Submitted:
31 July 2025
Posted:
31 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
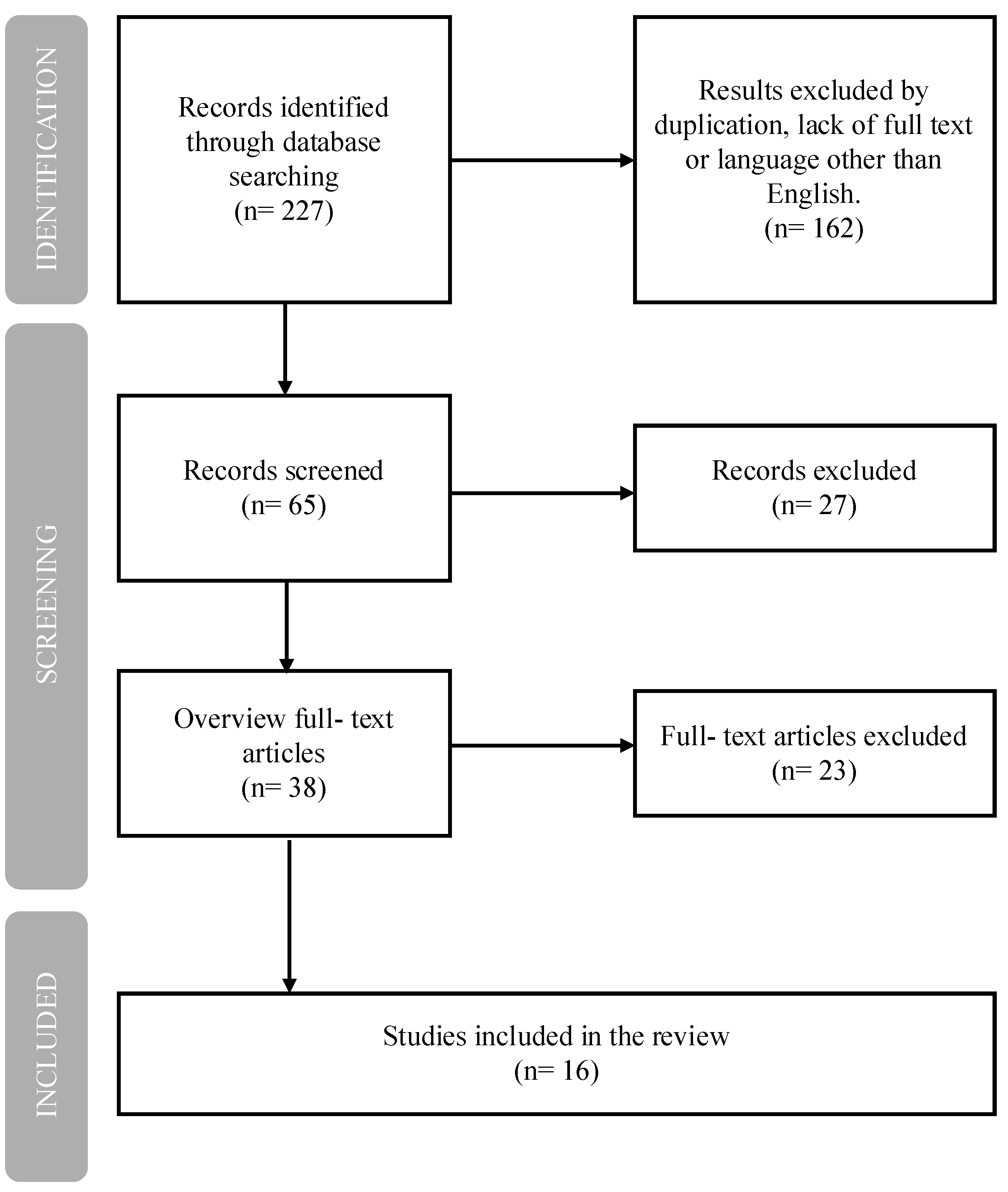
2. Methods
3. Results
3.1. TNF-Alpha Inhibitors
3.2. IL-6/IL-6R Inhibitors
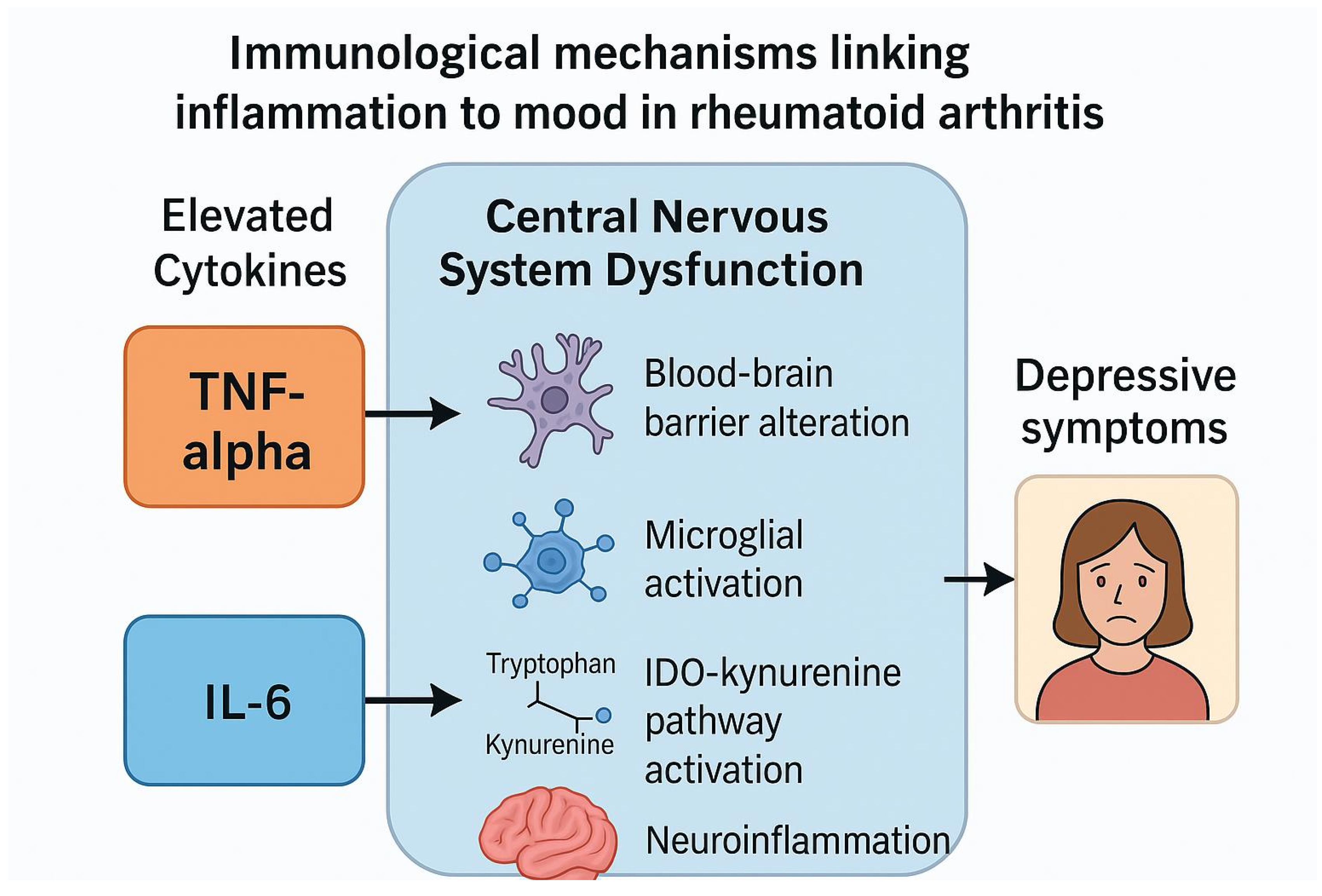
