Submitted:
30 July 2025
Posted:
31 July 2025
Read the latest preprint version here
Abstract
Keywords:
Introduction
Efferocytosis in the CNS
Efferocytosis in CNS development
Recognized Phases in EF
“Find me” Signals”
“Eat me” Molecules
Phagocytic Engulfment Receptors That Recognize PS
TAM Receptors
Triggering Receptor Expressed on Myeloid cells-2 (TREM2)
Engulfment Receptors Involved in EF Target Cell Adhesion
Spoiled for Choice: Engulfment Receptor Diversity in EF
Efferocytosis-Mediated Immune Modulation
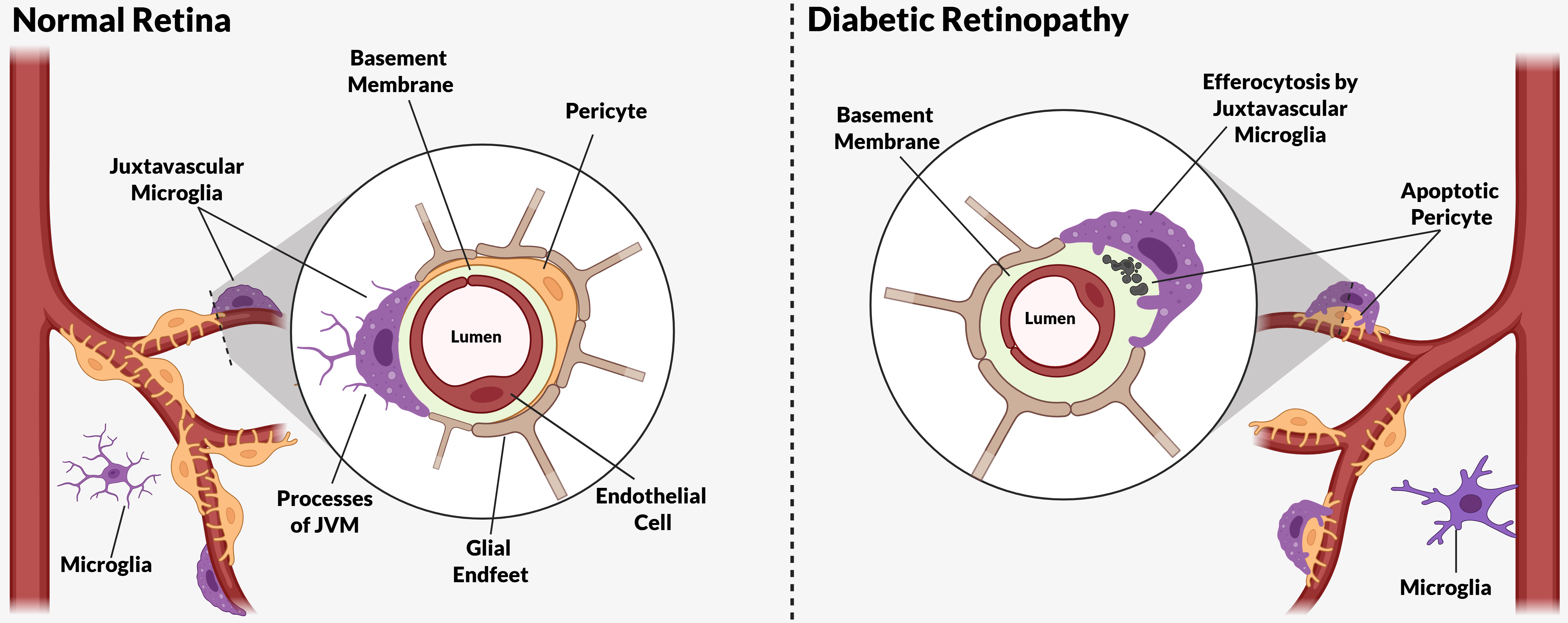
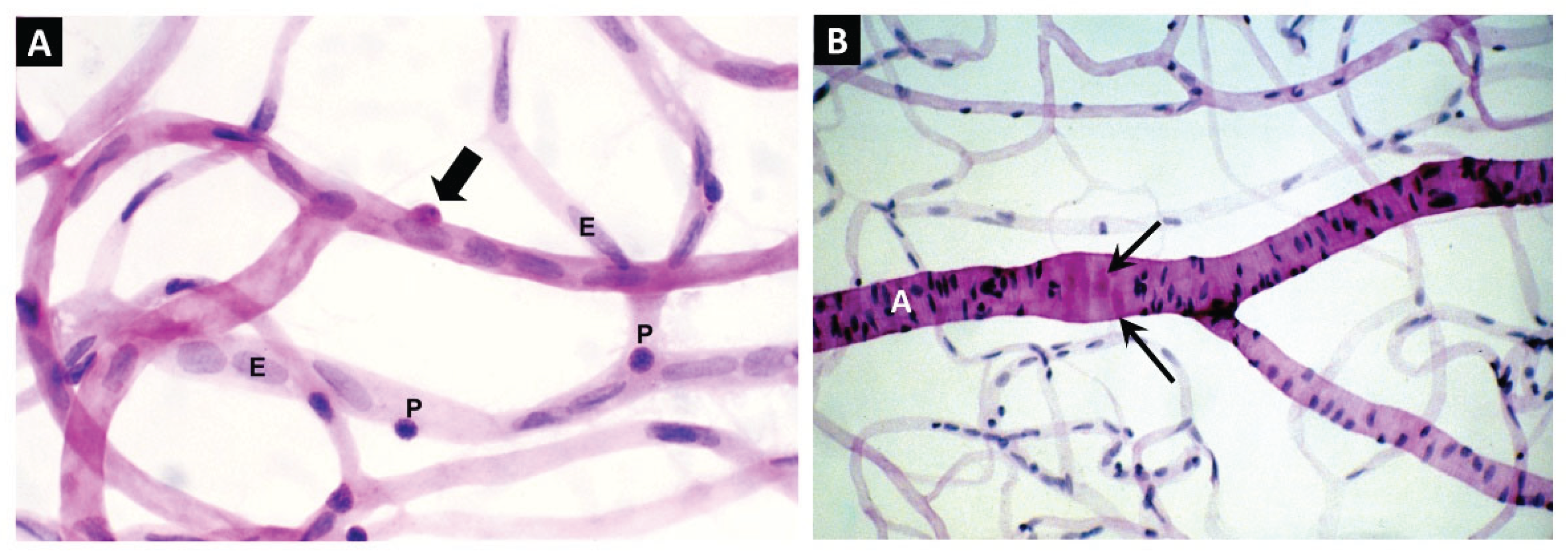
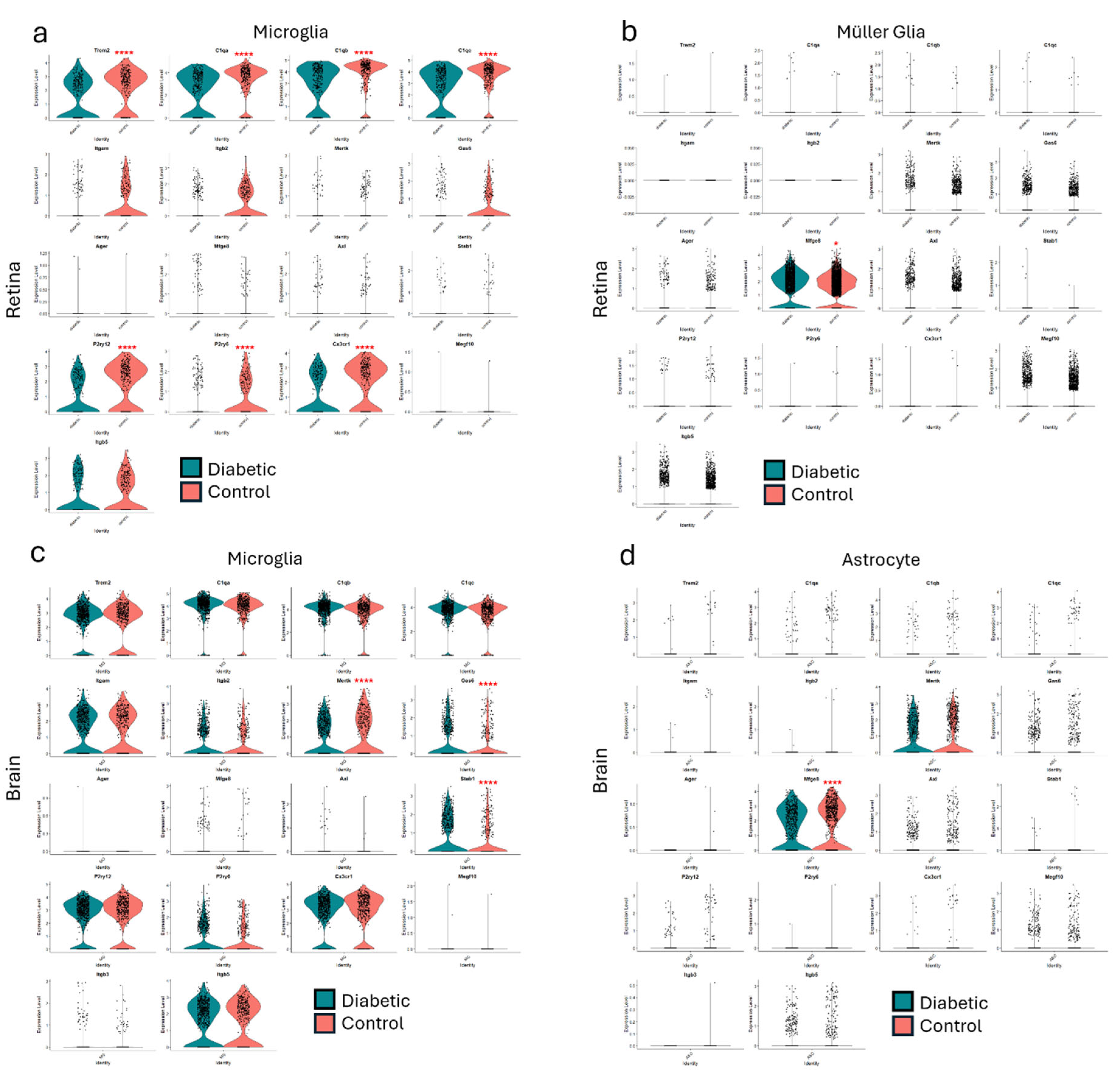
Impaired Efferocytosis in Diabetic Retinopathy
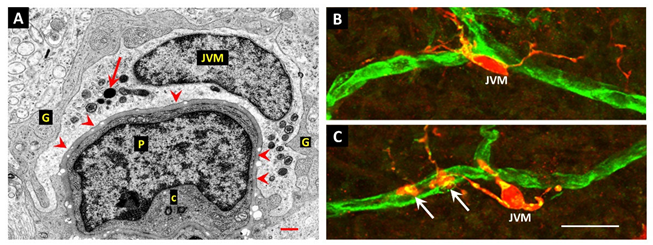
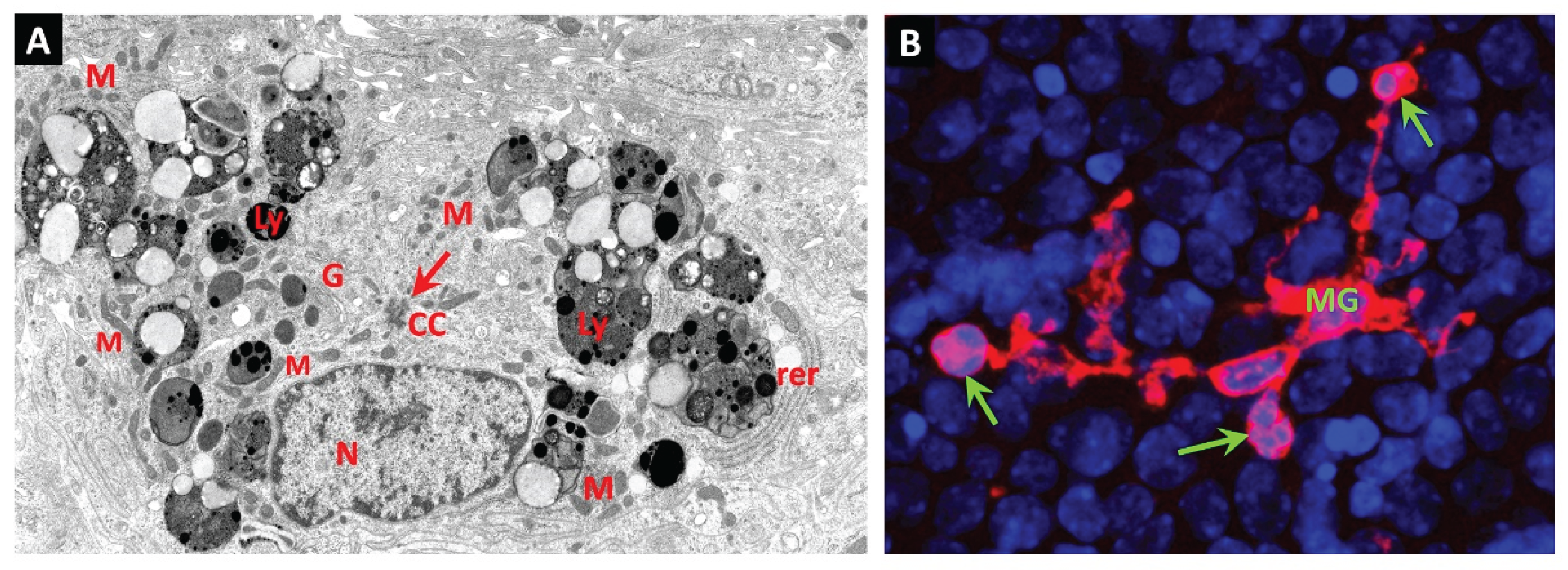
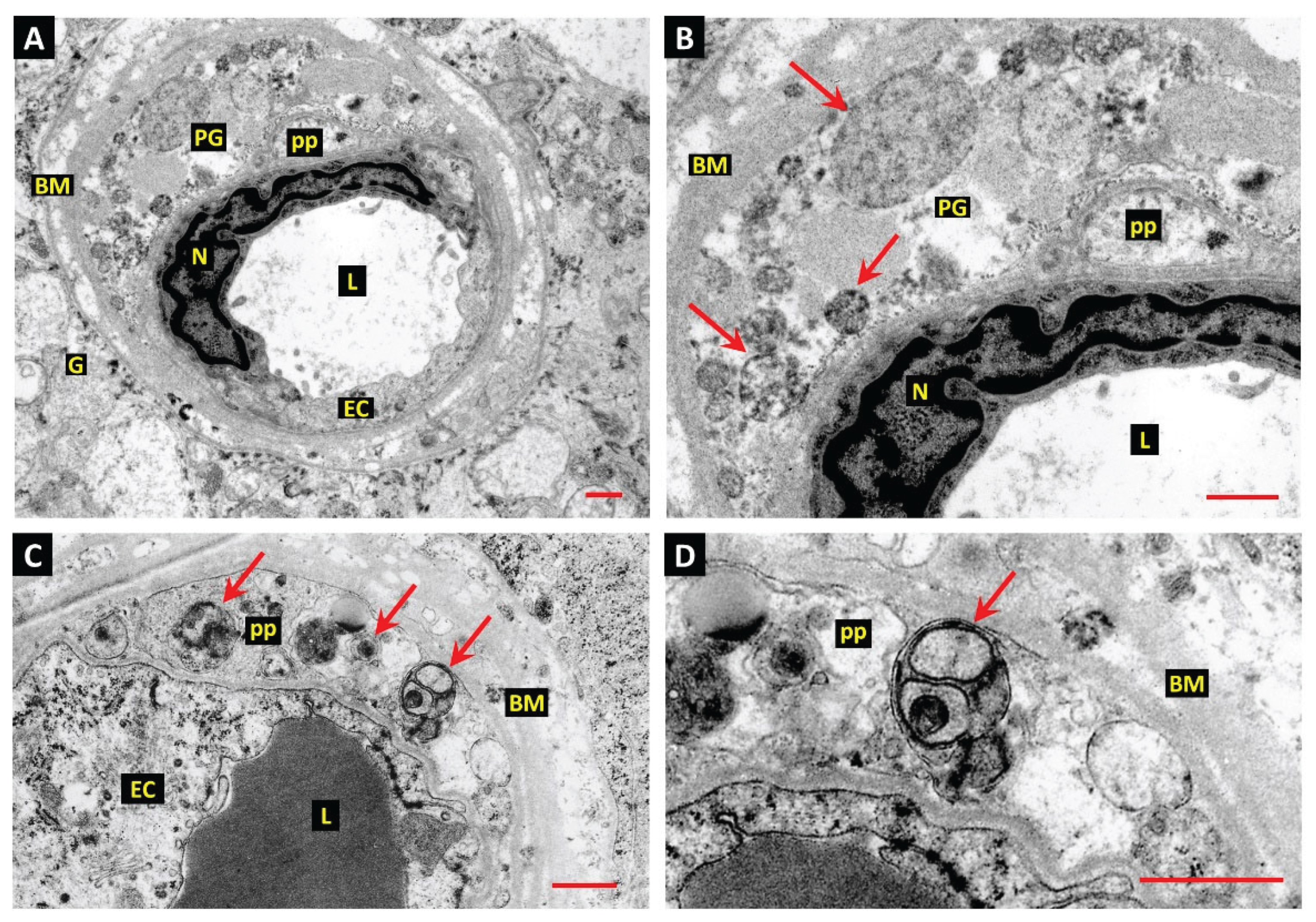
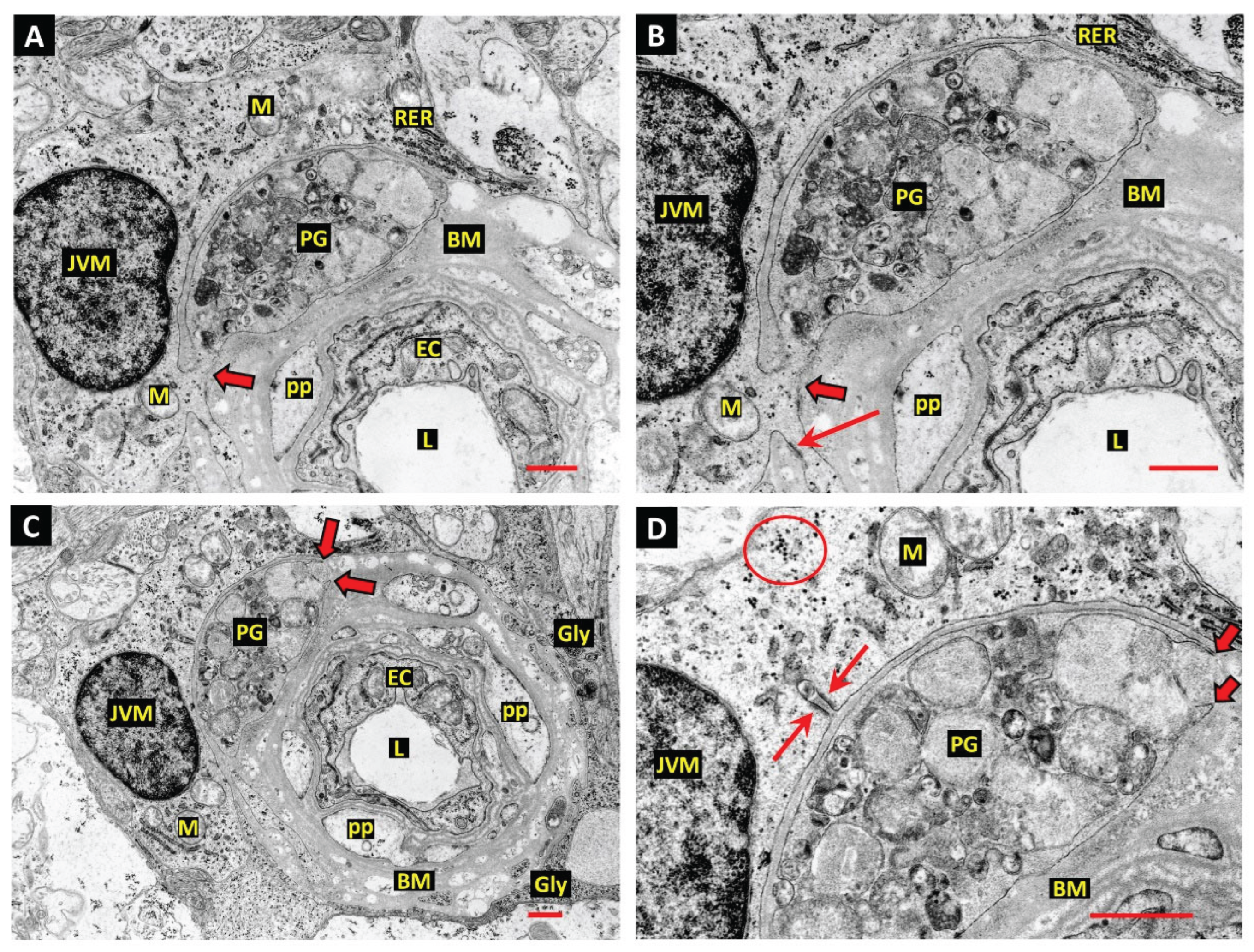
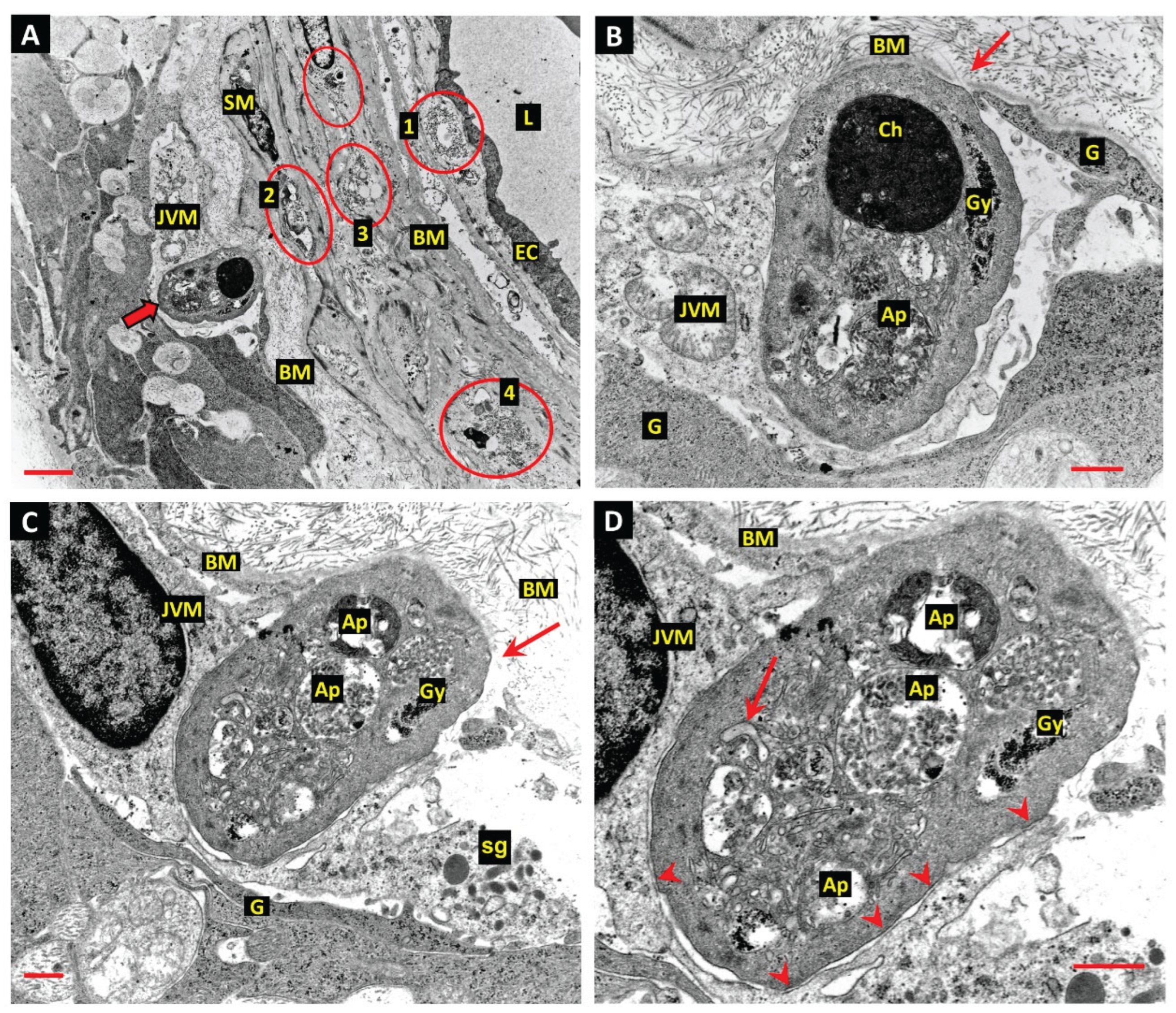
Juxtavascular Microglia (JVM) as Efferocytes of Dead Pericytes/VSMCs and their Inhibition in DR
Box 1. Juxtavascular Microglia
The Challenge of Efferocyte Access to the Vascular Basement Membranes in DR
Other Candidates for the Role of Efferocytes in EF of Apoptotic Pericytes and VSMCs
Proinflammatory Consequences of Failed EF in DR
Further Insights from Published Data
Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADAM17 | A Disintegrin and Metalloprotease 17 |
| AGE | advanced glycation endproduct |
| ApoE | apolipoprotein-E |
| ATP | Adenosine triphosphate |
| AXL | AXL receptor tyrosine kinase |
| BAI1 | brain-specific angiogenesis inhibitor 1 |
| BM | basement membrane |
| C1q | complement component-1q |
| C3 | Complement component-3 |
| CNS | central nervous system |
| CSF1R | colony-stimulating factor-1 receptor |
| CX3CL1 | CX3C motif chemokine ligand-1 (fractalkine) |
| GAL3 | Galectin-3 |
| GAS6 | growth arrest specific 6 |
| GL | glia limitans |
| HMGB1 | high mobility group box-1 |
| IL10 | Interleukin-10 |
| IL34 | interleukin-34 |
| IRF7 | interferon regulatory factor-7 |
| JVM | juxtavascular microglia |
| LPS | lysophosphatidylcholine |
| PS | phosphatidylserine |
| MEGF10 | multiple epidermal growth factor-like domains protein 10 |
| MerTK | mer proto-oncogene tyrosine kinase |
| MFGE8 | milk-fat globule-EGF factor E8 |
| MS | multiple sclerosis |
| NCAM | neural cell adhesion molecule |
| NVU | neurovascular unit |
| PTX3 | pentraxin-3 |
| PVM | perivascular macrophages |
| RAGE | receptor for advanced glycation endproducts |
| SLE | systemic lupus erythematosus |
| TAM | Tyro, Axl & MerTK receptor family |
| TGFβ | transforming growth factor beta |
| TREM2 | triggering receptor expressed on myeloid cells-2 |
| TYRO3 | tyrosine protein kinase receptor 3 |
| S1P | sphingosine-1 phosphate |
| TIM1/4 | T-cell immunoglobulin and mucin domain-containing protein 1/4 |
| UTP | uridine-5-triphosphate |
| VSMC | vascular smooth muscle cell |
References
- deCathelineau, A.M.; Henson, P.M. The final step in programmed cell death: phagocytes carry apoptotic cells to the grave. Essays in Biochemistry 2003, 39, 105–117. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: the natural outcome of the complete apoptotic program. FEBS Lett 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, W.; Zhao, M.; Liu, J.; Xu, Y.; Wan, J.; Wang, M. Mechanisms of efferocytosis in determining inflammation resolution: Therapeutic potential and the association with cardiovascular disease. British Journal of Pharmacology 2022, 179, 5151–5171. [Google Scholar] [CrossRef]
- Morioka, S.; Maueröder, C.; Ravichandran, K.S. Living on the Edge: Efferocytosis at the Interface of Homeostasis and Pathology. Immunity 2019, 50, 1149–1162. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol 2017, 198, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Arandjelovic, S.; Ravichandran, K.S. Phagocytosis of apoptotic cells in homeostasis. Nature Immunology 2015, 16, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, A.; Krejčová, G. On the origin of the functional versatility of macrophages. Front Physiol 2023, 14, 1128984. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.-m. Efferocytosis and Its Role in Inflammatory Disorders. Frontiers in Cell and Developmental Biology, 2022. [Google Scholar] [CrossRef]
- Abdolmaleki, F.; Farahani, N.; Gheibi Hayat, S.M.; Pirro, M.; Bianconi, V.; Barreto, G.E.; Sahebkar, A. The Role of Efferocytosis in Autoimmune Diseases. Frontiers in Immunology, 2018. [Google Scholar] [CrossRef]
- Mehrotra, P.; Ravichandran, K.S. Drugging the efferocytosis process: concepts and opportunities. Nature Reviews Drug Discovery 2022, 21, 601–620. [Google Scholar] [CrossRef]
- Chen, Y.; Kou, Y.; Ni, Y.; Yang, H.; Xu, C.; Fan, H.; Liu, H. Microglia efferocytosis: an emerging mechanism for the resolution of neuroinflammation in Alzheimer’s disease. Journal of Neuroinflammation 2025, 22, 96. [Google Scholar] [CrossRef]
- Xing, J.; Wang, K.; Xu, Y.-c.; Pei, Z.-j.; Yu, Q.-x.; Liu, X.-y.; Dong, Y.-l.; Li, S.-f.; Chen, Y.; Zhao, Y.-j.; et al. Efferocytosis: Unveiling its potential in autoimmune disease and treatment strategies. Autoimmunity Reviews 2024, 23, 103578. [Google Scholar] [CrossRef]
- Li, F.; Huang, Q.; Chen, J.; Peng, Y.; Roop, D.R.; Bedford, J.S.; Li, C.-Y. Apoptotic Cells Activate the “Phoenix Rising” Pathway to Promote Wound Healing and Tissue Regeneration. Science Signaling 2010, 3, ra13–ra13. [Google Scholar] [CrossRef]
- Faust, T.E.; Gunner, G.; Schafer, D.P. Mechanisms governing activity-dependent synaptic pruning in the developing mammalian CNS. Nature Reviews Neuroscience 2021, 22, 657–673. [Google Scholar] [CrossRef]
- Chung, W.-S.; Clarke, L.E.; Wang, G.X.; Stafford, B.K.; Sher, A.; Chakraborty, C.; Joung, J.; Foo, L.C.; Thompson, A.; Chen, C.; et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature 2013, 504, 394–400. [Google Scholar] [CrossRef]
- Mike, J.K.; Ferriero, D.M. Efferocytosis Mediated Modulation of Injury after Neonatal Brain Hypoxia-Ischemia. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Dorothy P. ; Lehrman, Emily K.; Kautzman, Amanda G.; Koyama, R.; Mardinly, Alan R.; Yamasaki, R.; Ransohoff, Richard M.; Greenberg, Michael E.; Barres, Ben A.; Stevens, B. Microglia Sculpt Postnatal Neural Circuits in an Activity and Complement-Dependent Manner. Neuron 2012, 74, 691–705. [Google Scholar] [CrossRef]
