Submitted:
29 July 2025
Posted:
29 July 2025
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
2.2.1. Preparation of LMNO Cathode Material
2.2.2. Preparation of Composite Cathodes with SN-Based Modified Layers
2.2.3. Preparation of PVHLi-1.1 Membranes
2.2. Materials Characterization
2.3. Electrochemical measurement
2.3.1. Ion Conductivity Testing
2.3.2. Linear Sweep Voltammetry Testing
2.3.3. Battery Performance Evaluation
3. Results and discussion
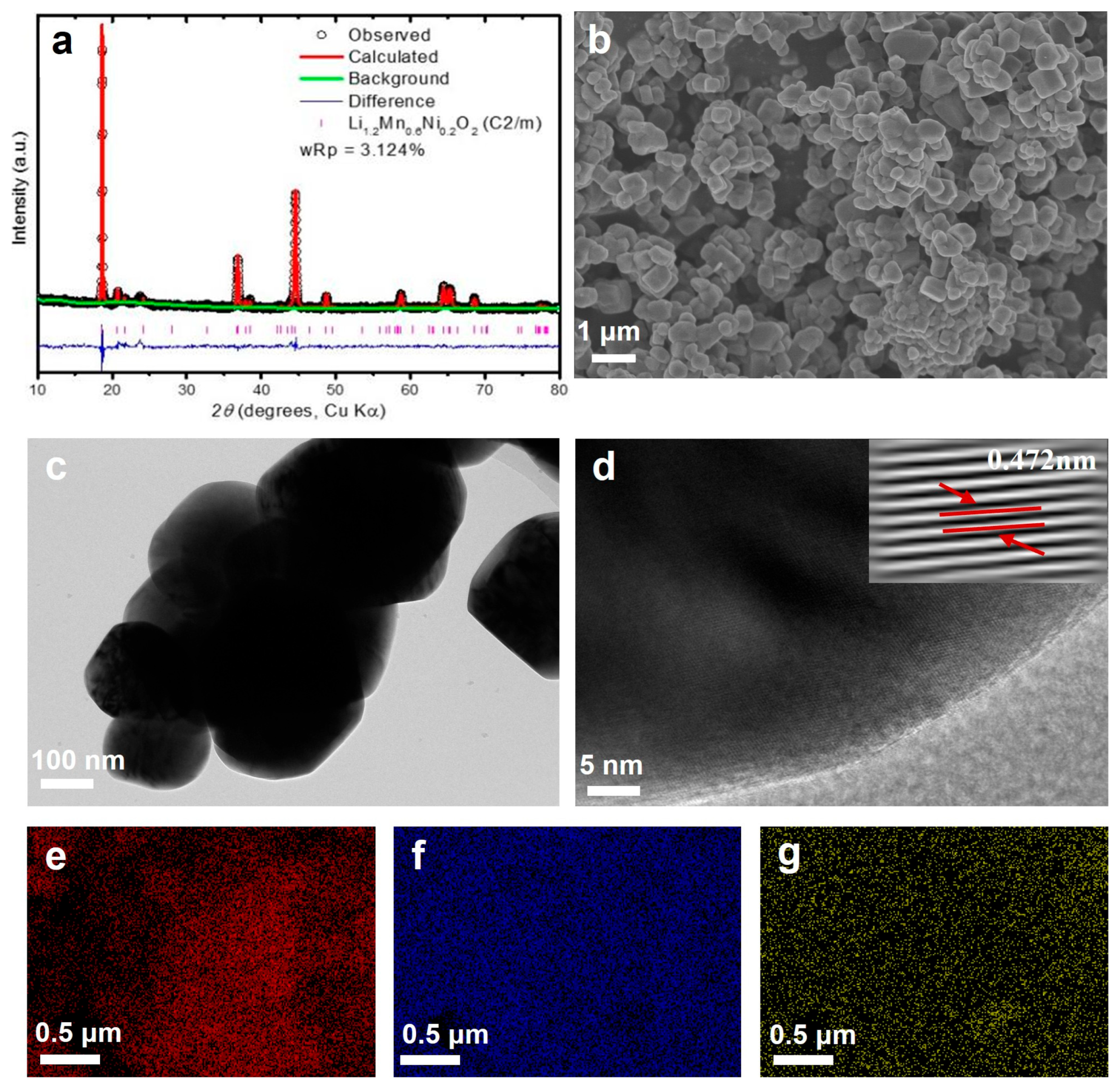
3.1. Characterization of LMNO Cathode
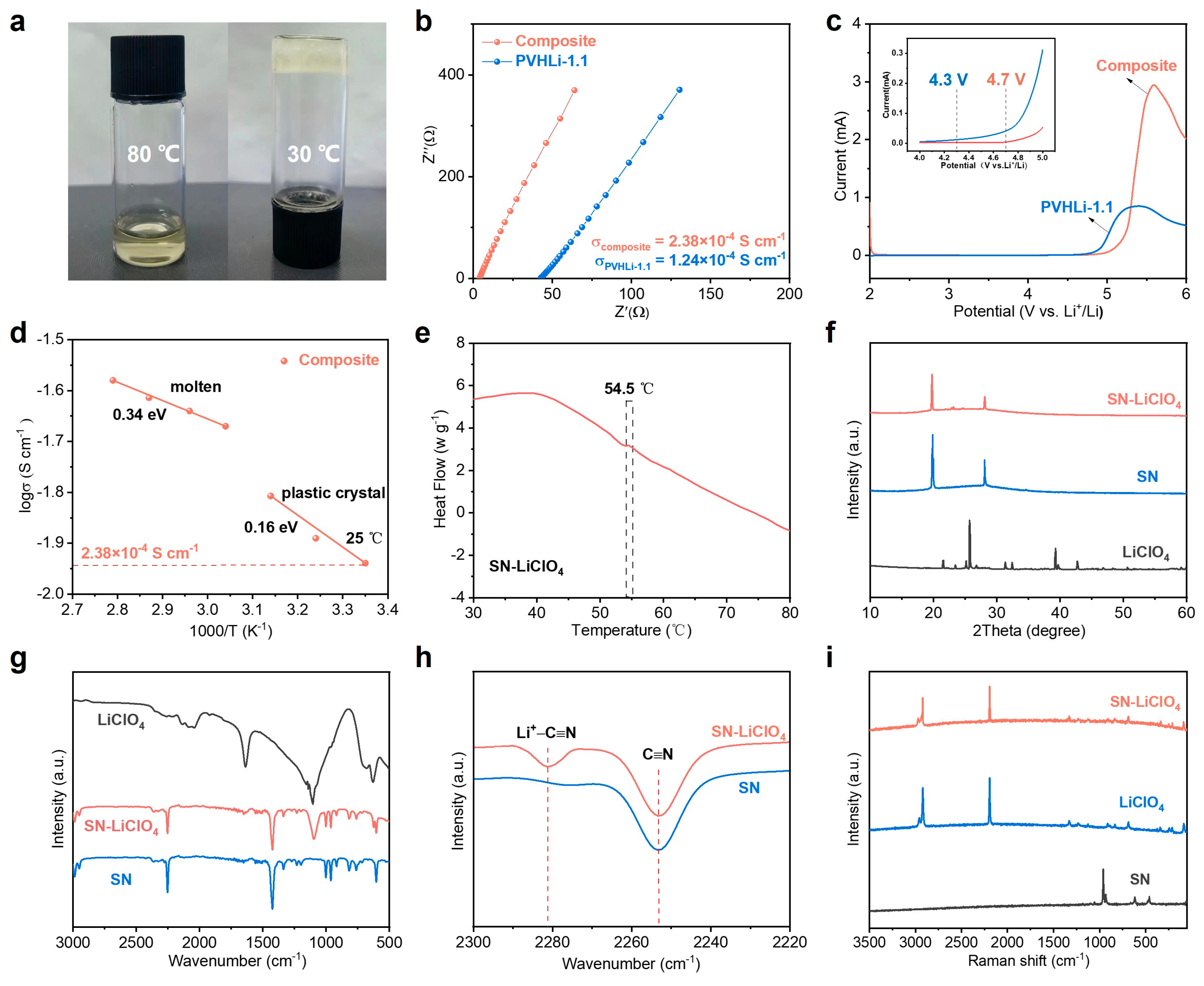