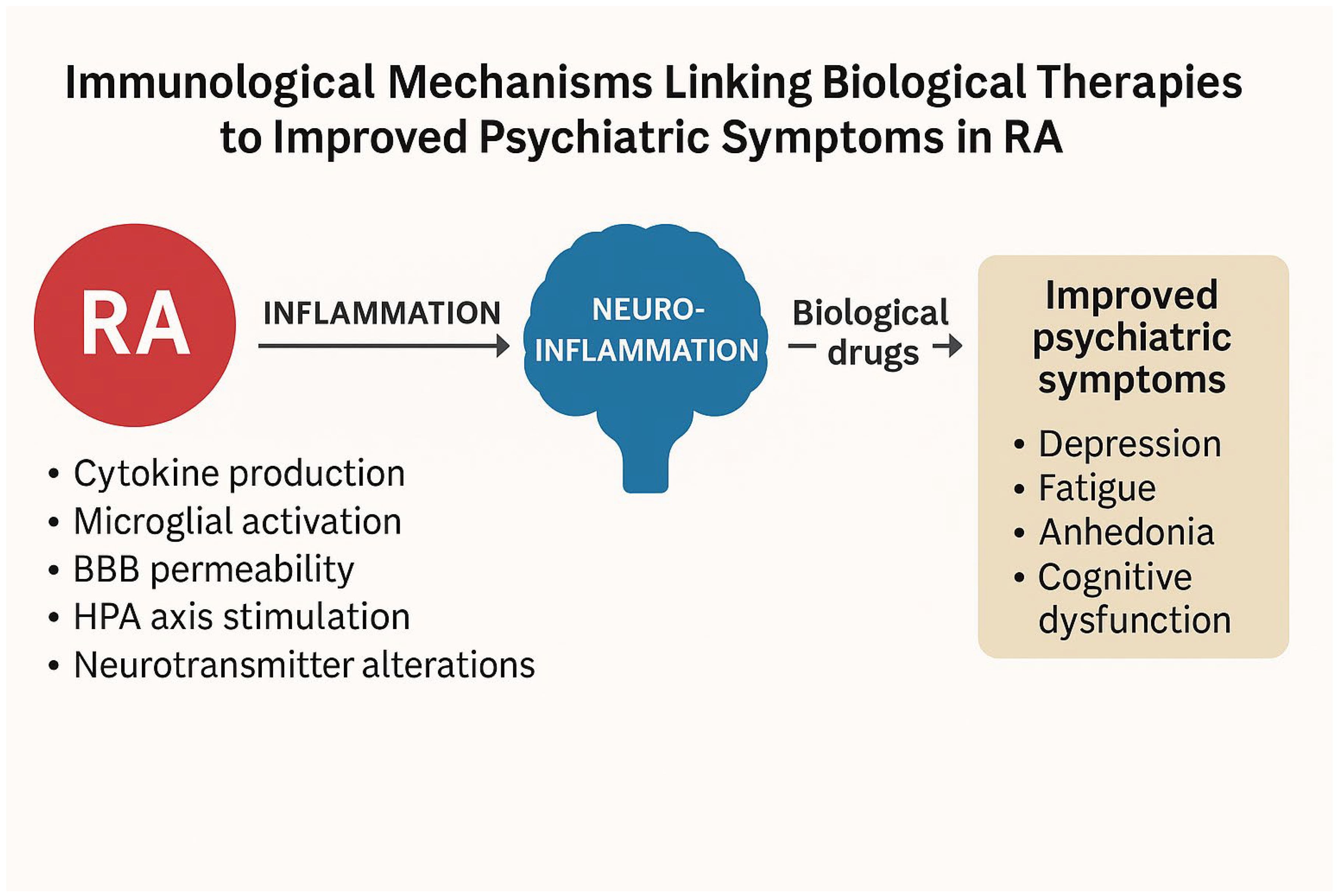
4. Discussion
4.1. TNF-α Inhibitors and Psychiatric Outcomes
4.2. IL-6/IL-6R Inhibitors and Psychiatric Outcomes
4.3. Proposed Mechanisms Linking Biological Therapies to Psychiatric Outcomes
- 1.
- Blood-Brain Barrier (BBB) Dysfunction
- 2.
- Microglial Activation and Neuroinflammation
- 3.
- Hypothalamic-Pituitary-Adrenal (HPA) Axis Dysregulation
- 4.
- Kynurenine Pathway Activation
- 5.