- Stevens, B.; Allen, N.J.; Vazquez, L.E.; Howell, G.R.; Christopherson, K.S.; Nouri, N.; Micheva, K.D.; Mehalow, A.K.; Huberman, A.D.; Stafford, B.; et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell 2007, 131, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Choi, Y.; Jung, E.; Lee, S.H.; Sohn, J.W.; Chung, W.S. Microglial MERTK eliminates phosphatidylserine-displaying inhibitory post-synapses. Embo j 2021, 40, e107121. [Google Scholar] [CrossRef]
- Damisah, E.C.; Hill, R.A.; Rai, A.; Chen, F.; Rothlin, C.V.; Ghosh, S.; Grutzendler, J. Astrocytes and microglia play orchestrated roles and respect phagocytic territories during neuronal corpse removal in vivo. Science Advances 2020, 6, eaba3239. [Google Scholar] [CrossRef]
- Halassa, M.M.; Fellin, T.; Haydon, P.G. The tripartite synapse: roles for gliotransmission in health and disease. Trends in Molecular Medicine 2007, 13, 54–63. [Google Scholar] [CrossRef]
- Freeman, M.R. Specification and Morphogenesis of Astrocytes. Science 2010, 330, 774–778. [Google Scholar] [CrossRef]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the Brain: Homeostasis and Disease. Frontiers in Immunology, 2019. [Google Scholar] [CrossRef]
- Bejarano-Escobar, R.; Sánchez-Calderón, H.; Otero-Arenas, J.; Martín-Partido, G.; Francisco-Morcillo, J. Müller glia and phagocytosis of cell debris in retinal tissue. Journal of Anatomy 2017, 231, 471–483. [Google Scholar] [CrossRef]
- Ginhoux, F.; Prinz, M. Origin of Microglia: Current Concepts and Past Controversies. Cold Spring Harbor Perspectives in Biology 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Alliot, F.; Godin, I.; Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Developmental Brain Research 1999, 117, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Chow, A.; Noizat, C.; Teo, P.; Beasley, Mary B. ; Leboeuf, M.; Becker, Christian D.; See, P.; Price, J.; Lucas, D.; et al. Tissue-Resident Macrophages Self-Maintain Locally throughout Adult Life with Minimal Contribution from Circulating Monocytes. Immunity 2013, 38, 792–804. [Google Scholar] [CrossRef]
- Prinz, M.; Jung, S.; Priller, J. Microglia Biology: One Century of Evolving Concepts. Cell 2019, 179, 292–311. [Google Scholar] [CrossRef]
- Möller, K.; Brambach, M.; Villani, A.; Gallo, E.; Gilmour, D.; Peri, F. A role for the centrosome in regulating the rate of neuronal efferocytosis by microglia in vivo. eLife 2022, 11, e82094. [Google Scholar] [CrossRef]
- Thion, M.S.; Garel, S. On place and time: microglia in embryonic and perinatal brain development. Current Opinion in Neurobiology 2017, 47, 121–130. [Google Scholar] [CrossRef]
- Wu, S.; Xue, R.; Hassan, S.; Nguyen, T.M.L.; Wang, T.; Pan, H.; Xu, J.; Liu, Q.; Zhang, W.; Wen, Z. Il34-Csf1r Pathway Regulates the Migration and Colonization of Microglial Precursors. Developmental Cell 2018, 46, 552–563.e554. [Google Scholar] [CrossRef]
- Francisco-Morcillo, J.; Bejarano-Escobar, R.; Rodríguez-León, J.; Navascués, J.; Martín-Partido, G. Ontogenetic Cell Death and Phagocytosis in the Visual System of Vertebrates. Developmental Dynamics 2014, 243, 1203–1225. [Google Scholar] [CrossRef]
- Ranawat, N.; Masai, I. Mechanisms underlying microglial colonization of developing neural retina in zebrafish. Elife 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.M.; Calvente, R.; Tassi, M.; Carrasco, M.-C.; Martín-Oliva, D.; Marín-Teva, J.L.; Navascués, J.; Cuadros, M.A. Embryonic and postnatal development of microglial cells in the mouse retina. Journal of Comparative Neurology 2008, 506, 224–239. [Google Scholar] [CrossRef] [PubMed]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The Cell Biology of Phagocytosis. Annual Review of Pathology: Mechanisms of Disease 2012, 7, 61–98. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.M.; Ravichandran, K.S. Putting the brakes on phagocytosis: “don’t-eat-me” signaling in physiology and disease. EMBO reports 2021, 22, e52564. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nature Reviews Neuroscience 2014, 15, 209–216. [Google Scholar] [CrossRef]
- Neher, J.J.; Emmrich, J.V.; Fricker, M.; Mander, P.K.; Théry, C.; Brown, G.C. Phagocytosis executes delayed neuronal death after focal brain ischemia. Proceedings of the National Academy of Sciences 2013, 110, E4098–E4107. [Google Scholar] [CrossRef]
- Brown, G.C.; Neher, J.J. Eaten alive! Cell death by primary phagocytosis: 'phagoptosis'. Trends in Biochemical Sciences 2012, 37, 325–332. [Google Scholar] [CrossRef]
- Park, S.-Y.; Kim, I.-S. Engulfment signals and the phagocytic machinery for apoptotic cell clearance. Experimental & Molecular Medicine 2017, 49, e331–e331. [Google Scholar] [CrossRef]
- Doran, A.C.; Yurdagul, A.; Tabas, I. Efferocytosis in health and disease. Nature Reviews Immunology 2020, 20, 254–267. [Google Scholar] [CrossRef]
- Lam, A.L.; Heit, B. Having an Old Friend for Dinner: The Interplay between Apoptotic Cells and Efferocytes. Cells 2021, 10, 1265. [Google Scholar] [CrossRef]
- Angelopoulou, E.; Paudel, Y.N.; Shaikh, M.F.; Piperi, C. Fractalkine (CX3CL1) signaling and neuroinflammation in Parkinson’s disease: Potential clinical and therapeutic implications. Pharmacological Research 2020, 158, 104930. [Google Scholar] [CrossRef]
- Sokolowski, J.D.; Chabanon-Hicks, C.N.; Han, C.Z.; Heffron, D.S.; Mandell, J.W. Fractalkine is a "find-me" signal released by neurons undergoing ethanol-induced apoptosis. Front Cell Neurosci 2014, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, D.; Church, K.A.; Pietramale, A.N.; Cardona, S.M.; Vanegas, D.; Rorex, C.; Leary, M.C.; Muzzio, I.A.; Nash, K.R.; Cardona, A.E. Fractalkine isoforms differentially regulate microglia-mediated inflammation and enhance visual function in the diabetic retina. Journal of Neuroinflammation 2024, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Kielbik, M.; Szulc-Kielbik, I.; Klink, M. Calreticulin-Multifunctional Chaperone in Immunogenic Cell Death: Potential Significance as a Prognostic Biomarker in Ovarian Cancer Patients. Cells 2021, 10. [Google Scholar] [CrossRef] [PubMed]
