ann. Oncol. 2024, 35, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.; Hu, W.; Zheng, C.; Wang, W.; Zheng, G.; Feng, X.; Guo, S.; Ma, L.; Chen, Y.; Zhang, L.; et al. Clinical features of extragastrointestinal stromal tumor compared with gastrointestinal stromal tumor: a retrospective, multicenter, real-world study. J. Oncol. 2021, 2021, 1460131. [Google Scholar] [CrossRef] [PubMed]
- Stavrou, N.; Memos, N.; Filippatos, C.; Sergentanis, T.N.; Zagouri, F.; Gavriatopoulou, M.; Psaltopoulou, T.; Dimopoulos, M.A. Neoadjuvant imatinib in recurrent/metastatic gastrointestinal stromal tumors: a systematic review and meta-analysis of proportions. J. Gastrointest. Cancer 2025, 56, 88. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).