- Direct Actions of IL-6
4.4. Role of Biological Therapies
4.5. Limitations
4.6. Conclusions
4.7. Clinical Implications
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Alamanos, Y., Voulgari, P.V, Drosos, A.A.: Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum. 2006, 36, 182-8. [CrossRef]
- Smolen, J.S,; Aletaha, D.; McInnes, I.B:. Rheumatoid arthritis. Lancet 2016, 388, 2023-2038. [CrossRef]
- Meade, T.; Manolios, N.; Cumming, R.; Conaghan, P.G.; Katz, P. Cognitive Impairment in Rheumatoid Arthritis: A Systematic Review. Arthritis Care Res (Hoboken) 2018, 70, 39-52. PMID: 28371512. [CrossRef]
- Panjrattan, C.; Chauhan, V.S.; Nath, S.; et al.: Depression among rheumatoid arthritis patients and barriers to seeking professional help: An observational study. Ind Psychiatry J. 202332(Suppl 1):S136-S140. [CrossRef]
- Katz, P,; Pedro, S.; Michaud, K. Sleep Disorders Among Individuals With Rheumatoid Arthritis. Arthritis Care Res (Hoboken) 2023, 75, 1250-1260. Epub 2023 Jan 30. PMID: 35997482. [CrossRef]
- Maloley, P.M.; England, B.R.; Sayles, H. et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis. Semin Arthritis Rheum. 2019, 49, 229-235. [CrossRef]
- Albeltagy, E.S.; Elaziz, S.YA.; Abozaid, S.Y.; et.al.: Interleukin 6, interleukin 17, disease-related and contextual factor association with depression, and its severity in patients with rheumatoid arthritis. Clin Rheumatol. 2021, 40, 895-904. [CrossRef]
- Liu, Y.; Ho, R.C.; Mak, A.: The role of interleukin (IL)-17 in anxiety and depression of patients with rheumatoid arthritis. Int J Rheum Dis. 2012, 15, 183-7. [CrossRef]
- El-Tantawy, A.M.; El-Sayed, A.E.; Kora, B.A.; Amin, R.T. Psychiatric morbidity associated with some cytokines (IL-1beta, IL-12, IL-18 and TNF-alpha) among rheumatoid arthritis patients. Egypt J Immunol. 2008, 15, 1-11.
- Li, YC.; Chou, YC.; Chen.; HC, et. Al.: Interleukin-6 and interleukin-17 are related to depression in patients with rheumatoid arthritis. Int J Rheum Dis. 2019, 22,980-985. [CrossRef]
- Cheng, Y.; Desse, S.; Martinez, A.; et.al.: TNFα disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav Immun. 2018, 69, 556-567. [CrossRef]
- Xu, D,.; Xu, Y.; Gao, X.; et.al: Potential value of Interleukin-6 as a diagnostic biomarker in human MDD and the antidepressant effect of its receptor antagonist tocilizumab in lipopolysaccharide-challenged rats. Int Immunopharmacol. 2023, 124(Part B), 110903. [CrossRef]
- Cathomas, F.; Fuertig, R.; Sigrist, H.; et. al: Hengerer, B et al. CD40-TNF activation in mice induces extended sickness behavior syndrome co-incident with but not dependent on activation of the kynurenine pathway. Brain Behav Immun.2015,50:125-140. [CrossRef]
- Brown, E.; Mc Veigh, C.J.; Santos, L.; et al.: TNFα-dependent anhedonia and upregulation of hippocampal serotonin transporter activity in a mouse model of collagen-inducedarthritis. Neuropharmacology. 2018,137:211220. [CrossRef]
- Bingham, C.O., III.; Black, S.; Shiff, N.J.; et al.: J.R. Response to Treatment with Intravenous Golimumab or Infliximab in Rheumatoid Arthritis Patients: PROMIS Results from the Real-World Observational Phase 4 AWARE Study. Rheumatol Ther. 2023, 10, 659-678. [CrossRef]
- Hsieh, S.C.; Tsai, P.H.; Kuo, C.F.; et al.: Health-related quality of life improvement by adalimumab therapy in patients with rheumatoid arthritis in Taiwan: A nationwide prospective study. J Chin Med Assoc. 2023, 86, 366-374. [CrossRef]
- Tiosano, S,; Yavne, Y.; Watad, A.; et al.: The impact of tocilizumab on anxiety and depression in patients with rheumatoid arthritis. European Journal of Clinical Investigation 2020, 50, e13268. [CrossRef]