- Vorselen, D. Dynamics of phagocytosis mediated by phosphatidylserine. Biochemical Society Transactions 2022, 50, 1281–1291. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. The Journal of Immunology 1992, 148, 2207–2216. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nature Reviews Immunology 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Freeman, G.J.; Casasnovas, J.M.; Umetsu, D.T.; DeKruyff, R.H. TIM genes: a family of cell surface phosphatidylserine receptors that regulate innate and adaptive immunity. Immunological Reviews 2010, 235, 172–189. [Google Scholar] [CrossRef]
- Mazaheri, F.; Breus, O.; Durdu, S.; Haas, P.; Wittbrodt, J.; Gilmour, D.; Peri, F. Distinct roles for BAI1 and TIM-4 in the engulfment of dying neurons by microglia. Nature Communications 2014, 5, 4046. [Google Scholar] [CrossRef]
- Hudson, B.I.; Lippman, M.E. Targeting RAGE Signaling in Inflammatory Disease. Annual Review of Medicine 2018, 69, 349–364. [Google Scholar] [CrossRef]
- Derk, J.; MacLean, M.; Juranek, J.; Schmidt, A.M. The Receptor for Advanced Glycation Endproducts (RAGE) and Mediation of Inflammatory Neurodegeneration. J Alzheimers Dis Parkinsonism 2018, 8. [Google Scholar] [CrossRef] [PubMed]
- Alí-Ruiz, D.; Vitureira, N.; Peluffo, H. Microglial CD300f immune receptor contributes to the maintenance of neuron viability in vitro and after a penetrating brain injury. Scientific Reports 2023, 13, 16796. [Google Scholar] [CrossRef] [PubMed]
- Lemke, G. Biology of the TAM receptors. Cold Spring Harb Perspect Biol 2013, 5, a009076. [Google Scholar] [CrossRef] [PubMed]
- Caberoy, N.B.; Alvarado, G.; Bigcas, J.-L.; Li, W. Galectin-3 is a new MerTK-specific eat-me signal. Journal of Cellular Physiology 2012, 227, 401–407. [Google Scholar] [CrossRef]
- Nomura, K.; Vilalta, A.; Allendorf, D.H.; Hornik, T.C.; Brown, G.C. Activated Microglia Desialylate and Phagocytose Cells via Neuraminidase, Galectin-3, and Mer Tyrosine Kinase. The Journal of Immunology 2017, 198, 4792–4801. [Google Scholar] [CrossRef]
- Mendonça, H.R.; Carvalho, J.N.A.; Abreu, C.A.; Mariano de Souza Aguiar dos Santos, D.; Carvalho, J.R.; Marques, S.A.; da Costa Calaza, K.; Martinez, A.M.B. Lack of Galectin-3 attenuates neuroinflammation and protects the retina and optic nerve of diabetic mice. Brain Research 2018, 1700, 126–137. [Google Scholar] [CrossRef]
- Uehara, F.; Ohba, N.; Ozawa, M. Isolation and Characterization of Galectins in the Mammalian Retina. Investigative Ophthalmology & Visual Science 2001, 42, 2164–2172. [Google Scholar]
- Lew, D.S.; McGrath, M.J.; Finnemann, S.C. Galectin-3 Promotes Müller Glia Clearance Phagocytosis via MERTK and Reduces Harmful Müller Glia Activation in Inherited and Induced Retinal Degeneration. Frontiers in Cellular Neuroscience, 2022. [Google Scholar] [CrossRef]
- Lemke, G.; Rothlin, C.V. Immunobiology of the TAM receptors. Nature Reviews Immunology 2008, 8, 327–336. [Google Scholar] [CrossRef]
- Zagórska, A.; Través, P.G.; Lew, E.D.; Dransfield, I.; Lemke, G. Diversification of TAM receptor tyrosine kinase function. Nature Immunology 2014, 15, 920–928. [Google Scholar] [CrossRef]
- Shen, K.; Reichelt, M.; Kyauk, R.V.; Ngu, H.; Shen, Y.-A.A.; Foreman, O.; Modrusan, Z.; Friedman, B.A.; Sheng, M.; Yuen, T.J. Multiple sclerosis risk gene Mertk is required for microglial activation and subsequent remyelination. Cell Reports 2021, 34. [Google Scholar] [CrossRef]
- Fourgeaud, L.; Través, P.G.; Tufail, Y.; Leal-Bailey, H.; Lew, E.D.; Burrola, P.G.; Callaway, P.; Zagórska, A.; Rothlin, C.V.; Nimmerjahn, A.; et al. TAM receptors regulate multiple features of microglial physiology. Nature 2016, 532, 240–244. [Google Scholar] [CrossRef]
- Gautier, E.L.; Shay, T.; Miller, J.; Greter, M.; Jakubzick, C.; Ivanov, S.; Helft, J.; Chow, A.; Elpek, K.G.; Gordonov, S.; et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nature Immunology 2012, 13, 1118–1128. [Google Scholar] [CrossRef]
- Duncan, J.L.; LaVail, M.M.; Yasumura, D.; Matthes, M.T.; Yang, H.; Trautmann, N.; Chappelow, A.V.; Feng, W.; Earp, H.S.; Matsushima, G.K.; et al. An RCS-Like Retinal Dystrophy Phenotype in Mer Knockout Mice. Investigative Ophthalmology & Visual Science 2003, 44, 826–838. [Google Scholar] [CrossRef]
- Gal, A.; Li, Y.; Thompson, D.A.; Weir, J.; Orth, U.; Jacobson, S.G.; Apfelstedt-Sylla, E.; Vollrath, D. Mutations in MERTK, the human orthologue of the RCS rat retinal dystrophy gene, cause retinitis pigmentosa. Nature Genetics 2000, 26, 270–271. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, P.M.; Yasumura, D.; Weir, J.; Matthes, M.T.; Abderrahim, H.; LaVail, M.M.; Vollrath, D. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Human Molecular Genetics 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hu, L.; Li, J.; Ruan, W.; Cao, Y.; Zhuang, J.; Xu, H.; Peng, Y.; Zhang, Z.; Xu, C.; et al. AXL kinase-mediated astrocytic phagocytosis modulates outcomes of traumatic brain injury. Journal of Neuroinflammation 2021, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiang, X.; Li, Y.; Bu, G.; Chen, X.-F. TREM2 and sTREM2 in Alzheimer’s disease: from mechanisms to therapies. Molecular Neurodegeneration 2025, 20, 43. [Google Scholar] [CrossRef]
- Colonna, M. The biology of TREM receptors. Nature Reviews Immunology 2023, 23, 580–594. [Google Scholar] [CrossRef]
- Gao, H.; Di, J.; Clausen, B.H.; Wang, N.; Zhu, X.; Zhao, T.; Chang, Y.; Pang, M.; Yang, Y.; He, R.; et al. Distinct myeloid population phenotypes dependent on TREM2 expression levels shape the pathology of traumatic versus demyelinating CNS disorders. Cell Reports 2023, 42. [Google Scholar] [CrossRef]
- Deczkowska, A.; Weiner, A.; Amit, I. The Physiology, Pathology, and Potential Therapeutic Applications of the TREM2 Signaling Pathway. Cell 2020, 181, 1207–1217. [Google Scholar] [CrossRef]
- Karch, C.M.; Goate, A.M. Alzheimer's disease risk genes and mechanisms of disease pathogenesis. Biol Psychiatry 2015, 77, 43–51. [Google Scholar] [CrossRef]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer's disease. N Engl J Med 2013, 368, 107–116. [Google Scholar] [CrossRef]
- Guerreiro, R.; Wojtas, A.; Bras, J.; Carrasquillo, M.; Rogaeva, E.; Majounie, E.; Cruchaga, C.; Sassi, C.; Kauwe, J.S.K.; Younkin, S.; et al. TREM2 Variants in Alzheimer's Disease. New England Journal of Medicine 2013, 368, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Yuan, P.; Condello, C.; Keene, C.D.; Wang, Y.; Bird, Thomas D. ; Paul, Steven M.; Luo, W.; Colonna, M.; Baddeley, D.; Grutzendler, J. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 2016, 90, 724–739. [Google Scholar] [CrossRef]