3.2. Characterizations of SN-LiClO4
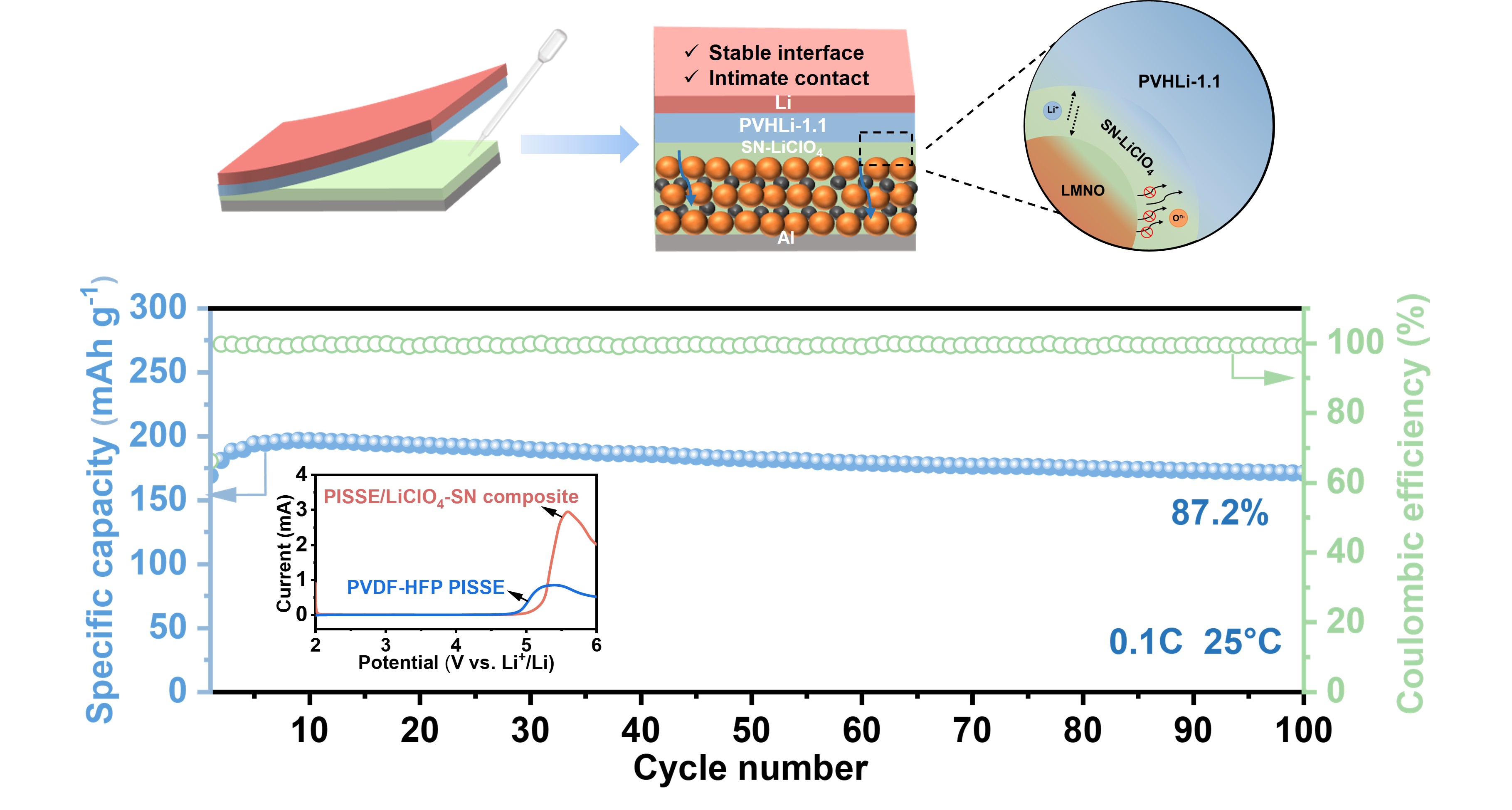
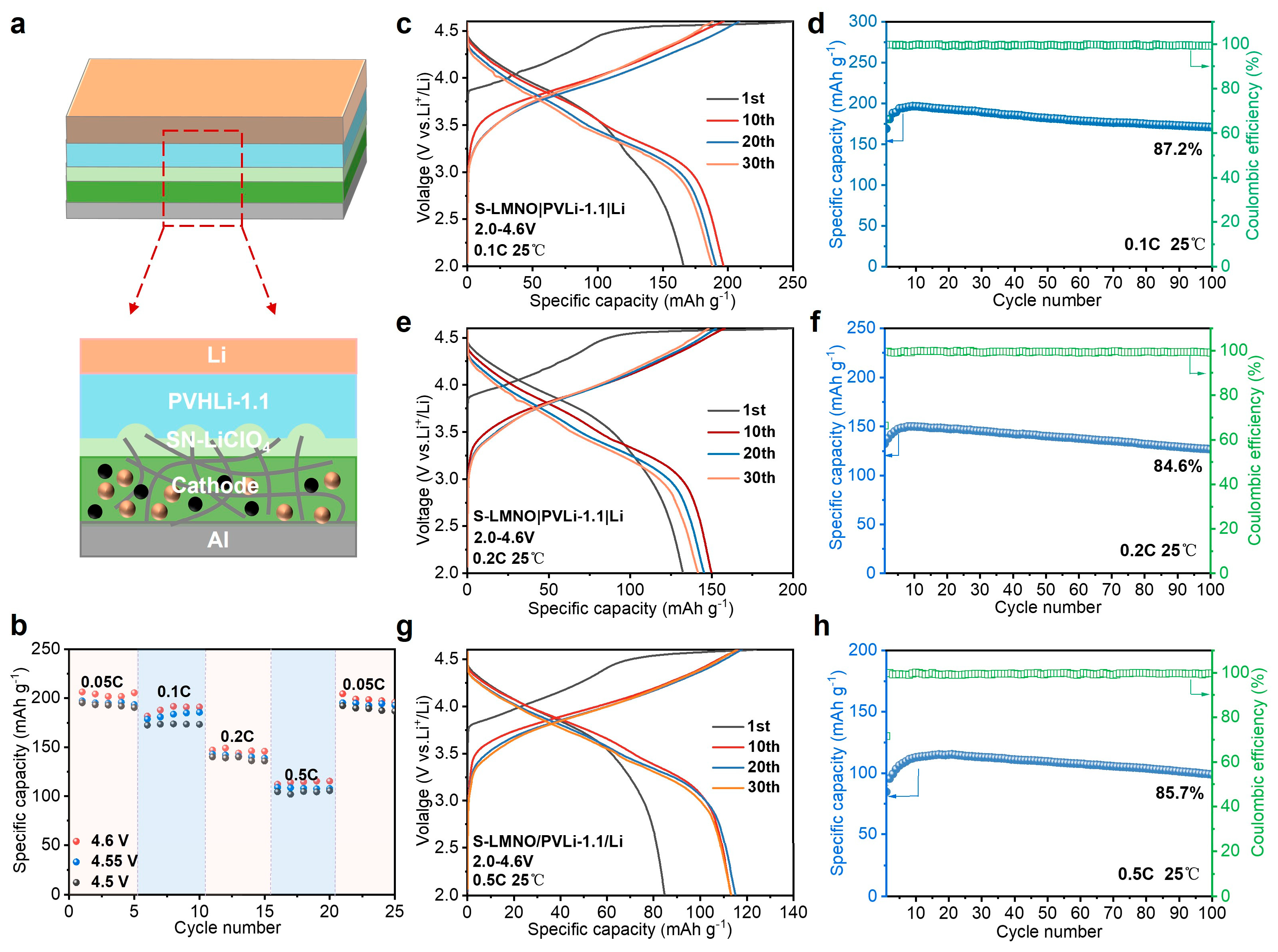
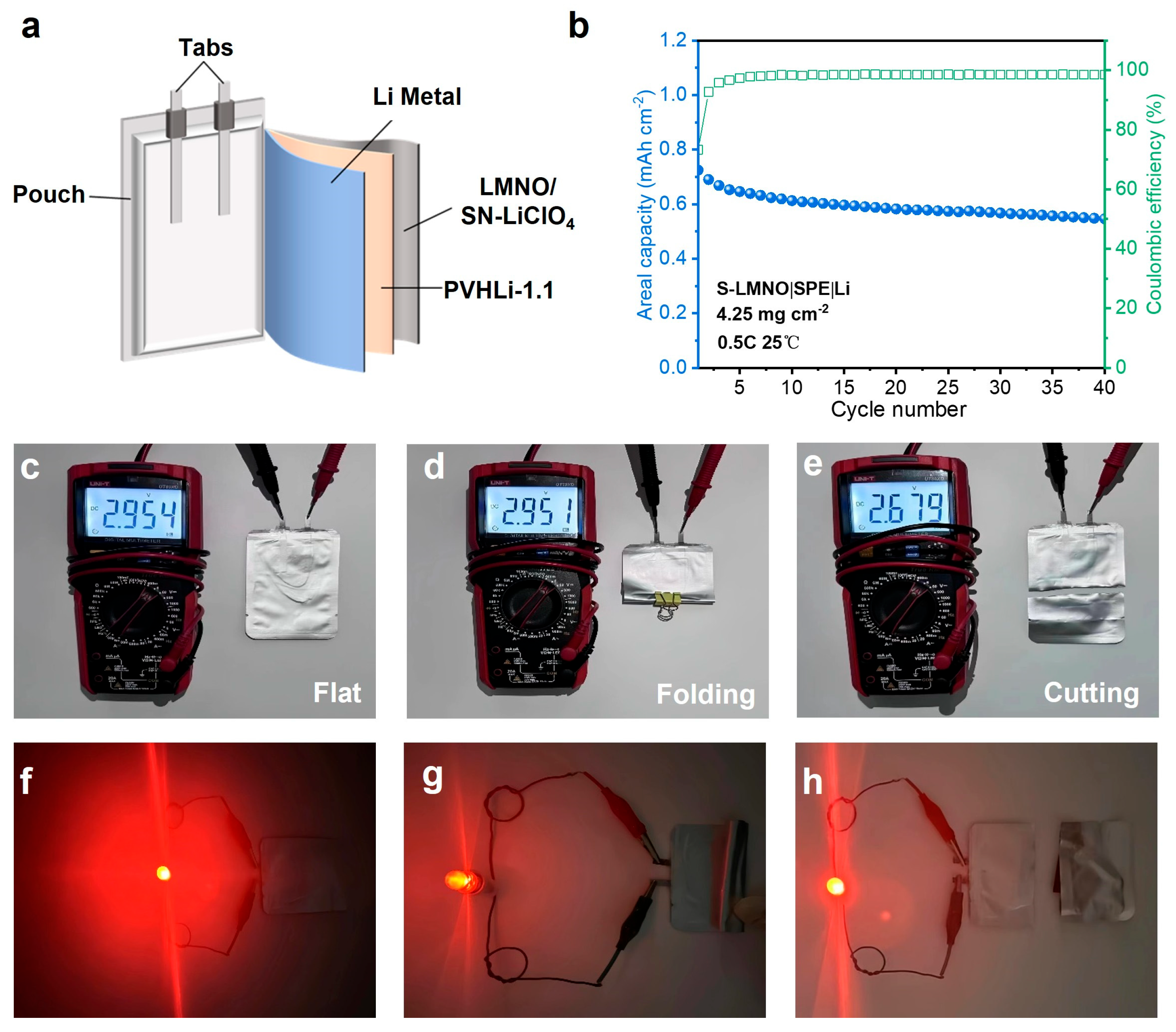
3.3. High-Voltage LNMO|PVHLi-1.1-SN–LiClO₄|Li ASSLB Performance Test
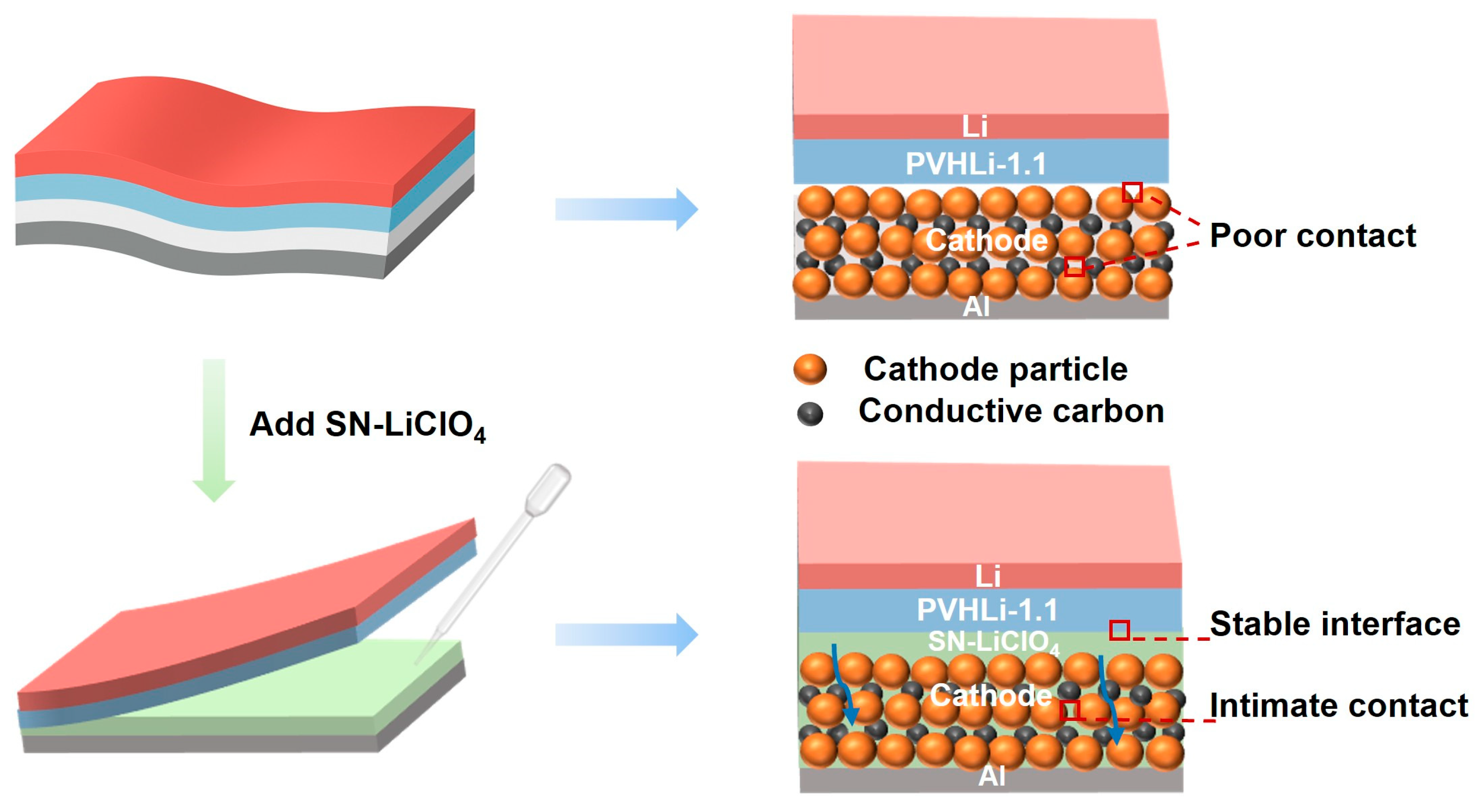
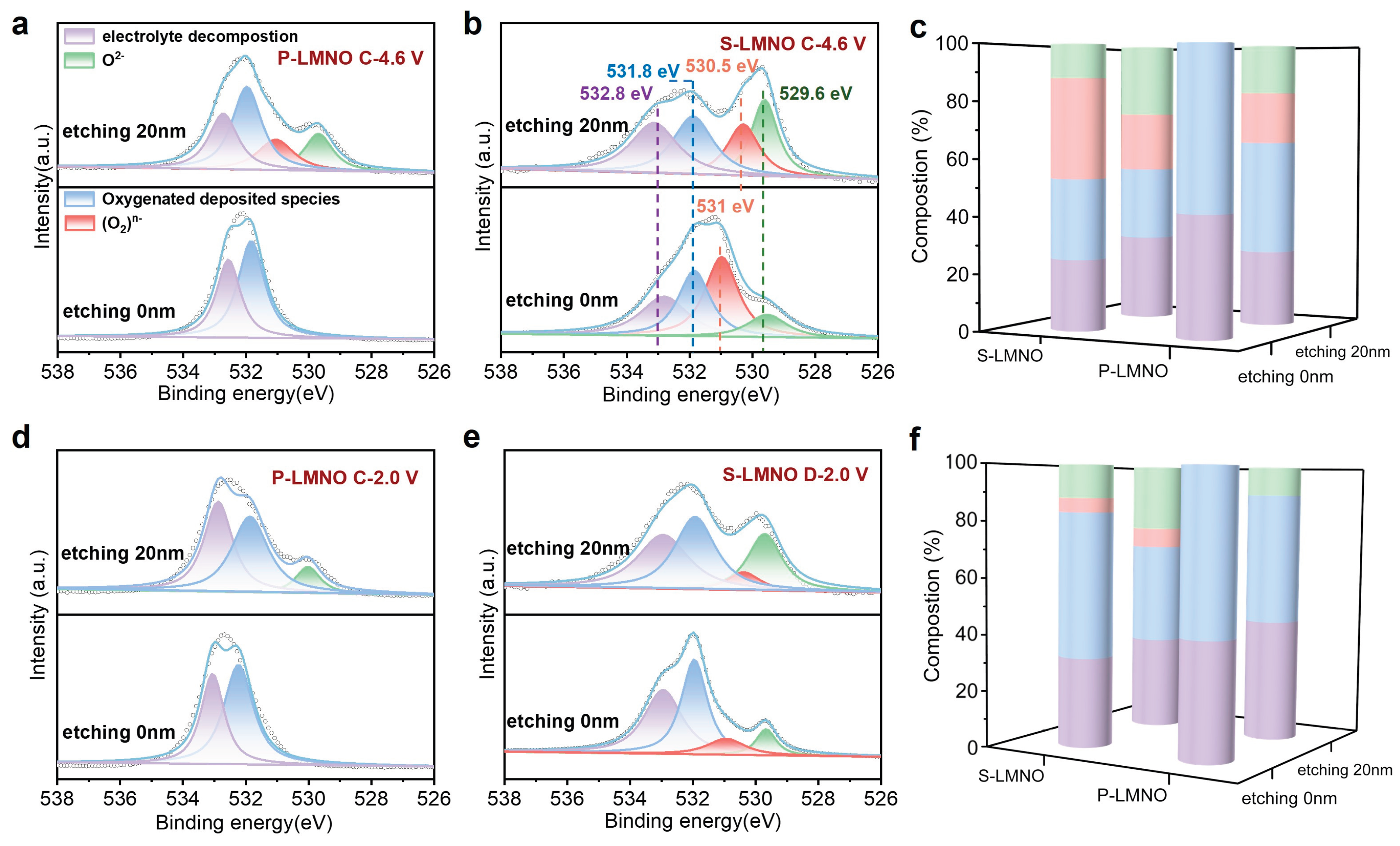
3.4. Interface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Zhang, X.; Yu, H. Design of high-energy-density lithium batteries: Liquid to all solid state. eTransportation 2024, 23. [CrossRef]