- Strand, V.; Boklage, S.H.; Kimura, T,; et. al: High levels of interleukin-6 in patients with rheumatoid arthritis are associated with greater improvements in health-related quality of life for sarilumab compared with adalimumab. Arthritis Res Ther. 2020, 22,250. [CrossRef]
- Edwards, J.C., Leandro, M.J.: Cambridge, G. B: Lymphocyte depletion therapy with rituximab in rheumatoid arthritis. Rheum Dis Clin North Am. 2004, 30 393-403. [CrossRef]
- Genovese, M.C.; Becker, J.-C.; Schiff, M.; Luggen, M.; Sherrer, Y.; Kremer, J.; Birbara, C.; Box, J.; Natarajan, K.; Nuamah, I.; et al. Abatacept for Rheumatoid Arthritis Refractory to Tumor Necrosis Factor α Inhibition. New Engl. J. Med. 2005, 353, 1114–1123. [CrossRef]
- Yirmiya, R.; Goshen, I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behav. Immun. 2011, 25, 181–213. [CrossRef]
- Dantzer, R.; O'COnnor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [CrossRef]
- Miwa, Y.; Nishimi, A.; Nishimi, S.; Saito, M.; Tokunaga, T.; Yanai, R.; Takahashi, R.; Wakabayashi, K.; Kasama, T.; Hosaka, M. Combined infliximab and methotrexate treatment improves the depressive state in rheumatoid arthritis patients more effectively than methotrexate alone. Eur. J. Rheumatol. 2014, 1, 147–149. [CrossRef]
- Miwa, Y.; Isojima, S.; Saito, M.; Ikari, Y.; Kobuna, M.; Hayashi, T.; Takahashi, R.; Kasama, T.; Hosaka, M.; Sanada, K. Comparative Study of Infliximab Therapy and Methotrexate Monotherapy to Improve the Clinical Effect in Rheumatoid Arthritis Patients. Intern. Med. 2016, 55, 2581–2585. [CrossRef]
- Mathias, S.D.; Colwell, H.H.; Miller, D.P.; Moreland, L.W.; Buatti, M.; Wanke, L. Health-related quality of life and functional status of patients with rheumatoid arthritis randomly assigned to receive etanercept or placebo. Clin. Ther. 2000, 22, 128–139. [CrossRef]
- Bae, S.-C.; Gun, S.C.; Mok, C.C.; Khandker, R.; Nab, H.W.; Koenig, A.S.; Vlahos, B.; Pedersen, R.; Singh, A. Improved health outcomes with Etanercept versus usual DMARD therapy in an Asian population with established rheumatoid arthritis. BMC Musculoskelet. Disord. 2013, 14, 13–13. [CrossRef]
- Machado, D.A.; Guzman, R.M.; Xavier, R.M.; Simon, J.A.; Mele, L.; Pedersen, R.; Ferdousi, T.; Koenig, A.S.; Kotak, S.; Vlahos, B. Open-Label Observation of Addition of Etanercept Versus a Conventional Disease-Modifying Antirheumatic Drug in Subjects With Active Rheumatoid Arthritis Despite Methotrexate Therapy in the Latin American Region. Am. J. Clin. Oncol. 2014, 20, 25–33. [CrossRef]
- Kekow, J.; Moots, R.J.; Emery, P.; Durez, P.; Koenig, A.; Singh, A.; Pedersen, R.; Robertson, D.; Freundlich, B.; Sato, R. Patient-reported outcomes improve with etanercept plus methotrexate in active early rheumatoid arthritis and the improvement is strongly associated with remission: the COMET trial. Ann. Rheum. Dis. 2010, 69, 222–225. [CrossRef]
- Kekow, J.; Moots, R.; Khandker, R.; Melin, J.; Freundlich, B.; Singh, A. Improvements in patient-reported outcomes, symptoms of depression and anxiety, and their association with clinical remission among patients with moderate-to-severe active early rheumatoid arthritis. Rheumatology 2010, 50, 401–409. [CrossRef]
- Curtis, J.R.; Herrem, C.; Ndlovu, ’.N.; O’bRien, C.; Yazici, Y. A somatization comorbidity phenotype impacts response to therapy in rheumatoid arthritis: post-hoc results from the certolizumab pegol phase 4 PREDICT trial. Arthritis Res. Ther. 2017, 19, 1–11. [CrossRef]
- Harrold, L.R.; John, A.; Reed, G.W.; Haselkorn, T.; Karki, C.; Li, Y.; Best, J.; Zlotnick, S.; Kremer, J.M.; Greenberg, J.D. Impact of Tocilizumab Monotherapy on Clinical and Patient-Reported Quality-of-Life Outcomes in Patients with Rheumatoid Arthritis. Rheumatol. Ther. 2017, 4, 405–417. [CrossRef]