- Wang, Y.; Ulland, T.K.; Ulrich, J.D.; Song, W.; Tzaferis, J.A.; Hole, J.T.; Yuan, P.; Mahan, T.E.; Shi, Y.; Gilfillan, S.; et al. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J Exp Med 2016, 213, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wu, X.; Li, X.; Jiang, L.L.; Gui, X.; Liu, Y.; Sun, Y.; Zhu, B.; Piña-Crespo, J.C.; Zhang, M.; et al. TREM2 Is a Receptor for β-Amyloid that Mediates Microglial Function. Neuron 2018, 97, 1023–1031.e1027. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.L.; Koike, M.; Spusta, S.C.; Niemi, E.C.; Yenari, M.; Nakamura, M.C.; Seaman, W.E. A role for TREM2 ligands in the phagocytosis of apoptotic neuronal cells by microglia. Journal of Neurochemistry 2009, 109, 1144–1156. [Google Scholar] [CrossRef]
- Takahashi, K.; Rochford, C.D.P.; Neumann, H. Clearance of apoptotic neurons without inflammation by microglial triggering receptor expressed on myeloid cells-2. Journal of Experimental Medicine 2005, 201, 647–657. [Google Scholar] [CrossRef]
- Wang, Y.; Cella, M.; Mallinson, K.; Ulrich, Jason D. ; Young, Katherine L.; Robinette, Michelle L.; Gilfillan, S.; Krishnan, Gokul M.; Sudhakar, S.; Zinselmeyer, Bernd H.; et al. TREM2 Lipid Sensing Sustains the Microglial Response in an Alzheime's Disease Model. Cell 2015, 160, 1061–1071. [Google Scholar] [CrossRef]
- Bailey, C.C.; DeVaux, L.B.; Farzan, M. The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E. J Biol Chem 2015, 290, 26033–26042. [Google Scholar] [CrossRef]
- Atagi, Y.; Liu, C.-C.; Painter, M.M.; Chen, X.-F.; Verbeeck, C.; Zheng, H.; Li, X.; Rademakers, R.; Kang, S.S.; Xu, H.; et al. Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2) *. Journal of Biological Chemistry 2015, 290, 26043–26050. [Google Scholar] [CrossRef]
- Grainger, D.J.; Reckless, J.; McKilligin, E. Apolipoprotein E Modulates Clearance of Apoptotic Bodies In Vitro and In Vivo, Resulting in a Systemic Proinflammatory State in Apolipoprotein E-Deficient Mice1. The Journal of Immunology 2004, 173, 6366–6375. [Google Scholar] [CrossRef]
- Mazaheri, F.; Snaidero, N.; Kleinberger, G.; Madore, C.; Daria, A.; Werner, G.; Krasemann, S.; Capell, A.; Trümbach, D.; Wurst, W.; et al. TREM2 deficiency impairs chemotaxis and microglial responses to neuronal injury. EMBO reports 2017, 18, 1186–1198. [Google Scholar] [CrossRef]
- Jaumouillé, V.; Waterman, C.M. Physical Constraints and Forces Involved in Phagocytosis. Frontiers in Immunology, 2020. [Google Scholar] [CrossRef]
- Henson, P.M.; Hume, D.A. Apoptotic cell removal in development and tissue homeostasis. Trends in Immunology 2006, 27, 244–250. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Canton, J.; Furuya, W.; Glogauer, M.; Grinstein, S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Mol Biol Cell 2014, 25, 1511–1522. [Google Scholar] [CrossRef] [PubMed]
- Mylvaganam, S.; Freeman, S.A.; Grinstein, S. The cytoskeleton in phagocytosis and macropinocytosis. Current Biology 2021, 31, R619–R632. [Google Scholar] [CrossRef] [PubMed]
- Park, B.; Lee, J.; Moon, H.; Lee, G.; Lee, D.H.; Hoon Cho, J.; Park, D. Co-receptors are dispensable for tethering receptor-mediated phagocytosis of apoptotic cells. Cell Death & Disease 2015, 6, e1772–e1772. [Google Scholar] [CrossRef]
- Dransfield, I.; Zagórska, A.; Lew, E.D.; Michail, K.; Lemke, G. Mer receptor tyrosine kinase mediates both tethering and phagocytosis of apoptotic cells. Cell Death & Disease 2015, 6, e1646–e1646. [Google Scholar] [CrossRef]
- Sokolova, D.; Ghansah, S.A.; Puletti, F.; Georgiades, T.; De Schepper, S.; Zheng, Y.; Crowley, G.; Wu, L.; Rueda-Carrasco, J.; Koutsiouroumpa, A.; et al. Astrocyte-derived MFG-E8 facilitates microglial synapse elimination in Alzheimer's disease mouse models. bioRxiv 2024. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Cambier, S.; Proost, P.; Marques, P.E. Complement Receptors and Their Role in Leukocyte Recruitment and Phagocytosis. Front Cell Dev Biol 2021, 9, 624025. [Google Scholar] [CrossRef]
- Scott-Hewitt, N.; Perrucci, F.; Morini, R.; Erreni, M.; Mahoney, M.; Witkowska, A.; Carey, A.; Faggiani, E.; Schuetz, L.T.; Mason, S.; et al. Local externalization of phosphatidylserine mediates developmental synaptic pruning by microglia. The EMBO Journal 2020, 39, e105380. [Google Scholar] [CrossRef] [PubMed]
- Païdassi, H.; Tacnet-Delorme, P.; Garlatti, V.; Darnault, C.; Ghebrehiwet, B.; Gaboriaud, C.; Arlaud, G.r.J.; Frachet, P. C1q Binds Phosphatidylserine and Likely Acts as a Multiligand-Bridging Molecule in Apoptotic Cell Recognition1. The Journal of Immunology 2008, 180, 2329–2338. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Chu, S.-H.; Hernandez, M.X.; Fang, M.J.; Modarresi, L.; Selvan, P.; MacGregor, G.R.; Tenner, A.J. Cell-specific deletion of C1qa identifies microglia as the dominant source of C1q in mouse brain. Journal of Neuroinflammation 2017, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Dickson, B.H.; Tasnim, T.; Lam, A.L.; Vreize, A.; Blythe, E.N.; Dekaban, G.A.; Heit, B. MERTK Coordinates Efferocytosis by Regulating Integrin Localization and Activation. bioRxiv, 2007. [Google Scholar] [CrossRef]
- Flannagan, R.S.; Jaumouillé, V.; Grinstein, S. The cell biology of phagocytosis. Annu Rev Pathol 2012, 7, 61–98. [Google Scholar] [CrossRef]
- Ling, L.; Templeton, D.; Kung, H.-J. Identification of the Major Autophosphorylation Sites of Nyk/Mer, an NCAM-related Receptor Tyrosine Kinase*. Journal of Biological Chemistry 1996, 271, 18355–18362. [Google Scholar] [CrossRef]
- Gordon, S. Phagocytosis: An Immunobiologic Process. Immunity 2016, 44, 463–475. [Google Scholar] [CrossRef]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. The Journal of Clinical Investigation 1998, 101, 890–898. [Google Scholar] [CrossRef]
- Noda, M.; Doi, Y.; Liang, J.; Kawanokuchi, J.; Sonobe, Y.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Fractalkine Attenuates Excito-neurotoxicity via Microglial Clearance of Damaged Neurons and Antioxidant Enzyme Heme Oxygenase-1 Expression. Journal of Biological Chemistry 2011, 286, 2308–2319. [Google Scholar] [CrossRef]