- Xu, J.; Sun, M.; Qiao, R.; Renfrew, S.E.; Ma, L.; Wu, T.; Hwang, S.; Nordlund, D.; Su, D.; Amine, K.; et al. Elucidating anionic oxygen activity in lithium-rich layered oxides. Nat. Commun. 2018, 9, 1–10. [CrossRef]
- Wang, H.; Geng, X.; Hu, L.; Wang, J.; Xu, Y.; Zhu, Y.; Liu, Z.; Lu, J.; Lin, Y.; He, X. Efficient direct repairing of lithium- and manganese-rich cathodes by concentrated solar radiation. Nat. Commun. 2024, 15, 1–13. [CrossRef]
- Yuan, X.; Dong, T.; Liu, J.; Cui, Y.; Dong, H.; Yuan, D.; Zhang, H. Bi-affinity Electrolyte Optimizing High-Voltage Lithium-Rich Manganese Oxide Battery via Interface Modulation Strategy. Angew. Chem. Int. Ed. Engl. 2023, 62, e202304121. [CrossRef]
- Liu, W.; Li, J.; Li, W.; Xu, H.; Zhang, C.; Qiu, X. Inhibition of transition metals dissolution in cobalt-free cathode with ultrathin robust interphase in concentrated electrolyte. Nat. Commun. 2020, 11, 1–11. [CrossRef]
- Betz, J.; Brinkmann, J.; Nölle, R.; Lürenbaum, C.; Kolek, M.; Stan, M.C.; Winter, M.; Placke, T. Cross Talk between Transition Metal Cathode and Li Metal Anode: Unraveling Its Influence on the Deposition/Dissolution Behavior and Morphology of Lithium. Adv. Energy Mater. 2019, 9. [CrossRef]