- Manning-Bennett, A.T.; Hopkins, A.M.; Sorich, M.J.; Proudman, S.M.; Foster, D.J.; Abuhelwa, A.Y.; Wiese, M.D. The association of depression and anxiety with treatment outcomes in patients with rheumatoid arthritis – a pooled analysis of five randomised controlled trials. Ther. Adv. Musculoskelet. Dis. 2022, 14. [CrossRef]
- Behrens, F.; Burmester, G.-R.; Hofmann, M.W.; Aringer, M.; Kellner, H.; Liebhaber, A.; Wassenberg, S.; Peters, M.A.; Zortel, M.; Amberger, C. Sustained effectiveness and safety of subcutaneous tocilizumab over two years in the ARATA observational study. Clin. Exp. Rheumatol. 2022, 41, 1463–1472. [CrossRef]
- Sun, Y.; Wang, D.; Salvadore, G.; Hsu, B.; Curran, M.; Casper, C.; Vermeulen, J.; Kent, J.M.; Singh, J.; Drevets, W.C.; et al. The effects of interleukin-6 neutralizing antibodies on symptoms of depressed mood and anhedonia in patients with rheumatoid arthritis and multicentric Castleman’s disease. Brain, Behav. Immun. 2017, 66, 156–164. [CrossRef]
- Dhillon, S. Intravenous Tocilizumab: A Review of Its Use in Adults with Rheumatoid Arthritis. BioDrugs 2013, 28, 75–106. [CrossRef]
- Persoons, P.; Vermeire, S.; Demyttenaere, K.; Fischler, B.; Vandenberghe, J.; VAN Oudenhove, L.; Pierik, M.; Hlavaty, T.; VAN Assche, G.; Noman, M.; et al. The impact of major depressive disorder on the short- and long-term outcome of Crohn's disease treatment with infliximab. Aliment. Pharmacol. Ther. 2005, 22, 101–110. [CrossRef]
- Minderhoud, I.M. Crohn’s disease, fatigue, and infliximab: Is there a role for cytokines in the pathogenesis of fatigue?. World J. Gastroenterol. 2007, 13, 2089–93. [CrossRef]
- Krishnan, R.; Cella, D.; Leonardi, C.; Papp, K.; Gottlieb, A.; Dunn, M.; Chiou, C.; Patel, V.; Jahreis, A. Effects of etanercept therapy on fatigue and symptoms of depression in subjects treated for moderate to severe plaque psoriasis for up to 96 weeks. Br. J. Dermatol. 2007, 157, 1275–1277. [CrossRef]
- Tyring, S.; Gottlieb, A.; Papp, K.; Gordon, K.; Leonardi, C.; Wang, A.; Lalla, D.; Woolley, M.; Jahreis, A.; Zitnik, R.; et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006, 367, 29–35. [CrossRef]
- Uzzan, S.; Azab, A.N. Anti-TNF-α Compounds as a Treatment for Depression. Molecules 2021, 26, 2368. [CrossRef]
- Ertenli, I.; Ozer, S.; Kiraz, S.; Apras, S.B.; Akdogan, A.; Karadag, O.; Calguneri, M.; Kalyoncu, U. Infliximab, a TNF-alpha antagonist treatment in patients with ankylosing spondylitis: the impact on depression, anxiety and quality of life level. Rheumatol. Int. 2010, 32, 323–330. [CrossRef]
- Webers, C.; Stolwijk, C.; Schiepers, O.; Schoonbrood, T.; van Tubergen, A.; Landewé, R.; van der Heijde, D.; Boonen, A. Infliximab treatment reduces depressive symptoms in patients with ankylosing spondylitis: an ancillary study to a randomized controlled trial (ASSERT). Arthritis Res. Ther. 2020, 22, 1–11. [CrossRef]
- Karson, A.; Demirtaş, T.; Bayramgürler, D.; Balcı, F.; Utkan, T. Chronic Administration of Infliximab (TNF-α Inhibitor) Decreases Depression and Anxiety-like Behaviour in Rat Model of Chronic Mild Stress. Basic Clin. Pharmacol. Toxicol. 2012, 112, 335–340. [CrossRef]
- Bayramgürler, D.; Karson, A.; Özer, C.; Utkan, T. Effects of long-term etanercept treatment on anxiety- and depression-like neurobehaviors in rats. Physiol. Behav. 2013, 119, 145–148. [CrossRef]
- Mansur, R.B.; Delgado-Peraza, F.; Subramaniapillai, M.; Lee, Y.; Iacobucci, M.; Rodrigues, N.; Rosenblat, J.D.; Brietzke, E.; Cosgrove, V.E.; Kramer, N.E.; et al. Extracellular Vesicle Biomarkers Reveal Inhibition of Neuroinflammation by Infliximab in Association with Antidepressant Response in Adults with Bipolar Depression. Cells 2020, 9, 895. [CrossRef]