- Mizuno, T.; Kawanokuchi, J.; Numata, K.; Suzumura, A. Production and neuroprotective functions of fractalkine in the central nervous system. Brain Research 2003, 979, 65–70. [Google Scholar] [CrossRef]
- Bonnefoy, F.; Gauthier, T.; Vallion, R.; Martin-Rodriguez, O.; Missey, A.; Daoui, A.; Valmary-Degano, S.; Saas, P.; Couturier, M.; Perruche, S. Factors Produced by Macrophages Eliminating Apoptotic Cells Demonstrate Pro-Resolutive Properties and Terminate Ongoing Inflammation. Frontiers in Immunology, 2018. [Google Scholar] [CrossRef]
- Stitt, A.W.; Curtis, T.M.; Chen, M.; Medina, R.J.; McKay, G.J.; Jenkins, A.; Gardiner, T.A.; Lyons, T.J.; Hammes, H.-P.; Simó, R.; et al. The progress in understanding and treatment of diabetic retinopathy. Progress in Retinal and Eye Research 2016, 51, 156–186. [Google Scholar] [CrossRef]
- Curtis, T.M.; Gardiner, T.A.; Stitt, A.W. Microvascular lesions of diabetic retinopathy: clues towards understanding pathogenesis? Eye (Lond) 2009, 23, 1496–1508. [Google Scholar] [CrossRef]
- Feenstra, D.J.; Yego, E.C.; Mohr, S. Modes of Retinal Cell Death in Diabetic Retinopathy. J Clin Exp Ophthalmol 2013, 4, 298. [Google Scholar] [CrossRef] [PubMed]
- Cogan, D.G.; Toussaint, D.; Kuwabara, T. Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 1961, 66, 366–378. [Google Scholar] [CrossRef]
- Barber, A.J.; Gardner, T.W.; Abcouwer, S.F. The Significance of Vascular and Neural Apoptosis to the Pathology of Diabetic Retinopathy. Investigative Ophthalmology & Visual Science 2011, 52, 1156–1163. [Google Scholar] [CrossRef]
- Gastinger, M.J.; Singh, R.S.J.; Barber, A.J. Loss of Cholinergic and Dopaminergic Amacrine Cells in Streptozotocin-Diabetic Rat and Ins2Akita-Diabetic Mouse Retinas. Investigative Ophthalmology & Visual Science 2006, 47, 3143–3150. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.-T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and Blood-Retinal Barrier-Preserving Effects of Cannabidiol in Experimental Diabetes. The American journal of pathology 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Barber, A.J.; Antonetti, D.A.; Kern, T.S.; Reiter, C.E.N.; Soans, R.S.; Krady, J.K.; Levison, S.W.; Gardner, T.W.; Bronson, S.K. The Ins2Akita Mouse as a Model of Early Retinal Complications in Diabetes. Investigative Ophthalmology & Visual Science 2005, 46, 2210–2218. [Google Scholar] [CrossRef]
- Martin, P.M.; Roon, P.; Van Ells, T.K.; Ganapathy, V.; Smith, S.B. Death of Retinal Neurons in Streptozotocin-Induced Diabetic Mice. Investigative Ophthalmology & Visual Science 2004, 45, 3330–3336. [Google Scholar] [CrossRef]
- Kanamori, A.; Makoto, N.; Hirokazu, M.; Hidetaka, M.; and Negi, A. Diabetes has an additive effect on neural apoptosis in rat retina with chronically elevated intraocular pressure. Current eye research 2004, 28, 47–54. [Google Scholar] [CrossRef]
- Zeng, X.-X.; Ng, Y.-K.; Ling, E.-A. Neuronal and microglial response in the retina of streptozotocin-induced diabetic rats. Visual Neuroscience 2000, 17, 463–471. [Google Scholar] [CrossRef]
- Barber, A.J.; Lieth, E.; Khin, S.A.; Antonetti, D.A.; Buchanan, A.G.; Gardner, T.W. Neural apoptosis in the retina during experimental and human diabetes. Early onset and effect of insulin. The Journal of Clinical Investigation 1998, 102, 783–791. [Google Scholar] [CrossRef]
- Shahror, R.A.; Shosha, E.; Morris, C.; Wild, M.; Mu, S.; Csanyi, G.; Boerma, M.; Rusch, N.J.; Fouda, A.Y. Deletion of myeloid HDAC3 promotes efferocytosis to ameliorate retinal ischemic injury. Journal of Neuroinflammation 2024, 21, 170. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Archer, D.B.; Curtis, T.M.; Stitt, A.W. Arteriolar involvement in the microvascular lesions of diabetic retinopathy: implications for pathogenesis. Microcirculation 2007, 14, 25–38. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Stitt, A.W.; Anderson, H.R.; Archer, D.B. Selective loss of vascular smooth muscle cells in the retinal microcirculation of diabetic dogs. Br J Ophthalmol 1994, 78, 54–60. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Stitt, A.W. Pericyte and Vascular Smooth Muscle Death in Diabetic Retinopathy Involves Autophagy. International Journal of Translational Medicine 2022, 2, 26–40. [Google Scholar] [CrossRef]
- Gardiner, T.A.; Stitt, A.W. Juxtavascular Microglia Scavenge Dying Pericytes and Vascular Smooth Muscle Cells in Diabetic Retinopathy. International Journal of Translational Medicine 2022, 2, 41–50. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Vandenabeele, P.; Abrams, J.; Alnemri, E.S.; Baehrecke, E.H.; Blagosklonny, M.V.; El-Deiry, W.S.; Golstein, P.; Green, D.R.; et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death Differ 2009, 16, 3–11. [Google Scholar] [CrossRef]
- Kroemer, G.; El-Deiry, W.S.; Golstein, P.; Peter, M.E.; Vaux, D.; Vandenabeele, P.; Zhivotovsky, B.; Blagosklonny, M.V.; Malorni, W.; Knight, R.A.; et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death. Cell Death Differ 2005, 12 Suppl 2, 1463–1467. [Google Scholar] [CrossRef]
- Baehrecke, E.H. Autophagy: dual roles in life and death? Nat Rev Mol Cell Biol 2005, 6, 505–510. [Google Scholar] [CrossRef]
- Lieberthal, W.; Menza, S.A.; Levine, J.S. Graded ATP depletion can cause necrosis or apoptosis of cultured mouse proximal tubular cells. Am J Physiol 1998, 274, F315–327. [Google Scholar] [CrossRef]
- Lelli, J.L.; Becks, L.L.; Dabrowska, M.I.; Hinshaw, D.B. ATP converts necrosis to apoptosis in oxidant-injured endothelial cells. Free Radical Biology and Medicine 1998, 25, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ 2018, 25, 486–541. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.M. Autophagic Cell Death During Development – Ancient and Mysterious. Frontiers in Cell and Developmental Biology, 2021. [Google Scholar] [CrossRef]
- Mills, J.C.; Stone, N.L.; Erhardt, J.; Pittman, R.N. Apoptotic membrane blebbing is regulated by myosin light chain phosphorylation. J Cell Biol 1998, 140, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, M.; Kern, T.S.; Lorenzi, M. Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 1996, 97, 2883–2890. [Google Scholar] [CrossRef]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef]