- Antony Jose, S.; Gallant, A.; Gomez, P. L.; Jaggers, Z.; Johansson, E.; LaPierre, Z.; Menezes, P. L. Solid-State Lithium Batteries: Advances, Challenges, and Future Perspectives. Batteries, 2025, 11, 90. [CrossRef]
- Hu, N.; Zhang, Y.; Yang, Y.; Wu, H.; Liu, Y.; Hao, C.; Zheng, Y.; Sun, D.; Li, W.; Li, J.; et al. Unraveling the Spatial Asynchronous Activation Mechanism of Oxygen Redox-Involved Cathode for High-Voltage Solid-State Batteries. Adv. Energy Mater. 2024, 14. [CrossRef]
- Yu, R.; Wang, C.; Duan, H.; Jiang, M.; Zhang, A.; Fraser, A.; Zuo, J.; Wu, Y.; Sun, Y.; Zhao, Y.; Liang, J.; Fu, J.; Deng, S.; Ren, Z.; Li, G.; Huang, H.; Li, R.; Chen, N.; Wang, J.; Li, X.; Singh, C. V.; Sun, X. Manipulating Charge-Transfer Kinetics of Lithium-Rich Layered Oxide Cathodes in Halide All-Solid-State Batteries. Adv. Mater. 2023, 35, 2207234. [CrossRef]
- Wu, Y.; Zhou, K.; Ren, F.; Ha, Y.; Liang, Z.; Zheng, X.; Wang, Z.; Yang, W.; Zhang, M.; Luo, M.; Battaglia, C.; Yang, W.; Zhu, L.; Gong, Z.; Yang, Y. Highly Reversible Li2RuO3 Cathodes in Sulfide-based All Solid-state Lithium Batteries. Energy Environ. Sci. 2022, 15, 3470-3482.