- Mansur, R.B.; Subramaniapillai, M.; Lee, Y.; Pan, Z.; Carmona, N.E.; Shekotikhina, M.; Iacobucci, M.; Rodrigues, N.; Nasri, F.; Rashidian, H.; et al. Leptin mediates improvements in cognitive function following treatment with infliximab in adults with bipolar depression. Psychoneuroendocrinology 2020, 120, 104779. [CrossRef]
- Mansur, R.B.; Subramaniapillai, M.; Lee, Y.; Pan, Z.; Carmona, N.E.; Shekotikhina, M.; Iacobucci, M.; Rodrigues, N.; Nasri, F.; Rosenblat, J.D.; et al. Effects of infliximab on brain neurochemistry of adults with bipolar depression. J. Affect. Disord. 2021, 281, 61–66. [CrossRef]
| Generic Name | Trade Name Original, Biosimilars |
Group | Molecule Type | Mechanism of Action | |
| ADL | Adalimumab | Humira, Amgevita, Hyrimoz, Idacio, Hulio, Amsparity, Imraldi | anti-TNF | Fully human monoclonal IgG1 antibody | Neutralizes TNF-α (soluble and membrane-bound forms); blocks p55/p75 receptors |
| CZP | Certolizumab Pegol | Cimzia | anti-TNF | Humanized Fab’ fragment conjugated with PEG | Neutralizes TNF-α; lacks Fc-mediated effects |
| IFX | Infliximab | Remicade, Inflectra, Remsima, Flixabi, Zessly | anti-TNF | Chimeric IgG1 monoclonal antibody (human-mouse) | Blocks TNF-α; inhibits inflammatory pathways |
| ETN | Etanercept | Enbrel, Benepali, Erelzi | anti-TNF | Fusion protein: p75 TNF receptor + Fc IgG1 | Binds TNF-α and TNF-β; acts as a decoy receptor |
| GLM | Golimumab | Simponi (s.c.), Simponi Aria (i.v.) | anti-TNF | Fully human monoclonal IgG1 antibody | Blocks TNF-α; inhibits inflammatory pathways |
| SRK | Sirukumab | None (discontinued, never approved) | anti-IL-6 | Fully human monoclonal IgG1 antibody | Neutralizes IL-6 (the cytokine, not the receptor) |
| SARI | Sarilumab | Kevzara | anti-IL-6R | Fully human monoclonal IgG1 antibody | Blocks IL-6 receptor (soluble and membrane-bound) |
| TCZ | Tocilizumab | RoActemra (EU), Actemra (USA) | anti-IL-6R | Humanized monoclonal IgG1 antibody | Blocks IL-6 receptor (soluble and membrane-bound) |
| * Only biologic drugs used in RA with documented effects on TNF-α or IL-6 pathways are included. Other biologics, such as rituximab (anti-CD20) or abatacept (T-cell costimulation inhibitor), were excluded as they are beyond the scope of this analysis focusing on neuropsychiatric outcomes. Abbreviations: ADL, adalimumab; CZP, certolizumab pegol; IFX, infliximab; ETN, etanercept; GLM, golimumab; SRK, sirukumab; SARI, sarilumab; TCZ, tocilizumab; TNF, tumor necrosis factor; IL-6, interleukin-6; IL-6R, interleukin-6 receptor; IgG1, immunoglobulin G1; Fab’, antigen-binding fragment; PEG, polyethylene glycol; Fc, crystallizable fragment of antibody; s.c., subcutaneous; i.v., intravenous. | |||||
| Study | Study Design (treatment length) | Biologic agent | Measure | Outcome |
| Miwa et al. 2014 [23] | Pilot study (30 weeks) | IFX (n=34) vs MTX (n=42) | SDS | IFX significantly improved depression vs. MTX |
| Miwa et al. 2016 [24] | Open-label cohort (6months) | IFX (n=60) vs MTX (n=53) | HAM-D | No significant difference between IFX and MTX |
| Bringham et al. 2023 [15] | Observational Phase 4 AWARE (52 weeks) | GAL (n=685) vs IFX (n= 585) | PROMIS | Improvement in all PROMIS domains incl. depression |
| Curtis et al. 2017 [ 30] | RCT Phase 4 PREDICT (52 weeks) | CZP (n=733) | Clinical data | SCP phenotype: lower treatment response, more AEs |
| Mathias et al. 2000 [25] | RCT Phase 3, double-blind (6 months) | ETN (n= 76) vs placebo (n=80) | SF-36 MOS | ETN > placebo in improving depressive symptoms |
| Bae et al. 2013[26] | Open label, multicentre (16 weeks) | ETN+MTX (n=197) vs DMARDs +MTX (n=103) | HADS | Greater improvements in ETN+MTX group (HADS) |