- Taylor, S.; Mehina, E.; White, E.; Reeson, P.; Yongblah, K.; Doyle, K.P.; Brown, C.E. Suppressing Interferon-γ Stimulates Microglial Responses and Repair of Microbleeds in the Diabetic Brain. The Journal of Neuroscience 2018, 38, 8707–8722. [Google Scholar] [CrossRef]
- Tang, J.; Kern, T.S. Inflammation in diabetic retinopathy. Progress in Retinal and Eye Research 2011, 30, 343–358. [Google Scholar] [CrossRef]
- Forrester, J.V.; Kuffova, L.; Delibegovic, M. The Role of Inflammation in Diabetic Retinopathy. Front Immunol 2020, 11, 583687. [Google Scholar] [CrossRef]
- Lou, N.; Takano, T.; Pei, Y.; Xavier, A.L.; Goldman, S.A.; Nedergaard, M. Purinergic receptor P2RY12-dependent microglial closure of the injured blood-brain barrier. Proc Natl Acad Sci U S A 2016, 113, 1074–1079. [Google Scholar] [CrossRef]
- Pathak, V.; Bertelli, P.M.; Pedrini, E.; Harkin, K.; Peixoto, E.; Allen, L.-D.; Mcloughlin, K.; Chavda, N.D.; Hamill, K.J.; Guduric-Fuchs, J.; et al. Modulation of diabetes-related retinal pathophysiology by PTX3. Proceedings of the National Academy of Sciences 2024, 121, e2320034121. [Google Scholar] [CrossRef]
- Ma, L.; Li, D.; Wen, Y.; Shi, D. Advances in understanding the role of pentraxin-3 in lung infections. Frontiers in Immunology, 2025. [Google Scholar] [CrossRef]
- van Rossum, A.P.; Fazzini, F.; Limburg, P.C.; Manfredi, A.A.; Rovere-Querini, P.; Mantovani, A.; Kallenberg, C.G.M. The prototypic tissue pentraxin PTX3, in contrast to the short pentraxin serum amyloid P, inhibits phagocytosis of late apoptotic neutrophils by macrophages. Arthritis & Rheumatism 2004, 50, 2667–2674. [Google Scholar] [CrossRef]
- Rovere, P.; Peri, G.; Fazzini, F.; Bottazzi, B.; Doni, A.; Bondanza, A.; Zimmermann, V.S.; Garlanda, C.; Fascio, U.; Sabbadini, M.G.; et al. The long pentraxin PTX3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 2000, 96, 4300–4306. [Google Scholar] [CrossRef]
- Qi, X.; Guo, H.; Xia, X.; Liu, Y.; Qiu, S.; Lin, T.; He, W.; Jin, L.; Cheng, J.; Hao, L.; et al. Paeoniflorin alleviated STZ-induced diabetic retinopathy via regulation of the PDI/ADAM17/MerTK pathway. International Immunopharmacology 2025, 155, 114571. [Google Scholar] [CrossRef]
- Thorp, E.; Vaisar, T.; Subramanian, M.; Mautner, L.; Blobel, C.; Tabas, I. Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). J Biol Chem 2011, 286, 33335–33344. [Google Scholar] [CrossRef] [PubMed]
- Cai, B.; Thorp, E.B.; Doran, A.C.; Subramanian, M.; Sansbury, B.E.; Lin, C.-S.; Spite, M.; Fredman, G.; Tabas, I. MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proceedings of the National Academy of Sciences 2016, 113, 6526–6531. [Google Scholar] [CrossRef] [PubMed]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger Function of Resident Autofluorescent Perivascular Macrophages and Their Contribution to the Maintenance of the Blood–Retinal Barrier. Investigative Ophthalmology & Visual Science 2009, 50, 5997–6005. [Google Scholar] [CrossRef]
- Joost, E.; Jordao, M.J.C.; Mages, B.; Prinz, M.; Bechmann, I.; Krueger, M. Microglia contribute to the glia limitans around arteries, capillaries and veins under physiological conditions, in a model of neuroinflammation and in human brain tissue. Brain Struct Funct 2019, 224, 1301–1314. [Google Scholar] [CrossRef]
- Lassmann, H.; Zimprich, F.; Vass, K.; Hickey, W.F. Microglial cells are a component of the perivascular glia limitans. J Neurosci Res 1991, 28, 236–243. [Google Scholar] [CrossRef]
- Mondo, E.; Becker, S.C.; Kautzman, A.G.; Schifferer, M.; Baer, C.E.; Chen, J.; Huang, E.J.; Simons, M.; Schafer, D.P. A Developmental Analysis of Juxtavascular Microglia Dynamics and Interactions with the Vasculature. J Neurosci 2020, 40, 6503–6521. [Google Scholar] [CrossRef]
- Grossmann, R.; Stence, N.; Carr, J.; Fuller, L.; Waite, M.; Dailey, M.E. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 2002, 37, 229–240. [Google Scholar] [CrossRef]
- Mato, M.; Ookawara, S.; Sakamoto, A.; Aikawa, E.; Ogawa, T.; Mitsuhashi, U.; Masuzawa, T.; Suzuki, H.; Honda, M.; Yazaki, Y.; et al. Involvement of specific macrophage-lineage cells surrounding arterioles in barrier and scavenger function in brain cortex. Proc Natl Acad Sci U S A 1996, 93, 3269–3274. [Google Scholar] [CrossRef]
- Gehrmann, J.; Matsumoto, Y.; Kreutzberg, G.W. Microglia: intrinsic immuneffector cell of the brain. Brain Res Brain Res Rev 1995, 20, 269–287. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, T.A.; Anderson, H.R.; Stitt, A.W. Inhibition of advanced glycation end-products protects against retinal capillary basement membrane expansion during long-term diabetes. J Pathol 2003, 201, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Stitt, A.W.; Anderson, H.R.; Gardiner, T.A.; Archer, D.B. Diabetic retinopathy: quantitative variation in capillary basement membrane thickening in arterial or venous environments. Br J Ophthalmol 1994, 78, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Roy, S.; John, H.; Kyle, T.; and Beglova, E. Vascular Basement Membrane Thickening in Diabetic Retinopathy. Current eye research 2010, 35, 1045–1056. [Google Scholar] [CrossRef]
- Stitt, A.W. AGEs and Diabetic Retinopathy. Investigative Ophthalmology & Visual Science 2010, 51, 4867–4874. [Google Scholar] [CrossRef]
- DeGroot, J.; Verzijl, N.; Budde, M.; Bijlsma, J.W.J.; Lafeber, F.P.J.G.; TeKoppele, J.M. Accumulation of Advanced Glycation End Products Decreases Collagen Turnover by Bovine Chondrocytes. Experimental Cell Research 2001, 266, 303–310. [Google Scholar] [CrossRef]
- Sloseris, D.; Forde, N.R. AGEing of collagen: The effects of glycation on collagen’s stability, mechanics and assembly. Matrix Biology 2025, 135, 153–160. [Google Scholar] [CrossRef]
- McDonald, D.M.; Coleman, G.; Bhatwadekar, A.; Gardiner, T.A.; Stitt, A.W. Advanced glycation of the Arg-Gly-Asp (RGD) tripeptide motif modulates retinal microvascular endothelial cell dysfunction. Mol Vis 2009, 15, 1509–1520. [Google Scholar]
- Li, H.; Zhang, X.; Guan, X.; Cui, X.; Wang, Y.; Chu, H.; Cheng, M. Advanced glycation end products impair the migration, adhesion and secretion potentials of late endothelial progenitor cells. Cardiovascular Diabetology 2012, 11, 46. [Google Scholar] [CrossRef]