- Sun, S.; Zhao, C.-Z.; Yuan, H.; Fu, Z.-H.; Chen, X.; Lu, Y.; Li, Y.-F.; Hu, J.-K.; Dong, J.; Huang, J.-Q.; et al. Eliminating interfacial O-involving degradation in Li-rich Mn-based cathodes for all-solid-state lithium batteries. Sci. Adv. 2022, 8, eadd5189. [CrossRef]
- Liu, B.; Hu, N.; Li, C.; Ma, J.; Zhang, J.; Yang, Y.; Sun, D.; Yin, B.; Cui, G. Direct Observation of Li-Ion Transport Heterogeneity Induced by Nanoscale Phase Separation in Li-rich Cathodes of Solid-State Batteries. Angew. Chem. Int. Ed. 2022, 61, e202209626. [CrossRef]
- Cao, C.; Carbone, M.R.; Komurcuoglu, C.; Shekhawat, J.S.; Sun, K.; Guo, H.; Liu, S.; Chen, K.; Bak, S.-M.; Du, Y.; et al. Atomic insights into the oxidative degradation mechanisms of sulfide solid electrolytes. Cell Rep. Phys. Sci. 2024, 5. [CrossRef]
- Li, J.; Luo, J.; Li, X.; Fu, Y.; Zhu, J.; Zhuang, X. Li Metal Anode Interface in Sulfide-based All-solid-state Li batteries. EcoMat 2023, 5, e12383. [CrossRef]
- Byeon, Y.-W.; Kim, H. Review on Interface and Interphase Issues in Sulfide Solid-State Electrolytes for All-Solid-State Li-Metal Batteries. Electrochem 2021, 2, 452–471. [CrossRef]
- Wang, H.; Yang, Y.; Gao, C.; Chen, T.; Song, J.; Zuo, Y.; Fang, Q.; Yang, T.; Xiao, W.; Zhang, K.; et al. An entanglement association polymer electrolyte for Li-metal batteries. Nat. Commun. 2024, 15, 1–12. [CrossRef]
- Chen, S.; Wang, S.; Peng, Q.; Wei, Z.; Cheng, S.; Fang, Z.; Duan, P.; Cheng, Y.; Cheng, Y.; Jin, K.; et al. In-situ fabricated succinonitrile-based composite electrolyte for high-performance and safe solid-state lithium batteries. J. Power Sources 2024, 604. [CrossRef]
- Chen, J.; Yang, Z.; Xu, X.; Qiao, Y.; Zhou, Z.; Hao, Z.; Chen, X.; Liu, Y.; Wu, X.; Zhou, X.; et al. Nonflammable Succinonitrile-Based Deep Eutectic Electrolyte for Intrinsically Safe High-Voltage Sodium-Ion Batteries. Adv. Mater. 2024, 36, e2400169. [CrossRef]
- Bao, D.; Tao, Y.; Zhong, Y.; Zhao, W.; Peng, M.; Zhang, H.; Sun, X. High-Performance Dual-Salt Plastic Crystal Electrolyte Enabled by Succinonitrile-Regulated Porous Polymer Host. Adv. Funct. Mater. 2023, 33, 2213211. [CrossRef]
- Das, S.; Prathapa, S.J.; Menezes, P.V.; Row, T.N.G.; Bhattacharyya, A.J. Study of Ion Transport in Lithium Perchlorate-Succinonitrile Plastic Crystalline Electrolyte via Ionic Conductivity and in Situ Cryo-Crystallography. J. Phys. Chem. B 2009, 113, 5025–5031. [CrossRef]
- Li, Y.; Yuan, W.; Hu, Z.; Shen, Y.; Wu, G.; Cong, F.; Fu, X.; Lu, F.; Li, Y.; Liu, P.; et al. Constructing PVDF-Based Polymer Electrolyte for Lithium Metal Batteries by Polymer-Induced Phase Structure Adjustment Strategy. Adv. Funct. Mater. 2025, 35. [CrossRef]
- Xiong, Z.; Wang, Z.; Zhou, W.; Liu, Q.; Wu, J.-F.; Liu, T.-H.; Xu, C.; Liu, J. 4.2V polymer all-solid-state lithium batteries enabled by high-concentration PEO solid electrolytes. Energy Storage Mater. 2023, 57, 171–179. [CrossRef]
- Li, H.; Du, Y.; Wu, X.; Xie, J.; Lian, F. Developing “Polymer-in-Salt” High Voltage Electrolyte Based on Composite Lithium Salts for Solid-State Li Metal Batteries. Adv. Funct. Mater. 2021, 31, 2103049. [CrossRef]
- Liu, W.; Yi, C.; Li, L.; Liu, S.; Gui, Q.; Ba, D.; Li, Y.; Peng, D.; Liu, J. Designing Polymer-in-Salt Electrolyte and Fully Infiltrated 3D Electrode for Integrated Solid-State Lithium Batteries. Angew. Chem. Int. Ed. Engl. 2021, 60, 12931–12940. [CrossRef]