| Machado et al. 2014 [27] | Open label, randomized (24 weeks) | ETN+MTX (n=281) vs DMARDs+MTX (n=142) | HADS | Improvements observed in ETN+MTX depressive domains |
| Kekow et al. 2010 [28] | RCT double-blind, COMET 104 weeks | ETN+MTX (n= 274) vs MTX (n= 268) | HADS | ETN+MTX better than MTX alone in PROs |
| Kekow et al. 2011 [29] | RCT double-blind, COMET⃰ 104 weeks | ETN+MTX vs MTX | HADS | Clinical remission reduced depressive symptoms |
| Hsieh et al. 2023 [16] | Observational 24 weeks | ADL (n=100) | EQ-5D -3L | Improvements from baseline to weeks 12, 24 |
| Abbreviations: IFX, infliximab; GAL, golimumab; CZP, certolizumab pegol; ETN, etanercept; ADL, adalimumab; MTX, methotrexate; DMARDs, disease-modifying anti-rheumatic drugs; SDS, Self-Rating Depression Scale; HAM-D, Hamilton Depression Rating Scale; PROMIS, Patient-Reported Outcomes Measurement Information System; SF-36 MOS, Medical Outcomes Study 36-Item Short Form Survey; HADS, Hospital Anxiety and Depression Scale; EQ-5D-3L, EuroQol 5 Dimensions 3 Level questionnaire; RCT, randomized controlled trial; AEs, adverse events; PROs, patient-reported outcomes. ⃰Note1: The studies by Kekow et al. (2010 and 2011) are based on the same COMET trial population Note2: The total sample includes 3,027 participants across all TNF-α inhibitor studies. Among them, approximately 2,211 patients received active treatment with a TNF-α inhibitor, either as monotherapy or in combination with methotrexate (MTX), compared to placebo, MTX alone, or standard care | ||||
| Study | Study Design/ duration | Biologic agent (n) | Measures | Outcome |
| Tiosano et al.2020 [17] | Observational 24weeks |
TCZ (n=91) | HDRS | 66% of patients achieved improvements in depressive domains. |
| Harrold et al. 2017[31] | Observational cohort study 1 year |
TCZ (n= 255) | EQ-5D | 20% to 36% of patients achieved improvements in depressive state. |
| Manning- Bennett et al. 2022[32] | 5 RCT | TCZ vs DMARDs (n=5502) | Clinical | Comorbid depression was associated with less frequent remission (CDAI and SDAI) |
| Behrens 2021 et al.[33] | Observational ARATA 52 weeks | TCZ (n= 1300) | BDI-II | Patients achieved improvements in DAS-28 and PROs; however, patients with depression presented lower response and higher adverse events rates. |
| Sun et al. 2017[34] | Post hoc analysis RCT 24 weeks |
sirukumab vs siltuximab (n=176) | PDMA includinngSF-36 | Baseline solute IL-6 receptors level predicted mental health benefit. The improvement in depressive state by sirukumab correlated positivieliy with the baseline solute IL-6R levels. |
| Strand et al. 2020 [18] | Post hoc analysis RCT MONARCH phase 3 and they were treated for 24 weeks | SARI or ADL (n=148) | SF-36 | IL-6 blockade > TNF-alpha in QoL gains no difference in the mental statate. high besline IL-6 levels better improvements in physical domains with SARI compared to ADL. |
| Abbreviations: HDRS (Hamilton Depression Rating Scale); EQ-5D (EuroQol-5 dimensions-5); DMARDs (Disease-Modifying Antirheumatic Drugs); CDAI (Clinical Disease Activity Index); SDAI (Simple Disease Activity Index); PROs (Patient- Reported Outocmes); BDI (Beck Depression Inventory), PDMA (Prevalent Depressed Mood and Anhedonia); SF-36 (Short Form Survey); n- number of participants. Note: The total sample includes 7,472 participants across all IL-6 or IL-6R inhibitor studies. Approximately 4,609 patients received active treatment with an IL-6 pathway inhibitor, either alone or in combination with MTX, and were compared to MTX, placebo, or conventional DMARDs. | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