- Kuzuya, M.; Asai, T.; Kanda, S.; Maeda, K.; Cheng, X.W.; Iguchi, A. Glycation cross-links inhibit matrix metalloproteinase-2 activation in vascular smooth muscle cells cultured on collagen lattice. Diabetologia 2001, 44, 433–436. [Google Scholar] [CrossRef]
- Chang, R.T.; Fisher, M.J.; Sumbria, R.K. Brain endothelial cells as phagocytes: mechanisms and implications. Fluids and Barriers of the CNS 2025, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Zheng, Y.; Sun, L.; Badea, S.R.; Jin, Y.; Liu, Y.; Rolfe, A.J.; Sun, H.; Wang, X.; Cheng, Z.; et al. Microvascular endothelial cells engulf myelin debris and promote macrophage recruitment and fibrosis after neural injury. Nature Neuroscience 2019, 22, 421–435. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Findley, A.P.; Mitchell, D.M. Intercellular contact and cargo transfer between Müller glia and to microglia precede apoptotic cell clearance in the developing retina. Development 2024, 151. [Google Scholar] [CrossRef]
- Thiel, W.A.; Blume, Z.I.; Mitchell, D.M. Compensatory engulfment and Müller glia reactivity in the absence of microglia. Glia 2022, 70, 1402–1425. [Google Scholar] [CrossRef]
- Vecino, E.; Rodriguez, F.D.; Ruzafa, N.; Pereiro, X.; Sharma, S.C. Glia–neuron interactions in the mammalian retina. Progress in Retinal and Eye Research 2016, 51, 1–40. [Google Scholar] [CrossRef]
- Carpi-Santos, R.; de Melo Reis, R.A.; Gomes, F.C.A.; Calaza, K.C. Contribution of Müller Cells in the Diabetic Retinopathy Development: Focus on Oxidative Stress and Inflammation. Antioxidants (Basel) 2022, 11. [Google Scholar] [CrossRef]
- Coughlin, B.A.; Feenstra, D.J.; Mohr, S. Müller cells and diabetic retinopathy. Vision Research 2017, 139, 93–100. [Google Scholar] [CrossRef]
- Zong, H.; Ward, M.; Madden, A.; Yong, P.H.; Limb, G.A.; Curtis, T.M.; Stitt, A.W. Hyperglycaemia-induced pro-inflammatory responses by retinal Müller glia are regulated by the receptor for advanced glycation end-products (RAGE). Diabetologia 2010, 53, 2656–2666. [Google Scholar] [CrossRef]
- Canning, P.; Glenn, J.V.; Hsu, D.K.; Liu, F.T.; Gardiner, T.A.; Stitt, A.W. Inhibition of advanced glycation and absence of galectin-3 prevent blood-retinal barrier dysfunction during short-term diabetes. Exp Diabetes Res 2007, 2007, 51837. [Google Scholar] [CrossRef]
- Vlassara, H.; Li, Y.M.; Imani, F.; Wojciechowicz, D.; Yang, Z.; Liu, F.-T.; Cerami, A. Identification of Galectin-3 As a High-Affinity Binding Protein for Advanced Glycation End Products (AGE): A New Member of the AGE-Receptor Complex. Molecular Medicine 1995, 1, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Manigrasso, M.B.; Juranek, J.; Ramasamy, R.; Schmidt, A.M. Unlocking the biology of RAGE in diabetic microvascular complications. Trends in Endocrinology & Metabolism 2014, 25, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Experimental & Molecular Medicine 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Steinle, J.J. Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy. Cell Signal 2020, 73, 109687. [Google Scholar] [CrossRef]
- Mohammad, G.; Siddiquei, M.M.; Othman, A.; Al-Shabrawey, M.; Abu El-Asrar, A.M. High-mobility group box-1 protein activates inflammatory signaling pathway components and disrupts retinal vascular-barrier in the diabetic retina. Experimental Eye Research 2013, 107, 101–109. [Google Scholar] [CrossRef]
- Santos, A.R.; Dvoriantchikova, G.; Li, Y.; Mohammad, G.; Abu El-Asrar, A.M.; Wen, R.; Ivanov, D. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PLoS One 2014, 9, e87574. [Google Scholar] [CrossRef]
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.-J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the αvβ3-integrin. American Journal of Physiology-Cell Physiology 2010, 299, C1267–C1276. [Google Scholar] [CrossRef]
- Yang, T.; Guo, R.; Zhang, F. Brain perivascular macrophages: Recent advances and implications in health and diseases. CNS Neurosci Ther 2019, 25, 1318–1328. [Google Scholar] [CrossRef]
- Silvin, A.; Qian, J.; Ginhoux, F. Brain macrophage development, diversity and dysregulation in health and disease. Cellular & Molecular Immunology 2023, 20, 1277–1289. [Google Scholar] [CrossRef]
- Mendes-Jorge, L.; Ramos, D.; Luppo, M.; Llombart, C.; Alexandre-Pires, G.; Nacher, V.; Melgarejo, V.; Correia, M.; Navarro, M.; Carretero, A.; et al. Scavenger function of resident autofluorescent perivascular macrophages and their contribution to the maintenance of the blood-retinal barrier. Invest Ophthalmol Vis Sci 2009, 50, 5997–6005. [Google Scholar] [CrossRef]
- Sharifiaghdam, M.; Shaabani, E.; Faridi-Majidi, R.; De Smedt, S.C.; Braeckmans, K.; Fraire, J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Molecular Therapy 2022, 30, 2891–2908. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.K.; Gardiner, T.A.; Archer, D.B. A morphologic and autoradiographic study of cell death and regeneration in the retinal microvasculature of normal and diabetic rats. American journal of ophthalmology 1985, 100, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Joussen, A.M.; Poulaki, V.; Le, M.L.; Koizumi, K.; Esser, C.; Janicki, H.; Schraermeyer, U.; Kociok, N.; Fauser, S.; Kirchhof, B.; et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB J 2004, 18, 1450–1452. [Google Scholar] [CrossRef] [PubMed]
- Moore, T.C.; Moore, J.E.; Kaji, Y.; Frizzell, N.; Usui, T.; Poulaki, V.; Campbell, I.L.; Stitt, A.W.; Gardiner, T.A.; Archer, D.B.; et al. The role of advanced glycation end products in retinal microvascular leukostasis. Invest Ophthalmol Vis Sci 2003, 44, 4457–4464. [Google Scholar] [CrossRef]
- Wen, R.X.; Shen, H.; Huang, S.X.; Wang, L.P.; Li, Z.W.; Peng, P.; Mamtilahun, M.; Tang, Y.H.; Shen, F.X.; Tian, H.L.; et al. P2Y6 receptor inhibition aggravates ischemic brain injury by reducing microglial phagocytosis. CNS Neurosci Ther 2020, 26, 416–429. [Google Scholar] [CrossRef]
- Anwar, S.; Pons, V.; Rivest, S. Microglia Purinoceptor P2Y6: An Emerging Therapeutic Target in CNS Diseases. Cells 2020, 9. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Yang, X.; Wang, J.-K.; Yan, X.-X.; Liu, F.; Zuo, Y.-C. MFGE8 promotes adult hippocampal neurogenesis in rats following experimental subarachnoid hemorrhage via modifying the integrin β3/Akt signaling pathway. Cell Death Discovery 2024, 10, 359. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Zhang, Z.H.; Zhuang, Z.; Lu, Y.; Wu, L.Y.; Ye, Z.N.; Zhang, X.S.; Chen, C.L.; Li, W.; Hang, C.H. Recombinant milk fat globule-EGF factor-8 reduces apoptosis via integrin β3/FAK/PI3K/AKT signaling pathway in rats after traumatic brain injury. Cell Death Dis 2018, 9, 845. [Google Scholar] [CrossRef]