- Liu, Q.; Yu, Q.; Li, S.; Wang, S.; Zhang, L.; Cai, B.; Zhou, D.; Li, B. Safe LAGP-based all solid-state Li metal batteries with plastic super-conductive interlayer enabled by in-situ solidification. Energy Storage Mater. 2020, 25, 613–620. [CrossRef]
- Hu, Z.; Xian, F.; Guo, Z.; Lu, C.; Du, X.; Cheng, X.; Zhang, S.; Dong, S.; Cui, G.; Chen, L. Nonflammable Nitrile Deep Eutectic Electrolyte Enables High-Voltage Lithium Metal Batteries. Chem. Mater. 2020, 32, 3405–3413. [CrossRef]
- Wang, Q.; Fan, H.; Fan, L.-Z.; Shi, Q. Preparation and performance of a non-ionic plastic crystal electrolyte with the addition of polymer for lithium ion batteries. Electrochimica Acta 2013, 114, 720–725. [CrossRef]
- Shen, C.; Liu, Y.; Hu, L.; Li, W.; Liu, X.; Shi, Y.; Jiang, Y.; Zhao, B.; Zhang, J. Regulating anionic redox activity of lithium-rich layered oxides via LiNbO3 integrated modification. Nano Energy 2022, 101. [CrossRef]
- Zhao, B.; Yang, M.; Li, J.; Li, S.; Zhang, G.; Liu, S.; Cui, Y.; Liu, H. Cellulose-Based Plastic Crystal Electrolyte Membranes with Enhanced Interface for Solid-State Lithium Batteries. Energy Technol. 2021, 9, 2100114. [CrossRef]
- Arunkumar, R.; Babu, R.S.; Rani, M.U.; Kalainathan, S. Effect of PBMA on PVC-based polymer blend electrolytes. J. Appl. Polym. Sci. 2017, 134. [CrossRef]
- Bian, X.; Liang, J.; Tang, X.; Li, R.; Kang, L.; Su, A.; Su, X.; Wei, Y. A boron nitride-polyvinylidene fluoride-co-hexafluoropropylene composite gel polymer electrolyte for lithium metal batteries. J. Alloy. Compd. 2019, 803, 1075–1081. [CrossRef]
- Zhang, L.; Xu, X.; Jiang, S.; Wei, L.; Xi, K.; Lei, Y.; Cheng, X.; Yin, J.; Gao, Y. Halloysite nanotubes modified poly(vinylidenefluoride-co-hexafluoropropylene)-based polymer-in-salt electrolyte to achieve high-performance Li metal batteries. J. Colloid Interface Sci. 2023, 645, 45–54. [CrossRef]
- Sathiya, M.; Rousse, G.; Ramesha, K.; Laisa, C.P.; Vezin, H.; Sougrati, M.T.; Doublet, M.-L.; Foix, D.; Gonbeau, D.; Walker, W.; et al. Reversible anionic redox chemistry in high-capacity layered-oxide electrodes. Nat. Mater. 2013, 12, 827–835. [CrossRef]
- Naylor, A.J.; Makkos, E.; Maibach, J.; Guerrini, N.; Sobkowiak, A.; Björklund, E.; Lozano, J.G.; Menon, A.S.; Younesi, R.; Roberts, M.R.; et al. Depth-dependent oxygen redox activity in lithium-rich layered oxide cathodes. J. Mater. Chem. A 2019, 7, 25355–25368. [CrossRef]
- Shimoda, K.; Minato, T.; Nakanishi, K.; Komatsu, H.; Matsunaga, T.; Tanida, H.; Arai, H.; Ukyo, Y.; Uchimoto, Y.; Ogumi, Z. Oxidation behaviour of lattice oxygen in Li-rich manganese-based layered oxide studied by hard X-ray photoelectron spectroscopy. J. Mater. Chem. A 2016, 4, 5909–5916. [CrossRef]
| Li1.2Mn0.6Ni0.2O2, C2/m, a = 4.954(6) Å, b = 8.563(3) Å, c = 5.030(6) Å , V = 201.4(2), β = 109.25(1)o, Rwp = 3.124% | ||||||
| Atom | Position | Occupancy | Uiso | Multiplicity | ||
| Li1 | 0 | 0.1607(11) | 0 | 0.3 | 0.029(3) | 4 |
| Mn1 | 0 | 0.1607(11) | 0 | 0.7 | 0.029(3) | 4 |
| Ni1 | 0 | 0.5 | 0 | 0.6 | 0.012(4) | 2 |
| Mn2 | 0 | 0.5 | 0 | 0.4 | 0.012(4) | 2 |
| Li3 | 0 | 0 | 0.5 | 1 | 0.035 | 2 |
| Li4 | 0 | 0.66602 | 0.5 | 1 | 0.035 | 4 |
| O1 | 0.238(4) | 0 | 0.257(3) | 1 | 0.013(2) | 4 |
| O2 | 0.232(3) | 0.346(1) | 0.213(1) | 1 | 0.013(1) | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