- Liu, F.; Chen, Y.; Hu, Q.; Li, B.; Tang, J.; He, Y.; Guo, Z.; Feng, H.; Tang, J.; Zhang, J.H. MFGE8/Integrin β3 pathway alleviates apoptosis and inflammation in early brain injury after subarachnoid hemorrhage in rats. Experimental Neurology 2015, 272, 120–127. [Google Scholar] [CrossRef]
- Liu, F.; Hu, Q.; Li, B.; Manaenko, A.; Chen, Y.; Tang, J.; Guo, Z.; Tang, J.; Zhang, J.H. Recombinant Milk Fat Globule–EGF Factor-8 Reduces Oxidative Stress via Integrin β3/Nuclear Factor Erythroid 2–Related Factor 2/Heme Oxygenase Pathway in Subarachnoid Hemorrhage Rats. Stroke 2014, 45, 3691–3697. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.; Li, Z.; Chen, J.; Chang, Y.; Li, Y.; Zeng, S.; Pan, S.; Pan, S.; Huang, K. Potentiating microglial efferocytosis by MFG-E8 improves survival and neurological outcome after successful cardiopulmonary resuscitation in mice. Brain Pathology 2025, 35, e13327. [Google Scholar] [CrossRef] [PubMed]
- Haage, V.; Bautista, A.R.; Tuddenham, J.; Marshe, V.; Chiu, R.; Liu, Y.; Lama, T.; Kelly, S.S.; Parghi, N.A.; Park, J.; et al. A proteogenomic tool uncovers protein markers for human microglial states. bioRxiv, 2003. [Google Scholar] [CrossRef]
- Chen, P.; Guan, X.; Zhao, X.; Chen, F.; Yang, J.; Wang, Y.; Hu, Y.; Lian, Q.; Chen, H. Characterization and differentiation of CD51+ Stem Leydig cells in adult mouse testes. Molecular and Cellular Endocrinology 2019, 493, 110449. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.R.; Zheng, S.; Park, D.; Woodson, R.I.; Reardon, M.A.; Juncadella, I.J.; Kinchen, J.M.; Zhang, J.; Lysiak, J.J.; Ravichandran, K.S. Unexpected requirement for ELMO1 in clearance of apoptotic germ cells in vivo. Nature 2010, 467, 333–337. [Google Scholar] [CrossRef]
- Elliott, Michael R. ; Ravichandran, Kodi S. The Dynamics of Apoptotic Cell Clearance. Developmental Cell 2016, 38, 147–160. [Google Scholar] [CrossRef]
- Sierra, A.; Encinas, J.M.; Deudero, J.J.P.; Chancey, J.H.; Enikolopov, G.; Overstreet-Wadiche, L.S.; Tsirka, S.E.; Maletic-Savatic, M. Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2010, 7, 483–495. [Google Scholar] [CrossRef]
- Iram, T.; Ramirez-Ortiz, Z.; Byrne, M.H.; Coleman, U.A.; Kingery, N.D.; Means, T.K.; Frenkel, D.; El Khoury, J. Megf10 Is a Receptor for C1Q That Mediates Clearance of Apoptotic Cells by Astrocytes. J Neurosci 2016, 36, 5185–5192. [Google Scholar] [CrossRef]
- Hickman, S.E.; Kingery, N.D.; Ohsumi, T.K.; Borowsky, M.L.; Wang, L.-c.; Means, T.K.; El Khoury, J. The microglial sensome revealed by direct RNA sequencing. Nature Neuroscience 2013, 16, 1896–1905. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced Glycation End-Products (AGEs): Formation, Chemistry, Classification, Receptors, and Diseases Related to AGEs. Cells 2022, 11. [Google Scholar] [CrossRef]
- Harley, O.; Amelia, Y.S.; Gustianty, E.; Soetedjo, N.N.M.; Kartasasmita, A.S. Retinal Microglia: Revealing New Opportunities for Identifying Early Biomarkers of Diabetic Retinopathy. Current eye research 2025, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Yu, Z.-W.; Li, H.-Y.; Yuan, Y.; Gao, X.-Y.; Kuang, H.-Y. Retinal microglia polarization in diabetic retinopathy. Visual Neuroscience 2021, 38, E006. [Google Scholar] [CrossRef]
- Silveira, A.S.d.A.; Alves, A.C.d.A.; Gimenes, G.M.; Quessada, P.d.S.; Lobato, T.B.; Dias, B.B.; Pereira, A.C.G.; Iser-Bem, P.N.; Pereira, J.N.B.; Hatanaka, E.; et al. Evidence for a Pro-Inflammatory State of Macrophages from Non-Obese Type-2 Diabetic Goto-Kakizaki Rats. International Journal of Molecular Sciences 2024, 25, 10240. [Google Scholar] [CrossRef]
- Torres-Castro, I.; Arroyo-Camarena, Ú.D.; Martínez-Reyes, C.P.; Gómez-Arauz, A.Y.; Dueñas-Andrade, Y.; Hernández-Ruiz, J.; Béjar, Y.L.; Zaga-Clavellina, V.; Morales-Montor, J.; Terrazas, L.I.; et al. Human monocytes and macrophages undergo M1-type inflammatory polarization in response to high levels of glucose. Immunology Letters 2016, 176, 81–89. [Google Scholar] [CrossRef]
- Kowluru, R.A.; Kowluru, A.; Mishra, M.; Kumar, B. Oxidative stress and epigenetic modifications in the pathogenesis of diabetic retinopathy. Prog Retin Eye Res 2015, 48, 40–61. [Google Scholar] [CrossRef]
- Edgar, L.; Akbar, N.; Braithwaite, A.T.; Krausgruber, T.; Gallart-Ayala, H.; Bailey, J.; Corbin, A.L.; Khoyratty, T.E.; Chai, J.T.; Alkhalil, M.; et al. Hyperglycemia Induces Trained Immunity in Macrophages and Their Precursors and Promotes Atherosclerosis. Circulation 2021, 144, 961–982. [Google Scholar] [CrossRef]
- Mitroulis, I.; Ruppova, K.; Wang, B.; Chen, L.-S.; Grzybek, M.; Grinenko, T.; Eugster, A.; Troullinaki, M.; Palladini, A.; Kourtzelis, I.; et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell 2018, 172, 147–161.e112. [Google Scholar] [CrossRef] [PubMed]
- Engerman, R.L.; Kern, T.S. Progression of Incipient Diabetic Retinopathy During Good Glycemic Control. Diabetes 1987, 36, 808–812. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Huang, G.; Wang, Z.; Wang, L.; Gao, Q. IRF7: role and regulation in immunity and autoimmunity. Frontiers in Immunology, 2023. [Google Scholar] [CrossRef]
- Cohen, M.; Matcovitch, O.; David, E.; Barnett-Itzhaki, Z.; Keren-Shaul, H.; Blecher-Gonen, R.; Jaitin, D.A.; Sica, A.; Amit, I.; Schwartz, M. Chronic exposure to TGFβ1 regulates myeloid cell inflammatory response in an IRF7-dependent manner. The EMBO Journal 2014, 33, 2906–2921. [Google Scholar] [CrossRef] [PubMed]
- Stevens, S.L.; Leung, P.Y.; Vartanian, K.B.; Gopalan, B.; Yang, T.; Simon, R.P.; Stenzel-Poore, M.P. Multiple Preconditioning Paradigms Converge on Interferon Regulatory Factor-Dependent Signaling to Promote Tolerance to Ischemic Brain Injury. The Journal of Neuroscience 2011, 31, 8456–8463. [Google Scholar] [CrossRef]
- Barry-Carroll, L.; Gomez-Nicola, D. The molecular determinants of microglial developmental dynamics. Nature Reviews Neuroscience 2024, 25, 414–427. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




